Abstract
Background
Healthcare workers (HCWs) are at risk for coronavirus disease 2019 (COVID-19), and for spreading severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) amongst colleagues and patients.
Aim
To study the presence of SARS-CoV-2 RNA and possible onward transmission by HCWs upon return to work after COVID-19, and association with disease severity and development of antibodies over time.
Methods
Unvaccinated HCWs with positive SARS-CoV-2 reverse transcriptase polymerase chain reaction (RT-PCR) were recruited prospectively. Data on symptoms were collected via telephone questionnaires on days 2, 7, 14 and 21 after a positive test. Upon return to work, repeat SARS-CoV-2 RT-PCR was performed and serum was collected. Repeat serum samples were collected at weeks 4, 8, 12 and 16 to determine antibody dynamics over time. Phylogenetic analysis was conducted to investigate possible transmission events originating from HCWs with a positive repeat RT-PCR.
Findings
Sixty-one (84.7%) participants with mild/moderate COVID-19 had a repeat SARS-CoV-2 RT-PCR performed upon return to work (median 13 days after symptom onset), of which 30 (49.1%) were positive with a median cycle threshold (Ct) value of 29.2 (IQR 26.9–29.9). All HCWs developed antibodies against SARS-CoV-2. No significant differences in symptomatology and presence of antibodies were found between repeat RT-PCR-positive and -negative HCWs. Eleven direct colleagues of six participants with a repeat RT-PCR Ct value <30 tested positive after the HCW returned to work. Phylogenetic and epidemiologic analysis did not indicate onward transmission through HCWs who were SARS-CoV-2 RNA positive upon return to work.
Conclusions
HCWs regularly return to work with substantial SARS-CoV-2 RNA loads. However, this study found no evidence for subsequent in-hospital transmission.
Keywords: SARS-CoV-2, COVID-19, Healthcare worker, Infectious disease transmission
Introduction
Healthcare workers (HCWs) play a critical role in the response against the ongoing coronavirus disease 2019 (COVID-19) pandemic. Multiple studies have shown higher infection rates in HCWs compared with the general population, suggesting an occupational risk [[1], [2], [3]]. As for all confirmed cases, COVID-19 in HCWs requires measures to prevent transmission, including quarantine. Hereby, (long) periods of absence can increase the strain on the healthcare system.
During this study, hospital guidelines prescribed that HCWs with confirmed COVID-19 could return to work 24 h after symptom resolution. National and international guidelines generally recommend a minimal duration of isolation of 7–10 days after onset of COVID-19 symptoms, and 24 h to 5 days after improvement or resolution of symptoms [[4], [5], [6], [7]]. Some guidelines mention the option of retesting before returning to work in specific circumstances (e.g. for HCWs with severe immune deficiencies) [5,6,8], but standard retesting before returning to work is not recommended by other guidelines as the assumed risk of transmission is considered to be negligible after these time periods [9,10].
On the other hand, severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) RNA can be detected in upper respiratory tract samples for prolonged periods, even without symptoms [11]. These cases are not considered to be infectious, as studies in mild cases of COVID-19 have found that no viable virus could be detected in individuals with prolonged shedding of SARS-CoV-2 RNA [12,13]. However, in these studies, samples were collected from 14 to 30 days after diagnosis, whereas most HCWs may resume work sooner. In addition, in these studies, viral culture was performed to determine infectivity and corresponding risk of transmission. As the standard procedure for HCWs returning to work in Dutch hospitals after SARS-CoV-2 infection does not include reverse transcriptase polymerase chain reaction (RT-PCR) or viral culture, viral loads at that time are not determined, and the risk of transmission by mild cases who may return to work sooner remains unclear.
Repeat RT-PCR testing could further examine the risk of transmission of HCWs upon return to work. Furthermore, the presence of SARS-CoV-2-specific antibodies has been negatively correlated with the presence of infectious virus [14,15]. Therefore, antibody dynamics could be valuable in determining the risk of transmission upon return to work, and subsequent re-infection in this population with increased occupational risk.
The aim of this prospective observational study was to assess the presence of SARS-CoV-2 RNA and corresponding cycle threshold (Ct) values upon resolution of symptoms in HCWs infected with SARS-CoV-2, and its relation to disease severity, antibody dynamics and risk of transmission.
Methods
Study design
Participants
Amsterdam University Medical Centres (Amsterdam UMC), The Netherlands, offers SARS-CoV-2 RT-PCR testing of combined nasopharyngeal and oropharyngeal swab specimens for HCWs with COVID-19-like symptoms (coughing, pharyngitis, dyspnoea, rhinitis, and anosmia or dysgeusia). HCWs that tested positive in routine testing between May and September 2020, during the national ‘second wave’ and before the national vaccination campaign started, were invited to participate in this prospective observational study.
Sampling process
On day 2 after the positive SARS-CoV-2 RT-PCR, a telephone questionnaire was administered regarding signs and symptoms at the time of disease onset, as well as at the present time. The presence of 14 predefined symptoms (coughing, pharyngitis, dyspnoea, rhinitis, abdominal pain, diarrhoea, nausea, vomiting, anorexia, fever, myalgia, headache, fatigue, and anosmia or dysgeusia) was determined. Follow-up symptomatology questionnaires were conducted on days 7, 14 and 21, as long as participants reported experiencing symptoms.
Repeat nasopharyngeal and oropharyngeal swabs and initial serum were collected when HCWs returned to work. Hospital guidelines for returning to work required that all respiratory symptoms had to be resolved for >24 h. Anosmia, dysgeusia and fatigue were not required to be resolved upon returning to work. Repeat serum samples were collected at weeks 4, 8, 12 and 16 after the initial positive RT-PCR. All sera were stored at -20 °C until serological tests were performed.
The nasopharyngeal and oropharyngeal swabs were collected in E-swab or UTM viral transport medium (COPAN Diagnostics, Murrieta, CA, USA).
Laboratory assays
SARS-CoV-2 RNA was extracted using the MagNA Pure 96 system (Roche, Penzberg, Germany). RT-PCR targeting the SARS-CoV-2 E gene was performed according to a previously published protocol [16]. The presence of antibodies was determined by the enzyme-linked-immunosorbent-assay-based Wantai SARS-CoV-2 double antigen sandwich total antibody assay (Wantai Biological Pharmacy, Beijing, China).
Contact tracing in HCWs that returned to work
Standard contact tracing was performed for every SARS-CoV-2-positive HCW (or patient) by the Infection Control Department. To investigate the risk of transmission of HCWs with a positive repeat PCR, potential secondary infections were identified using data from the Occupational Health and Infection Control Department. Potential secondary infections were defined as contacts within the same department that tested positive for SARS-CoV-2 within 7 days after study participants with a repeat RT-PCR Ct value <30 returned to work.
Viral genomes of specimens of study participants and return-to-work contacts were amplified using the Ion AmpliSeq SARS-CoV-2 Research Panel, and sequenced on an Ion GeneStudio S5 system (both from ThermoFisher Scientific, Waltham, MA, USA). Sequences were analysed phylogenetically to infer relatedness in a background of contemporaneous SARS-CoV-2 viral genomes from the Netherlands, derived from the GISAID database (Table S1, see online supplementary material). A maximum-likelihood phylogeny was constructed using the Augur pipeline [17]. This study used procedures taken from [github.com/nextstrain/ncov] including the clock rate, reference genome and site masking. Trees were visualized using ggtree [18] as implemented in R (R Core Team, Vienna, Austria).
Ethics and consent
Informed consent was obtained from all participants. The study was reviewed and approved by the Amsterdam UMC Institutional Review Board, and conducted in accordance with the Declaration of Helsinki, and national and institutional standards.
Statistical analysis
Unknown or missing answers in the symptomatology questionnaires were considered as absent. Fatigue and anosmia/dysgeusia were not included to determine disease duration. Sera with an absorbance/cut-off ratio (s/c) >1.1 were considered positive, and samples with an s/c <0.9 were considered negative. An s/c between 0.9 and 1.1 was considered indeterminate.
Data were analysed using RStudio (R Core Team) and Graphpad Prism Version 9.0.2 for Mac (GraphPad Software, San Diego, CA, USA). Normality checks were performed using the Shapiro–Wilk test. Descriptive analyses were made on baseline characteristics and the number of observations, presented as number and percentage. For descriptive statistics, quantitative variables that did not follow a normal distribution are presented as median and interquartile range (IQR). Binomial logistic regression was used to calculate odds ratios and 95% confidence intervals for evaluating the association of the presence of symptoms with seroprevalence and presence of viral RNA. P-values <0.05 were considered to indicate significance.
Results
Participants
In total, 72 HCWs were included in this study. Demographics are shown in Table I . One HCW was admitted to hospital (1.4%). Upon study inclusion, 20.8% of the HCWs reported that they had worked while having COVID-like symptoms before they tested positive. Experiencing mild symptoms that were not directly recognized was the most common explanation.
Table I.
Descriptive statistics of the study cohort
| Characteristic | Value |
|---|---|
| Age, median (IQR) | 33 (26.0–45.0) |
| Female, N (%) | 54 (75.0) |
| Body mass index, median (IQR) | 23 (20.5–26.9) |
| Profession, N (%) | |
| Direct patient contact | 44 (61.1) |
| Physician | 10 (15.3) |
| Nurse | 20 (27.8) |
| Medical intern | 8 (11.1) |
| Clinical assistant | 4 (5.6) |
| Other | 2 (2.8) |
| No direct patient contact | 28 (38.9) |
| Researcher | 10 (13.9) |
| Pharmacy staff/assistant | 5 (6.9) |
| Laboratory technician | 2 (2.8) |
| Other | 11 (15.3) |
| Comorbidities, N (%) | |
| High blood pressure | 3 (4.2) |
| Diabetes | 1 (1.4) |
| Cardiovascular disease | 1 (1.4) |
| Asthma | 4 (5.6) |
| Other | 4 (5.6) |
| Continued to work while having symptoms, N (%) | |
| Yesa | 15 (20.8) |
| No knowledge of regulations | 0 (0.0) |
| Mild symptoms | 12 (80.0) |
| Devoted symptoms to another cause | 7 (40.0) |
| Work pressure/sense of responsibility | 3 (20.0) |
| No | 48 (66.7) |
| Don't know | 3 (4.2) |
| Unknown | 6 (8.3) |
IQR, interquartile range.
Multiple answers were possible.
Symptomatology
The median time between disease onset and time of initial RT-PCR was 1 day (range 1–7 days). The median duration of symptoms was 10 days (range 0–41 days). Symptoms decreased over time (Table II ). Fever and dyspnoea were not reported frequently. At disease onset, rhinitis, headache and fatigue were observed most frequently. Gastrointestinal symptoms were reported in a minority of HCWs. On day 21, 43% of HCWs still reported symptoms. Fatigue and anosmia or dysgeusia most frequently persisted by day 21. The majority (80.6%) of HCWs had a self-reported mild experience of COVID-19. No significant differences in symptomatology were found between repeat RT-PCR-positive and repeat RT-PCR-negative HCWs (data not shown).
Table II.
Detailed symptomatology in healthcare workers with coronavirus disease 2019 confirmed by reverse transcriptase polymerase chain reaction
| Symptom | Time of interview |
||||
|---|---|---|---|---|---|
| Disease onset (N=72) | Day 2 (N=72) | Day 7 (N=71) | Day 14 (N=71) | Day 21 (N=71) | |
| Respiratory symptoms | |||||
| Coughing | 22 (30.6) | 39 (54.9) | 27 (38.0) | 12 (16.9) | 9 (12.7) |
| Pharyngitis | 21 (29.2) | 19 (26.8) | 7 (9.9) | 6 (8.5) | 3 (4.2) |
| Dyspnoea | 7 (9.7) | 11 (15.5) | 11 (15.5) | 5 (7.0) | 9 (12.7) |
| Rhinitis | 30 (41.7) | 48 (67.6) | 29 (40.8) | 11 (15.5) | 8 (11.3) |
| Gastrointestinal symptoms | |||||
| Abdominal pain | 4 (5.6) | 7 (9.9) | 3 (4.2) | 2 (2.8) | 0 (0.0) |
| Diarrhoea | 7 (9.7) | 8 (11.1) | 2 (2.8) | 1 (1.4) | 1 (1.4) |
| Nausea | 3 (4.2) | 7 (9.9) | 2 (2.8) | 2 (2.8) | 3 (4.2) |
| Vomiting | 1 (1.4) | 3 (4.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Anorexia | 12 (16.7) | 26 (36.6) | 20 (28.2) | 5 (7.0) | 4 (5.6) |
| Other symptoms | |||||
| Fever | 13 (18.1) | 18 (25.4) | 4 (5.6) | 1 (1.4) | 0 (0.0) |
| Myalgia | 19 (26.4) | 23 (32.4) | 9 (12.7) | 3 (4.2) | 3 (4.2) |
| Headache | 37 (51.4) | 39 (54.9) | 16 (22.5) | 12 (16.9) | 9 (12.7) |
| Fatigue | 32 (44.4) | 49 (69.0) | 35 (49.3) | 22 (31.0) | 18 (25.4) |
| Anosmia or dysgeusia | 13 (18.9) | 25 (35.2) | 36 (50.7) | 22 (31.0) | 17 (23.9) |
| No symptoms experienced | 0 (0.0) | 0 (0.0) | 15 (21.1) | 36 (50.7) | 40 (56.3) |
Virology
The median Ct value of the initial RT-PCR was 21.1 (IQR 18.0–26.0). Sixty-one (84.7%) participants had a repeat RT-PCR performed upon return to work, at a median of 13 days (range 6–42 days) after symptom onset. Thirty (49.1%) of them were positive, with a median Ct value of 29.2 (IQR 26.9–29.9). Eleven participants did not have a repeat RT-PCR performed.
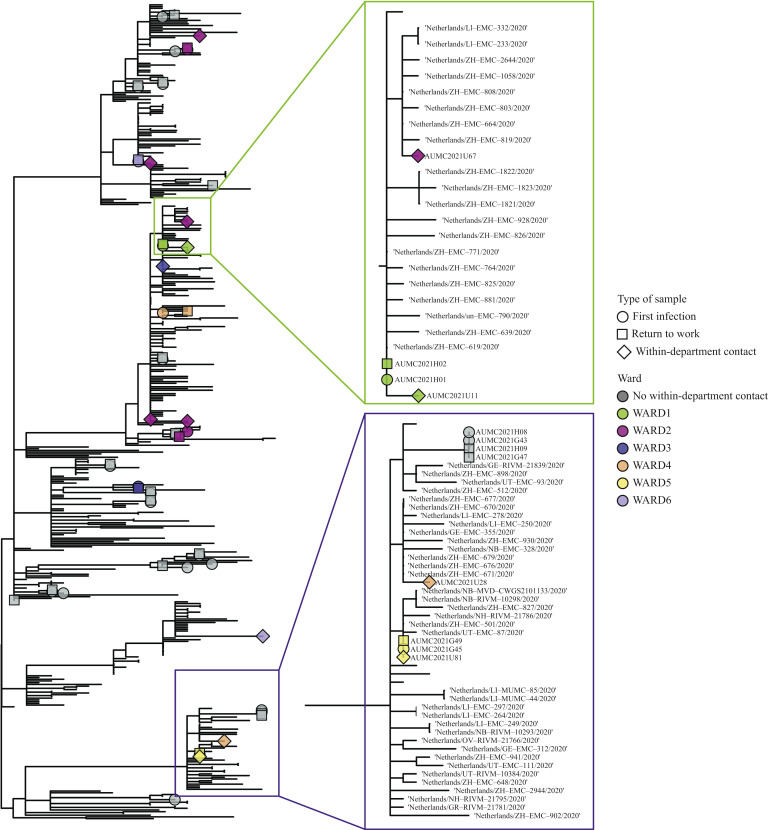
Twenty-two of the 30 repeat RT-PCR-positive participants (73.3%) had a repeat RT-PCR specimen with a Ct value <30 (corresponding to 36% of all HCWs for whom repeat RT-PCR results were available). Of these 22 participants, 11 SARS-CoV2 RNA-positive within-department contacts were identified as potential secondary transmissions. Specimens of these 11 within-department contacts were sequenced (Figure 1 ).
Figure 1.
Maximum likelihood phylogeny of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) sequences with identified potential transmission clusters. A condensed maximum-likelihood phylogeny of SARS-CoV-2 sequences that were collected (marked with tip shapes) and a random sample of contemporaneous reference sequences (no tips) circulating within the Netherlands. Tip shapes are coloured according to the wards on which the healthcare workers (circle and square tips) and their within-department contacts (diamond tips) were working. The figure zooms in on two potential transmission clusters that were found.
Phylogenetic analysis revealed one pair of identical viral genomes of return-to-work and corresponding within-department contact, and one pair that differed by two single-nucleotide polymorphisms (SNPs). Contact tracing and epidemiological data of these two pairs showed no indications of onward transmission. Eight return-to-work and corresponding within-department contact pairs had pairwise genetic distances that were not compatible with direct transmission (minimal pairwise genetic distance of five SNPs).
Serology
All HCWs from whom serum was collected developed antibodies during the follow-up period (data not shown). Upon symptom resolution, antibodies were detected in 42 of 48 (87.5%) HCWs from whom serum was collected at this time point. At 16 weeks, antibodies were detected in 97.5% of HCWs. Two HCWs seroreverted (from positive to negative antibody status) during the follow-up period, within 8 weeks of disease onset. No significant difference in the presence of antibodies was found between repeat RT-PCR-positive and repeat RT-PCR-negative HCWs.
Discussion
HCWs are at increased risk for SARS-CoV-2 infection and onward transmission to colleagues and patients. Guidelines are inconsistent on the timing for SARS-CoV-2-positive HCWs to return to work. This study investigated symptoms, repeated RT-PCR, risk of transmission and antibody dynamics in HCWs when returning to work. A generally mild course of COVID-19 was found, and despite high SARS-CoV-2 RNA viral loads, no evidence for transmission from returning HCWs upon resolution of symptoms was identified.
Surprisingly, almost 50% of repeat RT-PCR assays when returning to work were positive, with Ct values suggesting the possibility of replicating virus. This study showed RT-PCR positivity up to 38 days after symptom onset, which is in line with the now well-established experience that RNA may be detected for longer periods after SARS-CoV-2 infection [[9], [10], [11]]. Relatively high viral loads (Ct values <30) were found in 36% of HCWs upon return to work in this study, raising the question of whether the study hospital guidelines are sufficiently stringent to prevent nosocomial transmission, especially as national and international guidelines generally recommend a longer duration of isolation after COVID-19 in HCWs [[4], [5], [6], [7]].
Ct values were used as a surrogate marker for infectivity in accordance with previous studies, as they correlate well with the ability to culture (viable) virus; a cut-off value of 30 is associated with inability to culture virus [19,20]. Viral sequencing was performed to investigate whether onward transmission occurred by HCWs who returned to work. Phylogenetic analysis showed one pair of identical viral sequences of a return-to-work study participant and within-department contact, and one pair that differed by two SNPs. For the pair with identical sequences, the probability of direct transmission was deemed negligible after assessment of the contact tracing data, as the index HCW worked from home for 1 month after his infection and there was no contact with other HCWs at that time. Epidemiological assessment of the pair differing by two SNPs suggested that direct transmission was unlikely, as the return-to-work HCW remained home for 14 days after symptom onset, had no symptoms on return to work, and the HCWs did not know each other. Thus, despite the high numbers of positive specimens with theoretically viable virus in this study, no evidence was found for onward transmission at work from returning HCWs upon resolution of symptoms. However, the possibility of HCW-to-HCW transmission cannot be ruled out completely, as onward transmission may have occurred in this study but remained undiagnosed in asymptomatic individuals.
A possible explanation for the identical viral genomes found in one return-to-work and corresponding within-department contact pair may be exposure to comparable genomes circulating in the Netherlands at that time [as evidenced by identical genomes detected in contemporaneous SARS-CoV-2 viral genomes from the Netherlands (Table S1, see online supplementary material)]. Although direct transmission could not definitely be ruled out for one pair in this study, a symptom-based strategy for determining when HCWs with SARS-CoV-2 infection could return to work as in the current hospital guidelines are considered adequate and safe. Nevertheless, as this study was performed before emergence of the Alpha variant, the emergence of new circulating variants associated with higher transmissibility [21,22] may require re-evaluation of guidelines. Moreover, as study participation was on a voluntary basis, the included HCWs may have been more compliant with social distancing rules and personal protection guidelines. This could explain, in part, the absence of documented transmission by HCWs after returning to work. Infection prevention measures such as physical distancing, personal protective equipment and vaccination should remain a priority for SARS-CoV-2 in-hospital infection control, as there is evidence that HCW-to-HCW transmission is an important route of nosocomial infection [[22], [23], [24], [25]], and transmissions generally occur before a HCW tests positive.
Despite low symptomatology, all HCWs in this cohort seroconverted. Comparable prospective studies showed similar but somewhat lower rates, possibly due to a shorter follow-up period [26,27] or because immunoglobulin G alone was measured [28]. Further research is needed to determine long-term protection, and protection against new variants. The presence of antibodies did not seem to be associated with repeat RT-PCR positivity, indicating that even mild infections with faster viral clearance result in an antibody response. The majority of participants (87.5%) had already developed antibodies when returning to work, which further reduces the assumed risk of transmission at this time point given the negative correlation with SARS-CoV-2-specific antibodies and the presence of infectious virus [14,15].
The main limitation of this study is that infectivity of the HCWs when returning to work could not be determined. In addition, the small sample size of this study, especially the limited number of HCWs returning to work with high viral loads, may have influenced the conclusions about the risk of transmission. However, extensive phylogenetic as well as background analyses in combination with contact tracing data showed no evidence for direct transmission.
A strength of this study is that it was conducted prospectively in confirmed SARS-CoV-2-positive HCWs. Most studies in HCWs are retrospective seroprevalence studies in which it is impossible to evaluate symptomatology accurately, or determine the antibody responses in this specific population. Furthermore, all analyses were performed in the same laboratory, making it possible to compare Ct values between participants.
To conclude, this study found relatively high viral loads in SARS-CoV-2-positive HCWs when returning to work after symptom resolution. As no evidence for secondary HCW-to-HCW transmission after returning to work was found, a symptom-based approach appears to be adequate for the prevention of SARS-CoV-2 infection from returning HCWs. As HCW-to-HCW transmission is a common source of nosocomial SARS-CoV-2 infection, infection prevention measures and guideline adherence should remain priorities when shaping future hospital policy and practice.
Acknowledgements
The authors wish to thank the laboratory team, including Dr. Matthijs Welkers, Dr. Robin van Houdt, Dr. Rosa van Mansfeld and Sjoerd Rebers, for conducting the (sequencing) analyses, and the team of the Department of Occupational Health for providing information to the (possible) participants and obtaining the specimens.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhin.2022.02.024.
Conflict of interest statement
None declared.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Contemporaneous severe acute respiratory syndrome coronavirus-2 viral genomes from the Netherlands, derived from the GISAID database.
References
- 1.Nguyen L.H., Drew D.A., Graham M.S., Joshi A.D., Guo C.G., Ma W., et al. Risk of COVID-19 among front-line health-care workers and the general community: a prospective cohort study. Lancet Public Health. 2020;5:e475–e483. doi: 10.1016/S2468-2667(20)30164-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rudberg A.S., Havervall S., Manberg A., Jernbom Falk A., Aguilera K., Ng H., et al. SARS-CoV-2 exposure, symptoms and seroprevalence in healthcare workers in Sweden. Nat Commun. 2020;11:5064. doi: 10.1038/s41467-020-18848-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mutambudzi M., Niedwiedz C., Macdonald E.B., Leyland A., Mair F., Anderson J., et al. Occupation and risk of severe COVID-19: prospective cohort study of 120 075 UK Biobank participants. Occup Environ Med. 2020;78:307–314. doi: 10.1136/oemed-2020-106731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Health Service Executive (HSE) HSE; Dublin: 2021. COVID-19 telephone assessment, testing pathway and return to work of symptomatic healthcare workers version 9.https://www.hse.ie/eng/staff/workplace-health-and-wellbeing-unit/covid-19-guidance/assessment-testing-and-return-to-work-of-symptomatic-healthcare-worker1.pdf Available at: [Google Scholar]
- 5.World Health Organization . WHO; Geneva: 2020. Prevention, identification and management of health worker infection in the context of COVID-19.https://www.who.int/publications/i/item/10665-336265 Available at: [Google Scholar]
- 6.Centers for Disease Control and Prevention. Return to work criteria for healthcare personnel with SARS-CoV-2 infection (Interim Guidance) CDC; Atlanta, GA: 2021. https://www.cdc.gov/coronavirus/2019-ncov/hcp/return-to-work.html Available at: [Google Scholar]
- 7.GGZ Standaarden. Richtlijn Corona . GGZ Standaarden; Utrecht: 2021. https://www.ggzstandaarden.nl/richtlijnen/ggz-en-corona-richtlijn/richtlijn/verantwoord-inzetten-van-zorg-professionals Available at: [Google Scholar]
- 8.European Centre for Disease Prevention and Control. Infection prevention and control and preparedness for COVID-19 in healthcare settings - sixth update. ECDC; Stockholm: 2021. https://www.ecdc.europa.eu/en/publications-data/infection-prevention-and-control-and-preparedness-covid-19-healthcare-settings Available at: [Google Scholar]
- 9.van Kampen JJA, van de Vijver D., Fraaij P.L.A., Haagmans B.L., Lamers M.M., Okba N., et al. Duration and key determinants of infectious virus shedding in hospitalized patients with coronavirus disease-2019 (COVID-19) Nat Commun. 2021;12:267. doi: 10.1038/s41467-020-20568-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bullard J., Dust K., Funk D., Strong J.E., Alexander D., Garnett L., et al. Predicting infectious severe acute respiratory syndrome coronavirus 2 from diagnostic samples. Clin Infect Dis. 2020;71:2663–2666. doi: 10.1093/cid/ciaa638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walsh K.A., Jordan K., Clyne B., Rohde D., Drummond L., Byrne P., et al. SARS-CoV-2 detection, viral load and infectivity over the course of an infection. J Infect. 2020;81:357–371. doi: 10.1016/j.jinf.2020.06.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sohn Y., Jeong S.J., Chung W.S., Hyun J.H., Baek Y.J., Cho Y., et al. Assessing viral shedding and infectivity of asymptomatic or mildly symptomatic patients with COVID-19 in a later phase. J Clin Med. 2020;9 doi: 10.3390/jcm9092924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laferl H., Kelani H., Seitz T., Holzer B., Zimpernik I., Steinrigl A., et al. An approach to lifting self-isolation for health care workers with prolonged shedding of SARS-CoV-2 RNA. Infection. 2021;49:95–101. doi: 10.1007/s15010-020-01530-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glans H., Gredmark-Russ S., Olausson M., Falck-Jones S., Varnaite R., Christ W., et al. Shedding of infectious SARS-CoV-2 by hospitalized COVID-19 patients in relation to serum antibody responses. BMC Infect Dis. 2021;21:494. doi: 10.1186/s12879-021-06202-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim Y.I., Kim S.M., Park S.J., Kim E.H., Yu K.M., Chang J.H., et al. Critical role of neutralizing antibody for SARS-CoV-2 reinfection and transmission. Emerg Microbes Infect. 2021;10:152–160. doi: 10.1080/22221751.2021.1872352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K., et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25:2000045. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hadfield J., Megill C., Bell S.M., Huddleston J., Potter B., Callender C., et al. Nextstrain: real-time tracking of pathogen evolution. Bioinformatics. 2018;34:4121–4123. doi: 10.1093/bioinformatics/bty407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu G., Smith D.K., Huachen Zhu Y.G., Lam T.T.-Y. ggtree: an R package for visualization and annotation of phylogenetic trees with their covariates and other associated data. Methods Ecol Evol. 2017;8:28–36. [Google Scholar]
- 19.Igloi Z., Velzing J., van Beek J., van de Vijver D., Aron G., Ensing R., et al. Clinical evaluation of Roche SD Biosensor rapid antigen test for SARS-CoV-2 in municipal health service testing site, the Netherlands. Emerg Infect Dis. 2021;27:1323–1329. doi: 10.3201/eid2705.204688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gniazdowski V., Paul Morris C., Wohl S., Mehoke T., Ramakrishnan S., Thielen P., et al. Repeated coronavirus disease 2019 molecular testing: correlation of severe acute respiratory syndrome coronavirus 2 culture with molecular assays and cycle thresholds. Clin Infect Dis. 2021;73:e860–e869. doi: 10.1093/cid/ciaa1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou B., Thao T.T.N., Hoffmann D., Taddeo A., Ebert N., Labroussaa F., et al. SARS-CoV-2 spike D614G change enhances replication and transmission. Nature. 2021;592:122–127. doi: 10.1038/s41586-021-03361-1. [DOI] [PubMed] [Google Scholar]
- 22.Sabino E.C., Buss L.F., Carvalho M.P.S., Prete C.A., Jr., Crispim M.A.E., Fraiji N.A., et al. Resurgence of COVID-19 in Manaus, Brazil, despite high seroprevalence. Lancet. 2021;397:452–455. doi: 10.1016/S0140-6736(21)00183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sikkens J.J., Buis D.T.P., Peters E.J.G., Dekker M., Schinkel M., Reijnders T.D.Y., et al. Serologic surveillance and phylogenetic analysis of SARS-CoV-2 infection among hospital health care workers. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.18554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schneider S., Piening B., Nouri-Pasovsky P.A., Kruger A.C., Gastmeier P., Aghdassi S.J.S. SARS-coronavirus-2 cases in healthcare workers may not regularly originate from patient care: lessons from a university hospital on the underestimated risk of healthcare worker to healthcare worker transmission. Antimicrob Resist Infect Control. 2020;9:192. doi: 10.1186/s13756-020-00848-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koopsen J., Dekker M., Thung P., Jonges M., Vennema H., Leenstra T., et al. Rapid reinfection with SARS-CoV-2 variant-of-concern Alpha detected in a nurse during an outbreak at a non-COVID inpatient ward: lessons learned. Antimicrob Resist Infect Control. 2021;10:137. doi: 10.1186/s13756-021-01008-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fafi-Kremer S., Bruel T., Madec Y., Grant R., Tondeur L., Grzelak L., et al. Serologic responses to SARS-CoV-2 infection among hospital staff with mild disease in eastern France. EBioMedicine. 2020;59:102915. doi: 10.1016/j.ebiom.2020.102915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brandstetter S., Roth S., Harner S., Buntrock-Dopke H., Toncheva A.A., Borchers N., et al. Symptoms and immunoglobulin development in hospital staff exposed to a SARS-CoV-2 outbreak. Pediatr Allergy Immunol. 2020;31:841–847. doi: 10.1111/pai.13278. [DOI] [PubMed] [Google Scholar]
- 28.Fill Malfertheiner S., Brandstetter S., Roth S., Harner S., Buntrock-Dopke H., Toncheva A.A., et al. Immune response to SARS-CoV-2 in health care workers following a COVID-19 outbreak: a prospective longitudinal study. J Clin Virol. 2020;130:104575. doi: 10.1016/j.jcv.2020.104575. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.