Abstract
Background:
Guidelines recommend lowering systolic blood pressure below 130 mmHg, irrespective of previous strokes. However, there is a concern that lowering systolic blood pressure in people with low baseline diastolic blood pressure might increase the risk of stroke.
Methods:
We conducted a secondary analysis of the Secondary Prevention of Small Subcortical Strokes trial that randomly assigned participants with a history of subcortical strokes to an intensive (<130 mmHg) (N = 1519) or standard (130–149 mmHg) (N = 1501) systolic targets. We examined the effects of blood pressure intervention on stroke and cardiovascular composite across the range of baseline diastolic blood pressure in spline regression models and tested for interaction of baseline diastolic blood pressure with the intervention on outcomes.
Results:
Mean baseline systolic and diastolic blood pressures were 143±19 and 78±11 mmHg, respectively. Within each baseline diastolic blood pressure tertile, the achieved diastolic was lower in the intensive versus standard arm. There were 275 stroke events over 10889 years of follow-up. Lower baseline diastolic blood pressure was associated with increased risk of stroke in an observational spline regression model. Hazard ratios relating blood pressure intervention with the risk of stroke in the lowest (HR 0.78, 95% CI 0.52 to 1.16) and the highest (HR 0.80, 95% CI 0.53 to 1.21) baseline diastolic tertiles were similar (p = 0.95). Results were similar for the cardiovascular composite.
Conclusions:
Intensive systolic control does not appear to increase the risk of stroke in those with low baseline diastolic blood pressure and prior stroke.
Keywords: BP intervention, SPS-3, stroke, J curve, systolic blood pressure, diastolic blood pressure
Introduction
With an annual incidence of nearly ~800,000 per year, stroke is the fourth leading cause of death and is the major cause of disability in the United States.1–3 It is estimated that the financial impact of strokes is ~ $34 billion/year in healthcare expenses, medication, and missed days of work in the US1.
Hypertension is a leading risk factor for stroke4, 5. The Secondary Prevention of Small Subcortical Strokes (SPS3) Trial tested in patients with recent lacunar stroke (n = 3020), a systolic-blood-pressure (SBP) target of < 130 mmHg versus 130 to 149 mmHg on the incidence of subsequent stroke6; the results were inconclusive with a statistically non-significant reduction for stroke (hazard ratio 0·81, 95% CI 0·64–1·03, p=0·08). However, the subsequent, larger Systolic Blood Pressure Intervention Trial (SPRINT) (n = 9,361) which tested an even lower SBP target of < 120 mmHg versus standard SBP goal of < 140 mmHg demonstrated 25% reduction in cardiovascular composite and 27% reduction in all-cause mortality7. Hence, the current AHA/ACC guidelines recommend a target SBP of <130 mmHg in people with high blood pressure including those with previous stroke8.
However, in those with lower baseline diastolic blood pressure (DBP), there are concerns that lowering SBP can decrease mean arterial pressure (MAP) leading to ischemia with increased risk of stroke9, 10. Indeed, lower DBP has been associated with increased risk of coronary events9, 11–13, stroke14, 15 and all-cause mortality16, 17 in observational studies. The fundamental question is whether the associations of low baseline DBP with increased risk of stroke and other cardiovascular outcomes is causal or whether it represents an epiphenomenon due to confounding from underlying conditions like increased arterial stiffness and atherosclerosis. In the current study, we performed a secondary analysis of SPS-3 data to examine the hypothesis that lowering SBP in those with lower baseline DBP and previous stroke will lead to increased risk of subsequent stroke and worse cardiovascular outcomes.
Methods:
Limited access SPS3 dataset is available from the National Institute of Neurological Disorders and Stroke18 (https://www.ninds.nih.gov/Current-Research/Research-Funded-NINDS/Clinical-Research/Archived-Clinical-Research-Datasets). We used this dataset in the current analysis.
Details of the SPS3 trial have been previously published.6, 19, 20 In brief, 3020 participants with recent, confirmed lacunar stroke in 81 locations throughout North America, Central America, and Spain were randomly assigned by a two-by-two factorial design to SBP target of < 130 mmHg versus 130 to 149 mmHg, and to antiplatelet therapy of 325mg aspirin plus 75mg of clopidogrel or 325mg aspirin plus placebo per day. Randomization was done using a computerized schedule, randomized block design, and stratified by clinical center and baseline hypertension status. Participants underwent written informed consent with approval by local Institutional Review Boards in accordance with the Declaration of Helsinki.
SPS3 trial population:
Normotensive or hypertensive participants were recruited from March 2003 to April 2011 if they were at least 30 years old and had a recent MRI-confirmed lacunar stroke in the previous 180 days. Participants were considered to have baseline hypertension if they had either SBP >135 mm Hg or DBP >85 mm Hg at two consecutive visits, or a history of hypertension before the index stroke and taking antihypertensive medications at the time of the baseline visit. Major exclusion criteria were previous disabling stroke, intracranial hemorrhage, cortical ischemic stroke, anticipated requirement for long-term use of anticoagulants or other antiplatelet agents and intolerance or contraindications to aspirin or clopidogrel.
SPS3 SBP intervention:
Following a standardized study protocol20, BP was measured by trained staff while minimizing physical contact with an automated sphygmomanometer (Press-Mate BP-8800C model, Colin Medical Instruments, San Antonio, TX, USA). The average of 3-readings were used. Antihypertensive regimen was based on national guidelines and involved step-wise titration of medications until the SBP targets of <130 mmHg (intensive therapy) or 130–149 mmHg (standard therapy) were achieved. Normotensive participants were only assigned antihypertensive drugs if they later became hypertensive. Participants were seen monthly until they reached the assigned SBP goals, and thereafter every three months.
SPS-3 Outcomes:
Participants were followed until April 2012. The primary outcome was time to occurrence of any stroke6. Ischemic stroke was clinically defined as a focal neurological deficit persisting for longer than 24 hours, without hemorrhage as confirmed by neuroimaging. Intracerebral, subdural or epidural, and subarachnoid hemorrhages as defined by neuroimaging were included as hemorrhagic stroke. We additionally defined a cardiovascular disease (CVD) composite outcome as time to the main stroke outcome, hospitalization for myocardial infarction (defined acute by standard criteria as compatible clinical history with changes on ECG or in cardiac enzyme concentrations), congestive heart failure, or vascular death. In sensitivity analyses, we also examined the time to acute myocardial infarction.
Statistical Methods:
We compared numeric baseline characteristics between baseline DBP tertiles ≤73, 74–82, or ≥83 mmHg) using 1-way ANOVA and categorical variables using χ2 tests. We calculated follow-up blood pressures by averaging scheduled visits from month 3 to end of study. Box plots were used to display mean follow-up SBP, DBP, and MAP by baseline DBP tertiles and SBP treatment groups.
In an observational Cox regression analyses adjusted for age, gender, race, and BP intervention arm, we used cubic spline terms for baseline DBP with baseline DBP of 70 mmHg as the reference, for a graphical display of the associations of baseline DBP on the hazard for the stroke outcome. Next, we used a model with cubic terms for baseline DBP to display graphical models of the hazard ratio for the intensive vs standard SBP group across the range of baseline DBP. We further tested for modification of the effects of the intervention on stroke by baseline DBP with two different approaches. We tested for significance of the product term of baseline DBP and the intervention in a Cox regression model that includes baseline DBP and BP intervention as additional terms. We also compared the regression coefficients of the effects of the BP intervention on stroke outcome from separate Cox regression models in the lowest and highest baseline DBP tertiles. We repeated the above analyses for the CVD composite outcome as well as for myocardial infarction.
In additional analyses, we repeated the above models replacing baseline DBP with baseline MAP. In further sensitivity models, we repeated all the above models in the subgroup with treated baseline hypertension or SBP>135 or DBP>85.
We analyzed all data using STATA version MP 15.1 or SAS version 9.4. We conducted hypothesis testing using 2-sided α=0.05 without adjustment for multiple comparisons. We performed analyses of scaled Schoenfeld residuals21 that indicated no evidence of non-proportional hazards.
Results:
In the 3020 SPS3 trial participants who were randomized, those in the lowest DBP tertile (DBP≤73 mmHg) were older, more likely to be male, white, not currently employed, and to have a history of diabetes, hypertension, statin use and lower baseline LDL-cholesterol (Table 1).
Table 1.
Baseline Characteristics$ by diastolic blood pressure tertiles (N=3020)
| Characteristic | ≤ 73 mmHg | 74–82 mmHg | ≥83 mmHg | p-value |
|---|---|---|---|---|
| N=1,009 | N=1,041 | N=970 | ||
| Diastolic blood pressure, mmHg | 67 ± 5 | 78 ± 3 | 90 ± 7 | |
| Diastolic blood pressure (hypertensive subgroup), mmHg | 67 ± 5 | 78 ± 3 | 90 ± 7 | |
| Diastolic blood pressure (normotensive subgroup), mmHg | 66 ± 5 | 77 ± 3 | 84 ± 1 | |
| Age, years | 66 ± 11 | 63 ± 10 | 59 ± 10 | <0.001 |
| Male (%) | 56 | 64 | 70 | <0.001 |
| Black(%) | 12 | 14 | 21 | <0.001 |
| Hispanic ethnicity (%) | 24 | 22 | 20 | 0.14 |
| College educated (%) | 36 | 35 | 36 | 0.84 |
| Currently employed (%) | 36 | 49 | 56 | <0.001 |
| Married (%) | 63 | 67 | 64 | 0.19 |
| Ever smoked (%) | 59 | 59 | 63 | 0.065 |
| Diabetes (%) | 36 | 35 | 28 | <0.001 |
| Baseline hypertension* (%) | 78 | 92 | 99 | <0.001 |
| Myocardial infarction (%) | 6 | 5 | 5 | 0.63 |
| Congestive heart failure (%) | 1 | 1 | 1 | 0.95 |
| Coronary artery bypass graft/ stent (%) | 5 | 6 | 4 | 0.17 |
| Chronic obstructive pulmonary disease (%) | 4 | 3 | 2 | 0.099 |
| Peripheral vascular disease | 4 | 3 | 3 | 0.60 |
| Statin use (%) | 77 | 73 | 69 | <0.001 |
| ACE-I or ARB use (%) | 65 | 66 | 70 | 0.062 |
| Body mass index, kg/m2 | 28.6 ± 5.9 | 29.2 ± 6.0 | 29.2 ± 5.7 | 0.034 |
| Systolic blood pressure, mmHg | 130 ± 14 | 142 ± 14 | 158 ± 18 | <0.001 |
| Systolic blood pressure (hypertensive subgroup), mmHg | 133 ± 14 | 143 ± 13 | 158 ± 17 | <0.001 |
| Systolic blood pressure (normotensive subgroup), mmHg | 120 ± 7 | 125 ± 4 | 127 ± 2 | <0.001 |
| Mean arterial blood pressure, mmHg | 88 ± 7 | 99 ± 5 | 113 ± 9 | <0.001 |
| eGFR, ml/min/1.73 m2 | 78 ± 19 | 81 ± 19 | 82 ± 19 | <0.001 |
| Serum LDL-cholesterol | 108 ± 37 | 114 ± 42 | 118 ± 39 | <0.001 |
| Serum HDL-cholesterol | 45 ± 16 | 46 ± 23 | 45 ± 17 | 0.58 |
| Serum triglycerides | 157 ± 101 | 171 ± 129 | 169 ± 118 | 0.027 |
Mean ± SD for continuous variables and N (%) for categorical variables are presented
History of hypertension or antihypertensive use or baseline elevated BP (>135/85)
Achieved blood pressures by baseline DBP tertiles:
Boxplots reflecting the effects of intensive SBP control on post-3 months mean follow-up values of SBP, DBP and MAP by baseline DBP tertiles are shown in Figure 1. The achieved mean follow-up SBP was <130mmHg with intensive control irrespective of baseline DBP (Figure 1, panel A). The achieved DBP and MAP within each baseline DBP tertile in the intensive group were lower than the standard group (Figure 1, panels B and C). However, within the intensive arm, the achieved mean follow-up DBP (65 ± 7 versus 74 ± 7 mmHg, p < 0.001) and MAP (85 ± 7 versus 92 ± 8 mmHg, p < 0.001) were much lower in the lowest baseline DBP tertile compared to the highest DBP tertile.
Figure 1.
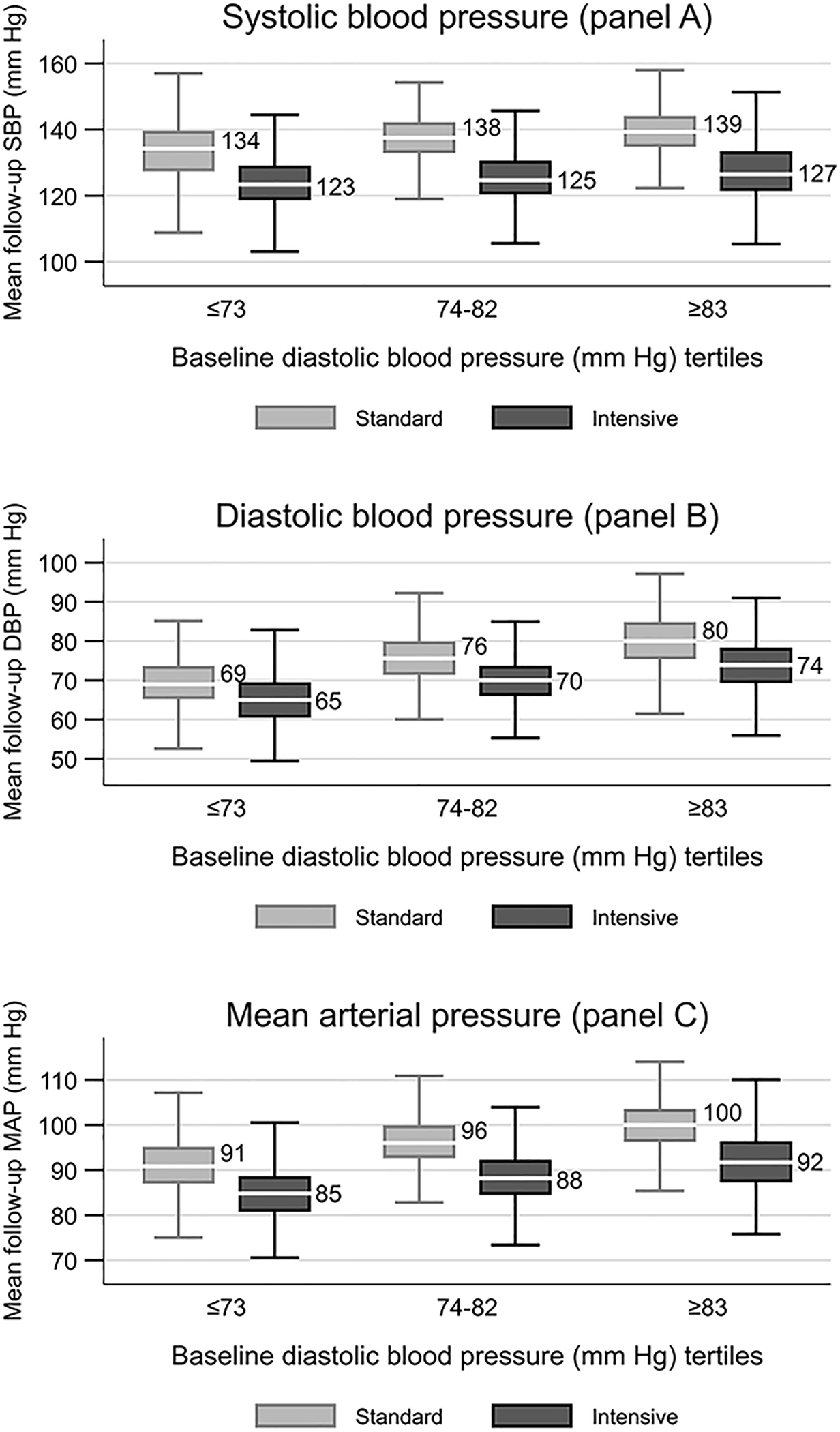
Box plots of mean follow-up SBP (Panel A), DBP (Panel B), and, MAP(Panel C) by SBP intervention and baseline DBP tertiles. Shows 1st quartile – 1.5 IQR, 1st quartile, median, 3rd quartile and 3rd quartile + 1.5 IQR, IQR=3rd quartile - 1st quartile. 71 participants (40 in standard and 31 in intensive BP arm) had missing follow-up blood pressure data after 3 months and were not included.
Associations of baseline DBP and MAP with stroke and the effects of intensive SBP lowering on stroke across the range of baseline DBP and MAP:
There were 124 stroke events over 5480 years of follow-up in the intensive SBP group and 151 stroke events over 5410 years of follow-up in the standard SBP group. In an observational spline regression model, using baseline DBP of 70 mmHg as the reference, lower baseline DBP was associated with increased risk of stroke (Figure 2, panel A). Despite this observational association, there was no evidence that intensive SBP lowering significantly increased the risk of stroke in those with lower baseline DBP in a spline regression model relating intensive SBP intervention with the risk of stroke across the range of baseline DBP (Figure 2, panel B).
Figure 2.
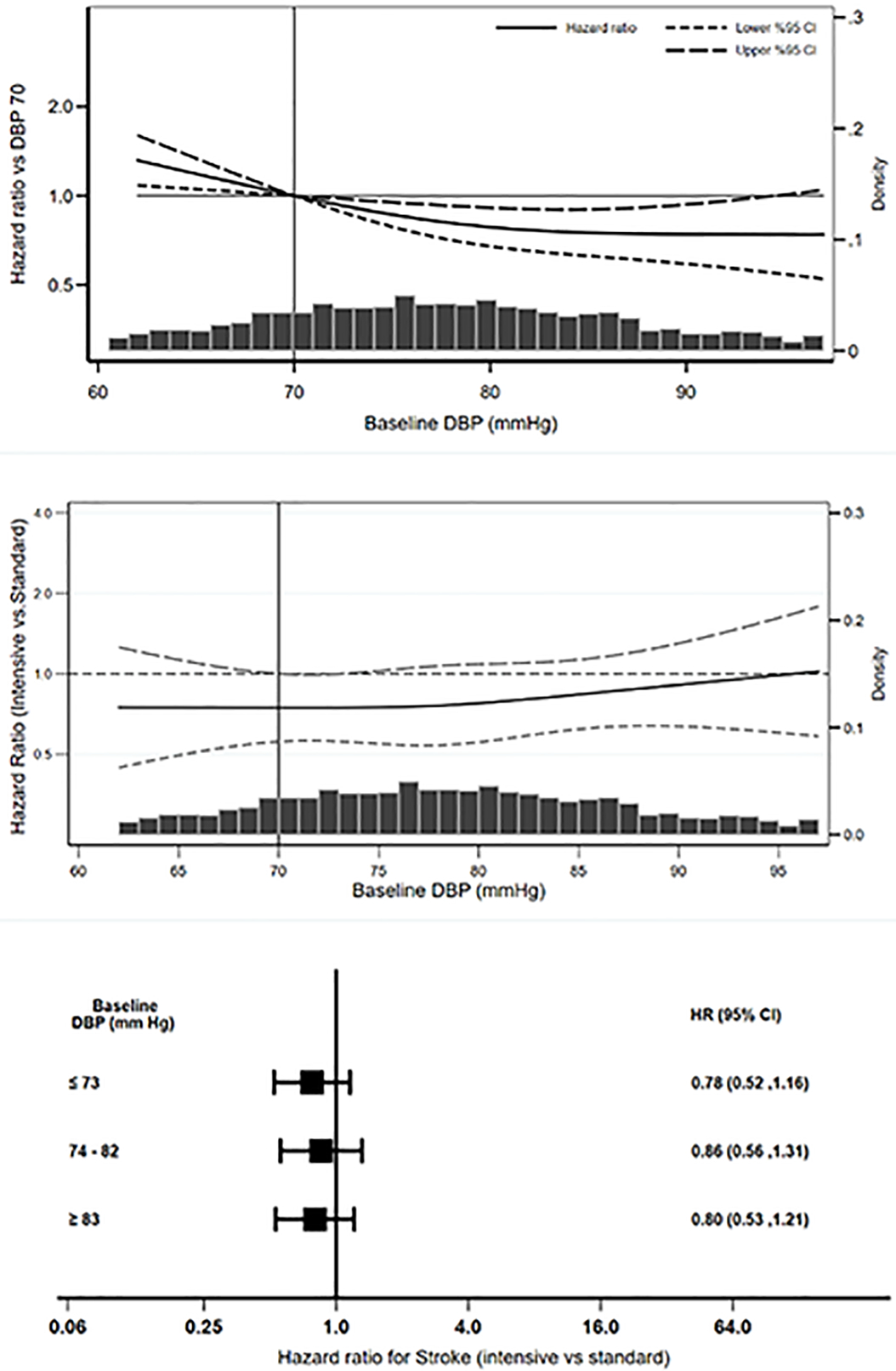
Observational associations of baseline DBP with stroke (Panel A) and effects of BP intervention on stroke across the range of baseline DBP (Panel B) and baseline DBP tertiles (Panel C)
In a Cox model, hazard ratios for stroke for the BP intervention and each 10 mmHg higher baseline DBP were 0.80 (95% CI 0.63 to 1.02) and 0.93 (95% CI 0.83 to 1.05), respectively (Table 2). Interaction product term of baseline DBP and SBP intervention for the stroke outcome was non-significant (p = 0.50).
Table 2.
Interactions of baseline diastolic blood pressure and mean arterial pressure with BP intervention on outcomes.
| Outcome by BP parameter | Intensive versus standard | For each 10 mm Hg increase | Interaction term* | ||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | P value | |
| Diastolic BP | |||||
| Stroke | 0.80 (0.63, 1.02) | 0.074 | 0.93 (0.83, 1.05) | 0.24 | 0.50 |
| CVD composite | 0.86 (0.70, 1.06) | 0.15 | 0.99 (0.89, 1.09) | 0.76 | 0.87 |
| Myocardial Infarction | 0.93 (0.59, 1.46) | 0.75 | 1.14 (0.93, 1.41) | 0.21 | 0.31 |
| Mean arterial BP | |||||
| Stroke | 0.81 (0.64, 1.03) | 0.084 | 0.98 (0.89, 1.08) | 0.68 | 0.34 |
| CVD composite | 0.87 (0.71, 1.07) | 0.18 | 1.04 (0.95, 1.13) | 0.39 | 0.75 |
| Myocardial Infarction | 0.93 (0.59, 1.47) | 0.76 | 1.15 (0.96, 1.38) | 0.12 | 0.20 |
p-value for product term of BP intervention and each 10 mm Hg higher baseline DBP or MAP
In subgroup analyses by baseline DBP tertiles (Figure 2, panel C), in separate Cox regression models, hazard ratios for relating intensive SBP intervention with the risk of stroke in the lowest (HR 0.78, 95% CI 0.52 to 1.16) and the highest (HR 0.80, 95% CI 0.53 to 1.21) DBP tertiles were similar (p = 0.95 for comparison of the regression coefficients). Similar findings were observed with regards to MAP (Table 2 and Figure 3, panels A-C) with an Interaction p = 0.34 for the product term of baseline MAP and SBP intervention for the stroke outcome.
Figure 3.
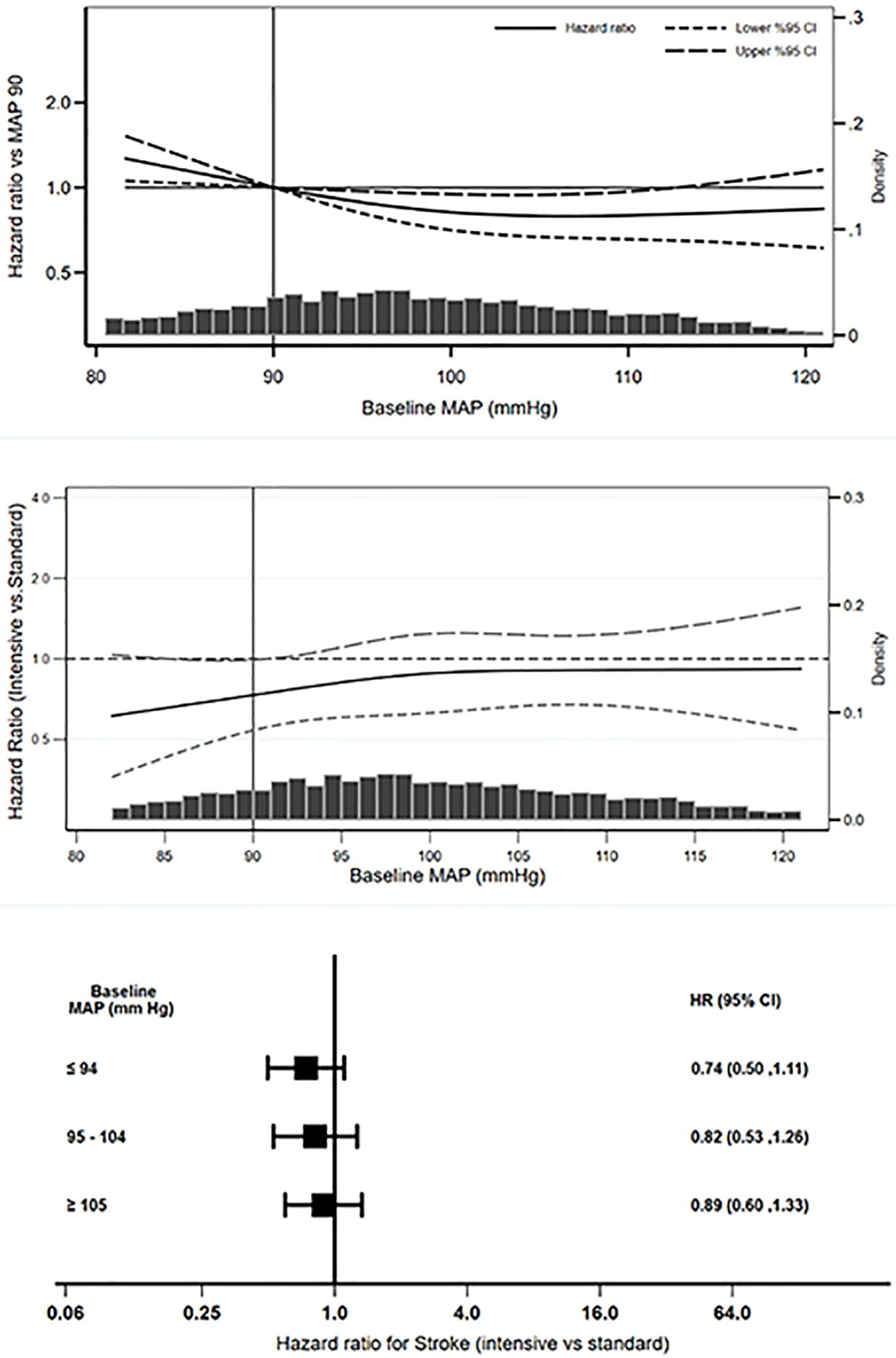
Observational associations of baseline MAP with stroke (Panel A) and effects of BP intervention on stroke across the range of baseline MAP (Panel B) and baseline MAP tertiles (Panel C)
Associations of baseline DBP and MAP with cardiovascular composite outcome and the effects of intensive SBP lowering on cardiovascular outcome across the range of baseline DBP and MAP:
There were 172 cardiovascular composite events over 5423 years of follow-up in the intensive arm and 197 cardiovascular composite events over 5358 years of follow-up in the standard arm. The corresponding numbers for myocardial infarction events in the intensive and standard arms were 36 events over 5725 years of follow-up and 39 events over 5667 years of follow-up, respectively. The associations of baseline DBP (Table 2 and Figure S1, panels A-C) and MAP (Table 2 and Figure S2, panels A-C) with cardiovascular composite outcome and the effects of the SBP intervention on the cardiovascular composite outcome across the range of baseline DBP and MAP were similar to those observed with stroke outcome). The interaction p-values of baseline DBP and MAP with SBP intervention for cardiovascular composite outcome were 0.87 and 0.75, respectively. There was also no evidence of interaction between the BP intervention and baseline DBP for myocardial infarction outcome (Table 2, Figures S3 and S4).
Sensitivity analyses limiting to those with baseline hypertension (N = 2709):
These are presented in Table S1 and Figures S5–10. The results were similar to the main analyses.
Discussion:
The results of the current study indicate that in observational spline regression analyses, baseline DBP < 70 mmHg was associated with increased risk of stroke and cardiovascular events in persons with documented lacunar stroke. In those in the lowest baseline DBP tertile, intensive SBP lowering further lowered DBP to an achieved mean DBP of 65 ± 7 mmHg. However, there was no evidence that intensive control of blood pressure increased the risk of stroke recurrence or CVD composite in those with a low baseline DBP.
The associations of both low and high DBP with increased risk of myocardial infarction was described as “J-curve” phenomenon in the 1970’s22 and subsequently noted in several observational analyses23–28. Such associations of low DBP with increased risk of stroke have been reported as well14, 15. Biological plausibility of the associations of low DBP with increased risk of stroke can be explained by potential decrease in cerebral perfusion. As MAP is estimated to be the sum of 1/3rd of SBP and 2/3rd of DBP, in those with low DBP, lowering SBP can lead to decrease in MAP resulting in cerebral hypo-perfusion. This could be particularly relevant in patients with persistent hypertension that damages the vascular endothelium and thickens the vessel walls leading to predisposition towards subcortical ischemia29, 30. Thus, based on the concerns about decreasing cerebral perfusion in those with low DBP, it has been argued that intensive SBP lowering could have adverse neurological effects10.
Our analyses suggest that intensely controlling SBP to <130 mmHg in persons with baseline DBP <70 mmHg does not appear to increase the risk for stroke or CVD composite outcomes. Thus, the observational associations of low baseline DBP with stroke likely represents confounding due to factors such as increased arterial stiffness due to atherosclerosis which can result in low baseline DBP as well as increased risk of stroke.
Intensive SBP goal of < 120 mmHg compared to standard SBP goal of < 140 mmHg has been shown to reduce cardiovascular events and all-cause mortality in persons without diabetes in SPRINT7. The Action to Control Cardiovascular Risk in Diabetes Blood Pressure (ACCORD BP) trial tested similar SBP target as in SPRINT in addition to intensive or standard glycemic control in a 2 X2 factorial design in persons with type 2 diabetes mellitus31. In the primary analysis that combined the intensive and standard glycemia arms, ACCORD BP reported a nonsignificant 12% decreased risk of the primary cardiovascular composite, along with a significant 41% decreased risk of stroke for intensive versus standard SBP arms. A post-hoc analysis of ACCORD BP noted an interaction between intensive glycemia and intensive SBP interventions and that intensive SBP control significantly reduced the risk of primary cardiovascular composite in those on standard glycemic control32. In the subsequent Strategy of Blood Pressure Intervention in the Elderly Hypertensive Patients (STEP) trial, intensive SBP treatment target of 110 to <130 mm Hg compared to standard SBP treatment target of 130 to <150 mm Hg in persons with and without diabetes, intensive SBP lowering reduced cardiovascular events and incidence of stroke33. Thus, data from recent large randomized controlled trials support the current guidelines on SBP goal of < 130 mmHg in persons with and without diabetes.
The results from the current analyses on stroke from the SPS3 trial is consistent with emerging data from secondary analyses of BP interventional trials34–36 as well as Mandelian randomization studies37 that support the hypothesis that the associations of lower DBP with worse outcomes represents confounding and that low baseline DBP did not modify the beneficial effects of intensive SBP lowering on cardiovascular events and all-cause mortality in persons without diabetes34 and in those with diabetes on standard glycemic control35. Furthermore, In a randomized controlled trial of standard (systolic=130–140 mmHg) (N=56) or intensive (systolic<125 mmHg) (N=55) SBP lowering, intensive SBP had no significant effects on white matter integrity in patients with small vessel disease and history of lacunar stroke suggesting the safety of BP lowering in those with severe small vessel disease of the brain38. Hence, low baseline DBP should not be an impediment to guidelines8 recommended SBP goal of < 130 mmHg in persons with previous history of stroke.
While post-hoc analysis is a limitation of the current study, these analyses by examining whether baseline DBP modified the effects of the SBP intervention, still preserves the randomized comparisons. Nonetheless, as randomized controlled trials are not powered to detect interactions of the intervention with baseline factors, the power might be low to identify such interactions. As a result of low power, for comparison of the effect of the BP intervention on the risk of stroke in those in the lowest DBP tertile, the point estimate of the hazard ratio was 0.78 with 95% CI of 0.52 to 1.16. While, spline regression analysis suggested a possible reduction in stroke risk with the BP intervention in those with baseline DBP < 70 mmHg, up to a 16% higher risk of stroke with intensive SBP lowering cannot be excluded in those in the lowest DBP tertile. Furthermore, these results may not be generalizable to those with elevated SBP but DBP much lower than the range of DBP in the current study. In addition, these data may not apply to those in the acute or subacute period of stroke as the SPS3 trial included patients who had a lacunar stroke in the past 180 days.
In summary, while there are observational associations of low baseline DBP with increased risk of stroke, there was no evidence that low baseline DBP modified the effects of a SBP intervention on stroke or cardiovascular outcomes.
Perspectives:
Even though, low baseline DBP was associated with higher risk of stroke, this association is unlikely to be causal as a SBP intervention that further lowered DBP did not appear to increase the risk of stroke in those with low baseline DBP. Thus, low baseline DBP should not be a deterring factor for guidelines8 recommended SBP goal of < 130 mmHg in those with previous history of stroke.
Supplementary Material
Pathophysiologic Novelty and Significance.
What is new?
While there is an observational association of low baseline diastolic blood pressure with higher risk of stroke, intensive SBP goal of < 130 mmHg compared to SBP goal of < 140 mmHg did not increase the risk of stroke in people with low baseline diastolic blood pressure and a history of lacunar stroke.
What is Relevant?
It is unlikely that the “J-Curve” phenomenon of the associations of low baseline diastolic blood pressure with higher risk of stroke is a causal phenomenon.
What are the Pathophysiological Implications?
Low baseline diastolic blood pressure should not be an impediment in lowering systolic blood pressure to guidelines recommended goal of < 130 mmHg in those previous history of stroke.
Acknowledgements:
Statistical analyses and preparation of this manuscript were funded by grants from National Heart, Lung and Blood Institute (R21HL145494), National Institute of Diabetes, Digestive and Kidney Diseases (R01DK118219 and R21DK10657), and Veterans Administration Office of Rural Health (VA ORH-14398).
This research is based on the National Institute of Neurologic Disease and Stroke’s Archived Clinical Research data (Secondary Prevention of Small Subcortical Strokes Trial, Oscar Benavente, MD, and U01NS038529) received from the Archived Clinical Research Dataset web site (https://www.ninds.nih.gov/Current-Research/Research-Funded-NINDS/Clinical-Research/Archived-Clinical-Research-Datasets).
Footnotes
Disclosures:
The authors declare that they have no other relevant financial interests.
References:
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. Heart disease and stroke statistics-2017 update: A report from the american heart association. Circulation. 2017;135:e146–e603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ovbiagele B, Nguyen-Huynh MN. Stroke epidemiology: Advancing our understanding of disease mechanism and therapy. Neurotherapeutics. 2011;8:319–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang Q, Tong X, Schieb L, Vaughan A, Gillespie C, Wiltz JL, et al. Vital signs: Recent trends in stroke death rates—United States, 2000–2015. MMWR. Morbidity and mortality weekly report. 2017;66:933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics-2020 update: A report from the American Heart Association. Circulation. 2020;141:e139–e596 [DOI] [PubMed] [Google Scholar]
- 5.Hall MJ, Levant S, DeFrances CJ. Hospitalization for stroke in U.S. Hospitals, 1989–2009. NCHS Data Brief. 2012:1–8 [PubMed] [Google Scholar]
- 6.Benavente OR, Coffey CS, Conwit R, Hart RG, McClure LA, Pearce LA, et al. Blood-pressure targets in patients with recent lacunar stroke: The SPS3 randomised trial. Lancet. 2013;382:507–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sprint Research Group. A randomized trial of intensive versus standard blood-pressure control. New England Journal of Medicine. 2015;373:2103–2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Journal of the American College of Cardiology. 2018;71:e127–e248 [DOI] [PubMed] [Google Scholar]
- 9.McEvoy JW, Chen Y, Rawlings A, Hoogeveen RC, Ballantyne CM, Blumenthal RS, et al. Diastolic blood pressure, subclinical myocardial damage, and cardiac events: Implications for blood pressure control. Journal of the American College of Cardiology. 2016;68:1713–1722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saper CB. How low can you go? Ann Neurol. 2015;78:665–666 [DOI] [PubMed] [Google Scholar]
- 11.Cruickshank JM, Thorp JM, Zacharias FJ. Benefits and potential harm of lowering high blood pressure. Lancet. 1987;1:581–584 [DOI] [PubMed] [Google Scholar]
- 12.Farnett L, Mulrow CD, Linn WD, Lucey CR, Tuley MR. The j-curve phenomenon and the treatment of hypertension. Is there a point beyond which pressure reduction is dangerous? JAMA. 1991;265:489–495 [PubMed] [Google Scholar]
- 13.Messerli FH, Mancia G, Conti CR, Hewkin AC, Kupfer S, Champion A, et al. Dogma disputed: Can aggressively lowering blood pressure in hypertensive patients with coronary artery disease be dangerous? Annals of Internal Medicine. 2006;144:884–893 [DOI] [PubMed] [Google Scholar]
- 14.Irie K, Yamaguchi T, Minematsu K, Omae T. The j-curve phenomenon in stroke recurrence. Stroke. 1993;24:1844–1849 [DOI] [PubMed] [Google Scholar]
- 15.Rothwell PM, Howard SC, Spence JD. Relationship between blood pressure and stroke risk in patients with symptomatic carotid occlusive disease. Stroke. 2003;34:2583–2590 [DOI] [PubMed] [Google Scholar]
- 16.Kimm H, Mok Y, Lee SJ, Lee S, Back JH, Jee SH. The j-curve between diastolic blood pressure and risk of all-cause and cardiovascular death. Korean Circ J. 2018;48:36–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lip S, Tan LE, Jeemon P, McCallum L, Dominiczak AF, Padmanabhan S. Diastolic blood pressure j-curve phenomenon in a tertiary-care hypertension clinic. Hypertension. 2019;74:767–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Archived clinical research datasets. U.S. Department of Health and Human Services. at https://www.ninds.nih.gov/Current-Research/Research-Funded-NINDS/Clinical-Research/Archived-Clinical-Research-Datasets.) [Google Scholar]
- 19.Benavente OR, Hart RG, McClure LA, Szychowski JM, Coffey CS, Pearce LA. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012;367:817–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benavente OR, White CL, Pearce L, Pergola P, Roldan A, Benavente MF, et al. The secondary prevention of small subcortical strokes (SPS3) study. International Journal of Stroke. 2011;6:164–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526 [Google Scholar]
- 22.Stewart IM. Relation of reduction in pressure to first myocardial infarction in patients receiving treatment for severe hypertension. Lancet. 1979;1:861–865 [DOI] [PubMed] [Google Scholar]
- 23.Cruickshank J, Thorp J, Zacharias FJ. Benefits and potential harm of lowering high blood pressure The Lancet.1987;1:581–584 [DOI] [PubMed] [Google Scholar]
- 24.Messerli FH, Mancia G, Conti C, et al. Dogma disputed: Can aggressively lowering blood pressure in hypertensive patients with coronary artery disease be dangerous? Annals of Internal Medicine. 2006;144:884–893 [DOI] [PubMed] [Google Scholar]
- 25.Vidal-Petiot E, Ford I, Greenlaw N, Ferrari R, Fox KM, Tardif JC, et al. Cardiovascular event rates and mortality according to achieved systolic and diastolic blood pressure in patients with stable coronary artery disease: An international cohort study. Lancet. 2016;388:2142–2152 [DOI] [PubMed] [Google Scholar]
- 26.Sim JJ, Shi J, Kovesdy CP, Kalantar-Zadeh K, Jacobsen SJ. Impact of achieved blood pressures on mortality risk and end-stage renal disease among a large, diverse hypertension population. Journal of the American College of Cardiology. 2014;64:588–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bangalore S, Messerli FH, Wun C-C, Zuckerman AL, DeMicco D, Kostis JB, et al. J-curve revisited: An analysis of blood pressure and cardiovascular events in the Treating to New Targets (TNT) Trial. European Heart Journal. 2010;31:2897–2908 [DOI] [PubMed] [Google Scholar]
- 28.Kang YY, Wang JG. The j-curve phenomenon in hypertension. Pulse. 2016;4:49–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wardlaw JM. What causes lacunar stroke? Journal of Neurology, Neurosurgery & Psychiatry. 2005;76:617–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lammie GA. Pathology of small vessel stroke. British Medical Bulletin. 2000;56:296–306 [DOI] [PubMed] [Google Scholar]
- 31.Accord Study Group, Cushman WC, Evans GW, Byington RP, Goff DC Jr., Grimm RH Jr., et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beddhu S, Chertow GM, Greene T, Whelton PK, Ambrosius WT, Cheung AK, et al. Effects of intensive systolic blood pressure lowering on cardiovascular events and mortality in patients with type 2 diabetes mellitus on standard glycemic control and in those without diabetes mellitus: Reconciling results from ACCORD BP and SPRINT. J Am Heart Assoc. 2018;7:e009326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang W, Zhang S, Deng Y, Wu S, Ren J, Sun G, et al. Trial of intensive blood-pressure control in older patients with hypertension. New England Journal of Medicine. 2021;385:1268–1279 [DOI] [PubMed] [Google Scholar]
- 34.Beddhu S, Chertow GM, Cheung AK, Cushman WC, Rahman M, Greene T, et al. Influence of baseline diastolic blood pressure on effects of intensive compared to standard blood pressure control. Circulation. 2018;137:134–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ilkun OL, Greene T, Cheung AK, Whelton PK, Wei G, Boucher RE, et al. The influence of baseline diastolic blood pressure on the effects of intensive blood pressure lowering on cardiovascular outcomes and all-cause mortality in type 2 diabetes. Diabetes Care. 2020;43:1878–1884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kalkman DN, Brouwer TF, Vehmeijer JT, Berger WR, Knops RE, de Winter RJ, et al. J curve in patients randomly assigned to different systolic blood pressure targets: An experimental approach to an observational paradigm. Circulation. 2017;136:2220–2229 [DOI] [PubMed] [Google Scholar]
- 37.Arvanitis M, Qi G, Bhatt DL, Post WS, Chatterjee N, Battle A, et al. Linear and nonlinear mendelian randomization analyses of the association between diastolic blood pressure and cardiovascular events: The j-curve revisited. Circulation. 2021;143:895–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Markus HS, Egle M, Croall ID, Sari H, Khan U, Hassan A, et al. PRESERVE: Randomized trial of intensive versus standard blood pressure control in small vessel disease. Stroke. 2021;52:2484–2493 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


