Abstract
Background
Polyethylene glycol (PEG) and polysorbate reactions were initially implicated as a likely risk factor for reacting to coronavirus disease 2019 (COVID-19) vaccines and remain a source of vaccine hesitancy despite increasing evidence that they do not pose an increased risk for COVID-19 vaccine reactions.
Objective
To investigate COVID-19 vaccine safety outcomes in patients with reported reactions to PEG- and polysorbate-containing medications and vaccines.
Methods
COVID-19 vaccine safety was reviewed in patients with PEG or polysorbate reactions documented in their electronic medical records at a tertiary academic medical center (cohort 1) and patients referred to Allergy and Immunology with reported PEG or polysorbate reactions (cohort 2). COVID-19 vaccine safety was also reviewed following reported symptoms (onset ≤ 12 hours) to first-dose PEG-containing messenger RNA (mRNA) COVID-19 vaccine (cohort 3).
Results
Of 252 patients in cohort 1 (n = 202) and cohort 2 (n = 50), 236 (94%) received mRNA COVID-19 vaccines (106 Pfizer, 130 Moderna); 235 received both doses. Only 3 patients from cohort 2 developed mild rash following vaccination. None of the 44 patients in cohort 3 with acute symptoms following first-dose mRNA COVID-19 vaccine (27 Pfizer, 17 Moderna) had previously reported PEG or polysorbate reactions. Of these 44 patients, 43 received the second dose and all 3 who developed symptoms following the second dose (1 required epinephrine) had negative PEG skin testing.
Conclusion
Patients with reported reactions to PEG and polysorbate safely received COVID-19 vaccines. PEG and polysorbate skin testing did not identify patients at risk for first dose or recurrent reactions to COVID-19 vaccines. Screening for PEG and polysorbate allergy may only increase vaccine hesitancy without identifying patients at risk for COVID-19 vaccine reactions.
Introduction
Anaphylaxis to the messenger RNA (mRNA) coronavirus disease 2019 (COVID-19) vaccine has been reported to both Pfizer-BioNTech and Moderna mRNA COVID-19 vaccines, primarily after receipt of the first dose.1, 2, 3, 4 Allergic reactions to polyethylene glycol (PEG) and polysorbate were implicated as a risk factor for an allergic reaction to COVID-19 vaccines because PEG is the only known potentially allergenic component in the COVID-19 mRNA vaccines and polysorbate is the only known potentially allergenic component in the COVID-19 Johnson & Johnson (J&J) vaccine.5, 6, 7, 8
Current guidance of the Centers for Disease Control and Prevention (CDC) highlights allergic reactions to PEG as a contraindication to receiving mRNA COVID-19 vaccine and allergic reactions to polysorbate as a contraindication to receiving J&J COVID-19 vaccine.9 Published guidance following CDC recommendations for first-dose risk stratification has suggested that (1) patients with a possible PEG and polysorbate allergy be referred to an allergist for possible PEG or polysorbate skin testing, (2) patients with a possible PEG allergy receive the J&J COVID-19 vaccine or be referred to an allergist for possible PEG or polysorbate skin testing, and (3) patients with a possible polysorbate allergy receive any COVID-19 vaccine if they have subsequently tolerated a polysorbate-containing vaccine or receive mRNA COVID-19 vaccine if they have not.4
Although this strategy provides a vaccine option for most patients, vaccination sites are still left with the important but arduous task of screening for PEG and polysorbate allergy, because PEG and polysorbate can be found in numerous medications and vaccines.10 Differentiating between allergic and non-allergic reactions can also pose challenges. Furthermore, a national survey found that vaccine efficacy has the largest influence on people's reported likelihood of receiving a vaccine, and patients cleared to receive J&J COVID-19 vaccine may still prefer allergy evaluation for clearance to receive mRNA COVID-19 vaccines owing to reports of higher efficacy with these vaccines.11, 12, 13, 14
Consequently, assessing COVID-19 vaccination safety in patients with history of PEG or polysorbate reactions has become a question that allergists face often. A recently published International Consensus document on the risk of allergic reactions to COVID-19 vaccines recommends that patients with past medical history of reaction to a COVID-19 vaccine excipient are referred to allergy for assessment of vaccination safety.15 A COVID-19 Vaccination Approach Survey found that 82% of allergists reported evaluating patients to clear them for their first dose of Pfizer or Moderna vaccine, with many performing PEG and polysorbate skin testing.16 Studies investigating the safety of COVID-19 vaccination in patients with a history of PEG and polysorbate reactions are needed.
To investigate this, we reviewed COVID-19 vaccine safety outcomes in patients with reported reactions to PEG- and polysorbate-containing medications and vaccines.
Methods
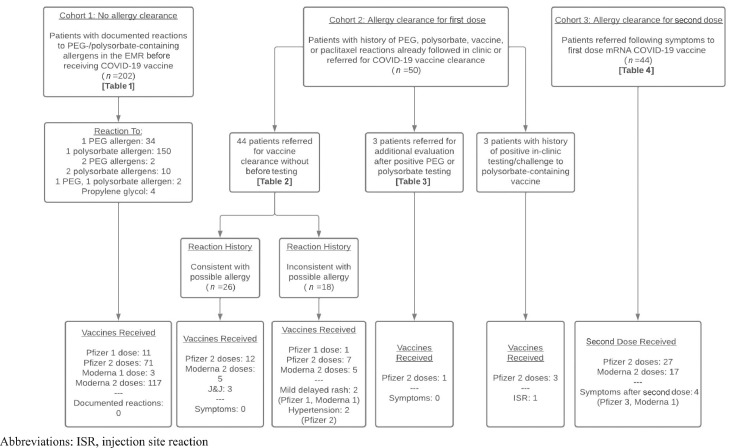
We reviewed demographics, documented allergies, PEG and polysorbate reaction histories, allergy evaluations, and vaccine safety outcomes in 3 patient cohorts with possible PEG and polysorbate reactions (Fig. 1). This study was approved by our institution's institutional review board (IRB #21-33623).
Figure 1.
Overview of 3 patient cohorts reviewed. The patient cohorts corresponding to the 4 tables are shown, as are the vaccines received in each patient cohort. Symptoms documented or experienced after vaccination are shown at the bottom underneath the “—.” COVID-19, coronavirus disease 2019; EMR, electronic medical record; ISR, injection site reaction; mRNA, messenger RNA; PEG, polyethylene glycol.
Cohort 1
We queried the electronic health system database at a tertiary academic medical center for patients with PEG and polysorbate reaction(s) documented in their electronic medical record (EMR) between May 4, 2011, and August 23, 2021, who subsequently received COVID-19 vaccine. We excluded patients with documented reactions to mRNA COVID-19 vaccine and patients referred to Allergy and Immunology. Documented reaction symptoms obtained from the EMR were categorized as follows:
-
•
Anaphylaxis: anaphylaxis
-
•
Cutaneous: hives, itching, rash, swelling
-
•
Respiratory: shortness of breath
-
•
Neurologic: lightheadedness
-
•
Gastrointestinal: abdominal pain, diarrhea, nausea only, nausea and vomiting
-
•
Other: other, unknown
Cohort 2
Patients with a history of PEG, polysorbate, vaccine, or paclitaxel reactions already established in the allergy and immunology clinic or referred for COVID-19 vaccine clearance between December 1, 2020, and August 31, 2021, were included in this cohort. Patients with a history of reactions to any vaccine were included because it was not always clear which vaccine patients had reacted to. Patients with a history of paclitaxel reactions were included because paclitaxel contains a PEG derivative that is structurally similar to the excipient mRNA COVID-19 vaccines.17
Available reaction comments and descriptions of anaphylaxis, allergic reaction, erythema or redness, flushing, rash, urticaria or hives, angioedema or swelling, wheezing, shortness of breath, coughing, throat tightness, and difficulty in swallowing were categorized as a history possibly consistent with allergy. Reaction comments and descriptions of body or muscle aches, chills, fever, Guillain-Barré syndrome, hand-foot syndrome, injection site reactions, mania, migraine, nausea, neuropathy, and vasculitis were categorized as a history not consistent with allergy.
Cohort 3
Patients referred between December 1, 2020, and August 31, 2021, to Allergy and Immunology after experiencing symptoms following first-dose COVID-19 vaccine were included in this cohort. Patients with symptom onset less than 20 hours after vaccine receipt were included because a prior report of anaphylaxis cases from the Vaccine Safety Datalink found that symptom onset when documented occurred within 20 hours.18 Patients referred for symptoms following second-dose COVID-19 vaccine were excluded from this review. Reported symptoms were categorized as follows:
-
•
Cutaneous: diaphoresis, erythema, flushing, hives, itching, rash, swelling, warm sensation
-
•
Lower respiratory: chest tightness, shortness of breath, wheezing
-
•
Cardiovascular: chest pain, hypertension, palpitations, tachycardia
-
•
Neurologic: dizziness, headache, lightheadedness, numbness, tingling
-
•
Gastrointestinal: dry heaving, nausea, vomiting
-
•
Upper airway: difficulty in swallowing, voice change
-
•
Tongue or throat sensation: throat closing, throat sensation not otherwise specified, throat swelling, throat tightness, tongue pain, tongue swelling, tongue tingling
Medication and Vaccine Classification
Medications and vaccines were classified into those that contained PEG or polysorbate based on published package inserts of the Food and Drug Administration. Influenza, tetanus, pneumococcal, and meningococcal vaccines were considered to potentially contain polysorbate unless it was clearly documented that the patient had received a vaccine type that did not contain polysorbate.
Testing
PEG 3350 and polysorbate testing were performed closely following guidance outlined in Banerji et al.4 Triamcinolone IDT 40 mg/mL was not performed owing to our previous clinical experience with false positives at that dilution. Testing with Refresh eyedrops was not performed. The highest dilutions used for testing are shown in eTable 1.
eTable 1.
Dilutions Used for PEG and Polysorbate Skin Prick and Intradermal Tests
| Medication | Skin prick test (highest dilution tested) | Intradermal test (highest dilution tested) |
|---|---|---|
| Miralax (PEG 3350) | 170 mg/mL | NA |
| Methylprednisolone acetate (PEG 3350) | 40 mg/mL | 4 mg/mL |
| Methylprednisolone succinate (control) | 40 mg/mL | 4 mg/mL |
| Triamcinolone acetate (polysorbate 80) | 40 mg/mL | 4 mg/mL |
| Prevnar-13 (polysorbate 80) | 1:10 | 1:100 |
Abbreviations: NA, not available; PEG, polyethylene glycol.
Results
Cohort 1
There were 202 patients with PEG and polysorbate reactions documented in the EMR before receiving COVID-19 vaccines, and none had documented reactions to the COVID-19 vaccine received: Moderna in 120 (59%), Pfizer in 82 (41%), 2 doses in 188 (93%). No patients were documented as having received J&J. No patients were documented as having received an allergy evaluation before receiving the COVID-19 vaccine. Characteristics are shown in Table 1 .
Table 1.
Characteristics of Patients in Cohort 1. Patients with documented reactions to PEG and polysorbate in the EMR before receiving COVID-19 vaccines did not have documented reactions to the COVID-19 vaccine. Patients could have more than one PEG or polysorbate reaction documented as shown in "Documented Reactions(s)", resulting in a combined total percentage of individual allergens >100%.
| Characteristics | Received COVID-19 vaccine (n = 202) |
|---|---|
| Age in y, median (range) | 75 (14-93) |
| Female sex at birth, n (%) | 147 (73) |
| Race and Ethnicity, n (%) White Hispanic or Latino Black Asian American Indian Multiracial Other or unknown |
137 (68) 13 (6) 7 (4) 33 (16) — — 12 (6) |
| Documented reaction(s), n (%) 1 PEG allergen 1 Polysorbate allergen 2 PEG allergens 2 Polysorbate allergens 1 PEG, 1 polysorbate allergen Propylene glycol |
34 (17) 150 (74) 2 (1) 10 (5) 2 (1) 4 (2) |
| # PEG allergens, n (%) Bimatoprost (PEG, unspecified) Doxorubicina (PEG 2000) Methylprednisolone acetate (PEG 3350) Neulasta (20-kD monomethoxy PEG) PEG, unspecified type PEG 300 PEG 400 PEG 3350 PEG-asparaginase (PEG 5000) Perflutren (PEG 5000) Trastuzumab (PEG 3350) |
39 (19) 8 (4) 4 (2) 1 (0) 2 (1) 10 (5) 1 (0) 2 (1) 4 (2) 3 (1) 1 (0) 3 (1) |
| # Polysorbate allergens, n (%) Influenza vaccine, unspecified type Influenza quadrivalent vaccine (polysorbate 80) Hepatitis A vaccine (polysorbate 20) Pneumococcal vaccine, unspecified type Pneumococcal conjugated vaccine (Prevnar 13) (polysorbate 80) Tetanus vaccine (polysorbate 80) Zoster vaccine (polysorbate 80) |
172 (85) 47 (23) 5 (2) 2 (1) 17 (8) 12 (6) 66 (33) 23 (11) |
| Documented reaction symptoms, n (%) Anaphylaxis Cutaneous Respiratory Neurologic Gastrointestinal Other/unknown Missing |
9 (4) 72 (36) 1 (0) 1 (0) 7 (3) 21 (10) 105 (52) |
Abbreviations: COVID-19, coronavirus disease 2019; EMR, electronic medical record; PEG, polyethylene glycol.
Note: The bolded n (%) indicate total n (%) for all PEG allergens and all polysorbate allergens.
Included as doxorubicin can be PEGylated in liposomal form.
There were 39 patients with PEG and 172 patients with polysorbate reactions documented in the EMR prior to COVID-19 vaccine receipt. The specific PEG- and polysorbate-containing medications and vaccines documented as allergies are shown in Table 1. Reactions were documented a median of 1010 days (interquartile range, 458-2059) before receipt of the first COVID-19 vaccine dose. A polysorbate-containing vaccine had been administered in 72% of the patients after their reaction was first documented and before receipt of the first COVID-19 vaccine dose. Although details regarding reaction history were not available for more than half of the documented reactions, cutaneous symptoms were the most common documented symptom (Table 1).
Taken together, these findings suggest that patients with documented reactions to medications and vaccines containing PEG or polysorbate can receive mRNA COVID-19 vaccines safely.
Cohort 2
There were 50 patients referred to Allergy and Immunology with a history of PEG, polysorbate, vaccine, or paclitaxel reaction. There were 44 patients referred without any prior PEG or polysorbate excipient testing, 3 patients referred for repeat evaluation after positive excipient testing, and 3 patients who had a history of positive in-clinic testing to polysorbate-containing vaccines. All were cleared to receive COVID-19 vaccines. Of these patients, 37 received COVID-19 vaccines (24 Pfizer, 10 Moderna, 3 J&J). The only adverse effects reported were mild delayed rash (n = 2; Pfizer 1, Moderna 1), mild immediate rash around the injection site (n = 1; Pfizer), and hypertension (n = 2; Pfizer 2) as described further below.
Of 44 patients referred without any prior excipient testing, 33 received COVID-19 vaccines. Demographics and vaccines received are shown in Table 2 . There were 26 patients whose reaction history was consistent with allergy and 18 patients whose reaction was clearly inconsistent with allergy. Of 26 patients whose reaction history was consistent with allergy, 20 patients received COVID-19 vaccines and none of them developed symptoms following vaccination (12 Pfizer, 5 Moderna, 3 J&J). Five patients with reaction history consistent with allergy to PEG-containing medications underwent Miralax (n = 5), methylprednisolone acetate (n = 5), methylprednisolone succinate (n = 4), triamcinolone (n = 1), and Prevnar-13 (n = 1) skin testing; all had negative results. Among 18 patients with a history inconsistent with allergy, 13 subsequently received COVID-19 vaccines, of whom 4 reported symptoms. Furthermore, 2 patients reported mild delayed rash: one after first dose only of Moderna and one after second dose only of Pfizer. Two patients who reported experiencing hypertension, chest tightness, lightheadedness, dizziness, and tachycardia after receiving flu vaccines also reported hypertension after receiving COVID-19 vaccines (both Pfizer; one after first dose only, one after both doses).
Table 2.
Characteristics of Patients in Cohort 2. Characteristics of patients referred for COVID-19 vaccine clearance following PEG (not including mRNA vaccines), polysorbate, vaccine, and paclitaxel reactions are shown. The number of documented allergies ranges from zero as not all reported reactions were documented as an allergy in the EMR.
| Characteristics | All (n = 44) | Received vaccine (n = 33) |
|---|---|---|
| Age in y at time of referral, median (range) | 51 (18-80) | 48 (18-80) |
| Female sex at birth, n (%) | 37 (84) | 28 (85) |
| Race and Ethnicity, n (%) White Hispanic or Latino Black Asian American Indian Multiracial Other or unknown |
28 (64) — 2 (5) 3 (7) 1 (2) — 10 (23) |
21 (64) — 2 (6) 3 (9) — — 7 (21) |
| Primary language, n (%) English Farsi Russian |
42 (96) 1 (2) 1 (2) |
32 (97) 1 (3) — |
| Documented allergies in EMR, median (range) | 2 (0-52) | 2 (0-25) |
| Comorbidities, n (%) Drug allergy Food allergy Environmental allergy Asthma History of anaphylaxis Atopic dermatitis Urticaria/angioedema Anxiety |
35 (80) 8 (18) 20 (46) 14 (32) 21 (48) 7 (16) 7 (16) 30 (68) |
27 (82) 8 (24) 15 (46) 12 (36) 16 (49) 6 (18) 7 (21) 22 (67) |
| Reaction history consistent with | ||
| Allergy to medication containing PEG, n | 9 | 8 (5 Pfizer, 2 Moderna, 1 J&J) |
| Allergy to vaccine or medication containing polysorbate, n | 11 | 7 (4 Pfizer, 2 Moderna, 1 J&J) |
| Allergy to vaccine without polysorbate, n | 3 | 2 (1 Pfizer, 1 J&J) |
| Allergy to paclitaxel, n | 3 | 3 (2 Pfizer, 1 Moderna) |
| Inconsistent with allergy, n | 18 | 13 (8 Pfizera, 5 Moderna) |
Abbreviations: COVID-19, coronavirus disease 2019; EMR, electronic medical record; GBS, Guillain-Barré syndrome; mRNA, messenger RNA.
One patient with history of GBS after influenza vaccine declined second dose. All other patients received at least 2 doses of mRNA COVID-19 vaccine.
There were 3 patients referred for repeat evaluation after positive testing, as shown in Table 3 . Of these patients, 2 had repeat testing at our clinic that was negative and 1 declined repeat testing. Only one of these patients subsequently received COVID-19 vaccine.
Table 3.
Patients Referred for Evaluation After Positive Excipient Testing.
| Patient | Comorbidities | Initial reaction | First Positive testing | Second testing | COVID-19 vaccine |
|---|---|---|---|---|---|
| 1 | Drug allergy Environmental allergy Urticaria Anxiety |
Miralax prep complicated by vomiting and abdominal pain | (+) Miralax IDT 1:100 (+) Refresh eyedrops IDT 1:10 |
Declined | Declined |
| 2 | Drug allergy Food allergy Environmental allergy Asthma Anaphylaxis Atopic dermatitis Anxiety |
Documented reactions to 52 allergens in EMR | Reportedly (+) PEG testing | (−) Miralax SPT 1:1 (170 mg/mL) (−) Methylprednisolone acetate IDT 1:10 (4 mg/mL) |
Received Pfizer |
| 3 | Drug allergy Anaphylaxis Anxiety |
Syncope following methylprednisolone acetate, lidocaine, and bupivacaine injection | (+) Miralax SPT 1:100, 1:10, 1:1 (+) Methylprednisolone acetate SPT 1:10, 1:1 |
(−) Miralax SPT 1:1 (170 mg/mL) (−) Methylprednisolone acetate IDT 1:10 (4 mg/mL) Negative local anesthetic testing Tolerated sequential challenges with methylprednisolone acetate 16 mg intramuscularly, lidocaine 10 mg subcutaneously, bupivacaine 7.5 mg subcutaneously |
Declined |
Abbreviations: COVID-19, coronavirus disease 2019; EMR, electronic medical record; PEG, polyethylene glycol; SPT, skin prick test.
Three patients who had positive in-clinic testing to polysorbate-containing vaccines (one had positive challenge to Tdap vaccine, one had positive challenge to influenza vaccine, and one had positive skin testing and history of urticaria following influenza vaccine) were able to receive both doses of mRNA COVID-19 vaccine (Pfizer). One patient developed an acute localized rash around the injection site after both doses of Pfizer vaccine.
Taken together, these findings suggest that patients with reported reactions to to medications and vaccines containing PEG or polysorbate, even those with a history strongly suggestive of true allergy, can receive COVID-19 vaccines safely.
Cohort 3
There were 44 patients referred for evaluation of symptoms that occurred within the first 20 hours following first dose of Pfizer (n = 27) or Moderna (n = 17) COVID-19 vaccination. Demographics, reaction history, and testing results are shown in Table 4 . All 44 patients reported symptom onset within 12 hours. Although patients with symptom onset less than 20 hours after vaccine receipt were included per a prior report of anaphylaxis cases from Vaccine Safety Datalink, it is important to note that symptom onset less than 4 hours is considered immediate per CDC guidance and the 2 cases with symptom onset 5 to 12 hours after vaccination would be considered delayed.9 , 18 The 2 delayed cases occurred after Moderna (n = 1) and Pfizer (n = 1) vaccination. Of note, although 57% reported at least one drug allergy at time of evaluation, none had previously reported a reaction to PEG or polysorbate. All received the second dose.
Table 4.
Characteristics of Patients in Cohort 3. Characteristics of patients referred for evaluation of symptoms following 1st dose mRNA COVID-19 vaccine are shown. All patients received 2nd dose. Both columns show symptoms and treatment following 1st dose. Details regarding 2nd dose symptoms and treatment are reported in the manuscript.
| Characteristics | All patients (n = 44) | Patients with symptoms after second dose (n = 4) |
|---|---|---|
| Age in y, median (range) | 45 (24-78) | 44 (34-52) |
| Female sex at birth, n (%) | 39 (89) | 4 (100) |
| Race and Ethnicity, n (%) White Hispanic or Latino Black Asian American Indian Multiracial Other or declined or unknown |
22 (50) 6 (14) 1 (2) 10 (23) — 1 (2) 4 (9) |
— 1 (25) — 2 (50) — — 1 (25) |
| Primary language, n (%) English Mandarin |
43 (98) 1 (2) |
4 (100) — |
| Documented allergies in EMR, median (range) | 2 (0-11) | 6 (5-11) |
| Comorbidities Drug allergy Food allergy Environmental allergy Asthma History of anaphylaxis Atopic dermatitis Urticaria/angioedema Anxiety |
25 (57) 16 (36) 27 (61) 22 (50) 8 (18) 8 (18) 5 (11) 24 (55) |
4 (100) 3 (75) 4 (100) 4 (100) 3 (75) 2 (50) 1 (25) 4 (100) |
| Symptoms onset <30 min (Pfizer 19, Moderna 11) 30-60 min (Pfizer 5, Moderna 4) 1-4 h (Pfizer 2, Moderna 1) 5-12 h (Pfizer 1, Moderna 1) |
— 30 (68) 9 (20) 3 (7) 2 (5) |
— 4 (100) — — — |
| Symptoms Cutaneous Lower respiratory Cardiovascular Neurologic Gastrointestinal Upper airway Tongue/throat sensation |
— 31 (70) 10 (23) 5 (11) 15 (34) 6 (14) 3 (7) 15 (34) |
— 4 (100) 2 (50) — — — — 3 (75) |
| Treatment Antihistamines (H1) Corticosteroids Intramuscular epinephrine ED visit only Hospitalization |
— 14 (32) 2 (5) 6 (14) 12 (27) 1 (2) |
— 4 (100) — 2 (50) 2 (50) 1 (25) |
Abbreviations: COVID-19, coronavirus disease 2019; ED, emergency department; EMR, electronic medical record; mRNA, messenger RNA.
Fourteen patients underwent testing for Miralax (n = 14), methylprednisolone acetate (n = 14), methylprednisolone succinate (n = 9), triamcinolone (n = 13), and Prevnar-13 (n = 12). All testing results were negative except for 1 questionable positive result to methylprednisolone succinate (IDT 1:10 dilution) and 2 questionable positive results to Prevnar-13 (IDT 1:100 dilution). One patient had a questionable irritant reaction to Miralax (SPT 17 mg/mL) but repeat testing was negative to Miralax at a higher dilution (SPT 170 mg/mL) and methylprednisolone acetate, highlighting the fallibility of PEG 3350 testing.
Following the second dose, 4 patients (9%, 4 of 44) reported symptoms that were visible or required treatment (Pfizer 3, Moderna 1). All occurred after negative excipient skin testing. All 4 patients had reported cutaneous symptoms with first dose and had received treatment with H1 antihistamines (Table 4). In addition, 2 patients had reported lower respiratory and 3 tongue/throat sensation symptoms after first dose and 2 had received epinephrine (Table 4). Reported symptoms following second dose were: (1) coughing and rashes treated with 2 doses of intramuscular epinephrine; (2) acute throat tickling and difficulty in swallowing treated with H1 antihistamines; (3) facial erythema and shaking for 10 minutes resolving without treatment; and (4) eyelid and facial angioedema, throat sensation, and difficulty in swallowing for which 5 doses of intramuscular epinephrine were administered (2 initially and then 3 for ongoing throat tightness) and the patient was admitted to the hospital for observation from the emergency department.
Taken together, these findings demonstrate that the patients who experienced symptoms following first-dose COVID-19 vaccine would not have been identified by screening for reactions to PEG or polysorbate and that PEG and polysorbate excipient testing did not predict those who were able to safely receive a second dose.
Discussion
Our study found that patients with documented reactions to medications and vaccines containing PEG or polysorbate can indeed receive COVID-19 vaccines safely. This study adds to the growing body of evidence that screening for PEG or polysorbate allergy before administering COVID-19 vaccines only serves to increase vaccine hesitancy without identifying patients at risk for COVID-19 vaccine reactions.
A recently published study found that 8 patients with history of anaphylaxis to injectable medications or vaccines containing PEG or polysorbate safely tolerated mRNA COVID-19 vaccine at a large academic hospital employee vaccination program. Of these patients, 7 were cleared by an allergist for vaccination based on their history of having tolerated PEG or polysorbate and 1 was cleared with testing by an allergist.19 We add to this with our cohort 1 data by specifically investigating first-dose COVID-19 vaccine safety without any allergy evaluation in patients who reported reactions to multiple different medications and vaccines containing PEG or polysorbate, 57 of whom had no documentation of having tolerated polysorbate-containing vaccines before receiving a COVID-19 vaccine.
We found that both patients with reactions to PEG or polysorbate documented in the EMR (cohort 1) or reported to patients’ providers (cohort 2) received COVID-19 vaccines safely. No specific pattern of atopy was observed in cohort 2. Although EMR documentation and patient reports of reactions to PEG or polysorbate are not reliable indicators of a true allergy, they are the initial reference point for patients, providers, and vaccine administrators determining COVID-19 vaccine safety. In addition, the finding that COVID-19 vaccines were safe remained true in the subset of patients with reaction histories consistent with potentially IgE-mediated allergic reactions, and even among patients who had positive challenge results to polysorbate-containing vaccines. This finding is in line with prior reports of patients with PEG-asparaginase reactions who tolerated mRNA COVID-19 vaccines and patients with PEG allergy who tolerated both polysorbate-containing vaccines and PEG-containing COVID-19 vaccines.20, 21, 22, 23, 24 This suggests that patients do not need to be screened for PEG or polysorbate allergy before receiving COVID-19 vaccines.
IgE-mediated allergy to PEG or polysorbate did not seem to be the cause of reactions to mRNA COVID-19 vaccines in our cohort. Patients with reactions to mRNA COVID-19 vaccines (cohort 3) did not have a preexisting history of PEG or polysorbate allergy or any specific pattern of atopy. This finding is in line with the CDC reports of anaphylaxis to the mRNA COVID-19 vaccine occurring in patients without a history of PEG or polysorbate allergy.2 , 3 In addition, as observed in prior reports, the results of excipient testing did not correspond with whether patients experienced symptoms with COVID-19 vaccines.25 , 26 Indeed, all the patients who had a recurrent reaction to the second dose, including those requiring epinephrine, had negative PEG testing results.
A non–IgE-mediated mechanism may explain why most patients with symptoms following first-dose COVID-19 vaccine are able to tolerate the second dose, as observed in our patient cohort 3 and in previous reports.27, 28, 29 There is evidence that PEGylated nanoparticles can activate the complement system depending on the form and structure of PEGylation.30 Further investigation into the mechanism by which reactions occur to mRNA COVID-19 vaccines is needed.
Non–IgE-mediated anxiety-related adverse events have been reported to COVID-19 vaccines.31 In cohort 2, an anxiety diagnosis was the second most commonly observed comorbidity following drug allergy, and in cohort 3, it was the third most commonly observed after drug allergy and environmental allergy. This highlights the need for avenues to provide adequate patient reassurance before COVID-19 vaccine administration. These avenues would ideally extend beyond the allergy and immunology clinic, as general practitioners and vaccine administration sites are often first line in discussing vaccine safety concerns with patients.
Although negative PEG and polysorbate excipient allergy testing could provide reassurance for patients with histories of reactions to PEG or polysorbate, it is not routinely recommended.15 Not only is IgE-mediated hypersensitivity to PEG likely not responsible for most mRNA COVID-19 vaccine reactions, but excipient testing is fraught with false positives. A prior report found that Refresh eyedrops are irritating.25 Skin testing with COVID-19 vaccines has been studied but may carry similar risk for false positives.32 , 33 We additionally found that Prevnar-13 intradermal testing and possibly methylprednisolone succinate testing at 4 mg/mL are likely irritating. Positive testing, even if followed by repeat negative testing, understandably increases vaccine hesitancy and we observed this in patients referred to our clinic. Taken together, our findings support the recommendation that PEG and polysorbate excipient skin testing is not routinely performed to evaluate COVID-19 vaccine safety, even in patients with histories of reactions to PEG or polysorbate. Larger studies are needed to identify whether there are clinical factors that would more accurately identify patients at risk for developing symptoms from COVID-19 vaccines.
There are limitations to our study. Chart review and data extracted from the EMR do not always accurately reflect true reaction history as documentation in the allergy section of the electronic medical record can be incomplete and inaccurate.34 However, documented and reported reactions are often the initial reference point for providers determining COVID-19 vaccine safety and we believe that the information provided in this study will still be of use to vaccine administration sites. In addition, in the cohort of patients with documented reactions to PEG or polysorbate in the EMR, we were only able to capture COVID-19 vaccine administrations recorded at our institution. However, this would, if anything, underestimate the number of patients who received COVID-19 vaccines safely.
In summary, most patients with documented or reported reactions to PEG or polysorbate can safely receive COVID-19 vaccines. These findings do not negate the possibility that there are patients with PEG allergy who would react to PEG contained in COVID-19 vaccines. However, screening for PEG and polysorbate reactions may not be the optimal method for identifying patients who experience reactions to COVID-19 vaccines and may only serve to increase vaccine hesitancy. To improve vaccine protocols, further investigation into what clinical factors identify patients at risk for COVID-19 vaccine symptoms is needed.
Acknowledgments
We thank Mark Marino, David Pines, and Stephanie Villanueva for their guidance and ongoing partnership in incorporating rapidly changing clinical practices for COVID-19 vaccine reaction management.
Supplementary Data
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.anai.2022.03.006.
Footnotes
Disclosures: The authors have no conflicts of interest to report.
Funding: The authors have no funding sources to report.
References
- 1.Gee J, Marquez P, Su J, Calvert GM, Liu R, Myers T, et al. First month of COVID-19 vaccine safety monitoring - United States, December 14, 2020-January 13, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(8):283–288. doi: 10.15585/mmwr.mm7008e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shimabukuro T, Nair N. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine. JAMA. 2021;325(8):780–781. doi: 10.1001/jama.2021.0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shimabukuro T. Allergic reactions including anaphylaxis after receipt of the first dose of Moderna COVID-19 vaccine - United States, December 21, 2020-January 10, 2021. Am J Transplant. 2021;21(3):1326–1331. doi: 10.1111/ajt.16517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banerji A, Wolfson AR, Wickner PG, Cogan AS, McMahon AE, Saff R, et al. COVID-19 vaccination in patients with reported allergic reactions: updated evidence and suggested approach. J Allergy Clin Immunol Pract. 2021;9(6):2135–2138. doi: 10.1016/j.jaip.2021.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stone CA, Jr, Liu Y, Relling MV, Krantz MS, Pratt AL, Abreo A, et al. Immediate hypersensitivity to polyethylene glycols and polysorbates: more common than we have recognized. J Allergy Clin Immunol Pract. 2019;7(5):1533–1540. doi: 10.1016/j.jaip.2018.12.003. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou ZH, Stone CA, Jr, Jakubovic B, Phillips EJ, Sussman G, Park J, et al. Anti-PEG IgE in anaphylaxis associated with polyethylene glycol. J Allergy Clin Immunol Pract. 2021;9(4):1731–1733. doi: 10.1016/j.jaip.2020.11.011. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krantz MS, Liu Y, Phillips EJ, Stone CA., Jr. Anaphylaxis to pegylated liposomal echocardiogram contrast in a patient with IgE-mediated macrogol allergy. J Allergy Clin Immunol Pract. 2020;8(4):1416–1419. doi: 10.1016/j.jaip.2019.12.041. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cabanillas B, Akdis CA, Novak N. Allergic reactions to the first COVID-19 vaccine: a potential role of polyethylene glycol? Allergy. 2021;76(6):1617–1618. doi: 10.1111/all.14711. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. COVID-19 vaccines for people with allergies. Available at: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/specific-groups/allergies.html. Accessed October 7, 2021.
- 10.Banerji A, Wickner PG, Saff R, Stone CA, Jr, Robinson LB, Long AA, et al. mRNA vaccines to prevent COVID-19 disease and reported allergic reactions: current evidence and suggested approach. J Allergy Clin Immunol Pract. 2021;9(4):1423–1437. doi: 10.1016/j.jaip.2020.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. Pfizer-BioNTech COVID-19 vaccine overview and safety. Available at: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/Pfizer-BioNTech.html. Accessed October 7, 2021.
- 12.Centers for Disease Control and Prevention. Moderna COVID-19 vaccine overview and safety. Available at: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/Moderna.html. Accessed October 7, 2021.
- 13.Centers for Disease Control and Prevention. Johnson & Johnson's Janssen COVID-19 vaccine overview and safety. Available at: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/janssen.html. Accessed October 7, 2021.
- 14.Kaplan RM, Milstein A. Influence of a COVID-19 vaccine's effectiveness and safety profile on vaccination acceptance. Proc Natl Acad Sci U S A. 2021;118(10) doi: 10.1073/pnas.2021726118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greenhawt M, Abrams EM, Shaker M, Chu DK, Khan D, Akin C, et al. The risk of allergic reaction to SARS-CoV-2 vaccines and recommended evaluation and management: a systematic review, meta-analysis, GRADE assessment, and international consensus approach. J Allergy Clin Immunol Pract. 2021;9(10):3546–3567. doi: 10.1016/j.jaip.2021.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Journal of Allergy and Immunology: in Practice. COVID-19 vaccination approach survey. Available at: https://www.jaci-inpractice.org/covid-19. Accessed Oct 12, 2021.
- 17.Banerji A, Wolfson AR, Robinson LB, McMahon AE, Cogan AS, Saff RR, et al. COVID-19 vaccines tolerated in patients with paclitaxel and docetaxel allergy. Allergy. 2022;77(3):1048–1051. doi: 10.1111/all.15178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McNeil MM, Weintraub ES, Duffy J, Sukumaran L, Jacobsen SJ, Klein NP, et al. Risk of anaphylaxis after vaccination in children and adults. J Allergy Clin Immunol. 2016;137(3):868–878. doi: 10.1016/j.jaci.2015.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krantz MS, Stone CA, Jr, Rolando LA, Nobis AE, Koo G, Corey KB, et al. An academic hospital experience screening mRNA COVID-19 vaccine risk using patient allergy history. J Allergy Clin Immunol Pract. 2021;9(10):3807–3810. doi: 10.1016/j.jaip.2021.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mark C, Gupta S, Punnett A, Upton J, Orkin J, Atkinson A, et al. Safety of administration of BNT162b2 mRNA (Pfizer-BioNTech) COVID-19 vaccine in youths and young adults with a history of acute lymphoblastic leukemia and allergy to PEG-asparaginase. Pediatr Blood Cancer. 2021;68(11):e29295. doi: 10.1002/pbc.29295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ieven T, Van Weyenbergh T, Vandebotermet M, Devolder D, Breynaert C, Schrijvers R. Tolerability of polysorbate 80 containing COVID-19 vaccines in confirmed PEG allergic patients. J Allergy Clin Immunol Pract. 2021;9(12):4470–4472. doi: 10.1016/j.jaip.2021.09.039. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koo G, Anvari S, Friedman DL, Zarnegar-Lumley S, Szafron V, Kahwash BM, et al. mRNA COVID-19 vaccine safety in patients with previous immediate hypersensitivity to pegaspargase. J Allergy Clin Immunol Pract. 2022;10(1):322–325. doi: 10.1016/j.jaip.2021.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rush C, Faulk KE, Bradley ZK, Turner A, Krumins M, Greenhawt M. The safety of SARS-CoV-2 vaccines in persons with a known history of pegaspargase allergy: a single institution experience. J Allergy Clin Immunol Pract. 2022;10(2):630–632. doi: 10.1016/j.jaip.2021.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Picard M, Drolet JP, Masse MS, Filion CA, ALMuhizi F, Fein M, et al. Safety of COVID-19 vaccination in patients with polyethylene glycol allergy: a case series. J Allergy Clin Immunol Pract. 2022;10(2):620–625. doi: 10.1016/j.jaip.2021.11.021. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolfson AR, Robinson LB, Li L, McMahon AE, Cogan AS, Fu X, et al. First-dose mRNA COVID-19 vaccine allergic reactions: limited role for excipient skin testing. J Allergy Clin Immunol Pract. 2021;9(9):3308–3320. doi: 10.1016/j.jaip.2021.06.010. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carpenter T, Konig J, Hochfelder J, Siegel S, Gans M. Polyethylene glycol and polysorbate testing in 12 patients before or after coronavirus disease 2019 vaccine administration. Ann Allergy Asthma Immunol. 2022;128(1):99–101. doi: 10.1016/j.anai.2021.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krantz MS, Kwah JH, Stone CA, Jr, Phillips EJ, Ortega G, Banerji A, et al. Safety evaluation of the second dose of messenger RNA COVID-19 vaccines in patients with immediate reactions to the first dose. JAMA Intern Med. 2021;181(11):1530–1533. doi: 10.1001/jamainternmed.2021.3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robinson LB, Landman AB, Shenoy ES, Hashimoto D, Fu X, Jr Camargo CA, et al. Allergic symptoms after mRNA COVID-19 vaccination and risk of incomplete vaccination. J Allergy Clin Immunol Pract. 2021;9(8):3200–3202. doi: 10.1016/j.jaip.2021.05.031. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krantz MS, Bruusgaard-Mouritsen MA, Koo G, Phillips EJ, Jr Stone CA, Garvey LH. Anaphylaxis to the first dose of mRNA SARS-CoV-2 vaccines: don't give up on the second dose! Allergy. 2021;76(9):2916–2920. doi: 10.1111/all.14958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pannuzzo M, Esposito S, Wu LP, Key J, Aryal S, Celia C, et al. Overcoming nanoparticle-mediated complement activation by surface PEG pairing. Nano Lett. 2020;20(6):4312–4321. doi: 10.1021/acs.nanolett.0c01011. [DOI] [PubMed] [Google Scholar]
- 31.Hause AM, Gee J, Johnson T, Jazwa A, Marquez P, Miller E, et al. Anxiety-related adverse event clusters after Janssen COVID-19 vaccination - five U.S. mass vaccination sites, April 2021. MMWR Morb Mortal Wkly Rep. 2021;70(18):685–688. doi: 10.15585/mmwr.mm7018e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kohli-Pamnani A, Zapata K, Gibson T, Kwittken PL. Coronavirus disease 2019 vaccine hypersensitivity evaluated with vaccine and excipient allergy skin testing. Ann Allergy Asthma Immunol. 2022;128(1):97–98. doi: 10.1016/j.anai.2021.08.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pienkowski MM, Pienkowski SM. Evaluation of anaphylaxis risk by skin testing with coronavirus disease 2019 messenger RNA vaccines on patients with anaphylaxis. Ann Allergy Asthma Immunol. 2022;128(1):101–103. doi: 10.1016/j.anai.2021.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramsey A, Macy E, Chiriac AM, Blumenthal KG. Drug allergy labels lost in translation: from patient to charts and backwards. J Allergy Clin Immunol Pract. 2021;9(8):3015–3020. doi: 10.1016/j.jaip.2021.02.005. [DOI] [PubMed] [Google Scholar]