Abstract
We aimed to investigate a sexually dimorphic role of calcitonin gene-related peptide (CGRP) in rodent models of pain. Based on findings in migraine where CGRP has a preferential pain-promoting effect in female rodents, we hypothesized that CGRP antagonists and antibodies would attenuate pain sensitization more efficaciously in female than male mice and rats. In hyperalgesic priming induced by activation of interleukin 6 signaling, CGRP receptor antagonists olcegepant and CGRP8-37 both given intrathecally, blocked, and reversed hyperalgesic priming only in females. A monoclonal antibody against CGRP, given systemically, blocked priming specifically in female rodents but failed to reverse it. In the spared nerve injury model, there was a transient effect of both CGRP antagonists, given intrathecally, on mechanical hypersensitivity in female mice only. Consistent with these findings, intrathecally applied CGRP caused a long-lasting, dose-dependent mechanical hypersensitivity in female mice but more transient effects in males. This CGRP-induced mechanical hypersensitivity was reversed by olcegepant and the KCC2 enhancer CLP257, suggesting a role for anionic plasticity in the dorsal horn in the pain-promoting effects of CGRP in females. In spinal dorsal horn slices, CGRP shifted GABAA reversal potentials to significantly more positive values, but, again, only in female mice. Therefore, CGRP may regulate KCC2 expression and/or activity downstream of CGRP receptors specifically in females. However, KCC2 hypofunction promotes mechanical pain hypersensitivity in both sexes because CLP257 alleviated hyperalgesic priming in male and female mice. We conclude that CGRP promotes pain plasticity in female rodents but has a limited impact in males.
SIGNIFICANCE STATEMENT The majority of patients impacted by chronic pain are women. Mechanistic studies in rodents are creating a clear picture that molecular events promoting chronic pain are different in male and female animals. We sought to build on evidence showing that CGRP is a more potent and efficacious promoter of headache in female than in male rodents. To test this, we used hyperalgesic priming and the spared nerve injury neuropathic pain models in mice. Our findings show a clear sex dimorphism wherein CGRP promotes pain in female but not male mice, likely via a centrally mediated mechanism of action. Our work suggests that CGRP receptor antagonists could be tested for efficacy in women for a broader variety of pain conditions.
Keywords: CGRP, dorsal horn, hyperalgesic priming, neuropathic pain, olcegepant, pain
Introduction
Pathways that mediate the development and maintenance of chronic pain are increasingly recognized as distinct in males and females (Mogil, 2020). Underlying mechanisms governing these sex differences are now becoming clear. In males, it has been demonstrated that microglia and macrophage activation contribute strongly to the development of chronic pain, because either blocking microglia activation or depleting animals of microglia can prevent the development of mechanical hypersensitivity after injury in mice (Sorge et al., 2015; Taves et al., 2016; Echeverry et al., 2017; Mapplebeck et al., 2018; Paige et al., 2018; Yu et al., 2020). In females, the depletion of microglia does not reverse nerve injury-induced hypersensitivity, and it appears that T cells may play a critical role in promoting chronic pain (Sorge et al., 2015), although there is also evidence that T cells promote pain resolution in both sexes (Krukowski et al., 2016; Laumet et al., 2019). Another female-specific pain-promoting mechanism is prolactin signaling, which sensitizes female nociceptors through a direct action on the prolactin receptor (Patil et al., 2013, 2019b; Paige et al., 2020). Two mechanisms could underlie this sex difference in responses to prolactin, a sexually dimorphic expression pattern for the prolactin receptor in subtypes of dorsal root ganglion (DRG) neurons (Patil et al., 2019a), and/or a female-specific translation of the prolactin receptor at central and peripheral terminals of nociceptors (Patil et al., 2019b; Paige et al., 2020).
One of the most striking sex differences in medicine is the far greater incidence of migraine headache in women than in men (Ashina et al., 2021). A number of new therapeutics for migraine have recently been approved. These new drugs target calcitonin gene-related peptide (CGRP) by sequestering the peptide with an antibody or blocking the receptor with a small-molecule antagonist or a function-blocking antibody (Dodick et al., 2014; Moreno-Ajona et al., 2020). Work aiming to better understand the mechanisms through which CGRP acts in migraine headache demonstrated a dramatic leftward shift in the dose-dependent effects of CGRP in promoting pain when applied to the dura of female mice (Avona et al., 2019). Other studies have also demonstrated a sex difference in the expression of CGRP receptor components in the trigeminal (TG) nucleus (Ji et al., 2019). However, the pain-promoting effects of CGRP are not limited to the trigeminal region. Previous experiments demonstrate that CGRP antiserum given intrathecally increases nociceptive thresholds in a rat model of arthritis (Kuraishi et al., 1988). Moreover, CGRP applied to the dorsal horn induces signaling that increases synaptic efficacy, suggesting a direct action on dorsal horn neurons (Sun et al., 2004). To our knowledge, potential sex differences in the effects of CGRP on the DRG or spinal dorsal horn have not been assessed.
Anionic plasticity is an important contributor to chronic pain (Kaila et al., 2014; Price and Prescott, 2015; Lorenzo et al., 2020). Changes in Cl– gradients in dorsal horn neurons can lead to decreased inhibitory efficacy enabling non-noxious stimuli to gain access to the ascending nociceptive pathway (Coull et al., 2003, 2005; Keller et al., 2007; Ferrini et al., 2013). This is an important cause of mechanical allodynia, a prominent feature of pain after injury. Decreased expression or function of the Cl– extrusion transporter KCC2 is the best understood mechanism for anionic plasticity in the dorsal horn (Coull et al., 2003; Miletic and Miletic, 2008; Asiedu et al., 2012; Ferrini et al., 2013; Li et al., 2016; Dedek et al., 2019; Ferrini et al., 2020; Locke et al., 2020; Lorenzo et al., 2020). While previous studies have demonstrated a key role for KCC2 in mechanical allodynia after injury in both male and female rodents (Mapplebeck et al., 2019), the brain-derived neurotrophic factor (BDNF) microglia signaling mechanism promoting neuropathic pain is engaged only in male mice (Sorge et al., 2015). BDNF promotes hyperalgesic priming in the body only in male mice (Moy et al., 2019) but regulates priming in the cephalic region in both sexes (Burgos-Vega et al., 2016). Given this literature, a goal of our work was to determine whether CGRP may influence anionic plasticity in the spinal dorsal horn in a sex-specific fashion.
We show that two structurally distinct CGRP antagonists block and reverse the development of mechanical hypersensitivity specifically in females in hyperalgesic priming, incision, and spared nerve injury (SNI)-induced neuropathic pain. Likewise, CGRP monoclonal antibodies (mAbs) block the establishment of hyperalgesic priming in female but not male rats and mice. Intrathecal CGRP causes increased and temporally prolonged hindpaw hypersensitivity in female mice when compared with male mice. This effect is reversed by intrathecal CLP257, a KCC2 activator (Gagnon et al., 2013), suggesting a link between CGRP signaling and KCC2 in the dorsal horn. In direct support of this hypothesis, CGRP applied to the spinal cord causes a depolarization of the GABAA receptor reversal potential (EGABA) in female but not male mice. This effect appears to be linked to a change in KCC2 function, but not trafficking. Our findings point to a previously unexplored central role of CGRP in promoting pain in female rodents.
Materials and Methods
Animals
All animal procedures were approved by the Institutional Animal Care and Use Committee at the University of Texas at Dallas, the Canadian Council on Animal Care, and the Committee for Animal Protection of Université Laval (CPAUL). Both male and female animals were used in all experiments unless otherwise noted. Experimenters were blinded to each experimental group for all experiments. Animals used per group are noted in each figure. For mouse behavioral experiments, all animals were bred in the Animal Research Facility at the University of Texas at Dallas and were 7–12 weeks old at the time of the experiment. Each group was either Swiss Webster or C57BL/6. Electrophysiological experiments were performed in C57BL/6NCrL male and female mice housed under the same conditions in the animal facility at the CERVO Brain Research Center. Animals were housed in same-sex groups of two to four on a 12 h light/dark cycle, and food and water were available ad libitum. Mice were assigned to experimental groups using a random number generator.
For rat experiments, Sprague Dawley rats were purchased from Taconic to arrive weighing ∼250 g each. Once rats arrived in the facility, they were habituated for a minimum of 5 d. Before experiments, animals were handled for a minimum of 15 min/d over 3 d. Animals were housed in same-sex groups of two on a 12 h light/dark cycle with access to food and water ad libitum. All rats were assigned to their experimental group using a random number generator, with no more than one animal per experimental group in each housing cage. Experimenters were blinded to treatment groups for all behavioral experiments.
Drugs
Recombinant soluble human interleukin 6 receptor (IL-6r) and recombinant human IL-6 were obtained from R&D Systems. We have previously shown that these IL-6 signaling components produce equivalent effects in hyperalgesic priming (Paige et al., 2018). Prostaglandin E2 (PGE2) was obtained from Cayman Chemical. Rat ∞-CGRP8-37 and rat α-CGRP were obtained from Bachem or Tocris Bioscience. CLP257 was ordered from Tocris Bioscience. IL-6 and IL-6r stock solutions were made in sterile 1× PBS and did not undergo multiple freeze/thaw cycles. PGE2 stock solution was made in 100% ethanol. Rat α-CGRP8-37 and rat α-CGRP were made in sterile filtered 1× PBS for in vivo experiments and in double distilled H2O for electrophysiological recordings. Monoclonal mAbs were shipped to University of Texas at Dallas from Alder BioPharmaceuticals (which has been acquired by Lundbeck) at the working concentrations for injection. The vehicle for both the CGRP mAb and control mAb was 25 mm histidine and 250 mm sorbitol at pH 6.0. All drugs were diluted to the final concentration in sterile filtered 0.9% saline and kept on ice until immediately before the injections.
von Frey testing
Before testing, animals were placed in an acrylic box with mesh flooring and allowed to acclimate for a minimum of 1 h on the day of testing. Baseline paw withdrawal thresholds were determined before any experimental work. During experiments, if time points were earlier than 1 h post-treatment injection, animals were allowed to habituate for 1 h, injections were given, and animals were placed back into the acrylic boxes. The paw withdrawal threshold was then determined using calibrated von Frey filaments using the up-down method (Chaplan et al., 1994).
Hyperalgesic priming
To establish hyperalgesic priming, 0.1 ng of IL-6 or IL-6r was injected intraplantarly into the left hindpaw, as noted for each experiment. Paw withdrawal threshold was then measured at 3, 24, and 72 h, and then 7 d or until mice returned to their baseline mechanical withdrawal threshold. For experiments in rats, animals were tested every other day after the 72 h time point until they had returned to baseline. Animals were then injected intraplantarly with 100 ng of PGE2, and the mechanical paw withdrawal threshold was measured at 3 and 24 h after injection.
SNI
Animals were anesthetized using 4% isoflurane gas and kept on a heating pad for the entire length of surgery. A small incision was made in the left leg, and the tibial and common peroneal branches of the sciatic nerve were ligated and cut (Decosterd and Woolf, 2000). The sural nerve was left intact. The incision was closed using two staples, and animals were given a subcutaneous injection of 1 mg/ml gentamicin. Mice were returned to their home cage and were tested at 21 d postsurgery to determine whether mechanical hypersensitivity had developed. Drug testing was performed after animals were confirmed to show mechanical hypersensitivity in the affected paw.
Brennan incision model
Mice were anesthetized using 4% isoflurane gas. An incision was made using a scalpel through the skin and underlying fascia of the left hindpaw. Two sutures were used to close the incision, and animals were allowed to recover in their home cages for 24 h before any behavioral testing was performed (Banik et al., 2006).
Intrathecal injections
Animals were anesthetized using isoflurane gas—4% for induction, 1.5% for maintenance anesthesia. Injections were performed as described by Hylden and Wilcox (1980). A total volume of 5 μl was injected for each drug using a 50 μl Hamilton syringe with an attached 0.5 inch, 30 gauge needle. Animals were allowed to recover in their home cage for a minimum of 10 min before any behavioral testing was performed.
Immunohistochemistry analysis
Tissue preparation and immunohistochemistry.
Mice were anesthetized with xylazine (12 mg/kg) and ketamine (80 mg/kg) administered intraperitoneally. Animals were then transcardially perfused with 10% PFA in 0.1 m PB at a pH of 7.4. Spinal cords were dissected and postfixed in 4% PFA for 2 h at room temperature (RT). Fixed spinal cords were then moved to a 25% sucrose suspension at 4°C for a minimum of 24 h. These spinal cords were then stored in an antifreeze solution at −20°C until processed for immunohistochemistry.
Mice spinal cord explants were obtained by laminectomy from ketamine/xylazine anesthetized C57BL/6N male and female mice. The whole spinal cord was immersed in ice-cold sucrose-based artificial CSF (S-ACSF), and the lumbar enlargement was isolated and divided in two pieces (rostral and caudal). Each section was left to recover in ACSF at 34°C for 30 min. Consecutively, the two lumbar pieces from each animal were transferred separately to RT ACSF supplemented with 50 nm CGRP or vehicle. Rostral–caudal fractions were randomly assigned to the treatments to prevent differences arising from spinal segment variability. After 3 h, spinal cords were fixed in 4% PFA for 20 h at 4°C. Fixed spinal cords were moved to 25% sucrose solution at 4°C for a minimum of 24 h.
The fixed spinal cord tissue was sectioned transversally using a vibratome (model VT1200S, Leica) in 50-μm-thick slices. Sections were permeabilized in PBS, pH 7.4, with 0.2% Triton X-100 (PBST) for 10 min; washed twice in PBS; and incubated overnight at 4°C in primary anti-KCC2 raised in rabbit (1:1000; catalog #07–432, Millipore), anti-CGRP raised in mouse (1:5000; catalog #C7113, Sigma-Aldrich), and anti-NeuN raised in chicken (1:1000; catalog #6B9155, EMD Millipore Sigma) antibodies diluted in PBST containing 10% normal goat serum. After washing in PBS, the sections were incubated for 2 h at RT in a solution containing a mixture of goat-Cy3 anti-rabbit (1:500; catalog #111–165–144, Jackson ImmunoResearch), goat anti-chicken Alexa Fluor 647 (1:500; catalog #A-21449, Thermo Fisher Scientific), goat anti-mouse Alexa Fljor 405 (1:500; catalog #A-31553, Thermo Fisher Scientific), and IB4 conjugated with Alexa Fluor 488 (1:500; catalog #I21411, Thermo Fisher Scientific) diluted in PBST, pH 7.4, containing 10% normal goat serum. Upon completion of this step, sections were washed three times with PBS, and then were mounted on Fisherbrand Superfrost Plus glass slides (catalog #10149870, Thermo Fisher Scientific) and coverslipped (catalog #12–544-E, Thermo Fisher Scientific) using fluorescence mounting medium (catalog #S3023, Dako).
Confocal microscopy imaging.
Images were captured using a confocal microscope (model LSM 700, Zeiss). Appropriate filters were selected for the separate detection of Alexa Fluor 405, Alexa Fluor 488, Cy3, and Alexa Fluor 647, using a multitrack scanning method. Eight-bit images were taken with a 63×/1.4 oil-immersion objective lens. To ensure consistency among samples, all parameters of laser power, pinhole size, and image detection were kept unchanged between the image acquisitions of different samples. The chosen parameters were set so that the detection of the staining was maximal while avoiding pixel saturation. For the subsequent quantification, the channels corresponding to each staining were exported separately as TIFF files (grayscale). The channel corresponding to KCC2 staining was further analyzed using a custom-made MATLAB code.
Image analysis and KCC2 quantification
To calculate the intracellular and membrane KCC2 levels, a custom-made MATLAB code was developed for the analysis, as previously described (Dedek et al., 2019; Ferrini et al., 2020; Lorenzo et al., 2020). Briefly, the homemade MATLAB routines used for analyzing the membrane and intracellular KCC2 provide a profiling plot of the membranes of selected neurons within the region of interest (lamina I and lamina II based on the CGRP and IB4 staining, respectively). The peak of the plot defines the membrane (position 0). The values on the left-hand side of the position 0 on the x-axis stand for the extracellular space while the values on the right-hand side of the position 0, stand for the intracellular space. For the final plotting, to obtain one curve per experimental group, the KCC2 values per position per animal belonging in the same group were merged. To obtain the data for the membrane KCC2, we grouped the values at position 0 for each group.
Electrophysiology
Tissue preparation.
C57BL/6N mice were deeply anesthetized with ketamine/xylazine. After decapitation, the vertebral column was swiftly removed and immersed in ice-cold oxygenated (95% O2, 5% CO2) S-ACSF containing the following (in mm): 252 sucrose, 2.5 KCl, 2 MgCl2, 2 CaCl2, 1.25 NaH2PO4, 26 NaHCO3, 10 glucose, and 5 kynurenic acid. A laminectomy was performed in ice-cold S-ACSF to extract the spinal cord. The lumbar enlargement was isolated and parasagittal slices (300 µm thick) were obtained in the same solution with a Leica vibratome. Slices were allowed to recover for 30 min at 34°C in oxygenated ACSF containing the following (in mm): 126 NaCl, 2.5 KCl, 2 MgCl2, 2 CaCl2, 1.25 NaH2PO4, 26 NaHCO3, and 10 glucose. Slices were then moved to RT oxygenated ACSF supplemented with 50 nm rat CGRP (Tocris Bioscience) or vehicle and maintained in this solution for a minimum of 2 h.
Cl– extrusion measurements.
After incubation, slices were transferred to a recording chamber where they were continuously perfused (2–3 ml/min) with oxygenated ACSF containing 1 μm tetrodotoxin (Alomone Labs), 1 μm strychnine (Sigma-Aldrich), 10 μm 6-cyano-7-nitroquinoxaline-2,3-dione (Sigma-Aldrich), 40 μm aminophosphonovalerate (Tocris Bioscience), and 50 nm rat CGRP or vehicle. Neurons were identified using a 40× water-immersion objective. Neurons from laminae I and II were selected for the experiment, maintaining an approximate ratio of 1:2, respectively. Lamina I was identified as a narrow dark band of gray matter with a typical reticulated appearance in the edge of the dorsal white matter, and lamina II as a wider translucent band below lamina I.
Differences in KCC2 extrusion were indirectly measured by estimation of EGABA under a challenging intracellular Cl– load, as previously described (Lorenzo et al., 2020). Whole-cell patch-clamp recordings were performed with a MultiClamp 700B amplifier (Molecular Devices). Borosilicate pipettes (3–5 MΩ) were filled with an intracellular solution containing the following (in mm): 115 K-methylsulfate, 25 KCl, 2 MgCl2, 10 HEPES, 4 ATP-Na, and 0.4 GTP-Na, adjusted with KOH to a pH of 7.2. Only cells with a stable access resistance <20 MΩ and a resting potential more negative than −50 mV were included for the analysis. The GABAA receptor agonist muscimol was freshly dissolved in HEPES-buffered ACSF to a concentration of 500 μm and was briefly applied (30 ms, 10 psi) at increasing holding potentials (12.5 mV steps). Intervals of 30 s at a holding voltage of −60 mV were allowed between puffs. Data were analyzed using Clampfit 10.2 (Molecular Devices), and membrane potentials were corrected offline for liquid junction potential (8 mV). GABA I–V curves were obtained from an average of three protocol recordings, and EGABA was estimated as the x-axis intercept of the derived linear equation using Prism. Two different experimenters collected the data yielding to the same result. Experimenters were blinded to the animal sex.
Statistics
All statistical analysis was performed using Prism version 8.0, and all data are shown as the mean ± SEM. Differences between each group of animals in behavioral experiments was determined using a two-way ANOVA with Bonferroni's post hoc test and α = 0.05. Differences between slopes in dose–response curve comparisons were determined using an ANCOVA and α = 0.05. Differences in EGABA were determined using an unpaired t test after passing normality with a D'Agostino–Pearson test. A Wilcoxon signed-rank test was used for KCC2 quantification in spinal cord explants.
Results
Olcegepant attenuates mechanical hypersensitivity associated with hyperalgesic priming and SNI only in female mice
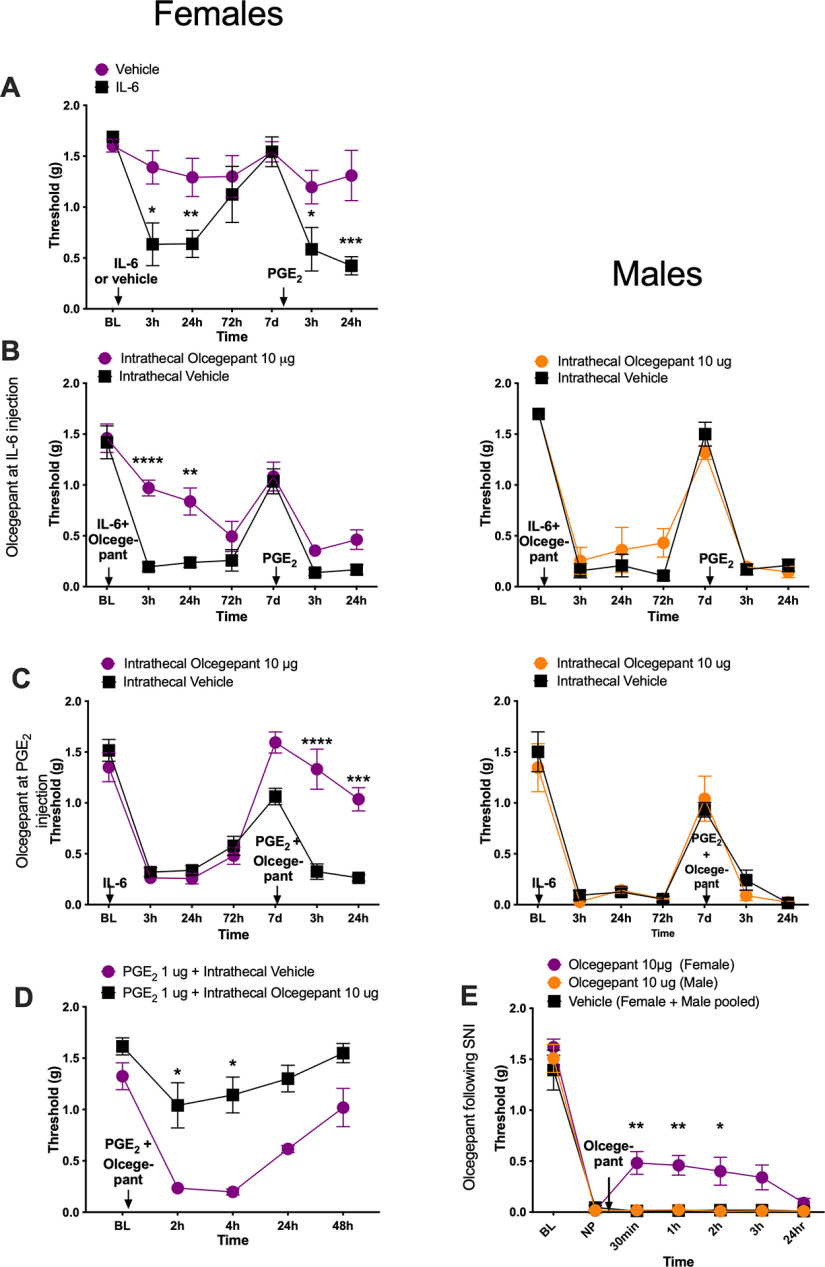
We first tested the effect of intrathecally injected CGRP receptor antagonists on IL-6r-induced hyperalgesic priming (Dina et al., 2008; Paige et al., 2018). In this testing paradigm, animals are first challenged with 0.1 ng of IL-6r injected intraplantarly, and then mechanical sensitivity is measured. Following the resolution of initial mechanical hypersensitivity, 100 ng of PGE2 is injected intraplantarly as a second stimulus. Animals that received a previous injection of IL-6r show enhanced responses to the subsequent PGE2 injection, demonstrating the presence of hyperalgesic priming. We have previously shown that 100 ng of PGE2 is a subthreshold stimulus in male mice (Paige et al., 2018). Here we confirmed that in female mice intraplantarly injected with vehicle there was no response to a 100 ng PGE2 injection. However, the intraplantarly IL-6r-injected female mice did show a mechanical hypersensitivity response to the 100 ng dose of PGE2 (Fig. 1A; time effect: F(3.167,28.51) = 8.21, p = 0.0004). Having determined an equivalent priming effect in male and female mice, we then tested the effect of a CGRP receptor antagonist in this model. We administered an intrathecal injection of 10 μg of olcegepant, a CGRP receptor antagonist, or vehicle immediately before IL-6r injection to assess the effect of CGRP receptor blockade on the response to IL-6r and the development of hyperalgesic priming. Olcegepant attenuated the initial mechanical hypersensitivity in response to IL-6r injection in female mice but had no effect in male mice (Fig. 1B; female olcegepant effect: F(1,42) = 31.27, p < 0.0001; time effect: F(6,42) = 34.53, p < 0.0001). Olcegepant administered intrathecally immediately before IL-6r injection did not have any effect on the development of hyperalgesic priming in either male or female mice (Fig. 1B). Therefore, the blocking of spinal CGRP receptors at the time of the initial stimulus has an effect on acute hypersensitivity in female mice, but priming is not affected in either sex.
Figure 1.
Intrathecal administration of olcegepant reduces mechanical hypersensitivity specifically in female mice. All graphs display mechanical withdrawal threshold. A, Female mice received an intraplantar injection of IL-6r or vehicle followed by the injection of 100 ng of PGE2 after the initial hypersensitivity had resolved (vehicle, n = 6; IL-6r, n = 5). B, Mice received an intrathecal injection of 10 μg of olcegepant before 0.1 ng intraplantar injection of IL-6r. A second intraplantar injection of 100 ng of PGE2 was given after initial mechanical sensitivity to IL-6r had resolved (n = 4 mice/group). C, Animals received an intraplantar injection of 0.1 ng of IL-6r. After initial mechanical hypersensitivity had resolved, animals received an intrathecal injection of 10 μg of olcegepant and an intraplantar injection of 100 ng PGE2 (n = 4 mice/group). D, Female mice received an intraplantar injection of PGE2 (10 µg) followed directly by an intrathecal dose of vehicle or olcegepant (vehicle, n = 6; olcegepant, n = 5). E, Animals underwent an SNI surgery. Twenty-one days postinjury, animals received a single intrathecal injection of 10 μg of olcegepant (males, n = 3; females, n = 4) or vehicle (pooled males and females, n = 5). Differences between groups were measured using a two-way ANOVA with Bonferroni's post hoc test: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
In a separate set of animals, we tested the ability of 10 μg of olcegepant to reverse established hyperalgesic priming. An injection of 0.1 ng of IL-6r was administered intraplantarly, and mechanical hypersensitivity was measured (Fig. 1C). After the initial hypersensitivity was resolved following IL-6r injection, animals received 10 μg of either olcegepant or vehicle intrathecally immediately before receiving an intraplantar injection of 100 ng of PGE2. Olcegepant reversed hyperalgesic priming in female mice but had no effect in male mice (Fig. 1C; female olcegepant effect: F(1,42) = 27.77, p < 0.0001; time effect: F(6,42) = 45.44, p < 0.0001). We also used a higher dose of PGE2 (1 µg), which causes mechanical hypersensitivity without a priming stimulus in female mice (Tavares-Ferreira et al., 2022). This higher dose of intraplantarly injected PGE2 caused mechanical hypersensitivity in female mice, and this effect was completely reversed by intrathecal treatment of 10 µg of olcegepant given just after the PGE2 injection (Fig. 1D; olcegepant effect: F(1,9) = 48.79, p < 0.0001; time effect: F(2.68,24.1) = 18.6, p < 0.0001).
We then tested the impact of intrathecal olcegepant in the SNI model of neuropathic pain in mice. Following the establishment of neuropathic pain, animals were given a single intrathecal injection of 10 μg of olcegepant or vehicle. In female mice, olcegepant reduced mechanical hypersensitivity at 30 min, 1 h, and 2 h following injection, but the drug had no effect in male mice (Fig. 1E; olcegepant effect: F(2,63) = 25.26, p < 0.0001; time effect: F(6,63) = 123.6, p < 0.0001). These experiments demonstrate a female-specific effect of olcegepant on mechanical hypersensitivity in hyperalgesic priming and neuropathic pain models.
CGRP8–37 attenuates hyperalgesic priming, postsurgical pain, and neuropathic pain only in female mice
CGRP8-37 is a peptide antagonist of the CGRP receptor (Chiba et al., 1989). We chose to test a second CGRP antagonist to reduce the probability that an off-target effect explains our observations of a sex-specific effect of blocking the CGRP receptor. Animals received an intrathecal injection of either 1 μg of CGRP8-37 or vehicle immediately before receiving an intraplantar injection of 0.1 ng of IL-6r. In female mice, CGRP8-37 reduced mechanical hypersensitivity induced by IL-6r and blocked the development of hyperalgesic priming as revealed by the lack of response to 100 ng of PGE2 injection (Fig. 2A; CGRP8-37 effect: F(1,126) = 88.54, p < 0.0001; time effect: F(8,126) = 12.30, p < 0.0001). There was no effect of CGRP8-37 in male mice (Fig. 2A). In our next experiment, 0.1 ng of IL-6r was injected intraplantarly to establish priming. When the initial hypersensitivity had resolved, 1 μg of CGRP8-37 was injected intrathecally immediately before intraplantar injection of 100 ng of PGE2. Again, in female mice CGRP8-37 was able to reverse established hyperalgesic priming but had no impact on male mice (Fig. 2B; CGRP8-37 effect in females: F(1,126) = 41.25, p < 0.0001; time effect in females: F(8,126) = 45.44, p <0.0001).
Figure 2.
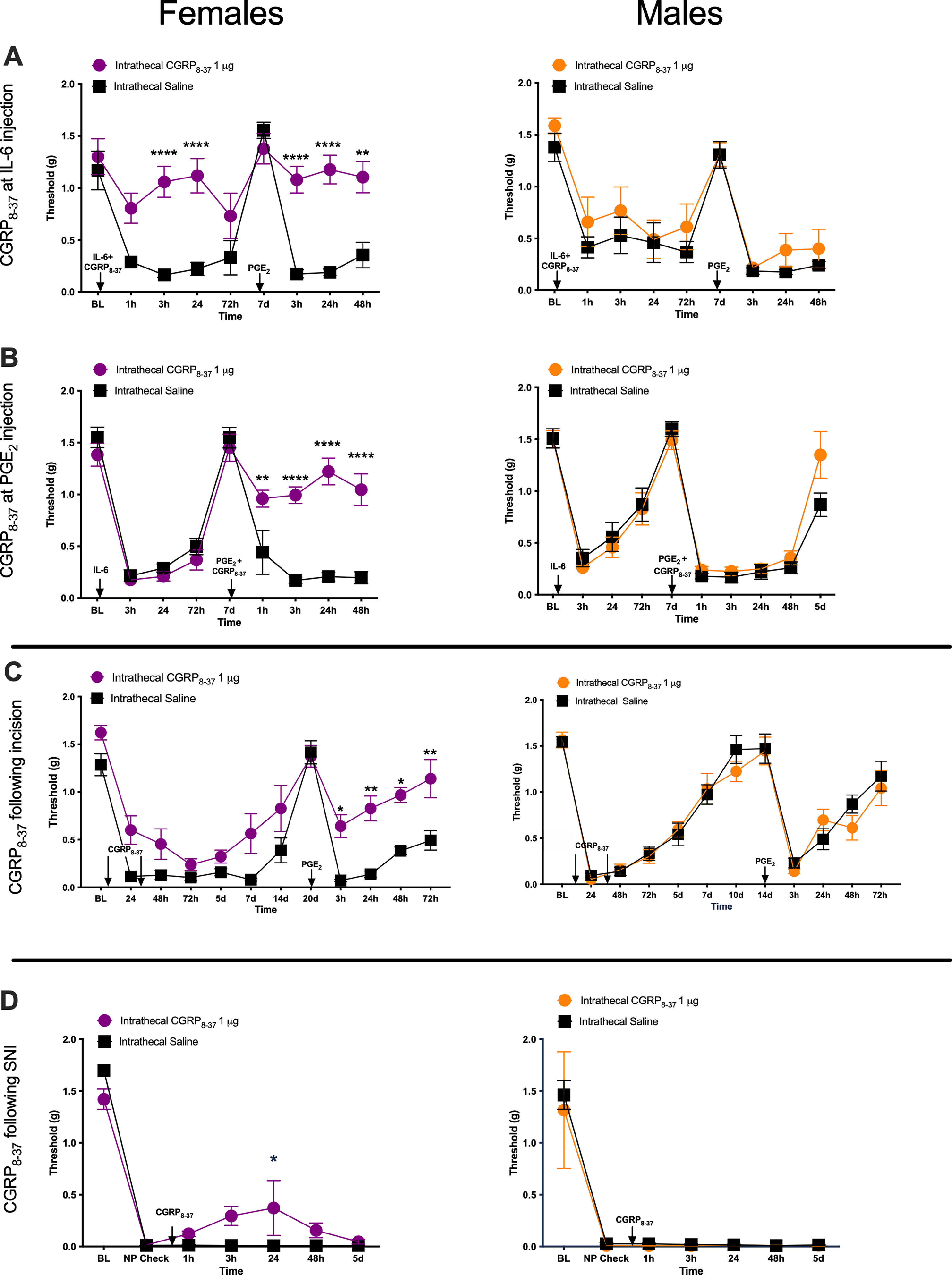
Intrathecal administration of CGRP8-37 reduces mechanical hypersensitivity specifically in female mice in three pain models. A, Animals received a single intrathecal injection of 1 μg of CGRP8-37 immediately before an intraplantar injection of 0.1 ng of IL-6r. Once initial hypersensitivity to intraplantar injection of IL-6r had resolved, animals received a second intraplantar injection of 100 ng of PGE2 (n = 8 mice/group). B, Animals received an intraplantar injection of 0.1 ng of IL-6r and then 7 d later an injection of 100 ng of PGE2. Immediately before the PGE2 injection, animals received an intrathecal injection of 1 μg of CGRP8-37 (n = 7–8 mice/group). C, Animals were given a hindpaw incision and an intrathecal injection of 1 μg of CGRP8-37 at the time of incision and then 24 h postincision. After the initial hypersensitivity following the incision had returned to baseline levels, animals received an intraplantar injection of 100 ng of PGE2 to test for hyperalgesic priming (n = 5–6 mice/group). D, Mice were given an SNI surgery and then a single intrathecal injection of 1 μg of CGRP8-37 21 d after initial injury (males, n = 3 mice/group; females, n = 4 mice/group). NP, Neuropathic pain. Differences between groups were measured using a two-way ANOVA with Bonferroni's post hoc test: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
We then tested to see whether the female-specific effects of a CGRP antagonist would generalize to hyperalgesic priming established using a single hindpaw incision instead of an IL-6r injection. Animals were given a single incision in the hindpaw and received two sutures. At the time of surgery, animals received a first intrathecal injection of 1 μg of CGRP8-37 or vehicle, and then a second injection of 1 μg of CGRP8-37 24 h postsurgery. We gave two drug treatments in this incision-induced priming paradigm because our previous studies indicated that two treatments are necessary to block hyperalgesic priming in this model (Tillu et al., 2012). This is consistent with the duration of ongoing activity that is induced in nociceptors by the incision (Xu and Brennan, 2010). Intrathecal CGRP8-37 administration did not have a significant impact on mechanical hypersensitivity following the incision in either sex, but the development of hyperalgesic priming was partially attenuated in female but not male mice (Fig. 2C; CGRP8-37 effect in females: F(1,108) = 64.40, p < 0.0001; time effect in females: F(11,108) = 24.54, p < 0.0001).
We then tested the effect of intrathecal CGRP8-37 in the SNI model. Following the establishment of neuropathic pain, on day 21 after injury, animals received a single intrathecal injection of 1 μg of CGRP8-37 or vehicle. In female mice, CGRP8-37 had a small, but significant, effect on mechanical hypersensitivity at 24 h postinjection, but there was no effect at any time point in male mice (Fig. 2C; CGRP8-37 effect in females: F(1,42) = 4.762, p = 0.035; time effect in females: F(6,42) = 93.18, p < 0.0001).
CGRP-sequestering mAb blocks IL-6-induced mechanical hypersensitivity and development of hyperalgesic priming in female mice and rats
CGRP receptor antagonists given intrathecally can act on the spinal dorsal horn or on the DRGs since the intrathecal space bathes both of these tissues. We sought to determine whether the sex-specific CGRP antagonist effect was mediated in the peripheral nervous system or CNS using CGRP sequestering mAbs that do not cross the blood–brain barrier (BBB; Edvinsson, 2015). The CGRP mAb was given intraperitoneally 24 h before human IL-6. These antibody experiments were performed in both mice and rats to determine whether there was a species-specific effect of the CGRP mAb. We chose to use IL-6 rather than IL-6r for these experiments because, while we have shown that IL-6 and IL-6r produce equivalent effects in hyperalgesic priming in mice (Paige et al., 2018), only IL-6 has been tested in rats.
In mice, animals were given an intraperitoneal injection of 20 mg/kg CGRP mAb, 20 mg/kg control mAb, or mAb vehicle (see Materials and Methods). Twenty-four hours post-intraperitoneal injections, animals then received intraplantar injections of 0.1 ng of either IL-6 or vehicle. Following the resolution of initial hypersensitivity, animals received a second injection of control or CGRP mAbs 24 h before a 100 ng intraplantar injection of PGE2. In female mice, the double CGRP mAb treatment blocked mechanical hypersensitivity following intraplantar injection of IL-6 and hyperalgesic priming (Fig. 3A; CGRP mAb effect: F(2,15) = 68.23, p < 0.0001; time effect: F(2.7,40.4) = 43.20, p < 0.0001). However, when CGRP mAb was given only before PGE2 injection, but not at the time of IL-6 injection, there was no effect on hyperalgesic priming (Fig. 3A). In males, there was no effect of CGRP mAbs (Fig. 3A).
Figure 3.
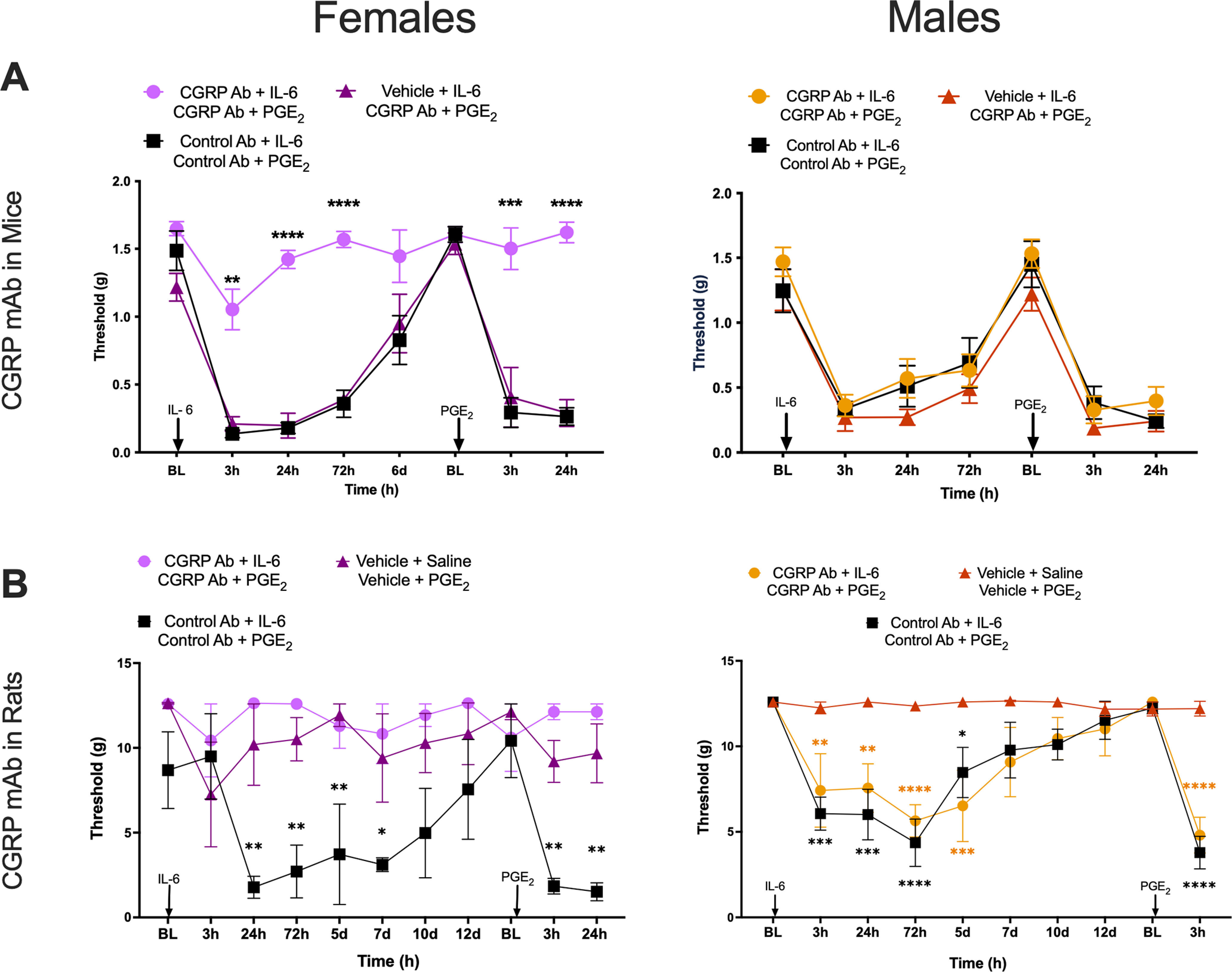
CGRP mAbs block IL-6-induced mechanical hypersensitivity and development of hyperalgesic priming in female mice and rats. A, Mice were given an intraperitoneal injection of 20 mg/kg CGRP mAb, 20 mg/kg control mAb, or vehicle 24 h before receiving an intraplantar injection of 0.1 ng of IL-6. After initial hypersensitivity had resolved, animals received an intraperitoneal injection of either CGRP mAb or control mAb at the doses described above 24 h before a 100 ng PGE2 intraplantar injection (n = 6 mice/group). Stars show significant differences versus control Ab. B, Rats received an intraperitoneal injection of 20 mg/kg CGRP mAb, 20 mg/kg control mAb, or vehicle 24 h before an intraplantar injection of 0.1 ng of IL-6 or saline, and, once mechanical hypersensitivity had resolved, animals received an intraperitoneal injection of the same drug or vehicle 24 h before intraplantar injection of 100 ng of PGE2 (n = 6 rats/group). Stars show significant differences versus vehicle treatment, color coded by group. Differences between groups were measured using a two-way ANOVA with Bonferroni's post hoc test: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
To eliminate the possibility of a mouse-specific effect, we tested the CGRP mAb in male and female rats using the same stimulus and hyperalgesic priming paradigm. Animals received an intraperitoneal injection of 20 mg/kg CGRP mAb, 20 mg/kg control mAb, or vehicle 24 h before intraplantar injection of 0.1 ng of IL-6. Following the resolution of mechanical hypersensitivity, animals received a second intraperitoneal injection of CGRP mAb, control mAb, or vehicle 24 h before an intraplantar injection of 100 ng of PGE2. CGRP mAb blocked IL-6-induced mechanical hypersensitivity in female rats in a manner similar to that observed in female mice (Fig. 3B; treatment effect: F(2,9) = 13.9, p = 0.0018; time effect: F(10,90) = 2.35, p = 0.016). There was no effect of CGRP mAb in males (treatment effect: F(2,9) = 14.0, p = 0.0017; time effect: F(9,81) = 13.34, p = 0.0001). Therefore, in female mice and rats, CGRP mAb treatment blocks IL-6-induced mechanical hypersensitivity and hyperalgesic priming. Because receptor antagonists were effective at both stages, these findings suggest that the initial effects of CGRP in these models may be peripherally mediated, while the later effects on the maintenance of hyperalgesic priming are potentially CNS driven.
Intrathecal CGRP causes prolonged mechanical hypersensitivity only in female mice
We next gave intrathecal CGRP to both male and female mice. Previous work has demonstrated that intrathecal CGRP in male mice and rats causes transient pain hypersensitivity, but it has not been directly compared with effects in females (Cridland and Henry, 1988, 1989; Rogoz et al., 2014; Yokai et al., 2016). In the TG system in rodents, CGRP-induced pain-promoting effects are far more potent and efficacious in female animals (Avona et al., 2019, 2021). Therefore, we tested increasing doses of α-CGRP (0.1, 0.3, and 1.0 nmol) given intrathecally to male and female mice. In male mice, these doses of intrathecal CGRP created transient hindpaw hypersensitivity that resolved quickly, while these same three doses had a greater magnitude and prolonged effect in female mice (Fig. 4A,B). Across a range of 5 CGRP doses (vehicle, and 0.01, 0.03, 0.1, 0.3, and 1 nmol) with mechanical hypersensitivity measured only at 60 min after intrathecal injection, CGRP showed increased potency (female EC50 = 0.0166 nmol; male EC50 = 0.0655 nmol) and efficacy (female Emax threshold = 0.13 g; male Emax threshold = 0.64 g) in female versus male mice (Fig. 4C). Differences between curve fits were determined using an ANCOVA test (p-value = 0.0013, F(2,7.931).
Figure 4.
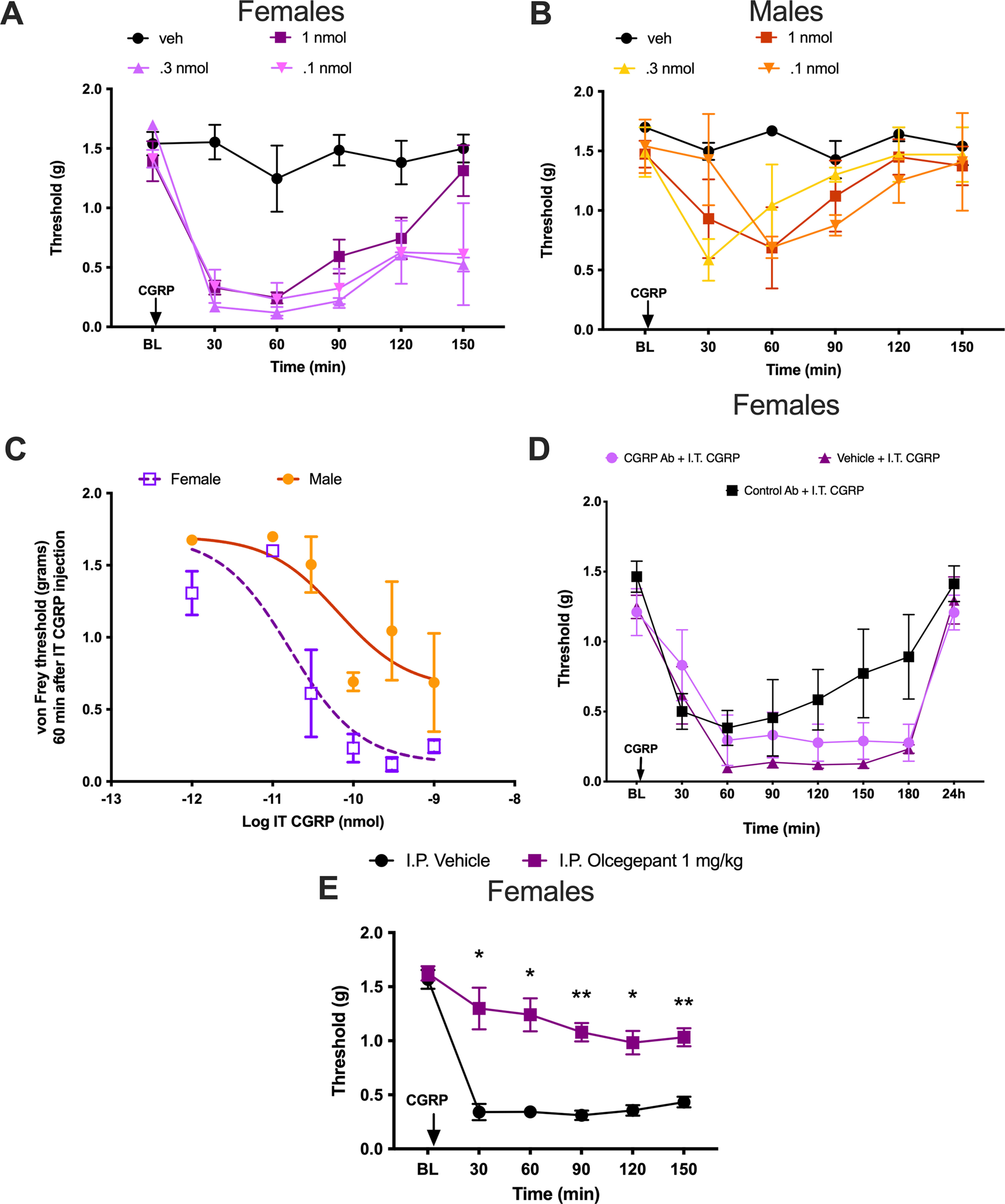
Intrathecal CGRP causes an increased response in female mice that is not blocked by systemic CGRP mAb but is blocked by systemic olcegepant. A, Female mice received an intrathecal injection of CGRP in half-log step increments of 0.1, 0.3, or 1 nmol rat α-CGRP (n = 2–4 mice/dose). B, Male mice received intrathecal injections of rat α-CGRP in the same doses as female mice (n = 2–4 mice/dose). C, Dose–response curve of male and female mice at 60 min post-intrathecal injection of CGRP. Six increasing doses of rat α-CGRP were administered to both males and females, as follows: 0.01, 0.03, 0.1, 0.3, and 1 nmol. Differences between slopes were determined using an ANCOVA: p-value = 0.0013 [F = 7.931 (df of the numerator = 2; df of the denominator = 39); n = 11 mice for female dose–response curve; n = 12 mice for male dose–response curve]. D, Female mice received an intraperitoneal injection of 20 mg/kg CGRP mAb, 20 mg/kg control mAb, or vehicle 24 h before intrathecal injection of 0.1 nmol rat α-CGRP, and mechanical hypersensitivity was measured following these intrathecal injections (n = 6 mice/group). E, Female mice received an intraperitoneal injection of 1 mg/kg olcegepant or vehicle 30 min before intrathecal injection of 0.1 nmol rat α-CGRP, and mechanical hypersensitivity was measured following these intrathecal injections (vehicle, n = 8; olcegepant, n = 5). Differences between groups were measured using a two-way ANOVA with Bonferroni's post hoc test: *p < 0.05, **p < 0.01. I.P., Intraperitoneal; I.T., intrathecal; veh, vehicle.
To test whether the effects of intrathecal CGRP were likely centrally or peripherally mediated, we gave female mice an intraperitoneal injection of 20 mg/kg CGRP mAb, 20 mg/kg control mAb, or vehicle injection 24 h before receiving an intrathecal injection of 0.1 nmol CGRP. The systemically administered CGRP mAb was not able to block the hindpaw hypersensitivity resulting from the intrathecal CGRP injection (Fig. 4D). We also assessed the effect of olcegepant in this testing paradigm. Unlike the monoclonal antibody, systemic pretreatment with 1 mg/kg olcegepant completely blocked the effect of intrathecal CGRP injection in female mice (Fig. 4E; treatment effect: F(1,11) = 73.10, p < 0.0001; time effect: F(3,33) = 44.0, p < 0.0001). We did not test the effect of olcegepant alone because multiple previous studies have shown a lack of effect of the drug on baseline pain responses in both male and female rodents (Michot et al., 2012; Munro et al., 2018; Christensen et al., 2019; Kopruszinski et al., 2021). These results suggest that CGRP has increased potency and efficacy in promoting mechanical hypersensitivity in female mice via a CNS-mediated mechanism of action.
CGRP depolarizes the GABAA reversal potential in female dorsal horn neurons
The EGABA for ionic currents is critical for effective inhibition (Doyon et al., 2011, 2016). In CNS neurons, the K+-Cl– cotransporter KCC2 is the primary contributor to setting intracellular Cl–, hence EGABA (Doyon et al., 2011). Previous work has demonstrated that decreased KCC2 expression and/or increased KCC2 internalization in dorsal horn neurons following peripheral nerve injury is a causative factor in mechanical hypersensitivity (Coull et al., 2003; Ferrini et al., 2013, 2020; Lorenzo et al., 2020). This mechanism is engaged in male and female rodents; however, there is a sex difference in the underlying mechanisms regulating KCC2. In males, this involves BDNF signaling (Sorge et al., 2015; Moy et al., 2019), but in females the mechanism is not clearly elucidated. Since our finding suggests that CGRP affects aspects of nociceptive behavior in females by acting on central neurons (Fig. 4D,E), we sought to determine whether CGRP signaling regulates KCC2 extrusion capacity in the spinal dorsal horn (laminae I and II) of female and male mice. Detection of differences in KCC2 activity requires challenging the transporter, so we measured EGABA in whole-cell configuration by imposing a Cl– load (29 mm Cl–) through the recording pipette (Ferrini et al., 2020) while recording from laminae I and II dorsal horn neurons. Interestingly, while there was no difference in EGABA between females and males at resting conditions, bath incubation of CGRP (50 ng/ml, >2 h) induced a significant depolarization of EGABA exclusively in females (female EGABA: control = −43.68 ± 1.06 mV; CGRP = −40.54 ± 0.37 mV; unpaired t test, t = 2.795, p = 0.011; male EGABA: control = −43.19 ± 0.93 mV; CGRP = −45.27 ± 1.13 mV; unpaired t test, t = 1.406, p = 0.17; Fig. 5A,B).
Figure 5.
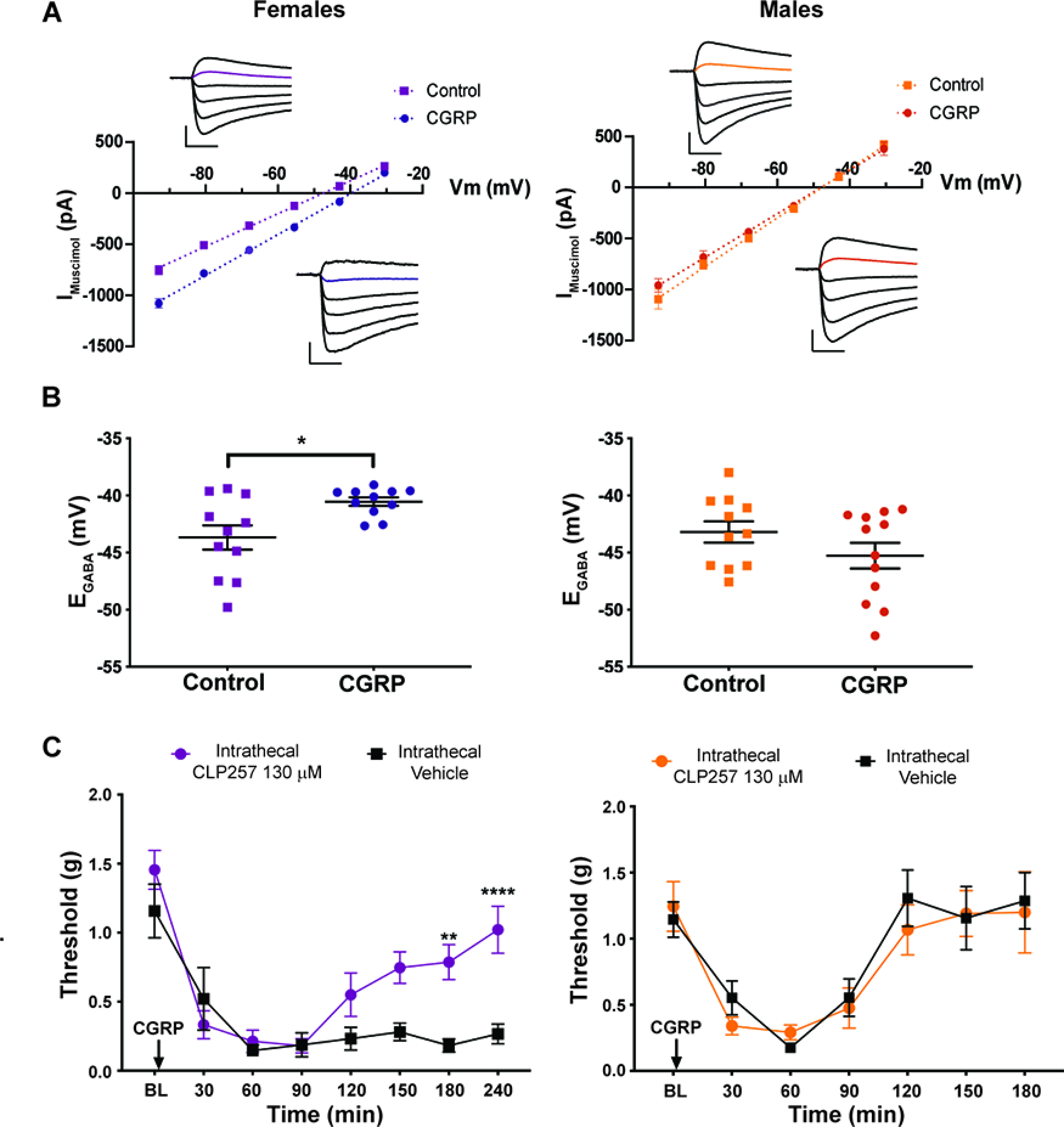
CGRP reduces Cl– extrusion capacity in spinal dorsal horn neurons of female mice. A, I–V plots of a representative neurons of each experimental group. Insets, Electrophysiological traces of the currents generated by 500 μmol muscimol puffs at different holding voltage steps (93–30.5 mV). Calibration: 300 pA, 100 ms. B, KCC2 activity in laminae I and II neurons was estimated from the GABAA I–V curve under 29 mm Cl– load. Incubation of spinal cord slices with 50 ng/ml CGRP for >2 h induced a depolarization of EGABA only in female tissue (n = 11–12 neurons/group). C, Male and female mice received an intrathecal injection of 130 μmol CLP257 immediately before intrathecal injection of 0.1 nmol CGRP. Mechanical hypersensitivity was measured using von Frey filament testing (n = 4 animals/group). Differences between groups were measured using a two-way ANOVA with Bonferroni's post hoc test: **p < 0.01, ***p <0.001, ****p < 0.0001.
We then used CLP257, a KCC2 enhancer (Gagnon et al., 2013), to determine whether augmenting KCC2 activity could reverse CGRP-evoked mechanical hypersensitivity in female mice. We administered intrathecal injection of 130 μmol CLP257 immediately before intrathecal injection of 0.1 nmol CGRP. CLP257 was able to decrease hindpaw hypersensitivity caused by intrathecal CGRP in female mice, but we did not observe an effect in male mice (Fig. 5C; female CLP257 effect: F(1,6) = 10.46, p = 0.018; time effect: F(7,42) = 20.36, p < 0.0001). Of note, hindpaw sensitivity caused by intrathecal CGRP returned to baseline levels in male mice by the same time point CLP257 began to have an effect in female mice. These findings link the effect of CGRP on KCC2 to mechanical hypersensitivity in female mice.
CLP257 inhibits hyperalgesic priming in male and female mice
To determine whether KCC2 potentiation could block or reverse hyperalgesic priming, we gave male and female mice intrathecal injection of CLP257 either before IL-6 injection or before PGE2 injection in previously primed mice. Intrathecal injection of CLP257 (130 μmol) immediately before intraplantar injection of 0.1 ng of IL-6 effectively blocked IL-6-induced mechanical hypersensitivity in both sexes (Fig. 6A; female CLP257 effect: F(1,6) = 42.21, p = 0.0006; time effect: F(6,36) = 17.56 p < 0.0001; male CLP257 effect: F(1,6) = 61.10, p = 0.0002; time effect: F(6,36) = 8.45, p < 0.0001). After mechanical hypersensitivity had resolved, animals received a second intraplantar injection, this time 100 ng of PGE2. In both males and females that previously received CLP257, the development of hyperalgesic priming was blocked (Fig. 6A).
Figure 6.
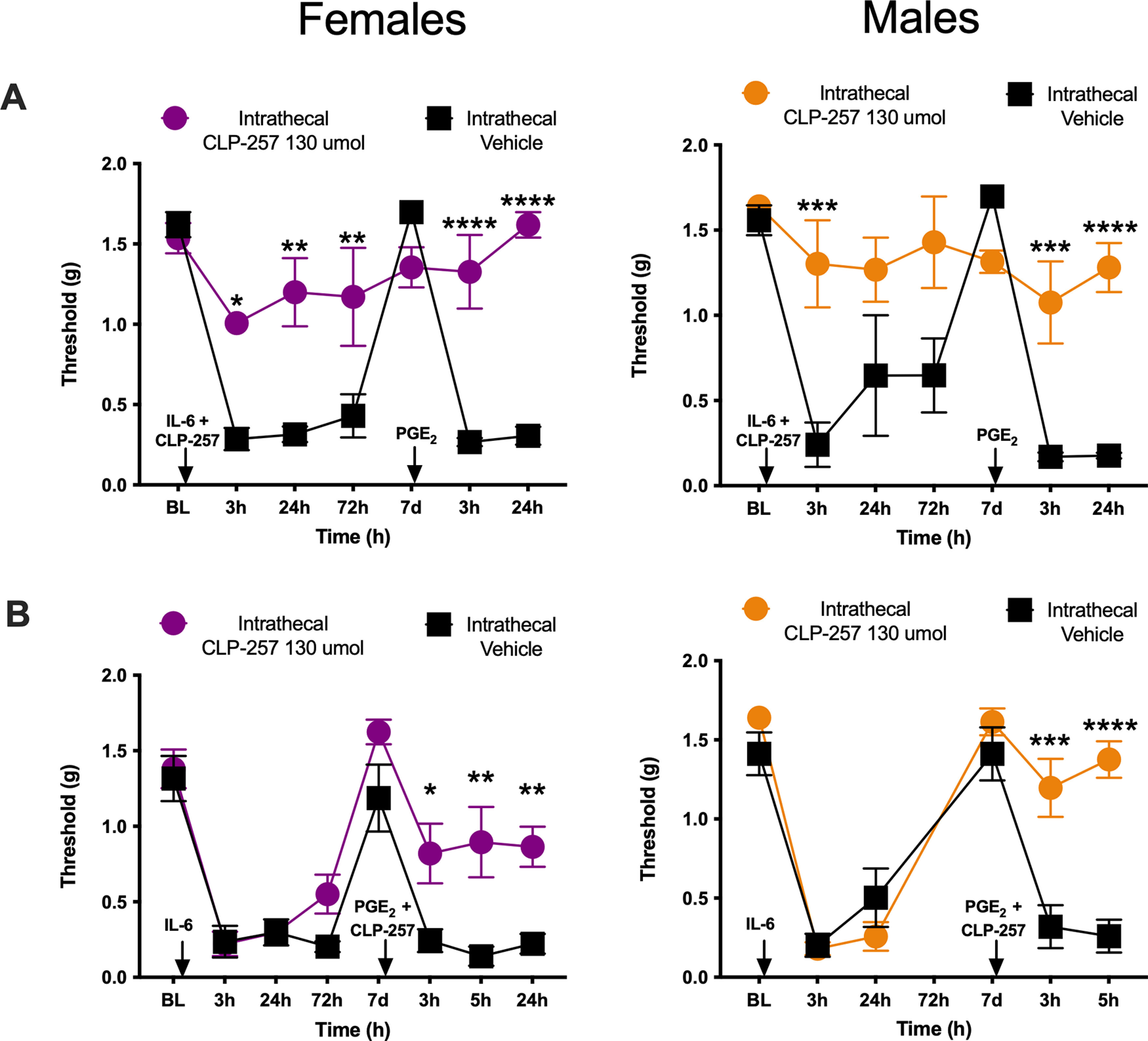
CLP257 blocks and reverses hyperalgesic priming in both male and female mice. A, Animals received an intrathecal injection of 130 μmol CLP257 immediately before an intraplantar injection of 0.1 ng of IL-6. After initial mechanical hypersensitivity to IL-6 had resolved, mice received an intraplantar injection of 100 ng of PGE2 (n = 4 mice/group). B, An intraplantar injection of 0.1 ng of IL-6 was given to animals and immediately before the intraplantar injection of 100 ng of PGE2 injection animals received an intrathecal injection of 130 μmol CLP257 (n = 4 mice/group). Differences between groups were measured using a two-way ANOVA with Bonferroni's post hoc test: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
In a separate set of experiments, priming was established using intraplantar injection of 0.1 ng of IL-6, and after animals had returned to baseline mechanical hypersensitivity, they received an intrathecal injection of CLP257, at the same dose, immediately before intraplantar injection of 100 ng of PGE2. CLP257 was able to reverse hyperalgesic priming in both male and female mice (Fig. 6B; female CLP257 effect: F(1,6) = 12.9, p = 0.012; time effect: F(7,42) = 31.84, p < 0.0001; male CLP257 effect: F(1,6) = 80.68, p < 0.0001; time effect: F(5,30) = 36.15, p < 0.0001). These results suggest that KCC2 dysregulation is an important factor in the development and maintenance of hyperalgesic priming in both mouse sexes. While our previous experiments point to CGRP as a potential causative factor in females, a distinct mechanism, such as BDNF (Moy et al., 2019), is potentially responsible for KCC2 regulation in male mice.
CGRP does not induce KCC2 internalization in female dorsal horn neurons
EGABA depolarization is usually associated with reduced membrane expression of KCC2 (Kaila et al., 2014; Ferrini et al., 2020). We therefore hypothesized that CGRP may cause KCC2 internalization in dorsal horn neurons. To test this, we gave intrathecal injections of 0.1 nmol CGRP or vehicle and killed animals 1 h later to remove the spinal cord. We then processed these spinal cords to examine KCC2 localization. Contrary to our hypothesis, we did not note any evidence of enhanced KCC2 internalization in response to CGRP treatment in either sex (Fig. 7A–F). To examine whether changes in KCC2 membrane expression may occur under conditions where CGRP application can be more precisely controlled, and at a time point when the difference between male and female is most apparent, we replicated the electrophysiological conditions at which we observed a significant shift in EGABA. No difference was found in KCC2 membrane expression in either female or male spinal cord explants after 3 h of incubation of 50 nm CGRP, indicating that CGRP did not induce KCC2 internalization in females (Fig. 8A–C; Wilcoxon signed-rank test: males: W = 12, p = 0.46; females: W = −10, p = 0.47). These results suggest that CGRP downstream mechanisms can alter intracellular Cl– and EGABA through mechanisms that do not involve apparent changes in KCC2 membrane localization. This observation is consistent with recent findings that, in males, BDNF-signaling per se, in the absence of NMDA signaling, causes a decrease in KCC2 function without internalization (Plasencia-Fernandez et al., 2019). The ability of CLP257 to enhance KCC2 function under these conditions is also consistent with previous findings that CLP257 can enhance KCC2-mediated transport even in the absence of enhanced membrane expression, as shown in an overexpression assay (oocytes) where membrane KCC2 was saturated (Gagnon et al., 2013).
Figure 7.
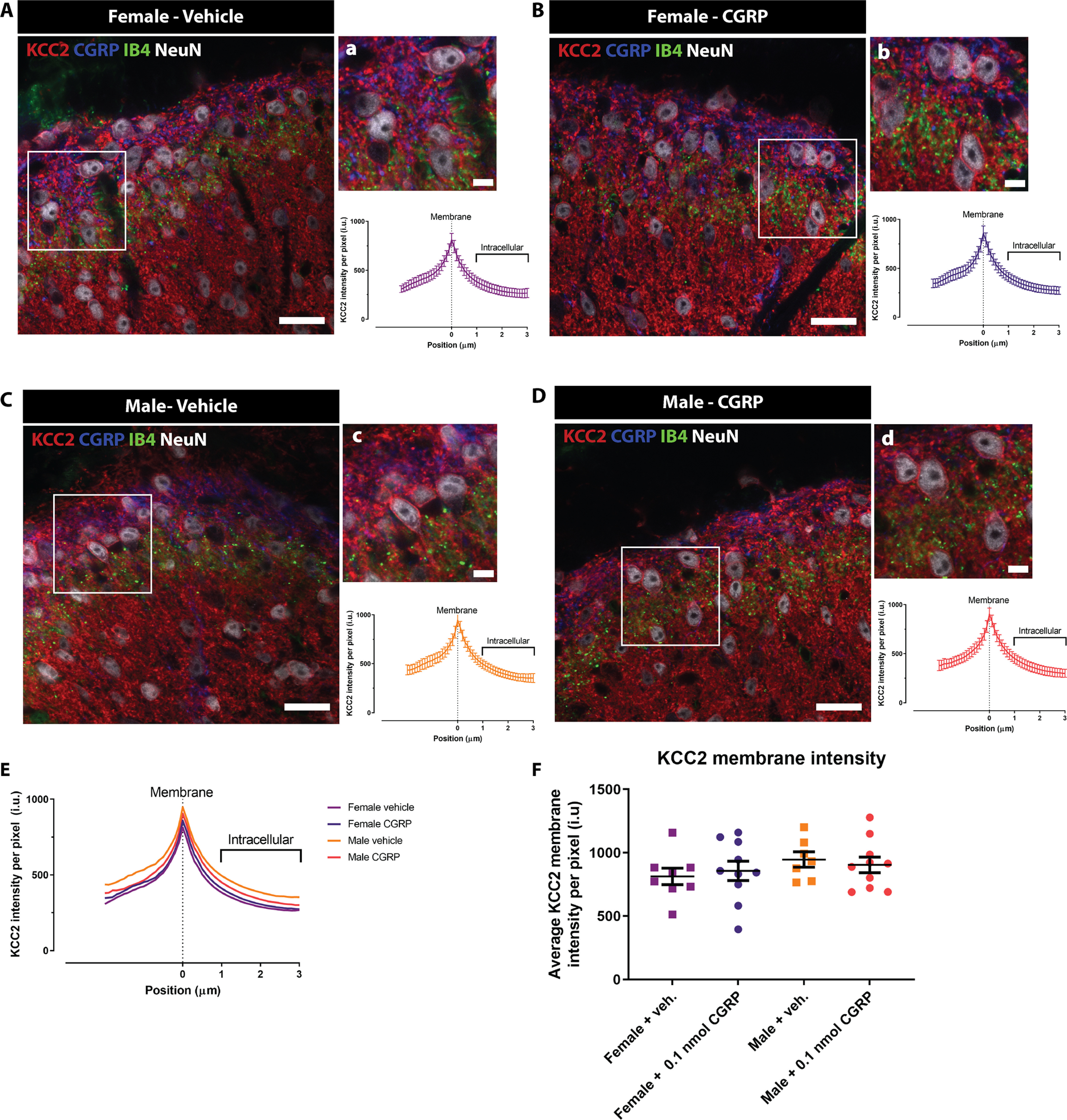
CGRP administration does not provoke KCC2 internalization in dorsal horn neurons in either female or male mice. A–D, Representative confocal images of the dorsal horn of spinal cord in female and male mice treated with either 0.1 nmol CGRP or vehicle showing CGRP, IB4, KCC2, and NeuN staining. Scale bars: A–D, 20 μm. a–d, High-magnification images of the areas highlighted in the white rectangle in A–D showing neurons expressing KCC2 (top) together with the average pixel KCC2 intensity plots versus distance to the membrane profile (bottom graphs). Scale bars: a–d, 5 μm. E, Average KCC2 intensity profiles from dorsal horn neurons of female mice treated with vehicle (violet line; n = 8 mice), female mice treated with 0.1 nmol CGRP (purple line; n = 10 mice), male mice treated with vehicle (orange line; n = 7 mice), and male mice treated with 0.1 nmol CGRP (red line; n = 10 mice). Results are presented as the mean per group for visualization purposes since the curves are duplications of the graphs in a–d. F, Membrane KCC2 intensity of the four experimental groups calculated at position zero. No significant differences were observed between vehicle or CGRP-treated female or male mice with Welch's ANOVA; n = 7-10 mice/group.
Figure 8.
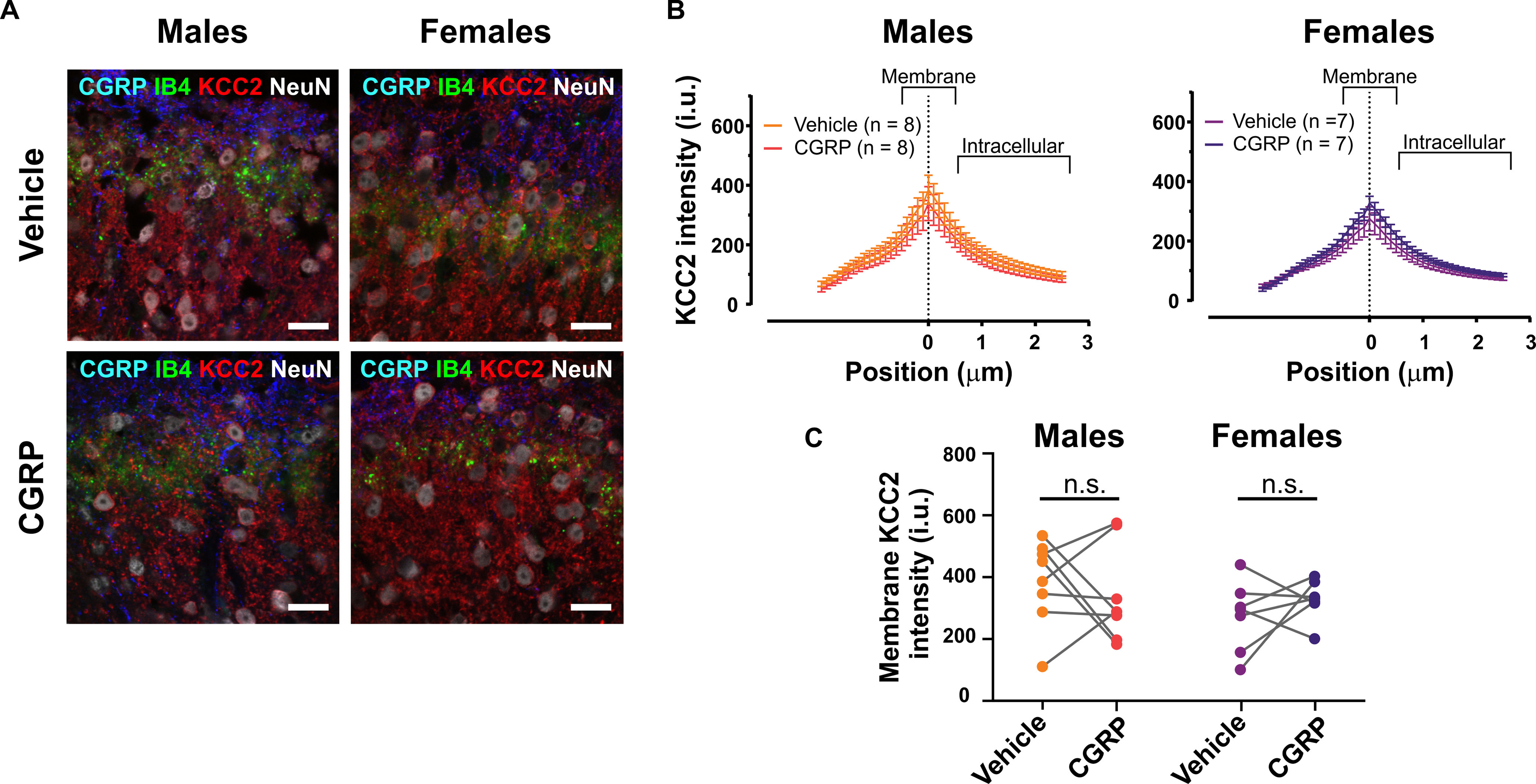
CGRP incubation for 3 h does not cause KCC2 internalization in superficial dorsal horn neurons in either female or male mice spinal cord explants. A, Representative confocal images of the dorsal horn of spinal cord explants from female and male mice incubated with either 50 nm CGRP or vehicle for 3 h, showing CGRP, IB4, KCC2, and NeuN staining. Scale bar, 20 μm. B, Average KCC2 intensity profiles from dorsal horn neurons of male (left; n = 8 mice) and female (right; n = 7 mice) mice explants incubated with vehicle (orange and violet lines) or 50 nm CGRP (red and purple lines). Results are presented as the mean per group. C, Membrane KCC2 intensity of the four experimental groups calculated at position zero. No significant differences were observed between vehicle- and CGRP-treated female or male mice spinal cord explants.
Discussion
Our findings support the conclusion that CGRP promotes mechanical sensitization in the DRG/spinal cord system, but primarily in female rodents. This adds to a growing body of evidence identifying signaling pathways that promote pain specifically in female animals in numerous models of chronic pain (Patil et al., 2013, 2019a,b; Sorge et al., 2015; Avona et al., 2019; Paige et al., 2020; Avona et al., 2021; Luo et al., 2021). We found that CGRP, acting both in the periphery and CNS, regulates early pain signaling in female mice and rats, but that more persistent effects, such as those involved in the maintenance of hyperalgesic priming, require CNS CGRP signaling. The finding that intrathecally applied CGRP evoked mechanical hypersensitivity was more potent and efficacious in females and was not blocked by a peripherally restricted CGRP-targeting mAb further supports this contention. Olcegepant was able to block this effect, suggesting that this drug likely enters the CNS under the experimental conditions used here. Collectively, our study raises the possibility that CGRP receptor-blocking therapies, in particular small-molecule antagonists that enter the CNS, may be effective for nonmigraine pain in women. This hypothesis can be tested in future clinical trials as the number of approved drugs targeting the CGRP system has expanded dramatically in recent years (Yuan et al., 2019).
We demonstrated that CGRP depolarizes EGABA in the neurons of the dorsal horn of the spinal cord specifically in female mice. EGABA is controlled by the expression and localization of KCC2 in dorsal horn neurons (Coull et al., 2003; Kaila et al., 2014; Price and Prescott, 2015; Doyon et al., 2016). Previous work has demonstrated some distinct mechanisms underlying KCC2 function in male and female mice and rats. While peripheral nerve injury causes KCC2 downregulation in both sexes (Mapplebeck et al., 2019), and KCC2 activators show efficacy in male and female rodents in neuropathic pain models, a microglial P2X4-BDNF-TrkB-mediated mechanism of KCC2 downregulation is engaged only in male rodents (Mapplebeck et al., 2018, 2019). In our experiments, while we saw a change in EGABA in female mice, we did not observe any sign of CGRP-regulated internalization of KCC2. Over the time course of our experiments, it is unlikely that transcriptional downregulation could explain the observed effect, so we did not examine KCC2 expression with quantitative PCR, but we cannot rule out such effects. It may be that a different mechanism downstream of CGRP receptors controls KCC2 function in female mice. Possibilities include phosphorylation-regulated transporter function (Friedel et al., 2015; Kahle and Delpire, 2016) or targeted degradation of KCC2 pools (Plasencia-Fernandez et al., 2019). These mechanisms should be tested in future experiments. Nevertheless, the female-specific effects of CGRP appear to involve KCC2 function because its acute administration in slices affects EGABA, and its systemic action can be reversed by CLP257. Interestingly, and in agreement with previous studies in neuropathic pain models (Mapplebeck et al., 2019), the KCC2 enhancer CLP257 could both block and reverse the establishment of hyperalgesic priming in male and female mice. This indicates that while different upstream mechanisms governing KCC2 function are likely engaged in male and female mice, the cotransporter appears to play an important role in hyperalgesic priming in both sexes.
We addressed the following two issues around the pharmacology of CGRP in hyperalgesic priming in female mice and rats using distinct CGRP antagonism approaches: (1) whether CGRP antagonism could prevent and/or reverse hyperalgesic priming; and (2) whether these effects were likely centrally or peripherally mediated. We found that the CGRP mAb was able to block only hyperalgesic priming from occurring, but intrathecal injection of both olcegepant and CGRP8-37 was able to reverse established priming in female animals when given near the time of the PGE2 injection. There was less efficacy of olcegepant versus CGRP8-37 in preventing the establishment of hyperalgesic priming when it was given at the same time as the priming stimulus IL-6r. This might be explained by shorter receptor occupancy for the small-molecule versus the peptide antagonist, but this would need to be assessed in additional studies. Nevertheless, systemically administered olcegepant blocked the effect of intrathecally administered CGRP in female mice while the CGRP antibody had no effect. This strongly supports the conclusion that centrally administered CGRP promotes pain preferentially in female mice, and this effect can only be blocked by antagonist approaches that enter the CNS. The penetration of olcegepant across the BBB is controversial (Tfelt-Hansen and Olesen, 2011). Some studies demonstrate a CNS effect of the compound (Sixt et al., 2009), but others have failed to make such an observation (Hirsch et al., 2013; Christensen et al., 2020, 2021; Ernstsen et al., 2021). It is notable that the studies reporting negative findings have all relied entirely on the use of male animals (Hirsch et al., 2013; Christensen et al., 2020, 2021; Ernstsen et al., 2021). Our results are consistent with a key role of centrally acting CGRP inducing mechanical sensitization in female, but not male, rodents. This is likely mediated by an action on CGRP receptors that are found on dorsal horn neurons. The consequence of acting on these receptors is a reduction in the Cl– extrusion capacity of these cells, reducing the efficacy of spinal inhibition. Reducing this pathologic effect in female rodents, and potentially in female chronic pain patients, would best be achieved with small-molecule receptor antagonists that can target these CNS receptors.
There are several limitations to our study. First, we acknowledge that the sample size for many of our mouse behavioral experiments is small. We designed our mouse behavioral experiments with a group size of eight per treatment with an equal split of male and female mice. We found a striking sex difference despite the small sample size that was consistent across experiments with different CGRP receptor antagonists. Second, we have not discovered a mechanism that explains the enhanced in vivo potency and efficacy for CGRP in female rodents. Future studies will need to explore how this occurs. A possibility could be sex hormone effects on receptor signaling coupling. Finally, we have not controlled for fluctuations in the sex hormone cycle in female rodents. Given that the time course of our hyperalgesic priming experiments temporally cover several cycles, it is unlikely that changes in hormone concentration over the cycle affect our observations.
It is estimated that 20% of the population in the United States suffers from chronic pain, and women make up the majority of the patients seeking treatment for their pain (Dahlhamer et al., 2018). A potential explanation for this phenomenon is that currently available analgesics are ineffective in female patients because they target molecular mechanisms that are specific to males (Mogil, 2020; Shansky and Murphy, 2021). This creates a strong case for developing pain drugs that could be used specifically in each sex. An example of this would be repurposing recently approved CGRP-targeting mAbs and/or CGRP receptor antagonists that have been approved for the treatment of migraine (Yuan et al., 2019), a pain state that disproportionately impacts female patients (Ashina et al., 2021), for the treatment of other pain states in women. Our work suggests that small-molecule CGRP receptor antagonists would be the better choice for such clinical trials because these drugs likely enter the CNS, while mAbs do not. Our work adds support to the hypothesis that mechanisms underlying chronic pain are at least partially distinct in males and females, and points to CGRP as a target that can be further explored for the development of female-specific analgesics.
Footnotes
This work was supported by National Institutes of Health (NIH)/National Institute of Neurological Disorders and Stroke (NINDS) Grants NS-102161 (to T.J.P. and A.N.A.), NS-065926 (to T.J.P.), NS-113457 (to C.P.), and NS-104200 (to A.N.A. and G.D.); Alder BioPharmaceuticals (T.J.P.); and Canadian Institutes of Health Research Grant FDN159906 and Canada Research Chair in Chronic Pain and Related Brain Disorders (to Y.D.K.).
A.L.F. and L.F.G.-M. were employees of Alder BioPharmaceuticals. The authors declare no other competing financial interests.
References
- Ashina M, Katsarava Z, Do TP, Buse DC, Pozo-Rosich P, Özge A, Krymchantowski AV, Lebedeva ER, Ravishankar K, Yu S, Sacco S, Ashina S, Younis S, Steiner TJ, Lipton RB (2021) Migraine: epidemiology and systems of care. Lancet 397:1485–1495. 10.1016/S0140-6736(20)32160-7 [DOI] [PubMed] [Google Scholar]
- Asiedu MN, Mejia G, Ossipov MK, Malan TP, Kaila K, Price TJ (2012) Modulation of spinal GABAergic analgesia by inhibition of chloride extrusion capacity in mice. J Pain 13:546–554. 10.1016/j.jpain.2012.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avona A, Burgos-Vega C, Burton MD, Akopian AN, Price TJ, Dussor G (2019) Dural calcitonin gene-related peptide produces female-specific responses in rodent migraine models. J Neurosci 39:4323–4331. 10.1523/JNEUROSCI.0364-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avona A, Mason BN, Burgos-Vega C, Hovhannisyan AH, Belugin SN, Mecklenburg J, Goffin V, Wajahat N, Price TJ, Akopian AN, Dussor G (2021) Meningeal CGRP-prolactin interaction evokes female-specific migraine behavior. Ann Neurol 89:1129–1144. 10.1002/ana.26070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banik RK, Woo YC, Park SS, Brennan TJ (2006) Strain and sex influence on pain sensitivity after plantar incision in the mouse. Anesthesiology 105:1246–1253. 10.1097/00000542-200612000-00025 [DOI] [PubMed] [Google Scholar]
- Burgos-Vega CC, Quigley LD, Avona A, Price T, Dussor G (2016) Dural stimulation in rats causes brain-derived neurotrophic factor-dependent priming to subthreshold stimuli including a migraine trigger. Pain 157:2722–2730. 10.1097/j.pain.0000000000000692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL (1994) Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53:55–63. 10.1016/0165-0270(94)90144-9 [DOI] [PubMed] [Google Scholar]
- Chiba T, Yamaguchi A, Yamatani T, Nakamura A, Morishita T, Inui T, Fukase M, Noda T, Fujita T (1989) Calcitonin gene-related peptide receptor antagonist human CGRP-(8-37). Am J Physiol 256:E331–E335. 10.1152/ajpendo.1989.256.2.E331 [DOI] [PubMed] [Google Scholar]
- Christensen SL, Petersen S, Kristensen DM, Olesen J, Munro G (2019) Targeting CGRP via receptor antagonism and antibody neutralisation in two distinct rodent models of migraine-like pain. Cephalalgia 39:1827–1837. 10.1177/0333102419861726 [DOI] [PubMed] [Google Scholar]
- Christensen SL, Ernstsen C, Olesen J, Kristensen DM (2020) No central action of CGRP antagonising drugs in the GTN mouse model of migraine. Cephalalgia 40:924–934. 10.1177/0333102420914913 [DOI] [PubMed] [Google Scholar]
- Christensen SL, Rasmussen RH, Ernstsen C, La Cour S, David A, Chaker J, Haanes KA, Christensen ST, Olesen J, Kristensen DM (2021) CGRP-dependent signalling pathways involved in mouse models of GTN- cilostazol- and levcromakalim-induced migraine. Cephalalgia 41:1413–1426. 10.1177/03331024211038884 [DOI] [PubMed] [Google Scholar]
- Coull JA, Boudreau D, Bachand K, Prescott SA, Nault F, Sík A, De Koninck P, De Koninck Y (2003) Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature 424:938–942. 10.1038/nature01868 [DOI] [PubMed] [Google Scholar]
- Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De Koninck Y (2005) BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature 438:1017–1021. 10.1038/nature04223 [DOI] [PubMed] [Google Scholar]
- Cridland RA, Henry JL (1988) Effects of intrathecal administration of neuropeptides on a spinal nociceptive reflex in the rat: VIP, galanin, CGRP, TRH, somatostatin and angiotensin II. Neuropeptides 11:23–32. 10.1016/0143-4179(88)90024-8 [DOI] [PubMed] [Google Scholar]
- Cridland RA, Henry JL (1989) Intrathecal administration of CGRP in the rat attenuates a facilitation of the tail flick reflex induced by either substance P or noxious cutaneous stimulation. Neurosci Lett 102:241–246. 10.1016/0304-3940(89)90085-2 [DOI] [PubMed] [Google Scholar]
- Dahlhamer J, Lucas J, Zelaya C, Nahin R, Mackey S, DeBar L, Kerns R, Von Korff M, Porter L, Helmick C (2018) Prevalence of chronic pain and high-impact chronic pain among adults - United States, 2016. MMWR Morb Mortal Wkly Rep 67:1001–1006. 10.15585/mmwr.mm6736a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decosterd I, Woolf CJ (2000) Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain 87:149–158. 10.1016/S0304-3959(00)00276-1 [DOI] [PubMed] [Google Scholar]
- Dedek A, Xu J, Kandegedara CM, Lorenzo LE, Godin AG, De Koninck Y, Lombroso PJ, Tsai EC, Hildebrand ME (2019) Loss of STEP61 couples disinhibition to N-methyl-d-aspartate receptor potentiation in rodent and human spinal pain processing. Brain 142:1535–1546. 10.1093/brain/awz105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dina OA, Green PG, Levine JD (2008) Role of interleukin-6 in chronic muscle hyperalgesic priming. Neuroscience 152:521–525. 10.1016/j.neuroscience.2008.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodick DW, Goadsby PJ, Silberstein SD, Lipton RB, Olesen J, Ashina M, Wilks K, Kudrow D, Kroll R, Kohrman B, Bargar R, Hirman J, Smith J (2014) Safety and efficacy of ALD403, an antibody to calcitonin gene-related peptide, for the prevention of frequent episodic migraine: a randomised, double-blind, placebo-controlled, exploratory phase 2 trial. Lancet Neurol 13:1100–1107. 10.1016/S1474-4422(14)70209-1 [DOI] [PubMed] [Google Scholar]
- Doyon N, Prescott SA, Castonguay A, Godin AG, Kröger H, De Koninck Y (2011) Efficacy of synaptic inhibition depends on multiple, dynamically interacting mechanisms implicated in chloride homeostasis. PLoS Comput Biol 7:e1002149. 10.1371/journal.pcbi.1002149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyon N, Vinay L, Prescott SA, De Koninck Y (2016) Chloride regulation: a dynamic equilibrium crucial for synaptic inhibition. Neuron 89:1157–1172. 10.1016/j.neuron.2016.02.030 [DOI] [PubMed] [Google Scholar]
- Echeverry S, Shi XQ, Yang M, Huang H, Wu Y, Lorenzo LE, Perez-Sanchez J, Bonin RP, De Koninck Y, Zhang J (2017) Spinal microglia are required for long-term maintenance of neuropathic pain. Pain 158:1792–1801. 10.1097/j.pain.0000000000000982 [DOI] [PubMed] [Google Scholar]
- Edvinsson L (2015) CGRP receptor antagonists and antibodies against CGRP and its receptor in migraine treatment. Br J Clin Pharmacol 80:193–199. 10.1111/bcp.12618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernstsen C, Christensen SL, Olesen J, Kristensen DM (2021) No additive effect of combining sumatriptan and olcegepant in the GTN mouse model of migraine. Cephalalgia 41:329–339. 10.1177/0333102420963857 [DOI] [PubMed] [Google Scholar]
- Ferrini F, Trang T, Mattioli TA, Laffray S, Del'Guidice T, Lorenzo LE, Castonguay A, Doyon N, Zhang W, Godin AG, Mohr D, Beggs S, Vandal K, Beaulieu JM, Cahill CM, Salter MW, De Koninck Y (2013) Morphine hyperalgesia gated through microglia-mediated disruption of neuronal Cl(-) homeostasis. Nat Neurosci 16:183–192. 10.1038/nn.3295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrini F, Perez-Sanchez J, Ferland S, Lorenzo LE, Godin AG, Plasencia-Fernandez I, Cottet M, Castonguay A, Wang F, Salio C, Doyon N, Merighi A, De Koninck Y (2020) Differential chloride homeostasis in the spinal dorsal horn locally shapes synaptic metaplasticity and modality-specific sensitization. Nat Commun 11:3935. 10.1038/s41467-020-17824-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedel P, Kahle KT, Zhang J, Hertz N, Pisella LI, Buhler E, Schaller F, Duan J, Khanna AR, Bishop PN, Shokat KM, Medina I (2015) WNK1-regulated inhibitory phosphorylation of the KCC2 cotransporter maintains the depolarizing action of GABA in immature neurons. Sci Signal 8:ra65. 10.1126/scisignal.aaa0354 [DOI] [PubMed] [Google Scholar]
- Gagnon M, Bergeron MJ, Lavertu G, Castonguay A, Tripathy S, Bonin RP, Perez-Sanchez J, Boudreau D, Wang B, Dumas L, Valade I, Bachand K, Jacob-Wagner M, Tardif C, Kianicka I, Isenring P, Attardo G, Coull JA, De Koninck Y (2013) Chloride extrusion enhancers as novel therapeutics for neurological diseases. Nat Med 19:1524–1528. 10.1038/nm.3356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch S, Corradini L, Just S, Arndt K, Doods H (2013) The CGRP receptor antagonist BIBN4096BS peripherally alleviates inflammatory pain in rats. Pain 154:700–707. 10.1016/j.pain.2013.01.002 [DOI] [PubMed] [Google Scholar]
- Hylden JL, Wilcox GL (1980) Intrathecal morphine in mice: a new technique. Eur J Pharmacol 67:313–316. 10.1016/0014-2999(80)90515-4 [DOI] [PubMed] [Google Scholar]
- Ji Y, Rizk A, Voulalas P, Aljohani H, Akerman S, Dussor G, Keller A, Masri R (2019) Sex differences in the expression of calcitonin gene-related peptide receptor components in the spinal trigeminal nucleus. Neurobiol Pain 6:100031. 10.1016/j.ynpai.2019.100031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahle KT, Delpire E (2016) Kinase-KCC2 coupling: Cl– rheostasis, disease susceptibility, therapeutic target. J Neurophysiol 115:8–18. 10.1152/jn.00865.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaila K, Price TJ, Payne JA, Puskarjov M, Voipio J (2014) Cation-chloride cotransporters in neuronal development, plasticity and disease. Nat Rev Neurosci 15:637–654. 10.1038/nrn3819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller AF, Beggs S, Salter MW, De Koninck Y (2007) Transformation of the output of spinal lamina I neurons after nerve injury and microglia stimulation underlying neuropathic pain. Mol Pain 3:27. 10.1186/1744-8069-3-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopruszinski CM, Navratilova E, Swiokla J, Dodick DW, Chessell IP, Porreca F (2021) A novel, injury-free rodent model of vulnerability for assessment of acute and preventive therapies reveals temporal contributions of CGRP-receptor activation in migraine-like pain. Cephalalgia 41:305–317. 10.1177/0333102420959794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krukowski K, Eijkelkamp N, Laumet G, Hack CE, Li Y, Dougherty PM, Heijnen CJ, Kavelaars A (2016) CD8+ T cells and endogenous IL-10 are required for resolution of chemotherapy-induced neuropathic pain. J Neurosci 36:11074–11083. 10.1523/JNEUROSCI.3708-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuraishi Y, Nanayama T, Ohno H, Minami M, Satoh M (1988) Antinociception induced in rats by intrathecal administration of antiserum against calcitonin gene-related peptide. Neurosci Lett 92:325–329. 10.1016/0304-3940(88)90611-8 [DOI] [PubMed] [Google Scholar]
- Laumet G, Edralin JD, Dantzer R, Heijnen CJ, Kavelaars A (2019) Cisplatin educates CD8+ T cells to prevent and resolve chemotherapy-induced peripheral neuropathy in mice. Pain 160:1459–1468. 10.1097/j.pain.0000000000001512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Chen SR, Chen H, Wen L, Hittelman WN, Xie JD, Pan HL (2016) Chloride homeostasis critically regulates synaptic NMDA receptor activity in neuropathic pain. Cell Rep 15:1376–1383. 10.1016/j.celrep.2016.04.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke S, Yousefpour N, Mannarino M, Xing S, Yashmin F, Bourassa V, Ribeiro-da-Silva A (2020) Peripheral and central nervous system alterations in a rat model of inflammatory arthritis. Pain 161:1483–1496. 10.1097/j.pain.0000000000001837 [DOI] [PubMed] [Google Scholar]
- Lorenzo LE, Godin AG, Ferrini F, Bachand K, Plasencia-Fernandez I, Labrecque S, Girard AA, Boudreau D, Kianicka I, Gagnon M, Doyon N, Ribeiro-da-Silva A, De Koninck Y (2020) Enhancing neuronal chloride extrusion rescues α2/α3 GABAA-mediated analgesia in neuropathic pain. Nat Commun 11:869. 10.1038/s41467-019-14154-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X, Chen O, Wang Z, Bang S, Ji J, Lee SH, Huh Y, Furutani K, He Q, Tao X, Ko MC, Bortsov A, Donnelly CR, Chen Y, Nackley A, Berta T, Ji RR (2021) IL-23/IL-17A/TRPV1 axis produces mechanical pain via macrophage-sensory neuron crosstalk in female mice. Neuron 109:2691–2706.e5. 10.1016/j.neuron.2021.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapplebeck JCS, Dalgarno R, Tu Y, Moriarty O, Beggs S, Kwok CHT, Halievski K, Assi S, Mogil JS, Trang T, Salter MW (2018) Microglial P2X4R-evoked pain hypersensitivity is sexually dimorphic in rats. Pain 159:1752–1763. 10.1097/j.pain.0000000000001265 [DOI] [PubMed] [Google Scholar]
- Mapplebeck JCS, Lorenzo LE, Lee KY, Gauthier C, Muley MM, De Koninck Y, Prescott SA, Salter MW (2019) Chloride dysregulation through downregulation of KCC2 mediates neuropathic pain in both sexes. Cell Rep 28:590–596.e4. 10.1016/j.celrep.2019.06.059 [DOI] [PubMed] [Google Scholar]
- Michot B, Bourgoin S, Viguier F, Hamon M, Kayser V (2012) Differential effects of calcitonin gene-related peptide receptor blockade by olcegepant on mechanical allodynia induced by ligation of the infraorbital nerve vs the sciatic nerve in the rat. Pain 153:1939–1948. 10.1016/j.pain.2012.06.009 [DOI] [PubMed] [Google Scholar]
- Miletic G, Miletic V (2008) Loose ligation of the sciatic nerve is associated with TrkB receptor-dependent decreases in KCC2 protein levels in the ipsilateral spinal dorsal horn. Pain 137:532–539. 10.1016/j.pain.2007.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogil JS (2020) Qualitative sex differences in pain processing: emerging evidence of a biased literature. Nat Rev Neurosci 21:353–365. 10.1038/s41583-020-0310-6 [DOI] [PubMed] [Google Scholar]
- Moreno-Ajona D, Pérez-Rodríguez A, Goadsby PJ (2020) Gepants, calcitonin-gene-related peptide receptor antagonists: what could be their role in migraine treatment? Curr Opin Neurol 33:309–315. 10.1097/WCO.0000000000000806 [DOI] [PubMed] [Google Scholar]
- Moy JK, Szabo-Pardi T, Tillu DV, Megat S, Pradhan G, Kume M, Asiedu MN, Burton MD, Dussor G, Price TJ (2019) Temporal and sex differences in the role of BDNF/TrkB signaling in hyperalgesic priming in mice and rats. Neurobiol Pain 5:100024. 10.1016/j.ynpai.2018.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro G, Petersen S, Jansen-Olesen I, Olesen J (2018) A unique inbred rat strain with sustained cephalic hypersensitivity as a model of chronic migraine-like pain. Sci Rep 8:1836. 10.1038/s41598-018-19901-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paige C, Maruthy GB, Mejia G, Dussor G, Price T (2018) Spinal inhibition of P2XR or p38 signaling disrupts hyperalgesic priming in male, but not female, mice. Neuroscience 385:133–142. 10.1016/j.neuroscience.2018.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paige C, Barba-Escobedo PA, Mecklenburg J, Patil M, Goffin V, Grattan DR, Dussor G, Akopian AN, Price TJ (2020) Neuroendocrine mechanisms governing sex differences in hyperalgesic priming involve prolactin receptor sensory neuron signaling. J Neurosci 40:7080–7090. 10.1523/JNEUROSCI.1499-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil MJ, Green DP, Henry MA, Akopian AN (2013) Sex-dependent roles of prolactin and prolactin receptor in postoperative pain and hyperalgesia in mice. Neuroscience 253:132–141. 10.1016/j.neuroscience.2013.08.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil M, Hovhannisyan AH, Wangzhou A, Mecklenburg J, Koek W, Goffin V, Grattan D, Boehm U, Dussor G, Price TJ, Akopian AN (2019a) Prolactin receptor expression in mouse dorsal root ganglia neuronal subtypes is sex-dependent. J Neuroendocrinol 31:e12759. 10.1111/jne.12759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil M, Belugin S, Mecklenburg J, Wangzhou A, Paige C, Barba-Escobedo PA, Boyd JT, Goffin V, Grattan D, Boehm U, Dussor G, Price TJ, Akopian AN (2019b) Prolactin regulates pain responses via a female-selective nociceptor-specific mechanism. iScience 20:449–465. 10.1016/j.isci.2019.09.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plasencia-Fernandez I, Bergeron MJ, De Koninck Y (2019) TrkB receptors engage different signaling cascades regulating respectively KCC2 function, trafficking and degradation. IBRO Rep 6:S262. 10.1016/j.ibror.2019.07.815 [DOI] [Google Scholar]
- Price TJ, Prescott SA (2015) Inhibitory regulation of the pain gate and how its failure causes pathological pain. Pain 156:789–792. 10.1097/j.pain.0000000000000139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogoz K, Andersen HH, Kullander K, Lagerström MC (2014) Glutamate, substance P, and calcitonin gene-related peptide cooperate in inflammation-induced heat hyperalgesia. Mol Pharmacol 85:322–334. 10.1124/mol.113.089532 [DOI] [PubMed] [Google Scholar]
- Shansky RM, Murphy AZ (2021) Considering sex as a biological variable will require a global shift in science culture. Nat Neurosci 24:457–464. 10.1038/s41593-021-00806-8 [DOI] [PubMed] [Google Scholar]
- Sixt ML, Messlinger K, Fischer MJ (2009) Calcitonin gene-related peptide receptor antagonist olcegepant acts in the spinal trigeminal nucleus. Brain 132:3134–3141. 10.1093/brain/awp168 [DOI] [PubMed] [Google Scholar]
- Sorge RE, Mapplebeck JCS, Rosen S, Beggs S, Taves S, Alexander JK, Martin LJ, Austin J-S, Sotocinal SG, Chen D, Yang M, Shi XQ, Huang H, Pillon NJ, Bilan PJ, Tu Y, Klip A, Ji R-R, Zhang J, Salter MW, et al. (2015) Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat Neurosci 18:1081–1083. 10.1038/nn.4053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun RQ, Tu YJ, Lawand NB, Yan JY, Lin Q, Willis WD (2004) Calcitonin gene-related peptide receptor activation produces PKA- and PKC-dependent mechanical hyperalgesia and central sensitization. J Neurophysiol 92:2859–2866. 10.1152/jn.00339.2004 [DOI] [PubMed] [Google Scholar]
- Tavares-Ferreira D, Ray PR, Sankaranarayanan I, Mejia GL, Wangzhou A, Shiers S, Uttarkar R, Megat S, Barragan-Iglesias P, Dussor G, Akopian AN, Price TJ (2022) Sex differences in nociceptor translatomes contribute to divergent prostaglandin signaling in male and female mice. Biol Psychiatry 91:129–140. 10.1016/j.biopsych.2020.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taves S, Berta T, Liu DL, Gan S, Chen G, Kim YH, Van de Ven T, Laufer S, Ji RR (2016) Spinal inhibition of p38 MAP kinase reduces inflammatory and neuropathic pain in male but not female mice: sex-dependent microglial signaling in the spinal cord. Brain Behav Immun 55:70–81. 10.1016/j.bbi.2015.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tfelt-Hansen P, Olesen J (2011) Possible site of action of CGRP antagonists in migraine. Cephalalgia 31:748–750. 10.1177/0333102411398403 [DOI] [PubMed] [Google Scholar]
- Tillu DV, Melemedjian OK, Asiedu MN, Qu N, De Felice M, Dussor G, Price TJ (2012) Resveratrol engages AMPK to attenuate ERK and mTOR signaling in sensory neurons and inhibits incision-induced acute and chronic pain. Mol Pain 8:5. 10.1186/1744-8069-8-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Brennan TJ (2010) Guarding pain and spontaneous activity of nociceptors after skin versus skin plus deep tissue incision. Anesthesiology 112:153–164. 10.1097/ALN.0b013e3181c2952e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokai M, Kurihara T, Miyata A (2016) Spinal astrocytic activation contributes to both induction and maintenance of pituitary adenylate cyclase-activating polypeptide type 1 receptor-induced long-lasting mechanical allodynia in mice. Mol Pain 12:174480691664638. 10.1177/1744806916646383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Liu H, Hamel KA, Morvan MG, Yu S, Leff J, Guan Z, Braz JM, Basbaum AI (2020) Dorsal root ganglion macrophages contribute to both the initiation and persistence of neuropathic pain. Nat Commun 11:264. 10.1038/s41467-019-13839-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H, Spare NM, Silberstein SD (2019) Targeting CGRP for the prevention of migraine and cluster headache: a narrative review. Headache 59 [Suppl 2]:20–32. 10.1111/head.13583 [DOI] [PubMed] [Google Scholar]