Abstract
Active biodegradable packaging are being developed from biodegradable biopolymers which may solve the environmental problems caused by petroleum-based materials (plastics), as well as improving the shelf life, quality, nutritional profile, and safety of packaged food. The functional performance of active ingredients in biodegradable packaging can be extended by controlling their release profiles. This can be achieved by incorporating active ingredients in sandwich-structured packaging including multilayer and composite packaging. In multilayer materials, the release profile can be controlled by altering the type, structure, and thickness of the different layers. In composite materials, the release profile can be manipulated by altering the interactions of active ingredients with the surrounding biopolymer matrix. This article reviews the preparation, properties, and applications of multilayer and composite packaging for controlling the release of active ingredients. Besides, the basic theory of controlled release is also elaborated, including diffusion, swelling, and biodegradation. Mathematical models are presented to describe and predict the controlled release of active ingredients from thin films, which may help researchers design packaging materials with improved functional performance.
Subject terms: Biomaterials, Biochemistry, Biological techniques
Introduction
In recent years, increasing attention has been paid to the problem of environmental pollution caused by the fabrication and disposal of synthetic plastic-based packaging1. The food industry is one of the major users of these materials as they are particularly effective at protecting foods from spoilage during storage and transport2. For this reason, researchers have focused on the development of innovative biodegradable packaging for application in the food industry to replace plastic packaging3. These biodegradable materials are designed to reduce the negative environmental impacts of plastics, while still increasing the shelf life, quality, and safety of packaged food. Biodegradable packaging are typically constructed from food biopolymers (such as proteins and polysaccharides), as well as other functional ingredients (such as lipids, phospholipids, and inorganic particles)4. This new generation of packaging is typically greener, safer and more biodegradable than traditional plastics. Moreover, active ingredients such as antimicrobials, antioxidants, and nutrients can be incorporated into biodegradable packaging to gain additional functions (Table 1), such as inhibiting microbial growth, reducing lipid oxidation, and enhancing nutritional value5.
Table 1.
Active ingredients commonly used in food packaging.
| Active ingredients | Functions | Stability | Application | |
|---|---|---|---|---|
| Essential oils and plant extracts | Oregano oil | Able to achieve antibacterial, anti-oxidation, antifungal, sweating, and pain relief101; improving the barrier properties and mechanical properties of film102 | Volatile, easy to lose in storage |
Pastry103; ground beef104; beef105 |
| Cinnamon essential oil | Be antimicrobial antifungal, antioxidant, anti-inflammatory, and antidiabetic106 | Be low solubility, irritations, and allergic reactions107 |
Pork meat balls108; dry tofu109; strawberry110 |
|
| Vanilla | Antioxidant and antimicrobial activities, act as a flavor enhancer, cross-linking agent; improving the barrier performance of packaging111 | thermal instability and volatile nature112 |
Doodhpeda (milk-based solid soft sweet), biscuit, and skimmed milk powder111; crab stick113; smoked chicken breast114 |
|
| Green tea extract (Tea polyphenols) | Antioxidant, antibacterial, anti-inflammatory, anti-tumor, and anticancer115 | Unstable in alkaline and high temperature environments116 |
Pork117; marinated anchovies118 mushrooms119; raw chicken meat120 |
|
| Tannic acid |
Be used as a cross-linking agent, nonsurfactant template, metal chelating ligands; antidiarrheal, astringent and hemostasis, anti-mutagenesis, antivirus and anticancer121 |
Susceptible to oxidation in alkaline solution122 |
Linseed oil123; fresh-cut apples124 |
|
| Carvacrol | Be as flavor and fragrance agent, antioxidant, antibacterial, antifungal, acaricidal, and anticancer125 | High volatility, low water solubility, and stability126 |
Beef127; blackberries and raspberries128; ham129 |
|
| Organic acid and their salts | Potassium sorbate | With antibacterial, inhibit mold and corrupt bacteria, hygroscopic | Unstable in air and easily oxidized and colored |
Butter cake130; soft cheese131; lasagna pasta132 |
| Benzoic acid | Be as preservative and flavoring agent133 | High stability | Cheese and toasted bread134; | |
| Enzymes | Lysozyme |
Antibacterial; acts as a natural antibiotic, and enhances the efficacy of other antibiotics, strengthens the immune system135 |
Easy to be destroyed by alkali, acid environment; heat stability is very strong |
Pork136; pear juice and rice milk-based smoothie137; ground beef patties138 |
| Bacteriocins | Nisin | A wide antibacterial, antifungal, and antiviral activity | Sensitivity to the environmental, stresses, susceptibility to proteolysis139; easy to interact with food components such as proteins and fat particles140 |
Beef141; ham142; hot dog143; pork144 |
| Pediocin | Display antimicrobial activity against a wide spectrum of Gram-positive bacteria | Stable in dilute aqueous solutions145 |
Sliced ham146; raw sliced pork147 |
|
| Inorganic nanoparticles or microparticles | Silver nanoparticles | Antimicrobial properties against a wide range of microorganisms, including bacteria, yeast, and mould; low effects on the sensory attributes in food148 | Easy to get aggregation and reaction due to the high surface energy, surface passivating reagent, and capping reagent149 |
Litchi150; walnuts, hazelnuts, almonds and pistachios151; red grapes152 |
| Titanium dioxide (TiO2) nanoparticles | Can be used in food additives, pigments, photocatalysis, and personal care products; for sterilization and industrial photolytic processes regarding the decomposing of organic matters153 | High chemical stability, biocompatibility, and a robust photocatalytic activity153 |
Cherry tomato154; banana155; pork156; margarine157 |
|
Nevertheless, biodegradable packaging should be carefully designed to retain these active ingredients and control their release. Many bioactive ingredients may be rapidly released from the film matrix due to their small molecular dimensions, which reduces their efficacy and shortens the shelf life of the food6. Thus, it is extremely important to slow down the migration and realize the controlled release of active ingredients during food storage and transportation. The retention and release kinetics depend on the composition and structure of the film matrix6,7, while some researchers have achieved controlled release by utilizing multilayer or composite systems8–11. However, many studies only focus on the characterization of the macroscopic properties of these materials but ignore the molecular and physicochemical mechanisms that influence their release behaviors. Understanding the underlying mechanisms of controlled release is helpful for researchers in designing new materials and processing methods, which is therefore of great importance to the advance of the food packaging area. For this reason, this paper provides an overview of controllable release from switch structured multilayer and composite active packaging materials, with a focus on the impact of the sandwich structure on controlling the release of active components. The preparation and application of these advanced packaging materials are also reviewed. In addition, the latest advances in this area are summarized, which helps to identify knowledge gaps and to stimulate further research in this important area.
Advanced packaging with sandwich structure
Multilayer packaging
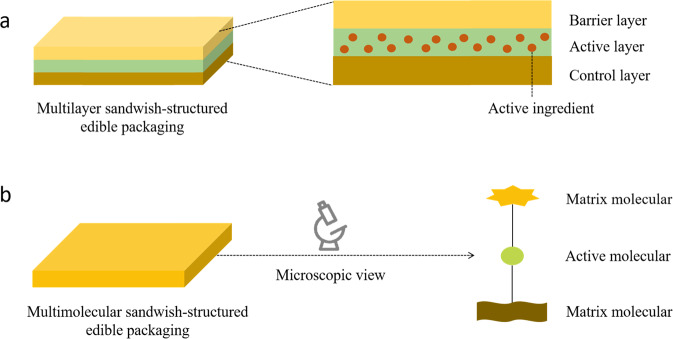
The utilization of multilayers often leads to improved or extended functional performance over monolayer materials12. This type of packaging material often consists of three distinct layers, which are the barrier, active, and control layers6, as shown in Fig. 1a.
Fig. 1. Schematic representation of biodegradable packaging materials with sandwich structure.
a Multilayer and b composite packaging.
The barrier layer is the outermost one in the sandwich-like structure and is therefore in direct contact with the external environment. It is often designed to act as a barrier to substances that cause degradation of packaged food, such as moisture, oxygen, and microorganisms. The barrier layer may also be designed to ensure good retention and protection of the active ingredients located within the interior of the packaging material.
The active layer is located in the middle of the sandwich-like structure and usually contains the active ingredients, such as antioxidants and antimicrobials13. It is important to design the system so that the active ingredients are retained in a stable form within the active layer until required, and then diffuse through the control layer into the food at the required rate7.
The control layer is the innermost one in the sandwich-like structure, which is directly in contact with food. It is designed to control the rate at which the active ingredients move into the food, as well as to protect the food and active ingredients.
The functional performance of each layer can be controlled by adjusting material properties, such as composition, pore size, thickness, polarity, swelling capacity, and solubility. The release of active ingredients from multilayer packaging can be influenced by various internal and external factors. The internal factors refer to the properties of the biodegradable packaging material itself, including the nature of the active ingredients (such as molecular weight, size, polarity, and concentration) and the properties of the surrounding matrix (such as polarity, thickness, pore size, glass transition temperature, additives, plasticizing, cross-linking degree, crystallization, and swelling)14–17. The external factors refer to the influence of the environment on the release profile, including storage time, temperature, relative humidity, and food properties.
Many studies have shown that the structure of multilayer packaging can be manipulated to control the release of active ingredients, so as to prolong their action time. For instance, multilayer biodegradable films have been produced by layer-by-layer solvent-casting using zein as the barrier layer, tea polyphenols as the active layer, and gelatin as the control layer11. By changing the ratio of zein/gelatin in the active layer, the water barrier properties and release time of the tea polyphenols could be controlled. The release time of the tea polyphenols from multilayer systems is longer than that from control groups. Other researchers have used electrospinning and coating technologies to prepare multilayer films from polylactic acid (PLA)18. These films consist of an active layer containing gallic acid sandwiched between two PLA layers and could be designed to control the release profile of gallic acid into food. Sequential electrospinning has been used to fabricate multilayer films consisting of a curcumin-loaded active layer sandwiched between layers formed from ethylcellulose nanofibers and gelatin nanofibers19. The curcumin could be continuously released from these multilayer films and maintain its antioxidant activity throughout this time. Representative examples of research on the controlled release films with sandwich-like structures are summarized in Table 2.
Table 2.
Representative examples of previous research on degradable multilayer packaging materials with different compositions.
| Composition & structure: barrier/active/control layers | Preparation methods | Functional properties |
|---|---|---|
| Zein/zein-gelatin-tea polyphenol/gelatin | Layer-by-layer solvent-casting | Tea polyphenol release was slower from multilayer than monolayer films11. |
| Polylactide/gallic acid/polylactide | Electrospinning | Multilayer films could prolong polyphenol release for more than 1000 h18. |
| Ethylcellulose/gelatin-curcumin/ethylcellulose | Sequential electrospinning | Multilayer films released curcumin continuously for 96 h and maintained its antioxidant activity19. |
| Cellulose acetate/potassium sorbate/cellulose acetate | Dry phase inversion technique | Potassium sorbate release was slower from multilayer than monolayer films28. |
|
PHBV/zein-cinnamaldehyde/PHBV Alginate/zein-cinnamaldehyde/PHBV |
Electrospinning | Multilayer films could be designed with good antibacterial activity67,158. |
| Chitosan/chitosan-cinnamon oil/sodium alginate | Layer-by-layer electrostatic deposition technique | Multilayer coatings had better antimicrobial activity than monolayer coatings68. |
| Sodium alginate/chitosan-cinnamon essential oil/sodium alginate | Layer-by-layer solvent- casting | Multilayer films had better retention and sustained release than monolayer films68. |
| Balangu seed gum/gelatin-menthol/balangu seed gum | Electrospinning | Multilayer films were designed to control the release of menthol159. |
| PUR/PVA-gentamicin/PUR | Needleless electrospinning technology | Multilayer films with good antimicrobial activity could be designed66. |
|
Zein/zein-thymol/zein Zein-spelt bran/zein-hymol/zein-spelt bran |
Layer-by-layer solvent-casting | The thymol release rate could be controlled by altering film thickness and bran content160. |
| Zein-gelatin/zein-gelatin-oregano oil/zein-gelatin | Continuous casting method | Tri-layer films with oregano oil in intermediate and/or upper layer exhibited a high retention rate161. |
| Alginate/chitosan-sodium benzoate alginate beads/alginate | Layer-by-layer solvent-casting | Alginate beads could be used to control the release rate of sodium benzoate162. |
| PVOH/PVOH-lysozyme/PVOH | Layer-by-layer solvent-casting | The release rate of lysozyme from the films could be controlled163. |
| Bacterial cellulose/PVA-bacterial cellulose-sorbic acid/bacterial cellulose | Layer-by-layer assembly | Sorbic acid release was slower from multilayer than monolayer films164. |
| Bacterial cellulose/PVA-bacterial cellulose-vanillin/bacterial cellulose | Layer-by-layer solvent-casting | A bacterial cellulose control layer delayed vanillin release and prevented PVA dissolution in food165. |
| Starch/PCL-carvacrol/starch | Electrospinning | Multilayer films prolonged antimicrobial action and reduced water vapor permeability compared to starch films86. |
| Bacterial cellulose/ bacterial cellulose-Scrophularia striata/β-cyclodextrin | Layer-by-layer solvent-casting | The release rate of Scrophularia striata was reduced in food simulant with multilayer films166. |
Composite packaging
This type of packaging material is comprised of a blend of active and matrix molecules that are linked together by covalent or noncovalent bonds, such as electrostatic, hydrophobic, hydrogen bonding, or van der Waals interactions20, as shown in Fig. 1b. Covalent bonds involve the sharing of electron pairs between two atoms21. As a result, they tend to be stronger and more stable than noncovalent bonds22. The formation of new covalent bonds between the molecules used to form packaging is usually achieved through specific chemical or enzymatic reactions23. Some of the most commonly utilized reactions, such as esterification, etherification, amidation, and glycosylation, occur between particular functional groups on active and matrix molecules24. Noncovalent bonds also rely on the presence of particular functional groups on these molecules, such as the nonpolar groups for hydrophobic interactions, polar groups for hydrogen bonding interactions, and charged groups for electrostatic interactions20.
The bonds formed between the active and matrix molecules restrict the migration of the active ingredients in the packaging, which can be used to control their retention and release25. As an example, the formation of bonds between thyme extract polyphenols and the chitosan/pea starch in composite films has been reported to reduce the release rate of polyphenols26. Furthermore, the incorporation of tannic acid into the films could reduce polyphenol release by cross-linking the chitosan molecules in the biopolymer matrix. The release rate of active molecules from this kind of packaging material can also be modulated by altering the properties of the film matrix, such as the porosity, swelling, and biodegradation6. In another study, composite packaging was assembled from two active ingredients (tea polyphenol and oregano oil) and two matrix molecules (zein and gelatin), which was designed to control the release of active ingredients27. It was reported that hydrogen and hydrophobic bonds were formed between these active ingredients and matrix molecules. The microstructure of the films changed after the active ingredients were incorporated, with evidence of small spherical droplets being dispersed within a biopolymer matrix. Other examples of research on the retention and release of active ingredients from composite packaging are summarized in Table 3.
Table 3.
Advanced examples of active degradable packaging materials assembled from sandwich-like molecules.
| Matrix composition | Active ingredients | Perspectives |
|---|---|---|
| Chitosan, pea starch | Thyme extract polyphenols | The interaction between polyphenols and the matrix inhibited polyphenol release; solvent polarity affected polyphenol release and antioxidant activity of the film26. |
| Zein, gelatin | Tea polyphenol, oregano essential oil | Hydrogen and hydrophobic bonding occurred between active ingredient and the matrix; the retention of active ingredient promoted by simultaneous loading of tea polyphenols and oregano oil in composite film27. |
| Pullulan, gelatin | Potassium sorbate | The release mechanism of potassium sorbate depended on dissolution and swelling of matrix; release rate could be adjusted by changing pullulan and gelatin ratio167. |
| Oligomeric proanthocyanidins, gelatin | Lysozyme | Increasing oligomeric proanthocyanidin cross-linking retarded lysozyme release78. |
| Cinnamaldehyde, gliadin | Lysozyme | Increasing biopolymer matrix cross-linking retarded lysozyme release79. |
| Methylcellulose, glutaraldehyde | Maqui (Aristotelia chilensis) berry fruit extract | Maqui extracts reacted with glutaraldehyde through phenolic or glycoside-hydroxyl groups; the release amount of antioxidant compounds increased with increasing glutaraldehyde concentration80. |
| Soy protein isolate/poly(ethylene oxide) blend, poly(lactic acid) | Allyl isothiocyanate | The release of allyl isothiocyanate could be controlled by changing the relative humidity168. |
| Low methoxyl pectin | Lysozyme | Low methoxyl pectin formed insoluble complexes with lysozyme, mainly due to electrostatic attraction, which controlled the release of antimicrobial lysozyme169. |
Preparation of advanced packaging
Preparation of multilayer packaging
The preparation of packaging materials with the appropriate functional attributes requires the selection of appropriate ingredients, film-forming techniques, and preparation conditions12,28. This section describes the main preparation technologies and conditions that are typically used to prepare biodegradable packaging materials with different structures and functional properties. Based on literature, the most widely used approaches for preparing multilayer packaging materials are the layer-by-layer (LbL), electrohydrodynamic, and coextrusion methods, while the most widely used ones for creating composite packaging materials are extrusion and solution casting.
Layer-by-layer (LbL) assembly
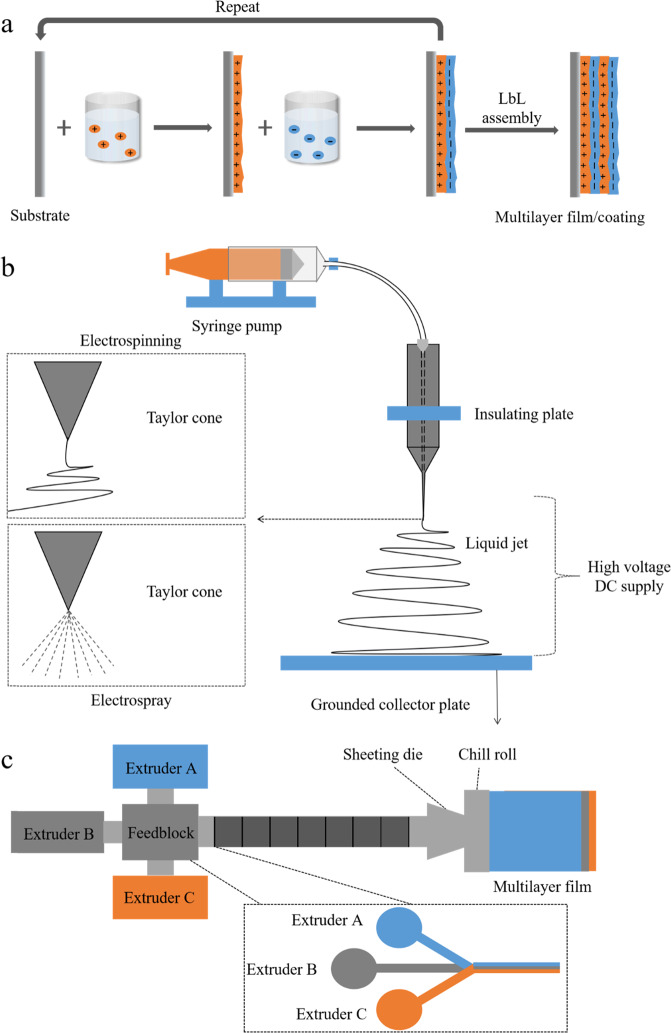
This is a method based on electrostatic attraction, hydrogen bonding, hydrophobic attraction, or entanglements between molecules in neighboring layers. In general, this method involves the formation of multilayer films or coatings by sequential deposition of numerous film-forming materials onto a surface29. The utilization of electrostatic attraction between successive layers is one of the most commonly used methods for assembling this type of film or coating (Fig. 2a). It involves immersing a substrate with a negative surface charge into a solution containing positively charged substances, which causes these substances to be attracted to the surface of the substrate. After washing off the excess solution, the positively charged substrate formed is then immersed in another solution containing negatively charged substances, which causes these substances to form a new layer. This process can be repeated a number of times to produce multilayered films or coatings30. Typically, the materials used in this electrostatic LbL deposition process are water-dispersible and electrically charged biopolymers or colloidal particles. The properties of the films or coatings formed (such as their composition, thickness, and charge) can be manipulated by altering the type, number, and sequence of the charged substances used to assemble the layers.
Fig. 2. Preparation of multilayer edible films.
a Electrostatic layer-by-layer (LbL) deposition. b Electrospinning or electrospraying. c Coextrusion.
In addition to electrostatic attraction, other intermolecular interactions can also be used to assemble LbL films or coatings, such as hydrogen bonding, covalent bonding, and hydrophobic interactions9. The interactions involved depend on the nature of the substances used to prepare the biodegradable packaging. For instance, proteins often form cross-links with neighboring molecules through a combination of hydrophobic, electrostatic, covalent, and hydrogen bonds31,32.
The LbL method has a number of potential advantages over other fabrication techniques for preparing multilayer packaging29:
The preparation process is simple and inexpensive, requiring no specialized equipment;
Films with different functional attributes can be created by altering their physicochemical and structural properties (such as composition, thickness, and charge) by selecting different types, numbers, and sequences of film-forming materials;
The preparation of multilayer films or coatings is not limited to flat surfaces and can be applied to food with different shapes.
Electrohydrodynamic process
The electrohydrodynamic process involves the spraying of polymer solutions into microscale or nanoscale fibers or particles by applying a high-voltage electric field. There are two common methods used in the electrohydrodynamic process: electrospinning and electrospraying33. The packaging formed using electrospinning tends to have a high porosity, high polymer orientation, and large specific surface area, which is advantageous for some food applications34. The utilization of electrospinning to form the layers in multilayer packaging has been shown to enhance the mechanical, optical, and/or functional properties35.
The apparatus typically used to create packaging using the electrospinning technique consists of four main parts: a high-voltage power supply, an injection pump, a capillary tube with a tip, and a metal collector (Fig. 2b). In detail, a polymer solution is placed into the capillary tube and then a high-voltage electric field is applied between the tip of the tube and collection plate. The polymer solution will be drawn out of the tip and form a jet of fluid in the form of a twisted Taylor cone. During its passage from the tip to the collection plate, the solvent is rapidly evaporated, which leads to the formation of polymer-rich nanofibers that are deposited onto the collector34,36,37.
Electrospraying works in much the same way as electrospinning, but the polymer solution and operating conditions used are designed so that the Taylor cone jet produced remains stable and does not lengthen38,39. As a result, nanoparticles are formed by electrospraying rather than the nanofibers formed by electrospinning. It has been reported that electrospraying is more suitable for preparing biodegradable coatings, while electrospinning is more suitable for preparing biodegradable films33. Compared to other methods of preparing packaging, electrohydrodynamic methods have the following potential advantages:
Polymer nanofibers can be prepared directly, continuously, and on a large scale, which is beneficial for commercial applications;
The conditions used to form films or coatings are relatively mild, allowing a broad range of raw materials utilized;
The production equipment is relatively inexpensive and easy to operate (but caution must be taken because of the high voltages used);
The morphology and functional performance of the films or coatings produced can be manipulated by altering the ingredients and processing operations used;
Fibers or particles with nanometer-scale dimensions can be fabricated, which leads to films or coatings with large specific surface areas and high porosities.
Coextrusion
Films with multilayer structures can be prepared using the coextrusion method by heating two or more different kinds of polymer materials above their glass transition temperature and then extruding them separate through a specially designed nozzle40. The coextrusion process generally includes four main steps: feeding, melting, stream confluence, and coextrusion. Based on the way that the different polymer streams are brought together in the film-forming process, coextrusion can be divided into two main types: die and feed block12. In die coextrusion, two or more materials are fed separately into the extruder and heated, and then the different material melt streams are brought together at the exit of the die (Fig. 2c). In feed block coextrusion, two or more materials are brought together before the extrusion die, then made to form a laminated layer of melt stream, which is then extruded through the die. Coextrusion has been widely used for the industrial production of polymer-based packaging. Compared to other technologies, coextrusion has the following potential advantages:
It is beneficial for commercial applications with relatively short processing time and low energy consumption;
Powdered film-forming materials can be used as the input to the extruder, which means that there is no need to remove any solvents after film formation;
The range of operating conditions is relatively wide, which means that film-forming materials with different melting points can be utilized;
The mechanical and optical properties of biodegradable packaging can be improved41,42;
The mixing degree of material and the thickness of films formed can be accurately controlled43.
Preparation of composite packaging
This type of biodegradable packaging material typically consists of active and matrix molecules44. In this case, the whole of the packaging material can therefore be regarded as a carrier system for the active ingredients. The structure and properties of these films can be controlled by altering the composition and processing methods used to fabricate them, which leads to different functional attributes. In general, there are two major methods that are currently used for preparing composite biodegradable films: the dry and wet methods45. Typically, extrusion is used to produce films from solids, whereas solvent-casting is used to produce films from solutions (Fig. 3)5.
Fig. 3. Preparation of composite edible films/coatings.
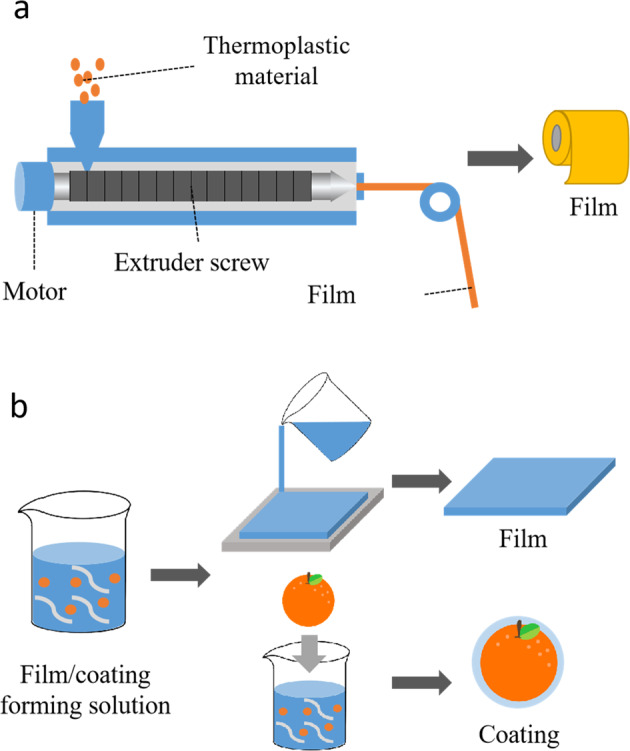
a Extrusion. b Solvent-casting.
Extrusion
The extrusion method mainly utilizes the thermoplastic properties of polymer materials to prepare packaging. In this method, a solid material with low water content is heated above its glass transition temperature to form a melt, thoroughly mixed, and then passed through a suitably-shaped nozzle. The resulting material is then cooled leading to the formation of a film. The use of the extrusion method to prepare biodegradable films typically involves several steps: feeding, mixing, hydration, and extrusion. Thermoplastic food materials, such as powdered polysaccharides (e.g., starch or wheat gluten)46,47 and proteins (e.g., zein, soy protein, whey protein, or gelatin) are typically used as starting materials for extrusion41,42,48.
The various steps in the extrusion process are briefly summarized here42. First, the polymers and plasticizers are introduced into the feed zone and air compression is used to reduce the moisture content of these materials. The materials then enter the kneading zone where they are subjected to a high-temperature/high-pressure kneading treatment. They are then heated to above the glass transition temperature to convert into a melt form, which is extruded through the nozzle due to forces generated by the rotation of the extrusion screw. Unlike coextrusion, the polymer materials used to produce films that are mixed and not layered during the fabrication process. Compared to the other film-forming methods, extrusion has a number of potential advantages, including high throughput, low cost, continuous production, and the ability to process a wide range of materials.
Solvent-casting
Solvent-casting is the most commonly used method for preparing biodegradable films in laboratories. It generally includes three steps: solution preparation, mold casting, and drying49. Since it is difficult to form a biodegradable film with all the functional properties required using a single material, solution preparation usually involves selecting and mixing multiple film-forming materials and functional additives together in an appropriate solvent. The various components used may be molecules dissolved in solution or particles suspended in solution. This process is typically carried out at room temperature but may be carried out at other temperatures if required. The resulting system is then poured into a suitable mold and the solution is dried by removing the solvent through evaporation, which may involve simple air-drying or the use of drying equipment. During the drying process, the interactions and arrangement of the molecules in the system will change. Compared to the extrusion method, the solvent-casting method does not require the use of complicated equipment or expensive cost, and the preparation process is simple. At the same time, the absence of high-temperature heating avoids the degradation of heat-sensitive active ingredients. However, the preparation process of the solvent-casting method takes a long time and is not suitable for mass production50.
Controlled release types and mathematical models for active packaging
The release of active ingredients from food packaging may involve controlled or sustained release mechanisms, which can be achieved by regulating the parameters affecting the movement of the active molecules through the film matrix. In general, the release rate is influenced by the nature of the active ingredient, the properties of the matrix materials, the film-forming method used, the properties of the release media, and the environmental conditions. The main objective of sustained release is to prolong the release of the actives from the film so that the desired activity is maintained over an extended period. The main objective of controlled release is to create a specific release profile that depends on the application, which could involve, burst, triggered, or sustained release. Researchers in this area aim to establish the impact of specific active and matrix properties on the release profile so that they can manipulate it for the required application51.
Diffusion-controlled release
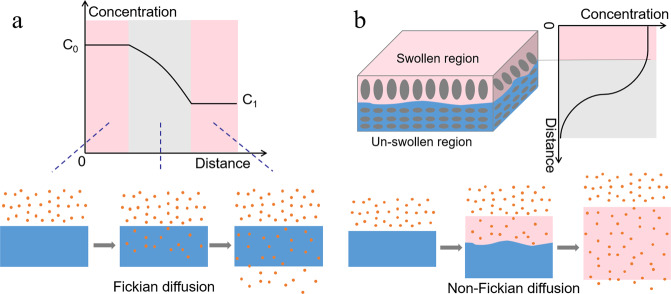
Diffusion refers to the process where active ingredients in food packaging move from areas of high to low concentration as a result of the concentration gradient. Diffusion is present in all controlled release systems, and only systems with diffusion as the dominant release mechanism are referred to as “diffusion-controlled” release systems. Diffusion-controlled release is usually described by Fick’s law (Fig. 4). When the relaxation time of the matrix material is much longer than the characteristic solvent diffusion time, the controlled release system conforms to Fick’s law51. It should be noted that Fick’s law only applies to simple shaped systems where time, space, and concentration are independent of diffusivity. Unlike the erosion and biodegradation mechanisms, the degradation of matrix materials is not obvious during diffusion release. Typically, this diffusion release mechanism can be implemented using either reservoir or matrix systems.
Fig. 4. Distribution models of active ingredients in the film/coating during diffusion.
a Fickian model b non-Fickian model.
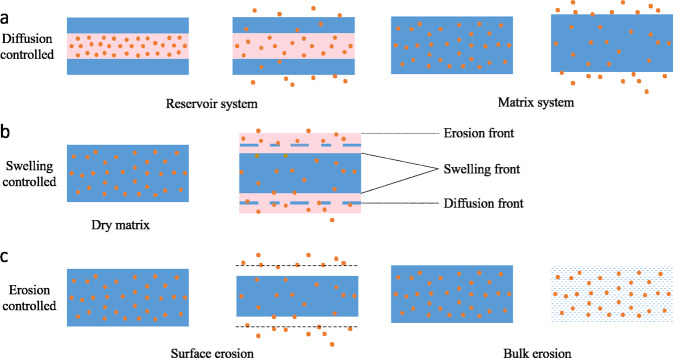
The reservoir system typically consists of an active layer and a barrier layer, with the active ingredient located inside the active layer, as shown in Fig. 5. In a reservoir system, the diffusion rate of the active ingredient depends on the properties of the barrier layer, including its thickness, area, and permeability7. Generally, the release of active ingredients involves three processes: the diffusion of solvent, the dissolution of active ingredients and the diffusion of active ingredients. In the case of ignoring the boundary layer resistance, the release rate is mainly controlled by the active ingredient diffusion step. When there is enough active ingredient in the packaging material, diffusion follows zero-order kinetics, that is, the active ingredient maintains a constant release rate, thus achieving the purpose of controlled release52.
Fig. 5. Three common release mechanisms utilized in active packaging.
a Diffusion-controlled release. b Swelling-controlled release. c Erosion-controlled release.
Under appropriate conditions, Fick’s first law of diffusion can be used to describe the release of active ingredients from reservoir systems using the following mathematical model19.
| 1 |
Here, dM/dt is the release rate of the active ingredient at time t, and A and L are the surface area and thickness of the layers respectively. D is the diffusion coefficient through the matrix material; K is the distribution coefficient of the active ingredient between the film and environment; and ΔC is the solubility of the active ingredient. When D, L, K, and ΔC remain unchanged, the ΔM/dt is stable, which results in a constant release rate of the active ingredients. When the active ingredient is released, it may affect the integrity of the barrier layer, causing collapse or cracks and resulting in changes in the surface area, thickness, or permeability of the material, and the release process no longer conforms to the zero-order release kinetics, making the release rate unstable53. Therefore, the selection of barrier materials in the reservoir system is very important for the controlled release of active ingredients. Generally, materials that are not easily degraded or swelled are selected as the barrier layer.
In the matrix system, the active ingredients are uniformly distributed within a non-biodegradable and non-erodible matrix. The release rate is related to the shape of the system, the type of matrix material used, and the properties of the active ingredient. In a matrix system, the release steps of the active ingredient include dissolution of the active ingredient in the matrix, diffusion of the active ingredient through matrix, and the removal of active ingredient from the surface of the matrix. Among them, the rate-limiting step for the release of active ingredient is usually diffusion through the matrix. Since there is no barrier layer, the matrix system will have a burst release in the early stages, and then the release rate will gradually decrease over time. The release rate of active ingredients in the matrix system generally follows Fick diffusion, and the release rate varies with the shape of the system. The shape of the system can be assumed to be an infinite flat plate with a thickness of δ, and the change of the release rate of active ingredients with time can be described by the following mathematical model54.
| 2 |
Here, Mt/M∞ represents the fractional solute release of the active ingredient at time t, and Dm represents the diffusion coefficient of the active ingredient through the matrix. At the initial stage of the release, when the fractional solute release is less than 60%, the release rate of the active ingredient is given by the following mathematical model:
| 3 |
In this formula, Mt/M∞ is proportional to t−0.5, thus it is difficult to maintain a stable release rate of the active ingredient in the matrix system with a simple flat plate structure. Instead, controlled release from matrix systems is often achieved by controlling the shape of the matrix structure, e.g., cylindrical or spherical55,56. In contrast, reservoir systems vary the properties of the barrier layers to control the release of active ingredients, e.g., their thickness or material properties. Compared to the matrix system, the reservoir system is easier to obtain stable diffusion rates, so it is more widely used in food active packaging.
Swelling-induced release
Swelling-controlled release systems are based on changes in the morphology of the polymer matrix when it comes into contact with certain solvents. In this case, when the matrix contacts with the solvent, it absorbs solvent molecules, which causes it to swell. This process increases the spaces between the polymer molecules in the matrix, thereby allowing the active molecules to be released. More specifically, the matrix material undergoes a glass transition when it absorbs solvent molecules, which changes the structure of the matrix and dissolves active ingredients. As a result, the diffusion coefficient of active ingredient increases, which allows it to move through the polymer matrix and be released into the surroundings. During this process, the matrix material is not degraded or eroded, so the packaging material maintains its original shape. The process of swelling-controlled release systems therefore involves several steps: solvent absorption, volume swelling, active dissolution, and active diffusion19.
The release rate depends on which step is the rate-limiting one. If the solvent absorption, dissolution or diffusion steps are the rate-limiting steps, the release of active ingredient conforms to Fickian diffusion. Conversely, if the volume swelling step is the rate-limiting one, the release of the active ingredient is non-Fickian diffusion. In this case, the relevant parameters of the volume swelling need to be considered57.
After the matrix absorbs the solvent, it undergoes a transition from the glassy to the rubbery state, which increases the molecular mobility of the polymer chains and the porosity of the network structure. This process is usually described by the following simple mathematical model58:
| 4 |
In this formula, Mt/M∞ represents the fractional solute release of the active ingredient; k represents the rate constant; and n is a number that depends on the release mechanism: 0.5 < n < 1 for non-Fickian diffusion; n = 0.5 for Fickian diffusion (as shown in Eq. (3)); n = 1, Fickian and non-Fickian diffusion occur simultaneously, which is called case II transport7. When the release rate of the active ingredient only depends on the swelling rate of polymer, the release rate of the active ingredient is positively correlated with the amount of solvent entering the system.
For non-Fickian diffusion, the following mathematical model of expansion dynamics can be used considering the influence of polymer viscosity on osmotic pressure, assuming the sheet plate model59,60:
| 5 |
In this formula, V1 is the molecular volume of the solvent; NA is the Avogadro constants; V is the concentration of the active ingredient; A is the activity of the solvent; and dV/dt is the concentration of the active ingredient. By numerical integration of this equation, the function of the swelling part of the glassy polymer in the thin plate with time can be obtained. On this basis, it has been reported that the release of active ingredients depends on the dynamic change of substrate thickness. For instance, for the swelling of matrix tablets, either erosion or diffusion front motion could inhibit the release kinetics of active ingredients, depending on their relative rates61. The matrix materials used in swelling-controlled release systems are generally hydrophilic polymers such as proteins or polysaccharides that can undergo glassy to rubbery transitions in the presence of solvents (typically water).
Degradation-induced release
Degradation refers to the process of chemical degradation of polymer molecules and/or physically disruption of polymer matrix, which leads to the simultaneous breakdown of packaging and release of active ingredients62. When the substrate is a biodegradable material and the erosion rate of the substrate is much less than the diffusion rate, the diffusion is considered as the rate-limiting step, and the release kinetics of the active ingredients is mainly characterized by diffusion, which is described by Fick’s law (see Eqs. (1–3)). Conversely, when substrate erosion is the main rate-limiting step, the controlled release rate of active ingredients is mainly affected by the degradation rate of matrix materials54. Depending on the degradation mechanism, either surface or bulk erosion can occur63.
Surface erosion involves degradation that occurs only at the surface of polymer matrix that is in direct contact with the surrounding solvent, and often involves polymers that contain functional groups that undergo rapid hydrolysis63. The release rate of active ingredients is affected by the shape and surface area/volume ratio of system. In the absence of boundary effects, the release rate of active ingredients from flat films is given by:
| 6 |
In this equation, dM/dt is the release rate of the active ingredient; C0 represents the content of the active ingredient per unit area; B represents the surface degradation rate of the polymer; and A is the surface area of the film. When the surface area remains constant, a steady release rate of active ingredient can be achieved by ensuring that C0*B also remains constant. In this case, the degradation rate of polymer surface should be inversely proportional to the content of active ingredient per unit area.
When C0*B is constant, the dM/dt is proportional to the surface area A, which is in line with the Hopfenberg empirical model64:
| 7 |
In this formula, Mt/M∞ represents the fractional solute release of the active ingredient; k0 is the erosion rate constant; C0 is the initial concentration of the active ingredient in the system; a represents 1/2 of the slice thickness; and n is related to the system’s shape: for a thin slice, n = 1; for a cylinder, n = 2; and for a sphere, n = 3. Since the Flake model is mainly used in food packaging, n = 1. Consequently, the release rate can be described by the following expression:
| 8 |
In this case, the release of active ingredients conforms to zero-order release kinetics, so that a controlled release of active ingredients can be realized.
When the diffusion rate of solvent in the system is greater than the degradation rate of the matrix, then the degradation mode is bulk erosion. In this case, the solvent uniformly disperses throughout the polymer matrix, causing the polymer chains to break or dissociate and the system to erode uniformly7. The release rate of the active ingredient is not related to the surface area or volume of the system but depends on the solvent diffusion rate and the decomposition/dissociation rate of the polymer. Generally speaking, the release rate in the early stage of bulk erosion is relatively small, then increases rapidly with the rapid diffusion of the solvent and the continuous degradation of the matrix.
However, both surface and bulk erosion mechanisms are in ideal conditions. In practice, polymer matrices are degraded or dissociated by a mixture of both surface and bulk erosion. In this case, the situation is more complex, and an erosion parameter Ɛ can be defined63:
| 9 |
In this model, represents the average diffusion distance; λ is a constant; Deff is the effective diffusivity of water in the polymer; is the average molecular weight of the polymer; NA is the Avogadro constant; N is the average number of monomers per polymer chain; and ρ represents the density of the polymer matrix. It can be seen from this equation that the type of polymer and the shape of the system are the main factors affecting the release rate of the active ingredients. When applying this model to food packaging, the value of can be manipulated by adjusting the thickness of the film and changing the polymer type to achieve the appropriate erosion parameters.
When the polymer is hydrophilic or contains highly reactive functional groups, water can rapidly spread throughout the polymer matrix, leading to predominantly bulk erosion. Conversely, when the polymer is hydrophobic or contains less reactive functional groups, it is more prone to surface erosion52. One of the main advantages of the surface erosion mechanism is that the release rate can be controlled by altering the surface area of matrix material54.
Controlled release mechanisms of advanced active packaging
Multilayer packaging
The controlled release of active ingredients in food packaging depends on many factors, with the most important ones depending on the dominant release mechanism. The composition and structure of packaging can be controlled to manipulate these factors. In this section, we consider the use of packaging with multilayer structures, which have been shown to be better at controlling the release of active ingredients65. The mechanisms of which multilayers can improve the release characteristics of films are described in the following text.
There are more options for controlling the release properties of multilayer films than other films. For instance, the composition, properties, and thickness of each of the different layers used can be manipulated. Initially, we consider the impact of increasing the thickness of the layers. In diffusion-induced release reservoir systems, it can be seen from Eq. (1) that the film thickness (L) is inversely proportional to the release rate. In swelling-induced release systems, the release of active ingredients depends on the increase in the film thickness over time. In the biodegradable-release induced system, the average diffusion distance of active ingredients in Eq. (9) affects the erosion parameters of the system. Thus, the distance between the active ingredients and food surface affects their release profile. The impact of layer thickness on the release profile of gentamicin from multilayer materials (PUR/PVA/PUR) assembled from polyvinyl alcohol (PVA) and polyurethane (PUR) nanofibers have been studied66. The results show that the retention time of gentamicin could be prolonged by increasing the thickness of the layers. In another study, the release of potassium sorbate from cellulose acetate/potassium sorbate/cellulose acetate (CA/Psb/CA) multilayer films was compared to that from the monolayer films28. The release rate of potassium sorbate is slower from the multilayer films than the monolayer ones. In the monolayer films, potassium sorbate release is controlled by Fickian diffusion at the beginning but then dissolution of potassium sorbate crystals at the later stages. In contrast, for the multilayer films, there are no crystals present and so the release is only controlled by diffusion.
In a sandwich-like multilayer structure, the protective effect of barrier layer can reduce the loss of active ingredients, which can improve the retention and activity of active ingredients. In another study, cinnamaldehyde was loaded into multilayer materials formed from zein, poly(hydroxybutyrate-co-hydroxyvalerate) (PHBV), and/or sodium alginate films67. The cinnamaldehyde was trapped in the zein layer. The release rate and release mechanism of the cinnamaldehyde could be manipulated by altering the number and type of additional layers used. In particular, the release rate decreased as the number of layers was increased. In another study, cinnamon oil (CO) was encapsulated within multilayer materials assembled from chitosan (CS) and sodium alginate (SA) films68. The release profile of this essential oil was compared for monolayer films (CS–CO), bilayer films (SA/[CS–CO]), and multilayer films (SA/[CS–CO]/SA). The results showed that the multilayer films were the most effective at retaining the antimicrobial active ingredients after 10 days storage, with less than 40% of the cinnamon oil being lost. In contrast, as much as 70% of the cinnamon oil was lost from the monolayer films.
The presence of the control layer in multilayer sandwich-like films is important for inhibiting the burst release of active ingredients from packaging. Without the control layer, the active ingredient would be directly in contact with the food surfaces and therefore rapidly diffuse into the food, which would reduce its action time. The control layer, located between the active ingredient and the food surface, is a layer through which the active ingredient should pass to reach the food surface. Therefore, the thickness, chemical composition, and diffusion properties of the control layer are important factors related to the release of active ingredients in these multilayer structures6. As an example, silver nanoparticles (AgNPs) have been incorporated into multilayer films assembled from chitosan and graphene (GO) layers: CS/[GO@AgNPs]/CS69. This study showed that the release of the nanoparticles from the multilayer films was considerably slower than from monolayer films (GO@AgNPs). Catechins have also been placed within the middle layer of biodegradable multilayer packaging, so that they are not directly in contact with the surfaces of the food, which can extend their antioxidant activity under aerobic conditions and reduce the rancidity of packaged food70.
Composite packaging
The active agents are dispersed within a polymer matrix in composite packaging. The retention and release of the active molecules can then be controlled by altering the interactions between the active and matrix molecules, as well as by the properties of the polymer network71. In general, this kind of system can be divided into two types (network-limited system and interaction-limited system) depending on the nature of the dominant release mechanism. For a network-limited system, the release of the active ingredients mainly depends on the properties of the three-dimensional polymer network inside the packaging material, such as its porosity and stability to environmental changes. For an interaction-limited system, the release of the active ingredients mainly depends on the strength of the attractive and repulsive interactions between the active molecules and the polymer matrix.
The molecules in packaging are cross-linked by covalent or noncovalent bonds to form a three-dimensional polymer network structure25,72. These cross-links are usually formed by physical, chemical, or enzymatic mechanisms. Cross-linking can be carried out during or after the film structure is formed73. Typically, active molecules form cross-links with the matrix molecules during the preparation of film or coating materials. The formation of these cross-links can be used to control the retention and release of the active ingredients in packaging. A number of physicochemical mechanisms can be utilized to control the release profile including: (1) controlling the pore size of the original polymer network; (2) altering the swelling properties of the polymer network; (3) reducing the biodegradation and erosion of the polymer network; (4) controlling the physical or chemical binding of the active ingredient to the polymer network6,73–77. As an example, lactoferrin (an antimicrobial protein) has been incorporated into composite packaging assembled by cross-linking gelatin with oligomeric proanthocyanidins (OPCs)78. The observed initial burst release of lysozyme from the packaging could be reduced by increasing the OPCs concentration so as to induce greater cross-linking of the polymer network. In addition, it was reported that the OPCs restricted lysozyme release much less in acidic and alkaline environments than in neutral ones, suggesting that these packaging may be used for the pH-triggered release of lysozyme.
In another study, the effect of different concentrations of cinnamaldehyde on the release of lysozyme from gliadin films was studied, where the cinnamaldehyde was used as a cross-linking agent and glycerol was used as a plasticizer79. At pH 2, gliadin films containing 1.5%, 3%, and 5% cinnamaldehyde released 48%, 18%, and 8% of the active ingredient after 115 h, respectively. This result was attributed to greater cross-linking of the polymer network at higher cinnamaldehyde concentrations, which would have reduced the pore size and inhibited lysozyme diffusion. In a different experiment, biodegradable active packaging containing maqui berry fruit extract (an antioxidant) were prepared by cross-linking methylcellulose with glutaraldehyde80. The quantity of polyphenols released decreased as the cross-linking agent concentration increased, which can again be attributed to a reduction in the pore size of the polymer network.
For interaction-limited systems, the controlled release of active ingredients mainly depends on the covalent and noncovalent bonds formed between the active and matrix molecules. Generally, covalent bonds are stronger than noncovalent ones and therefore lead to a stronger attachment of the active molecules to the polymer matrix. A method has recently been developed that uses covalent bonds to connect active molecules to polysaccharides and then apply them in biodegradable coatings81. In this study, a covalent bond was formed through a Schiff base and reductive amination reaction, which combined vanillin, trans-cinnamaldehyde, and chitosan to form a biodegradable coating with good adhesion. The results showed that the formation of a covalent bond overcomes the dissolution problem of lipophilic vanillin and cinnamaldehyde in the reaction, as well as reducing their volatility. The resulting biodegradable coating exhibited better antibacterial activity and shelf-life extension of melon than the control groups. The authors concluded that the advantages of using covalent bonds rather than noncovalent ones are: (1) solubility problems associated with binding active lipophilic reagents in aqueous media can be overcome; (2) the release of volatile compounds can be inhibited; (3) coating adhesion to the fruit can be improved. Thus, covalent bonds are highly effective at limiting the release of active molecules. Other authors have also demonstrated that covalently attaching active molecules to matrix molecules is effective at restricting the release of active ingredients82,83. Nevertheless, there is clearly a need for more research on the utilization of both covalent and noncovalent bonds specifically designed to modulate the release profile of active ingredients from biodegradable composite packaging in a more systematic fashion.
Properties of advanced packaging materials
Compared to petroleum-based packaging materials, biodegradable films that are composed of biopolymers usually have a worse barrier and mechanical properties. Consequently, researchers are looking at approaches to address this problem, including adding crosslinkers, plasticizers, and fillers to the films, as well as changing their structural organization.
Barrier properties
The barrier properties are an important part of food packaging materials, such as their water vapor permeability, gas permeability, and light transmittance properties. The main parameters affecting mass transfer of substances across packaging materials are diffusivity, solubility, and permeability, which are closely related to the composition and structure of the polymer matrix84.
Research has shown that multilayer structures can improve the barrier properties of biodegradable packaging materials. For instance, the mechanical strength and gas barrier properties of multilayer films (PLA/FG/PLA) and monolayer films (FG) assembled from polylactic acid (PLA) and fish gelatin (FG) were compared85. The oxygen permeability and water vapor permeability of the three-layer films were 8 and 11 times lower than those of the monolayer films, respectively. Moreover, the tensile strength of the three-layer films increased by around 17.5 MPa, which was appreciably higher than that of the monolayer ones. In another study, starch/PCL-carvacrol/starch multilayer films were prepared by electrospinning using starch and polycaprolactone (PCL) as the matrix materials and carvacrol as the active ingredient86. The water vapor permeability of these multilayer films (190 g m−1 pa−1 s−1) was considerably lower than that of starch films (570 g m−1 pa−1 s−1), which highlights the efficacy of the multilayer structures to improve the barrier properties of packaging materials.
Research has also been carried out on composite active packaging materials. For example, biodegradable films have been prepared by incorporating eugenol (active ingredient) into starch and chitosan mixtures (matrix materials) using solvent-casting and solvent-evaporation methods87. Optimization of the matrix composition was shown to decrease the WVP of the films from around 2.00 × 10−10 g m−1 pa−1 s−1 to 1.29 × 10−10 g m−1 pa−1 s−1. Moreover, the addition of eugenol significantly improved the flexibility, hydrophobicity, antimicrobial activity, and antioxidant properties of the films. The authors obtained information about the molecular interactions between the active ingredient and matrix materials including the hydrogen bonding or other covalent bonding.
Mechanical properties
The mechanical properties of packaging materials are critical for many of their applications, including the elongation at break, tensile strength, yield stress, yield strain, and Young’s modulus12. The layer structure of packaging materials has been shown to have an important effect on their mechanical properties88. The overall mechanical properties of multilayer systems depend on the properties of the individual layers they are comprised of, which are usually proteins and/or polysaccharides in biodegradable packaging materials49,89. For instance, kafirin/kafirin-gelatin/gelatin films have been prepared using a layer-by-layer solvent-casting method90. These multilayer films were shown to have a higher tensile strength (6.3 MPa) and better resistance to water migration than blended films made from kafirin and gelatin.
The mechanical properties of biodegradable films are also related to the interactions between the molecules, which may be enhanced by the incorporation of some active ingredients. As an example, the impact of citric acid concentration on the properties of potato starch-chitosan (PS–CS) composite films prepared by a solution blending-casting method have been examined91. Citric acid was found to act as a cross-linker, which significantly enhanced the mechanical properties of the composite films. The performance of the composite films was found to depend on the citric acid concentration used. When the amount of citric acid added was increased from 0 to 15%, the tensile strength of the films increased from around 9.7–12.6 MPa, but when it was increased further the tensile strength decreased. This effect may be due to the plasticizing effect of the citric acid at high concentrations, which reduced the attractive interactions between the matrix molecules. In some studies, it has been reported that incorporation of active ingredients can decrease the mechanical strength of biodegradable films. For example, incorporation of apple polyphenols into chitosan films reduced the tensile strength and elongation at break, but increased the antibacterial and antioxidant properties92. Consequently, it is important to elucidate the impact of specific additive ingredients on the mechanical properties of specific biopolymer matrices when developing effective active biodegradable packaging materials.
Degradability
In many published studies, the characterization of active packaging materials mainly focuses on their bioactivity but neglects their degradability45. In general, the degradation of packaging materials may occur through various mechanisms, which can be classified as photodegradable, oxidatively degradable, biodegradable, and hydrolytically degradable93. It should be noted, however, that different types of packaging materials are affected differently by their environment, such as pH, temperature, and humidity. Consequently, the rate and extent of the degradation of packaging materials may depend on precisely where they are disposed, e.g., a hot humid country near the Equator or a cold dry country near the Artic circle. Indeed, the degradation of many of the proteins and polysaccharides used to assemble packaging materials is strongly affected by pH, temperature, and humidity. Consequently, it is important to establish the degradation of a new packaging material under the conditions it will be disposed of.
Application of advanced packaging materials in preservation of fruits and meats
Fresh fruits, vegetables, and meat products are easily spoiled due to their high moisture content, so food packaging is needed to extend their shelf life, improve their quality, and ensure their safety. Compared to plastic packaging materials, advanced biodegradable packaging can include active ingredients that have antibacterial and antioxidant functions, thus providing a substantial advantage in extending the quality, shelf life and safety of packaged food.
Multilayer packaging
In general, multilayer packaging materials can be designed to have better performance, such as enhanced barrier properties, mechanical strength, and protective properties. In particular, active ingredients (such as antioxidants and antimicrobials) can be added to one or more layers of packaging materials to enhance their ability to protect food94.
Studies have shown that adding antibacterial agents to multilayer active materials can increase the storage time and the quality of food. For instance, biodegradable composite packaging has been developed using a layer-by-layer assembly technique to prepare multilayer films containing chitosan and polyvinyl alcohol95. The performance of these films could be enhanced by adding Cu2O-based antibacterial particles to the matrix. These films improve the quality attributes of cherry tomatoes during storage by reducing texture and moisture loss96. In another study, multilayer biodegradable films were created to control the respiration of fruits and vegetables by releasing 1-methylcyclopropene (1-MCP) to inhibit ethylene production. The films consist of hydrophobic ethylcellulose as the outer layer, 1-MCP and palladium carbon (1-MCP-Pd/C) as the middle layer, and a hydrophilic hydrogel as the inner layer. The inner hydrophilic gel was designed to absorb excess water from the fruits and vegetables, thereby avoiding the accumulation of condensed water. The inner hydrogel permeability was changed after absorbing moisture, which promotes the diffusion of antiseptic and antibacterial inner components. The film was then applied to mushroom preservation, which delays softening, browning, and weight loss during storage97.
Meat products are rich in proteins and lipids, which makes them susceptible to chemical and microbial degradation. Food packaging materials have been developed to overcome these problems by including antibacterial and antioxidant ingredients. For instance, tea polyphenol has been incorporated into multilayer films formed from poly(L-lactic acid), polyvinyl alcohol, and poly (ε-caprolactone) using a lamination technique, which can act as an antioxidant and antimicrobial that increased the shelf life and quality of packaged meat products98.
Composite packaging
There have also been many examples of the application of composite packaging materials for food applications. In this section, we discuss a few examples to highlight the potential of these kinds of materials. A composite film fabricated from egg proteins and cellulose nanomaterials has been shown to increase the shelf lives of banana, avocado, papaya, and strawberry99. Films prepared from polyvinyl alcohol, ethylcellulose and tea polyphenols (PVA/EC-TP) using an electrostatic spinning technology were shown to extend the shelf life of packaged pork by 3 days, which was mainly attributed to the antioxidant and antimicrobial activity of the polyphenols100. The shelf life of packaging food has also been increased by controlling gas penetration using composite materials assembled from organic porous chitosan microspheres embedded in a poly(L-lactic acid) matrix95. The microspheres were used as gas “switches” to regulate the permeability of the films to O2, CO2, and H2O and the selectivity of CO2/O2. This packaging material was then applied to citrus, mango, cherry, waxberry, and strawberry to form coatings or films. The results show that the films/coatings could prolong the shelf life and improve the quality of the fruits.
Future trends
Due to environmental pollution and non-renewable petroleum resources, it is likely that researchers will continue to investigate and develop advanced biodegradable materials to replace plastic ones. At present, the share of biodegradable packaging materials in the food packaging market is very low. Indeed, many of these advanced materials are still in the research and development stage, and feasibility studies of their commercial viability have not been performed. To further advance the use of active biodegradable materials, the following problems need to be addressed:
The materials and equipment required to manufacture active biodegradable packaging materials should be available, affordable, and capable of large-scale economic production. Many of the materials and processes described in the scientific literature do not currently meet these requirements;
The performance of new packaging materials in real situations needs to be systematically addressed. Many of the materials described in the literature may be difficult to apply to real food products, may adversely affect their quality attributes, or may not survive during storage, distribution, and utilization;
Government regulations need to be developed globally to address the appropriate creation and utilization of novel kinds of packaging materials.
Consumers need to be educated about the potential advantages of new packaging materials to the environment.
In particular, it is important to address the amount of advanced packaging required to ensure that it can meet the market demand. The global output of plastics was around 368 million tonnes in 2019 (https://www.plasticseurope.org/en/resources/market-data). Demand for plastic is on the rise especially with the development of takeout and express delivery services. However, the growth in plastic production has been matched by poor recycling, such as in the case of the European Union, which produced about 61.8 million tonnes of plastic in 2018, while only 9.4 million tonnes were recycled (https://www.plasticseurope.org/en/resources/market-data). As a result, it will be important that any new packaging materials can be produced on these scales if it is going to have a significant environmental benefit. As mentioned earlier, many of the methods developed to produce biodegradable packaging materials in the literature are not currently appropriate to meet these large demands. For instance, many researchers use solvent-casting to produce advanced packaging materials, which are unsuitable for large-scale industrial production. In addition, the active ingredients used in many studies (such as polyphenols, essential oils, or nanofibers) are expensive or have practical challenges to their utilization. For instance, essential oils may evaporate or chemically degrade during storage, thereby reducing their activity. Consequently, a better understanding of the performance of advanced packaging materials under real conditions is required. Thus, there is a need to develop more commercially viable methods for producing biodegradable active packaging materials on a large scale, as well as to rigorously test their performance under realistic application conditions.
Countries around the world are introducing policies to regulate the use of petroleum-based plastics, so as to reduce their negative environmental impacts, such as the European Committee for Standardization (EN13432-2000), American Society for Testing and Materials (ASTM D6400-2004), and the Deutsches Institut für Normung (DIN V 54900). As an example, some of the common standards that have been developed for biodegradable plastics are: (1) passing an aerobic biodegradation test; (2) passing a composting and biodegradation test; (3) passing an ecological non-toxic test; (4) passing a heavy metal content test. New biodegradable materials should also be specifically designed to pass all of these tests. Although the use of active ingredients in food has gradually been regulated in recent years, there is still no unified authority to issue relevant specifications for active biodegradable packaging, and there is still a long way to go in market supervision.
Consumer acceptance of advanced packaging is also an important factor in their promotion. Surveys have shown that the price and quality of food are more important than green packaging, and consumers are only willing to pay a small premium for biodegradable packaging (https://www.plasticseurope.org/en/resources/market-data). To improve consumer acceptance, it will therefore be important to reduce the costs of new biodegradable packaging materials, create government policies (such as taxes and subsidies) that reduce the price differential between biodegradable and non-biodegradable packaging materials, and to inform consumers of environmental benefits.
Conclusions
There is growing interest in the design and production of biodegradable packaging to replace synthetic plastics so as to reduce the negative environmental impacts associated with their production and disposal. In this review, we focus on biodegradable packaging materials with either multilayer or composite structures. In particular, we focus on the preparation and release mechanisms of these kinds of materials. In addition, the basic release mechanisms of controlled release packaging and the potential impact of active ingredients on the mechanical and barrier properties of films and coatings are reviewed. At present, active biodegradable packaging materials have been successfully designed, characterized, and applied in research laboratories. However, many factors still need to be addressed before the widespread commercial success of these materials, including demonstrating their efficacy in real-life applications, developing large-scale economic manufacturing processes, establishing appropriate government regulations, and gaining consumer acceptance.
Author contributions
All authors contributed to the inception and development of this paper. Q.W. collected the literature and wrote the original draft. W.C. and W.Z. revised the initial manuscript. D.J.M. revised the final manuscript critically and improved the manuscript accordingly. X.L. revised the final manuscript before submission. F.L. drafted the outline and revised the manuscript.
Data availability
Data sharing is not applicable. This is a review article and no new datasets were generated or analyzed during this article.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Muncke J. Tackling the toxics in plastics packaging. PLoS Biol. 2021;19:e3000961. doi: 10.1371/journal.pbio.3000961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Motelica, L. et al. Biodegradable antimicrobial food packaging: Trends and perspectives. Foods9, 1–36 (2020). [DOI] [PMC free article] [PubMed]
- 3.Debeaufort F, Voilley J-AQ-GA. Edible films and coatings: tomorrow’s packagings: a review. Food Sci. Nutr. 2010;38:299–313. doi: 10.1080/10408699891274219. [DOI] [PubMed] [Google Scholar]
- 4.Barbosa CH, Andrade MA, Vilarinho F, Fernando AL, Silva AS. Active edible packaging. Encyclopedia. 2021;1:360–370. [Google Scholar]
- 5.Ribeiro AM, Estevinho BN, Rocha F. Preparation and incorporation of functional ingredients in edible films and coatings. Food Bioprocess Technol. 2021;14:209–231. [Google Scholar]
- 6.Almasi, H., Jahanbakhsh Oskouie, M. & Saleh, A. A review on techniques utilized for design of controlled release food active packaging. Crit. Rev. Food Sci. Nutri.61, 2601–2621 (2020). [DOI] [PubMed]
- 7.Mastromatteo M, Mastromatteo M, Conte A, Del Nobile MA. Advances in controlled release devices for food packaging applications. Trends Food Sci. Technol. 2010;21:591–598. [Google Scholar]
- 8.Aloui H, Khwaldia K. Natural antimicrobial edible coatings for microbial safety and food quality enhancement. Compr. Rev. Food Sci. Food Saf. 2016;15:1080–1103. doi: 10.1111/1541-4337.12226. [DOI] [PubMed] [Google Scholar]
- 9.Arnon-Rips H, Poverenov E. Improving food products’ quality and storability by using layer by layer edible coatings. Trends Food Sci. Technol. 2018;75:81–92. [Google Scholar]
- 10.Falguera V, Quintero JP, Jiménez A, Muñoz JA, Ibarz A. Edible films and coatings: Structures, active functions and trends in their use. Trends Food Sci. Technol. 2011;22:292–303. [Google Scholar]
- 11.Xia C, Wang W, Wang L, Liu H, Xiao J. Multilayer zein/gelatin films with tunable water barrier property and prolonged antioxidant activity. Food Packaging Shelf Life. 2019;19:76–85. [Google Scholar]
- 12.Anukiruthika T, et al. Multilayer packaging: advances in preparation techniques and emerging food applications. Compr. Rev. Food Sci. Food Saf. 2020;19:1156–1186. doi: 10.1111/1541-4337.12556. [DOI] [PubMed] [Google Scholar]
- 13.Sharma R, Jafari SM, Sharma S. Antimicrobial bio-nanocomposites and their potential applications in food packaging. Food Control. 2020;112:107086. [Google Scholar]
- 14.Cozzolino CA, et al. Exploiting the nano-sized features of microfibrillated cellulose (MFC) for the development of controlled-release packaging. Colloids Surf. B: Biointerfaces. 2013;110:208–216. doi: 10.1016/j.colsurfb.2013.04.046. [DOI] [PubMed] [Google Scholar]
- 15.Narayanan A, Mallesha N, Ramana KV. Synergized antimicrobial activity of eugenol incorporated polyhydroxybutyrate films against food spoilage microorganisms in conjunction with pediocin. Appl. Biochem. Biotechnol. 2013;170:1379–1388. doi: 10.1007/s12010-013-0267-2. [DOI] [PubMed] [Google Scholar]
- 16.Neo YP, et al. Evaluation of gallic acid loaded zein sub-micron electrospun fibre mats as novel active packaging materials. Food Chem. 2013;141:3192–3200. doi: 10.1016/j.foodchem.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 17.Wu YM, Wang ZW, Hu CY, Nerín C. Influence of factors on release of antimicrobials from antimicrobial packaging materials. Crit. Rev. Food Sci. Nutr. 2018;58:1108–1121. doi: 10.1080/10408398.2016.1241215. [DOI] [PubMed] [Google Scholar]
- 18.Quiles-Carrillo, L., Montanes, N., Lagaron, J. M., Balart, R. & Torres-Giner, S. Bioactive multilayer polylactide films with controlled release capacity of gallic acid accomplished by incorporating electrospun nanostructured coatings and interlayers. Appl. Sci.9, 533 (2019).
- 19.Wang, S., Liu, R., Fu, Y., & Kao, W. J. Release mechanisms and applications of drug delivery systems for extended-release. Expert Opin. Drug Del.17, 1289–1304 (2020). [DOI] [PubMed]
- 20.Jakobek L. Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chem. 2015;175:556–567. doi: 10.1016/j.foodchem.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 21.Bacskay GB, Reimers JR, Nordholm S. The mechanism of covalent bonding. J. Chem. Educ. 1997;74:1494–1502. [Google Scholar]
- 22.Li S, et al. Development of antibacterial nanoemulsions incorporating thyme oil: layer-by-layer self-assembly of whey protein isolate and chitosan hydrochloride. Food Chem. 2021;339:128016. doi: 10.1016/j.foodchem.2020.128016. [DOI] [PubMed] [Google Scholar]
- 23.Liu, F., Ma, C., Gao, Y. & McClements, D. J. Food-grade covalent complexes and their application as nutraceutical delivery systems: A review. Compr. Rev. Food Sci. Food Saf.16, 76–95 (2017). [DOI] [PubMed]
- 24.Deng L, et al. Development of disulfide bond crosslinked gelatin/ε-polylysine active edible film with antibacterial and antioxidant activities. Food Bioprocess Technol. 2020;13:577–588. [Google Scholar]
- 25.Benbettaïeb N, Karbowiak T, Debeaufort F. Bioactive edible films for food applications: influence of the bioactive compounds on film structure and properties. Crit. Rev. Food Sci. Nutr. 2019;59:1137–1153. doi: 10.1080/10408398.2017.1393384. [DOI] [PubMed] [Google Scholar]
- 26.Talón E, Trifkovic KT, Vargas M, Chiralt A, González-Martínez C. Release of polyphenols from starch-chitosan based films containing thyme extract. Carbohydr. Polym. 2017;175:122–130. doi: 10.1016/j.carbpol.2017.07.067. [DOI] [PubMed] [Google Scholar]
- 27.Chen X, Xiao J, Cai J, Liu H. Phase separation behavior in zein-gelatin composite film and its modulation effects on retention and release of multiple bioactive compounds. Food Hydrocoll. 2020;109:106105. [Google Scholar]
- 28.Uz M, Alsoy S. Development of mono and multilayer antimicrobial food packaging materials for controlled release of potassium sorbate. LWT - Food Sci. Technol. 2011;44:2302–2309. [Google Scholar]
- 29.Zhang X, Chen H, Zhang H, Chen H. Layer-by-layer assembly: from conventional to unconventional methods. Chem. Commun. 2007;14:1395–1405. doi: 10.1039/b615590a. [DOI] [PubMed] [Google Scholar]
- 30.Decher G, Hong JD. Buildup of ultrathin multilayer films by a self-assembly process: II. consecutive adsorption of anionic and cationic bipolar amphiphiles and polyelectrolytes on charged surfaces. Ber. der Bunsenges. f.ür. physikalische Chem. 1991;95:1430–1434. [Google Scholar]
- 31.Calva-Estrada SJ, Jiménez-Fernández M, Lugo-Cervantes E. Protein-based films: advances in the development of biomaterials applicable to food packaging. Food Eng. Rev. 2019;11:78–92. [Google Scholar]
- 32.Hassan, B., Chatha, S. A. S., Hussain, A. I., Zia, K. M. & Akhtar, N. Recent advances on polysaccharides, lipids and protein based edible films and coatings: A review. Int. J. Biol. Macromolecules109, 1095–1107 (2018). [DOI] [PubMed]
- 33.Anu Bhushani J, Anandharamakrishnan C. Electrospinning and electrospraying techniques: potential food based applications. Trends Food Sci. Technol. 2014;38:21–33. [Google Scholar]
- 34.Zhao L, et al. Electrospun functional materials toward food packaging applications: a review. Nanomaterials. 2020;10:1–31. doi: 10.3390/nano10010150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fabra MJ, Busolo MA, Lopez-Rubio A, Lagaron JM. Nanostructured biolayers in food packaging. Trends Food Sci. Technol. 2013;31:79–87. [Google Scholar]
- 36.Kriegel C, Arrechi A, Kit K, McClements DJ, Weiss J. Fabrication, functionalization, and application of electrospun biopolymer nanofibers. Crit. Rev. Food Sci. Nutr. 2008;48:775–797. doi: 10.1080/10408390802241325. [DOI] [PubMed] [Google Scholar]
- 37.Reneker DH, Yarin AL. Electrospinning jets and polymer nanofibers. Polymer. 2008;49:2387–2425. [Google Scholar]
- 38.Brandenberger H, Nüssli D, Piëch V, Widmer F. Monodisperse particle production: a method to prevent drop coalescence using electrostatic forces. J. Electrost. 1999;45:227–238. [Google Scholar]
- 39.Jaworek A, Sobczyk AT. Electrospraying route to nanotechnology: an overview. J. Electrost. 2008;66:197–219. [Google Scholar]
- 40.Messin T, et al. Confinement effect in PC/MXD6 multilayer films: impact of the microlayered structure on water and gas barrier properties. J. Membr. Sci. 2017;525:135–145. [Google Scholar]
- 41.Hernandez-Izquierdo VM, Krochta JM. Thermoplastic processing of proteins for film formation—a review. J. Food Sci. 2008;73:30–39. doi: 10.1111/j.1750-3841.2007.00636.x. [DOI] [PubMed] [Google Scholar]
- 42.Andreuccetti C, et al. Functional properties of gelatin-based films containing Yucca schidigera extract produced via casting, extrusion and blown extrusion processes: a preliminary study. J. Food Eng. 2012;113:33–40. [Google Scholar]
- 43.Liu H, Xie F, Yu L, Chen L, Li L. Thermal processing of starch-based polymers. Prog. Polym. Sci. 2009;34:1348–1368. [Google Scholar]
- 44.Lacoste A, Schaich KM, Zumbrunnen D, Yam KL. Advancing controlled release packaging through smart blending. Packaging Technol. Sci. 2005;18:77–87. [Google Scholar]
- 45.Chen, W. et al. Fortification of edible films with bioactive agents: a review of their formation, properties, and application in food preservation. Crit. Rev. Food Sci. Nutr. 1–27. 10.1080/10408398.2021.1881435 (2021). [DOI] [PubMed]
- 46.Li M, et al. Extrusion processing and characterization of edible starch films with different amylose contents. J. Food Eng. 2011;106:95–101. [Google Scholar]
- 47.Türe H, Gällstedt M, Hedenqvist MS. Antimicrobial compression-moulded wheat gluten films containing potassium sorbate. Food Res. Int. 2012;45:109–115. [Google Scholar]
- 48.Ciannamea EM, Stefani PM, Ruseckaite RA. Physical and mechanical properties of compression molded and solution casting soybean protein concentrate based films. Food Hydrocoll. 2014;38:193–204. [Google Scholar]
- 49.Rhim JW, Mohanty KA, Singh SP, Ng PKW. Preparation and properties of biodegradable multilayer films based on soy protein isolate and poly(lactide) Ind. Eng. Chem. Res. 2006;45:3059–3066. [Google Scholar]
- 50.Suhag R, Kumar N, Petkoska AT, Upadhyay A. Film formation and deposition methods of edible coating on food products: a review. Food Res. Int. 2020;136:109582. doi: 10.1016/j.foodres.2020.109582. [DOI] [PubMed] [Google Scholar]
- 51.Fu Y, Kao WJ. Drug release kinetics and transport mechanisms of non-degradable and degradable polymeric delivery systems. Expert Opin. Drug Deliv. 2010;7:429–444. doi: 10.1517/17425241003602259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pothakamury UR, Barbosa-Cánovas GV. Fundamental aspects of controlled release in foods. Trends Food Sci. Technol. 1995;6:397–406. [Google Scholar]
- 53.Lecomte F, Siepmann J, Walther M, MacRae RJ, Bodmeier R. pH-sensitive polymer blends used as coating materials to control drug release from spherical beads: elucidation of the underlying mass transport mechanisms. Pharm. Res. 2005;22:1129–1141. doi: 10.1007/s11095-005-5421-2. [DOI] [PubMed] [Google Scholar]
- 54.Peppas, N. Chemical and physical structure of polymers as carriers for controlled release of bioactive agents: A review. J. Macromol. Sci. Part C23, 61–126 (1983).
- 55.Jahromi AK, Shieh H, Saadatmand M. Theoretical study of diffusional release of a dispersed solute from cylindrical polymeric matrix: a novel configuration for providing zero-order release profile. Appl. Math. Model. 2019;73:136–145. [Google Scholar]
- 56.Siegel RA. Theoretical analysis of inward hemispheric release above and below drug solubility. J. Controlled Release. 2000;69:109–126. doi: 10.1016/s0168-3659(00)00292-3. [DOI] [PubMed] [Google Scholar]
- 57.Lee PI. Kinetics of drug release from hydrogel matrices. J. Controlled Release. 1985;2:277–288. [Google Scholar]
- 58.Vahabzadeh, F., Najafi, A. & Zivdar M. Micro encapsulation of orange oil by complex coacervation and its release behavior. Int J Eng-iran. 17, 325–334 (2004).
- 59.Thomas NL, Windle AH. A theory of case II diffusion. Polymer. 1982;23:529–542. [Google Scholar]
- 60.Papanu JS, Soane Soong DS, Bell AT, Hess DW. Transport models for swelling and dissolution of thin polymer films. J. Appl. Polym. Sci. 1989;38:859–885. [Google Scholar]
- 61.Colombo P, Bettini R, Santi P, De Ascentiis A, Peppas NA. Analysis of the swelling and release mechanisms from drug delivery systems with emphasis on drug solubility and water transport. J. Controlled Release. 1996;39:231–237. [Google Scholar]
- 62.Siepmann J, Göpferich A. Mathematical modeling of bioerodible, polymeric drug delivery systems. Adv. Drug Deliv. Rev. 2001;48:229–247. doi: 10.1016/s0169-409x(01)00116-8. [DOI] [PubMed] [Google Scholar]
- 63.von Burkersroda F, Schedl L, G.opferich A. Why degradable polymers undergo surface erosion or bulk erosion. Biomaterials. 2002;23:4221–4231. doi: 10.1016/s0142-9612(02)00170-9. [DOI] [PubMed] [Google Scholar]
- 64.Hopfenberg, H. B. Controlled release from erodible slabs, cylinders, and spheres. In (ACS Symposium Series), Controlled Release Polymeric Formulations. vol 3, 26–32 (1976).
- 65.Wang P, et al. Characterization and antioxidant activity of trilayer gelatin/dextran-propyl gallate/gelatin films: Electrospinning versus solvent casting. LWT - Food Sci. Technol. 2020;128:109536. [Google Scholar]
- 66.Sirc J, et al. Controlled gentamicin release from multi-layered electrospun nanofibrous structures of various thicknesses. Int. J. Nanomed. 2012;7:5315–5325. doi: 10.2147/IJN.S35781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cerqueira MA, et al. Use of electrospinning to develop antimicrobial biodegradable multilayer systems: encapsulation of cinnamaldehyde and their physicochemical characterization. Food Bioprocess Technol. 2016;9:1874–1884. [Google Scholar]
- 68.Zhang W, Shu C, Chen Q, Cao J, Jiang W. The multi-layer film system improved the release and retention properties of cinnamon essential oil and its application as coating in inhibition to penicillium expansion of apple fruit. Food Chem. 2019;299:125109. doi: 10.1016/j.foodchem.2019.125109. [DOI] [PubMed] [Google Scholar]
- 69.Gu B, et al. A sandwich-like chitosan-based antibacterial nanocomposite film with reduced graphene oxide immobilized silver nanoparticles. Carbohydr. Polym. 2021;260:117835. doi: 10.1016/j.carbpol.2021.117835. [DOI] [PubMed] [Google Scholar]
- 70.Carrizo D, Taborda G, Nerín C, Bosetti O. Extension of shelf life of two fatty foods using a new antioxidant multilayer packaging containing green tea extract. Innovative Food Sci. Emerg. Technol. 2016;33:534–541. [Google Scholar]
- 71.Chen X, Chen M, Xu C, Yam KL. Critical review of controlled release packaging to improve food safety and quality. Crit. Rev. Food Sci. Nutr. 2019;59:2386–2399. doi: 10.1080/10408398.2018.1453778. [DOI] [PubMed] [Google Scholar]
- 72.Dušek K, Dušková-Smrčková M. Network structure formation during crosslinking of organic coating systems. Prog. Polym. Sci. 2000;25:1215–1260. [Google Scholar]
- 73.Garavand F, Rouhi M, Razavi SH, Cacciotti I, Mohammadi R. Improving the integrity of natural biopolymer films used in food packaging by crosslinking approach: a review. Int. J. Biol. Macromolecules. 2017;104:687–707. doi: 10.1016/j.ijbiomac.2017.06.093. [DOI] [PubMed] [Google Scholar]
- 74.Balaguer MP, Gómez-Estaca J, Gavara R, Hernandez-Munoz P. Functional properties of bioplastics made from wheat gliadins modified with cinnamaldehyde. J. Agric. Food Chem. 2011;59:6689–6695. doi: 10.1021/jf200477a. [DOI] [PubMed] [Google Scholar]
- 75.Menzel C, et al. Molecular structure of citric acid cross-linked starch films. Carbohydr. Polym. 2013;96:270–276. doi: 10.1016/j.carbpol.2013.03.044. [DOI] [PubMed] [Google Scholar]
- 76.Azeredo HMC, Waldron KW. Crosslinking in polysaccharide and protein films and coatings for food contact—a review. Trends Food Sci. Technol. 2016;52:109–122. [Google Scholar]
- 77.Ma W, Tang CH, Yin SW, Yang XQ, Qi JR. Genipin-crosslinked gelatin films as controlled releasing carriers of lysozyme. Food Res. Int. 2013;51:321–324. [Google Scholar]
- 78.Zhu JY, et al. Development and characterization of multifunctional gelatin-lysozyme films via the oligomeric proanthocyanidins (OPCs) crosslinking approach. Food Biophysics. 2017;12:451–461. [Google Scholar]
- 79.Fajardo P, Pau M, Gomez-estaca J, Gavara R, Hernandez-munoz P. Chemically modified gliadins as sustained release systems for lysozyme. Food Hydrocoll. 2014;41:53–59. [Google Scholar]
- 80.López De Dicastillo C, Rodríguez F, Guarda A, Galotto MJ. Antioxidant films based on cross-linked methyl cellulose and native Chilean berry for food packaging applications. Carbohydr. Polym. 2016;136:1052–1060. doi: 10.1016/j.carbpol.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 81.Arnon-Rips H, Cohen Y, Saidi L, Porat R, Poverenov E. Covalent linkage of bioactive volatiles to a polysaccharide support as a potential approach for preparing active edible coatings and delivery systems for food products. Food Chem. 2021;338:127822. doi: 10.1016/j.foodchem.2020.127822. [DOI] [PubMed] [Google Scholar]
- 82.Basu S, Pacelli S, Paul A. Self-healing DNA-based injectable hydrogels with reversible covalent linkages for controlled drug delivery. Acta Biomaterialia. 2020;105:159–169. doi: 10.1016/j.actbio.2020.01.021. [DOI] [PubMed] [Google Scholar]
- 83.Carli S, et al. Biochemically controlled release of dexamethasone covalently bound to PEDOT. Chem. - A Eur. J. 2018;24:10300–10305. doi: 10.1002/chem.201801499. [DOI] [PubMed] [Google Scholar]
- 84.Miller KS, Krochta JM. Oxygen and aroma barrier properties of edible films: a review. Trends Food Sci. Technol. 1997;8:228–237. [Google Scholar]
- 85.Hosseini SF, Javidi Z, Rezaei M. Efficient gas barrier properties of multi-layer films based on poly(lactic acid) and fish gelatin. Int. J. Biol. Macromolecules. 2016;92:1205–1214. doi: 10.1016/j.ijbiomac.2016.08.034. [DOI] [PubMed] [Google Scholar]
- 86.Tampau A, González-Martínez C, Chiralt A. Release kinetics and antimicrobial properties of carvacrol encapsulated in electrospun poly-(ε-caprolactone) nanofibres. Application in starch multilayer films. Food Hydrocoll. 2018;79:158–169. [Google Scholar]
- 87.Zheng K, et al. Chitosan-acorn starch-eugenol edible film: physico-chemical, barrier, antimicrobial, antioxidant and structural properties. Int. J. Biol. Macromolecules. 2019;135:344–352. doi: 10.1016/j.ijbiomac.2019.05.151. [DOI] [PubMed] [Google Scholar]
- 88.Meyers MA, Chen PY, Lin AYM, Seki Y. Biological materials: structure and mechanical properties. Prog. Mater. Sci. 2008;53:1–206. doi: 10.1016/j.jmbbm.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 89.Cerqueira, M. A., Torres-Giner, S. & Lagaron, J. M. Chapter 6-Nanostructured multilayer films. Editor(s): Cerqueira, M. A., Lagaron J. M., Castro L. M., Vicente A. A. In Nanomaterials for Food Packaging, 147–171 (Elsevier, 2018).
- 90.Wang W, et al. Fabrication and characterization of multilayered kafirin/gelatin film with one-way water barrier property. Food Hydrocoll. 2018;81:159–168. [Google Scholar]
- 91.Wu H, et al. Effect of citric acid induced crosslinking on the structure and properties of potato starch/chitosan composite films. Food Hydrocoll. 2019;97:105208. [Google Scholar]
- 92.Sun L, et al. Preparation and characterization of chitosan film incorporated with thinned young apple polyphenols as an active packaging material. Carbohydr. Polym. 2017;163:81–91. doi: 10.1016/j.carbpol.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 93.Pillai, C. K. S. Recent advances in biodegradable polymeric materials. Materi. Sci. Tech-lond. 30, 558–566 (2014).
- 94.Ke, C. L., Deng, F. S., Chuang, C. Y. & Lin, C. H. Antimicrobial actions and applications of chitosan. Polymers13, 904 (2021). [DOI] [PMC free article] [PubMed]
- 95.Zhou Z, et al. A plant leaf-mimetic membrane with controllable gas permeation for efficient preservation of perishable products. ACS Nano. 2021;15:8742–8752. doi: 10.1021/acsnano.1c00997. [DOI] [PubMed] [Google Scholar]
- 96.Yan J, et al. Preparation and property studies of chitosan-PVA biodegradable antibacterial multilayer films doped with Cu2O and nano-chitosan composites. Food Control. 2021;126:108049. [Google Scholar]
- 97.Wu W, Ni X, Shao P, Gao H. Novel packaging film for humidity-controlled manipulating of ethylene for shelf-life extension of Agaricus bisporus. LWT - Food Sci. Technol. 2021;145:111331. [Google Scholar]
- 98.Dong T, et al. Evaluation of the effects of prepared antibacterial multilayer film on the quality and shelf-life stability of chilled meat. J. Food Process. Preservation. 2017;41:1–10. [Google Scholar]
- 99.Jung, S. et al. Multifunctional bio-nanocomposite coatings for perishable fruits. Adv. Mater.32, 1908291 (2020). [DOI] [PubMed]
- 100.Yang Y, et al. Preparation and functional properties of poly(vinyl alcohol)/ethyl cellulose/tea polyphenol electrospun nanofibrous films for active packaging material. Food Control. 2021;130:108331. [Google Scholar]
- 101.Jeyakumari A, Zynudheen AA, Binsi PK, Parvathy U, Ravishankar CN. Microencapsulation of fish oil-oregano essential oil blends by spray drying and its oxidative stability. J. Agric. Sci. Technol. 2017;19:1–12. [Google Scholar]
- 102.Oliveira SPLF, Bertan LC, De Rensis CMVB, Bilck AP, Vianna PCB. Whey protein-based films incorporated with oregano essential oil. Polimeros. 2017;27:158–164. [Google Scholar]
- 103.Balan GC, et al. Production of wheat flour/PBAT active films incorporated with oregano oil microparticles and its application in fresh pastry conservation. Food Bioprocess Technol. 2021;14:1587–1599. [Google Scholar]
- 104.Kodal Coşkun B, Çalikoǧlu E, Karagöz Emiroǧlu Z, Candoǧan K. Antioxidant active packaging with soy edible films and oregano or thyme essential oils for oxidative stability of ground beef patties. J. Food Qual. 2014;37:203–212. [Google Scholar]
- 105.Zinoviadou KG, Koutsoumanis KP, Biliaderis CG. Physico-chemical properties of whey protein isolate films containing oregano oil and their antimicrobial action against spoilage flora of fresh beef. Meat Sci. 2009;82:338–345. doi: 10.1016/j.meatsci.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 106.Rao, P. V. & Gan, S. H. Cinnamon: a multifaceted medicinal plant. Evidence-based Complementary Alternative Med.2014, 642942 (2014). [DOI] [PMC free article] [PubMed]
- 107.Amiri S, Rahimi A. Poly(ε-caprolactone) electrospun nanofibers containing cinnamon essential oil nanocapsules: a promising technique for controlled release and high solubility. J. Ind. Text. 2019;48:1527–1544. [Google Scholar]
- 108.Iamareerat B, Singh M, Sadiq MB, Anal AK. Reinforced cassava starch based edible film incorporated with essential oil and sodium bentonite nanoclay as food packaging material. J. Food Sci. Technol. 2018;55:1953–1959. doi: 10.1007/s13197-018-3100-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu H, et al. Preparation of soy protein isolate (SPI)-pectin complex film containing cinnamon oil and its effects on microbial growth of dehydrated soybean curd (Dry Tofu) J. Food Process. Preservation. 2014;38:1371–1376. [Google Scholar]
- 110.Ferreira RR, Souza AG, Quispe YM, Rosa DS. Essential oils loaded-chitosan nanocapsules incorporation in biodegradable starch films: a strategy to improve fruits shelf life. Int. J. Biol. Macromolecules. 2021;188:628–638. doi: 10.1016/j.ijbiomac.2021.08.046. [DOI] [PubMed] [Google Scholar]
- 111.Yang Z, Peng H, Wang W, Liu T. Blending of low-density polyethylene with vanillin for improved barrier and aroma-releasing properties in food packaging. J. Appl. Polym. Sci. 2010;116:2658–2667. [Google Scholar]
- 112.Kayaci F, Uyar T. Solid inclusion complexes of vanillin with cyclodextrins: their formation, characterization, and high-temperature stability. J. Agric. Food Chem. 2011;59:11772–11778. doi: 10.1021/jf202915c. [DOI] [PubMed] [Google Scholar]
- 113.Lee KY, Lee JH, Yang HJ, Song KB. Characterization of a starfish gelatin film containing vanillin and its application in the packaging of crab stick. Food Sci. Biotechnol. 2016;25:1023–1028. doi: 10.1007/s10068-016-0165-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yang HJ, Lee JH, Lee KY, Song K, Bin Antimicrobial effect of an Undaria pinnatifida composite film containing vanillin against Escherichia coli and its application in the packaging of smoked chicken breast. Int. J. Food Sci. Technol. 2017;52:398–403. [Google Scholar]
- 115.Zhang, W., Jiang, H., Rhim, J. W., Cao, J. & Jiang, W. Tea polyphenols (TP): a promising natural additive for the manufacture of multifunctional active food packaging films. Crit. Rev. Food Sci. Nutri. 1–14. 10.1080/10408398.2021.1946007 (2021). [DOI] [PubMed]
- 116.Namal Senanayake SPJ. Green tea extract: chemistry, antioxidant properties and food applications—a review. J. Funct. Foods. 2013;5:1529–1541. [Google Scholar]
- 117.Yang HJ, Lee JH, Won M, Song KB. Antioxidant activities of distiller dried grains with solubles as protein films containing tea extracts and their application in the packaging of pork meat. Food Chem. 2016;196:174–179. doi: 10.1016/j.foodchem.2015.09.020. [DOI] [PubMed] [Google Scholar]
- 118.Bilgin Fıçıcılar B, Gençcelep H, Özen T. Effects of bay leaf (Laurus nobilis) and green tea (Camellia sinensis) extracts on the physicochemical properties of the marinated anchovies with vacuum packaging. CYTA - J. Food. 2018;16:848–858. [Google Scholar]
- 119.Wrona M, Bentayeb K, Nerín C. A novel active packaging for extending the shelf-life of fresh mushrooms (Agaricus bisporus) Food Control. 2015;54:200–207. [Google Scholar]
- 120.Mujeeb Rahman P, Abdul Mujeeb VM, Muraleedharan K. Chitosan–green tea extract powder composite pouches for extending the shelf life of raw meat. Polym. Bull. 2017;74:3399–3419. [Google Scholar]
- 121.Lu, R. et al. Medical applications based on supramolecular self-assembled materials from tannic acid. Front. Chem.8, 871 (2020). [DOI] [PMC free article] [PubMed]
- 122.Ringwald C, Ball V. Step-by-step deposition of type B gelatin and tannic acid displays a peculiar ionic strength dependence at pH 5. RSC Adv. 2016;6:4730–4738. [Google Scholar]
- 123.Hazer B, Ashby RD. Synthesis of a novel tannic acid-functionalized polypropylene as antioxidant active-packaging materials. Food Chem. 2021;344:128644. doi: 10.1016/j.foodchem.2020.128644. [DOI] [PubMed] [Google Scholar]
- 124.Zhang C, et al. Physical properties and bioactivities of chitosan/gelatin-based films loaded with tannic acid and its application on the preservation of fresh-cut apples. LWT - Food Sci. Technol. 2021;144:111223. [Google Scholar]
- 125.Suntres ZE, Coccimiglio J, Alipour M. The bioactivity and toxicological actions of carvacrol. Crit. Rev. Food Sci. Nutr. 2015;55:304–318. doi: 10.1080/10408398.2011.653458. [DOI] [PubMed] [Google Scholar]
- 126.Yildiz ZI, Celebioglu A, Kilic ME, Durgun E, Uyar T. Fast-dissolving carvacrol/cyclodextrin inclusion complex electrospun fibers with enhanced thermal stability, water solubility, and antioxidant activity. J. Mater. Sci. 2018;53:15837–15849. [Google Scholar]
- 127.Wang L, Heising J, Fogliano V, Dekker M. Fat content and storage conditions are key factors on the partitioning and activity of carvacrol in antimicrobial packaging. Food Packaging Shelf Life. 2020;24:100500. [Google Scholar]
- 128.Velázquez-Contreras F, et al. Effect of pla active packaging containing monoterpene-cyclodextrin complexes on berries preservation. Polymers. 2021;13:1–17. doi: 10.3390/polym13091399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lim GO, Hong YH, Song KB. Application of gelidium corneum edible films containing carvacrol for ham packages. J. Food Sci. 2010;75:90–93. doi: 10.1111/j.1750-3841.2009.01431.x. [DOI] [PubMed] [Google Scholar]
- 130.Sangsuwan J, Rattanapanone N, Pongsirikul I. Development of active chitosan films incorporating potassium sorbate or vanillin to extend the shelf life of butter cake. Int. J. Food Sci. Technol. 2015;50:323–330. [Google Scholar]
- 131.Marcuzzo E, Peressini D, Sensidoni A. Shelf life of short ripened soft cheese stored under various packaging conditions. J. Food Process. Preservation. 2013;37:1094–1102. [Google Scholar]
- 132.Sousa GM, Yamashita F, Soares Júnior MS. Application of biodegradable films made from rice flour, poly(butylene adipate-co-terphthalate), glycerol and potassium sorbate in the preservation of fresh food pastas. LWT - Food Sci. Technol. 2016;65:39–45. [Google Scholar]
- 133.del Olmo A, Calzada J, Nuñez M. Benzoic acid and its derivatives as naturally occurring compounds in foods and as additives: Uses, exposure, and controversy. Crit. Rev. Food Sci. Nutr. 2017;57:3084–3103. doi: 10.1080/10408398.2015.1087964. [DOI] [PubMed] [Google Scholar]
- 134.Dobiáš J, Chudackova K, Voldrich M, Marek M. Properties and polyethylene films with incorporated benzoic anhydride and/or ethyl and propyl esters of 4-hydroxybenzoic acid and their suitability for food packaging. Food Addit. Contam. 2000;17:1047–1053. doi: 10.1080/02652030010014394. [DOI] [PubMed] [Google Scholar]
- 135.Leśnierowski G, Yang T. Lysozyme and its modified forms: a critical appraisal of selected properties and potential. Trends Food Sci. Technol. 2021;107:333–342. [Google Scholar]
- 136.Cegielska-Radziejewska R, et al. The effect of modified lysozyme treatment on the microflora, physicochemical and sensory characteristics of pork packaged in preservative gas atmospheres. Coatings. 2021;11:1–14. [Google Scholar]
- 137.Glicerina V, et al. Efficacy of biodegradable, antimicrobial packaging on safety and quality parameters maintenance of a pear juice and rice milk-based smoothie product. Food Control. 2021;128:108170. [Google Scholar]
- 138.Ünalan IU, Korel F, Yemenicioǧlu A. Active packaging of ground beef patties by edible zein films incorporated with partially purified lysozyme and Na2EDTA. Int. J. Food Sci. Technol. 2011;46:1289–1295. [Google Scholar]
- 139.Bahrami A, Delshadi R, Jafari SM, Williams L. Nanoencapsulated nisin: an engineered natural antimicrobial system for the food industry. Trends Food Sci. Technol. 2019;94:20–31. [Google Scholar]
- 140.Gharsallaoui A, Joly C, Oulahal N, Degraeve P. Nisin as a food preservative: part 2: antimicrobial polymer materials containing nisin. Crit. Rev. Food Sci. Nutr. 2016;56:1275–1289. doi: 10.1080/10408398.2013.763766. [DOI] [PubMed] [Google Scholar]
- 141.Tu L, Mustapha A. Reduction of Brochothrix thermosphacta and Salmonella serotype Typhimurium on vacuum-packaged fresh beef treated with nisin and nisin combined with EDTA. J. Food Sci. 2002;67:302–306. [Google Scholar]
- 142.Yang Y, Liu H, Wu M, Ma J, Lu P. Bio-based antimicrobial packaging from sugarcane bagasse nanocellulose/nisin hybrid films. Int. J. Biol. Macromolecules. 2020;161:627–635. doi: 10.1016/j.ijbiomac.2020.06.081. [DOI] [PubMed] [Google Scholar]
- 143.Franklin NB, Cooksey KD, Getty KJK. Inhibition of Listeria monocytogenes on the surface of individually packaged hot dogs with a packaging film coating containing nisin. J. Food Prot. 2004;67:480–485. doi: 10.4315/0362-028x-67.3.480. [DOI] [PubMed] [Google Scholar]
- 144.Leelaphiwat P, Pechprankan C, Siripho P, Bumbudsanpharoke N, Harnkarnsujarit N. Effects of nisin and EDTA on morphology and properties of thermoplastic starch and PBAT biodegradable films for meat packaging. Food Chem. 2021;369:130956. doi: 10.1016/j.foodchem.2021.130956. [DOI] [PubMed] [Google Scholar]
- 145.Rodríguez JM, Martínez MI, Kok J. Pediocin PA-1, a wide-spectrum bacteriocin from lactic acid bacteria. Crit. Rev. Food Sci. Nutr. 2002;42:91–121. doi: 10.1080/10408690290825475. [DOI] [PubMed] [Google Scholar]
- 146.Santiago-Silva P, et al. Antimicrobial efficiency of film incorporated with pediocin (ALTA® 2351) on preservation of sliced ham. Food Control. 2009;20:85–89. [Google Scholar]
- 147.Woraprayote W, et al. Anti-listeria activity of poly(lactic acid)/sawdust particle biocomposite film impregnated with pediocin PA-1/AcH and its use in raw sliced pork. Int. J. Food Microbiol. 2013;167:229–235. doi: 10.1016/j.ijfoodmicro.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 148.Kraśniewska, K., Galus, S. & Gniewosz, M. Biopolymers-based materials containing silver nanoparticles as active packaging for food applications—a review. Int. J. Mol. Sci.21, 698 (2020). [DOI] [PMC free article] [PubMed]
- 149.Pal J, Deb MK, Deshmukh DK. Microwave-assisted synthesis of silver nanoparticles using benzo-18-crown-6 as reducing and stabilizing agent. Appl. Nanosci. 2014;4:507–510. [Google Scholar]
- 150.Wu Z, Huang X, Li YC, Xiao H, Wang X. Novel chitosan films with laponite immobilized Ag nanoparticles for active food packaging. Carbohydr. Polym. 2018;199:210–218. doi: 10.1016/j.carbpol.2018.07.030. [DOI] [PubMed] [Google Scholar]
- 151.Tavakoli H, Rastegar H, Taherian M, Samadi M, Rostami H. The effect of nano-silver packaging in increasing the shelf life of nuts: an in vitro model. Ital. J. Food Saf. 2017;6:156–161. doi: 10.4081/ijfs.2017.6874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kumar S, Shukla A, Baul PP, Mitra A, Halder D. Biodegradable hybrid nanocomposites of chitosan/gelatin and silver nanoparticles for active food packaging applications. Food Packaging Shelf Life. 2018;16:178–184. [Google Scholar]
- 153.Shabbir S, Kulyar MFeA, Bhutta ZA, Boruah P, Asif M. Toxicological consequences of titanium dioxide nanoparticles (TiO2NPs) and their jeopardy to human population. BioNanoScience. 2021;11:621–632. doi: 10.1007/s12668-021-00836-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cao C, et al. Poly(butylene adipate-co-terephthalate)/titanium dioxide/silver composite biofilms for food packaging application. LWT - Food Sci. Technol. 2020;132:109874. [Google Scholar]
- 155.Ezati P, Riahi Z, Rhim JW. CMC-based functional film incorporated with copper-doped TiO2 to prevent banana browning. Food Hydrocoll. 2022;122:107104. [Google Scholar]
- 156.Zhu Z, Cai H, Sun DW, Wang HW. Photocatalytic effects on the quality of pork packed in the package combined with TiO2 coated nonwoven fabrics. J. Food Process Eng. 2019;42:1–10. [Google Scholar]
- 157.Pirsa S, Asadi S. Innovative smart and biodegradable packaging for margarine based on a nano composite polylactic acid/lycopene film. Food Additives Contaminants Part A. 2021;38:856–869. doi: 10.1080/19440049.2021.1891299. [DOI] [PubMed] [Google Scholar]
- 158.Souza MP, et al. Construction of a biocompatible and antioxidant multilayer coating by layer-by-layer assembly of κ-carrageenan and quercetin nanoparticles. Food Bioprocess Technol. 2018;11:1050–1060. [Google Scholar]
- 159.Rezaeinia H, Ghorani B, Emadzadeh B, Mohebbi M. Prolonged-release of menthol through a superhydrophilic multilayered structure of balangu (Lallemantia royleana)-gelatin nanofibers. Mater. Sci. Eng. C. 2020;115:111115. doi: 10.1016/j.msec.2020.111115. [DOI] [PubMed] [Google Scholar]
- 160.Mastromatteo M, Barbuzzi G, Conte A, Del Nobile MA. Controlled release of thymol from zein based film. Innovative Food Sci. Emerg. Technol. 2009;10:222–227. [Google Scholar]
- 161.Cai J, Xiao J, Chen X, Liu H. Essential oil loaded edible films prepared by continuous casting method: Effects of casting cycle and loading position on the release properties. Food Packaging Shelf Life. 2020;26:100555. [Google Scholar]
- 162.Conte, A., Lecce, L., Iannetti, M. & Del Nobile, M. A. Study on the influence of bio-based packaging system on sodium benzoate release kinetics. Foods9, 1010 (2020). [DOI] [PMC free article] [PubMed]
- 163.Buonocore GG, Conte A, Corbo MR, Sinigaglia M, Del Nobile MA. Mono- and multilayer active films containing lysozyme as antimicrobial agent. Innovative Food Sci. Emerg. Technol. 2005;6:459–464. [Google Scholar]
- 164.Jipa IM, Stoica-Guzun A, Stroescu M. Controlled release of sorbic acid from bacterial cellulose based mono and multilayer antimicrobial films. LWT - Food Sci. Technol. 2012;47:400–406. [Google Scholar]
- 165.Stroescu M, Stoica-Guzun A, Jipa IM. Vanillin release from poly(vinyl alcohol)-bacterial cellulose mono and multilayer films. J. Food Eng. 2013;114:153–157. [Google Scholar]
- 166.Sukhtezari, S., Almasi, H., Pirsa, S., Zandi, M. & Pirouzifard, M. K. Development of bacterial cellulose based slow-release active films by incorporation of Scrophularia striata Boiss. extract. Carbohydrate Polymers156, 340–350 (2017). [DOI] [PubMed]
- 167.Kowalczyk, D. et al. Release kinetics and antimicrobial properties of the potassium sorbate-loaded edible films made from pullulan, gelatin and their blends. Food Hydrocoll101, 105539 (2020).
- 168.Vega-Lugo AC, Lim LT. Controlled release of allyl isothiocyanate using soy protein and poly(lactic acid) electrospun fibers. Food Res. Int. 2009;42:933–940. [Google Scholar]
- 169.Bayarri M, Oulahal N, Degraeve P, Gharsallaoui A. Properties of lysozyme/low methoxyl (LM) pectin complexes for antimicrobial edible food packaging. J. Food Eng. 2014;131:18–25. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable. This is a review article and no new datasets were generated or analyzed during this article.