Abstract
Background
Enthusiasm for precision oncology may obscure the psychosocial and ethical considerations associated with the implementation of tumor genetic sequencing.
Methods
Patients with advanced cancer undergoing tumor‐only genetic sequencing in the National Cancer Institute Molecular Analysis for Therapy Choice (MATCH) trial were randomized to a web‐based genetic education intervention or usual care. The primary outcomes were knowledge, anxiety, depression, and cancer‐specific distress collected at baseline (T0), posteducation (T1) and after results (T2). Two‐sided, 2‐sample t tests and univariate and multivariable generalized linear models were used.
Results
Five hundred ninety‐four patients (80% from NCI Community Oncology Research Program sites) were randomized to the web intervention (n = 293) or usual care (n = 301) before the receipt of results. Patients in the intervention arm had greater increases in knowledge (P for T1‐T0 < .0001; P for T2‐T0 = .003), but there were no significant differences in distress outcomes. In unadjusted moderator analyses, there was a decrease in cancer‐specific distress among women (T0‐T1) in the intervention arm but not among men. Patients with lower health literacy in the intervention arm had greater increases in cancer‐specific distress and less decline in general anxiety (T0‐T1) and greater increases in depression (T0‐T2) in comparison with those receiving usual care.
Conclusions
Web‐based genetic education before tumor‐only sequencing results increases patient understanding and reduces distress in women. Refinements to the intervention could benefit low‐literacy groups and men.
Keywords: genetic education, online education intervention, precision oncology, tumor sequencing
Short abstract
In the Communication and Education in Tumor Profiling (COMET) study, patients with advanced cancer undergoing tumor genetic sequencing in the National Cancer Institute Molecular Analysis for Therapy Choice (MATCH) trial have been randomized to a web‐based genetic education intervention or usual care. Web‐based genetic education has resulted in increased patient understanding and reduced distress in female patients with cancer.
Introduction
With the advent of massively parallel sequencing, genetic aberrations in tumors can be used to identify targeted therapies to improve cancer outcomes. 1 , 2 , 3 Yet, enthusiasm for precision oncology may obscure the complex psychosocial and ethical considerations associated with implementing tumor sequencing in precision oncology care. 4 , 5
Although pretest genetic counseling with a genetic provider is recommended for germline testing, there are no guidelines for pretest counseling before tumor genetic testing. The American Society of Clinical Oncology and others have recommended that the potential for identifying incidental germline findings be shared with patients in advance of tumor testing, 6 , 7 but how best to communicate this remains unclear. Several studies suggest that patients with advanced cancer have poor genetic knowledge and understanding of the difference between tumor and germline testing and high expectations of benefit. 8 , 9 , 10 , 11 , 12 Qualitative studies have reported patient disappointment, distress, and loss of hope after tumor genetic testing. 10 , 12 This suggests a need for improved pretest education for tumor genetic testing to align expectations and improve patient outcomes. 9 , 10 , 11 The limited workforce of genetic providers and the time sensitivity of results for treatment planning require novel and timely genetic delivery strategies to deliver patient‐centered, high‐quality precision oncology care.
To address this need, we developed a web‐based genetic education intervention for patients with advanced cancer undergoing tumor genetic testing to increase genetic knowledge and decrease distress and to reduce the burden on oncology providers.
Materials and Methods
The study protocol was approved by the appropriate institutional review board. Communication and Education in Tumor Profiling (COMET) was a randomized trial of web‐based genetic education versus usual care in patients with advanced cancer undergoing tumor‐only sequencing coordinated by the ECOG‐ACRIN Cancer Research Group (NCT02823652) and conducted through the ECOG‐ACRIN NCI Community Oncology Research Program (NCORP) Research Base. The COMET intervention is a theoretically informed, user‐tested, self‐directed, mobile‐ready genetic education intervention. Based on the tiered‐binned model, the intervention includes “indispensable” tier 1 information for all users and tier 2 information providing additional material to support variable information needs (see Table 1). 13
TABLE 1.
The Multimodality COMET eHealth Education Intervention
| Module | Tier 1 Written Content (No. of Screens) | Tier 2 Written Content (No. of Screens) | Tier 2 Videos [min] a |
|---|---|---|---|
| 1. Introduction |
|
|
|
| 2. Genetics and cancer |
|
|
|
| 3. Tumor genetic testing |
|
|
|
| 4. Results and implications |
|
|
|
| 5. Benefits, risks, and limitations |
|
|
|
| 6. Review |
|
Abbreviation: COMET, Communication and Education in Tumor Profiling.
The intervention is informed by the tiered‐binned model for genetic education and informed consent and was user‐tested with 7 patients with advanced cancer (age range of 39‐73 years, 5 females and 2 males, 1 non‐White patient, 6 patients with less than a college degree, and a range of cancer types). The linear intervention includes 6 modules and 4 optional videos, but participants can view modules for as long and as many times as desired and go back to previously viewed topics.
Videos include a genetic counselor explaining specific topics. The content in the videos is intentionally redundant to tier 1 and tier 2 content and is designed to provide an alternative method for reviewing content for patients with different learning preferences.
A link is provided to publicly available educational information (National Cancer Institute website).
A link is provided to publicly available educational information (Penn Medicine Diagnostics).
The primary aim was to evaluate the efficacy of web‐based genetic education to increase genetic knowledge and decrease distress in comparison with usual care. The secondary aim was to evaluate potential moderators of changes in knowledge and distress.
Recruitment
A total of 594 patients enrolled from September 2016 to May 2019. The first 194 patients (145 from NCORP community sites, 28 from academic sites, and 21 from other National Clinical Trials Network–affiliated sites) from a total of 74 sites were recruited through the National Cancer Institute Molecular Analysis for Therapy Choice (NCI‐MATCH) trial (EAY131, MATCH cohort). 3 , 14 English‐speaking adults who were enrolled in NCI‐MATCH with email and web access and had not received their tumor genetic test results were eligible. In July 2017, NCI‐MATCH met its sequencing goal, and patients enrolled after July 2017 received their sequencing results before enrollment and thus were not eligible for COMET. 3 To meet the accrual goal, COMET recruited an additional 400 patients (including 328 from NCORP sites, 69 from academic sites, and 3 from National Clinical Trials Network–affiliated sites) who met NCI‐MATCH eligibility criteria, consented to site‐based tumor sequencing, and had not yet received tumor sequencing results from 37 sites (non‐MATCH cohort).
Study Procedures
Eligible patients were identified by research staff at the time of their enrollment in MATCH or at their consent to tumor sequencing. Participants completed informed consent forms and were registered in the Oncology Patient Enrollment Network and ECOG‐ACRIN's Systems for Easy Entry of Patient‐Reported Outcomes for the completion of patient‐reported outcome (PRO) measures. Participants were randomly assigned 1:1 (stratified by gender [male vs female], race [White vs others], age [≤65 vs >65 years], and education level [high school or less vs some college vs post‐bachelor]) to the intervention or usual‐care arm via a permuted block design and were asked to complete a baseline survey (T0).
Arm A (intervention) participants received an email to log in to the intervention and to access a posteducation survey (T1) after the web education intervention. For those who did not complete the web education, the survey was sent to participants 9 days after completion of the T0 survey.
Arm B (usual care) was intended as a real‐world comparison group and consisted of any usual‐care education from providers or information sought through usual resources. Arm B participants received the T1 survey 6 days after the baseline survey to align with the arm A postintervention survey (which was also completed by arm A participants who did not access the intervention) and account for changes in outcomes that could occur with time and usual‐care information.
The disclosure of tumor test results occurred according to usual clinical practice and was not prescribed per protocol because of a lack of established standards for the sharing of results. Results were typically provided by the oncologist, although other staff may have shared results. The postdisclosure survey (T2) was completed after site research staff recorded result disclosure. If disclosure was not entered, the survey was launched 49 days after T0 to allow 2 to 3 weeks for results to return and for providers to disclose results. Before T2 survey completion, patients were asked if they had received their results. If they responded yes, they proceeded with survey completion. If they said no, they were asked to return to complete the survey after disclosure and were sent 3 weekly reminders to reassess if disclosure had occurred. They could also complete the T2 survey if results were not disclosed.
Primary Outcomes
Outcomes were informed by our theoretical model, which was informed by the Self‐Regulation Theory of Health Behavior 15 , 16 and included potential benefits and risks of receiving genetic information (eg, misunderstanding of results, distress, and uncertainty; Supporting Table 1a). Primary outcomes included the following:
-
1.
Genetic knowledge (T0‐T2) was evaluated with an adapted version of the ClinSeq knowledge scale 17 modified for tumor genetic testing (see Supporting Table 1b). Tumor sequencing was novel when this trial was designed, and this necessitated the adaptation of an existing scale.
-
2.
General anxiety and
-
3.
depression (T0‐T2) were assessed with 4‐item short forms of the Patient‐Reported Outcomes Measurement Information System (PROMIS). 18 , 19
-
4.
State anxiety (T0‐T2) was assessed with 20 items from the State‐Trait Anxiety Inventory. 20
-
5.
Cancer‐specific distress (T0‐T2) was measured with an adapted 14‐item version of the Impact of Events Scale. 21 , 22
Moderators and Secondary Outcomes
Health literacy (T0) was assessed with a 3‐item scale. 23 Higher scores indicated lower health literacy.
Uncertainty about tumor genetic testing (T0‐T2) was assessed with a 3‐item scale adapted for tumor genetic testing from the uncertainty subscale of the Multi‐Dimensional Impact of Cancer Risk Assessment questionnaire. 24
The perceived utility of tumor genetic testing (T0‐T2) was assessed with 10 items assessing patient perceptions of the utility of genetic information and used in related research. 25
Satisfaction with the disclosure of genetic results (T2 only) was assessed with an adapted 9‐item scale used in related research. 25 , 26
Analysis
The primary end points included changes in genetic knowledge and distress (anxiety, depression, state anxiety, and cancer‐specific distress) from the baseline to the postintervention period (from T0 to T1) and from the baseline to the postdisclosure period (from T0 to T2). Preliminary data from the COMET pilot study (state anxiety, cancer‐specific distress, and knowledge), together with published data on PROMIS depression and anxiety, 27 were used to calculate power. A sample size of 200 patients (with evaluable paired data) per arm would provide at least 91% power for differences between arms in mean change scores for all outcomes from T0 to T1. With a lower response rate expected at T2, 150 patients (with evaluable paired data) per arm would give 72% power for differences in changes in knowledge and 95% power for differences in changes in cancer‐specific distress from T0 to T2. The aforementioned calculations used t tests at a 2‐sided significance level of .005 (adjusted for 10 primary analyses with a Bonferroni correction). Supporting Table 2 provides mean change scores and standard deviations for the primary end points.
Only patients who completed the baseline survey were included in the data analysis. For each of the 10 primary outcome measures, the change was defined as the T1 score or T2 score minus the T0 score. A 2‐sample t test was used to evaluate the intervention effect on the changes in cognitive and affective responses. A P value < .005 was considered statistically significant for tests on these primary outcomes (in light of the Bonferroni correction described previously). Per the study protocol, an intention‐to‐treat approach was used to evaluate the intervention effect, regardless of intervention uptake, unless otherwise specified. Linear regression was used to further evaluate the intervention effect, with adjustments made for potential baseline confounders (defined as factors unbalanced between arms at a P value < .1). To evaluate potential moderator effects on these change scores, 2‐way interactions of arm and patient factors, including age (>65 vs ≤65 years), sex, race (White vs others), highest education (high school or less vs some college vs post‐bachelor), health literacy score at the baseline (on a continuous scale), and its individual factor, were fitted into generalized linear models separately. Models with significant 2‐way interaction terms were further confirmed individually, with adjustments made for covariates (including patient characteristics and the target PRO outcome score at the baseline). Fisher exact tests were used to test the association between categorical variables. Two‐sample t tests were used to test differences in continuous variables. A P value of .05 was considered statistically significant in the analysis of moderators and secondary outcomes because these were exploratory in nature. All P values were 2‐sided. SAS 9.4 (SAS Institute, Inc, Cary, North Carolina) was used for analyses.
Results
Because there were no significant differences in the baseline characteristics and primary outcomes between the cohort enrolled in MATCH and those after MATCH closed (the non‐MATCH cohort), the results reported here are based on patients across cohorts.
Study Participants
Five hundred ninety‐four participants were randomized (293 to the intervention arm and 301 to the usual‐care arm), and 473 (80%) were from NCORP sites. Seventy‐nine percent of the participants completed the baseline survey, with no difference seen in response rates between arms (see Fig. 1). The median age of the participants was 64 years, 56% were women, 94% were White, 45% had a common cancer, and 36% reported a high school education or less (Table 2). There were no significant differences in baseline characteristics between arms.
Figure 1.
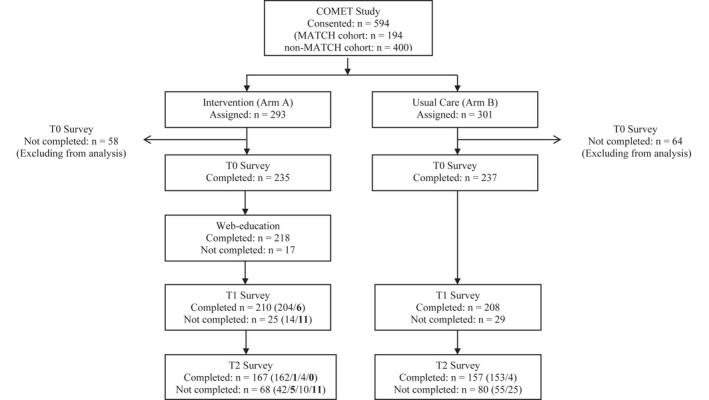
Consolidated Standards of Reporting Trials diagram. Values in parentheses refer to the number of patients from preceding boxes, with each value corresponding to the row in order. Bolded values refer to the number of participants who did not receive the assigned web education intervention. COMET indicates Communication and Education in Tumor Profiling; MATCH, Molecular Analysis for Therapy Choice; T0, baseline; T1, posteducation; T2, after result disclosure.
TABLE 2.
Participant Characteristics at the Baseline by Arm Assigned and by Accrual Source
| Patient Characteristic | Arm Assigned | Accrual Source | Total (n = 472) | ||
|---|---|---|---|---|---|
| Web Education (n = 235) | Usual Care (n = 237) | MATCH (n = 145) | Non‐MATCH (n = 327) a | ||
| Health literacy, mean (SD) b | 2.5 (2.6) | 2.9 (2.7) | 2.9 (2.6) | 2.7 (2.7) | 2.7 (2.7) |
| Age, mean (SD), y | 62.6 (10.5) | 63.0 (11.3) | 61.0 (10.8) c | 63.6 (10.9) c | 62.8 (10.9) |
| Age, No. (%) | |||||
| >65 y | 104 (44.3) | 106 (44.7) | 54 (37.2) d | 156 (47.7) d | 210 (44.5) |
| ≤65 y | 131 (55.7) | 131 (55.3) | 91 (62.8) | 171 (52.3) | 262 (55.5) |
| Sex, No. (%) | |||||
| Male | 101 (43.0) | 107 (45.1) | 56 (38.6) | 152 (46.5) | 208 (44.1) |
| Female | 134 (57.0) | 130 (54.9) | 89 (61.4) | 175 (53.5) | 264 (55.9) |
| Race, No. (%) | |||||
| White | 218 (94.0) | 221 (94.4) | 133 (91.7) | 306 (95.3) | 439 (94.2) |
| African American | 8 (3.4) | 9 (3.9) | 8 (5.5) | 9 (2.8) | 17 (3.7) |
| Other | 6 (2.6) | 4 (1.7) | 4 (2.8) | 6 (1.9) | 10 (2.1) |
| Unknown | 3 (—) | 3 (—) | 0 (—) | 6 (—) | 6 (—) |
| Ethnicity, No. (%) | |||||
| Hispanic | 1 (0.4) | 5 (2.1) | 2 (1.4) | 4 (1.2) | 6 (1.3) |
| Non‐Hispanic | 229 (99.6) | 229 (97.9) | 142 (98.6) | 316 (98.8) | 458 (98.7) |
| Unknown | 5 (—) | 3 (—) | 1 (—) | 7 (2.1) | 8 (—) |
| Highest education, No. (%) | |||||
| High school or less | 82 (34.9) | 88 (37.1) | 49 (33.8) | 121 (37.0) | 170 (36.0) |
| Some college/bachelor | 123 (52.3) | 119 (50.2) | 81 (55.9) | 161 (49.2) | 242 (51.3) |
| Post‐bachelor | 30 (12.8) | 30 (12.7) | 15 (10.3) | 45 (13.8) | 60 (12.7) |
| Cancer type, No. (%) | |||||
| Common cancers | |||||
| Lung (NSCLC/NOS) | 33 (14.0) | 37 (15.6) | 14 (9.7) | 56 (17.1) | 70 (14.8) |
| Colorectal | 37 (15.7) | 30 (12.6) | 32 (22.1) | 35 (10.7) | 67 (14.2) |
| Breast | 22 (9.4) | 31 (13.1) | 17 (11.7) | 36 (11.0) | 53 (11.2) |
| Prostate | 11 (4.7) | 13 (5.5) | 3 (2.1) | 21 (6.4) | 24 (5.1) |
| Common cancer subtotal | 103 (43.8) | 111 (46.8) | 66 (45.5) | 148 (45.3) | 214 (45.3) |
| Uncommon cancers | |||||
| Pancreatic | 13 (5.5) | 21 (8.9) | 9 (6.2) | 25 (7.6) | 34 (7.2) |
| Ovarian | 13 (5.5) | 22 (9.3) | 11 (7.6) | 24 (7.3) | 35 (7.4) |
| Head and neck | 14 (6.0) | 11 (4.6) | 6 (4.1) | 19 (5.8) | 25 (5.3) |
| Esophageal/GE junction/gastric | 14 (6.0) | 6 (2.5) | 5 (3.4) | 15 (4.6) | 20 (4.2) |
| Endometrial/uterine (nonsarcoma) | 13 (5.5) | 7 (3.0) | 4 (2.8) | 16 (4.9) | 20 (4.2) |
| Kidney/renal cell | 9 (3.8) | 9 (3.8) | 3 (2.1) | 15 (4.6) | 18 (3.8) |
| GYN, other | 10 (4.3) | 4 (1.7) | 8 (5.5) | 6 (1.8) | 14 (3.0) |
| Sarcoma | 8 (3.4) | 5 (2.1) | 3 (2.1) | 10 (3.1) | 13 (2.8) |
| Neuroendocrine | 4 (1.7) | 8 (3.4) | 7 (4.8) | 5 (1.5) | 12 (2.6) |
| Other | 24 (10.2) | 27 (11.4) | 15 (10.3) | 36 (11.0) | 51 (10.8) |
| Primary site not specified | 10 (4.3) | 6 (2.5) | 8 (5.5) | 8 (2.4) | 16 (3.4) |
| Uncommon cancer subtotal | 132 (56.2) | 126 (53.2) | 79 (54.5) | 179 (54.7) | 258 (54.7) |
Abbreviations: GE, gastroesophageal; GYN, gynecological; NCI‐MATCH, National Cancer Institute Molecular Analysis for Therapy Choice; NOS, not otherwise specified; NSCLC, non–small cell lung carcinoma; SD, standard deviation.
This table represents data of participants who completed the baseline survey.
Four hundred patients who met NCI‐MATCH eligibility criteria, consented to site‐based tumor sequencing, and had not yet received tumor sequencing results from 37 sites.
Scores were missing for 12 patients (5 in arm A and 7 in arm B). The possible range was 0 to 12; higher scores indicated lower health literacy.
There was a significant difference in age between MATCH and non‐MATCH patients (P = .02).
There was a significant difference in age distribution between MATCH and non‐MATCH patients (P = .04).
Among the participants in the intervention arm who completed the T0 survey, 93% (n = 218) completed web education. The T1 survey was completed by 210 and 208 participants in arm A (89%) and arm B (88%), respectively. Before the T2 survey, 72% of the participants reported having received test results (73% in arm A and 70% in arm B; P = .56). In total, 167 participants (71%) in arm A and 157 participants (66%) in arm B completed the T2 survey (P = .28).
Efficacy of the Web‐Based Intervention to Increase Knowledge and Decrease Distress
In our primary intention‐to‐treat analyses for the T0 to T1 change, there was a significant increase in knowledge in the intervention arm (P < .0001; Fig. 2A). There were no significant differences in distress outcomes (Fig. 2A). Findings were similar in primary intention‐to‐treat analyses for the T0 to T2 change (P = .003 for knowledge; Fig. 2B). Because none of the baseline patient characteristics met the predefined criteria for potential confounders, no multivariable analysis adjusting for covariates was performed. Mean scores of the primary outcomes by arm and time point are shown in Supporting Table 3; mean change scores by arm are shown in Supporting Table 4; and outcomes by age, sex, and education are shown in Supporting Table 5. Results were similar when we conducted sensitivity analyses accounting for survey timing and the as‐treated approach (ie, intervention received; results not shown).
Figure 2.
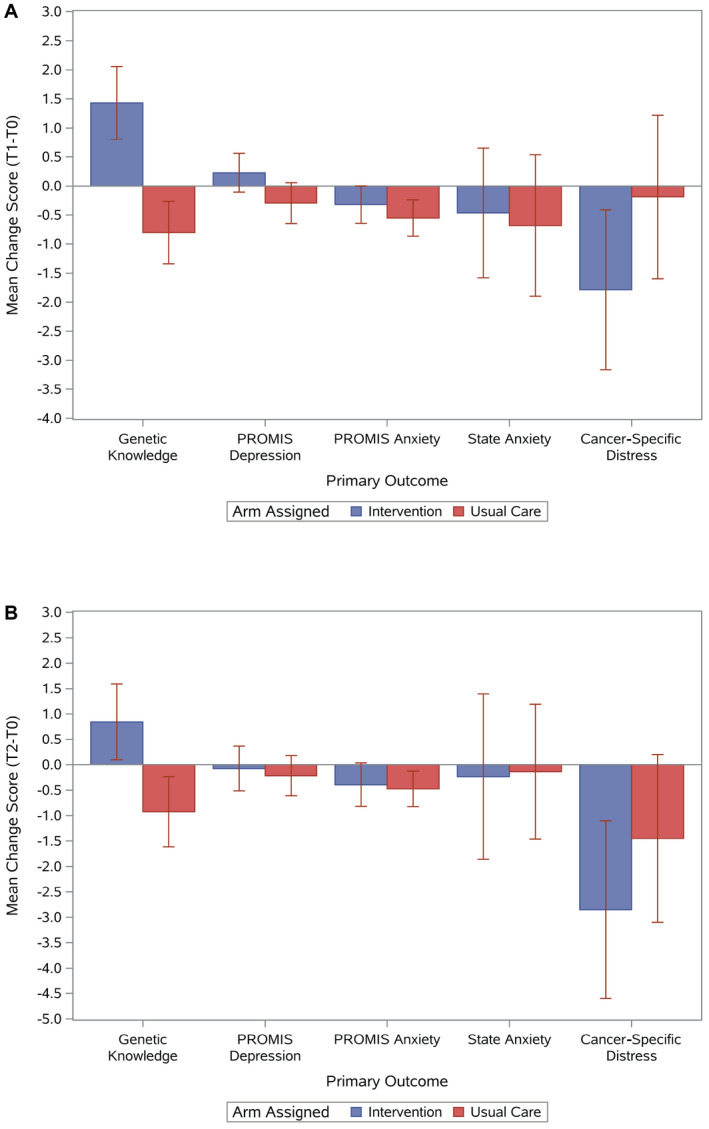
Mean change scores and 95% confidence intervals by assigned arm and outcome: (A) mean change scores (T1‐T0) and (B) mean change scores (T2‐T0). Positive scores are a favorable outcome for genetic knowledge (eg, increased knowledge), whereas negative scores are favorable for depression, anxiety, and cancer‐specific distress (eg, reduced depression, anxiety, or distress).
Moderators of Changes in Knowledge and Distress
In unadjusted moderator analyses evaluating patient factors that could affect changes in cognitive and affective outcomes, only outcomes with significant moderators are further described here. There was a greater decrease in cancer‐specific distress for women from T0 to T1 among those in the intervention arm versus those receiving usual care (−2.4 vs 0.9; P = .01), but there was no effect in men (P = .052 for the 2‐way interaction; Fig. 3). Patients with lower health literacy in the intervention arm had 1) more increase you in cancer‐specific distress (slope = 0.85; P = .003 against 0; R 2 = 0.053)—but there were no significant changes in the usual‐care arm (slope = −0.26; P = .32 against 0; R 2 = 0.005; P = .004 for the 2‐way interaction)—and 2) no significant changes in general anxiety (slope = 0.06; P = .32 against 0; R 2 = 0.005)—but there were slight decreases in the usual‐care arm (slope = −0.11; P = .07 against 0; R 2 = 0.018; P = .049 for the 2‐way interaction)—from T0 to T1 (Fig. 4). Patients with lower health literacy in the intervention arm had greater increases in depression (slope = 0.22; P = .01 against 0; R 2 = 0.037), but there were no changes in the usual‐care arm (slope = −0.06; P = .47 against 0; R 2 = 0.004; P = .02 for the 2‐way interaction), from T0 to T2. These conclusions were further confirmed with controlling for covariates and in the as‐treated analysis (results not shown). Baseline cancer‐specific distress and anxiety were higher for those with lower health literacy than those with higher health literacy (slope = 1.09 and slope = 0.24, respectively; both P values < .0001 against 0). In secondary models including baseline distress scores, the 2‐way interaction of health literacy and treatment arm remained significant (P = .03), and this suggests that the literacy moderator effect cannot be attributed to associated anxiety/depression or uncertainty.
Figure 3.
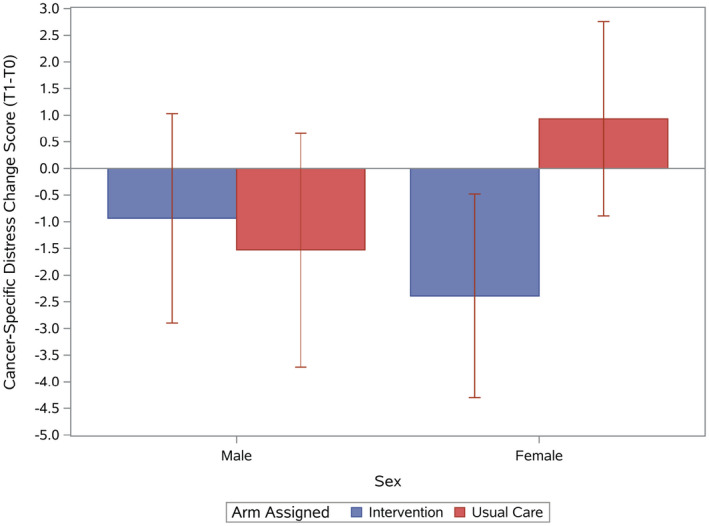
Cancer‐specific distress mean change scores (T1‐T0) and 95% confidence intervals by sex and assigned arm.
Figure 4.
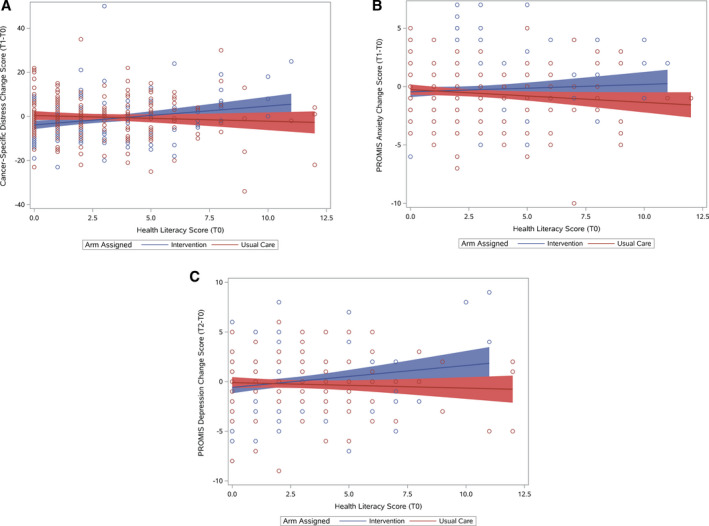
Affective outcomes by health literacy and arm: (A) cancer‐specific distress mean change scores (T1‐T0) by health literacy and arm, (B) PROMIS anxiety mean change scores (T1‐T0) by health literacy and arm, and (C) PROMIS depression mean change scores (T2‐T0) by health literacy and arm. Regression lines with 95% confidence intervals are shown by assigned arm. PROMIS indicates Patient‐Reported Outcomes Measurement Information System.
Secondary Outcomes by Arm
There were no significant differences in the change in uncertainty or perceived utility by arm (Supporting Table 2). Satisfaction did not differ significantly between arms in the intention‐to‐treat analysis or among those who received results (by research staff or patient report separately).
Discussion
In this large, multicenter, randomized trial, which included a representative patient population in community and academic practices, we have demonstrated that web‐based genetic education increases knowledge by providing a clinically relevant eHealth tool to increase patient understanding of tumor genetic testing. Additionally, high intervention use demonstrates that it is a salient and easily accessible intervention for patients with advanced cancer.
Although there is a large literature on outcomes with genetic counseling for germline testing, there are no prospective data on patient outcomes with tumor genetic testing. Although we did not find a reduction in distress with the intervention, secondary analyses identified a reduction in cancer‐specific distress for women in the intervention arm. Studies have shown that 24% to 45% of patients with advanced cancer have distress. 28 , 29 , 30 Furthermore, screening for distress and interventions targeted to reduce distress in this population have had variable results. 31 , 32 Thus, it may be difficult to achieve distress reductions in patients with advanced cancer through genetic education alone. Although there are no quantitative studies evaluating distress after tumor genetic results, data in germline testing show transient increases in cancer‐specific distress after the receipt of a positive result and reductions for patients receiving negative results. 33 Our data, showing reduced cancer‐specific distress for women in the intervention arm but not in the cohort as a whole, point to opportunities for refinements to the intervention to better address the affective needs of patients with advanced cancer receiving tumor genetic results.
We also found that patients with lower health literacy in the intervention group had transient increases in cancer‐specific distress and less reduction in anxiety. Although these differences were seen only from the baseline to the postintervention period, if the intervention were to be used clinically, careful attention to psychosocial responses among lower literacy patients would be recommended. It is possible that the complexity of information in the intervention generated confusion and led to greater distress. Alternatively, the intervention emphasized that testing may, or may not, help to guide treatment, and a better understanding of the limitations of testing could have led to distress. Lower literacy patients also had higher distress at the baseline, and this suggests that this population may benefit from additional support in general. Additional research focused on understanding the experience of lower literacy patients with cancer could inform modifications to address their unique needs. 9
Another clinically important finding in this study is the complexity of evaluating how and when tumor genetic results are shared in clinical practice. Because these results are critical to treatment decisions, they are often shared by the medical oncologist. Although this study allowed us to evaluate usual practices across a national representation of clinical practices, there likely was variability in how practices shared results. We found that patients frequently did not recall “disclosure of results.” Additional research evaluating provider variability, patient preferences, and how best to share results is needed. Furthermore, high use of this web‐based intervention indicates that eHealth platforms could help to support providers and patients in sharing genetic results.
Although the study has several strengths, including the large, representative population of patients, the majority of whom were treated in community‐based practices, the randomized design, and robust PROs, we acknowledge several limitations. We could not collect information on decliners, we excluded those without internet access, and representation among minority non‐White groups was low. MATCH had relatively few actionable results, and outcomes could vary by test result. 4 , 34 The intervention was designed for tumor‐only sequencing, and outcomes could be different with paired sequencing or germline testing. It was difficult to assess result disclosure, and this resulted in lower completion rates for the postdisclosure outcomes and could have affected PROs. Given the lack of an existing knowledge scale for tumor sequencing, we had to adapt an existing scale used for germline testing. The magnitude of the increase in knowledge was small, and further validation of this measure, including defining thresholds for meaningful change, would be valuable.
In summary, web‐based genetic education before the receipt of tumor genetic test results increased patient understanding. Although the intervention did not significantly reduce distress, women had reductions in cancer‐specific distress. Future refinements to the web intervention could benefit low‐literacy groups and men.
Funding Support
This study was conducted by the ECOG‐ACRIN Cancer Research Group (Peter J. O'Dwyer, MD, and Mitchell D. Schnall, MD, PhD, group cochairs) and was supported by the National Cancer Institute of the National Institutes of Health under the following award numbers: UG1CA189816, UG1CA189819, UG1CA189828, UG1CA189830, UG1CA189863, UG1CA189956, UG1CA190140, UG1CA233160, UG1CA233180, UG1CA189809, and U10CA180821. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Mention of trade names, commercial products, or organizations does not imply endorsement by the US government.
Conflict of Interest Disclosures
Angela R. Bradbury reports research support and consulting fees from AstraZeneca and Merck. Angela M. DeMichele reports institutional research support from Pfizer, Genentech, Novartis, and Calithera. Kara N. Maxwell reports payment to her institution from the National Cancer Institute (K08CA215312). Keith T. Flaherty reports grants or contracts from Novartis and Sanofi; consulting fees from Loxo Oncology, Clovis Oncology, Strata Oncology, Vivid Biosciences, Checkmate Pharmaceuticals, Kinnate Pharmaceuticals, Scorpion Therapeutics, X4 Pharmaceuticals, PIC Therapeutics, Sanofi, Amgen, Asana, Adaptimmune, Aeglea, Shattuck Labs, Tolero, Apricity, Oncoceutics, Fog Pharma, Neon, Tvardi, xCures, Monopteros, Vibliome, ALX Oncology, Lilly, Novartis, Genentech, Bristol‐Myers Squibb, Merck, Takeda, Verastem, Boston Biomedical, Pierre Fabre, and Debiopharm; participation on boards for X4 Pharmaceuticals, PIC Therapeutics, Sanofi, Amgen, Asana, Adaptimmune, Aeglea, Shattuck Labs, Tolero, Apricity, Oncoceutics, Fog Pharma, Neon, Tvardi, xCures, Monopteros, Vibliome, ALX Oncology, Lilly, Novartis, Genentech, Bristol‐Myers Squibb, Merck, Takeda, Verastem, Boston Biomedical, Pierre Fabre, and Debiopharm; leadership or fiduciary roles with Loxo Oncology, Clovis Oncology, Strata Oncology, Vivid Biosciences, Checkmate Pharmaceuticals, Kinnate Pharmaceuticals, and Scorpion Therapeutics; and stock or stock options in Loxo Oncology, Clovis Oncology, Strata Oncology, Vivid Biosciences, Checkmate Pharmaceuticals, Kinnate Pharmaceuticals, Scorpion Therapeutics, X4 Pharmaceuticals, PIC Therapeutics, Apricity, Oncoceutics, Fog Pharma, Tvardi, xCures, Monopteros, Vibliome, and ALX Oncology. Stefan C. Grant reports funding support from Guardant Health and a stipend for serving as vicechair of a steering committee for the Precision Medicine Exchange Consortium. Ruth C. Carlos reports travel reimbursement from ECOG‐ACRIN, RSNA, ARRS, and ARBIR; participation on a data safety monitoring board for ECOG‐ACRIN; stock or stock options in ARBIR; and salary support from JACR. Lynne I. Wagner reports consulting fees from Celgene (Bristol‐Myers Squibb) and Athenex. The other authors made no disclosures.
Author Contributions
Angela R. Bradbury: Study concept and design; drafting of the manuscript; acquisition, analysis, or interpretation of data; and critical revision of the manuscript for important intellectual content. Ju‐Whei Lee: Study concept and design; drafting of the manuscript; statistical analysis; acquisition, analysis, or interpretation of data; and critical revision of the manuscript for important intellectual content. Jill Bennett Gaieski: Drafting of the manuscript; acquisition, analysis, or interpretation of data; and critical revision of the manuscript for important intellectual content. Shuli Li: Acquisition, analysis, or interpretation of data and critical revision of the manuscript for important intellectual content. Ilana F. Gareen: Acquisition, analysis, or interpretation of data and critical revision of the manuscript for important intellectual content. Keith T. Flaherty: Acquisition, analysis, or interpretation of data and critical revision of the manuscript for important intellectual content. Benjamin A. Herman: Acquisition, analysis, or interpretation of data and critical revision of the manuscript for important intellectual content. Susan M. Domchek: Acquisition, analysis, or interpretation of data and critical revision of the manuscript for important intellectual content. Angela M. DeMichele: Acquisition, analysis, or interpretation of data and critical revision of the manuscript for important intellectual content. Kara N. Maxwell: Acquisition, analysis, or interpretation of data and critical revision of the manuscript for important intellectual content. Adedayo A. Onitilo: Acquisition, analysis, or interpretation of data and critical revision of the manuscript for important intellectual content. Shamsuddin Virani: Acquisition, analysis, or interpretation of data and critical revision of the manuscript for important intellectual content. SuJung Park: Acquisition, analysis, or interpretation of data and critical revision of the manuscript for important intellectual content. Bryan A. Faller: Acquisition, analysis, or interpretation of data and critical revision of the manuscript for important intellectual content. Stefan C. Grant: Acquisition, analysis, or interpretation of data and critical revision of the manuscript for important intellectual content. Ryan C. Ramaekers: Acquisition, analysis, or interpretation of data and critical revision of the manuscript for important intellectual content. Robert J. Behrens: Acquisition, analysis, or interpretation of data and critical revision of the manuscript for important intellectual content. Gopakumar S. Nambudiri: Acquisition, analysis, or interpretation of data and critical revision of the manuscript for important intellectual content. Ruth C. Carlos: Acquisition, analysis, or interpretation of data and critical revision of the manuscript for important intellectual content. Lynne I. Wagner: Study concept and design; drafting of the manuscript; acquisition, analysis, or interpretation of data; and critical revision of the manuscript for important intellectual content.
Supporting information
Table S1A
Table S1B
Table S2
Table S3
Table S4
Table S5
Bradbury AR, Lee J‐W, Gaieski JB, Li S, Gareen IF, Flaherty KT, Herman BA, Domchek SM, DeMichele AM, Maxwell KN, Onitilo AA, Virani S, Park S, Faller BA, Grant SC, Ramaekers RC, Behrens RJ, Nambudiri GS, Carlos RC, Wagner LI. A randomized study of genetic education versus usual care in tumor profiling for advanced cancer in the ECOG‐ACRIN Cancer Research Group (EAQ152). Cancer.2022. 10.1002/cncr.34063
See editorial on pages 1359‐1360, this issue.
Beth McCarty‐Wood, CGC, Neeraja Reedy, CGC, Dana Clark, CGC, Danielle McKenna, CGC, Mary O'Connor, and Mary Lou Smith contributed to the development and editing of the educational content.
This trial has been registered at ClinicalTrials.gov (NCT02823652).
References
- 1. MacConaill LE. Existing and emerging technologies for tumor genomic profiling. J Clin Oncol. 2013;31:1815‐1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stadler ZK, Schrader KA, Vijai J, Robson ME, Offit K. Cancer genomics and inherited risk. J Clin Oncol. 2014;32:687‐698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen AP, Eljanne M, Harris L, Malik S, Seibel NL. National Cancer Institute basket/umbrella clinical trials: MATCH, LungMAP, and beyond. Cancer J. 2019;25:272‐281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Strzebonska K, Waligora M. Umbrella and basket trials in oncology: ethical challenges. BMC Med Ethics. 2019;20:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Marchiano EJ, Birkeland AC, Swiecicki PL, Spector‐Bagdady K, Shuman AG. Revisiting expectations in an era of precision oncology. Oncologist. 2018;23:386‐388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Robson ME, Bradbury AR, Arun B, et al. American Society of Clinical Oncology policy statement update: genetic and genomic testing for cancer susceptibility. J Clin Oncol. 2015;33:3660‐3667. [DOI] [PubMed] [Google Scholar]
- 7. ACMG Board of Directors . ACMG policy statement: updated recommendations regarding analysis and reporting of secondary findings in clinical genome‐scale sequencing. Genet Med. 2015;17:68‐69. [DOI] [PubMed] [Google Scholar]
- 8. Best MC, Bartley N, Jacobs C, et al. Patient perspectives on molecular tumor profiling: “why wouldn't you?”. BMC Cancer. 2019;19:753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Davies G, Butow P, Napier CE, et al. Advanced cancer patient knowledge of and attitudes towards tumor molecular profiling. Transl Oncol. 2020;13:100799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Miller FA, Hayeems RZ, Bytautas JP, et al. Testing personalized medicine: patient and physician expectations of next‐generation genomic sequencing in late‐stage cancer care. Eur J Hum Genet. 2014;22:391‐395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Roberts JS, Gornick MC, Le LQ, et al. Next‐generation sequencing in precision oncology: patient understanding and expectations. Cancer Med. 2019;8:227‐237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gray SW, Hicks‐Courant K, Lathan CS, Garraway L, Park ER, Weeks JC. Attitudes of patients with cancer about personalized medicine and somatic genetic testing. J Oncol Pract. 2012;8:329‐335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bradbury AR, Patrick‐Miller L, Long J, et al. Development of a tiered and binned genetic counseling model for informed consent in the era of multiplex testing for cancer susceptibility. Genet Med. 2015;17:485‐492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Flaherty KT, Gray R, Chen A, et al. The Molecular Analysis for Therapy Choice (NCI‐MATCH) trial: lessons for genomic trial design. J Natl Cancer Inst. 2020;112:1021‐1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shiloh S. Illness representations, self‐regulation, and genetic counseling: a theoretical review. J Genet Couns. 2006;15:325‐337. [DOI] [PubMed] [Google Scholar]
- 16. Leventhal H, Benyamini Y, Brownlee S, et al. Illness representations: theoretical foundations. In: Petrie KJ, Weinman JA, eds. Perceptions of Health and Illness: Current Research and Applications. Harwood Academic Publishers; 1997:19‐45. [Google Scholar]
- 17. Kaphingst KA, McBride CM, Wade C, et al. Patients' understanding of and responses to multiplex genetic susceptibility test results. Genet Med. 2012;14:681‐687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cella D, Riley W, Stone A, et al. The Patient‐Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self‐reported health outcome item banks: 2005‐2008. J Clin Epidemiol. 2010;63:1179‐1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schalet BD, Pilkonis PA, Yu L, et al. Clinical validity of PROMIS Depression, Anxiety, and Anger across diverse clinical samples. J Clin Epidemiol. 2016;73:119‐127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Speilberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State‐Trait Anxiety Inventory. Consulting Psychologists Press; 1983. [Google Scholar]
- 21. Sundin EC, Horowitz MJ. Impact of Event Scale: psychometric properties. Br J Psychiatry. 2002;180:205‐209. [DOI] [PubMed] [Google Scholar]
- 22. Thewes B, Meiser B, Hickie IB. Psychometric properties of the Impact of Event Scale amongst women at increased risk for hereditary breast cancer. Psychooncology. 2001;10:459‐468. [DOI] [PubMed] [Google Scholar]
- 23. Chew LD, Bradley KA, Boyko EJ. Brief questions to identify patients with inadequate health literacy. Fam Med. 2004;36:588‐594. [PubMed] [Google Scholar]
- 24. Cella D, Hughes C, Peterman A, et al. A brief assessment of concerns associated with genetic testing for cancer: the Multidimensional Impact of Cancer Risk Assessment (MICRA) questionnaire. Health Psychol. 2002;21:564‐572. [PubMed] [Google Scholar]
- 25. Bradbury AR, Patrick‐Miller LJ, Egleston BL, et al. Patient feedback and early outcome data with a novel tiered‐binned model for multiplex breast cancer susceptibility testing. Genet Med. 2016;18:25‐33. [DOI] [PubMed] [Google Scholar]
- 26. Demarco TA, Peshkin BN, Mars BD, Tercyak KP. Patient satisfaction with cancer genetic counseling: a psychometric analysis of the Genetic Counseling Satisfaction Scale. J Genet Couns. 2004;13:293‐304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hinds PS, Nuss SL, Ruccione KS, et al. PROMIS pediatric measures in pediatric oncology: valid and clinically feasible indicators of patient‐reported outcomes. Pediatr Blood Cancer. 2013;60:402‐408. [DOI] [PubMed] [Google Scholar]
- 28. Zabora J, BrintzenhofeSzoc K, Curbow B, Hooker C, Piantadosi S. The prevalence of psychological distress by cancer site. Psychooncology. 2001;10:19‐28. [DOI] [PubMed] [Google Scholar]
- 29. Carlson LE, Angen M, Cullum J, et al. High levels of untreated distress and fatigue in cancer patients. Br J Cancer. 2004;90:2297‐2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bultz BD, Carlson LE. Emotional distress: the sixth vital sign in cancer care. J Clin Oncol. 2005;23:6440‐6441. [DOI] [PubMed] [Google Scholar]
- 31. Schuurhuizen C, Braamse AMJ, Beekman ATF, et al. Screening and stepped care targeting psychological distress in patients with metastatic colorectal cancer: the TES cluster randomized trial. J Natl Compr Canc Netw. 2019;17:911‐920. [DOI] [PubMed] [Google Scholar]
- 32. Faller H, Schuler M, Richard M, Heckl U, Weis J, Kuffner R. Effects of psycho‐oncologic interventions on emotional distress and quality of life in adult patients with cancer: systematic review and meta‐analysis. J Clin Oncol. 2013;31:782‐793. [DOI] [PubMed] [Google Scholar]
- 33. Hamilton JG, Lobel M, Moyer A. Emotional distress following genetic testing for hereditary breast and ovarian cancer: a meta‐analytic review. Health Psychol. 2009;28:510‐518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Harris LN, Chen A, O'Dwyer PJ, et al. Update on the NCI–Molecular Analysis for Therapy Choice (NCI‐MATCH/EAY131) precision medicine trial. Mol Cancer Ther. 2018;7(suppl):B080. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1A
Table S1B
Table S2
Table S3
Table S4
Table S5


