Abstract
Background
Non-antiviral therapeutic options are required for the treatment of hospitalised patients with COVID-19. CD24Fc is an immunomodulator with potential to reduce the exaggerated inflammatory response to tissue injuries. We aimed to evaluate the safety and efficacy of CD24Fc in hospitalised adults with COVID-19 receiving oxygen support.
Methods
We conducted a randomised, double-blind, placebo-controlled, phase 3 study at nine medical centres in the USA. Hospitalised patients (age ≥18 years) with confirmed SARS-CoV-2 infection who were receiving oxygen support and standard of care were randomly assigned (1:1) by site-stratified block randomisation to receive a single intravenous infusion of CD24Fc 480 mg or placebo. The study funder, investigators, and patients were masked to treatment group assignment. The primary endpoint was time to clinical improvement over 28 days, defined as time that elapsed between a baseline National Institute of Allergy and Infectious Diseases ordinal scale score of 2–4 and reaching a score of 5 or higher or hospital discharge. The prespecified primary interim analysis was done when 146 participants reached the time to clinical improvement endpoint. Efficacy was assessed in the intention-to-treat population. Safety was assessed in the as-treated population. This study is registered with ClinicalTrials.gov, NCT04317040.
Findings
Between April 24 and Sept 22, 2020, 243 hospitalised patients were assessed for eligibility and 234 were enrolled and randomly assigned to receive CD24Fc (n=116) or placebo (n=118). The prespecified interim analysis was done when 146 participants reached the time to clinical improvement endpoint among 197 randomised participants. In the interim analysis, the 28-day clinical improvement rate was 82% (81 of 99) for CD24Fc versus 66% (65 of 98) for placebo; median time to clinical improvement was 6·0 days (95% CI 5·0–8·0) in the CD24Fc group versus 10·0 days (7·0–15·0) in the placebo group (hazard ratio [HR] 1·61, 95% CI 1·16–2·23; log-rank p=0·0028, which crossed the prespecified efficacy boundary [α=0·0147]). 37 participants were randomly assigned after the interim analysis data cutoff date; among the 234 randomised participants, median time to clinical improvement was 6·0 days (95% CI 5·0–9·0) in the CD24Fc group versus 10·5 days (7·0–15·0) in the placebo group (HR 1·40, 95% CI 1·02–1·92; log-rank p=0·037). The proportion of participants with disease progression within 28 days was 19% (22 of 116) in the CD24Fc group versus 31% (36 of 118) in the placebo group (HR 0·56, 95% CI 0·33–0·95; unadjusted p=0·031). The incidences of adverse events and serious adverse events were similar in both groups. No treatment-related adverse events were observed.
Interpretation
CD24Fc is generally well tolerated and accelerates clinical improvement of hospitalised patients with COVID-19 who are receiving oxygen support. These data suggest that targeting inflammation in response to tissue injuries might provide a therapeutic option for patients hospitalised with COVID-19.
Funding
Merck & Co, National Cancer Institute, OncoImmune.
Introduction
COVID-19 is characterised by a wide range of clinical manifestations, ranging from asymptomatic or mild influenza-like illness (eg, cough, myalgia, fatigue, low-grade fever, headache, and diarrhoea) to severe disease including viral pneumonia (eg, dyspnoea and hypoxaemia) that can rapidly progress to critical illness involving acute respiratory distress syndrome, coagulopathy, shock, multiorgan failure, and death.1, 2 Although the exact mechanism underlying the pathobiology of severe COVID-19 is not known, viral replication causes necroptosis of lung epithelial cells and release of proinflammatory cytokines.3 Neighbouring pneumocytes and resident macrophages recognise components released from the necroptotic cells and inflammatory cytokines and initiate a hyperinflammatory signalling cascade resulting in the uncontrolled production and release of cytokines or chemokines (otherwise known as a cytokine storm), including interleukin (IL)-6, IL-2, IL-10, tumour necrosis factor α, and interferon γ.4
Research in context.
Evidence before this study
We searched PubMed and ClinicalTrials.gov from Jan 15, 2020, to Nov 12, 2021, for papers in English, using the terms “CD24Fc and COVID-19” and “randomised clinical trials and COVID-19”, and found no previous clinical studies on soluble CD24 appended to heavy chains 2 and 3 of human immunoglobulin G1, CD24Fc, in COVID-19. Drugs that inhibit inflammatory responses, including dexamethasone, tocilizumab, and baricitinib, have been approved for use in patients with COVID-19. CD24Fc has been tested for safety and tolerability in a phase 1 clinical study in healthy volunteers (NCT02650895). A phase 2 trial of CD24Fc for the prevention of acute graft-versus-host disease following myeloablative allogeneic haematopoietic stem cell transplantation has been completed (NCT02663622; the data are being summarised for publication).
Added value of this study
In this randomised, multicentre, double-blind, placebo-controlled, phase 3 study, we examined the therapeutic effect of CD24Fc for hospitalised patients with COVID-19 who were receiving oxygen support. We found that CD24Fc had significant efficacy in accelerating clinical improvement and in reducing disease progression compared with placebo.
Implications of all the available evidence
Our findings suggest a possible new framework for the treatment of COVID-19—ie, targeting an innate immune checkpoint of host response to tissue injury. This work might lead to new approaches to address diseases associated with inflammation.
To date, there are few treatment options for hospitalised patients with COVID-19. Dexamethasone, a broad-acting anti-inflammatory corticosteroid, has shown a significant reduction in mortality in a large study of hospitalised patients with COVID-19.5 The survival benefit associated with dexamethasone appeared to be greatest in patients receiving mechanical ventilation and lesser among patients requiring oxygen support.5 The intravenous antiviral agent remdesivir has been shown to significantly shorten time to clinical recovery in hospitalised patients with moderate-to-severe COVID-19.6, 7, 8, 9 Effects on mortality were variable in the remdesivir trials. Other agents under investigation for the treatment of severe COVID-19 include passive immunotherapy with convalescent plasma, monoclonal antibodies, IL-6 inhibitors, IL-1 pathway inhibitors, and immunoglobulins.10 To date, virus-neutralising antibodies, administered either as antigen-specific monoclonal antibodies or more broadly antigen-reactive convalescent serum, have not shown a clear benefit in hospitalised patients with severe COVID-19.11, 12 However, the monoclonal antibodies could be sensitive to viral mutations, as monoclonal antibodies such as bamlanivimab and etesevimab are no longer recommended for use in the treatment of the omicron (B.1.1.529) SARS-CoV-2 variant.
Furthermore, antibodies or targeted therapeutics against cytokine and chemokine receptors have been tested in several randomised trials. Although tocilizumab and sarilumab, two IL-6 receptor antagonists, were previously shown to be ineffective at reducing disease progression and accelerating clinical recovery among hospitalised patients with COVID-19,13, 14 a more recent study in critically ill patients hospitalised with COVID-19 found them both to be effective at extending the number of organ-support-free days (by 10–11 days) and in improving overall survival compared with standard of care.15 Baricitinib, a Janus kinase inhibitor, showed only a small benefit in clinical efficacy when used in combination with remdesivir, with a 1-day improvement in clinical recovery overall and potentially more in the subgroup receiving high-flow oxygen or non-invasive ventilation, although the effect was not tested in combination with dexamethasone.16
Because viral lysis of host cells might contribute to COVID-19 pathogenesis and enhanced severity of disease,17, 18, 19 reducing the innate host injury response might serve as another potential target for anti-COVID-19 therapeutics. CD24 is a small glycosylphosphatidylinositol-anchored cell surface glycoprotein that appears to suppress host cell response to injury through its interaction with immune-inhibitory transmembrane receptors called sialic acid-binding immunoglobulin-type lectins (Siglec) by downregulation of NF-κB activation.20, 21 As a major CD24 receptor, Siglec-10, has been shown to be downregulated in the lungs of patients with COVID-19;22 it is of interest to determine whether fortifying the CD24-Siglec pathway might lead to clinical benefit for patients.
Soluble CD24 appended to heavy chains 2 and 3 of human immunoglobulin G1, CD24Fc, is in development for the treatment of diseases associated with inflammation.23 Preclinical studies have suggested that CD24Fc reduces the risk of graft-versus-host disease in recipients of allogeneic haematopoietic stem cell transplants.24, 25 More recently, it has been shown that CD24Fc confers therapeutic benefit against pneumonia and diarrhoea induced by simian immunodeficiency virus infection in Chinese rhesus monkey models.26, 27 The safety and clinical activity of CD24Fc has been evaluated in multiple phase 1 (NCT02650895) and phase 2 (NCT02663622) clinical trials, but CD24Fc has not yet been evaluated in patients with COVID-19. We aimed to evaluate the safety and efficacy of CD24Fc in hospitalised adults with COVID-19 who were requiring oxygen support and were at risk of progression to severe and critical illness.
Methods
Study design and participants
We conducted a randomised, double-blind, placebo-controlled, phase 3 trial at nine medical centres in the USA. Eligible participants were hospitalised adults (age ≥18 years) with a diagnosis of COVID-19 and confirmed SARS-CoV-2 infection by PCR, who were requiring oxygen support (oxygen saturations <94%). Initially, the protocol specified that eligible participants included adults with COVID-19 and a National Institute of Allergy and Infectious Diseases (NIAID) 8-point ordinal scale (NIAID-OS) score of 3 (ie, receiving non-invasive ventilation or high-flow oxygen) or 4 (ie, receiving supplemental oxygen), regardless of acute respiratory distress syndrome. After the event number required for interim analysis was reached, the protocol was amended to also allow the enrolment of participants with critical COVID-19, defined by an NIAID-OS score of 2 (ie, receiving invasive mechanical ventilation or extracorporeal membrane oxygenation [ECMO]) if intubation occurred within 7 days from randomisation.
Exclusion criteria were previous enrolment in a CD24Fc clinical trial, women who were pregnant or breastfeeding, and patients with severe liver disease (Child-Pugh score C or aspartate aminotransferase more than five times the upper limit of normal) or renal impairment (creatinine clearance ≤30 mL/min or receiving renal replacement therapy).
The study protocol and all amendments were approved by the US Food and Drug Administration (FDA) and a central institutional review board (Western Institutional Review Board, Seattle, WA, USA) and accepted by the institutional review board at each study centre. The study was conducted in accordance with the protocol, protocol amendments, Good Clinical Practice, and the Declaration of Helsinki. All participants provided written informed consent before enrolment. The protocol is included in the appendix (pp 10–99).
Randomisation and masking
Eligible participants were randomly assigned (1:1) to receive a single intravenous infusion of either CD24Fc 480 mg or placebo over 60 min on day 1. The study drug and placebo were diluted to 100 mL with normal saline by local hospital pharmacists. The study funder, investigators, and patients were masked to treatment group assignment. In addition to the assigned treatment, all participants received the standard of care and other experimental therapeutics at the treating physician's discretion following local institutional guidelines for COVID-19 treatment. Randomisation was stratified by study site. The block randomisation schedule, with a block size of four, was produced by a computerised random list generator and was securely provided to trained, unmasked pharmacists at each site.
Procedures
The protocol-specified primary outcome was the time to sustained clinical improvement in COVID-19 disease status from randomisation (day 1) to day 29, defined as the time that elapsed between a baseline NIAID-OS score of 2–4 and a score of 5 or higher (ie, not requiring supplemental oxygen, based on oxygen saturation threshold of 93%) without a drop in score to less than 5zthin 28 days. The clinical status of each participant was evaluated by the treating physician through day 29 using the NIAID-OS (NCT04280705, ClinicalTrials.gov entry on March 20, 2020, by the NIAID ACTT-1 Study Team; appendix pp 13–17). Participants who were discharged from hospital before day 29 were interviewed by telephone or telemedicine weekly (plus or minus 3 days). The date when the participant showed sustained clinical improvement with an NIAID-OS score of 5 or higher or hospital discharge date, whichever came first, was used as the date of clinical improvement. If the participant was rehospitalised for respiratory distress, a record of rehospitalisation with oxygen therapy was used to modify the clinical improvement date. The new time to clinical improvement was modified to the time from randomisation to the last improvement to a NIAID-OS score of 5 or higher or the last date of hospital discharge, whichever came first.
Prespecified key secondary outcomes reported herein were the proportion of participants who had died or had respiratory failure (ie, defined as the need for mechanical ventilation, ECMO, non-invasive ventilation, or high-flow oxygen devices) by day 29, time to hospital discharge, hospital discharge without readmission, time to intubation, time to disease progression (ie, time to progression from NIAID-OS score of 3 or 4 to 1 or 2, or from 2 to 1, from randomisation to day 28), and all-cause mortality (at days 15 and 29).
Other secondary endpoints, including proportion of participants with clinical relapse (ie, return to oxygen support for at least 1 day after initial recovery within 28 days after treatment), conversion rate of clinical status on days 8 and 15 (ie, proportion of participants who changed from NIAID-OS score of 2–4 to 5 or higher within 28 days after randomisation), and duration of hospitalisation were not reported in the manuscript as their effects are integrated into the primary endpoint analysis.
The general safety and tolerability profile of CD24Fc was assessed by monitoring adverse events (grade 3–5) during the 28-day treatment period and comparing the incidences of specific adverse events by system organ classes in both treatment groups. The study investigators rated the severity (mild, moderate, or severe) and relatedness (possibly, probably, or definitely related to study medication) of each adverse event.
Statistical analysis
The primary endpoint was assessed in the intention-to-treat population and was analysed according to the treatment assigned at randomisation, regardless of the actual treatment received. For the analysis of the primary endpoint (time to clinical improvement), all participants without clinical improvement or who died within 28 days were censored on the day 29 visit.
The sample size of the trial was determined on the basis of the analysis of the primary endpoint. A total of 208 events in the intention-to-treat population would provide 80% power with a two-sided significance level of 0·05 to detect a hazard ratio (HR) of 1·54 for time to clinical improvement with CD24Fc versus placebo, assuming the median time to clinical recovery was 7·14 days in the CD24Fc group and 11·00 days in the placebo group, using a log-rank test. The assumption of median time to clinical recovery of 11·00 days in the placebo group in this study is consistent with reported outcomes seen in previous studies in patients receiving remdesivir.6, 7 An interim analysis of the primary efficacy endpoint of time to clinical improvement was prespecified to occur when 146 participants reached the time to clinical improvement endpoint (70% of the planned 208 total events), with a two-sided α level of 0·0147 (ie, the predefined stopping boundary) computed by Lan-DeMets function approximating the O'Brien-Fleming boundary.28
Subgroups of patients with baseline NIAID-OS score of either 2–4 or 3–4 and those who received or did not receive standard-of-care concomitant therapies were assessed for clinical improvement. The log-rank test was used to compare survival curves for the primary analysis of time to event endpoints and was used to compute related p values. The Kaplan-Meier method was used to estimate the cumulative probability of sustained clinical improvement, as well as to calculate the median time from randomisation to clinical improvement for each group. The Brookmeyer-Crowley method was used to construct the 95% CIs for the medians. HRs and 95% CIs were estimated by the Cox proportional hazard model.
All participants enrolled and randomised before the final database lock date (Jan 31, 2021) were included for a supportive analysis of the time to clinical improvement endpoint and for all efficacy analyses of secondary endpoints.
For the analysis of the secondary endpoints using proportions, Fisher's exact test was used to test differences in the proportions who died or had respiratory failure between the treatment groups and for the all-cause mortality endpoint. The log-rank test was used to compare the time to disease progression between the two groups. Analyses of secondary endpoints were exploratory, not adjusted for multiplicity, and their associated p values were considered nominal. All randomised participants who received at least one dose of study therapy (as-treated population) were included in the safety analyses.
We performed post-hoc sensitivity analyses using site as a stratification factor. To evaluate potential overestimation and bias caused by censored deaths possibly related to COVID-19, we performed a post-hoc analysis using the Fine-Gray method.29
SAS version 9.4 TS1M6 was used for the statistical analyses. This study is registered with ClinicalTrials.gov, NCT04317040.
Role of the funding source
The funders of the study had a role in study design, data collection, data analysis, data interpretation, and writing of the report.
Results
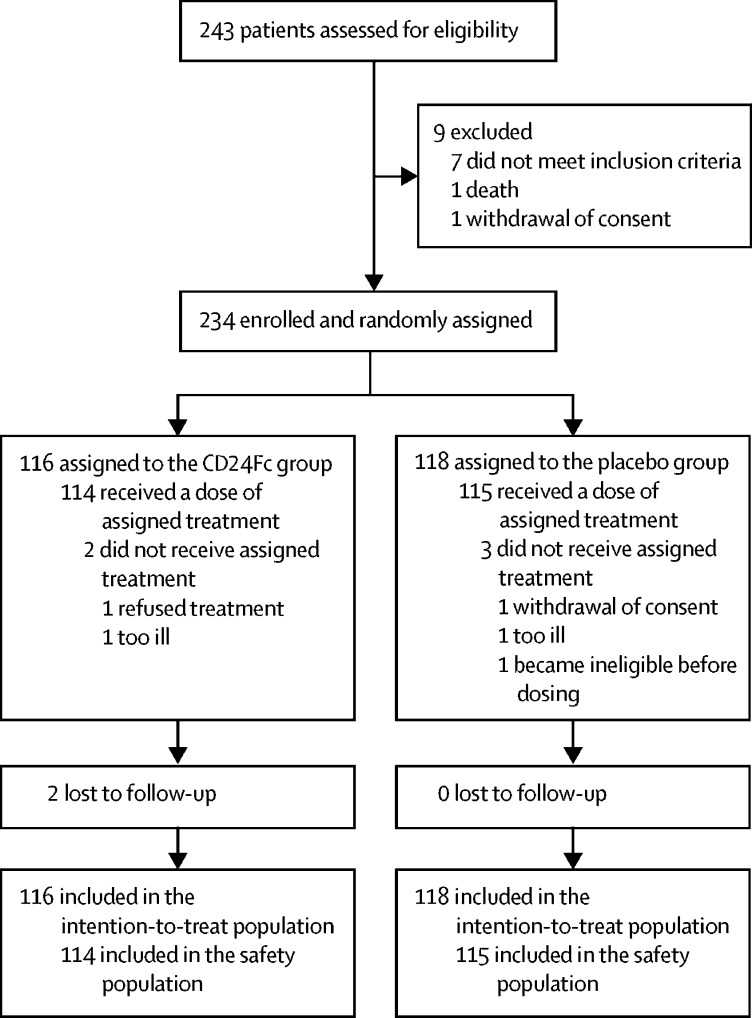
Between April 24 and Sept 22, 2020, 243 hospitalised patients were assessed for eligibility (figure 1 ). 234 eligible participants were enrolled and randomly assigned to receive CD24Fc (n=116) or placebo (n=118) and were included in the intention-to-treat population. Five participants were not dosed because they became ineligible before dosing (two participants in the CD24Fc group, three in the placebo group). Data from all randomised participants who were enrolled before the interim analysis cutoff date (Aug 21, 2020) were included in the interim primary analysis of time to clinical improvement (n=197). After the interim analysis cutoff date, the study entry criteria were modified to allow enrolment of participants with critical COVID-19 disease (ie, NIAID-OS score of 2, receiving mechanical ventilation or ECMO). 37 participants were enrolled after the interim analysis cutoff date (17 participants in the CD24Fc group and 20 in the placebo group) until the final database lock date (ie, enrolled between Aug 21, 2020, and the end of enrolment on Sept 22, 2020); of these, three participants in the CD24Fc group and one in the placebo group had an NIAID-OS score of 2 at study entry. Two additional participants were enrolled and randomly assigned in error; these participants were not included in the intention-to-treat population as they were determined to be ineligible in hindsight.
Figure 1.
Study profile
The treatment groups were generally well balanced with respect to baseline demographics and disease characteristics; the total number of comorbidities was balanced between the two groups, although specific comorbidities differed (diabetes and chronic obstructive pulmonary disease were more frequent but obesity and asthma were less frequent in the CD24Fc group than in the placebo group; table 1 ). The median age among the intention-to-treat population was 59·0 years (IQR 48·0–68·0), 145 (62%) of 234 participants were male and 89 (38%) were female, and 111 (47%) were White. At randomisation, 121 (52%) of 234 participants were receiving high-flow oxygen or non-invasive mechanical ventilation (ie, NIAID-OS score of 3) and four (2%) were receiving invasive mechanical ventilation or ECMO (ie, NIAID-OS score of 2). There were no meaningful differences between the treatment groups in any of these baseline parameters.
Table 1.
Baseline characteristics of all randomised participants
| CD24Fc group (n=116) | Placebo group (n=118) | |
|---|---|---|
| Age, years | ||
| Mean (SD) | 57·8 (14·0) | 57·8 (14·2) |
| Median (IQR) | 58·0 (48·0–69·0) | 60·5 (48·0–67·0) |
| Range | 26–86 | 23–91 |
| <65 | 75 (65%) | 80 (68%) |
| ≥65 | 41 (35%) | 38 (32%) |
| Gender | ||
| Male | 71 (61%) | 74 (63%) |
| Female | 45 (39%) | 44 (37%) |
| Race | ||
| White | 53 (46%) | 58 (49%) |
| Black or African American | 28 (24%) | 22 (19%) |
| Asian | 2 (2%) | 2 (2%) |
| Other | 33 (28%) | 34 (29%) |
| Mixed | 0 | 2 (2%) |
| Ethnicity | ||
| Hispanic or Latino | 41 (35%) | 44 (37%) |
| Not Hispanic or Latino | 75 (65%) | 74 (63%) |
| Time from disease onset to randomisation, days | ||
| Participants with defined onset data, n | 111 | 114 |
| Median (IQR) | 10·0 (6·0–13·0) | 10·0 (7·0–12·0) |
| Range | 1–90 | 2–29 |
| Time from hospitalisation to randomisation, days | ||
| Median (IQR) | 3·0 (1·0–4·0) | 3·0 (1·0–4·0) |
| Range | 0–59 | 0–22 |
| NIAID-OS score at baseline | ||
| 2 | 3 (3%) | 1 (1%) |
| 3 | 57 (49%) | 64 (54%) |
| 4 | 56 (48%) | 52 (44%) |
| ≥5 | 0 | 1 (1%) |
| Comorbidities | ||
| ≥2 | 65 (56%) | 68 (58%) |
| <2 | 51 (44%) | 50 (42%) |
| Hypertension | 64 (55%) | 64 (54%) |
| Diabetes | 26 (22%) | 24 (20%) |
| Obesity | 12 (10%) | 24 (20%) |
| Chronic obstructive pulmonary disease | 4 (3%) | 0 |
| Asthma | 9 (8%) | 13 (11%) |
| Concomitant medications | ||
| Remdesivir | 70 (60%) | 90 (76%) |
| Systemic corticosteroids | 97 (84%) | 98 (83%) |
| Remdesivir or dexamethasone, or both | 98 (84%) | 101 (86%) |
| Convalescent plasma therapy | 63 (54%) | 64 (54%) |
| Antithrombotic agents | 108 (93%) | 113 (96%) |
| Hydroxychloroquine | 1 (1%) | 2 (2%) |
| C-reactive protein concentration, nmol/L | ||
| Participants with available data, n | 106 | 110 |
| Median (IQR) | 1035·9 (454·3–1381·0) | 958·4 (552·4–1638·1) |
Data are n (%) unless otherwise stated. NIAID-OS=National Institute of Allergy and Infectious Diseases 8-point ordinal scale.
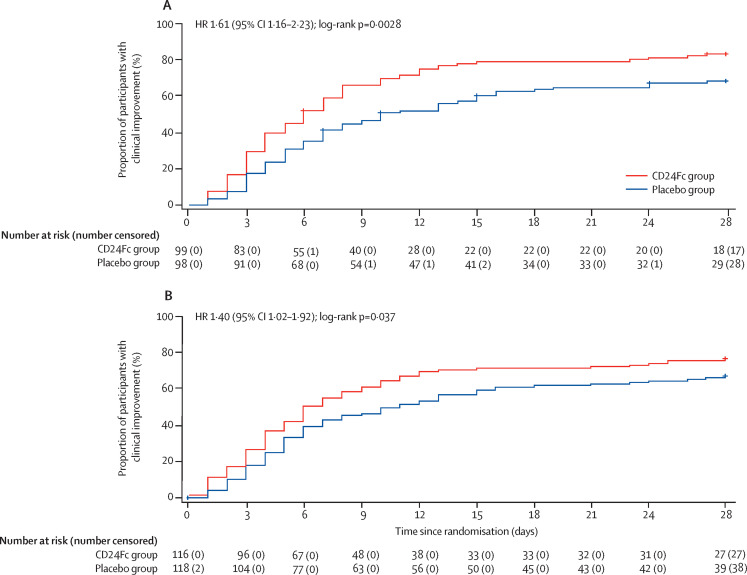
Of the 197 participants in the interim analysis, 99 were in the CD24Fc group and 98 were in the placebo group. The 28-day improvement rate was 81 (82%) of 99 participants in the CD24Fc group and 65 (66%) of 98 in the placebo group. Median time to clinical improvement was 6·0 days (95% CI 5·0–8·0) in the CD24Fc group versus 10·0 days (7·0–15·0) in the placebo group (HR 1·61, 95% CI 1·16–2·23; log-rank p=0·0028; table 2 , figure 2A ). The stopping criterion for efficacy was met based on the prespecified efficacy boundary of 0·0147 and the study was declared a success on the basis of the interim analysis results. The post-hoc sensitivity analysis using site as a stratification factor showed an HR of 1·61 (95% CI 1·15–2·25; p=0·0035), which supported the primary analysis. A comprehensive analysis of efficacy parameters in the interim population, including secondary endpoints and subgroup analyses of time to clinical improvement, showed clinical benefit of CD24Fc (appendix pp 6, 8–9).
Table 2.
Primary and secondary outcomes by analysis population and subgroup
| Point estimate (95% CI) | |
|---|---|
| Primary endpoint, time to clinical improvement | |
| Interim analysis (n=197) | 1·61 (1·16–2·23) |
| All randomised participants (n=234) | 1·40 (1·02–1·92) |
| Subgroup analyses of time to clinical improvement for all randomised participants | |
| Baseline NIAID-OS score of 2 | NA (NA–NA)* |
| Baseline NIAID-OS score of 3 | 1·21 (0·75–1·94) |
| Baseline NIAID-OS score of 4 | 1·85 (1·19–2·87) |
| Baseline NIAID-OS score of 3 or 4 | 1·46 (1·06–2·00) |
| Concomitant corticosteroids | 1·46 (1·02–2·09) |
| Concomitant remdesivir | 1·44 (0·98–2·14) |
| No concomitant corticosteroids or remdesivir | 1·51 (0·50–4·55) |
| Secondary time-to-event analyses for all randomised participants | |
| Time to hospital discharge | 1·42 (1·03–1·95) |
| Time to disease progression† | 0·56 (0·33–0·95) |
| Time to intubation† | 0·53 (0·29–0·94) |
| Death or respiratory failure by day 29 in all randomised participants | |
| CD24Fc group | 26 (22%; 15–31) |
| Placebo group | 33 (28%; 20–37) |
| All-cause mortality by day 29 in all randomised participants | |
| CD24Fc group | 16 (14%; 8–21) |
| Placebo group | 18 (15%; 9–23) |
| Participants requiring intubation by day 29 in all randomised participants | |
| CD24Fc group | 18 (15%; NA–NA) |
| Placebo group | 32 (27%; NA–NA) |
Point estimates are HR or n (%). HRs are for CD24Fc group versus placebo group. NIAID-OS=National Institute of Allergy and Infectious Diseases 8-point ordinal scale. NA=not available. HR=hazard ratio.
Analysis was not performed because of small number of participants (n=4) with baseline NIAID-OS score of 2.
Time to event analysed as rate of events comparison; HR less than 1 favours CD24Fc.
Figure 2.
Kaplan-Meier curves for time to clinical improvement
(A) Primary endpoint; time to clinical improvement among randomised participants included in the interim analysis (n=197). (B) Supportive analysis; time to clinical improvement among all randomised participants (n=234). HR=hazard ratio.
In the supportive analysis of time to clinical improvement in the entire randomised population, median time to clinical improvement was 6·0 days (95% CI 5·0–9·0) in the CD24Fc group versus 10·5 days (7·0–15·0) in the placebo group (HR 1·40, 95% CI 1·02–1·92; p=0·037; table 2, figure 2B). The post-hoc sensitivity analysis using site as a stratification factor showed an HR of 1·42 (95% CI 1·03–1·96; p=0·034). The 28-day improvement rate was 89 (77%) of 116 participants in the CD24Fc group and 78 (66%) of 118 in the placebo group. The post-hoc analysis using the Fine-Gray method showed an HR of 1·37 (95% CI 1·02–1·83), which was similar to that based on the Cox model.
In participants with NIAID-OS scores of 3 or 4 at baseline, median time to clinical improvement was 6·0 days in the CD24Fc group versus 10·0 days in the placebo group (HR 1·46, 95% CI 1·06–2·00; p=0·019). The 28-day improvement rate was 89 (79%) of 113 participants in the CD24Fc group versus 78 (67%) of 116 in the placebo group.
222 (95%) of 234 participants received background standard of care treatment with remdesivir, systemic corticosteroid treatment, or convalescent plasma therapy, or a combination thereof. Participants who received at least one of the standard-of-care therapies were distributed nearly equally between the CD24Fc (107 [92%] of 116) and placebo (115 [97%] of 118) groups. The treatment effect on time to clinical improvement was generally consistent in the subgroup of participants who received standard-of-care therapies compared with the entire randomised population (appendix p 7, table 2). In participants who received systemic corticosteroids during the study (97 in the CD24Fc group and 98 in the placebo group), the HR for time to clinical improvement favoured CD24Fc versus placebo (HR 1·46, 95% CI 1·02–2·09; table 2). Among those who received dexamethasone (81 in the CD24Fc group and 77 in the placebo group), the HR was 1·82 (95% CI 1·22–2·72). Among those who received the current standard daily dose of 6·0 mg dexamethasone5 (61 in the CD24Fc group and 59 in the placebo group), the HR was 1·64 (1·05–2·55). In participants who received remdesivir during the study, the HR was 1·44 (0·98–2·14; table 2). The number of participants who did not receive background treatment with remdesivir, systemic corticosteroids, or convalescent plasma, or a combination thereof, was too few for a reliable analysis (n=12; table 1). In general, treatment with CD24Fc accelerated clinical improvement compared with placebo in other subgroups examined by age, race, and sex (appendix p 7).
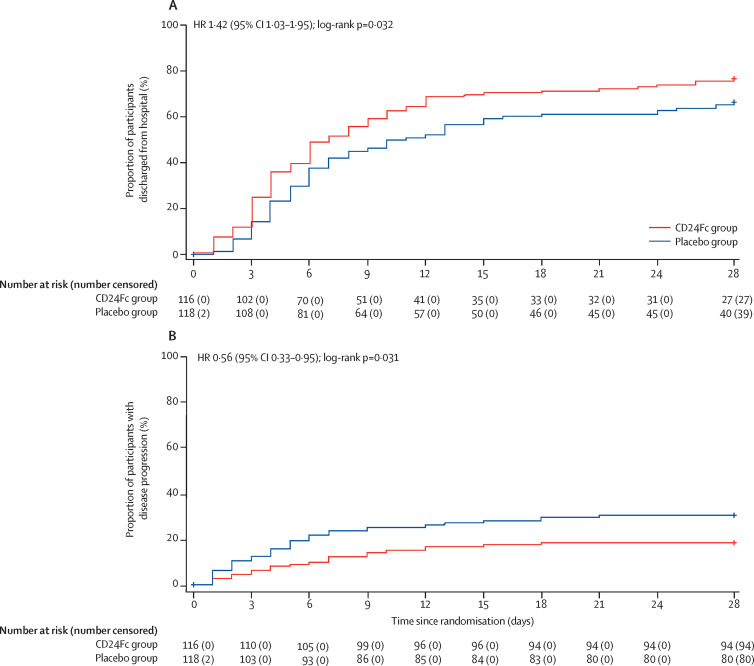
Among 234 randomised participants, the rate of hospital discharge without readmission during the 28-day post-treatment period was 77% (89 of 116) in the CD24Fc group and 65% (77 of 118) in the placebo group; the median time from randomisation to hospital discharge was 7·0 days (95% CI 6·0–9·0) in the CD24Fc group and 10·5 days (7·0–15·0) in the placebo group (HR 1·42; 95% CI 1·03–1·95; p=0·032; figure 3A ; table 2). Among participants with NIAID-OS scores of 3 or 4 at baseline, the rate of hospital discharge without readmission was 79% (89 of 113) in the CD24Fc group versus 66% (77 of 116) in the placebo group; median time from randomisation to hospital discharge was 6·0 days (95% CI 5·0–9·0) in the CD24Fc group and 10·0 days (7·0–15·0) in the placebo group (HR 1·48, 95% CI 1·07–2·03; p=0·016; table 2).
Figure 3.
Kaplan-Meier estimates of secondary endpoints
(A) Time to hospital discharge among all randomised participants (n=234). (B) Time to disease progression (defined by death, invasive mechanical ventilation, or extracorporeal membrane oxygenation) among all randomised participants (n=234). HR=hazard ratio.
Median time to disease progression was not estimated due to the low rates of progression; among 234 randomised participants, the 28-day rates of disease progression were 19% (22 of 116) in the CD24Fc group versus 31% (36 of 118) in the placebo group (HR 0·56, 95% CI 0·33–0·95; p=0·031; figure 3B, table 2). Among participants with NIAID-OS scores of 3 or 4 at baseline, the rates of disease progression were 18% (20 of 113) in the CD24Fc group versus 31% (36 of 116) in the placebo group (0·51, 0·30–0·89; p=0·015).
The proportion of participants who died or had respiratory failure by day 29 was 22% (26 of 116) in the CD24Fc group versus 28% (33 of 118) in the placebo group (between-group difference –6%, 95% CI –17 to 6; p=0·33; table 2). Among participants with NIAID-OS scores of 3 or 4 at baseline, the corresponding proportions of participants with respiratory failure or death at day 29 were 20% (23 of 113) in the CD24Fc group versus 28% (33 of 116) in the placebo group.
By day 29, all-cause mortality had occurred in 16 (14%) of 116 participants in the CD24Fc group versus 18 (15%) of 118 in the placebo group (risk difference –2%, 95% CI –11 to 8; table 2). For the subgroup of participants with NIAID-OS scores of 3 or 4 at baseline, all-cause mortality had occurred in 14 (12%) in the CD24Fc group versus 18 (16%) in the placebo group (risk difference –3%, 95% CI –12 to 6).
32 (28%) of 114 participants who received CD24Fc and 35 (30%) of 115 who received placebo had at least one adverse event (table 3 ). Adverse events were generally balanced between the treatment groups. The incidence of serious adverse events was similar in both treatment groups (37 events in 26 [23%] participants who received CD24Fc vs 32 events in 27 [24%] participants who received placebo). There were no treatment-related adverse events. The most frequently observed adverse events in both treatment groups were in the system organ class of respiratory, thoracic, and mediastinal disorders. The incidence of respiratory failure-associated adverse events was higher in participants who received placebo (15 [13%] participants) than in those who received CD24Fc (five [4%]; table 3).
Table 3.
Adverse events
| CD24Fc group (n=114) | Placebo group (n=115) | ||
|---|---|---|---|
| Total adverse events | 69 | 52 | |
| Participants with adverse events | 32 (28%) | 35 (30%) | |
| Treatment-related adverse events | 0 | 0 | |
| Participants with treatment-related adverse events | 0 | 0 | |
| Total serious adverse events | 37 | 32 | |
| Participants with serious adverse events | 26 (23%) | 27 (24%) | |
| Deaths | 16 (14%) | 18 (15%) | |
| Total fatal serious adverse events | 17 | 19 | |
| Participants with fatal serious adverse events | 15 (13%) | 18 (16%) | |
| Fatal serious adverse events* | |||
| Respiratory, thoracic, and mediastinal disorders | 7 (6%) | 13 (11%) | |
| Respiratory failure | 5 (4%) | 12 (10%) | |
| Cardiac disorders | 6 (5%) | 3 (3%) | |
| Cardiac arrest | 3 (3%) | 2 (2%) | |
| Total non-fatal serious adverse events | 22 | 15 | |
| Participants with non-fatal serious adverse events | 17 (15%) | 12 (10%) | |
| Non-fatal serious adverse events* | |||
| Respiratory, thoracic, and mediastinal disorders | 7 (6%) | 7 (6%) | |
| Respiratory failure | 0 | 3 (3%) | |
| Blood and lymphatic system disorders | 3 (3%) | 1 (1%) | |
| Anaemia | 3 (3%) | 1 (1%) | |
| Renal and urinary disorders | 3 (3%) | 1 (1%) | |
| Acute kidney injury | 3 (3%) | 1 (1%) | |
| Grade ≥3 adverse events by system organ class* | |||
| Blood and lymphatic system disorders | |||
| Acute anaemia | 0 | 1 (1%) | |
| Acute exacerbation of chronic anaemia | 1 (1%) | 0 | |
| Anaemia | 5 (4%) | 1 (1%) | |
| Worsening of anaemia | 1 (1%) | 0 | |
| Cardiac disorders | |||
| Asystole arrest | 1 (1%) | 0 | |
| Cardiac arrest | 2 (2%) | 2 (2%) | |
| Renal and urinary disorders | |||
| Acute kidney injury | 1 (1%) | 1 (1%) | |
| Acute renal failure | 4 (3%) | 1 (1%) | |
| Oliguric acute renal failure with hyperkalaemia | 0 | 1 (1%) | |
| Respiratory, thoracic, and mediastinal disorders | |||
| Pneumothorax | 2 (2%) | 1 (1%) | |
| Right pneumothorax | 1 (1%) | 0 | |
| Exacerbation of respiratory failure | 3 (3%) | 7 (6%) | |
| Hypoxic respiratory failure | 0 | 2 (2%) | |
| Hypoxic respiratory failure secondary to COVID-19 | 0 | 1 (1%) | |
| Respiratory failure | 4 (3%) | 6 (5%) | |
Data are n or n (%). Adverse event assessment period was 28 days from randomisation.
With incidence rates of 2% or greater in one or more treatment group, by system organ class in all participants who received study treatment.
The most frequent grade 3 or worse adverse event in the CD24Fc group was anaemia and in the placebo group was exacerbation of respiratory failure (table 3). All anaemia-related adverse events in this study were deemed either not related or unlikely to be related to study medication by the study investigators. No infusion-related adverse events occurred. None of the deaths in this study were attributed to study treatment. Additional investigator-reported deaths were reported by some sites outside of the 28-day protocol-specified reporting period. Of these deaths, one was in the CD24Fc group and six were in the placebo group. Deaths outside of the 28-day protocol-specified period were not uniformly reported across study sites, therefore these findings should be interpreted with caution.
Discussion
This study showed that, among hospitalised patients with COVID-19 who were requiring oxygen support, the administration of a single intravenous infusion of CD24Fc 480 mg led to robust, significant, and sustained improvements in time to clinical improvement compared with placebo over the 28-day period after treatment.
When time to clinical improvement was analysed by NIAID-OS score at baseline, the magnitude of the between-group difference in response was numerically larger in the subgroup of participants with less severe disease at baseline (ie, NIAID-OS score of 4 vs NIAID-OS score of 3), which suggests that CD24Fc is more effective when administered early in the course of SARS-CoV-2 infection. Beneficial effects of CD24Fc on clinical status at day 28 were seen across all other subgroups examined, including by age, race, and sex. Taken together, the results from this study show that CD24Fc substantially and meaningfully improved time to clinical improvement, based on either reduction of oxygen support or hospitalisation duration, compared with placebo in hospitalised participants with COVID-19 who had varied baseline demographics.
During the course of this study, use of remdesivir, systemic corticosteroids, or convalescent plasma therapy, or a combination thereof, became more widespread for the treatment of COVID-19, due to positive signals reported in the medical literature and emergency use authorisations issued by the FDA.6, 7 Most participants in this study (95%) received at least one concomitant COVID-19 therapy during the randomised treatment period, and dexamethasone and remdesivir were the most common. The benefit of CD24Fc versus placebo on time to clinical improvement was observed across subgroups of participants receiving many standard-of-care therapies. These findings suggest that CD24Fc could be a beneficial addition to the treatment armamentarium in hospitalised patients with COVID-19, leading to reduced hospital stays and improved clinical status. A limitation of this study was that convalescent plasma therapy was considered as part of standard of care during the enrolment period, before randomised studies with convalescent plasma therapy failed to show efficacy in patients with COVID-19.12
In addition to beneficial effects on time to clinical improvement, secondary analyses showed that treatment with CD24Fc also reduced the likelihood of disease progression, defined by death or need for invasive mechanical ventilation, by 44% compared with placebo, and shortened the time to hospital discharge by 3·5 days. Consistent with the reduced rate of intubation in the CD24Fc group, there was a numerical reduction in death and respiratory failure in the CD24Fc group compared with placebo at day 29 (22% vs 28%, respectively) that did not reach significance. The between-group differences in all-cause mortality by day 29 were –2% (p=0·75) in the overall population and –12% (p=0·030) in patients requiring intubation (table 2). No apparent benefit with respect to survival at 4 weeks was seen in this study, which might be due to the small sample size or short observation period. These results should be considered exploratory in nature because adjustments for multiple comparisons were not prespecified in the study protocol.
The overall adverse event profiles were similar in the CD24Fc and placebo groups. The incidences and types of adverse events, treatment-related adverse events, and serious adverse events were generally similar across the treatment groups. The adverse events observed were consistent with a population of hospitalised patients with COVID-19. The most frequently observed adverse events in both treatment groups were in the system organ class of respiratory, thoracic, and mediastinal disorders. The incidences of anaemia-related adverse events were higher in the CD24Fc group than in the placebo group, but none were considered related to study treatment. Consistent with the beneficial effect of CD24Fc on clinical improvement, the incidence of respiratory failure-related adverse events was numerically higher in the placebo group than in the CD24Fc group.
Previous studies with IL-6 receptor antagonists have shown mixed results with respect to improvements in clinical recovery and delayed disease progression in hospitalised patients with COVID-19.13, 14, 15 By comparison, this study showed that CD24Fc accelerates clinical recovery and reduces disease progression among hospitalised patients with COVID-19. The observed effects on clinical status observed in this trial are likely to be due to the novel mechanism of action of CD24Fc that suppresses production of multiple inflammatory cytokines and decreases inflammation in multiple tissues, including the lungs.25, 26 Through interactions with Siglec-G in mice and Siglec-10 in humans, CD24 functions as an innate immune checkpoint surveillance molecule that suppresses activation of NF-κB caused by tissue injury—namely, danger-associated molecular patterns such as HMGB1 and HSP70/90.20, 21 This pathway might be defective in patients with SARS-CoV-2 infection, as Siglec-10 has been shown to be selectively downregulated in lung tissue in patients with COVID-19.22 Exogenous HMGB1 also induces the expression of SARS-CoV-2 entry receptor ACE2 in alveolar epithelial cells.30 The broad impact of CD24Fc on inflammation in patients with COVID-19, as described elsewhere,31 is consistent with this notion.
We did our primary analysis in the intention-to-treat population as this is the standard approach, although there are some potential drawbacks of this approach that have been described previously.32 After the prespecified interim analysis, this study was stopped on the basis of the efficacy criteria for stopping enrolment being met. Although this resulted in a small patient population, the robust results in both primary and secondary outcomes, as well as in subgroup analyses, are supportive of the prespecified stopping point being correctly designed into the trial to provide a reliable measure of treatment effect. An additional independent trial would be valuable to confirm the clinical benefit found in this study and could be designed with a longer study duration and the power to evaluate survival benefits. Because this study enrolled very few patients who were receiving invasive mechanical ventilation or ECMO, the effect of CD24Fc in these patients requires further study. Finally, as the trial was conducted in 2020, the effect of CD24Fc against more recent SARS-CoV-2 variants has not been tested. However, as CD24Fc targets the host immune system, its therapeutic activity would be less likely to be affected by viral mutations.
Treatment with CD24Fc significantly accelerated clinical improvement. It also reduced disease progression and shortened the length of hospital stay among participants with COVID-19 receiving oxygen support. CD24Fc had an acceptable safety and tolerability profile compared with placebo.
Data sharing
The data sharing policy, including restrictions, of Merck Sharp & Dohme, a subsidiary of Merck & Co, is available at http://engagezone.msd.com/ds_documentation.php. Requests for access to the clinical study data can be submitted through the Engage Zone website or via email to dataaccess@merck.com.
Declaration of interests
JW, JDP, ATC, CDM, EDW, DB, OKG, JEL, and SK had clinical trial agreements with OncoImmune. JDP reports payment for Baptist Medical Health COVID update (CME event). EDW was on the speaker's bureau at Merck & Co (before Merck's acquisition of OncoImmune). JEL is a consultant to Merck & Co for HIV-related studies. SK reports a grant from OncoImmune, during the conduct of the study; and grants from Merck & Co, Gilead Sciences, and Arbutus Pharmaceuticals, outside of the submitted work. ZL serves as a scientific advisory board member for Alphamab, Hengenix, and Ikonisys; and receives grants from Heat Biologics, National Institutes of Health, and Pelotonia, outside of the submitted work. EL, JiC, and XZ are employees of Edetek, the clinical data management and biostats service provider for OncoImmune. DB, DG, and AK are employees of Merck Sharp & Dohme, a subsidiary of Merck & Co, and could hold stocks or stock options in Merck & Co. H-YC, MD, RT, YL, and PZ are employees of OncoImmune, and received grants from National Institutes of Health and National Cancer Institute (R44CA246991-02S1), during the conduct of the study. MD, YL, and PZ have a patent or a patent pending. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
CD24Fc has been acquired by Merck & Co (Kenilworth, NJ, USA). OncoImmune (Rockville, MD, USA), a wholly-owned subsidiary of Merck & Co, funded this trial, with partial support from a grant from the National Cancer Institute (R44CA246991-02S1), provided the trial drugs, and collaborated with the academic authors and consultants on the trial design as well as the collection, analysis, and interpretation of the data. ClinSmart (Newtown, PA, USA) conducted the trial according to the protocol and collected the data. A data and safety monitoring committee reviewed safety and efficacy data with assistance from an unmasked statistical team at Edetek (Princeton, NJ, USA). The statisticians at Edetek analysed the data with assistance from Merck & Co (Kenilworth, NJ, USA) and OncoImmune. All authors vouch for the accuracy and completeness of the data and the fidelity of the trial to the protocol. We thank Amy O Johnson-Levonas, Jennifer Rotonda, and Michele McColgan, employees of Merck Sharp & Dohme Corp (Whitehouse Station, NJ, USA), a subsidiary of Merck & Co, for their assistance with editing and preparing this paper for publication.
Acknowledgments
Contributors
All authors are responsible for the work described in this paper. JW, JVC, KH, MD, SK, YL, and PZ conceived, designed, and planned the study. JW, JDP, ATC, CDM, ZL, JBC, EDW, DB, OKG, JEL, JVC, RT, SK, YL, and PZ acquired the data. ZL, EL, JiC, XZ, KH, DG, AK, JaC, H-YC, MD, SK, YL, and PZ analysed the data. EL, JiC, XZ, DG, and AK accessed and verified the data. JW, KH, DG, AK, SK, YL, and PZ interpreted the results. YL and PZ wrote the first draft of the manuscript. All authors critically reviewed or revised the manuscript for important intellectual content and provided final approval of the version to be published, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors had access to the data package. PZ, YL, SK, DG, and AK are responsible for the decision to submit the paper for publication.
Contributor Information
SAC-COVID Study Team:
Pan Zheng, Yang Liu, Martin Devenport, Raymond Touomou, Hung-Yen Chou, Jai Thakor, Imaan Khan, Nicole Do, Josephine Faragalla, Andrea Hook, Sarah Kern, Janira V. Ramos, Jason Ward, Jamie Chen, John Higson, Meena Dam, Dawn Serkin, Pooja Karloopia, Wendy Moore, Mark Scofield, David Jeffery Childers, Jeffrey S. Cantrell, Millie Corgan, Ella Li, Jian Chen, Xiang Zhou, Jing Liu, Denise Redvers-Higgins, Hua Han, Jiyun Hou, Yudi Pan, Karyn Tucker, Xiaoyan Zhang, Shyamasundaran Kottilil, Joel V. Chua, Jennifer Husson, Shivakumar Narayanan, Jaqueline Bran, Ka Wing Joyce Lam, Alicia Jeffrey, Olivia K. Giddings, Jennie Pexa, Mario Becerra, James Welker, Kathleen W. Gray, Nicole Richmond, Chukwuemeka Nzelibe, Carlos D. Malvestutto, Susan Koletar, Mahdee Sobhanie, Jan Clark, Zihai Li, Kelsi Reynolds, Karthik Chakravarthy, Kevin Weller, Mohamed Yusuf, Jennifer Severing, Kelley Barley, Juan D. Pulido, Jennifer C. Fulton, William Gil, M.D. Jeanine, Richmond R.N., Sandy Jones, Kristina Clemmer, Dana Byrne, Lisa Pedroza, Emily Nicole Davidson, Amanda Logan, Katie Grant, Eric D. Whitman, Jason Kessler, Robert Roland, Rosemary Stefiniw, Molly Maurer, Salome Geene, Christopher F. Buck, Debra Connolly, Patrice Light, Sunanda Baviskar, Yee Won Low, Kyra Michalski, Pamela Giordano, Jennifer Chao, Michelle Williams, Amulya Makkapati, Andrew T. Catanzaro, Jonathan B. Cohen, Mehad Musbah, Pramila Jaladanki, Ying Yuan, Shilpa Rele, Desirae Stewart, Starlet Lewis, Ian Sankar, Nabulungi Kasumba, Kaylia Biney, Elham Hekmat, Jordan E. Lake, Bindu Akkanti, Melissa J. Reimer-McAtee, and Marisel Negret Hernandez
Supplementary Material
References
- 1.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li S, Zhang Y, Guan Z, et al. SARS-CoV-2 triggers inflammatory responses and cell death through caspase-8 activation. Signal Transduct Target Ther. 2020;5:235. doi: 10.1038/s41392-020-00334-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luo W, Li YX, Jiang LJ, Chen Q, Wang T, Ye DW. Targeting JAK-STAT signaling to control cytokine release syndrome in COVID-19. Trends Pharmacol Sci. 2020;41:531–543. doi: 10.1016/j.tips.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with COVID-19—preliminary report. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of COVID-19—final report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spinner CD, Gottlieb RL, Criner GJ, et al. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial. JAMA. 2020;324:1048–1057. doi: 10.1001/jama.2020.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldman JD, Lye DCB, Hui DS, et al. Remdesivir for 5 or 10 days in patients with severe COVID-19. N Engl J Med. 2020;383:1827–1837. doi: 10.1056/NEJMoa2015301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pan H, Peto R, Henao-Restrepo AM, et al. Repurposed antiviral drugs for COVID-19—interim WHO Solidarity Trial results. N Engl J Med. 2021;384:497–511. doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berlin DA, Gulick RM, Martinez FJ. Severe COVID-19. N Engl J Med. 2020;383:2451–2460. doi: 10.1056/NEJMcp2009575. [DOI] [PubMed] [Google Scholar]
- 11.Lundgren JD, Grund B, Barkauskas CE, et al. A neutralizing monoclonal antibody for hospitalized patients with COVID-19. N Engl J Med. 2021;384:905–914. doi: 10.1056/NEJMoa2033130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simonovich VA, Burgos Pratx LD, Scibona P, et al. A randomized trial of convalescent plasma in COVID-19 severe pneumonia. N Engl J Med. 2021;384:619–629. doi: 10.1056/NEJMoa2031304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stone JH, Frigault MJ, Serling-Boyd NJ, et al. Efficacy of tocilizumab in patients hospitalized with COVID-19. N Engl J Med. 2020;383:2333–2344. doi: 10.1056/NEJMoa2028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lescure FX, Honda H, Fowler RA, et al. Sarilumab in patients admitted to hospital with severe or critical COVID-19: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med. 2021;9:522–532. doi: 10.1016/S2213-2600(21)00099-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gordon AC, Mouncey PR, Al-Beidh F, et al. Interleukin-6 receptor antagonists in critically ill patients with COVID-19. N Engl J Med. 2021;384:1491–1502. doi: 10.1056/NEJMoa2100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalil AC, Patterson TF, Mehta AK, et al. Baricitinib plus remdesivir for hospitalized adults with COVID-19. N Engl J Med. 2021;384:795–807. doi: 10.1056/NEJMoa2031994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teuwen LA, Geldhof V, Pasut A, Carmeliet P. COVID-19: the vasculature unleashed. Nat Rev Immunol. 2020;20:389–391. doi: 10.1038/s41577-020-0343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20:363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao X. COVID-19: immunopathology and its implications for therapy. Nat Rev Immunol. 2020;20:269–270. doi: 10.1038/s41577-020-0308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen GY, Chen X, King S, et al. Amelioration of sepsis by inhibiting sialidase-mediated disruption of the CD24-SiglecG interaction. Nat Biotechnol. 2011;29:428–435. doi: 10.1038/nbt.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen GY, Tang J, Zheng P, Liu Y. CD24 and Siglec-10 selectively repress tissue damage-induced immune responses. Science. 2009;323:1722–1725. doi: 10.1126/science.1168988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blanco-Melo D, Nilsson-Payant BE, Liu WC, et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181:1036–1045. doi: 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng X, Wu W, Liu Y, Zheng P. United States Patent and Trademark Office; Alexandria, VA: 2019. Methods of use of soluble CD24 for therapy of rheumatoid arthritis. US20190016783. [Google Scholar]
- 24.Toubai T, Hou G, Mathewson N, et al. Siglec-G-CD24 axis controls the severity of graft-versus-host disease in mice. Blood. 2014;123:3512–3523. doi: 10.1182/blood-2013-12-545335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toubai T, Rossi C, Oravecz-Wilson K, et al. Siglec-G represses DAMP-mediated effects on T cells. JCI Insight. 2017;2 doi: 10.1172/jci.insight.92293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tian RR, Zhang MX, Liu M, et al. CD24Fc protects against viral pneumonia in simian immunodeficiency virus-infected Chinese rhesus monkeys. Cell Mol Immunol. 2020;17:887–888. doi: 10.1038/s41423-020-0452-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tian RR, Zhang MX, Zhang LT, et al. CD24 and Fc fusion protein protects SIVmac239-infected Chinese rhesus macaque against progression to AIDS. Antiviral Res. 2018;157:9–17. doi: 10.1016/j.antiviral.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 28.Lan KK, DeMets DL. Discrete sequential boundaries for clinical trials. Biometrika. 1983;70:659–663. [Google Scholar]
- 29.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 30.Chen R, Huang Y, Quan J, et al. HMGB1 as a potential biomarker and therapeutic target for severe COVID-19. Heliyon. 2020;6 doi: 10.1016/j.heliyon.2020.e05672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song NJ, Allen C, Vilgelm AE, et al. Treatment with soluble CD24 attenuates COVID-19-associated systemic immunopathology. J Hematol Oncol. 2022;15:5. doi: 10.1186/s13045-021-01222-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greenland S, Lanes S, Jara M. Estimating effects from randomized trials with discontinuations: the need for intent-to-treat design and G-estimation. Clin Trials. 2008;5:5–13. doi: 10.1177/1740774507087703. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sharing policy, including restrictions, of Merck Sharp & Dohme, a subsidiary of Merck & Co, is available at http://engagezone.msd.com/ds_documentation.php. Requests for access to the clinical study data can be submitted through the Engage Zone website or via email to dataaccess@merck.com.