Abstract
Abstract
Purpose of Review
Stroke is the leading cause of permanent motor disability in the United States (US), but there has been little progress in developing novel, effective strategies for treating post-stroke motor deficits. The past decade has seen the rapid development of many promising, gamified neurorehabilitation technologies; however, clinical adoption remains limited. The purpose of this review is to evaluate the recent literature surrounding the adoption and use of gamification in neurorehabilitation after stroke.
Recent Findings
Gamification of neurorehabilitation protocols is both feasible and effective. Deployment strategies and scalability need to be addressed with more rigor. Relationship between engaged time on task and rehabilitation outcomes should be explored further as it may create benefits beyond repetitive movement.
Summary
As gamification becomes a more common and feasible way of delivering exercise-based therapies, additional benefits of gamification are emerging. In spite of this, questions still exist about scalability and widespread clinical adoption.
Keywords: Stroke, Neurological rehabilitation, Virtual reality, Video games, Stroke rehabilitation
Introduction
Stroke is the leading cause of permanent motor disability in the United States (US), affecting over seven million Americans and costing the nation approximately $34,000,000,000 per year [1]. Furthermore, as the prevalence of stroke is increasing as the US population continues to age at an unprecedented rate, the total expected burden of stroke in the US from 2005 to 2050 is currently set at more than 2.2 trillion dollars [2]. Thus, the development of effective strategies to deal with this upcoming burden of care must be considered a national priority. A major contributor to the lifetime burden of stroke is the commonality of serious long-term motor disabilities associated with stroke, resulting in decreased functional independence [1]. Stroke-related motor impairments are persistent, disabling, and difficult to treat [3]. Over 15 years of follow-up, roughly two-thirds of stroke survivors will consistently report significant disability that interferes with independent performance of activities of daily living [4]. In addition, this functional impairment is associated with depression, anxiety, and decreased quality of life in chronic stroke survivors [4]. Despite the enormous public health cost of stroke, there are few approaches that have succeeded in integrating affordable and accessible technologies into stroke recovery.
Post-stroke recovery is complex and may be mediated by numerous neurobiological factors such as size and location of the lesion, age, comorbid conditions, presence of cognitive deficits, overall symptom severity, active medications, and genetic components to name just a few [5] The results of multiple meta-analyses demonstrate that no single therapeutic approach has emerged as a reliably effective therapy for functional recovery in chronic stroke survivors [3, 6, 7]. In a well-powered retrospective analysis, Ward et al. showed impressive therapeutic outcomes in chronic stroke survivors who underwent intensive (90 hours over 9 weeks) rehabilitation that took advantage of advanced technologies such as neuromodulation and rehabilitation robotics [8••]. By contrast, in a large (n=770) stroke rehabilitation randomized controlled trial, Rodgers et al. showed no difference between conventional rehabilitation techniques compared with other forms of advanced upper limb rehabilitation [9]. However, despite the size of the population, the results of this study are difficult to interpret due to the wide range in participant time post-stroke (1–260 weeks), the relatively low protocol-allowed dosage of the advanced therapeutic interventions (2.25 hours/week over 12 weeks), and the use of a single robotic device that is not ideal for training upper limb functional movements. Historically, literature from animal models advocates for high repetitions of task-specific movements that also involve an active learning component to promote training with intention. High repetitions, alone, are not sufficient to drive plasticity in chronic stroke recovery [10]. In addition, the importance of an enriched environment in combination with task-specific training has been shown to be essential for optimizing recovery in animal models [11]. Unfortunately, human studies of improvement in chronic stroke survivors are less consistent: although there is moderate evidence that at least 20 hours of repetitive task training is important for improving arm function post-stroke [3], increased therapeutic intensity and therapy time does not always equate to a better outcome [7]. While the current consensus in the stroke rehabilitation community is primarily supportive of intensive doses of neurorehabilitation for stroke survivors, given the inconsistencies in the literature, it is often difficult to justify the logistical and economical challenges associated with the delivery of such high doses of rehabilitation. Thus, due to increasing economic pressures and inconclusive evidence for appropriate rehabilitation, stroke survivors will often experience a progressive retraction of services over time, resulting in decreased accessibility to rehabilitation services [12, 13].
Much of the hesitance that surrounds the delivery of high doses of neurorehabilitation is focused on the resource intensive nature of traditional neurorehabilitation. Conventional therapeutic approaches to outpatient stroke rehabilitation suffer from several logistical and ideological flaws. They often require regular clinical visits, which are expensive and often inconvenient. Alternatively, outpatient rehabilitation may involve self-directed home exercises, which are often monotonous and difficult to complete correctly without feedback. In both cases, an essential contributor in ensuring that rehabilitation exercises are completed with adequate intensity and regularity appears to be the enjoyment of the stroke survivor. There is evidence to suggest that encouraging exercises in a highly engaged state can significantly improve therapeutic outcome [14]. Furthermore, maintaining high levels of motivation in stroke survivors also appears to be an important feature of successful stroke rehabilitation intervention [15].
On paper, the gamification of neurorehabilitation has the potential to address many of the issues associated with the delivery of therapeutic exercises that are highly intense, engaging, and low-cost. Recent advances in motion capture technology and gaming development software have made such approaches highly feasible [16]. Despite this, gamified approaches to neurorehabilitation are still far from standard of care. Thus, the role of this review is to investigate the current state-of-the-science surrounding gamification of neurorehabilitation: both its efficacy and viability as a highly scalable, future-proof rehabilitation service deployment strategy that can meet the growing need for long-term neurorehabilitation.
The Spectrum of Gamified Rehabilitation
Central to the study of the gamification of neurorehabilitation is the question of efficacy. The first step for gamified therapy to be recognized as a viable therapeutic tool is that it must be shown to be at least as effective as conventional therapy. This is an emerging field, and thus many papers have been published recently that attempt to investigate the efficacy and non-inferiority of gamified neurorehabilitation [8••, 17••]. A systematic approach to interrogating databases such as PubMed, Cinahl, and Google Scholar reveals more than 15,000 articles published on the topic of gamified stroke rehabilitation with more than 50% of those articles published within the last 5 years. In addition to this recent growth, another evident trend in the literature is that the scope, definitions, and nomenclature surrounding the field of gamified therapy are rapidly broadening.
To that end, we will begin the narrative review with some considerations regarding the nomenclature and core concepts surrounding different gamified neurorehabilitation styles, technologies, and techniques. For instance, gaming consoles such as the Nintendo Wii and Microsoft Xbox Kinect are often classified as Virtual Reality. Although in the strictest sense, these technologies meet the technological definition of “virtual reality,” it is important that we acknowledge that the diversity of technology represented by this term has expanded to the point where specialized nomenclature is required. In fact, that these non-immersive gaming modalities are still referred to as VR by the neurorehabilitation community highlights the need for advancements in the field. By collapsing videogame consoles and immersive neurorehabilitation systems into the same “VR” category, systematic reviews and meta-analyses may draw inaccurate conclusions about the relative efficacies of different approaches to gamified neurorehabilitation. This nomenclature exists, however, does not fully capture the finite differences within these categories. Expanding upon the existing nomenclature, a distinction can be made between exergaming and serious games. Exergaming has been defined across the literature as games in which an individual interacts with a gaming scenario through movement [18, 19]. Exergames are commercially available gaming systems that can be adopted for rehabilitation or for purely recreational use. On the contrary, serious games are systems designed specifically for rehabilitation purposes [20]. Similarly, when considering robotic neurorehabilitation, one must be aware that much of the existing literature fails to differentiate between robotic intervention with and without gamified elements. This leaves us unable to determine the role that gamification plays in driving functional improvements compared with benefits derived from active-assisted motions controlled or enabled by the robotic device. Finally, understanding the distinction between the concepts of synchronous and asynchronous telerehabilitation is an important concept to explore. Synchronous telerehabilitation encompasses evaluation and treatment performed remotely by a clinician and often utilizes gamified rehabilitation platforms as a medium for intervention. By contrast, in asynchronous telerehabilitation, patients are assigned a set of exercises by the treating clinician to be performed in an unsupervised environment. With the advancement of telerehabilitation technology, asynchronous sessions can be logged, and can provide the patient with real-time feedback about task performance. However, optimal ratios of synchronous to asynchronous telerehabilitation have not been adequately explored and could have great implications for value-based care. Furthermore, a lack of established parameters for asynchronous and synchronous care fails to identify the threshold of skilled therapy intervention to impart a meaningful clinical and functional difference. These nomenclature considerations are important factors in establishing a comprehensive understanding of the literature.
For the purposes of this review, we have identified three categories of gamified neurorehabilitation: (1) Non-Robotic Gamified Neurorehabilitation (NRGN), where actions in the game are controlled by consumer electronic devices; (2) Robot-enabled Gamified Neurorehabilitation (RGN) where actions in the video games are controlled by limb movements that are enabled or resisted by a robotic device; and (3) Immersive Virtual Reality modality (IVR), where the user experiences a fully artificial environment through virtual reality headsets. Although we acknowledge that mixed reality and augmented reality approaches to gamified neurorehabilitation are exciting emerging approaches, due to lack of literature, these technologies will not be discussed in this review (Table 1).
Table 1.
Different styles of gamified neurorehabilitation and their levels of evidence
| Gamification medium | Working definition | Strengths | Weaknesses | Examples | Level of evidence |
|---|---|---|---|---|---|
| Non-Robotic Gamified Neurorehabilitation (NRGN) | Gamified neurorehabilitation systems where actions in the game are controlled by consumer electronic devices (i.e Microsoft Kinect, Nintendo Wii, Logitech Adaptive Controller) |
- Lower cost - High potential for scalability - Can be combined with aerobic training - Uncomplicated technology setup |
- Often not appropriate for stroke survivors with severe impairment - Insufficient guidance for/measurement of limb positioning |
“Serious game” MindMotion Go (Mindmaze, Switzerland) “Exergame”: Wii Fit (Nintendo, USA) |
1A |
| Robot-Enabled Gamified Neurorehabilitation (RGN) | Traditional video games where actions in the game are controlled by limb movements that are enabled or resisted by a robotic device |
- Usable by stroke survivors at most functional ability levels and ROM - Enables high numbers of movement repetitions in highly impaired patients |
- Expensive technology - Large physical footprint; not accessible for many homes - Regulatory issues prevent home use in many cases |
Robot assists with movement: InMotion (Motion Technologie, USA) Robot provides gravity support only: Armeo Spring, (Hocoma, Switzerland) |
2A* |
| Immersive Virtual Reality Neurorehabilitation (IVR) | Gaming environment where the user experiences a fully artificial environment through virtual reality headsets | -Immersive environments permit action observation activities in patients with complete paralysis |
-Can cause dizziness and motion sickness - Often requires a large amount of dedicated space and close activity supervision |
Serious games: Virtual Reality Rehabilitation Game (Tsatsis et al., 2017) Exergames: Oculus Quest (Facebook Technologies, USA) |
2A |
*Although robotic neurorehabilitation for stroke can be considered to have 1A evidence, fewer studies have investigated the specific role of gamification vs. robot-enabled rehabilitation without gamification to understand their relative contributions to functional improvements
Non-Robotic Gamified Neurorehabilitation (NRGN)
Non-Robotic Gamified Neurorehabilitation (NRGN) platforms encompass a wide variety of gamified neurorehabilitation technologies. NRGN systems may be specifically designed for rehabilitation purposes with targeted therapeutic interventions implemented in a gamified medium (Mind Motion Go, Mind Maze). In other cases, commercially available gaming systems may be adopted within the scope of rehabilitation interventions (Nintendo Wii, Sony PlayStation). In both cases, NRGN platforms utilize a variety of technologies to allow end users to interact with the interventions including motion capture, controller-based accelerometers and gyroscopes, and force plates. While these differences are noted in the clinical space, the literature often fails to discreetly characterize and differentiate between the two. For the purposes of this narrative, we will consider NRGN as the above detailed categories.
In a recent Cochrane review by Laver et al., 42% of RCTS included NRGN exergaming platforms, including Nintendo Wii [n=15], GestureTek IREX [n=8], Microsoft Kinect [n=4], and Playstation EyeToy [n=1] [21]. Despite the reportedly high number of NRGN platforms identified, the review failed to differentiate between purely NRGN systems and IVR. Both modalities were included together with no subgroup analysis completed to compare forms of therapy. This consistent misnomer in the literature fails to clearly distinguish the benefits of NRGN from other gamified mediums including VR.
Overall, NRGN was shown to be safe and effective in improving arm motor function and activities of daily living after stroke with clinically significant effects and reasonable effect sizes [21]. The use of Sony Playstation EyeToy2 and Nintendo Wii has been widely investigated for post-stroke rehabilitation [22–27] and both present low-cost and accessible options with promising results for stroke recovery. Playstation 2-based therapy is shown to be feasible and enjoyable [22, 27], leading to clinically significant improvements in motor function post-stroke [23, 28]. Reported shortcomings described include physical limitations experienced by those with moderate to severe motor impairments that may make interaction with NRGN platforms more difficult. This increased task difficulty may be frustrating for some end users. As such, it has been suggested that NRGN may be more appropriate for those with higher levels of mobility [22]. Furthermore, platform reported performance metrics may not be sufficiently detailed to inform progress and provide adequate feedback to patients and therapists alike. Nintendo Wii-based therapy (using the Wii-Fit and the Wii-Balance board) is also shown to be feasible and effective for post-stroke rehabilitation even in individuals of older age and with limited function [24, 25, 29, 30]. It has been associated with significant improvements in arm motor function [24, 25, 29] and higher levels of patient engagement and compliance when compared to usual care [25]. Wii-based targeted upper limb rehabilitation has potential to improve cardiovascular fitness [26], mobility, as well as dynamic and static balance, which has important clinical relevance for reducing fall-risk post-stroke. Using surface electromyography (EMG), Trihn et al. also showed physiological evidence for facilitated muscle activation and lower-limb improvements even after an UE-targeted problem, highlighting the importance of standing during stroke therapy [26].
Similar to Wii and Playstation, the Nintendo Xbox Kinect also has positive evidence in improving motor function and performance in activities of daily living, but showed no evidence of improving motor strength [31]. Multiple other gaming setups have been tested, and despite depicting varied sample sizes, most show promising results: Donoso-Brown tested a EMG-controlled video game setup and found that it may facilitate muscle activation; Carabeo et al. tested an Android-based tablet game (FINDEX) and found improvements in hand dexterity [32], Kim et al. investigated the a portable augmented reality gaming system (IREX) and found that patients in the gaming group had significant improvements in static and dynamic balance and increased ambulation function in comparison with the conventional physical therapy group [29].
Novel approaches to NRGN continue to emerge in the neurorehabilitation space. A recent study by Krakauer et al. investigated a novel NRGN approach, neuroanimation therapy (NAT) [33]. In this multi-center, single-blinded parallel randomized controlled trial, Krakauer et al. compared high-intensity upper limb rehabilitation using NAT, traditional occupation therapy, and historical controls in individuals with subacute stroke. They found that high-intensity upper limb rehabilitation emphasizing movement quality was superior to gains demonstrated by those receiving standard physical therapy in the historical control group.
NRGN is motivating gamification and may be more engaging than standard therapy exercises [25, 32, 34]. Large RCTs show high adherence rates [17••, 22, 35] to NRGN interventions when compared to standard of care. In addition to improved motor function, gamified rehabilitation strategies result in optimal levels of compliance [36], usability [16], and enjoyment [16, 37], directly influencing rehabilitation outcomes [37]. NRGN platforms present a feasible option to harness the multitude of facets needed to drive optimized motor performance in a salient, enjoyable medium. Despite this globally positive evidence, a lack of differentiating between exergames and serious games in the NRGN space presents a limitation in interpretation. Because of this, superiority of one intervention vs. another cannot be clearly discerned. More attention and investigation of the distinct difference in exergame NRGN and serious game NRGN is warranted.
The ultimate goal should be to narrow the gap between exergames and serious games, to ensure that gamified rehabilitation is, at a minimum, as enjoyable as playing mainstream video games. By adopting concepts of universal design, technologies such as the QuadStick controller (QuadStick, USA), the Logitech Adaptive Gaming Kit (Logitech, USA), and the XBox Microsoft, USA) are making this possible [38].
Immersive Virtual Reality (IVR)
IVR deploys the use of headsets that display highly realistic multisensory simulated environments, allowing patients to interact with the virtual environments. When combined with treadmill or hand-held controllers, it encourages life-like movements, maximizing task-oriented intense exercise repetitions [39, 40]. Moreover, IVR may involve visual, audio, and vibrotactile cueing and real-time feedback, which can facilitate cortical reorganization and promote body control entrainment and proprioception [40, 41].
One of the biggest challenges related to evaluating the quality of evidence for IVR stroke rehabilitation therapies stems from the fact that most systematic reviews and meta-analyses include both immersive and non-immersive VR modalities, making it difficult to accurately determine the true efficacy or effectiveness of IVR [21, 42]. However, IVR systems specifically built for rehabilitation are likely more effective for upper limb recovery than commercially available IVR systems because they incorporate key principles of neurorehabilitation underlying recovery and brain repair: task-specific practice, multisensory feedback, increasing difficulty, variable practice, and mechanisms to promote the use of the paretic limb [43]. Additionally, IVR may be implemented as a medium of mental practice and action observation in upper extremity recovery of stroke. While not a game, per se, the evidence supporting the use of mental practice in post-stroke recovery can be facilitated more readily through the use of IVR [44, 45].
The only meta-analysis to specifically evaluate IVR therapies for stroke rehabilitation found that patients who received IVR rehabilitation showed a trend toward significantly greater improvements compared to those receiving traditional or conventional therapies [46•], showing benefits in treating both lower and upper extremity impairments. However, studies had limited statistical power (10 participants per experimental group on average) and limited design (no long-term follow-up), showing weak to moderate quality of evidence. In addition, studies included in the meta-analysis exclusively targeted chronic stroke, limiting generalizability of the findings [46•].
With only one meta-analysis available on this topic, it is still unclear whether IVR rehabilitation is more beneficial to stroke-related upper or lower extremity impairments. Individuals receiving IVR therapy showed statistically and clinically significant improvements in mobility (measured by the TUG test) when compared with the control group [40, 46•]. Moreover, IVR paired with treadmill training resulted in positive effects on gait and balance (measured by the 6MWT, 10MWT and TUG), and was shown to be superior to standard treadmill training in regards to improvements in medial-lateral dynamic balance control and sit-to-stand transfers [47]. Evidence supporting the efficacy of IVR rehabilitation for the upper extremity is more limited [48, 49]. Although some positive results were described [48], other studies also failed to show significant improvements in upper extremity function or impairment in response to IVR rehabilitation [48]. When considering the efficacy of IVR rehabilitation, realism of the virtual environments and immersion of each user can have significant effects on the overall efficacy of the therapy [46•, 50]. Further research is required to both create truly immersive IVR rehabilitation environments that simulate real-world task performance, and better understand how these features modulate the therapeutic effect of IVR rehabilitation.
Robot-Enabled Gamified Neurorehabilitation (RGN)
Robot-mediated rehabilitation for stroke is supported by a substantial body of literature and is shown to enable high-intensity, high-repetition, adaptive, and quantifiable motor training. Of the available robotic technologies, most come standard with gamification. Despite this, however, the literature fails to control for the significant differences in gamification mediums within robotic-enabled gamified neurorehabilitation. The extent of gamification is highly variable ranging from simplistic interactive therapeutic games (i.e., Fig. 1) to more engaging exergames (i.e., Fig. 2). Few studies have investigated the efficacy of robotic rehabilitation and gamification compared with robotic rehabilitation without gamification.
Fig. 1.
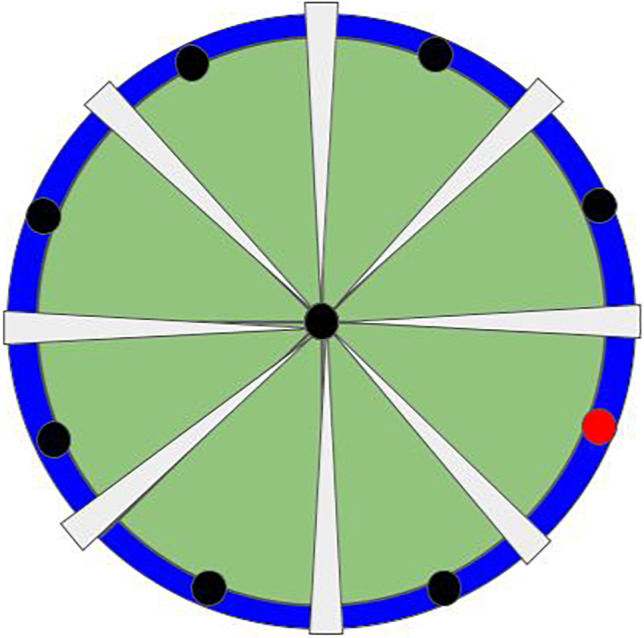
Rendering of a simple interactive game commonly integrated in robotic therapies. The goal of this game would be to hit the target (red dot) by using robot-enabled upper extremity motor control
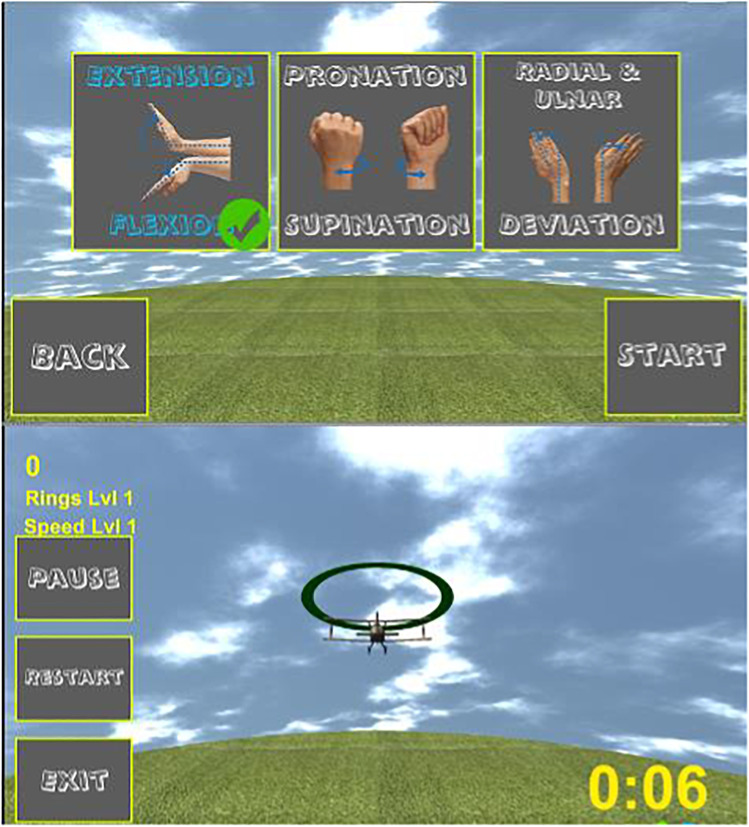
Fig. 2.
Example of an exergame, where wrist movements (flexion/extension, pronation/supination, radial/ulnar deviation) control an airplane’s speed and direction, moving it toward a specific target
Multiple reviews and meta-analyses have analyzed the outcomes of upper extremity robotic rehabilitation and indicated substantial improvements in arm function (as measured by the Fugl-Meyer assessment) across acute, subacute, and chronic stroke populations, with especially clinical meaningful effects in chronic stroke survivors [51–53]. Bertani et al. analyzed 14 randomized trials, systematic reviews, and meta-analysis and concluded that robotic therapy after stroke involving intensive, repetitive, and task-oriented exercises may drive positive reorganization in the motor cortex and lead to improved outcomes [51]. The most recent Cochrane review found that robot-assisted rehabilitation had high-quality evidence for improving ADL and arm muscle strength [53]. Similar to other gamified rehabilitation systems, heterogeneity across study populations and designs limits the clinical interpretation of these positive findings and despite overall positive outcomes, superiority against standard care is still controversial [9, 51]. Of note, RATULS, the largest randomized controlled trial published to date, involved 770 stroke survivors and found that improvements in upper limb impairment after robot-assisted training using the MIT-Manus did not translate into clinical function and improvements in ADL, showing no superiority against a dose-matched usual care. Importantly, the trial highlighted that neither robot-assisted training nor usual care was cost-effective and concluded that robot-assisted therapy might benefit from combined approaches with functionally orientated therapies [9].
Robotics for lower extremity rehabilitation, particularly gait training, has been well reported in the literature [53–55]. Despite this level of evidence for robotic gait training in stroke, there exists a significant limitation in generalization and clinical application. The literature fails to distinguish between gamified robotic gait training (i.e., Lokomat, Hocoma) and non-gamified robotic gait training (i.e., EKSO NR Exoskeleton, EKSO Bionics, ReWalk Exoskeleton, ReWalk Robotics). This lack of distinction limits the body of evidence and clinical application of such findings as the literature demonstrates the neuroplastic benefits of gamified interventions [42].
Behavioral and Neurophysiological Effects of Gamified Therapy
Playing video games is shown to have positive meaningful behavioral effects across cognitive, motor, and affective domains [42]. Although neurophysiological processes induced by playing video games are not fully established, they are likely related to alterations of neural processing in the ventral striatum (VS), the area involved with dopaminergic pathways and associated with reward processing and motivation [56]. PET studies showed that playing video games led to a substantial increase in endogenous striatal dopamine release, comparable to amphetamine and methamphetamine injections [57], and other neuroimaging studies support that idea by showing that video game playing is associated with structural alterations in the striatal reward system [58].
Improved attention, visual-motor, and cognitive-motor performance also have been described, with potentially durable improvements that can transfer beyond gaming environments into daily living [42]. Overall gaming literature reports on better integration between attentional and sensorimotor areas and improvements in peripheral visual attention. Volumetric increases in hippocampus and temporal lobe suggest improvements in visuospatial and navigation skills [59] and changes in prefrontal areas are linked to improvements in cognitive control and working memory. Lastly, gamified therapies can lead to increased cortical recruitment to optimize neuroplasticity, especially when combined with aerobic exercise and motor skill practice [60].
It stands to reason that all of the aforementioned effects of gamification have the potential to enhance the efficacy of neurorehabilitation if they can be successfully integrated into existing stroke rehabilitation paradigms. However, in the domain of rehabilitation, serious games, and exergames, the term “gamification” is often used broadly. Many rehabilitation programs claim to be “gamified,” when they are simply providing a user interface that provides patients with interactive and continuous feedback about task performance parameters. Such simple attempts at gamification are much less likely to produce the neurobiological responses to gamification that have been discussed, which may not produce the desired outcome of gamification as has been observed in other fields [61–63] Thus, as studies begin to explore the utility and efficacy of gamification in stroke rehabilitation, it is crucial that patient feedback related to engagement is also included as a variable, since it has been shown to significantly modulate therapeutic outcome [16, 17••]. Literature evaluating the relationship between quality of gamification and its subsequent effect on the efficacy of gamified stroke rehabilitation is notably lacking and more research in this domain is required.
Overall Conclusions for Gamified Therapy Efficacy
Overall, literature on gamified therapies has shown exponential growth in the last few years. Despite limited sample sizes and limited data to enable comparisons across different gaming modalities and stroke populations, the available literature favors gamified rehabilitation when compared to conventional rehabilitation [42, 64, 65]. Gamified therapies can promote major components of a successful rehabilitation program: high intensity of task-oriented and purposeful movements [22], optimal levels of enjoyment [16, 66], engagement [41, 67, 68], and motivation [68, 69]. Importantly, gamified therapy is shown to be effective and able to produce positive and long-lasting effects on motor and functional outcomes even at chronic phases post-stroke (>6 months), when typically spontaneous motor recovery tends to slow down and motor gains are more difficult to achieve [42, 51, 64]. Qualitative findings also show that gamified home-based therapy increases participation and independence in daily activities, alleviates social isolation, and gives patients a sense of ownership and control over the rehabilitation process [68].
Adherence, Motivation, and Potential to Increase Therapy Hours Through Asynchronous Therapy
High-intensity therapy is needed to induce neuroplastic changes and harness motor recovery after stroke [70] but traditional rehabilitation approaches may not produce adequate doses of task-specific practice [71]. Lack of motivation and adherence to therapy are major barriers to optimal rehabilitation after stroke [72], as it can significantly hinder therapy intensity and negatively impact the number of repetitions. Playing games enables repetitive exercises to be more engaging and motivating [68] and therefore patients are more likely to achieve sufficient rehabilitation dosage [69]. Lohse and colleagues [72] proposed that integrating games into rehabilitation therapies can increase time spent in therapy by increasing the likelihood of (1) starting a therapy session (engagement), (2) staying in therapy (increased engagement and delayed disengagement), and (3) coming back for another session (reengagement). With that in mind, optimizing gaming setups to be engaging and motivating can play a direct influence on gamified rehabilitation outcomes.
Patient’s adherence to gamified interventions depends on multiple personal factors including intrinsic motivation, technology literacy, and underlying mental or cognitive impairments. In addition, different gaming systems will lead to different levels of engagement and enjoyment, as not all games are made the same [67]. Current evidence indicates that gaming features such as fantasy (versus realistic or mixed) scenarios and team (versus individual) activities are correlated to larger effect sizes [65] and that playing competitive exercises, especially against non-impaired adversaries, may lead to higher enjoyment and motivation [73]. Janssen et al. determined that a context- and interest-related game results in increased engagement and motivation, which can be utilized to activate reward systems in the brain, encouraging more therapy. More work needs to be done to determine optimal gaming settings.
Cases Against Integration of Gamified Therapy into the HEP
Key Barriers to Adoption in Current-Day Clinics
The long-standing time gap between innovation and adoption, often referred to as “the Valley of Death,” has proven to be a significant barrier to the acceptance and application of novel treatment approaches across the healthcare ecosystem. While advances continue to be made across the biomedical research space, the time of technology transfer from bench to bedside is an estimated 17 years [74]. This significant lag in time to adoption has resulted in inferior intervention strategies that fail to capture the necessary intensity or repetition required to support fundamental principles of motor learning, neuroplasticity, and ultimately, motor recovery.
Despite a growing body of evidence to support the utilization of gamified rehabilitation technology in the care of patients following stroke, translation into standard of care physical therapy practice and home-based telerehabilitation programs remains limited. A number of barriers have been identified in adoption of gamified telerehabilitation in clinical/institutional and patient facing domains of clinical practice. The acceptance rate of novel technology by physical therapists and rehabilitation professionals has been stunted by a lack of technological competence and knowledge base among clinicians. Lack of technological fluency results in lower rate of adoption of telerehabilitation strategies and a greater degree of resistance to deviate from previously established in-clinic practice patterns [75]. Likewise, patient buy-in of both physical space and technology competence has further limited more widespread adoption despite supporting evidence. Without the guidance of a physical therapist either on site or virtually, patient compliance is at risk. These barriers must be accounted for in order to successfully implement a gamified telerehabilitation program.
Self-efficacy and technology competence are two major barriers to the clinician specific adoption of gamified rehabilitation and virtual reality rehabilitation technology in clinical practice [76•]. In a study by Levac et al., a clinician-reported survey assessing the barriers to adoption of virtual reality-based gamified rehabilitation in practice identified ease of use, lack of knowledge regarding virtual reality technology, and need for clinical support for successful adoption as primary personal factors limiting adoption of technology in practice [77]. This lack of knowledge may be due to lack of experience, or lack of access to continuing professional development with a focus in technology and clinical applications. The degree of technological competence of physical therapists in the neurorehabilitation space has become a primary target of knowledge translation (KT) task forces both at the institutional and personal levels [76•, 77]. The goal of such knowledge translation task forces should be to identify gaps in clinic-based utilization of technology to optimize adoption of technology in the home, telerehabilitation space [76•, 77]. Additional barriers faced by clinicians in adopting gamified telerehabilitation practice may be in preconceived attitudes and culture toward technological adoption [76•]. Optimal integration and adoption of gamified rehabilitation both in clinic and in the telerehabilitation space are largely influenced by institutional and cultural support of integration within the clinical setting. Clinical settings that provide clinicians access to gamified rehabilitation platforms with the appropriate education for use likely see an increase in utilization in patient care.
Cost has further been identified as a factor influencing the adoption of technology in clinician practice [42, 76•]. The initial upfront cost of gamified rehabilitation technology can be a significant expense for clinics. Combined with a lack of clinician driven adoption as discussed previously, organizations may be less incentivized to invest in technology as return on investment has been identified as cause for concern. Until recently, no current procedural terminology (CPT) codes existed to support the cost of home-based rehabilitation technology. In the 2022 Physician Fee Schedule, the Centers for Medicare and Medicaid (CMS) have proposed novel category III remote therapeutic monitoring (RTM) codes to support reimbursement for the setup, education, and asynchronous use of rehabilitation technology in the home. The advent of RTM CPT codes presents a needed solution to address cost disparities often identified as barrier to clinical adoption of advanced neurorehabilitation technologies.
Patient use of technology and the barriers of asynchronous technology may further limit adoption of gamified rehabilitation. In a systematic review by Chen et al., physical space and technical proficiency were identified as two primary limitations for patient compliance and adoption of in-home technology for rehabilitation [68]. Physical barriers included gamified rehabilitation devices that took up large amounts of space and those that posed as physical barriers within the home [68]. Technical proficiency of the patient and/or caregiver must be accounted for when designing and implementing a gamified telerehabilitation program. For many patients interacting with technology from device setup to utilization [68]. Devices that require specific sequencing for setup, turning on/off, and charging batteries may pose a challenge for patients who are unfamiliar with technology systems. Patient barriers further identified include limitations of not having a “live” therapist treating [68]. In a study by Hung et al., patients and clinicians were surveyed regarding opinions on rehabilitation [78]. When asked about the limitations of home-based rehabilitation versus rehabilitation provided in clinic, patients identified “No therapists’ instructions,” “Lack of facility modalities,” “Tend to slack off,” and “No corrections on posture” as the primary drawbacks of a telerehabilitation program when compared to face-to-face rehabilitation following stroke [78]. The limitations of asynchronous participation in gamified therapy are further underscored by findings of Cramer et al. in which the clinical decision making of a skilled therapist was highlighted to optimize adoption of gamified technology [17••]. Identification of these barriers emphasizes the necessity for gamified rehabilitation to be patient centered and adopted into clinical practice rather than arbitrarily given to patients without guidance.
In light of pre-existing barriers to adoption identified above, the COVID-19 pandemic presented an opportunity for the rapid adoption and implementation of technology-enabled rehabilitation and telemedicine solutions across the continuum of care. Uptake of technology-based care and delivery substantially increased from the onset of the COVID-19 pandemic through present time [79]. This acceleration in clinical utility of technology-based solutions far surpassed the aforementioned 17-year time gap of standard practice. As such, an opportunity presents to further defy barriers of adoption to accelerate clinician and patient acceptance of advanced technology solutions for optimized care.
How Do Gamification and Conventional PT Co-exist
As the role and practice patterns of physical therapists in neurorehabilitation and the healthcare space have continued to develop over time, cultural shifts and education are paramount to ensure that gamified rehabilitation and conventional physical therapy paradigms co-exist symbiotically. The advancement of biotechnology as it pertains to gamified rehabilitation is dependent on the physical therapy profession’s ability to work alongside scientists, engineers, and researchers to develop and support new rehabilitation technologies from design to implementation [80]. The American Physical Therapy Association (APTA)’s Physical Therapy and Society Summit (PASS) Meeting in 2006 clearly outlined the profession’s commitment to the adoption and integration of novel technology into clinical practice [81]. The PASS Steering Committee emphasized the collaborative, interdisciplinary role physical therapists across the continuum of practice play in technology and gamification. The Steering Committee emphasized the concept that therapists should not merely “use” technology but rather serve to collaborate to become leaders in the development, testing, and implementation [81]. This allows therapists to utilize their skillset, clinical experience, and expertise in movement science and rehabilitation to identify barriers to practice and facilitate technology and gamification as adjuncts to existing therapeutic and motor control paradigms implemented in conventional practice. As highlighted in the APTA’s Vision Statement, innovation across practice settings, paradigms, education, and research is of central importance for the advancement of the field [5].
In the clinical space, acceptance and adoption of technology will allow for advancement of both gamified treatment modality development and clinical practice in its entirety. Technology and gamified rehabilitation does not aim to replace clinical decision making, clinical practice, and skillset of a physical therapist. Gamified rehabilitation and rehabilitation technology serve as augmentative tools and platforms that augment clinical practice, allowing for optimized interventions. The successful coexistence of gamified rehabilitation and technology in physical therapy practice is dependent on the clinician’s ability to view technology as a compliment to clinical practice as opposed to a replacement.
Conclusion
The body of literature surrounding gamified rehabilitation therapy is rapidly expanding and there is strong evidence suggesting efficacy. However, in spite of the continued clinical and research development, there remain challenges to adoption and widespread implementation. As research into gamification becomes more sophisticated, further research is required to evaluate the specific neurobiological underpinnings of gamified environments that are being used in neurorehabilitation. In addition, in the field of rehabilitation robotics, no studies have attempted to control for the effects of gamification paired with robotics compared to robotics alone. A more detailed understanding of these issues would likely result in more efficacious gamified neurorehabilitation environments and a stronger argument for mainstream adoption.
Availability of Data and Material
Not applicable.
Code Availability
Not applicable.
Declarations
Human and Animal Rights
This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of Interest
The authors declare no competing interests.
Footnotes
This article is part of the Topical Collection on Neurorehabilitation and Recovery
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. Heart Disease and Stroke Statistics—2017 update: a report from the American Heart Association. Circulation [Internet]. 2017 [cited 2020 Aug 11];135. Available from: https://www.ahajournals.org/doi/10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed]
- 2.Langhorne P, Sandercock P, Prasad K. Evidence-based practice for stroke. Lancet Neurol. 2009;8:308–309. doi: 10.1016/S1474-4422(09)70060-2. [DOI] [PubMed] [Google Scholar]
- 3.Pollock A, Baer G, Campbell P, Choo PL, Forster A, Morris J, et al. Physical rehabilitation approaches for the recovery of function and mobility following stroke. Cochrane Stroke Group, editor. Cochrane Database Syst Rev [Internet]. 2014 [cited 2020 Aug 11]; Available from: http://doi.wiley.com/10.1002/14651858.CD001920.pub3. [DOI] [PMC free article] [PubMed]
- 4.Crichton SL, Bray BD, McKevitt C, Rudd AG, Wolfe CDA. Patient outcomes up to 15 years after stroke: survival, disability, quality of life, cognition and mental health. J Neurol Neurosurg Psychiatry. 2016;87:1091–1098. doi: 10.1136/jnnp-2016-313361. [DOI] [PubMed] [Google Scholar]
- 5.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, et al. Heart disease and stroke statistics—2008 update. Circulation. 2008;117:e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 6.Winstein CJ, Stein J, Arena R, Bates B, Cherney LR, Cramer SC, et al. Guidelines for adult stroke rehabilitation and recovery: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke [Internet]. 2016 [cited 2020 May 26];47. Available from: https://www.ahajournals.org/doi/10.1161/STR.0000000000000098. [DOI] [PubMed]
- 7.Dobkin BH. Spinal and supraspinal plasticity after incomplete spinal cord injury: correlations between functional magnetic resonance imaging and engaged locomotor networks. Prog Brain Res [Internet]. Elsevier; 2000 [cited 2019 Jun 19]. p. 99–111. Available from: http://www.sciencedirect.com/science/article/pii/S0079612300280102. [DOI] [PubMed]
- 8.Ward NS, Brander F, Kelly K. Intensive upper limb neurorehabilitation in chronic stroke: outcomes from the Queen Square programme. J Neurol Neurosurg Psychiatry. 2019;90:498–506. doi: 10.1136/jnnp-2018-319954. [DOI] [PubMed] [Google Scholar]
- 9.Rodgers H, Bosomworth H, Krebs HI, van Wijck F, Howel D, Wilson N, et al. Robot assisted training for the upper limb after stroke (RATULS): a multicentre randomised controlled trial. Lancet. 2019;394:51–62. doi: 10.1016/S0140-6736(19)31055-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Plautz EJ, Milliken GW, Nudo RJ. Effects of Repetitive Motor Training on Movement Representations in Adult Squirrel Monkeys: Role of Use versus Learning. Neurobiol Learn Mem. 2000;74:27–55. doi: 10.1006/nlme.1999.3934. [DOI] [PubMed] [Google Scholar]
- 11.Biernaskie J, Corbett D. Enriched rehabilitative training promotes improved forelimb motor function and enhanced dendritic growth after focal ischemic injury. J Neurosci. 2001;21:5272–5280. doi: 10.1523/JNEUROSCI.21-14-05272.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rajsic S, Gothe H, Borba HH, Sroczynski G, Vujicic J, Toell T, et al. Economic burden of stroke: a systematic review on post-stroke care. Eur J Health Econ. 2019;20:107–134. doi: 10.1007/s10198-018-0984-0. [DOI] [PubMed] [Google Scholar]
- 13.Medford-Davis LN, Fonarow GC, Bhatt DL, Xu H, Smith EE, Suter R, et al. Impact of insurance status on outcomes and use of rehabilitation services in acute ischemic stroke: findings from get with the guidelines-stroke. J Am Heart Assoc. 5:e004282. [DOI] [PMC free article] [PubMed]
- 14.Kleim JA, Jones TA. Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage. J Speech Lang Hear Res [Internet]. 2008 [cited 2021 May 28];51. [DOI] [PubMed]
- 15.Oyake K, Suzuki M, Otaka Y, Momose K, Tanaka S. Motivational strategies for stroke rehabilitation: a Delphi study. Arch Phys Med Rehabil. 2020;101:1929–1936. doi: 10.1016/j.apmr.2020.06.007. [DOI] [PubMed] [Google Scholar]
- 16.Putrino D, Zanders H, Hamilton T, Rykman A, Lee P, Edwards DJ. Patient Engagement Is Related to Impairment Reduction During Digital Game-Based Therapy in Stroke. Games Health J. 2017;6:295–302. doi: 10.1089/g4h.2016.0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.•• Cramer SC, Dodakian L, Le V, See J, Augsburger R, McKenzie A, et al. Efficacy of home-based telerehabilitation vs in-clinic therapy for adults after stroke: a randomized clinical trial. JAMA Neurol. 2019. Randomized blinded noninferiority clinical trial showed that telerehabilitation showed comparable efficacy to usual care for improving motor status following stroke. [DOI] [PMC free article] [PubMed]
- 18.Henrique PPB, Colussi EL, Marchi ACBD. Effects of Exergame on Patients’ Balance and Upper Limb Motor Function after Stroke: A Randomized Controlled Trial. J Stroke Cerebrovasc Dis. 2019;28:2351–2357. doi: 10.1016/j.jstrokecerebrovasdis.2019.05.031. [DOI] [PubMed] [Google Scholar]
- 19.Mat Rosly M, Mat Rosly H, Davis OAMGM, Husain R, Hasnan N. Exergaming for individuals with neurological disability: a systematic review. Disabil Rehabil. 2017;39:727–735. doi: 10.3109/09638288.2016.1161086. [DOI] [PubMed] [Google Scholar]
- 20.Sardi L, Idri A, Fernández-Alemán JL. A systematic review of gamification in e-Health. J Biomed Inform. 2017;71:31–48. doi: 10.1016/j.jbi.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 21.Laver KE, Lange B, George S, Deutsch JE, Saposnik G, Crotty M. Virtual reality for stroke rehabilitation. Cochrane Stroke Group, editor. Cochrane Database Syst Rev [Internet]. 2017 [cited 2020 Aug 18]; Available from: http://doi.wiley.com/10.1002/14651858.CD008349.pub4. [DOI] [PMC free article] [PubMed]
- 22.Rand D, Givon N, Weingarden H, Nota A, Zeilig G. Eliciting upper extremity purposeful movements using video games: a comparison with traditional therapy for stroke rehabilitation. Neurorehabil Neural Repair. 2014;28:733–739. doi: 10.1177/1545968314521008. [DOI] [PubMed] [Google Scholar]
- 23.Yavuzer G, Senel A, Atay MB, Stam HJ. “‘Playstation eyetoy games’” improve upper extremity-related motor functioning in subacute stroke: a randomized controlled clinical trial. Eur J Phys Rehabil Med. 2008;44:237–244. [PubMed] [Google Scholar]
- 24.Saposnik G, Teasell R, Mamdani M, Hall J, McIlroy W, Cheung D, et al. Effectiveness of virtual reality using wii gaming technology in stroke rehabilitation: a pilot randomized clinical trial and proof of principle. Stroke. 2010;41:1477–1484. doi: 10.1161/STROKEAHA.110.584979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McNulty PA, Thompson-Butel AG, Faux SG, Lin G, Katrak PH, Harris LR, et al. The efficacy of Wii-based Movement Therapy for upper limb rehabilitation in the chronic poststroke period: a randomized controlled trial. Int J Stroke Off J Int Stroke Soc. 2015;10:1253–1260. doi: 10.1111/ijs.12594. [DOI] [PubMed] [Google Scholar]
- 26.Trinh T, Scheuer SE, Thompson-Butel AG, Shiner CT, McNulty PA. Cardiovascular fitness is improved post-stroke with upper-limb Wii-based Movement Therapy but not dose-matched constraint therapy. Top Stroke Rehabil. 2016;23:208–216. doi: 10.1080/10749357.2016.1138672. [DOI] [PubMed] [Google Scholar]
- 27.Neil A, Ens S, Pelletier R, Jarus T, Rand D. Sony PlayStation EyeToy elicits higher levels of movement than the Nintendo Wii: implications for stroke rehabilitation. Eur J Phys Rehabil Med. 2013;49:13–21. [PubMed] [Google Scholar]
- 28.Flynn S, Palma P, Bender A. Feasibility of using the Sony PlayStation 2 gaming platform for an individual poststroke: a case report. J Neurol Phys Ther JNPT. 2007;31:180–189. doi: 10.1097/NPT.0b013e31815d00d5. [DOI] [PubMed] [Google Scholar]
- 29.Kim EK, Kang JH, Park JS, Jung BH. Clinical feasibility of interactive commercial nintendo gaming for chronic stroke rehabilitation. J Phys Ther Sci. 2012;24:901–903. doi: 10.1589/jpts.24.901. [DOI] [Google Scholar]
- 30.Golla A, Müller T, Wohlfarth K, Jahn P, Mattukat K, Mau W. Home-based balance training using Wii FitTM: a pilot randomised controlled trial with mobile older stroke survivors. Pilot Feasibil Stud. 2018;4:143. doi: 10.1186/s40814-018-0334-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee HS, Park YJ, Park SW. The effects of virtual reality training on function in chronic stroke patients: a systematic review and meta-analysis. Biomed Res Int. 2019;2019:1–12. doi: 10.1155/2019/7595639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carabeo CGG, Dalida CMM, Padilla EMZ, Rodrigo MMT. Stroke patient rehabilitation: a pilot study of an android-based game. Simul Gaming. 2014;45:151–166. doi: 10.1177/1046878114531102. [DOI] [Google Scholar]
- 33.Krakauer JW, Kitago T, Goldsmith J, et al. Comparing a novel neuroanimation experience to conventional therapy for high-dose intensive upper-limb training in subacute stroke: the SMARTS2 randomized trial. Neurorehabil Neural Repair. 2021;35(5):393–405. doi: 10.1177/15459683211000730. [DOI] [PubMed] [Google Scholar]
- 34.Webster D, Celik O. Systematic review of Kinect applications in elderly care and stroke rehabilitation. J NeuroEngineering Rehabil. 2014;11:108. doi: 10.1186/1743-0003-11-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rogers JM, Duckworth J, Middleton S, Steenbergen B, Wilson PH. Elements virtual rehabilitation improves motor, cognitive, and functional outcomes in adult stroke: evidence from a randomized controlled pilot study. J NeuroEngineering Rehabil [Internet]. 2019 [cited 2020 Aug 10];16. Available from: https://jneuroengrehab.biomedcentral.com/articles/10.1186/s12984-019-0531-y. [DOI] [PMC free article] [PubMed]
- 36.Dodakian L, McKenzie AL, Le V, See J, Pearson-Fuhrhop K, Burke Quinlan E, et al. A home-based telerehabilitation program for patients with stroke. Neurorehabil Neural Repair. 2017;31:923–933. doi: 10.1177/1545968317733818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goršič M, Cikajlo I, Goljar N, Novak D. A multisession evaluation of an adaptive competitive arm rehabilitation game. J NeuroEngineering Rehabil [Internet]. 2017 [cited 2020 Aug 18];14. Available from: https://jneuroengrehab.biomedcentral.com/articles/10.1186/s12984-017-0336-9. [DOI] [PMC free article] [PubMed]
- 38.Tabacof L, Dewil S, Herrera JE, Cortes M, Putrino D. Adaptive esports for people with spinal cord injury: new frontiers for inclusion in mainstream sports performance. Front Psychol [Internet]. Frontiers; 2021 [cited 2021 Apr 21];12. Available from: https://www.frontiersin.org/articles/10.3389/fpsyg.2021.612350/full?&utm_source=Email_to_authors_&utm_medium=Email&utm_content=T1_11.5e1_author&utm_campaign=Email_publication&field=&journalName=Frontiers_in_Psychology&id=612350. [DOI] [PMC free article] [PubMed]
- 39.Cortés-Pérez I, Nieto-Escamez FA, Obrero-Gaitán E. Immersive virtual reality in stroke patients as a new approach for reducing postural disabilities and falls risk: a case series. Brain Sci [Internet]. 2020 [cited 2021 May 27];10. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7287864/. [DOI] [PMC free article] [PubMed]
- 40.Corbetta D, Imeri F, Gatti R. Rehabilitation that incorporates virtual reality is more effective than standard rehabilitation for improving walking speed, balance and mobility after stroke: a systematic review. J Physiother. 2015;61:117–124. doi: 10.1016/j.jphys.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 41.Saposnik G, Levin M. for the Stroke Outcome Research Canada (SORCan) Working Group. Virtual reality in stroke rehabilitation: a meta-analysis and implications for clinicians. Stroke. 2011;42:1380–1386. doi: 10.1161/STROKEAHA.110.605451. [DOI] [PubMed] [Google Scholar]
- 42.Lohse KR, Hilderman CGE, Cheung KL, Tatla S, Van der Loos HFM. Virtual reality therapy for adults post-stroke: a systematic review and meta-analysis exploring virtual environments and commercial games in therapy. Quinn TJ, editor. PLoS ONE. 2014;9:e93318. [DOI] [PMC free article] [PubMed]
- 43.Maier M, Ballester BR, Verschure PFMJ. Principles of neurorehabilitation after stroke based on motor learning and brain plasticity mechanisms. Front Syst Neurosci [Internet]. 2019 [cited 2021 May 27];13. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6928101/. [DOI] [PMC free article] [PubMed]
- 44.Braun SM, Beurskens AJ, Borm PJ, Schack T, Wade DT. The effects of mental practice in stroke rehabilitation: a systematic review. Arch Phys Med Rehabil. 2006;87:842–852. doi: 10.1016/j.apmr.2006.02.034. [DOI] [PubMed] [Google Scholar]
- 45.Decety J, Jeannerod M. Mentally simulated movements in virtual reality: does Fitts’s law hold in motor imagery? Behav Brain Res. 1995;72:127–134. doi: 10.1016/0166-4328(96)00141-6. [DOI] [PubMed] [Google Scholar]
- 46.• Palacios-Navarro G, Hogan N. Head-mounted display-based therapies for adults post-stroke: a systematic review and meta-analysis. Sensors [Internet]. 2021 [cited 2021 Mar 3];21. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7915338/. Meta-analysis that specifically evaluated immersive virtual reality therapies for stroke rehabilitation found that patients who received IVR rehabilitation found a trend toward significantly greater improvements compared to those receiving traditional or conventional therapies. [DOI] [PMC free article] [PubMed]
- 47.Yang Y-R, Tsai M-P, Chuang T-Y, Sung W-H, Wang R-Y. Virtual reality-based training improves community ambulation in individuals with stroke: a randomized controlled trial. Gait Posture. 2008;28:201–206. doi: 10.1016/j.gaitpost.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 48.Crosbie JH, Lennon S, Basford JR, McDonough SM. Virtual reality in stroke rehabilitation: still more virtual than real. Disabil Rehabil. 2007;29:1139–1146. doi: 10.1080/09638280600960909. [DOI] [PubMed] [Google Scholar]
- 49.Henderson A, Korner-Bitensky N, Levin M. Virtual reality in stroke rehabilitation: a systematic review of its effectiveness for upper limb motor recovery. Top Stroke Rehabil. 2007;14:52–61. doi: 10.1310/tsr1402-52. [DOI] [PubMed] [Google Scholar]
- 50.Subramanian SK, Levin MF. Viewing medium affects arm motor performance in 3D virtual environments. J NeuroEngineering Rehabil. 2011;8:36. doi: 10.1186/1743-0003-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bertani R, Melegari C, De Cola MC, Bramanti A, Bramanti P, Calabrò RS. Effects of robot-assisted upper limb rehabilitation in stroke patients: a systematic review with meta-analysis. Neurol Sci. 2017;38:1561–1569. doi: 10.1007/s10072-017-2995-5. [DOI] [PubMed] [Google Scholar]
- 52.Veerbeek JM, Langbroek-Amersfoort AC, van Wegen EEH, Meskers CGM, Kwakkel G. Effects of robot-assisted therapy for the upper limb after stroke. Neurorehabil Neural Repair. 2017;31:107–121. doi: 10.1177/1545968316666957. [DOI] [PubMed] [Google Scholar]
- 53.Mehrholz J, Thomas S, Kugler J, Pohl M, Elsner B. Electromechanical-assisted training for walking after stroke. Cochrane Database Syst Rev [Internet]. 2020 [cited 2021 May 27]; Available from: https://www.readcube.com/articles/10.1002%2F14651858.CD006185.pub5. [DOI] [PMC free article] [PubMed]
- 54.Tedla JS, Dixit S, Gular K, Abohashrh M. Robotic-assisted gait training effect on function and gait speed in subacute and chronic stroke population: a systematic review and meta-analysis of randomized controlled trials. Eur Neurol. 2019;81:103–111. doi: 10.1159/000500747. [DOI] [PubMed] [Google Scholar]
- 55.Bruni MF, Melegari C, De Cola MC, Bramanti A, Bramanti P, Calabrò RS. What does best evidence tell us about robotic gait rehabilitation in stroke patients: a systematic review and meta-analysis. J Clin Neurosci. 2018;48:11–17. doi: 10.1016/j.jocn.2017.10.048. [DOI] [PubMed] [Google Scholar]
- 56.Lorenz RC, Gleich T, Gallinat J, Kühn S. Video game training and the reward system. Front Hum Neurosci [Internet]. 2015 [cited 2021 May 27];9. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4318496/. [DOI] [PMC free article] [PubMed]
- 57.Koepp MJ, Gunn RN, Lawrence AD, Cunningham VJ, Dagher A, Jones T, et al. Evidence for striatal dopamine release during a video game. Nature. 1998;393:266–268. doi: 10.1038/30498. [DOI] [PubMed] [Google Scholar]
- 58.Kühn S, Romanowski A, Schilling C, Lorenz R, Mörsen C, Seiferth N, et al. The neural basis of video gaming. Transl Psychiatry. 2011;1:e53–e53. doi: 10.1038/tp.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Palaus M, Marron EM, Viejo-Sobera R, Redolar-Ripoll D. Neural basis of video gaming: a systematic review. Front Hum Neurosci [Internet]. 2017 [cited 2021 May 27];11. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5438999/. [DOI] [PMC free article] [PubMed]
- 60.Zhang Y, Du G, Yang Y, Qin W, Li X, Zhang Q. Higher integrity of the motor and visual pathways in long-term video game players. Front Hum Neurosci [Internet]. Frontiers; 2015 [cited 2021 May 27];9. Available from: https://www.frontiersin.org/articles/10.3389/fnhum.2015.00098/full. [DOI] [PMC free article] [PubMed]
- 61.Landers RN, Bauer KN, Callan RC, Armstrong MB. Psychological theory and the gamification of learning. Gamification Educ Bus. 2015:165–86.
- 62.Caponetto I, Earp J, Ott M. Gamification and Education: a Literature Review. Proc 8th Eur Conf Games-Based Learn - ECGBL 2014. 2014;1:50–7.
- 63.Brian S. Bovee. A gamification technique to increase engagement in asynchronous online discussions. Issues Inf Syst [Internet]. 2020 [cited 2021 May 27]; Available from: https://iacis.org/iis/2020/3_iis_2020_20-30.pdf.
- 64.Karamians R, Proffitt R, Kline D, Gauthier LV. Effectiveness of virtual reality- and gaming-based interventions for upper extremity rehabilitation poststroke: a meta-analysis. Arch Phys Med Rehabil. 2020;101:885–896. doi: 10.1016/j.apmr.2019.10.195. [DOI] [PubMed] [Google Scholar]
- 65.Tăut D, Pintea S, Roovers J-PWR, Mañanas M-A, Băban A. Play seriously: effectiveness of serious games and their features in motor rehabilitation. A meta-analysis. NeuroRehabilitation. 2017;41:105–118. doi: 10.3233/NRE-171462. [DOI] [PubMed] [Google Scholar]
- 66.Donoso Brown EV, McCoy SW, Fechko AS, Price R, Gilbertson T, Moritz CT. Preliminary investigation of an electromyography-controlled video game as a home program for persons in the chronic phase of stroke recovery. Arch Phys Med Rehabil. 2014;95:1461–1469. doi: 10.1016/j.apmr.2014.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Burke JW, McNeill MDJ, Charles DK, Morrow PJ, Crosbie JH, McDonough SM. Optimising engagement for stroke rehabilitation using serious games. Vis Comput. 2009;25:1085–1099. doi: 10.1007/s00371-009-0387-4. [DOI] [Google Scholar]
- 68.Chen Y, Abel KT, Janecek JT, Chen Y, Zheng K, Cramer SC. Home-based technologies for stroke rehabilitation: a systematic review. Int J Med Inform. 2019;123:11–22. doi: 10.1016/j.ijmedinf.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jurkiewicz MT, Marzolini S, Oh P. Adherence to a home-based exercise program for individuals after stroke. Top Stroke Rehabil. 2011;18:277–284. doi: 10.1310/tsr1803-277. [DOI] [PubMed] [Google Scholar]
- 70.Nudo RJ. Recovery after brain injury: mechanisms and principles. Front Hum Neurosci [Internet]. Frontiers; 2013 [cited 2021 May 27];7. Available from: https://www.frontiersin.org/articles/10.3389/fnhum.2013.00887/full. [DOI] [PMC free article] [PubMed]
- 71.Lang CE, MacDonald JR, Reisman DS, Boyd L, Jacobson Kimberley T, Schindler-Ivens SM, et al. Observation of amounts of movement practice provided during stroke rehabilitation. Arch Phys Med Rehabil. 2009;90:1692–1698. doi: 10.1016/j.apmr.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lohse K, Shirzad N, Verster A, Hodges N, Van der Loos HFM. Video games and rehabilitation: using design principles to enhance engagement in physical therapy. J Neurol Phys Ther. 2013;37:166–175. doi: 10.1097/NPT.0000000000000017. [DOI] [PubMed] [Google Scholar]
- 73.Goršič M, Cikajlo I, Goljar N, Novak D. A multisession evaluation of an adaptive competitive arm rehabilitation game. J NeuroEng Rehabil. 2017;14:128. doi: 10.1186/s12984-017-0336-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Morris ZS, Wooding S, Grant J. The answer is 17 years, what is the question: understanding time lags in translational research. J R Soc Med. 2011;104:510–520. doi: 10.1258/jrsm.2011.110180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Glegg SMN, Levac DE. Barriers, facilitators and interventions to support virtual reality implementation in rehabilitation: a scoping review. PM R. 2018;10:1237–1251.e1. doi: 10.1016/j.pmrj.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.• Bower KJ, Verdonck M, Hamilton A, Williams G, Tan D, Clark RA. What factors influence clinicians’ use of technology in neurorehabilitation? A multisite qualitative study. Phys Ther. 2021;101:pzab031. This study found that although clinicians perceive technology as having a beneficial role in improving health outcomes, self-efficacy and technology competence are two major barriers to scalability and widespread clinical adoption. [DOI] [PubMed]
- 77.Levac D, Glegg S, Colquhoun H, Miller P, Noubary F. Virtual reality and active videogame-based practice, learning needs, and preferences: a cross-Canada survey of physical therapists and occupational therapists. Games Health J. 2017;6:217–228. doi: 10.1089/g4h.2016.0089. [DOI] [PubMed] [Google Scholar]
- 78.Hung Y-X, Huang P-C, Chen K-T, Chu W-C. What do stroke patients look for in game-based rehabilitation: a survey study. Medicine. 2016;95:e3032. doi: 10.1097/MD.0000000000003032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mann DM, Chen J, Chunara R, Testa PA, Nov O. COVID-19 transforms health care through telemedicine: evidence from the field. J Am Med Inform Assoc. 2020;27:1132–1135. doi: 10.1093/jamia/ocaa072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Trumbower RD, Wolf SL. A forward move: interfacing biotechnology and physical therapy in and out of the classroom. Phys Ther. 2019;99:519–525. doi: 10.1093/ptj/pzz008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kigin CM, Rodgers MM, Wolf SL. The physical therapy and society summit (PASS) meeting: observations and opportunities. Phys Ther. 2010;90:1555–1567. doi: 10.2522/ptj.20100138. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.