Abstract
Combinatorial conjugation of organ-on-a-chip platforms with additive manufacturing technologies is rapidly emerging as a disruptive approach for upgrading cancer-on-a-chip systems towards anatomic-sized dynamic in vitro models. This valuable technological synergy has potential for giving rise to truly physiomimetic 3D models that better emulate tumor microenvironment elements, bioarchitecture, and response to multi-dimensional flow dynamics. Gathering on this, herein we showcase the most recent advances on bioengineering 3D-bioprinted cancer-on-a-chip platforms and provide a comprehensive discussion on design guidelines and possibilities for high-throughput analysis. Such hybrid platforms represent a new generation of highly sophisticated 3D tumor models with improved biomimicry and predictability of therapeutics performance.
Keywords: In vitro tumor models, 3D bioprinting, Cancer-on-a-chip, Preclinical drug screening
Bottom-up bioengineering 3D tumor models – Deconstructing key tumor elements
Cancer is a complex, dynamic, and heterogeneous disease that is inherently influenced by its surrounding environment.[1,2] In recent decades, evidences have been mounting on the reality that human cancer models cannot be limited to cancer cells alone, since the heterogeneous tumor microenvironment (TME, see Glossary) and its components have been recognized to play a key role in tumor growth, multi-resistance to therapeutics, and metastasis (Box 1).[3,4]
Box 1 – Human tumor microenvironment elements and their role in cancer progression.
Human TME is highly complex and comprised by multifarious building blocks that together contribute for disease progression. Naturally, the tumor site is nourished by irregular/leaky blood vessels, lymphatic networks, as well as densely populated by cancer and stromal cells (e.g., immune cells, cancer-associated fibroblasts (CAFs), etc.), dwelling in a supporting extracellular matrix (ECM) comprising key proteins, glycosaminoglycans, and soluble biomolecular mediators (e.g., growth factors, cytokines, etc.), which all act synergistically to drive tumor progression and influence drug response.[98]
In the tumor microenvironment (TME) milieu, CAFs are characterized by an overexpression of alpha-smooth muscle actin (α-SMA+), of platelet-derived growth factor (PDGF+), and exhibit a myofibroblast-like phenotype.[99,100] These stromal components interact with tumor cells via autocrine and paracrine signaling, producing key biomolecular mediators including: (i) growth factors (basic fibroblast growth factor (bFGF), transforming growth factor (TGF), epidermal growth factor (EGF)); (ii) interleukins (e.g., interleukin (IL)-1β, IL-6, IL-8), and (iii) chemokines, that participate in various disease-related processes such as tumor metastasis.[101] Moreover, CAFs abundantly secrete ECM components in the tumor site, mainly collagen and hyaluronic acid, playing a key role on desmoplasia, as well as in the tumor metabolic shift.[102,103] Adding to this, the tumor stroma is also populated by several immune cells, such as tumor-associated macrophages (TAMs) that orchestrate and upkeep an immunosuppressive microenvironment, fueling tumor growth. TAMs also promote cancer cells survival through the secretion of key biomolecular mediators (e.g., IL-10, IL-8, TGF-β, vascular endothelial growth factor (VEGF)), contribute for ECM remodeling, angiogenesis, and the maintenance of the immunosuppressive microenvironment in a prolonged time-frame.[104,105]
In the tumor niche, although cancer cells can secrete residual amounts of ECM, CAFs remain the key players in de novo ECM production.[5,106] As an end result, the abundant ECM deposition leads to increased tumor stiffness (desmoplastic reaction), to the accumulation of mechanical stress, and to the establishment of a protective biophysical barrier. Recapitulating this phenomenon in in vitro models in a tumor-specific mode is paramount to model the mechanical cues and the decrease in oxygen supply, as well as the establishment of hypoxic and acidic environments in solid tumors. In feedback, the hypoxia onset then promotes TAM and CAF recruitment accelerating the dynamic ECM remodeling and angiogenesis.[3,107]
On the other hand, peritumoral vasculature plays a key role in providing nutrients/metabolites or oxygen, it influences anti-cancer therapeutics bioavailability, and it is also a key-mediator in cancer-immune system cells crosstalk and ultimately constitutes the supporting go-to escape route for cells to metastasize.[108,109] By realizing the importance of such components, different biofabrication and cancer-on-a-chip technologies are being actively developed to capture tumor-specific blood vessels leaky and spatially aberrant features that are generally encountered in vivo.[12,110–113] Alongside, the ubiquitous and intricate network of lymphatic vessels is another key aspect to consider owing to its contribute for metastasis and antitumor drugs recycling in vivo.[46,114] Interestingly, despite its importance, most preclinical in vitro models fail to recapitulate lymphatic components.
Recapitulating key TME elements is crucial in the design stages of bioengineered 3D tumor models since tumor ECM [5], blood and lymphatic vessels, as well as stromal elements such as immune system cells and cancer-associated fibroblasts (CAFs) take the leading role as instrumental effectors in prompting tumor cell survival and invasion/metastasis in vivo, whilst also bioinstructing the genetic landscape and shaping the biophysical environment via tumor-ECM deposition and influencing drug resistance (Box 1).[6] Owing to non-malignant cells’ influence on tumor evolution, several in vitro co-culture platforms combining cancer and stromal cells at different ratios have been developed to emulate such tumor-stroma interactions. This intricate relationship is particularly relevant in cancers where the stromal compartment represents more than the malignant cells in the TME, as in the case of pancreatic cancer.[7]
Identifying and deconstructing these fundamental tumor building blocks and hallmarks that influence therapeutics response such as anatomic scale, 3D bioarchitecture, multi-cellular heterogeneity, hypoxic/necrotic niches, tumor-specific ECM biomolecular composition/mechanical properties, as well as tumor-specific fluid dynamics and blood/lymphatic vasculature, opens new avenues for generating truly physiomimetic models in an in vitro setting (Figure 1). Gathering on this, several efforts have been put forth toward bottom-up engineering of the next-generation preclinical tumor models that reproduce major TME building blocks, as well as the inherent complexity, multidimensionality, and spatiotemporal dynamics of native tumors in a more comprehensively mode. Herein, we will discuss the gain-of-function provided by the recent combinations of advanced 3D biofabrication and dynamic organ-on-a-chip technologies, and demonstrate their potential to level-up in vitro tumor modelling beyond the available standard, static-cultured 3D models. Materializing such all-encompassing organotypic models is envisioned to improve preclinical screening and to accelerate innovative therapeutics translation from the bench to the bedside.
Figure 1.
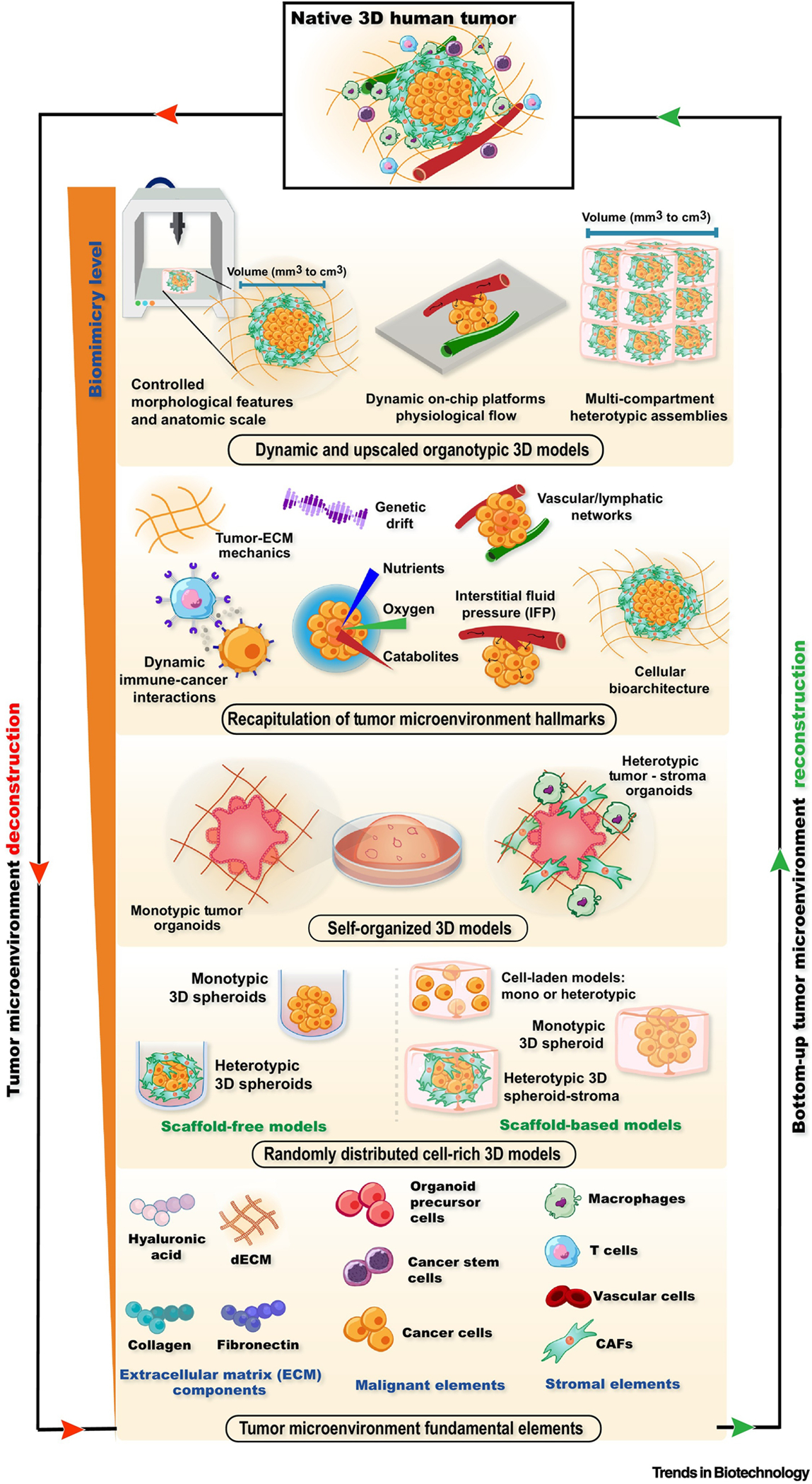
Deconstruction and bottom-up reconstruction of organotypic 3D tumor models by including key TME building blocks and hallmarks. The rationale built-up of such all-encompassing tumor surrogate models is envisioned to improve preclinical screening and to accelerate innovative therapeutics translation from the bench to the bedside. Abbreviations: Extracellular matrix (ECM), Cancer-associated fibroblasts (CAFs), Tumor microenvironment (TME), Static 3D In vitro models – Self-assembled and self-organized living microtumors.
Standard 3D in vitro Tumor Models to Recreate the Native Tumor Microenvironment
Research in cell-rich static 3D in vitro tumor models has already demonstrated potential for improving the discovery and preclinical screening of candidate anti-cancer therapeutics, especially when compared to their more simplistic 2D counterparts.[8] Such 3D platforms enable researchers to seamlessly emulate the complex TME including key cellular, biochemical, and biophysical cues, in a cost-effective and high-throughput amenable mode, while contributing to reducing drug development costs and laboratory animal use. The most common approaches for bioengineering cell-rich organotypic 3D tumor models from the bottom-up rely on self-assembled 3D spheroids or self-organized organoids.[9,10] Specifically, 3D spheroids, comprising scaffold-free, autonomously assembled cellular aggregates, are amidst the most widely used microtumor platforms owing to their ability for accurately recapitulating key solid tumor hallmarks in vitro including 3D cell-cell physical contacts, gene expression patterns, and growth kinetics, while also enabling the inclusion of different cellular populations (e.g., heterotypic culture models). Larger 3D spheroids (c.a. 300–500 μm in diameter) are further able to recapitulate the limited oxygen/nutrients diffusion that in turn originate hypoxic/necrotic regions encountered in several of human solid tumors, a key aspect that ultimately influences resistance to therapeutics.[11] Classically, 3D micro-spheroids are generated via forced cell self-aggregation in high-throughput and in a cost-effective mode through a number of different methodologies such as the hanging-drop technique or by exploiting forced floating (e.g., ultra-low adhesion surfaces).[12] Although these easy-to-assemble and well-established platforms have provided advances in our understanding of human tumors pathophysiology in vitro and contributed to improve preclinical screening at a certain level, they still fail to recapitulate pre-existing ECM or native tumor cellular heterogeneity.[13]
Recently an evolution toward enhancing in vitro human tumor models has been materialized with the establishment of advanced tumor-derived living organoids. Leveraging on their autonomous biological pre-programming, tumor organoids self-organize in culture, providing a unique opportunity to recapitulate 3D tumor-like architecture, function, and cancer cell heterogeneity, being considered more physiomimetic platforms and to be positioned beyond standard 3D cell aggregates that artificially and randomly self-agglomerate.[14] These living microtissues also preserve the genetic and phenotypic biomarkers of the original tumors, ultimately maintaining the pathophysiological traits of human cancers in an in vitro setting. Cancer organoids can be generally derived from pre-programmed induced pluripotent stem cells (iPSCs) or adult stem cells (aSCs), or directly from patient tumors. Engineered iPSC-based organoids are commonly genetically programmed for giving rise to living models with specific phenotypic traits that can match specific tumor mutations.[15,16] Although such platforms open the possibility for precisely recreating human tumor genetic traits and carcinogenesis, their complex processing often hampers their widespread use for anti-cancer drug-screening tests.[14] On the other hand, patient-derived tumor organoids established from surgically resected tissues are highly promising for personalized medicine approaches. Despite their proven predictive potential and possibility to be employed in personalized medicine applications, living organoid 3D models are still generally cultured in animal-origin supporting hydrogels (e.g., Matrigel™, collagen), and mostly lack immune and stromal components, as well as perivascular network components. [17,18] The absence of key tumor stroma elements is recently being addressed via advanced co-culturing of matured 3D tumor organoids with tumor-associated stromal cells such as CAFs or immune cells, and in vascular competent setups involving endothelialized tubular structures (i.e., seeded with vascular cells and/or pericytes).[19,20] These elegant approaches provide the so-desired recapitulation of highly influential TME elements, yet key aspects such as 3D bio-architecture, fluid dynamics, and culture in tumor-physiomimetic ECM remain challenging to implement in vitro.
Given that tumor ECM is a key TME component that instructs cells bioactivity and tumor survival, embedding cells in hydrogel biomaterials that resemble the native tumor ECM enables researchers to better emulate the dynamic cell-ECM interactions and the biological barriers to therapeutics diffusion found in vivo.[21] Such supporting biomaterials must promote cellular attachment features, stimulate cellular proliferation, as well as promote 3D model assembly in a TME-mimetic setting. To better simulate tumor ECM, researchers have engineered ECM-mimetic natural-derived biomaterials that provide a suitable biochemical and biomechanical environment to promote cell adhesion, proliferation, and differentiation.[22,23]. Proteinaceous ECM-mimetic biomaterials such as collagen, gelatin, or fibroin, as well as decellularized tumor tissues, or in vivo found biopolymers hyaluronic acid have been commonly explored to fabricate ECM-mimetic cell-laden hydrogels.[24] These biomaterials are generally present in the TME, are highly bioactive, and can be easily functionalized with chemical moieties (e.g., methacrylate, thiol, etc.) or conjugated with synthetic biomaterials (e.g., polyethylene glycol (PEG), polycaprolactone (PCL)), rendering them ideal tools to generate tunable hydrogels that better mimic tumor ECM-specific biophysical properties.[25]. The chemical versatility of ECM-mimetic biomaterials not only accounts for their biological effect, but also unlocks their multi-combination. This engineering freedom has recently enabled the establishment of tunable and mechano-dynamic ECM-mimetic double-network hydrogels comprising two key tumor ECM components - thiolated-hyaluronic acid and norbornene-hydroxyphenylacetic acid dually functionalized gelatin.[26] These hydrogel precursors were combined to emulate ECM stiffening in vitro in an on-demand mode recapitulating the fibrotic stroma, gene expression profiles and epithelial-to-mesenchymal transition as those observed in native tumors.[40] In view of such advances, it becomes clear that the biomimicry potential of bioengineered cell-rich and matrix-rich 3D static tumor models is exponentially increasing and major aspects of human neoplasia are now possible to be modeled in a high-throughput/high-content mode, unlocking the so-desired switch to large-scale screening and big data generation.
Looking to building-up models that further upgrade the in vitro/in vivo correlation of 3D testing platforms, the field is now rapidly moving toward mimicking additional aspects of the TME including fluid dynamics and living tumors anatomic scale/bioarchitecture in an attempt to introduce such additional factors which are well-recognized to influence patients’ responses.
Next-generation organotypic 3D models – Bioengineering anatomic scale, bioarchitecture and fluid dynamics
Leveraging different 3D bioprinting technologies (Box 2) for human disease modelling applications has recently opened new possibilities to advance the manufacture of human tumor-sized and anatomically complex constructs that are build-up from rationally designed tumor-mimetic bioinks combining tumor/stroma cells with a wide range ECM-mimetic biomaterials.[12] This unique combination enables an automated and rapid biofabrication of biomimetic microtumors with: (i) a spatially controlled cellular organization in a user programmed mode, (ii) a high resolution and tunable scale resembling complex human-sized structures (i.e., vasculature, tumor mass, stromal compartments), and (iii) comprising key biochemical and biophysical properties, all of which ultimately influence the therapy screening process and data output.[27] Various types of biomaterials including collagen, gelatin, hyaluronic acid, alginate, and chitosan, as well as tissue-derived decellularized ECM (dECM), among many others are currently being explored to formulate bioactive bioinks.[28] Several advances on the engineering of suitable biomaterial inks for accommodating living cells, have enabled the fabrication of architecturally complex constructs with high fidelity (Box 2). Gathering on these advances, extrusion-based 3D bioprinting was leveraged to fabricate 3D mini-brain models of glioblastoma combining cancer and immune system cells (i.e., macrophages) in a more anatomically relevant scale when compared to standard 3D spheroids.[29] This elegant approach enabled researchers to probe key intercellular interactions and the contribution of macrophages to cancer cells proliferation, migration, and resistance/sensitivity to therapeutics. On a different take, advanced stereolithographic 3D bioprinting and programmed digital masks were employed to generate highly complex and biomimetic 3D hepatic models that recapitulate the hexagonal lobular structure found in the native human tissue, as well as key biomarkers expression.[30] Precisely patterning cellular spatial organization is a key advantage of 3D-bioprinted models in comparison to other technologies and could be a useful platform for investigating tumor-specific metastasis to liver tissues in an integrative approach.
Box 2 -. 3D-bioprinting technologies.
3D bioprinting is an additive manufacturing technology used for assembling computer-aided designed (CAD) living and non-living 3D biologically relevant constructs with controlled architectures employing different building blocks such as living cells, biomaterials and/or biomolecules.[115] Currently the most widespread 3D bioprinting modalities include: (i) Extrusion-based bioprinting typically requiring a viscous or shear-thinning bioink loaded in a cartridge and deposited layer-by-layer in a very precise mode over a cell culture plate. Following proper bioink deposition, the constructs usually need to be crosslinked through chemical, thermal, or photo-crosslinking; (ii) inkjet-based bioprinting where a bioink is deposited as droplets generating patterned layers; (iii) stereolithography (SLA)/digital light processing (DLP) bioprinting in which a user-defined 2D pattern is projected into a bioink enabling the fabrication of complex 3D architectures along the z-axis in a layer-by-layer based assembly, or (iv) laser-induced forward transfer (LIFT) where a pulsed laser beam is focused towards a donor substrate creating high-pressure bubbles and promoting a very localized ink release, among several others that have been widely described in detail in key seminal reports.[116–119] More recently, acoustic bioprinting, magnetic bioprinting and volumetric tomographic bioprinting reviewed in very interesting review articles have emerged as promising techniques to produce 3D bioprinted tumor models.[41,50,120]
Currently, extrusion-based 3D bioprinting has been widely applied to reproduce the TME in complex 3D tumor models. ECM-mimetic bioinks including collagen, gelatin, hyaluronic acid, alginate, chitosan, and dECM biomaterials have been used to formulate bioactive bioinks and bioprinted living systems.[28] Besides the inherently required biocompatibility and bioactivity, to undergo successful bioprinting, formulated bioinks must possess suitable rheological properties to generate models with optimal shape-fidelity and structural stability.[58,121] To assure the latter, numerous types of chemical functionalization routes have been explored to install different functional groups (e.g., methacryloyl, boronic, azide-DBCO, etc.), in pristine polymers/protein biomaterials as a strategy to improve their in-/post-printing processability into structurally stable 3D constructs.[122] Particularly, chemical functionalization methods that enable on-demand crosslinking have been widely explored to date, including dynamic/static light, as well as enzyme- and click-chemistry-mediated crosslinking, among others.[123] Adding to this, new approaches are further introducing dynamic covalent chemistries and formulating nanocomposite bioinks to incorporate dynamic viscoelastic features and tunable physicochemical properties found in native ECM.[28,124,125] Recent advances in bioprinting technologies including microparticle- or polymer-based supporting baths are further contributing towards improving the processability and fabrication of truly freeform 3D constructs and full-human size models with controlled bio-architectures.[126–128]
Although such reports emphasize the valuable contribution of 3D bioprinting in healthy/tumor tissues modelling, the majority of these approaches lack the introduction of dynamic fluid cues naturally present in the TME. Including fluid flow and its associated shear stress, has been shown to influence cancer cells proliferation, as well as affect their phenotype.[31] In fact, recent evidences point out that cancer cells cultured in a 3D setting exhibit higher drug resistance and more aggressive phenotypes, when compared to their static cultured counterparts.[32] Engineering approaches for seamlessly introducing such features have been mainly accomplished to date through the development of microfluidic cancer on-a-chip systems.[33]
3D cancer-on-a-chip platforms (Box 3) enable researchers to recreate human tumor-stroma interactions and the inclusion of relevant ECM-mimetic matrices, while dynamically manipulating key biochemical and biophysical parameters such as pH, oxygen, biomolecular gradients, and most importantly the flow of nutrients/metabolites, and/or cells (i.e., immune system cells, mesenchymal/stromal stem cells, etc.).[34] These microphysiological platforms allow a channel-independent and precise control over applied mechanical forces, emulating key biophysical cues/mechanical stimulation patterns found in specific human organs.[35–40] Moreover, by varying design layouts it is possible generate spatially controlled chemical gradients and analyze the consequent impact on cancer cells invasion.
Box 3 -. Cancer-on-a-chip technologies.
Microfluidic cancer-on-a-chip platforms display perfusable compartments and channels populated by living cells aiming to recapitulate human pathophysiology. These systems are commonly manufactured with optically clear plastic, glass, or flexible materials such as polydimethylsiloxane (PDMS). Such devices generally integrate small, perfused channels with sizes ranging from tens to hundreds of micrometers and allowing the precise control over cellular, physical, and biochemical microenvironment. Such platforms enable high-resolution, real-time imaging acquisition and evaluation of biochemical and metabolic factors through the integration of biosensors. Cancer-on-a-chip 3D cell culture platforms provide the necessary control over fluid flow, allowing researchers to model important processes such as shear stress, interstitial pressure, and chemical (e.g., cytokines) complex gradients and to study their effect on 3D tumor models in real time. Particularly, organ-on-a-chip systems have been designed to allow high-resolution, real-time imaging acquisition and evaluation of biochemical and metabolic factors by integrating specific sensors with capacity to detect biomarkers in real time, providing an advanced preclinical platform for cancer diagnosis and the discovery/screening of novel therapeutics.
Despite being a useful platform to recapitulate key human cancer hallmarks under dynamic conditions, there is still a need to introduce key aspects such as anatomic scale, controlled spatial cellular positioning, and culture in tunable cell-laden ECM-mimetic hydrogels. In this sense, 3D bioprinting merged with microfluidics arises as a promising technology to overcome such issues, opening new possibilities to deposit tumor/stroma-laden biomaterial in a very controlled mode.
Despite that these platforms recapitulate the biomimetic stimuli and dynamic biochemical/fluidic cues existing in vivo, the establishment of intricate, spatially controlled 3D vascular networks and the integration of human-scaled 3D tumor models into organ-on-a-chip systems remains a highly pursued upgrade.[41] To overcome this, synergistic combinations of 3D bioprinting technologies with microfluidics platforms are rapidly emerging as promising solutions to emulate such complex TME hallmarks, further improving the biomimicry potential of 3D in vitro tumor models.[42] Besides unlocking the capacity to create geometrically relevant and heterogenous 3D tumor models with high precision, bioprinting-on-a-chip also allows the engineering of vessel-like tubular constructs for assembling 3D vascular competent models on the fly during biofabrication.[43–46] This boosts vascularized microfluidic channel personalization in real time, going beyond the pre-programmed channel architectures that are introduced in standard microfluidic chips production process (i.e., stereolithography-produced). Such technological combination also enables researchers to introduce complex, tortuous/multi-sized vessel structures in a single construct. The latter is critical, considering the deregulated/aberrant vasculature encountered in different tumors and provides the so-desired design versatility if dynamic tumor-vasculature personalized 3D model engineering is envisioned. Leveraging bioprinting to introduce perfusable microvasculature-like channels with different tortuosity, controlled/homogeneous channel dimensions, programmed multi-scale branching, and/or multi-layered vasculature (e.g., comprising human umbilical vein endothelial cells (HUVECs) and supporting fibroblast/pericyte cells), as well as including ECM/basement membrane components with tunable mechanical properties, significantly upgrades the current possibilities offered by standard microphysiological platforms for tumor vasculature modelling.[47,48] In this context, perfusable PDMS microfluidic devices were recently fabricated by using nanoclay-reinforced pluronic F-127 ink as a sacrificial biomaterial to print blood vessel-on-a-chip with channels comprising uniform diameters and circular cross-sections.[44] Adding to this, user-programmed versatility and manufacturing control, 3D bioprinting-on-chip also enables the inclusion of ECM-mimetic hydrogel biomaterials, and the spatially controlled deposition of cell-laden ECM-mimetic hydrogel bioinks within a microfluidic device, eliminating the challenges/variability imposed by traditional cell seeding methods in microfluidic chips (e.g., via manual or with a syringe pump). 3D-bioprinted cancer-on-a-chip platforms represent a significant breakthrough and enclose an enormous potential for giving rise to next-generation physiomimetic 3D cancer models (Figure 2) that fully recapitulate key TME building blocks, otherwise unable to be introduced with standard 3D culture approaches.
Figure 2.

Bioengineering next-generation cancer models through rational combination of advanced biofabrication technologies and microfluidic chips with programmed designs as a strategy to give rise to highly physiomimetic 3D-bioprinted organ-on-a-chip platforms recapitulating fluid dynamics and TME heterogeneity, ultimately providing unique breakthroughs in in vitro cancer modelling.
General design considerations for 3D-bioprinted cancer-on-a-chip organotypic models
To materialize the 3D bioprinting cancer-on-a-chip synergy, various design parameters related to model pathophysiological features, cytoarchitecture, cellular heterogeneity, biomechanical cues, microfluidic chip designs, fluid dynamics, and 3D co-culture conditions, must be considered at early design stages.[49] When designing the tumor components, a fundamental knowledge of the native TME and its deconstruction into its functional elements including cancer cells, cancer stem cells, and stromal cells, as well as the supporting tumor ECM and blood/lymphatic-like vessels is key. Recognizing and introducing these building blocks at physiological cell ratios that capture their proportions in in vivo tumors is critical to assure a better in vitro/in vivo correlation.[50] Efforts to better recapitulate tumor-stromal ratios in in vitro 3D models have been underway, yet their full implementation in 3D-bioprinted tumor models remains to be fully explored.[7,51,52] Advanced quantifications technologies based on digital image analysis and artificial intelligence algorithms are emerging as valuable predictors for this specific parameter and can in the future aid researchers in making more informative design choices for this aspect.[53,54] Moreover, at the design stage of the tumor elements one must also consider patient personalization level and whether the designed models are more broadly applicable or if their key purpose is to recapitulate patient-specific microenvironments. If the latter is envisioned, the pre-design exploitation of highly informative tumor characterization technologies such as in-depth single cell transcriptomics, or multiplexed mass cytometric imaging of tumor tissue slices will surely provide the so-desired information of key building blocks volumetric organization as recently demonstrated.[55,56]
Factors that should be taken into consideration to fabricate physiomimetic 3D-bioprinted cancer-on-a-chip platforms include not only the cellular distribution of tumor elements in a suitable spatial distribution as discussed before, but also the composition and mechanical properties of the ECM. In fact, one of the major design considerations for 3D bioprinting is the selection of bioink(s) to be used and the 3D architecture to be fabricated. Tumor ECM-mimetic biomaterials ideally should provide suitable rheological properties to maximize printing performance and enable a suitable resolution and construct shape fidelity post-printing. Specifically, bioink properties such as viscosity, stiffness and gelation kinetics, are important factors that must be considered for the accurate printing process as well as to ensure high construct fidelity, resolution, and shape maintenance. While inkjet-based and laser-assisted bioprinting require bioink viscosities in the range of 10 mPa.s and 1–300 mPa.s, respectively, for the most widespread extrusion bioprinting technology, formulations with higher viscosities are required (30–6 × 107 mPa s).[57] Adding to this, polymer/protein chemical functionalization for enabling covalent crosslinking strategies (via for example, Michael-type addition reactions, click chemistry, or photopolymerization) have been applied to formulate bioinks with tunable mechanical properties and printability.[57,58] Adding to this tumor ECM-mimetic bioinks, the biological, biophysical, and biochemical cues similar to those found in tumor tissues must be offered.[58,59]
To date, bioinks comprising collagen, gelatin methacryloyl (GelMA), hyaluronic acid, or alginate and their combinations thereof have been widely used to bioprint 3D tumor models.[33] Recent efforts have been made to process tissue-specific bioinks by using dECM. Although temperature-mediated self-gelling dECM hydrogels provide cells with tissue-specific biomechanical and biomolecular signatures, such biomaterials generally present poor mechanical properties and printability.[60] To improve dECM printability and enable researchers to precisely tune mechanical properties, recent attempts have focused on merging dECM with other mechanically robust hydrogels such as hyaluronic acid or alginate.[61] Following similar strategies found in other proteinaceous hydrogels, researchers have also been focusing on installing functional chemical moieties into dECM to obtain photocrosslinking or stimuli-responsive enzyme-based crosslinking.[62,63] On this front, dECM modified with methacryloyl groups enabled to produce photocrosslinkable bioinks with modular mechanical properties and suitable for 3D cell culture.[64] These findings may open new avenues for bioprinting 3D tumor models with native ECM biochemical and biophysical cues increasing the biomimicry potential of these models.
In a more challenging perspective, the 3D bioprinting of complex tumor models may require the assembly of different tumor components in different ECM-mimetic bioinks that recapitulate the biophysical features of the tumor, stroma and vasculature compartments, in which ECM composition is well known to differ. In this scenario the development of customized bioinks and the use of advanced multi-nozzle/multi-printhead configurations for continuous fabrication of multi-material constructs may enhance the accuracy and complexity of the engineered platforms, while upkeeping the fabrication speed and scalability.[65–67]
Adding to the identification of the unitary cellular elements and the selection of a suitable supporting matrix, during the design stages one can also consider the inclusion of additional levels of complexity and biomimicry. In particular, the 3D bioprinting of patient-personalized 3D tumor masses with anatomic scale and full volume, recapitulating patient’s own tumors at the detection stage could provide a valuable opportunity for generating in vitro models that can be used as improved human surrogates for screening candidate therapeutics. In this regard, sophisticated approaches aimed at bioprinting 3D tumor masses with anatomic scale may be materialized by processing in vivo human tumors bioimaging data (e.g., computed tomography (CT) or magnetic resonance imaging (MRI)), and converting this information into a printable 3D model by using computer-aided design/medical bioimaging software.[43] This strategy has tremendous potential for enabling accurate bioink deposition and originate the fabrication of highly personalized 3D tumor models with specific bioarchitecture, anatomic scale, and spatially controlled cellular organization.
Another key design consideration of merging 3D bioprinting with cancer-on-a-chip models is their assembly methodology. These platforms can generally be manufactured via two different approaches. One is single-step production in which all components of the 3D tumor models, namely the microfluidic chips, hollow channels, and the 3D tumor tissues, are directly manufactured in a user-defined/fully-customized mode, where microfluidic chips with tunable inlets/outlets and designs as well as 3D-bioprinted constructs can be fabricated. This can be materialized by using multi-modal systems equipped with multiple printing heads allowing both 3D-printing and 3D-bioprinting [68–70]. On the other hand, these platforms can be generated via post-integrated production in which 3D tumor components can be directly 3D-bioprinted in the pre-fabricated device, or separately bioprinted and inserted into user pre-produced or commercially available microfluidic devices (e.g., VasKit perfusion device - CELLINK®, Multi-organ-chips – TissUse™, among several others). The latter can be more time-consuming and generally offers less personalization potential. Traditionally, organ-on-a-chip platforms have been manufactured by casting into a master mold previously fabricated through micromachining, soft lithography, photopolymerization, or other manufacturing techniques. Such techniques are often considered technically limiting owing to their labor-intensive, time-consuming, and costly features. In this regard, 3D printing has also shown to be a very valuable fabrication technique as it enables user-defined, cost-effective, and high-precision production of microfluidic chips for various biomedical applications, including organ-on-a-chip devices.[68,70–72] Recently, a 3D-bioprinted liver-on-a-chip was engineered in an one step approach.[73] Such a strategy seems to facilitate the bioprocessing steps; however, currently available 3D bioprinters still make the process slow and hardly applicable for high-throughput assays. The development of 3D printers/bioprinters capable of manufacturing the microfluidic device and enable 3D tumor tissue bioprinting with volumetric freedom and in an expeditious way could open new avenues in this field.[33] Gathering on this, 3D-bioprinted cancer-on-chip platforms have been exploited to modulate key TME-related parameters such as the hypoxic conditions established in solid tumors, which is a particularly challenging parameter to be modulated in standard 3D scaffold-free models and is known directly influence cellular responses to therapeutics.[74]
An important aspect related to microfluidic chip fabrication is the selection of suitable, and ideally, inert materials for their manufacture. To date, microfluidic chips have been most commonly fabricated by using polydimethylsiloxane (PDMS). Although such materials offer great optical transparency, flexibility, and gas permeation facilities, PDMS can non-specifically absorb small hydrophobic molecules, such as anti-cancer drugs and other soluble molecules including proteins.[75] Recent advances in quantification strategies and correction approaches for this phenomenon have been described providing the grounds for further use of PDMS.[76] Alongside, researchers have also been investigating alternative fabrication materials including polycaprolactone, poly(methyl methacrylate) (PMMA), among others.[77,78] However the need to discover new materials with optical clarity that overcome non-specific molecule absorption, which alongside enable proper on-chip fixation/adherence of 3D-bioprinted ECM-mimetic hydrogel biomaterials when subjected to fluid flow-associated shear stress is an active field of research.[79] Due to their high water content and bioactivity, hydrogels have also been explored microfluidic fabrication. Nonetheless, there is still a plethora of challenges to surpass especially considering the challenges in processing hydrogels for fabrication of chips with complex designs and their inherent swelling/molecule diffusion features.[80] The rational introduction of such parameters (i.e., cellular elements, ECM-mimetic biomaterials, chip design, and manufacturing materials) are envisioned to contribute to improving the biomimicry and predictive potential of 3D-bioprinted on-a-chip disease surrogates. Up to date, several reports have been successful in installing important TME features on such systems, overcoming the limitations posed by static 3D mono- or heterotypic microtumor models as it will be discussed in the following sections.
3D bioprinted cancer-on-chip models – Key developments and applications
The combination of 3D bioprinting technologies with microfluidic systems has already proven valuable to bioengineering a new generation of upgraded 3D tumor models that more faithfully recapitulate TME building blocks in a spatially controlled mode, whilst enabling culture in a dynamic environment. The impact of such synergy was recently demonstrated with the preclinical bioperformance screening of Metuzumab, an anti-CD147 monoclonal antibody that was administered into a 3D-bioprinted hepatoma-on-a-chip model and compared to both static 3D-bioprinted living architectures and 2D monolayered in vitro models.[81] Both static and dynamic 3D in vitro models displayed increased therapy resistance when compared to their 2D monolayer counterparts. These findings emphasize the relevance of integrating fluid flow dynamics in 3D-bioprinted models and their effect in model’s pathophysiological features.
Increasing complexity of these dynamic models has also been materialized by the introduction of lymphatic capillary mimics in the juxta-tumoral compartment in 3D-bioprinted cancer-on-a-chip models. It is important to emphasize, that lymphatic system introduction in 3D models has remained rather underexplored with most platforms developed to date encompassing the introduction of lymphatic cells that are then expected to self-assemble into primitive vessel-like structures in culture.[82–84] Despite the successful combination with cancer cells and the establishment of perfusable structures with this approach, the 3D spatial distribution, the size, tortuosity, and length of lymphatic structures is difficult to control and to reproduce across several batches, impairing the potential of this strategy and its scalability.[83] To overcome these drawbacks and aiming to simultaneously recapitulate the vascular and lymphatic vessels, as well as their fluid dynamics present in the TME, a 3D-bioprinted cancer-on-a-chip device comprising bioprinted blood/lymphatic compartments was recently reported.[46] In this seminal approach, the canceron-a-chip platform comprising a 3D-bioprinted blood and a lymphatic vessel pair was assembled by 3D bioprinting both perfusable channels using tunable bioinks (i.e., alginate, GelMA, PEG combinations) and a multi-layered coaxial nozzle. The control offered by the 3D bioprinting equipment enabled the fabrication of hollow, perfusable vascular and lymphatic networks surrounding the tumor component in a microfluidic device. This elegant system was used to simulate the mass transport mechanisms of different macromolecules and chemotherapeutics in this TME mimetic platform. The successful inclusion of the lymphatic system component in such 3D tumor models could also prove to be a valuable approach for providing useful insights regarding its role on human tumors development, invasion, and resistance.
Taking advantage of 3D bioprinting to construct complex tissues and of microfluidic systems to introduce a dynamic environment, a patient-derived glioblastoma (GBM)-on-a-chip was also recently developed to evaluate chemoradiotherapy treatments.[85] Using a rationale deconstruction/reconstruction bottom-up design, researchers leveraged 3D bioprinting to fabricate ring-like structures emulating GBM key components. Particularly, researchers designed a patient-derived tumor component comprising GBM cells and brain-derived dECM. This personalized element was then surrounded by a user-programmed vascular network mimetic channel and the whole model enclosed by a gas-permeable enclosure. The engineered platform was then exploited to evaluate the biochemical and biophysical cues in GBM progression and responses to therapeutics. The in vitro generated data from patient-personalized platforms was then correlated with clinical data, providing the so desired predictive/follow-up potential to aid medical decision. In fact, an informed selection of several candidate drug combinations fitted that better fitted patient response was identified. Such encouraging achievement demonstrates the synergy of bioprinting/microfluidic platforms and paves the way for a new generation of technologies for patient-personalized medical treatment discovery.
It is important to emphasize that microfluidic platforms also offer the possibility for simultaneously screening several anti-cancer drugs/drug combinations, via the manufacture of multiplexed channels or via chips parallelization, as a strategy to increase data output.[86,87] Recently the inclusion of on-chip (bio)sensing possibilities may also open new avenues to further improve 3D-bioprinted cancer-on-a-chip platforms potential for high-throughput/high-content screening in real time via continued monitoring, accelerating the drug screening process and aiding in medical decision making.[88] The fabrication speed and patient-personalization potential of these platforms is a long-desired feature if one considers that patient-derived xenograft models have low throughput, encompass high costs and can require up to 6 months or longer to be properly established.[89,90]
3D-bioprinted cancer-on-a-chip – Metastasis models
Dynamic 3D-bioprinted cancer-on-a-chip systems have also a unique potential to evaluate other key aspects of human neoplasia such as the metastization process. Engineering platforms to model such complex biological process is a major advantage from a therapeutic perspective considering that the extravasation of cancer cells from the primary tumor niche to secondary organs is the cause of about 90% of cancer-related mortality.[91] To metastasize and form secondary tumors in distant organs, primary tumor cells must invade the tumor-surrounding vascular network (intravasation) and then travel to secondary tissues. Such an intricate biological process is generally driven by: (i) TME physical traits including mechanical forces and biochemical signals, and (ii) the interactions between cancer and stromal cells that trigger vasculature formation around the tumor enabling the formation of dissemination routes to secondary organs.[92]
Cancer-on-a-chip devices have been proposed as powerful platforms to recreate the invasion/metastatic process.[93] These systems containing continuously perfusable microfluid channels, enable to mimic tumor mechanical forces such as shear stress from the dynamic flow recapitulating the blood flow, tension from the solid tumor, and stiffness variation of ECM.[94] On the other hand, as previously discussed, 3D bioprinting has emerged as an amenable technique to produce perusable hollow channels with complex designs in one-step process, enabling the precisely reproduction of neo-blood vessels formation and study angiogenesis or metastasis. Therefore, the 3D bioprinting of blood vessels and 3D cancer tissue in a microfluidic device as recently emerged as a remarkable improvement to engineer relevant 3D-bioprinted cancer-on-a-chip models and investigate the metastatic process.
The 3D bioprinting-assisted engineering of blood vessel-like structures can be performed through sacrificial hydrogel bioprinting, embedded bioprinting, and co-axial bioprinting. Such approaches allow precise and efficient control over hydrogel deposition to obtain complex vascular systems on a chip. In this context, recently a vascular network was produced by printing agarose fibers as sacrificial material and surrounding them by a cell-laden GelMA hydrogel inserted in the chip.[46] The sacrificial material was then removed, resulting in hollow channels that were then perfused with endothelial cells to form a perfusable and endothelialized vessel. By using a similar strategy and aiming to study the influence of neutrophils in ovarian cancer invasion, an advanced bioprinted tumor-on-chip was established by integrating ovarian tumor spheroid-laden collagen matrix in a bioprinted microfluidic device fabricated on a porous membrane and carrying neutrophiles.[95] In this rationally designed approach, 3D bioprinting enabled to print microchannels using a sacrificial ink (i.e., Pluronic-based hydrogel) to create a tubular mold that is further removed leaving a perfusable hollow channel. This design enabled to recreate the interplay between neutrophils and cancer cells, and the 3D tumor invasion into the collagen matrix, modulated by chemotaxis and generation of neutrophil extracellular traps (NETs). In order to better simulate breast cancer metastasis to bone compartments, a tri-culture metastatic model comprising by cancer, bone, and vascular cells was recently created.[96] This 3D-bioprinted system allowed to study trans-endothelial migration and the colony-forming behavior of metastatic cancer cells, being a valuable tool for studying metastatic breast cancer progression in bone.
Outlook and Future Perspectives
Human tumors exhibit highly complex and dynamic environments that ultimately dictate disease progression and metastasis. Simulating the complex TME and its key hallmarks in 3D in vitro platforms requires the integration of both architectural and compositionally relevant microtissues into the microfluidic devices to provide dynamic cancer-stroma interactions. Herein, 3D bioprinting can emerge as a unique technology to be integrated in organ-on-a-chip field as it allows rapid, automated, and controlled fabrication of complex and reproducible anatomic-sized tumor models, while organ-on-a-chip systems enable to emulate key physiological, biophysical and biochemical cues found in vivo. The establishment of 3D-bioprinted cancer-on-a-chip platforms by integration of both technologies is emerging as a promising and revolutionary approach for understanding human tumor pathophysiology and for screening more effective treatments in a more physiomimetic and scalable mode, surpassing the limitations of currently available static 3D tumor models. Although such models recapitulate key TME cellular and non-cellular building blocks, immune system elements are seldomly included. Researchers are now actively focusing on developing immunocompetent cancer-on-a-chip models to better evaluate tumor-immune system interactions and screen candidate anti-cancer immunotherapies (see Box 4).[89,97] These immune-active platforms have been rather underexplored and one can conceive that fabricating 3D-bioprinted cancer-on-a-chip devices comprising the anatomic scale of the TME, while accommodating multiple tumor-associated immune system cells and soluble factors in a dynamic environment, may facilitate immunotherapies screening under flow and provide more realistic data outputs. To this end, the biological complexity of tumor-immune cell interactions, the long-term viability of immune cells under flow, as well as their phenotype along time in culture are some of the key parameters that must be carefully addressed in such setups. Advances in this direction are expected in upcoming years.
Box 4 – Targeting the cancer immune system.
Cancer immunotherapy has rapidly emerged as a promising cancer treatment that aims at exploiting patients’ immune system (re)activation. Such an approach intends to stimulate immune cells to fight cancer through various strategies including immune system modulation, via administration of exogenous cytokines or antibodies (e.g., Programed death 1/programmed death-ligand (PD-1/PD-L1) among others, or through the injection of engineered immune cells in the bloodstream (i.e., engineered chimeric antigen receptor (CAR) T-cells and T-cell receptor engineered T-cells, natural killer (NK)-cells, etc.). In the human body, immune system cells can be present in tissues (e.g., tissue-resident macrophages, etc.) but are most generally living in suspension, travelling through the blood vessels network, under dynamic flow conditions. In specific scenarios, immune cells extravasate from the vasculature and infiltrate into the TME, communicating with cancer/stromal cells. Aiming to investigate several mechanisms underlying cancer immunotherapy, different microfluidic cancer on-chip systems have been established. To evaluate the effect of NK-cell based immunotherapies and antibody-dependent cell cytotoxicity in breast cancer, breast cancer spheroids and NK-cells were co-cultured in a collagen matrix.[129] Such model was integrated in a microfluidic device comprised by two laterally perfused vascular channels which enabled cancer cell-targeting antibodies perfusion into the collagen hydrogel. In this platform antibody extravasation and penetration in the tumor matrix was slower and hampered by tumor spheroid mass. Whereas, NK-cells rapidly penetrated the tumor spheroid, destroying cancer cells. Furthermost, combining antibody-cytokine conjugates and NK-cells increased cytotoxicity demonstrating the usefulness of the engineered model to study candidate immunotherapies. From a personalized medicine point-of-view, patient-derived organotypic tumor microtissues retaining autologous immune cells were cultured in a commercial microfluidic device and the response to PD-1/CTLA-4 monoclonal antibody targeting immune checkpoints were already evaluated.[130] This system enabled researchers to identify the optimal parameters for maximizing immune-mediated tumor cell killing by effector CD8+ T via PD-1 and CTLA-4 blockage. This platform revealed to be highly promising for evaluating novel combinatorial therapies and its upscaling to 3D-bioprinted tumor compartments/channels may provide an added value in the future.
Overall, the synergistic combination of both technologies may unlock the generation of human tumor models that mimic tumor, stroma, and vascular components, as well as the fluid/cellular transport dynamics that are important for cancer aggressiveness and resistance to therapeutics. Although 3D-bioprinted cancer-on chip is a promising strategy to fabricate complex constructs, the field is in still its infancy and there is a large room for progress and evolution toward high-throughput and fully patient-personalized systems (see Outstanding Questions). The optimization of the bioprinting processes on-a-chip and the inclusion of ECM-mimetic biomaterials such tumor-derived decellularized matrices that more accurately reproduce the native tissues still remain challenging. Moreover, the integration of computer-controlled sensors-on-chip that may give valuable real-time data regarding gas exchange, pH levels, and/or metabolic markers (e.g., glucose or lactate levels in culture media) need to be standardized. Such advances enable to obtain real-time information about the bioprinted models through the detection of key biomarkers and rapid extraction of valuable quantitative readouts from the culture media under circulation. In the future it is also envisioned that the optimization of evermore mimetic bioinks and physiomimetic chip designs, will contribute for a broad adoption of 3D-bioprinted cancer-on-a-chip platforms for both fundamental tumor biology studies and advanced therapeutic screening.
Outstanding Questions.
How can computational modelling of flow dynamics be combined with 3D bioprinting of organotypic models to provide an increase degree of similarity to the in vivo setting and in a tumor-specific mode?
How can advanced biofabrication techniques be improved for expediting a one-step manufacture of 3D-bioprinted organ-on-a-chip models comprising several TME components?
Can manufacturing and cell expansion technologies be upgraded to facilitate the fabrication of anatomic-scale, tumor cell-dense, and high-throughput 3D-bioprinted cancer-on-a-chip devices?
How can bioimaging and omics-based patient tumor analysis contribute for fabricating patient-specific and physiomimetic 3D-bioprinted cancer-on-a-chip platforms and unlock personalized medicine approaches?
How can biosensors be included in 3D-bioprinted cancer-on-a-chip platforms to extract more relevant information regarding therapeutics performance in real time and reduce chips manipulation?
Supplementary Material
Highlights:
The development of 3D in vitro models recapitulating key tumor microenvironment hallmarks is in high demand due to their potential for fostering new discoveries in cancer pathophysiology and for improving preclinical drug screening.
Tumor models combining biofabrication and organ-on-a-chip technologies open unique avenues in bioengineered tumor surrogates, which enclose key elements that are generally overlooked in standard 3D platforms.
Biofabricated 3D in vitro models on perfusable chips have provided significant advances, yet, addressing untapped microenvironment-specific designs and bioengineering parameters will be key for further improving biomimicry.
Next-generation patient-derived 3D-bioprinted cancer-on-a-chip platforms are expected to level-up current approaches, ultimately enabling the generation of increasingly physiomimetic tumor models for precision/personalized medicine.
Acknowledgements
This work was developed within the scope of the project CICECO-Aveiro Institute of Materials, UIDB/50011/2020 & UIDP/50011/2020, financed by national funds through the Portuguese Foundation for Science and Technology/MCTES. This work was also supported by the Programa Operacional Competitividade e Internacionalização (POCI), in the component FEDER, and by national funds (OE) through FCT/MCTES, in the scope of the projects PANGEIA (PTDC/BTM-SAL/30503/2017). The authors acknowledge the financial support by the Portuguese Foundation for Science and Technology (FCT) through a Doctoral Grant (DFA/BD/7692/2020, Maria V. Monteiro) and trough a Junior Researcher contract (CEEC/1048/2019, Vítor M. Gaspar). Y.S.Z. acknowledges the National Institutes of Health (R21EB025270, R00CA201603, R01EB028143) and the Brigham Research Institute.
Glossary
- 3D-Bioprinting
An additive manufacturing technology in which a bioink (i.e., cell laden biomaterial) is deposited in a precise, automated mode to produce a user-defined 3D construct.
- Decellularized Extracellular Matrix (dECM)
ECM devoid of cellular components that maintains native tissue architecture and composition. The process of decellularization encompasses chemical-based approaches (e.g., detergents, enzymatic, hypertonic, or acid/base solutions), or physical processes (e.g., stirring, sonication, high hydrostatic pressure, supercritical CO2, or freeze-thawing).
- Epithelial-to-mesenchymal transition (EMT)
A biological process that allows epithelial cells to adopt a mesenchymal phenotype, losing their cell polarity and cell–cell adhesions, and promoting their migration/invasion capacity.
- Extracellular matrix (ECM)
A 3D network composed of proteins (collagen, elastin, fibronectin, laminin), glycoproteins, glycosaminoglycans (hyaluronic acid), and soluble factors that support tumor growth, progression, and resistance.
- Hydrogels
A polymer network that can undergo photo-, enzymatic, thermal, physical, or/and ionic photocrosslinking resulting in a highly hydrated scaffolds with the ability to support cell growth in a native-mimetic way.
- Hypoxia
Deprivation of adequate oxygen supply at the tissue level. Integrins: heterodimeric cell transmembrane receptors that attach to the cytoskeleton and the ECM, and mediate cell adhesion and multiple signal transduction pathways related to cell movement, growth, differentiation, survival, and apoptosis.
- Tumor microenvironment (TME)
dynamic and complex environment composed of cancer cells, stromal cells (e.g., fibroblasts, endothelial cells, immune cells, mesenchymal stem cells), blood and lymphatic vessels hosted in the ECM.
- Matrigel
A mixture of ECM proteins derived from Engelbreth-Holm-Swarm (EHS) mouse sarcoma cells.
- Organ-on-a-chip
A microfluidic platform with continuously perfused channels inhabited by living cells cultured under dynamic conditions arranged to recapitulate organ-level physiology.
- Organoids
3D culture models that are distinct from spheroids because they originate through the proliferation and self-organization of a progenitor cell source that generates aggregates of cells resembling the architecture and functionality of the native tissue they derive from.
- Spheroids
3D multicellular tumor aggregates that can be composed by both cancer cells and stromal cells. Commonly, 3D tumor spheroids are assembled by seeding the proper cell suspension in low adhesion substrates to force cells aggregation in a microtumor mass.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Whiteside TL (2008) The tumor microenvironment and its role in promoting tumor growth. Oncogene 27, 5904–5912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duan Q et al. (2020) Turning Cold into Hot: Firing up the Tumor Microenvironment. Trends in Cancer 6, 605–618 [DOI] [PubMed] [Google Scholar]
- 3.Nia HT et al. (2020) Physical traits of cancer. Science (80-. ). 370, 6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Emon B et al. (2018) Biophysics of Tumor Microenvironment and Cancer Metastasis - A Mini Review. Comput. Struct. Biotechnol. J 16, 279–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nazemi M and Rainero E (2020) Cross-Talk Between the Tumor Microenvironment, Extracellular Matrix, and Cell Metabolism in Cancer. Front. Oncol 10, 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalluri R (2016) The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 16, 582–598 [DOI] [PubMed] [Google Scholar]
- 7.Monteiro MV et al. (2021) Stratified 3D Microtumors as Organotypic Testing Platforms for Screening Pancreatic Cancer Therapies. Small Methods 5, 2001207. [DOI] [PubMed] [Google Scholar]
- 8.Wang C et al. (2014) Three-dimensional in vitro cancer models: A short review. Biofabrication 6, 022001. [DOI] [PubMed] [Google Scholar]
- 9.Yang L et al. (2019) Tumor organoids: From inception to future in cancer research. Cancer Lett. 454, 120–133 [DOI] [PubMed] [Google Scholar]
- 10.Nunes AS et al. (2019) 3D tumor spheroids as in vitro models to mimic in vivo human solid tumors resistance to therapeutic drugs. Biotechnolody Bioeng. 116, 206–226 [DOI] [PubMed] [Google Scholar]
- 11.Ferreira LP et al. (2018) Design of spherically structured 3D in vitro tumor models - Advances and prospects. Acta Biomater. 75, 11–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Radhakrishnan J et al. (2020) Organotypic cancer tissue models for drug screening: 3D constructs, bioprinting and microfluidic chips. Drug Discov. Today 25, 879–890 [DOI] [PubMed] [Google Scholar]
- 13.Ferreira LP et al. (2018) Bioinstructive microparticles for self-assembly of mesenchymal stem Cell-3D tumor spheroids. Biomaterials 185, 155–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fan H et al. (2019) Emerging organoid models: Leaping forward in cancer research. J. Hematol. Oncol 12, 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heinrich MA et al. (2021) Translating complexity and heterogeneity of pancreatic tumor: 3D in vitro to in vivo models. Adv. Drug Deliv. Rev 174, 265–293 [DOI] [PubMed] [Google Scholar]
- 16.Li X et al. (2014) Oncogenic transformation of diverse gastrointestinal tissues in primary organoid culture. Nat. Med 20, 769–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kratochvil MJ et al. (2019) Engineered materials for organoid systems. Nat. Rev. Mater 4, 606–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tuveson D and Clevers H (2019) Cancer modeling meets human organoid technology. Science (80-. ) 364, 952–955 [DOI] [PubMed] [Google Scholar]
- 19.Skardal A et al. (2016) Organoid-on-a-chip and body-on-a-chip systems for drug screening and disease modeling. Drug Discov. Today 21, 1399–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsai S et al. (2018) Development of primary human pancreatic cancer organoids, matched stromal and immune cells and 3D tumor microenvironment models. BMC Cancer 18, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monteiro MV et al. (2020) Chapter 2 - Bioinspired biomaterials to develop cell-rich spherical microtissues for 3D in vitro tumor modeling. In Biomaterials for 3D Tumor Modeling pp. 43–65 [Google Scholar]
- 22.Hinderer S et al. (2016) ECM and ECM-like materials — Biomaterials for applications in regenerative medicine and cancer therapy. Adv. Drug Deliv. Rev 97, 260–269 [DOI] [PubMed] [Google Scholar]
- 23.Fong ELS et al. (2016) Heralding a new paradigm in 3D tumor modeling. Biomaterials 108, 197–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liaw CY et al. (2018) Engineering 3D Hydrogels for Personalized In Vitro Human Tissue Models. Adv. Healthc. Mater 7, 1701165. [DOI] [PubMed] [Google Scholar]
- 25.Caliari SR and Burdick JA (2016) A practical guide to hydrogels for cell culture. Nat. Methods 13, 405–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu HY et al. (2018) Biomimetic and enzyme-responsive dynamic hydrogels for studying cell-matrix interactions in pancreatic ductal adenocarcinoma. Biomaterials 160, 24–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Langer EM et al. (2019) Modeling Tumor Phenotypes In Vitro with Three-Dimensional Bioprinting. Cell Rep. 26, 608–623.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morgan FLC et al. (2020) Dynamic bioinks to advance bioprinting. Adv. Healthc. Mater 9, 1901798. [DOI] [PubMed] [Google Scholar]
- 29.Heinrich MA et al. (2019) 3D-bioprinted mini-brain: a glioblastoma model to study cellular interactions and therapeutics. Adv. Mater 31, 1806590. [DOI] [PubMed] [Google Scholar]
- 30.Ma X et al. (2016) Deterministically patterned biomimetic human iPSC-derived hepatic model via rapid 3D bioprinting. Proc. Natl. Acad. Sci 113, 2206–2211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang S-F et al. (2008) Tumor cell cycle arrest induced by shear stress: Roles of integrins and Smad. Proc. Natl. Acad. Sci 105, 3927–3932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Azimi T et al. (2020) Cancer cells grown in 3D under fluid flow exhibit an aggressive phenotype and reduced responsiveness to the anti-cancer treatment doxorubicin. Sci. Rep 10, 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu F and Choudhury D (2019) Microfluidic bioprinting for organ-on-a-chip models. Drug Discov. Today 24, 1248–1258 [DOI] [PubMed] [Google Scholar]
- 34.Hachey S and Hughes C (2018) Applications of Tumor Chip Technology. Lab Chip 18, 2893–2912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thompson CL et al. (2020) Mechanical Stimulation: A Crucial Element of Organ-on-Chip Models. Front. Bioeng. Biotechnol 8, 1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaarj K and Yoon J-Y (2019) Methods of delivering mechanical stimuli to organ-on-a-chip. Micromachines 10, 700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ergir E et al. (2018) Small force, big impact: next generation organ-on-a-chip systems incorporating biomechanical cues. Front. Physiol 9, 1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huh D et al. (2010) Reconstituting organ-level lung functions on a chip. Science (80-. ). 328, 1662–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huh D et al. (2012) A human disease model of drug toxicity–induced pulmonary edema in a lung-on-a-chip microdevice. Sci. Transl. Med 4, 159ra147–159ra147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Phelps AS et al. (2019) Modelling cancer in microfluidic human organs-on-chips. Nat. Rev. Cancer 19, 65–81 [DOI] [PubMed] [Google Scholar]
- 41.Ma J et al. (2018) Bioprinting of 3D tissues/organs combined with microfluidics. RSC Adv. 8, 21712–21727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang YS and Khademhosseini A (2020) Engineering in vitro human tissue models through bio-design and manufacturing. Bio-Design Manuf. 3, 155–159 [Google Scholar]
- 43.Park JY et al. (2018) 3D Bioprinting and its application to organ-on-a-chip. Microelectron. Eng 200, 1–11 [Google Scholar]
- 44.Klak M et al. (2020) Novel strategies in artificial organ development: What Is the future of medicine? Micromachines 11, 1–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grigoryan B et al. (2019) Multivascular networks and functional intravascular topologies within biocompatible hydrogels. Science (80-. ). 364, 458–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cao X et al. (2019) A Tumor-on-a-Chip System with Bioprinted Blood and Lymphatic Vessel Pair. Adv. Funct. Mater 29, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sasmal P et al. (2018) 3D bioprinting for modelling vasculature. Microphysiological Syst. 2, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kolesky DB et al. (2016) Three-dimensional bioprinting of thick vascularized tissues. Proc. Natl. Acad. Sci 113, 3179–3184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li J et al. (2020) Improving bioprinted volumetric tumor microenvironments in vitro. Trends in Cancer 6, 745–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gaspar VM et al. (2019) Advanced Bottom-Up Engineering of Living Architectures. Adv. Mater 32, 1903975. [DOI] [PubMed] [Google Scholar]
- 51.Moorman AM et al. (2012) The prognostic value of tumour-stroma ratio in triple-negative breast cancer. Eur. J. Surg. Oncol 38, 307–313 [DOI] [PubMed] [Google Scholar]
- 52.Tanaka HY et al. (2020) Heterotypic 3D pancreatic cancer model with tunable proportion of fibrotic elements. Biomaterials 251, 120077. [DOI] [PubMed] [Google Scholar]
- 53.Zhao K et al. (2020) Artificial intelligence quantified tumour-stroma ratio is an independent predictor for overall survival in resectable colorectal cancer. EBioMedicine 61, 103054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Millar EKA et al. (2020) Tumour Stroma Ratio Assessment Using Digital Image Analysis Predicts Survival in Triple Negative and Luminal Breast Cancer. Cancers (Basel). 12, 3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Irmisch A et al. (2021) The tumor profiler study: integrated, multi-omic, functional tumor profiling for clinical decision support. Cancer Cell 39, 288–293 [DOI] [PubMed] [Google Scholar]
- 56.Zanotelli VRT et al. (2020) A quantitative analysis of the interplay of environment, neighborhood, and cell state in 3D spheroids. Mol. Syst. Biol 16, e9798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hölzl K et al. (2016) Bioink properties before, during and after 3D bioprinting. Biofabrication 8, 032002. [DOI] [PubMed] [Google Scholar]
- 58.Gopinathan J and Noh I (2018) Recent trends in bioinks for 3D printing. 22, 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gungor-Ozkerim PS et al. (2018) Bioinks for 3D bioprinting: an overview. Biomater. Sci 6, 915–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ferreira LP et al. (2020) Decellularized Extracellular Matrix for Bioengineering Physiomimetic 3D in Vitro Tumor Models. Trends Biotechnol. 38, 1–18 [DOI] [PubMed] [Google Scholar]
- 61.Curley CJ et al. (2019) An injectable alginate/extra cellular matrix (ECM) hydrogel towards acellular treatment of heart failure. Drug Deliv. Transl. Res 9, 1–13 [DOI] [PubMed] [Google Scholar]
- 62.Yin J et al. (2018) 3D Bioprinting of Low-Concentration Cell-Laden Gelatin Methacrylate (GelMA) Bioinks with a Two-Step Cross-linking Strategy. ACS Appl. Mater. Interfaces 10, 6849–6857 [DOI] [PubMed] [Google Scholar]
- 63.Lin CC and Korc M (2018) Designer hydrogels: Shedding light on the physical chemistry of the pancreatic cancer microenvironment. Cancer Lett. 436, 22–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ali M et al. (2019) A Photo-Crosslinkable Kidney ECM-Derived Bioink Accelerates Renal Tissue Formation. Adv. Healthc. Mater 8, 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Skylar-Scott MA et al. (2019) Voxelated soft matter via multimaterial multinozzle 3D printing. Nature 575, 330–335 [DOI] [PubMed] [Google Scholar]
- 66.Cameron T et al. (2020) Development of a disposable single-nozzle printhead for 3D bioprinting of continuous multi-material constructs. Micromachines 11, 459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang YS and Khademhosseini A (2020) Systems and methods for in vivo multi-material bioprinting., U.S. Patent Application No. 16/091,193 [Google Scholar]
- 68.Miri AK et al. (2019) Bioprinters for organs-on-chips. Biofabrication 11, 42002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Behroodi E et al. (2020) A combined 3D printing/CNC micro-milling method to fabricate a large-scale microfluidic device with the small size 3D architectures: an application for tumor spheroid production. Sci. Rep 10, 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Knowlton S et al. (2016) Towards single-step biofabrication of organs on a chip via 3D printing. Trends Biotechnol. 34, 685–688 [DOI] [PubMed] [Google Scholar]
- 71.Datta P et al. (2020) 3D bioprinting for reconstituting the cancer microenvironment. npj Precis. Oncol 4, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sun H et al. (2020) Combining additive manufacturing with microfluidics: an emerging method for developing novel organs-on-chips. Curr. Opin. Chem. Eng 28, 1–9 [Google Scholar]
- 73.Lee H and Cho DW (2016) One-step fabrication of an organ-on-a-chip with spatial heterogeneity using a 3D bioprinting technology. Lab Chip 16, 2618–2625 [DOI] [PubMed] [Google Scholar]
- 74.Park W et al. (2021) 3D Cell-Printed Hypoxic Cancer-on-a-Chip for Recapitulating Pathologic Progression of Solid Cancer. J. Vis. Exp. Jove [DOI] [PubMed] [Google Scholar]
- 75.Gokaltun A et al. (2017) Recent advances in nonbiofouling PDMS surface modification strategies applicable to microfluidic technology. Technology 5, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Grant J et al. (2021) Simulating drug concentrations in PDMS microfluidic organ chips. Lab Chip [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pourmand A et al. (2018) Fabrication of whole-thermoplastic normally closed microvalve, micro check valve, and micropump. Sensors Actuators B Chem. 262, 625–636 [Google Scholar]
- 78.Ali S et al. (2018) Rapid prototyping of whole-thermoplastic microfluidics with built-in microvalves using laser ablation and thermal fusion bonding. Sensors Actuators B Chem. 255, 100–109 [Google Scholar]
- 79.Campbell SB et al. (2021) Beyond Polydimethylsiloxane: Alternative Materials for Fabrication of Organ-on-a-Chip Devices and Microphysiological Systems. ACS Biomater. Sci. Eng 7, 2880–2899 [DOI] [PubMed] [Google Scholar]
- 80.Nie J et al. (2020) Hydrogels: The Next Generation Body Materials for Microfluidic Chips? Small 2003797, 1–26 [DOI] [PubMed] [Google Scholar]
- 81.Li Y et al. (2019) 3D bioprinting of hepatoma cells and application with microfluidics for pharmacodynamic test of Metuzumab. Biofabrication 11, 34102. [DOI] [PubMed] [Google Scholar]
- 82.Asano Y et al. (2014) Ultrastructure of blood and lymphatic vascular networks in three-dimensional cultured tissues fabricated by extracellular matrix nanofilm-based cell accumulation technique. Microscopy 63, 219–226 [DOI] [PubMed] [Google Scholar]
- 83.Nishiguchi A et al. (2018) In vitro 3D blood/lymph-vascularized human stromal tissues for preclinical assays of cancer metastasis. Biomaterials 179, 144–155 [DOI] [PubMed] [Google Scholar]
- 84.Liu T et al. (2021) Investigating lymphangiogenesis in a sacrificially bioprinted volumetric model of breast tumor tissue. Methods 190, 72–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yi HG et al. (2019) A bioprinted human-glioblastoma-on-a-chip for the identification of patient-specific responses to chemoradiotherapy. Nat. Biomed. Eng 3, 509–519 [DOI] [PubMed] [Google Scholar]
- 86.Horowitz LF et al. (2020) Multiplexed drug testing of tumor slices using a microfluidic platform. NPJ Precis. Oncol 4, 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chang TC et al. (2014) Parallel microfluidic chemosensitivity testing on individual slice cultures. Lab Chip 14, 4540–4551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Aleman J et al. (2021) Microfluidic integration of regeneratable electrochemical affinity-based biosensors for continual monitoring of organ-on-a-chip devices. Nat. Protoc [DOI] [PubMed] [Google Scholar]
- 89.Maulana TI et al. (2021) Immunocompetent Cancer-on-Chip models to assess immuno-oncology therapy. Adv. Drug Deliv. Rev [DOI] [PubMed] [Google Scholar]
- 90.Choi Y et al. (2018) Studying cancer immunotherapy using patient-derived xenografts (PDXs) in humanized mice. Exp. Mol. Med 50, 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Barney LE et al. (2016) The predictive link between matrix and metastasis. Curr. Opin. Chem. Eng 11, 85–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Urosevic J and Gomis RR (2018) Organ-specific metastases. Nat. Biomed. Eng 2, 347–348 [DOI] [PubMed] [Google Scholar]
- 93.Kühlbach C et al. (2018) A Microfluidic System for the Investigation of Tumor Cell Extravasation. Bioengineering 5, 1–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Duinen V. Van et al. (2015) Microfluidic 3D cell culture : from tools to tissue models. Curr. Opin. Biotechnol 35, 118–126 [DOI] [PubMed] [Google Scholar]
- 95.Surendran V et al. (2021) A novel tumor-immune microenvironment (TIME)-on-Chip mimics three dimensional neutrophil-tumor dynamics and neutrophil extracellalar traps (NETs)-mediated collective tumor invasion. Biofabrication [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cui H et al. (2020) Engineering a novel 3D printed vascularized tissue model for investigating breast cancer metastasis to bone. Adv. Healthc. Mater 9, 1900924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Maharjan S et al. (2020) 3D Immunocompetent Organ-on-a-Chip Models. Small Methods 4, 2000235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang M et al. (2017) Role of tumor microenvironment in tumorigenesis. J. Cancer 8, 761–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schnittert J et al. (2019) Targeting Pancreatic Stellate Cells in Cancer. Trends in Cancer 5, 128–142 [DOI] [PubMed] [Google Scholar]
- 100.Bu L et al. (2019) Biological heterogeneity and versatility of cancer-associated fibroblasts in the tumor microenvironment. Oncogene 38, 4887–4901 [DOI] [PubMed] [Google Scholar]
- 101.Sun Q et al. (2018) The impact of cancer-associated fibroblasts on major hallmarks of pancreatic cancer. Theranostics 8, 5072–5087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sazeides C and Le A (2018) Metabolic Relationship between Cancer- - Associated Fibroblasts and Cancer Cells. In Advances in Experimental Medicine and Biology pp. 149–165 [DOI] [PubMed] [Google Scholar]
- 103.Erdogan B and Webb DJ (2017) Cancer-associated fibroblasts modulate growth factor signaling and extracellular matrix remodeling to regulate tumor metastasis. Biochem Soc Trans 45, 229–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Torphy RJ et al. (2020) Understanding the immune landscape and tumor microenvironment of pancreatic cancer to improve immunotherapy. Mol. Carcinog 59, 775–782 [DOI] [PubMed] [Google Scholar]
- 105.De Jaeghere EA et al. (2019) Fibroblasts Fuel Immune Escape in the Tumor Microenvironment. Trends in Cancer 5, 704–723 [DOI] [PubMed] [Google Scholar]
- 106.Xu S et al. (2019) The role of collagen in cancer: from bench to bedside. J. Transl. Med 17, 309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pereira BA et al. (2019) CAF Subpopulations: A New Reservoir of Stromal Targets in Pancreatic Cancer. Trends in Cancer 5, 724–741 [DOI] [PubMed] [Google Scholar]
- 108.Pradhan S et al. (2018) A Microvascularized Tumor-mimetic Platform for Assessing Anti-cancer Drug Efficacy. Sci. Rep 8, 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hachey SJ and Hughes CCW (2018) Applications of Tumor Chip Technology. Lab Chip 18, 2893–2912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ma X et al. (2018) 3D bioprinting of functional tissue models for personalized drug screening and in vitro disease modeling. Adv. Drug Deliv. Rev 132, 235–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hermida MA et al. (2020) Three dimensional in vitro models of cancer: Bioprinting multilineage glioblastoma models. Adv. Biol. Regul 75, 100658. [DOI] [PubMed] [Google Scholar]
- 112.Mao S et al. (2020) Bioprinting of in vitro tumor models for personalized cancer treatment: a review. Biofabrication 12, 42001. [DOI] [PubMed] [Google Scholar]
- 113.Datta P et al. (2020) 3D bioprinting for reconstituting the cancer microenvironment. npj Precis. Oncol 4, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Albrecht I and Christofori G (2011) Molecular mechanisms of lymphangiogenesis in development and cancer. Int. J. Dev. Biol 55, 483–494 [DOI] [PubMed] [Google Scholar]
- 115.Groll J et al. (2016) Biofabrication: reappraising the definition of an evolving field. Biofabrication 8, 13001. [DOI] [PubMed] [Google Scholar]
- 116.Mota C et al. (2020) Bioprinting: from tissue and organ development to in vitro models. Chem. Rev 120, 10547–10607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Heinrich MA et al. (2019) 3D bioprinting: from benches to translational applications. Small 15, 1805510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Moroni L et al. (2018) Biofabrication strategies for 3D in vitro models and regenerative medicine. Nat. Rev. Mater 3, 21–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kang Y et al. (2020) 3D Bioprinting of Tumor Models for Cancer Research. ACS Appl. Bio Mater 3, 5552–5573 [DOI] [PubMed] [Google Scholar]
- 120.Sánchez-Salazar MG et al. (2021) Advances in 3D bioprinting for the biofabrication of tumor models. Bioprinting 21, e00120 [Google Scholar]
- 121.Dzobo K et al. (2019) Recent trends in decellularized extracellular matrix bioinks for 3D printing: An updated review. Int. J. Mol. Sci 20, 4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Muir VG and Burdick JA (2020) Chemically Modified Biopolymers for the Formation of Biomedical Hydrogels. Chem. Rev [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Aldana AA et al. (2021) Trends in Double Networks as Bioprintable and Injectable Hydrogel Scaffolds for Tissue Regeneration. ACS Biomater. Sci. Eng [DOI] [PubMed] [Google Scholar]
- 124.Lee M et al. (2020) Nanocomposite bioink exploits dynamic covalent bonds between nanoparticles and polysaccharides for precision bioprinting. Biofabrication 12, 25025. [DOI] [PubMed] [Google Scholar]
- 125.Lyu J et al. (2020) Instant Gelation System as Self-Healable and Printable 3D Cell Culture Bioink Based on Dynamic Covalent Chemistry. ACS Appl. Mater. Interfaces 12, 38918–38924 [DOI] [PubMed] [Google Scholar]
- 126.McCormack A et al. (2020) 3D printing in suspension baths: keeping the promises of bioprinting afloat. Trends Biotechnol. 38, 584–593 [DOI] [PubMed] [Google Scholar]
- 127.Mirdamadi E et al. (2020) FRESH 3D Bioprinting a Full-Size Model of the Human Heart. ACS Biomater. Sci. Eng 6, 6453–6459 [DOI] [PubMed] [Google Scholar]
- 128.Hinton TJ et al. (2015) Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci. Adv 1, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ayuso JM et al. (2019) Evaluating natural killer cell cytotoxicity against solid tumors using a microfluidic model. Oncoimmunology 8, 1553477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Aref AR et al. (2018) 3D Microfluidic Ex Vivo Culture of Organotypic Tumor Spheroids to Model Immune Checkpoint Blockade Amir. Lab Chip 18, 3129–3143 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


