Abstract
Convalescent plasma (CP) therapy has been suggested as a treatment for emerging viral diseases. Moreover, many studies have been conducted to evaluate the efficacy of COVID-19 CP therapy, with some of them indicating that CP may be a promising treatment for the disease. However, the evidence for CP therapy's effectiveness in severe COVID-19 cases is limited. So, this study aimed to assess the probable effects of CP therapy in patients diagnosed with severe COVID-19. The study was designed as a single-arm, retrospective cohort of patients with severe COVID. Demographic data, laboratory test reports, and convalescent plasma transfusion doses were collected from medical records for patients before and after convalescent plasma transfusion. The clinical outcomes were hospital discharge and death. Also, laboratory parameters considered secondary outcomes.
After CP therapy, some symptoms improved, especially in patients under 55 years old, as follows. Respiratory function was significantly enhanced after convalescent plasma transfusion, and the inflammatory biomarkers' values decreased significantly (p < 0.05). Moreover, the estimated median of partial thromboplastin time (PTT) and Prothrombin time (PT) in patients did not change after CP therapy (p > 0.05). Regarding COVID-19 mortality, a strong association was found between older ages and death (p < 0.001). Also, CP transfusion in the early days of admission was effective in treatment outcomes (p = 0.023). Other characteristics, including sex, blood group, number of CP transfusions, and preexisting conditions, did not significantly correlate with mortality.
In conclusion, this study demonstrates the effectiveness of CP therapy in patients under the age of 55. Despite some improvement, we could not say that they were entirely due to the CP treatment. More extensive randomized clinical trials that cover different stages of the disease are needed.
Keywords: Convalescent plasma, CP therapy, COVID-19, SARS-CoV-2
1. Introduction
In December 2019, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged in Wuhan, China. The virus spreads to other cities in China and also other countries. The disease caused by this virus is currently identified as coronavirus disease-2019 (COVID-19) [1]. Based on Worldometer on August 31, 2021, the cumulative number of infected people is over 218 million cases and more than 4.5 million deaths [2]. Currently, various treatment strategies like antiviral drugs, anti-SARS-CoV-2 monoclonal antibodies, anti-inflammatory drugs, and immunomodulators agents are available. Some treatment procedures seem to be beneficial, most have failed, and many treatment strategies are under assessment. However, no licensed specific antiviral agent can efficiently target the virus, and treatment of COVID-19 remains obscure [[3], [4], [5]]. Moreover, several studies are designed to assess convalescent plasma (CP) therapy as a treatment for the disease with the CP obtained from individuals who have recovered from COVID- 19 [6].
SARS-CoV-2 spike protein is responsible for cell attachment via the ACE-2 receptor, and neutralizing antibodies can block the S protein as well as viral entry. It has been suggested that CP therapy can suppress viremia in patients by neutralizing antibodies present in plasma, which enhance viral clearance [7,8]. Neutralizing antibodies are detectable in the patient's plasma around 10–15 days following the initial SARS-CoV-2 infection [9].
CP therapy generally has not been associated with adverse results and improves outcomes in patients with other infectious agents [10]. Moreover, many studies have been conducted to evaluate the efficacy of COVID-19 CP therapy, with some of them indicating that CP may be a promising treatment for COVID-19. The evidence for the efficacy of CP therapy in severe COVID-19 cases is, however, limited. So, this study was designed to assess the probable effects of convalescent plasma therapy in patients diagnosed with severe COVID-19.
2. Methods
This research was carried out in Amir Alam hospital, Tehran, Iran, from Jun 2020 to August 2021. Because of limitations associated with the use of historical controls [11], the study was designed as a single-arm, retrospective cohort of patients with severe COVID-19 who have received routine medications without any clear clinical improvement. According to the WHO's most recent guidelines, all of the patients had severe COVID-19 pneumonia. Patients were eligible for CP transfusion if they met one or more of the following criteria: 1) respiratory distress with a respiration rate of fewer than 30 breaths per minute; 2) oxygen saturation less than 90%; 3) 50% or more progression in lung infiltrates; and 4) respiratory failure requiring ventilation (non-invasive or mechanical ventilation).
2.1. Donors
The Blood Transfusion Organization of Iran conducted activities to collect CP from donors. Donor assessments were performed according to the Eligibility Criteria for COVID-19 Convalescent Plasma established by the Iranian Ministry of Health as follows: 1) The CP donors must range from 18 to 60 years old; 2) All donors should have been previously diagnosed with laboratory-confirmed COVID-19; 3) Symptoms must have subsided for at least 28 days before donation and negative tests for SARS-CoV-2 and HIV, hepatitis B, hepatitis C, and syphilis; And 4) Specific antibody titer against SARS-CoV-2 must be greater than 1:1000. Following the donation, 500 mL of CP was collected from each donor by apheresis and then stored at −25 °C.
2.2. Data collection
Age, sex, comorbidities, blood groups, laboratory test reports, and convalescent plasma transfusion dose were all collected from medical records for patients before and after convalescent plasma transfusion. The clinical outcomes were hospital discharge and death. Also, laboratory parameters including complete blood count (CBC), coagulation and inflammation factors, biochemical tests, and arterial blood gasses (ABGs) were considered secondary outcomes.
3. Statistical analysis
Continuous and categorical variables were shown as median (IQR) and n (%), respectively. To examine differences between independent groups, the Wilcoxon rank-sum test was applied. Also, Wilcoxon signed-rank test was used to compare before and after interventions. Kaplan–Meier estimates from the time of CP transfusion were used to assess the overall survival. The log-rank test was used to compare survival curves between the groups that received CP therapy in the early days of admission (within six days) and those that received it in the late days of admission (after six days). A two-sided α of less than 0·05 was considered statistically significant. Statistical analyses were performed using R version 4.1.0 (2021-05-18).
4. Results
This study included 89 confirmed severe COVID-19 pneumonia patients who received CP transfusion (34 females and 55 males). The median age of the subjects was 60 years (IQR: 47 - 70). The median time from hospital admission to CP transfusion was five days. Moreover, the median time from CP transfusion to hospital discharge or death was eight days and seven days, respectively. Also, there were 46 cases with comorbidities, including hypertension, coronary heart disease, diabetes, and other preexisting medical conditions (Table 1 ). The SARS-CoV-2 IgG antibody titer in all convalescent plasma units was higher than 1:1000. Thirty-six patients received one unit (500 mL), while the other 53 received two units of convalescent plasma transfusion. Finally, 24 patients died, while 65 were discharged alive.
Table 1.
Demographic and clinical characteristics of study subjects.
| Variable | Total | N = 89a |
|---|---|---|
| Sex | 89 | |
| Female | 34 (38%) | |
| Male | 55 (62%) | |
| Age group (Years) | 89 | |
| Under 55 | 34 (38%) | |
| ≥55 | 55 (62%) | |
| Blood Group | 89 | |
| A | 31 (35%) | |
| AB | 4 (4.5%) | |
| B | 19 (21%) | |
| O | 35 (39%) | |
| Number of transfusions | 89 | |
| One unit | 36 (40%) | |
| Two units | 53 (60%) | |
| Outcome | 89 | |
| Alive | 65 (73%) | |
| Dead | 24 (27%) | |
| Comorbidity (all causes) | 89 | 46 (52%) |
| Hypertension | 24 (27%) | |
| Coronary heart disease (CHD) | 10 (11%) | |
| Diabetes | 12 (13%) | |
| Other preexisting conditions | 28 (31%) | |
| Days from admission to CP transfusion | 89 | 5 (3, 11) |
| Days from CP transfusion to discharge | 65 | 7 (5, 15) |
| Days from CP transfusion to death | 24 | 7 (3, 14) |
N (%), Median (IQR).
Lymphocytopenia is a common symptom of COVID-19 patients. As a result, the lymphocyte count in these patients can be used to assess treatment outcomes. In the present study, the CBC findings, including WBC, neutrophil, and lymphocyte, were not significantly different before and after convalescent plasma transfusion (p > 0.05) (Table 2 A; Fig. 1 ).
Table 2.
Laboratory findings in patients with severe COVID-19 pneumonia before and after convalescent plasma transfusion.
| Laboratory test | N | Before transfusiona | After transfusiona | p-valueb |
|---|---|---|---|---|
| A) CBC | ||||
| WBC | 89 | 10.4 (7.9, 14.3) | 11.5 (8.8, 14.5) | 0.2 |
| Lymphocyt | 89 | 9 (5, 13) | 8 (5, 14) | 0.4 |
| Neutrophil | 89 | 88 (83, 93) | 90 (83, 93) | 0.066 |
| Plt | 89 | 248 (191, 320) | 264 (194, 322) | 0.6 |
| B) Coagulation markers | ||||
| PT | 89 | 13. (12.4, 13.7) | 13.03 (11.9, 13.6) | 0.4 |
| PTT | 89 | 28.1 (25.7, 31.3) | 26.8 (25.0, 32.2) | 0.5 |
| C) Inflammation markers | ||||
| CRP | 89 | 29 (10, 70) | 12 (4, 33) | <0.001c |
| ESR | 89 | 44 (19, 67) | 30 (14, 44) | <0.001c |
| D) Biochemical indexes | ||||
| SGOT | 89 | 36 (27, 52) | 32 (25, 49) | 0.063 |
| SGPT | 89 | 46 (31, 68) | 48 (30, 66) | 0.12 |
| LDH | 89 | 1110 (899, 1266) | 972 (721, 1254) | 0.033c |
| Bilirubin-Total | 89 | 0.76 (0.50, 1.26) | 0.85 (0.60, 1.22) | 0.3 |
| Alb | 89 | 3.16 (2.85, 3.43) | 3.20 (2.96, 3.37) | 0.5 |
| Creatinine | 89 | 1.03 (0.90, 1.23) | 0.99 (0.87, 1.17) | 0.011c |
| E) Arterial Blood Gases | ||||
| BEB | 89 | −0.11 (−1.62, 1.63) | 1.30 (−0.48, 3.90) | <0.001c |
| BEecf | 89 | −1.0 (−2.7, 1.6) | 1.7 (−1.0, 3.8) | <0.001c |
| HCO3- | 89 | 24.2 (22.7, 27.2) | 26.7 (24.3, 29.7) | <0.001c |
| PCO2 | 89 | 40 (35, 46) | 42 (37, 51) | 0.005c |
| pH | 89 | 7.40 (7.29, 7.46) | 7.41 (7.28, 7.44) | 0.6 |
| PO2 | 89 | 44 (30, 52) | 46 (34, 59) | 0.058 |
| SO2 | 89 | 77 (62, 87) | 75 (64, 88) | 0.4 |
Abbreviations: CBC, Complete blood count; WBC, White blood cells; Plt, Platelets; PT, Prothrombin time; PTT, Partial thromboplastin time; CRP, C-reactive protein; ESR, Erythrocyte sedimentation rate; SGOT, Serum glutamic oxaloacetic transaminase; SGPT, Serum glutamic pyruvic transaminase; LDH, Lactate dehydrogenase; Alb, Albumin; BEB, Base excess; BEEcf, Base excess in the extracellular fluid compartment; HCO3-, Bicarbonate; PCO2, Partial pressure of carbon dioxide; pH, Power of hydrogen; PO2, Partial pressure of oxygen; SO2, Oxygen saturation.
Median (IQR).
Wilcoxon rank-sum test; Wilcoxon rank-sum exact test.
Statistically significant.
Fig. 1.
CBC findings in patients before and after convalescent plasma transfusion.
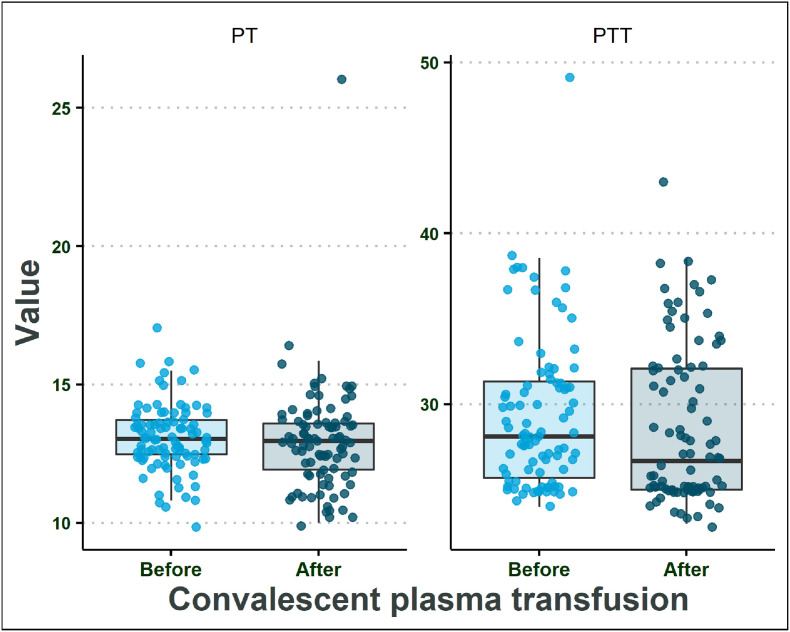
It has been reported that another important factor associated with covid-19 mortality is the elevation in coagulation markers like PT and PTT [12]. According to the findings of our study, the estimated median of PTT and PT in patients was in the normal range before CP transfusion and did not change significantly after the CP transfusion (Table 2B; Fig. 2 ).
Fig. 2.
Coagulation parameters in patients before and after convalescent plasma transfusion.
Inflammation-related parameters have previously been shown to be elevated during acute phases of COVID-19. In this study, after the CP therapy, the values of the inflammatory biomarkers like CRP and ESR decreased significantly (p < 0.001) (Table 2C; Fig. 3 ).
Fig. 3.
Inflammation-related parameters in patients before and after convalescent plasma transfusion.
Also, many studies suggested various biochemical tests as important prognostic factors associated with COVID-19 severity. The results of our study showed that except for creatinine that decreased slightly (p = 0.011), the median value of biochemical factors, including SGOT, SGPT, LDH, bilirubin-total, and albumin, were not significantly different before and after CP transfusion (p > 0.05) (Table 2D; Fig. 4 ).
Fig. 4.
Biochemical tests in patients before and after convalescent plasma transfusion.
Finally, the arterial blood gases (ABGs) report showed no significant differences in Po2, PH, and So2 before and after CP therapy, while Beb, BEecf, HCO3-, and Pco2 improved significantly after convalescent plasma transfusion (p < 0.05) (Table 2E; Fig. 5 ).
Fig. 5.
Arterial Blood Gases (ABG) in patients before and after convalescent plasma transfusion.
Regarding COVID-19 mortality, a strong association was found between older ages and death (p < 0.001). Also, CP transfusion in the early days of admission was found to be effective in treatment outcomes and patients survival (p = 0.023) (Fig. 6 ). Other characteristics, including sex, blood group, number of CP transfusions, and preexisting conditions, did not significantly correlate with mortality (Table 3 ).
Fig. 6.
Kaplan-Meyer survival analysis after convalescent plasma transfusion based on early and late use of CP transfusion.
Table 3.
Demographic and clinical characteristics of patients with mortality.
| Variable | Alive, N = 65a | Dead, N = 24a | p-valueb |
|---|---|---|---|
| Sex | 0.3 | ||
| Female | 27 (42%) | 7 (29%) | |
| Male | 38 (58%) | 17 (71%) | |
| Age group (years) | <0.001c | ||
| Under 55 | 32 (49%) | 2 (8.3%) | |
| >55 | 33 (51%) | 22 (92%) | |
| Blood group | 0.5 | ||
| A | 20 (31%) | 11 (46%) | |
| AB | 3 (4.6%) | 1 (4.2%) | |
| B | 16 (25%) | 3 (12%) | |
| O | 26 (40%) | 9 (38%) | |
| Number of transfusions | 0.3 | ||
| One unit | 24 (37%) | 12 (50%) | |
| Two unit | 41 (63%) | 12 (50%) | |
| Comorbidity (all causes) | 0.2 | ||
| Negative | 34 (52%) | 9 (38%) | |
| Positive | 31 (48%) | 15 (62%) | |
| Hypertension | 0.8 | ||
| Negative | 48 (74%) | 17 (71%) | |
| Positive | 17 (26%) | 7 (29%) | |
| Coronary heart disease | >0.9 | ||
| Negative | 58 (89%) | 21 (88%) | |
| Positive | 7 (11%) | 3 (12%) | |
| Diabetes | 0.7 | ||
| Negative | 57 (88%) | 20 (83%) | |
| Positive | 8 (12%) | 4 (17%) | |
| Other preexisting conditionsd | 0.076 | ||
| Negative | 48 (74%) | 13 (54%) | |
| Positive | 17 (26%) | 11 (46%) | |
| Days form admission to CP transfusion | 4.0 (3.0, 8.0) | 7.5 (4.8, 12.0) | 0.023c |
n (%); Median (IQR).
Pearson's Chi-squared test; Fisher's exact test; Wilcoxon rank-sum test.
Statistically significant.
Include different types of cancer, liver disease, and thyroid disorders.
5. Discussion
This retrospective study examined the efficacy of CP therapy in 89 subjects with severe COVID-19. Clinical symptoms of most patients with less than 55 years old improved after CP therapy. Also, the respiratory function was slightly improved, and patients' need for oxygen supply was reduced. Moreover, coagulation and inflammation parameters significantly decreased in patients. These findings suggest that antibodies found in CP may help to reduce immune system inflammation and improve clinical outcomes.
Recent findings on COVID-19 discovered that lymphocyte counts in the peripheral blood were significantly lower and plasma levels of inflammatory cytokines were significantly higher in severe patients compared to patients with moderate COVID-19 [13,14]. In this study, the lymphocyte count did not change in the early days after CP therapy, while CRP and ESR levels were significantly reduced, which shows that this therapy may decrease the cytokine storm [15,16].
Our data suggest that the dose of injected CP has no effects on its therapeutic impact. This may be due to our study's lack of data on neutralizing antibody titration for stratified analysis. In contrast, Zeng and colleagues in their study, observed a relation between clinical outcome and the volume of CP infused [17].
The treatment time point is the essential component that influences CP-therapy efficacy. Our results showed that patients who received CP in the early days of admission had a better treatment outcome (p-value: 0.023), emphasizing the necessity of early CP therapy. The Median time from the admission and the CP transfusion in the survived patients was four days compared to 7.5 days in dead patients, which was consistent with earlier findings [17,18].
COVID-19 has been known to be deadly in older people [19]. Most of the subjects in this study who did not survive despite receiving early CP therapy were also more than 55 years old. This result suggests that CP therapy is probably ineffective in the elderly with severe disease.
Duan et al. used 200 mL of CP with neutralizing antibody >1:640 in a prospective cohort of 10 severe patients and ten historical controls. They conclude that CP therapy could potentially improve clinical outcomes in severe COVID-19 cases [18].
In a multicenter retrospective study of critically ill patients (6 in the intervention group and 15 controls), Zeng et al. found that CP therapy can prevent SARS-CoV-2 shedding but not decrease mortality in critically ill patients in the end stages of the disease [20].
Although there was a sign for improved outcomes among the elderly, Rogers et al. in a cohort study with 64 patients in the intervention group and 177 matched controls, found no significant reduction in in-hospital mortality or increased hospital discharge rate associated with the use of CP [21].
In a large multicenter prospective study with propensity score-matched controls, Salazar et al. concluded that CP use in severe and critically ill patients with COVID-19 might improve survival if delivered early in the course of disease [22].
In a multicenter non-randomized study involving 115 moderate to severe patients in the intervention group and 74 controls, Abolghasemi et al. discovered that CP therapy significantly improved patients' survival, reduced hospitalization time considerably, and reduced the need for intubation in COVID-19 patients when compared to the control group [23].
Bradfute et al. in a single-arm trial in 12 COVID-19 patients with severe to life-threatening disease, concluded CP infusion did not develop recipient neutralizing antibody titers. They also stated that pre-screening of CP is essential for selecting donors with high neutralizing antibodies [24].
6. Limitations
Also, our research had some limitations. 1) As mentioned before, the study was designed as a single-arm, retrospective cohort because of limitations associated with historical controls. So, it is critical to conduct well-designed randomized clinical trials with larger patient cohorts and COVID-19 stages. 2) We were unable to obtain antibody titers from CP recipients due to the retrospective nature of the study. 3) Because the test for determining SARS-CoV-2 NAb was not available, convalescent donor testing for SARS-CoV-2 NAb was not performed. 4) Patients were given a combination of antiviral drugs. Corticosteroid treatment was also given to the most severe patients, which may have influenced the disease's outcome [25,26].
7. Conclusions
In conclusion, this study demonstrates the effectiveness of CP therapy in severe COVID-19 patients under the age of 55. Despite some improvement, we could not say that they were entirely due to the CP treatment. More extensive randomized clinical trials that cover different stages of the disease are needed.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Ladan Ghadami: Conceptualization, Writing – original draft. Mehrdad Hasibi: Writing – review & editing. Ali Asadollahi-Amin: Writing – review & editing. Behzad asanjarani: Writing – review & editing. Mohammad Farahmand: Formal analysis. Hamed Abdollahi: Writing – review & editing, Supervision.
Declaration of competing interest
The authors declared that they have no conflicts of interest to this work.
References
- 1.Wadood N., Toro A., Madan A. A review on COVID-19: the epidemiology, transmission, impact on pregnancy and reproductive Health and its prevention. J. Women's Health Care. 2021;10(514) 2167-0420.21. [Google Scholar]
- 2.Worldometers.info. COVID-19 CORONAVIRUS PANDEMIC Dover, Delaware, U.S.A. 2021 [updated 31 August, 2021. Available from: https://www.worldometers.info/coronavirus/.
- 3.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G., et al. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N. Engl. J. Med. 2020;382(19):1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Libster R., Pérez Marc G., Wappner D., Coviello S., Bianchi A., Braem V., et al. Early high-titer plasma therapy to prevent severe covid-19 in older adults. N. Engl. J. Med. 2021;384(7):610–618. doi: 10.1056/NEJMoa2033700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cascella M., Rajnik M., Aleem A., Dulebohn S., Di Napoli R. StatPearls. StatPearls Publishing; 2022. Features, Evaluation, and Treatment of Coronavirus (COVID-19)https://www.ncbi.nlm.nih.gov/books/NBK554776/ [PubMed] [Google Scholar]
- 6.Wang Y., Huo P., Dai R., Lv X., Yuan S., Zhang Y., et al. Convalescent plasma may be a possible treatment for COVID-19: a systematic review. Int. Immunopharm. 2021;91:107262. doi: 10.1016/j.intimp.2020.107262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harvala H., Robb M.L., Watkins N., Ijaz S., Dicks S., Patel M., et al. Convalescent plasma therapy for the treatment of patients with COVID-19: assessment of methods available for antibody detection and their correlation with neutralising antibody levels. Transfus. Med. 2021;31(3):167–175. doi: 10.1111/tme.12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coughlin M.M., Prabhakar B.S. Neutralizing human monoclonal antibodies to severe acute respiratory syndrome coronavirus: target, mechanism of action, and therapeutic potential. Rev. Med. Virol. 2012;22(1):2–17. doi: 10.1002/rmv.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu F., Wang A., Liu M., Wang Q., Chen J., Xia S., et al. 2020. Neutralizing Antibody Responses to SARS-CoV-2 in a COVID-19 Recovered Patient Cohort and Their Implications. [Google Scholar]
- 10.Joyner M.J., Wright R.S., Fairweather D., Senefeld J.W., Bruno K.A., Klassen S.A., et al. Early safety indicators of COVID-19 convalescent plasma in 5000 patients. J. Clin. Invest. 2020;130(9) doi: 10.1172/JCI140200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pandis N., Cobourne M.T. Clinical trial design for orthodontists. J. Orthod. 2013;40(2):93–103. doi: 10.1179/1465313313Y.0000000048. [DOI] [PubMed] [Google Scholar]
- 12.Pourbagheri-Sigaroodi A., Bashash D., Fateh F., Abolghasemi H. Laboratory findings in COVID-19 diagnosis and prognosis. Clin. Chim. Acta. 2020;510:475–482. doi: 10.1016/j.cca.2020.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hung I.F., To K.K., Lee C.K., Lee K.L., Chan K., Yan W.W., et al. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin. Infect. Dis. 2011;52(4):447–456. doi: 10.1093/cid/ciq106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu X., Hu C., Jiang D., Zuo Q., Li Y., Wang Y., et al. Effectiveness of convalescent plasma therapy for COVID-19 patients in Hunan, China. Dose Response. 2020;18(4) doi: 10.1177/1559325820979921. 1559325820979921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeng H., Wang D., Nie J., Liang H., Gu J., Zhao A., et al. The efficacy assessment of convalescent plasma therapy for COVID-19 patients: a multicenter case series. Signal Trans. Targeted Ther. 2020;5(1):219. doi: 10.1038/s41392-020-00329-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duan K., Liu B., Li C., Zhang H., Yu T., Qu J., et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc. Natl. Acad. Sci. Unit. States Am. 2020;117(17):9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Biswas M., Rahaman S., Biswas T.K., Haque Z., Ibrahim B. Association of sex, age, and comorbidities with mortality in COVID-19 patients: a systematic review and meta-analysis. Intervirology. 2021;64(1):36–47. doi: 10.1159/000512592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeng Q.-L., Yu Z.-J., Gou J.-J., Li G.-M., Ma S.-H., Zhang G.-F., et al. Effect of convalescent plasma therapy on viral shedding and survival in patients with coronavirus disease 2019. J. Infect. Dis. 2020;222(1):38–43. doi: 10.1093/infdis/jiaa228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rogers R., Shehadeh F., Mylona E.K., Rich J., Neill M., Touzard-Romo F., et al. Clinical Infectious Diseases; 2020. Convalescent Plasma for Patients with Severe Coronavirus Disease 2019 (COVID-19): A Matched Cohort Study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salazar E., Christensen P.A., Graviss E.A., Ngyuen D.T., Castillo B., Chen J., et al. medRxiv; 2020. Early Transfusion of a Large Cohort of COVID-19 Patients with High Titer Anti-SARS-CoV-2 Spike Protein IgG Convalescent Plasma Confirms a Signal of Significantly Decreased Mortality. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abolghasemi H., Eshghi P., Cheraghali A.M., Fooladi A.A.I., Moghaddam F.B., Imanizadeh S., et al. Clinical efficacy of convalescent plasma for treatment of COVID-19 infections: results of a multicenter clinical study. Transfus. Apher. Sci. 2020;59(5):102875. doi: 10.1016/j.transci.2020.102875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bradfute S.B., Hurwitz I., Yingling A.V., Ye C., Cheng Q., Noonan T.P., et al. Severe acute respiratory syndrome coronavirus 2 neutralizing antibody titers in convalescent plasma and recipients in New Mexico: an open treatment study in patients with coronavirus disease 2019. J. Infect. Dis. 2020;222(10):1620–1628. doi: 10.1093/infdis/jiaa505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu K., Chen Y., Yuan J., Yi P., Ding C., Wu W., et al. Factors associated with prolonged viral RNA shedding in patients with coronavirus disease 2019 (COVID-19) Clin. Infect. Dis. 2020;71(15):799–806. doi: 10.1093/cid/ciaa351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li T.Z., Cao Z.H., Chen Y., Cai M.T., Zhang L.Y., Xu H., et al. Duration of SARS‐CoV‐2 RNA shedding and factors associated with prolonged viral shedding in patients with COVID‐19. J. Med. Virol. 2021;93(1):506–512. doi: 10.1002/jmv.26280. [DOI] [PMC free article] [PubMed] [Google Scholar]