Summary
Previously either regarded as insignificant or feared as potential sources of infection, the bacteria living on our skin are increasingly recognized to benefit human health. Skin commensals modulate mucosal immune defenses and directly interfere with pathogens; however, their contribution to the skin’s physical integrity is less understood. Here we show that the abundant skin commensal Staphylococcus epidermidis contributes to skin barrier integrity. S. epidermidis secretes a sphingomyelinase that acquires essential nutrients for the bacteria but also assists the host in producing ceramides, the main constituent of the epithelial barrier that averts skin dehydration and aging. In mouse models, S. epidermidis significantly increases skin ceramide levels and prevents water loss of damaged skin in a fashion entirely dependent on its sphingomyelinase. Our findings reveal a symbiotic mechanism that demonstrates an important role of the skin microbiota in the maintenance of the skin’s protective barrier.
Keywords: Staphylococcus epidermidis, symbiosis, skin, skin barrier, skin microbiota, commensal, ceramides, probiotic
Graphical Abstract
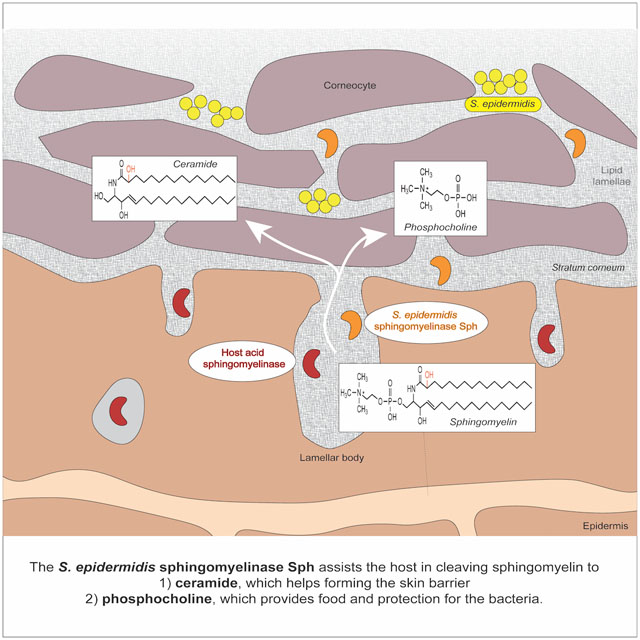
eTOC:
Ceramides are a key constituent of the skin barrier that prevent skin dehydration and aging. Zheng et al. show that the abundant skin commensal Staphylococcus epidermidis secretes a sphingomyelinase that facilitates host production of ceramides to help maintain skin integrity and prevent water loss of damaged skin.
Introduction
Over the last decade, it has been well established that the microbiota of the intestine plays a significant role for human health (Pflughoeft and Versalovic, 2012). Recent research indicates that also skin bacteria, in particular the skin commensal Staphylococcus epidermidis (Grice and Segre, 2011; Otto, 2009), exert a beneficial role by mediating mucosal immune tolerance (Leech et al., 2019; Scharschmidt et al., 2015), influencing immunological pathways and interacting with other microorganisms to modulate inflammation (Lai et al., 2009; Lima-Junior et al., 2021), and controlling overgrowth of pathogenic microorganisms via stimulation of innate host defense or direct bacterial interference (Cogen et al., 2008; Heilbronner et al., 2021; Liu et al., 2020a; Naik et al., 2015; Nakatsuji et al., 2017; Williams et al., 2019). For those reasons, S. epidermidis and related skin commensals are now frequently being promoted for potential probiotic-type topical application in order to maintain a healthy skin microbiome and promote the healing of skin diseases (Liu et al., 2020b; Nakatsuji et al., 2021; Williams et al., 2019).
In addition to harboring a series of immune cells that contribute to protection from infiltrating microorganisms (Kupper and Fuhlbrigge, 2004), the skin also forms a physical barrier whose integrity is critical for its defensive function (Proksch et al., 2008). Of premier importance to the skin’s physical integrity are its outer layers, which ascertain skin homeostasis by forming a permeability barrier that prevents dehydration and pathogen infiltration (Jungersted et al., 2008; Proksch et al., 2008). The outmost layer of the human epidermis, the stratum corneum, consists of corneocytes, which are flattened dead cells that develop from keratinocytes, after they proliferate, differentiate, and migrate towards the surface of the skin (Fig. 1A). Corneocytes are embedded in a matrix of lamellar lipid sheets that are essential for the skin’s permeability barrier function, preventing the loss of water and electrolytes and the infiltration of harmful substances (Elias and Ghadially, 2002; Proksch et al., 2008; Wertz, 2000).
Fig. 1. S. epidermidis skin colonization decreases water loss and increases stratum corneum ceramide content.
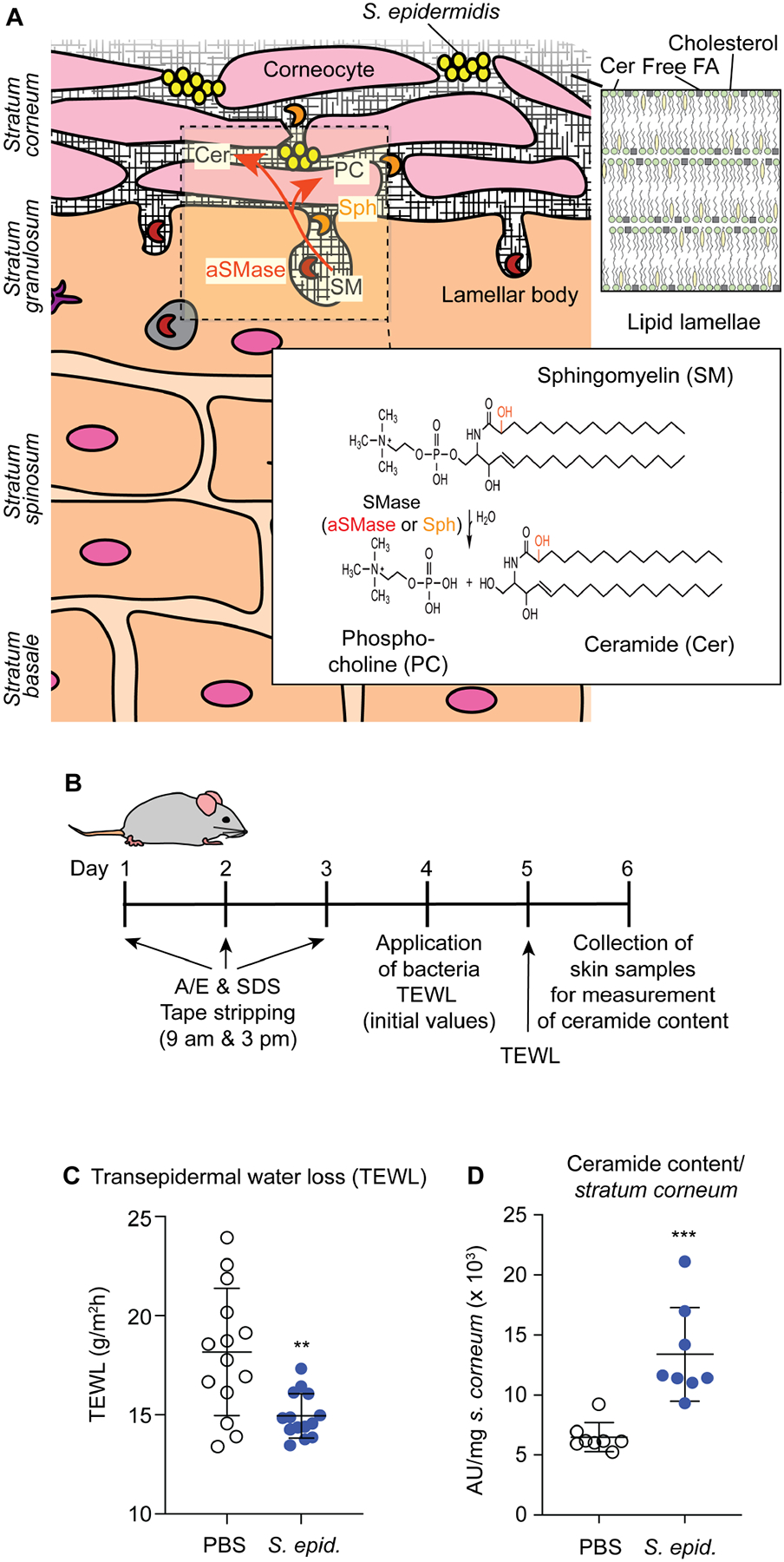
A, Epidermal architecture, role of aSMase, and proposed role of the S. epidermidis sphingomyelinase Sph in homeostasis of the skin protective barrier. The graph shows the different layers of the epidermis, with live keratinocytes in the lower and dead corneocytes in the outmost stratum corneum layer. The acid sphingomyelinase (aSMase) enzyme, which produces phosphocholine (PC) and ceramide (Cer) from sphingomyelin is produced in keratinocytes and secreted via exocytosis in lamellar bodies. As described in the present study, S. epidermidis colonizes the skin surface and secretes an enzyme, Sph, with the same activity, assisting aSMase in the production of the skin’s protective barrier that is composed of lipid lamellae of ceramides, cholesterol, and free fatty acids (FA). The SM and Cer type shown in the insert is the most abundant Cer 2 type (d18:1/16:0), with the hydroxyl group shown in red present in the Cer 5 type. d, 1,3-dihydroxy. B, Setup of mouse model. A/E, acetone/diethyl ether. SDS, sodium dodecyl sulfate. TEWL, transepidermal water loss. TEWL measurements 24 h after skin compromise and association of mice with the indicated bacteria or PBS as control. Treatment groups were formed by evenly distributing mice according to their initial TEWL levels due to high mouse-specific variations in TEWL background levels. n=14/group. C, Total stratum corneum ceramide amount after association of compromised skin of live mice for 48 h with S. epidermidis or PBS control (n=8/group). C,D, Statistical analysis is by unpaired t-tests. Error bars show the mean ± SD.
Ceramides, a heterogeneous group of sphingolipids, constitute more than 50% of the lipids in the lamellar sheets, with the remaining constituents being cholesterol and free fatty acids (van Smeden et al., 2011). While ceramides can also be synthesized de novo and regenerated from glycosphingolipids via glycosidases, a considerable portion of the ceramides in the lipid lamellae (~ 20 – 40 %, ceramide types 2 and 5) is produced by cleavage from the abundant membrane lipid, sphingomyelin (SM) (t’Kindt et al., 2012; Uchida et al., 2000; Wertz et al., 1985). This reaction is catalyzed by the Zn2+-dependent acid sphingomyelinase (aSMase), which is secreted from keratinocytes in lamellar bodies via exocytosis, and which cleaves SM into ceramides and phosphocholine (PC) (Kornhuber et al., 2015) (Fig. 1A). Ceramides may then be further modified by acylation and covalently attached to stratum corneum proteins (Kihara, 2016). Deficiency in aSMase is associated with skin aging (Jensen et al., 2005), as are low levels of ceramides and other stratum corneum lipids (Ghadially et al., 1995; Rogers et al., 1996). Furthermore, ceramide deficiency causes dry skin (xerosis) (Vyumvuhore et al., 2018), which is typically observed with skin aging (Paul et al., 2011), and contributes to a variety of skin disorders, including psoriasis and atopic dermatitis (Bhattacharya et al., 2019; Kleuser and Japtok, 2013; Sahle et al., 2015). For those reasons, ceramides are major ingredients of a multitude of topical skin restoration and anti-aging products (Lueangarun et al., 2019; Spada et al., 2018).
While considerable knowledge has recently been gained about how commensal bacteria affect the skin’s immune system and interact within the skin microbiome, it is not known whether the colonizing microbiota also plays a direct role in contributing to the physical integrity of the skin and its function as a defensive permeability barrier. Furthermore, previous reports on beneficial functions of skin bacteria have not included an analysis of how these mechanisms serve the bacteria, lacking evolutionary explanation of why they developed. Moreover, analysis of the bacterial molecules that contribute to bacterial survival on the skin has focused on pathogen colonization during skin disease, for example by investigating factors that allow S. aureus to colonize the skin in the context of atopic dermatitis (Nakamura et al., 2020). In contrast, our understanding of the mechanisms underlying asymptomatic skin colonization by commensals, including those that have a potential benefit to the host, has remained very limited.
In the present study, we show that the skin commensal S. epidermidis contributes to skin barrier homeostasis by assisting the host to produce ceramides and identify activity of a secreted S. epidermidis sphingomyelinase as the underlying mechanism. Furthermore, we show that this sphingomyelinase serves to produce nutrients for S. epidermidis and significantly promotes skin colonization. Our study thus demonstrates an important role of the skin microbiota in promoting skin integrity in a host-commensal symbiotic fashion.
Results
S. epidermidis reduces water loss and increases stratum corneum ceramide content in a mouse model.
We assumed that under normal conditions, the skin produces sufficient stratum corneum lipids to maintain its barrier function, while any contribution of the microbiota to that process would manifest after periods of insults to the skin, which regularly occur such as by exposure to dryness, physical harm, or dehydrating agents. Therefore, to investigate a potential impact of the skin commensal S. epidermidis on the skin’s permeability barrier, we developed a mouse model in which we compromised the skin with a regimen including detergent, organic solvent, and tape stripping (Fig. 1B). We then applied defined amounts of S. epidermidis, and measured transepidermal water loss (TEWL) 24 h afterwards. Notably, the treatment was optimized to result in significant compromise of the skin permeability barrier (as determined by a significant increase in TEWL), but no visible compromise to skin integrity upon histological examination. We used hairless mice as they represent a well-established model for dermatological research (Benavides et al., 2009) and because preparation of the stratum corneum for the measurement of lipid contents is severely hampered by hair growth. Remarkably, we found that application of S. epidermidis under those conditions significantly lowered water loss through the skin, indicating a beneficial role of S. epidermidis in maintaining skin barrier homeostasis (Fig. 1C).
We then prepared the stratum corneum from compromised mouse skin treated with S. epidermidis, and measured ceramide content, hypothesizing that the observed impact of the commensal bacteria is due to an effect on the concentration of ceramides as the main constituent of the stratum corneum lipid lamellae. Indeed, ceramide levels of compromised skin were significantly increased after colonization with S. epidermidis as compared to control (Fig. 1D).
S. epidermidis produces a potent sphingomyelinase expressed on human skin.
To identify the mechanism underlying the observed contribution of S. epidermidis to skin barrier homeostasis, we hypothesized that S. epidermidis produces an SMase that catalyzes the formation of ceramides from SM during colonization. It is known that some bacteria produce SMases, which in contrast to mammalian aSMase have neutral pH optima and are Mg2+- or Mn2+-dependent (Goni and Alonso, 2002). In several pathogenic species such as S. aureus, where the SMase is called β-toxin, they have been implicated in virulence (Flores-Diaz et al., 2016; Herrera et al., 2016). While genes homologous to S. aureus β-toxin are annotated in the genomes of S. epidermidis and other staphylococci (Fig. S1), these putative SMases have not gained any attention by researchers and their function has remained unknown.
We first performed experiments to investigate the enzymatic characteristics of the putative S. epidermidis SMase (hereafter referred to as Sph, and its gene as sph). To that end, we over-expressed sph in the heterologous host Staphylococcus carnosus and purified Sph (Fig. S2). We found that Sph is a potent neutral sphingomyelinase (Fig. 2A, Fig. S2). It produced predominantly type-2 ceramides (Fig. S3), which mostly have d(1,3-dihydroxy)18:1-type sphingosine and 16:0 fatty-acid side chains (Uchida et al., 2000; Valsecchi et al., 2007), cleaving all except very short-chain fatty acid sphingomyelin species (Fig. 2A, Fig. S2). Activity was dependent on Mg2+, Ca2+, or Mn2+ (Fig. S2).
Fig. 2. Enzymatic activity, species-wide and in-vivo expression of Sph.
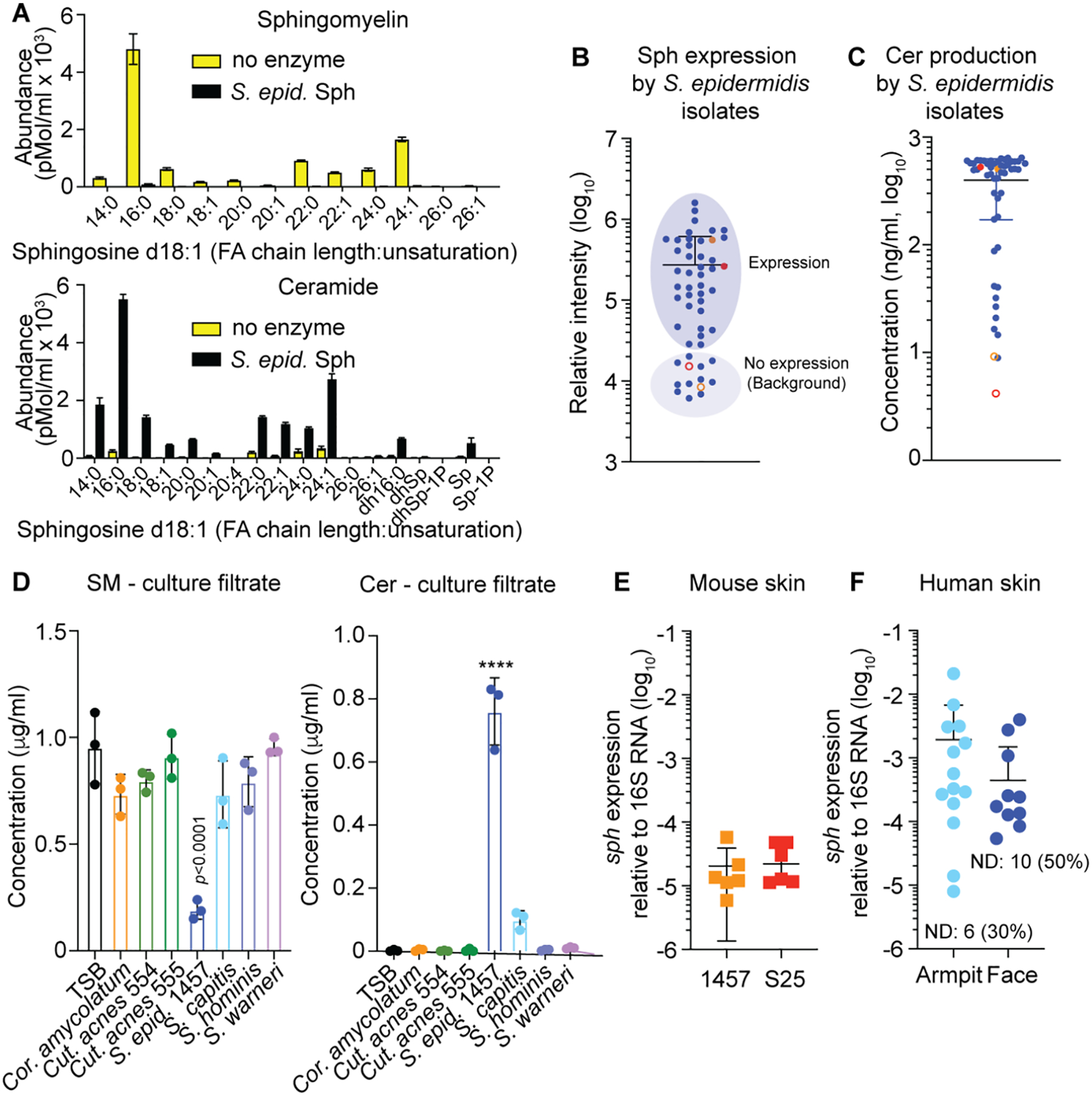
A, Cleavage of sphingomyelin from human keratinocytes by purified Sph (100 μg/ml). Shown is the Tandem-MS analysis of the major sphingomyelin type with d18:1 sphingosine, producing type-2 ceramide. FA, fatty acid; Sp, sphingosine (d18:1) of undetermined FA chain length; dh, dihydro (d18:0 instead of d18:1); P, phosphate. n=3. See Fig. S2 for results with other tissue sources using RP-HPLC/ESI-MS. B, In-vitro expression of Sph by Western blot and relative densitometric analysis in a collection of S. epidermidis isolates. Filled colored circles show data obtained with strains 1457 (red) and S25 (orange), open circles those with strains 1457 Δsph (red) and S25 Δsph (orange). See Fig. S4 for Western blot images. C, Production of ceramides by culture filtrates of a collection of S. epidermidis isolates. Filled colored circles show data obtained with strains 1457 (red) and S25 (orange), open circles those with strains 1457 Δsph (red) and S25 Δsph (orange). D, SM degradation and Cer production by other skin-colonizing bacteria compared to S. epidermidis. n=3. Statistical analysis is by 1-way ANOVA with Dunnett’s post-test via data obtained with TSB. E, Expression of the sph gene on mouse skin associated with the S. epidermidis strains used in this study. Expression was determined 1 day after association. n=6/group. F, Expression of the sph gene in swabs obtained from armpits and faces of human volunteers. n=20. Computation of mean and SD includes 0 values from individuals without detectable expression (ND). A-F, Error bars show the mean ± SD. S. epid., S. epidermidis; Cut. acnes, Cutibacterium acnes; Cor. amycolatum, Corynebacterium amycolatum.
To analyze the distribution of sph in S. epidermidis as a species, we analyzed a collection of 48 S. epidermidis skin isolates (Gu et al., 2005) by analytical PCR and found virtual omnipresence of the sph gene (47/48; 98%). These findings are in accordance with those from previous whole-genome sequencing analyses, according to which sph is present in most S. epidermidis strains (Conlan et al., 2012; Meric et al., 2018). Predicted Sph protein sequences from published S. epidermidis genomes are highly similar, differing maximally in two amino acids. Of note, in accordance with our PCR results, analysis of publicly available S. epidermidis genomes revealed that the sph gene is generally not interrupted by a phage, which is often the case for its S. aureus homolog, the hlb gene encoding β-toxin, and which abolishes enzyme production (Coleman et al., 1991). Accordingly, we detected Sph expression by Western blot of culture filtrates in 37 (77%) and considerable sphingomyelinase activity in most isolates of the skin isolate collection (Fig. 2B,C, Fig. S4).
We then investigated whether similar enzymatic activity to degrade SM and produce ceramides is also present in other major skin commensals, including Corynebacterium ssp., Cutibacterium acnes, and three further predominant skin-colonizing staphylococci (Grice and Segre, 2011; Kloos and Musselwhite, 1975). In contrast to S. epidermidis, no other non-staphylococcal bacteria we investigated were able to produce ceramides; and among other staphylococci, we only detected low activity in one strain (S. capitis) (Fig. 2D).
To analyze whether the sph gene is expressed in vivo, we determined expression during skin colonization in live mice (without compromise) using two strains, S. epidermidis 1457, an S. epidermidis standard strain (Mack et al., 1994), and S. epidermidis S25, a strain from our skin isolate collection. In-vitro expression and ceramide production capacity of these strains was representative for the species (Fig. 2B,C). Furthermore, we analyzed whether sph is expressed in the human skin microbiome in human volunteers. Our analyses demonstrated that the sph gene is expressed during experimental colonization of mouse skin and at even higher average levels in the skin microbiome of human volunteers (Fig. 2E,F). In humans, sph expression was found in 14 of 20 subjects (70%) in armpits and 10 of 20 subjects (50%) on facial skin, with higher frequency and extent of expression in armpits being in accordance with published data on body site dependence of S. epidermidis colonization (Kloos and Musselwhite, 1975; Oh et al., 2014).
These data show that S. epidermidis Sph is a potent SMase and the sph gene is present and expressed in a majority of S. epidermidis strains, notably including in the human skin microbiome and during experimental colonization in mice, without restriction to a specific genetic background. Furthermore, they indicate that SMase activity is limited to S. epidermidis among major skin-colonizing bacteria.
Sph in S. epidermidis does not harm the host.
A potentially beneficial role of a commensal factor implies that it does not have any deleterious effects on host cells. The S. aureus and B. cereus homologues of S. epidermidis Sph have been reported to exert virulence phenotypes, namely cytotoxicity and contribution to biofilm formation (Bernheimer et al., 1974; Huseby et al., 2010). Before analyzing potential beneficial effects, we therefore first determined whether the sph gene in S. epidermidis contributes to those virulence phenotypes. To that end, we constructed an isogenic S. epidermidis sph deletion mutant. The sph gene did not have measurable effects on cytolysis of keratinocytes or human or sheep erythrocytes either in its natural background in S. epidermidis, or when over-expressed in S. carnosus (Fig. 3A,B; Fig. S5). Furthermore, it did not have an effect on biofilm formation (Fig. 3C; Fig. S5). On the other hand, SMase activity of S. epidermidis Sph was even higher than that of S. aureus β-toxin (Fig. 3D), as was binding to liposomes and keratinocytes (Fig. 3E).
Fig. 3. S. epidermidis Sph does not harm the host.

A, Lysis of keratinocytes by the indicated bacterial strains. Bacteria were seeded at a ratio of 2:1 to keratinocytes. After 1-day incubation, the final ratio of bacteria to keratinocytes was about 700:1. Cytotoxicity is expressed as percent relative to complete lysis with triton X100 (100% lysis). n=3/group. B, Lysis of human erythrocytes by the indicated bacteria as measured by heme release. The bacteria to erythrocyte ratio was 5000:1. n=8/group. See Fig. S5 for results with sheep erythrocytes and bacterial culture filtrates. C, Biofilm formation in TSB/0.125% glucose. Biofilms were grown for 24 h in microtiter plates, stained with crystal violet, and absorption at 560 nm was measured. n=8/group. See Fig. S5 for results with different glucose concentrations. D, Comparison of enzymatic activities (sphingomyelin cleavage) of purified Sph and S. aureus β-toxin at equal concentrations (1.5 μg/ml). n=3/group. E, Binding of S. epidermidis Sph and S. aureus β-toxin to liposomes and keratinocytes. Binding was determined and measured via Western Blots and densitometry of positive bands. n=6/group. Statistical analysis is by two-tailed, unpaired t-tests. F, Structure of S. epidermidis Sph modeled using SWISS MODEL (https://swissmodel.expasy.org) based on the closest related sequence available (PDB 2uyr.1.A; B. cereus sphingomyelinase N57A). The three non-conserved amino acids of the edge metal binding site are shown with side chains (K91, V132, S133; see Fig. S1). The solvent-exposed loop is highlighted in yellow. Illustrations of the model obtained by SWISS MODEL were performed using Protean 3D (Lasergene 17). G, Structure comparison of S. epidermidis Sph with B. cereus Sph (PDB 2uyr.1.A) and S. aureus β-toxin (PDB 3i5v.1.A). Blue color designates conserved, red color divergent parts. A-E, Error bars show the mean ± SD. S. e., S. epid., S. epidermidis; S. c., S. carnosus.
These findings indicate that there are inherent differences between the virulence-associated B. cereus or S. aureus enzymes and S. epidermidis Sph that cannot be explained by differences in enzymatic activity, expression, or binding to cells. We therefore compared their amino acid sequences and modeled the structure of Sph based on the published structures of the S. aureus and B. cereus enzymes. Notably, despite considerable overall sequence similarity and structural conservation as compared to the enzymes from B. cereus and S. aureus, we observed poor conservation in the S. epidermidis enzyme (and those from other non-S. aureus staphylococci) of several specific sequence characteristics, namely the edge metal binding site, solvent-exposed loop, and β-hairpin previously implicated in target cell binding in virulence-associated SMases (Ago et al., 2006) (Fig. 3F,G; Fig. S1). Altogether, these data show that – while Sph is a potent sphingomyelinase - it has no detectable impact on virulence phenotypes, which may be related to specific structural characteristics.
Sph contributes to S. epidermidis skin colonization.
Next, we explored whether Sph has a role in asymptomatic skin colonization. In the extremely nutrient-limited environment on the surface of the human skin, the acquisition of carbon and particularly nitrogen is a severe problem for commensals. On the other hand, as a major component of corneocytes, SM is an abundant potential nutrient source on the skin but not readily usable owing to its high hydrophobicity. We thus hypothesized that Sph directly benefits the bacteria by producing PC as a nutrient source. PC contains carbon and nitrogen and is well water-soluble in contrast to SM or the other product of the SMase-catalyzed degradation of SM, ceramide. In media with limited nitrogen and carbon sources, we observed that supplementation with SM increased growth in a manner dependent on the presence of the sph gene, as SM significantly stimulated growth of the wild-type strain in contrast to the isogenic Δsph mutant, which grew significantly less both in the presence and absence of SM (Fig. 4A). Genetic complementation or addition of PC, the cleavage product of SMase-catalyzed cleavage of SM we hypothesized to be the relevant SM-derived nutrient source, reversed this growth deficiency (Fig. 4B,C). This finding shows that S. epidermidis can use SM as nutrient source based on the enzymatic activity expressed by the sph gene that produces metabolizable PC.
Fig. 4. Sph promotes S. epidermidis growth under skin-like conditions and during in-vivo skin colonization.
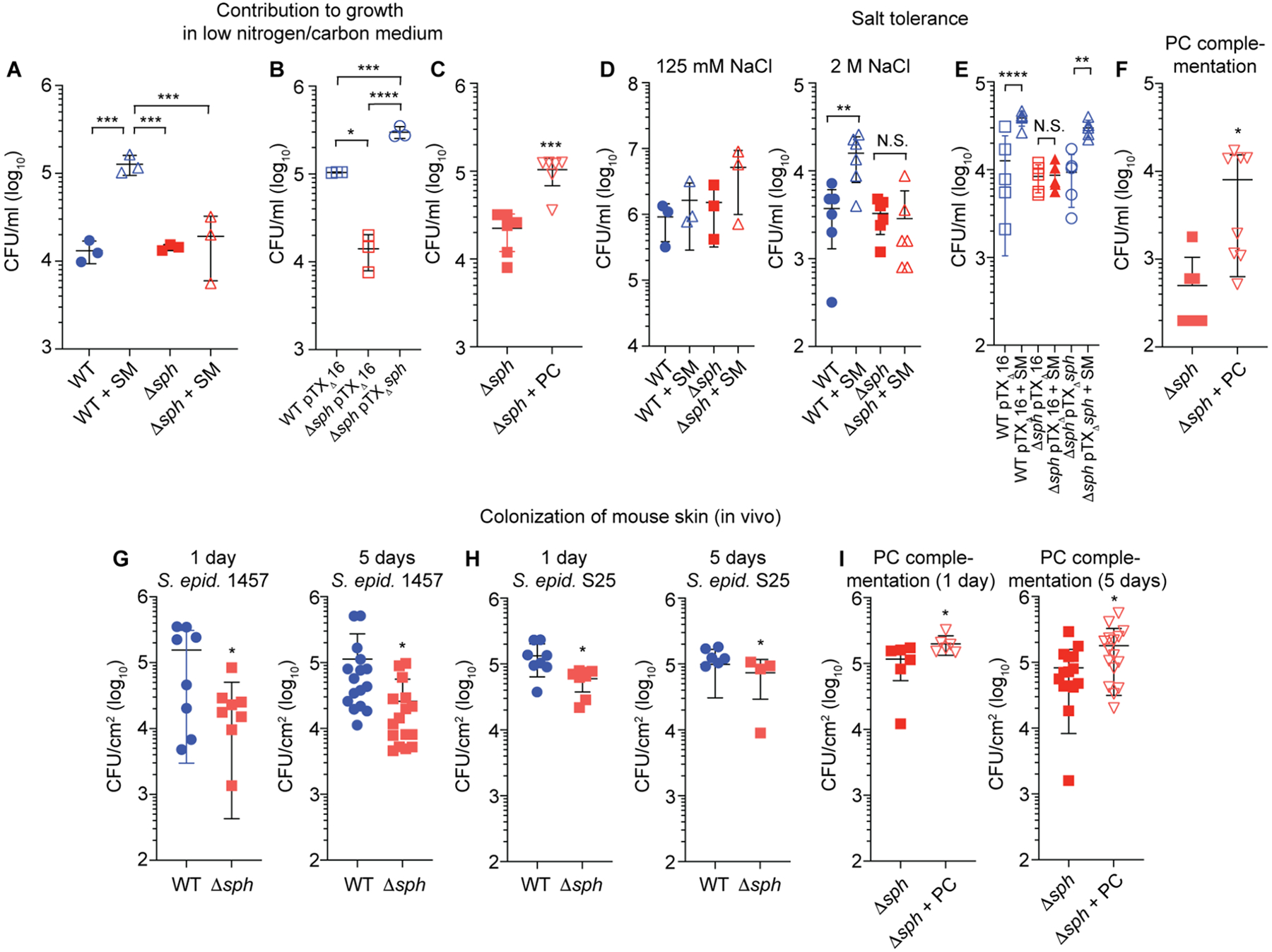
A, Growth in synthetic medium (SNLM) under low-nutrient conditions of S. epidermidis 1457 wild-type (WT) and isogenic sph mutant (Δsph), with or without sphingomyelin (SM) (n=3/group). B, Effect of genetic complementation under the same conditions (with SM; n=3/group). C, Growth of Δsph mutant under the same conditions (without SM) with and without addition of PC; n=6/group. D, Growth of WT and Δsph mutant in SNLM at physiological (125 mM, n=3/group) and high (2 M, n=6/group) concentrations of NaCl with or without SM. E, Growth of genetic complementation and corresponding control strains in SNLM with 2 M NaCl (n=5/group) with or without SM. F, Growth of Δsph mutant in SNLM with 2 M NaCl with and without addition of PC (without SM). n=8/group. G,H, CFU measurements 1 day (24 h) or 5 days (120 h) after association of mice with equal CFU (2 × 104/cm2) of WT or isogenic Δsph bacteria of strains S. epidermidis 1457 (n=8/group, 1 day; n=16/group, 5 days) or S25 (n=8/group, 1 day; n=4–6/group, 5 days). I, Complementation of 1457 Δsph strain in-vivo growth deficiency with PC. 50 μg of PC was applied to the skin of mice. (n=6/group, 1 day; n=16/group, 5 days). B,E, Plasmid-harboring strains were grown with addition of tetracycline (12.5 μg/ml). C,D,F,G,H, I, Statistical analysis is by unpaired, two-tailed t-tests. A,B,E, Statistical analysis is by 1-way ANOVAs with Tukey’s post-tests. A-I, Error bars show the mean ± SD. S. epid., S. epidermidis.
In addition, because PC is a precursor to the osmoprotectant glycine betaine (Boch et al., 1994), we hypothesized that this mechanism may also contribute to S. epidermidis resistance to the high-salt stress conditions that frequently develop on the skin. In support of this hypothesis, we detected a significant contribution of SM to growth at high salt concentrations in the wild-type but not Δsph strain, while genetic complementation or addition of PC reversed the deficiency observed in the Δsph strain (Fig. 4D–F). These findings indicate that Sph-mediated degradation of SM to the osmoprotectant precursor PC also functions to facilitate growth of S. epidermidis under the salt stress conditions encountered on the human skin.
To test for the biological relevance of these in-vitro findings, we used an in-vivo skin colonization model in mice (without compromise). Absence of sph led to significantly reduced colonization of S. epidermidis 1457 (Fig. 4G). We confirmed these findings using a further S. epidermidis isolate, the skin isolate S. epidermidis S25 from our collection, and an isogenic sph mutant (S25Δsph) that we constructed. Findings obtained with S25 and S25Δsph were consistent with those obtained with the 1457/1457Δsph strain pair (Fig. 4H), ruling out strain dependence of the observed effects. Finally, we could complement the observed in vivo growth deficiency of the S. epidermidis 1457 Δsph mutant strain by adding PC to the skin of live mice colonized with S. epidermidis 1457 Δsph, linking the growth phenotype directly to the PC product of Sph catalytic activity and confirming our in vitro results (Fig. 4I). These results indicate that Sph has a significant role in S. epidermidis skin colonization, which our data suggest is due to nutrient acquisition and osmostabilization in that extremely hostile environment.
S. epidermidis increases ceramide content of the skin via Sph activity.
We then asked whether the impact of S. epidermidis on water loss and ceramide content in the stratum corneum that we had observed (Fig. 1C,D) is due to the SMase activity encoded sph. To that end, we first determined whether Sph in S. epidermidis can produce ceramides from skin cells. To that end, we measured the major SM-derived ceramides (ceramide 2, d18:1/16:0), as measurement of total ceramides via TLC is not amenable to a high number of samples. Wild-type S. epidermidis 1457 and S25, but not the corresponding isogenic sph-deficient S. epidermidis (Δsph) mutant strains, had the capacity to produce that abundant ceramide type when added to keratinocyte cultures, and the deficiency of the Δsph strains was overcome by complementation with a plasmid over-expressing Sph (Fig. 5A). In contrast to the considerably increased production of ceramides, relative reduction of the SM substrate after application of wild-type S. epidermidis 1457 was minor in extent, likely due to SM abundance in cells (Fig. 5B). It was much more pronounced in the over-expression clone as well as with strain S25, in accordance with stronger Sph production in that strain (see Fig. 2B). These results demonstrate that S. epidermidis Sph produces ceramides from skin cells in the natural bacterial background.
Fig. 5. S. epidermidis produces ceramides via Sph from host cells and on the skin in vivo.
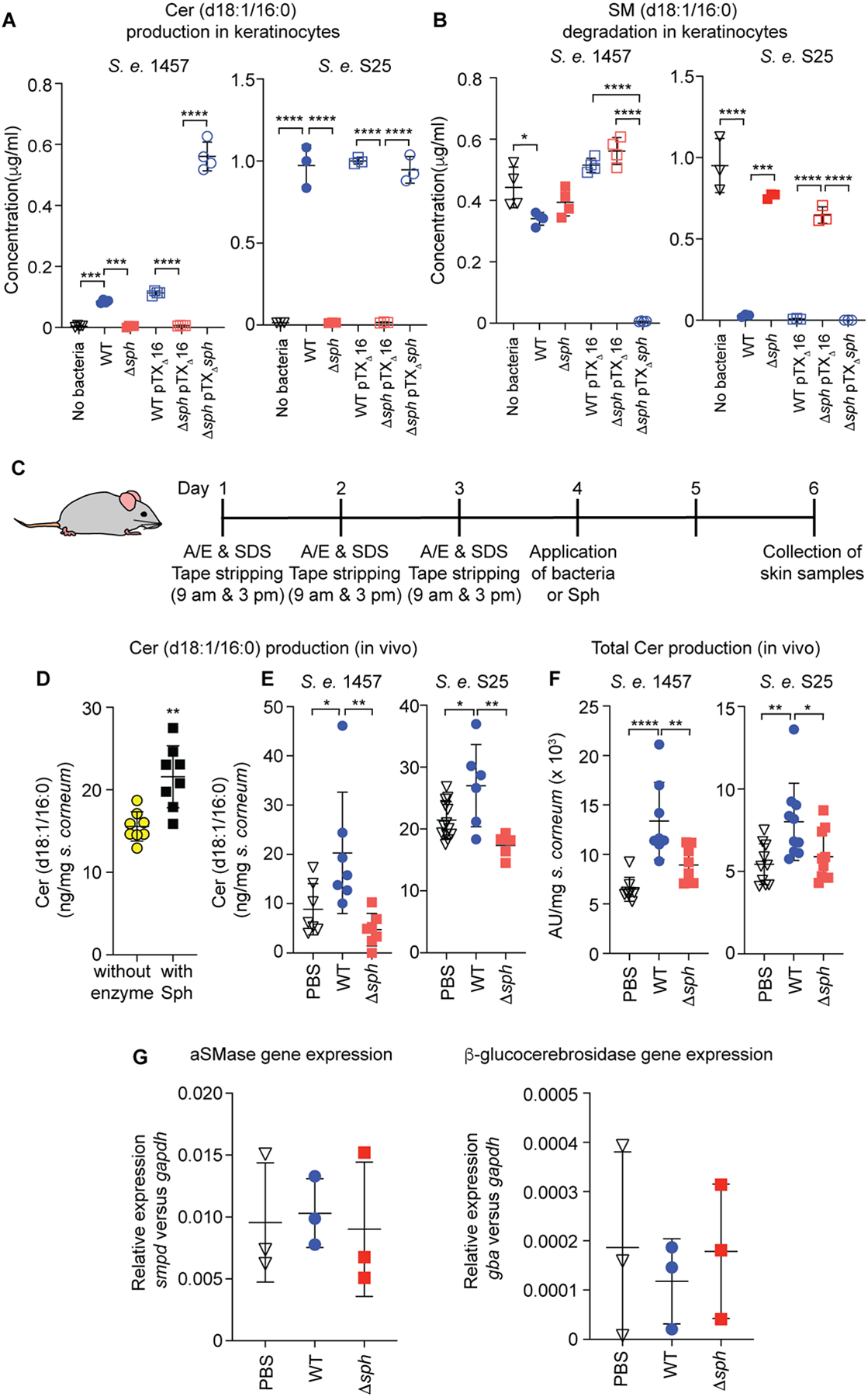
A, SM degradation and B, ceramide production [measuring the main SMase-derived ceramide type 2 (d18:1/16:0, see Figs. 1,2)] in human keratinocyte culture by equal CFU (107) of S. epidermidis wild-type (WT), sph isogenic mutant (Δsph), sph-complemented and control strains in S. epidermidis 1457 and S25 strain backgrounds. n=4/group (1457); n=3/group (S25). C, Setup of mouse experiment used for the results shown in panels D-F. A/E, acetone/diethyl ether. Mouse picture is from Stockio.com. D, Stratum corneum ceramide (d18:1/16:0) production by purified Sph on the compromised skin of live mice. n=8/group. E, Stratum corneum ceramide 2 (d18:1/16:0) production, after association with compromised skin of live mice for 24 or 48 h (n=7), respectively, with the indicated bacteria or PBS as control. F, Total stratum corneum ceramide production after association of compromised mouse skin for 48 h with the indicated bacteria (n=8/group; measurement by TLC). G, Expression of host ceramide biosynthesis genes. n=3/group. D, Statistical analysis is by unpaired, two-tailed t test. A,B,E,F,G, statistical analysis is by one-way ANOVAs with Tukey’s post-tests. A,B,D,E,F,G, Error bars show the mean ± SD.
To determine whether the Sph-mediated capacity to produce ceramides from skin cells manifests in a natural environment, we used our mouse model with pre-treatment to compromise the skin barrier (Fig. 5C). We first applied purified Sph, which significantly increased d18:1/16:0 ceramide levels on compromised skin of live mice as compared to mice to which no enzyme had been applied (Fig. 5D). Then, we tested for contribution of sph in the natural S. epidermidis background, using both S. epidermidis 1457 and S25 wild-type versus Δsph strain pairs. S. epidermidis wild-type but not Sph-deficient (Δsph) bacteria significantly increased d18:1/16:0 as well as total stratum corneum ceramide levels in the compromised mouse skin model (Fig. 5E,F). Comparison with control mice that only received PBS also revealed that the observed differences in ceramide levels caused by S. epidermidis colonization on the skin were entirely due to sph (Fig. 5E,F). Furthermore, expression of the genes that are central to host production of ceramides (aSMase, gene smpd; β-glucocerebrosidase; gene gba) indicated that these changes were not due to a hypothetical impact of Sph in a direct or indirect fashion on the host’s pathways to produce ceramides, as expression of these genes was not significantly impacted by sph (Fig. 5G). Of note, expression of these host ceramide production genes was also generally not impacted by application of S. epidermidis (Fig. 5G).
S. epidermidis prevents skin dehydration via Sph activity.
To test whether the Sph-mediated capacity to increase ceramide levels on compromised skin leads to a protective effect that is directly associated with an impact on the skin barrier and the prevention of skin dehydration, we tested TEWL. Dehydration effects as measured by TEWL were significantly decreased in skin-compromised live mice to which Sph was applied as compared to control mice (Fig. 6A).
Fig. 6. S. epidermidis promotes skin rehydration after compromise via Sph production in vivo.
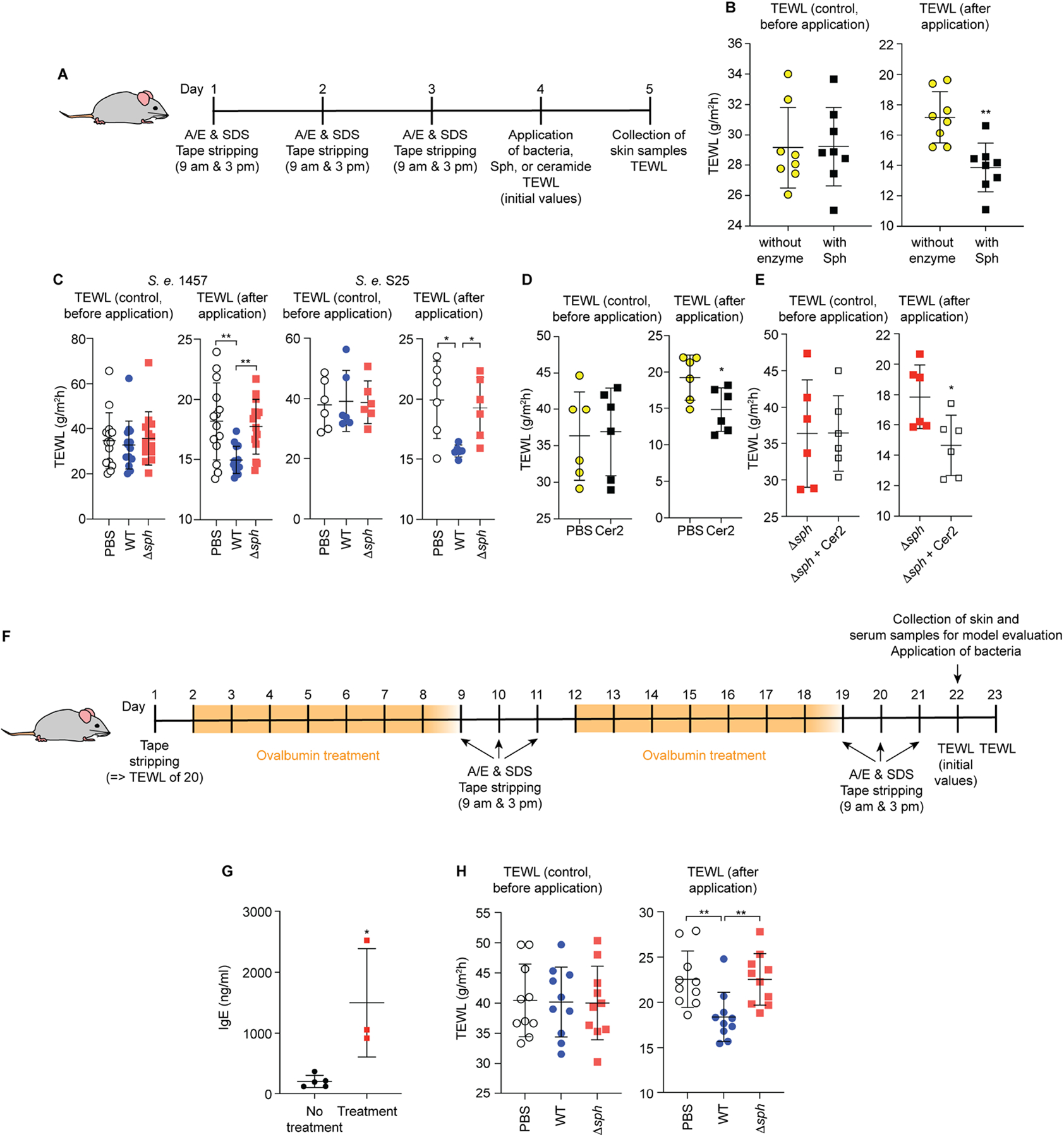
A, Setup of mouse model used for the results shown in panels B-E. A/E, acetone/diethyl ether. B, Transepidermal water loss (TEWL) measurements 24 h after application of Sph. n=8/group. C, TEWL 24 h after association of mice with the indicated bacteria or PBS as control. n=14/group (1457); n=6/group (S25). D, Effect of treatment of compromised skin of live mice with ceramide 2 or PBS as control, TEWL measurements. n=6/group. E, Effect of treatment of compromised skin of live mice previously associated with S. epidermidis 1457 Δsph with ceramide 2, TEWL measurements. n=6/group. F, Atopic dermatitis (AD) model used for the results shown in panels G and H. Mice were treated to develop inflammatory phenotypes typical for AD before application of bacteria and TEWL was measured 24 h afterwards. G, IgE levels on day 22 before application of bacteria. n=5 (control group), n=3 (treatment group). See Fig. S6 and Table S1 for histological evaluation of the AD model. H, TEWL 24 h after association of mice with the indicated bacteria or PBS as control. n=10/group A-E, H, Treatment groups were formed by evenly distributing mice according to their initial TEWL levels due to high mouse-specific variations in TEWL background levels. Statistical analysis is by unpaired t-tests (A,C,D,G) or 1-way ANOVAs with Tukey post-tests (B,E,H). Error bars show the mean ± SD.
Then, we determined whether Sph-mediated water loss prevention also manifests in the natural bacterial background. We observed significantly reduced TEWL after application of S. epidermidis Sph enzyme (Fig. 6B) and wild-type, but not Δsph bacteria using both 1457 and S25 wild-type/Δsph strain pairs (Fig. 6C). As further control linking the observed effects to production of the ceramide 2 product of the Sph-catalyzed reaction, application of ceramide 2 to the compromised skin of live mice decreased TEWL (Fig. 6D), including when the mice had been associated with Sph-deficient S. epidermidis (Δsph) (Fig. 6E).
Our mouse model was set up to mimic the sort of physical harm to which otherwise healthy skin is commonly exposed. To also evaluate the impact of S. epidermidis and Sph on skin health during pathological skin conditions that knowingly are associated with ceramide depletion, we used a model of atopic dermatitis (AD) following the setup described by Uberoi et al. (Uberoi et al., 2021). We verified that the treatment we applied (Fig. 6F) led to significantly increased skin IgE levels (Fig. 6G) and, using histological examination, the signs of increased inflammation that are characteristic for AD (Fig. S6, Table S1). In the AD model, application of S. epidermidis led to a significant, Sph-dependent decrease in TEWL (Fig. 6H), similar to the results we had obtained in healthy, physically compromised skin. These findings reveal a significant contribution of S. epidermidis colonization to skin permeability homeostasis and the prevention of skin dehydration after deleterious insults or in diseased skin that is mediated by the sph gene.
Discussion
For over a century, the interests of microbiologists, biomedical researchers, and physicians in bacteria has focused on pathogens and the mechanisms that underlie bacterial diseases. More recently, the beneficial roles of colonizing bacteria have also gained considerable attention, in part prompted by the technical advances in microbiome sequencing that allow detailed insight into the composition of the bacterial communities that colonize human epithelial surfaces (Weinstock, 2012).
Initially focused on the large bacterial gut communities, analyses of commensal-human interactions now also comprise studies on the skin microbiome and the roles that skin commensals play for human health (Cogen et al., 2008; Grice and Segre, 2011; Nakatsuji et al., 2018). Major advances have been made in our understanding for example of how skin commensals elicit specific immune responses that combat pathogens or limit inflammation (Belkaid and Hand, 2014). However, a considerable part of the protective role the skin plays is not only due to epithelial immune mechanisms but the physical skin barrier, which is composed of ceramides and other lipid molecules that maintain barrier homeostasis and prevent dehydration, skin deterioration, and bacterial infiltration (Proksch et al., 2008; Sahle et al., 2015; Wertz, 2000). Whether skin commensals assist in the maintenance of the physical skin barrier has remained unknown.
Most studies on the role of skin commensal bacteria have focused on S. epidermidis, which is generally recognized as one of the most important and frequent skin colonizers (Grice and Segre, 2011; Kloos and Musselwhite, 1975; Otto, 2009). Like many other colonizing bacteria, this species can cause opportunistic infections, which is why molecular studies on S. epidermidis and other colonizers until recently have almost exclusively focused on factors that are involved in causing disease (Becker et al., 2014; Otto, 2012). More recently, S. epidermidis has been used as an example organism to study the interaction between the skin’s immune system and the skin-colonizing microbiota (Harrison et al., 2019; Lima-Junior et al., 2021; Linehan et al., 2018; Naik et al., 2015). However, despite the considerably increased interest in bacterial colonization, no specific bacterial factors that promote asymptomatic colonization of the skin have yet been identified conclusively by use of defined deletion mutants.
In this study we describe the mechanism of a host-commensal symbiotic interaction that promotes asymptomatic colonization by S. epidermidis, while also facilitating maintenance and reestablishment of skin integrity. Our study provides direct in-vivo and genetic evidence using defined mutants to (i) establish a role in colonization of a skin commensal gene and (ii) demonstrate a direct impact of a bacterial commensal on the maintenance of a healthy skin barrier via a specific bacterial factor. Our results indicate that a healthy skin microbiota plays a significant role in the prevention of ceramide depletion and skin dehydration, processes that have been linked to skin aging and the resistance to skin diseases.
Importantly, we found that the gene underlying the mechanism we describe is widespread in S. epidermidis. This contrasts many other S. epidermidis-expressed mechanisms reported to benefit the host, such as bacteriocin-mediated bacterial interference mechanisms, which are highly strain-specific (Heilbronner et al., 2021; Liu et al., 2020a), or a neoplasia-preventing molecule in S. epidermidis, which has only been detected in one strain (Nakatsuji et al., 2018). Furthermore, we found that sph is expressed in vivo on human skin at levels considerably exceeding those observed during experimental colonization of mice. This suggests that in humans, Sph-mediated effects may be even higher than those detected in our mouse experiments. Finally, we did not detect ceramide-producing activity in several other bacteria that are abundant on human skin, although our limited selection certainly does not reflect the complexity of the skin microbiome.
Several recent studies have revealed specific mechanisms of how commensal skin bacteria benefit the host (Lai et al., 2009; Leech et al., 2019; Lima-Junior et al., 2021; Linehan et al., 2018; Liu et al., 2020a; Naik et al., 2015; Nakatsuji et al., 2018; Nakatsuji et al., 2017). However, except for those based on direct bacterial interference, it has remained poorly understood why these mechanisms developed from an evolutionary biology point of view, for which there also must be a beneficial aspect for the bacteria. In studies such as that by Naik et al. (Naik et al., 2015), which have linked pathogen exclusion phenomena to immune stimulation, evidence to show a benefit to the stimulating bacteria is currently difficult to obtain because the responsible bacterial genes have remained elusive - despite recent evidence for the involvement of formylated peptides in that specific case (Linehan et al., 2018). The fact that the mechanism we describe is based on a single bacterial gene made it possible to directly analyze whether this gene benefits the bacteria. We found that Sph significantly contributes to S. epidermidis survival on the skin, which as we show is likely due to the production of phosphocholine as a nutrient and osmoprotectant precursor. The evolution of an enzyme to degrade SM makes biological sense given that SM is one of the most abundant molecules on the skin surface but inaccessible to the bacteria as a nutrient without enzymatic degradation.
The symbiotic character of the host-commensal interaction we describe is directly connected to the interesting finding that the S. epidermidis Sph enzyme lacks the virulence-associated effects of its previously characterized homologues in pathogenic bacteria (Flores-Diaz et al., 2016; Herrera et al., 2016). Most importantly, we showed that S. epidermidis, in contrast to S. aureus β-toxin, does not cause cytolysis of live keratinocytes, suggesting that even when penetrating into deeper skin layers and not only degrading SM from dead keratinocytes on the skin surface, S. epidermidis Sph does not harm the host. While the detailed assessment of the molecular underpinnings of this functional difference between the S. aureus and S. epidermidis SMase enzymes is beyond the scope of the present study, our results suggest that they are related to structural differences in areas of the enzyme believed to be important for target cell binding. Our findings thus suggest that in agreement with the notion of generally benevolent interaction of S. epidermidis with the host (Otto, 2009), the Sph enzyme has evolved not to damage the host in contrast to its homologue in S. epidermidis’ pathogenic cousin, S. aureus, which has been shown to contribute to colonization of diseased skin (Katayama et al., 2013). However, it is possible that other cell- and tissue-damaging virulence factors that are present in S. aureus but absent from S. epidermidis, and some of which synergize with β-toxin (Cheung et al., 2012; Otto, 2004; 2009; 2014), also contribute to that the functional difference between those enzymes.
Limitations of our study include details in the setup of our mouse experiments that were technically imperative but may to a certain extent influence how well those experiments reflect the situation in humans. This includes the use of occlusion in the bacterial colonization model, which is indispensable and common when CFUs are to be counted (Nakamura et al., 2013; Nakamura et al., 2020). Occlusion may generally impact skin barrier parameters (Zhai and Maibach, 2001), but it is unlikely that this influences the relative effect of sph on bacterial colonization. Importantly, occlusion was not used in the model evaluating skin barrier parameters (ceramide levels and TEWL). In that model, we applied S. epidermidis in numbers (2 × 108/cm2, 4 × 107/cm2, respectively) that slightly exceed those found on human skin, where numbers differ extensively depending on body site (~ 102 - 107/cm2) (Kloos and Musselwhite, 1975). It is common to colonize mice with bacterial CFU that exceed human colonization patterns in order to analyze physiological mechanisms. In comparison with other studies with S. epidermidis (Naik et al., 2015; Scharschmidt et al., 2015), the numbers we used are similar or even lower. We thus believe it is fair to assume that the mechanism we describe contributes to skin homeostasis under real-life conditions in humans, although the extent may significantly vary at different sites of the human body. In the bacterial colonization model, we used much lower CFU (2 × 104/cm2) for a better analysis of the impact of sph on growth in vivo. Only for qRT-PCR determination of bacterial in-vivo gene expression, CFU had to be further increased to extract sufficient mRNA. Finally, the use of hairless mice in our study was mandatory as hair growth, even after shaving, interferes with stratum corneum preparation. While mouse skin generally does not exactly represent human skin, we believe that the low epilation of human skin is mimicked by hairless mice as used in our study at least as well as by furred or shaved mice.
In conclusion, our study underlines the potential translational use of S. epidermidis in a probiotic fashion to promote skin health. Probiotics are drugs containing live microorganisms. Frequently promoted and marketed for intestinal health and application, recent studies have also suggested the use of topically applied skin-colonizing bacteria for example to eradicate potentially harmful co-colonizers (Liu et al., 2020b; Nakatsuji et al., 2021; Williams et al., 2019). Our findings indicate potential therapeutic use of S. epidermidis to promote homeostasis of the physical skin barrier and thereby prevent skin deterioration and its consequences. With S. epidermidis being a natural skin colonizer, such a strategy could involve screening of the existing skin microbiome composition for a deficiency in sph expression and application of high-Sph producing S. epidermidis probiotic strains, potentially in combination with direct bacteriocin- or signaling-mediated bacterial interference mechanisms to additionally eliminate S. aureus and other harmful pathogens.
STAR Methods
RESOURCE AVAILABILITY
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Michael Otto (motto@niaid.nih.gov).
Materials availability
All data is available in the main text or the supplementary materials. Unique biological material is available subject to completion of simple transfer agreements (MTAs) with the NIAID.
Data and code availability
All data reported in this paper will be shared by the lead contact upon request.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Human studies
Isolation of bacterial samples from human skin was performed at Shanghai Renji Hospital and approved by the ethics committee of Renji Hospital, School of Medicine, Shanghai Jiaotong University (protocol approval KY2021–044-B). All participants were male, between 18 and 94 years old. Informed consent was obtained from all participants. Human blood was obtained under approved protocols at the NIH Blood Bank.
Cell lines
The human keratinocyte cell line HaCaT (RRID:CVCL_0038) was purchased from AddexBio, authenticated and proven to be free of contamination including by mycoplasma by the company. It was banked upon arrival in frozen form for future experiments.
Animal studies
Animal studies were approved by the Institutional Animal Care and Use Committee of the NIAID (Protocol LB1E). Animal work was conducted adhering to the institution’s guidelines for animal use and followed the guidelines and basic principles in the United States Public Health Service Policy on Humane Care and Use of Laboratory Animals, and the Guide for the Care and Use of Laboratory Animals by certified staff in an Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) International accredited facility. For antibody production, female, 8 – 10 week-old C57BL-6Ncrl mice (Jackson Laboratories;) were used. For all other experiments, SKH1-Hrhr (hairless) mice were purchased from Charles River and then bred in the animal facility at NIH. Female littermates of SKH1-Hrhr mice that were 10 – 14 week-old were randomly assigned for experiments.
Bacterial strains and constructs
S. epidermidis strains used in this study comprised strain 1457 and a series of strains from a collection of skin isolates obtained from heathy individuals in Shanghai (Gu et al., 2005). A further S. epidermidis strain, the lantibiotic epidermin producer Tu3298 (Allgaier et al., 1986), which was used in recent studies on immune tolerance (Leech et al., 2019; Scharschmidt et al., 2015), was also used for comparison of Sph production. Analytical PCR for ica, fdh, and sph genes was performed using isolated genomic DNA for all strains. See Table S2 for oligonucleotides. S. epidermidis strain 1457 was generally used in this study, including as background for sph deletion and complementation. Strain 1457 is an infection isolate that is positive for the exopolysaccharide ica genes (Heilmann et al., 1996; Mack et al., 1994) and negative for the fdh gene, identifying the strain as a member of the predominant A/C clonal group of S. epidermidis (Conlan et al., 2012; Meric et al., 2015). We also produced and analyzed an sph mutant in a second strain, strain S25, a member of the Shanghai skin isolate collection. This strain was identified as ica- and fdh-negative. The Δsph (sph deletion) strains were constructed using an allelic replacement strategy with plasmid pKOR1, as described (Bae and Schneewind, 2006), leading to deletion of the entire sph gene without replacement. Strain 1457 received the allelic replacement plasmid via electroporation and strain S25 via phage transduction as described (Augustin and Gotz, 1990; Winstel et al., 2015). No growth differences were observed between Δsph and corresponding parental strains (Fig. S5). For constitutive over-expression of sph, the gene was cloned into plasmid pTXΔ (Wang et al., 2007) via BamH1 and Mlu1 restriction sites, and the resulting plasmid pTXΔsph was electroporated into S. carnosus TM300 (Schleifer and Fischer, 1982) (for purification) and S. epidermidis Δsph (for genetic complementation). For production of His-tagged S. aureus β-toxin, hlb was amplified from genomic DNA of RN4220 (Kreiswirth et al., 1983) introducing a region coding for a C-terminal 6His-tag via the used oligonucleotides (Table S2), and the PCR product was cloned via introduced BamH1 and Mlu1 restriction sites into pTXΔ.
Corynebacterium amycolatum strain SK46 and Cutibacterium acnes strains HM-554 (HL110PA3) and HM-555 (HL110PA4) (Fitz-Gibbon et al., 2013) were isolated from normal human skin and obtained from BEI Resources. Staphylococcus capitis (ATCC27840), Staphylococcus hominis (ATCC27844) and Staphylococcus warneri (ATCC17917) were purchased from ATCC.
Culture conditions for in vitro systems
Bacteria were routinely grown in tryptic soy broth (TSB) with the appropriate antibiotics added for plasmid-harboring strains (tetracycline, 12.5 μg/ml; ampicillin, 100 μg/ml; chloramphenicol, 10 μg/ml) at 37 °C, unless otherwise noted. Cell lines were cultured in DMEM supplemented with 10% FBS. Sphingomyelin nutrient/salt stress experiments were performed in synthetic nasal medium (SNLM) with increased glucose concentration as described (Krismer et al., 2014), with alterations as indicated.
METHOD DETAILS
Sphingomyelinase purification
To purify large amounts of the S. epidermidis sphingomyelinase Sph, S. carnosus TM300 pTXΔsph was grown in 200 ml TSB supplemented with 12.5 μg/ml tetracycline for 2 days. Culture supernatant containing Sph was obtained by centrifugation at 4200 × g for 15 min and applied to a self-packed HR 16/10 column filled with Resource 30S (GE Healthcare) material (column volume 20 ml) using an AKTA Pure 25 chromatography system (GE Healthcare). After washing with 5 column volumes of buffer A (20 mM Tris-HCl, pH 7.0), a 15-column volume gradient from 0% to 100% buffer B (20 mM Tris-HCl, pH 7.0, 1M NaCl) was applied at a flow rate of 5 ml/min. The peak eluting at 45% buffer B was collected, diluted with buffer A, and applied to a Source 15S PE 4.6/100 column (GE Healthcare). After washing with 3 column volumes of buffer A, a 15-column volume gradient from 0% to 100% buffer B was applied at a flow rate of 3 ml/min. The peak eluting at 42% buffer B containing Sph was collected (Fig. S2).
Owing to insufficient purity of commercially available β-toxin, β-toxin was purified as His-tagged fusion protein after cloning in a staphylococcal expression plasmid (pTXΔ), yielding plasmid pTXΔhlb-6His, and over-expression in S. carnosus. See Table S2 for oligonucleotides used. For purification, culture filtrate of S. aureus RN4220 pTXΔhlb-6His was applied to a HisTrap HP column (5 ml, GE Healthcare). The column was washed with 10 column volumes of buffer A (20 mM sodium phosphate, pH 7.0). His-tagged β-toxin was eluted with a 5-column volume gradient from 0% to 100% buffer B (20 mM sodium phosphate, pH 7.0, 250 mM imidazole) at a flow rate of 5 ml/min.
Genomic DNA extraction
S. epidermidis strains were cultured in TSB overnight. Bacteria were collected by centrifugation at 20,000 × g for 5 min. Bacterial pellets were resuspended in 400 μl P1 buffer (Qiagen) supplemented with RNAse and 40 μl of 0.5 mg/ml lysostaphin. After incubation at 37 °C for 15 min, 20 μl of saturated SDS (dissolved in 45% EtOH) was added and vortexed. The mixture was incubated at 37 °C for 5 min. 130 μl sodium perchlorate (5 M) and 600 μl phenol/chloroform/isoamylalcohol (Sigma-Aldrich) were added and vortexed. After centrifugation at 20,000 × g for 10 min, the upper phase was transferred to new tube. Genomic DNA was precipitated by addition of 800 μl cold ethanol and centrifugation at 20,000 × g for 10 min. DNA pellets were washed twice with 500 μl of cold 70% ethanol. Purified genomic DNA was dissolved in 100 μl water and used for PCR reactions.
Sph expression in mouse and human skin
Mouse skin:
Mice were associated with 5 × 1010 CFU on the back. After 1 day, the skin was swabbed with swabs premoistened with PBS and immediately submerged in 1 ml PBS. The bacterial CFU in the obtained PBS dilution was determined using measurement of the OD at 600 nm and 109 CFU S. epidermidis were subjected to RNA isolation. Staphylococcal RNA was isolated using a QIAGEN RNeasy Mini Kit according to manufacturer protocols. RNA integrity was determined on an Agilent 2100 Bioanalyzer using RNA 6000 Nano Chips (Agilent Technologies) according to manufacturer protocols. Quantitative Real-Time PCR was performed directly on the mRNA transcripts in triplicate using a Superscript III Platinum SYBR Green One-Step qRT-PCR Kit (Invitrogen Life Technologies) and a 7500 Fast Real-Time PCR System (Applied Biosystems). See Table S2 for oligonucleotides. Expression of S. epidermidis 16S rRNA and the sph gene was quantified using a standard curve of known quantities of S. epidermidis genomic DNA. Expression levels of the sph gene are expressed as normalized ratios of the quantity of sph to 16S rRNA.
Human skin:
20 healthy adults were recruited for the study. Exclusion criteria were use of any antimicrobials within the last two weeks and use of cosmetics within the preceding 24 h. The participants were unrelated. Separate mucosal swabs were collected from the face and armpit of each participant, and the swabs were immediately submerged in 1 ml normal saline. All samples were kept at −80°C until further use. For RNA isolation, swab samples were vortexed for 2 min and centrifuged at 13,000 × g for 10 min at 4 °C. The bacterial pellets were lyzed with a Mini-Beadbeater (Biospec Products) at maximum speed for 30 s. After incubation on ice for 5 min, the samples were centrifuged; and the supernatants were used to isolate total RNA using a Qiagen RNeasy kit (Qiagen 74106) according to the manufacturer’s instructions. Genomic DNA was removed by a wipeout buffer (Qiagen 205311) and approximately 0.2 μg of total RNA was converted to cDNA using a reverse transcription kit (Qiagen 205311). The obtained cDNA was used as a template for qRT-PCR using a SYBR-green PCR reagent (Roche). See Table S2 for oligonucleotides. The 16S rRNA gene of S. epidermidis was used as a housekeeping gene control. Reactions were performed in MicroAmp Optical 96-well reaction plates and fluorescence signals were detected by a 7500 Sequence Detector (Applied Biosystems).
Anti-Sph antibody production and Western blot
To obtain anti-Sph antibody, 1 ml of 0.1 mg/ml pure Sph was mixed with Freund’s Complete Adjuvant (CFA). After emulsification, 0.1 ml of the Sph-CFA emulsion was injected under the skin of 4-week-old female C57BL-6Ncrl mice (Charles River). A total of 15 mice were used for antibody production. 2 weeks later, the mice were injected with the same volume and concentration of Sph in Freund’s Incomplete Adjuvant (IFA) emulsion. After another two weeks, injections were repeated using the Sph-IFA emulsion. One week after the final injection, blood was collected from each mouse. Serum containing anti-Sph antibody was separated and stored at − 20 °C.
For Western blots, proteins or culture supernatants were loaded onto 12% SDS–PAGE gels and separated at 130 V for 90 min. Proteins were stained by Imperial Protein Stain (Thermo Scientific) or transferred to nitrocellulose membranes for Western blot using an iBlot dry blotting system (Invitrogen). Membranes were blocked in Odyssey blocking buffer for 1 h at room temperature before addition of anti-Sph antibody (1:1,000 dilution). After overnight shaking at 4 °C, primary antibody was washed off with wash buffer containing Tris-buffered saline (TBS) pH 7.4 and 0.1% Tween 20. Membranes were then incubated in blocking buffer with Cy5-labelled goat anti-mouse IgG (1:5,000 dilution, Invitrogen) in the dark for 2 h at room temperature. Membranes were scanned using a Typhoon scanner (GE Healthcare).
For concentration of culture supernatants, 265 μl of trichloroacetic acid (TCA, 100% w/v) was added to 1.5 ml culture supernatant. Samples were mixed and incubated at 4 °C overnight. After centrifugation at 20,000 × g for 20 min, the supernatant was discarded. The pellet was washed with 500 μl of cold acetone twice and dried in a SpeedVac for 30 min. The pellet was dissolved in 50 μl SDS gel-loading buffer. 20-μl samples were separated on a 12 % SDS-PAGE for subsequent Western blotting.
Sphingomyelinase activity assays
Sphingomyelinase activity assays were performed using the Amplex® Red Sphingomyelinase Assay Kit (Invitrogen) following the manufacturer’s instructions, with additional ingredients added as indicated. Fluorescence was read using a Tecan Safire multimode microtiter plate reader.
Lipid extraction and Sph digestion of lipids
Sphingomyelin from avian egg and porcine brain was purchased from Avanti Polar Lipids, Inc. Sheep blood was purchased from Quad Five. Human heparinized whole blood was obtained from the NIH blood bank. Lipids from sheep erythrocytes, human erythrocytes and human keratinocytes were extracted using a modified Bligh and Dyer method (Bligh and Dyer, 1959). Erythrocytes and keratinocytes were washed with PBS 3 times. 200 μl cells were mixed with 200 μl chloroform and 400 μl methanol and transferred to lysing matrix tubes (MP Biomedicals). After homogenization at 1800 oscillations/min for 2 min in a FastPrep 96 homogenizer (MP Biomedicals), 200 μl chloroform and 200 μl water were added. The mixture was homogenized again for 1 min under the same conditions, incubated on ice for 5 min and then centrifuged at 20,000 × g for 5 min at 4 °C. The chloroform layer was transferred to a 1.5-ml reaction tube and dried in vacuo. Lipid extracts were digested with 100 μg/ml Sph or β-toxin in digestion buffer (40 μM Tris-HCl, pH 7.5, 5 mM MgCl2, 0.4% triton X100) with 20 μg of sphingomyelin or extracted lipids. Reaction products were analyzed by thin layer chromatography (TLC) or LC/MS.
Thin layer chromatography (TLC)
Lipids that were extracted as above were redissolved in chloroform and applied onto Silica gel 60 thin-layer chromatography (TLC) plates (EMD Millipore). TLC was performed twice in chloroform/methanol/acetic acid (190:9:1) as described (Imokawa et al., 1991). The plates were air-dried, stained with 10% CuSO4 and 8% H3PO4 aqueous solution and charred on a hot plate. Ceramide standards were purchased from Avanti Polar Lipids, Inc. For background subtraction during spot quantification, the rolling ball method in the Typhoon scanner ImageQuant operating software was used with a radius of 200.
RP-HPLC/ESI-MS
Sphingomyelins and ceramides were routinely quantified by reversed-phase chromatography/electrospray ionization mass spectrometry (RP-HPLC/ESI-MS), measuring the most abundant ceramide 2 type d18:1/16:0. To that end, extracted lipids were dissolved in 200 μl of chloroform/methanol (1:1) and 100 μl sample was injected onto a ZORBAX Eclipse Plus 95Å C18, 4.6 × 100 mm, 3.5 μm HPLC column (Agilent) using an Agilent 1260 Infinity Liquid Chromatography system coupled to a 6120 Single Quadrupole mass spectrometer. Buffer A was isopropanol/methanol/water (5:1:4) supplemented with 0.2% formic acid, 0.028% ammonia and 5 μM phosphoric acid. Buffer B was isopropanol supplemented with 0.2% formic acid and 0.028% ammonia (Ogiso et al., 2014). After sample injection, the column was run with the following steps at a flow rate of 0.5 ml/min: 2 column-volume gradient from 30% to 50% B, 8 column-volume gradient from 50% to 80% B, 1 column-volume gradient from 80% to 95% B, 0.5 column-volume gradient from 95% to 5% B, 5% B for 1 column volume and 30% B in 4 column volumes. Absolute amounts of d18:1/16:0 ceramide and sphingomyelin were determined by using a calibration curve obtained with the corresponding commercial lipids.
HPLC-MS/MS (Tandem MS) analysis
Separations of sphingolipids were also performed by HPLC-MS/MS analyses at the MUSC Lipidomics Shared Resource (Bielawski et al., 2009). The equipment consisted of a Thermo Scientific Vanquish μHPLC system coupled to a Thermo Scientific Quantum Access Max triple quadrupole mass spectrometer equipped with an ESI probe operating in the multiple reaction monitoring positive ion mode (MRM).
Chromatographic separations were obtained under a gradient elution on a C8 column using a mobile phase with ammonium formate, formic acid in water and methanol, as previously described (Bielawski et al., 2009). Prior to analysis, samples underwent an ethyl acetate/isopropanol liquid-liquid extraction. Quantitative analyses of sphingolipids were based on eight-point calibration curves generated for each target analyte. The synthetic standards along with a set of internal standards were spiked into an artificial matrix; they were then subjected to an extraction procedure identical to that used for the biological samples. These extracted standards were then analyzed with the samples by HPLC-MS/MS. Peaks for the target analytes and internal standards were recorded and processed using the instrument’s software. Plotting the analyte/internal standard peak area ratios against analyte concentrations generates the sphingolipid specific calibration curves. Any sphingolipid for which no standards were available were quantitated using the calibration curve of its closest counterpart.
Measurement of biofilm formation
Overnight cultures of bacteria were diluted in TSB supplemented with 0.125%, 0.25% or 0.5% glucose to OD600 of 0.1. 200 μl of bacteria was added to wells of 96-well flat-bottom plates. The plates were incubated in 37 °C for 1, 2, or 3 days. Supernatant containing planktonic bacteria was gently removed and wells were washed with distilled water twice. 125 μl of 0.1% aqueous solution of crystal violet was added to wells, which were stained at room temperature for 15 min. The plates were rinsed with distilled water 3 times and dried for 3 h. 125 μl of 30% acetic acid was added to each well to solubilize crystal violet. 125 μl of the solubilized CV was transferred to a new flat bottom plate and absorbance at 550 nm was read using a Tecan Spark multimode microtiter plate reader.
Cytolysis of erythrocytes and keratinocytes
To measure lysis of erythrocytes, 1 ml defibrinated sheep blood (VWR International) or human blood (Department of Transfusion Medicine, NIH) was washed with 49 ml cold PBS three times. After washing, cells were resuspended in 25 ml PBS, distributed into 96-well plates at 100 μl/well, and 100 μl of culture filtrate or 109 CFU/ml of bacteria were added. 2% triton X100, which lyses cells completely, was used as positive, and PBS as negative control. After incubation for 1 h at 37 °C and centrifugation at 450 × g for 10 min at 4 °C, 100 μl of supernatant from each well was transferred to a new 96-well flat bottom plate, which was read at 540 nm using a Tecan Spark multimode microplate reader.
To measure lysis of keratinocytes, a lactate dehydrogenase (LDH) assay was performed using a cytotoxicity detection kit (Roche). HaCat keratinocyte cells were cultured in Dulbeccos’ Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) containing phenol red in 12-well plates for 1 week. One day before the assay, the medium was changed to fresh DMEM with no phenol red indicator and a reduced FBS content of 1%. 100 μg/ml sphingomyelinase solution or 107 CFU/ml of bacteria were added to the cell culture. After 24-h incubation, cells were removed by centrifugation at 450 × g for 15 min and then bacteria were removed by centrifugation at 20,000 × g for 5 min. Supernatants were collected for the LDH assay, which was performed following the protocol included in the kit.
Ceramide quantification in keratinocyte culture
Keratinocyte (HaCat) cells were cultured in in 12-well flat bottom plates in 2 ml DMEM supplemented with 10% FBS for 1 week. S. epidermidis, S. carnosis, S. capitis, S. hominis and S. warneri were cultured in TSB overnight. C. amycolatum was cultured in TSB for 48 h. C. acnes was cultured in Reinforced Clostridial Medium (RCM) for 4 days in an anaerobic chamber. Culture supernatants were collected by centrifugation at 20,000 × g for 5 min. 107 CFU/ml of bacteria or 1 ml of supernatant were added to the HaCat cell culture. After 24-h incubation, cells and bacteria were removed by centrifugation at 20,000 × g for 5 min and supernatants were collected for LC-MS analysis (see above).
To prepare keratinocyte supernatant samples for LC-MS/MS analysis, HaCat cells were cultured in 6-well flat bottom plates in 3 ml DMEM supplemented with 10% FBS for 1 week. PBS or 9 μg Sph enzyme were added to the cell culture. After 24-h incubation, cells were removed by centrifugation at 450 × g for 15 min and then cellular debris was removed by centrifugation at 20,000 × g for 5 min. 2-ml supernatants were transferred to 15-ml Falcon tubes and sent to the MUSC Lipidomics Shared Resource for LC-MS/MS analysis (see above).
Binding to liposomes or keratinocytes
The phospholipids 1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-(1’-rac-glycerol) (sodium salt) (POPG), 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine (POPE), 1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-L-serine (sodium salt) (POPS) and 1-palmitoyl-2-oleoyl-glycero-3-phosphocholine (POPC) and sphingomyelin N-palmitoyl-D-erythro-sphingosylphosphorylcholine were purchased from Avanti Polar Lipids, Inc. 2.5 mg of each kind of phospholipid or sphingomyelin were mixed in an 1.5-ml reaction tube and resuspended in 500 μl extrusion buffer (250 mM raffinose pentahydrate, 25 mM Tris-HCl, pH 7.5, 1 mM dithiothreitol). Lipid mixtures were extruded 13 times through 0.1-μm membranes using an Avanti Mini Extruder. The obtained uniform liposome suspensions were stored at 4 °C before the binding experiment. Keratinocytes were cultured in DMEM supplemented with 10% FBS, dissociated by trypsin on the day of the binding experiment, and washed with PBS three times before use.
Before the binding experiment, 0.2-mg/ml protein solutions were centrifuged at 16,000 × g for 30 min to remove any insoluble material. Then, liposomes and protein solutions were pre-heated to 37 °C. 25 μl each of the liposome and protein solutions were mixed and incubated at 37 °C for 30 min. Liposomes with bound proteins were separated from unbound proteins by centrifugation at 16,000 × g for 30 min. Pellets and supernatants were loaded onto 12% SDS-PAGE gels and protein bands were visualized by Western blot. The fluorescence intensities of bands were quantified using ImageQuant TL 8.1. The keratinocyte binding experiment was performed in the same way, except that the centrifugation step was performed at 450 × g.
Sphingomyelin nutrient/salt stress experiments
S. epidermidis strains 1457 WT and 1457 Δsph were cultured in TSB overnight and sub-cultured in synthetic nasal medium (SNLM) with increased glucose concentration as described (Krismer et al., 2014), in which the solution containing individual amino acids was replaced by 0.2% casamino acid solution. Six days later, bacteria were pelleted by centrifugation at 20,000 × g for 5 min and washed twice by PBS. For the nutrient-limiting experiment, 107 CFU/ml bacteria were resuspended in SNLM without urea and with only 0.0025% casamino acids. For the salt stress experiment, 107 CFU/ml bacteria were resuspended in SNLM with different amounts of NaCl. CFU after 6 days, at the beginning of the experiments, were ~ 2 ×106. 0.083 mg/ml sphingomyelin or 0.036 mg/ml phosphocholine was added where indicated. Bacteria were grown for 24 h at 37 °C with shaking and plated on TSA for CFU counting.
Skin colonization mouse model
Bacteria were grown in TSB overnight, harvested by centrifugation at 20,000 × g for 5 min, and washed with PBS three times. 105 CFU of bacteria (2 × 104/cm2) were applied on the back of SKH1-Hrhr hairless mice. The application sites were covered by Tegaderm film (3M Medical).
Elizabethan collars were used to prevent self-grooming and scratching. Mice were singly housed for 1 day or 5 days. After euthanizing mice, skin tissue around the application site was excised and collected together with the respective Tegaderm film in tubes containing PBS. Then, the samples were sonicated for 5 min, vortexed for 15 min, homogenized twice at 1800 oscillations/min for 1 min in a FastPrep 96 homogenizer, and plated on TSA for CFU counting. 50 μg PC was applied onto the skin where indicated.
Compromised skin mouse model
Ceramide quantification:
To mimic dehydrated skin conditions, we compromised the skin of mice. To that end, 2 ml of acetone/diethyl ether (1:1) was applied to the dorsum using cotton pads for 2 min, followed by tape stripping. Then, the skin was washed with 5% SDS solution and rinsed with water. The entire process of skin treatment was performed twice per day for a total of 3 days. On the fourth day, 109 CFU (2 × 108/cm2) of bacteria or 2.7 μg Sph protein were applied onto the compromised skin. Elizabethan collars were used to protect application sites. Mice were euthanized after 48 h and 24 h for bacteria and Sph applications, respectively, and skin samples were collected. The stratum corneum was separated by trypsin digestion and prepared for lipid extraction. Extracted lipids were analyzed by LC/MS and TLC (see above).
TEWL measurement and qRT-PCR of ceramide synthesis enzymes:
Transepidermal water loss (TEWL) was measured using a VapoMeter (Delfin Technologies, Finland). Two sites per mouse were used for TEWL measurements. TEWL values were determined by triplicate measurements and the mean was defined as the TEWL value of every measurement site. After 3 days with skin washes as described above, TEWL was measured and mice were distributed into groups, so that the average TEWL of all groups was similar. On the fourth day, 2 × 108 CFU (4 × 107/cm2) of bacteria or 2.7 μg Sph protein or 50 μg ceramide d18:1/16:0 (N-palmitoyl-D-erythro-sphingosine, Avanti Polar Lipids, Inc.) were applied onto the compromised skin. TEWL was measured again 24 h later. For qRT-PCR of ceramide synthesis enzymes, skin tissues at the application sites were cut and saved in 1 ml of RNAlater solution (Invitrogen) at 4 °C. 50 mg tissue was cut up into small pieces, mixed with 1 ml of QIAzol Lysis Reagent and homogenized using Lysing matrix A (MP Biomedicals) twice at 1800 oscillations/min for 1 min in a FastPrep 96 homogenizer. RNA was extracted following the Qiazol Handbook (Qiagen). Then, the air dried RNA pellet was purified using an RNeasy Plus kit (Qiagen) according to the manufacturer’s protocol. qRT-PCR was performed with a SuperScript III Platinum SYBR Green One-Step qRT-PCR kit (Invitrogen). See Table S2 for oligonucleotides.
AD mouse model
The skin on the back of mice was tape-stripped on day 1. On days 2 to 8, 100 μg ovalbumin (Sigma Aldrich) was applied daily to the same area. On days 9 to 11; a combination treatment consisting of application of acetone/diethyl ether (1:1), tape stripping and application of 5% SDS was performed on the same area twice daily at 9 am and 3 pm. On days 12 to 18, the ovalbumin treatment was repeated and on days 19 to 21 the combination treatment. On day 22, mice were assigned to three groups according to their TEWL readings. 2 × 108 CFU (4 × 107/cm2) of bacteria were applied onto the compromised skin. TEWL was measured 24 h later (on day 23). Serum samples were collected on day 22 before application of bacteria for IgE analysis. IgE was measured using an IgE Mouse Uncoated ELISA Kit (Invitrogen) following the manufacturer’s protocol. Histology slide preparation, H&E stain and pathologist reading services were provided by Histoserv, Inc.
QUANTIFICATION AND STATISTICAL ANALYSIS
Statistical analysis was performed using GraphPad Prism Version 8 using 1-way ANOVA for the comparison of more than two groups, and unpaired two-tailed Student’s t-tests for the comparison of two groups. In ANOVAs, Tukey’s or Dunnett’s post-tests were used, as indicated, which correct for multiple comparisons using statistical hypothesis testing. All error bars show the mean and standard deviation (SD). All replicates are biological. Sample numbers are given in the figure legends. No statistical methods were used to predetermine sample sizes. No blinding was used.
Supplementary Material
Fig. S1. Comparison of Sph-like sequences in staphylococci and bacilli. Related to Fig. 2.
Fig. S2. Purification, sphingomyelin cleavage activity, and pH and metal dependence of S. epidermidis Sph. Related to Fig. 2.
Fig. S3. Thin-layer chromatography (TLC) of lipids from different sources before and after digestion with purified Sph. Related to Fig. 2.
Fig. S4. Sph production in S. epidermidis isolates. Related to Fig. 2.
Fig. S5. Impact of S. epidermidis Sph on cytotoxicity, biofilm formation, and in-vitro growth. Related to Fig. 3.
Fig. S6. Histological evaluation of AD model. Related to Fig. 6.
Tab. S1. Histological evaluation of AD model. Related to Fig. 6.
Tab. S2. Oligonucleotides used in this study. Related to STAR Methods.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-Sph antibody | This paper | N/A |
| Goat anti-Mouse IgG (H+L) Cross-Adsorbed Secondary Antibody, Cyanine5 | Invitrogen | CAT#A10524; RRID:AB_2534033 |
| Bacterial and virus strains | ||
| Staphylococcus epidermidis 1457 | Mack et al., 1994 | |
| Staphylococcus epidermidis 1457 Δsph | This paper | N/A |
| Staphylococcus epidermidis 1457 Δsph pTXΔsph | This paper | N/A |
| Staphylococcus epidermidis 1457 pTXΔ16 | This paper | N/A |
| Staphylococcus epidermidis 1457 Δsph pTXΔ16 | This paper | N/A |
| Staphylococcus epidermidis Tu3298 | Allgaier et al., 1986 | |
| Staphylococcus carnosus pTXΔ16 | This paper | N/A |
| Staphylococcus carnosus pTXΔsph | This paper | N/A |
| Staphylococcus carnosus TM300 | Schleifer and Fischer, 1982 | |
| Staphylococcus aureus RN4220 | Kreiswirth et al., 1983 | |
| Staphylococcus capitis | ATCC | ATCC27840 |
| Staphylococcus hominis | ATCC | ATCC27844 |
| Staphylococcus warneri | ATCC | ATCC17917 |
| Staphylococcus epidermidis skin isolate 10 | Gu et al., 2005 | N/A |
| Staphylococcus epidermidis skin isolate 1 | Gu et al., 2005 | N/A |
| Staphylococcus epidermidis skin isolate 12 | Gu et al., 2005 | N/A |
| Staphylococcus epidermidis skin isolate 13 | Gu et al., 2005 | N/A |
| Staphylococcus epidermidis skin isolate 14 | Gu et al., 2005 | N/A |
| Staphylococcus epidermidis skin isolate 17 | Gu et al., 2005 | N/A |
| Staphylococcus epidermidis skin isolate 19 | Gu et al., 2005 | N/A |
| Staphylococcus epidermidis skin isolate 20 | Gu et al., 2005 | N/A |
| Staphylococcus epidermidis skin isolate 21 | Gu et al., 2005 | N/A |
| Staphylococcus epidermidis skin isolate 23 | Gu et al., 2005 | N/A |
| Staphylococcus epidermidis skin isolate 25 (S25) | Gu et al., 2005 | N/A |
| Staphylococcus epidermidis skin isolate 26 | Gu et al., 2005 | N/A |
| Staphylococcus epidermidis skin isolate 27 | Gu et al., 2005 | N/A |
| Staphylococcus epidermidis skin isolate 30 | Gu et al., 2005 | N/A |
| Staphylococcus epidermidis skin isolate 31 | Gu et al., 2005 | N/A |
| Staphylococcus epidermidis skin isolate 34 | Gu et al., 2005 | N/A |
| Staphylococcus epidermidis skin isolate 36 | Gu et al., 2005 | N/A |
| Staphylococcus epidermidis skin isolate 37 | Gu et al., 2005 | N/A |
| Staphylococcus epidermidis skin isolate 38 | Gu et al., 2005 | N/A |
| Staphylococcus epidermidis skin isolate 39 | Gu et al., 2005 | N/A |
| Staphylococcus epidermidis skin isolate 4 | Gu et al., 2005 | N/A |
| Staphylococcus epidermidis skin isolate 40 | Gu et al., 2005 | N/A |
| Staphylococcus epidermidis skin isolate 41 | Gu et al., 2005 | N/A |
| Staphylococcus epidermidis skin isolate 48 | Gu et al., 2005 | N/A |
| Staphylococcus epidermidis skin isolate 5 | Gu et al., 2005 | N/A |
| Staphylococcus epidermidis skin isolate 50 | Gu et al., 2005 | N/A |
| Staphylococcus epidermidis skin isolate 52 | Gu et al., 2005 | N/A |
| Staphylococcus epidermidis skin isolate 54 | Gu et al., 2005 | N/A |
| Staphylococcus epidermidis skin isolate 55 | Gu et al., 2005 | N/A |
| Staphylococcus epidermidis skin isolate 56 | Gu et al., 2005 | N/A |
| Staphylococcus epidermidis skin isolate 57 | Gu et al., 2005 | N/A |
| Staphylococcus epidermidis skin isolate 59 | Gu et al., 2005 | N/A |
| Staphylococcus epidermidis skin isolate 6 | Gu et al., 2005 | N/A |
| Staphylococcus epidermidis skin isolate 62 | Gu et al., 2005 | N/A |
| Staphylococcus epidermidis skin isolate 65 | Gu et al., 2005 | N/A |
| Staphylococcus epidermidis skin isolate 72 | Gu et al., 2005 | N/A |
| Staphylococcus epidermidis skin isolate 75 | Gu et al., 2005 | N/A |
| Staphylococcus epidermidis skin isolate 77 | Gu et al., 2005 | N/A |
| Staphylococcus epidermidis skin isolate 78 | Gu et al., 2005 | N/A |
| Staphylococcus epidermidis skin isolate 79 | Gu et al., 2005 | N/A |
| Staphylococcus epidermidis skin isolate 80 | Gu et al., 2005 | N/A |
| Staphylococcus epidermidis skin isolate 81 | Gu et al., 2005 | N/A |
| Staphylococcus epidermidis skin isolate 82 | Gu et al., 2005 | N/A |
| Staphylococcus epidermidis skin isolate 84 | Gu et al., 2005 | N/A |
| Staphylococcus epidermidis skin isolate 85 | Gu et al., 2005 | N/A |
| Staphylococcus epidermidis skin isolate 86 | Gu et al., 2005 | N/A |
| Staphylococcus epidermidis skin isolate 9 | Gu et al., 2005 | N/A |
| Corynebacterium amycolatum SK46 | BEI | HM-109 |
| Cutibacterium acnes 554 | Fitz-Gibbon et al., 2013 | HL110PA3 |
| Cutibacterium acnes 555 | Fitz-Gibbon et al., 2013 | HL110PA4 |
| Biological samples | ||
| Human skin swabs | This paper | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| Sphingomyelin (Egg, Chicken) | Avanti Polar Lipids | CAT#860061C-200mg, LOT#860061C-200MG-D-118 |
| Sphingomyelin (Brain, Porcine) | Avanti Polar Lipids | CAT#860052P-25mg, LOT#860052P-25MG-C-028 |
| CER1 (d18:1/26:0/18:1), N-[26-oleoyloxy hexacosanoyl]-D-erythro-sphingosine | Avanti Polar Lipids | CAT#860850P-1mg, LOT#860850P-1MG-B-010 |
| C16 Ceramide (d18:1/16:0), N-palmitoyl-D-erythro-sphingosine | Avanti Polar Lipids | CAT#860516P, LOT#160CER-20 |
| C24 Ceramide (d18:1/24:0), N-lignoceroyl-D-erythro-sphingosine | Avanti Polar Lipids | CAT#860524P-5mg, LOT#860524P-5MG-C-015 |
| 16:0(2R-OH) Ceramide, N-(2’-(R)-hydroxypalmitoyl)-D-erythro-sphingosine | Avanti Polar Lipids | CAT#860815P-5MG, LOT#860815P-5MG-A-010 |
| 16:0 SM (d18:1/16:0), N-palmitoyl-D-erythro-sphingosylphosphorylcholine | Avanti Polar Lipids | CAT#860584P-5mg, LOT#160SM-17 |
| 16:0–18:1 PS (POPS), 1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-L-serine (sodium salt) | Avanti Polar Lipids | CAT#840034C |
| 16:0–18:1 PE, 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine | Avanti Polar Lipids | CAT#850757C |
| 16:0–18:1 PG, 1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-(1’-rac-glycerol) (sodium salt) | Avanti Polar Lipids | CAT#840457C |
| 16:0–18:1 PC (POPC), 1-palmitoyl-2-oleoyl-glycero-3-phosphocholine | Avanti Polar Lipids | CAT#850457C |
| Phenol/chloroform/isoamylalcohol | Fisher | CAT#BP1753I-100, LOT#167637 |
| Phosphocholine chloride calcium salt tetrahydrate | Sigma Aldrich | CAT#P0378–5G |
| Tetracycline | Sigma Aldrich | CAT#T3383, LOT#31K1763 |
| Ampicillin | Sigma Aldrich | CAT#A9393–25G, Lot#087M4861V |
| Chloramphenicol | Sigma Aldrich | CAT#C0378–100G, Batch#015K0562 |
| Trichloroacetic acid | Sigma Aldrich | CAT#T6399 |
| Freund′s Adjuvant, Complete | Sigma Aldrich | CAT#F5881, LOT#SLBH7316V |
| Freund′s Adjuvant, Incomplete | Sigma Aldrich | CAT#F5506, Lot# J9000V |
| Imperial Protein Stain | Thermo Scientific | CAT#24615 |
| Odyssey Blocking Buffer (PBS) | Li-Cor | CAT#927–70001 |
| 20% SDS | Quality Biological | CAT#351-066-721, LOT#720611 |
| Ovalbumin, Albumin from chicken egg white | Sigma Aldrich | CAT#A5503–5G |
| RNALater stabilization Solution | Invitrogen | CAT#AM7020, LOT#00990043 |
| QIAzol Lysis Reagent | Qiagen | CAT#79306, LOT#56603546 |
| Critical commercial assays | ||
| Amplex® Red Sphingomyelinase Assay Kit | ThermoFisher | CAT# A12220 |
| IgE Mouse Uncoated ELISA Kit with Plates | Invitrogen | CAT#88–50460 |
| SuperScript™ III Platinum™ SYBR™ Green One-Step qRT-PCR Kit | Invitrogen | CAT#11736–059 LOT#2236309 |
| Plasmid Midi Kit | Qiagen | CAT#12145 |
| RNeasy Plus Kit | Qiagen | CAT#74106 |
| QuantiTect Reverse Transcription Kit | Qiagen | CAT#205311 |
| RNA 6000 Nano Kit | Agilent | CAT#5067–1511 |
| iBlot™ 2 Transfer Stacks, nitrocellulose, regular size | Invitrogen | CAT#IB23001 |
| Cytotoxicity Detection Kit (LDH) | Roche | CAT#11644793001 |
| Experimental models: Cell lines | ||
| Human keratinocyte cell line HaCat | Addexbio | CAT#T0020001, LOT#003798, RRID:CVCL_0038 |
| Experimental models: Organisms/strains | ||
| Mouse SKH1-Hrhr | Charles River Laboratories | Strain code 477, RRID:IMSR_CRL:477 |
| Mouse C57BL-6Ncrl | Charles River Laboratories | Strain code 027, RRID:IMSR_CRL:027 |
| Oligonucleotides | ||
| See Table S2. | ||
| Recombinant DNA | ||
| pTXΔ16 plasmid | Wang et al, 2007 | N/A |
| Software and algorithms | ||
| OpenLab ChemStation | Agilent | N/A |
| MassHunter | Agilent | N/A |
| Prism | GraphPad | N/A |
| ImageQuant | GE Healthcare | N/A |
| Unicorn | GE Heathcare | N/A |
| Other | ||
| Source 30S | GE Healthcare (Cytiva) | CAT#17-1273-01 |
| Source 15S PE 4.6/100 column | GE Healthcare (Pharmacia Biotech) | Id#9647018 |
| HisTrap HP column | GE Healthcare | CAT#17-5248-02. LOT#10257327 |
| ZORBAX RR Eclipse Plus C18 column | Agilent | CAT# 959961–902 |
| Lysing Matrix A, 2mL tube | MP | CAT#6910100, LOT#152082 |
| Lysing Matrix B, 2mL tube | MP | CAT#6911100, LOT#127966 |
| TLC Silica gel 60 F254 | EMD | CAT#155341 |
| Elizabethan collar for mouse | Kent Scientific Corporation | CAT#EC201V-10 |
| Tegaderm roll | 3M | CAT#16002 |
| Vapometer | Delfin Technologies Ltd | S/N SWL5233 |
Highlights:
Commensal Staphylococcus epidermidis contributes to skin barrier homeostasis.
S. epidermidis produces a sphingomyelinase that helps generate protective ceramides.
Sphingomyelinase contributes to S. epidermidis skin colonization.
S. epidermidis prevents skin dehydration via its sphingomyelinase activity.
Acknowledgments
The authors thank Justin S. Bae for technical assistance and Seth W. Dickey for critically reading the manuscript. This study was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases (NIAID), U.S. National Institutes of Health (NIH), project number 1 ZIA AI000904 (to M. O.), and the National Natural Science Foundation of China, grant numbers 81861138043 (to M. L.) and 82072235 (to Q. L.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that there are no conflicts of interest.
References
- Ago H, Oda M, Takahashi M, Tsuge H, Ochi S, Katunuma N, Miyano M, and Sakurai J (2006). Structural basis of the sphingomyelin phosphodiesterase activity in neutral sphingomyelinase from Bacillus cereus. J Biol Chem 281, 16157–16167. 10.1074/jbc.M601089200. [DOI] [PubMed] [Google Scholar]
- Allgaier H, Jung G, Werner RG, Schneider U, and Zahner H (1986). Epidermin: sequencing of a heterodetic tetracyclic 21-peptide amide antibiotic. Eur J Biochem 160, 9–22. 10.1111/j.1432-1033.1986.tb09933.x. [DOI] [PubMed] [Google Scholar]
- Augustin J, and Gotz F (1990). Transformation of Staphylococcus epidermidis and other staphylococcal species with plasmid DNA by electroporation. FEMS Microbiol Lett 54, 203–207. 10.1016/0378-1097(90)90283-v. [DOI] [PubMed] [Google Scholar]
- Bae T, and Schneewind O (2006). Allelic replacement in Staphylococcus aureus with inducible counter-selection. Plasmid 55, 58–63. 10.1016/j.plasmid.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Becker K, Heilmann C, and Peters G (2014). Coagulase-negative staphylococci. Clin Microbiol Rev 27, 870–926. 10.1128/CMR.00109-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkaid Y, and Hand TW (2014). Role of the microbiota in immunity and inflammation. Cell 157, 121–141. 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benavides F, Oberyszyn TM, VanBuskirk AM, Reeve VE, and Kusewitt DF (2009). The hairless mouse in skin research. J Dermatol Sci 53, 10–18. 10.1016/j.jdermsci.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernheimer AW, Avigad LS, and Kim KS (1974). Staphylococcal sphingomyelinase (beta-hemolysin). Ann N Y Acad Sci 236, 292–306. 10.1111/j.1749-6632.1974.tb41499.x. [DOI] [PubMed] [Google Scholar]
- Bhattacharya N, Sato WJ, Kelly A, Ganguli-Indra G, and Indra AK (2019). Epidermal Lipids: Key Mediators of Atopic Dermatitis Pathogenesis. Trends Mol Med 25, 551–562. 10.1016/j.molmed.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielawski J, Pierce JS, Snider J, Rembiesa B, Szulc ZM, and Bielawska A (2009). Comprehensive quantitative analysis of bioactive sphingolipids by high-performance liquid chromatography-tandem mass spectrometry. Methods Mol Biol 579, 443–467. 10.1007/978-1-60761-322-0_22. [DOI] [PubMed] [Google Scholar]
- Bligh EG, and Dyer WJ (1959). A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37, 911–917. 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Boch J, Kempf B, and Bremer E (1994). Osmoregulation in Bacillus subtilis: synthesis of the osmoprotectant glycine betaine from exogenously provided choline. J Bacteriol 176, 5364–5371. 10.1128/jb.176.17.5364-5371.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung GY, Duong AC, and Otto M (2012). Direct and synergistic hemolysis caused by Staphylococcus phenol-soluble modulins: implications for diagnosis and pathogenesis. Microbes Infect 14, 380–386. 10.1016/j.micinf.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogen AL, Nizet V, and Gallo RL (2008). Skin microbiota: a source of disease or defence? Br J Dermatol 158, 442–455. 10.1111/j.1365-2133.2008.08437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman D, Knights J, Russell R, Shanley D, Birkbeck TH, Dougan G, and Charles I (1991). Insertional inactivation of the Staphylococcus aureus beta-toxin by bacteriophage phi 13 occurs by site- and orientation-specific integration of the phi 13 genome. Mol Microbiol 5, 933–939. 10.1111/j.1365-2958.1991.tb00768.x. [DOI] [PubMed] [Google Scholar]
- Conlan S, Mijares LA, Program NCS, Becker J, Blakesley RW, Bouffard GG, Brooks S, Coleman H, Gupta J, Gurson N, et al. (2012). Staphylococcus epidermidis pan-genome sequence analysis reveals diversity of skin commensal and hospital infection-associated isolates. Genome Biol 13, R64. 10.1186/gb-2012-13-7-r64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias PM, and Ghadially R (2002). The aged epidermal permeability barrier: basis for functional abnormalities. Clin Geriatr Med 18, 103–120, vii. 10.1016/s0749-0690(03)00037-5. [DOI] [PubMed] [Google Scholar]
- Fitz-Gibbon S, Tomida S, Chiu BH, Nguyen L, Du C, Liu M, Elashoff D, Erfe MC, Loncaric A, Kim J, et al. (2013). Propionibacterium acnes strain populations in the human skin microbiome associated with acne. J Invest Dermatol 133, 2152–2160. 10.1038/jid.2013.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Diaz M, Monturiol-Gross L, Naylor C, Alape-Giron A, and Flieger A (2016). Bacterial Sphingomyelinases and Phospholipases as Virulence Factors. Microbiol Mol Biol Rev 80, 597–628. 10.1128/MMBR.00082-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghadially R, Brown BE, Sequeira-Martin SM, Feingold KR, and Elias PM (1995). The aged epidermal permeability barrier. Structural, functional, and lipid biochemical abnormalities in humans and a senescent murine model. J Clin Invest 95, 2281–2290. 10.1172/JCI117919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goni FM, and Alonso A (2002). Sphingomyelinases: enzymology and membrane activity. FEBS Lett 531, 38–46. 10.1016/s0014-5793(02)03482-8. [DOI] [PubMed] [Google Scholar]
- Grice EA, and Segre JA (2011). The skin microbiome. Nat Rev Microbiol 9, 244–253. 10.1038/nrmicro2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J, Li H, Li M, Vuong C, Otto M, Wen Y, and Gao Q (2005). Bacterial insertion sequence IS256 as a potential molecular marker to discriminate invasive strains from commensal strains of Staphylococcus epidermidis. J Hosp Infect 61, 342–348. 10.1016/j.jhin.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Harrison OJ, Linehan JL, Shih HY, Bouladoux N, Han SJ, Smelkinson M, Sen SK, Byrd AL, Enamorado M, Yao C, et al. (2019). Commensal-specific T cell plasticity promotes rapid tissue adaptation to injury. Science 363. 10.1126/science.aat6280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbronner S, Krismer B, Brotz-Oesterhelt H, and Peschel A (2021). The microbiome-shaping roles of bacteriocins. Nat Rev Microbiol. 10.1038/s41579-021-00569-w. [DOI] [PubMed] [Google Scholar]
- Heilmann C, Schweitzer O, Gerke C, Vanittanakom N, Mack D, and Gotz F (1996). Molecular basis of intercellular adhesion in the biofilm-forming Staphylococcus epidermidis. Mol Microbiol 20, 1083–1091. 10.1111/j.1365-2958.1996.tb02548.x. [DOI] [PubMed] [Google Scholar]
- Herrera A, Vu BG, Stach CS, Merriman JA, Horswill AR, Salgado-Pabon W, and Schlievert PM (2016). Staphylococcus aureus beta-Toxin Mutants Are Defective in Biofilm Ligase and Sphingomyelinase Activity, and Causation of Infective Endocarditis and Sepsis. Biochemistry 55, 2510–2517. 10.1021/acs.biochem.6b00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huseby MJ, Kruse AC, Digre J, Kohler PL, Vocke JA, Mann EE, Bayles KW, Bohach GA, Schlievert PM, Ohlendorf DH, and Earhart CA (2010). Beta toxin catalyzes formation of nucleoprotein matrix in staphylococcal biofilms. Proc Natl Acad Sci U S A 107, 14407–14412. 10.1073/pnas.0911032107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imokawa G, Abe A, Jin K, Higaki Y, Kawashima M, and Hidano A (1991). Decreased level of ceramides in stratum corneum of atopic dermatitis: an etiologic factor in atopic dry skin? J Invest Dermatol 96, 523–526. 10.1111/1523-1747.ep12470233. [DOI] [PubMed] [Google Scholar]
- Jensen JM, Forl M, Winoto-Morbach S, Seite S, Schunck M, Proksch E, and Schutze S (2005). Acid and neutral sphingomyelinase, ceramide synthase, and acid ceramidase activities in cutaneous aging. Exp Dermatol 14, 609–618. 10.1111/j.0906-6705.2005.00342.x. [DOI] [PubMed] [Google Scholar]
- Jungersted JM, Hellgren LI, Jemec GB, and Agner T (2008). Lipids and skin barrier function--a clinical perspective. Contact Dermatitis 58, 255–262. 10.1111/j.1600-0536.2008.01320.x. [DOI] [PubMed] [Google Scholar]
- Katayama Y, Baba T, Sekine M, Fukuda M, and Hiramatsu K (2013). Beta-hemolysin promotes skin colonization by Staphylococcus aureus. J Bacteriol 195, 1194–1203. 10.1128/JB.01786-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihara A (2016). Synthesis and degradation pathways, functions, and pathology of ceramides and epidermal acylceramides. Prog Lipid Res 63, 50–69. 10.1016/j.plipres.2016.04.001. [DOI] [PubMed] [Google Scholar]
- Kleuser B, and Japtok L (2013). Sphingolipids and inflammatory diseases of the skin. Handb Exp Pharmacol, 355–372. 10.1007/978-3-7091-1511-4_18. [DOI] [PubMed] [Google Scholar]
- Kloos WE, and Musselwhite MS (1975). Distribution and persistence of Staphylococcus and Micrococcus species and other aerobic bacteria on human skin. Appl Microbiol 30, 381–385. 10.1128/am.30.3.381-395.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornhuber J, Rhein C, Muller CP, and Muhle C (2015). Secretory sphingomyelinase in health and disease. Biol Chem 396, 707–736. 10.1515/hsz-2015-0109. [DOI] [PubMed] [Google Scholar]
- Kreiswirth BN, Lofdahl S, Betley MJ, O’Reilly M, Schlievert PM, Bergdoll MS, and Novick RP (1983). The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature 305, 709–712. 10.1038/305709a0. [DOI] [PubMed] [Google Scholar]
- Krismer B, Liebeke M, Janek D, Nega M, Rautenberg M, Hornig G, Unger C, Weidenmaier C, Lalk M, and Peschel A (2014). Nutrient limitation governs Staphylococcus aureus metabolism and niche adaptation in the human nose. PLoS Pathog 10, e1003862. 10.1371/journal.ppat.1003862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupper TS, and Fuhlbrigge RC (2004). Immune surveillance in the skin: mechanisms and clinical consequences. Nat Rev Immunol 4, 211–222. 10.1038/nri1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y, Di Nardo A, Nakatsuji T, Leichtle A, Yang Y, Cogen AL, Wu ZR, Hooper LV, Schmidt RR, von Aulock S, et al. (2009). Commensal bacteria regulate Toll-like receptor 3-dependent inflammation after skin injury. Nat Med 15, 1377–1382. 10.1038/nm.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech JM, Dhariwala MO, Lowe MM, Chu K, Merana GR, Cornuot C, Weckel A, Ma JM, Leitner EG, Gonzalez JR, et al. (2019). Toxin-Triggered Interleukin-1 Receptor Signaling Enables Early-Life Discrimination of Pathogenic versus Commensal Skin Bacteria. Cell Host Microbe 26, 795–809 e795. 10.1016/j.chom.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima-Junior DS, Krishnamurthy SR, Bouladoux N, Collins N, Han SJ, Chen EY, Constantinides MG, Link VM, Lim AI, Enamorado M, et al. (2021). Endogenous retroviruses promote homeostatic and inflammatory responses to the microbiota. Cell 184, 3794–3811 e3719. 10.1016/j.cell.2021.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linehan JL, Harrison OJ, Han SJ, Byrd AL, Vujkovic-Cvijin I, Villarino AV, Sen SK, Shaik J, Smelkinson M, Tamoutounour S, et al. (2018). Non-classical Immunity Controls Microbiota Impact on Skin Immunity and Tissue Repair. Cell 172, 784–796 e718. 10.1016/j.cell.2017.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Liu Q, Meng H, Lv H, Liu Y, Liu J, Wang H, He L, Qin J, Wang Y, et al. (2020a). Staphylococcus epidermidis Contributes to Healthy Maturation of the Nasal Microbiome by Stimulating Antimicrobial Peptide Production. Cell Host Microbe 27, 68–78 e65. 10.1016/j.chom.2019.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Liu Y, Du Z, Zhang L, Chen J, Shen Z, Liu Q, Qin J, Lv H, Wang H, et al. (2020b). Skin microbiota analysis-inspired development of novel anti-infectives. Microbiome 8, 85. 10.1186/s40168-020-00866-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lueangarun S, Tragulplaingam P, Sugkraroek S, and Tempark T (2019). The 24-hr, 28-day, and 7-day post-moisturizing efficacy of ceramides 1, 3, 6-II containing moisturizing cream compared with hydrophilic cream on skin dryness and barrier disruption in senile xerosis treatment. Dermatol Ther 32, e13090. 10.1111/dth.13090. [DOI] [PubMed] [Google Scholar]
- Mack D, Nedelmann M, Krokotsch A, Schwarzkopf A, Heesemann J, and Laufs R (1994). Characterization of transposon mutants of biofilm-producing Staphylococcus epidermidis impaired in the accumulative phase of biofilm production: genetic identification of a hexosamine-containing polysaccharide intercellular adhesin. Infect Immun 62, 3244–3253. 10.1128/iai.62.8.3244-3253.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meric G, Mageiros L, Pensar J, Laabei M, Yahara K, Pascoe B, Kittiwan N, Tadee P, Post V, Lamble S, et al. (2018). Disease-associated genotypes of the commensal skin bacterium Staphylococcus epidermidis. Nat Commun 9, 5034. 10.1038/s41467-018-07368-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meric G, Miragaia M, de Been M, Yahara K, Pascoe B, Mageiros L, Mikhail J, Harris LG, Wilkinson TS, Rolo J, et al. (2015). Ecological Overlap and Horizontal Gene Transfer in Staphylococcus aureus and Staphylococcus epidermidis. Genome Biol Evol 7, 1313–1328. 10.1093/gbe/evv066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik S, Bouladoux N, Linehan JL, Han SJ, Harrison OJ, Wilhelm C, Conlan S, Himmelfarb S, Byrd AL, Deming C, et al. (2015). Commensal-dendritic-cell interaction specifies a unique protective skin immune signature. Nature 520, 104–108. 10.1038/nature14052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Oscherwitz J, Cease KB, Chan SM, Munoz-Planillo R, Hasegawa M, Villaruz AE, Cheung GY, McGavin MJ, Travers JB, et al. (2013). Staphylococcus delta-toxin induces allergic skin disease by activating mast cells. Nature 503, 397–401. 10.1038/nature12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Takahashi H, Takaya A, Inoue Y, Katayama Y, Kusuya Y, Shoji T, Takada S, Nakagawa S, Oguma R, et al. (2020). Staphylococcus Agr virulence is critical for epidermal colonization and associates with atopic dermatitis development. Sci Transl Med 12. 10.1126/scitranslmed.aay4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsuji T, Chen TH, Butcher AM, Trzoss LL, Nam SJ, Shirakawa KT, Zhou W, Oh J, Otto M, Fenical W, and Gallo RL (2018). A commensal strain of Staphylococcus epidermidis protects against skin neoplasia. Sci Adv 4, eaao4502. 10.1126/sciadv.aao4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsuji T, Chen TH, Narala S, Chun KA, Two AM, Yun T, Shafiq F, Kotol PF, Bouslimani A, Melnik AV, et al. (2017). Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Sci Transl Med 9. 10.1126/scitranslmed.aah4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsuji T, Hata TR, Tong Y, Cheng JY, Shafiq F, Butcher AM, Salem SS, Brinton SL, Rudman Spergel AK, Johnson K, et al. (2021). Development of a human skin commensal microbe for bacteriotherapy of atopic dermatitis and use in a phase 1 randomized clinical trial. Nat Med 27, 700–709. 10.1038/s41591-021-01256-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogiso H, Taniguchi M, Araya S, Aoki S, Wardhani LO, Yamashita Y, Ueda Y, and Okazaki T (2014). Comparative Analysis of Biological Sphingolipids with Glycerophospholipids and Diacylglycerol by LC-MS/MS. Metabolites 4, 98–114. 10.3390/metabo4010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh J, Byrd AL, Deming C, Conlan S, Program NCS, Kong HH, and Segre JA (2014). Biogeography and individuality shape function in the human skin metagenome. Nature 514, 59–64. 10.1038/nature13786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto M (2004). Virulence factors of the coagulase-negative staphylococci. Front Biosci 9, 841–863. 10.2741/1295. [DOI] [PubMed] [Google Scholar]
- Otto M (2009). Staphylococcus epidermidis--the ‘accidental’ pathogen. Nat Rev Microbiol 7, 555–567. 10.1038/nrmicro2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto M (2012). Molecular basis of Staphylococcus epidermidis infections. Semin Immunopathol 34, 201–214. 10.1007/s00281-011-0296-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto M (2014). Staphylococcus aureus toxins. Curr Opin Microbiol 17, 32–37. 10.1016/j.mib.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul C, Maumus-Robert S, Mazereeuw-Hautier J, Guyen CN, Saudez X, and Schmitt AM (2011). Prevalence and risk factors for xerosis in the elderly: a cross-sectional epidemiological study in primary care. Dermatology 223, 260–265. 10.1159/000334631. [DOI] [PubMed] [Google Scholar]
- Pflughoeft KJ, and Versalovic J (2012). Human microbiome in health and disease. Annu Rev Pathol 7, 99–122. 10.1146/annurev-pathol-011811-132421. [DOI] [PubMed] [Google Scholar]
- Proksch E, Brandner JM, and Jensen JM (2008). The skin: an indispensable barrier. Exp Dermatol 17, 1063–1072. 10.1111/j.1600-0625.2008.00786.x. [DOI] [PubMed] [Google Scholar]
- Rogers J, Harding C, Mayo A, Banks J, and Rawlings A (1996). Stratum corneum lipids: the effect of ageing and the seasons. Arch Dermatol Res 288, 765–770. 10.1007/BF02505294. [DOI] [PubMed] [Google Scholar]
- Sahle FF, Gebre-Mariam T, Dobner B, Wohlrab J, and Neubert RH (2015). Skin diseases associated with the depletion of stratum corneum lipids and stratum corneum lipid substitution therapy. Skin Pharmacol Physiol 28, 42–55. 10.1159/000360009. [DOI] [PubMed] [Google Scholar]
- Scharschmidt TC, Vasquez KS, Truong HA, Gearty SV, Pauli ML, Nosbaum A, Gratz IK, Otto M, Moon JJ, Liese J, et al. (2015). A Wave of Regulatory T Cells into Neonatal Skin Mediates Tolerance to Commensal Microbes. Immunity 43, 1011–1021. 10.1016/j.immuni.2015.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleifer KH, and Fischer U (1982). Description of a New Species of the Genus Staphylococcus: Staphylococcus carnosus. Int. J. Syst. Evol. Microbiol 32, 153–156. [Google Scholar]
- Spada F, Barnes TM, and Greive KA (2018). Skin hydration is significantly increased by a cream formulated to mimic the skin’s own natural moisturizing systems. Clin Cosmet Investig Dermatol 11, 491–497. 10.2147/CCID.S177697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- t’Kindt R, Jorge L, Dumont E, Couturon P, David F, Sandra P, and Sandra K (2012). Profiling and characterizing skin ceramides using reversed-phase liquid chromatography-quadrupole time-of-flight mass spectrometry. Anal Chem 84, 403–411. 10.1021/ac202646v. [DOI] [PubMed] [Google Scholar]
- Uberoi A, Bartow-McKenney C, Zheng Q, Flowers L, Campbell A, Knight SAB, Chan N, Wei M, Lovins V, Bugayev J, et al. (2021). Commensal microbiota regulates skin barrier function and repair via signaling through the aryl hydrocarbon receptor. Cell Host Microbe 29, 1235–1248 e1238. 10.1016/j.chom.2021.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida Y, Hara M, Nishio H, Sidransky E, Inoue S, Otsuka F, Suzuki A, Elias PM, Holleran WM, and Hamanaka S (2000). Epidermal sphingomyelins are precursors for selected stratum corneum ceramides. J Lipid Res 41, 2071–2082. [PubMed] [Google Scholar]
- Valsecchi M, Mauri L, Casellato R, Prioni S, Loberto N, Prinetti A, Chigorno V, and Sonnino S (2007). Ceramide and sphingomyelin species of fibroblasts and neurons in culture. J Lipid Res 48, 417–424. 10.1194/jlr.M600344-JLR200. [DOI] [PubMed] [Google Scholar]
- van Smeden J, Hoppel L, van der Heijden R, Hankemeier T, Vreeken RJ, and Bouwstra JA (2011). LC/MS analysis of stratum corneum lipids: ceramide profiling and discovery. J Lipid Res 52, 1211–1221. 10.1194/jlr.M014456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyumvuhore R, Michael-Jubeli R, Verzeaux L, Boudier D, Le Guillou M, Bordes S, Libong D, Tfayli A, Manfait M, and Closs B (2018). Lipid organization in xerosis: the key of the problem? Int J Cosmet Sci 40, 549–554. 10.1111/ics.12496. [DOI] [PubMed] [Google Scholar]
- Wang R, Braughton KR, Kretschmer D, Bach TH, Queck SY, Li M, Kennedy AD, Dorward DW, Klebanoff SJ, Peschel A, et al. (2007). Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat Med 13, 1510–1514. 10.1038/nm1656. [DOI] [PubMed] [Google Scholar]
- Weinstock GM (2012). Genomic approaches to studying the human microbiota. Nature 489, 250–256. 10.1038/nature11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertz PW (2000). Lipids and barrier function of the skin. Acta Derm Venereol Suppl (Stockh) 208, 7–11. 10.1080/000155500750042790. [DOI] [PubMed] [Google Scholar]
- Wertz PW, Miethke MC, Long SA, Strauss JS, and Downing DT (1985). The composition of the ceramides from human stratum corneum and from comedones. J Invest Dermatol 84, 410–412. 10.1111/1523-1747.ep12265510. [DOI] [PubMed] [Google Scholar]
- Williams MR, Costa SK, Zaramela LS, Khalil S, Todd DA, Winter HL, Sanford JA, O’Neill AM, Liggins MC, Nakatsuji T, et al. (2019). Quorum sensing between bacterial species on the skin protects against epidermal injury in atopic dermatitis. Sci Transl Med 11. 10.1126/scitranslmed.aat8329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstel V, Kuhner P, Krismer B, Peschel A, and Rohde H (2015). Transfer of plasmid DNA to clinical coagulase-negative staphylococcal pathogens by using a unique bacteriophage. Appl Environ Microbiol 81, 2481–2488. 10.1128/AEM.04190-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai H, and Maibach HI (2001). Effects of skin occlusion on percutaneous absorption: an overview. Skin Pharmacol Appl Skin Physiol 14, 1–10. 10.1159/000056328. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Comparison of Sph-like sequences in staphylococci and bacilli. Related to Fig. 2.
Fig. S2. Purification, sphingomyelin cleavage activity, and pH and metal dependence of S. epidermidis Sph. Related to Fig. 2.
Fig. S3. Thin-layer chromatography (TLC) of lipids from different sources before and after digestion with purified Sph. Related to Fig. 2.
Fig. S4. Sph production in S. epidermidis isolates. Related to Fig. 2.
Fig. S5. Impact of S. epidermidis Sph on cytotoxicity, biofilm formation, and in-vitro growth. Related to Fig. 3.
Fig. S6. Histological evaluation of AD model. Related to Fig. 6.
Tab. S1. Histological evaluation of AD model. Related to Fig. 6.
Tab. S2. Oligonucleotides used in this study. Related to STAR Methods.
Data Availability Statement
All data reported in this paper will be shared by the lead contact upon request.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.


