Abstract
Nosocomial pneumonia is a notable cause of morbidity and mortality and leads to increases in lengths of hospital stays and institutional expenditures. Aminoglycosides are used to treat patients with these infections, but few data on the doses and schedules required to achieve optimal therapeutic outcomes exist. We analyzed aminoglycoside treatment data for 78 patients with nosocomial pneumonia to determine if optimization of aminoglycoside pharmacodynamic parameters results in a more rapid therapeutic response (defined by outcome and days to leukocyte count resolution and temperature resolution). Cox proportional hazards, Classification and Regression Tree (CART), and logistic regression analyses were applied to the data. By all analyses, the first measured maximum concentration of drug in serum (Cmax)/MIC predicted days to temperature resolution and the second measured Cmax/MIC predicted days to leukocyte count resolution. For days to temperature resolution and leukocyte count resolution, CART analyses produced breakpoints, with an 89% success rate at 7 days of therapy for a Cmax/MIC of >4.7 and an 86% success rate at 7 days of therapy for a Cmax/MIC of >4.5, respectively. Logistic regression analyses predicted a 90% probability of temperature resolution and leukocyte count resolution by day 7 if a Cmax/MIC of ≥10 is achieved within the first 48 h of aminoglycoside therapy. Aggressive aminoglycoside dosing immediately followed by individualized pharmacokinetic monitoring would ensure that Cmax/MIC targets are achieved early in therapy. This would increase the probability of a rapid therapeutic response for pneumonia caused by gram-negative bacteria and potentially decreasing durations of parenteral antibiotic therapy, lengths of hospitalization, and institutional expenditures, a situation in which both the patient and the institution benefit.
Nosocomial pneumonia is the second most common nosocomial infection in the United States and causes the highest rates of morbidity and mortality (10, 13, 18, 19). Approximately 30 to 50% of deaths among patients with nosocomial pneumonia are directly attributable to the infection, with the highest mortality rates seen for patients with Pseudomonas aeruginosa or Acinetobacter infection or patients with concurrent bacteremia (6, 14). Nosocomial infections also result in increased lengths of hospitalization (15), which increase institutional expenditures and result in a net loss of revenue under prospective payment systems.
Aminoglycosides have been used for over 30 years for the treatment of nosocomial pneumonia caused by gram-negative bacteria. However, few data exist on the optimization of aminoglycoside doses and schedules for the enhancement of therapeutic outcomes. Although many factors have been evaluated, controversy about which aminoglycoside pharmacokinetic and pharmacodynamic variables are linked to outcome persists. Data from in vitro studies and studies with animal models suggest that the peak concentration in serum (Cmax) and total aminoglycoside exposure predict efficacy. Few trials have adequately examined these elements in the clinical setting. Cmax (9, 11–14) and Cmax/MIC for the organism (Cmax/MIC) (21, 24) are thought to be the best predictors of efficacy; this hypothesis is consistent with an antibiotic with concentration-dependent killing activity. Antibiotic “therapeutic ranges” are usually inadequate for drugs with concentration-dependent killing activity, because the range of MICs for the bacteria will generally be larger than the therapeutic range. The current therapeutic range for aminoglycosides was derived from a small number of inadequately controlled studies. These studies have varied in their definitions of pharmacokinetic parameters (i.e., time of Cmax), have not been designed to optimally examine relationships between other pharmacokinetic and pharmacodynamic parameters, have not consistently examined concomitant antibiotic therapy, and have used in the same analysis sets of patients with different sites of infection. Although more sensitive clinical markers of therapeutic response may exist, previous studies have used only final classifications of cure and failure or improvement and no improvement as outcome determinants.
Temperature and leukocyte count are commonly used to judge improvement in patients with pneumonia, with resolution of these parameters defining treatment course and duration (1). The analysis described here was performed to determine if aminoglycoside pharmacokinetic and pharmacodynamic parameters could be used to optimize the outcome and the therapeutic response in patients with nosocomial pneumonia caused by gram-negative bacteria. If this is the case, potential reductions in the rates of patient morbidity and mortality, as well as lengths of hospitalization and institutional expenditures, may be realized.
(This study was presented in part at the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy, New Orleans, La., 15 to 18 September 1996.)
MATERIALS AND METHODS
Study population.
Adult (ages, ≥18 years) medical and surgical patients who were admitted to Bassett Healthcare from October 1981 to May 1995 and who received gentamicin or tobramycin for ≥72 h for documented first-episode pneumonia caused by gram-negative bacteria were eligible for analysis. A diagnosis of pneumonia was made according to the criteria of the Centers for Disease Control and Prevention (7), as follows: (i) a new, unexplained pulmonary infiltrate on chest radiograph, (ii) growth of a sole pathogenic organism in a culture of a purulent sputum sample, and (iii) leukocytosis and/or fever. Patients with pulmonary exacerbation of cystic fibrosis, neutropenic fever, or human immunodeficiency virus type 1 infection were excluded.
Data acquisition. (i) Clinical evaluation.
Prospectively collected therapeutic response data included days to temperature resolution (i.e., number of days after antibiotic treatment initiation until the patient’s temperature remained ≤37.9°C during the remainder of the hospital stay) and days to leukocyte count resolution (i.e., number of days after antibiotic treatment initiation until the patient’s leukocyte count remained 5,000 to 10,000/mm3 during the remainder of the hospital stay). Patients were classified as “cured” if they achieved both temperature and leukocyte count resolution during aminoglycoside therapy with eradication of the symptoms of infection (i.e., decreased sputum production and purulence), along with clinical improvement. Since repeat cultures were not performed consistently for these patients, eradication of the initial isolated pathogen was not included in the definition of “cured.”
(ii) Predictor variables.
Prospectively collected predictor variables included age, sex, weight, presence of shock, presence of comorbid conditions, estimated prognosis (23), intensive care unit (ICU) admission, laboratory test results, fluid intake and output, albumin and nutritional status, and organism culture and organism susceptibility data (Microscan; Dade, West Sacramento, Calif.), concurrent pharmacotherapy, concurrent antibiotic therapy, type and duration of aminoglycoside therapy, total aminoglycoside dose, and aminoglycoside dose/total and ideal body weight. Shock was defined as a systolic blood pressure of <80 mm Hg with urine output of <500 ml/day or a systolic blood pressure decrease of >50 mm Hg with a drop in the systolic blood pressure to <100 mm Hg with the decrease (28). Nutritional status was classified as normal (serum albumin concentration, ≥3.5 g/dl), mild depletion (serum albumin concentration, 2.8 to 3.4 g/dl), or moderate to severe depletion (serum albumin concentration, ≤2.7 g/dl) (17). Estimated patient prognosis was classified as rapidly fatal, ultimately fatal, or nonfatal by the method of McCabe and Jackson (23).
(iii) Toxicity evaluation.
Aminoglycoside-associated nephrotoxicity was defined as a rise in the serum creatinine concentration of ≥0.5 mg/dl if the initial serum creatinine concentration was <3.0 mg/dl or a rise of 1.0 mg/dl if the initial serum creatinine concentration was ≥3.0 mg/dl (3). A change in the serum creatinine concentration was determined by subtracting the initial concentration from the highest serum creatinine concentration attained during therapy or within 3 to 5 days after the discontinuation of aminoglycoside therapy. If the serum creatinine concentration began to rise after therapy was completed, it was monitored until it peaked.
Aminoglycoside pharmacokinetics.
Clinical and pharmacokinetic data were prospectively collected in a database by the Clinical Pharmacy Service (CPS) of Bassett Healthcare. The initial aminoglycoside dosing regimens were chosen by the patient’s physician. These regimens were compared to empiric calculations performed by CPS by using hospital-specific population pharmacokinetic parameters (4) and were altered only if there was a significant (i.e., ≥25%) discrepancy between the prescribed dose and the dose determined by empiric calculations.
Aminoglycoside pharmacokinetic analysis was performed within 72 h of the initiation of therapy. After a predose serum aminoglycoside sample was obtained, the dose was infused over 30 min and the exact infusion duration was recorded. One postdistributional serum sample was obtained at least 60 min after completion of the infusion. A second postdose serum sample was obtained at least one estimated half-life later. Samples were immediately spun and were assayed or frozen until analysis. Concentrations were analyzed in duplicate by the fluorescence polarization immunoassay technique (TDX; Abbott Laboratories, Abbott Park, Ill.).
Data on the concentration in serum were fitted to a one-compartment, intravenous-infusion model by the method of Sawchuk and Zaske (31). Cmax values were extrapolated to those that would be found 30 min after the end of the 30-min infusion, and trough concentrations (Cmin) were extrapolated to those that would be found immediately before administration of the next dose. The area under the concentration-time curve from time zero to 24 h (AUC0–24) was calculated by dividing the total daily dose (dose24) by the calculated aminoglycoside clearance (CL). Doses and intervals were modified to achieve a Cmax of 7 to 10 μg/ml and a Cmin of <2 μg/ml. Concentrations in serum were redetermined 24 to 72 h after adjustment of the initial dose. If patients had stable renal function and volume status, repeat two-point pharmacokinetic studies were performed. For patients with unstable clinical status or renal function, repeat three-point studies were performed. CPS ensured that any concurrent anti-infective agents were given at the appropriate doses according to the patient’s disease state, renal function, and hepatic function. Serum creatinine concentrations were determined before aminoglycoside therapy, every 2 days during therapy, and for 3 to 5 days after the completion of aminoglycoside therapy. Creatinine CL was calculated by the method of Cockcroft and Gault (8).
The pharmacokinetic variables analyzed included the first and the second measured aminoglycoside Cmax and Cmin, AUC0–24, AUC0–48, and AUC0–72, and the time-averaged AUC over 24 h for the entire course of therapy (AUC24). The pharmacodynamic variables analyzed included the first and the second measured Cmax/MIC, the first and the second measured Cmin/MIC, AUC0–24/MIC, AUC0–48/MIC, AUC0–72/MIC, and time-averaged AUC24/MIC.
Data analysis.
Data were analyzed with SAS software, version 6.08 (30), and Classification and Regression Tree (CART) software (34). To determine significant predictors for time to temperature resolution and leukocyte count resolution, univariate and multivariate Cox proportional hazards model (step in-step out procedure) analyses were performed. CART software was also used to determine significant predictors of therapeutic response and their breakpoints.
To determine the probability of time to temperature resolution and leukocyte count resolution by a specified day of aminoglycoside therapy (Yi) for each of the significant predictors determined (see above), days to temperature resolution and leukocyte count resolution were converted to categorical variables (response or nonresponse) for the specified day. Logistic regression analyses were performed for these categorical variables and each significant predictor of response in order to determine the following regression equation: Yi = constant + slope · predictor variable. The probability of response for each specified day of aminoglycoside therapy was then defined by the equation P = 1/(1 + e−Yi), where P is the probability of temperature or leukocyte count resolution by Yi.
Concurrent antibiotic therapy was assessed by means of a five-variable categorical set, run as a covariate, which included concurrent β-lactam therapy active against the isolated organism, concurrent β-lactam therapy inactive against the isolated organism, other antibiotic therapy active against the isolated organism, other antibiotic therapy inactive against the isolated organism, or no concurrent antibiotic therapy. Following the initial Cox proportional hazards model analyses with individual variables, further analyses were performed with interaction terms.
Statistical significance was defined as a P value of ≤0.05. Unless otherwise noted, all data are presented as medians (25th to 75th percentiles).
RESULTS
A total of 275 patients admitted from 1981 to 1995 met the criteria of the Centers for Disease Control and Prevention for pneumonia. Patients were excluded due to (i) the presence of pneumonia caused by fungal pathogens or gram-positive bacteria (n = 125), (ii) performance of only Kirby-Bauer susceptibility testing (n = 62), or (iii) alteration of antibiotic regimens shortly after the first 72 h of therapy which resulted in patients being deemed unevaluable for clinical response (n = 10). Therefore, 78 consecutively treated patients who had pneumonia caused by gram-negative bacteria and who were admitted from February 1983 to November 1993 were included in the study. Due to the exclusion of patients for whom Kirby-Bauer susceptibility testing was performed, the majority (95%) of patients were admitted within the last 6 years of the acquisition window (1987 to 1993). The four patients treated from 1983 to 1986 did not differ with respect to antibiotic therapy or medical intervention which would influence outcome and thus were included in all subsequent analyses. Patient demographics and summary characteristics are presented in Table 1. Most patients received concomitant β-lactam therapy. No patients were receiving corticosteroids or other immunosuppressive agents. Organisms isolated from the patients’ purulent sputum were those typical as causes of nosocomial pneumonia (1, 7), with P. aeruginosa predominating (58%). All isolated organisms were susceptible to the aminoglycoside used (MIC interquartile range, 1 to 4 μg/ml). Pneumonia developed a median of 11 days (7 to 21 days) after hospitalization.
TABLE 1.
Demographics and characteristics of 78 patients with nosocomial pneumonia caused by gram-negative bacteria
| Characteristic | Value |
|---|---|
| Age (yr)a | 69 (58–75) |
| Sex (no. [%] of patients) | |
| Male | 52 (67) |
| Female | 26 (33) |
| Wt (kg)a | |
| Total body wt | 65.6 (55.0–79.0) |
| Ideal body wt | 65.0 (54.2–73.0) |
| Year of hospital admission (no. [%] of patients) | |
| 1983–1986 | 4 (5) |
| 1987–1990 | 47 (60) |
| 1991–1993 | 27 (35) |
| Aminoglycoside used (no. [%] of patients) | |
| Gentamicin | 38 (49) |
| Tobramycin | 40 (51) |
| Concurrent β-lactam therapy (no. [%] of patients) | |
| Sensitive organism | 56 (72) |
| Resistant organism | 17 (22) |
| None | 5 (6) |
| ICU admission (no. [%] of patients) | |
| Medical service | 19 (24) |
| Surgical service | 21 (27) |
| Organisms isolated from sputum (no. [%] of patients) | |
| P. aeruginosa | 45 (58) |
| Enterobacter species | 9 (12) |
| Klebsiella species | 9 (12) |
| Serratia species | 6 (8) |
| Citrobacter species | 5 (6) |
| Acinetobacter species | 1 (1) |
| Escherichia coli | 1 (1) |
| Proteus species | 1 (1) |
| Stenotrophomonas species | 1 (1) |
Values are medians (interquartile ranges).
The aminoglycoside dosing regimens and the calculated pharmacokinetic and pharmacodynamic variables are presented in Tables 2 and 3, respectively. The median time to individualized pharmacokinetic monitoring (IPM) was 2.5 days (2 to 3 days) after antibiotic initiation. Patients were treated with an aminoglycoside for a median of 11 days (8 to 14 days). For 60 patients serum aminoglycoside concentrations were redetermined for IPM following initial dosage adjustment.
TABLE 2.
Aminoglycoside dosing characteristics for 78 patients with pneumonia caused by gram-negative bacteria
| Variable | Before IPM (n = 78) | After IPM (n = 60) |
|---|---|---|
| Aminoglycoside dose (mg)a | 105 (90–140) | 230 (175–320) |
| Dosing interval (no. [%] of patients) | ||
| 6 h | 1 (1) | 0 (0) |
| 8 h | 16 (21) | 15 (19) |
| 12 h | 49 (63) | 37 (48) |
| 18 h | 4 (5) | 8 (10) |
| 24 h | 8 (10) | 17 (22) |
| 36 h | 0 (0) | 1 (1) |
| Cmax (μg/ml)a | 5.3 (3.9–6.3) | 6.7 (5.2–7.6) |
| Cmin (μg/ml)a | 0.6 (0.3–1.1) | 0.8 (0.5–1.1) |
Values are medians (interquartile ranges).
TABLE 3.
Aminoglycoside pharmacokinetic and pharmacodynamic variables for 78 patients with pneumonia caused by gram-negative bacteria
| Variable | Median (interquartile range) |
|---|---|
| Aminoglycoside CL (ml/min/1.73 m2) | 71.5 (50.4–91.3) |
| Aminoglycoside half-life (h) | 3.5 (2.6–5.0) |
| AUC0–24 (μg · h/ml) | 52.2 (34.5–77.5) |
| AUC0–48 (μg · h/ml) | 104.9 (81.9–142.9) |
| AUC0–72 (μg · h/ml) | 168.8 (130.7–219.0) |
| AUC24 (μg · h/ml) | 56.5 (42.9–75.3) |
| First Cmax/MIC | 3.6 (1.4–6.2) |
| First Cmin/MIC | 0.3 (0.2–0.7) |
| Second Cmax/MIC | 3.7 (1.9–6.9) |
| Second Cmin/MIC | 0.4 (0.2–0.8) |
| AUC0–24/MIC (SIT−1 · 24 h) | 38.7 (13.9–67.2) |
| AUC0–48/MIC (SIT−1 · 24 h) | 77.0 (25.8–131.2) |
| AUC0–72/MIC (SIT−1 · 24 h) | 113.6 (40.8–199.9) |
| AUC24/MIC (SIT−1 · 24 h) | 38.9 (14.6–60.0) |
The patients’ median initial temperature was 39.2°C (37.8–40.1°C). The fever resolved in 5 days (0 to 10 days). The initial leukocyte count was 12,000/mm3 (9,000 to 17,000/mm3) and resolved in a median of 6.5 days (0 to 10 days). Seventy-two (92%) patients had resolution of fever, leukocyte count, and signs of infection and were classified as cures. Six (8%) patients did not meet these criteria and were classified as failures. Five failures were among patients receiving a combination β-lactam–aminoglycoside regimen to which the isolated organism was sensitive, and one failure occurred in a patient receiving a concomitant β-lactam to which the isolated organism was resistant.
The median maximum change in the serum creatinine concentration was 0.1 mg/dl (−1.4 to 1.7 mg/dl), while the median maximum change in creatinine CL was 0.35 ml/min/1.73 m2 (−67 to 124 ml/min/1.73 m2). Eight (10.3%) patients treated with an aminoglycoside for 12 days (range, 8 to 15 days) met the nephrotoxicity criteria, with one patient having a rising serum creatinine concentration prior to aminoglycoside therapy and 7 (9%) patients developing nephrotoxicity during aminoglycoside therapy.
Analyses of therapeutic response variables. (i) Days to temperature resolution.
Cox proportional hazards model analysis was performed for each clinical, pharmacokinetic, and pharmacodynamic predictor variable. Statistically significant variables included first Cmax, second Cmax, first Cmax/MIC, second Cmax/MIC, AUC0–24/MIC, AUC0–48/MIC, AUC0–72/MIC, first Cmin, and first Cmin/MIC. The total aminoglycoside dose, total AUC, duration of therapy, the MIC for the organism, age, prognosis, ICU admission, ventilator status, nutritional status, and Pseudomonas infection were not significant. Concurrent antibiotic therapy, defined as a categorical set and run as a covariate, was not a significant predictor of therapeutic response. The results for the multivariate Cox proportional hazards model are as follows. For all patients (n = 78), the first Cmax/MIC was the most predictive of response for time to temperature resolution (P = 0.01). For patients with repeat IPM, the second Cmax/MIC was the most predictive of response for both time to temperature resolution (P = 0.004) and time to leukocyte count resolution (P = 0.001).
CART analysis was performed with the significant predictors of response determined by the multivariate Cox proportional hazards model analysis. The model was as follows: temperature resolution by Yi = first Cmax/MIC + second Cmax/MIC + AUC0–24/MIC + AUC24/MIC. Data from treatment days 5, 7, and 9 were chosen for evaluation. Data for days 5 and 9 represented the 95% confidence intervals for the patients’ mean response times. Data for day 7 represented the allotted diagnosis-related group length of stay for complicated pneumonia (7.6 days). By this analysis, the variable with the highest sum of improvements was first Cmax/MIC. CART analysis was also performed for each individual significant predictor variable (as determined by Cox proportional hazards model analyses) for days 5, 7, and 9 to determine their breakpoints. The first Cmax/MIC breakpoint for temperature resolution by day 7 of therapy was 4.7.
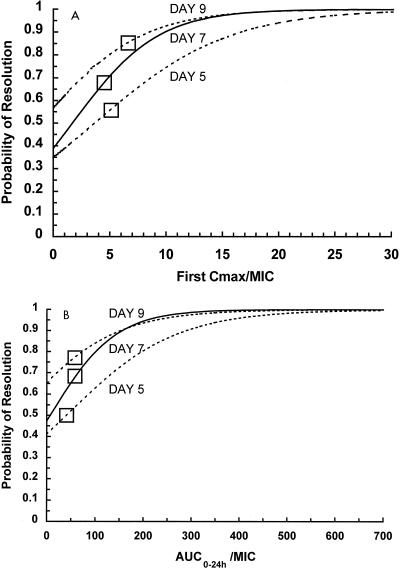
Figure 1 represents the logistic regression-derived equations for the probability of achieving temperature resolution. This illustrates the fact that higher aminoglycoside target values for the first Cmax/MIC are needed to effect an earlier therapeutic response. The first Cmax/MIC of ≥10 is associated with a ≥90% probability of temperature resolution by day 7 of therapy. AUC0–24/MIC was a less important predictor. An AUC0–24/ MIC serum inhibitory titer (SIT) of ≥150 SIT−1 · 24 h is associated with a ≥90% probability of temperature resolution by day 7 of therapy. In our patients dosed with IPM to achieve traditional ranges of concentrations in serum, an MIC breakpoint of approximately 0.3 μg/ml would have been needed in order to attain these Cmax/MIC and AUC0–24/MIC target values.
FIG. 1.
Probability of temperature resolution by days 5, 7, and 9 of aminoglycoside therapy as determined by logistic regression analysis. (A) Use of first Cmax/MIC as a predictor variable. (B) Use of AUC0–24/MIC as a predictor variable. □, breakpoints for the significant predictors as determined by CART analysis.
(ii) Days to leukocyte count resolution.
Cox proportional hazards model analysis for days to leukocyte count resolution found the following significant variables: second Cmax, AUC0–72, first Cmax/MIC, second Cmax/MIC, AUC24/MIC, AUC0–24/MIC, AUC0–48/MIC, AUC0–72/MIC, first Cmin, first Cmin/MIC, duration of aminoglycoside therapy, and total aminoglycoside dose. Nonsignificant variables included total AUC, organism MIC, age, prognosis, ICU admission, ventilator status, nutritional status, and Pseudomonas infection. Concurrent antibiotic therapy, defined as a categorical set and run as a covariate, was not a significant predictor of therapeutic response. The results for the multivariate Cox proportional hazards model analyses are as follows. For all patients (n = 78), the AUC0–72 was most predictive of response (P = 0.03). For patients with repeat IPM, the second Cmax/MIC was the most predictive of response (P = 0.001).
CART analysis was performed with the following model (by using the significant predictors of response determined by multivariate Cox proportional hazards model analysis): leukocyte count resolution by Yi = first Cmax/MIC + second Cmax/MIC + AUC0–24MIC + AUC24/MIC. The variable with the highest sum of improvements was second Cmax/MIC. CART analysis was performed for each significant predictor variable for days 5, 7, and 9 of aminoglycoside therapy to determine their breakpoints for time to leukocyte count resolution. The second Cmax/MIC breakpoint for leukocyte count resolution by day 7 of therapy was 3.5.
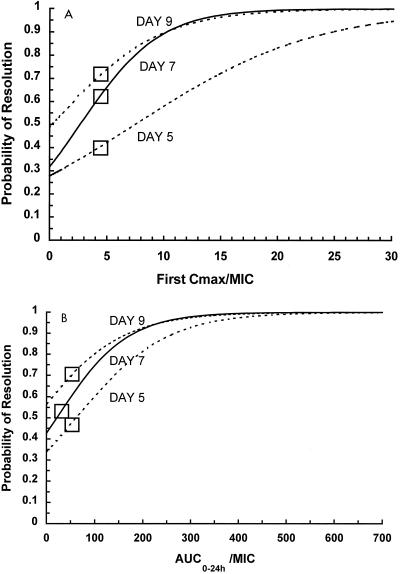
Figure 2 represents the logistic regression-derived equations for the probability of achieving leukocyte count resolution. Higher target values for predictor variables are needed in order to effect an earlier therapeutic response. By these equations, a first measured Cmax/MIC of ≥10 and a second measured Cmax/MIC of ≥12.5 are associated with a ≥90% probability of leukocyte count resolution by day 7 of aminoglycoside therapy. The probability of response for AUC0–24/MIC revealed similar relationships: an AUC0–24/MIC of ≥175 SIT−1 · 24 h is associated with a ≥90% probability of leukocyte count resolution by day 7 of aminoglycoside therapy. With the dosing regimens used for our group of patients, an MIC breakpoint of approximately 0.3 μg/ml would have been needed in order to attain these Cmax/MIC and AUC0–24/MIC target values.
FIG. 2.
Probability of leukocyte count resolution by days 5, 7, and 9 of aminoglycoside therapy as determined by logistic regression analysis. (A) Use of first Cmax/MIC as a predictor variable. (B) Use of AUC0–24/MIC as a predictor variable. □, breakpoints for the significant predictors as determined by CART analysis.
DISCUSSION
Nosocomial infections result in significant morbidity and mortality and prolong the length of hospitalization by an average of 14 days (15). These complications may also result in institutional financial loss under prospective payment systems. Rapid response to antibiotic therapy is desirable to improve patient outcomes and reduce financial losses. Antibiotic therapy is commonly individualized according to the resolution of clinical response variables such as elevated leukocyte count, fever, and sputum production and purulence. Our study is the first analysis that provides data that correlate a rapid (i.e., day 7) clinical response in patients with nosocomial pneumonia caused by gram-negative bacteria to aminoglycoside pharmacodynamic variables.
Aminoglycosides are effective, inexpensive antibiotics, making them attractive for the treatment of a variety of infections caused by gram-negative bacteria. However, concerns regarding ototoxicity, nephrotoxicity, and uncertain optimal pharmacokinetic and pharmacodynamic targets for efficacy have made newer, broad-spectrum β-lactam and quinolone antibiotics enticing options, despite their increased expense and their propensity to select resistant mutants. At issue for aminoglycoside therapy is the identification of specific pharmacodynamic targets for therapeutic response for various pathogens, infection sites, and patient immune function.
Previous trials have investigated aminoglycoside pharmacodynamic relationships incompletely (11, 24, 25, 29, 33). Each of these investigations used a cure and fail endpoint or an improvement and no improvement endpoint rather than other, more clinically relevant outcome parameters which often define parenteral antibiotic treatment course and duration. This is particularly important in the current era of cost containment, since symptom resolution affects pharmacotherapy modification (1) and the length of hospitalization.
We have demonstrated an important relationship between aminoglycoside concentrations, organism susceptibility, and therapeutic response. This is the first analysis that may be considered relevant to current practice since combination antibiotic therapy was consistently evaluated. Note that when multiple patient factors were analyzed for both outcome and time to temperature resolution and leukocyte count resolution, only aminoglycoside pharmacodynamic variables were significant. We were unable to derive any statistical relationship between concomitant antibiotic therapy and temperature or leukocyte count resolution. Because the concentrations of the concomitantly administered antibiotics in serum were not measured, we could not specifically assess β-lactam pharmacokinetic and pharmacodynamic parameter influences on the therapeutic response. However, with 28% of our patients essentially receiving aminoglycoside monotherapy (6% of patients received monotherapy and 22% received therapy with a β-lactam to which the infecting organism was resistant), any significant associations between concomitant antibiotic therapy and therapeutic response should have been evident. These results are consistent with a literature review which concluded that more data in support of aminoglycoside monotherapy than β-lactam monotherapy for the treatment of pneumonia caused by gram-negative bacteria exist, with few prospective data suggesting the superiority of combination therapy over monotherapy (9).
There is no dispute that aminoglycosides in combination with β-lactam antibiotics are effective in treating bacillary pneumonia caused by gram-negative bacteria. However, the question of how quickly a response can be effected with these agents remains. The use of more sensitive markers of therapeutic response (e.g., days to temperature resolution) may be more appropriate for the determination of optimal pharmacokinetic and pharmacodynamic goals. Since aminoglycosides kill bacteria in a concentration-dependent manner and β-lactams operate in a time-dependent fashion, the aminoglycosides may primarily be responsible for the early therapeutic response seen with combination antibiotic therapy. Because the aminoglycoside concentrations in the bronchial secretions of our patients were not measured, this investigation assumes but cannot demonstrate that high concentrations in serum result in high concentrations in pulmonary secretions. However, the concept of a shorter time to bacterial eradication with the quinolone ciprofloxacin (concentration-dependent killing effect) in comparison to that with the antibiotic cefmenoxime (time-dependent killing effect) at equal measures of exposure has previously been described for patients with nosocomial pneumonia (16).
An important strength of our investigation is that all statistical analyses of predictor variables were concordant. Cmax/MIC was most predictive of days to temperature resolution and leukocyte count resolution by Cox proportional hazards model, logistic regression, and CART analyses. CART analyses determined that achievement of Cmax/MIC of >4.7 within 48 h of the initiation of aminoglycoside therapy results in a temperature resolution success rate of 89% and a leukocyte count resolution success rate of 86% by day 7. By logistic regression analysis, we determined that to achieve a 90% probability of therapeutic response by day 7, a Cmax/MIC ratio of ≥10 must be achieved within the first 48 h of therapy.
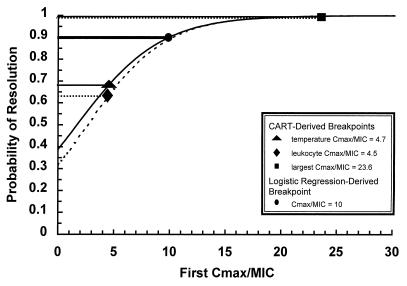
The difference in Cmax/MIC ratios that predict similar success rates between logistic regression (Cmax/MIC of 10 producing a 90% success rate) and CART analyses (Cmax/MIC ratios of 4.7 and 4.5 producing success rates of 86 and 89%, respectively) can be explained by the CART methodology. While regression analysis allows for specific predictions by yielding a summary for the averages of the distributions corresponding to a set of Cmax/MIC ratios, CART analysis determines the point which maximizes the probability of the correct classification of subjects as responders or nonresponders. Thus, for temperature resolution by day 7, all patients for which the Cmax/MIC was >4.7 are combined, and the average of the probabilities of response is calculated. As indicated in Fig. 3, a Cmax/MIC of 4.7 corresponds to a 68% probability of response, while a Cmax/MIC of 23.6 (the highest value seen for our 78 patients) corresponds to a 99% probability of therapeutic response; the average of this probability range is 89%. Similarly, for leukocyte count resolution by day 7, a Cmax/MIC breakpoint of 4.5 by CART analysis corresponds to a 63% probability of response, while the maximum Cmax/MIC seen for our patients (23.6) corresponds to a 99% probability of response; the average of this range is 86%.
FIG. 3.
Probability of therapeutic response by day 7 of aminoglycoside therapy by using first Cmax/MIC as the predictor variable: comparison of logistic regression- and CART-derived breakpoints. ———, temperature resolution data; –––, leukocyte count resolution data; ————————, temperature resolution and leukocyte count resolution probability as determined by logistic regression analysis.
Our data demonstrate that initial dosing choices for patients with nosocomial pneumonia caused by gram-negative bacteria often result in suboptimal Cmax/MIC ratios. Optimal concentrations in serum may best be achieved by giving large loading doses of aminoglycosides immediately followed by IPM with the first dose (21). If the pathogen and/or the MIC is unknown at the time of IPM, target concentrations can be determined empirically by using institutional MIC data for potential causative bacteria. We caution against extrapolating these data to single daily dosing regimens with aminoglycosides since the majority of our patients were dosed two to three times daily, and thus, optimal Cmax/MIC ratios were obtained multiple times within a 24-h interval.
Other advantages to using pharmacodynamic targets to affect the therapeutic response include the potential effect on toxicity. The duration of aminoglycoside therapy is an important predictor of nephrotoxicity (3, 32) and ototoxicity (2, 27). Maximization of the probability of a therapeutic response with aminoglycoside therapy for pneumonia caused by gram-negative bacteria through optimization of the Cmax/MIC ratio may result in shorter courses of aminoglycoside therapy and may minimize the risk of toxicity.
In conclusion, the present analysis demonstrates that early optimization of aminoglycoside pharmacodynamic targets may shorten the time to clinical improvement in patients with nosocomial pneumonia caused by gram-negative bacteria. This approach is both clinically and economically advantageous. Aminoglycosides are inexpensive, effective agents for the treatment of infections caused by gram-negative bacteria. A rapid clinical response may result in earlier extubation of ventilated patients and shortened stays in an ICU. A clinical response may also be an indicator for shorter courses of intravenous antibiotic therapy, quicker conversion to oral antibiotic regimens, and earlier discharge from the hospital. Additionally, shortened courses of aminoglycoside therapy minimize the risk of nephrotoxicity and ototoxicity. Rapid bacterial eradication can also decrease the risk of emergence of resistance (5). Although the data used in this analysis were collected prospectively in a database, this remains a retrospective analysis, and prospective studies are needed to validate our findings.
ACKNOWLEDGMENT
This work was supported by Abbott Diagnostics, Inc.
REFERENCES
- 1.American Thoracic Society. Hospital-acquired pneumonia in adults: diagnosis, assessment of severity, initial antimicrobial therapy, and preventative strategies. A consensus statement. Am J Respir Crit Care Med. 1995;153:1711–1725. doi: 10.1164/ajrccm.153.5.8630626. [DOI] [PubMed] [Google Scholar]
- 2.Bendush C L, Senior S L, Wooller H O. Evaluation of nephrotoxic and ototoxic effects of tobramycin in worldwide study. Med J Aust Spec Suppl. 1977;2:22–26. doi: 10.5694/j.1326-5377.1977.tb113913.x. [DOI] [PubMed] [Google Scholar]
- 3.Bertino J S, Jr, Booker L A, Franck P A, Jenkins P L, Franck K R, Nafziger A N. Incidence of and significant risk factors for aminoglycoside-associated nephrotoxicity in patients dosed by using individualized pharmacokinetic monitoring. J Infect Dis. 1993;167:173–179. doi: 10.1093/infdis/167.1.173. [DOI] [PubMed] [Google Scholar]
- 4.Bertino J S, Jr, Booker L A, Franck P, Rybicki B. Gentamicin pharmacokinetics in patients with malignancies. Antimicrob Agents Chemother. 1991;35:1501–1503. doi: 10.1128/aac.35.7.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blaser J, Stone B B, Groner M C, Zinner S H. Comparative study with enoxacin and netilmicin in a pharmacodynamic model to determine importance of ratio of antibiotic pharmacokinetic concentrations to MIC for bacterial activity and emergence of resistance. Antimicrob Agents Chemother. 1987;31:1055–1060. doi: 10.1128/aac.31.7.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bryan C S, Reynolds K L. Bacteremic nosocomial pneumonia. Am Rev Respir Dis. 1984;129:668–671. doi: 10.1164/arrd.1984.129.5.668. [DOI] [PubMed] [Google Scholar]
- 7.Center for Disease Control and Prevention. National nosocomial infection surveillance study. Hospital Infections Program. Atlanta, Ga: Centers for Disease Control and Prevention; 1994. [Google Scholar]
- 8.Cockcroft D W, Gault M H. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 9.Collins T, Gerding D N. Aminoglycosides versus β-lactams in gram-negative pneumonia. Semin Respir Infect. 1991;6(3):136–146. [PubMed] [Google Scholar]
- 10.Craven D E, Steeger K A, Barber T W. Preventing nosocomial pneumonia: state of the art and perspectives for the 1990’s. Am J Med. 1991;91:44S–53S. doi: 10.1016/0002-9343(91)90343-v. [DOI] [PubMed] [Google Scholar]
- 11.Deziel-Evans L M, Murphy J E, Job M L. Correlation of pharmacokinetic indices with therapeutic outcome in patients receiving aminoglycosides. Clin Pharm. 1986;5:319–324. [PubMed] [Google Scholar]
- 12.Donowitz G R, Mandell G L. Acute pneumonia. In: Mandell G L, Bennett J E, Dolin R, editors. Principles and practice of infectious diseases. New York, N.Y: Churchill Livingstone; 1995. [Google Scholar]
- 13.Fagon J Y, Chastre Y, Domart Y, Trouillet J L, Pierre J, Carne C, Gibert C. Nosocomial pneumonia in patients receiving continuous mechanical ventilation. Am Rev Respir Dis. 1989;139:877–884. doi: 10.1164/ajrccm/139.4.877. [DOI] [PubMed] [Google Scholar]
- 14.Fagon J Y, Chastre J, Hance A, Montravers P, Novara A, Gibert C. Nosocomial pneumonia in ventilated patients: a cohort study evaluating attributable mortality and hospital stay. Am J Med. 1993;94:281–288. doi: 10.1016/0002-9343(93)90060-3. [DOI] [PubMed] [Google Scholar]
- 15.Freeman J, Rosner B A, McGowan J E., Jr Adverse effects of nosocomial infection. J Infect Dis. 1979;140:732–740. doi: 10.1093/infdis/140.5.732. [DOI] [PubMed] [Google Scholar]
- 16.Goss T F, Forrest A, Nix D E, Ballow C H, Birmingham M C, Cumbo T J, Schentag J J. Mathematical examination of dual individualization principles. II. The rate of bacterial eradication at the same area under the inhibitory curve is more rapid for ciprofloxacin than for cefmenoxime. Ann Pharmacother. 1994;28:863–868. doi: 10.1177/106002809402800707. [DOI] [PubMed] [Google Scholar]
- 17.Grant J P. Handbook of total parenteral nutrition. Philadelphia, Pa: The W. B. Saunders Co.; 1980. [Google Scholar]
- 18.Gross P A, New H C, Aswapokee P, Van Antwerpen C, Aswapokee N. Deaths from nosocomial infections: experience in a university hospital and a community hospital. Am J Med. 1980;68:219–223. doi: 10.1016/0002-9343(80)90357-5. [DOI] [PubMed] [Google Scholar]
- 19.Gross P A, Van Antwerpen C. Nosocomial infections and hospital deaths: a case-control study. Am J Med. 1983;75:658–662. doi: 10.1016/0002-9343(83)90453-9. [DOI] [PubMed] [Google Scholar]
- 20.Jackson G G, Riff L J. Pseudomonas bacteremia: pharmacologic and other bases for failure of treatment with gentamicin. J Infect Dis. 1971;124(Suppl.):S185–S191. doi: 10.1093/infdis/124.supplement_1.s185. [DOI] [PubMed] [Google Scholar]
- 21.Kashuba A D M, Bertino J S, Jr, Nafziger A N. Dosing of aminoglycosides to rapidly attain pharmacodynamic goals and hasten therapeutic response by using individualized pharmacokinetic monitoring of patients with pneumonia caused by gram-negative organisms. Antimicrob Agents Chemother. 1998;42:1842–1844. doi: 10.1128/aac.42.7.1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klastersky J, Daneau D, Swings G, Weerts D. Antibacterial activity in serum and urine as a therapeutic guide in bacterial infections. J Infect Dis. 1974;129:187–193. doi: 10.1093/infdis/129.2.187. [DOI] [PubMed] [Google Scholar]
- 23.McCabe W R, Jackson G G. Gram-negative bacteremia. I. Etiology and ecology. Arch Intern Med. 1962;110:83–91. [Google Scholar]
- 24.Moore R D, Lietman P S, Smith C R. Clinical response to aminoglycoside therapy: importance of the ratio of peak concentration to minimal inhibitory concentration. J Infect Dis. 1987;155:93–99. doi: 10.1093/infdis/155.1.93. [DOI] [PubMed] [Google Scholar]
- 25.Moore R D, Smith C R, Lietman P S. Association of aminoglycoside plasma levels with therapeutic outcome in gram-negative pneumonia. Am J Med. 1984;77:657–662. doi: 10.1016/0002-9343(84)90358-9. [DOI] [PubMed] [Google Scholar]
- 26.Moore R D, Smith C R, Lietman P S. The association of aminoglycoside plasma levels with mortality in patients with gram-negative bacteremia. J Infect Dis. 1984;149:443–448. doi: 10.1093/infdis/149.3.443. [DOI] [PubMed] [Google Scholar]
- 27.Moore R D, Smith C R, Lietman P S. Risk factors for the development of auditory toxicity in patients receiving aminoglycosides. J Infect Dis. 1984;149:23–30. doi: 10.1093/infdis/149.1.23. [DOI] [PubMed] [Google Scholar]
- 28.Moore R D, Smith C R, Lipsky J J, Mellits E D, Lietman P S. Risk factors for nephrotoxicity in patients treated with aminoglycosides. Ann Intern Med. 1984;100:352–357. doi: 10.7326/0003-4819-100-3-352. [DOI] [PubMed] [Google Scholar]
- 29.Noone P, Parsons T M C, Pattison J R, Slack R C B, Garfield-Davies D, Hughes K. Experience in monitoring gentamicin therapy during treatment of serious gram-negative sepsis. Br Med J. 1974;1:477–481. doi: 10.1136/bmj.1.5906.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.SAS Institute. SAS user’s guide. Statistics. Cary, N.C: SAS Institute; 1985. [Google Scholar]
- 31.Sawchuk R J, Zaske D E. Pharmacokinetics of dosing regimens which utilize multiple intravenous infusions: gentamicin in burn patients. J Pharmacokinet Biopharm. 1976;4:183–195. doi: 10.1007/BF01086153. [DOI] [PubMed] [Google Scholar]
- 32.Sawyers C L, Moore R D, Lerner S A, Smith C R. A model for predicting nephrotoxicity in patients treated with aminoglycosides. J Infect Dis. 1986;33:1062–1068. doi: 10.1093/infdis/153.6.1062. [DOI] [PubMed] [Google Scholar]
- 33.Schentag J J, Nix D E, Adelman M H. Mathematical examination of dual individualization principles. I. relationships between AUC above MIC and area under the inhibitory curve for cefmenoxime, ciprofloxacin, and tobramycin. DICP Ann Pharmacother. 1991;25:1050–1057. doi: 10.1177/106002809102501003. [DOI] [PubMed] [Google Scholar]
- 34.Steinberg D, Colla P. CART: tree-structured nonparametric data analysis. San Diego, Calif: Salford Systems; 1995. [Google Scholar]
- 35.Zaske D E, Bootman J L, Solem L B, Strate R G. Increased burn patient survival with individualized dosages of gentamicin. Surgery. 1982;91:142–149. [PubMed] [Google Scholar]