Abstract
Treatment of septicemia caused by Escherichia coli with ceftazidime (CAZ) may be associated with the development of septic shock due to the release of bacterial lipopolysaccharide. We examined the suppressive effect of clindamycin (CLDM) on CAZ-induced release of endotoxin by cultured E. coli and the subsequent production of inflammatory cytokines (tumor necrosis factor alpha [TNF-α] and interleukin-1β [IL-1β]). E. coli ATCC 12014 was incubated in inactivated horse serum with or without CLDM for 1, 4, or 18 h, followed by the addition of CAZ and collection of the culture supernatant at 0, 1, and 2 h. The concentration of endotoxin in each sample was measured by a chromogenic Limulus test. Another portion of the culture supernatant was added to THP-1 cell culture and incubated for 4 h, and the concentrations of TNF-α and IL-1β in the supernatant were measured by an enzyme-linked immunosorbent assay. In the control group (no CLDM), CAZ administration resulted in significant increases in endotoxin, TNF-α, and IL-1β concentrations. Pretreatment of E. coli with CLDM for 4 or 18 h before the addition of CAZ significantly suppressed the concentrations of endotoxin, TNF-α, and IL-1β in a time-dependent manner. In addition, CAZ treatment transformed E. coli from rod-shaped bacteria to filament-like structures, as determined by electron microscopy, while pretreatment with CLDM prevented these morphological changes. Our in vitro studies showed that CAZ-induced release of large quantities of endotoxin by E. coli could be suppressed by prior administration of CLDM.
Septicemia and septic shock are life-threatening conditions characterized by fever (sometimes, hypothermia), organ failure, and low blood pressure. Despite recent advances in antimicrobial therapy, the mortality rate due to sepsis caused by gram-negative bacteria remains high at present. For example, about 300,000 people suffer from septicemia every year in the United States and about 20 to 30% of these patients die of septic shock caused by gram-negative bacterial infections (2, 10, 16). In a survey conducted between January 1993 and April 1994, about 2 of every 100 hospitalized patients had symptoms related to sepsis, about 40% of these patients had gram-negative bacterial infections, and 25% of them eventually developed septic shock. In addition, the mortality rates were 38 and 45% in those patients who were hospitalized for 28 days and 5 months, respectively (23).
An important determinant of mortality in septic shock is the release of endotoxin. Endotoxin is a structural component (lipopolysaccharide [LPS]) of the cell wall of gram-negative bacteria (13, 31). When LPS is released from bacteria, its active-site lipid A is exposed, leading to the activation of complement and blood coagulation systems (5, 6, 9, 11, 18, 20, 28). At the same time, macrophages and monocytes are activated and large quantities of inflammatory cytokines (e.g., tumor necrosis factor alpha [TNF-α] or interleukin-1β [IL-1β]) are released into the circulatory system (17). These changes trigger a variety of systemic inflammatory reactions mediated by a complicated cytokine network. Furthermore, in severe septicemia, several systemic changes lead to circulatory disorders and multiorgan failure (32).
Although antibiotics are indispensable for the treatment of septicemia, they may indirectly induce septic shock due to the rapid lysis of gram-negative bacteria, which enhances the release of large quantities of endotoxin into the circulatory system (22). For example, β-lactams with a high affinity to penicillin binding protein 3 (PBP-3) are known to change the morphology of bacterial cells to a filament form and result in the release of large quantities of endotoxin (3, 4, 8, 25).
Clindamycin (CLDM) is effective against gram-positive and anaerobic bacteria, but it is not effective against Escherichia coli. However, it has recently been reported that subminimal inhibitory concentrations of CLDM may influence bacterial viability, toxin production, and host response. For example, CLDM promotes phagocytosis of bacteria by neutrophils (29, 30), suppresses the production of hemolysin by E. coli (1), inhibits the proinflammatory interactions of Pseudomonas aeruginosa-derived pigments by neutrophils (21), and reduces the amount of exotoxin released by Clostridium perfringens (26). On the other hand, ceftazidime (CAZ) is an effective antibiotic against E. coli, but when E. coli is killed by CAZ, large quantities of endotoxin are released (3, 4, 10, 25).
Using cultures of E. coli, we examined the suppressive effect of CLDM on CAZ-induced release of endotoxin by E. coli and the subsequent production of inflammatory cytokines (TNF-α and IL-1β) from THP-1 cells.
MATERIALS AND METHODS
Organism and preparation of bacterial suspension.
A standard strain of E. coli ATCC 12014 (E. coli O55:B5) was used in the present experiments. The organism was stored in 10% skim milk at −70°C. One day before the experiment, the organism was incubated overnight on Mueller-Hinton II agar (MHA; Becton Dickinson Microbiology Systems, Cockeysville, Md.) at 37°C. One of the resultant colonies was inoculated in Mueller-Hinton broth (Becton Dickinson Microbiology Systems) and incubated at 37°C until the logarithmic growth phase of bacteria was reached. The inoculation dose of E. coli O55:B5 was adjusted so that the microorganism was in the logarithmic growth phase (about 107 CFU/ml) following preincubation in the absence of any antibiotics. Thus, 107, 5 × 105, and 104 CFU/ml were used in 1-, 4-, and 18-h preincubation experiments, respectively.
Antibiotics.
CAZ (Tanabe Seiyaku, Osaka, Japan) and CLDM (Pharmacia & Upjohn, Tokyo, Japan) were used in this study. The MICs of CAZ and CLDM were 0.39 and 200 μg/ml, respectively, as determined by the standard microdilution procedure recommended by the National Committee for Clinical Laboratory Standards (19).
Pretreatment with CLDM.
Horse serum (Life Technologies Oriental, Tokyo, Japan) was inactivated at 58°C for 15 min and used as the culture medium. CLDM was added to the medium at a concentration of 25 μg/ml. This concentration is equivalent to that in the pulmonary tissue of adult patients following a bolus intravenous dose of 600 mg of CLDM (7). Five milliliters of E. coli O55:B5 inoculum was added to 45 ml of a medium containing CLDM, and the culture was incubated at 37°C for 1, 4, or 18 h. Control experiments were also performed by incubation of a similar volume of E. coli O55:B5 inoculum in inactivated horse serum without CLDM.
Determination of viable cell counts and endotoxin release.
In the next step, 38 ml of each culture (with or without CLDM) was divided into two portions and each was placed in a sterile test tube. In one tube, 1 ml of horse serum containing CAZ (final concentration, 10 μg/ml) was added, while 1 ml of horse serum alone was added in the other tube. Thus, four experimental groups were prepared: (i) control group containing E. coli in which no antibiotics were added, (ii) CAZ group in which E. coli was treated with only CAZ and not preincubated with CLDM, (iii) CLDM group in which E. coli was preincubated with CLDM but not treated with CAZ, and (iv) CLDM-CAZ group in which E. coli was preincubated with CLDM and subsequently treated with CAZ.
Viable cell counts were determined in control, CAZ, CLDM, and CLDM-CAZ groups at 0, 1, 2, and 4 h after the addition of CAZ by quantitative cultivation on MHA plates. To determine the concentration of endotoxin, control and test culture samples (5 ml) were collected at 0, 1, or 2 h after the addition of CAZ. Each sample was centrifuged at 1,000 × g for 15 min at 4°C and gently subjected to mechanical sterilization with a 0.2-μm-pore-size filter (EB-DISK 25; Kanto Chemical, Tokyo, Japan). Samples were stored at −20°C until measurement of endotoxin concentration.
Measurement of endotoxin concentrations.
The concentration of endotoxin in each sample was measured by a chromogenic endotoxin-specific assay with Limulus amoebocyte lysate (LAL) coagulation enzyme. Briefly, 50 μl of each sample was warmed with 100 μl of perchloric acid solution (Toxicolor system PCA-1 set; Seikagaku Corp., Tokyo, Japan) for 20 min at 37°C to remove any serum inhibitory activity against coagulation reaction of LAL. After centrifugation, the supernatant was neutralized with NaOH solution and diluted appropriately with endotoxin-free distilled water. Ten microliters of each diluted solution was mixed with 100 μl of the main reagent (ES-200 set; Seikagaku Corp.) in each well of a endotoxin-free 96-well plate (Toxipet Plate 96F; Seikagaku Corp.). After incubation for 30 min at 37°C, 50 μl each of sodium nitrite solution, ammonium sulfamate solution, and N-(1-naphthyl) ethylenediamine dihydrochloride solution were added to complete the diazo coupling reaction (Toxicolor system DIA-MP set, Seikagaku Corp.), and A545 of each well was measured with a spectrophotometer (Multiscan Multisoft; Labsystems Japan K.K., Tokyo, Japan). Endotoxin levels were calculated relative to the level of the reference endotoxin, E. coli O111:B4 LPS (Toxicolor system Et-2 set; Seikagaku Corp.).
Production of TNF-α and IL-1β by endotoxin-stimulated THP-1 cells.
A human acute monocytic leukemia cell line (THP-1) was used in the present experiments. THP-1 cells were obtained from a child with acute monocytic leukemia. This cell line is known to produce TNF-α and IL-1β in response to purified endotoxin (14, 27). The cells were maintained in RPMI 1640 medium containing 10% inactivated fetal calf serum, 5 × 10−6 M 2-mercaptoethanol, and 0.5% l-glutamine. To induce cytokine production, 2 ml of THP-1 cells (2 × 106 cells) was seeded into a pyrogen-free 24-well plate (Nuncron; Nunc, Roskilde, Denmark), and 100 μl of each bacterial filtrate sample was added. The plate was incubated at 37°C under 5% CO2 for 4 h. The resultant culture was centrifuged at 250 × g for 2 min at room temperature, and the supernatant was stored at −20°C until measurement of TNF-α and IL-1β.
Direct effect of CLDM on THP-1 cells.
To investigate the direct effect of CLDM on TNF-α production, CLDM was added into the medium of THP-1 cells (2 × 106 cells) at various concentrations (3.13, 6.25, 12.5, 25, and 50 μg/ml) or not added (control). After the cultures were incubated for 1, 4, or 18 h at 37°C under 5% CO2, purified E. coli O55:B5 LPS (Sigma-Aldrich Corp., Tokyo, Japan) at a final concentration of 1 μg/ml was added to each well and the cells were incubated for another 4 h. The resultant culture was centrifuged at 250 × g for 2 min at room temperature, and the supernatant was stored at −20°C until measurement of TNF-α.
Measurement of TNF-α and IL-1β.
The concentrations of TNF-α and IL-1β in the supernatant of THP-1 cell culture were determined by using an enzyme-linked immunosorbent assay commercial kit (Cytoscreen; BioSource International, Camarillo, Calif.) according to the protocols of the manufacturer.
Morphological examination.
Bacterial cultures (100 μl) were collected from each group before and after 18 h of incubation with CLDM, as well as 1 h after the addition of CAZ. The sample was placed on a 0.4-μm-pore-size filter (Isopore Track-Etched Membrane Filter; Millipore Co., Bedford, Mass.) and fixed first in 2% glutaraldehyde solution for 1 h and then in 1% osmium tetroxide for 1 h. The samples were then dehydrated with serial concentrations of ethyl alcohol (50, 70, 80, 90, 95, and 100%) and t-butyl alcohol and subjected to critical point drying. The filter was fixed on a sample table, and after gold palladium coating, the morphological features of E. coli were examined at ×5,000 magnification with a scanning electron microscope (Hitachi S-800: Hitachi Co., Tokyo, Japan).
Statistical analysis.
For each experiment, the concentrations of endotoxin, TNF-α, and IL-1β were measured in two wells. All experiments were repeated more than twice on different days. Differences between values for the groups were tested for statistical significance by the Mann-Whitney test. A P value of <0.05 denoted the presence of a significant statistical difference.
RESULTS
Antibacterial activity of CAZ against E. coli.
In a series of preliminary experiments, we first determined the antibacterial effects of CAZ against E. coli O55:B5. For this purpose, about 107 CFU of E. coli per ml was treated with 2.5, 5, 10, or 20 μg of CAZ per ml (Table 1). Viable cell count was reduced in a concentration-dependent manner after the addition of CAZ for 4 h (up to 10 μg/ml).
TABLE 1.
Effects of different concentrations of CAZ on viable cell counts of E. coli O55:B5
| CAZ concn (μg/ml) | Viable cell count (log10 CFU/ml)a at the following time (h) after treatment:
|
|||
|---|---|---|---|---|
| 0 | 1 | 2 | 4 | |
| 0 | 7.3 ± 0.1 | 7.1 ± 0.2 | 7.6 ± 0.1b | 8.4 ± 0.1c |
| 2.5 | 7.0 ± 0.1c | 7.1 ± 0.1b | 6.9 ± 0.1b | |
| 5 | 7.0 ± 0.1b | 6.9 ± 0.1c | 6.2 ± 0.2c | |
| 10 | 6.9 ± 0.1c | 6.2 ± 0.1c | 5.5 ± 0.1c | |
| 20 | 6.6 ± 0.2c | 5.9 ± 0.1c | 5.3 ± 0.1c | |
Values are reported as mean ± standard deviations (n = 4).
Significantly different from the value obtained with the control (0 h) (P < 0.05 by the Mann-Whitney test).
Significantly different from the value obtained with the control (0 h) (P < 0.0001 by the Mann-Whitney test).
Effects of CLDM and CAZ on viable cell counts of E. coli.
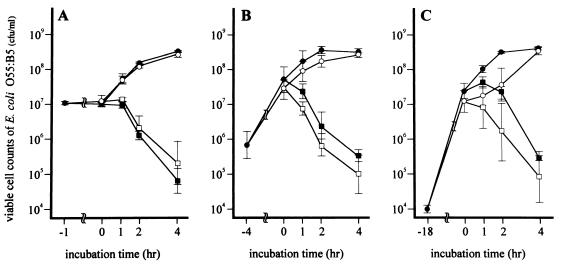
After preincubation, the viable cell count of E. coli with CLDM did not significantly decrease from that of the control, irrespective of the duration of the preincubation period (Fig. 1). In addition, further incubation of the control and CLDM groups for 1 to 4 h in the absence of CAZ increased the number of viable bacteria to more than 108 (Fig. 1). In contrast, CAZ resulted in a rapid decrease in viable cell counts, amounting to 50- to 100-fold in 4 h, in control and CLDM-pretreated cultures (CAZ and CLDM-CAZ groups [Fig. 1]).
FIG. 1.
Effects of CLDM and CAZ on viable cell counts of E. coli O55:B5. E. coli O55:B5 was pretreated with or without CLDM (25 μg/ml) for 1 (A), 4 (B), or 18 (C) h and then incubated for another 4 h with or without CAZ (10 μg/ml). Symbols: closed circles, control group (no antibiotics used); closed squares, CAZ group (CAZ treatment only); open circles, CLDM group (pretreatment with CLDM only); open squares, CLDM-CAZ group (pretreatment with CLDM followed by CAZ). Data are the means ± standard deviations for four experiments.
Effect of CLDM on CAZ-induced release of endotoxin.
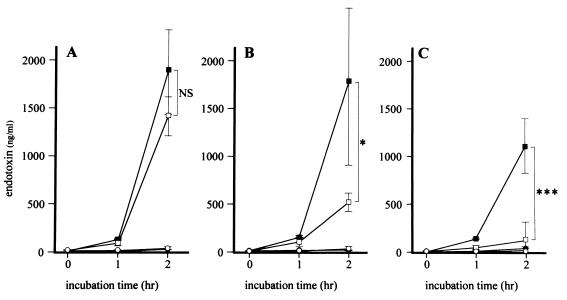
The concentration of endotoxin released by E. coli after 2 h of incubation was <50 ng/ml in the control and CLDM groups (Fig. 2). The addition of CAZ to the culture medium increased the concentration of endotoxin to >1,000 ng/ml at 2 h (Fig. 2). However, pretreatment with CLDM before the addition of CAZ resulted in suppression of the effect of CAZ (Fig. 2). This effect was dependent on the duration of preincubation with CLDM. There was no significant difference in the amount of endotoxin released by the addition of CAZ following 1 h of pretreatment with CLDM (1,352 ± 414 versus 1,800 ± 898 ng/ml [Fig. 2A]) compared to those following CLDM-free pretreatment. However, the amount of endotoxin released by the addition of CAZ was reduced following 4 h (517 ± 97 versus 1,784 ± 833 ng/ml [P < 0.05] [Fig. 2B]) and 18 h (144 ± 160 versus 1,196 ± 290 ng/ml [P < 0.001] [Fig. 2C]) of pretreatment with CLDM.
FIG. 2.
Kinetics of endotoxin released by E. coli O55:B5 after treatment with CAZ. After pretreatment with CLDM for 1 (A), 4 (B), or 18 (C) h, samples were collected from the control group (closed circles), CAZ group (closed squares), CLDM group (open circles), and CLDM-CAZ group (open squares) at 0, 1, and 2 h after the addition of CAZ and then mechanically sterilized. Data are the means ± standard deviations for four experiments. Statistical significance by the Mann-Whitney test is indicated as follows: NS, not significant (P > 0.05); ∗, P < 0.05; ∗∗∗, P < 0.001.
TNF-α and IL-1β concentrations released by THP-1 cells.
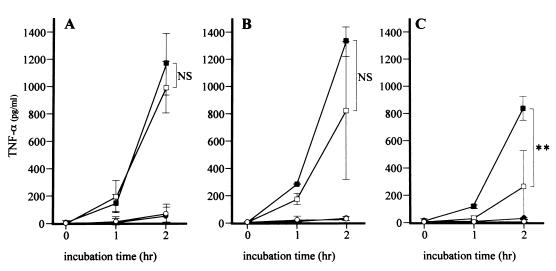
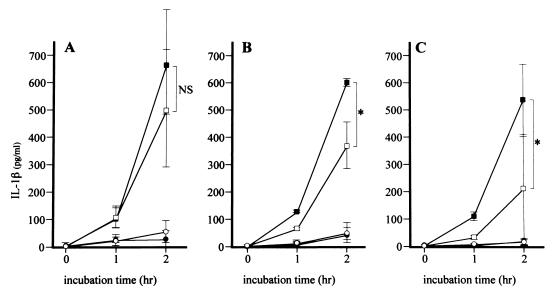
In the control group, the concentrations of TNF-α and IL-1β in the culture supernatant were <100 pg/ml. Incubation with CLDM alone resulted in small but insignificant changes in the concentrations of TNF-α and IL-1β at 1 and 2 h (Fig. 3 and 4). In contrast, addition of CAZ significantly increased the concentrations of TNF-α and IL-1β at 2 h (Fig. 3 and 4). However, pretreatment with CLDM before the addition of CAZ resulted in suppression of the effects of CAZ (Fig. 3 and 4). This effect was dependent on the duration of preincubation with CLDM. The concentrations of TNF-α following the addition of CAZ were not significantly different after 1 and 4 h of pretreatment with CLDM (957 ± 190 versus 1,171 ± 229 pg/ml and 822 ± 509 versus 1,326 ± 109 pg/ml [Fig. 3A and B, respectively]) compared to those following CLDM-free pretreatment. However, the concentrations of TNF-α following the addition of CAZ were reduced after 18 h (266 ± 264 versus 854 ± 88 pg/ml [P < 0.01] [Fig. 3C]) of pretreatment with CLDM. In the same manner, the concentrations of IL-1β following the addition of CAZ were not significantly different after 1 h of pretreatment with CLDM (494 ± 214 versus 677 ± 192 pg/ml [Fig. 4A]) compared to those following CLDM-free pretreatment, but they were reduced after 4 h (367 ± 86 versus 603 ± 15 pg/ml [P < 0.05] [Fig. 4B]) and 18 h (216 ± 204 versus 537 ± 135 pg/ml [P < 0.05] [Fig. 4C]) of pretreatment with CLDM.
FIG. 3.
Kinetics of TNF-α released by stimulated THP-1 cells. After pretreatment with CLDM for 1 (A), 4 (B), or 18 (C) h, samples were collected from the control group (closed circles), CAZ group (closed squares), CLDM group (open circles), and CLDM-CAZ group (open squares) at 0, 1, and 2 h after the addition of CAZ and then mechanically sterilized. THP-1 cells were stimulated by these samples for 4 h, and the concentration of TNF-α in THP-1 cell cultures was determined. Data are the means ± standard deviations for four experiments. Statistical significance by the Mann-Whitney test is indicated as follows: NS, not significant (P > 0.05); ∗∗, P < 0.01.
FIG. 4.
Kinetics of IL-1β released by stimulated THP-1 cells. After pretreatment with CLDM for 1 (A), 4 (B), or 18 (C) h, samples were collected from the control group (closed circles), CAZ group (closed squares), CLDM group (open circles), and CLDM-CAZ group (open squares) at 0, 1, and 2 h after the addition of CAZ and then mechanically sterilized. THP-1 cells were stimulated by these samples for 4 h, and the concentration of IL-1β in THP-1 cell cultures was determined. Data are the means ± standard deviations for four experiments. Statistical significance by the Mann-Whitney test is indicated as follows: NS, not significant (P > 0.05); ∗, P < 0.05.
On the other hand, 3.13 and 6.25 μg of CLDM per ml did not significantly reduce the concentration of TNF-α in the medium of THP-1 cells stimulated with purified E. coli O55:B5 LPS regardless of pretreatment time (Table 2). However, 25 and 50 μg/ml of CLDM significantly reduced TNF-α concentrations after pretreatment for 4 or 18 h (Table 2).
TABLE 2.
Direct effect of CLDM on TNF-α production by LPS-stimulated THP-1 cellsa
| CLDM concn (μg/ml) | TNF-α concn (pg/ml)b for cells with the following pretreatment time (h) with CLDM:
|
||
|---|---|---|---|
| 1 | 4 | 18 | |
| 0 | 562 ± 41 | 557 ± 58 | 564 ± 42 |
| 3.13 | 531 ± 57 | 581 ± 218 | 567 ± 101 |
| 6.25 | 448 ± 190 | 403 ± 100 | 624 ± 107 |
| 12.5 | 417 ± 119 | 376 ± 117c | 497 ± 109 |
| 25 | 457 ± 79c | 390 ± 95c | 393 ± 82c |
| 50 | 500 ± 49 | 379 ± 75c | 371 ± 77c |
The cells were incubated with or without CLDM (0, 3.13, 6.25, 12.5, 25, and 50 μg/ml) for 1, 4, or 18 h and stimulated with purified E. coli O55:B5 LPS (1 μg/ml) for another 4 h.
Values are reported as mean ± standard deviations (n = 4).
Significantly different from the value obtained with the control (no CLDM) (P < 0.05 by the Mann-Whitney test).
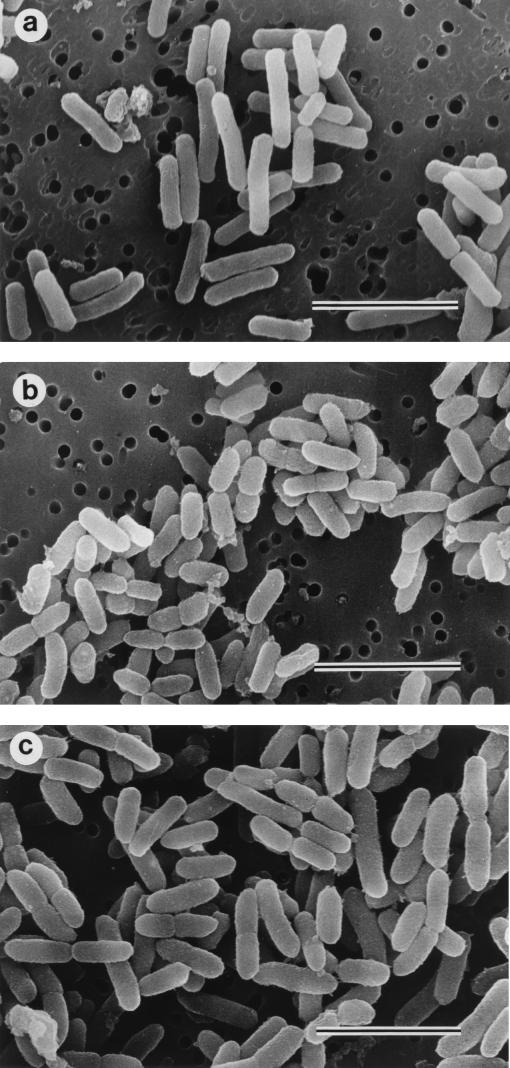
Morphological examination.
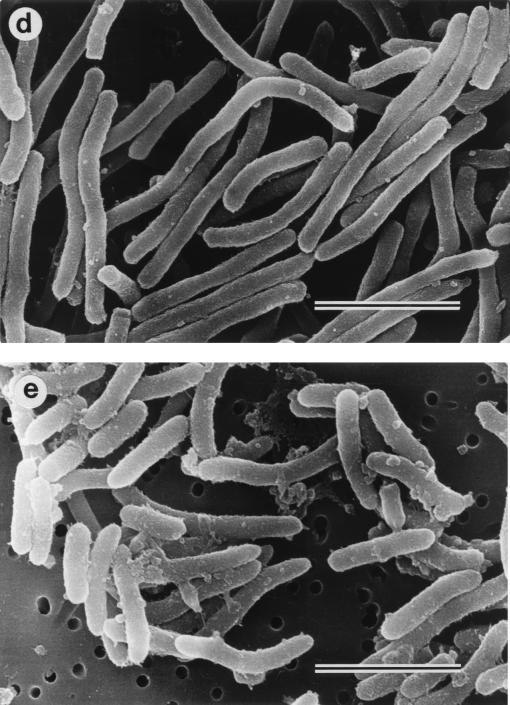
Morphological examination showed rod-shaped E. coli cells when not treated (control) (Fig. 5a). The morphological features of E. coli were similar to those of the control after 18 h of preincubation with CLDM (Fig. 5b and c). In contrast, the addition of CAZ resulted in elongation of the cells within the first hour (Fig. 5d). However, elongation of the cells was clearly suppressed when the cells were pretreated with CLDM followed by the addition of CAZ (CLDM-CAZ group) (Fig. 5e).
FIG. 5.
Photomicrographs of E. coli O55:B5 examined by electron microscopy. (a) Fresh sample of E. coli before incubation (control), (b) E. coli after 18 h of preincubation in CLDM-free medium, (c) E. coli after 18 h of preincubation with 25 μg of CLDM per ml, (d) E. coli preincubated in CLDM-free medium shown 1 h after the addition of 10 μg of CAZ per ml, and (e) E. coli preincubated with 25 μg of CLDM per ml shown 1 h after the addition of 10 μg/ml of CAZ. Note transformation of E. coli to filament-like structures in panel d. Bars, 3 μm.
DISCUSSION
The major finding of this study was that pretreatment of cultured E. coli with CLDM for 4 to 18 h reduced the amount of CAZ-induced increase in bacterial endotoxin levels and prevented the associated rise in TNF-α and IL-1β production. We also showed that these changes were associated with elongation of the bacteria. The mechanisms of CLDM-induced suppression of CAZ-enhanced endotoxin release are not clear at present but may be due, at least in part, to the morphological changes described above. It is possible that the suppression of filament formation reduces the surface area of lysed bacteria, which in turn decreases the amount of endotoxin released by E. coli. Several studies have reported that β-lactam antibiotics with a high affinity to PBP-3 (e.g., CAZ) enhance elongation of gram-negative bacteria and that due to subsequent bacteriolysis, large quantities of endotoxin are released in the supernatant during the early phase of incubation (3, 4, 25). Jackson and Kropp (8) compared the effects of two β-lactam antibiotics (imipenem [IPM] and CAZ that show a strong affinity toward PBP-2 and PBP-3, respectively) on P. aeruginosa and reported that CAZ released 10 to 40 times more endotoxin than IPM. In addition, Dofferhoff et al. (3, 4) examined the effects of various antibiotics on E. coli cultured in a solution containing human monocyte cells by measuring the concentrations of endotoxin and TNF-α. They reported higher concentrations of endotoxin and TNF-α in cultures treated with low concentrations of CAZ, aztreonam, and cefuroxime than in cultures treated with IPM. Simon et al. (25) also reported results similar to those described above with THP-1 cells, which we used in this study. They reported that incubating these cells with supernatant of E. coli treated with CAZ, cefotaxime, or aztreonam was associated with higher concentrations of TNF-α than in THP-1 cells treated with a supernatant of E. coli treated with IPM or no antibiotics.
Our results also showed a fast bactericidal effect for CAZ against E. coli. CAZ rapidly killed E. coli O55:B5, and more than 1,000 ng/ml of endotoxin was released after 2 h of incubation. In addition, there was no significant difference in viable cell count between CAZ and CLDM-CAZ groups, i.e., 25 μg of CLDM per ml did not affect the growth of E. coli or the bactericidal activity of CAZ. Furthermore, 18 h of preincubation with CLDM did not change the amount of endotoxin released by E. coli. In contrast, the addition of CAZ enhanced the production of endotoxin to more than 1,000 ng/ml. However, pretreatment of such cultures with CLDM for 4 and 18 h significantly reduced the amount of endotoxin to 517 and 144 ng/ml, respectively. Similar changes were also observed for TNF-α and IL-1β released into the supernatant; the concentrations of both cytokines were higher following CLDM-free pretreatment than following pretreatment with CLDM for 4 and 18 h. Previous studies have shown that CLDM suppresses the production of α-toxin by C. perfringens (26) and hemolysin by E. coli (1). These toxins are proteins, but endotoxin itself is a LPS. Nonetheless, due to the suppression of enzymes that inhibit the synthesis of cell walls, the amount of endotoxin may be secondarily decreased.
CLDM is also known to enhance the cytoplasmic function of polymorphonuclear leukocytes (11), accelerate phagocytosis (24), promote antibody production by lymphocytes (15), and suppress the production of superoxide and myeloperoxidase in human neutrophils when stimulated by pyocyanin or 1-hydroxyphenazine, both of which are produced by P. aeruginosa (21). These findings suggest that CLDM is an effective immunomodulator. In fact, Stevens et al. (26) reported that CLDM suppressed the production of TNF-α by LPS-stimulated human monocytes. In the present study, 3.13 μg of CLDM per ml failed to suppress the production of TNF-α when THP-1 cells were stimulated with purified LPS. This level of CLDM was larger than the level in the bacterial filtrate sample added into the THP-1 cell culture in this study. Nevertheless, 25- and 50-μg/ml concentrations of CLDM, which are attainable in pulmonary tissue clinically (7), significantly reduced TNF-α concentrations after pretreatment for 4 or 18 h. Therefore, excess production of inflammatory cytokines such as TNF-α induced by release of LPS endotoxin may be suppressed.
In conclusion, we have shown that pretreatment with CLDM suppresses CAZ-enhanced release by E. coli of endotoxin, TNF-α, and IL-1β. From these findings, we intend to study further the suppressive effect of CLDM against endotoxin release by gram-negative bacteria in in vitro and in vivo models.
ACKNOWLEDGMENTS
We thank F. G. Issa from the Department of Medicine, University of Sydney, Sydney, Australia, for the careful reading and editing of the manuscript.
REFERENCES
- 1.Boe N M, Dellinger E P, Minshew B H. Effect of clindamycin on growth and haemolysin production by Escherichia coli. J Antimicrob Chemother. 1983;12(Suppl. C):105–116. doi: 10.1093/jac/12.suppl_c.105. [DOI] [PubMed] [Google Scholar]
- 2.Bone R C. Gram-negative sepsis: a dilemma of modern medicine. Clin Microbiol Rev. 1993;6:57–68. doi: 10.1128/cmr.6.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dofferhoff A S M, Nijland J H, de Vries-Hospers H G, Mulder P O M, Weits J, Bom V J J. Effects of different types and combinations of antimicrobial agents on endotoxin release from gram-negative bacteria: an in-vitro and in-vivo study. Scand J Infect Dis. 1991;23:745–754. doi: 10.3109/00365549109024303. [DOI] [PubMed] [Google Scholar]
- 4.Dofferhoff A S M, Esselink M T, de Vries-Hospers H G, Zanten A V, Bom V J J, Weits J, Vellenga E. The release of endotoxin from antibiotic-treated Escherichia coli and the production of tumor necrosis factor by human monocytes. J Antimicrob Chemother. 1993;31:373–384. doi: 10.1093/jac/31.3.373. [DOI] [PubMed] [Google Scholar]
- 5.Fealon D T, Ruddy S, Schur P H, McCabe W R. Activation of the properdin pathway of complement in patients with gram-negative bacteremia. N Engl J Med. 1975;292:937–940. doi: 10.1056/NEJM197505012921802. [DOI] [PubMed] [Google Scholar]
- 6.Homma J Y, Matsuura M, Kumazawa Y. Structure-activity relationship of chemically synthesized nonreducing parts of lipid A analogs. Adv Exp Med Biol. 1990;256:101–119. doi: 10.1007/978-1-4757-5140-6_6. [DOI] [PubMed] [Google Scholar]
- 7.Ikeda T, Sakai T, Kikuchi K, Mizuwatari T. Concentration of clindamycin phosphate in lung tissue. Jpn J Antibiot. 1985;38:3477–3480. [PubMed] [Google Scholar]
- 8.Jackson J J, Kropp H. Beta-lactam antibiotic-induced release of endotoxin: in vitro comparison of penicillin-binding protein (PBP) 2 specific imipenem and PBP-3 specific ceftazidime. J Infect Dis. 1992;165:103–141. doi: 10.1093/infdis/165.6.1033. [DOI] [PubMed] [Google Scholar]
- 9.Kimball H R, Melmon K L, Wolff S M. Endotoxin-induced kinin production in man. Proc Soc Exp Biol Med. 1972;139:1078–1082. doi: 10.3181/00379727-139-36302. [DOI] [PubMed] [Google Scholar]
- 10.Kirikae T, Nakano M, Morrison D C. Antibiotic-induced endotoxin release from bacteria and its clinical significance. Microbiol Immunol. 1997;41:285–294. doi: 10.1111/j.1348-0421.1997.tb01203.x. [DOI] [PubMed] [Google Scholar]
- 11.Koga H. High-performance liquid chromatography measurement of antimicrobial concentrations in polymorphonuclear leukocytes. Antimicrob Agents Chemother. 1987;31:1904–1908. doi: 10.1128/aac.31.12.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kotani S, Takada H. Structural requirements of lipid A for endotoxicity and biological activities: an overview. Adv Exp Med Biol. 1990;256:13–43. doi: 10.1007/978-1-4757-5140-6_2. [DOI] [PubMed] [Google Scholar]
- 13.Michie H R, Manogue K R, Spriggs D R, Revhaug A, O’Dwyer S, Dinarello C A, Cerami A, Wolff S M, Wilmore D W. Detection of circulating tumor necrosis factor after endotoxin administration. N Engl J Med. 1988;318:1481–1486. doi: 10.1056/NEJM198806093182301. [DOI] [PubMed] [Google Scholar]
- 14.Molina J, Scadden D T, Bym R, Dinarello C A, Groopman J E. Production of tumor necrosis factor-alpha and interleukin 1-beta by monocytic cells infected with human immunodeficiency virus. J Clin Invest. 1989;84:733–737. doi: 10.1172/JCI114230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mori K. Influence of antibiotics on IgG production by cultured human lymphocytes. Kurume Med J. 1993;56:1314–1326. [Google Scholar]
- 16.Morrison D C, Bucklin S E, Norimatsu M. Contribution of soluble endotoxin released from gram-negative bacteria by antibiotics to pathogenesis of experimental sepsis in mice. J Endotoxin Res. 1996;3:237–243. [Google Scholar]
- 17.Morrison D C, Ryan J L. Endotoxin and disease mechanisms. Annu Rev Med. 1987;38:417–432. doi: 10.1146/annurev.me.38.020187.002221. [DOI] [PubMed] [Google Scholar]
- 18.Nakano M, Asou H, Yamamoto I. Stimulation of phagocytic activity in the reticuloendothelial systems of mice by lipid A complexed with homologous or heterologous proteins. Infect Immun. 1975;11:592–594. doi: 10.1128/iai.11.3.592-594.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. 3rd ed. Approved standard M7-A3. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1993. [Google Scholar]
- 20.Nies A S, Forsyth R P, Williams H E, Melmon K L. Contribution of kinins to endotoxin shock in unanesthetized rhesus monkeys. Circ Res. 1968;22:155–164. doi: 10.1161/01.res.22.2.155. [DOI] [PubMed] [Google Scholar]
- 21.Ras G J, Anderson R, Taylor G W, Savage J E, van Niekerk E, Joone G, Koornhof H J, Saunders J, Wilson R, Cole P J. Clindamycin, erythromycin, and roxithromycin inhibit the proinflammatory interactions of Pseudomonas aeruginosa pigments with human neutrophils in vitro. Antimicrob Agents Chemother. 1992;36:1236–1240. doi: 10.1128/aac.36.6.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reilly J, Compagnon A, Tournier P, Buit H D. Les accidents du traitment des fievres typhoides par la chloromycetine. Ann Med (Paris) 1950;51:597–629. [PubMed] [Google Scholar]
- 23.Sands K E, Bates D W, Lanken P N, et al. Epidemiology of sepsis syndrome in 8 academic medical centers. JAMA. 1997;278:234–240. [PubMed] [Google Scholar]
- 24.Santos J I, Arbo A, Pavia N. In vitro and in vivo effects of clindamycin on polymorphonuclear leukocyte function. Clin Ther. 1992;14:578–594. [PubMed] [Google Scholar]
- 25.Simon D M, Koenig G, Trenholme G M. Differences in release of tumor necrosis factor from THP-1 cells stimulated by filtrates of antibiotic-killed Escherichia coli. J Infect Dis. 1991;164:800–802. doi: 10.1093/infdis/164.4.800. [DOI] [PubMed] [Google Scholar]
- 26.Stevens D L, Bryant A E, Hackett S P. Antibiotic effects on bacterial viability, toxin production, and host response. Clin Infect Dis. 1995;20(Suppl. 2):154–157. doi: 10.1093/clinids/20.supplement_2.s154. [DOI] [PubMed] [Google Scholar]
- 27.Tsuchiya S, Yamabe M, Yamaguchi Y, Kobayashi Y, Konno T, Tada K. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1) Int J Cancer. 1980;26:171–176. doi: 10.1002/ijc.2910260208. [DOI] [PubMed] [Google Scholar]
- 28.Ulevitch R J, Cochran C G, Henson P M, Morrison D C, Doe W F. Mediation systems in bacterial lipopolysaccharide-induced hypotension and disseminated intravascular coagulation. I. The role of complement. J Exp Med. 1975;142:1570–1590. doi: 10.1084/jem.142.6.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Veringa E M, Lambe D W, Jr, Ferguson D A, Jr, Verhoel J. Enhancement of opsonophagocytosis of Bacteroides spp. by clindamycin in subinhibitory concentration. J Antimicrob Chemother. 1989;23:577–587. doi: 10.1093/jac/23.4.577. [DOI] [PubMed] [Google Scholar]
- 30.Veringa E M, Verhoef J. Clindamycin at subinhibitory concentrations enhances antibody- and complement-dependent phagocytosis by human polymorphonuclear leukocytes of Staphylococcus aureus. Chemotherapy. 1987;33:243–249. doi: 10.1159/000238502. [DOI] [PubMed] [Google Scholar]
- 31.Wolf S M. Biological effects of bacterial endotoxins in man. J Infect Dis. 1973;128(Suppl.):259–264. doi: 10.1093/infdis/128.supplement_1.s259. [DOI] [PubMed] [Google Scholar]
- 32.Young L S. Gram-negative sepsis. In: Mandell G L, Douglas R G Jr, Benett J E, editors. Principles and practice of infectious disease. 3rd ed. New York, N.Y: Churchill Livingstone; 1990. pp. 611–636. [Google Scholar]