Abstract
To identify psychological antecedents of COVID-19 vaccine hesitancy among healthcare personnel (HCP). We surveyed 4603 HCP to assess psychological antecedents of their vaccination decisions (the ‘5 Cs’) for vaccines in general and for COVID-19 vaccines. Most HCP accept vaccines, but many expressed hesitancy about COVID-19 vaccines for the psychological antecedents of vaccination: confidence (vaccines are effective), complacency (vaccines are unnecessary), constraints (difficult to access), calculation (risks/benefits), collective responsibility (need for vaccination when others vaccinate). HCP who were hesitant only about COVID-19 vaccines differed from HCP who were consistently hesitant: those with lower confidence were more likely to be younger and women, higher constraints were more likely to have clinical positions, higher complacency were more likely to have recently cared for COVID-19 patients, and lesser collective responsibility were more likely to be non-white. These results can inform interventions to encourage uptake of COVID-19 vaccines in HCP.
Keywords: COVID-19, Healthcare personnel, Vaccines, Vaccination attitudes, Vaccine refusal
Introduction
Over 77 million Americans have been infected with COVID-19, and more than 900,000 have died since the first case was identified in the US in January 2020 [1]. Healthcare personnel (HCP) have been disproportionately vulnerable to infection and death [2], in addition to mental health issues associated with caring for COVID-19 patients [3]. Accordingly, the preservation of healthcare capacity has been a cornerstone of pandemic response efforts [4], especially in the context of new, highly transmissible variants, including Omicron [5].
Several COVID-19 vaccines have received emergency use authorization (EUA) by the Food and Drug Administration [6], and the Pfizer vaccine has received full approval [7]. HCP were among the first groups to be prioritized for vaccine distribution [8], and they were among the first to receive ‘booster’ doses [9]. While vaccination rates among HCP have generally been high [10, 11], vaccine hesitancy and refusal among HCP remain a problem [12, 13]. In response, many healthcare organizations have announced COVID-19 vaccine mandates, and more have done so following full approval of the Pfizer vaccine. But staffing shortages, especially of nurses, have caused other healthcare employers to choose not to implement mandates [14], and efforts by state governments to prohibit private employer mandates may have a similar effect [15]. It is also preferable, from an ethical point of view, for HCP to voluntarily vaccinate than to do so only because vaccination is a condition of employment [16].
Our survey of HCP at a large US healthcare system was conducted to identify and quantify the beliefs and attitudes of HCP related to COVID-19 vaccines. A previous paper reported participants’ vaccination status, their intention to vaccinate, and the reasons for their vaccination decisions [17]. The current paper focuses on those HCP who accept vaccines in general, but are hesitant about COVID-19 vaccines; knowledge about this group may inform interventions aimed to increase acceptance of COVID-19 vaccines in HCP as well as others in their social and professional networks. This group is of interest because many HCP who have received COVID-19 vaccines may continue to be hesitant about COVID-19 vaccines, and their hesitancy may be a barrier to their receipt of additional COVID-19 vaccines in the future, or of their willingness to advocate for COVID-19 vaccination in their social networks.
Methods
A survey was conducted at a large, eight-hospital health system in Michigan, USA. This survey, fielded from 9 April to 4 May of 2021, coincided with the third COVID-19 surge in Michigan. Of the 37,695 invited HCP (by email), 4603 completed the survey (12.2% response rate). The study was approved by the health system Institutional Review Board.
In addition to demographic questions, this survey assessed vaccine attitudes using the validated 5C Psychological Antecedents of Vaccination Scale [18, 19]. Respondents were also asked about their fear of COVID-19 infection, their anticipated likelihood of COVID-19 infection, their history of COVID-19 diagnosis, and their workplace exposure to COVID-19 patients. Participants were asked to identify their reasons for and against receiving a COVID-19 vaccine.
Measures
Respondents were asked their age, gender (male, female, prefer not to specify) and race (coded as white yes/no for present analyses), as well as vaccination status, mask use outside of their work environment, whether they had a clinical work role, whether they worked with COVID-19 patients in the past three months (yes/no), whether they had a history of COVID-19 diagnosis (yes/no), how severe they believed COVID-19 infection would be for them (0–100, from less to more severe), and whether they were afraid of COVID-19 infection (0–100, from less to more afraid) [20].
The 5-item version of the 5C Psychological Antecedents of Vaccination Scale was used for vaccines in general and modified to assess antecedents for COVID-19 vaccines (10 total items). There is one item for each of the five Cs: Confidence, Complacency, Constraints, Calculation, and Collective Responsibility, rated on a 7-point Likert scale, from Strongly Disagree to Strongly Agree:
“I am completely confident that vaccines are safe.” (Confidence)
“Vaccination is unnecessary because vaccine preventable diseases are not common anymore.” (Complacency)
“Getting vaccinated is a hassle.” (Constraints)
“When I think about getting vaccinated, I weigh benefits and risks to make the best decision possible.” (Calculation)
“When everyone is vaccinated, I don’t have to get vaccinated, too.” (Collective responsibility) [19]
The Calculation subscale was not included in analyses as there was a limited range of responses (i.e., nearly all participants reported they weigh the risks and benefits in making decisions about vaccination) for both vaccines in general and COVID-19 vaccines, as would be anticipated. Accordingly, we subsequently refer to the ‘4 Cs’ (i.e. confidence, complacency, constraints, and collective responsibility).
Dichotomizing 4Cs into Hesitancy or Acceptance
The 7-point Likert scale can be divided into “agree” (strongly agree, moderately agree, agree), “disagree” (strongly disagree, moderately disagree, disagree), and “neutral” (mid-point on Likert scale). Each item was individually recoded as accepting or hesitant (accounting for reverse coded items). Neutral responses were excluded from analyses for confidence, constraints, and collective responsibility, which allowed us to focus on respondents who shifted clearly from acceptance to hesitancy between vaccines in general and COVID-19 vaccines. Neutrals were included in the “hesitant” category for the complacency item because neutrality on the significance of vaccine preventable disease is typically more representative of hesitancy. (The absence of positive concern about vaccine-preventable disease is sufficient reason to refuse vaccines.)
We then compared participants’ responses to general vaccines and COVID-19 vaccines for each of the 4Cs separately. We recoded participants as: (1) consistent acceptance (accepting for both vaccines in general and COVID-19 vaccines on the specific subscale); (2) consistent hesitancy (hesitant for both vaccines in general and COVID-19 vaccines on the specific subscale); and (3) COVID-19 specific hesitancy (accepting of vaccines in general and hesitant of COVID-19 vaccines). Very few HCP were COVID-19 specific accepting, but vaccine hesitant in general, thus analyses could not be conducted on these individuals.
Analyses
Differences in 4C Scores Across Demographic, Work, and COVID-19 Specific Characteristics
Analyses were performed using SPSS 28.0 (Armonk, NY) [21]. Analyses were first conducted to examine the direct relationships between demographic, work, and COVID-19 specific experiences (e.g., work with COVID-19 patients, personal history of COVID-19 diagnosis) and COVID-19 specific 4Cs scores. T-tests were conducted for categorical values (e.g., gender, personal history of COVID-19 diagnosis, etc.) with each of the 4Cs as the dependent variable (with full response scales, non-dichotomized). Correlations were conducted for continuous variables (e.g., correlation between age and complacency).
Differences Across Consistently Accepting, Consistently Hesitant, and COVID-19 Specific Hesitant Groups
Analyses were then conducted to determine group differences on key variables amongst participants who were consistently accepting, consistently hesitant, and COVID-19 specific hesitant. ANOVAs were conducted for continuous variables of age, vaccination status, self-rated belief in severity if infected with COVID-19, fear of COVID-19 infection, and the number of reasons endorsed for and concerns about COVID-19 vaccination. Bonferroni post-hoc tests were conducted to determine intergroup differences on significant findings. Chi-square analyses were conducted for dichotomous variables of gender, race, clinical work position, personal history of COVID-19 diagnosis, and past 3 months work status with COVID-19 patients.
Results
Demographics
HCP included in analyses were 17.1% men with a mean age of 46.09 years (SD = 12.98). Nearly 86% of HCP identified as vaccinated and included those vaccinated (85.4%) or scheduled to be vaccinated (0.3%). Almost 15% of HCP reported a previous COVID-19 infection, 49.6% worked in a clinical care position, 19.7% worked with COVID-19 patients in the previous three months, and 62.0% always wore masks around persons outside of their family.
The COVID-19 4Cs varied significantly based on demographics, work, and COVID-19 specific variables; see Table 1. Women had higher confidence in COVID-19 vaccines than men did. Whites had higher confidence, lower complacency, and higher levels of collective responsibility. HCP who had not been diagnosed with COVID-19 reported higher confidence, lower complacency, and fewer constraints. HCP who worked with COVID-19 patients in the previous three months had higher confidence, less complacency, fewer constraints, and higher levels of collective responsibility. HCP in clinical care positions reported more constraints. Notably, vaccination status was predictive of substantial differences on all of the 4Cs; differences in the mean scores between vaccinated and non-vaccinated people were between 3.65 points on the Likert scale (confidence) and 1.30 points on the Likert scale (complacency).
Table 1.
Relationship of demographic, work experience, and COVID-19 experiences and beliefs to 4C scores
| Confidence | Complacency | Constraints | Collective responsibility | |||||
|---|---|---|---|---|---|---|---|---|
| M (SD) | t, p | M (SD) | t, p | M (SD) | t, p | M (SD) | t, p | |
| Gender | ||||||||
| Men (n = 771) | 5.44 (1.77) | 6.54, 0.001 | 1.28 (0.81) | − 0.40, 0.69 | 2.53 (1.86) | 1.76, 0.08 | 1.56 (1.22) | 0.80, 0.42 |
| Women (n = 3666) | 5.89 (1.59) | 1.29 (0.85) | 2.41 (1.79) | 1.53 (1.17) | ||||
| Race (% white) | ||||||||
| White (n = 3826) | 5.55 (1.72) | − 6.60, 0.001 | 1.28 (0.82) | 4.85, 0.001 | 2.45 (1.81) | − 0.26, 0.80 | 1.50 (1.14) | 7.54, 0.001 |
| Non-White (n = 740) | 5.08 (2.03) | 1.45 (1.07) | 2.44 (1.85) | 1.87 (0.03) | ||||
| Diagnosed with COVID-19 | ||||||||
| Yes (n = 673) | 4.99 (2.01) | − 7.70, 0.001 | 1.48 (1.07) | 6.02, 0.001 | 2.69 (1.89) | 3.67, 0.001 | 1.82 (1.43) | − 0.95, 0.17 |
| No (n = 3885) | 5.56 (1.73) | 1.27 (0.83) | 2.41 (1.80) | 1.17 (0.02) | ||||
| 3 Mos Work w/COVID-19 Patients | ||||||||
| Yes (n = 898) | 5.56 (1.71) | 6.69, 0.001 | 1.27 (0.81) | − 5.31, 0.001 | 2.39 (1.79) | − 4.35, 0.001 | 1.51 (1.15) | − 5.83, 0.001 |
| No (n = 3668) | 5.12 (2.03) | 1.45 (1.07) | 2.69 (1.89) | 1.77 (1.45) | ||||
| Vaccinated | ||||||||
| Yes (n = 3966) | 5.96 (1.26) | − 64.55, 0.001 | 1.14 (0.59) | 38.90, 0.001 | 2.26 (1.73) | 18.55, 0.001 | 1.29 (0.79) | 48.01, 0.001 |
| No (n = 600) | 2.31 (1.49) | 2.44 (1.43) | 3.69 (1.86) | 3.40 (1.85) | ||||
| Clinical care position | ||||||||
| Yes (n = 2241) | 5.47 (1.81) | 0.98, 0.33 | 1.32 (0.90) | − 1.60, 0.11 | 2.54 (1.84) | − 3.52, 0.001 | 1.55 (1.20) | 0.40, 0.69 |
| No (n = 2274) | 5.52 (1.74) | 1.28 (0.82) | 2.35 (1.78) | 1.56 (1.23) | ||||
| Always mask | ||||||||
| Yes (n = 2798) | 5.79 (1.56) | − 14.84, 0.001 | 1.18 (0.65) | 12.63, 0.001 | 2.26 (1.76) | 9.33, 0.001 | 1.36 (0.96) | 14.34, 0.001 |
| No (n = 1717) | 5.00 (2.00) | 1.51 (1.10) | 2.77 (1.87) | 1.88 (1.49) | ||||
| Correlations | ||||||||
| Anticipated likelihood of infection | 0.18** | 0.04* | 0.10** | 0.07** | ||||
| Anticipated severity of infection | 0.08** | − 0.05** | 0.03 | 0.003 | ||||
| Fear of infection | − 0.10** | − 0.12** | − 0.03 | − 0.11** | ||||
| # Vaccine concerns endorsed | 0.43** | 0.21** | 0.27** | 0.26** | ||||
| # Vaccine reasons endorsed | − 0.44** | − 0.27** | − 0.17** | − 0.31** | ||||
Table represents the differences in 4C scores across key characteristics in the present study. Bold text indicates a significant difference. For the correlations: * = significant difference of 0.05 or less. ** = significant difference of 0.01 or less. Participants were allowed to not answer any item, thus response rates across some items in Table 1 reflect the lower response (e.g., gender and clinical care position)
COVID-19 Vaccines vs. Vaccines in General
For each of the 4Cs that we analyzed we report the frequency and attributes of respondents from three different groups: (1) consistent acceptance, (2) consistent hesitancy, and (3) COVID-19 specific hesitancy. Most participants were not in any of the COVID-19 specific hesitancy groups (83.4%; n = 3838). No HCP were in all 4 of the COVID-19 specific hesitancy groups, 0.5% (n = 23) were in three of the groups, 2.6% (119) were in 2 of the groups, and 13.5% (n = 623) individuals were in one of the COVID-19 specific hesitancy groups. In light of the minimal overlap between the COVID-19 specific hesitancy groups, we treat these groups as if their memberships are distinct; see Table 2. Furthermore, the fact that there is very little overlap between these groups suggests that, for the 4Cs we analyzed, each of the Cs tracks a distinct psychological antecedent of vaccination.
Table 2.
Number of HCP in COVID-specific hesitancy groups and overlap amongst COVID-specific hesitancy groups
| Confidence (n) | Constraints (n) | Collective responsibility (n) | Complacency (n) | |
|---|---|---|---|---|
| Confidence (n) | 445 | 107 | 66 | 51 |
| Constraints (n) | 453 | 41 | 32 | |
| Collective responsibility (n) | 154 | 46 | ||
| Complacency (n) | 115 |
Crosstabs represent the number of individuals in each COVID-Specific Hesitancy group (e.g., 445 HCP were in the Confidence COVID-Specific Hesitancy group) or the overlap between two groups (e.g., there were 107 HCP who were in both the Confidence and Constraints COVID-Specific Hesitancy groups)
Similarities Across 4Cs
For each of the confidence, constraints, and collective responsibility measures, there were some stable differences between the members of the consistent acceptance, consistent hesitancy, and COVID-19 specific hesitancy groups (note: complacency is presented separately because of the different coding approach); see Table 3. On all three measures, HCP with COVID-19 specific hesitancy were younger, more likely to be women, less likely to be vaccinated, and more likely to have worked with COVID-19 patients in the past three months compared to the consistent acceptance group. For each of these subscales, HCP with COVID-19 specific hesitancy had greater anticipated likelihood of infection and had lower rates of masking around non-family than did the consistent acceptance groups. Individuals who had COVID-19 specific hesitancy also reported more concerns about vaccines and fewer number of reasons to vaccinate, than did members of the consistent acceptance groups. Members of the COVID-19 specific hesitancy groups generally resembled members of the consistent hesitancy groups on these variables.
Table 3.
Differences amongst consistent accepting, consistent non-accepting, and COVID-specific non-accepting
| Confidence | Constraints | Collective Responsibility | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Consistent acceptance | Consistent hesitancy | COVID-specific hesitancy | Consistent acceptance | Consistent hesitancy | COVID-specific hesitancy | Consistent acceptance | Consistent hesitancy | COVID-specific Hesitancy | |
| Age | 46.97 (13.07)a | 44.95(12.68)b | 40.93 (11.55)c | 46.89(12.95)a | 45.44 (12.82)b | 44.05 (13.01)b | 46.64 (13.01)a | 42.54 (12.65)b | 44.02 (12.74)b |
| Gender (% women) | 80.9% (2745)a | 87.7% (299)b | 88.5% (377)b | 83.4% (2561)a | 78.2% (413)b | 84.6% (369)a | 82.8% (3267)a | 77.8% (77)a | 85.8% (121)a |
| Race (% white) | 85.9% (2956)a | 75.5% (284)b | 79.6% (354)b | 83.6% (2610)a | 85.4% (468)a | 85.0% (385)a | 85.2% (3420)a | 72.2% (78)b | 71.4% (110)b |
| Diagnosed w/ COVID-19 | 12.7% (437)a | 23.4% (88)b | 22.5% (100)b | 13.6% (425)a | 18.6% (102)b | 14.8% (67)ab | 13.8% (552)a | 21.3% (23)b | 24.8% (38)b |
| Vaccinated | 98.5% (3389)a | 30.3% (114)b | 46.5% (207)c | 92.6% (2891)a | 79.4% (435)b | 73.1% (331)c | 93.6% (3758)a | 26.9% (29)b | 32.5% (50)b |
| Clinical care position | 50.1% (1713)a | 50.1% (183)a | 52.8% (230)a | 48.1% (1489)a | 53.5% (289)b | 56.8% (254)b | 49.9% (1987)a | 47.6% (50)a | 49.7% (75)a |
| Work with COVID-19 patients | 18.0% (620)a | 28.7% (108)b | 24.3% (108)b | 18.2% (568)a | 21.9% (120)b | 24.3% (110)b | 18.5% (741)a | 34.3% (37)b | 30.5% (47)b |
| Anticipated Likelihood of infection | 35.81 (0.52)a | 47.12 (1.71)b | 50.47 (1.50)b | 36.34 (28.36)a | 45.18 (29.49)b | 43.41 (27.87)b | 37.78 (28.43)a | 47.82 (32.73)b | 46.62 (30.89)b |
| Anticipated Severity of infection | 32.30 (0.46)a | 37.19 (1.52)b | 38.83 (1.33)b | 33.05 (25.46)a | 36.51 (24.88)b | 35.22 (23.91)ab | 33.75 (25.23)a | 31.96 (26.93)a | 36.09 (25.05)a |
| Fear of infection | 48.03 (0.61)a | 35.02 (2.05)b | 39.89 (1.79)c | 47.44 (34.11)a | 45.10 (33.73)a | 45.37 (33.45)a | 48.09 (33.81)a | 28.05 (31.14)b | 33.91 (32.04)b |
| # Vaccine Concerns | 2.50 (0.04)a | 6.11 (0.14)b | 5.49 (0.12)c | 2.61 (2.28)a | 4.60 (3.05)b | 4.35 (2.94)b | 2.86 (2.41)a | 5.60 (3.02)b | 5.78 (3.06)b |
| # Vaccine reasons | 6.28 (0.04)a | 2.14 (0.15)b | 3.61 (0.13)c | 5.96 (2.53)a | 5.01 (2.86)b | 4.92 (2.98)b | 5.96(2.49)a | 2.36 (3.22)b | 2.34 (2.48)b |
| Always mask w/NonFamily | 66.2% (2269)a | 40.0% (148)b | 44.9%(198)b | 65.5%(2033)a | 55.0%(301)b | 52.8%(238)b | 64.8%(2589)a | 31.8%(34)b | 44.1%(67)c |
Non-matching superscripts across rows indicate a significance difference between groups. Rows which contain superscripts of a, b, and c, indicate that all three groups were significantly different from one another. Groups with a superscript of “ab” indicate that the group did not differ from either of the other two groups. Significance indicates a p-value less than 0.05
Confidence Specific Differences
HCP who had COVID-19 specific hesitancy for “confidence” were less likely to be white (X2 = 36.41; p = 0.001) and more likely to have been diagnosed with COVID-19 infection (X2 = 53.93; p = 0.001) than the confidence consistent acceptance group; they also reported a higher anticipated severity of infection (F = 13.48; p = 0.001) and a lower fear of infection (F = 29.52; p = 0.001) than the members of the confidence consistent acceptance group. Furthermore, HCP with COVID-19 specific hesitancy on “confidence” were younger (F = 36.87; p = 0.001) and more likely to be vaccinated (X2 = 2125.92; p = 0.001) than the confidence consistent hesitancy group.
Constraints Specific Differences
HCP with COVID-19 specific hesitancy on “constraints” were more likely to be in a clinical care position (X2 = 15.13; p = 0.001) than were members of the consistent acceptance group. Also, this is the only one of the three Cs for which the COVID-19 specific hesitancy group has no race-based difference compared to the corresponding consistent acceptance group. Furthermore, HCP with COVID-specific hesitancy on “constraints” were less likely to be vaccinated (X2 = 206.34; p = 0.001) than the constraints consistent hesitancy group.
Collective Responsibility Specific Differences
HCP with COVID-19 specific hesitancy on “collective responsibility” were less likely to be white (X2 = 33.48; p = 0.001) and had less fear of infection (F = 10.23; p = 0.001) than did members of the collective responsibility consistent acceptance group. Furthermore, HCP with COVID-19 specific hesitancy on “collective responsibility” were more likely to wear a mask with non-family (X2 = 333.78; p = 0.001) than HCP in the corresponding consistent hesitancy group.
Complacency Specific Differences
Participants infrequently endorsed the attitude that vaccines were unnecessary, thus the following groups were created for analyses: consistently accepting, consistently non-accepting (including both hesitancy and neutral), and COVID-19 specific non-accepting; see Table 4. HCP with COVID-19 specific non-acceptance on “complacency” were less likely to be white (X2 = 6.07; p = 0.04), less likely to be vaccinated (X2 = 528.04; p = 0.001), and more likely to have worked with COVID-19 patients in the previous three months (X2 = 15.78; p = 0.001) than HCP who were consistently accepting on “complacency”. They also had less fear of infection (F = 26.09; p = 0.001), expressed more concerns about vaccination (F = 69.25; p = 0.001), endorsed fewer reasons to vaccinate (F = 118.30; p = 0.001), and were less likely to mask with non-family (X2 = 275.80; p = 0.001) than were members of the complacency consistent acceptance group. Furthermore, HCP with COVID-19 specific non-acceptance on “complacency” were less likely to be vaccinated (X2 = 528.04; p = 0.001) than HCP who were consistently non-accepting on “complacency”.
Table 4.
Differences amongst complacency consistent accepting, consistent non-accepting, and COVID-specific non-accepting
| Complacency | |||
|---|---|---|---|
| Consistent acceptance | Consistent non-acceptance | COVID-specific non-acceptance | |
| Age | 46.04 (13.02)a | 45.16 (11.50)a | 43.51 (12.98)a |
| Gender (% women) | 82.7% (3374)a | 78.9% (71)a | 85.8% (91)a |
| Race (% white) | 84.7% (3526)a | 70.3% (71)b | 78.3% (90)b |
| Diagnosed with COVID-19 | 14.2% (590)a | 26.3% (26)b | 21.7% (25)b |
| Vaccinated | 90.9% (3784)a | 20.8% (21)b | 28.7% (33)b |
| Clinical care position | 50.0% (2062)a | 50.0% (48)a | 56.1% (64)a |
| Work with COVID-19 patients past 3 months | 18.7% (779)a | 39.6% (40)b | 29.6% (34)c |
| Anticipated likelihood of infection | 38.20 (28.68)a | 44.64 (33.27)ab | 46.45 (30.88)b |
| Anticipated severity of infection | 33.81 (25.00)a | 29.38 (26.19)b | 28.42 (23.33)b |
| Fear of infection | 47.54 (33.80)a | 18.63 (27.36)b | 25.46 (28.88)b |
| # Vaccine concerns endorsed | 2.35 (1.52)a | 3.20 (1.40)b | 3.43 (1.21)b |
| # Vaccine reasons endorsed | 3.51 (1.10)a | 0.59 (1.22)b | 1.31 (1.62)c |
| Always mask with non-family | 63.6% (2633)a | 25.3% (25)b | 33.0% (38)b |
Non-matching superscripts across rows indicate a significance difference in the groups (i.e., Consistent Acceptance, Consistent Non-Acceptance, or COVID-Specific Non-Acceptance). Rows which contain superscripts of a, b, and c, indicate that all three groups were significantly different from one another. Groups with a superscript of “ab” indicate that the group did not differ from either of the other two groups. Significance indicates a p-value less than 0.05
Discussion
Previous studies have found that each of the 5Cs can be associated with vaccine acceptance and vaccination status [18, 19], for the seasonal influenza vaccine in particular [22]. Research has also shown that confidence and collective responsibility [23]—and complacency [22]—can predict an intention to receive COVID-19 vaccines among HCP. Our study is the first to track relationships between the 5Cs and COVID-19 vaccination attitudes and behaviors among HCP after COVID-19 vaccines were available. Consequently, the fact that we also found that the constraints item predicts vaccination status may indicate that participants in other studies, who were asked about prospective barriers to future vaccination decisions, could not accurately identify barriers to COVID-19 vaccination in advance.
A large number of the HCP we surveyed were accepting of vaccines in general, and agreed to receive a COVID-19 vaccine, but were nonetheless hesitant about COVID-19 vaccines along at least one of the 5Cs. An individual can be vaccine hesitant even if they agree to be vaccinated [24], and HCP are no exception. Vaccine hesitant HCP are less likely to advocate for vaccines with patients, family, and friends [25], which may undermine community vaccine acceptance and uptake, given the high levels of trust that people place in HCP, both in general [26] and about healthcare information in particular [27, 28]. Also, a vaccine-hesitant HCP may be unwilling to accept booster vaccinations in the future.
It can be very difficult to change the minds of people who refuse or are hesitant about receiving all vaccines [29]. Accordingly, when someone refuses COVID-19 vaccines because they refuse all vaccines, it may be especially challenging to increase their acceptance of COVID-19 vaccines. In contrast, someone who generally accepts vaccines, but has concerns only about COVID-19 vaccines, may be easier to persuade to accept COVID-19 vaccines, including boosters.
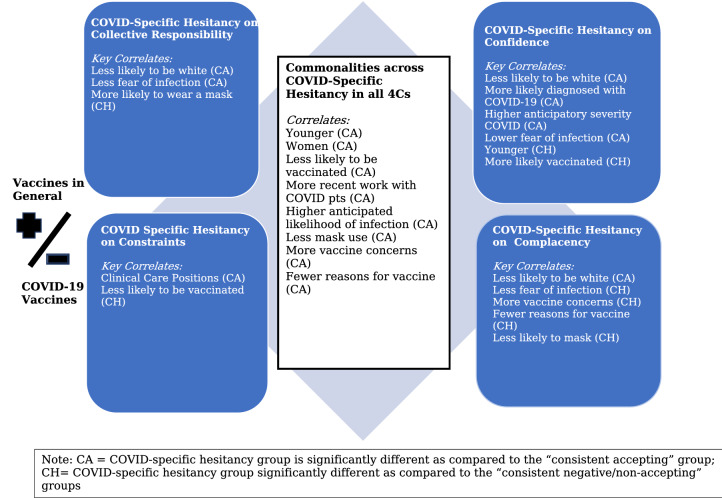
Accordingly, our analyses focused on groups that may be more responsive to efforts to increase acceptance of COVID-19 vaccine; see Fig. 1. The members of the COVID-19 specific hesitancy (or non-acceptance) groups generally have high confidence in vaccines and low complacency about vaccine-preventable diseases; they identify few constraints to vaccination, while embracing a high level of collective responsibility for controlling disease. The hesitancy of these HCP therefore seems to be related to something particular about COVID-19 infection or COVID-19 vaccines. Our research provides some indication about the sources of their COVID-19 specific hesitancy.
Fig. 1.
Characteristics of and potential interventions for individuals more hesitant to COVID-19
First, the similarities between the confidence, constraints, and collective responsibility COVID-19 specific hesitancy groups are informative. The fact that these HCP are younger and more likely to be women than their corresponding consistent acceptance groups indicates that concerns about fertility or pregnancy may be driving some instances of vaccine hesitancy in these groups. This is consistent with other studies about people’s safety concerns about COVID-19 vaccines [30]. Also, the fact that members of these three groups are more likely to have worked with COVID-19 patients in the past three months compared to the consistent acceptance groups perhaps indicates that direct experiences of COVID-19 disease contributes to hesitancy, perhaps by making HCP less concerned about infection.
Second, the differences between the different COVID-19 specific hesitancy groups are also instructive. The fact that there is very little overlap in the membership of the four COVID-19 specific hesitancy groups indicates that members of each group had different motivations for their hesitancy and were not merely expressing a general hesitancy about COVID-19 vaccines. The differences between other attributes of the COVID-19 specific hesitancy groups can indicate specific origins of vaccine hesitancy and potential sites for pro-vaccination interventions.
Less Confidence in COVID-19 Vaccines
Among people who are generally accepting of vaccines, non-white HCP were more likely to worry about the safety of COVID-19 vaccines. There is abundant evidence that non-white persons often have lower trust in government and healthcare institutions [31, 32], and in COVID-19 vaccines, in particular [33]. At the time that this survey was conducted, COVID-19 vaccines had been available for only a few months, such that people could rationally believe those vaccines were safe only if they had high levels of trust in government and healthcare institutions. In contrast, someone who did not have high levels of trust in government or healthcare institutions could nonetheless have confidence in other vaccines, since they would have had many direct personal experiences of their safety and effectiveness. This interpretation of our results provides some reason to hope that non-white HCP will develop greater confidence in COVID-19 vaccines over time, i.e. as they accumulate additional personal experiences and scientific evidence of the safety and effectiveness of these vaccines. The recent history of sustained increases in vaccination rates among Black, Asian, and Hispanic Americans may support such optimism [34].
Greater Constraints for COVID-19 Vaccination
When this survey was distributed, COVID-19 vaccines were available only at the hospital system’s administrative offices, which are located 30–45 min from some HCP’s work sites. In contrast, the HCP we surveyed can receive seasonal influenza vaccine in their workplace from Occupational Health services, and they can receive other vaccines from primary care physicians. Therefore, it is understandable that some HCP likely faced substantial constraints on receiving COVID-19 vaccine compared to receiving vaccines in general.
Officially, all HCP at this healthcare institution could have received compensated release time to travel to receive the COVID-19 vaccine, and they could have also taken sick days if they experienced side effects. Nonetheless, HCP with clinical care positions may have faced substantial barriers to receiving a COVID-19 vaccine, and these additional barriers may explain why these HCP were more likely to be members of the COVID-specific hesitancy group for constraints. Staffing requirements for patient care are generally much less flexible than staffing requirements for other work roles, such as administration or food service work. Also, Michigan was experiencing a surge of COVID-19 infections when this survey was administered, such that clinical care workers would have been especially burdened by their caretaking responsibilities. Even clinical care staff who were not working on COVID-19 units would have been burdened by the surge, as they were often short-staffed, either because of a general staffing shortage or because staff from their units had been transferred to COVID-19 units during surge conditions.
Less Collective Responsibility for Controlling COVID-19
The COVID-19 specific hesitancy respondents for the collective responsibility measure were less likely to be white. This fact may indicate a disturbing relationship between race, social solidarity, and decision making about COVID-19 vaccines. Non-white persons, especially Blacks/African Americans, have been disproportionately vulnerable to hospitalization and death from COVID-19 disease, due to their increased vulnerability to chronic illness, their high levels of employment in service jobs, and their inadequate access to health resources [35, 36]. This population has also been subject to historical and ongoing forms of healthcare injustice [37], in Michigan in particular [38]. So, members of the collective responsibility COVID-19 specific hesitancy group have disproportionately been harmed by COVID-19 infection and have been least helped by society. When dominant social groups fail to respect and care for members of disadvantaged social groups—but instead leave them to face burdens and injustices on their own—it can be reasonable for members of disadvantaged groups to be skeptical about subsequent appeals for ‘solidarity’ and ‘social responsibility’ [39]. Furthermore, the fact that members of this group were more likely than the collective responsibility consistent hesitant group members to wear masks around non-family indicates that this group takes seriously their responsibility to avoid directly harming other persons by transmitting COVID-19 infection, while they nonetheless remained unmotivated by the abstract rhetoric of collective responsibility.
More Complacency About COVID-19
On the complacency measure, the COVID-19 specific non-acceptance respondents were more likely to have worked with COVID-19 patients in the previous three months than had the members of the complacency consistent acceptance group. This indicates that HCP who have worked with COVID-19 patients may be experiencing ‘survivorship bias’ when it comes to their judgments about the severity of COVID-19 infection. This bias is a tendency to focus on those who successfully make it through a selection process, while ignoring those who did not make it, in ways that distort one’s judgment about that process [40]. In this case, the HCP in the COVID-19 specific non-acceptance group may have concluded—based on the fact that they did not become seriously ill or die after workplace exposure to COVID-19—that exposure to COVID-19 infection is not especially dangerous and that vaccination is therefore not necessary.
Limitations
This study was conducted at a single health system, so the results may not be generalizable to other US healthcare systems. There may also be a response bias in HCP who were willing to complete the survey. However, the large sample size, and the range of demographics, work status, COVID-19 experiences, and 4Cs responses suggest that the results are meaningful.
Conclusion
It can be complicated and difficult to persuade vaccine-hesitant persons to vaccinate, especially if there is insufficient understanding of the origins of their hesitancy [41, 42]. Even physicians and nurses are often frustrated in their attempts to promote vaccination with hesitant patients and families [43, 44]. However, there are evidence-backed techniques for effective pro-vaccination communication. For example, motivational interviewing techniques have been used with great success by Canadian vaccination counsellors and immunization nurses [45, 46]. Other evidence-backed techniques include empathic and reflective listening, positive pro-vaccination personal stories, and the avoidance of hard persuasion or inundating people with vaccine information [47–50].
This study identifies areas for future research to better understand HCP vaccine hesitancy and for potential interventions to promote vaccination among hesitant HCP.
First, it is essential for US healthcare institutions to continue to promote vaccine acceptance, in light of the substantial number of vaccine-hesitant HCP, even among those who have for now agreed to receive COVID-19 vaccines. While some hesitant HCP agreed to receive COVID-19 vaccines (at the time of the survey), perhaps because they anticipated a future vaccine mandate, their hesitancy may lead them to reject recommended boosters, especially if they discover that their institutions’ vaccine mandates are less stringent than they originally thought they would be. In the context of a nationwide nursing shortage [51], and a widespread commitment to provide religious and medical exemptions to COVID-19 vaccine mandates [52]—both of which place pressure on healthcare institutions to weaken their vaccine mandates—healthcare institutions should not assume that past compliance is a good indication of their staff’s future vaccination decisions. Recent decisions by some health systems to suspend their vaccine mandates—because of staffing shortages—make the ongoing hesitancy of HCP about COVID-19 vaccines an even more pressing problem [53].
Second, healthcare institutions should focus pro-vaccination interventions on members of the COVID-19 specific hesitancy groups we discussed. These HCP are not committed vaccine refusers—they accept other vaccines—but they have concerns that are particular to COVID-19 vaccines. Therefore, this group may be more easily reached than committed vaccine refusers. The kinds of interventions that may work best with them will need to be tailored to facts about COVID-19 infection or about COVID-19 vaccines, rather than to general facts or values about vaccines or vaccine-preventable diseases.
Third, this paper’s discussion of the similarities among the COVID-19 specific hesitancy groups can identify groups of HCP that healthcare institutions should target with pro-vaccination interventions. For all of the 4Cs, younger HCP, women HCP, and HCP who had recently worked with COVID-19 patients were more likely to be hesitant about COVID-19 vaccines, even though they were generally accepting of vaccines. These groups represent low-hanging fruit for general pro-vaccination interventions.
Fourth, differences among COVID-19 specific hesitancy groups may identify different areas for tailored pro-vaccination interventions. In particular, messaging about the safety of COVID-19 vaccines may focus on overcoming worries about the potential impact of COVID-19 vaccines on fertility and pregnancy, while efforts to facilitate vaccination by HCP may focus on removing barriers faced by clinical staff, Finally, efforts to promote a sense that all HCP have a collective responsibility to vaccinate should take account of the many ways our political and social institutions have failed to demonstrate solidarity with nonwhite and otherwise less privileged members of our society.
Author Contributions
MN: Conceptualization, Methodology, Writing—Original Draft; LO: Conceptualization, Methodology, Formal analysis, Investigation, Data Curation, Writing—Review & Editing; VL, NA, RK, HK, and LW: Conceptualization, Methodology, Writing—Review & Editing; TM: Writing—Conceptualization, Methodology, Review & Editing; Supervision.
Funding
None.
Data Availability
The data that support the findings of this study are available from the corresponding author upon request.
Code Availability
SPSS 28.0 (Armonk, NY).
Declarations
Conflict of interest
The authors declared that they have no conflict of interest to disclose.
Ethical Approval
Beaumont Health IRB (Reference # 039235).
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Johns Hopkins Medicine. (2022, February 8). Coronavirus Resource Center. Retrieved February 8, 2022, from https://coronavirus.jhu.edu/
- 2.Smith C. The structural vulnerability of healthcare workers during COVID-19: Observations on the social context of risk and the equitable distribution of resources. Social Science & Medicine. 2020;258:113119. doi: 10.1016/j.socscimed.2020.113119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pappa S, Ntella V, Giannakas T, Giannakoulis VG, Papoutsi E, Katsaounou P. Prevalence of depression, anxiety, and insomnia among healthcare workers during the COVID-19 pandemic: A systematic review and meta-analysis. Brain, Behavior, and Immunity. 2020;88:901–907. doi: 10.1016/j.bbi.2020.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO. (2020, June 1). Maintaining essential health services: operational guidance for the COVID-19 context, interim guidance, 1 June 2020. World Health Organization. Retrieved September 22, 2021, from https://www.who.int/publications/i/item/WHO-2019-nCoV-essential_health_services-2020.2
- 5.CDC. (2021, December 20). Omicron Variant: What You Need to Know. Centers for Disease Control and Prevention. Retrieved January 2, 2022, from https://www.cdc.gov/coronavirus/2019-ncov/variants/omicron-variant.html
- 6.FDA. (2021, September 21). COVID-19 Vaccines. Food and Drug Administration. FDA. Retrieved September 22, 2021, from https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/covid-19-vaccines
- 7.FDA. (2021, August 23). FDA Approves First COVID-19 Vaccine. Food and Drug Administration. FDA. Retrieved September 22, 2021, from https://www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-vaccine
- 8.Dooling K. The Advisory committee on immunization practices’ updated interim recommendation for allocation of COVID-19 vaccine—United States, December 2020. MMWR. Morbidity and Mortality Weekly Report. 2021;69:1847. doi: 10.15585/mmwr.mm695152e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas, K. (2021, September 20). F.D.A. Decision on Covid Booster Shots Is Expected In Days - The New York Times. New York Times. Retrieved September 22, 2021, from https://www.nytimes.com/2021/09/20/science/covid-booster-FDA.html
- 10.Lee JT, Althomsons SP, Wu H, Budnitz DS, Kalayil EJ, Lindley MC, Pingali C, Bridges CB, Geller AP. Disparities in COVID-19 vaccination coverage among health care personnel working in long-term care facilities, by job category, National Healthcare Safety Network—United States, March 2021. Morbidity and Mortality Weekly Report. 2021;70(30):1036. doi: 10.15585/mmwr.mm7030a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schrading WA, Trent SA, Paxton JH, Rodriguez RM, Swanson MB, Mohr NM, Talan DA, Network PC. Vaccination rates and acceptance of SARS-CoV-2 vaccination among US emergency department health care personnel. Academic Emergency Medicine. 2021;28:455. doi: 10.1111/acem.14236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ledda C, Costantino C, Cuccia M, Maltezou HC, Rapisarda V. Attitudes of healthcare personnel towards vaccinations before and during the COVID-19 Pandemic. International Journal of Environmental Research and Public Health. 2021;18(5):2703. doi: 10.3390/ijerph18052703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biswas N, Mustapha T, Khubchandani J, Price JH. The nature and extent of COVID-19 vaccination hesitancy in healthcare workers. Journal of Community Health. 2021 doi: 10.1007/s10900-021-00984-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weber, L., & Kaiser Health News. (2021, August 30). Some hospitals are foregoing vaccine mandates to avert staffing shortages. Fortune. Retrieved September 22, 2021, from https://fortune.com/2021/08/30/health-care-hospital-worker-shortage-vaccine-mandates-staff-retention/
- 15.NASHP. (2021, October 1). State Efforts to Ban or Enforce COVID-19 Vaccine Mandates and Passports. National Academy for State Health Policy. Retrieved October 10, 2021, from https://www.nashp.org/state-lawmakers-submit-bills-to-ban-employer-vaccine-mandates/
- 16.Omer SB, Betsch C, Leask J. Mandate vaccination with care. Nature. 2019;571(7766):469–472. doi: 10.1038/d41586-019-02232-0. [DOI] [PubMed] [Google Scholar]
- 17.Oberleitner, L., Lucia, V., Navin, M., Ozdych, M., Afonso, N., Kennedy, R., Keil, H., Wu, L., & Mathew, T. (2022). Will understanding specific vaccination concerns help guide future COVID-19 vaccination efforts?: A survey of US healthcare personnel. Unpublished Manuscript.
- 18.Betsch C, Habersaat KB, Deshevoi S, Heinemeier D, Briko N, Kostenko N, Kocik J, Böhm R, Zettler I, Wiysonge CS, Dubé È. Sample study protocol for adapting and translating the 5C scale to assess the psychological antecedents of vaccination. British Medical Journal Open. 2020;10(3):e034869. doi: 10.1136/bmjopen-2019-034869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Betsch C, Schmid P, Heinemeier D, Korn L, Holtmann C, Böhm R. Beyond confidence: Development of a measure assessing the 5C psychological antecedents of vaccination. PLoS ONE. 2018;13(12):e0208601. doi: 10.1371/journal.pone.0208601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caserotti M, Girardi P, Rubaltelli E, Tasso A, Lotto L, Gavaruzzi T. Associations of COVID-19 risk perception with vaccine hesitancy over time for Italian residents. Social Science & Medicine. 2021;272:113688. doi: 10.1016/j.socscimed.2021.113688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.IBM Corp . IBM SPSS statistics for windows, Version 26.0. IBM Corp; 2019. [Google Scholar]
- 22.Kwok KO, Li K-K, Wei WI, Tang A, Wong SYS, Lee SS. Influenza vaccine uptake, COVID-19 vaccination intention and vaccine hesitancy among nurses: A survey. International Journal of Nursing Studies. 2021;114:103854. doi: 10.1016/j.ijnurstu.2020.103854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wismans A, Thurik R, Baptista R, Dejardin M, Janssen F, Franken I. Psychological characteristics and the mediating role of the 5C Model in explaining students’ COVID-19 vaccination intention. PLoS ONE. 2021;16(8):e0255382. doi: 10.1371/journal.pone.0255382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larson HJ, Cooper LZ, Eskola J, Katz SL, Ratzan S. Addressing the vaccine confidence gap. The Lancet. 2011;378:526–535. doi: 10.1016/S0140-6736(11)60678-8. [DOI] [PubMed] [Google Scholar]
- 25.European Centre for Disease Prevention and Control. (2015). Vaccine hesitancy among healthcare workers and their patients in Europe: A Qualitative Study. Stockholm: ECDC. Retrieved from https://www.ecdc.europa.eu/sites/default/files/media/en/publications/Publications/vaccine-hesitancy-among-healthcare-workers.pdf
- 26.Gallup. (2020, December 22). U.S. Ethics Ratings Rise for Medical Workers and Teachers. Gallup.com. Retrieved September 22, 2021, from https://news.gallup.com/poll/328136/ethics-ratings-rise-medical-workers-teachers.aspx
- 27.Swoboda CM, Van Hulle JM, McAlearney AS, Huerta TR. Odds of talking to healthcare providers as the initial source of healthcare information: Updated cross-sectional results from the Health Information National Trends Survey (HINTS) BMC Family Practice. 2018;19(1):146. doi: 10.1186/s12875-018-0805-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cutilli CC. Seeking health information: What sources do your patients use? Orthopaedic Nursing. 2010;29(3):214–219. doi: 10.1097/NOR.0b013e3181db5471. [DOI] [PubMed] [Google Scholar]
- 29.Navin MC, Wasserman JA, Ahmad M, Bies S. Vaccine education, reasons for refusal, and vaccination behavior. American Journal of Preventive Medicine. 2019;56(3):359–367. doi: 10.1016/j.amepre.2018.10.024. [DOI] [PubMed] [Google Scholar]
- 30.Male V. Are COVID-19 vaccines safe in pregnancy? Nature Reviews Immunology. 2021;21(4):200–201. doi: 10.1038/s41577-021-00525-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bogart LM, Ojikutu BO, Tyagi K, Klein DJ, Mutchler MG, Dong L, Lawrence SJ, Thomas DR, Kellman S. COVID-19 related medical mistrust, health impacts, and potential vaccine hesitancy among Black Americans living with HIV. Journal of Acquired Immune Deficiency Syndromes (1999) 2021;86(2):200. doi: 10.1097/QAI.0000000000002570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williamson LD, Smith MA, Bigman CA. Does discrimination breed mistrust? Examining the role of mediated and non-mediated discrimination experiences in medical mistrust. Journal of Health Communication. 2019;24(10):791–799. doi: 10.1080/10810730.2019.1669742. [DOI] [PubMed] [Google Scholar]
- 33.Funk, C., & Tyson, A. (2020, December 3). Intent to get a COVID-19 vaccine rises to 60% as confidence in research and development process increases. Pew Research Center Science & Society. Retrieved September 22, 2021, from https://www.pewresearch.org/science/2020/12/03/intent-to-get-a-covid-19-vaccine-rises-to-60-as-confidence-in-research-and-development-process-increases/
- 34.CDC. (2021, October 22). COVID Data Tracker. Centers for Disease Control and Prevention. Retrieved October 22, 2021, from https://covid.cdc.gov/covid-data-tracker
- 35.Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among black patients and white patients with Covid-19. New England Journal of Medicine. 2020;382(26):2534–2543. doi: 10.1056/NEJMsa2011686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.CDC. (2021, September 9). Risk for COVID-19 infection, hospitalization, and death by race/ethnicity. Centers for Disease Control and Prevention. Retrieved September 22, 2021, from https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-race-ethnicity.html
- 37.Sabatello M, Jackson Scroggins M, Goto G, Santiago A, McCormick A, Morris KJ, Daulton CR, Easter CL, Darien G. Structural racism in the COVID-19 pandemic: Moving forward. The American Journal of Bioethics. 2021;21(3):56–74. doi: 10.1080/15265161.2020.1851808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Benz TA. Toxic cities: Neoliberalism and environmental racism in Flint and Detroit Michigan. Critical Sociology. 2019;45(1):49–62. doi: 10.1177/0896920517708339. [DOI] [Google Scholar]
- 39.Mills CW. The racial contract. Cornell University Press; 1999. [Google Scholar]
- 40.Buckley JP, Keil AP, McGrath LJ, Edwards JK. Evolving methods for inference in the presence of healthy worker survivor bias. Epidemiology. 2015;26(2):204–212. doi: 10.1097/EDE.0000000000000217. [DOI] [PubMed] [Google Scholar]
- 41.Kempe A, O’Leary ST, Kennedy A, Crane LA, Allison MA, Beaty BL, Hurley LP, Brtnikova M, Jimenez-Zambrano A, Stokley S. Physician response to parental requests to spread out the recommended vaccine schedule. Pediatrics. 2015;135:2014–3474. doi: 10.1542/peds.2014-3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berry NJ, Henry A, Danchin M, Trevena LJ, Willaby HW, Leask J. When parents won’t vaccinate their children: A qualitative investigation of Australian primary care providers’ experiences. BMC Pediatrics. 2017;17:19. doi: 10.1186/s12887-017-0783-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Edwards KM, Hackell JM, AAP Committee on Infectious Diseases. AAP Committee on Practice and Ambulatory Medicine Countering vaccine hesitancy. Pediatrics. 2016;138(3):e20162146. doi: 10.1542/peds.2016-2146. [DOI] [PubMed] [Google Scholar]
- 44.Deem MJ. Nurses’ voices matter in decisions about dismissing vaccine-refusing families. AJN The American Journal of Nursing. 2018;118(8):11. doi: 10.1097/01.NAJ.0000544142.09253.e0. [DOI] [PubMed] [Google Scholar]
- 45.Gagneur A, Lemaître T, Gosselin V, Farrands A, Carrier N, Petit G, Valiquette L, De Wals P. A postpartum vaccination promotion intervention using motivational interviewing techniques improves short-term vaccine coverage: PromoVac study. BMC Public Health. 2018;18(1):811. doi: 10.1186/s12889-018-5724-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gagneur A, Bergeron J, Gosselin V, Farrands A, Baron G. A complementary approach to the vaccination promotion continuum: An immunization-specific motivational-interview training for nurses. Vaccine. 2019;37(20):2748–2756. doi: 10.1016/j.vaccine.2019.03.076. [DOI] [PubMed] [Google Scholar]
- 47.Leask J, Kinnersley P, Jackson C, Cheater F, Bedford H, Rowles G. Communicating with parents about vaccination: A framework for health professionals. BMC Pediatrics. 2012;12(1):154. doi: 10.1186/1471-2431-12-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Randall S, Leask J, Robinson P, Danchin M, Kinnersley P, Witteman H, Trevena L, Berry N. Underpinning of the sharing knowledge about immunisation (SKAI) communication approach: A qualitative study using recorded observations. Patient Education and Counseling. 2020;103(6):1118–1124. doi: 10.1016/j.pec.2019.12.014. [DOI] [PubMed] [Google Scholar]
- 49.Kaufman J, Attwell K, Tuckerman J, O'Sullivan J, Omer SB, Leask J, Regan A, Marshall H, Lee KJ, Snelling T, Perrett K. Feasibility and acceptability of the multi-component P3-MumBubVax antenatal intervention to promote maternal and childhood vaccination: A pilot study. Vaccine. 2020;38:4024. doi: 10.1016/j.vaccine.2020.04.010. [DOI] [PubMed] [Google Scholar]
- 50.Bednarczyk RA, Chamberlain A, Mathewson K, Salmon DA, Omer SB. Practice-, provider-, and patient-level interventions to improve preventive care: Development of the P3 model. Preventive Medicine Reports. 2018;11:131–138. doi: 10.1016/j.pmedr.2018.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.ANA. (n.d.). Workforce. American Nurses Association. Retrieved September 22, 2021, from https://www.nursingworld.org/practice-policy/workforce/
- 52.Hirsch, L., Cowley, S., & Scheiber, N. (2021, September 10). Biden’s Vaccine Mandate Leaves Businesses Relieved but Full of Questions. The New York Times. Retrieved from https://www.nytimes.com/2021/09/10/business/vaccine-mandate-business.html
- 53.Gooch, K. (2021, December 15). 17 healthcare organizations suspending COVID-19 vaccination mandates. Becker’s Hospital Review. Retrieved December 19, 2021, from https://www.beckershospitalreview.com/workforce/5-health-systems-suspending-vaccination-mandates.html
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon request.
SPSS 28.0 (Armonk, NY).