Abstract
Background:
National guidelines recommend that maternity systems provide patient-centered access to immediate postpartum long-acting reversible contraception (i.e., insertion of an intrauterine device or implant during the delivery hospitalization). Hospitals face significant barriers to offering these services, and efforts to improve peripartum contraception care quality have met with mixed success. Implementation toolkits—packages of resources and strategies to facilitate implementation of new services—are a promising approach for guiding clinical practice change.
Objective:
To develop a theory-informed toolkit, evaluate the feasibility of toolkit-based implementation of immediate postpartum long-acting reversible contraception care in a single site, and refine the toolkit and implementation process for future effectiveness testing.
Study Design:
We conducted a single-site feasibility study of toolkit-based implementation of immediate postpartum contraception services at a large academic medical center in 2017–2020. Based on prior qualitative work, we developed a theory-informed implementation toolkit. A Stakeholder Panel selected toolkit resources to use in a multicomponent implementation intervention at the study site. These resources included tools and strategies designed to optimize implementation conditions (i.e., implementation leadership, planning and evaluation; the financial environment; engagement of key stakeholders; patient needs; compatibility with workflow; and clinician and staff knowledge, skills, and attitudes). The implementation intervention was executed from January 2018-April 2019. Study outcomes included implementation outcomes (i.e., provider perceptions of the implementation process and implementation tools [assessed via online provider survey]) and healthcare quality outcomes (i.e., trends in prenatal contraceptive counseling, trends in immediate postpartum long-acting reversible contraceptive utilization [both ascertained by institutional administrative data], and the patient experience of contraceptive care [assessed via serial, cross-sectional, online patient survey items adapted from the National Quality Forum-endorsed, validated Person-Centered Contraceptive Counseling measure].
Results:
Implementation Process: Among 172/401 (43%) of eligible clinicians participating in surveys, 70% were “extremely” or “somewhat” satisfied with the implementation process overall. Prenatal Contraceptive Counseling: Among 4960 individuals undergoing childbirth at the study site in 2019, 1789 (36.1%) had documented prenatal counseling about postpartum contraception. Documented counseling rates increased overall across 2019 (Q1, 12.5%; Q4, 51.0%) but varied significantly by clinic site (Q4, range 30%−79%). Immediate Postpartum Long-Acting Reversible Contraception Utilization: Utilization increased across the study period (pre-implementation, 5.46% of deliveries; during implementation, 8.95%; post-implementation, 8.58%). Patient Experience of Contraceptive Care: Patient survey respondents (response rate 15–29%) were largely white (344/425, 81%) and highly educated (309/425, 73% with at least a 4-year college degree), reflecting the study site population. Scores were poor across settings, with modest improvements in the hospital setting from 2018 to 2020 (prenatal visits, 67% to 63%; hospital, 45% to 58%; outpatient postpartum, 69% to 65%). Based on these findings, toolkit refinements included additional resources designed to routinize prenatal contraceptive counseling and to support a more patient-centered experience of contraceptive care.
Conclusion:
A toolkit-based process to implement immediate postpartum long-acting reversible contraceptive services at a single academic center was associated with high acceptability, but mixed healthcare quality outcomes. Toolkit resources were added to optimize counseling rates and the patient experience of contraceptive care. Future research should formally test effectiveness of the refined toolkit in a multi-site, prospective trial.
Keywords: postpartum contraception, long-acting reversible contraception, LARC, implementation, toolkit, quality improvement
Introduction:
National guidelines recommend that maternity systems provide access to immediate postpartum long-acting reversible contraception (LARC).1–4 Medicaid programs began reporting immediate postpartum LARC utilization as a national quality measure5,6 in the Centers for Medicare and Medicaid Services (CMS)’s Core Measure set in 2019,7 documenting very low rates of utilization (median 0.8%, range, 0.4–1.9%) across 29 states.8 A national sample of commercially insured individuals demonstrated similarly low utilization rates.9 Statewide work in Michigan,10 New Mexico,11 and South Carolina12 has demonstrated that many birthing hospitals do not offer immediate postpartum LARC care. Together, these data suggest that access barriers may prevent some interested individuals from initiating immediate postpartum LARC.13 However, healthcare systems face significant challenges to offering these services,14–18 and those attempting to implement evidence-based peripartum contraceptive services have achieved mixed success—highlighting the need for more effective implementation interventions.11,19–22
Patient-centeredness—a core domain of healthcare quality23—is another key outcome for contraceptive quality improvement (QI) efforts. The U.S. healthcare system has a history—including in contemporary practice—of limiting the reproductive autonomy of some people, including those living on low incomes and people of color.24–26 Additionally, multiple studies suggest that contraceptive care is affected by healthcare workers’ biases, sometimes resulting in failures to center patient needs and preferences and in patients feeling subtly or overtly pressured in their contraceptive decision-making.27–31
Implementation toolkits are one promising approach to improving peripartum contraceptive care quality. Implementation refers to efforts to embed evidence-based practices, such as immediate postpartum LARC access, patient-centered contraception counseling, and shared decision-making, into routine care delivery. Implementation may include activities to newly adopt or address underutilization of a recommended clinical practice. Toolkits are packages of resources to support clinical practice change and may include tools for informing institutional policy, training practitioners and staff, mapping workflow, and auditing performance to guide ongoing improvement efforts. A recent systematic review found that toolkits can effectively support efforts to integrate evidence-based clinical practices into routine care, but called for future toolkits to be a) informed by high-quality evidence and theory, and b) rigorously evaluated for acceptability and effectiveness.32
Multiple entities have released immediate postpartum LARC implementation toolkits.33,34 None to date, however, have been developed and evaluated using rigorous implementation science and behavior change theory. We aimed to develop a theory-informed toolkit, evaluate the feasibility and acceptability of toolkit-based implementation of patient-centered, immediate postpartum LARC services, and refine the toolkit for future effectiveness testing.
Methods
We conducted a single-site feasibility study of toolkit-based implementation of immediate postpartum LARC services. To guide toolkit refinements, we measured implementation process outcomes, including provider perceptions of implementation tools and the implementation process, and healthcare quality outcomes, including institutional trends in prenatal contraceptive counseling rates, immediate postpartum LARC utilization rates, and patients’ reported experience of contraceptive care. The study was approved by our institutional review board (HUM00126810) and registered on Clinicaltrials.gov (NCT03774797), though implementation studies are not clinical trials.
Setting:
The study site is a midwestern academic medical center with over 150 maternity providers (including over 60 resident physicians), over 250 maternity nurses, and approximately 4800 pregnant patients receiving care at 12 ambulatory clinics annually. Before implementation began, LARC devices were on the inpatient formulary but not routinely offered to all pregnant individuals. Prenatal contraceptive counseling was documented in progress notes, with no institutional capacity to measure counseling rates or patient contraceptive preferences.
Toolkit Development:
We previously conducted a qualitative multiple case study of immediate postpartum LARC implementation at 11 U.S. early adopter hospitals,18 where we collected implementation artifacts (e.g., clinical practice guidelines, provider training materials) and interviewed key informants during site visits. Data collection and analysis were informed by behavior change theories captured in the Consolidated Framework for Implementation Research (CFIR) and the Expert Recommendations for Implementing Change.35,36 This prior work identified a menu of promising tools and strategies for optimizing local conditions for successful implementation of patient-centered contraceptive services for pregnant and postpartum individuals. Based on these findings, we generated a collection of resources in a publicly available online toolkit10 to guide the integration of immediate postpartum LARC services into widespread clinical practice. Appendix A summarizes a) key conditions for successful implementation, guided by the CFIR, and b) corresponding toolkit resources, including tools and strategies.
Implementation Process:
Study activities occurred over a three-year period from 2017–2020 (Figure 1). We convened a 22-person Stakeholder Panel that included midwives, family physicians, obstetricians, resident physicians, nurses, pharmacy and billing staff, and trained patient advocates to lead implementation efforts. The panel met regularly during 2017 and 2018 to plan implementation activities. To inform the panel’s work, MW and MHM conducted a series of key informant interviews with 11 providers and nine patients. Interviews were rapidly analyzed37–40 to identify local barriers and facilitators to immediate postpartum LARC implementation and patient preferences for care delivery. Panel members reviewed interview findings and selected tools and implementation resources to optimize local conditions for implementation. For example, to optimize clinician knowledge, skills, and attitudes (condition), the intervention included educational resources (tools) and mandatory education and simulation sessions (strategy). Selected toolkit tools and strategies were bundled into a multicomponent implementation intervention (Figure 2). The intervention was executed during January 2018-April 2019 and included clinician and staff training activities (e.g., Grand Rounds on national guidelines for patient-centered, evidence-based peripartum contraceptive care; post-placental IUD insertion simulation sessions); electronic medical record (EMR) modifications; adoption of new clinical protocols and processes for ordering, stocking, and billing for LARC devices; and dissemination of patient resources. Implementation evaluation activities occurred during 2019–2020. Formative focus groups (e.g., at division meetings and team huddles) helped identify care delivery workflow challenges and inform corresponding process refinements.
Figure 1.
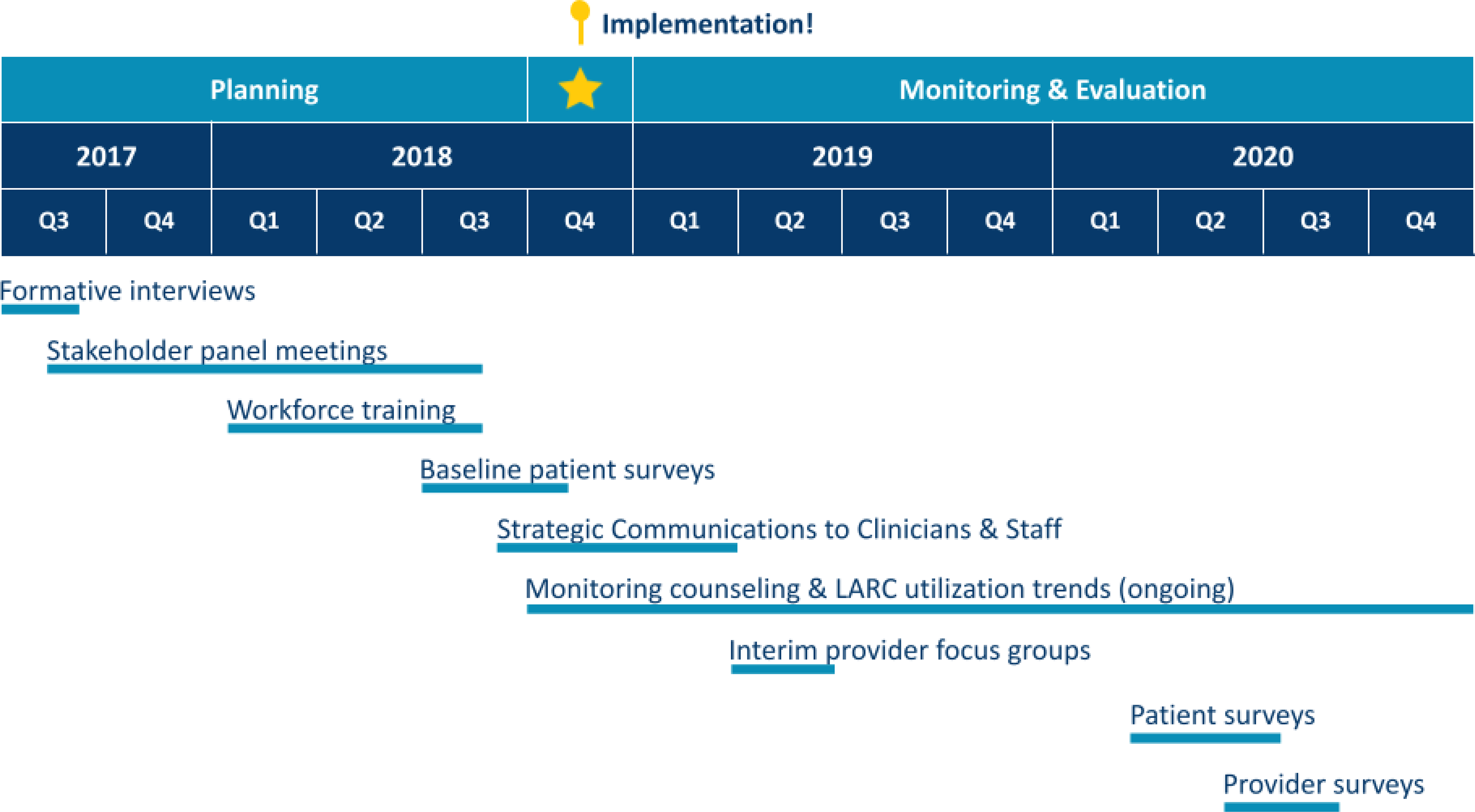
Study timeline
Figure 2.
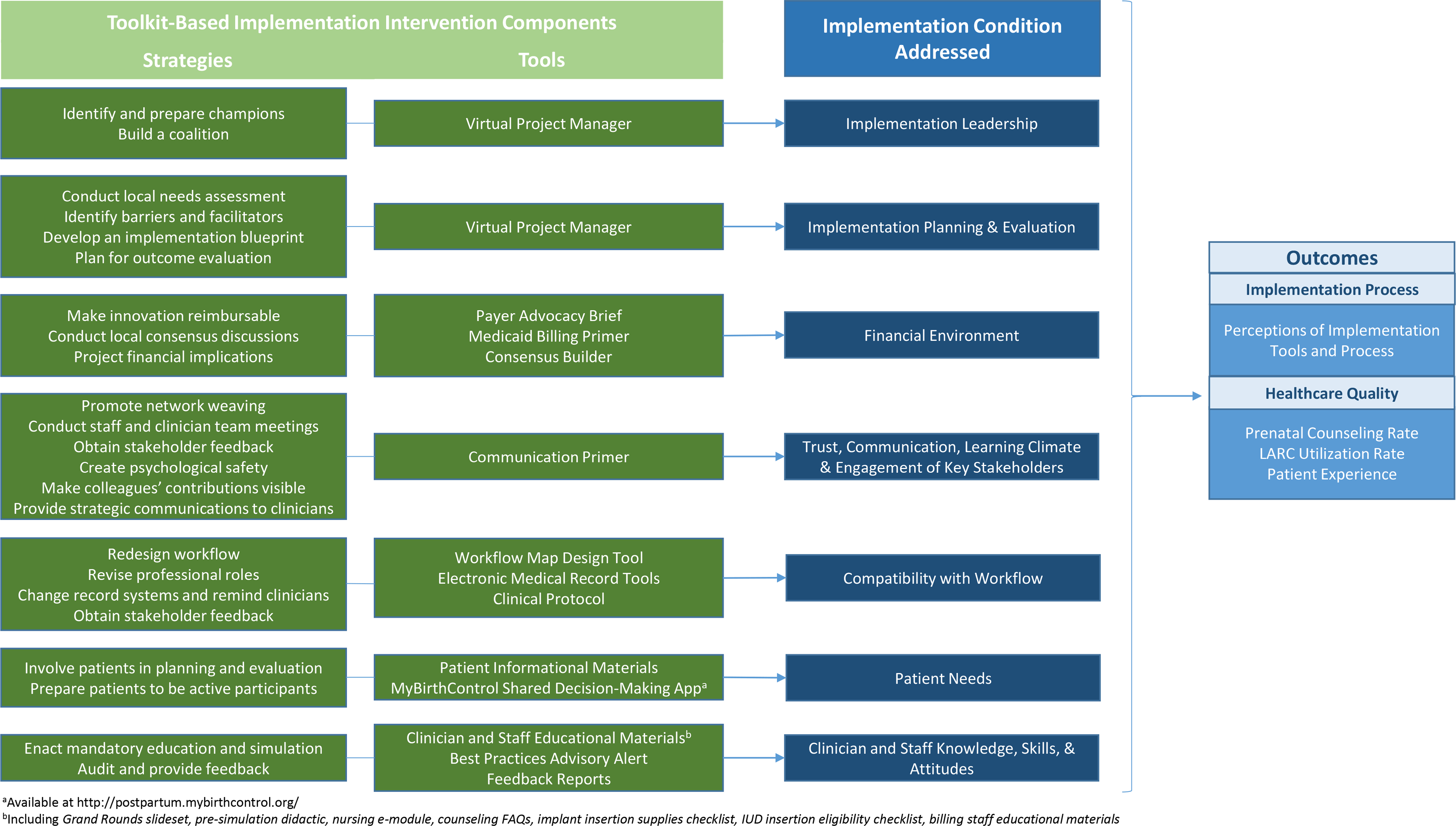
Implementation strategies and tools selected for a multicomponent intervention to increase provision of evidence-based peripartum contraceptive care
Outcomes:
We examined implementation and healthcare quality outcomes and toolkit refinements using provider and patient surveys and institutional administrative data (Table 1). Survey instruments—one each for providers and patients—were built in Qualtrics software, using validated measures where available. Experts in survey methodology, maternity care innovations, and our institution’s interdisciplinary Program on Women’s Healthcare Effectiveness Research pilot-tested and refined surveys prior to deployment.
Table 1.
Study outcomes
| Outcomes | Data Source | Timing of Data Collection |
|---|---|---|
| Implementation Process | ||
| Perceptions of implementation tools and process | Provider surveys | Spring 2020 |
| Healthcare Quality | ||
| Peripartum contraceptive counseling rate | Electronic Medical Record | January through December 2019 |
| Immediate postpartum LARC utilization rate | Electronic Medical Record | January 2017 through Dec 2019 |
| Patient experience of care | Patient surveys | Fall 2018; Spring 2020 |
| Toolkit Refinements | ||
| Key modifications to toolkit resources | Implementation process log | Throughout implementation |
LARC=long-acting reversible contraception
Implementation process outcomes:
Provider perceptions of the implementation process and implementation tools were assessed via an online post-implementation survey (July 8-August 16, 2020). All maternity care clinicians (generalist obstetricians, Maternal Fetal Medicine and family physicians, certified nurse midwives, resident obstetrician-gynecologists, and inpatient maternity nurses) were invited to participate via email. Clinicians were reminded about institutional changes in peripartum contraceptive care and then asked about satisfaction with and usefulness of toolkit resources (Figure 3). Survey items invited clinicians to provide free-text responses about their perceptions.
Figure 3.
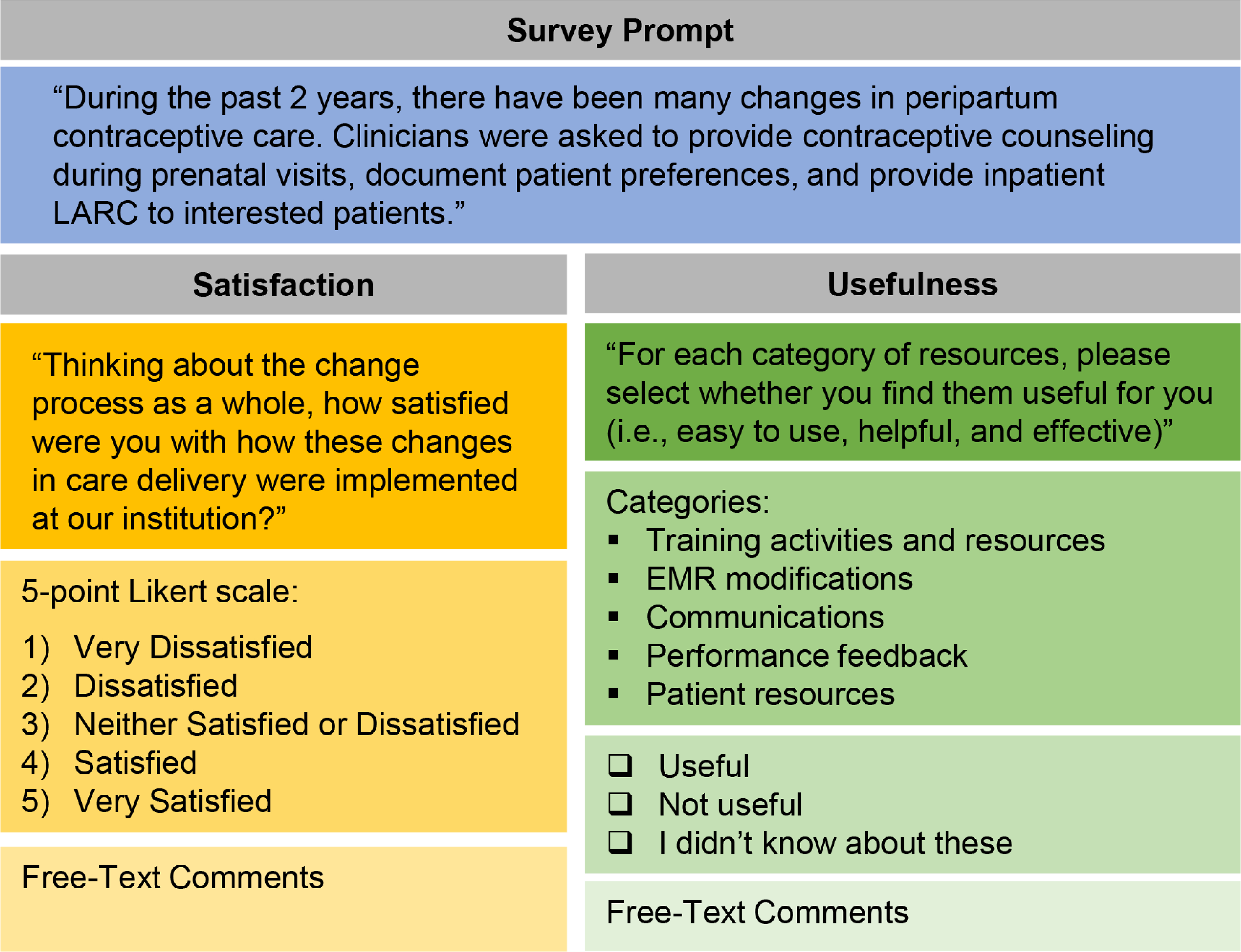
Provider survey questions
Healthcare quality outcomes:
Monthly prenatal contraceptive counseling rates (i.e., proportion of delivering individuals with documented counseling) were ascertained from EMR data in a standardized documentation element (Appendix B). This provided a feasible indicator of clinicians’ provision of a recommended service (i.e., prenatal counseling and documentation about a patient’s reproductive life goals and contraceptive method preferences). Immediate postpartum LARC utilization rates were ascertained by identifying LARC diagnostic and procedural codes in institutional administrative data (Appendix C). We measured monthly rates of LARC utilization (i.e., count of LARCs placed per total deliveries in a month) in three time periods: pre-implementation (January-December 2017), during implementation (January 2018-April 2019), and post-implementation (May-December 2019). We monitored these outcomes because the Office of Population Affairs designates LARC utilization rates as one tool for evaluating potential contraceptive access barriers.6 However, we recognized the limitations of utilization measures—where trends toward higher utilization could reflect removal of access barriers, but could also reflect non-patient centered care, where patients feel subtle or overt pressure to utilize contraceptive methods. We therefore also collected a balancing measure of patient-centeredness: the patient experience of peripartum contraceptive care, including whether individuals receive respectful care and the method they prefer, if any at all.41,42 We collected serial cross-sectional surveys of a convenience sample of pregnant and postpartum patients receiving care at the study site. We recruited approximately 100 pregnant and 100 postpartum individuals during each survey fielding period (early implementation: July-October 2018; post-implementation: February-July 2020) to provide at least 80% statistical power when testing for moderately-sized effects (Cohen’s D=0.40, odds ratio=2.0). All English-speaking adult patients receiving outpatient maternity care at our institution and meeting eligibility as pregnant (32+ weeks gestation) or postpartum (4–8 weeks post-childbirth) received up to three invitations (via email or telephone) to participate. The patient experience of care item was adapted from the National Quality Forum-endorsed, validated Person-Centered Contraceptive Counseling measure,43 with input from its creating team, for use in peripartum populations (Figure 4).44 The measure is scored dichotomously (all items “excellent” vs. any non-“excellent” responses). Surveys assessed the patient experience of contraceptive care in three settings: prenatal visits (pregnant sample), the childbirth hospitalization (postpartum sample), and postpartum visits (postpartum sample).
Figure 4.

Patient survey questions
Toolkit Refinements:
An implementation process log was used to track key modifications to toolkit resources, guided by observations from the Stakeholder Panel and the authors’ interpretation of implementation process and healthcare quality outcomes.
Analysis:
Quantitative administrative data and survey responses were summarized using descriptive statistics. Survey results are reported for the total number of patients or providers answering each question.45 Using a rapid analysis approach, free-text responses from providers were qualitatively coded based on emerging themes by two authors (MM, MW), with input from additional authors (AFP, MH). Discrepancies were rare and discussed until consensus was reached. Provider ratings and free-text responses were visually merged into a joint display presenting mean quantitative scores for each category of toolkit resources and representative qualitative quotes.46 Contraceptive counseling rates were summarized monthly. Monthly immediate postpartum LARC utilization trends before, during, and after pilot implementation were compared using a simple interrupted time series analysis. This logistic regression contained months, time period indicators, and their interactions. Statistical analyses were performed using Stata (StataCorp. 2019. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC.).
Results:
Implementation Process Outcomes
Of eligible clinicians, 172/401 (43%) participated in surveys (physicians: 62/86, 72%; obstetrics and gynecology residents: 12/25, 48%; midwives: 20/33, 61%; nurses: 90/257, 35%), with 70% being “extremely” or “somewhat” satisfied with the implementation process overall. Clinician perceptions about toolkit resources are presented in Figure 5. Clinicians found trainings to be helpful, but also described being unaware of some of the content covered in trainings and resources provided. EMR tools were praised as easy to use and helpful. Strategic communications largely targeted physicians and midwives and were perceived as helpful by half of respondents, while more than half were unaware that institutional-level performance feedback had been disseminated. Only one in three respondents found distributed feedback helpful, and some sought individualized performance feedback. Perceptions of patient educational resources were similar, with half of respondents unaware of these tools and many describing uncertainty about how to disseminate them.
Figure 5.
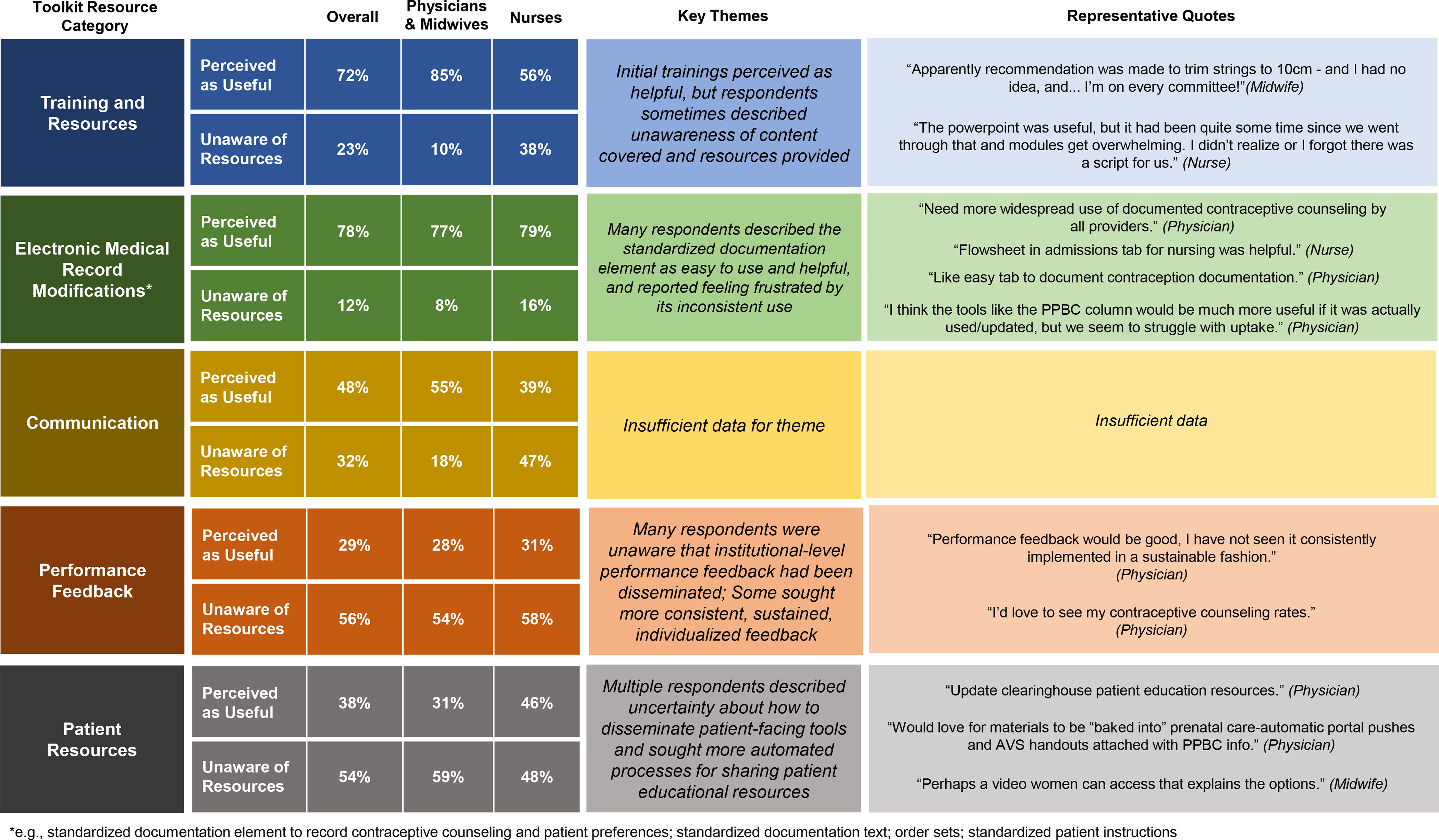
Clinician perceptions of implementation toolkit resources and processes
Healthcare Quality Outcomes
Prenatal Contraceptive Counseling:
Among 4960 individuals undergoing childbirth at the study site in 2019, 1789 (36.1%) had documented prenatal counseling about postpartum contraception. Documented counseling rates increased overall across 2019 (Q1, 12.5%; Q4, 51.0%) but varied significantly by clinic site (range 30–79% in Q4; Figure 6).
Figure 6.
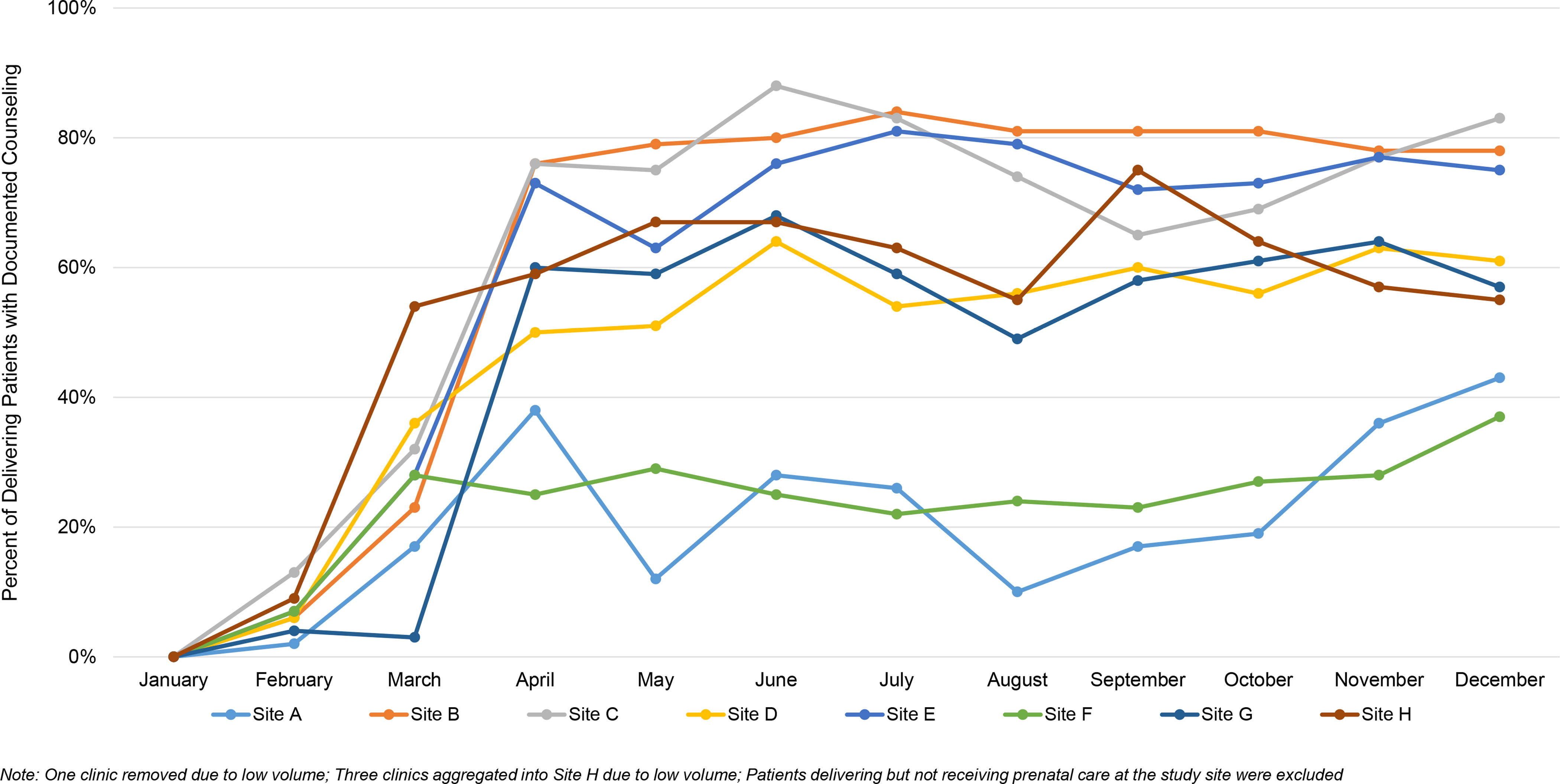
Prenatal contraceptive counseling rates over time, by clinic, 2019
Immediate Postpartum LARC Utilization:
The overall proportion of individuals utilizing immediate postpartum LARC increased across the study period (5.46% of deliveries pre-implementation, 8.95% during implementation, 8.58% post-implementation). An increasing monthly trend in immediate postpartum LARC utilization was observed during implementation (0.35% monthly increase, 95% CI [0.18%, 0.53%], p<0.001). The pre-implementation and post-implementation monthly trends, however, were not statistically different from zero (Pre: 0.36%, 95% CI [−0.2%,0.9%], p=0.206; Post: 0.1%, 95% CI [−0.19%, 0.41%], p=0.474) (Figure 7).
Figure 7.
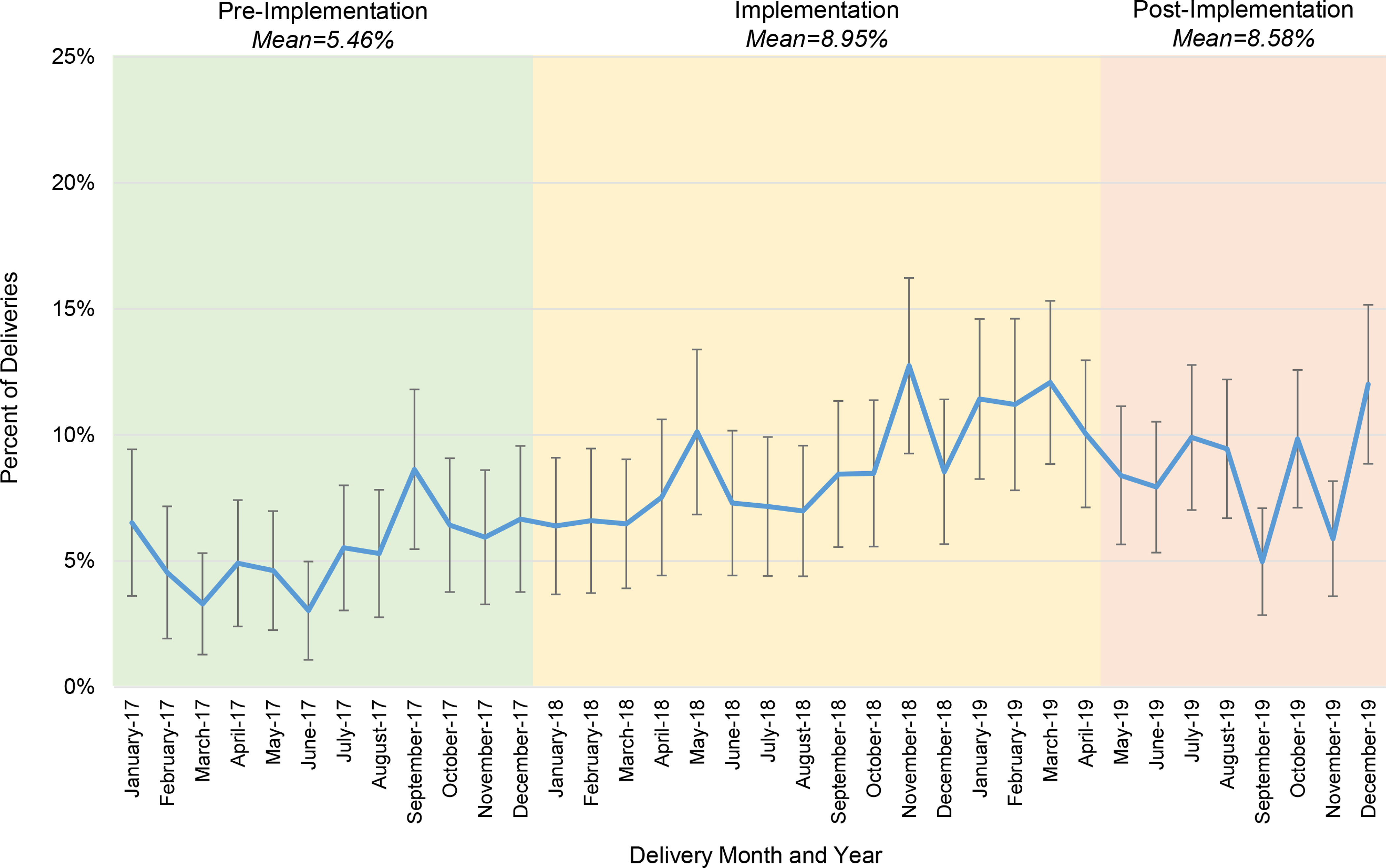
Immediate postpartum long-acting reversible contraceptive utilization rates, over time
Patient Experience of Contraceptive Care:
More eligible pregnant than postpartum individuals participated in surveys (2018: 26%, 117/459 pregnant vs 29%, 97/338 postpartum; 2020: 15%, 109/731 pregnant vs. 21%, 102/477 postpartum). Patient respondents were largely white (344/425, 81%) and highly educated (309/425, 73% with at least 4-year college degree), reflecting the study site population (Table 2). The proportion of respondents giving an “excellent” rating for all four items in the adapted Person-Centered Contraceptive Counseling measure was low in all settings in 2018 and 2020, with lower scores in the hospital setting (prenatal visits, 67%, 63%; hospital, 45%, 58%; outpatient postpartum, 69%, 65%).
Table 2.
Demographic characteristics of patient survey respondents
| Characteristic | 2018 Pregnant (n=117) | 2018 Postpartum (n=97) | 2020 Pregnant (n=109) | 2020 Postpartum (n=102) |
|---|---|---|---|---|
|
| ||||
| Age group | ||||
| 18–24 | 13 (11.1) | 11 (11.3) | 5 (4.6) | 4 (3.9) |
| 25–34 | 71 (60.7) | 57 (58.8) | 74 (67.9) | 68 (66.7) |
| 35–44 | 32 (27.4) | 20 (20.6) | 24 (22.0) | 25 (24.5) |
| Unknown | 1 (0.9) | 9 (9.3) | 6 (5.5) | 5 (4.9) |
| Education | ||||
| 8th grade or less | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Some high school, high school graduate, or GED | 7 (6.0) | 4 (4.1) | 4 (3.7) | 3 (2.9) |
| Some college or 2-year degree | 25 (21.4) | 15 (15.5) | 19 (17.4) | 18 (17.6) |
| 4-year college degree | 31 (26.5) | 23 (23.7) | 32 (29.4) | 32 (31.4) |
| More than 4-year college degree | 53 (45.3) | 46 (47.4) | 48 (44.0) | 44 (43.1) |
| Unknown | 1 (0.9) | 9 (9.3) | 6 (5.5) | 5 (4.9) |
| Ethnicity | ||||
| Hispanic or Latino | 2 (1.7) | 7 (7.2) | 4 (3.7) | 4 (3.9) |
| Not Hispanic or Latino/Unknown | 115 (98.3) | 90 (92.8) | 105 (96.3) | 98 (96.1) |
| Race | ||||
| White | 99 (84.6) | 72 (74.2) | 90 (82.6) | 83 (81.4) |
| Black or African-American | 7 (6.0) | 7 (7.2) | 4 (3.7) | 5 (4.9) |
| Asian | 5 (4.3) | 4 (4.1) | 4 (3.7) | 5 (4.9) |
| All othera | 6 (5.1) | 14 (14.4) | 11 (10.1) | 9 (8.8) |
Data presented as n (%)
Includes Native Hawaiian or Other Pacific Islander, American Indian or Alaska Native, Other, Unknown, and patients choosing more than one race
GED=General Educational Development Test
Toolkit Refinements:
Refinements focused on generating additional resources to routinize prenatal contraceptive counseling and support a more patient-centered experience of contraceptive care. We now provide a quarterly feedback report, with data on trends in prenatal contraceptive counseling and immediate postpartum LARC utilization rates and patient experience of care scores (now administered to all study site patients as part of routine QI efforts), with a goal of driving improvements in counseling rates and patient-centered care. The report also contains a “resource round-up” section reminding clinicians about educational materials to maintain knowledge and skills. The refined toolkit also incorporates multiple resources and strategies to optimize the patient experience of care. First, patient educational handouts are now automatically disseminated through the EMR’s after-visit patient instructions, based on gestational age. These materials now include a new shared decision-making tool, the MyBirthControl app,47–49 designed to improve the patient-centeredness of peripartum contraceptive counseling and decision-making. Second, we created a patient video about options for the timing of LARC insertion, to ensure that pregnant individuals interested in utilizing postpartum LARC methods receive comprehensive, accurate, and balanced information about trade-offs with immediate versus outpatient postpartum LARC insertion. Third, we developed additional training sessions for clinicians, including a Grand Rounds focused on the patient experience of care, reproductive justice, and findings from the current study, and annual training in immediate postpartum LARC for newly hired staff and trainees.
Comment
Principal Findings:
In this feasibility study at a single academic center, a toolkit-based process to implement evidence-based, patient-centered peripartum contraceptive services was associated with high clinician acceptability, but mixed healthcare quality outcomes: immediate postpartum LARC utilization increased; documented prenatal contraceptive counseling rates varied across ambulatory sites; and the patient experience of care remained poor. The refined toolkit thus now includes additional resources designed to routinize prenatal contraceptive counseling and support a more patient-centered experience of contraceptive care.
Results in Context:
Our findings build on and extend prior studies with public health officials and healthcare systems to improve immediate postpartum LARC implementation in maternity settings.11,18,21,22,50–53 Implementation challenges have been documented; one study of 11 healthcare systems implementing immediate postpartum LARC programs found that sites utilized, on average, 18 distinct implementation strategies (range, 11–22).18 Another study of five sites found that immediate postpartum LARC implementation efforts cost, on average, $14,433.94 (range $9955.61–$23,690.49), with higher costs at sites with more barriers to implementation.50 An implementation toolkit may help increase the efficiency and effectiveness of implementation efforts. The current study’s findings support the feasibility and acceptability of our theory-based implementation toolkit. Healthcare workers were pleased with toolkit resources, especially clinician training and EMR tools. Study findings provide preliminary support for this theory-based toolkit’s effectiveness in improving the quality of peripartum contraceptive care. We observed an encouraging improvement in rates of documented perinatal contraceptive counseling across the study period—particularly in some high-performing clinics—and potentially enhanced access to immediate postpartum LARC for interested patients. Toolkit refinements to support ongoing clinician education, more effective patient education and engagement in shared decision-making, and more timely clinician performance feedback may help ensure that recommended practices (i.e., patient-centered prenatal counseling about postpartum contraception, and provision of immediate postpartum LARC to interested, eligible patients) are fully routinized and improve the patient experience of care.
Our findings underscore the importance of routinely monitoring the patient experience of contraceptive care as a key indicator of peripartum healthcare quality. The National Quality Forum recently endorsed the Person-Centered Contraceptive Care survey as a patient-reported outcome performance measure for use in tandem with claims-based measures of contraceptive utilization to monitor the quality of contraceptive care among reproductive-aged women. Our findings suggest the pressing need for this measure to be formally adapted and validated specifically in peripartum populations, as well as need for more robust processes to involve patients and families in maternity care QI efforts. Patients and family members provide a vital, unique perspective about the healthcare system and can significantly enhance the success of QI efforts.54
Clinical implications:
Improving contraceptive access and the patient-centeredness of contraceptive services are crucial activities for supporting people in achieving their reproductive and broader educational, professional, and economic goals. Our study’s findings highlight the importance of including patient-centered outcomes in evaluation of peripartum contraceptive care to ensure it is fully responsive to individual patients’ preferences and needs.
Research Implications:
Prospective, multi-site trials are needed to evaluate the effectiveness of toolkit-based multicomponent implementation interventions in heterogeneous contexts (e.g., academic centers, community-based hospitals, hospitals serving rural populations). In addition to monitoring trends in quality outcomes, future work should also evaluate the sustainability and costs of quality improvements achieved with a toolkit-based implementation process. Our findings call for the development and inclusion of validated measures of the patient experience of contraceptive care in any implementation intervention evaluation. If successfully executed, such implementation research may help drive improvements in peripartum contraceptive care quality. More broadly, such implementation research in maternity contexts may identify promising strategies to address adverse birth outcomes, including unacceptable inequities in these outcomes.55
Strengths and Limitations:
Key study strengths include the use of rigorous formative research and behavior change theory to inform toolkit design, a stakeholder-engaged process for selection of toolkit items, and evaluation of both implementation outcomes and preliminary quality outcomes. Additionally, this prospective study included measurement of the patient experience as a key indicator of peripartum contraceptive care quality. Limitations include a single-site study with a principal goal of assessing feasibility and acceptability. Both provider and patient survey findings are limited by low response rates. Finally, the study site’s patient population is largely white and college-educated, limiting generalizability in strict methodologic terms, but we believe still providing insights relevant to more diverse patient populations. The consistency of our observed patient survey outcomes suggests to us that something about our institution—our care delivery processes, our culture, our communication, and our interactions with patients—is undermining the patient experience of care. If this is true for our patient population—a largely resourced, highly educated group—we believe it suggests that circumstances may be equally bad or worse in marginalized populations more likely to suffer from interpersonal biases based on race or class and structural inequities like structural racism.
Conclusions:
Toolkit-based implementation of recommended postpartum contraceptive care was associated with provider acceptability, variable improvement in contraceptive counseling, increased immediate postpartum LARC utilization, and no improvements in the patient care experience. Toolkit modifications were made with a goal of optimizing contraceptive counseling and the patient experience of peripartum contraceptive care. A rigorous, fully-powered, multi-site trial of the refined toolkit’s effectiveness is now needed. Additional work is also needed to develop a validated measure of the patient experience of peripartum contraceptive care and to identify and promote the widespread adoption of best practices for involving patients and communities in perinatal care QI efforts.
AJOG At A Glance.
A. Why was this study conducted?
Implementation toolkits—packages of resources and strategies to facilitate organizational change—are promising but relatively understudied.
B. What are the key findings?
A toolkit-based process to implement immediate postpartum long-acting reversible contraception (LARC) services at a single academic center was associated with a) positive provider perceptions; b) increasing documented prenatal contraception counseling rates (2019: Q1, 12.5%; Q4, 51.0%); c) increased inpatient LARC utilization (pre-implementation, 5.46%, post-implementation 8.58%); and d) no improvement in the proportion of patients reporting an excellent experience of care (baseline vs post-implementation: prenatal visits, 67% vs. 63%; childbirth hospitalization, 45% vs. 58%; outpatient postpartum, 69% vs. 65%).
C. What does this study add to what is already known?
A toolkit-based process to implement immediate postpartum LARC was feasible and acceptable. A rigorous, fully powered trial of the refined toolkit’s effectiveness is needed.
Funding:
Dr. Moniz is supported by the AHRQ, grant #K08 HS025465. Dr. Heisler is funded by the National Institutes of Health (NIH), including Grant Number P30DK092926 (MCDTR) from the National Institute of Diabetes and Digestive and Kidney Diseases. The AHRQ and NIH played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Conflicts of Interest:
Michelle Moniz is a paid consultant for RAND, NIDA, and the Society of Family Planning. Dr. Dalton has received grant funding from the Agency for Healthcare Research and Quality (AHRQ), American Association of Obstetricians and Gynecologists Foundation, the Laura and John Arnold Foundation, National Institute for Reproductive Health, Blue Cross Blue Shield of Michigan Foundation, the Society of Family Planning and the National Institutes for Health outside the submitted work. She is also a contributing editor for the Medical Letter and an author for Up-to-Date. She is also a consultant for Bind and expert witness for Merck. Christine Dehlendorf is an author for Up To Date. The remaining authors report no conflicts of interest.
Appendix A.
Overview of an Evidence-based Toolkit for Implementing Immediate Postpartum Long-Acting Reversible Contraception Services
| Conditions for Successful Implementation1 | Description1 | Implementation Strategies1 | Implementation Tools2 |
|---|---|---|---|
| Effective Implementation Champions | Effective champions use their influence in the organization, persuasiveness, grit, and participative leadership style to overcome institutional siloing, leverage professional networks, create tension for change, cultivate a positive learning climate, optimize workflows, engage key stakeholders, and lead implementation planning and evaluation processes. Clinical champions benefit from an interprofessional team of co-champions. Champions with competing demands on their time and insufficient support for project management may struggle to be effective. |
Identify and prepare champions
Build a coalition Conduct local needs assessment Assess for readiness and identify barriers and facilitators Tailor strategies Develop a blueprint Obtain feedback about the implementation plan Stage scale up Facilitation Plan for outcome evaluation Develop processes for quality monitoring Evaluate implementation |
Online Toolkit
Virtual Project Manager |
| Enabling Financial Environment | Non-reimbursement by major payers or evidence of financial losses can undermine implementation efforts, while written policies or audits documenting public and private payer reimbursement for services facilitates implementation. |
Conduct local consensus discussions
Project financial implications Access new funding Place innovation on inpatient formulary |
Consensus Builder
Medicaid Billing Primer Payer Advocacy Brief |
| Hospital Administrator Engagement | Disengaged leaders may not provide permission and support for the initiative to proceed, while engaged leaders who perceive the initiative as aligned with their mission and vision can act as vocal sponsors who increase the initiative’s visibility and priority in the organization. | Conduct local consensus discussions | Consensus Builder |
| Trust and Effective Communication | A lack of pre-existing relationships and communications processes across clinicians and operations staff may impede implementation success. Mistrust among individuals with divergent expertise, priorities, and reporting structures hamper communication and shared problem-solving. Conversely, strong relationships and robust communication norms across stakeholders promote collaboration necessary to address implementation challenges and ensure efficient frontline care delivery. |
Promote network weaving
Organize clinician and staff team meetings for shared problem-solving and accountability |
Communication Primer
Virtual Project Manager |
| Alignment with Stakeholders’ Professional Values | Individuals perceiving the initiative as aligned with their professional mission and values are more likely to promote its success. Individuals seeing the initiative as at odds with professional values may resist change and significantly stall institutional progress. |
Conduct local consensus discussions
Project financial implications |
Consensus Builder |
| Perception of Meeting Patients’ Needs | Perceptions that offering inpatient LARC services better meets patients’ needs strongly promotes provider and staff willingness to make services available to interested patients. Because multiple studies document real tensions between enhancing access to contraceptive care and ensuring that patients have a high-quality, patient-centered care experience, it is crucial to include patients in implementation planning and evaluation to ensure that care delivery changes are truly aligned with patient needs. |
Involve patients in implementation planning and evaluation
Prepare patients to be active participants Engage community resources |
MyBirthControl App
3
Patient Informational Material (handouts, posters, educational videos) |
| Robust Learning Climate | Robust learning climates, where providers and staff feel essential and empowered to shape change, catalyze implementation. Frontline providers and staff feel motivated to design new workflows, overcome challenges, and make real-time refinements to care delivery. |
Facilitation
Obtain stakeholder feedback Create psychological safety Make colleagues’ contributions visible |
Communication Primer |
| Compatibility with Workflow | Embedding inpatient LARC into daily care delivery routines requires steps to minimize workflow disruptions, including establishing communication processes across teams and settings, making devices available, optimizing medical records for documentation and device ordering, and streamlining billing and coding processes. Prospectively designing these processes promotes success, while letting workflow evolve organically can lead to inefficiencies, introduce confusion and frustration, and cause interruptions to service provision to patients. |
Assess and redesign workflow
Obtain stakeholder feedback Change record systems to streamline documentation Change physical structure and equipment Remind clinicians Make billing easier |
Clinical Protocol
Consent Forms Workflow Map Design Tool Electronic Medical Record Tools (tipsheet, standardized documentation) |
| Knowledge, Attitudes and Beliefs About the Clinical Practice | Providers may have significant knowledge and skill gaps, as many do not receive implant or post-placental IUD insertion training in residency. Providers with negative attitudes about the initiative can seriously undermine service delivery to patients and colleagues’ engagement in the initiative. Opinion leaders supportive of the initiative can strongly influence colleagues’ perceptions and motivation to participate. |
Dynamic training and education for clinicians and staff
Develop and distribute educational materials Conduct ongoing training Clinical supervision Conduct clinician and staff team meetings Engage local opinion leaders Audit and provide feedback about performance |
Provider Training Tools (Grand Rounds slideset, pre-simulation didactic, nursing e-module, counseling FAQs, implant insertion supplies checklist, IUD insertion eligibility checklist)
Communication Primer |
Content based on findings in Moniz et al. Implementing Immediate Postpartum Contraception: A Comparative Case Study at 11 Hospitals, in Implementation Science Communications, 2021: https://implementationsciencecomms.biomedcentral.com/artides/10.1186/s43058-021-00136-7
All tools are publicly available in an online toolkit at contraceptiveaccess.org
Available at http://postpartum.mybirthcontrol.org/
Appendix B.

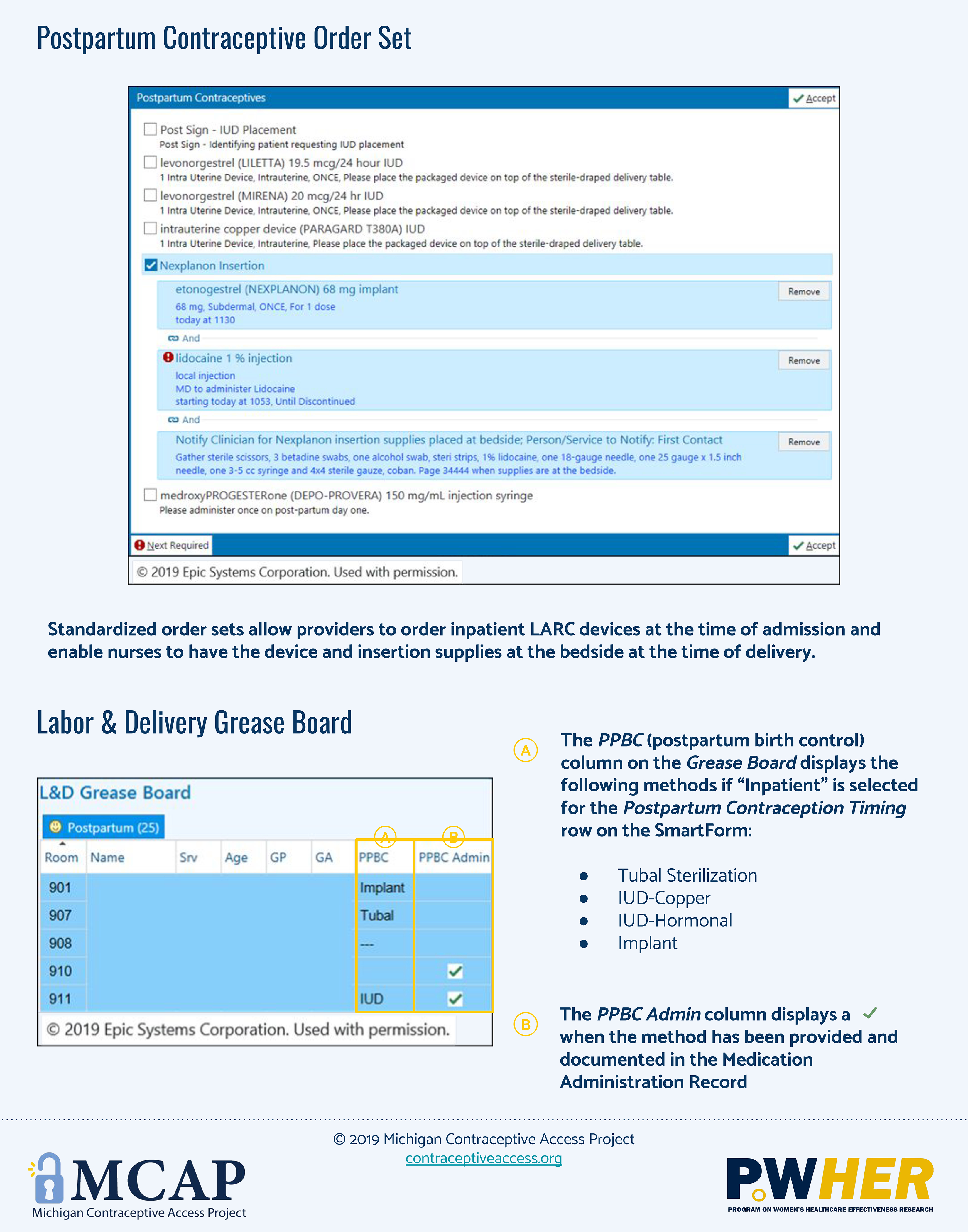
Appendix C.
Diagnostic and procedural codes used to identify immediate postpartum LARC utilization rates
| Deliveries | ||
| ICD-9 Dx | ICD-10 Dx | |
| Include | V27, V27.0, V27.1, V27.2, V27.3, V27.4, V27.5, V27.6, V27.7, V27.9, 650 | Z37, Z37.0, Z37.1, Z37.2, Z37.3, Z37.4, Z37.5, Z37.59, Z37.6, Z37.69, Z37.7, Z37.9, 080 |
| Exclude | 630, 631, 632, 633, 633.0, 633.00, 633.01, 633.1, 633.10, 633.11, 633.2, 633.20, 633.21, 633.8, 633.80, 633.81, 633.9, 633.90, 633.91, 634, 634.x, 635, 635.x, 636, 636.x, 637, 637.x, 638.x, 639, 639.0, 639.1, 639.2, 639.3, 639.4, 639.5, 639.6, 639.8, 639.9, V24, V24.0, V24.1, V24.2 | A34, O00, O00.0, O00.00, O00, O00.1, O00.2, O00.8, O00.9, O01.9, O02, O02.1, O03, O03.0, O03.1, O03.2, O03.30, O03.31, O03.32, O03.33, O03.34, O03.34, O03.39, O03.4, O03.5, O03.6, O03.7, O03.8x, O03.9, O04.5, O04.6, O04.7, O04.8x, O07.0, O07.1, O07.2, O07.3x, O07.4, O08.0, O08.1, O08.2, O08.3, O08.4, O08.5, O08.6, O08.7, O08.81, O08.83, O08.89, O08.9, Z33.2, Z39, Z39.0, Z39.1, Z39.2 |
| ICD-9 Px | ICD-10 Px | |
| Include | 72, 72.x, 73, 73.01, 73.09, 73.1, 73.2, 73.21, 73.22, 73.3, 73.4, 73.5, 73.51, 73.59, 73.6, 73.8, 73.9, 73.92, 73.93, 73.94, 73.99, 74, 74.1, 74.2, 74.4, 74.9, 74.99 | 0Q820ZZ, 0Q823ZZ, 0Q824ZZ, 0Q830ZZ, 0Q833ZZ, 0Q834ZZ, 0U7C7ZZ, 0W8NXZZ, 10A07ZZ, 10A08ZZ, 10D00Z0, 10D00Z1, 10D00Z2, 10D07Z3, 10D07Z4, 10D07Z5, 10D07Z6, 10D07Z7, 10D07Z8, 10E0XZZ, 10J07ZZ, 10S07ZZ, 10S0XZZ, 10900ZC, 10903ZC, 10904ZC, 10907ZA, 10907ZC, 10908ZA, 10908ZC, 3E030VJ, 3E033VJ, 3E040VJ, 3E043VJ, 3E050VJ, 3E053VJ, 3E060VJ, 3E063VJ, 3E0DXGC, 3E0P7GC |
| Exclude | 69.01, 69.51, 74.91, 75.0 | N/A |
| DRG | CPT | |
| Include | 370, 371, 372, 373, 374, 375 765, 766, 767, 768, 774, 775, 766 |
59400, 59409, 59410, 59610,59612, 59614, 59510, 59514, 59515, 59618, 59620, 59622 |
| Inpatient LARC | ||
| ICD-9 Dx | ICD-10 Dx | |
| Include | V25.11, V25.13, V25.5 | Z30.430, Z30.433, Z30.431, Z30.8 |
| ICD-10 Px | ||
| Include | 0UH97HZ, 0UH98HZ, 0UHC7HZ, 0UHC8HZ, 0UH90HZ | |
Dx=Diagnosis codes, Px=Procedure codes; DRG=Diagnosis Related Group codes; CPT=Current Procedural Terminology codes; NDC=National Drug Code codes; HCPCS= Healthcare Common Procedure Coding System codes; LARC=long-acting reversible contraception
Footnotes
Condensation: Toolkit-based implementation of postpartum contraception care was associated with provider acceptability, variable improvement in contraception counseling, increased immediate postpartum LARC utilization, and no improvement in patient care experience.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice. Committee Opinion No. 670: Immediate Postpartum Long-Acting Reversible Contraception. Obstet Gynecol 2016;128(2):e32–37. [DOI] [PubMed] [Google Scholar]
- 2.American College of Obstetricians Gynecologists’ Committee on Obstetric Practice, Association of Women’s Health Obstetric Neonatal Nurses. Committee Opinion No. 666: Optimizing Postpartum Care. Obstet Gynecol 2016;127(6):e187–192. [DOI] [PubMed] [Google Scholar]
- 3.American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 121: Long-acting reversible contraception: Implants and intrauterine devices. Obstet Gynecol 2011;118(1):184–196. [DOI] [PubMed] [Google Scholar]
- 4.Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep 2016;65(3):1–103. [DOI] [PubMed] [Google Scholar]
- 5.Moniz MH, Gavin LE, Dalton VK. Performance Measures for Contraceptive Care: A New Tool to Enhance Access to Contraception. Obstet Gynecol 2017;130(5):1121–1125. [DOI] [PubMed] [Google Scholar]
- 6.U.S. Department of Health & Human Services Office of Population Affairs. Contraceptive Care Measures. [Internet]. 2016; https://www.hhs.gov/opa/performance-measures/index.html. Accessed May 28, 2021.
- 7.Centers for Medicare & Medicaid Services. Adult Health Care Quality Measures. 2020; https://www.medicaid.gov/medicaid/quality-of-care/performance-measurement/adult-and-child-health-care-quality-measures/adult-health-care-quality-measures/index.html. Accessed May 28, 2021.
- 8.Centers for Medicare & Medicaid Services. Quality of Care for Adults in Medicaid: Findings from the 2018 Adult Core Set. 2019; https://www.medicaid.gov/medicaid/quality-of-care/downloads/performance-measurement/2019-adult-chart-pack.pdf. Accessed May 28, 2021.
- 9.Moniz MH, Soliman AB, Kolenic GE, et al. Cost Sharing and Utilization of Postpartum Intrauterine Devices and Contraceptive Implants Among Commercially Insured Women. Womens Health Issues 2019;29(6):465–470. [DOI] [PubMed] [Google Scholar]
- 10.Michigan Contraceptive Access Project. 2020; https://www.contraceptiveaccess.org/. Accessed May 28, 2021.
- 11.Palm HC, Degnan JH, Biefeld SD, Reese AL, Espey E, Hofler LG. An initiative to implement immediate postpartum long-acting reversible contraception in rural New Mexico. Am J Obstet Gynecol 2020;222(4S):S911 e911–S911 e917. [DOI] [PubMed] [Google Scholar]
- 12.Steenland MW, Pace LE, Sinaiko AD, Cohen JL. Medicaid Payments For Immediate Postpartum Long-Acting Reversible Contraception: Evidence From South Carolina. Health Aff (Millwood) 2021;40(2):334–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.U.S. Department of Health and Human Services Office of Population Affairs. Long-Acting Reversible Contraceptive (LARC) Methods. 2016; https://www.hhs.gov/opa/performance-measures/long-acting-reversible-contraceptive-methods/index.html. Accessed May 28, 2021.
- 14.Holland E, Michelis LD, Sonalkar S, Curry CL. Barriers to Immediate Post-placental Intrauterine Devices among Attending Level Educators. Womens Health Issues 2015;25(4):355–358. [DOI] [PubMed] [Google Scholar]
- 15.Kroelinger CD, Waddell LF, Goodman DA, et al. Working with State Health Departments on Emerging Issues in Maternal and Child Health: Immediate Postpartum Long-Acting Reversible Contraceptives. J Womens Health (Larchmt) 2015;24(9):693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castleberry NM, Stark L, Schulkin J, Grossman D. Implementing best practices for the provision of long-acting reversible contraception: a survey of obstetrician-gynecologists. Contraception 2019;100(2):123–127. [DOI] [PubMed] [Google Scholar]
- 17.Moniz MH, Chang T, Davis MM, Forman J, Landgraf J, Dalton VK. Medicaid Administrator Experiences with the Implementation of Immediate Postpartum Long-Acting Reversible Contraception. Womens Health Issues 2016;26(3):313–320. [DOI] [PubMed] [Google Scholar]
- 18.Moniz MH, Bonawitz K, Wetmore MK, et al. Implementing immediate postpartum contraception: a comparative case study at 11 hospitals. Implement Sci Commun 2021;2(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown JA, Greenfield LT, Rapkin RB. Special report: implementing immediate postpartum LARC in Florida. Am J Obstet Gynecol 2020;222(4S):S906–S909. [DOI] [PubMed] [Google Scholar]
- 20.Harper KD, Loper AC, Louison LM, Morse JE. Stage-based implementation of immediate postpartum long-acting reversible contraception using a reproductive justice framework. Am J Obstet Gynecol 2020;222(4S):S893–S905. [DOI] [PubMed] [Google Scholar]
- 21.Hofler LG, Cordes S, Cwiak CA, Goedken P, Jamieson DJ, Kottke M. Implementing Immediate Postpartum Long-Acting Reversible Contraception Programs. Obstet Gynecol 2017;129(1):3–9. [DOI] [PubMed] [Google Scholar]
- 22.Okoroh EM, Kane DJ, Gee RE, et al. Policy change is not enough: engaging provider champions on immediate postpartum contraception. Am J Obstet Gynecol 2018;218(6):590 e591–590 e597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, D.C.: The National Academies Press; 2001. [PubMed] [Google Scholar]
- 24.Stern AM. Sterilized in the name of public health: race, immigration, and reproductive control in modern California. Am J Public Health 2005;95(7):1128–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gold RB. Guarding Against Coercion While Ensuring Access: A Delicate Balance. 2014; https://www.guttmacher.org/sites/default/files/article_files/gpr170308.pdf. Accessed March 25, 2021.
- 26.Harris LH, Wolfe T. Stratified reproduction, family planning care and the double edge of history. Curr Opin Obstet Gynecol 2014;26(6):539–544. [DOI] [PubMed] [Google Scholar]
- 27.Gomez AM, Wapman M. Under (implicit) pressure: young Black and Latina women’s perceptions of contraceptive care. Contraception 2017;96(4):221–226. [DOI] [PubMed] [Google Scholar]
- 28.Higgins JA, Kramer RD, Ryder KM. Provider Bias in Long-Acting Reversible Contraception (LARC) Promotion and Removal: Perceptions of Young Adult Women. Am J Public Health 2016;106(11):1932–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dehlendorf C, Ruskin R, Grumbach K, et al. Recommendations for intrauterine contraception: a randomized trial of the effects of patients’ race/ethnicity and socioeconomic status. Am J Obstet Gynecol 2010;203(4):319 e311–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mann ES, White AL, Rogers PL, Gomez AM. Patients’ experiences with South Carolina’s immediate postpartum Long-acting reversible contraception Medicaid policy. Contraception 2019;100(2):165–171. [DOI] [PubMed] [Google Scholar]
- 31.Sznajder K, Carvajal DN, Sufrin C. Patient perceptions of immediate postpartum long-acting reversible contraception: A qualitative study. Contraception 2020;101(1):21–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamada J, Shorkey A, Barwick M, Widger K, Stevens BJ. The effectiveness of toolkits as knowledge translation strategies for integrating evidence into clinical care: a systematic review. BMJ Open 2015;5(4):e006808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baldwin SB, Wergeles M, Martinez Abad E, Chun K, Los Angeles County Department of Public Health, Office of Women’s Health. Immediate Postpartum Long-Acting Reversible Contraception Toolkit. 2019; http://publichealth.lacounty.gov/owh/LARC/LARCToolkit2019.webversion-fullpdf.pdf. Accessed February 15, 2021.
- 34.American College of Obstetricians and Gynecologists. Postpartum Contraceptive Access Initiative: Other Resources. 2017; https://pcainitiative.acog.org/resource-library/other-resources/. Accessed May 28, 2021.
- 35.Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci 2009;4:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Powell BJ, Waltz TJ, Chinman MJ, et al. A refined compilation of implementation strategies: results from the Expert Recommendations for Implementing Change (ERIC) project. Implement Sci 2015;10:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McNall M, Foster-Fishman PG. Methods of rapid evaluation, assessment, and appraisal. Am J Eval 2007;28(2):151–168. [Google Scholar]
- 38.Keith RE, Crosson JC, O’Malley AS, Cromp D, Taylor EF. Using the Consolidated Framework for Implementation Research (CFIR) to produce actionable findings: a rapid-cycle evaluation approach to improving implementation. Implement Sci 2017;12(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miles MB, Huberman AM, Saldaña J. Qualitative Data Analysis: A Methods Sourcebook. 3rd ed. Thousand Oaks, CA: PAGE Publications; 2014. [Google Scholar]
- 40.Anker M, Guidotti RJ, Orzeszyna S, Sapirie SA, Thuriaux MC. Rapid evaluation methods (REM) of health services performance: methodological observations. Bull World Health Organ 1993;71(1):15–21. [PMC free article] [PubMed] [Google Scholar]
- 41.Moniz MH, Spector-Bagdady K, Heisler M, Harris LH. Inpatient Postpartum Long-Acting Reversible Contraception: Care That Promotes Reproductive Justice. Obstet Gynecol 2017;130(4):783–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holt K, Reed R, Crear-Perry J, Scott C, Wulf S, Dehlendorf C. Beyond same-day long-acting reversible contraceptive access: a person-centered framework for advancing high-quality, equitable contraceptive care. Am J Obstet Gynecol 2020;222(4S):S878 e871–S878 e876. [DOI] [PubMed] [Google Scholar]
- 43.University of California San Francisco. The Person-Centered Contraceptive Couseling Measure. https://pcccmeasure.ucsf.edu/. Accessed May 28, 2021.
- 44.Dehlendorf C, Fox E, Silverstein IA, et al. Development of the Person-Centered Contraceptive Counseling scale (PCCC), a short form of the Interpersonal Quality of Family Planning care scale. Contraception 2021;103(5):310–315. [DOI] [PubMed] [Google Scholar]
- 45.Schoep ME, Nieboer TE, van der Zanden M, Braat DDM, Nap AW. The impact of menstrual symptoms on everyday life: a survey among 42,879 women. Am J Obstet Gynecol 2019;220(6):569 e561–569 e567. [DOI] [PubMed] [Google Scholar]
- 46.Guetterman TC, Fetters MD, Creswell JW. Integrating Quantitative and Qualitative Results in Health Science Mixed Methods Research Through Joint Displays. Ann Fam Med 2015;13(6):554–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.University of California San Francisco, Person-Centered Reproductive Health Program, California Preterm Birth Initiative. MyBirthControl. http://postpartum.mybirthcontrol.org/. Accessed May 28, 2021. [Google Scholar]
- 48.Dehlendorf C, Reed R, Fitzpatrick J, Kuppermann M, Steinauer J, Kimport K. A mixed-methods study of provider perspectives on My Birth Control: a contraceptive decision support tool designed to facilitate shared decision making. Contraception 2019;100(5):420–423. [DOI] [PubMed] [Google Scholar]
- 49.Holt K, Kimport K, Kuppermann M, Fitzpatrick J, Steinauer J, Dehlendorf C. Patient-provider communication before and after implementation of the contraceptive decision support tool My Birth Control. Patient Educ Couns 2020;103(2):315–320. [DOI] [PubMed] [Google Scholar]
- 50.Ling VB, Levi EE, Harrington AR, et al. The cost of improving care: a multisite economic analysis of hospital resource use for implementing recommended postpartum contraception programmes. BMJ Qual Saf 2020;Sep 2:bmjqs-2020–011111 (Online ahead of print). [DOI] [PubMed] [Google Scholar]
- 51.Bonawitz K, Wetmore M, Heisler M, et al. Champions in context: which attributes matter for change efforts in healthcare? Implement Sci 2020;15(1):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kroelinger CD, Morgan IA, DeSisto CL, et al. State-Identified Implementation Strategies to Increase Uptake of Immediate Postpartum Long-Acting Reversible Contraception Policies. J Womens Health (Larchmt) 2019;28(3):346–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.DeSisto CL, Estrich C, Kroelinger CD, et al. Using a multi-state Learning Community as an implementation strategy for immediate postpartum long-acting reversible contraception. Implement Sci 2017;12(1):138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kostal G, Shah A. Putting improvement in everyone’s hands: opening up healthcare improvement by simplifying, supporting and refocusing on core purpose. British Journal of Healthcare Management 2021;27(2). [Google Scholar]
- 55.Callaghan-Koru JA, Moniz MH, Hamm RF. Prioritize implementation research to effectively address the maternal health crisis. Am J Obstet Gynecol 2021;Feb 7: Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]


