Amiodarone-induced thyrotoxicosis (AIT) is a potentially life-threatening condition, due to the coexistence of severe thyrotoxicosis (often requiring long courses of medical therapy to restore euthyroidism) and compromised heart function, resulting in high morbidity and mortality rates [1]. Total thyroidectomy has been recognized as beneficial over long-term medical therapies in the setting of moderate-to-severe left ventricular systolic dysfunction, especially when performed without delay [2–4]. SARS-CoV-2 (Covid-19) infection is associated with high rates of morbidity and mortality, especially in patients with cardiovascular comorbidities [5, 6], obesity [7], and diabetes mellitus [8]. The relationship between SARS-CoV-2 infection and the thyroid is ambiguous: on the one side, the development of subacute thyroiditis (SAT) following SARS-CoV-2 infection has been extensively reported [9]; on the other side, histopathological studies on deceased patients reported evidences of SARS-CoV-2 infection in most tissues but not in the thyroid samples [10]. Moreover, SARS-CoV-2 has not been researched on histological samples from destructive thyroiditis to date, since SAT is a benign and often self-limiting condition, which does not require a surgical management [11].
A 62-year-old man was admitted to our outpatient facility with a diagnosis of newly-onset AIT. His past medical history revealed overweight-obesity (age 30), arterial hypertension (age 47), and type 2 diabetes (age 51) treated with oral antidiabetic medications with inadequate glycemic control. The patient had a history of ST-elevated myocardial infarction (age 54) treated with percutaneous coronary intervention, and atrial fibrillation (AF, age 56). At 58, post-ischemic dilated cardiomyopathy with mild systolic dysfunction (left ventricular ejection fraction, LVEF, 46%) was diagnosed; in the same year amiodarone (200 mg/d) was started for the recurrence of AF with high ventricular drive, and an ICD was implanted. Two weeks before admission to our clinic the patient-reported tachycardia, sweating, weight loss (−7 Kg) and fatigue. Thyroid function tests showed FT4 44.3 pg/mL (7–17), FT3 9.27 pg/mL (2.7–5.7), TSH 0.01 µU/mL (0.4–4). Amiodarone was withdrawn before referral. Neck ultrasound showed a normal-sized (14.2 mL), disomogeneous and mildly hypoechoic thyroid gland, without nodules. Doppler sonography revealed absent vascularization, and radioactive iodine uptake with 131-I was low (3 h:0.9%; 24 h:1.1%). Cardiac evaluation identified an overall adequate hemodynamic compensation (NYHA-II); ECG confirmed the presence of AF (HR 90 bpm); ecocardiography showed dilated cardiomyopathy with a mild reduction of the LVEF compared to previous measurements (42%). Sotalol (160 mg/d in two doses) was started with a good control of HR.
The patient received diagnosis of type 2 AIT, and oral methylprednisolone (40 mg/d) was started. After an initial drop of thyroid hormones concentrations (Fig. 1A), from week 4 the patient underwent a relapse of thyrotoxicosis with a significant worsening of symptoms, including tachycardia (HR 110 bpm) and mild dyspnea (NYHA-III). The patient was admitted to the inpatient ward after a negative nasopharyngeal buffer for SARS-CoV-2. During the hospital stay, oral glucocorticoids were continued and the HR was controlled by iv beta-blockers. One week following hospital admission, the patient developed mild respiratory symptoms with worsening of dyspnea; the nasopharyngeal buffer turned out positive for SARS-CoV-2. The patient was thus isolated in the Covid-19 ward. He remained paucisymptomatic for the whole course of the infection, with mild respiratory symptoms, although the radiological evidence of mild interstitial pneumonia. The patient did not report any subjective symptom of thyroid involvement, such as antero-cervical pain or discomfort, but thyroid hormone concentrations underwent a progressive increase, as shown in Fig. 1A. Ten days later, due to the further worsening of thyrotoxicosis with initial hemodynamic instability (estimated bed-side LVEF 28%), the patient was addressed to salvage thyroidectomy.
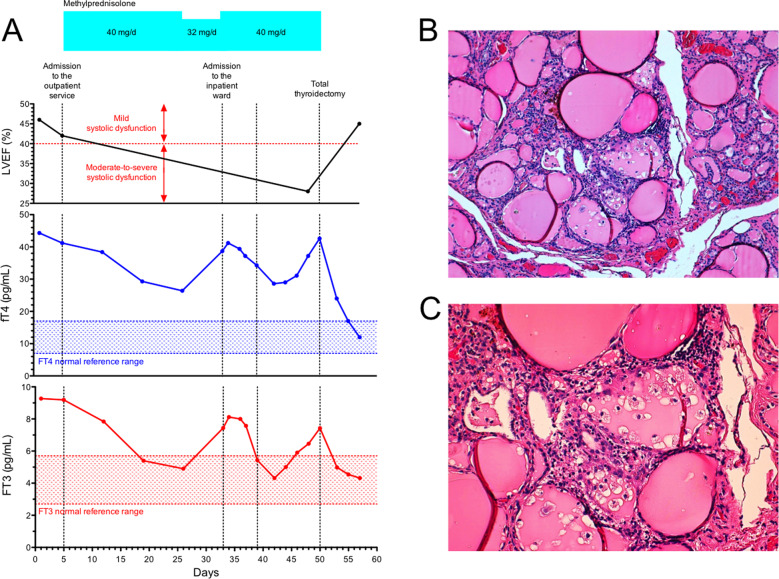
Fig. 1.
A Left ventricular ejection fraction and thyroid hormone concentrations over the course of the disease. Upper plot: LVEF, expressed as number %. Medium plot: FT4 concentrations, expressed in pg/mL. Lower plot FT3 concentrations, expressed in pg/mL. X axis reports the time (days); key moments in the patient’s clinical history are identified by vertical dotted lines. Y axis reports the appropriate variable for each panel, together with the respective reference ranges. Blue squares indicate methylprednisolone dosages. LVEF left ventricular ejection fraction; FT4 free thyroxine; FT3 free triiodothyronine. B, C Representative histopathological images from thyroid surgical specimens (hematoxylin and eosin staining). The focal disruption of the thyroid follicles is shown with small collections of macrophages intermingled with scattered lymphocytes (original magnification, ×10 and ×20, for B, C, respectively)
Total thyroidectomy was performed in a SARS-CoV-2-dedicated theater, under general anesthesia according to SIAARTI operational directives [12]. Surgery was performed by traditional cervical approach. The operative time was 45 min and no intraoperative complication was noted. Postoperatively the patient stayed in the ICU for 2 days, with a regular course. Total postoperative blood loss at drain removal was 30 mL. Despite the presence of AF, HR remained persistently within the normal range (64–76 bpm) without signs of hemodynamic instability. Levo-Thyroxine and hydrocortisone were started, and one week later he was discharged to home.
The histological sample was consistent with type 2 AIT, displaying typical features such as thyroid follicles disruption and macrophage infiltration [13] (Fig. 1B, C). The presence of SARS-CoV-2 was searched on the histological sample: in details, RNA was isolated from two 10 µm-thick formalin-fixed paraffin-embedded sections using the RNeasy FFPE kit (Qiagen, Hilden, Germany); RNA concentration and quality were assessed by spectrophotometry (Xpose, Gentbrugge, Belgium). About 150 ng of RNA was used for the RT-PCR assay to detect the SARS-CoV-2 genome, with an assay designed to target the viral nucleocapsid (N) and the RNA-dependent RNA Polymerases (RdRp) genes (Easy SARS-CoV-2 WE kit, Diatech Pharmacogenetics, Jesi,Italy). The assay was run in duplicate. No presence of SARS-CoV-2 genome was observed.
Nine months following total thyroidectomy the patient is alive, and continues the outpatient follow-up. He did not develop any complication from the total thyroidectomy. His heart conditions improved and despite the persistence of chronic AF with normal HR his LVEF went back to the values reported before the occurrence of AIT.
Discussion
SARS-CoV-2 infection has emerged as an important cause of mortality, and the underlying comorbidities displayed by our patient are well-known risk factors for a worse clinical outcome [5–8]. However, AIT mortality risk in case of worsening cardiac condition (i.e. thyrotoxicosis-induced rhythm or hemodynamic instability) is so high that it may be appropriate to consider a life-saving surgery while Covid-positive rather than a wait-and-see approach. Thyroid surgery in the setting of SARS-CoV-2 infection requires per sè some careful considerations: on the one hand, radiofrequency devices are expected to increase the risk of aerosolizing the virus by burning tissue microparticles, compared to the standard clamp-and-tie technique [14]; on the other hand, energy-based devices may reduce operative time, intraoperative blood loss, drain output [15]: in this context, a shorter operative time can lead to a lower infective risk for the operators and to a lower risk of hemodynamic instability [16], and a better hemostasis may reduce the risk of postoperative hematoma, an important point for patients with left ventricular systolic dysfunction and acute respiratory syndrome [17].
Typically, histological features of SAT include marked inflammatory infiltration with neutrophils, lymphocytes and foamy histiocytes, and variable degree of fibrosis with formation of giant cell granulomas in response to the release of colloid from ruptured follicles [18]. Here, we found only images of focal disruption of the thyroid follicles with small collection of macrophages and lymphocytes, in absence of acute inflammation and in line with histological findings described in type 2 AIT [13, 19]. Outside the setting of SAT, some studies on patients deceased for SARS-CoV-2 failed to identify the viral genome on thyroid tissue [10], whereas others reported the detection of SARS-CoV-2 antigens and genome at least in severe cases [20, 21], with the expression of the ACE-2 mRNA reported as a potential clue for the entry of the virus into the follicular cells [22]. SARS-CoV-2-infected thyroid tissues showed marked infiltration of neutrophils and macrophages as well as the activation of type I and type II interferon pathways [23]. These changes are consistent with SAT. Beside the typical aspect of amiodarone-induced destructive thyroiditis emerging from the analysis of the histological sample [13, 19], an important point is the absence of SARS-CoV-2 genome on thyroid tissue. SAT often develops after upper respiratory tract infections; the temporal correlation between SAT and Covid-19 infection allowed drawing a cause-relation nexus, even though the putative mechanism of Covid-induced SAT has not been elucidated to date: for instance, it is not clear whether SARS-CoV-2 colonizes thyroid tissue, or acts by triggering a cytokine-mediated damage. The absence of pathology studies due to the benignity of SAT, which does not require surgical management, is a limitation to the comprehension of the pathophysiology of this condition. According to our knowledge, our study is the first reporting the research of SARS-CoV-2 genome on the histological samples from a destructive thyroiditis of a Covid-positive patient, and it provides a negative result. Anyway, by taking into account the timing of the reported events, it is logical to assume that in our patient the occurrence of the destructive thyroiditis preceded SARS-CoV-2 infection. Whether this or the globally low viral title may explain the negative results of our research for SARS-CoV-2 genome by PCR on the histological samples will require more data from larger series.
Acknowledgments
Author contributions
D.C., E.A., L.D.N., G.M., and F.Bo. were involved in the direct care of the patient; L.T., A.M.P., F.Ba. were involved in the analysis of the surgical specimen and in the viral genome research; D.C., L.T., and F.Bo. were involved in the ideation of the report; D.C., L.T., P.P., A.M.P., E.A., and L.D.N. were involved in the bibliographic research; D.C., L.T., P.P. were responsible for the writing of the original draft; L.T. and A.M.P. were rensponsible for the drawing of the histology figures; G.M., F.Ba., and F.Bo. were responsible for the initial revision; all the authors were subsequently involved in the revision of the draft and all the authors gave the consent for the publication of the manuscript in this current form.
Funding
This work was supported by an unrestricted grant from the University of Pisa.
Compliance with ethical standards
Conflict of Interest
The authors declare no competing interests.
Consent
Written informed consent for participation to this research study and for publication of the case and the results of the analysis was obtained from the patient and is available upon request.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bartalena L, Bogazzi F, Chiovato L, Hubalewska-Dydejczyk A, Links TP, Vanderpump M. 2018 European Thyroid Association (ETA) guidelines for the management of amiodarone-associated thyroid dysfunction. Eur. Thyroid J. 2018;7(2):55–66. doi: 10.1159/000486957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.D. Cappellani, P. Papini, A. Pingitore, L. Tomisti, M. Mantuano, A.M. Di Certo et al. Comparison between total thyroidectomy and medical therapy for amiodarone-Induced thyrotoxicosis. J. Clin. Endocrinol. Metab. 105(1), 242–251(2020) [DOI] [PubMed]
- 3.D. Cappellani, P. Papini, A.M. Di Certo, R. Morganti, C. Urbani, L. Manetti et al. Duration of exposure to thyrotoxicosis increases mortality of compromised AIT patients: the role of early thyroidectomy. J Clin. Endocrinol. Metab. 105(9), e3427–e3436 (2020) [DOI] [PubMed]
- 4.Kotwal A, Clark J, Lyden M, McKenzie T, Thompson G, Stan MN. Thyroidectomy for amiodarone-induced thyrotoxicosis: mayo clinic experience. J. Endocr. Soc. 2018;2(11):1226–35.. doi: 10.1210/js.2018-00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chilazi M, Duffy EY, Thakkar A, Michos ED. COVID and cardiovascular disease: what we know in 2021. Curr. Atheroscler. Rep. 2021;23(7):37. doi: 10.1007/s11883-021-00935-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(7):811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tartof SY, Qian L, Hong V, Wei R, Nadjafi RF, Fischer H, et al. Obesity and mortality among patients diagnosed with COVID-19: results from an integrated health care organization. Ann. Intern. Med. 2020;173(10):773–81.. doi: 10.7326/M20-3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Apicella M, Campopiano MC, Mantuano M, Mazoni L, Coppelli A, Del Prato S. COVID-19 in people with diabetes: understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol. 2020;8(9):782–92.. doi: 10.1016/S2213-8587(20)30238-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.A. Brancatella, D. Ricci, D. Cappellani, N. Viola, D. Sgrò, F. Santini et al. Is subacute thyroiditis an underestimated manifestation of SARS-CoV-2 infection? Insights from a case series. J. Clin. Endocrinol. Metab. 105(10), e3742–e3746 (2020) [DOI] [PMC free article] [PubMed]
- 10.Bradley BT, Maioli H, Johnston R, Chaudhry I, Fink SL, Xu H, et al. Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: a case series. Lancet. 2020;396(10247):320–32.. doi: 10.1016/S0140-6736(20)31305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fatourechi V, Aniszewski JP, Fatourechi GZ, Atkinson EJ, Jacobsen SJ. Clinical features and outcome of subacute thyroiditis in an incidence cohort: Olmsted County, Minnesota, study. The. J. Clin. Endocrinol. Metab. 2003;88(5):2100–2105. doi: 10.1210/jc.2002-021799. [DOI] [PubMed] [Google Scholar]
- 12.Coccolini F, Perrone G, Chiarugi M, Di Marzo F, Ansaloni L, Scandroglio I, et al. Surgery in COVID-19 patients: operational directives. World J. Emerg. Surg. 2020;15(1):25. doi: 10.1186/s13017-020-00307-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D. Cappellani, G. De Marco, E. Ferrarini, L. Torregrossa, A.M. Di Certo, G. Cosentino, et al. Identification of two different phenotypes of patients with amiodarone-induced thyrotoxicosis and positive thyrotropin receptor antibody tests. Thyroid 31(10), 1463–1471 (2021) [DOI] [PubMed]
- 14.Zakka K, Erridge S, Chidambaram S, Beatty JW, Kynoch M, Kinross J, et al. Electrocautery, diathermy, and surgical energy devices: are surgical teams at risk during the COVID-19 pandemic? Ann. Surg. 2020;272(3):e257–e62.. doi: 10.1097/SLA.0000000000004112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bakkar S, Papavramidis TS, Aljarrah Q, Materazzi G, Miccoli P. Energy-based devices in thyroid surgery-an overview. Gland Surg. 2020;9(Suppl 1):S14–s7. doi: 10.21037/gs.2019.08.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.G.A. Brat, S. Hersey, K. Chhabra, A. Gupta, J. Scott, Protecting surgical teams during the COVID-19 outbreak: a narrative review and clinical considerations. Ann. Surg. (2020). (Ahead of print) [DOI] [PMC free article] [PubMed]
- 17.Materazzi G, Ambrosini CE, Fregoli L, De Napoli L, Frustaci G, Matteucci V, et al. Prevention and management of bleeding in thyroid surgery. Gland Surg. 2017;6(5):510–515. doi: 10.21037/gs.2017.06.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ranganath R, Shaha MA, Xu B, Migliacci J, Ghossein R, Shaha AR. de Quervain’s thyroiditis: a review of experience with surgery. Am. J. Otolaryngol. 2016;37(6):534–537. doi: 10.1016/j.amjoto.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LiVolsi VA, Baloch ZW. The pathology of hyperthyroidism. Front Endocrinol. 2018;9:737. doi: 10.3389/fendo.2018.00737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D.W.L. Wong, B.M. Klinkhammer, S. Djudjaj, S. Villwock, M.C. Timm, E.M. Buhl et al., Multisystemic Cellular tropism of SARS-CoV-2 in autopsies of COVID-19 patients. Cells. 10(8), 1900 (2021) [DOI] [PMC free article] [PubMed]
- 21.Poma AM, Bonuccelli D, Giannini R, Macerola E, Vignali P, Ugolini C, et al. COVID-19 autopsy cases: detection of virus in endocrine tissues. J. Endocrinol. Investig. 2022;45(1):209–14.. doi: 10.1007/s40618-021-01628-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rotondi M, Coperchini F, Ricci G, Denegri M, Croce L, Ngnitejeu ST, et al. Detection of SARS-COV-2 receptor ACE-2 mRNA in thyroid cells: a clue for COVID-19-related subacute thyroiditis. J. Endocrinol. Investig. 2021;44(5):1085–90.. doi: 10.1007/s40618-020-01436-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poma AM, Basolo A, Bonuccelli D, Proietti A, Macerola E, Ugolini C, et al. Activation of type I and type II interferon signaling in SARS-CoV-2-positive thyroid tissue of patients dying from COVID-19. Thyroid. 2021;31(12):1766–1775. doi: 10.1089/thy.2021.0345. [DOI] [PubMed] [Google Scholar]