Abstract
Background:
Although prenatal chemical exposures influence neurobehavior, joint exposures are not well explored as risk factors for internalizing disorders through adolescence.
Objective:
To evaluate associations of prenatal organochlorine and metal exposures, considered individually and as a mixture, with mid-childhood and adolescent internalizing symptoms.
Methods:
Participants were 468 children from a prospective cohort recruited at birth (1993-1998) in New Bedford, Massachusetts. Organochlorines (hexachlorobenzene, p,p'-dichlorodiphenyl dichloroethylene, polychlorinated biphenyls) and metals (lead, manganese) were analyzed in cord blood. Internalizing symptoms (anxiety, depressive, somatic) were assessed via multiple informants on the Conners’ Rating Scale (CRS) at 8-years and Behavior Assessment System for Children, Second Edition (BASC-2) at 15-years; higher T-scores indicate greater symptoms. Overall and sex-specific covariate-adjusted associations were evaluated using Bayesian Kernel Machine Regression (BKMR) and five-chemical linear regression models.
Results:
The cohort was socioeconomically diverse (35% household income <$20,000; 55% maternal ≤high school education at birth). Most chemical concentrations were consistent with background levels [e.g., median(range) cord blood lead: 1.1(0-9.4) μg/dL]. BKMR suggested linear associations and no interactions between chemicals. The overall mixture was positively associated with Conners’ Parent Rating Scale (CPRS) and BASC-2 Self Report of Personality (SRP) anxiety and depressive symptoms, and negatively with somatic symptoms. Prenatal lead was positively associated with adolescent anxiety symptoms [1.56 (95% CI: 0.50,2.61) BASC-2 SRP Anxiety score increase per doubling lead]. For CRPS and BASC-2 SRP, a doubling of cord blood manganese was positively associated with internalizing symptoms for girls [e.g., 3.26 (95% CI: 0.27,6.25) BASC-2 SRP Depression score increase], but not boys. Organochlorine exposures were not adversely associated with internalizing symptoms.
Discussion:
Low-level prenatal lead exposure was positively associated with adolescent anxiety symptoms, and prenatal manganese exposure was positively associated with internalizing symptoms for girls from mid-childhood through adolescence. In utero neurotoxicant metal exposures may contribute to the emergence of anxiety and depression.
Keywords: prenatal exposure, organochlorines, metals, chemical mixtures, internalizing symptoms, children’s health
1. Introduction
Approximately one in five people in the U.S. and worldwide are expected to experience a mental disorder in their lifetime, with anxiety and depressive disorders being two of the most commonly diagnosed (Merikangas et al. 2010; Steel et al. 2014). Development of an internalizing disorder (e.g., anxiety, depressive, somatic) (Liu et al. 2011) in childhood is of particular concern because childhood anxiety and depression are associated with social, emotional, and academic impairment, as well as increased risk for mental health problems through adulthood (Koenen et al. 2013; Woodward and Fergusson 2001). Recent epidemiologic research suggests that 4.4 million U.S. children have a current diagnosed anxiety disorder and 1.9 million meet criteria for depression (Ghandour et al. 2018). Thus, childhood internalizing disorders are a major public health problem, necessitating an understanding of their etiology.
Although there is a breadth of literature on sociodemographic and psychosocial risk factors for childhood internalizing problems (Merikangas 2005; Thapar et al. 2012), fewer studies have explored the extent to which these are associated with prenatal exposures to environmental neurotoxicants, specifically organochlorines and metals. During fetal development, the limbic system, which consists of brain structures integral to emotional and behavioral function, is rapidly developing and susceptible to toxicant exposures (Sokolowski and Corbin 2012). Based on evidence from animal literature, in utero organochlorine and metal exposures are hypothesized to induce later development of internalizing symptoms via numerous biological pathways, including dysregulation of dopaminergic function and neuroendocrine hormones, heightened oxidative stress, and increased inflammatory responses (Addae et al. 2013; Bell 2014; Betharia and Maher 2012; de Souza Lisboa et al. 2005; Mason et al. 2014; van den Bosch and Meyer-Lindenberg 2019); however, the potential mental health impacts of these chemicals, which readily cross the placenta and blood-brain barrier, have not been well characterized in epidemiologic research. Prior studies of prenatal chemical exposures and child neurobehavioral outcomes have largely focused on externalizing symptoms (Bellinger 2013; Korrick and Sagiv 2008), even though anxiety and depression are mental disorders that contribute substantially to the global burden of disease and disability (Rehm and Shield 2019). Studies including childhood internalizing symptoms have provided mixed evidence for associations with prenatal exposure to organochlorines (Jedynak et al. 2021; Maitre et al. 2021; Plusquellec et al. 2010; Rosenquist et al. 2017; Sioen et al. 2013; Strom et al. 2014), lead (Pb) (Arbuckle et al. 2016a; Bellinger et al. 1994; Horton et al. 2018; Plusquellec et al. 2010; Sioen et al. 2013), and manganese (Mn) (Horton et al. 2018; Mora et al. 2015). Most of this literature considered exposures independently, despite humans not experiencing chemical exposures in isolation, and only examined symptoms through mid-childhood. No single study has considered the joint effect of these chemical classes with anxiety, depressive, and somatic symptoms in childhood and adolescence.
In our prospective study, we evaluated associations of prenatal exposure to organochlorines and metals, as a mixture and as individual chemicals controlling for coexposure confounding, with internalizing symptoms during mid-childhood and adolescence. With limited prior literature on the combined impact of these two chemical classes, we hypothesized that greater prenatal exposure to these chemicals would be associated with increased internalizing symptoms, especially by adolescence when the onset and exacerbation of these symptoms is more likely compared to childhood. We also explored the potential for sex-specific associations as 1) internalizing disorders are more common in females by adolescence (Koenen et al. 2013; Liu et al. 2011) and 2) associations between these prenatal chemical exposures and neurobehavioral outcomes have been shown to sometimes differ by sex (Bauer et al. 2017; Cowell et al. 2021; Desrochers-Couture et al. 2018; Nakajima et al. 2017), although sex-specific susceptibility has varied by exposure, outcome, and/or study population.
2. Methods
2.1. Study population
This study utilized data from the New Bedford Cohort (NBC), a prospective cohort of mother-child pairs who were recruited after delivery at St. Luke’s Hospital (New Bedford, Massachusetts) from 1993 to 1998. The cohort was initially established to examine associations of prenatal exposure to organochlorines and metals with neurodevelopmental outcomes among children residing near the New Bedford Harbor (NBH) Superfund Site. All women recruited into the study were ≥18 years old, English- and/or Portuguese-speaking, and living in one of four towns (New Bedford, Acushnet, Fairhaven, Dartmouth) surrounding the NBH, which was highly contaminated with polychlorinated biphenyls (PCBs) and heavy metals between 1940 and late 1970s. Approximately 10% of women delivering at St. Luke’s Hospital met study eligibility criteria and gave birth when a study examiner was on site for recruitment. Infants were excluded if they were born by cesarean section because their mothers were unable to consent (care included post-section narcotic analgesia) or were unable to undergo a study-required neonatal examination. In total, 788 mother-infant pairs were consented and enrolled (Korrick et al. 2000; Sagiv et al. 2012).
Follow-up assessments have included detailed neurodevelopmental and psychological assessments via in-person visits conducted in mid-childhood at approximately 8 years of age (n=607) and in adolescence at approximately 15 years of age (n=528). For the current analyses, 468 participants had available data to be included. Children were eligible for in-person visits if they lived within commuting distance of New Bedford study offices, did not have neurologic impairment that precluded participation in assessments, and had at least one biomarker of prenatal chemical exposure available.
The study protocol was approved by the human subjects committee of the Brigham and Women’s Hospital (Boston, Massachusetts). Written consent was obtained from all participating parents and assent from adolescent participants prior to study evaluations.
2.2. Chemical exposure assessment
Umbilical cord blood samples for organochlorines and metals analyses were collected at birth. Prenatal organochlorine exposure was estimated by measuring levels of hexachlorobenzene (HCB), p,p’-dichlorodiphenyl dichloroethylene (p,p’-DDE), and 51 PCB congeners in cord serum on a wet-weight basis via high-resolution gas chromatography with electron capture detection and use of primary and confirmatory capillary columns at the Harvard T.H. Chan School of Public Health Organic Chemistry Laboratory (Boston, Massachusetts) (Korrick et al. 2000). In the current analysis, we used the sum of the four most prevalent PCBs (ΣPCB4; congeners 153, 118, 138, 180), as these were measured with the least error and are frequently used when exploring congener-specific effects. Due to insufficient serum sample volume, lipid concentrations could not be measured for study participants, and thus organochlorine concentrations were not lipid corrected. Cord serum lipid measurements were done in 12 randomly selected anonymous samples from the study hospital. Results demonstrated relative homogeneity of lipid concentration across samples [mean (SD) total lipid concentration: 1.7 (0.3) g/L], supporting the utility of wet-weight concentrations (Sagiv et al. 2012). The limits of detection (LOD) for HCB, p,p’-DDE, and individual PCB congeners were 0.01, 0.07, and 0.01 ng/g serum, respectively. Reproducibility was excellent for organics analyses, with a 3% within-batch coefficient of variation (CV) and 20% between-batch CV for the ΣPCBs and a similar performance for p,p’-DDE; HCB was similar with respect to within-batch, but had a 39% between-batch CV (Korrick et al. 2000).
Prenatal Pb and Mn exposures were estimated by measuring concentrations in cord whole blood at the Harvard T.H. Chan School of Public Health Trace Metals Laboratory (Boston, Massachusetts). Analyses were done using isotope dilution inductively coupled plasma mass spectrometry for Pb measurement (ICP-MS, Sciex Elan 5000, Perkin Elmer, Norwalk, CT) and external calibration on a dynamic reaction cell-inductively coupled plasma-mass spectrometer for Mn measurement (DRC-ICP-MS, Elan 6100, Perkin Elmer, Norwalk, CT). Concentrations were reported as the mean of five replicate measurements. The LOD for Pb and Mn was 0.02 μg/dL whole blood. Laboratory recovery rates for quality control standards ranged from 90-110%; precision was >95%.
We did not collect biosamples to assess postnatal chemical exposures in the NBC. However, infant and childhood blood Pb levels were abstracted from pediatric medical record reports of annual childhood Pb exposure screenings. Peak early childhood blood Pb [an available postnatal exposure index in the NBC that has been associated with cognitive deficits at low-level Pb exposure (Canfield et al. 2003; Lanphear et al. 2005)] was calculated as the maximum value between ages 12-36 months.
In the present analyses, machine concentration readings <LOD for organochlorines and metals were used to optimize statistical power and avoid biased exposure estimates due to censoring at the detection limit (Kim et al. 1995).
2.3. Outcome assessments
At the 8-year assessment, participants’ parents and teachers completed the revised Conners’ Rating Scale (CRS). Both the Conners’ Parent Rating Scale (CPRS), an 80-item questionnaire, and the Conners’ Teacher Rating Scale (CTRS), a 59-item questionnaire, ask respondents to rank relative frequency of observed symptoms using a four-point Likert scale (Conners 1997). For this analysis, we selected scales related to internalizing symptoms (i.e., anxiety, depressive, or somatic): Anxious-Shy from CPRS and CTRS and Psychosomatic from CPRS. CRS scores are standardized to age- and sex-adjusted T-scores, with a mean±standard deviation (SD) of 50±10; a higher score indicates greater symptoms. As previously described (Sagiv et al. 2010), some participants had multiple CTRS assessments (n=14 for this analysis). These duplicate assessments did not differ by completeness or by school grade. In cases where a primary and secondary teacher from the same grade completed the CTRS, the assessment from the primary teacher was selected (n=2 tests from secondary teachers were excluded). If two primary teachers from the same grade completed assessments, then a mean score was calculated (tests averaged: n=12).
At the 15-year assessment, participants, their parents, and their teachers completed the Behavior Assessment System for Children, Second Edition (BASC-2) (Reynolds and Kamphaus 2004). The BASC-2 Self Report of Personality (SRP), validated for ages 12-21 years, is a questionnaire comprising 176 items. The Parent Rating Scale (PRS) and Teacher Rating Scale (TRS) questionnaires have 150 and 139 items, respectively. On all forms, behaviors/symptoms are rated on a four-point Likert scale for frequency. For this analysis, we focused on the subset of internalizing symptom scales that were assessed across the three reporters. These included the clinical subscales of Anxiety, Depression, and Somatization, as well as the Internalizing Problems composite, which for BASC-2 PRS and TRS contains the above three scales, and for SRP includes four additional scales (Atypicality, Locus of Control, Sense of Inadequacy, Social Stress). BASC-2 scores are standardized to age- and sex-adjusted T-scores, with a mean±SD of 50±10; a higher score specifies greater symptoms. In a few cases, participants in this analysis had multiple BASC-2 PRS (n=3) or TRS assessments (n=6); in these instances, the most complete questionnaire administered closest to the participant’s 15-year assessment was included (test excluded due to completeness: 1 for BASC-2 TRS; tests excluded due to timing: 1 for BASC-2 PRS and 5 for BASC2-TRS). If two parent or teacher assessments had the same completeness and were within one-month of the adolescent’s study visit, their mean score was calculated (tests averaged: 2 for BASC-2 PRS).
In primary analyses, we focused on CPRS and BASC-2 SRP outcomes. In mid-childhood, parents are viewed as more accurate judges of internalizing symptoms as compared to teachers (Achenbach et al. 1987; Smith 2007). However, in adolescence, the child is seen as an optimal informant, as symptoms are less disruptive and oftentimes kept to oneself, and therefore less visible to parents and teachers (Koenen et al. 2013; Smith 2007).
2.4. Covariate assessment
After birth, information on the mother’s pregnancy and delivery, and the infant’s race/ethnicity, sex, and birth weight were abstracted from hospital records by a trained study nurse. At a home visit occurring approximately two weeks postpartum, mothers were interviewed for information on parental sociodemographics (maternal age, marital status, parity, maternal and paternal education), household income, and maternal smoking, alcohol consumption during pregnancy, pre-pregnancy weight, height, and gestational weight gain. We calculated pre-pregnancy body mass index (BMI) via self-reported weight and height. Additionally, participants reported information on their diet during pregnancy via a modified food frequency questionnaire (Salvini et al. 1989). At the 8- and 15-year assessments, sociodemographic characteristics and child health information were updated. Using information on parental and household sociodemographic factors at birth, we developed a prenatal social disadvantage index (PNSDI), adapting methods from prior research that constructed childhood social disadvantage indices (Gilman et al. 2017; Non et al. 2014). The data used for the PNSDI included 1) maternal age (≥20, <20 years); 2) maternal education level and 3) paternal education level (post-high school education, ≤high school graduate); 4) maternal marital status (married, not married); and 5) annual household income (≥$20,000, <$20,000). Each item was scored as (0, 1), respectively, and summed to produce a composite measure ranging from 0 to 5, with higher scores suggesting a greater degree of disadvantage. We derived a dichotomized variable in which ≥3 was considered high disadvantage, as this cut-point was particularly sensitive to greater risk of other environmental and social stressors, such as maternal smoking during pregnancy.
The 8- and 15-year assessments included a home visit during which quality of the home environment and parenting skills were assessed via the Home Observation for Measurement of the Environment (HOME) (Caldwell and Bradley 1985). On the 8- and 15-year study-visit questionnaires, parents reported their child’s medication use for behavioral disorder(s) and, at the 15-year assessment, reported on history of mental illness among biologically-related family members (i.e., has ever: had a serious mental illness, emotional problem, or nervous breakdown; seen a health professional or been given medicine for a psychological or emotional problem; been diagnosed or given medication by a physician or psychologist for a behavioral disorder; and/or stayed overnight at a facility due to mental or emotional problems). On the 8-year questionnaire, parents reported if their child ever had a “diagnosis of an emotional illness (e.g., depression)” based on information provided by a physician. At the 15-year assessment, parents reported if, during the prior five years, their child ever had a diagnosis of an anxiety disorder or depression by a physician, psychologist, or school professional. Outpatient pediatric medical records were also reviewed at both time points, noting if the participant ever had these diagnoses; and this information along with parent-reported information was utilized to define participants with these diagnosed internalizing disorders. Maternal IQ was assessed using the Kaufman Brief Intelligence Test at either the 8-year or 15-year assessment (Kaufman and Kaufman 1990).
2.5. Statistical analysis
In our analyses examining associations of prenatal chemical exposures with CRS and BASC-2 internalizing symptoms, we log2-transformed skewed chemical concentrations. In cases where chemical concentrations were zero, we imputed values as one-tenth of the non-zero minimum. In the overall analytic cohort, 0.2% (n=1) of HCB and of Pb concentrations were zero, while all other biomarkers had no zero values. Based on prior literature, we used directed acyclic graphs (Greenland et al. 1999) to select a priori potential confounders and predictors of internalizing symptoms for inclusion in models (See: Figure S2) (Liu et al. 2011; Merikangas 2005; Sagiv et al. 2010; Valeri et al. 2017). The covariates included in all models were parental characteristics at time of delivery [maternal age (continuous), PNSDI score (<3, ≥3)] or maternal characteristics during pregnancy [smoking (no, yes), alcohol consumption (≤1, >1 serving/week), average servings/week seafood consumption (continuous)]; child characteristics [race/ethnicity (Non-Hispanic White, Hispanic, Other), sex (female, male), age at CRS or BASC-2 (continuous)]; HOME score at 8-year (CRS analyses) or 15-year assessment (BASC-2 analyses) (continuous); and maternal IQ (continuous). For BASC-2 analyses, in cases where HOME score at 15 years was missing, we used the score at 8 years when available (n=15 observations).
Among the 607 children assessed at 8 years, 606 had complete CPRS and/or CTRS data. Of those 606 participants, 589 had cord serum organochlorine measures, 545 of these had cord blood Pb and Mn measures, and 448 of these had complete covariate data. Participants with at least one complete outcome, in addition to complete exposure and covariate data (n=448), were included in CRS analyses. For the 528 children assessed at 15 years, all had complete BASC-2 data for at least one reporter. Of those 528 participants, 519 had cord serum organochlorine measures, 477 of these had cord blood Pb and Mn measures, and 389 of these had complete covariate data. Participants with at least one complete outcome, as well as complete exposure and covariate data (n=389), were included in BASC-2 analyses. The overall analytic cohort consisted of 468 participants included in at least one complete case CRS and/or BASC-2 analysis (see flow chart: Figure S1).
We used Unix SAS version 9.1.4 (Cary, North Carolina) for data management and descriptive statistics, and R 4.0.3 (R Core Team, Vienna, Austria) for all regression analyses.
2.5.1. Bayesian Kernel Machine Regression
We first used Bayesian Kernel Machine Regression (BKMR) to explore possible nonlinearity and non-additive interactive effects for associations of the prenatal chemical mixture and internalizing symptom subscales. Briefly, BKMR is a supervised, flexible approach, which uses a kernel function to estimate the joint health effects of multivariate exposures, while adjusting for covariates (Bobb et al. 2015; Bobb et al. 2018). We applied BKMR with the component variable selection procedure, which provides variable-specific posterior inclusion probabilities (PIPs) to quantify relative importance of each exposure to the outcome. Our BKMR analyses used 50,000 Markov chain Monte Carlo iterations, in which the first 10,000 iterations were used as burn-in and then model convergence was evaluated with trace plots. Models were run in the overall analytic cohort, as well as stratified by sex.
2.5.2. Linear regression models
After visually assessing BKMR exposure-response functions, we largely observed no evidence of non-linear associations or interactions between exposures, and thus, in primary analyses, we fit covariate-adjusted five-chemical (i.e., HCB, p,p’-DDE, ΣPCB4, Pb, Mn) linear regression models. As exposures were log2-transformed, estimates are interpreted as the T-score difference per doubling prenatal chemical concentration. We examined possible sex-specific associations of chemical concentrations with internalizing symptoms by including product interaction terms between each chemical and child sex in models. We investigated magnitude and precision of sex-specific associations within strata and via product interaction term p-values.
2.5.3. Secondary analyses
As it is less common to have data for multiple reporters of child behavioral outcomes in environmental epidemiologic studies, we conducted secondary analyses using scores from parents (BASC-2 PRS) and teachers (CTRS, BASC-2 TRS). As prior studies have noted weak correlations and poor agreement among informants for adolescent internalizing symptoms (Reynolds and Kamphaus 2004; Sourander et al. 1999), we hypothesized that directions of associations may differ across BASC-2 reporters. We also explored binary outcomes that may indicate more severe psychopathology, with suggestive clinically significant symptoms defined by diagnostic cut points from each instrument’s a priori specification of clinical risk – the CRS dichotomizes risk at the 86th percentile (T-score≥61) and the BASC-2 dichotomizes risk at the 85th percentile (T-score≥60) (Conners 1997; Reynolds and Kamphaus 2004). Since these outcomes were not rare (Tables S1, S2), we used multivariable modified Poisson models with robust error variance to calculate risk ratios.
For a sensitivity analysis, we utilized inverse probability of censoring weighting (IPCW) (Cole and Hernán 2008; Hernán et al. 2004) to assess potential selection bias due to exclusion of participants with incomplete outcome and/or covariate data. We first ran logistic regression models to determine probability of inclusion in complete case analyses for CPRS and BASC-2 SRP as the dependent variable, and covariates collected at birth or the two-week postpartum home visit as independent variables. Based on predictors of inclusion in complete case analyses (Table S3), we used a subset of 622 participants (79% of the enrolled cohort) to assign weights to each observation. Covariates chosen for weighting at one or both follow-up points included: prenatal chemical concentrations (HCB, p,p’-DDE, £PCB4, Pb); parental and household characteristics at birth (maternal age, education, marital status, smoking during pregnancy; paternal education; household income) and child characteristics (sex, race/ethnicity, birth weight). To ensure that bias was not evident in the subset of 622, we compared distributions of several covariates with the enrolled 788 and saw no appreciable differences (Table S3). Because unstablized weights, defined as the inverse of the estimated probability of inclusion, may have extreme observations due to individuals with a rare combination of covariates, we stabilized weights with a numerator based on the probability of inclusion predicted by the exposures in the model (Cole and Hernán 2008). We additionally truncated the stabilized weights at the 2.5th and 97.5th percentiles (Hernán et al. 2004).
We used postnatal blood Pb measures from pediatric medical records to investigate whether a certain exposure window (i.e., prenatal versus postnatal Pb) was more strongly related to internalizing symptoms. Specifically, we used hierarchical variable selection with BKMR, in which the five prenatal chemicals were treated as one group and peak childhood Pb as another group (Valeri et al. 2017), and assessed group PIPs to see if the prenatal exposure mixture versus postnatal Pb was relatively more important with respect to the outcome. Furthermore, we ran BKMR models with a mixture that only included prenatal and peak childhood blood Pb.
In other sensitivity analyses for CPRS and BASC-2 SRP, we evaluated raw score instead of T-score outcomes to assess whether the potential truncation of T-scores impacted the direction and magnitude of associations. To elucidate whether clinical cases were driving associations or if results were generalizable to non-clinical samples, we ran models excluding those on prescription medications for behavioral problems and those with a diagnosis of an emotional illness at 8 years (for CPRS) and diagnosis of anxiety and/or depression at 15 years (for BASC-2 SRP). In those with available data, we adjusted models for family history of mental illness. Additionally, while maternal weight gain was not predictive of child internalizing symptoms in our analytic cohort, prior studies have found gestational weight gain to be associated with lower blood concentrations of lipophilic compounds (Lee et al. 2017) and child behavioral problems (Pugh et al. 2016). Therefore, we further adjusted models for gestational weight gain, as a categorical variable (insufficient, adequate, excessive) based on prepregnancy BMI-specific recommendations from the U.S. Institute of Medicine (Institute of Medicine and National Research Council Committee to Reexamine I. O. M. Pregnancy Weight Guidelines 2009). For CPRS, we ran models restricted to participants also included in BASC-2 SRP complete case analyses.
3. Results
3.1. Study population characteristics
Of 468 mother-child pairs in the analytic dataset, a substantial proportion of child participants were born to mothers who had no post-high school education (n=257; 55%), were unmarried (n=184; 39%), and/or had an annual household income <$20,000 (n=162; 35%). Approximately one-third of mothers reported smoking during pregnancy (n=149; 32%). The majority of study children were Non-Hispanic White (n=324; 69%), and 50% (n=234) were female. The mean±SD age of participants at the CPRS assessment was 8.1±0.7 years and at the BASC-2 SRP assessment was 15.5±0.6 years (Table 1).
Table 1.
Sociodemographic and health characteristics of 468 New Bedford Cohort participants included in at least one complete case analyses a for the revised Conners’ Rating Scale outcomes at age 8 years and/or the Behavior Assessment System for Children, Second Edition (BASC-2), outcomes at age 15 years; and associations of these predictors with Conners’ Parent Rating Scale (CPRS) Anxious-Shy and BASC-2 Self-Report of Personality (SRP) Anxiety T-scores
| Mean ± SD or N (%) |
Difference in CPRS Anxious-Shy T-score [β (95% CI)] b |
Difference in BASC-2 SRP Anxiety T-score [β (95% CI)] b |
|
|---|---|---|---|
| Parental and household characteristics | |||
| Maternal age at child’s birth (years) | 26.5 ± 5.4 | −0.34 (−0.52, −0.16) | −0.17 (−0.38, 0.03) |
| High prenatal social disadvantage index score (≥3 on 0-5 scale) | 185 (40) | 4.42 (2.46, 6.37) | 1.99 (−0.27, 4.25) |
| Maternal age at child’s birth < 20 years c | 57 (12) | 2.57 (−0.40, 5.53) | 0.70 (−2.79, 4.20) |
| Maternal high school graduate or less c | 257 (55) | 2.88 (0.94, 4.82) | 2.00 (−0.17, 4.16) |
| Paternal high school graduate or less c | 325 (69) | 2.24 (0.12, 4,35) | 0.25 (−2.06, 2.56) |
| Annual household income <$20,000 at birth c | 162 (35) | 4.01 (1.99, 6.03) | 3.86 (1.55, 6.17) |
| Not married at child’s birth c | 184 (39) | 4.16 (2.20, 6.13) | 0.13 (−2.12, 2.38) |
| Maternal prenatal smoking | 149 (32) | 2.30 (0.23, 4.37) | 3.01 (0.61, 5.40) |
| Prenatal alcohol consumption >1 serving/week | 23 (5) | −3.53 (−7.95, 0.88) | −4.13 (−9.58, 1.32) |
| Prenatal seafood consumption (servings/week) | 3.8 ± 4.3 | 0.03 (−0.20, 0.25) | −0.11 (−0.36, 0.13) |
| Maternal IQ | 98.4 ± 10.6 | −0.11 (−0.21, −0.02) | −0.14 (−0.24, −0.04) |
| HOME score (0-59 scale) at 8-year assessment d | 45.6 ± 5.3 | −0.27 (−0.45, −0.09) | - |
| HOME score (0-60 scale) at 15-year assessment d, e | 43.9 ± 6.3 | - | −0.17 (−0.34, 0.00) |
| Gestational weight gain f | |||
| Insufficient | 89 (20) | 0.53 (−2.19, 3.25) | 0.16 (−2.91, 3.23) |
| Adequate | 164 (37) | Reference | Reference |
| Excessive | 193 (43) | 0.82 (−1.38, 3.01) | −0.60 (−3.03, 1.83) |
| Child characteristics | |||
| Age at CPRS (years) | 8.1 ± 0.7 | 1.14 (−0.34, 2.63) | - |
| Age at BASC-2 SRP (years) | 15.5 ± 0.6 | - | −0.58 (−2.27, 1.10) |
| Female | 234 (50) | 1.19 (−0.76, 3.14) | 5.80 (3.70, 7.90) |
| Race/ethnicity | |||
| Non-Hispanic White | 324 (69) | Reference | Reference |
| Hispanic | 40 (9) | 4.63 (1.10, 8.16) | 1.93 (−1.91, 5.77) |
| Other | 104 (22) | 1.05 (−1.32, 3.41) | 0.10 (−2.61, 2.81) |
| Family history of mental illness | 181 (47) | 2.48 (0.37, 4.60) | 2.93 (0.77, 5.09) |
| Medication use for a behavioral disorder at 8-year assessment | 37 (8) | 4.22 (0.65, 7.79) | - |
| Medication use for a behavioral disorder at 15-year assessment | 39 (10) | - | 4.76 (1.18, 8.34) |
| Diagnosis of an emotional illness at 8-year assessment | 9 (2) | 15.64 (8.83, 22.45) | - |
| Diagnosis of an anxiety disorder and/or depression at 15-year assessment | 54 (14) | - | 6.13 (3.05, 9.21) |
Abbreviations: BASC-2 SRP: Behavior Assessment System for Children, 2nd Edition, Self-Report of Personality; CI: confidence interval; CPRS: Conners’ Parent Rating Scale; HOME: Home Observation for Measurement of the Environment; SD: standard deviation
Bolded effect estimates: p<0.05
For participants included in any of the complete case analyses, the following covariates have missing data (N missing): 20 with HOME score at 8-year assessment; 79 with HOME score at 15-year assessment; 2 with gestational weight gain; 11 with age at CPRS, medication use for a behavioral disorder at 8-year assessment, and diagnosis of an emotional illness at 8-year assessment; and 80 with age at BASC-2 SRP, family history of mental illness, medication use for a behavioral disorder at 15-year assessment, and diagnosis of an anxiety disorder and/or depression at 15-year assessment
Univariate regression models using complete case CPRS analytic cohort (n=448) and complete case BASC-2 SRP analytic cohort (n=388)
These parental and household characteristics contribute towards the total score for the prenatal social disadvantage index
A higher score on the scale indicates a higher quality of home environment and better parenting skills
For 15 participants in BASC-2 analyses who were missing the HOME score at the 15-year assessment, the HOME score from the 8-year assessment was used
Gestational weight gain is represented as a three-category (insufficient, adequate, excessive) variable based on maternal pre-pregnancy BMI. These categories are based on recommendations for total weight gain from the U.S. Institute of Medicine. For singleton pregnancies, adequate weight gain is BMI<18.5 kg/m2: 12.5-18.0 kg; BMI 18.5-24.9 kg/m2: 11.5-16.0 kg; BMI 25.0-29.9 kg/m2: 7.0-11.5 kg; and BMI≥30.0 kg/m2: 5.0-9.0 kg. In the case of twin pregnancies (n=2), the total weight gain guidelines are BMI 18.5-24.9 kg/m2: 17-25 kg; BMI 14.0-23.0 kg/m2: 7.0-11.5 kg; and BMI≥30.0 kg/m2: 11.0-19.0 kg (and no guidelines for BMI<18.5 kg/m2 with multiple fetuses).
Compared to those excluded from the analytic cohort, children included were younger at study assessments and more likely to be female, Non-Hispanic White, and have a low PNSDI score. On average, included participants had lower cord blood Pb levels. CPRS complete case participants had lower prenatal HCB levels, whereas those in BASC-2 SRP analyses had higher p,p’-DDE levels (Table S3).
3.2. Organochlorine and metal exposures
The median concentrations of organochlorines measured in cord serum were 0.02 ng/g for HCB, 0.31 ng/g for p,p’-DDE, and 0.18 ng/g for ΣPCB4. For cord blood metals, median concentrations were 1.1 ng/dL for Pb and 4.0 μg/dL for Mn. Mean concentrations of organochlorines were greater in girls compared to boys, whereas median concentrations were similar across sexes. In contrast, mean and median metal concentrations were higher in boys than in girls (Table 2). The organochlorines were positively and moderately correlated, with Spearman’s rank correlation coefficients (rs) ranging from 0.37 (HCB and p,p’-DDE) to 0.67 (p,p’-DDE and ΣPCB4. Cord blood Pb and Mn had a weaker, positive rs of 0.16, and were not strongly correlated with organochlorines (rs: −0.03 to 0.20). The correlation between cord blood and peak childhood Pb was moderate and positive (rs: 0.33) (Figure S3).
Table 2.
Distributions of biomarkers of prenatal organochlorine and metal exposure a among New Bedford Cohort participants included in at least one complete case analyses for the revised Conners’ Rating Scale (CRS) outcomes at age 8 years and/or the Behavior Assessment System for Children, Second Edition (BASC-2), outcomes at age 15 years (n=468) b
| Total cohort (n=468) | Boys (n=234) | Girls (n=234) | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Mean ± SD | Median | Range | Mean ± SD | Median | Range | Mean ± SD | Median | Range | |
| Organochlorines | |||||||||
| HCB (ng/g) | 0.03 ± 0.02 | 0.02 | 0.00 – 0.14 | 0.02 ± 0.02 | 0.02 | 0.00 – 0.14 | 0.03 ± 0.02 | 0.02 | 0.00 – 0.13 |
| p,p'-DDE (ng/g) | 0.53 ± 1.12 | 0.31 | 0.02 – 14.9 | 0.49 ± 0.82 | 0.32 | 0.02 – 10.3 | 0.57 ±1.35 | 0.31 | 0.06 – 14.9 |
| ΣPCB4 c (ng/g) | 0.26 ± 0.32 | 0.18 | 0.01 – 4.41 | 0.23 ± 0.21 | 0.17 | 0.01 – 1.69 | 0.28 ± 0.41 | 0.19 | 0.01 – 4.41 |
| Metals | |||||||||
| Pb (μg/dL) | |||||||||
| Cord blood | 1.38 ± 0.94 | 1.14 | 0.00 – 9.39 | 1.41 ± 0.86 | 1.21 | 0.13 – 5.25 | 1.36 ± 1.02 | 1.08 | 0.00 – 9.39 |
| Peak childhood d | 6.66 ± 3.93 | 6.00 | 1.00 – 38.0 | 6.51 ± 3.70 | 6.00 | 1.00 – 19.2 | 6.83 ± 4.16 | 6.00 | 1.00 – 38.0 |
| Mn (μg/dL) | 4.21 ± 1.53 | 3.96 | 0.66 – 14.6 | 4.34 ± 1.51 | 4.15 | 0.66 – 11.2 | 4.08 ± 1.54 | 3.72 | 1.75 – 14.6 |
Abbreviations: HCB: hexachlorobenzene; Mn: manganese; Pb: lead; PCB: polychlorinated biphenyl; p,p'-DDE: p,p’-dichlorodiphenyldichloroethylene; SD: standard deviation
Organochlorines were measured in cord serum; prenatal Pb and Mn were measured in cord whole blood; peak childhood Pb was measured in whole blood
Sample sizes: 468 for cord serum organochlorines, cord blood Pb, and cord blood Mn; 434 for peak childhood Pb
Sum of four PCB congeners (118, 138, 153, 180)
Peak childhood blood lead levels from ages 12 to 36 months
3.3. Internalizing symptoms in mid-childhood and adolescence
For the 8-year assessment, study children’s average internalizing symptoms were qualitatively slightly higher (worse) than the standardization samples, with mean±SD T-scores of 52.8±10.6 for CPRS Anxious-Shy and 53.7±11.1 for CPRS Psychosomatic scales. Boys and girls had similar distributions of scores. For CPRS, a larger percentage of girls than boys were categorized as having potentially clinically significant Anxious-Shy symptoms, while a higher percentage of boys than girls were classified this way by teachers. Overall, approximately 20% of participants had potentially clinically significant CPRS Anxious-Shy and/or Psychosomatic symptoms. CPRS Anxious-Shy and Psychosomatic scores were positively and moderately correlated (rs: 0.34), but CPRS and CTRS Anxious-Shy scores were not correlated (rs: 0.06) (Table S1).
In contrast to CPRS assessment, adolescents’ average scores were qualitatively slightly lower (better) than those of the standardization sample. Specifically, for the 15-year assessment, mean±SD T-scores for the BASC-2 SRP scales were 49.2±10.9 for Anxiety, 47.8±10.7 for Depression, 49.4±10.2 for Somatization, and 47.9±10.8 for the Internalizing Problems composite scales. For BASC-2 SRP, on average, girls had higher Anxiety, Depression, and Somatization scores than boys; this sex-specific pattern also was present with BASC-2 PRS and TRS. Between 14-17% of participants had potentially clinically significant self-reported symptoms (Table S2).
Concordance between reporters for internalizing symptoms was relatively low. Of 58 adolescents who were categorized with potentially clinically significant Anxiety problems by self-report, 22% were also defined this way by BASC-2 PRS and 7% by TRS. BASC-2 SRP and PRS were in stronger agreement for Depression (55%) and Somatization (44%) than for Anxiety (22%), and agreement between these sub-scales was low (range: 7-23%) for BASC-2 SRP and TRS (Table S4). While BASC-2 SRP subscale scores were positively and moderately correlated (rs: 0.48 to 0.64), correlations across different reporters were weak (e.g., Anxiety rs: 0.26 for SRP and PRS; 0.07 for SRP and TRS; Table S2).
In univariate regression models examining predictors of CPRS Anxious-Shy and BASC-2 SRP Anxiety scores, higher T-scores were associated with a high PNSDI score, maternal smoking during pregnancy, younger maternal age at birth, and lower maternal IQ. With respect to child characteristics, Hispanic race/ethnicity, female sex, poorer HOME score, behavioral disorder medication use, family history of mental illness, and diagnosis of an emotional illness (for CPRS) and anxiety and/or depression (for BASC-2 SRP) were associated with greater anxiety-related scores (Table 1).
3.4. Covariate-adjusted regression models
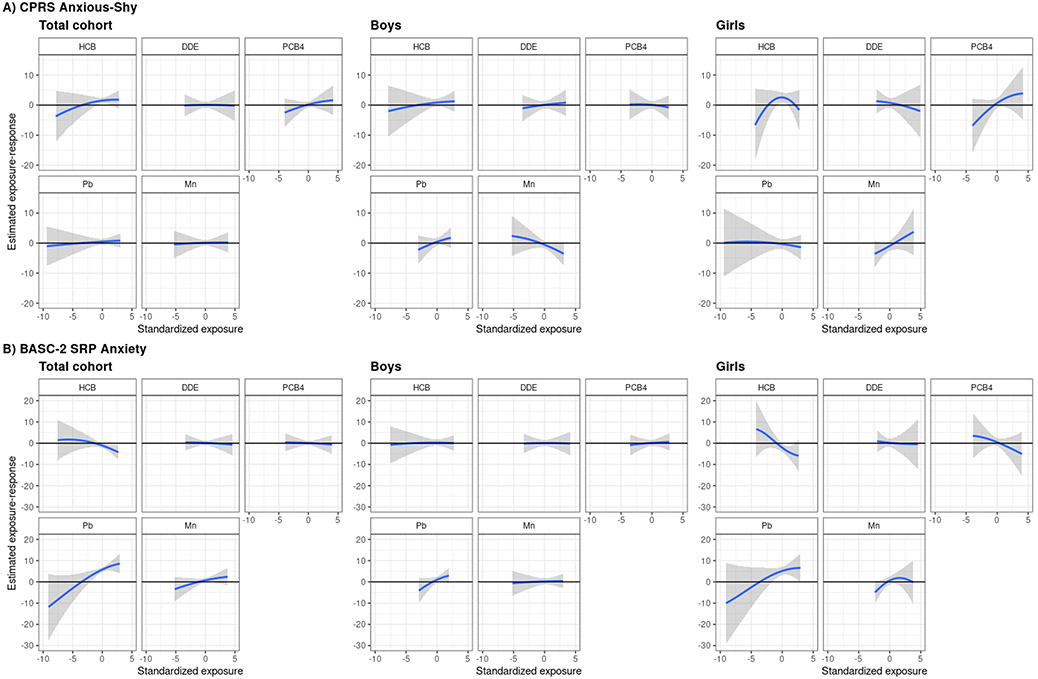
In exploratory BKMR analyses, univariate and bivariate exposure-response functions suggested linear associations of p,p’-DDE, ΣPCB4, Pb, and Mn with CPRS Anxious-Shy and Psychosomatic outcomes and BASC-2 SRP Anxiety, Depression, and Somatization outcomes (Figures 1, S4) and no interactions among the five chemicals (Figures S5, S6). For HCB, associations were also linear, except in the cases of CPRS Anxious-Shy for girls and BASC-2 SRP Somatization overall in which the exposure-response functions suggested an inverted U-shape (Figures 1, S4). However, because the credible intervals were wide at the lower-tail due to associations being driven by the minimum HCB concentration and because inclusion of a quadratic terms for HCB in these regression models did not significantly improve model fit, we treated HCB as a linear term.
Figure 1.
Univariate exposure-response functions and 95% credible intervals for each of the five chemicals, when the other chemicals are fixed at their medians, with A) the CPRS Anxious-Shy T-score and B) the BASC-2 SRP Anxiety T-score. Models adjusted for parental and household characteristics (prenatal social disadvantage index score; maternal age at birth; prenatal smoking; seafood, alcohol intake during pregnancy; maternal IQ; Home Observation for Measurement of the Environment score) and child characteristics (sex, race/ethnicity, age at assessment). Abbreviations: BASC-2 SRP, Behavior Assessment System for Children, Second Edition, Self-Report of Personality; CPRS, revised Conners’ Parent Rating Scale; DDE, p,p'-dichlorodiphenyl dichloroethylene; HCB, hexachlorobenzene; Pb, lead; PCB4, sum of four polychlorinated biphenyl congeners; Mn, manganese.
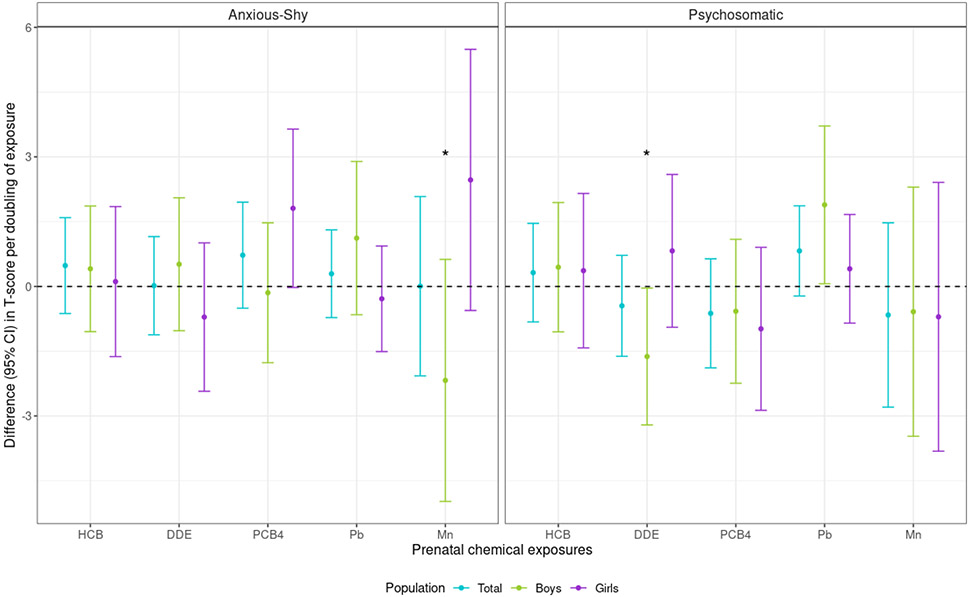
In five-chemical linear regression models, effect modification by sex was present for the association between prenatal Mn and CPRS Anxious-Shy score (Mn-sex interaction p-value=0.03) (Figure 2; Table S5). Although confidence intervals (CI) crossed the null, prenatal Mn was positively (i.e., adversely) associated with CPRS Anxious-Shy score for girls and negatively associated for boys in mid-childhood. Specifically, a doubling in prenatal Mn was associated with a 2.47 (95% CI: −0.56, 5.49) score difference for girls and −2.18 (95% CI: −4.98, 0.63) difference for boys. There were no associations of HCB, p,p’-DDE, or Pb with CPRS Anxious-Shy score. Prenatal ΣPCB4 was positively associated with CPRS Anxious-Shy score for girls, but the CI crossed the null [1.81 (95% CI: −0.02, 3.64) per doubling ΣPCB4]. For CPRS Psychosomatic score, there was a positive association with Pb, which was stronger for boys than girls [1.89 (95% CI: 0.06, 3.72) versus 0.41 (95% CI: −0.85, 1.67) per doubling Pb]. Per doubling prenatal p,p’-DDE, there was a negative association for boys [−1.62 (95% CI: −3.21, −0.04)], but not girls [0.82 (95% CI: −0.94, 2.59)] (p,p’-DDE-sex interaction p-value=0.04) (Figure 2; Table S5)
Figure 2.
Difference [95% confidence interval (CI)] in CPRS internalizing symptom T-scores associated with a doubling of each chemical, among New Bedford Cohort participants. Models adjusted for parental and household characteristics (prenatal social disadvantage index score; maternal age at birth; prenatal smoking; seafood, alcohol intake during pregnancy; maternal IQ; Home Observation for Measurement of the Environment score at 8-year assessment), child characteristics (sex, race/ethnicity, age at CPRS), and the other chemicals. Asterisks indicate sex-chemical interaction terms with a p-value <0.05. Abbreviations: CPRS, revised Conners’ Parent Rating Scale; DDE, p,p'-dichlorodiphenyl dichloroethylene; HCB, hexachlorobenzene; Pb, lead; PCB4, sum of four polychlorinated biphenyl congeners; Mn, manganese.
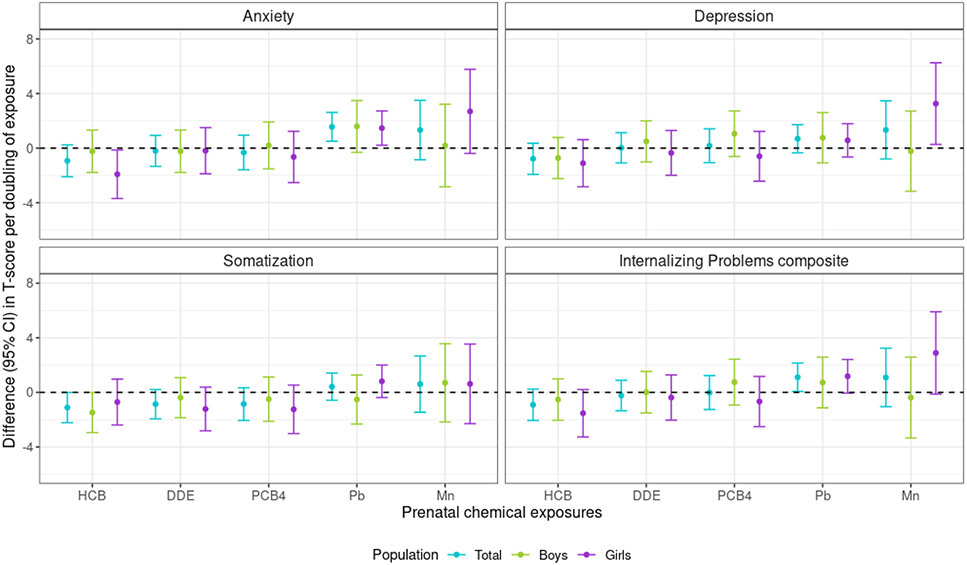
Prenatal Pb was positively associated with anxiety symptoms for the total cohort in adolescence, with a doubling of Pb exposure associated with a 1.56 (95% CI: 0.50, 2.61) BASC-2 SRP Anxiety score difference. The association of Pb with BASC-2 SRP Depression was also positive, although weaker than with Anxiety score and the CI crossed the null [0.69 (95% CI: −0.35, 1.72) per doubling Pb]. Prenatal Mn was positively associated with BASC-2 SRP Depression score for girls, for whom a doubling in Mn was associated with a 3.26 (95% CI: 0.27, 6.25) score difference, and null for boys [−0.23 (−3.16, 2.71)]. This pattern was also apparent for BASC-SRP Anxiety score and prenatal Mn, although the CI crossed the null [2.69 (95% CI: −0.39, 5.77) per doubling Mn for girls]. Additionally, a doubling in prenatal HCB was negatively associated with BASC-2 SRP Somatization score for the overall cohort [−1.11 (95% CI: −2.22, −0.01)], and negatively associated with Anxiety score for girls [−1.92 (95% CI: −3.69, −0.14)]. No other prenatal chemical was associated with BASC-2 SRP Somatization score. The BASC-2 SRP Internalizing Problems composite was strongly influenced by Anxiety and Depression scales (rs: 0.80, 0.84, respectively; Table S2), as the composite score was positively associated with prenatal Pb overall and Mn for girls (Figure 3; Table S6).
Figure 3.
Difference [95% confidence interval (CI)] in BASC-2 SRP internalizing symptom T-scores associated with a doubling of each chemical, among New Bedford Cohort participants. Models adjusted for parental and household characteristics (prenatal social disadvantage index score; maternal age at birth; prenatal smoking; seafood, alcohol intake during pregnancy; maternal IQ; Home Observation for Measurement of the Environment score at 15-year assessment), child characteristics (sex, race/ethnicity, age at BASC-2 SRP), and the other chemicals; no sex-chemical interaction terms had p-values <0.05. Abbreviations: BASC-2 SRP, Behavior Assessment System for Children, Second Edition, Self-Report of Personality; DDE, p,p'-dichlorodiphenyl dichloroethylene; HCB, hexachlorobenzene; Pb, lead; PCB4, sum of four polychlorinated biphenyl congeners; Mn, manganese.
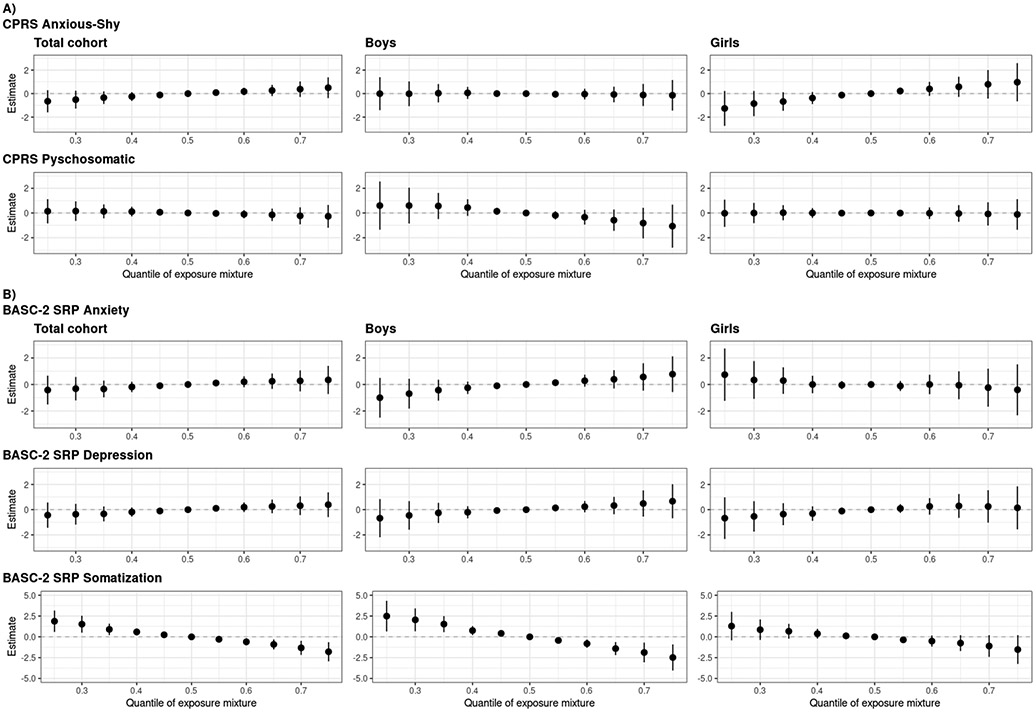
In addition to utilizing BKMR to inform model specification, we used this method to evaluate the relationship of the overall mixture with CPRS and BASC-2 SRP outcomes. For anxiety and depressive symptoms (CPRS Anxious-Shy; BASC-2 SRP Anxiety, Depression), the overall mixture was positively and linearly associated with symptoms for the total cohort, although credible intervals crossed the null. However, in sex-stratified models, the mixture was positively associated with CPRS Anxious-Shy score for girls and had no association for boys, whereas the mixture was positively associated with BASC-2 SRP Anxiety and Depression scores for boys and had no association for girls. The overall mixture was negatively and linearly associated with somatic symptoms (CPRS Psychosomatic, BASC-2 SRP Somatization) for the total cohort, with stronger associations in adolescence and for boys (Figure 4). These overall mixture associations reflect the sex-specific univariate exposure-response functions; for example, with BASC-2 SRP Anxiety, the null association of the mixture for girls is likely due to the positive associations of prenatal metal exposures counteracting the negative associations with the organochlorines, whereas the positive association of the mixture for boys is reflective of the positive association with prenatal Pb and null associations with all other chemicals (Figure 1).
Figure 4.
Overall effect of the five chemical mixture (hexachlorobenzene, p,p'-dichlorodiphenyl dichloroethylene, sum of four polychlorinated biphenyl congeners, lead, manganese) and 95% credible intervals, relative to the 50th percentile of the mixture, with A) the CPRS and B) the BASC-2 SRP internalizing symptom T-scores. Models adjusted for parental and household characteristics (prenatal social disadvantage index score; maternal age at birth; prenatal smoking; seafood, alcohol intake during pregnancy; maternal IQ; Home Observation for Measurement of the Environment score) and child characteristics (sex, race/ethnicity, age at assessment). Abbreviations: BASC-2 SRP, Behavior Assessment System for Children, Second Edition, Self-Report of Personality; CPRS, revised Conners’ Parent Rating Scale.
3.5. Secondary analyses
When comparing across informants, patterns of association were similar for parents and teachers on the CRS, but more variable for self-report versus parent- and teacher-report on the BASC-2. CTRS Anxious-Shy score was positively associated with Mn for girls, as it was for CPRS (Table S5). Likewise, when CPRS and CTRS scores were dichotomized, directions of associations were generally similar for risk ratios and linear regression estimates, although magnitude and precision varied, particularly with a weak and imprecise positive association for prenatal Mn and potentially clinically significant CPRS Anxious-Shy symptoms for girls (Figure S7). For BASC-2 continuous and dichotomized score outcomes, prenatal Pb was consistently associated with higher Anxiety score; otherwise, directions and magnitude of associations with chemicals differed based on informant. For example, the associations of prenatal Mn with BASC-2 PRS and TRS Depression continuous and dichotomized scores for girls were either negative or null [e.g., BASC-2 TRS T-score: −2.60 (95% CI: −5.59, 0.39) per doubling Mn], whereas the BASC-2 SRP associations with Mn were positive for girls (Table S6, Figure S8).
In BKMR models that additionally included peak childhood Pb in the mixture, univariate exposure-response functions suggested no evidence of associations of either prenatal or peak childhood Pb with CPRS Anxious-Shy score at 8 years for the total cohort (Figure S9). For the 15-year assessment, univariate exposure-response functions showed positive, linear associations for both prenatal and childhood Pb with BASC-2 SRP Anxiety score for the total cohort, but the prenatal Pb curve had a steeper slope (i.e., larger magnitude estimate) (Figure S10). Specifically, in the total cohort BASC-2 SRP Anxiety model, the group PIP was 0.28 for the five prenatal chemicals and 0.21 for childhood Pb; when only prenatal and postnatal Pb were included in the mixture, the PIP for prenatal Pb was 0.24 and childhood Pb was 0.11. Neither prenatal nor childhood Pb were associated with BASC-2 SRP Depression and Somatization scores (Figure S10).
Sensitivity analyses using raw scores instead of T-scores as outcomes (Table S7) and adjusting for family history of mental illness (Table S8) or gestational weight gain (Table S9) did not change associations. Overall, the direction and magnitude of associations did not appreciably change when calculated by IPCW, although in the case of the CPRS Anxious-Shy outcome, the positive associations of prenatal Mn for girls were slightly strengthened [e.g., 3.09 (95% CI: 0.08, 6.10) per doubling Mn versus 2.47 (95% CI: −0.56, 5.49) complete case] (Table S10). When excluding those taking medications for a behavioral disorder (Table S11), those diagnosed with an emotional illness by age 8, or those diagnosed with anxiety and/or depression by age 15 (Table S12), associations of most chemicals with CPRS and BASC-2 SRP outcomes did not substantially change. The exceptions were in analyses where participants on behavioral disorder medications at respective ages were excluded, the negative association between p,p’-DDE and CPRS Psychosomatic score for boys was attenuated, and the positive associations of Mn with CPRS Anxious-Shy and BASC-2 SRP Anxiety and Depression scores for girls were strengthened (Table S11). In CPRS analyses restricted to those participants with BASC-2 SRP follow-up, directions of associations did not change (Table S13).
4. Discussion
In our study of a socioeconomically diverse prospective cohort of mother-child pairs, we found evidence of adverse associations between prenatal metal exposures and internalizing symptoms. In models accounting for co-exposure confounding, positive, linear associations of prenatal Mn with internalizing symptoms for girls persisted from mid-childhood through adolescence. Although not present with mid-childhood outcomes, our findings also support positive associations between prenatal Pb and anxiety symptoms in adolescence. In analyses excluding participants on behavioral medications or with diagnosed anxiety or depression, associations with these metals persisted, suggesting that the findings are relevant to those with sub-clinical symptoms. We did not find consistent associations between organochlorines and internalizing symptoms at either time point. Furthermore, we observed that the overall mixture of prenatal organochlorines and metals was positively associated with child and adolescent anxiety and depressive symptoms, but negatively associated with somatic symptoms.
Prenatal Pb and Mn levels were similar to those observed in other United States/Canadian populations in the 1990s (Ettinger et al. 2020; Smargiassi et al. 2002), although cord blood Pb levels were higher than those measured more recently (Arbuckle et al. 2016b; Ettinger et al. 2020). Most prior studies of prenatal Pb exposure have assessed internalizing symptoms in early or mid-childhood and reported no associations (Arbuckle et al. 2016a; Bellinger et al. 1994; Horton et al. 2018; Plusquellec et al. 2010; Sioen et al. 2013). In our analyses, although an association with prenatal Pb was not present with mid-childhood anxiety-related symptoms, we observed an adverse association with CPRS Psychosomatic score. Previous studies have focused on internalizing problems as a composite scale and not specifically on somatic symptoms as an individual outcome. Somatic symptoms consist of physical complaints, often thought to be manifestations of underlying emotional problems in the absence of other health issues. The presentation and recognition of internalizing psychopathology in childhood may vary based on sociocultural factors (Liu et al. 2011), and thus, consideration of somatic symptoms can help identify at-risk children who are not scored highly on anxiety- or depressive-related scales because such symptoms are more typically manifested as somatic symptoms. These symptoms may also be comorbid with anxiety or depression (Silber and Pao 2003), or predictive of the development of a mental disorder in adulthood (Bohman et al. 2018), possibly signaling that associations between Pb exposure and anxiety or depressive problems may present later in the life course. During adolescence, a vulnerable period for the onset or exacerbation of internalizing symptoms (Kessler et al. 2005; Koenen et al. 2013) and when symptoms are best characterized by the individual experiencing them (Koenen et al. 2013; Smith 2007), we observed strong, positive associations of prenatal Pb exposure with continuous and dichotomized BASC-2 SRP Anxiety scores.
Prior studies of Pb exposure in infancy and childhood have shown more consistent associations with adverse neurobehavioral outcomes (Lanphear et al. 2005; Téllez-Rojo et al. 2006), as compared to Pb exposure prenatally. With use of deciduous teeth biomarkers, associations have been observed for postnatal, but not prenatal, Pb exposure with mid-childhood internalizing symptoms (Bellinger et al. 1994; Horton et al. 2018). For example, a study using distributed lag models and lagged weighted quantile sum regression for mixtures of Pb, Mn, and zinc found that early postnatal Pb, from birth to 12 months, was positively associated with BASC-2 PRS Anxiety and Depression scores at ages 8-11 years (Horton et al. 2018). However, in our BKMR model that evaluated prenatal versus peak childhood Pb, prenatal Pb appeared to have a stronger positive association than postnatal Pb with BASC-2 SRP Anxiety score in adolescence. Our study differs from prior analyses in the use of cord blood and childhood blood as biomarkers of exposure. Although cord blood and deciduous teeth reflect mid- to late-pregnancy exposures, our use of peak whole blood Pb captures maximum exposure from 12-36 months, whereas the dentine biomatrix allows for exploration of more specific exposure timing from birth through early childhood. Thus, we do not have data on the exposure window of birth to 12 months and this may account for differences in our findings versus those in prior studies.
There is limited literature on prenatal Mn exposure and child internalizing symptoms. In a Mexico City birth cohort, prenatal Mn was not associated with BASC-2 PRS Anxiety score in mid-childhood, but sex-specific associations were not considered (Horton et al. 2018). In contrast to our study, an analysis of prenatal Mn dentine levels observed a positive association with mid-childhood BASC-2 PRS Internalizing Problems score for boys but not girls (Mora et al. 2015). With sexual dimorphism in neural, as well as placental, structure and function, it is plausible that the absorption or toxicity of chemical insults, such as Mn, may differ based on sex (Cowell and Wright 2017; Sacher et al. 2013). Although investigation of sex-specific effects of prenatal Mn and internalizing symptoms is limited, adverse associations of Mn with other neurobehavioral outcomes have been observed just for girls. Specifically, high prenatal Mn exposure was adversely associated with visuospatial learning and working memory for girls aged 11-14 years (Bauer et al. 2017), and postnatal Mn exposure was negatively associated with long-term memory/learning (Torres-Agustín et al. 2013) and intellectual functioning for girls in mid-childhood (Riojas-Rodríguez et al. 2010). There is evidence that genetic variation in Mn transporter genes SLC30A10 and SLC39A8 may lead to dysregulation of excess brain Mn levels (Chen et al. 2015; Wahlberg et al. 2016), with girls being genetically less efficient in regulating Mn (Broberg et al. 2019).
Animal studies have observed increased anxiety-like behaviors in mice (Elnar et al. 2012) and depressive-like behaviors in rats (Lilienthal et al. 2014) following perinatal PCB exposure. PCBs, along with other organochlorines, may dysregulate glutamate and dopamine-mediated functions, which play essential roles in the limbic system (Bell 2014). However, our mainly null findings assessing prenatal organochlorine exposures, as a mixture with metal components, and internalizing symptoms in mid-childhood and adolescence are consistent with observations in a small number of prior epidemiologic studies, mostly focused on individual exposures and/or symptoms through mid-childhood. In single-chemical models, prenatal 2,2′,4,4′,5,5′-hexachlorobiphenyl (PCB-153) and p,p’-DDE were not associated with odds of mid-childhood abnormal emotional symptoms (Rosenquist et al. 2017; Sioen et al. 2013) or depression diagnosis through early adulthood (Strom et al. 2014). Additionally, studies considering co-exposure to a wide range of prenatal environmental exposures, including HCB, p,p’-DDE, and PCB congeners, found no associations with internalizing behaviors at 3-7 years (Jedynak et al. 2021) or 6-11 years (Maitre et al. 2021). Prior studies also did not observe associations between prenatal HCB and internalizing problems (Sioen et al. 2013; Strom et al. 2014). In a population with high prenatal PCB exposure, a study showed a positive association between prenatal PCB-153 and early childhood anxiety behaviors (Plusquellec et al. 2010), with behaviors assessed in a testing session via the Bayley Scales of Infant Development rather than more natural environments reflected in parent and teacher observations as well as self-report via behavioral check lists. Notably, prenatal organochlorine levels in the NBC were relatively low compared to other high risk exposure cohorts (Korrick et al. 2000; Longnecker et al. 2003), despite residence near the NBH Superfund Site. Thus, this study may have been underpowered to detect more modest effects.
For both mid-childhood and adolescent internalizing outcomes, we observed associations in which HCB or p,p’-DDE appeared protective against anxiety or somatic symptoms. This unexpected finding has also been reported in a few studies of prenatal or postnatal organochlorine exposure in relation to mid-childhood behaviors (Jedynak et al. 2021; Lenters et al. 2019; Maitre et al. 2021). As several of the proposed biological mechanisms for the effects of organochlorines on the neuroendocrine system are hypothesized to increase susceptibility to internalizing symptoms (Bell 2014), it is plausible that apparent protective associations are due to negative confounding bias by dietary factors (Choi et al. 2008) or live birth bias (Liew et al. 2015). Although we controlled for prenatal seafood consumption, a major source of organochlorines is diet (Schecter et al. 2010); thus, measurement error in self-reported responses may lead to residual confounding. Furthermore, a study of perinatal HCB exposure and childhood ADHD observed protective associations (Lenters et al. 2019), and proposed that if high organochlorine exposure increases likelihood of pregnancy loss (Jarrell et al. 1998; Longnecker et al. 2005), then this could possibly cause a protective association due to collider stratification bias (i.e., live birth bias) (Liew et al. 2015). However, NBC enrollment was based on live births, so we cannot quantify the impact of fetal loss.
We observed no evidence of interactions among our targeted organochlorines and metals in associations with internalizing symptoms. Several prior studies have observed that prenatal or postnatal Pb exposures modify the association of Mn and neurodevelopmental outcomes in early- or mid-childhood, with toxicity of one metal being stronger at higher levels of the other (Claus Henn et al. 2012; Kim et al. 2009; Lin et al. 2013; Menezes-Filho et al. 2018; Mora et al. 2015). The lack of interaction could be due to relatively low cord blood Pb levels in our cohort, a sample size insufficient to detect more moderate interactions, or simply no true effect modification.
Limitations of our study include potential for selection bias due to cohort attrition. We aimed to address potential selection bias with IPCW, which showed minimal bias, as complete case and IPCW effect estimates were similar. However, residual bias may remain, as some predictors of dropout may not have been measured or had too much missingness to be included in creating weights. Our results also may be prone to residual bias from measurement error of confounding covariates, as prenatal maternal behaviors (i.e., diet and smoking) were assessed retrospectively at approximately two weeks postpartum. Furthermore, while our sample size was relatively large in comparison to prior studies with follow up in mid-childhood and adolescence, we may still have been limited in our power to detect potential effect modification by sex in linear regression models. Additionally, we did not correct for multiple comparisons in our linear regression models, and thus some associations may be false positives. However, with correlated internalizing outcomes and expected modest differences in internalizing symptom T-scores per doubling of chemical level, we prioritized risk of false positive over false negative results. Replication in future studies is needed, particularly for findings on sex-specific associations. Generalizability was possibly limited due to NBC eligibility criteria of English- and/or Portuguese-speaking women, maternal residence near the NBH, and vaginal births, although the socioeconomic and demographic variability of our cohort would be expected to aid its generalizability to diverse populations. Lastly, except for Pb, we were only able to explore prenatal, not postnatal, chemical mixtures and thus were unable to elucidate which is a more susceptible window of exposure. Prior studies that used deciduous tooth dentine as a biomarker have been able to evaluate both prenatal and early postnatal chemical exposures (Horton et al. 2018; Mora et al. 2015), although sample sizes are often much smaller due to difficulties with teeth collection. Although not applied to the complete mixture, we used BKMR to investigate the impact of prenatal versus peak childhood Pb exposure and found that when associations with Pb were present, prenatal Pb appeared to have a stronger, positive association with mid-childhood and adolescent internalizing symptoms, even though exposure levels were lower during pregnancy than in early childhood.
Even with these limitations, our study had a number of strengths. This is the first study to use a flexible mixture approach to consider joint exposure to organochlorines and metals in relation to internalizing symptoms throughout childhood. With ascertainment of symptoms at two timepoints, we could explore whether associations change or persist from childhood to adolescence. Use of self-report in adolescence provides insight into symptoms at a life stage when anxiety and depressive disorders are more likely to present as compared to mid-childhood (Kessler et al. 2005; Koenen et al. 2013; National Research Council et al. 2009). While a few prior studies have explored simultaneous prenatal organochlorine or metal exposures (Horton et al. 2018; Jedynak et al. 2021; Maitre et al. 2021), or internalizing outcomes assessed beyond mid-childhood (Strom et al. 2014), to the best of our knowledge, no prior study has done both. Our study addresses this limitation in prior research by leveraging both comprehensive chemical biomarker data and data from multiple study visits to evaluate how exposure to a chemical mixture, rather than exposures in isolation, influence anxiety, depressive, and somatic symptoms from mid-childhood through adolescence, the latter being a sensitive period for onset or exacerbation of internalizing disorders. As a fetus is exposed to numerous chemicals and the toxicity of some chemicals may depend on the presence of others, it is important to optimize characterization of exposure risk in vulnerable populations, including children exposed in utero (Claus Henn et al. 2014; Mitro et al. 2015). Additionally, our use of continuous outcome scores has some advantages over clinical diagnosis, as it allows for detection of early or milder manifestations of internalizing disorders and enhanced power to detect exposure effects (Bellinger 2004). Furthermore, in a select few cases where we had multiple informants per child for teacher or parent report, we made the decision to average scores. This approach may induce outcome measurement error; however, with a continuous outcome, precision may be impacted but effect estimates are not biased. We also had access to assessments from multiple reporters, which permitted us to focus on informants known to better judge internalizing symptoms at each age, as well as compare patterns of associations across reporters. As anticipated based on prior research (Achenbach et al. 1987; Reynolds and Kamphaus 2004; Sourander et al. 1999), symptoms were weakly to moderately correlated across reporters. Moreover, in some cases, directions of association differed based on reporter. We also observed possible bias in how informants rate symptoms based on sex and/or context. For instance, teachers reported more CRS Anxious-Shy problems for boys whereas parents did so for girls. With the BASC-2, teachers classified relatively few participants as having potentially clinically significant symptoms. Such findings highlight the importance of considering the source of data for internalizing symptoms, which are often not easily observable by “outsiders.”
In summary, our results support a positive association of prenatal metals, but not organochlorines, with internalizing symptoms in mid-childhood and adolescence. Anxiety and depression are two of the most common psychiatric disorders, and emergence of these conditions in childhood increases lifelong risk of morbidity (Koenen et al. 2013; National Research Council et al. 2009; Woodward and Fergusson 2001). Thus, it is imperative to assess modifiable early life risk factors, such as prenatal chemical exposures, that can be intervened upon to reduce lifetime risk of internalizing disorders. These findings suggest that prenatal Pb and Mn exposures may contribute to susceptibility for anxiety and depressive symptoms and that future studies should explore associations in a sex-specific manner, particularly with respect to Mn exposure.
Supplementary Material
Acknowledgments:
Support for this research was provided by grants (P42ES005947, R01ES014864, P30ES000002) from the National Institute of Environmental Health Sciences (NIEHS/NIH). L.B.R. was additionally supported by the CDC/NIOSH Harvard Education and Research Center (T42OH008416).
Abbreviations:
- BASC-2
Behavior Assessment System for Children, Second Edition
- BKMR
Bayesian Kernel Machine Regression
- CI
confidence interval
- CRS
Conners’ Rating Scale
- CPRS
Conners’ Parent Rating Scale
- CTRS
Conners’ Teacher Rating Scale
- CV
coefficient of variation
- HCB
hexachlorobenzene
- HOME
Home Observation for Measurement of the Environment
- IPCW
inverse probability of censoring weighting
- LOD
limit of detection
- Mn
manganese
- NBC
New Bedford Cohort
- NBH
New Bedford Harbor
- Pb
lead
- PCB
polychlorinated biphenyl
- PIP
posterior inclusion probability
- PNSDI
prenatal social disadvantage index
- p,p’-DDE
p,p'-dichlorodiphenyl dichloroethylene
- PRS
Parent Rating Scales
- rs
Spearman’s rank correlation coefficient
- SD
standard deviation
- SRP
Self Report of Personality
- TRS
Teacher Rating Scales
Footnotes
Declarations of competing interests:
The authors have no financial or other conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achenbach TM, McConaughy SH, Howell CT. 1987. Child/adolescent behavioral and emotional problems: implications of cross-informant correlations for situational specificity. Psychol Bull 101:213–232. [PubMed] [Google Scholar]
- Addae C, Cheng H, Martinez-Ceballos E. 2013. Effect of the environmental pollutant hexachlorobenzene (HCB) on the neuronal differentiation of mouse embryonic stem cells. Int J Environ Res Public Health 10:5244–5256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbuckle TE, Davis K, Boylan K, Fisher M, Fu J. 2016a. Bisphenol A, phthalates and lead and learning and behavioral problems in Canadian children 6-11 years of age: CHMS 2007-2009. Neurotoxicology 54:89–98. [DOI] [PubMed] [Google Scholar]
- Arbuckle TE, Liang CL, Morisset AS, Fisher M, Weiler H, Cirtiu CM, et al. 2016b. Maternal and fetal exposure to cadmium, lead, manganese and mercury: The MIREC study. Chemosphere 163:270–282. [DOI] [PubMed] [Google Scholar]
- Bauer JA, Claus Henn B, Austin C, Zoni S, Fedrighi C, Cagna G, et al. 2017. Manganese in teeth and neurobehavior: Sex-specific windows of susceptibility. Environ Int 108:299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell MR. 2014. Endocrine-disrupting actions of PCBs on brain development and social and reproductive behaviors. Curr Opin Pharmacol 19:134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger D, Leviton A, Allred E, Rabinowitz M. 1994. Pre- and postnatal lead exposure and behavior problems in school-aged children. Environ Res 66:12–30. [DOI] [PubMed] [Google Scholar]
- Bellinger DC. 2004. What is an adverse effect? A possible resolution of clinical and epidemiological perspectives on neurobehavioral toxicity. Environ Res 95:394–405. [DOI] [PubMed] [Google Scholar]
- Bellinger DC. 2013. Prenatal Exposures to Environmental Chemicals and Children's Neurodevelopment: An Update. Saf Health Work 4:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betharia S, Maher TJ. 2012. Neurobehavioral effects of lead and manganese individually and in combination in developmentally exposed rats. Neurotoxicology 33:1117–1127. [DOI] [PubMed] [Google Scholar]
- Bobb JF, Valeri L, Claus Henn B, Christiani DC, Wright RO, Mazumdar M, et al. 2015. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics (Oxford, England) 16:493–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobb JF, Claus Henn B, Valeri L, Coull BA. 2018. Statistical software for analyzing the health effects of multiple concurrent exposures via Bayesian kernel machine regression. Environ Health 17:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohman H, Låftman SB, Cleland N, Lundberg M, Päären A, Jonsson U. 2018. Somatic symptoms in adolescence as a predictor of severe mental illness in adulthood: a long-term community-based follow-up study. Child Adolesc Psychiatry Ment Health 12:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broberg K, Taj T, Guazzetti S, Peli M, Cagna G, Pineda D, et al. 2019. Manganese transporter genetics and sex modify the association between environmental manganese exposure and neurobehavioral outcomes in children. Environ Int 130:104908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell BM, Bradley RH. 1985. Home Observation for Measurement of the Environment. New York:Dorsey. [Google Scholar]
- Canfield RL, Henderson CR Jr., Cory-Slechta DA, Cox C, Jusko TA, Lanphear BP. 2003. Intellectual impairment in children with blood lead concentrations below 10 microg per deciliter. N Engl J Med 348:1517–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Chakraborty S, Mukhopadhyay S, Lee E, Paoliello MM, Bowman AB, et al. 2015. Manganese homeostasis in the nervous system. J Neurochem 134:601–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi AL, Cordier S, Weihe P, Grandjean P. 2008. Negative confounding in the evaluation of toxicity: the case of methylmercury in fish and seafood. Crit Rev Toxicol 38:877–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus Henn B, Schnaas L, Ettinger AS, Schwartz J, Lamadrid-Figueroa H, Hernández-Avila M, et al. 2012. Associations of early childhood manganese and lead coexposure with neurodevelopment. Environ Health Perspect 120:126–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus Henn B, Coull BA, Wright RO. 2014. Chemical mixtures and children's health. Curr Opin Pediatr 26:223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SR, Hernán MA. 2008. Constructing inverse probability weights for marginal structural models. American journal of epidemiology 168:656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners CK. 1997. Conners’ Rating Scales-Revised Technical Manual. North Tonawanda, NY:Multi-Health Systems. [Google Scholar]
- Cowell W, Colicino E, Levin-Schwartz Y, Enlow MB, Amarasiriwardena C, Andra SS, et al. 2021. Prenatal metal mixtures and sex-specific infant negative affectivity. Environ Epidemiol 5:e147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowell WJ, Wright RJ. 2017. Sex-Specific Effects of Combined Exposure to Chemical and Non-chemical Stressors on Neuroendocrine Development: a Review of Recent Findings and Putative Mechanisms. Curr Environ Health Rep 4:415–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza Lisboa SF, Gonçalves G, Komatsu F, Queiroz CA, Almeida AA, Moreira EG. 2005. Developmental lead exposure induces depressive-like behavior in female rats. Drug Chem Toxicol 28:67–77. [DOI] [PubMed] [Google Scholar]
- Desrochers-Couture M, Oulhote Y, Arbuckle TE, Fraser WD, Séguin JR, Ouellet E, et al. 2018. Prenatal, concurrent, and sex-specific associations between blood lead concentrations and IQ in preschool Canadian children. Environ Int 121:1235–1242. [DOI] [PubMed] [Google Scholar]
- Elnar AA, Diesel B, Desor F, Feidt C, Bouayed J, Kiemer AK, et al. 2012. Neurodevelopmental and behavioral toxicity via lactational exposure to the sum of six indicator non-dioxin-like-polychlorinated biphenyls (Σ6 NDL-PCBs) in mice. Toxicology 299:44–54. [DOI] [PubMed] [Google Scholar]
- Ettinger AS, Egan KB, Homa DM, Brown MJ. 2020. Blood Lead Levels in U.S. Women of Childbearing Age, 1976-2016. Environ Health Perspect 128:17012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghandour RM, Sherman LJ, Vladutiu CJ, AN MM, Lynch SE, Bitsko RH, et al. 2018. Prevalence and Treatment of Depression, Anxiety, and Conduct Problems in US Children. J Pediatr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman SE, Hornig M, Ghassabian A, Hahn J, Cherkerzian S, Albert PS, et al. 2017. Socioeconomic disadvantage, gestational immune activity, and neurodevelopment in early childhood. Proc Natl Acad Sci U S A 114:6728–6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenland S, Pearl J, Robins JM. 1999. Causal diagrams for epidemiologic research. Epidemiology 10:37–48. [PubMed] [Google Scholar]
- Hernán MA, Hernández-Díaz S, Robins JM. 2004. A structural approach to selection bias. Epidemiology 15:615–625. [DOI] [PubMed] [Google Scholar]
- Horton MK, Hsu L, Claus Henn B, Margolis A, Austin C, Svensson K, et al. 2018. Dentine biomarkers of prenatal and early childhood exposure to manganese, zinc and lead and childhood behavior. Environ Int 121:148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine, National Research Council Committee to Reexamine I. O. M. Pregnancy Weight Guidelines. 2009. The National Academies Collection: Reports funded by National Institutes of Health. In: Weight Gain During Pregnancy: Reexamining the Guidelines, (Rasmussen KM, Yaktine AL, eds). Washington (DC):National Academies Press (US) Copyright © 2009, National Academy of Sciences. [Google Scholar]
- Jarrell J, Gocmen A, Foster W, Brant R, Chan S, Sevcik M. 1998. Evaluation of reproductive outcomes in women inadvertently exposed to hexachlorobenzene in southeastern Turkey in the 1950s. Reprod Toxicol 12:469–476. [DOI] [PubMed] [Google Scholar]
- Jedynak P, Maitre L, Guxens M, Gützkow KB, Julvez J, López-Vicente M, et al. 2021. Prenatal exposure to a wide range of environmental chemicals and child behaviour between 3 and 7 years of age - An exposome-based approach in 5 European cohorts. Sci Total Environ 763:144115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman A, Kaufman N. 1990. Kaufman Brief Intelligence Test. Circle Pines, MN:American Guidance Service. [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. 2005. Lifetime Prevalence and Age-of-Onset Distributions of DSM-IV Disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry 62:593–602. [DOI] [PubMed] [Google Scholar]
- Kim R, Aro A, Rotnitzky A, Amarasiriwardena C, Hu H. 1995. K x-ray fluorescence measurements of bone lead concentration: the analysis of low-level data. Phys Med Biol 40:1475–1485. [DOI] [PubMed] [Google Scholar]
- Kim Y, Kim BN, Hong YC, Shin MS, Yoo HJ, Kim JW, et al. 2009. Co-exposure to environmental lead and manganese affects the intelligence of school-aged children. Neurotoxicology 30:564–571. [DOI] [PubMed] [Google Scholar]
- Koenen KC, Rudenstine S, Susser E, Galea S. 2013. A Life Course Approach to Mental Disorders. Oxford, U.K.:Oxford University Press. [Google Scholar]
- Korrick SA, Altshul LM, Tolbert PE, Burse VW, Needham LL, Monson RR. 2000. Measurement of PCBs, DDE, and hexachlorobenzene in cord blood from infants born in towns adjacent to a PCB-contaminated waste site. Journal of exposure analysis and environmental epidemiology 10:743–754. [DOI] [PubMed] [Google Scholar]
- Korrick SA, Sagiv SK. 2008. Polychlorinated biphenyls, organochlorine pesticides and neurodevelopment. Curr Opin Pediatr 20:198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanphear BP, Hornung R, Khoury J, Yolton K, Baghurst P, Bellinger DC, et al. 2005. Low-level environmental lead exposure and children's intellectual function: an international pooled analysis. Environ Health Perspect 113:894–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YM, Kim KS, Jacobs DR Jr., Lee DH. 2017. Persistent organic pollutants in adipose tissue should be considered in obesity research. Obes Rev 18:129–139. [DOI] [PubMed] [Google Scholar]
- Lenters V, Iszatt N, Forns J, Čechová E, Kočan A, Legler J, et al. 2019. Early-life exposure to persistent organic pollutants (OCPs, PBDEs, PCBs, PFASs) and attention-deficit/hyperactivity disorder: A multi-pollutant analysis of a Norwegian birth cohort. Environ Int 125:33–42. [DOI] [PubMed] [Google Scholar]
- Liew Z, Olsen J, Cui X, Ritz B, Arah OA. 2015. Bias from conditioning on live birth in pregnancy cohorts: an illustration based on neurodevelopment in children after prenatal exposure to organic pollutants. Int J Epidemiol 44:345–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilienthal H, Heikkinen P, Andersson PL, van der Ven LT, Viluksela M. 2014. Dopamine-dependent behavior in adult rats after perinatal exposure to purity-controlled polychlorinated biphenyl congeners (PCB52 and PCB180). Toxicol Lett 224:32–39. [PubMed] [Google Scholar]
- Lin CC, Chen YC, Su FC, Lin CM, Liao HF, Hwang YH, et al. 2013. In utero exposure to environmental lead and manganese and neurodevelopment at 2 years of age. Environ Res 123:52–57. [DOI] [PubMed] [Google Scholar]
- Liu J, Chen X, Lewis G. 2011. Childhood internalizing behaviour: analysis and implications. J Psychiatr Ment Health Nurs 18:884–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longnecker MP, Wolff MS, Gladen BC, Brock JW, Grandjean P, Jacobson JL, et al. 2003. Comparison of polychlorinated biphenyl levels across studies of human neurodevelopment. Environ Health Perspect 111:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longnecker MP, Klebanoff MA, Dunson DB, Guo X, Chen Z, Zhou H, et al. 2005. Maternal serum level of the DDT metabolite DDE in relation to fetal loss in previous pregnancies. Environ Res 97:127–133. [DOI] [PubMed] [Google Scholar]
- Maitre L, Julvez J, López-Vicente M, Warembourg C, Tamayo-Uria I, Philippat C, et al. 2021. Early-life environmental exposure determinants of child behavior in Europe: A longitudinal, population-based study. Environ Int 153:106523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason LH, Harp JP, Han DY. 2014. Pb neurotoxicity: neuropsychological effects of lead toxicity. Biomed Res Int 2014:840547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menezes-Filho JA, Carvalho CF, Rodrigues JLG, Araújo CFS, Dos Santos NR, Lima CS, et al. 2018. Environmental Co-Exposure to Lead and Manganese and Intellectual Deficit in School-Aged Children. Int J Environ Res Public Health 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR. 2005. Vulnerability factors for anxiety disorders in children and adolescents. Child and adolescent psychiatric clinics of North America 14:649–679, vii. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, He JP, Burstein M, Swanson SA, Avenevoli S, Cui L, et al. 2010. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication--Adolescent Supplement (NCS-A). J Am Acad Child Adolesc Psychiatry 49:980–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitro SD, Johnson T, Zota AR. 2015. Cumulative Chemical Exposures During Pregnancy and Early Development. Curr Environ Health Rep 2:367–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora AM, Arora M, Harley KG, Kogut K, Parra K, Hernandez-Bonilla D, et al. 2015. Prenatal and postnatal manganese teeth levels and neurodevelopment at 7, 9, and 10.5 years in the CHAMACOS cohort. Environ Int 84:39–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima S, Saijo Y, Miyashita C, Ikeno T, Sasaki S, Kajiwara J, et al. 2017. Sex-specific differences in effect of prenatal exposure to dioxin-like compounds on neurodevelopment in Japanese children: Sapporo cohort study. Environ Res 159:222–231. [DOI] [PubMed] [Google Scholar]
- National Research Council, Institute of Medicine Committee on the Prevention of Mental Disorders and Substance Abuse Among Children, Youths and Young Adults : Research Advances and Promising Interventions. 2009. The National Academies Collection: Reports funded by National Institutes of Health. In: Preventing Mental, Emotional, and Behavioral Disorders Among Young People: Progress and Possibilities, (O'Connell ME, Boat T, Warner KE, eds). Washington (DC):National Academies Press (US), National Academy of Sciences. [Google Scholar]
- Non AL, Rewak M, Kawachi I, Gilman SE, Loucks EB, Appleton AA, et al. 2014. Childhood social disadvantage, cardiometabolic risk, and chronic disease in adulthood. American journal of epidemiology 180:263–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plusquellec P, Muckle G, Dewailly E, Ayotte P, Bégin G, Desrosiers C, et al. 2010. The relation of environmental contaminants exposure to behavioral indicators in Inuit preschoolers in Arctic Quebec. Neurotoxicology 31:17–25. [DOI] [PubMed] [Google Scholar]
- Pugh SJ, Hutcheon JA, Richardson GA, Brooks MM, Himes KP, Day NL, et al. 2016. Gestational weight gain, prepregnancy body mass index and offspring attention-deficit hyperactivity disorder symptoms and behaviour at age 10. Bjog 123:2094–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm J, Shield KD. 2019. Global Burden of Disease and the Impact of Mental and Addictive Disorders. Curr Psychiatry Rep 21:10. [DOI] [PubMed] [Google Scholar]
- Reynolds CR, Kamphaus RW. 2004. Behavior Assessment System for Children, Second Edition (BASC-2). Circle Pines, MN:American Guidance Service. [Google Scholar]
- Riojas-Rodríguez H, Solís-Vivanco R, Schilmann A, Montes S, Rodríguez S, Ríos C, et al. 2010. Intellectual function in Mexican children living in a mining area and environmentally exposed to manganese. Environ Health Perspect 118:1465–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenquist AH, Hoyer BB, Julvez J, Sunyer J, Pedersen HS, Lenters V, et al. 2017. Prenatal and Postnatal PCB-153 and p,p'-DDE Exposures and Behavior Scores at 5-9 Years of Age among Children in Greenland and Ukraine. Environ Health Perspect 125:107002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacher J, Neumann J, Okon-Singer H, Gotowiec S, Villringer A. 2013. Sexual dimorphism in the human brain: evidence from neuroimaging. Magn Reson Imaging 31:366–375. [DOI] [PubMed] [Google Scholar]
- Sagiv SK, Thurston SW, Bellinger DC, Tolbert PE, Altshul LM, Korrick SA. 2010. Prenatal organochlorine exposure and behaviors associated with attention deficit hyperactivity disorder in school-aged children. American journal of epidemiology 171:593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagiv SK, Thurston SW, Bellinger DC, Altshul LM, Korrick SA. 2012. Neuropsychological measures of attention and impulse control among 8-year-old children exposed prenatally to organochlorines. Environ Health Perspect 120:904–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvini S, Hunter DJ, Sampson L, Stampfer MJ, Colditz GA, Rosner B, et al. 1989. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol 18:858–867. [DOI] [PubMed] [Google Scholar]
- Schecter A, Colacino J, Haffner D, Patel K, Opel M, Päpke O, et al. 2010. Perfluorinated compounds, polychlorinated biphenyls, and organochlorine pesticide contamination in composite food samples from Dallas, Texas, USA. Environ Health Perspect 118:796–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silber TJ, Pao M. 2003. Somatization disorders in children and adolescents. Pediatr Rev 24:255–264. [DOI] [PubMed] [Google Scholar]
- Sioen I, Den Hond E, Nelen V, Van de Mieroop E, Croes K, Van Larebeke N, et al. 2013. Prenatal exposure to environmental contaminants and behavioural problems at age 7-8years. Environ Int 59:225–231. [DOI] [PubMed] [Google Scholar]
- Smargiassi A, Takser L, Masse A, Sergerie M, Mergler D, St-Amour G, et al. 2002. A comparative study of manganese and lead levels in human umbilical cords and maternal blood from two urban centers exposed to different gasoline additives. Sci Total Environ 290:157–164. [DOI] [PubMed] [Google Scholar]
- Smith SR. 2007. Making sense of multiple informants in child and adolescent psychopathology - A guide for clinicians. Journal of Psychoeducational Assessment 25:139–149. [Google Scholar]
- Sokolowski K, Corbin JG. 2012. Wired for behaviors: from development to function of innate limbic system circuitry. Front Mol Neurosci 5:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sourander A, Helstelä L, Helenius H. 1999. Parent-adolescent agreement on emotional and behavioral problems. Soc Psychiatry Psychiatr Epidemiol 34:657–663. [DOI] [PubMed] [Google Scholar]
- Steel Z, Marnane C, Iranpour C, Chey T, Jackson JW, Patel V, et al. 2014. The global prevalence of common mental disorders: a systematic review and meta-analysis 1980-2013. Int J Epidemiol 43:476–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strom M, Hansen S, Olsen SF, Haug LS, Rantakokko P, Kiviranta H, et al. 2014. Persistent organic pollutants measured in maternal serum and offspring neurodevelopmental outcomes--a prospective study with long-term follow-up. Environ Int 68:41–48. [DOI] [PubMed] [Google Scholar]
- Téllez-Rojo MM, Bellinger DC, Arroyo-Quiroz C, Lamadrid-Figueroa H, Mercado-García A, Schnaas-Arrieta L, et al. 2006. Longitudinal associations between blood lead concentrations lower than 10 microg/dL and neurobehavioral development in environmentally exposed children in Mexico City. Pediatrics 118:e323–330. [DOI] [PubMed] [Google Scholar]
- Thapar A, Collishaw S, Pine DS, Thapar AK. 2012. Depression in adolescence. Lancet 379:1056–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Agustín R, Rodríguez-Agudelo Y, Schilmann A, Solís-Vivanco R, Montes S, Riojas-Rodríguez H, et al. 2013. Effect of environmental manganese exposure on verbal learning and memory in Mexican children. Environ Res 121:39–44. [DOI] [PubMed] [Google Scholar]
- Valeri L, Mazumdar MM, Bobb JF, Claus Henn B, Rodrigues E, Sharif OIA, et al. 2017. The Joint Effect of Prenatal Exposure to Metal Mixtures on Neurodevelopmental Outcomes at 20-40 Months of Age: Evidence from Rural Bangladesh. Environ Health Perspect 125:067015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bosch M, Meyer-Lindenberg A. 2019. Environmental Exposures and Depression: Biological Mechanisms and Epidemiological Evidence. Annu Rev Public Health 40:239–259. [DOI] [PubMed] [Google Scholar]
- Wahlberg K, Kippler M, Alhamdow A, Rahman SM, Smith DR, Vahter M, et al. 2016. Common Polymorphisms in the Solute Carrier SLC30A10 are Associated With Blood Manganese and Neurological Function. Toxicol Sci 149:473–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward LJ, Fergusson DM. 2001. Life course outcomes of young people with anxiety disorders in adolescence. J Am Acad Child Adolesc Psychiatry 40:1086–1093. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.