Abstract
IMPORTANCE
A growing body of evidence suggests that adverse pregnancy outcomes (APOs), including hypertensive disorders of pregnancy, gestational diabetes (GD), preterm birth, and intrauterine growth restriction, are associated with increased risk of cardiometabolic disease and cardiovascular disease (CVD) later in life. Adverse pregnancy outcomes may therefore represent an opportunity to intervene to prevent or delay onset of CVD. The objective of this review was to summarize the current evidence for targeted postpartum interventions and strategies to reduce CVD risk in women with a history of APOs.
OBSERVATIONS
A search of PubMed and Ovid for English-language randomized clinical trials, cohort studies, descriptive studies, and guidelines published from January 1, 2000, to April 30, 2021, was performed. Four broad categories of interventions were identified: transitional clinics, lifestyle interventions, pharmacotherapy, and patient and clinician education. Observational studies suggest that postpartum transitional clinics identify women who are at elevated risk for CVD and may aid in the transition to longitudinal primary care. Lifestyle interventions to increase physical activity and improve diet quality may help reduce the incidence of type 2 diabetes in women with prior GD; less is known about women with other prior APOs. Metformin hydrochloride may prevent development of type 2 diabetes in women with prior GD. Evidence is lacking in regard to specific pharmacotherapies after other APOs. Cardiovascular guidelines endorse using a history of APOs to refine CVD risk assessment and guide statin prescription for primary prevention in women with intermediate calculated 10-year CVD risk. Research suggests a low level of awareness of the link between APOs and CVD among both patients and clinicians.
CONCLUSIONS AND RELEVANCE
These findings suggest that transitional clinics, lifestyle intervention, targeted pharmacotherapy, and clinician and patient education represent promising strategies for improving postpartum maternal cardiometabolic health in women with APOs; further research is needed to develop and rigorously evaluate these interventions. Future efforts should focus on strategies to increase maternal postpartum follow-up, improve accessibility to interventions across diverse racial and cultural groups, expand awareness of sex-specific CVD risk factors, and define evidence-based precision prevention strategies for this high-risk population.
A growing body of literature suggests that adverse pregnancy outcomes (APOs) such as gestational diabetes (GD), preterm birth, intrauterine growth restriction (IUGR), and the hypertensive disorders of pregnancy (HDP) (ie, gestational hypertension; preeclampsia; eclampsia; and hemolysis, elevated liver enzymes, and low platelet count syndrome) are associated with an elevated risk of cardiometabolic disease and cardiovascular disease (CVD), both in the early postpartum period1-4 and throughout the life span.5-7 Adverse pregnancy outcomes are common: more than 15% of child-bearing women in the US experience an HDP at least once,8 6% to 9% develop GD,9 10% experience preterm birth,10 and 10% to 15% experience IUGR.11 Rates of APOs are disproportionately elevated among American Indian, Asian, Black, Hispanic, and Pacific Islander women,2,12,13 highlighting a critical need to address the causes and consequences of these disparities.
Women who experience APOs face an elevated risk of developing adverse cardiometabolic traits such as chronic hypertension,14,15 hypercholesteromia,14,15 metabolic syndrome,16 and type 2 diabetes (T2D),3,15 as well as overt CVD such as ischemic heart disease, heart failure and cardiomyopathy, and valvular heart disease.6-8,10,17,18 Pregnancy thus provides a critical window into a woman’s future cardiometabolic health.1 As such, history of APOs is now recognized as a risk-enhancing factor for the development of atherosclerotic CVD (ASCVD) to refine risk assessment and inform statin allocation in the multisociety cardiovascular guidelines for the management of cholesterol and primary ASCVD prevention.19
The mechanisms linking APOs to future CVD and cardiometabolic disease remain incompletely understood. Prepregnancy cardiometabolic risk factors (eg, chronic hypertension, obesity, and T2D) are strongly associated with development of APOs but are not present in many affected women.20 Mounting evidence suggests that genetic polymorphisms predisposing to hypertension and obesity are enriched in women with HDP,21,22 implying that HDP may reflect latent genetic cardiometabolic risk. Similarly, GD and T2D appear to share several genetic risk loci.23 Whether similar genetic associations exist between cardiometabolic traits and other APOs is unclear. The extent to which APOs may also be directly causal for future CVD (eg, via endothelial injury, oxidative stress) remains unknown.24
To our knowledge, no reviews to date have comprehensively integrated evidence about targeted interventions or strategies to reduce CVD risk in women with prior APOs. A recent American Heart Association statement endorsed close monitoring of CVD risk factors in the first year post partum.25 Beyond incorporation of APOs as a risk-enhancing factor to guide statin prescribing, no other specific guidelines exist to direct long-term CVD risk reduction in women with APOs. Consequently, despite the well-described association between APOs and CVD, pregnancy history is incorporated into postpregnancy preventive care inconsistently. Herein, we review the evidence for targeted postpartum risk-reducing interventions, with the goal of identifying optimal care for this high-risk population of women as well as key areas of future investigation.
We searched PubMed and Ovid for English-language randomized clinical trials (RCTs), cohort studies, and descriptive studies, as well as relevant guideline documents and scientific statements, that were published between January 1, 2000, and April 30, 2021. Our preliminary literature search yielded transitional clinics, lifestyle interventions, pharmacotherapy, and education as the broad categories of interventions hypothesized to reduce long-term cardiometabolic risk after an APO. In follow-up searches, we paired these categories of potential interventions with variations of HDP, GD, preterm birth, and IUGR. The literature search was conducted from June 1, 2020, to April 30, 2021.
Observations
Early Postpartum Care
Although standard care of the pregnant woman includes frequent visits in late pregnancy, care is thereafter episodic and often limited for the postpartum mother, who remains at elevated risk for pregnancy-related morbidity and mortality as long as 1 year.26 The American College of Obstetricians and Gynecologists recommends that all postpartum women undergo comprehensive medical evaluation within 12 weeks, including chronic disease management, health care maintenance, and future reproductive planning.27 For women with APOs, the American College of Obstetricians and Gynecologists also recommends risk factor screening consisting of a thorough medical history, physical examination, nutritional assessment, and laboratory assessment, including measurement of cholesterol, glycemia, and urine microalbumin levels.28 However, postpartum care often occurs as a single obstetrics visit at 6 weeks, and although the American College of Obstetricians and Gynecologists recommends a transition to a primary care physician/clinician (PCP) within 4 to 12 weeks post partum, such transitions frequently do not occur.27
Transitional Clinics
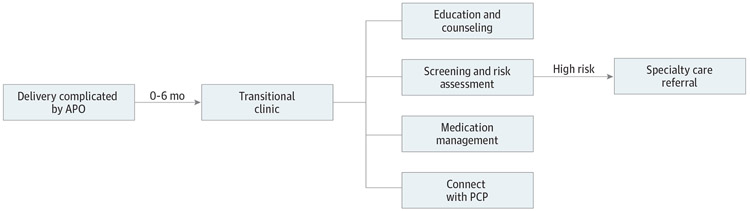
Postpartum transitional clinics have historically aimed to perform CVD risk assessment and counseling after an APO, ensure blood pressure stability in women with HDP, and facilitate the transition to longitudinal primary care (Figure). Although other such clinics have been established, the experiences from 4 postpartum transitional clinics in the US and Canada have been published as peer-reviewed studies29-32 (Table 1).
Figure. Overview of Postpartum Transitional Clinics for Women With Adverse Pregnancy Outcomes (APOs).
Postpartum transitional clinics have historically aimed to perform risk assessment for cardiovascular disease and counseling after an APO, ensure postpartum blood pressure stability in women with hypertensive disorders of pregnancy, and facilitate the transition to longitudinal primary care. PCP indicates primary care physician/clinician.
Table 1.
Transitional Clinics for Women With HDP Published in Peer-Reviewed Literature
| Transitional clinic |
Population and timing | No. of participants |
Methods | Reported outcomes | Conclusions | Limitations and challenges |
|---|---|---|---|---|---|---|
| MHC29 (Canada) | HDP, GD, preterm birth, IUGR, and placental abruption at approximately 6 mo post partum (control group: women with normal blood pressure from a separate study who did not participate in the MHC) | 157 | Provide risk assessment; communicate risk with PCP; make specialty referrals | 30-y CVD risk was 7.5% (IQR, 5.9%-12.0%) in MHC participants and 5.3% (IQR, 4.0%-7.0%) in controls (P < .001); lifetime CVD risk was optimal in 16% of MHC patients and 54% of controls (P < .001) | MHC identifies women in early postpartum period at elevated CVD risk | Of patients who were referred to the MHC, 41% attended their appointment, including 86% of White women in the MHC group and 100% of White women in the control group |
| MCRRC30 (Canada) | High-risk MHC patients, approximately 5 mo from MHC referral to MCRRC appointment | 48 | High-risk MHC patients referred to cardiology for follow-up and consideration of cardiac rehabilitation | Median 30-y CVD risk score was 10.8% (IQR, 7.5%-17.0%) in MCRRC participants and 6.5% (IQR, 5.0%-10.0%) in nonreferred MHC participants (P < .001); lifetime CVD risk was optimal in 2% of MCRRC participants and 28% of nonreferred MHC participants (P < .001); 28 of 48 patients were eligible for cardiac rehabilitation referral, 19 of 28 eligible patients were referred, and 5 of 19 referred patients attended their appointment | Follow-up care with existing systems in place does not meet needs of high-risk postpartum women | Small sample size; predominantly White women |
| PPEC31 (Canada) | Preeclampsia at approximately 2 mo post partum; follow-up visits every 3-6 mo for 1 y | 21 | Educate patients about increased CVD risk; identify modifiable risk factors; work with an interdisciplinary team to address risk factors | Reported physical activity increased from 14% prepregnancy to 76% at 4.4 mo (P < .05); nonsignificant weight loss (−0.4 kg) and BMI reduction (−0.1) | Increased physical activity; nonsignificant improvements in weight and BMI | Small sample size; low referral rate to the PPEC; 75% of patients who were referred attended their first clinic visit; race and ethnicity were not reported |
| CMC32 (US) | HDP at approximately 16 d post partum with option to continue | 412 | Antihypertensive medication management; educate patients and clinicians about CVD risk and heart-healthy lifestyles; transition patients to PCP; provide referral to a nutritionist | Modification of antihypertensive medications in 48% of women; 87% of women attended a consultation with a nutritionist; 80% of women within the CMC health care system followed up with PCP | CMC aided in transfer of care; engagement with long-term clinicians (PCPs and nutritionists) | Of patients who were referred to the CMC, 25% did not attend their appointment; PCP follow-up data were unavailable for women outside of the CMC health care system |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CMC, Cardiometabolic Clinic; CVD, cardiovascular disease; GD, gestational diabetes; HDP, hypertensive disorders of pregnancy; IUGR, intrauterine growth restriction; MCRRC, Maternal Cardiovascular Risk Reduction Clinic; MHC, Maternal Health Clinic; PCP, primary care physician/clinician; PPEC, Postpartum Preeclampsia Clinic.
The Maternal Health Clinic29 in Ontario, Canada, assessed CVD risk at 6 months post partum for women who had experienced APOs, communicated findings to PCPs, and referred high-risk patients to specialty care (eg, cardiology, endocrinology). Obstetricians both referred women to the Maternal Health Clinic and evaluated women during their visit. A Maternal Cardiovascular Risk Reduction Clinic,30 staffed by a cardiologist, was established within the Maternal Health Clinic to provide intensive risk reduction for women judged by treating clinicians to have especially high CVD risk (Table 1). Compared with other Maternal Health Clinic patients, women who were referred to the Maternal Cardiovascular Risk Reduction Clinic had elevated 30-year and lifetime CVD risk. Notably, only 50% of women referred attended a first Maternal Cardiovascular Risk Reduction Clinic visit, and of the 28 patients deemed eligible for cardiac rehabilitation by a cardiologist, 19 were referred and 5 attended the first visit.30
The Postpartum Preeclampsia Clinic31 in Alberta, Canada, employed a multidisciplinary team, including dieticians, pharmacists, obstetricians, and nurse practitioners, to educate postpartum women with preeclampsia about their CVD risk and address their modifiable risk factors.31 Participants were followed up by the clinic for at least 6 months. By 4.4 months post partum, women had significantly increased their physical activity and demonstrated improvements in body mass index and weight.31
The Cardiometabolic Clinic32 in Boston, Massachusetts, operates in an academic medical center and cares for women with HDP in the early postpartum period, aiming to prevent hypertensive postpartum readmissions, educate patients and clinicians about CVD risk, and ensure long-term PCP follow-up.33 Among a racially and ethnically diverse population (46.0% Asian, 33.0% Black, and 26.5% Hispanic), 75.0% of referred patients attended the Cardiometabolic Clinic at least once, 86.8% attended a nutrition consultation, and 79.5% receiving primary care within the same hospital network followed up with their PCP.
These observational studies have limitations, such as short follow-up duration and lack of a control group (eg, women with APOs who did not attend transitional clinics). Nonetheless, the available data suggest that postpartum transitional clinics may provide risk assessment and increase physical exercise and primary care follow-up among attendees. These findings are notable given claims-based studies34,35 suggesting that a small proportion of high-risk women receive recommended postpartum primary care follow-up (5.7% at 6 months in women with GD and 18.0% in women with HDP). Although women with complications during pregnancy may be more likely to return for postpartum visits than other women without APOs, follow-up rates are still low.36 In a retrospective cohort study, only 52% of women with severe preeclampsia attended postpartum follow-up, at which time 21% still had hypertension.37 Black and Hispanic women are at particularly heightened risk for loss to follow-up.34,35 Emerging data suggest that virtual care and telehealth strategies may help overcome barriers to follow-up for some postpartum women, although such strategies require further assessment.38
Lifestyle Modification
Lifestyle modification, including a heart-healthy diet, exercise, and weight loss, is the cornerstone of preventive care in patients with elevated CVD risk.19 Although few large RCTs have tested postpartum lifestyle modification in women after APOs, physical activity and weight loss decrease the risk of chronic hypertension and obesity, which are established mediators of CVD in women with APOs.6 Elevated body mass index superimposed on a history of HDP is associated with significantly higher risk for developing chronic hypertension.39 In a prospective study of women with prior GD,40 self-reported increase in physical activity was associated with reduced risk of T2D. Furthermore, postpartum weight gain and unhealthy lifestyle may partially mediate the association between GD and future CVD.7
Lifestyle Modification After HDP
Two studies41,42 examined lifestyle modification in women with HDP. Scholten et al41 studied an intensive exercise program in which women with recent preeclampsia and matched parous controls (all 6-12 months post partum) participated in a supervised, intensive 12-week exercise program. The program improved peripheral endothelial and autonomic function and indices of metabolic syndrome in both groups, although these indices did not completely normalize in women with preeclampsia.41 The Health Heart 4 Moms RCT42 included women within 5 years post partum with prior preeclampsia. Participants were randomized to the intervention arm, with online educational modules about CVD and lifestyle modification plus telephone-based nutritional counseling, or to the control group, in which participants received access to an educational website. The intervention group demonstrated greater knowledge about CVD risk factors, increased self-efficacy for an improved diet, and decreased self-reported physical inactivity.42
Lifestyle Modification After GD
Substantially more data exist on lifestyle modification in the population with GD.43-47 Interventions focused primarily on dietary modification and/or physical activity and were often paired with health education and personalized goal setting. Most interventions began less than 1 year post partum, had fewer than 100 participants, and included predominantly White women. Some studies had additional inclusion criteria, such as being overweight.43,45,46 Meta-analyses found that lifestyle interventions led to a moderate decrease in postpartum weight, waist circumference, and plasma triglyceride concentration43 as well as a trend toward reduction in incident T2D.45,46
Four RCTs48-51 are worth highlighting (Table 2). The Diabetes Prevention Program (DPP)48 randomized individuals with prediabetes to metformin hydrochloride, intensive lifestyle modification, or placebo during a 3-year period. Some women had a remote history of GD, and subgroup analyses in 350 women with prior GD and 1416 parous women without GD were subsequently performed.48,49 Compared with placebo, lifestyle modification and metformin each reduced incidence of T2D by approximately 50% in women with prior GD and prediabetes.48 This benefit persisted in the DPP Outcomes Study 10-year follow-up,50 which followed up participants for an additional 7 years and showed that intensive lifestyle modification and use of metformin reduced T2D incidence among those with prior GD by 35% and 40%, respectively.50 These studies suggest that lifestyle modification and metformin both mitigate T2D risk in prediabetic women with prior GD; as such, the American Diabetes Association recommends metformin and/or lifestyle modification in this population.51 However, women with GD have the highest relative risk for developing T2D within 3 to 6 years post partum, and participants in the DPP were already 12 years post partum at the time of enrollment.3
Table 2.
RCTs of Lifestyle and/or Pharmacological Interventions for Women With GD
| Intervention | Population and timing | Duration of follow-up |
Sample size | Control group | Intervention group | Outcome(s) | Conclusions | Limitations |
|---|---|---|---|---|---|---|---|---|
| DPP48 | GD and prediabetes at approximately 12 y post partum | 2.8 y | 1416 Without prior GD (487 to placebo, 464 to metformin, 465 to ILS); 350 women with prior GD (122 to placebo, 111 to metformin, 117 to ILS) | Placebo plus standard lifestyle recommendations |
Metformin group received metformin plus standard lifestyle recommendations; ILS group received education about diet and exercise with goal to reduce weight by 7% and increase physical activity to 150 min/wk | Prior GD: ILS and metformin reduced T2D incidence by approximately 50% vs placebo group | Metformin and ILS equally effective at delaying and preventing progression to T2D in women with prior GD | Median 12 y between pregnancy and prediabetes onset; prior GD group median age, 43 y vs 52 y in women without prior GD |
| BAB52 | GD at approximately 6 wk post partum | 6 wk to 12 mo Post partum | 36 in Intervention group, 39 in control group | “It’s Never Too Early to Prevent Diabetes” handout distributed at recruitment | DPP-styled intervention with website; 12 weekly modules about diet and exercise; communications with lifestyle coach | Intervention group gained significantly less weight post partum vs prepregnancy weight (mean, −0.7 kg in intervention group vs +4.0 kg in controls) | Intervention led to less weight gain vs prepregnancy weight | Self-reported prepregnancy weight; single-center study; small sample size |
| GEM53 | GD at approximately 6 wk post partum | Intervention of 6 wk to 6 mo; 7-12 mo postpartum maintenance | 44 Medical facilities: 22 with intervention (1087 women), 22 with standard care (1193 women) | Sent information about GD and lifestyle modifications | DPP-styled intervention with workbook about healthy diet and physical activity; telephone calls with dietician | At 12 mo, intervention group had 1.28-fold odds of achieving weight goal vs control group | Intervention moderately reduced postpartum weight retention; feasible in clinical setting | 26% missed 6-mo clinic-measured weight; 33% missed 12-mo clinic-measured weight |
| PAIGE54 | GD plus BMI ≥25 at approximately 4-6 wk post partum | 4-6 wk to 6 mo Post partum | 29 in Intervention group, 31 in control group | Watched a DVD about GD | 3-mo Free membership to Slimming World; telephone and text support with health educator; pedometer access plus standard of care | Intervention group lost a mean of 3.9 kg and control group gained a mean of 3.8 kg; no significant difference in blood glucose levels | Intervention led to significant weight loss at 6 mo post partum compared with controls | Small sample size; challenges with recruitment due to time constraints, lack of child care and not wanting to leave the infant; predominantly White sample; low pedometer use |
Abbreviations: BAB, Balance After Baby; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); DPP, Diabetes Prevention Program; GD, gestational diabetes; GEM, Gestational Diabetes’ Effects on Moms; ILS, intensive lifestyle modification; PAIGE, Postnatal Lifestyle Intervention Program for Overweight Women with Previous Gestational Diabetes Mellitus; RCT, randomized clinical trial; T2D, type 2 diabetes.
To address this gap, 3 studies modeled on the DPP49 enrolled participants within the first year post partum. Balance After Baby52 was a small-scale RCT in a single academic medical center. Gestational Diabetes’ Effects on Moms53 was a pragmatic cluster RCT within a larger health care system (Kaiser Permanente Northern California) with facility-level randomization and a large racially and socioeconomically diverse sample.53 Both programs were predominantly home based and distributed printed or online educational modules that focused on dietary changes and physical activity paired with telephone-based coaching. In both the Gestational Diabetes’ Effects on Moms and Balance After Baby RCTs, lifestyle intervention led to significant weight loss compared with usual care. The Postnatal Lifestyle Intervention Program for Overweight Women With Previous Gestational Diabetes Mellitus54 program in the UK was also modeled on the DPP, but unlike the Gestational Diabetes’ Effects on Moms and Balance After Baby RCTs, it randomized women with prediabetes and prior GD to a commercial weight-loss program for 3 months plus telephone and text-based counseling vs usual care.54 Participants in the Postnatal Lifestyle Intervention Program for Overweight Women With Previous Gestational Diabetes Mellitus program randomized to intensive lifestyle modification experienced more weight loss at 6 months than those randomized to traditional postpartum care.54
Recruitment and/or retention rates were low across almost all studies in both post-HDP and post-GD populations, highlighting the challenge of engaging the postpartum population in lifestyle interventions. In focus groups, postpartum women indicated that child care responsibilities, fatigue, lack of health literacy around CVD risks, limited time, and job constraints make participating in lifestyle modification interventions challenging.55-57 Women expressed a preference for programs that could accommodate their demanding schedules and permit participation from home.55-57
Breastfeeding and APOs
Observational studies58 suggest that breastfeeding may be protective against development of T2D, hypertension, and CVD. Among women with prior GD, breastfeeding was associated with reduced T2D risk.59 A prospective observational study60 found that among lactating women with prior GD, T2D incidence in the first 2 years after pregnancy was inversely related to increased duration and intensity of breastfeeding. Mounting evidence indicates that lactation may be beneficial in women with prior GD, and more research is needed in women with preterm birth, IUGR, and HDP.
Several ongoing studies61-69 are testing new approaches to postpartum cardiometabolic risk reduction (Table 3). We hope that the results of these studies elucidate the most effective interventions for at-risk women with a history of APOs.
Table 3.
Ongoing Clinical Trials of Postpartum Interventions in Women With APOs
| Clinical trial | APO | Intervention type | Projected sample size |
Intervention | Primary outcome(s) |
|---|---|---|---|---|---|
| Heart Health 4 New Moms: A Randomized Trial in the First Year After Preeclampsia61 | Preeclampsia and overweight/obesity | Lifestyle modification | 150 | Web-based lifestyle intervention and home blood pressure monitoring during first year post partum | Weight loss |
| Virtual Cardiac Wellness Program Following Hypertensive Disorders of Pregnancy62 | HDP | Lifestyle modification | 100 | Virtual cardiac wellness program beginning 0 to 6 wk post partum for 6 mo | Weight loss |
| Lifestyle Intervention for Women With Recent Pre-eclampsia or Gestational Diabetes Mellitus (“Mom’s Healthy Heart”)63 | Preeclampsia, GD | Lifestyle modification | 40 | Web-based lifestyle intervention and personalized dietician coaching 3-15 mo post partum | Retention and adherence to program |
| Fit After Baby: Increasing Postpartum Weight Loss in Women at Increased Risk for Cardiometabolic Disease (FAB)64 | HDP, GD, IUGR, and preterm birth | Lifestyle modification | 82 | Mobile application and communications with a lifestyle coach to increase exercise and weight loss during first year post partum | Weight loss and postpartum weight retention |
| Alternative Lifestyle Interventions for Vulnerable Ethnic Groups (ALIVE)65 | GD | Lifestyle modification | 435 | Address the increased risk of complications in Black women with prior GD (<4 wk post partum) by assessing the effectiveness of a DPP-styled telehealth intervention with community doulas | Completion of recommended T2D screening 6-12 wk post partum |
| A Pragmatic Approach to Lowering the Risk of Diabetes Mellitus After a Diagnosis of Gestational Diabetes Mellitus66 | GD | Pharmacotherapy | 200 | Metformin given 0-12 mo post partum | HbA1c level |
| Empagliflozin and the Preservation of Beta-cell Function in Women With Recent Gestational Diabetes (EMPA post-GDM)67 | GD | Pharmacotherapy | 120 | Empagliflozin given 6-36 mo post partum | Insulin secretion-sensitivity index-2 |
| The Impact of Liraglutide on Glucose Tolerance and the Risk of Type 2 Diabetes in Women With Previous Pregnancy-Induced Diabetes68 | GD | Pharmacotherapy | 105 | Subcutaneous liraglutide given daily for 5 y in women within 5 y post partum | Change in glucose tolerance |
| Postpartum Low-Dose Aspirin After Preeclampsia for Optimization of Cardiovascular Risk (PAPVASC)69 | Severe preeclampsia | Pharmacotherapy | 44 | Aspirin given 0-6 mo post partum | Endothelium-dependent dilation |
Abbreviations: APO, adverse pregnancy outcome; HbA1c, hemoglobin A1c; HDP, hypertensive disorders of pregnancy; IUGR, intrauterine growth restriction; T2D, type 2 diabetes.
Pharmacological Interventions
After HDP
Although the American College of Obstetricians and Gynecologists provides recommendations for management of acute preeclampsia and severe gestational hypertension, there are no standardized recommendations for postpartum medication titration.70,71 Few studies71 have evaluated the pharmacological management of persistent hypertension after HDP in the early postpartum period. Therefore, the effect of specific antihypertensive pharmacotherapies or degree of blood pressure control on subsequent CVD risk in this population remains unknown. Furthermore, no large RCTs have evaluated pharmacotherapy to prevent chronic hypertension, cardiometabolic disease, or CVD in women with normal blood pressure and prior HDP. The Postpartum Low-Dose Aspirin After Preeclampsia for Optimization of Cardiovascular Risk trial69 is evaluating whether aspirin after severe preeclampsia affects endothelial and vascular function (Table 3). As mentioned, guidelines encourage statin use in women with prior HDP and intermediate calculated 10-year ASCVD risk.19 No trials to date have specifically evaluated statins in women with prior HDP.
After GD
In the DPP—the largest study to evaluate pharmacological treatment of women with prior GD—metformin reduced T2D incidence by approximately 50% at 3 years of treatment and by 40% at 10 years of treatment in women with remote GD, with similar magnitude of benefit as intensive lifestyle modification.48,50 This finding may be particularly relevant to women with prior GD, for whom lifestyle interventions have been less effective, especially in the early postpartum period.55 As such, the American Diabetes Association recommends that women with prediabetes and a history of GD take metformin or implement lifestyle modifications.51
Recent small RCTs72-74 (<100 participants in each trial) evaluating novel diabetes medications in the early postpartum period after GD have shown promise. Studies suggest additive benefits of sitagliptin phosphate, liraglutide, and dapagliflozin when added to metformin with respect to glycemic excursion, T2D risk, and adiposity measures. Limitations include small sample sizes and short duration of follow-up.
As noted, there is a paucity of high-quality, well-powered studies evaluating pharmacotherapy for women with prior GD in the early postpartum period. In addition, pharmacotherapy options may be limited in this population by lack of data in breastfeeding women. Ongoing trials (Table 3) may help to bridge this evidence gap, including 1 investigating the effects of metformin on T2D risk in the first year post partum66 and another assessing the effects of empagliflozin on beta cell function in postpartum women after GD.67
Education
Knowledge about the link between APOs and elevated risk for CVD and cardiometabolic disease is lacking among clinicians and patients.33,75,76 In a recent scoping review including 402 women with prior HDP,75 patients reported little to no knowledge about the association between HDP and CVD. Focus groups with women with prior preeclampsia confirmed that they were seldom informed of the association between HDP and CVD.55 Similarly, a recent study of 79 women who experienced GD and/or preeclampsia77 found that more than 50% of women did not receive counseling about future CVD risk. Women with prior preeclampsia received less counseling than women with prior GD, and perceived risk of future CVD was low in both groups.77
Clinicians also appear to lack adequate knowledge about the association between APOs and CVD. In a recent review that included 1215 health care clinicians,75 most clinicians had little knowledge about future CVD risk in women with HDP. On average, obstetricians/gynecologists appear to be more aware of this link than internists.75 One study33 found that obstetricians/gynecologists are more likely than internists to ask about APOs when assessing cardiovascular risk; however, on learning of a patient’s APO history, both types of clinicians were likely to screen for hypertension with women with preeclampsia and for dysglycemia in women with prior GD. Obstetricians/gynecologists were less likely to follow up with additional risk factor assessment.33 Obstetricians/gynecologists may be more aware of the association between CVD and IUGR/preterm birth than internists.33
Future Directions
Women with a history of APOs represent a high-risk group for future CVD. High-quality evidence to guide precision prevention and treatment in this population is needed. There is a particular lack of data on risk-reducing strategies in women with prior preterm birth and IUGR, and studies to date have not evaluated the effect of postpartum interventions on long-term CVD outcomes. The Box summarizes key questions for future research in this area.
Ensuring successful transitions to longitudinal care for ongoing cardiometabolic risk screening and prevention is essential. To this end, novel strategies to facilitate and improve rates of postpartum follow-up are also needed, such as substituting telehealth for in-person visits, pairing transitional clinics with obstetric or pediatric visits, or providing child care during the visit. Ensuring these clinics are equally accessible to historically marginalized populations deserves particular attention, including overcoming cultural and language barriers.78 In addition, critical questions about the optimal design and implementation of transitional clinics remain to be addressed, such as whether to refer all women with APOs (vs a subset with higher risk) to transitional clinics; when women should be referred in their postpartum course; how frequently they should be seen; what type of clinician(s) should staff such clinics; and which transitional clinic patients may benefit from long-term follow-up with a cardiologist (Box). It is likely that such clinics will need to be adapted to specific practice settings, clinical expertise, and local population needs.
Box. Key Questions for Future Research on Cardiometabolic Risk Reduction After APOs.
General Questions
What are the key barriers to postpartum preventive care and health maintenance?
How frequently should postpartum women be screened for CVD risk factors?
How can we effectively address racial disparities in access to postpartum care?
Are early postpartum or delayed postpartum interventions more effective?
How do we increase recruitment and retention in postpartum interventions?
Can 10-y, 30-y, and lifetime CVD risk calculators effectively risk-stratify women with a history of APOs?
What are the effects of postpartum interventions on long-term CVD risk?
Transitional Clinics
What are the essential components of transitional clinics?
How often should patients be seen at transitional clinics?
What services should be provided at transitional clinics?
Who should staff transitional clinics?
Are transitional clinics effective at preventing CVD in the long term?
Are transitional clinics cost-effective for cardiometabolic risk reduction?
How can transitional clinics be built most effectively and sustained in the setting of bundled payments for pregnancy care?
Lifestyle Modifications
How do we increase engagement in lifestyle modifications?
What types of lifestyle modifications are most effective at reducing CVD risk in women with APOs?
Are DPP-styled interventions effective in women with non-GD APOs?
Pharmacotherapy
What are optimal blood pressure and blood glucose level thresholds for initiating pharmacotherapy in women with prior APOs? Should treatment targets be more aggressive in women with APOs?
Can mechanistic insights about key pathways linking APOs to CVD lead to novel, targeted preventive therapies?
Education
What are the most effective strategies at increasing clinician awareness about CVD/cardiometabolic disease risk after APOs?
What are the most effective strategies at increasing patient awareness about CVD/cardiometabolic disease risk in women with prior APOs?
Although the DPP and similarly structured programs have shown success in reducing T2D incidence among women with prior GD,48,50,52-54 further research is required to evaluate whether similar interventions are effective after other APOs. Subpar recruitment and retention rates in lifestyle modification programs among non-White populations merits further attention; Dulce Mothers, a peer-led, culturally tailored DPP-styled intervention for postpartum Latina mothers with prior GD, represents 1 effort that is currently underway.79 Similarly, pharmacological strategies to prevent CVD or cardiometabolic disease in women with prior APOs remain understudied and warrant further research. Although newer diabetes drugs have shown promise,72-74 pharmacotherapy options may be limited by lack of data in breastfeeding women. Because breastfeeding lowers T2D risk, the relative efficacy of a pharmacological intervention that requires cessation of breastfeeding warrants dedicated study. Of note, no large RCTs have assessed pharmacotherapy specifically for women with prior HDP, preterm birth, or IUGR. The well-described associations between HDP and diverse CVD outcomes, the early progression to chronic hypertension, and the mediating role of blood pressure for excess CVD risk in affected women highlight the need to assess specific blood pressure targets and test new pharmacological strategies to optimize cardiometabolic health and slow vascular aging (Box).80
To date, limited studies81,82 have suggested that incorporating HDP history in 10-year ASCVD risk prediction models yields minimal improvement in net risk reclassification among middle-aged women, perhaps because HDP-associated CVD risk is largely captured in traditional CVD risk factors already included in prediction models.81 Incorporating history of APOs may be most useful in discriminating risk among younger women81 who have low calculated short-term CVD risk due to their age and are less likely to have developed overt CVD risk factors. Future research should test the utility of HDP and other APOs in refining risk using longer-term (eg, lifetime) CVD risk models and in racially diverse populations.
Awareness of the link between APOs and CVD is now growing thanks to recent literature, guidelines, and scientific statements.25 Increasing education about CVD after APOs is also needed across medical school and graduate medical curricula. Joint society educational recommendations, formalized training in cardiology and endocrinology fellowships, and increased clinical exposure to women with APOs through multidisciplinary care teams all have the potential to increase clinician awareness and improve clinical care.83 Engaging multiple stakeholders through community partnership and social media campaigns also has the potential to increase patient awareness about CVD risk.83 Finally, ongoing research is necessary to elucidate the pathophysiology of APOs and their links to long-term CVD risk to inform novel precision prevention strategies in this high-risk population.
Conclusions
Transitional clinics, lifestyle intervention, targeted pharmacotherapy, and clinician and patient education represent promising strategies for improving postpartum maternal cardiometabolic health in women with APOs; further research is needed to develop and rigorously evaluate these interventions. Future efforts should focus on strategies to increase maternal postpartum follow-up, improve accessibility to interventions across diverse racial and cultural groups, expand awareness of sex-specific CVD risk factors, and define evidence-based precision prevention strategies for this high-risk population.
Funding/Support:
This study was supported by grants R01HL142711, R01HL148050, R01HL148565, and R01HL151283 from the NHLBI (Dr Natarajan); grant TNE-18CVD04 from Fondation Leducq (Dr Natarajan); a Hassenfeld Scholar Award from Massachusetts General Hospital (Dr Natarajan); grant 5U01DK123795-02 from the National Institute of Diabetes and Digestive and Kidney Diseases (Dr Powe); and grant T32HL094301-07 from the NHLBI (Dr Honigberg).
Role of the Funder/Sponsor:
The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Abbreviations:
- APO
adverse pregnancy outcome
- CVD
cardiovascular disease
- DPP
Diabetes Prevention Program
- GD
gestational diabetes
Footnotes
Conflict of Interest Disclosures: Dr Sarma reported receiving grants from the National Heart, Lung, and Blood Institute (NHLBI) and a CRICO Patient Safety Award outside the submitted work. Dr Michos reported receiving personal fees from the advisory boards of Novartis International AG, Amarin Corporation, Esperion, Therapeutics Inc, and AstraZeneca outside the submitted work. Dr Natarajan reported receiving grants from Amgen Inc, Apple Inc, Boston Scientific Corporation, and AstraZeneca; personal fees from Apple Inc, Blackstone Life Sciences, Novartis International AG, Genentech/Roche, AstraZeneca, and Foresite Labs; and having a spouse who is employed by Vertex Pharmaceuticals Inc outside the submitted work. No other disclosures were reported.
Contributor Information
Amanda R. Jowell, Currently a medical student at Harvard Medical School, Boston, Massachusetts.
Amy A. Sarma, Department of Medicine, Harvard Medical School, Boston, Massachusetts; Cardiology Division, Department of Medicine, Massachusetts General Hospital, Boston; Corrigan Women’s Heart Health Program, Massachusetts General Hospital, Boston.
Martha Gulati, Division of Cardiology, University of Arizona, Phoenix.
Erin D. Michos, Ciccarone Center for the Prevention of Cardiovascular Disease, Johns Hopkins University School of Medicine, Baltimore, Maryland; Division of Cardiology, Johns Hopkins University School of Medicine, Baltimore, Maryland.
Arthur J. Vaught, Division of Maternal-Fetal Medicine, Department of Gynecology and Obstetrics, Johns Hopkins University School of Medicine, Baltimore, Maryland; Division of Surgical Critical Care, Department of Surgery, Johns Hopkins University School of Medicine, Baltimore, Maryland.
Pradeep Natarajan, Department of Medicine, Harvard Medical School, Boston, Massachusetts; Cardiology Division, Department of Medicine, Massachusetts General Hospital, Boston; Program in Medical and Population Genetics and Cardiovascular Disease Initiative, Broad Institute of Harvard and MIT, Cambridge, Massachusetts; Cardiovascular Research Center, Massachusetts General Hospital, Boston.
Camille E. Powe, Department of Medicine, Harvard Medical School, Boston, Massachusetts; Diabetes Unit, Endocrine Division, Department of Medicine, Massachusetts General Hospital, Boston.
Michael C. Honigberg, Department of Medicine, Harvard Medical School, Boston, Massachusetts; Cardiology Division, Department of Medicine, Massachusetts General Hospital, Boston; Corrigan Women’s Heart Health Program, Massachusetts General Hospital, Boston; Program in Medical and Population Genetics and Cardiovascular Disease Initiative, Broad Institute of Harvard and MIT, Cambridge, Massachusetts; Cardiovascular Research Center, Massachusetts General Hospital, Boston.
REFERENCES
- 1.Cain MA, Salemi JL, Tanner JP, Kirby RS, Salihu HM, Louis JM. Pregnancy as a window to future health: maternal placental syndromes and short-term cardiovascular outcomes. Am J Obstet Gynecol. 2016;215(4):484.e1–484.e14. doi: 10.1016/j.ajog.2016.05.047 [DOI] [PubMed] [Google Scholar]
- 2.Jarvie JL, Metz TD, Davis MB, Ehrig JC, Kao DP. Short-term risk of cardiovascular readmission following a hypertensive disorder of pregnancy. Heart. 2018;104(14):1187–1194. doi: 10.1136/heartjnl-2017-312299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Song C, Lyu Y, Li C, et al. Long-term risk of diabetes in women at varying durations after gestational diabetes: a systematic review and meta-analysis with more than 2 million women. Obes Rev. 2018;19(3):421–429. doi: 10.1111/obr.12645 [DOI] [PubMed] [Google Scholar]
- 4.Rosenbloom JI, Stwalley D, Lindley KJ, Michael Nelson D, Olsen MA, Stout MJ. Latency of preterm hypertensive disorders of pregnancy and subsequent cardiovascular complications. Pregnancy Hypertens. 2020;21(May):139–144. doi: 10.1016/j.preghy.2020.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grandi SM, Filion KB, Yoon S, et al. Cardiovascular disease-related morbidity and mortality in women with a history of pregnancy complications. Circulation. 2019;139(8):1069–1079. doi: 10.1161/CIRCULATIONAHA.118.036748 [DOI] [PubMed] [Google Scholar]
- 6.Honigberg MC, Zekavat SM, Aragam K, et al. Long-term cardiovascular risk in women with hypertension during pregnancy. J Am Coll Cardiol. 2019;74(22):2743–2754. doi: 10.1016/j.jacc.2019.09.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tobias DK, Stuart JJ, Li S, et al. Association of history of gestational diabetes with long-term cardiovascular disease risk in a large prospective cohort of US women. JAMA Intern Med. 2017;177(12):1735–1742. doi: 10.1001/jamainternmed.2017.2790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garovic VD, White WM, Vaughan L, et al. Incidence and long-term outcomes of hypertensive disorders of pregnancy. J Am Coll Cardiol. 2020;75(18):2323–2334. doi: 10.1016/j.jacc.2020.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casagrande SS, Linder B, Cowie CC. Prevalence of gestational diabetes and subsequent type 2 diabetes among US women. Diabetes Res Clin Pract. 2018;141:200–208. doi: 10.1016/j.diabres.2018.05.010 [DOI] [PubMed] [Google Scholar]
- 10.Tanz LJ, Stuart JJ, Williams PL, et al. Preterm delivery and maternal cardiovascular disease in young and middle-aged adult women. Circulation. 2017;135(6):578–589. doi: 10.1161/CIRCULATIONAHA.116.025954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suhag A, Berghella V. Intrauterine growth restriction (IUGR): etiology and diagnosis. Curr Obstet Gynecol Rep. 2013;2(2):102–111. doi: 10.1007/s13669-013-0041-z [DOI] [Google Scholar]
- 12.Ferrara A Increasing prevalence of gestational diabetes mellitus: a public health perspective. Diabetes Care. 2007;30(suppl 2):S141–S146. doi: 10.2337/dc07-s206 [DOI] [PubMed] [Google Scholar]
- 13.Schaaf JM, Liem SMS, Mol BWJ, Abu-Hanna A, Ravelli ACJ. Ethnic and racial disparities in the risk of preterm birth: a systematic review and meta-analysis. Am J Perinatol. 2013;30(6):433–450. doi: 10.1055/s-0032-1326988 [DOI] [PubMed] [Google Scholar]
- 14.Stuart JJ, Tanz LJ, Missmer SA, et al. Hypertensive disorders of pregnancy and maternal cardiovascular disease risk factor development: an observational cohort study. Ann Intern Med. 2018;169(4):224–232. doi: 10.7326/M17-2740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanz LJ, Stuart JJ, Williams PL, et al. Preterm delivery and maternal cardiovascular disease risk factors: the Nurses’ Health Study II. J Womens Health (Larchmt). 2019;28(5):677–685. doi: 10.1089/jwh.2018.7150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith GN, Pudwell J, Walker M, Wen SW. Risk estimation of metabolic syndrome at one and three years after a pregnancy complicated by preeclampsia. J Obstet Gynaecol Can. 2012;34(9):836–841. doi: 10.1016/S1701-2163(16)35382-8 [DOI] [PubMed] [Google Scholar]
- 17.Crump C, Sundquist J, Howell EA, McLaughlin MA, Stroustrup A, Sundquist K. Pre-term delivery and risk of ischemic heart disease in women. J Am Coll Cardiol. 2020;76(1):57–67. doi: 10.1016/j.jacc.2020.04.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Søndergaard MM, Hlatky MA, Stefanick ML, et al. Association of adverse pregnancy outcomes with risk of atherosclerotic cardiovascular disease in postmenopausal women. JAMA Cardiol. 2020;5(12):1390–1398. doi: 10.1001/jamacardio.2020.4097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e596–e646. doi: 10.1161/CIR.0000000000000678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duckitt K, Harrington D. Risk factors for pre-eclampsia at antenatal booking: systematic review of controlled studies. BMJ. 2005;330(7491):565–567. doi: 10.1136/bmj.38380.674340.E0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Honigberg MC, Chaffin M, Aragam K, et al. Genetic variation in cardiometabolic traits and medication targets and the risk of hypertensive disorders of pregnancy. Circulation. 2020;142(7):711–713. doi: 10.1161/CIRCULATIONAHA.120.047936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gray KJ, Kovacheva VP, Mirzakhani H, et al. Risk of pre-eclampsia in patients with a maternal genetic predisposition to common medical conditions: a case-control study. BJOG. 2021;128(1):55–65. doi: 10.1111/1471-0528.16441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Powe CE, Kwak SH. Genetic studies of gestational diabetes and glucose metabolism in pregnancy. Curr Diab Rep. 2020;20(12):69. doi: 10.1007/s11892-020-01355-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lane-Cordova AD, Khan SS, Grobman WA, Greenland P, Shah SJ. Long-term cardiovascular risks associated with adverse pregnancy outcomes: JACC review topic of the week. J Am Coll Cardiol. 2019;73(16):2106–2116. doi: 10.1016/j.jacc.2018.12.092 [DOI] [PubMed] [Google Scholar]
- 25.Parikh NI, Gonzalez JM, Anderson CAM, et al. ; American Heart Association Council on Epidemiology and Prevention; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular and Stroke Nursing; and the Stroke Council. Adverse pregnancy outcomes and cardiovascular disease risk: unique opportunities for cardiovascular disease prevention in women. Circulation. 2021;143(18):e902–e916. doi: 10.1161/CIR.0000000000000961 [DOI] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and prevention (CDC). Pregnancy Mortality Surveillance System: trends in pregnancy-related deaths. Updated November 25, 2020. Accessed March 1, 2021. https://www.cdc.gov/reproductivehealth/maternal-mortality/pregnancy-mortality-surveillance-system.htm# [Google Scholar]
- 27.ACOG Committee opinion No. 736: optimizing postpartum care. Obstet Gynecol. 2018;131(736):140–150. doi: 10.1097/AOG.0000000000002633 [DOI] [PubMed] [Google Scholar]
- 28.ACOG practice bulletin No 212: pregnancy and heart disease. Obstet Gynecol. 2019;133(5):320–356. doi: 10.1097/AOG.0000000000003243 [DOI] [PubMed] [Google Scholar]
- 29.Cusimano MC, Pudwell J, Roddy M, Cho CKJ, Smith GN. The Maternal Health Clinic: an initiative for cardiovascular risk identification in women with pregnancy-related complications. Am J Obstet Gynecol. 2014;210(5):438.e1–438.e9. doi: 10.1016/j.ajog.2013.12.001 [DOI] [PubMed] [Google Scholar]
- 30.Gladstone RA, Pudwell J, Pal RS, Smith GN. Referral to cardiology following postpartum cardiovascular risk screening at the Maternal Health Clinic in Kingston, Ontario. Can J Cardiol. 2019;35(6):761–769. doi: 10.1016/j.cjca.2019.03.008 [DOI] [PubMed] [Google Scholar]
- 31.Janmohamed R, Montgomery-Fajic E, Sia W, et al. Cardiovascular risk reduction and weight management at a hospital-based postpartum preeclampsia clinic. J Obstet Gynaecol Can. 2015;37(4):330–337. doi: 10.1016/S1701-2163(15)30283-8 [DOI] [PubMed] [Google Scholar]
- 32.Celi AC, Seely EW, Wang P, Thomas AM, Wilkins-Haug LE. Caring for women after hypertensive pregnancies and beyond: implementation and integration of a postpartum transition clinic. Matern Child Health J. 2019;23(11):1459–1466. doi: 10.1007/s10995-019-02768-7 [DOI] [PubMed] [Google Scholar]
- 33.Wilkins-Haug L, Celi A, Thomas A, Frolkis J, Seely EW. Recognition by women’s health care providers of long-term cardiovascular disease risk after preeclampsia. Obstet Gynecol. 2015;125(6):1287–1292. doi: 10.1097/AOG.0000000000000856 [DOI] [PubMed] [Google Scholar]
- 34.Bernstein JA, Quinn E, Ameli O, et al. Follow-up after gestational diabetes: a fixable gap in women’s preventive healthcare. BMJ Open Diabetes Res Care. 2017;5(1):e000445. doi: 10.1136/bmjdrc-2017-000445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lewey J, Levine LD, Yang L, Triebwasser JE, Groeneveld PW. Patterns of postpartum ambulatory care follow-up care among women with hypertensive disorders of pregnancy. J Am Heart Assoc. 2020;9(17):e016357. doi: 10.1161/JAHA.120.016357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bennett WL, Chang HY, Levine DM, et al. Utilization of primary and obstetric care after medically complicated pregnancies: an analysis of medical claims data. J Gen Intern Med. 2014;29(4):636–645. doi: 10.1007/s11606-013-2744-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levine LD, Nkonde-Price C, Limaye M, Srinivas SK. Factors associated with postpartum follow-up and persistent hypertension among women with severe preeclampsia. J Perinatol. 2016;36(12):1079–1082. doi: 10.1038/jp.2016.137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cairns AE, Tucker KL, Leeson P, et al. ; SNAP-HT Investigators. Self-management of postnatal hypertension: the SNAP-HT trial. Hypertension. 2018;72(2):425–432. doi: 10.1161/HYPERTENSIONAHA.118.10911 [DOI] [PubMed] [Google Scholar]
- 39.Timpka S, Stuart JJ, Tanz LJ, Rimm EB, Franks PW, Rich-Edwards JW. Lifestyle in progression from hypertensive disorders of pregnancy to chronic hypertension in Nurses’ Health Study II: observational cohort study. BMJ. 2017;358:j3024. doi: 10.1136/bmj.j3024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bao W, Tobias DK, Bowers K, et al. Physical activity and sedentary behaviors associated with risk of progression from gestational diabetes mellitus to type 2 diabetes mellitus: a prospective cohort study. JAMA Intern Med. 2014;174(7):1047–1055. doi: 10.1001/jamainternmed.2014.1795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scholten RR, Thijssen DJH, Lotgering FK, Hopman MTE, Spaanderman MEA. Cardiovascular effects of aerobic exercise training in formerly preeclamptic women and healthy parous control subjects. Am J Obstet Gynecol. 2014;211(5):516.e1–516.e11. doi: 10.1016/j.ajog.2014.04.025 [DOI] [PubMed] [Google Scholar]
- 42.Rich-Edwards JW, Stuart JJ, Skurnik G, et al. Randomized trial to reduce cardiovascular risk in women with recent preeclampsia. J Womens Health (Larchmt). 2019;28(11):1493–1504. doi: 10.1089/jwh.2018.7523 [DOI] [PubMed] [Google Scholar]
- 43.Hewage SS, Wu S, Neelakantan N, Yoong J. Systematic review of effectiveness and cost-effectiveness of lifestyle interventions to improve clinical diabetes outcome measures in women with a history of GDM. Clin Nutr ESPEN. 2020;35:20–29. doi: 10.1016/j.clnesp.2019.10.011 [DOI] [PubMed] [Google Scholar]
- 44.Guo J, Chen JL, Whittemore R, Whitaker E. Postpartum lifestyle interventions to prevent type 2 diabetes among women with history of gestational diabetes: a systematic review of randomized clinical trials. J Womens Health (Larchmt). 2016;25(1):38–49. doi: 10.1089/jwh.2015.5262 [DOI] [PubMed] [Google Scholar]
- 45.Goveia P, Cañon-Montañez W, Santos DP, et al. Lifestyle intervention for the prevention of diabetes in women with previous gestational diabetes mellitus: a systematic review and meta-analysis. Front Endocrinol (Lausanne). 2018;9:583. doi: 10.3389/fendo.2018.00583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pedersen ALW, Terkildsen Maindal H, Juul L. How to prevent type 2 diabetes in women with previous gestational diabetes? a systematic review of behavioural interventions. Prim Care Diabetes. 2017;11(5):403–413. doi: 10.1016/j.pcd.2017.05.002 [DOI] [PubMed] [Google Scholar]
- 47.Gilinsky AS, Kirk AF, Hughes AR, Lindsay RS. Lifestyle interventions for type 2 diabetes prevention in women with prior gestational diabetes: a systematic review and meta-analysis of behavioural, anthropometric and metabolic outcomes. Prev Med Rep. 2015;2:448–461. doi: 10.1016/j.pmedr.2015.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ratner RE, Christophi CA, Metzger BE, et al. ; Diabetes Prevention Program Research Group. Prevention of diabetes in women with a history of gestational diabetes: effects of metformin and lifestyle interventions. J Clin Endocrinol Metab. 2008;93(12):4774–4779. doi: 10.1210/jc.2008-0772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Knowler WC, Barrett-Connor E, Fowler SE, et al. ; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aroda VR, Christophi CA, Edelstein SL, et al. ; Diabetes Prevention Program Research Group. The effect of lifestyle intervention and metformin on preventing or delaying diabetes among women with and without gestational diabetes: the Diabetes Prevention Program Outcomes Study 10-year follow-up. J Clin Endocrinol Metab. 2015;100(4):1646–1653. doi: 10.1210/jc.2014-3761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.American Diabetes Association. 14. Management of diabetes in pregnancy: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44(suppl 1):S200–S210. doi: 10.2337/dc21-S014 [DOI] [PubMed] [Google Scholar]
- 52.Nicklas JM, Zera CA, England LJ, et al. A web-based lifestyle intervention for women with recent gestational diabetes mellitus: a randomized controlled trial. Obstet Gynecol. 2014;124(3):563–570. doi: 10.1097/AOG.0000000000000420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ferrara A, Hedderson MM, Brown SD, et al. The comparative effectiveness of diabetes prevention strategies to reduce postpartum weight retention in women with gestational diabetes mellitus: the Gestational Diabetes’ Effects on Moms (GEM) cluster randomized controlled trial. Diabetes Care. 2016;39(1):65–74. doi: 10.2337/dc15-1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Holmes VA, Draffin CR, Patterson CC, et al. ; PAIGE Study Group. Postnatal lifestyle intervention for overweight women with previous gestational diabetes: a randomized controlled trial. J Clin Endocrinol Metab. 2018;103(7):2478–2487. doi: 10.1210/jc.2017-02654 [DOI] [PubMed] [Google Scholar]
- 55.Skurnik G, Roche AT, Stuart JJ, et al. Improving the postpartum care of women with a recent history of preeclampsia: a focus group study. Hypertens Pregnancy. 2016;35(3):371–381. doi: 10.3109/10641955.2016.1154967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seely EW, Rich-Edwards J, Lui J, et al. Risk of future cardiovascular disease in women with prior preeclampsia: a focus group study. BMC Pregnancy Childbirth. 2013;13:240. doi: 10.1186/1471-2393-13-240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nicklas JM, Zera CA, Seely EW, Abdul-Rahim ZS, Rudloff ND, Levkoff SE. Identifying postpartum intervention approaches to prevent type 2 diabetes in women with a history of gestational diabetes. BMC Pregnancy Childbirth. 2011;11:23. doi: 10.1186/1471-2393-11-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schwarz EB, Ray RM, Stuebe AM, et al. Duration of lactation and risk factors for maternal cardiovascular disease. Obstet Gynecol. 2009;113(5):974–982. doi: 10.1097/01.AOG.0000346884.67796.ca [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Feng L, Xu Q, Hu Z, Pan H. Lactation and progression to type 2 diabetes in patients with gestational diabetes mellitus: a systematic review and meta-analysis of cohort studies. J Diabetes Investig. 2018;9(6):1360–1369. doi: 10.1111/jdi.12838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gunderson EP, Hurston SR, Ning X, et al. ; Study of Women, Infant Feeding and Type 2 Diabetes After GDM Pregnancy Investigators. Lactation and progression to type 2 diabetes mellitus after gestational diabetes mellitus—a prospective cohort study. Ann Intern Med. 2015;163(12):889–898. doi: 10.7326/M15-0807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Heart Health 4 New Moms: a randomized trial in the first year after preeclampsia (HH4NM). ClinicalTrials.gov identifier: NCT03749746. Updated December 3, 2020. Accessed April 15, 2021. https://clinicaltrials.gov/ct2/show/NCT03749746 [Google Scholar]
- 62.Virtual cardiac wellness program following hypertensive disorders of pregnancy. ClinicalTrials.gov identifier: NCT04998942. Accessed April 15, 2021. https://clinicaltrials.gov/ct2/show/NCT04998942 [Google Scholar]
- 63.Lifestyle intervention for women with recent pre-eclampsia or gestational diabetes mellitus (“Mom's Healthy Heart”). ClinicalTrials.gov identifier: NCT03993145. Updated May 12, 2021. Accessed April 15, 2021. https://clinicaltrials.gov/ct2/show/NCT03993145 [Google Scholar]
- 64.Fit After Baby: increasing postpartum weight loss in women at increased risk for cardiometabolic disease (FAB). ClinicalTrials.gov identifier: NCT03215173. Updated March 8, 2021. Accessed April 15, 2021. https://clinicaltrials.gov/ct2/show/NCT03215173 [Google Scholar]
- 65.Alternative Lifestyle Interventions for Vulnerable Ethnic Groups (ALIVE). ClinicalTrials.gov identifier: NCT04406792. Updated September 22, 2021. Accessed April 15, 2021. https://www.clinicaltrials.gov/ct2/show/NCT04406792 [Google Scholar]
- 66.A pragmatic approach to lowering the risk of diabetes mellitus after a diagnosis of gestational diabetes mellitus. ClinicalTrials.gov identifier: NCT04615351. Accessed April 15, 2021. https://clinicaltrials.gov/ct2/show/NCT04615351 [Google Scholar]
- 67.Empagliflozin and the Preservation of Beta-cell Function in Women With Recent Gestational Diabetes (EMPA post-GDM). ClinicalTrials.gov identifier: NCT03215069. Accessed April 15, 2021. https://clinicaltrials.gov/ct2/show/NCT03215069 [Google Scholar]
- 68.The impact of liraglutide on glucose tolerance and the risk of type 2 diabetes in women with previous pregnancy-induced diabetes. ClinicalTrials.gov identifier: NCT01795248. Updated November 4, 2020. Accessed April 15, 2021. https://clinicaltrials.gov/ct2/show/NCT01795248 [Google Scholar]
- 69.Postpartum Low-Dose Aspirin After Preeclampsia for Optimization of Cardiovascular Risk (PAPVASC). ClinicalTrials.gov identifier: NCT04243278. Updated September 21, 2020. Accessed April 15, 2021. https://clinicaltrials.gov/ct2/show/NCT04243278 [Google Scholar]
- 70.ACOG practice bulletin No. 202: gestational hypertension and preeclampsia. Obstet Gynecol. 2019;133(1):1. doi: 10.1097/AOG.0000000000003018 [DOI] [PubMed] [Google Scholar]
- 71.Cairns AE, Pealing L, Duffy JMN, et al. Postpartum management of hypertensive disorders of pregnancy: a systematic review. BMJ Open. 2017;7(11):e018696. doi: 10.1136/bmjopen-2017-018696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Elkind-Hirsch KE, Shaler D, Harris R. Postpartum treatment with liraglutide in combination with metformin versus metformin monotherapy to improve metabolic status and reduce body weight in overweight/obese women with recent gestational diabetes: a double-blind, randomized, placebo-controlled study. J Diabetes Complications. 2020;34(4):107548. doi: 10.1016/j.jdiacomp.2020.107548 [DOI] [PubMed] [Google Scholar]
- 73.Elkind-Hirsch KE, Seidemann E, Harris R. A randomized trial of dapagliflozin and metformin, alone and combined, in overweight women after gestational diabetes mellitus. Am J Obstet Gynecol MFM. 2020;2(3):100139. doi: 10.1016/j.ajogmf.2020.100139 [DOI] [PubMed] [Google Scholar]
- 74.Elkind-Hirsch KE, Paterson MS, Shaler D, Gutowski HC. Short-term sitagliptin-metformin therapy is more effective than metformin or placebo in prior gestational diabetic women with impaired glucose regulation. Endocr Pract. 2018;24(4):361–368. doi: 10.4158/EP-2017-0251 [DOI] [PubMed] [Google Scholar]
- 75.Roth H, LeMarquand G, Henry A, Homer C. Assessing knowledge gaps of women and healthcare providers concerning cardiovascular risk after hypertensive disorders of pregnancy: a scoping review. Front Cardiovasc Med. 2019;6:178. doi: 10.3389/fcvm.2019.00178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Young B, Hacker MR, Rana S. Physicians’ knowledge of future vascular disease in women with preeclampsia. Hypertens Pregnancy. 2012;31(1):50–58. doi: 10.3109/10641955.2010.544955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sutherland L, Neale D, Henderson J, Clark J, Levine D, Bennett WL. Provider counseling about and risk perception for future chronic disease among women with gestational diabetes and preeclampsia. J Womens Health (Larchmt). 2020;29(9):1168–1175. doi: 10.1089/jwh.2019.7767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Marsiglia FF, Bermudez-Parsai M, Coonrod D. Familias Sanas: an intervention designed to increase rates of postpartum visits among Latinas. J Health Care Poor Underserved. 2010;21(3)(suppl):119–131. doi: 10.1353/hpu.0.0355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Philis-Tsimikas A, Fortmann AL, Dharkar-Surber S, et al. Dulce Mothers: an intervention to reduce diabetes and cardiovascular risk in Latinas after gestational diabetes. Transl Behav Med. 2014;4(1):18–25. doi: 10.1007/s13142-014-0253-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Honigberg MC, Natarajan P. Women’s cardiovascular health after hypertensive pregnancy: the long view from labor and delivery becomes clearer. J Am Coll Cardiol. 2020;75(18):2335–2337. doi: 10.1016/j.jacc.2020.01.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stuart JJ, Tanz LJ, Cook NR, et al. Hypertensive disorders of pregnancy and 10-year cardiovascular risk prediction. J Am Coll Cardiol. 2018;72(11):1252–1263. doi: 10.1016/j.jacc.2018.05.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Markovitz AR, Stuart JJ, Horn J, et al. Does pregnancy complication history improve cardiovascular disease risk prediction? findings from the HUNT study in Norway. Eur Heart J. 2019;40(14):1113–1120. doi: 10.1093/eurheartj/ehy863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sharma G, Zakaria S, Michos ED, et al. Improving cardiovascular workforce competencies in cardio-obstetrics: current challenges and future directions. J Am Heart Assoc. 2020;9(12):e015569. doi: 10.1161/JAHA.119.015569 [DOI] [PMC free article] [PubMed] [Google Scholar]