Abstract
Background
The diagnosis of arrhythmogenic cardiomyopathy (ACM) is challenging especially in children at risk of adverse events. Analysis of cardiac myocyte junctional protein distribution may have diagnostic and prognostic implications, but its utility is limited by the need for a myocardial sample. We previously reported that buccal mucosa cells show junctional protein redistribution similar to that seen in cardiac myocytes of adult patients with ACM.
Objectives
We aimed to determine when junctional protein distribution abnormalities first occur in children with ACM variants and whether they correlate with progression of clinically apparent disease.
Methods
We analyzed buccal mucosa samples of children and adolescents with a family history of ACM (n = 13) and age-matched controls (n = 13). Samples were immunostained for plakoglobin, desmoplakin, plakophilin-1 and connexin43 and analyzed by confocal microscopy. All participants were swabbed at least twice with an average interval of 12–18 months between samplings.
Results
Junctional protein re-localization in buccal mucosa cells did not correlate with the presence of ACM-causing variants but instead occurred with clinical onset of disease. No changes in protein distribution were seen unless and until there was clinical evidence of disease. In addition, progressive shifts in the distribution of key proteins correlated with worsening of the disease phenotype. Finally, we observed restoration of junctional signal for Cx43 in patient with a favorable response to anti-arrhythmic therapy.
Conclusions
Due to ethical concerns about obtaining heart biopsies in children with no apparent disease, it has not been possible to analyze molecular changes in cardiac myocytes with the onset/progression of clinical disease. Using buccal smears as a surrogate for the myocardium may facilitate future studies of mechanisms and pathophysiological consequences of junctional protein redistribution in ACM. Buccal cells may also be a safe and inexpensive tool for risk stratification and potentially monitoring response to treatment in children bearing ACM variants.
Keywords: Arrhythmogenic cardiomyopathy (ACM), Sudden cardiac death (SCD), Novel prognostic test, Cheek smear analysis, Desmosomal proteins, Connexin43 (Cx43)
1. Introduction
Arrhythmogenic cardiomyopathy (ACM) is a familial myocardial disease characterized by a high incidence of ventricular arrhythmias and sudden cardiac death particularly in young individuals and athletes [1]. The diagnosis of ACM can be challenging because of variable age-related expression of disease and incomplete genetic penetrance. This is especially true for the prospective identification of children and young adults at risk of adverse events.
ACM is often caused by variants in genes encoding proteins in desmosomes, cell-cell adhesion organelles in myocardial intercalated disks [3]. Several studies have demonstrated reduced amounts of immunoreactive signal for selected desmosomal proteins, gap junction proteins, and ion channel proteins at intercalated discs in myocardium from ACM patients. The most consistent reductions, seen in hearts of patients with classical right ventricular disease, involve the desmosomal protein plakoglobin and the major ventricular gap junction protein connexin43 (Cx43) [[4], [5], [6]]. The diagnostic and/or prognostic significance of such changes is the subject of ongoing investigation but the potential clinical utility of this approach is severely limited by the need for myocardial tissue for analysis. Recognizing that pathogenic variants in desmosomal genes cause disease in heart and skin [7], we previously investigated whether buccal mucosa cells, which can be easily and safely obtained at minimal cost, could be used as a surrogate for myocardium in ACM patients. We found that immunoreactive signals for plakoglobin and Cx43 were diminished at cell junctions in buccal cell smears from ACM patients [8]. Moreover, junctional signal for the desmosomal protein desmoplakin was reduced in buccal cells from patients with pathogenic variants in DSP, DSG2, or DSC2 but not PKP2 or JUP, whereas signal for the desmosomal protein plakophilin-1 (an isoform of plakophilin-2 expressed in outer stratified squamous epithelial cells) was depressed in patients with variants in PKP2 but not in other desmosomal genes [8]. Redistribution of plakoglobin appeared to occur preferentially in ACM as it was not seen in buccal smears obtained from patients with ischemic, dilated, or hypertrophic cardiomyopathy [8]. Moreover, buccal cells can be maintained in culture and used for personalized drug screening. For example, we showed that reduced cell surface signals for plakoglobin and Cx43 in cultured buccal cells from 6 adult ACM patients with documented desmosomal gene variants were normalized following exposure to SB216763, a GSK3β inhibitor that reverses ACM-related features in experimental models [[8], [9], [10]].
The earliest clinical manifestations of ACM do not usually become apparent until young adulthood or later [2]. It is not known, however, when changes in the distribution of cell-cell junction proteins first occur or whether they correlate with the onset or progression of clinically apparent disease. To address this question, we have analyzed buccal mucosal cells in children and adolescents from families with documented ACM variants. We found that changes in cell-cell junction proteins track with the clinical phenotype. No changes in junctional protein localization were seen in buccal smears until and unless there was evidence of disease. In addition, progressive shifts in the distribution of some proteins correlated with worsening of the disease phenotype. We also observed restoration of junctional Cx43 signal in one patient in response to bisoprolol treatment. These results suggest that analysis of buccal cells may be a safe, inexpensive and non-invasive tool to predict prognosis and potentially assess efficacy of pharmaceutical interventions in children and adolescents from families that carry pathogenic variants linked to ACM.
2. Methods and materials
2.1. Study group
Buccal mucosa smears were prepared from 12 children or adolescents (age range: 3–18) with a family history of ACM who were being clinically evaluated at the Great Ormond Street Hospital (GOSH) Centre for Inherited Cardiovascular Disease, London or the Nikos Protonotarios Medical Centre in Naxos, Greece. These individuals undergo comprehensive annual clinical phenotyping including resting, ambulatory and exercise electrocardiography (ECG); signal-averaged ECG (SA-ECG); advanced echocardiography and cardiac magnetic resonance imaging (MRI). Buccal smears were obtained during these annual hospital visits. All but one of these patients had documented pathogenic variants in desmosomal genes (JUP, PKP2, DSP, DSG2) including 8 with heterozygous dominant variants, 1 with compound heterozygous variants in DSP, and 1 each with homozygous recessive variants in JUP or PKP2. The remaining child showed no known pathogenic variants in a 96-gene cardiomyopathy panel. Gene variants in desmosomal genes, when identified, were classified as variants of uncertain significance (VUS), likely pathogenic or pathogenic using American College of Medical Genetics (ACMG) consensus guidelines. VUS with family history of ACM were considered as likely pathogenic/pathogenic in this study. Buccal smears were also obtained from 13 healthy children (age range: 3–18) with no clinical manifestations or family history of heart disease who served as normal controls. All subjects and/or their parents/guardians provided written informed consent. Sample collection protocols were approved by the National Health Service Health Research Authority (NHS/HRA) in the United Kingdom and appropriate institutional review boards in Greece. Standard confidentiality protocols were followed and database information was stored in password-encrypted files. All buccal mucosa samples were coded and analyzed in a blinded fashion. The majority of the study participants were swabbed twice with an average interval of 12–18 months between sampling.
2.2. Buccal mucosa sampling and preparation of smears
Clean, individually-wrapped cytology brushes were used for collection of buccal cells. Material was collected from the inside of the cheek by using slight rolling and scraping motions for about 30 s on each side. Immediately after collection, the buccal mucosa material was smeared on positively-charged microscopy slides, which were sprayed with a fixative (M-FIX, Merck) from a distance of ~30 cm. The slides were allowed to air-dry and stored at room temperature before being immunostained.
2.3. Immunohistochemistry
Buccal mucosa smears were immunostained with the following primary antibodies using established protocols: mouse monoclonal anti-plakoglobin (P8087, Sigma Aldrich), mouse monoclonal anti-plakophilin-1 (325700, Thermo Fisher Scientific), mouse monoclonal anti-desmoplakin (10R-D108AX, Fitzgerald), and mouse polyclonal anti-Cx43 (C8093, Sigma Aldrich). Slides were then incubated with Cy3-conjugated secondary antibodies (Jackson ImmunoResearch) for 2 h at room temperature and counterstained with DAPI to label nuclei. All immunostained preparations were imaged at x20 using a Nikon A1R confocal microscope.
We optimized our immunostaining protocol for each protein by first determining the lowest primary antibody concentration that produced strong signal in control buccal mucosa cells. These conditions were then applied throughout the study to determine whether cells from subjects from ACM families showed apparent reduction in junctional signal. Using this approach, we consistently observed either control levels of signal or a virtual absence of junctional signal for any given primary antibody. This ‘binary’ approach ensures reproducibility of our results and precludes the need for signal quantification. Each batch of ACM samples was immunostained alongside freshly-obtained smears from age-matched controls.
3. Results
3.1. Immunoreactive signals for selected junction proteins are reduced in buccal mucosa cells in children with clinical ACM
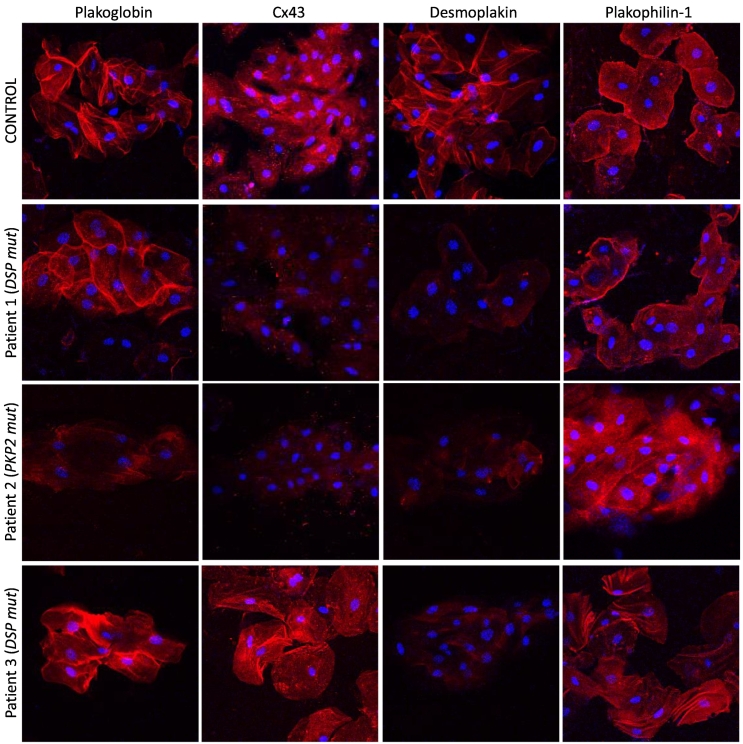
To determine whether changes in the distribution of cell-cell junction proteins occur in buccal mucosa cells in children with clinically manifest ACM, as previously observed in adults [8], we analyzed buccal smears from 3 children with documented ACM and pathogenic variants in desmosomal genes (Group A, Table 1). Patient 1 (currently 12 years of age) developed woolly hair and palmoplantar keratoderma during the first year of life. He was evaluated at age 2 years after genetic testing revealed compound heterozygosity for a Ser711fsX4 frameshift and an Arg2586X nonsense variant in DSP. Cardiomyopathy was first diagnosed at age 7 years. At the time the first buccal swab was obtained (2019), he had dilated cardiomyopathy (DCM) with significant left ventricular (LV) dilatation and systolic impairment on echocardiography and non-sustained ventricular tachycardia (NSVT) on Holter monitoring, in addition to a recent history of recurrent syncope. When the second buccal swab was obtained (2020) his condition was stable without significant progression of disease. Analysis of both sets of buccal smears showed strong membrane immunoreactive signals for plakoglobin and plakophilin-1 but severely depressed signals for desmoplakin and Cx43 compared to age-matched controls (Fig. 1).
Table 1.
Demographic features and mutation profiles of children.
| Patient number | Sex | Current age (years) | Gene | Nucleotide position | Protein change | Mutation type | Zygosity | Pathogenecity |
|
|---|---|---|---|---|---|---|---|---|---|
| ACMG classification | |||||||||
| 1 | M | 13 | DSP | c.2131_2132delAG | p.Ser711CysfsX4 | Frameshift | Compound heterozygous | Pathogenic | |
| c.7756C > T | p.Arg2586X | Nonsense | Pathogenic | ||||||
| Group A | 2 | F | 9 | PKP2 | c.2577G > T | p.Lys859Asn | Missense | Homozygous | Likely pathogenic |
| 3 | M | 9 | DSP | c.250C > T | p.Arg84X | Nonsense | Heterozygous | Pathogenic | |
| 4 | M | 4 | JUP | c.2038_2039del | p.Trp680GlyfsX11 | Frameshift | Homozygous | Pathogenic | |
| 5 | M | 16 | PKP2 | c.2489 + 1 G > A | – | Splicing variant | Heterozygous | Pathogenic | |
| Group B | 6 | M | 12 | DSG2 | c.1003A > G | p.Thr335Ala | Missense | Heterozygous | VUS |
| 7 | F | 18 | PKP2 | c.2146-1G > C | – | Splicing variant | Heterozygous | Pathogenic | |
| 8 | F | 12 | PKP2 | c.148_151delACAG | p.Thr50SerfsX61 | Frameshift | Heterozygous | Pathogenic | |
| 9 | M | 13 | PKP2 | c.148_151delACAG | p.Thr50SerfsX61 | Frameshift | Heterozygous | Pathogenic | |
| 10 | M | 16 | PKP2 | c.2146-1G > C | – | Splicing variant | Heterozygous | Pathogenic | |
| Group C | 11 | F | 10 | DSP | c.1865 T > C | p.Leu622Pro | Missense | Heterozygous (de novo) | Likely pathogenic |
| 12 | F | 16 | – | – | – | – | – | – |
Fig. 1.
Representative images of buccal mucosa smears from a control and 3 children with a clinical presentation of ACM. Patient 1 (DSP mut) shows depressed immunoreactive signal for Cx43 and DSP but control-like signal for PG and PKP1. Patient 2 (PKP2 mut) shows depressed immunoreactive signal for PG, Cx43 and DSP but not PKP1 compared to age-matched controls. Patient 3 (DSP mut) shows depressed signal for DSP but control-like signal for PG, Cx43 and PKP1. Protein localization was unaltered between serial swabs. Cell nuclei (blue) are stained with DAPI.
Patient 2 (currently 9 years of age) was diagnosed with ACM following presentation with symptoms of heart failure. Genetic screening identified homozygosity for the variant PKP2 p.Lys859Asn. At the time her buccal cells were obtained (2021), she showed severe biventricular dysfunction on echocardiography and cardiac MRI and NSVT on Holter monitoring requiring placement of a defibrillator (ICD). Analysis of her buccal smears showed strong membrane immunoreactive signal for plakophilin-1 but significantly depressed signals for plakoglobin, Cx43, and desmoplakin compared to age-matched controls (Fig. 1).
Patient 3 (currently 9 years of age) was first evaluated at age 6 years. He had a strong maternal history of ACM. His mother suffered an out-of-hospital cardiac arrest despite a non-diagnostic clinical assessment prior to the event. Genetic screening identified a nonsense variant DSP p.Arg84X. At the time buccal cells were obtained (in 2019 and 2021), the child showed left axis deviation on ECG but no structural abnormalities by imaging modalities. Analysis of both sets of buccal smears showed strong membrane immunoreactive signals for plakoglobin, Cx43, and plakophilin-1 but clearly depressed signal for desmoplakin compared to age-matched control (Fig. 1).
3.2. Buccal cell-cell junction protein localization is normal in unaffected children carrying ACM variants
Previous studies of myocardial junctional protein distribution have involved ACM patients who fulfilled diagnostic criteria and exhibited clinical manifestations of disease or who died suddenly and were diagnosed with ACM at autopsy [[4], [5], [6]]. Ethical considerations preclude myocardial sampling in subjects who carry ACM variants but are otherwise normal. To determine whether cell-cell junction proteins are mislocalized in children before clinical abnormalities arise, we analyzed serial buccal mucosa smears from 6 unaffected children with desmosomal gene variants.
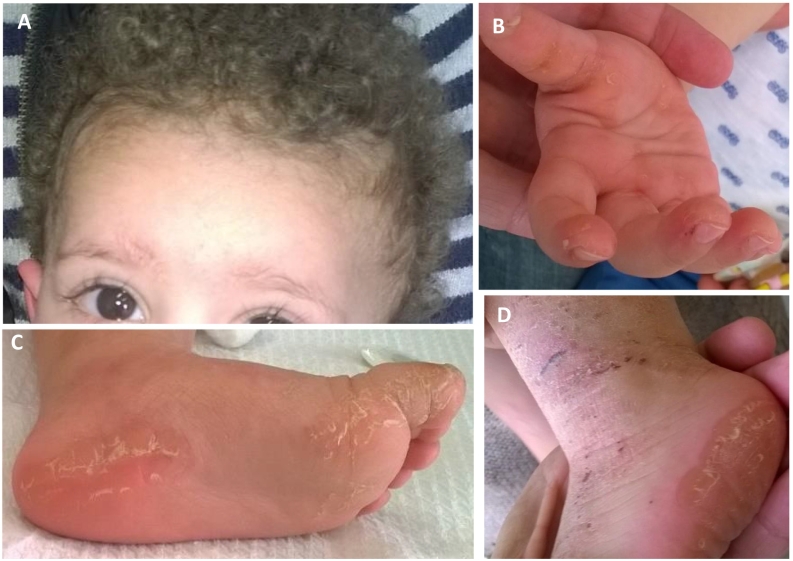
Patient 4 (currently 5 years of age) was first evaluated at age 14 months for hyperkeratotic palmar and plantar skin lesions which appeared in the second month of life. This was associated with woolly hair and curled eyelashes (Fig. 2). Genetic analysis showed homozygosity for frameshift variant JUP p.Trp680GlyfsX11 known to cause Naxos disease. Buccal cells were first obtained when the child was 3 years of age, at which time his physical examination, 12‑lead ECG, 24-hour Holter monitor, and transthoracic echocardiography were all normal. Analysis of the smears showed normal immunoreactive signals for plakoglobin, Cx43, desmoplakin, and plakophilin-1 (Fig. 3).
Fig. 2.
Cutaneous phenotype in a 18-year-old boy with Naxos disease (patient 4). Woolly hair (A), keratoderma over the tendon areas of the palms (B) and on the pressure areas of the soles (C). Eczematous lesions on the dorsal surface of the legs (D).
Fig. 3.
Representative images of buccal mucosa smears from a control and 3 asymptomatic children bearing desmosomal gene mutations. Two of the children are bearing autosomal dominant mutations in PKP2 and one is homozygous for a recessive mutation in JUP. All children have no clinical manifestation of ACM and they showed normal distribution for PG, Cx43, DSP and PKP1 in their buccal epithelium, indistinguishable from controls in both sets of samples (~18 months apart). Cell nuclei (blue) are stained with DAPI.
Patient 5 (currently 16 years of age) was first evaluated at age 9 years. His father had documented ACM with ICD implantation. Both are heterozygous for PKP2 c.2489 + 1 G > A, which is predicted to cause abnormal mRNA splicing. Patient 6 (currently 12 years of age) was first evaluated at age 10 years. He has a missense variant DSG2 p.Thr335Ala. A maternal uncle had clinically manifest ACM with an ICD. His mother carries the DSG2 variant and had a positive SA-ECG but showed no structural abnormalities on imaging modalities. Patient 7 (currently 18 years of age), first evaluated at age7 years, has a splice acceptor variant PKP2 c.2146-1G > C. Her father, a carrier of the same PKP2 variant, has ACM and an ICD. Patients 8 and 9 are siblings, currently 12 and 13 years of age, respectively. First evaluated aged 9 and 10, they both have a frameshift variant PKP2 p.Thr50SerfsX61. Their father and paternal grandfather carry the same pathogenic variant and both had been diagnosed with ACM. All of these children undergo annual electrocardiographic and imaging studies and none showed any abnormalities at the times buccal cells were obtained. Repeat buccal smears were prepared from each of them 12–18 months apart. All showed normal distribution for plakoglobin, Cx43, desmoplakin, and plakophilin-1. Demographic information and mutation status for these children are included in Table 1 (Group B) and representative confocal immunofluorescence images are shown in Fig. 3.
3.3. Changes in buccal cell-cell junction protein distribution correlate with onset of ACM clinical manifestations and pharmacological intervention
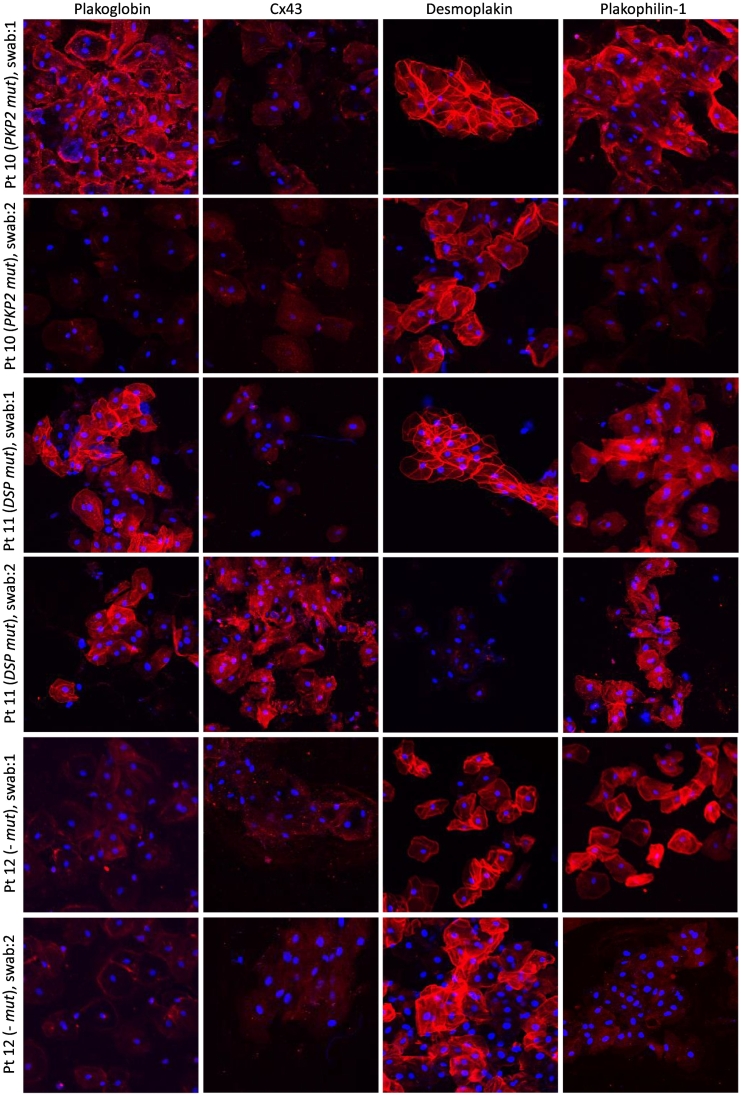
Patient 10 (currently 16 years of age) was first evaluated at age 3 years. His father had been diagnosed with ACM and received an ICD following a cardiac arrest. Both father and son were found to have a splice variant PKP2 c.2146–1 G > C, predicted to affect the acceptor splice site of intron 10. At age 14, the patient's ECG showed minor intraventricular conduction delay and T-wave inversions in lead V1 and flat T-waves in V4. He also had a positive SA-ECG in two vectors although imaging studies did not show any abnormalities. Immunohistochemical analysis of his buccal smears at this time showed normal distribution for plakoglobin, desmoplakin, and plakophilin-1 but severely depressed immunoreactive signal for Cx43 (Fig. 4, swab 1). When he was next evaluated at GOSH in 2021, his ECG showed fractionated QRS complexes in leads V1 and V2 with T-waves upright from V2 to V6. SA-ECG was positive for late potentials in all three vectors. On echocardiogram, there was mild left ventricular dilatation but with good biventricular systolic and diastolic function. Immunohistochemical analysis of a second set of buccal smears showed normal localization of desmoplakin but in contrast with the first set of samples, signals for plakoglobin, Cx43 and plakophilin-1 were now severely depressed (Fig. 4, swab 2).
Fig. 4.
Representative images of serial buccal smear samples from 3 children with ACM obtained ~18 months apart. All 3 children show changes in the localization of key junctional proteins over time correlating with deterioration of disease manifestation and pharmaceutical management.
Patient 11 (currently 10 years of age) was first evaluated at age 5 years and given a diagnosis of erythrokeratoderma-cardiomyopathy syndrome. She was found to have a de novo missense mutation DSP p.Leu622Pro. At age 9, she showed a severe phenotype of ACM with frequent ventricular ectopic beats, biventricular dilatation, and impaired LV systolic function (EF: 48%). Immunohistochemical analysis of her buccal smears at this time showed normal distribution for plakoglobin, desmoplakin, and plakophilin-1 but severely depressed immunoreactive signal for Cx43 (Fig. 4, swab 1). A year later, there was a significant increase in ventricular ectopy and triplets were evident on ECG and 24-h Holter monitoring. Echocardiography showed deterioration of systolic function and the EF was reduced to 38%. Worsening of both the arrhythmic and contractile phenotypes prompted implantation of an ICD and initiation of treatment with bisoprolol. Analysis of the second set of buccal smears showed normal distribution for plakoglobin and plakophilin-1 but in contrast to the first set of samples, immunoreactive signal for the mutant protein desmoplakin was now severely depressed. Interestingly, localization of Cx43 appeared normal with strong signal present on buccal cell membranes (Fig. 4, swab 2). Restoration of junctional Cx43 signal was associated with absence of ventricular ectopy on ECG at the time the second buccal mucosa sample was obtained. Moreover, Holter monitoring showed a reduction from an 8.2% arrhythmic burden (482 couplets, 8 triplets) at the time of the first swab to a 1.8% arrhythmic burden (26 couplets, 1 triplet) 6 weeks following establishment of an 2.5 mg daily dosage of bisoprolol.
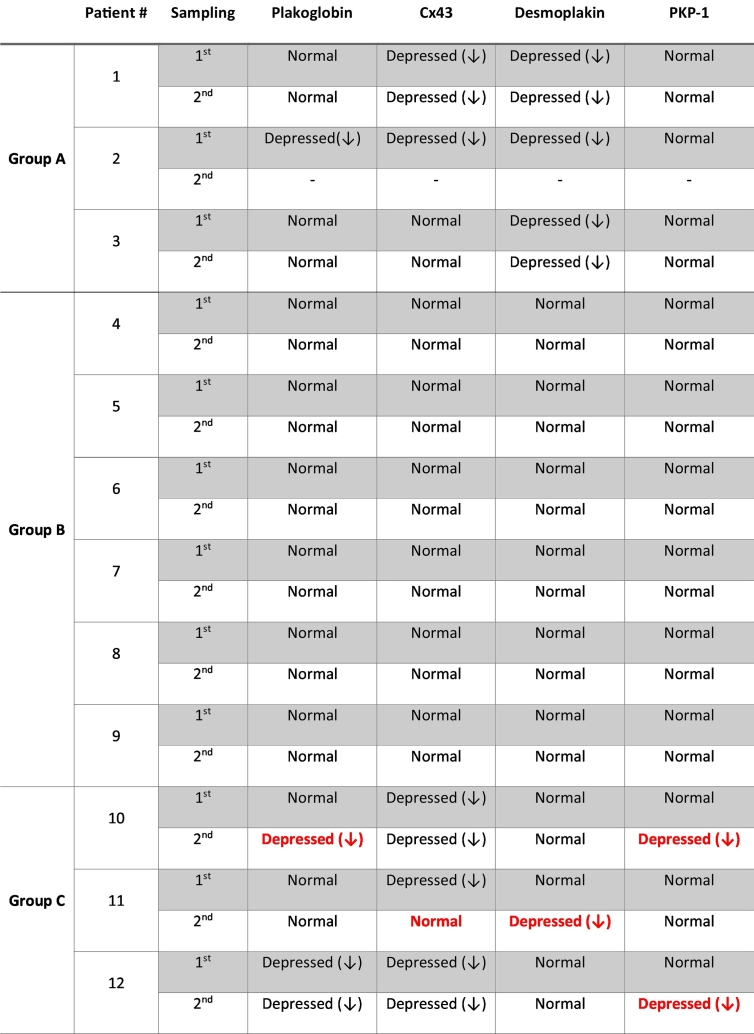
Patient 12 (currently 17 years of age) was first evaluated at age 16 years after developing symptoms of syncope and palpitations. She was found to have severe clinical manifestations of ACM including frequent syncopal/pre-syncopal episodes and persistent atrial flutter. Imaging studies showed a moderately dilated RV with impaired systolic function and an RVOT aneurysm. The LV was mildly dilated with preserved systolic function. Immunohistochemical analysis of her buccal smears at this time showed normal distribution for desmoplakin and plakophilin-1 while immunoreactive signals for plakoglobin and Cx43 were severely depressed at buccal cell membranes (Fig. 4, swab 1). She subsequently underwent atrial flutter ablation. Echocardiography a year after the initial buccal swab showed impaired biventricular systolic function with an EF of 45–50%, septal dyskinesia, and regional wall motion abnormalities at the apex. Immunohistochemical analysis of a second set of buccal smears showed normal localization for desmoplakin but this time in addition to plakoglobin and Cx43 immunoreactive signals being depressed, signal for plakophilin-1 was also severely depressed (Fig. 4, swab 2). The patient has undergone genetic testing but no pathogenic variants were identified on a 96-gene cardiomyopathy panel. Demographic information and mutation status for these children are included in Table 1 (Group C). A summary of immunohistochemical findings in buccal mucosa smears of children in all three groups is presented in Table 2.
Table 2.
Summary of immunohistochemical findings in buccal mucosa smears from patient groups A, B and C.
Changes in protein localization between serial samples are depicted in red.
4. Discussion
Loss of desmosomal, gap junction, and ion channel proteins at myocardial intercalated disks has been reported in ACM patients and in many experimental models of the disease [[4], [5], [6],[9], [10], [11]]. While it is likely that reduced electrical coupling and altered ion channel function contribute to arrhythmogenesis in ACM, the pathogenic implications of redistribution of desmosomal proteins are not so apparent. It is known, however, that desmosomal proteins participate, directly or indirectly, in major cell signaling pathways including Wnt/β-catenin and Hippo/Yap cascades in which alterations have been implicated in the pathogenesis of ACM [12,13]. The extent to which abnormal cell signaling may contribute to myocardial injury in ACM is not well understood but reduced immunohistochemical staining for junctional proteins appears to be a consistent feature of established disease [[4], [5], [6],9].
Because of the limited availability of myocardial tissue samples in ACM patients and ethical concerns about obtaining heart biopsies from gene carriers with no apparent disease, it has not been possible to correlate changes in the distribution of cell junction proteins with the onset and/or progression of clinical disease or responses to therapy. This is especially true for children and adolescents who carry ACM disease alleles. Using buccal mucosa cells as a surrogate for myocardium provides an opportunity to investigate these questions. Here, we show that changes in protein localization in buccal cells correlate with clinical onset of disease. Moreover, progressive changes in protein localization occurred with clinical deterioration, and normalization of junctional Cx43 signal was associated with a significant reduction in ventricular ectopy following therapy (78% drop in arrhythmic burden), albeit only in one patient. The fact that protein mislocalization does not occur until disease becomes clinically manifest obviously reduces the diagnostic value of buccal cell analysis. However, the fact that changes in these cells seem to reflect changes in cardiac myocytes suggests that analysis of buccal cells may be a useful tool for future studies to elucidate mechanisms responsible for junctional protein redistribution and to characterize their biochemical and pathophysiological consequences. Serial buccal mucosal sampling might also be a safe and inexpensive addition to the usual screening studies used in following ACM gene carriers at risk of developing clinical disease and an independent means of assessing response to therapy. Buccal mucosa cell cultures may provide an opportunity to experiment on cells from children at different stages of disease development for mechanistic and drug screening studies. This could be especially useful in characterizing alterations in signaling pathways in early ACM and evaluating potential therapies in patient-specific cells [8].
Some observations reported here in children and adolescents differ from our previous studies in adults with ACM. For example, in the present study we found normal protein distribution in 5 children who carried pathogenic variants but showed no overt disease, whereas we previously saw reduced junctional signals for plakoglobin and Cx43 in buccal mucosa cells from 3 adults who also carried variants but appeared well [8]. A possible explanation for this difference may be related to patient age. Alternatively, the adult gene carriers in the previous study may have exhibited subtle disease features at the time their buccal smears were analyzed, as they had not been monitored with the same comprehensive clinical evaluations performed in children in the present study.
We also saw some minor differences in patterns of protein distribution in adults and children with overt ACM. For example, affected adults with DSP variants showed reduced signal for desmoplakin but not plakophilin-1, while patients with PKP2 variants showed depressed signal for plakophilin-1 but not desmoplakin [8]. We saw the same results in the present study in 4 of 5 symptomatic children (cases 1, 3, 10, 11), but one child with a PKP2 variant (case 2) showed normal plakophilin-1 signal but reduced desmoplakin signal. However, this child was homozygous for a recessive C-terminal variant in PKP2 whereas the adults in the previous study were all heterozygous for variants in PKP2 associated with typical right ventricular disease. Thus, the severity of biophysical impact of a pathogenic variant, which is likely related to zygosity, could determine the specific pattern of abnormal protein distribution observed in buccal mucosa cells. Finally, loss of junctional plakoglobin signal in buccal mucosa cells was seen previously in all ACM adults regardless of the underlying variant [4,8], but 3 symptomatic children with DSP variants (cases 1, 3, and 11) showed normal plakoglobin signal (associated with abnormalities in other junctional proteins). One possible explanation for this difference is that the mutant protein (desmoplakin) might shift first from the membrane (as was seen in case 11) and changes in plakoglobin eventually follow. Alternatively, patterns of protein redistribution may vary in patients who show different clinical and pathological patterns of disease. Most previous studies of junctional protein redistribution in heart and buccal cells involved patients with classic right ventricular disease, the great majority of whom showed reduced signal for plakoglobin. However, DSP variants have been increasingly linked to biventricular or left-dominant ACM. A recent report described two DSP variant carriers with subclinical ACM who showed loss of desmoplakin but not plakoglobin signals in endomyocardial biopsies [14]. Similarly, a skin biopsy from a child with two DSP variants showed abnormal desmoplakin signal but normal plakoglobin distribution [15]. Indeed, a recent study of 107 patients with pathogenic DSP variants proposed that they exhibit “desmoplakin cardiomyopathy”, which was distinguished from classical ACM or DCM [16].
Analysis of serial buccal smears allowed correlation of changes in protein distribution with progression of clinical disease or in a single case, in response to therapy. For example, we observed initial loss of Cx43 signal and subsequent loss of one or more desmosomal proteins in two patients (cases 10, 11) whose heart disease progressed. This sequence may reflect the well documented clinical scenario in which ventricular ectopy is the initial clinical expression of disease with eventual progression to more severe features associated with structural remodeling of the heart. We also observed restoration of junctional Cx43 signal in a patient who showed frequent ventricular ectopy when the initial buccal smear was obtained but had responded to therapy and showed a significantly reduced arrhythmic burden when a repeat sample was analyzed. Although this hypothesis is based on observations rising from a single patient, there have been several studies in the past reporting drug modulation of gap junction remodeling. Dhein et al. showed that although Cx43 localizes at the lateral membranes in the left atrium of atrial fibrillation (AF) patients not receiving beta-blockers, it is shifted to the intercalated disks in those patients receiving metoprolol [17]. In line with these findings, metoprolol and propranolol up-regulate the junctional localization of Cx43 in a rat model of myocardial infarction through miR-1 inhibition [[18], [19]]. Finally, metoprolol increases Cx43 expression in cultured cardiomyocytes without alterations in mRNA levels suggesting a post-transcriptional regulatory mechanism [20].
A significant limitation of this study is the small number of subjects that were analyzed, and we acknowledge that robust conclusions cannot be supported by such a small sample size. ACM is a rare disease, and only a limited number of subjects were available, despite years long efforts to recruit study subjects. Additional work is required to validate and extend these observations. Our results may facilitate future studies using buccal cells to gain new insights into disease mechanisms and genotype-phenotype relationships in children and improved risk stratification in this vulnerable group.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We are grateful to the all the children and their parents/guardians for participating in this study. To complete this work, Dr. Asimaki was supported by a Rosetrees Foundation corn seed fund (M689) and a Children Cardiomyopathy Foundation Research Grant. Dr. Bueno is supported by a British Heart Foundation project grant (PG/18/27/33616). Professor Sheppard is supported by the Cardiac Risk in the Young (CRY) charity. Professor Behr is supported by the Robert Lancaster Memorial Fund. Dr. Kaski is supported by the Medical Research Council/National Institutes of Health Research Clinical Academic Research Partnership (MRC/NIHR CARP) and the Great Ormond Street Hospital Children's Charity and Max's Foundation. The funding sources had no involvement in the study design, collection, analysis and interpretation of data, the writing of the report or the decision to submit the article for publication.
References
- 1.Basso C., Corrado D., Marcus F.I., Nava A., Thiene G. Arrythmogenic right ventricular cardiomyopathy. Lancet. 2009;373:1289–1300. doi: 10.1016/S0140-6736(09)60256-7. [DOI] [PubMed] [Google Scholar]
- 2.Sen-Chowdhry S., Morgan R.D., Chambers J.C., McKenna W.J. Arrhythmogenic cardiomyopathy: etiology, diagnosis and treatment. Annu Rev Med. 2010;61:233–253. doi: 10.1146/annurev.med.052208.130419. [DOI] [PubMed] [Google Scholar]
- 3.Marcus F.I., Edson S., Towbin J.A. Genetics of arrhythmogenic right ventricular cardiomyopathy: a practical guide for physicians. J Am Coll Cardiol. 2013;61:1945–1948. doi: 10.1016/j.jacc.2013.01.073. [DOI] [PubMed] [Google Scholar]
- 4.Asimaki A., Tandri H., Huang H., Halushka M.K., Gautam S., Basso C., et al. A new diagnostic test for arrhythmogenic right ventricular cardiomyopathy. N Engl J Med. 2009;360:1075–1084. doi: 10.1056/NEJMoa0808138. [DOI] [PubMed] [Google Scholar]
- 5.Noorman M., Hakim S., Kessler E., Groeneweg J., Gpj Cox M., Asimaki A., et al. Remodeling of the cardiac sodium channel, Connexin43 and Plakoglobin at the intercalated disk in patients with arrhythmogenic cardiomyopathy. Heart Rhythm. 2012;10(3):412–419. doi: 10.1016/j.hrthm.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fidler L.M., Wilson G.J., Liu F., Cui X., Scherer S.W., Taylor G.P., et al. Abnormal connexin43 in arrhythmogenic right ventricular cardiomyopathy caused by plakophilin-2 mutations. J Cell Mol Med. 2009;13:4219–4228. doi: 10.1111/j.1582-4934.2008.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee J.Y.W., McGrath J.A. Mutations in genes encoding desmosomal proteins: spectrum of cutaneous and extracutaneous abnormalities. Br J Dermatol. 2019;184:596–605. doi: 10.1111/bjd.19342. [DOI] [PubMed] [Google Scholar]
- 8.Asimaki A., Protonotarios A., James C.A., Chelko S.P., Tichnell C., Murrat B., et al. Characterizing the molecular pathology of arrhythmogenic cardiomyopathy in buccal mucosa cells. Circ Arrhythm Electrophysiol. 2016 Feb;9(2) doi: 10.1161/CIRCEP.115.003688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asimaki A., Kapoor S., Plovie E., Arndt A.K., Adams E., Liu Z., et al. An in-vivo drug screen in zebrafish identifies a novel modulator of intercalated disc remodeling in arrhythmogenic cardiomyopathy. Sci. Transl. Med. 2014;6(240):240ra74. doi: 10.1126/scitranslmed.3008008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chelko S.P., Asimaki A., Andersen P., Bedja D., Amat-Alarcon N., DeMazumder D., et al. Central role for GSK3β in the pathogenesis of arrhythmogenic cardiomyopathy. JCI Insight. 2016 Apr 21;1(5) doi: 10.1172/jci.insight.85923. pii: e85923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chelko S.P., Asimaki A., Lowenthal J., Bueno-Beti C., Bedja D., Scalco A., et al. Therapeutic modulation of the immune response in arrhythmogenic cardiomyopathy. Circulation. 2019;140:1491–1505. doi: 10.1161/CIRCULATIONAHA.119.040676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia-Gras E., Lombardi R., Giocondo M.J., Willerson J.T., Schneider M.D., Khoury D.S., et al. Suppression of canonical Wnt/beta-catenin signalling by nuclear plakoglobin recapitulates phenotype of arrhythmogenic right ventricular cardiomyopathy. J Clin Invest. 2006;116:2012–2021. doi: 10.1172/JCI27751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen S.N., Gurha P., Lombardi R., Ruggiero A., Willerson J.T., Marian A.J. The hippo pathway is activated and is a causal mechanism for adipogenesis in arrhythmogenic cardiomyopathy. Circ Res. 2014;114:454–468. doi: 10.1161/CIRCRESAHA.114.302810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosset S., Medeiros Domingo A., Asimaki A., Graf D., Metzger J., Schitter J., et al. Reduced desmoplakin immunofluorescence signal in arrhythmogenic cardiomyopathy with epicardial right ventricular outflow tract tachycardia. Heart Rhythm Case Rep. 2018;5:57–62. doi: 10.1016/j.hrcr.2018.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whittock N.V., Wan H., Eady R.A.J., Morley S.M., Garzon M.C., Kristal L., et al. Compound heterozygosity for non-sense and mis-sense mutations in desmoplakin underlies skin fragility/woolly hair syndrome. J Invest Dermatol. 2002;118:232–238. doi: 10.1046/j.0022-202x.2001.01664.x. [DOI] [PubMed] [Google Scholar]
- 16.Smith E.D., Lakdawala N.K., Papouthisakis N., Aubert G., Mazzanti A., McCanta A.C., et al. Desmoplakin cardiomyopathy, a fibrotic and inflammatory form of cardiomyopathy distinct from typical dilated or arrhythmogenic right ventricular cardiomyopathy. Circulation. 2020;141:1872–1884. doi: 10.1161/CIRCULATIONAHA.119.044934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dhein S., Rothe S., Busch A., Gomez D.R., Boldt A., Reutemann A., et al. Effects of metoprolol therapy on cardiac gap junction remodelling and conduction in human chronic atrial fibrillation. Brit J Pharmacol. 2011;164:607–616. doi: 10.1111/j.1476-5381.2011.01460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu Y., Zhang Y., Shan H., Pan Z., Li X., Li B., et al. MicroRNA-1 downregulation by propranolol in a rat model of myocardial infarction: a new mechanism for ischaemic cardioprotection. Cardiovasc Res. 2009;84:434–441. doi: 10.1093/cvr/cvp232. [DOI] [PubMed] [Google Scholar]
- 19.Qin W., Zhang L., Li Z., Xiao D., Zhang Y., Yang H., et al. Metoprolol protects against myocardial infarction by inhibiting miR-1 expression in rats. J Pharm Pharmacol. 2020;72:76–83. doi: 10.1111/jphp.13192. [DOI] [PubMed] [Google Scholar]
- 20.Salameh A., Blanke K., Dhein S., Janousek J. Cardiac gap junction channels are upregulated by metoprolol: an unexpected effect of beta-blockers. Pharmacology. 2010;85:203–210. doi: 10.1159/000276982. [DOI] [PubMed] [Google Scholar]