Abstract
Abacavir (formerly 1592U89) is a potent 2′-deoxyguanosine analog reverse transcriptase inhibitor that has been demonstrated to have a favorable safety profile in initial clinical trials with adults with human immunodeficiency virus (HIV) type 1 infection. A phase I study was conducted to evaluate the pharmacokinetics and safety of abacavir following the administration of two single oral doses (4 and 8 mg/kg of body weight) to 22 HIV-infected children ages 3 months to 13 years. Plasma was collected for analysis at predose and at 0.5, 1, 1.5, 2, 2.5, 3, 5, and 8 h after the administration of each dose. Plasma abacavir concentrations were determined by high-performance liquid chromatography, and data were analyzed by noncompartmental methods. Abacavir was well tolerated by all subjects. The single abacavir-related adverse event was rash, which occurred in 2 of 22 subjects. After administration of the oral solution, abacavir was rapidly absorbed, with the time to the peak concentration in plasma occurring within 1.5 h postdosing. Pharmacokinetic parameter estimates were comparable among the different age groups for each dose level. The mean maximum concentration in plasma (Cmax) and the mean area under the curve from time zero to infinity (AUC0–∞) increased by 16 and 45% more than predicted, respectively, as the abacavir dose was doubled from 4 to 8 mg/kg (Cmax increased from 1.69 to 3.94 μg/ml, and AUC0–∞ increased from 2.82 to 8.09 μg · h/ml). Abacavir was rapidly eliminated, with a mean elimination half-life of 0.98 to 1.13 h. The mean apparent clearance from plasma decreased from 27.35 to 18.88 ml/min/kg as the dose increased. Neither body surface area nor creatinine clearance were correlated with pharmacokinetic estimates at either dose. The extent of exposure to abacavir appears to be slightly lower in children than in adults, with the comparable unit doses being based on body weight. In conclusion, this study showed that abacavir is safe and well tolerated in children when it is administered as a single oral dose of 4 or 8 mg/kg.
The predominant mode of pediatric human immunodeficiency virus (HIV) type 1 (HIV-1) infection is by the vertical transmission of HIV-1 from infected mothers to their infants (18). The Centers for Disease Control and Prevention (CDC) has estimated that 6,000 to 7,000 children have been born annually to HIV-1-infected women since 1978 (10, 19). The rapid evaluation of novel antiretroviral therapies alone and in combination with more established therapies with pediatric populations is therefore critical.
Currently, five antiretroviral agents are approved for use in children. Four of the agents are nucleoside analogs that act as competitive inhibitors or chain terminators of the reverse transcriptase enzyme of HIV-1 and include zidovudine, stavudine, and didanosine. The remaining agent, ritonavir, is an inhibitor of HIV-1 protease. Several of the currently available antiretroviral therapies have a limited duration of efficacy due to toxicities or the emergence of viral resistance.
The potent and selective anti-HIV activity of abacavir (formerly 1592U89), (−)-(1S,4R)-4-[2-amino-6-(cyclopropylamino)-9H-purin-9-yl]-2-cyclopentene-1-methanol, a nucleoside analog, has recently been described (7, 9, 11–14, 21, 22) and is summarized in the accompanying report of a single-dose study with adults (16). The pharmacokinetics of escalating single oral doses of abacavir in adults have been evaluated for the companion report (16). Abacavir is rapidly and well absorbed over the range of doses studied (100 to 1,200 mg), with the time to the peak concentration in plasma occurring at 1 to 1.7 h postdosing. All doses resulted in plasma abacavir concentrations that exceeded the mean 50% inhibitory concentration (IC50) of abacavir for clinical HIV isolates in vitro. Abacavir was well tolerated, with no treatment-limiting toxicities reported. On the basis of the favorable data, this single-dose phase I study (Glaxo Wellcome protocol CNAA1001) was initiated with children to examine the pharmacokinetics and safety of abacavir.
(This work was presented in part at the 3rd Conference on Retroviruses and Opportunistic Infections, Alexandria, Va., 1996 [14].)
MATERIALS AND METHODS
Study population.
Subjects between the ages of 3 months and 13 years and with HIV infection were eligible for the study. The study was approved by the institutional review board of each participating institution. Written informed consent was obtained from each child’s parent or legal guardian. Subjects were eligible for the study if they had HIV infection defined as positive results on two separate determinations (excluding a determination with cord blood) by one or more HIV detection tests (HIV culture, HIV PCR, or HIV p24 antigen) and met the criteria for an AIDS diagnosis based on the 1994 CDC AIDS surveillance case definition (5). Subjects who were 18 months of age or older were also eligible for the study if they tested positive for antibody to HIV-1.
Subjects were required to be clinically stable and to be free of active opportunistic infections. All subjects were required to have clinical laboratory values that were normal for their ages or that deviated from normal by no more than the values for grade 1 of the AIDS Clinical Trials Group adverse event grading table. These assessments included routine hematologic analysis (complete blood count with differential, mean corpuscular volume, and platelet count), serum chemistry analysis (electrolyte, aspartate aminotransferase, alanine aminotransferase, total bilirubin, creatinine, albumin, glucose, alkaline phosphatase, and serum amylase, cholesterol, and triglyceride levels), and urinalysis (dipstick for protein and blood).
Subjects were excluded from the study if their concomitant medications included those that could potentially interfere with the absorption or elimination of abacavir (such as rifampin and probenecid), other antiretroviral agents, other investigational agents, glucocorticoids, and those that are used for prophylaxis against Pneumocystis carinii pneumonia and that could not be temporarily discontinued during the dosing phase of the study (such as co-trimoxazole, dapsone, and atovaquone). Subjects were also excluded from the study if they were pregnant (females of childbearing potential) or had clinical evidence of disease progression, including a body weight of less than 5 kg, wasting or failure to thrive (as defined in the CDC guidelines [5]), and progressive encephalopathy. All prescription and over-the-counter medications were withheld for 48 h prior to dosing and for 24 h after dosing.
Study design.
To ensure an adequate distribution for pharmacokinetic analysis of both doses, at least six subjects were enrolled in each of the following cohorts, by age: 3 to 5 months, 6 to 23 months, 2 to 5 years, and 6 to 13 years. Subjects received single oral doses of 4 mg of abacavir solution per kg of body weight, followed by a second single dose of 8 mg/kg after at least a 14-day washout period. Those in the older-age cohorts received both doses of abacavir and completed the study before the youngest-age cohort (3 to 5 months) was enrolled. The doses selected for use in the current study were based on results from an escalating, single-oral-dose study of abacavir with HIV-infected adults (16). To ensure comparable exposures to abacavir, doses of 4 and 8 mg/kg (approximately equivalent to unit doses of 300 and 600 mg for adults, respectively) were evaluated and predicted to produce total drug concentrations in plasma greater than the mean in vitro IC50 of abacavir for clinical isolates of HIV-1 for approximately 3.5 to 4.5 h.
Abacavir was supplied as the lyophilized succinate salt containing 300 mg of free base equivalent and was reconstituted with 50 ml of sterile distilled water to a concentration of 6 mg/ml. The drug was administered via a 10-ml Exacta-Med Dispenser, provided by Glaxo Wellcome Inc., for volumes of less than 10 ml. The same dispenser was used to measure aliquots, which were combined in a dosing cup for dosing volumes of greater than 10 ml. Each dose was followed immediately by a minimum amount of water, given as follows: 1 oz (for subjects ages 3 to 5 months), 2 oz (for subjects ages 6 to 23 months), and 3 oz (for subjects ages 2 to 13 years).
Within 7 days of administration of the first dose, the subjects underwent a screening evaluation, including a medical history, physical examination, and measurement of clinical and laboratory parameters. All drugs were discontinued at least 48 h before dosing with abacavir and were not reinstituted until 24 h after the administration of each dose. The subjects fasted for at least 2 h before dosing and for 1 h after dosing. The subjects returned to the study site at least 14 days later to begin the next dosing period. At 10 to 14 days after completion of the last dosing period, subjects returned for a follow-up examination similar to that used for the screening evaluation.
Clinical and laboratory monitoring.
The safety and tolerability of abacavir were evaluated on the basis of adverse experience reports, measurements of vital signs and clinical laboratory test values, and the results of physical examinations. In each dosing period, the severity (mild, moderate, or severe), duration, and potential relationship to abacavir (unrelated or possibly, probably, or almost certainly related, according to the investigator) of any adverse events were recorded. Medical histories were obtained and complete physical examinations were performed at all visits. Vital sign determinations (sitting blood pressure and sitting pulse), electrocardiogram (ECG) studies, routine hematologic studies, serum chemistry studies, and urinalysis (dipstick for protein and blood) were performed at screening, at 48 h postdosing in each dosing period, and at each follow-up visit (10 to 14 days postdosing).
Blood sampling and analytical methods.
Blood samples (1.5 ml each) were collected by venipuncture and placed in a powdered EDTA-containing pediatric tube immediately before dosing and at 0.5, 1.0, 1.5, 2.0, 2.5, 3.0, 5.0, and 8.0 h postdosing. Blood samples were kept at 4°C upon collection and were centrifuged within 1 h of collection to separate the plasma, which was stored at −40°C until it was analyzed. The stability of abacavir in plasma samples has been validated at −40°C for 11 months, which covered the period from the time of sample collection to the time of analysis of the plasma samples.
Plasma abacavir concentrations were determined by a validated reversed-phase high-performance liquid chromatography (HPLC) assay with UV detection. Briefly, analytical stock standard and control solutions were prepared separately in HPLC-grade water. The appropriate volumes of the stock solutions were spiked into normal, blank, pooled human plasma to provide working standards or controls. The quantifiable range was 25 to 5,000 ng/ml, and the control concentrations were 40, 400, and 2,500 ng/ml. To 0.2 ml of standard, control, or unknown samples, 0.1 ml of 10% trichloroacetic acid was added, and the components were mixed by vortexing and were centrifuged at 8,800 × g for 10 min. The supernatants were transferred into injection vials (containing limited volume inserts) and were placed in an autosampler. The supernatants (0.1 ml) were injected at 15-min intervals, and the chromatographic separation was achieved on a Rainin C18 Microsorb MV column. The mobile phase consisted of 40% methanol and 0.3% triethylamine (vol/vol) (TEA) at a constant flow rate of 1.0 ml/min. Abacavir was detected by measuring the UV absorbance at 284 nm. The approximate retention time for abacavir was 9 min under these conditions. The interday precisions (percent coefficient of variation) calculated from the quality control samples was 9.1% at 0.04 μg/ml, 4.7% at 0.40 μg/ml, and 4.8% at 2.50 μg/ml; and the interday variabilities (biases) were 5.5, 3.4, and 4.6%, respectively.
Pharmacokinetic analysis.
The pharmacokinetic parameters for abacavir were determined by noncompartmental methods with the WinNonlin Noncompartmental Analysis Program (Scientific Consulting, Inc., Cary, N.C.). The peak concentration in plasma (Cmax) and the time to Cmax (Tmax) were obtained from direct inspection of the plasma concentration-time profile. Estimates for the apparent terminal elimination half-life (t1/2β) were calculated as ln(2)/λz, where λz is the terminal elimination rate constant and is a first-order rate constant determined from the negative of the slope of the linear regression line of the apparent terminal linear portion of the log concentration-versus-time curve. The WinNonlin program selected at least three points for inclusion in the linear regression. These points were visually inspected, with no changes made to the selected points. The area under the curve from time zero to time t (AUC0–t), where t is the last time point with a measurable concentration of abacavir, was calculated by the linear trapezoidal method. The AUC from time zero to infinity (AUC0–∞) was then determined as AUC0–t + Clast/λz, where Clast is the last measurable concentration. The apparent clearance from plasma (CL/F) was calculated as the dose divided by AUC0–∞ and was then normalized to body weight. The apparent volume of distribution (V/F) was calculated as the dose divided by the product of λz and AUC0–∞. Creatinine clearance was obtained as the product of 0.55 and the ratio of the subject’s height to serum creatinine value (20). Body surface area was calculated as the product of 9.8 and (body weight)0.67 (8).
Statistical analysis.
Differences between treatments with respect to Cmax, AUC0–∞, t1/2β, λz, CL/F, and V/F values were assessed by analysis of variance with PROC MIXED (or mixed effects linear models) from SAS (version 6.09). The model included age category as the fixed effect and subjects as the random effect. Analyses were performed for both log-transformed and untransformed data. Descriptive statistics, including geometric least-squares means and their 95% confidence intervals (CIs), were calculated for each treatment. Dose proportionality was determined by calculating the ratios of the geometric least-squares mean for the 8-mg/kg dose to the geometric least-squares mean for the 4-mg/kg dose and the resultant 90% CI of each parameter of interest. The 90% CI of the geometric least-squares ratios was used because this dose-proportionality comparison is essentially a bioequivalence comparison between the 4- and 8-mg/kg treatments in which the 90% CI is the standard for bioequivalence comparisons. Treatments were considered dose proportional if the resultant 90% CI for the ratios of the least-squares means of AUC0–∞ and Cmax contained 2.0. Treatments were considered similar if the 90% CIs for the ratios of the least-squares means of t1/2β, λz, CL/F, and V/F contained 1.0. Nonparametric methods were used to compute the 95% CI for the untransformed median Tmax values for each treatment. A 90% CI for the median Tmax difference between treatments was calculated, and the Wilcoxon signed rank test was used to compare differences between treatments. The Spearman test was used to determine the correlation between each log-transformed parameter (Cmax, AUC0–∞, and λz) and each demographic variable (age, weight, creatinine clearance, and body surface area). A stepwise procedure with PROC REG from SAS was used to select demographic variables that can be good predictors of the pharmacokinetic parameters.
RESULTS
Subject demographics and accountability.
Twenty-two subjects were actually enrolled in this study (Table 1). Three subjects were enrolled in the youngest-age cohort (3 to 5 months). Subjects in all cohorts except the cohort of subjects ages 6 to 23 months received both doses of abacavir. In the cohort of subjects ages 6 to 23 months, five subjects received both doses of abacavir, one subject received the 4-mg/kg dose, and another subject received the 8-mg/kg dose.
TABLE 1.
Characteristics of subjects at study entry
| Characteristic | Age group
|
|||
|---|---|---|---|---|
| 3–5 mo | 6–23 mo | 2–5 yr | 6–13 yr | |
| No. of subjects | 3 | 7 | 6 | 6 |
| Age (yr) | ||||
| Median | 5.4 | 14.64 | 2.72 | 12.92 |
| Range | 5.28–5.40 | 6.12–20.04 | 2.06–4.94 | 7.68–13.32 |
| Sex (no. of males/ no. of females) | 2/1 | 4/3 | 6/0 | 3/3 |
| Race (no. of subjects) | ||||
| White | 0 | 1 | 0 | 1 |
| Black | 3 | 5 | 6 | 5 |
| Other | 0 | 1 | 0 | 0 |
| Ht (cm) | ||||
| Median | 61 | 79 | 87 | 142 |
| Range | 59–62 | 54–84 | 75–104 | 127–146 |
| Wt (kg) | ||||
| Median | 5.5 | 9.9 | 11.8 | 31.2 |
| Range | 5.1–5.7 | 3.9–14.8 | 8.9–17.9 | 26.8–44.3 |
The following subjects had exceptions to the screening criteria but were enrolled with the approval of the sponsor. One subject (subject 17) did not meet the minimum weight requirement of 5 kg because her screening weight at 6 months was 3.9 kg. Two subjects (subjects 15 and 20) stopped concomitant therapy 24 h rather than 48 h prior to study drug administration, and two subjects received a dose of diphenhydramine hydrochloride (Benadryl) on the study day for the treatment of a rash.
One subject (subject 16) with nonspecific abnormalities on screening ECG was enrolled, and subsequent ECGs were normal. While most screening clinical laboratory and hematology values were within the normal range for age or deviated from the normal values by no more than the values for grade 1 of the AIDS Clinical Trials Group adverse event grading table, there were five exceptions to this general rule. At the screening evaluation two subjects (subjects 4 and 13) had elevated serum amylase levels of grade 3 (subsequent values were normal) and grade 2 (subsequent values were unchanged), respectively. At the screening evaluation subject 1 had a low blood glucose level that was grade 4 but that was thought to be due to incorrect handling. Subject 17 had a grade 2 elevation of the potassium level. Finally, one subject (subject 5) had a grade 2 reduced hemoglobin level at the screening evaluation.
Safety evaluation.
Abacavir was well tolerated. There were no withdrawals due to adverse events. Two subjects experienced four adverse events while receiving the 4-mg/kg dose, and three subjects experienced four adverse events while receiving the 8-mg/kg dose. None of the adverse events were serious, and all were considered mild or moderate in intensity. Three subjects each experienced a single event of rash, and for two subjects (ages 5 and 13 years), the rash was attributed to the 8-mg/kg dose of abacavir. The rash resolved within 24 h after administration of diphenhydramine hydrochloride. For the third subject, the rash was diagnosed as candidiasis and was treated with topical antifungal agents.
No clinically significant changes in hematologic findings, clinical chemistry findings, vital signs, physical examination findings, or urinalysis parameters attributable to abacavir were noted during the study. Hematologic changes were noted in several subjects: three subjects (4 mg/kg) and two subjects (8 mg/kg) had decreased hemoglobin levels (grade 2), one subject (4 mg/kg) had a decreased neutrophil count (grade 3) which returned to normal at follow-up, and one subject (8 mg/kg) also had a decreased neutrophil count (grade 2). Clinical chemistry changes were noted in two subjects. One subject had elevated alkaline phosphatase and aspartate aminotransferase (grade 1) values after taking the 4-mg/kg dose, but the values had decreased at subsequent follow-up visits. Another subject with elevated baseline alkaline phosphatase values had normal values after taking the 4-mg/kg dose but had elevated values again after taking the 8-mg/kg dose.
Pharmacokinetic evaluation.
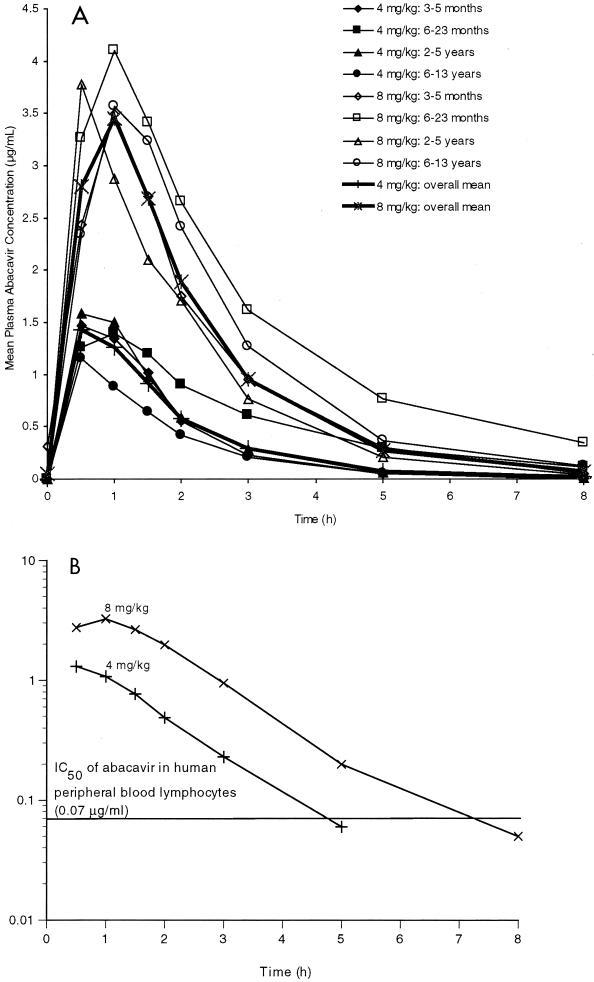
The mean plasma abacavir concentration-versus-time profiles by age category for the 4- and 8-mg/kg doses are presented in Fig. 1A. Following oral administration, abacavir was rapidly absorbed, with measurable concentrations in plasma recorded at the first postdose sampling time (30 min). Mean concentration-time curves were generally similar within each dose group, with a single peak value observed at 0.5 or 1 h. The median plasma abacavir concentration with respect to the in vitro IC50 for clinical HIV-1 isolates (0.07 μg/ml) is also presented in Fig. 1B. For the 4-mg/kg dose, the median plasma abacavir concentration was greater than 0.07 μg/ml from 0.5 h (first measurable concentration in plasma) to 3.0 h (last measurable concentration in plasma), or for a duration of at least 2.5 h. For the 8-mg/kg dose, the median plasma abacavir concentration was greater than 0.07 μg/ml from 0.5 h (first measurable concentration in plasma) to 5.0 h (last measurable concentration in plasma), or for a duration of at least 4.5 h.
FIG. 1.
(A) Mean plasma abacavir concentration-time profiles by age cohort and mean concentrations in plasma averaged for each dose following administration of oral doses of 4 and 8 mg/kg. (B) Log plot of median plasma abacavir concentration-time profiles for each dose.
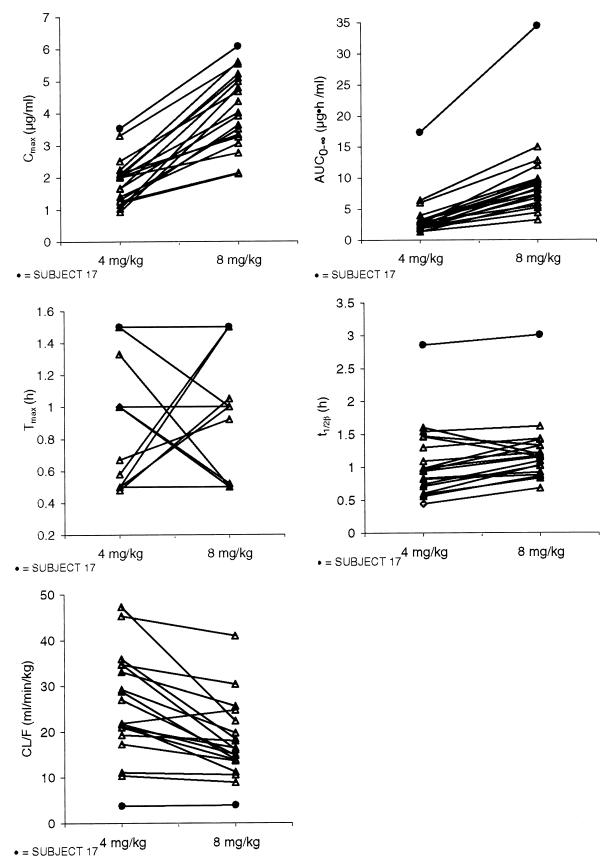
The pharmacokinetic parameter estimates for individual patients receiving both the 4- and 8-mg/kg doses are presented in Fig. 2. The results indicate that subject 17 (age 6 months) exhibited pharmacokinetic values that were clearly distinct from those for the other patients. For each treatment, parameter values for subject 17 were higher than those for all other subjects for AUC0–∞, Cmax, and t1/2β, but CL/F values were lower. Both AUC0–∞ and Cmax values clearly increased with dose, but the trends for the other parameters were much less distinct.
FIG. 2.
Pharmacokinetic parameter estimates for individual subjects receiving both doses (4 and 8 mg/kg) of abacavir.
The mean pharmacokinetic parameter estimates for the data presented in Fig. 2 (excluding the data for subject 17) are presented in Table 2. The results indicate that intersubject variability was moderately high for both treatments (35 to 48% for the 4-mg/kg treatment and 21 to 37% for the 8-mg/kg treatment).
TABLE 2.
Overall mean pharmacokinetic parameter estimates obtained following the administration of single oral doses of 4 and 8 mg/kg to HIV-infected childrena
| Dose (mg/kg) | Cmax (μg/ml) | AUC0–∞ (μg · h/ml) | Tmax (h) | t1/2β (h) | CL/F (ml/min/kg) | λz (1/h) |
|---|---|---|---|---|---|---|
| 4 | 1.69 (37)b | 2.82 (48) | 0.75 (0.48–1.50) | 0.98 (35) | 27.35 (37) | 0.80 (38) |
| 8 | 3.94 (28) | 8.09 (37) | 0.87 (0.50–1.50) | 1.13 (21) | 18.88 (41) | 0.64 (23) |
Data for subject 17 are excluded.
Values in parentheses are coefficients of variation (ranges for Tmax).
The 90% CIs for the geometric least-squares mean ratios of the log-transformed AUC0–∞ and Cmax values did not include the value 2, indicating that the pharmacokinetics of abacavir were not strictly proportional to the dose (Table 3). A twofold increase in dose from 4 to 8 mg/kg resulted in a 2.90-fold increase in AUC0–∞, and the AUC0–∞ value obtained with the 8-mg/kg dose (7.46 μg · h/ml) exceeded the expected AUC0–∞ value obtained from the 4-mg/kg dose (2.57 × 2 = 5.14 μg · h/ml) by 45%. Similarly, a twofold increase in dose from 4 to 8 mg/kg resulted in a 2.33-fold increase in Cmax, and the Cmax value obtained with the 8-mg/kg dose (3.68 μg/ml) exceeded the expected Cmax value obtained from the 4-mg/kg dose (1.58 × 2 = 3.16 μg/ml) by 16%. For Tmax, t1/2β, CL/F, and λz, the values were significantly different between treatments since their 90% CIs did not include 1.0. Of the untransformed parameters, neither Cmax nor AUC0–∞ was dose proportional, and the other parameters were significantly different between treatments.
TABLE 3.
Statistical analysis comparing the ratios of geometric least-squares mean values of 8- versus 4-mg/kg dosesa
| Parameter | LSM of 4-mg/kg dose | LSM of 8-mg/kg dose | Log-transformed ratio (90% CI) | Untransformed ratio (90% CI) |
|---|---|---|---|---|
| AUC0–∞ (μg · h/ml) | 2.57 | 7.46 | 2.91 (2.56–3.26) | 2.35 (2.11–2.58) |
| Cmax (μg/ml) | 1.58 | 3.68 | 2.33 (2.02–2.68) | 2.94 (2.64–3.24) |
| Tmax (h)b | 0.75 | 0.78 | 0.25 (0.00–0.5) | NAc |
| t1/2β (h) | 0.93 | 1.11 | 1.20 (1.09–1.32) | 1.15 (1.06–1.24) |
| CL/F (ml/min/kg) | 25.62 | 17.84 | 0.70 (0.62–0.78) | 0.70 (0.59–0.80) |
| λz (1/h) | 0.75 | 0.62 | 0.84 (0.76–0.92) | 0.80 (0.70–0.90) |
The units for the pharmacokinetic parameters apply only to the least-squares means (LSMs).
Untransformed median difference.
NA, not applicable.
Regression modeling.
No significant correlations (P > 0.05) between the demographic parameters and Cmax, AUC0–∞, or λz, with or without data for subject 17, were detected for either dose. Stepwise regression analysis did not generate consistent associations between the demographic variables and the pharmacokinetic parameters, with or without data for subject 17, for each dose level.
DISCUSSION
This is the first study to evaluate the safety and pharmacokinetics of abacavir in HIV-infected children. The results indicate that abacavir is rapidly absorbed following the administration of single oral doses of 4 and 8 mg/kg. The pharmacokinetics of abacavir are not strictly dose proportional in the population studied, as indicated by the greater than predicted increases in Cmax (16%) and AUC0–∞ (45%) observed when the administered dose was doubled from 4 to 8 mg/kg. Our study did not identify a strongly predictable relationship between measures of growth and development and the pharmacokinetic estimates of abacavir by stepwise regression analysis.
Abacavir was well tolerated by HIV-infected children. A rash attributed to abacavir occurred in 2 of 22 children who received the 8-mg/kg dose. The favorable safety profile of abacavir is well supported by preclinical toxicology studies with animals (7, 12, 13) and by an initial dose-escalation study with adults (16).
One child (subject 17) clearly presented values for all pharmacokinetic parameters that were very different from those for the other children in the study population. At the time of screening, this 6-month-old patient received a waiver of the exception criteria because she weighed less than 5 kg. Because abacavir is eliminated primarily by metabolism rather than by renal filtration, one possible reason for this subject’s results may be unusually underdeveloped metabolic pathways resulting in elevated AUC0–∞ and Cmax values or prolonged t1/2β values.
A comparison of the pharmacokinetic results obtained in the present study with those reported previously for adults (16) on a milligram-per-kilogram dose basis yield a number of observations. By using a mean body weight of 70 kg, comparable doses of abacavir in HIV-infected adults who received the 300- and 600-mg doses were approximately 4 and 8 mg/kg, respectively. At these doses, the least-squares mean values of AUC0–∞ were approximately 45 to 48% lower in children than in adults (2.57 or 7.46 versus 4.93 or 13.5 μg · h/ml), indicating lower bioavailability in children. The least-squares mean values of Cmax were approximately 15 to 34% lower in children than in adults (1.58 or 3.68 versus 2.39 or 4.36 μg/ml). The median Tmax occurred over the range of 0.5 to 1.5 h following the administration of abacavir as an oral solution formulation. These times are very similar to the Tmax range noted for adults (1.0 to 1.7 h) following the administration of abacavir as a tablet formulation, suggesting that absorption rates may be comparable between children and adults. The least-squares mean value of t1/2β was approximately 21 to 33% shorter for children than for adults (0.93 or 1.11 versus 1.17 or 1.66 h), which represents an actual difference of 44 min between the lowest and highest values. The lower AUC0–∞ and the shorter t1/2β were consistent with the more rapid apparent clearance from children than from adults (25.62 or 17.84 versus 12.55 or 10.14 ml/min/kg). These results suggest that children may need a higher dose of abacavir than adults on a milligram-per-kilogram basis in order to achieve the same exposure.
The differences in pharmacokinetics of abacavir between children and adults are consistent with the results reported in previous studies in which the pharmacokinetics of other reverse transcriptase inhibitors were evaluated in children with HIV infection. Balis et al. (3) reported that with the exception of oral bioavailability the pharmacokinetics of didanosine in children appeared to be comparable to those in adults. Oral bioavailability, however, was significantly lower in children (35 versus 19%) (3). Chadwick et al. (6) reported that the concentrations of zalcitabine in plasma were lower and that the t1/2 was shorter in children than in adults given comparable doses. Kline et al. (15) indicated that higher doses (on a milligram-per-kilogram basis) of stavudine than those administered to adults (0.5 or 1 mg/kg/day) were needed to achieve equivalent drug exposure in children (1 or 2 mg/kg/day) (15). Similarly, Lewis et al. (17) reported that consistently lower concentrations of lamivudine in serum were recorded in children compared with those that were recorded in adults, suggesting the need for the administration of higher doses to children to achieve doses equivalent to those achieved in adults. In addition, the t1/2 in serum tended to be shorter in children than in adults (1.7 versus 2.5 h). Studies have shown that the pharmacokinetics of zidovudine in children older than several months of age are similar to those in adults (1, 2). However, different pharmacokinetics have been reported in infants under 2 weeks of age, including a longer t1/2 in serum, greater bioavailability, and a lower rate of clearance (4).
The median plasma abacavir concentration exceeded the IC50 noted in studies of clinical isolates from zidovudine-naive patients with <300 CD4+ cells/mm3 for at least 2.5 and 4.5 h for the 4- and 8-mg/kg doses, respectively. These times are consistent with those obtained from a dose-ranging study of abacavir with adults (3.5 and 4.5 h for the 300- and 600-mg doses, respectively) (16).
Because of the small number of patients enrolled in this study, no strongly predictive associations between demographic and pharmacokinetic parameters were noted. A large study that uses the population approach to data analysis is warranted to further evaluate the demographic parameters that are predictors of abacavir pharmacokinetics.
In summary, the results of this study confirm the desirable pharmacokinetic properties and the favorable safety profile of abacavir for use in the treatment of HIV-infected children. Studies are under way to evaluate the clinical efficacy of the 8-mg/kg dose of abacavir for the treatment of HIV-1 infection in pediatric patients. Use of this dose is supported by the pharmacokinetic observations in the present study.
ACKNOWLEDGMENTS
This work was supported by a grant from Glaxo Wellcome Inc.
We gratefully acknowledge the assistance of the following study nurses at the indicated sites: Nana Howlett and Micki Roy of St. Jude Children’s Research Hospital, Nancy R. Calles of Baylor College of Medicine, and Deborah Fonken of Children’s Memorial Hospital. We also thank Laurene Wang for critical review of the manuscript, Michael J. O’Mara for performing the bioanalytical studies, and Laurel Adams for study monitoring.
REFERENCES
- 1.Balis F M, Pizzo P A, Murphy R F, Eddy J, Jarosinski P F, Falloon J, Broder S, Poplack D G. The pharmacokinetics of zidovudine administered by continuous infusion in children. Ann Intern Med. 1989;110:279–285. doi: 10.7326/0003-4819-110-4-279. [DOI] [PubMed] [Google Scholar]
- 2.Balis F M, Pizzo P A, Eddy J, Wilfert C, McKinney R, Scott G, Murphy R F, Jaroninski P F, Falloon J, Poplack D G. Pharmacokinetics of zidovudine administered intravenously and orally in children with human immunodeficiency virus infection. J Pediatr. 1989;114:880–884. doi: 10.1016/s0022-3476(89)80158-1. [DOI] [PubMed] [Google Scholar]
- 3.Balis F M, Pizzo P A, Butler K M, Hawkins M E, Brouwers P, Husson R N, Jacobsen F, Blaney S M, Gress J, Jaronski P, Poplack D G. Clinical pharmacology of 2′,3′-dideoxyinosine in human immunodeficiency virus-infected children. J Infect Dis. 1992;165:99–104. doi: 10.1093/infdis/165.1.99. [DOI] [PubMed] [Google Scholar]
- 4.Boucher F D, Modlin J F, Weller S, Ruff A, Mirochnick M, Pelton S, Wilfert C, McKinney R, Crain M J, Elkins M M, Blum M R, Prober C G. Phase I evaluation of zidovudine administered to infants exposed at birth to the human immunodeficiency virus. J Pediatr. 1993;122:137–144. doi: 10.1016/s0022-3476(05)83507-3. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Classification system for HIV infection in children under 13 years of age. Official authorized addenda: human immunodeficiency virus infection codes and official guidelines for coding and reporting ICD-9-M. Morbid Mortal Weekly Rep. 1994;43(No. RR-12):1–19. [Google Scholar]
- 6.Chadwick E G, Nazareno L A, Nieuwenhuis T J, Massarella J W, de Dennis S R K, Williams K, Yogev R. Phase I evaluation of zalcitabine administered to human immunodeficiency virus-infected children. J Infect Dis. 1995;172:1475–1479. doi: 10.1093/infdis/172.6.1475. [DOI] [PubMed] [Google Scholar]
- 7.Ching S V, Ayers K M, Dornsife R E, Grebe G L, Howard J L. Abstracts of the 34th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1994. Nonclinical toxicology and in vitro toxicity studies with the novel anti-HIV agent (1S,4R)-4-[2-amino-6-(cyclopropylamino)-9H-purin-9-yl]-2-cyclopentene-1-methanol (1592U89) succinate, abstr. I88; p. 92. [Google Scholar]
- 8.Conover W J. Practical nonparametric statistics. 2nd ed. New York, N.Y: John Wiley & Sons, Inc.; 1980. Some methods based on ranks; pp. 213–343. [Google Scholar]
- 9.Daluge S M, Good S S, Faletto M B, Miller W H, St. Clair M H, Boone L R, Tisdale M, Parry N R, Reardon J E, Dornsife R E, Averett D R, Krenitsky T A. 1592U89, a novel carbocyclic nucleoside analog with potent, selective anti-human immunodeficiency virus activity. Antimicrob Agents Chemother. 1997;41:1082–1093. doi: 10.1128/aac.41.5.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis S F, Beyers R H, Lindegren M L, Caldwell M B, Karon J M, Gwinn M. Prevalence and incidence of vertically acquired HIV infection in the United States. JAMA. 1995;274:952–955. [PubMed] [Google Scholar]
- 11.Faletto M B, Miller W H, Garvey E P, St. Clair M H, Daluge S M, Good S S. Unique intracellular activation of the potent anti-human immunodeficiency virus agent 1592U89. Antimicrob Agents Chemother. 1997;41:1099–1107. doi: 10.1128/aac.41.5.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Good S S, Owens B X, Faletto M B, Mahony W B, Domin B A. Abstracts of the 34th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1994. Disposition in monkeys and mice of (1S,4R)-4-[2-amino-6-(cyclopropylamino)-9H-purin-9-yl]-2-cyclopentene-1-methanol (1592U89) succinate, a potent inhibitor of HIV, abstr. I86; p. 92. [Google Scholar]
- 13.Good S S, Daluge S M, Ching S V, Ayers M M, Mahony W B, Falletto M B, Domin B A, Owens B S, Dornsife R E, McDowell J A, LaFon S W, Symonds W T. 1592U89 succinate-preclinical toxicological and disposition studies and preliminary clinical pharmacokinetics. Antivir Res. 1995;26:A229. [Google Scholar]
- 14.Hughes W, McDowell J, Adams L, Flynn P, Hetherington S, Kline M, Shenep J, Yogev R, LaFon S. Abstracts of the 3rd Conference on Retroviruses and Opportunistic Infections. Alexandria, Va: Infectious Diseases Society of American for the Foundation for Retrovirology and Human Health; 1996. Evaluation of the novel nucleoside 1592U89 in a phase I safety and pharmacokinetics (PK) study in HIV-infected infants and children, abstr. 195; p. 115. [Google Scholar]
- 15.Kline M W, Dunkle L M, Church J A, Goldsmith J C, Harris A T, Federici M E, Schultze M E, Woods L, Loewen D F, Kaul S, Cross A, Rutkiewicz V L, Rosenblatt H M, Hanson I C, Shearer W T. A phase I/II evaluation of stavudine (d4T) in children with human immunodeficiency virus infection. Pediatrics. 1995;96:247–252. [PubMed] [Google Scholar]
- 16.Kumar P N, Sweet D E, McDowell J A, Symonds W, Lou Y, Hetherington S, LaFon S. Safety and pharmacokinetics of abacavir (1592U89) following oral administration of escalating single doses in human immunodeficiency type 1-infected adults. Antimicrob Agents Chemother. 1999;43:603–608. doi: 10.1128/aac.43.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lewis L L, Venzon D, Church J, Farley M, Wheeler S, Keller A, Rubin M, Yuen G, Mueller B, Sloas M, Wood L, Balis F, Shearer G M, Brouwers P, Goldsmith J, Pizzo P A the National Cancer Institute Pediatric Branch Human Immunodeficiency Virus Working Group. Lamvidine in children with human immunodeficiency virus infection: a phase I/II study. J Infect Dis. 1996;174:16–25. doi: 10.1093/infdis/174.1.16. [DOI] [PubMed] [Google Scholar]
- 18.Pizzo P A, Wilfert C. Antiretroviral therapy for infection due to human immunodeficiency virus in children. Clin Infect Dis. 1994;19:177–196. doi: 10.1093/clinids/19.1.177. [DOI] [PubMed] [Google Scholar]
- 19.Rogers M F. Epidemiology of HIV/AIDS in women and children in the USA. Acta Paediatr Suppl. 1997;421:15–16. doi: 10.1111/j.1651-2227.1997.tb18313.x. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz G J, Haycock G B, Edelmann C M, Spitzer A. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics. 1976;58:259–263. [PubMed] [Google Scholar]
- 21.St. Clair M H, Millard J, Rooney J, Tisdale M, Parry N, Sadler B M, Blum M R, Painter G. In vitro antiviral activity of 141W94 (VX-478) in combination with other antiretroviral agents. Antivir Res. 1996;29:53–56. doi: 10.1016/0166-3542(95)00916-7. [DOI] [PubMed] [Google Scholar]
- 22.Tisdale M, Alnadaf T, Cousens D. Combination of mutations in human immunodeficiency virus type 1 reverse transcriptase required for resistance to the carbocyclic nucleoside 1592U89. Antimicrob Agents Chemother. 1997;41:1094–1098. doi: 10.1128/aac.41.5.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]