Abstract
BACKGROUND:
Current guidelines recommend restaging with MRI after neoadjuvant therapy for rectal cancer, but the accuracy of restaging MRI in estimating circumferential margin involvement requires additional clarification.
OBJECTIVE:
Measure the accuracy of circumferential resection margin assessment by MRI after neoadjuvant therapy and identify characteristics associated with accuracy.
DESIGN:
MRI data were retrospectively analyzed for concordance with the findings of whole-mount pathology analysis of the corresponding surgical specimens. Univariate and multivariate logistic regression analyses were performed to identify characteristics associated with accuracy.
SETTING
Comprehensive cancer center.
PATIENTS
Consecutive patients who underwent total mesorectal excision for rectal cancer between January 2018 and February 2020 after receiving neoadjuvant therapy and undergoing restaging with MRI.
MAIN OUTCOME MEASURES
Accuracy, sensitivity, specificity, and positive and negative predictive values for categorizing the circumferential resection margin as threatened; Mean and paired mean differences in proximity of the margin.
RESULTS:
Of the 94 patients included in the analysis, 39 (41%) had a threatened circumferential resection margin according to MRI at restaging, but only 17 (18%) had a threatened margin based on pathology. The accuracy of MRI in identifying a threatened margin was 63.8%, with margin proximity overestimated by 0.4 cm on average. In multivariate logistic regression, anterior location of the margin and tumor proximity to the anal verge were independently associated with reduced MRI accuracy.
LIMITATIONS
Retrospective design, single institution.
CONCLUSIONS:
The knowledge that MRI-based restaging after neoadjuvant therapy overestimates circumferential margin proximity may render some surgical radicality unnecessary and thereby help avoid the associated morbidity. With the recognition that MRI-based assessment of margin proximity may not be reliable for an anterior margin and for distal tumors, radiologists may want to use greater caution in interpreting images of tumors with these characteristics and to acknowledge the uncertainty in their reports. See Video Abstract at https://links.lww.com/DCR/B814.
Keywords: MRI, Neoadjuvant treatment, Rectal cancer, Resection margins, Whole mount pathology
Abstract
ANTECEDENTES:
Las pautas actuales recomiendan la re-estadificación por medio de la resonancia magnética luego de terapia neoadyuvante en los casos de cáncer de recto, pero la precisión de la reevaluación con la IRM para estimar el grado de implicación del margen circunferencial requiere aclaraciones adicionales.
OBJETIVO:
Medir el grado de exactitud en la evaluación del margen de resección circunferencial mediante resonancia magnética después de la terapia neoadyuvante e identificar las características asociadas con la precisión.
DISEÑO:
Se analizaron retrospectivamente los datos de resonancia magnética para determinar la concordancia entre los hallazgos del análisis de la pieza de anatamopatología y las muestras quirúrgicas correspondientes. Se realizó el análisis de regresión logística univariada y multivariada para identificar las características asociadas con la exactitud.
AJUSTE:
Centro oncológico integral.
PACIENTES:
Todos aquellos que se sometieron consecutivamente a una excisión total del mesorrecto por cáncer rectal entre Enero 2018 y Febrero 2020 luego de recibir terapia neoadyuvante y someterse a una re-estadificación por imágenes de resonancia magnética (IRM).
PRINCIPALES MEDIDAS DE RESULTADO:
La exactitud, la sensibilidad y especificidad; los valores predictivos positivos y negativos para categorizar el margen de resección circunferencial como amenazado; la diferencia media y las medias pareadas de proximidad a los margenes.
RESULTADOS:
De los 94 pacientes incluidos en el análisis, 39 (41%) tenían un margen de resección circunferencial amenazado según la resonancia magnética en la re-estadificación, pero solo 17 (18%) tenían un margen amenazado basado en la patología. La precisión de la resonancia magnética para identificar un margen amenazado fue del 63,8%, con la proximidad del margen sobreestimada en 0,4 cm en promedio. En la regresión logística multivariada, la ubicación anterior de los bordes de resección y la proximidad del tumor al margen anal se asociaron de forma independiente con la reducción en la precisión de la resonancia magnética.
LIMITACIONES:
Diseño retrospectivo en una institución única.
CONCLUSIONES:
El saber que la re-estadificación basada en la IRM, luego de terapia neoadyuvante sobreestima la proximidad de la lesión a los márgenes circunferenciales, hace innecesaria cierta radicalidad quirúrgica complementaria, lo que ayuda a evitar morbilidad asociada. Reconociendo que la evaluación de proximidad de los márgenes de resección basada en la resonancia magnética, no puede ser confiable en casos de márgenes anteriores y en casos de tumores distales. Los radiólogos recomiendan tener más precaución en la interpretación de imágenes de tumores con estas características y reconocen cierto desasosiego en sus informes.
Consulte Video Resumen en https://links.lww.com/DCR/B814. (Traducción—Dr. Xavier Delgadillo)
INTRODUCTION
The distance between tumor tissue and the circumferential resection margin (CRM) following total mesorectal excision of rectal cancer bears important prognostic implications. Distance of ≤0.1 cm is usually categorized as CRM involvement, and distance of ≤0.2 cm is usually categorized as threatened CRM. Both are associated with higher risk of local recurrence and with poor survival.1–3
Imaging plays a central role in the management of rectal cancer. Baseline rectal MRI is the standard modality for staging, prognostication, and identification of patients who would benefit from neoadjuvant therapy (NAT).4 At restaging after NAT, MRI is increasingly utilized to guide treatment decisions, including determining which patients are appropriate candidates for a potentially rectum-preserving management strategy, which patients can safely undergo R0 resection with standard total mesorectal excision, and which patients would benefit from additional intensification therapy. Current guidelines recommend restaging with MRI for all rectal cancer patients receiving NAT.5 Understanding the limitations of MRI for restaging is important for proper management of rectal cancer by multidisciplinary teams and by rectal cancer surgeons in particular.
While baseline MRI is reasonably accurate in characterizing the spatial relationship of the tumor to the CRM,6 the accuracy of post-NAT MRI in predicting CRM involvement (ymrCRM) is less clear. Assessing the degree of extramural tumor extension following NAT is challenging, since it can be difficult to distinguish viable tumor cells from tumor fibrosis and/or acellular mucin pools.7 Incorrectly interpreting the latter two as viable tumor can lead to overestimation of residual tumor and underestimation of response to NAT.8
Several studies found that the positive predictive value of ymrCRM is low, suggesting overdiagnosis of CRM involvement.9–13 Data from the MERCURY (Magnetic Resonance Imaging in Rectal Cancer European Equivalence Study) trial showed that CRM involvement was associated with local recurrence. However, the hazard ratio for local recurrence based on an involved CRM in pathology specimens (ypCRM) was nearly twice as high as the hazard ratio for local recurrence based on ymrCRM (8.80 vs. 4.25), also suggesting overdiagnosis of CRM involvement.14 Data on factors associated with the accuracy of ymrCRM are extremely limited.
While pathological examination of rectal cancer specimens has historically focused on the tumor and the adjacent area,15 the fixing, sectioning, and dyeing of the entire specimen (whole mount) can help identify pathology in other areas. The whole-mount approach also facilitates comparison with axial images. For these reasons comparison of MRI with whole mount pathology can be more informative than comparison with standard pathology. Most studies on the accuracy of ymrCRM did not use whole-mount pathology as a reference standard,9,11,12,16–19 and a study that did use whole-mount pathology had a relatively small sample size.13
The aim of this study was to measure the accuracy of ymrCRM and identify factors associated with accuracy. Whole-mount ypCRM was used as a reference standard. Our hypothesis was that similar to previous reports utilizing standard pathology as a reference, accuracy would be low, but that by using whole mount pathology specimens, we would be able to clearly identify predictors of accuracy.
PATIENTS AND METHODS
Patients
The study population consisted of consecutive patients at Memorial Sloan Kettering Cancer Center who underwent total mesorectal excision for rectal cancer (cT ≥ 3, and/or cN ≥ 1, and/or cM ≥ 1) between January 1, 2018, and March 1, 2020, after completing NAT and undergoing restaging with MRI. Patients for whom the results of whole-mount pathology analysis were not available were excluded. Data on patient, treatment, and tumor characteristics were collected with approval from the institutional review board.
MRI
The 3T MRI restaging protocol included T2-weighted fast spin echo sequences in the sagittal, coronal, and axial planes; axial T1-weighted images; and an axial diffusion-weighted image sequence. The axial T2-weighted and diffusion-weighted image sequences were performed in identical planes perpendicular to the rectal lumen at the site of the tumor (oblique axial plane). The CRM was measured, and the location of the shortest CRM was determined as part of routine post-NAT reporting at our institution. Each MRI scan was evaluated by one of 6 gastrointestinal radiologists with at least 5 years of experience as a board-certified attending radiologist.
Histopathology
CRMs were painted in ink before dissection. Samples were fixed in formalin and stained with hematoxylin and eosin. Specimens were then sectioned at 4-mm intervals, processed into whole-mount sections, and numbered proximal to distal. Histopathological evaluation including CRM measurement, following the guidelines of the American College of Pathologists, was performed by gastrointestinal oncology pathologists with at least 10 years of experience as a board-certified attending pathologist who were blinded in both cases to the post-NAT MRI results.
CRM
CRM proximity was defined as the shortest distance from the most radial point of the tumor to the mesorectal fascia on MRI, and the shortest distance from the most radial point of the tumor to the surgical margin on histopathology. Distance of ≤0.2 cm was categorized as threatened CRM. For patients with no extramural extension evident on post-NAT MRI and for patients with a pathologic complete response (no tumor) on pathology no CRM distance was reported, but the CRM was categorized as not threatened. The location of the shortest distance to the mesorectal fascia on post-NAT MRI was categorized (starting at 10 o’clock and proceeding clockwise) as right anterolateral, anterior midline, left anterolateral, left lateral, left posterolateral, posterior midline, right posterolateral, or right lateral. A CRM in one of the first three locations was categorized as an anterior CRM.
For determination of a threatened CRM on post-NAT MRI, diagnostic accuracy parameters—overall accuracy, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV)—were calculated by measuring concordance with ypCRM. These parameters were calculated for the cohort as a whole and for factors associated with accurate CRM determination. The mean difference and the paired mean difference in CRM distance for both modalities were also calculated.
Statistical Analysis
Categorical variables are presented as frequency (percent) and continuous variables are presented as mean ± standard deviation. Univariate analysis was performed to identify differences between groups (using the chi-square test or t-test) and to identify factors associated with ymrCRM accuracy. Factors with a statistical significance of p < 0.1 in univariate analysis were included in a multivariate logistic regression analysis. Statistical calculations were performed with SPSS software version 20 (IBM).
RESULTS
Ninety-four patients met the inclusion criteria (Fig. 1). Patient, tumor, and treatment characteristics are listed in Table 1. Mean age was 57.6 ± 14.1 years; 38 (40%) of the 94 patients were women. Mean distance of the tumor from the anal verge on post-NAT MRI was 7.66 ± 3.14 cm. The majority of patients (82%) received both chemoradiotherapy and systemic chemotherapy as part of NAT. Fourteen patients (15%) underwent extended resection that was wider than standard total mesorectal excision.
FIGURE 1.
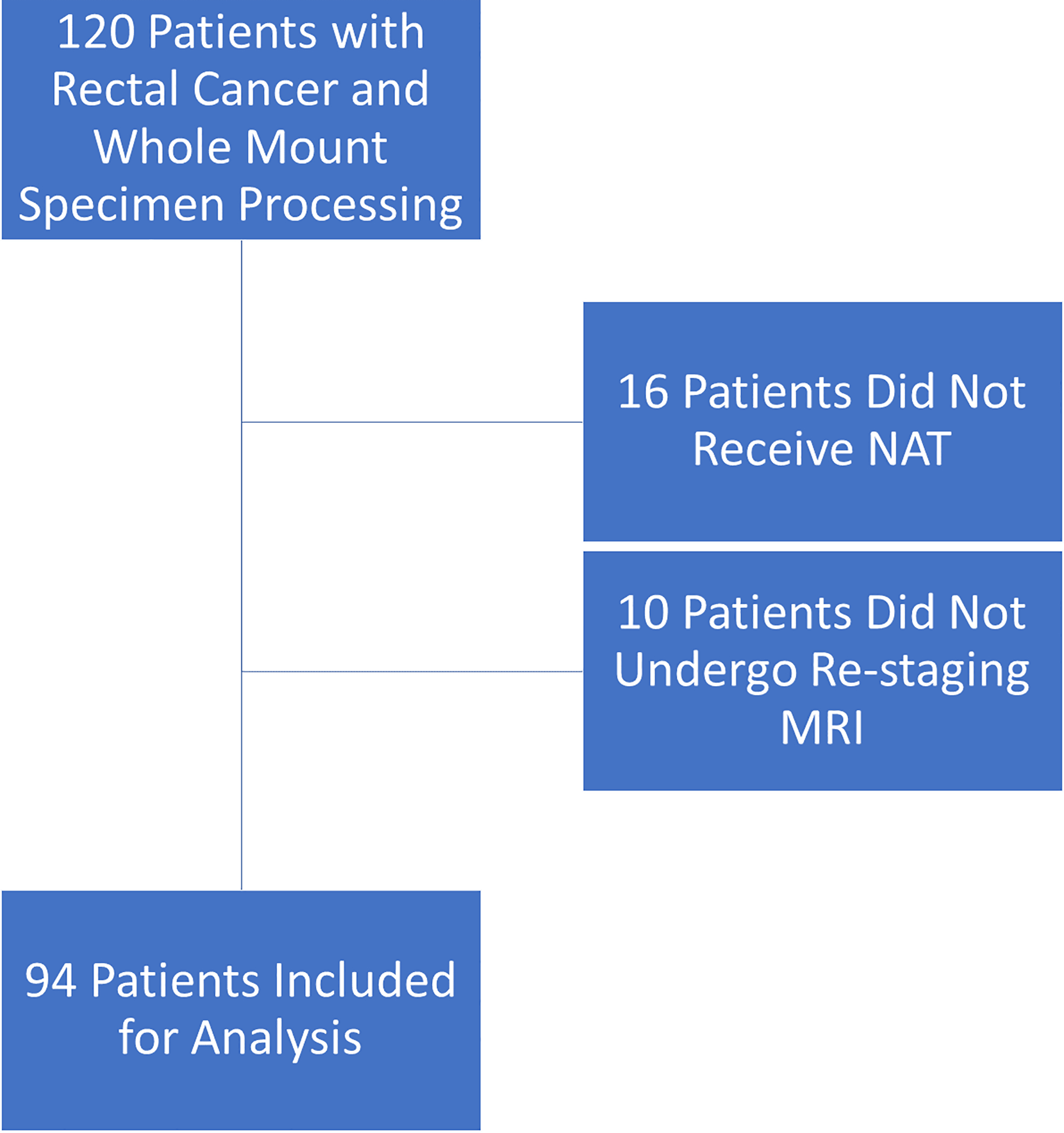
Inclusion and exclusion of patients.
TABLE 1.
Baseline patient, tumor, and treatment characteristics
| Characteristic | No. (%) of patients |
|---|---|
| Age, mean ± SD | 57.6 ± 14.1 yr |
| Female | 38 (40.4) |
| Distance from anal verge, mean ± SD | 7.66 ± 3.14 cm |
| Time from NAT to MRI, mean ± SD | 108.4 ± 142.8 days |
| Time from MRI to surgery, mean ± SD | 43.9 ± 57.8 days |
| Anterior CRM | 42 (44.7) |
| cT category | |
| 1/2 | 5 (5.3) |
| 3 | 73 (77.7) |
| 4 | 15 (16) |
| Missinga | 1 (1.1) |
| cN category | |
| 0 | 10 (10.6) |
| ≥1 | 72 (76.6) |
| x | 12 (12.7) |
| cM category | |
| 0 | 81 (86.2) |
| 1 | 13 (13.8) |
| Clinical stage | |
| II | 19 (20.2) |
| III | 62 (66.0) |
| IV | 13 (13.8) |
| Surgery | |
| Low anterior resection | 64 (68.1) |
| Abdominoperineal resection | 30 (31.9) |
| Neoadjuvant therapy | |
| Systemic chemotherapy only | 14 (14.9) |
| Chemoradiotherapy only | 3 (3.2) |
| Both | 77 (81.9) |
One patient with clear nodal disease identified by CT did not undergo MRI. NAT, neoadjuvant treatment; CRM, circumferential resection margins
CRM Distance
For the 80 patients with ymrCRM data available, the mean distance to CRM was 0.52 ± 0.66 cm. For the remaining 14 patients, who had no evidence of extramural extension and therefore no ymrCRM distance reported, the CRM was categorized as not threatened. For 12 of the 14 patients, no extramural extension was seen on histopathology, and ymrCRM categorization matched ypCRM categorization in 13 patients (93%); the 14th patient had a positive CRM (0 cm) on histopathology.
For the 87 patients with ypCRM data available, the mean distance to CRM was 1.01 ± 0.81 cm. The remaining 7 patients had a pathologic complete response and therefore no ypCRM measurement. ymrCRM and ypCRM differed significantly in mean distance to CRM (0.52 ± 0.66 and 1.01 ± 0.81 cm, respectively; p < 0.001). For the 73 patients with both ymrCRM and ypCRM data available, the paired mean CRM distance difference was 0.38 ± 0.84 cm shorter on ymrCRM (p < 0.001).
After exclusion of the 14 patients that underwent a wider than standard resection, ymrCRM and ypCRM still differed significantly in mean distance to CRM (0.55 ± 0.69 Cm and 1.09 ± 0.82 cm, respectively, p<0.001), and the paired mean difference was 0.44 ± 0.88 cm shorter on ymrCRM (p< 0.001, N=64).
CRM Categorization
CRM categorization by both ymrCRM and whole-mount histopathology (Fig. 2) was available for all 94 patients. Based on ymrCRM, 39 patients (42%) had a threatened CRM, whereas only 17 patients (18%) had a threatened CRM based on whole-mount histopathology (Table 2). The overall accuracy, sensitivity, and specificity of ymrCRM categorization were 63.8%, 64.7%, and 63.6%, respectively. PPV and NPV were 28.2% and 89.0%, respectively. For 28 patients (30%) ymrCRM categorization corresponded to overstaging, and for 6 patients (6%) it corresponded to understaging.
FIGURE 2.
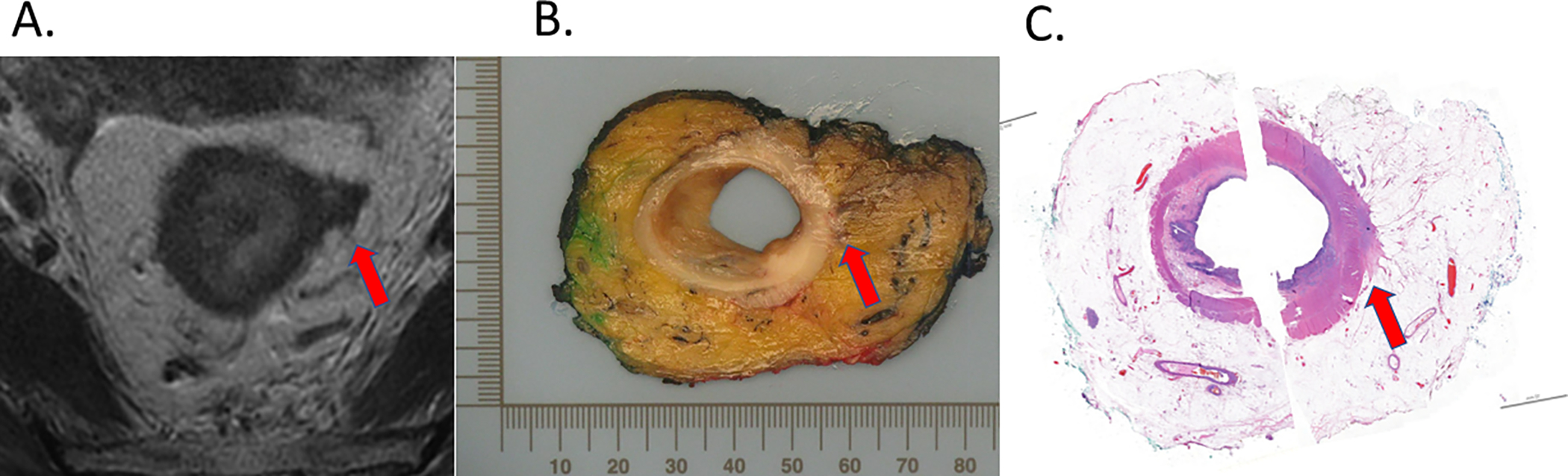
Post-NAT MRI (A), post-surgery whole-mount gross pathology (B), and post-surgery whole-mount histopathology (C). Maximal extramural extension is indicated (arrow).
TABLE 2.
ymrCRM categorization of the CRM as threatened (≤0.2 cm) compared with ypCRM categorization
| ymrCRM | No. of specimens in which ypCRM identified: |
Total | |
|---|---|---|---|
| Threatened CRM | Nonthreatened CRM | ||
| Threatened CRM | 11 | 28 | 39 |
| Nonthreatened CRM | 6 | 49 | 55 |
| Total | 17 | 77 | 94 |
In univariable analysis, the only baseline characteristic significantly associated with incorrect ymrCRM categorization was anterior CRM location (Table 3). In logistic regression analysis of the two variables with odds ratios at p < 0.1 on univariable analysis, both anterior CRM location and distance ≤ 7 cm from the anal verge were independent predictors of incorrect ymrCRM categorization (Table 3). Similar to previous publications, distance of ≤ 7 cm from the anal verge was chosen as the cutoff for distal rectal cancer.20
TABLE 3.
Analysis of factors potentially associated with accurate ymrCRM categorization
| Characteristic | Univariate analysis |
Multivariate logistic regression |
||
|---|---|---|---|---|
| Odds ratio (95% CI) | p | Odds ratio (95% CI) | p | |
| Age < 57 yr | 1.38 (0.59–320) | 0.459 | ||
| Female | 1.15 (0.49–2.73) | 0.745 | ||
| Distance from anal verge ≤ 7 cm | 0.45 (0.18–1.08) | 0.071 | 0.38 (0.15–0.96) | 0.040 |
| Anterior CRM | 0.33 (0.14–0.80) | 0.012 | 0.34 (0.13–0.85) | 0.021 |
| cT4 | 0.41 (0.14–1.27) | 0.115 | ||
| cN ≥ 1 | 0.87 (0.24–3.12) | 0.827 | ||
| cM1 | 1.34 (0.38–4.67) | 0.662 | ||
| Abdominoperineal resection | 0.52 (0.21–1.27) | 0.147 | ||
| Combination NATa | 0.69 (0.22–2.16) | 0.522 | ||
Chemoradiotherapy and systemic chemotherapy.
Anterior CRM
Forty-two patients (45%) had an anterior CRM. Nineteen (45%) of the 42 patients had a threatened CRM based on ymrCRM, but only 6 (14%) of the 42 patients had a threatened CRM based on whole-mount ypCRM. The overall accuracy, sensitivity, specificity, PPV, and NPV of ymrCRM categorization for patients with an anterior CRM are listed in Table 4. ymrCRM categorization corresponded to overstaging in 19 (45%) of the 42 patients and understaging in 2 patients (5%).
TABLE 4.
Accuracy of ymrCRM categorization
| Group (n) | % |
No. (%) of patients |
|||||
|---|---|---|---|---|---|---|---|
| Accuracy | Sensitivity | Specificity | PPV | NPV | Overstaging | Understaging | |
| Full cohort (94) | 63.8 | 64.7 | 63.6 | 28.2 | 89.0 | 28 (29.8) | 6 (6.4) |
| Anterior CRM (42) | 50.0 | 66.6 | 47.2 | 17.3 | 89.5 | 19 (45.2) | 2 (4.8) |
| Distal tumora (35) | 51.4 | 58.3 | 47.8 | 36.8 | 68.8 | 12 (34.3) | 5 (14.3) |
≤7 cm from the anal verge.
For the 37 patients (88% of 42) with both ymrCRM distance and ypCRM distance available, the paired mean CRM distance was 0.51 ± 0.83 cm shorter on ymrCRM than on ypCRM (p = 0.001). Of the 52 patients with a nonanterior CRM, 36 (69%) had both ymrCRM distance and ypCRM distance available. For these patients, the paired mean distance to the CRM was not meaningfully different between the two modalities: 0.25 ± 0.83 cm shorter on ymrCRM in comparison to ypCRM (p = 0.084).
Distal Tumor
Five (5%) of the 94 patients had no data on distance of the tumor from the anal verge at restaging after NAT; three of the five had a complete response, and two had poor-quality images of the sagittal view. Of the 35 patients (37% of 94) with a tumor ≤ 7 cm from the anal verge, 19 (54%) had a threatened CRM according to ymrCRM, but only 7 (20%) had a threatened CRM according to ypCRM. The overall accuracy, sensitivity, specificity, PPV, and NPV of ymrCRM categorization for patients with a tumor ≤ 7 cm from the anal verge are listed in Table 4. ymrCRM categorization corresponded to overstaging in 12 (34%) of the 35 patients and understaging in 5 patients (14%).
For the 29 patients (83% of 35) with both ymrCRM and ypCRM distance available, the paired mean CRM distance was 0.40 ± 0.66 cm closer on post treatment MRI than on pathology, (p = 0.003). Of the 54 patients with a tumor > 7cm from the anal verge, 43 (80%) had both ymrCRM distance and ypCRM distance available. For these patients, the paired mean difference was 0.37±0.96 Cm closer to the CRM on restaging MRI than on histopathology, (p=0.016).
DISCUSSION
The findings of our study—which is the largest to date on comparing ymrCRM and whole-mount ypCRM—indicate that post-NAT MRI overcalls the CRM as threatened in about 30% of patients (confirming previous reports10,12,17) and overestimates CRM proximity by 0.4 cm on average. The 63.8% accuracy of ymrCRM in identifying a threatened CRM was lower than the values reported in most9,12,13,19 (but not all17) previous studies. This difference may be explained partly by our focus on a threatened CRM (≤0.2 cm) rather than an involved CRM (≤0.1 cm), which was based on our reasoning that the category of threatened CRM includes more patients and still holds important prognostic information. Accuracy data were slightly different for involved CRMs (Supplemental Table 1). Measures of diagnostic accuracy are affected by prevalence in the study population, and the higher accuracies in previous studies may also be due to higher prevalence of threatened or involved CRMs.18,19,21
For the majority of patients in our study, NAT included both chemoradiotherapy and systemic chemotherapy, a treatment modality not used in previous studies on ymrCRM. Both the longer treatment duration and the relative aggressiveness of the NAT may be associated with development of severe fibrosis and desmoplasia, which may complicate ymrCRM. NAT type was not associated with diagnostic accuracy, but our analysis may have been underpowered, since only 3 patients received chemoradiotherapy alone and only 14 received systemic chemotherapy alone.
Both an anterior CRM and tumor proximity (≤7 cm) to the anal verge were associated with a decrease in ymrCRM accuracy but for different reasons. The sensitivity and NPV of ymrCRM in patients with an anterior CRM were similar to those for the full entire cohort, but specificity and PPV were lower (i.e., threatened CRMs were overcalled) and CRM proximity was consistently overestimated (by approximately 0.5 cm). This suggests that overestimation of CRM proximity can lead to overdiagnosis in patients with an anterior CRM. In patients with distal tumors, all accuracy parameters except PPV were lower than the accuracy parameters for the full cohort, and the difference between the rates of overdiagnosis and underdiagnosis was smaller than in patients with an anterior CRM. The surrounding anatomy of distal tumors (e.g., the funneling of the mesorectum and the proximity of the pelvic floor muscles) likely makes ymrCRM interpretation more difficult. The finding that an anterior CRM and distal tumor location are associated with lower ymrCRM accuracy is consistent with the data from studies by Kim et al.12 and Peschaud et al.,21 which did not use whole-mount pathology analysis.
The potential limitations of our study include retrospective design and a study population from a single, high-volume, specialized cancer center, which may limit generalizability. The fact that the ymrCRM and ypCRM assessments were performed as part of standard practice at our institution and not specifically for this study may have negatively impacted diagnostic accuracy (but may have also increased generalizability). Each MRI scan was interpreted by only one reader, and thus no data on interobserver agreement were available. Although ypCRM data may be affected by tissue shrinkage associated with paraffin fixation and devascularization,22 tissue shrinkage would decrease CRM proximity, which would only strengthen our finding of overdiagnosis by ymrCRM. Another potential factor is that rectal pressure gradient is approximately 80 mmHg in healthy individuals23 but is equal to atmospheric pressure in rectal surgical specimens. Although in vivo resting pressure is lower in patients undergoing NAT,24 it is possible that this pressure compressed the mesorectum and contributed to the overestimation of CRM proximity. Additionally, distance to CRM was measured to the mesorectal fascia on MRI but measured to the surgical margin on histopathology. It is possible that in cases of extended-total mesorectal excision, such as resection anterior to Denonvilliers’ fascia, a wider resection may have contributed to differences in CRM proximity. However, exclusion of patients that underwent wider resection did not change the study’s findings.
Notwithstanding the above limitations, our study provides strong evidence that ymrCRM overestimates CRM proximity, particularly for an anterior CRM and for distal tumors. ymrCRM is more accurate in categorizing the CRM as not threatened and in identifying the absence of extramural tumor extension. ymrCRM is reasonably accurate for nondistal tumors and in identifying a nonanterior threatened CRM.
ymrCRM is crucial for surgical planning. It helps surgeons determine whether total mesorectal excision or extended surgery should be performed and whether intraoperative radiotherapy should be administered. The knowledge that ymrCRM overestimates CRM proximity may render some surgical radicality unnecessary and thereby help avoid the associated morbidity. Nevertheless, in the United States rates of positive CRM remain high at approximately 16%,25 and surgeons should not hesitate to undertake a radical surgical approach if it is thought to benefit an individual patient. With the recognition that ymrCRM assessment of CRM distance may not be reliable for an anterior CRM and for distal tumors, radiologists may want to use greater caution in interpreting images of tumors with these characteristics and to acknowledge the uncertainty in their reports. Digitally capturing corresponding axial slices for MRI scans and whole-mount specimens may help elucidate the accuracy of post-NAT MRI in characterizing other features of rectal cancer response to treatment.
Supplementary Material
ACKNOWLEDGMENT
We thank Arthur Gelmis, BS, Department of Surgery, Memorial Sloan Kettering Cancer Center, for editing the manuscript.
Funding/Support:
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748. Dr. Yuval was supported in part by the NCI grant T32 CA009501.
Footnotes
Financial Disclosures: Dr. Garcia-Aguilar has received honoraria from Medtronic, Johnson & Johnson, and Intuitive Surgical.
Selected as a Podium Original Contribution (Presentation SP22) for the Annual Scientific Meeting of the American Society of Colon & Rectal Surgeons, San Diego, CA, April 24 to 28, 2021.
REFERENCES
- 1.Birbeck KF, Macklin CP, Tiffin NJ, et al. Rates of circumferential resection margin involvement vary between surgeons and predict outcomes in rectal cancer surgery. Ann Surg. 2002;235:449–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gosens MJ, Klaassen RA, Tan-Go I, et al. Circumferential margin involvement is the crucial prognostic factor after multimodality treatment in patients with locally advanced rectal carcinoma. Clin Cancer Res. 2007;13:6617–6623. [DOI] [PubMed] [Google Scholar]
- 3.Quirke P, Durdey P, Dixon MF, Williams NS. Local recurrence of rectal adenocarcinoma due to inadequate surgical resection. Histopathological study of lateral tumour spread and surgical excision. Lancet. 1986;2:996–999. [DOI] [PubMed] [Google Scholar]
- 4.Tudyka V, Blomqvist L, Beets-Tan RG, et al. EURECCA consensus conference highlights about colon & rectal cancer multidisciplinary management: the radiology experts review. Eur J Surg Oncol. 2014;40:469–475. [DOI] [PubMed] [Google Scholar]
- 5.Beets-Tan RGH, Lambregts DMJ, Maas M, et al. Magnetic resonance imaging for clinical management of rectal cancer: Updated recommendations from the 2016 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur Radiol. 2018;28:1465–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beets-Tan RG, Beets GL, Vliegen RF, et al. Accuracy of magnetic resonance imaging in prediction of tumour-free resection margin in rectal cancer surgery. Lancet. 2001;357:497–504. [DOI] [PubMed] [Google Scholar]
- 7.Nougaret S, Reinhold C, Mikhael HW, Rouanet P, Bibeau F, Brown G. The use of MR imaging in treatment planning for patients with rectal carcinoma: have you checked the “DISTANCE”? Radiology. 2013;268:330–344. [DOI] [PubMed] [Google Scholar]
- 8.van der Sande ME, Beets GL, Hupkens BJ, et al. Response assessment after (chemo)radiotherapy for rectal cancer: why are we missing complete responses with MRI and endoscopy? Eur J Surg Oncol. 2019;45:1011–1017. [DOI] [PubMed] [Google Scholar]
- 9.Kulkarni T, Gollins S, Maw A, Hobson P, Byrne R, Widdowson D. Magnetic resonance imaging in rectal cancer downstaged using neoadjuvant chemoradiation: accuracy of prediction of tumour stage and circumferential resection margin status. Colorectal Dis. 2008;10:479–489. [DOI] [PubMed] [Google Scholar]
- 10.Vliegen RF, Beets GL, Lammering G, et al. Mesorectal fascia invasion after neoadjuvant chemotherapy and radiation therapy for locally advanced rectal cancer: accuracy of MR imaging for prediction. Radiology. 2008;246:454–462. [DOI] [PubMed] [Google Scholar]
- 11.Barbaro B, Fiorucci C, Tebala C, et al. Locally advanced rectal cancer: MR imaging in prediction of response after preoperative chemotherapy and radiation therapy. Radiology. 2009;250:730–739. [DOI] [PubMed] [Google Scholar]
- 12.Kim IY, Cha SW, Ahn JH, Kim YW. Factors affecting the restaging accuracy of magnetic resonance imaging after preoperative chemoradiation in patients with rectal cancer. Eur J Surg Oncol. 2015;41:493–498. [DOI] [PubMed] [Google Scholar]
- 13.Jia X, Zhang Y, Wang Y, et al. MRI for restaging locally advanced rectal cancer: detailed analysis of discrepancies with the pathologic reference standard. AJR Am J Roentgenol. 2019;213:1081–1090. [DOI] [PubMed] [Google Scholar]
- 14.Patel UB, Taylor F, Blomqvist L, et al. Magnetic resonance imaging-detected tumor response for locally advanced rectal cancer predicts survival outcomes: MERCURY experience. J Clin Oncol. 2011;29:3753–3760. [DOI] [PubMed] [Google Scholar]
- 15.Quirke P, Dixon MF. The prediction of local recurrence in rectal adenocarcinoma by histopathological examination. Int J Colorectal Dis. 1988;3:127–131. [DOI] [PubMed] [Google Scholar]
- 16.van den Broek JJ, van der Wolf FS, Lahaye MJ, et al. Accuracy of MRI in restaging locally advanced rectal cancer after preoperative chemoradiation. Dis Colon Rectum. 2017;60:274–283. [DOI] [PubMed] [Google Scholar]
- 17.Patra A, Baheti AD, Ankathi SK, et al. Can post-treatment MRI features predict pathological circumferential resection margin (pCRM) involvement in low rectal tumors. Indian J Surg Oncol. 2020;11:720–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim SH, Lee JM, Park HS, Eun HW, Han JK, Choi BI. Accuracy of MRI for predicting the circumferential resection margin, mesorectal fascia invasion, and tumor response to neoadjuvant chemoradiotherapy for locally advanced rectal cancer. J Magn Reson Imaging. 2009;29:1093–1101. [DOI] [PubMed] [Google Scholar]
- 19.McGlone ER, Shah V, Lowdell C, Blunt D, Cohen P, Dawson PM. Circumferential resection margins of rectal tumours post-radiotherapy: how can MRI aid surgical planning? Tech Coloproctol. 2014;18:937–943. [DOI] [PubMed] [Google Scholar]
- 20.Habr-Gama A, São Julião GP, Vailati BB, et al. Organ preservation in cT2N0 rectal cancer after neoadjuvant chemoradiation therapy: the impact of radiation therapy dose-escalation and consolidation chemotherapy. Ann Surg. 2019;269:102–107. [DOI] [PubMed] [Google Scholar]
- 21.Peschaud F, Cuenod CA, Benoist S, et al. Accuracy of magnetic resonance imaging in rectal cancer depends on location of the tumor. Dis Colon Rectum. 2005;48:1603–1609. [DOI] [PubMed] [Google Scholar]
- 22.Goldstein NS, Soman A, Sacksner J. Disparate surgical margin lengths of colorectal resection specimens between in vivo and in vitro measurements. The effects of surgical resection and formalin fixation on organ shrinkage. Am J Clin Pathol. 1999;111:349–351. [DOI] [PubMed] [Google Scholar]
- 23.Oblizajek NR, Gandhi S, Sharma M, et al. Anorectal pressures measured with high-resolution manometry in healthy people-Normal values and asymptomatic pelvic floor dysfunction. Neurogastroenterol Motil. 2019;31:e13597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Nardi P, Testoni SG, Corsetti M, et al. Manometric evaluation of anorectal function in patients treated with neoadjuvant chemoradiotherapy and total mesorectal excision for rectal cancer. Dig Liver Dis. 2017;49:91–97. [DOI] [PubMed] [Google Scholar]
- 25.Patel SH, Hu CY, Massarweh NN, et al. Circumferential resection margin as a hospital quality assessment tool for rectal cancer surgery. J Am Coll Surg. 2020;230:1008–1018 e1005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


