Abstract
The purpose of this work was to evaluate the in vitro possibilities of ampicillin-ceftriaxone combinations for 10 Enterococcus faecalis strains with high-level resistance to aminoglycosides (HLRAg) and to assess the efficacy of ampicillin plus ceftriaxone, both administered with humanlike pharmacokinetics, for the treatment of experimental endocarditis due to HLRAg E. faecalis. A reduction of 1 to 4 dilutions in MICs of ampicillin was obtained when ampicillin was combined with a fixed subinhibitory ceftriaxone concentration of 4 μg/ml. This potentiating effect was also observed by the double disk method with all 10 strains. Time-kill studies performed with 1 and 2 μg of ampicillin alone per ml or in combination with 5, 10, 20, 40, and 60 μg of ceftriaxone per ml showed a ≥2 log10 reduction in CFU per milliliter with respect to ampicillin alone and to the initial inoculum for all 10 E. faecalis strains studied. This effect was obtained for seven strains with the combination of 2 μg of ampicillin per ml plus 10 μg of ceftriaxone per ml and for six strains with 5 μg of ceftriaxone per ml. Animals with catheter-induced endocarditis were infected intravenously with 108 CFU of E. faecalis V48 or 105 CFU of E. faecalis V45 and were treated for 3 days with humanlike pharmacokinetics of 2 g of ampicillin every 4 h, alone or combined with 2 g of ceftriaxone every 12 h. The levels in serum and the pharmacokinetic parameters of the humanlike pharmacokinetics of ampicillin or ceftriaxone in rabbits were similar to those found in humans treated with 2 g of ampicillin or ceftriaxone intravenously. Results of the therapy for experimental endocarditis caused by E. faecalis V48 or V45 showed that the residual bacterial titers in aortic valve vegetations were significantly lower in the animals treated with the combinations of ampicillin plus ceftriaxone than in those treated with ampicillin alone (P < 0.001). The combination of ampicillin and ceftriaxone showed in vitro and in vivo synergism against HLRAg E. faecalis.
The American Heart Association recommends 4 to 6 weeks of penicillin or ampicillin plus an aminoglycoside for treatment of enterococcal endocarditis (45). After the first reports, in the late 1970s, of clinical isolations of Enterococcus faecalis with high-level resistance to aminoglycosides (HLRAg) (23), the number of infections caused by HLRAg strains has been increasing. At the present time, E. faecalis with HLRAg occurs worldwide (5, 11, 35, 43). High-level resistance to streptomycin and gentamicin precludes bactericidal synergism with penicillins or glycopeptides (8, 11, 12). This fact causes a problem for the treatment of patients with endocarditis caused by these strains. Results from animal studies using the endocarditis model have provided controversial data about the efficacy of ampicillin administered by continuous intravenous (i.v.) infusion, and they are clearly not definitive (21, 40). A small number of patients have been cured with antibiotic treatment alone, and others have required valve replacement (1, 9, 14, 25, 26, 28, 29, 34, 36–38). To date, no proven therapy is known to be as effective as ampicillin or penicillin plus an aminoglycoside for infections caused by these strains when bactericidal activity is desirable, as in infective endocarditis; thus, new alternatives for treatment should be evaluated. Recently, Mainardi et al. (30) demonstrated a synergistic effect of amoxicillin and cefotaxime against 48 of 50 clinical strains of E. faecalis. This experience of amoxicillin-cefotaxime synergy against E. faecalis was limited to only two HLRAg strains.
The studies of antimicrobial efficacy in experimental models of infection provide an important basis for clinical investigative studies in humans, but antibiotic pharmacokinetics may differ greatly between humans and animals because of the higher elimination rate of the drugs in animals. In order to surmount this problem, different authors have used animal models of humanlike pharmacokinetics (6, 19, 31). In previous experiments, we described a mathematical model that determines the doses to be given to animals by a computer-controlled infusion pump system to obtain serum profiles for rabbits similar to those observed in humans after i.v. administration of a drug in an open one-compartment pharmacokinetic model (19). The first purpose of this study was to develop a new and amenable open two-compartment pharmacokinetics mathematical model that would enable us to determine the doses to be administered to rabbits by a computer-controlled infusion pump system to simulate the human kinetics of an antimicrobial. Thus, the elimination kinetics of ceftriaxone was examined in healthy rabbits, and thereafter, ceftriaxone was administered to the animals in a way that simulated the human serum profile following an i.v. bolus dose of 2 g.
The second purpose of this study was to confirm and enlarge the previous observations from the work of Mainardi et al. (30), by evaluating the in vitro effect of the combination of ampicillin plus ceftriaxone in a large number of HLRAg E. faecalis strains and applying complementary techniques, such as time-kill curves. In addition, we investigated the therapeutic outcome of the combination of ampicillin plus ceftriaxone, both given with humanlike pharmacokinetics, in the treatment of experimental endocarditis due to E. faecalis strains highly resistant to aminoglycosides.
(This work was presented in part at the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy, October 1996, New Orleans, La. [20].)
MATERIALS AND METHODS
In vitro studies. (i) Bacterial strains.
We studied 10 E. faecalis strains, originally isolated from patients with a clinically documented infection, which were susceptible to ampicillin or vancomycin and highly resistant to aminoglycosides. The strains were first identified by the API 20 STREP system (BioMérieux, La Balme-Les-Grottes, France) and later confirmed according to the criteria recommended by Facklam and Collins (13). The in vivo studies were performed with two HLRAg E. faecalis strains (V45 and V48) originally isolated from the blood of two patients with endocarditis. Working stock cultures were kept frozen at −70°C in double-strength skim milk (Difco Laboratories, Detroit, Mich.). Before each experiment, one aliquot was thawed and subcultured onto 5% sheep blood Columbia agar plates (BioMérieux).
(ii) Media and antibiotics.
Mueller-Hinton broth (MHB), Mueller-Hinton agar (MHA) plates, and brain heart infusion (BHI) agar plates (Difco Laboratories) were used. The antibiotics included were ampicillin (Antibioticos SA, Madrid, Spain); ceftriaxone (Roche SA, Madrid, Spain); and streptomycin, tobramycin, gentamicin, kanamycin, and amikacin (Sigma Chemical Co., St. Louis, Mo.). Ampicillin and ceftriaxone solutions were prepared fresh on the day used. Stock solutions of streptomycin, tobramycin, gentamicin, kanamycin, and amikacin were prepared and stored at −20°C.
(iii) In vitro antibiotic susceptibility tests.
MICs were determined on MHA by the standard agar dilution method (32). Plates were inoculated with a Steers replicator (104 CFU/spot) and incubated at 37°C for 18 h. MICs of ampicillin were determined on MHA alone and in combination with a fixed concentration of ceftriaxone (4 μg/ml). Strains with aminoglycoside (streptomycin, gentamicin, tobramycin, and kanamycin) MICs of ≥2,000 μg/ml were considered to be in the HLRAg category. To determine the bactericidal effect of ampicillin, the microdilution method was followed by using cation-adjusted MHB and an initial inoculum of approximately 5 × 105 CFU/ml. Ampicillin concentrations ranging from 0.06 to 128 μg/ml were assayed, and after 24 h of incubation at 37°C, an aliquot of 50 μl was spotted onto BHI agar plates supplemented with 1,000 IU of penicillinase (Difco Laboratories). Plates were incubated at 37°C for 48 h, and colonies were counted. An effect was considered bactericidal when a ≥99.9% reduction in colony counts was obtained with respect to the initial inoculum (27).
(iv) Synergy studies.
A qualitative estimation of the synergy between ampicillin and ceftriaxone (bacteriostatic interaction) was obtained by the double disk method (ampicillin, 10 μg; ceftriaxone, 30 μg) on BHI agar plates. To perform time-kill synergy studies, the method described by Sahm and Torres was followed (39). Prior to inoculation, each tube of fresh BHI broth was supplemented with ampicillin (final concentrations of 1 and 2 μg/ml) either alone or in combination with ceftriaxone (final concentrations, 5, 10, 20, 40, and 60 μg/ml). A positive growth tube without antibiotics was used as a control. Test tubes were inoculated (final concentration, 107 CFU/ml) and incubated at 37°C, and the number of CFU per milliliter was determined after 0, 4, and 24 h of incubation. The carryover effect was excluded by using BHI agar plates supplemented with penicillinase. Antimicrobial cooperation was defined as a ≥2 log10 decrease in CFU per milliliter between the combination and its most active agent alone after 24 h, with the number of surviving organisms in the presence of the combination ≥2 log10 CFU/ml below the starting inoculum. According to the American Society for Microbiology definition of synergy, one of the drugs must be present in a concentration which does not affect the growth curve of the test organism when used alone. The combination was considered to have a positive bactericidal activity when a ≥3 log10 reduction in colony counts was reached.
Pharmacokinetic studies.
Ampicillin and ceftriaxone were administered with a system to reproduce human serum pharmacokinetics in rabbits in order to mimic the human serum profile after an i.v. infusion of 2 g of ampicillin or ceftriaxone. A computer-controlled infusion pump system that delivered decreasing quantities of drug was employed (infusion pump, Alice King; the computer software was written by our group). This approach involved three steps: (i) estimation of ampicillin and ceftriaxone pharmacokinetic parameters in rabbits, (ii) application of a mathematical model to obtain the required infusion doses to simulate human kinetics in the animals, and (iii) in vivo experimental pharmacokinetic studies, done to simulate in rabbits the pharmacokinetic profile of ampicillin and ceftriaxone in humans.
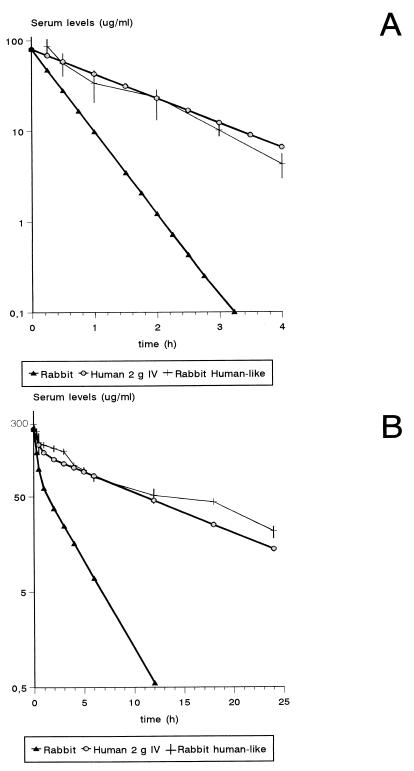
The pharmacokinetic studies which led to the humanlike pharmacokinetics of ampicillin in rabbits, including the explanation of the mathematical model used on the basis of an open one-compartment model, were previously described (19). The pharmacokinetic data of the human-adapted model of 2 g of ampicillin given i.v. in rabbits are shown in Table 1 and Fig. 1A. The serum profile and the pharmacokinetic parameters of ampicillin in rabbits administered with this model were similar to those of 2 g of i.v. ampicillin in humans.
TABLE 1.
| Parameter | Value (mean ± SD)
|
||
|---|---|---|---|
| Rabbit (n = 8)b | Human 2 g i.v.c | Rabbit humanlike (n = 7)d | |
| t1/2 (h) | 0.3 ± 0.03 | 1.1 | 0.99 ± 0.08 |
| kel (h−1) | 2.4 ± 0.29 | 0.63 | 0.71 ± 0.05 |
| AUC0–α(μg · h/ml) | 38.19 | 127.8 | 116.7 ± 31.83 |
The pharmacokinetic parameters were estimated on the basis of an open one-compartment model.
T1/2 and kel data are for healthy rabbits treated with 50 mg/kg i.v. The AUC0–α was calculated from the data of an ideal profile obtained by C0r of 80 μg/ml and the kel of ampicillin in rabbits.
kel, t1/2, and AUC0–α were obtained from an ideal serum human profile of 2 g of i.v. ampicillin.
Data are for humanlike pharmacokinetics of 2 g of ampicillin in rabbits.
FIG. 1.
Results of the pharmacokinetic studies with rabbits with humanlike pharmacokinetics of 2 g of ampicillin (A) or 2 g of ceftriaxone (B).
(i) Estimation of ceftriaxone pharmacokinetic parameters in rabbits.
To determine concentrations of ceftriaxone in serum, blood was drawn from a carotid catheter at 4, 8, 12, 16, 20, 25, 30, 45, 60, and 90 min and at 2, 2.5, 3, 3.5, 4, and 5 h after a single i.v. injection of 50 mg of ceftriaxone per kg of body weight. This study was done with eight healthy rabbits. Ceftriaxone concentrations were determined by the disk plate bioassay method (3) with Micrococcus luteus ATCC 9341 as the bioassay microorganism and antibiotic medium 5 (Difco Laboratories) as the growth medium. The serum samples from the rabbits were diluted with pooled rabbit serum so that their concentrations would be within the range of the standard curve. The standard samples were assayed in quintuplicate, and the serum samples were assayed in triplicate. Results were expressed as micrograms per milliliter of blood. The linearity (R2) of the standard curve was 0.98. The sensitivity of the assay was about 1.8 μg/ml of sample, and the between- and within-day coefficients of variation for replicates (n = 7) at 1 and 40 μg/ml were less than 5%.
The serum disposition constants (αr and βr), the zero-time intercept for the α phase (Ar), and the zero-time intercept for the β phase (Br) in rabbits were determined by using a nonlinear least-squares regression analysis of the concentration-time curve on the basis of an open two-compartment model.
(ii) Application of a mathematical model to obtain the required infusion doses to simulate human kinetics of ceftriaxone, as a drug with an open two-compartment model, in the animals.
The development of the mathematical model is shown in the Appendix of this article.
(iii) In vivo experimental pharmacokinetic studies.
These studies were done to simulate in rabbits the pharmacokinetic profile of 2 g of ceftriaxone in humans. Briefly, two polyethylene catheters (inside diameter, 0.81 mm; outside diameter, 1.27 mm; Portex SA, Hythe, Kent, England) were inserted, one through the carotid artery (sampling) and the other into the vena cava through the jugular vein (infusion), as previously described (19). The pump system was set up to deliver previously calculated flow rates of i.v. infusion to simulate the human kinetics of ceftriaxone. This study was done with five healthy rabbits.
To determine serum ceftriaxone concentrations, 2 ml of blood was sampled at 0, 0.5, 1, 2, 3, 4, 6, 12, 18, and 24 h after the start of infusion, through the carotid catheter. Ceftriaxone serum concentrations were assayed by the microbiological bioassay described above.
Different pharmacokinetic parameters were estimated on the basis of an open one-compartment model to compare the pharmacokinetics of ceftriaxone in rabbits, in the human-adapted model, and in humans. The half-life at the β phase of ceftriaxone in the rabbits with humanlike pharmacokinetics (t1/2) was calculated as ln 2/kel, where kel is the elimination rate constant. The elimination rate constant was determined as the slope obtained from a linear regression analysis of the terminal phase of the plasma concentration-time curve on the basis of an open one-compartment model. Thereafter, the area under the concentration-time curve from 0 h to α phase (AUC0–α) was calculated as C0/kel. The pharmacokinetic parameters of ceftriaxone in humans and rabbits were calculated as described above with a C0 of 256.8 μg/ml.
Establishing endocarditis and installation with the infusion pump system.
Experimental aortic valve infective endocarditis was induced in New Zealand rabbits (weight, approximately 2 to 2.1 kg) by the method of Garrison and Freedman (17), as modified by Durack and Beeson (10). The induction of nonbacterial thrombotic endocarditis was done as previously described (18, 19). Briefly, a polyethylene catheter was inserted through the right carotid artery into the left ventricular cavity and was left in place throughout the experiment. The same day, one or two catheters (inside diameter, 0.81 mm; outside diameter, 1.27 mm; Portex SA), depending on the treatment group, were placed into the inferior vena cava through the jugular vein by the same technique described previously (19), to administer ampicillin and ceftriaxone treatment. The infusion pump system was set up to deliver 2 ml of 0.9% saline per h to keep the catheter open until the beginning of antimicrobial administration. Twenty-four hours after placement of the intracardiac catheter, different groups of animals were inoculated via the jugular catheter with 1 ml of saline containing either 108 CFU of E. faecalis V45 or 105 CFU of E. faecalis V48 in the stationary phase of growth. The presence of endocarditis was confirmed by a blood culture yielding enterococci, obtained before starting antimicrobial therapy.
Treatment groups and estimation of therapeutic efficacy.
Antimicrobial therapy was initiated 48 h after infection and was continued for 3 days. The rabbits infected with either strain were randomized into the following treatment groups: group 1, control without treatment; group 2, ampicillin humanlike pharmacokinetics, 2 g every 4 h i.v.; group 3, ampicillin humanlike pharmacokinetics, 2 g every 4 h i.v. plus ceftriaxone humanlike pharmacokinetics, 2 g every 12 h.
After 3 days of treatment, the animals were sacrificed 6 h after ending the ampicillin and ceftriaxone infusion with a lethal i.v. injection of sodium pentothal. The chest was opened, the heart was excised and opened, and the aortic valve vegetations were removed aseptically. The animals included in the control group were sacrificed 48 h after induction of infection. The vegetations were rinsed with saline, weighed, and homogenized in 2 ml of tryptic soy broth (Difco Laboratories) in a tissue homogenizer (Stomacher 80). Homogenates were quantitatively cultured onto 5% sheep blood Columbia agar plates. The plates were incubated for 48 h at 37°C in room air. Results were expressed as the log10 CFU of E. faecalis V45 or V48 per gram of vegetation. Bacterial densities in valvular vegetations, calculated to be between 0 and 2 log CFU/g, were reported as log10 2 CFU/g rather than 0 because of potential errors associated with the low weight of the valvular tissue.
Analysis of results.
Results were expressed as the means, 95% confidence intervals of the means of grams of vegetations and log10 CFU of E. faecalis per gram of vegetation, and number of animals with sterile vegetations. Differences in log10 CFU of enterococci per gram of vegetation and the size of the vegetations (in grams) were compared by one-way analysis of variance. When the F value was significant, each treatment group was compared with the control group and with each of the other treatment groups by Scheffe’s test. Comparisons between the number of animals with sterile vegetations were made by Fisher’s exact test. P values equal to or less than 0.05 were considered significant.
RESULTS
In vitro antibiotic susceptibility tests.
MICs of ampicillin and ceftriaxone were determined by the agar dilution method for the 10 E. faecalis clinical strains used in the study (Table 2). All strains were ampicillin susceptible (MIC, 1 to 4 μg/ml) and ceftriaxone resistant (MIC, ≥256 μg/ml). The bactericidal effects of different ampicillin concentrations on the 10 strains tested after 24 h of incubation are shown in Table 3. The efficacy of intermediate ampicillin concentrations in reducing the number of viable cells was frequently higher than the efficacy of high concentrations. This result was consistently found in eight isolates and confirmed in three replicate experiments. The highest bactericidal effects were obtained at concentrations ranging from two to eight times the MIC. The window of ampicillin bactericidal activity in the different strains (reduction in viable bacterial counts exceeded 99.9%) corresponded to antibiotic concentrations ranging from 2 to 16 μg/ml. The bactericidal effect was not more detectable at ampicillin concentrations ranging from 4 to 32 times the MIC (8 to 32 μg/ml). All the E. faecalis strains showed high levels of resistance to gentamicin, tobramycin, kanamycin, and streptomycin, with MICs of ≥2,000 μg/ml. The presence of the aph(2")-aac(6′) and the aph(3′)-III genes was confirmed in all strains by the PCR method.
TABLE 2.
Susceptibilities to antibiotics of 10 high-level aminoglycoside-resistant E. faecalis strains and synergy by the double disk method
| E. faecalis strain | MIC (μg/ml)a
|
Synergy by double disk method | ||
|---|---|---|---|---|
| Ampicillin | Ceftriaxone | Ampicillin-ceftriaxoneb | ||
| E51 | 2 | 1,024 | 0.25 | + |
| E61 | 2 | >1,024 | 0.5 | + |
| V45 | 1 | 512 | 0.5 | + |
| E365 | 4 | 1,024 | 2 | + |
| E74 | 2 | 1,024 | 0.5 | + |
| E81 | 4 | >1,024 | 2 | + |
| E10 | 4 | >1,024 | 1 | + |
| E78 | 2 | 1,024 | 0.5 | + |
| E17 | 2 | 1,024 | 0.5 | + |
| V48 | 1 | 256 | 0.06 | + |
MIC determined by the agar dilution method.
MIC of ampicillin in MHA with 4 μg of ceftriaxone per ml.
TABLE 3.
Activity of different ampicillin concentrations with HLRAg E. faecalis strains
| E. faecalis strain | Ampicillin MIC (μg/ml)a | Change in log10 CFU/ml at different ampicillin concns (μg/ml)b
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | ||
| E51 | 1 | −0.6 | −3.4 | −4.6 | −4.2 | −3.8 | −1.7 | −1.7 | −1.6 |
| E61 | 2 | −1.5 | −3.8 | −3.7 | −1.9 | −1.5 | −1.7 | −1.7 | |
| V45 | 0.5 | −2.8 | −4.0 | −4.1 | −2.3 | −2.3 | −2.7 | −2.4 | −2.2 |
| E365 | 2 | −1.7 | −4.1 | −2.3 | −2.6 | −2.4 | −2.6 | −2.7 | |
| E74 | 1 | −0.9 | −3.6 | −4.2 | −4.1 | −2.8 | −2.3 | −2.4 | −2.4 |
| E81 | 2 | −0.8 | −2.0 | −2.2 | −2.2 | −2.3 | −2.8 | −2.8 | |
| E10 | 2 | −0.8 | −2.7 | −2.8 | −3.4 | −2.6 | −2.2 | −2.4 | |
| E78 | 2 | −0.3 | −4.1 | −4.2 | −1.7 | −2.8 | −3.9 | −4 | |
| E17 | 1 | −0.5 | −2.4 | −2.9 | −3.4 | −3.1 | −2.8 | −2.8 | −2.5 |
| V48 | −0.7 | −2.7 | −2.8 | −4.2 | −3.8 | −2.6 | −2.3 | −2.3 | |
MIC determined by microdilution method in MHB.
Change in log CFU per milliliter from the initial inoculum (24 h at 37°C). Boldface indicates window of bactericidal effect.
Synergy studies.
A reduction of 1 to 4 dilutions in ampicillin MICs was obtained when ampicillin was combined with a fixed subinhibitory ceftriaxone concentration of 4 μg/ml. This potentiating effect was also observed by the double disk method for all 10 strains (Table 2). When MICs were determined by the microdilution method in cation-adjusted MHB, ampicillin MICs were approximately 1 dilution lower (Table 3).
The results of the time-kill studies performed with 1 and 2 μg of ampicillin per ml alone or in combination with 5, 10, 20, 40, and 60 μg of ceftriaxone per ml are shown in Table 4. After 4 h of contact, ampicillin alone produced a bacteriostatic effect (≤0.3 log increase with respect to the original inoculum) in 4 of 10 strains with 1 μg of ampicillin per ml and in 9 of 10 with 2 μg/ml; similar data were obtained after 24 h: 3 of 10 and 9 of 10, respectively. At concentrations ranging from 5 to 60 μg/ml, ceftriaxone alone did not significantly alter the original bacterial count at 4 or 24 h. After 24 h of incubation, a ≥2 log10 decrease in CFU per milliliter between ceftriaxone plus ampicillin and ampicillin alone was found for all 10 E. faecalis strains studied. At 24 h, the majority of the ampicillin-ceftriaxone combined concentrations (70%) produced this effect, and in 36%, a bactericidal effect was observed (≥3 log10 killing with respect to the initial inoculum). Note that ceftriaxone alone did not affect the enterococcal bacterial counts after 24 h of incubation but slightly influenced the slope of the bacterial growth curve for eight strains, as shown in the 4-h counts. Thus, in these cases, the requirements for a strict definition of synergy were not fulfilled, but the results certainly suggest a situation similar to true synergy. This strong antimicrobial cooperation between the two drugs was obtained in seven strains with the combination of 2 μg of ampicillin per ml plus 10 μg of ceftriaxone per ml and in six strains with 5 μg of ceftriaxone per ml. After only 4 h of incubation, one-third of the ampicillin-ceftriaxone combined concentrations produced significant reductions in bacterial counts (≥2 log10 with respect to ampicillin alone or to the initial inoculum) in 9 of the 10 strains.
TABLE 4.
Results of time-kill experiments with 10 HLRAg E. faecalis strains
| Antimicrobial agentb | Change in log10 CFU/mla for strain:
|
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E10
|
E17
|
E51
|
E74
|
E78
|
V48
|
E365
|
V45
|
E81
|
E61
|
|||||||||||
| 4 h | 24 h | 4 h | 24 h | 4 h | 24 h | 4 h | 24 h | 4 h | 24 h | 4 h | 24 h | 4 h | 24 h | 4 h | 24 h | 4 h | 24 h | 4 h | 24 h | |
| Growth control | +1.5 | +1.8 | +1.7 | +2 | +1.9 | +2.3 | +2.0 | +2.3 | +1.9 | +2.4 | +1.2 | +2.1 | +1.7 | +2.0 | +0.7 | +1.8 | +1.6 | +1.9 | +1.7 | +2.1 |
| A (1) | −0.2 | +0.8 | +0.8 | +0.3 | +1 | +0.8 | +0.1 | +1.3 | +1.8 | +2.2 | −0.3 | +0.8 | +1 | +0.2 | +1.15 | +0.5 | +0.2 | −2 | +1 | +1 |
| A (2) | −0.4 | −0.6 | −0.1 | −2.2 | −0.1 | −0.1 | −1 | −1.2 | −0.8 | +0.8 | −2.5 | −0.9 | +0.2 | −1 | −1.5 | +0.2 | +0.5 | 0 | +0.1 | −1 |
| C (5) | +0.7 | +2.0 | +0.9 | +2.1 | +0.4 | +1.7 | +0.5 | +1.7 | +0.6 | +1.9 | +0.7 | +1.9 | +0.6 | +1.8 | +0.8 | +1.9 | +0.7 | +1.7 | +0.8 | +1.8 |
| C (10) | +0.5 | +1.9 | +0.5 | +1.9 | +0.6 | +1.6 | +0.6 | +1.8 | +0.7 | +1.7 | +0.9 | +2.0 | +0.8 | +2.0 | +0.9 | +2.0 | +0.6 | +1.8 | +0.7 | +1.9 |
| C (20) | +0.7 | +2.1 | +0.7 | +2.0 | +0.4 | +1.8 | +0.4 | +1.9 | +0.5 | +1.7 | +1.1 | +2.1 | +0.7 | +1.9 | +0.7 | +1.9 | +0.5 | +1.7 | +0.7 | +1.7 |
| C (40) | +0.7 | +1.8 | +0.7 | +1.9 | +0.4 | +1.7 | +0.7 | +1.7 | +0.8 | +1.8 | +0.95 | +1.9 | +0.8 | +1.7 | +0.8 | +1.8 | +0.6 | +1.9 | +0.6 | +1.9 |
| C (60) | +0.6 | +1.9 | +0.8 | +1.8 | +0.5 | +1.6 | +0.5 | +1.9 | +0.5 | +1.7 | +1.2 | +2.3 | +0.6 | +1.8 | +0.7 | +2.1 | +0.8 | +2.0 | +0.5 | +2.0 |
| A (1) + C (5) | −0.6 | −1.7 | +0.3 | −2.6 | −1.0 | −1 | −1.8 | −1.9 | +1.8 | +2.1 | −2.9 | −2.7 | +0.6 | −1 | −2.9 | +0.2 | +0.3 | −3 | +1 | −1.7 |
| A (1) + C (10) | −0.9 | −2 | +0.4 | −2.3 | −1.1 | −2.1 | −1.8 | −2.2 | +1.7 | +2.3 | −3.1 | −2.8 | +0.5 | −1 | −3.1 | −2.0 | +0.1 | −2.5 | +1 | −1.9 |
| A (1) + C (20) | −0.8 | −2 | +0.3 | −2.5 | −0.9 | −2.2 | −2 | −2.2 | +1.9 | +2.1 | −3.0 | −2.7 | +0.7 | −2.3 | −2.8 | −2.2 | +0.3 | −2.4 | +1 | −1.7 |
| A (1) + C (40) | −0.7 | −2.3 | −2.3 | −2.3 | −1.1 | −2.2 | −1.9 | −2.3 | −0.4 | +1 | −2.9 | −3.0 | −0.7 | −2.3 | −3.0 | −2.1 | −0.4 | −3 | +1 | −2 |
| A (1) + C (60) | −1 | −2.3 | −1.5 | −2.5 | −1.0 | −2.2 | −1.9 | −2.3 | −0.4 | +1.2 | −2.8 | −2.7 | −1 | −2.3 | −2.6 | −2.3 | −0.4 | −3 | +1 | −2 |
| A (2) + C (5) | −0.9 | −2.4 | −2.7 | −2.3 | −2.9 | −2.5 | −2.1 | −3.2 | −2 | −3 | −2.9 | −4.0 | −1 | −2.3 | −3.0 | −2.5 | −0.1 | −1.2 | −2 | −3.5 |
| A (2) + C (10) | −1.1 | −2.2 | −3 | −4.1 | −2.9 | −3.2 | −2.1 | −3.3 | −2 | −2 | −3.2 | −4.8 | −2.4 | −4.2 | −3.2 | −3.9 | −0.2 | −1.2 | −2 | −3.4 |
| A (2) + C (20) | −0.9 | −3 | −3 | −3.3 | −2.9 | −3.7 | −2 | −3.3 | −2 | −2.6 | −3.0 | −4.5 | −2.4 | −2.8 | −3.1 | −4.4 | −1.7 | −1.4 | −2 | −4 |
| A (2) + C (40) | −1.3 | −3.5 | −2.5 | −4.3 | −2.9 | −4.1 | −1.9 | −3.3 | −2 | −2.3 | −3.1 | −4.6 | −2.5 | −3 | −2.9 | −4.6 | −1.7 | −1.8 | −2.2 | −4 |
| A (2) + C (60) | −1.7 | −3.4 | −2.6 | −4.3 | −2.7 | −4 | −2 | −3.2 | −1.9 | −2.3 | −2.8 | −4.5 | −2.6 | −2.8 | −2.9 | −2.1 | −1.9 | −2.4 | −2 | −3 |
Change in log10 CFU per milliliter from the initial inoculum.
A, ampicillin; C, ceftriaxone. Numbers in parentheses are micrograms per milliliter.
Pharmacokinetic studies.
The rabbit pharmacokinetic data of ceftriaxone that we used in the mathematical model, determined with eight healthy rabbits that had received one i.v. bolus dose of 50 mg/kg, were as follows (shown as means ± standard deviations [SD]): αr, 3.7 ± 0.41 h−1; βr, 0.42 ± 0.07 h−1; k21r, 1.53 ± 0.3 h−1; k13r, 1.07 ± 0.3 h−1; and Vr, 0.16 ± 0.01 liters/kg. The profile in human serum produced by a 2-g i.v. injection of ceftriaxone was simulated in rabbits with the controlled-infusion pump system (Fig. 1B). This resulted in peak and trough ceftriaxone levels in rabbit serum (mean ± SD) of 242.7 ± 17.6 μg/ml at 15 min and 21.6 ± 2.9 μg/ml at 24 h, respectively (Fig. 1B). The pharmacokinetic parameters obtained from the human-adapted model were similar to those of 2 g of i.v. ceftriaxone in humans (Table 5). We carried out pharmacokinetic studies of ampicillin and ceftriaxone after six repeated injections of ampicillin and four of ceftriaxone in healthy rabbits and found no significant differences with respect to the results presented in Fig. 1.
TABLE 5.
Comparison of the pharmacokinetic parameters of ceftriaxonea
| Parameter | Value (mean ± SD)
|
||
|---|---|---|---|
| Rabbit (n = 8)b | Human 2 g i.v.c | Rabbit humanlike (n = 8)d | |
| t1/2 (h) | 1.66 ± 0.15 | 6.92 | 7.21 ± 0.19 |
| kel (h−1) | 0.4 ± 0.04 | 0.1 | 0.09 ± 0.002 |
| AUC0–α (μg · h/ml) | 260.72 | 1,506.3 | 1,819 ± 82.72 |
The pharmacokinetic parameters were estimated on the basis of an open one-compartment model.
t1/2 and kel data are for healthy rabbits treated with 50 mg/kg i.v. The AUC0–α was calculated from the data of an ideal profile obtained by C0 of 256.8 μg/ml and the kel of ceftriaxone in rabbits. Data were obtained from the work Patel et al. (33).
kel, t1/2, and AUC0–α were obtained from an ideal serum human profile of 2 g of ceftriaxone.
Data are for humanlike pharmacokinetics of 2 g of ceftriaxone in rabbits.
Treatment of established endocarditis.
Results of therapy of experimental endocarditis caused by E. faecalis V48 are shown in Table 6. After 3 days of treatment, residual bacterial titers in the cardiac vegetations were significantly lower in the treated animals than in the controls (P < 0.0001). Comparisons between the treated groups revealed that the combination of ampicillin with ceftriaxone was significantly more effective than ampicillin alone in reducing the number of bacteria in the vegetations (P < 0.001). The mean log10 CFU per gram of vegetation in the group receiving ampicillin plus ceftriaxone was 6.3 less than that of the control group and 3.7 less than that of the ampicillin group. Likewise, the size of the vegetations of the animals treated with the combination was significantly less than that found in the animals treated with ampicillin alone (P = 0.0001). None of the animals had sterile vegetations.
TABLE 6.
Treatment of experimental endocarditis caused by E. faecalis V48 or V45 with a humanlike profile of ampicillin alone or in combination with ceftriaxone
| Treatment group |
E. faecalis V48
|
E. faecalis V45
|
|||||
|---|---|---|---|---|---|---|---|
| No. dead/ no. total (%) | Wt of vegetation (g)b | Log CFU/g of vegetationb | No. dead/no. total (%) | No. sterilized/no. survived (%) | Wt of vegetation (g) | Log CFU/g of vegetation | |
| Control | 3/12 (25) | 11.6 ± 1 | 3/12 (25) | 10.5 ± 0.4 | |||
| Ampicillina | 6/19 (31) | 0.12 ± 0.057 | 7.9 ± 1.7c | 4/20 (20) | 0/16 (0) | 0.0607 ± 0.01 | 6.0 ± 1.4c |
| Ampicillina + ceftriaxonea | 3/17 (18) | 0.067 ± 0.024d | 5.3 ± 0.6ce | 3/20 (15) | 8/17 (47)f | 0.0421 ± 0.02 | 3.1 ± 1.3ce |
Ampicillin, 2 g/4 h i.v. at humanlike pharmacokinetics; ceftriaxone, 2 g/12 h at humanlike pharmacokinetics.
Results are shown as means ± SD.
P < 0.0001 versus control.
P < 0.0001 versus ampicillin.
P < 0.001 versus ampicillin.
P < 0.001 versus ampicillin.
In the animals with endocarditis due to E. faecalis V45, the treatment with ampicillin plus ceftriaxone was even more effective (Table 6). Ceftriaxone plus ampicillin was more effective than ampicillin alone in reducing enterococcal vegetation counts (P < 0.0001). Therefore, no animal treated with ampicillin alone had sterile vegetations, whereas treatment with ampicillin plus ceftriaxone resulted in sterilization of all the infected valves in 47% of the animals (8 of 17) with endocarditis due to E. faecalis V45 (P < 0.001). In order to study the possible antibiotic carryover effect, a microbiological bioassay of the homogenates with Micrococcus as the bioassay microorganism was carried out, and no traces of ampicillin or ceftriaxone were found. It is unlikely that E. faecalis (V45 or V48) growth inhibition could be produced by possible traces of ampicillin and ceftriaxone 6 h after final infusion, since they were unable to inhibit M. luteus growth. Moreover, taking into account that the trough concentration of ampicillin is 4 μg/ml and the trough concentration of ceftriaxone is 30 μg/ml, and the kel of ampicillin and ceftriaxone are 2.4 and 0.4 h−1, respectively, at 6 h after terminating infusion of the drugs, the concentrations in serum in the animals at the moment of sacrifice would be 0.000002 μg of ampicillin per ml and <2 μg of ceftriaxone per ml, levels that do not inhibit E. faecalis growth.
DISCUSSION
The first purpose of this study was to describe a mathematical model that determines the doses to be given to animals by a computer-controlled infusion pump system to obtain serum profiles in the rabbit similar to those observed in humans after i.v. administration of 2 g of ceftriaxone. In previous studies performed in our laboratory (19), we developed a model of humanlike pharmacokinetics to be used for drugs with an open one-compartment pharmacokinetic model (e.g., ampicillin). The results of the present study show that the model we developed is simple and adaptable for simulating the human pharmacokinetics of antimicrobials with an open two-compartment model.
It is a widespread belief that enterococci are naturally tolerant of the bactericidal effect of β-lactam agents, even though occasional nontolerant strains have been found (22). In this work, when the antibiotic effect of ampicillin was studied at various concentrations, a bactericidal effect (>3 log decrease in colony count) was found in 9 of 10 of our strains but only in a narrow range of ampicillin concentrations. In fact, in six of nine of these strains, this bactericidal window was shown only with one or two of all the ampicillin concentrations tested. At ampicillin concentrations above this bactericidal window, there is a predominant static effect (nonbactericidal) in the tested isolates. If only these concentrations were tested, all strains could be considered classically tolerant. Indeed, the skipped tubes tend to be ignored in most MBC determinations. The existence of a bactericidal window for most E. faecalis strains has been previously described by other groups for penicillin and amoxicillin (15, 16, 30). Interestingly, tolerance may have been developed by intermittent challenge with β-lactams (22). Experiments by our group suggest that pulse-exposure and, particularly, stepwise graded concentration regimens may produce a local selection of tolerant variants in local compartments with high and/or low concentrations, in this way closing the bactericidal window (4).
The data obtained in this study suggest that the association of ampicillin and ceftriaxone may show in vitro synergism against E. faecalis with HLRAg. In all of the tested E. faecalis isolates, a strong antibacterial cooperation between ampicillin and ceftriaxone was detectable with a ≥2 log10 decrease in CFU per milliliter between the combination and its most active agent alone after 24 h. The results may indicate that the combination could be useful in the infections caused by HLRAg E. faecalis strains. It can be suggested that nonbactericidal ampicillin concentrations move into the bactericidal range by association with ceftriaxone, enlarging the range of ampicillin’s bactericidal effect. This synergistic effect has been previously detected by Mainardi et al. (30) with amoxicillin and cefotaxime; these authors proposed that, at low amoxicillin concentrations, the low-molecular-weight penicillin-binding proteins (PBPs) 4 and 5 would be partially saturated, but the nonessential PBPs 2 and 3 could participate in building the cell wall; the combination with cefotaxime would totally saturate PBPs 2 and 3, producing the bactericidal synergistic effect. At higher concentrations, ampicillin alone may be able to inhibit the function of PBPs 4 and 5, producing an optimal bactericidal effect. Beyond a given (high) concentration, ampicillin could inhibit autolysins, again reducing bacteriolysis, as was suggested by Fontana et al. (15). Other β-lactam and/or β-lactam associations may produce a similar effect; for instance, an increase in the bactericidal effect of ampicillin by combination with imipenem in Enterococcus faecium has been recently reported (7). We were unable to find any synergistic activity between ampicillin and ceftriaxone against E. faecium (41), confirming previous results of Mainardi et al. (30).
In this work, all E. faecalis strains were significantly killed by low concentrations of ampicillin (1 or 2 μg/ml) when ceftriaxone was associated with it. The rate of killing was generally higher with 2 than with 1 μg of ampicillin per ml, independently of the ceftriaxone concentration, suggesting that the main bactericidal activity was due to ampicillin. It is important to note that strong antimicrobial cooperation and a bactericidal effect were obtained at low ampicillin concentrations, similar to those expected to be reached at the heart valve vegetations.
In rabbits, after 3 days of treatment, the ampicillin-ceftriaxone combination was more effective than ampicillin alone in decreasing the size of the vegetations and the bacterial concentrations of HLRAg E. faecalis inside the vegetations. Therefore, it is remarkable that close to half the animals infected by the V45 strain had no detectable CFU in their vegetations at the end of treatment with the combination. There is, thus, total accordance between the in vitro and the in vivo studies. In the animal model, ampicillin alone can decrease the enterococcal bacterial count from the valve, but to a much lesser extent than it can in the presence of ceftriaxone. It could be considered that the period during which the ampicillin concentrations needed for optimal killing are available in the host is too short to be effective. It is well known that β-lactam killing activity is proportional to the time of exposure (AUC) (24). Ceftriaxone, enlarging the range of ampicillin’s bactericidal concentrations, increases the period during which these concentrations are available, and thus increased bactericidal activity is expected to occur. Our results in the treatment of experimental endocarditis due to E. faecalis with ampicillin alone are not comparable to those of other studies mainly because we use humanlike pharmacokinetics of ampicillin which mimic an i.v. administration of 2 g every 4 h, and no other studies use this technique. The amount of drug, the time above MIC, the AUC, and the shape of this AUC are totally different from those for the administration of the drug with animal pharmacokinetics every 8 or 12 h as used in the other studies (21, 40), and as would be expected, the efficacy in our study was superior. In our study, ampicillin alone provided an important reduction of bacterial titers in the vegetations, but the combination was significantly superior.
Therapy of endocarditis due to HLRAg E. faecalis remains controversial. To date, there is no known effective medical treatment for patients with endocarditis in whom the infecting strain of E. faecalis is susceptible to ampicillin but highly resistant to aminoglycosides. Recently, Venditti et al. (44) described a patient with endocarditis due to HLRAg E. faecalis for whom treatment with ampicillin plus ceftriaxone failed. One possible explanation for this failure is that ceftriaxone had to be stopped after 3 weeks because of fever related to the administration of the drug. We have successfully treated two patients, one with the ampicillin-ceftriaxone combination and the second with cefotaxime instead of ceftriaxone, the case being a human immunodeficiency virus-infected patient with cholangitis due to Cryptosporidium spp. that precluded the use of ceftriaxone (2).
In conclusion, the results presented here show that strain-independent in vitro bactericidal synergism occurred against HLRAg E. faecalis. Likewise, in the treatment of HLRAg E. faecalis-induced experimental endocarditis, ceftriaxone combined with ampicillin, both given with humanlike kinetics of 2 g every 12 h and 2 g every 4 h, was an effective therapeutic choice, but it remains to be seen if this is going to be translated into superior clinical results. Further studies are needed to establish the efficacy of this combination in humans with HLRAg enterococcal endocarditis.
ACKNOWLEDGMENTS
We thank Celine Cavallo for English-language assistance.
This work was supported in part by Fondo Investigaciones Sanitarias de la Seguridad Social (FISS grant no. 96/0057-00) and Hospital General Vall d’Hebron [grant no. PR(HG) 67/97].
Appendix
Development of the mathematical model. The aim of this mathematical model was to determine the doses that would obtain the desired humanlike pharmacokinetics of ceftriaxone on the basis of an open two-compartment model in the animals.
The system we used to imitate human kinetics in rabbits is based on the administration of decreasing quantities of drug to counteract the higher elimination rate in the animal.
(a) To estimate the zero-time intercept for the α phase (Ah) and the zero-time intercept for the β phase (Bh) in humans for a maximum concentration of ceftriaxone (Cmax) in serum after a dose of 2 g in humans (Cmax = 256.9 μg/ml) (32), we used the following second-degree equation. For practical reasons, we have used Cmax as Ch0.
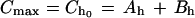 |
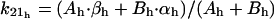 |
in which βh, αh, and k21h are known human constants, was calculated from the work of Patel et al. (33).
(b) At the end of the distribution phase (α phase), we estimated the concentration of the drug (Cdα) necessary to reach the human concentration at the end of the α phase (Chα) by subtracting it from the estimated serum level in animals (Crα):
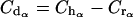 |
in which
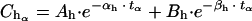 |
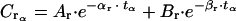 |
in which tα = time value of α phase in animals (hours), Ar = zero-time intercept for the α phase in rabbits (micrograms per milliliter) for C0 = 250 μg/ml, and Br = zero-time intercept for the β phase in rabbits (micrograms per milliliter) for C0 = 250 μg/ml.
In order to find Ar and Br, we used the following second-degree equation:
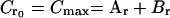 |
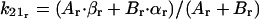 |
in which βr, αr, and k21r are known rabbit constants obtained previously.
(c) We divided the elimination phase (β phase) into time periods (Tx; T1...Tn). At the final limit point of each time period (Tx), the serum drug level is higher in human kinetics (Chx) than in rabbit kinetics (Crx). We determined the concentration of the drug (Cdx, Cd1...Cdn) required to counterbalance this difference by subtracting the desired concentration (Chx, Ch1...Chn) from the estimated serum level in the animal (Crx. Cr1...Crn) by the following mathematical formula, where x = number of the period:
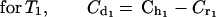 |
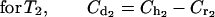 |
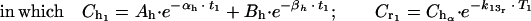 |
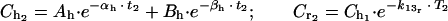 |
Thus, the general formula for Tx = 1, 2, 3, ....infinity is:
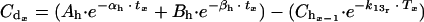 |
in which Tx = time value between periods. Time period is in hours; tx = time in human kinetics (hours); and k13r = rabbit elimination rate constant from the central compartment (per hour).
(d) The amount of drug (Qx, in milligrams per hour) that must be given by continuous i.v. infusion during the entire length of the α phase (tα) and the time periods (Tx) of the β phase to attain the desired concentration at the end of the tα (Chα) or at the end of Tx (Chx) was determined by the following mathematical fraction:
for α phase,
 |
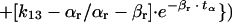 |
for β phase,
 |
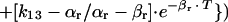 |
in which Qx = quantity of drug to be administered during Tx (milligrams per hour), Vr = volume of distribution of the drug in rabbits (liters per kilogram), and Wr = animal weight (kilograms).
(e) The infusion rate (Vx, in milliliters per hour) to use during the time period (Tx) in order to administer Qx depends on the concentration of the drug solution used (S, in milligrams per milliliter): Vx = Qx/S.
(f) The first i.v. dose was determined by the following formula:
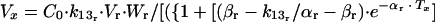 |
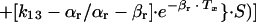 |
in which C0 is the maximum concentration of ceftriaxone.
REFERENCES
- 1.Almirante B, Tornos M P, Gurgui M, Pujol M, Miro J M. Prognosis of enterococcal endocarditis. Rev Infect Dis. 1991;13:1248–1249. doi: 10.1093/clinids/13.6.1248. [DOI] [PubMed] [Google Scholar]
- 2.Almirante B, Gavaldà J, Tornos M P, Ribera E, Pahissa A. Program and abstracts of the Fourth International Symposium on Modern Concepts in Endocarditis and Cardiovascular Infections, Yverdon-Les-Bains, Switzerland. 1997. Effectiveness of ampicillin plus ceftriaxone or cefotaxime in the treatment of high-level aminoglycoside resistant (HLAR) Enterococcus faecalis infective endocarditis, abstr. 179; p. 179. [Google Scholar]
- 3.Anhalt J P. Antimicrobial assays. In: Washington II J A, editor. Laboratory procedures in clinical microbiology. New York, N.Y: Springer-Verlag; 1985. pp. 691–729. [Google Scholar]
- 4.Baquero F, Negri M C. Selective compartments for resistant microorganisms in antibiotic gradients. Bioessays. 1997;19:731–736. doi: 10.1002/bies.950190814. [DOI] [PubMed] [Google Scholar]
- 5.Bartoloni A, Stefani S, Orsi A, Nicoletti A, Difonzo A R, Paradisi F. High-level aminoglycoside resistance among enterococci isolated from blood cultures. J Antimicrob Chemother. 1992;29:729–731. doi: 10.1093/jac/29.6.729. [DOI] [PubMed] [Google Scholar]
- 6.Blatter M, Fluckinger U, Entenza J, Glauser M P, Francioli P. Simulated human serum profiles of one daily dose of ceftriaxone plus netilmicin in treatment of experimental streptococcal endocarditis. Antimicrob Agents Chemother. 1993;31:971–976. doi: 10.1128/aac.37.9.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brandt C M, Rouse M S, Laue N W, Stratton N W, Wilson W R, Steckelberg J M. Effective treatment of multidrug-resistant enterococcal experimental endocarditis with combinations of cell wall-active agents. J Infect Dis. 1996;173:909–913. doi: 10.1093/infdis/173.4.909. [DOI] [PubMed] [Google Scholar]
- 8.Cercenado E, Eliopoulos G M, Wennersten C B, Moellering R C., Jr Influence of high-level gentamicin resistance and beta-hemolysis on susceptibility of enterococci to the bactericidal activities of ampicillin and vancomycin. Antimicrob Agents Chemother. 1992;36:2526–2528. doi: 10.1128/aac.36.11.2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Das S S, Anderson J R, Macdonald A A, Somerville K W. Endocarditis due to high level gentamicin resistant Enterococcus faecium. J Infect. 1994;28:185–191. doi: 10.1016/s0163-4453(94)95680-4. [DOI] [PubMed] [Google Scholar]
- 10.Durack D T, Beeson P B. Experimental bacterial endocarditis. I. Colonisation of a sterile vegetation. Br J Exp Pathol. 1972;53:44–49. [PMC free article] [PubMed] [Google Scholar]
- 11.Eliopoulos G M, Wennersten C, Zighelboim-Daum S, Reiszner S, Goldman F, Moellering R C., Jr High-level resistance to gentamicin in clinical isolates of Streptococcus (Enterococcus) faecium. Antimicrob Agents Chemother. 1988;32:1528–1532. doi: 10.1128/aac.32.10.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eliopoulos G M. Antibiotic resistance in Enterococcus species: an update. In: Remington J S, Swartz M N, editors. Current clinical topics in infectious diseases. Boston, Mass: Blackwell Science, Inc.; 1996. pp. 21–51. [PubMed] [Google Scholar]
- 13.Facklam R R, Collins M D. Identification of Enterococcus species isolated from human infections by a conventional test scheme. J Clin Microbiol. 1989;27:731–734. doi: 10.1128/jcm.27.4.731-734.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernandez-Guerrero M L, Barros C, Rodriguez Tudela J L, Fernandez Roblas R, Soriano F. Aortic endocarditis caused by gentamicin-resistant Enterococcus faecalis. Eur J Clin Microbiol Infect Dis. 1988;7:525–527. doi: 10.1007/BF01962605. [DOI] [PubMed] [Google Scholar]
- 15.Fontana R, Boaretti M, Grossato A, Tonin E A, Lleo M M, Satta G. Paradoxical response of Enterococcus faecalis to the bactericidal activity of penicillin is associated with reduced activity of one autolysin. Antimicrob Agents Chemother. 1990;34:314–320. doi: 10.1128/aac.34.2.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fontana R, Grossato A, Ligozzi M, Tonin E A. In vitro response to bactericidal activity of cell wall-active antibiotics does not support the general opinion that enterococci are naturally tolerant to these antibiotics. Antimicrob Agents Chemother. 1990;34:1518–1522. doi: 10.1128/aac.34.8.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garrison P, Freedman L R. Experimental endocarditis. I. Staphylococcal endocarditis in rabbits resulting from placement of a polyethylene catheter in right side of the heart. Yale J Biol Med. 1970;42:394–410. [PMC free article] [PubMed] [Google Scholar]
- 18.Gavaldà J, Pahissa A, Almirante B, Laguarda M, Crespo E, Pou L, Fernández F. Effect of gentamicin dosing interval on therapy of viridans streptococcal experimental endocarditis with gentamicin and penicillin. Antimicrob Agents Chemother. 1995;39:2098–2103. doi: 10.1128/aac.39.9.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gavaldà J, Cardona P J, Capdevila J A, Laguarda M, Almirante B, Pigrau C, Crespo E, Pahissa A. Treatment of experimental endocarditis due to Enterococcus faecalis using once-daily dosing regimen of gentamicin plus simulated profiles of ampicillin in human serum. Antimicrob Agents Chemother. 1996;40:173–178. doi: 10.1128/aac.40.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gavaldà J, Capdevila J A, Torres C, Laguarda M, Tenorio C, de Otero J, Pahissa A. Abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. Efficacy of ampicillin (A) and ceftriaxone (C) in the treatment of experimental endocarditis due to Enterococcus faecalis highly-resistant to aminoglycosides, abstr. B004; p. 33. [Google Scholar]
- 21.Hellinger W C, Rouse M S, Rabadan P M, Henry N K, Steckelberg J M, Wilson W R. Continuous intravenous versus intermittent ampicillin therapy of experimental endocarditis caused by aminoglycoside-resistant enterococci. Antimicrob Agents Chemother. 1992;36:1272–1275. doi: 10.1128/aac.36.6.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hodges T L, Zighelboim-Daum S, Eliopoulos G M, Wennersten C, Moellering R C., Jr Antimicrobial susceptibility changes in Enterococcus faecalis following various penicillin exposure regimens. Antimicrob Agents Chemother. 1992;36:121–125. doi: 10.1128/aac.36.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horodniceanu T, Bougueleret L, El-Solh N, Bieth G, Delbos F. High-level, plasmid-borne resistance to gentamicin in Streptococcus faecalis subsp. zymogenes. Antimicrob Agents Chemother. 1979;16:686–689. doi: 10.1128/aac.16.5.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hyatt J M, McKinnon P S, Zimmer G S, Schentag J J. The importance of pharmacokinetic/pharmacodynamic surrogate markers to outcome. Focus on antibacterial agents. Clin Pharmacokinet. 1995;28:143–160. doi: 10.2165/00003088-199528020-00005. [DOI] [PubMed] [Google Scholar]
- 25.Jones B L, Ludlam H A, Brown D F. High dose ampicillin for the treatment of high-level aminoglycoside resistant enterococcal endocarditis. J Antimicrob Chemother. 1994;33:891–892. doi: 10.1093/jac/33.4.891. [DOI] [PubMed] [Google Scholar]
- 26.Kathpalia S, Lolans V, Levandowski R, Jackson G G. Resistance to all aminoglycoside antibiotics in enterococcal endocarditis. Clin Res. 1984;32:372. [Google Scholar]
- 27.Knapp C, Moody J A. Tests to assess bactericidal activity. In: Isenberg H D, editor. Clinical Microbiology procedures handbook. Washington, D.C: American Society for Microbiology; 1992. pp. 5.16.1–5.16.33. [Google Scholar]
- 28.Libertin C R, McKinley K M. Gentamicin-resistant enterococcal endocarditis: the need for routine screening for high-level resistance to aminoglycosides. South Med J. 1990;83:458–460. [PubMed] [Google Scholar]
- 29.Lipman M L, Silva J., Jr Endocarditis due to Streptococcus faecalis with high-level aminoglycoside resistant enterococcal endocarditis. J Antimicrob Chemother. 1989;11:325–328. [Google Scholar]
- 30.Mainardi J L, Gutmann L, Acar J F, Goldstein F W. Synergistic effect of amoxicillin and cefotaxime against Enterococcus faecalis. Antimicrob Agents Chemother. 1995;39:1984–1987. doi: 10.1128/aac.39.9.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mizen L, Woodnutt G, Kernutt I, Catherall E. Simulation of human serum pharmacokinetics of ticarcillin-clavulanic acid and ceftazidime in rabbits and efficacy against experimental Klebsiella pneumoniae meningitis. Antimicrob Agents Chemother. 1989;33:693–699. doi: 10.1128/aac.33.5.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests on bacteria that grow aerobically. 4th ed. Approved standard M7-A4. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 33.Patel I H, Sugihara J G, Weinfeld R E, Wong E G, Siemsen A W, Berman S J. Ceftriaxone pharmacokinetics in patients with various degrees of renal impairment. Antimicrob Agents Chemother. 1984;33:438–442. doi: 10.1128/aac.25.4.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patterson J E, Colodny S M, Zervos M J. Serious infection due to β-lactamase-producing Streptococcus faecalis with high-level resistance to gentamicin. J Infect Dis. 1988;158:1144–1145. doi: 10.1093/infdis/158.5.1144. [DOI] [PubMed] [Google Scholar]
- 35.Patterson J E, Sweeney A H, Simms M, Carley N, Mangi R, Sabetta J, Lyons R W. An analysis of 110 serious enterococcal infections. Epidemiology, antibiotic susceptibility, and outcome. Medicine (Baltimore) 1990;74:191–200. doi: 10.1097/00005792-199507000-00003. [DOI] [PubMed] [Google Scholar]
- 36.Pesterl E, Graninger W, Georgopoulos A. The efficacy of teicoplanin in the treatment of endocarditis caused by gram-positive bacteria. J Antimicrob Chemother. 1993;31:755–766. doi: 10.1093/jac/31.5.755. [DOI] [PubMed] [Google Scholar]
- 37.Rice L B, Calderwood S B, Eliopoulos G M, Farber B F, Karchmer A W. Enterococcal endocarditis: a comparison of prosthetic and native valve disease. Rev Infect Dis. 1991;13:1–7. doi: 10.1093/clinids/13.1.1. [DOI] [PubMed] [Google Scholar]
- 38.Sacher H L, Miller W C, Landau S W, Sacher M L, Dixon W A, Dietrich K A. Relapsing native-valve enterococcal endocarditis: a unique cure with oral ciprofloxacin combination drug therapy. J Clin Pharmacol. 1991;31:719–721. doi: 10.1002/j.1552-4604.1991.tb03766.x. [DOI] [PubMed] [Google Scholar]
- 39.Sahm D F, Torres C. Effects of medium and inoculum variations on screening for high-level aminoglycoside resistance in Enterococcus faecalis. J Clin Microbiol. 1988;26:250–256. doi: 10.1128/jcm.26.2.250-256.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thauvin C, Eliopoulos G M, Willey S, Wennersten C, Moellering R C., Jr Continuous-infusion ampicillin therapy of enterococcal endocarditis in rats. Antimicrob Agents Chemother. 1987;31:139–143. doi: 10.1128/aac.31.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Torres C, Tenorio C, Gavaldà J, Zarazaga M, Pahissa A, Baquero F. Program and abstracts of the 7th Reunión Nacional de la Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica, Madrid, Spain. 1997. Ausencia de efecto sinérgico ampicilina-ceftriaxona en Enterococcus faecium, abstr. 24; p. 104. [Google Scholar]
- 42.van de Klundert J A M, Vliegenthart J S. PCR detection of genes coding for aminoglycoside-modifying enzymes. In: Persing D H, Smith T F, Tenover F C, White T J, editors. Diagnostic molecular microbiology: principles and applications. Washington, D.C: American Society for Microbiology; 1993. pp. 547–552. [Google Scholar]
- 43.van der Meer J T, van Vianen W, Hu E, van Leeuwen W B, Valkenburg H A, Thompson J, Michel M F. Distribution, antibiotic susceptibility and tolerance of bacterial isolates in culture-positive cases of endocarditis in The Netherlands. Eur J Clin Microbiol Infect Dis. 1991;10:728–734. doi: 10.1007/BF01972497. [DOI] [PubMed] [Google Scholar]
- 44.Venditti M, Brandimarte C, Cassone M, Galiè M, Tarasi A, Tarasi D. Program and abstracts of the Fourth International Symposium on Modern Concepts in Endocarditis and Cardiovascular Infections, Yverdon-Les-Bains, Switzerland. 1997. Endocarditis caused by an Enterococcus faecalis high-level resistant to aminoglycosides: failure of ampicillin and ceftriaxone combined therapy, abstr. 178; p. 178. [Google Scholar]
- 45.Wilson W R, Karchmer A W, Dajani A S, Aubert T K A, Bayer A, Kaye D, Bisno A L, Ferrieri P, Shulman S T, Durack D T. Antibiotic treatment of adults with infective endocarditis due to streptococci, enterococci, staphylococci, and HACEK microorganisms. American Heart Association. JAMA. 1995;274:1706–1713. [PubMed] [Google Scholar]