Abstract
Background:
Primary hyperparathyroidism and familial hypocalciuric hypercalcemia have similar biochemical profiles, and calcium-to-creatinine-clearance ratio helps distinguish the two. Additionally, 24-hour urine calcium >400 mg/day indicates surgery and guidelines recommend obtaining 24-hour urine calcium preoperatively. Our aim was to assess how 24-hour urine calcium altered care in the evaluation of suspected primary hyperparathyroidism.
Methods:
Consecutive patients assessed for primary hyperparathyroidism from 2018 to 2020 were reviewed. Primary hyperparathyroidism was diagnosed by 2016 American Association of Endocrine Surgeons Parathyroidectomy Guidelines criteria. 24-hour urine calcium-directed change in care was defined as familial hypocalciuric hypercalcemia diagnosis, surgical deferment for additional testing, or 24-hour urine calcium >400 mg/day as the sole surgical indication.
Results:
Of 613 patients, 565 (92%) completed 24-hour urine calcium and 477 (84%) had concurrent biochemical testing to calculate calcium-to-creatinine-clearance ratio. 24-hour urine calcium was <100 mg/day in 9% (49/565) and calcium-to-creatinine-clearance ratio was <0.01 in 17% (82/477). No patient had confirmed familial hypocalciuric hypercalcemia, although 1 had a CASR variant of undetermined significance. When calcium-to-creatinine-clearance ratio was <0.01, familial hypocalciuric hypercalcemia was excluded by 24-hour urine calcium >100 mg/day (56%), prior normal calcium (16%), renal insufficiency (11%), absence of familial hypercalcemia (3%), normal repeat 24-hour urine calcium (10%), or interfering diuretic (1%). 24-hour urine calcium-directed change in care occurred in 25 (4%), including 4 (1%) who had genetic testing. Four-gland hyperplasia was more common with calcium-to-creatinine-clearance ratio <0.01 (17% vs calcium-to-creatinine-clearance ratio ≥ 0.01, 4%, P < .001), but surgical failure rates were equivalent (P = .24).
Conclusion:
24-hour urine calcium compliance was high, and results affected management in 4%, including productive identification of hypercalciuria as the sole surgical indication in 2 patients. When calcium-to-creatinine-clearance ratio <0.01, clinical assessment was sufficient to exclude familial hypocalciuric hypercalcemia and only 1% required genetic testing. 24-hour urine calcium should be ordered judiciously during primary hyperparathyroidism assessment.
Introduction
Primary hyperparathyroidism (pHPT) is one of the most commonly diagnosed endocrine disorders and is due to inappropriate hypersecretion of parathyroid hormone (PTH) from 1 or more enlarged parathyroid glands.1,2 Diagnosis of pHPT is biochemical and is characterized by elevation of serum calcium levels with corresponding elevated or nonsuppressed PTH levels.3 pHPT can have a biochemical profile similar to that of familial hypocalciuric hypercalcemia (FHH), which is a rare, benign autosomal dominant disease. Clinically, FHH is characterized by lifelong and usually asymptomatic hypercalcemia and relatively low renal calcium excretion.4 Despite the biochemical similarities between pHPT and FHH, discrimination of the 2 diseases is of vital importance because pHPT usually necessitates parathyroidectomy, whereas FHH does not require surgical treatment.5
Twenty-four-hour urine calcium level (24UCa) with subsequent calculation of the calcium-to-creatinine clearance ratio (CCCR) is frequently used to distinguish pHPT from FHH.6 Current guidelines recommend obtaining 24UCa preoperatively, and although a range of results can be observed in FHH, a cut-off of CCCR <0.01 is concerning for FHH, whereas CCCR >0.02 is consistent with pHPT.3,6,7 Despite the widely accepted use of CCCR to discriminate pHPT from FHH, several studies have brought the utility of this diagnostic measure into question due to the overlap of CCCR in these 2 distinct disease entities.5,8 In fact, alternative methods of differentiating pHPT and FHH have been proposed, including a 2-step approach with CCCR and genetic testing (when CCCR <0.02) and the development of a novel predictive tool, “pro-FHH.”6,9 The first step in the surgical evaluation of any patient referred for possible operative management of pHPT is review and, if necessary, confirmation of the biochemical diagnosis.3
However, although the discriminative power and diagnostic utility of CCCR have been investigated, there is a paucity of data examining how 24UCa affects the preoperative management of patients referred for possible pHPT surgery. We aim to assess if, how, and how often 24UCa and CCCR alters patient care in the evaluation of suspected pHPT.
Methods
Study subjects and biochemical testing
After institutional QA/QI approval (QIIRB2843), all patients who were referred for endocrine surgery assessment of any type of hyperparathyroidism from 2018 to 2020 at a single institution were retrospectively reviewed. Patients with secondary hyperparathyroidism (n = 82), tertiary hyperparathyroidism (n = 19), an existing diagnosis of familial or MEN1-related hyperparathyroidism (n = 17), repeat biochemical testing results not consistent with hyperparathyroidism (n = 48), or recurrent or persistent hyperparathyroidism (n = 35) were excluded (Fig 1). Determination of pHPT symptoms (ostealgia, myalgias, fatigue, depression, neurocognitive slowing, memory loss, nephrolithiasis, nephrocalcinosis, bone demineralization, peptic ulcers, nocturia, polyuria, polydipsia, constipation, or headaches) and thiazide diuretic use was done in a standardized fashion at the initial visit. Biochemical evaluation with serum calcium (sCa), serum creatinine (sCr), vitamin D 25-OH (VitD25OH), serum parathyroid hormone (PTH), 24-hour urine calcium (24UCa), and 24-hour urine creatinine (24UCr) levels was routinely assessed; available data on estimated glomerular filtration rate (GFR) was also retrieved. Reference limits were determined by the laboratory that performed the biochemical testing. CCCR was calculated as [24UCa (mg/day) × sCr(mg/dL)/ 24UCr (mg/day) × sCa(mg/dL)], and pHPT was diagnosed by 2016 American Association of Endocrine Surgeons Parathyroidectomy Guidelines criteria.3
Fig 1.
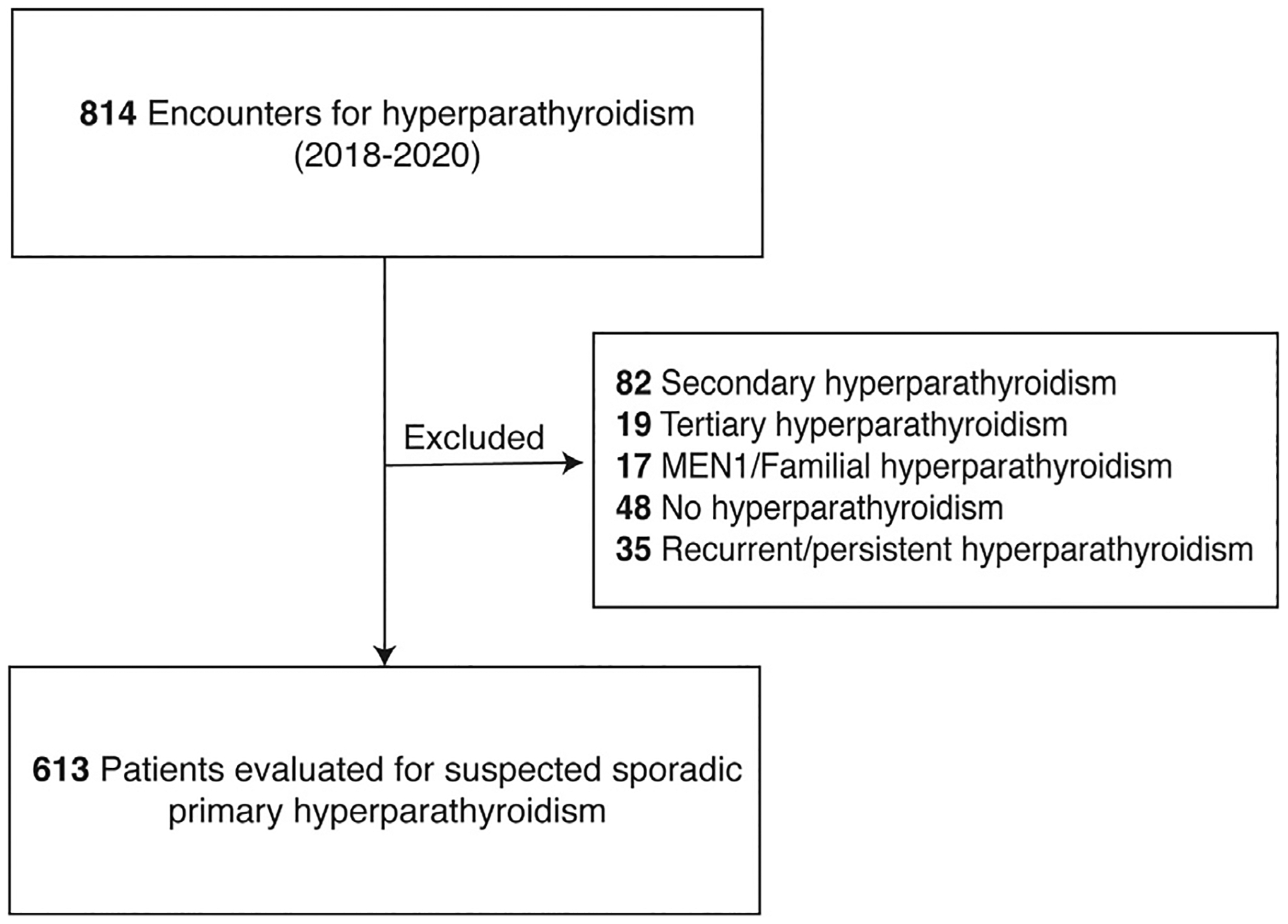
Patients assessed for possible primary hyperparathyroidism in endocrine surgery clinic.
Classification of 24UCa-directed change in care and exclusion of FHH
Subsequent to surgical referral, a 24UCa-directed change in care was defined as diagnosis of FHH, deferment of surgery for suspected FHH and additional testing, or identification of 24UCa >400 mg/day as the sole indication for surgery. In patients with CCCR <0.01, exclusion of FHH occurred using historical laboratory values, family history, medication history, and/or referral to endocrinology for additional input. If FHH was not able to be excluded, patients were referred to genetics for germline testing for CASR, GNA11, and AP2S1 mutations.
Surgical conduct and operative outcome
Before parathyroid exploration, imaging studies were performed using SPECT-CT and neck ultrasound. Unilateral or bilateral exploration was conducted according to imaging results, intraoperative findings, and intraoperative PTH results, which were interpreted using the dual criteria.3,10 Resected parathyroid tissue was weighed (normal gland ≤50 mg) and tissue identity confirmed by histologic evaluation.11 Postoperatively, sCa, PTH, and VitD25OH were routinely reassessed at ≥6 months, and operative cure or failure were defined according to the guidelines.3
Statistics
Continuous variables were reported as mean and standard deviation for normally distributed data. Non-normally distributed continuous variables were reported as median and interquartile range. Categorical variables were analyzed using Fisher exact test. STATA SE 15.1 (College Station, TX) was used to perform all statistical analyses.
Results
Patient and biochemical characteristics
A total of 613 patients with suspected sporadic pHPT were included in the analysis, of whom 78.1% were women. Mean age at diagnosis was 64 (standard deviation, 12.3) years, 95.8% of patients were symptomatic, and 15.2% were on thiazide diuretics at presentation. Biochemical and patient characteristics at initial evaluation are summarized in Table I. The majority (72%) of patients presented with 24UCa results on referral, whereas 28% had 24UCa ordered by the evaluating endocrine surgeon. In total, 565/613 (92%) completed 24UCa testing, and, of those, 477/565 (84%) had concurrent testing that allowed calculation of CCCR. 24UCa testing was not completed in 48 (8%) due to incontinence or physical limitation preventing adequate sample collection, interfering diuretic medications that could not be stopped, or deferment of surgery due to medical comorbidities.
Table I.
Baseline patient and biochemical characteristics of study patients
| Characteristic (N = 613) | |
|---|---|
| Age at diagnosis, mean (SD) | 64 (12.3) |
| Men, N (%) | 134 (21.9) |
| Symptomatic, N (%) | 587 (95.8) |
| Thiazide use, N (%) | 93 (15.2) |
| Serum Ca, median (IQR), mg/dL | 10.7 (10.4–11.1) |
| Serum Cr, median (IQR), mg/dL | 0.9 (0.7–1.0) |
| GFR, median (IQR), mL/min | 70.0 (60.0–89.0) |
| Vitamin D 25-OH, median (IQR), ng/mL | 36 (27.0–46.9) |
| Serum PTH, median (IQR), pg/mL | 108.6 (82.0–148.0) |
| 24-hour urine calcium, median (mg/24h) | 252.8 (162.0–379.9) |
| 24-hour urine creatinine, median (IQR), mg/24h | 1180.0 (936.0–1500.0) |
| 24-hour urine volume, median (IQR), L/24h | 1.9 (1.4–2.5) |
| CCCR, median (IQR) | 0.017 (0.012–0.023) |
| Repeat 24-hour urine obtained, N (%) | 25 (4.1) |
| Parathyroid exploration, N (%) | 553 (90.4) |
| Surgical failure, N (%) | 8 (1.8) |
IQR, interquartile range; Ca, calcium; Cr, creatinine; GFR, glomerular filtration rate; CCCR, calcium-to-creatinine ratio; PTH, parathyroid hormone; SD, standard deviation.
Altogether, 24UCa was <100 mg/day in 49 of 565 (9%), and CCCR was <0.01 in 82 of 477 (17%). CCCR consistent with pHPT was observed in 83%, including 225 (47%) with a CCCR 0.01 to 0.02 and 170 (36%) with a CCCR ≥0.02 (Fig 2).
Fig 2.
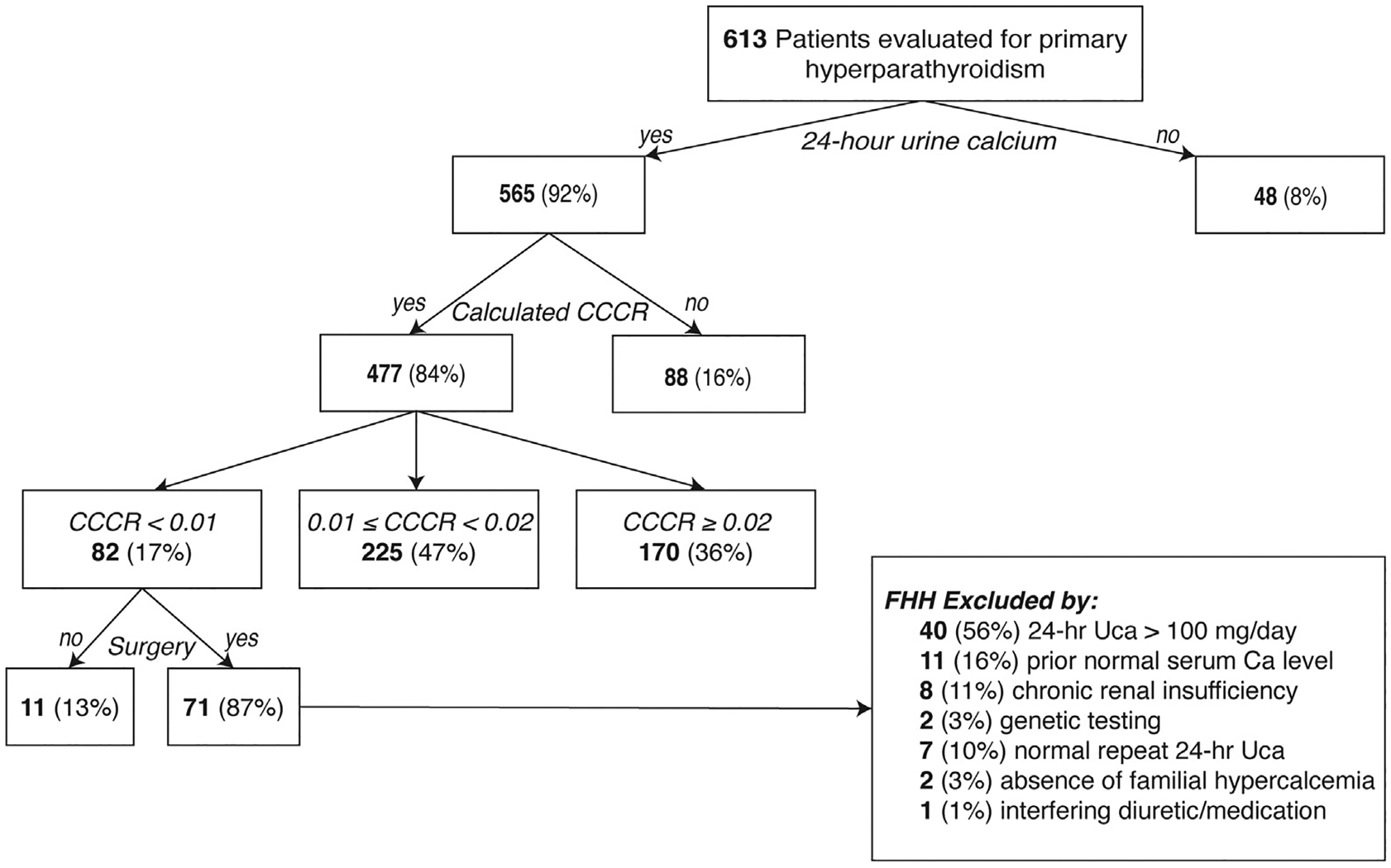
Classification of patients with suspected primary hyperparathyroidism based on 24-hour urine calcium and calcium-to-creatinine clearance ratio.
A repeat 24UCa was necessary in only 25 of 565 patients (4%)—22 (88%) for prior 24UCa <100 mg/day, 2 (8%) for transient correction of diuretic use, and 1 (4%) due to prolonged time since prior collection. Of the 22 patients who underwent repeat 24UCa testing for 24UCa values <100 mg/day, 6 were on a diuretic that was stopped temporarily for repeat testing, and, eventually, 18/22 (82%) had repeat 24UCa values that were then normal. Of the 4 patients with repeat low 24UCa testing, 3 had FHH ruled out by prior normal sCa levels, and 1 was referred to endocrinology and found to have potassium citrate interference with 24UCa results; all 4 patients ultimately underwent successful parathyroid exploration for parathyroid adenoma (N = 3) or multiglandular disease (N = 1) without evidence of surgical failure.
Exclusion and diagnosis of FHH
Of 82 patients with CCCR <0.01, 71 (87%) have undergone parathyroid exploration to date. In those who had surgery, FHH was excluded by 24UCa >100 mg/day (56%), documentation of prior normal serum calcium(s) (16%), chronic renal insufficiency (11%), negative genetic testing (3%), normal repeat 24UCa (10%), an absence of familial hypercalcemia (3%), or interfering diuretic/medication contributing to abnormal results (1%) (Fig 2). Of the 11 patients who did not undergo surgery, 4 (36%) had 24Uca >100 mg/day (1 is scheduled for surgery, 1 did not undergo surgery due to insurance issues, and 2 are pending cardiac evaluation), 2 (18%) had historical normal serum calcium (1 awaits surgery scheduling and 1 is being treated for cholangiocarcinoma), 1 (9%) had normal 24UCa on repeat testing but awaits scheduling, 2 (18%) are pending genetic testing, 1 (9%) had low 24UCa while on hydrochlorothiazide and is being managed medically given advanced age, and 1 (9%) who has a relative with a CASR variant of uncertain significance (VUS) (c.1644T>C) awaits genetic testing and is currently being managed nonoperatively (discussed further below). Of the 4 patients referred for genetic testing, 2 were negative and 2 are awaiting results.
Although to date none of the patients in the study cohort have had conventional FHH confirmed by genetic testing, a CASR VUS was identified in 1 of 2 patients from the same kindred who were not first-degree relatives. The first patient had a family history of parathyroid surgery in her mother, aunt, and uncle, and at age 48 presented with mild hypercalcemia and PTH elevation associated with a normal preoperative 24UCa (CCCR = 0.014). Despite single-focus imaging results, she had 4-gland hyperplasia and received subtotal parathyroidectomy with a decrease in her intraoperative PTH from 92 to 13 pg/mL (normal <65 pg/mL). She remains eucalemic 1.5 years later. She received genetic testing based on her family history and was found to carry a CASR VUS (c.1644T>C) with no mutations in MEN1, AP2S1, GNA11, or CDKN1B. In the interim, the daughter of her maternal cousin presented at age 30 with hypercalcemia and PTH elevation but had a low 24UCa (CCCR = 0.004); because of her hypocalciuria, family history, and the CASR results in her relative, surgery was deferred and genetic evaluation is pending.
24UCa-directed change in care
Overall, a 24UCa-directed change in care occurred in 25/565 (4%). This included 3 patients who had repeat 24UCa testing and referral for genetic testing, 1 who had referral for genetic testing due to initial low 24UCa, 19 who had repeat 24UCa testing alone, 2 asymptomatic patients with 24UCa >400 mg/day as the sole indication for surgery. Of the 23 patients who had deferment of surgery for additional testing, 19 (83%) ultimately had parathyroid exploration. Of those who did not undergo surgery, 1 is awaiting genetic testing results, 1 refused surgical intervention and is being managed medically, 1 was referred for genetic testing and was lost to follow-up, and 1 is being managed nonoperatively due to familial CASR VUS mutation (see earlier).
Pathology and surgical failure rates
After confirmation of biochemical diagnosis, 553 of 613 (90.4%) had parathyroid exploration. pHPT due to a single parathyroid adenoma was the most common finding (76%), followed by multiglandular disease (24%) including 6% with 4-gland hyperplasia (Table II). Multiglandular disease was more frequent in patients with CCCR <0.01 compared to those with CCCR ≥0.01 (35% vs 22%, P = .02), and pHPT due to 4-gland hyperplasia was 4 times more common in patients with CCCR <0.01 (17% vs 4%, P < .001). Surgical failure rates did not differ by CCCR (CCCR <0.01, 4% vs 1%, P = .24).
Table II.
Pathology results overall and stratified by CCCR level
| Overall (N = 552) | CCCR <0.01 (N = 71) | CCCR ≥0.01 (N = 366) | P value | |
|---|---|---|---|---|
| Pathology, N (%) | P < .001 | |||
| Single adenoma | 420 (76) | 46 (65) | 285 (78) | |
| Multiglandular disease | 132 (24) | 25 (35) | 81 (22) | |
| 4-gland hyperplasia | 31 (6) | 12 (17) | 16 (4) |
CCCR, calcium-to-creatinine clearance ratio.
Of the 48 patients without 24UCa collection, 37 (77%) went on to have surgery after FHH was ruled to be unlikely by normal prior serum calcium and lack of family history (23, 62%) or lack of family history alone (14, 38%). Compared with patients who had 24UCa collected, when 24UCa was not obtained preoperatively, there was an equivalent frequency of multiglandular disease (22% vs 24%, P = .81), 4-gland hyperplasia (11% vs 5%, P = .14), and surgical failure (3% vs 2%, P = .50).
Discussion
Although rare, FHH is clinically relevant owing to its overlap in presentation with pHPT, and due to distinct treatment algorithms, differentiation of the 2 diseases is important. In particular, missing FHH can result in unnecessary surgical intervention. Although several clinical characteristics can assist in differentiating pHPT from FHH, these characteristics are confounded by considerable diagnostic overlap between the 2 diseases. In the past, the presence of symptoms was reportedly less common in FHH than pHPT, but as a larger proportion of patients present today with seemingly asymptomatic pHPT due to identification of hypercalcemia by routine serum screening, such clinical criteria are less useful in the differentiation of the 2 diseases.9,12
The most discriminative feature between the 2 diseases is the difference in renal processing of calcium. In pHPT, elevated PTH levels stimulate renal parathyroid hormone receptor 1 to increase renal resorption of calcium. The resulting increased serum calcium level leads to CaSR-mediated renal calcium excretion in an effort to maintain calcium balance.13 FHH is due to a faulty CaSR mechanism resulting in paradoxically increased calcium resorption and subsequent hypocalciuria.4 Indices of renal calcium processing, including 24UCa excretion, 24-hour urine calcium/creatinine excretion ratio, and CCCR, have been previously examined and CCCR has demonstrated the highest discriminative power, with a sensitivity of 80% to 86% and specificity of 74% to 88% in diagnosing FHH using a cutoff value of 0.0115.5,6
Although CCCR has outperformed the other 2 renal indices, misclassification can still exist with its use, and its value in discriminating the 2 diseases is further limited by a large indeterminate range (0.01–0.02). In fact, a decade ago the insufficient diagnostic performance of CCCR led to recommendation of a 2-step screening process involving initial CCCR assessment and subsequent CASR genetic testing for patients with CCCR <0.02.6 However, current guidelines recommend the use of 24UCa or CCCR alone to exclude FHH.3,14 An interesting 2018 study by Moore et al investigated the use of CCCR in 1,000 patients who all received parathyroid surgery and concluded that CCCR was not helpful, at least in part because a large proportion (63%) of patients had CCCR <0.02 (ie, in the FHH or indeterminate range), but also because all patients in the series enjoyed surgical cure.8 The observed distribution of CCCR in our study was very similar, with 64% of patients having CCCR <0.02 and no confirmed cases of conventional FHH to date.
Although Moore et al did not identify differences in distribution of parathyroid histologic subtype between CCCR levels, we observed a significantly increased frequency of multiglandular disease and 4-gland hyperplasia in those with CCCR <0.01. Additionally, our rate of multiglandular disease (24%) was higher than a prior regional reported rate of 16%.15 Therefore, in patients with CCCR <0.01 after exclusion of FHH, the higher rate of multiglandular disease may be helpful for preoperative counseling and potentially guide a lower threshold for 4-gland exploration, but the majority still have single adenoma (65%) and are candidates for curative minimally invasive parathyroidectomy.
To date, there is also a lack of information surrounding the impact of routine use of 24-hour urine studies in the management of pHPT.5,6,8 In our study, compliance with 24UCa testing was high (92%). However, the incidence of 24UCa-directed change in care was relatively low (4%), and only 1% of patients required genetic testing. Additionally, 87% of patients with CCCR <0.01 ultimately underwent surgery, and there was no significant difference in surgical failure rates for those with CCCR <0.01 compared to CCCR ≥0.01, further confirming the diagnosis of pHPT. Thus, when CCCR <0.01, clinical assessment including prior normal serum calcium, lack of a family history of hypercalcemia, or exclusion of another reason for low 24UCa such as low vitamin D, low calcium intake, or improper specimen handling was sufficient to exclude FHH in most patients.
Interestingly, the small cohort of patients (n = 48) who did not have 24UCa collected preoperatively and had parathyroid exploration did not have significantly different rates of multiglandular disease (including 4-gland hyperplasia) or surgical failure compared to those with 24UCa collection. This finding further supports that 24UCa (or lack thereof) did not frequently alter management or outcomes in our study population. However, obtaining the 24UCa did successfully serve as a reminder to exclude FHH preoperatively. Prospective studies are necessary to assess if other methods of ruling out FHH are as effective as 24UCa.
The current analysis also found that 24UCa did productively identify hypercalciuria as the sole indication for surgery in 0.4% of patients. This supports the use of 24UCa especially in patients with asymptomatic pHPT or without other indications for surgery. Our findings do not support abandoning 24UCa, but rather more judicious use for selected patients. Other studies have suggested alternative methods to distinguish pHPT from FHH, including 2-step screening as described above and (in Europe) the development of an unsupervised risk equation (pro-FHH), which demonstrated improved area under a receiver operating characteristic curve compared to CCCR.6,9 However, the suggested 2-step screening in those with CCCR <0.02 would have required 64% of patients with calculated CCCR in our study to have genetic testing, which is neither practical nor cost-effective. And although “pro-FHH” appears to offer improved discrimination of pHPT from FHH compared to CCCR, it requires routine measurements of serum osteocalcin, which is not standard in the United States; moreover, prospective validation studies are still needed before clinical adoption.9
The major limitation of this study is its retrospective nature, precluding universal biochemical analysis in that not all patients had concurrent biochemical tests to calculate CCCR and therefore had to be excluded from comparative analysis of histological classification and surgical failure. The study patient population was also inherently biased because all patients had undergone initial evaluation for suspected hyperparathyroidism before referral. Therefore, patients with either clinical or biochemical evidence of suspected FHH may have not been referred for endocrine surgery evaluation. Additionally, because this study was performed at a single institution, access to outside records was limited and treatment patterns were specific to 1 high-volume endocrine surgery program. Lastly, the utilized exclusion criteria for FHH were clinical and not genetic for the majority of patients. Substantial genetic heterogeneity exists in FHH with more than 130 mutations identified in CASR alone and multiple reported novel mutations in FHH patients.16–19 Therefore, the genetic testing performed in this study could have missed a mutation, resulting in FHH, which, since none of the study cohort had genetically confirmed FHH, likely affected the frequency of 24UCa-directed change in care and limited conclusions about disease discrimination. Furthermore, not all patients with FHH have a low CCCR. However, FHH patients not appropriately diagnosed preoperatively would be expected to seemingly have multiglandular disease at parathyroid exploration but be hypercalcemic postoperatively. We observed a rate of operative failure that was low in short follow-up. The large patient population evaluated here without a confirmed case of conventional FHH suggests that it is uncommonly encountered in the initial evaluation of pHPT for surgical intervention.
In summary, in our geographical region of the United States the overall incidence of 24UCa-directed change in care was low, with an even lower observed frequency of need for genetic testing (1%) to exclude FHH. Compliance was high, suggesting that completion of the test can be readily accomplished. 24UCa was useful for excluding FHH and was the most frequently used method to rule out FHH in patients referred for surgical evaluation of pHPT. When CCCR was <0.01, clinical assessment, including repeat testing and/or patient history, was often adequate to rule out FHH, as shown by the infrequent referral for genetic testing. However, 24UCa did affect care in 4% of patients including providing the sole indication for surgery in some patients. Further, a low CCCR was associated with a 4-fold increased likelihood of anatomic multiglandular disease, which may be helpful for both preoperative counseling and surgical planning.
Discussion.
Dr. W. Barry Inabnet, III (Lexington): Did you analyze the volume of the 24-hour specimens collected? Low and high volume urine collections can influence the lab analysis.
Dr. Shimena Li: Yes. Thank you for that question. We did look at the volumes, and that was also evaluated by the endocrine surgeon. If they were inadequate, then the test was repeated.
Dr. David Schneider (Madison): Are there biochemical thresholds where you would not need a 24-hour urine? It is a cumber-some test to require routinely.
Dr. Shimena Li: That is a great question. The underlying question that we had for this study is whether there is a group of patients for whom 24-hour urine calcium does not need to be collected. From the data we collected, I don’t think we have a specific threshold biochemically, but I do think we need to be more judicious about ordering 24-hour urine calcium.
Dr. Philip Smith (Charlottesville): Can you comment on predictors of who would have a low CCCR and therefore those patients in whom it may be more useful?
Dr. Shimena Li: That’s another interesting question. I think what we have concluded from this study is that 24-hour urine calcium should be obtained in those who don’t have a prior indication for surgery as we did identify two patients with hypercalciuria. However, in terms of predictors, we didn’t specifically look at what would predict a low CCCR in our patient population, so I don’t have an answer to that question from our data review.
Dr. Naris Nilubol (Bethesda): For whom would you choose to send the CCCR?
Dr. Shimena Li: I think the clear reason for obtaining a CCCR is in patients who are asymptomatic and who do not have an indication for surgery. As I said before, we did productively identify two patients with hypercalciuria. We need further prospective studies to determine which symptomatic patients need CCCR measurement.
Dr. Rebecca Sippel (Madison): Your data suggest that there is little utility to routine urinary calcium testing. Have you modified your practice based on your data?
Dr. Shimena Li: I think this data adds to mounting evidence. Previous studies have suggested that the discriminatory value of CCCR is quite low in distinguishing the two diseases. To that, we are adding that there’s not a very frequent change in care based on these 24-hour urinary values. We haven’t changed our practice yet. I think one thing that the endocrine surgeons will use going forward is preoperative planning and counseling for those patients who have a CCCR of less than .01 as we did see an increased risk of multiglandular disease in those patients. There may be a lower threshold for bilateral exploration in those particular patients.
Dr. Christopher Mchenry (Cleveland): Can you describe more specifically what you mean by judiciously?
Dr. Shimena Li: What we mean by judiciously is perhaps the routine 24-hour urine collection for all patients being evaluated for primary hyperparathyroidism may not be necessary. I think we still need more prospective data to understand specifically which patient population needs 24-hour urinary calcium measurement but we need to think about obtaining it in specific populations instead of in all patients being evaluated for primary hyperparathyroidism.
Acknowledgments
SEC gratefully acknowledges the generous support of the William and Susan Johnson Fund for Endocrine Surgery Research.
Funding/Support
This research was supported by the grant 5T32HL0098036 from the National Heart, Lung, and Blood Institute (SRL). This grant had no role in the design and conduct of the study, data collection, analysis, or interpretation of the data. Preparation, review, and decision to submit this research for publication was independent from this grant.
Footnotes
Conflict of interest/Disclosure
There are no conflicts of interest or relevant financial interest related to the content of this article reported by the authors.
Presented at the American Association of Endocrine Surgeons (AAES) 2021 Virtual Annual Meeting, Podium Presentation, April 25–27, 2021.
References
- 1.Bilezikian JP, Bandeira L, Khan A, Cusano NE. Hyperparathyroidism. Lancet. 2018;391:168–178. [DOI] [PubMed] [Google Scholar]
- 2.Clarke BL. Epidemiology of primary hyperparathyroidism. J Clin Densitom. 2013;16:8–13. [DOI] [PubMed] [Google Scholar]
- 3.Wilhelm SM, Wang TS, Ruan DT, et al. The American Association of Endocrine Surgeons guidelines for definitive management of primary hyperparathyroidism. JAMA Surg. 2016;151:959–968. [DOI] [PubMed] [Google Scholar]
- 4.Hannan FM, Babinsky VN, Thakker RV. Disorders of the calcium-sensing receptor and partner proteins: insights into the molecular basis of calcium homeostasis. J Mol Endocrinol. 2016;57:R127–R142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhangu JS, Selberherr A, Brammen L, Scheuba C, Riss P. Efficacy of calcium excretion and calcium/creatinine clearance ratio in the differential diagnosis of familial hypocalciuric hypercalcemia and primary hyperparathyroidism. Head Neck. 2019;41:1372–1378. [DOI] [PubMed] [Google Scholar]
- 6.Christensen SE, Nissen PH, Vestergaard P, Heickendorff L, Brixen K, Mosekilde L. Discriminative power of three indices of renal calcium excretion for the distinction between familial hypocalciuric hypercalcaemia and primary hyperparathyroidism: a follow-up study on methods. Clin Endocrinol (Oxf). 2008;69:713–720. [DOI] [PubMed] [Google Scholar]
- 7.Bilezikian JP, Brandi ML, Eastell R, et al. Guidelines for the management of asymptomatic primary hyperparathyroidism: summary statement from the Fourth International Workshop. J Clin Endocrinol Metab. 2014;99: 3561–3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moore EC, Berber E, Jin J, Krishnamurthy V, Shin J, Siperstein A. Calcium creatinine clearance ratio is not helpful in differentiating primary hyperparathyroidism from familial hypercalcemic hypocalciuria: a study of 1000 patients. Endocr Pract. 2018;24:988–994. [DOI] [PubMed] [Google Scholar]
- 9.Bertocchio JP, Tafflet M, Koumakis E, et al. Pro-FHH: a risk equation to facilitate the diagnosis of parathyroid-related hypercalcemia. J Clin Endocrinol Metab. 2018;103:2534–2542. [DOI] [PubMed] [Google Scholar]
- 10.Wharry LI, Yip L, Armstrong MJ, et al. The final intraoperative parathyroid hormone level: how low should it go? World J Surg. 2014;38:558–563. [DOI] [PubMed] [Google Scholar]
- 11.McCoy KL, Yip L, Dhir M, Langenborg K, Seethala RR, Carty SE. Histologic hypercellularity in a biopsied normal parathyroid gland does not correlate with hyperfunction in primary hyperparathyroidism. Surgery. 2021;169:524–527. [DOI] [PubMed] [Google Scholar]
- 12.Shinall MC Jr, Dahir KM, Broome JT. Differentiating familial hypocalciuric hypercalcemia from primary hyperparathyroidism. Endocr Pract. 2013;19: 697–702. [DOI] [PubMed] [Google Scholar]
- 13.Hannan FM, Kallay E, Chang W, Brandi ML, Thakker RV. The calcium-sensing receptor in physiology and in calcitropic and noncalcitropic diseases. Nat Rev Endocrinol. 2018;15:33–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eastell R, Brandi ML, Costa AG, D’Amour P, Shoback DM, Thakker RV. Diagnosis of asymptomatic primary hyperparathyroidism: proceedings of the Fourth International Workshop. J Clin Endocrinol Metab. 2014;99: 3570–3579. [DOI] [PubMed] [Google Scholar]
- 15.Wang TS, Pasieka JL, Carty SE. Techniques of parathyroid exploration at North American endocrine surgery fellowship programs: what the next generation is being taught. Am J Surg. 2014;207:527–532. [DOI] [PubMed] [Google Scholar]
- 16.Hannan FM, Thakker RV. Calcium-sensing receptor (CaSR) mutations and disorders of calcium, electrolyte and water metabolism. Best Pract Res Clin Endocrinol Metab. 2013;27:359–371. [DOI] [PubMed] [Google Scholar]
- 17.Nissen PH, Christensen SE, Heickendorff L, Brixen K, Mosekilde L. Molecular genetic analysis of the calcium sensing receptor gene in patients clinically suspected to have familial hypocalciuric hypercalcemia: phenotypic variation and mutation spectrum in a Danish population. J Clin Endocrinol Metab. 2007;92:4373–4379. [DOI] [PubMed] [Google Scholar]
- 18.Mahajan A, Buse J, Kline G. Parathyroid hormone-dependent familial hypercalcemia with low measured PTH levels and a presumptive novel pathogenic mutation in CaSR. Osteoporos Int. 2020;31:203–207. [DOI] [PubMed] [Google Scholar]
- 19.Cordes M, Kuwert T, Haag C, Raue F. A novel mutation of the calcium sensing receptor gene in a franconian kindred: heterozygous mutation c.1697_1698delTG Exon 6. Horm Metab Res. 2017;49:142–146. [DOI] [PubMed] [Google Scholar]


