Abstract
Background
Age‐related decline in bone mass increases the risk of skeletal fractures, especially those of the hip, spine, and wrist. Steroidal contraceptives have been associated with changes in bone mineral density in women. Whether such changes affect the risk of fractures later in life is unclear. Hormonal contraceptives are among the most effective and most widely‐used contraceptives. Concern about fractures may limit the use of these effective contraceptives. Observational studies can collect data on premenopausal contraceptive use as well as fracture incidence later in life.
Objectives
We systematically reviewed the evidence from observational studies of hormonal contraceptive use for contraception and the risk of fracture in women.
Search methods
Through June 2015, we searched for observational studies. The databases included PubMed, POPLINE, Cochrane Central Register of Controlled Trials (CENTRAL), LILACS, EMBASE, CINAHL, and Web of Science. We also searched for recent clinical trials through ClinicalTrials.gov and the ICTRP. For other studies, we examined reference lists of relevant articles and wrote to investigators for additional reports.
Selection criteria
We included cohort and case‐control studies of hormonal contraceptive use. Interventions included comparisons of a hormonal contraceptive with a non‐hormonal contraceptive, no contraceptive, or another hormonal contraceptive. The primary outcome was the risk of fracture.
Data collection and analysis
Two authors independently extracted the data. One author entered the data into RevMan, and a second author verified accuracy. We examined the quality of evidence using the Newcastle‐Ottawa Quality Assessment Scale (NOS), developed for case‐control and cohort studies. Sensitivity analysis included studies of moderate or high quality based on our assessment with the NOS.
Given the need to control for confounding factors in observational studies, we used adjusted estimates from the models as reported by the authors. Where we did not have adjusted analyses, we calculated the odds ratio (OR) with 95% confidence interval (CI). Due to varied study designs, we did not conduct meta‐analysis.
Main results
We included 14 studies (7 case‐control and 7 cohort studies). These examined oral contraceptives (OCs), depot medroxyprogesterone acetate (DMPA), and the hormonal intrauterine device (IUD). This section focuses on the sensitivity analysis with six studies that provided moderate or high quality evidence.
All six studies examined oral contraceptive use. We noted few associations with fracture risk. One cohort study reported OC ever‐users had increased risk for all fractures (RR 1.20, 95% CI 1.08 to 1.34). However, a case‐control study with later data from a subset reported no association except for those with 10 years or more since use (OR 1.55, 95% CI 1.03 to 2.33). Another case‐control study reported increased risk only for those who had 10 or more prescriptions (OR 1.09, 95% CI 1.03 to 1.16). A cohort study of postmenopausal women found no increased fracture risk for OC use after excluding women with prior fracture. Two other studies found little evidence of association between OC use and fracture risk. A cohort study noted increased risk for subgroups, such as those with longer use or specific intervals since use. A case‐control study reported increased risk for any fracture only among young women with less than average use.
Two case‐control studies also examined progestin‐only contraceptives. One reported increased fracture risk for DMPA ever‐use (OR 1.44, 95% CI 1.01 to 2.06), more than four years of use (OR 2.16, 95% CI 1.32 to 3.53), and women over 50 years old. The other reported increased risk for any past use, including one or two prescriptions (OR 1.17, 95% CI 1.07 to 1.29) and for current use of 3 to 9 prescriptions (OR 1.36, 95% CI 1.15 to 1.60) or 10 or more (OR 1.54, 95% CI 1.33 to 1.78). For the levonorgestrel‐releasing IUD, one study reported reduced fracture risk for ever‐use (OR 0.75, 95% CI 0.64 to 0.87) and for longer use.
Authors' conclusions
Observational studies do not indicate an overall association between oral contraceptive use and fracture risk. Some reported increased risk for specific user subgroups. DMPA users may have an increased fracture risk. One study indicated hormonal IUD use may be associated with decreased risk. Observational studies need adjusted analysis because the comparison groups usually differ. Investigators should be clear about the variables examined in multivariate analysis.
Plain language summary
Hormonal birth control and fracture risk in observational studies
When bone mass declines with age, the risk of fractures increases. Birth control methods that have hormones may lead to changes in women’s bone density. Worry about fractures may limit the use of these effective methods. Observational studies can collect data on birth control use as well as fractures later in life. Through June 2015, we searched for such studies in several databases.
We included studies that looked at hormonal birth control use and fracture risk. We examined the quality of research methods using a tool for observational studies. With these types of studies, investigators need to control for differences in the study groups. We used the results from adjusted analyses as reported. Where we did not have adjusted analysis, we used the odds ratio to look at differences between groups.
We found 14 studies. Six of them had good quality results and looked at use of birth control pills. We did not find an overall difference in fracture risk for users and nonusers of birth control pills. One study found pill users were more likely to have fractures overall. Another had later data for a subset of those women. Pill use was not related to fracture risk except for 10 or more years since use. Still another study showed more risk when the woman had 10 or more prescriptions. When a study of postmenopausal women removed the women with prior fracture, pill users did not have higher fracture risk. Two more studies saw more fractures in pill users but only for certain subgroups.
Two studies looked at birth control methods that contain only the hormone progestin. They found that users of the injected ‘depo’ (depot medroxyprogesterone acetate) had more fractures as did women with longer current use. One showed more fractures for women with any past 'depo' use. Another study showed that women who had used the hormonal intrauterine device (IUD) were less likely to have a fracture.
These studies did not show that birth control pills are generally related to more fractures. Some studies reported greater risk for subgroups. Users of ‘depo’ may have more fracture risk. Observational studies need to examine differences between study groups. Investigators should be clear about the factors studied in the analysis.
Summary of findings
Background
Description of the condition
Age‐related decline in bone mass increases the risk of skeletal fractures, especially those of the hip, spine, and wrist (Howe 2011; Rachner 2011). Prevalence estimates for osteoporosis include the following: 30% for postmenopausal women and 70% for women aged 80 years or more in the USA; 29% of women in India; for Japanese women aged 50 to 79 years, 35% at the spine and 9.5% at the hip; and in Latin America, 12% to 18% for women at least 50 years old (Sanchez‐Riera 2010). The costs of osteoporosis‐related fractures can be substantial for the individual due to disability and to society for health and social care (Howe 2011). Steroidal contraceptives, particularly injectable contraceptives and combined oral contraceptives (COCs), have been associated with changes in bone mineral density in women. Whether such changes affect the risk of fractures later in life is unclear. Concern about bone health and fracture risk influences the recommendation and use of these effective contraceptives globally.
Description of the intervention
In the US, more than 60% of women in their childbearing years use contraceptives, and 40% of those women use hormonal contraceptives (Isley 2011). Steroidal contraceptives include combined contraceptives, containing both progestin and estrogen, as well as progestin‐only contraceptives. Combined hormonal contraceptives include a wide variety of pills, the vaginal ring, the transdermal patch, and combined injectables. Delivery methods for progestin‐only contraceptives include pills, injectables, implants, and a levonorgestrel‐releasing intrauterine device.
Depot medroxyprogesterone acetate (DMPA) is an effective contraceptive and the most widely‐used injectable (Goldberg 2011). First‐year failure rates for DMPA in the USA have been estimated at 0.2% for perfect use and 6% for typical use (Trussell 2011). Data from developing countries showed median failure rates of 2.4% for injectables versus 10.3% for condoms and 6.5% for pills (Cleland 2004). DMPA has attracted the most attention regarding bone health. This injectable may reduce bone mineral density (BMD), a potential concern for younger women who have not achieved peak bone mass and for perimenopausal women who may begin to lose bone mass. In 2004, the US Food and Drug Administration added a warning to DMPA labeling about the potential loss of BMD (FDA 2004), which might limit long‐term use. A systematic review of progestin‐only methods found an association between DMPA use and loss of bone mineral density (Curtis 2006). The clinical significance of this association was not clear. Evidence suggested that women gained BMD after discontinuation of DMPA. Another review concluded that adolescent users of DMPA do have decreases in BMD, but the loss can be recovered within one or two years after discontinuation (Isley 2011). Major health organizations have recommended not restricting DMPA use among women 18 to 45 years old (WHO 2006; Guilbert 2009; ACOG 2014). In Medical Eligibility Criteria (MEC) for contraceptive use, DMPA is category 1 (no restriction) for women aged 18 to 45 years. For women less than 18 and greater than 45 years of age, DMPA is category 2 (CDC 2010; WHO 2015a; WHO 2015b), which indicates the advantages of using the method generally outweigh the theoretical or proven risks.
Oral contraceptives (OCs) are the most commonly used reversible method in more developed regions (UN 2013). Intrauterine devices (IUDs) are widely used in developing areas, but most are non‐hormonal. Hormonal IUDs are not widely used. Failure rates for oral contraceptives in the USA (combined and progestin‐only) are estimated at 0.3% for perfect use and 9% for typical use in the first year (Trussell 2011). In a review of randomized controlled trials (RCTs) on steroidal contraceptives and bone health, combination contraceptives did not appear to have a negative effect, but no trials were placebo‐controlled and none had fracture as an outcome (Lopez 2014). An earlier review focused on combined hormonal contraceptives and bone health and included studies of varying designs (Martins 2006). Bone mineral density appeared to be affected by combined oral contraceptive (COC) use in adolescent and young women but not in premenopausal or postmenopausal women. A recent review noted that COCs have little effect on BMD (Isley 2011), and concluded that healthy women could use COCs without concerns regarding skeletal health.
How the intervention might work
The development of osteoporosis depends on tissue, cellular, and molecular interactions (Rachner 2011). Bone turnover involves a continuing process of formation and resorption (loss). Sex hormones help regulate bone metabolism (Herrmann 2010). Skeletal fragility, and the risk of fracture, results from low bone mass and deterioration of bone tissue (Sanchez‐Riera 2010). Low estrogen levels, whether related to progestin‐only contraceptives or menopause, can lead to increased bone turnover and bone loss (Isley 2011; Herrmann 2010). However, bone loss during contraceptive use may be temporary, similar to that which occurs during pregnancy or breastfeeding (ACOG 2014). Risk of future fractures after contraceptive use depends on whether the bone mass is restored or not.
Why it is important to do this review
Hormonal contraceptives are among the most effective and most widely‐used contraceptives. Concern about fractures may limit the use of these effective contraceptives. Women might switch to less effective methods or use nothing, and those alternatives could lead to increased rates of unintended pregnancy. Therefore, the question about an association between steroidal contraceptives and fractures is important to examine systematically with the available evidence.
Skeletal fragility fractures are rare in premenopausal women. Consequently, randomized controlled trials of contraceptive use may not be the best design for assessing fracture risk. Observational studies include case‐control studies as well as cohort studies. Such designs allow for collecting data on premenopausal contraceptive use as well as fracture incidence later in life. Reviewing observational studies does present additional challenges, including heterogeneity in study design and populations as well as increased risk of bias. However, a meta‐analysis compared estimates of intervention harm from studies of varying designs (Golder 2011). The investigators found the risk estimate of adverse effects to be similar from meta‐analyses of RCTs and from meta‐analyses of observational studies.
Objectives
We systematically reviewed the evidence from observational studies of hormonal contraceptive use for contraception and the risk of fracture in women.
Methods
Criteria for considering studies for this review
Types of studies
We considered cohort studies of contraceptive users as well as case‐control studies. Post hoc analysis from such studies was also considered. Randomized controlled trials were excluded, as they were reviewed elsewhere (Lopez 2014) and no RCT had fracture as an outcome. The Discussion contains pertinent results from the review of RCTs to provide context for the results here.
Types of participants
Participants were women who used steroidal contraceptives during their reproductive years or women in a comparison group who did not use hormonal contraceptives during their reproductive years. We excluded studies that focused on women with specific conditions or situations that can affect bone health, such as epilepsy because some medications have a negative influence and athletes given that exercise can have a positive influence (Howe 2011).
Types of interventions
Interventions included comparisons of a hormonal contraceptive with a non‐hormonal contraceptive, no contraceptive, or another hormonal contraceptive. The contraceptive must have been intended for contraception and not as treatment for another health condition, such as hormone replacement therapy for postmenopausal women. Interventions could also include a supplement for one group, such as another hormone or a vitamin or mineral preparation.
Types of outcome measures
The primary outcome was the risk of fracture, particularly fracture of the spine, hip, or wrist. We did not examine data on bone mineral density, which is considered a surrogate marker for fracture. Data from RCTs on hormonal contraceptives and bone mineral density have been reviewed elsewhere (Lopez 2014).
Search methods for identification of studies
Electronic searches
Through June 2015, we searched for studies of steroidal contraceptives and fractures. Databases included PubMed, POPLINE, Cochrane Central Register of Controlled Trials (CENTRAL), LILACS, and Web of Science. In addition, we searched for recent clinical trials through ClinicalTrials.gov and the International Clinical Trials Registry Platform (ICTRP). Details of the search strategy are given in Appendix 1. The previous search strategies, which also included EMBASE and CINAHL, can be found in Appendix 2.
Searching other resources
For other relevant studies, we examined reference lists of included studies as well as review articles. For the initial review, we wrote to investigators for information about other published or unpublished studies not identified in our search.
Data collection and analysis
Selection of studies
We assessed for inclusion all titles and abstracts identified during the literature search. In 2015, one author reviewed the search results for potentially eligible studies; another author checked for appropriate categorization. For the initial review, two authors independently examined the search results for eligible studies. We resolved any discrepancies by discussion. For studies that appeared to meet the criteria for this review, we obtained and examined the full‐text articles.
Data extraction and management
Two authors extracted the data. One author entered the data into Review Manager (RevMan 2014), and a second author verified accuracy. We resolved any discrepancies through discussion.
Assessment of risk of bias in included studies
We assessed the methodological quality of included studies by reviewing study design, implementation, and losses to follow‐up. We also examined the methods used for assessing the outcomes. To assess the observational data, we used the principles outlined in section 13.5 of Higgins 2011 and in the Newcastle‐Ottawa Scale for assessing the quality of non‐randomized studies (Wells 2011). The investigators reported that the content validity and inter‐rater reliability of this scale have been established and that they are currently examining criterion validity and intra‐rater reliability. The scale does not yet have an overall scoring or threshold for a 'good' or 'poor' study.
We adapted the Newcastle‐Ottawa Quality Assessment Scale (NOS) items for the interventions and outcomes in this review as per the developers' suggestions (Wells 2011). The scale has two versions; one is applicable to case‐control studies (Appendix 3) and one is pertinent to cohort studies (Appendix 4), although the criteria are similar for several items. Each version has eight items within three domains: selection (representativeness), comparability (due to design or analysis), and outcomes (assessment and follow‐up). For the risk of bias tables, we used headings appropriate to the Newcastle‐Ottawa Scale. Assessment of analysis included any adjustment for potential confounding factors related to fracture risk. The study groups could differ in ways related to the outcome, such as body mass index, exercise patterns, or use of steroids other than contraceptives. A study can receive one star (✸) for meeting each criterion. The exception is comparability (design or analysis), for which a study can receive a maximum of two stars. In this review, for one star under comparability, the study controlled for age. For two stars under comparability, the study also controlled for other important variables such as exercise, body mass index, use of hormone replacement therapy or use of other relevant drugs. We present study limitations in each area of the scale and considered them when interpreting results.
Measures of treatment effect
Given the need to control for confounding factors in observational studies, we used adjusted measures as the primary effect measures when available. We used the adjusted estimates from the models reported by the authors. Odds ratio (OR) is an appropriate effect measure for both cohort and case‐control studies and is commonly provided when adjusted analyses are obtained using logistic regression models. However, we considered other effect measures if an appropriate adjusted OR was not available from the report. The effect measure may have been an odds ratio, risk ratio, or hazard ratio.
Investigators used a variety of adjustment strategies. We specified whether confounding was considered in the design (e.g., matching, stratification). We provided the confounding factors considered in the design and analysis when presenting results. When investigators used multivariate models to adjust for potential confounding, we did not analyze the treatment effect as that would usually require individual participant data. Rather we presented the results from adjusted models as reported by the investigators. If no adjusted measures were given as part of the primary analysis, we used unadjusted measures. If data were available for unadjusted dichotomous outcomes, we calculated the OR with 95% confidence interval (CI).
Given the diversity of design features with observational studies, we did not conduct meta‐analysis for pooled estimates. We assessed sources of heterogeneity without pooling the data.
Unit of analysis issues
We did not encounter crossover studies or clustered designs for studies that met our inclusion criteria. However, if clustering was part of the design, we had planned to assess whether estimates were properly adjusted to account for clustering effects.
Dealing with missing data
If reports were missing data needed for analysis, we wrote to the authors. However, we limited our data requests to studies less than 10 years old. Investigators are unlikely to have access to data for older studies.
Assessment of heterogeneity
Due to varied study designs, we were unable to conduct meta‐analysis. Therefore, we did not need to assess statistical heterogeneity. However, we address heterogeneity due to differences in study design, analysis strategy (in particular the issue of confounding adjustments), and populations (Discussion).
Data synthesis
We intended to combine data from studies if they had similar designs, interventions, and outcome measures. Where we could analyze data, we used a fixed‐effect model for the dichotomous outcomes (Measures of treatment effect). Fixed‐effect and random‐effects models will give the same result if no heterogeneity exists and when a comparison does not involve a meta‐analysis, that is, has only one study (Higgins 2011). There is little consensus regarding the use of either model.
We organized the Results by the type of intervention (exposure) examined in the study. The major categories were oral contraceptives and progestin‐only injectables. Within those categories, we present results by outcome, that is, the type of fracture.
To assess the quality of the body of evidence, we tried to extrapolate our findings from the Newcastle‐Ottawa Scale (NOS) (Wells 2011) to the GRADE ratings that address confidence in the effect estimate (Balshem 2011). However, the GRADE approach has mainly focused on RCTs, given that much is based on the Risk of Bias tables (Higgins 2011). As noted earlier, the NOS does not have an overall scoring, but we wanted to synthesize results across studies. We assessed evidence from individual studies rather than from a meta‐analysis. Our approach is explained below (Sensitivity analysis).
Sensitivity analysis
We summarized the results from studies that provided at least moderate quality evidence. For inclusion as moderate quality evidence, studies had to meet at least six criteria of the Newcastle‐Ottawa Quality Assessment Scale (NOS) (Assessment of risk of bias in included studies).
Case‐control studies: selection of cases and controls (four items), comparability of cases and controls (at least one star), and exposure ascertainment (one item, i.e., method used).
Cohort studies: selection of exposed and non‐exposed cohorts (four items), comparability of cohorts (at least one star), and outcome assessment.
We downgraded the evidence a level for each criterion that was not met. A study might not have met the criteria due to design issues or insufficient information in the report. We upgraded the evidence by one level if the study had two stars for comparability.
Results
Description of studies
Results of the search
In 2015, the database searches produced 192 references. After we removed 62 duplicates electronically or by hand, we had 130 unduplicated references (Figure 1). The search for the initial review in 2012 yielded 429 unduplicated references for a grand total of 559. For this update, we identified one published article from a previously included study that had only had a conference presentation. We discarded the remaining citations based on title or abstract. Searches of clinical trials databases yielded only three unduplicated listings, none of which appeared relevant to this review.
1.
Study flow diagram.
Included studies
We included 14 studies plus 10 reports with additional analyses or design information (Characteristics of included studies). Studies could have examined more than one type of steroidal contraceptive; the 14 studies examined oral contraceptives (N = 12), DMPA (N = 4) and the hormonal IUD (N = 1). The type of fracture studied varied, e.g., first fracture, hip fracture, or forearm fracture. Details are provided in Effects of interventions. Designs included seven case‐control studies and seven cohort studies (of which two only analyzed baseline data). Six were conducted in the UK, two in Sweden and two in the USA, and one in each of Finland, Denmark, Italy, and Australia.
Excluded studies
We excluded 11 studies. Reasons included not having fracture as an outcome, not being a comparative study, or being a cross‐sectional study (Characteristics of excluded studies).
Risk of bias in included studies
We used the Newcastle‐Ottawa Scale (NOS) for assessing the quality of included studies (Appendix 3; Appendix 4). Assessments of case‐control studies are shown in Table 3. Assessments for cohort studies are shown in Table 4; we also included baseline assessments within cohort studies. We grouped the results from our Risk of bias tables into the main domains of the NOS.
1. Evidence quality assessment, case‐control studies.
| Studya | Selection | Comparability of cases and controls | Exposure | Evidence qualityb | |||||
| Case definition | Cases representative | Control selection | Control definition | Ascertainment method | Same ascertainment both groups | Nonresponse rate | |||
| La Vecchia 1999 | ✸ | ✸ | ‐ | ‐ | ✸ | ‐ | ✸ | ✸ | Very low |
| Mallmin 1994 | ✸ | ✸ | ✸ | ✸ | ✸ | ‐ | ✸ | ✸ | Low |
| Meier 2010 | ✸ | ✸ | ✸ | ‐ | ✸✸ | ✸ | ✸ | ✸ | Moderate |
| Memon 2011 | ✸ | ✸ | ✸ | ✸ | ✸✸ | ✸ | ✸ | ✸ | High |
| Michaelsson 1999 | ✸ | ✸ | ✸ | ‐ | ✸✸ | ‐ | ✸ | ✸ | Low |
| O'Neill 1996 | ✸ | ✸ | ✸ | ‐ | ✸✸ | ‐ | ✸ | ‐ | Low |
| Vestergaard 2006c | ✸ | ✸ | ✸ | ‐ | ✸✸ | ✸ | ✸ | ✸ | Moderate |
aNewcastle‐Ottawa Quality Assessment Scale (Appendix 3): 1 star (✸) for meeting each criterion, except comparability (design or analysis) can have 2 stars. For comparability in this review: 1 star if controlled for age; 2 stars if also controlled for other important variables, e.g., exercise, body mass index, use of hormone replacement therapy or other relevant drugs bModerate quality evidence: met criteria for selection (4 items), comparability (1 star; upgraded for 2 stars), and ascertainment method; downgrading due to design limitation or lack of information in report cVestergaard 2006 includes 3 reports: OC use (2006), OC use among young women (2008a); use of DMPA or hormonal IUD (2008b)
2. Evidence quality assessment, cohort studies.
| Studya | Selection | Comparability of cohorts | Outcome | Evidence qualityb | |||||
| Exposed cohort representative | Nonexposed cohort selection | Exposure ascertainment | Outcome not present at start | Assessment | Follow‐up length | Follow‐up adequacy | |||
| Barad 2005 | ✸ | ✸ | ✸ | ‐ | ✸✸ | ✸ | ‐ | ✸ | Moderate |
| Cooper 1993 | ✸ | ✸ | ✸ | ✸ | ✸ | ✸ | ✸ | ‐ | Moderate |
| Kaunitz 2006 | ‐ | ✸ | ‐ | ✸ | ‐ | ‐ | ✸ | ‐ | Very low |
| Lanza 2013 | ✸ | ✸ | ✸ | ‐ | ✸ | ✸ | ✸ | ‐ | Low |
| Tuppurainen 1994 | ✸ | ✸ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | Very Low |
| Vessey 1998 | ✸ | ✸ | ✸ | ✸ | ✸✸ | ✸ | ✸ | ✸ | High |
| Wei 2011 | ✸ | ✸ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | Very low |
aNewcastle‐Ottawa Quality Assessment Scale (Appendix 4): 1 star (✸) for meeting each criterion, except comparability (design or analysis) can have 2 stars. For comparability in this review: 1 star if controlled for age; 2 stars if also controlled for other important variables, e.g., exercise, body mass index, use of hormone replacement therapy or other relevant drugs bModerate quality evidence: met criteria for selection (4 items), comparability (1 star; upgraded for 2 stars), and outcome assessment. downgrading due to design limitation or lack of information in report.
Selection
This domain included four criteria, which differed between case‐control and cohort studies. All seven case‐control studies met the NOS criteria for case definition (having independent validation) and for representativeness of cases. Six studies met the criterion for control selection. For control definition, only two studies met the criterion, i.e., were clear about the controls not having a history of fracture.
The seven cohort studies met the criteria for representativeness of the exposed cohort and for selection of the non‐exposed cohort. Four met the criterion for exposure ascertainment; the others used written survey responses or did not report sufficient information. For the outcome not being present at study start, only three studies had such evidence and therefore met the criterion.
Exposure (case‐control)
Three criteria comprised this domain. Five of the case‐control studies met the criterion for ascertainment of exposure, which was based on the information source such as secure records or structured interview. Two did not meet the criterion due to insufficient information. All seven studies met the criterion for using the same ascertainment method for both cases and controls. Only three studies had comparable non‐response rates by group and overall non‐response rates less than 20%.
Comparability of study groups
This section addresses comparability based on design or analysis. All seven case‐control studies adjusted for age or matched on age. In addition, four adjusted for other important potential confounders. Another listed the important variables examined that reportedly had no association with the outcome (O'Neill 1996). The remaining two studies did not meet the criterion due to inadequate information on variables examined (La Vecchia 1999) or to conducting only univariate analysis (Mallmin 1994).
Of the cohort studies, two adjusted for age and other important potential confounders. Three did not have comparable cohorts or did not adjust for potential confounders (Tuppurainen 1994; Kaunitz 2006; Wei 2011). In addition, Cooper 1993 adjusted for age and parity but did not address other important variables. Lanza 2013 adjusted for age and stated that other (unspecified) variables were examined that did not make a meaningful difference after age.
An additional factor for this review was the type of OC used. Most studies had records of the specific pill type but analyzed the data as any OC. Vestergaard 2006 was the exception in examining the estrogen dose as well as the type of progestin for the 2008 paper on COCs.
Outcome (cohort)
This domain included three criteria. For outcome assessment, four cohort studies had an independent assessment or record linkage for the outcome. Of the other three studies, two were baseline assessments within cohort studies and one gathered fracture data as an adverse event.
The length of follow‐up appeared adequate in four of the seven studies. Those with shorter follow‐up included Barad 2005 with a 2.5 year mean as well as the two baseline assessments within cohort studies (Table 4). Losses were high however, so we assessed follow‐up as adequate (at least 80%) for only two studies.
Effects of interventions
for the main comparison.
| Oral contraceptive (OC) use compared with nonuse for contraceptiona | ||||
|
Patient or population: women Settings: hospital or clinical site Intervention: oral contraceptive (OC) use Comparison: no use of OC | ||||
| Outcomes | Relative effect (95% CI)b | Participants (study) | Quality of evidence (GRADE) | Participant ages Comparisons |
| All fractures | RR 1.20 (1.08 to 1.34) | 1365 (Cooper 1993) |
⊕⊕⊕⊝ moderate | Mean age 29 years; ranged from < 25 to > 65 OC use ever vs never |
| First fracture | OR 1.55 (1.03 to 2.33) | 819 (Memon 2011) |
⊕⊕⊕⊕ high | Age 20 to 87 years Last OC use > 10 years vs never |
| First fracture: radius or ulna; all sites | RR 1.5 (1.1 to 2.1); RR 1.2 (1.1 to 1.4) | 17,032 (Vessey 1998) |
⊕⊕⊕⊕ high | Recruited age 25 to 39 years; followed to 45 years OC use > 97 months vs no use |
| First fracture: radius or ulna; all sites | RR 2.5 (1.5 to 4.0); RR 1.3 (1.1 to 1.5) | 17,032 (Vessey 1998) |
⊕⊕⊕⊕ high | Recruited age 25 to 39 years; followed to 45 years Interval since use: 73 to 96 months vs no use (radius or ulna); < 12 months vs no use (all fractures) |
| First fracture | HR 1.07 (1.01 to 1.15); HR 1.09 (1.01 to 1.18) | 80,947 (Barad 2005) |
⊕⊕⊕⊝ moderate | Recruited age 50 to 74 years OC use: any vs none; < 5 years vs none |
| First fracture | OR 1.09 (1.03 to 1.16) | 87,627 (Meier 2010) |
⊕⊕⊕⊝ moderate | Age 20 to 44 years Current OC use > 10 prescriptions vs no use |
| Fracture, any | OR 1.50 (1.03 to 2.18); OR 1.30 (1.05 to 1.61) | 258,189 (Vestergaard 2006) |
⊕⊕⊕⊝ moderate | Mean age 51.7 years OC daily dose 0.3 to 0.99 tablet vs never user: < 15 years old; 15.1 to 17 years old |
| Fracture, any | OR 1.42 (1.09 to 1.84); OR 1.13 (1.05 to 1.22) | 258,189 (Vestergaard 2006) |
⊕⊕⊕⊝ moderate | Mean age 51.7 years OC ethinyl estradiol dose changed between 20 µg and > 30 µg vs no OC use: 15.1 to 17 years old; > 19 years old |
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||
aFrom sensitivity analysis (moderate or high quality evidence); significant differences in fracture risk bCI = confidence interval; RR = relative risk; OR = odds ratio; HR = hazard ratio
2.
| Progestin‐only contraceptive use compared with nonuse for contraceptiona | ||||
|
Patient or population: women Settings: hospital or clinical setting Intervention: use of progestin‐only contraceptive Comparison: nonuse of progestin‐only contraceptive | ||||
| Outcomes | Relative effect (95% CI)b | Participants (study) | Quality of evidence (GRADE) | Participant ages Comparisons |
| Fracture | OR 1.44 (1.01 to 2.06); OR 2.25 (1.14 to 4.42); 1.94 (1.09 to 3.45); OR 2.16 (1.32 to 3.53) | 258,189 (Vestergaard 2006) |
⊕⊕⊕⊝ moderate | Mean age 51.7 years DMPA use vs nonuse: ever use; use among women > 50 years old; daily dose > 1; use > 4 years |
| Fracture | OR 0.75 (0.64 to 0.87); OR 0.77 (0.59 to 0.99) | 258,189 (Vestergaard 2006) |
⊕⊕⊕⊝ moderate | Mean age 51.7 years Hormonal IUD use vs nonuse: ever use; use 1.6 to 4 years |
| First fracture | OR 1.36 (1.15 to 1.60); OR 1.54 (1.33 to 1.78) | 87,627 (Meier 2010) |
⊕⊕⊕⊝ moderate | Age 20 to 44 years DMPA current use vs nonuse: use 3 to 9 years; use > 10 years |
| First fracture | OR 1.17 (1.07 to 1.29); OR 1.23 (1.11 to 1.36); OR 1.30 (1.09 to 1.55) | 87,627 (Meier 2010) |
⊕⊕⊕⊝ moderate | Age 20 to 44 years DMPA past use (prescriptions) vs nonuse: 1 to 2; 3 to 9 ; > 10 |
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||
aFrom sensitivity analysis (moderate or high quality evidence); significant differences in fracture risk bCI = confidence interval; OR = odds ratio; DMPA = depot medroxyprogesterone acetate; IUD = intrauterine device
Most studies used adjusted analyses, so we were not able to analyze the data (Measures of treatment effect). We provide the results as reported by the investigators (Additional tables). For the few studies without adjusted analyses, we analyzed the data as noted below. Information on the specific confounders that the investigator considered are given in the Characteristics of included studies (Risk of bias, Comparability of groups).
Oral contraceptives
First fracture
Five studies examined first fractures for oral contraceptive users. Cooper 1993 was a prospective cohort study of OC users and nonusers. Data were from the Royal College of General Practitioners Oral Contraception Study in the UK. The same research group used a subset of these women with later data (Memon 2011). The relative risk (RR) was adjusted for some potential confounders. Ever users of OCs were more likely to have a fracture than never users (reported RR 1.20, 95% CI 1.08 to 1.34) (Table 5). Risk for forearm fracture did not differ between the groups. Standardized fracture rates were provided for years of OC use (Table 5). The rate for no use was 2.54; rates for users ranged from 3.10 (1 to 4 years) to 2.86 for 10 or more years.
3. Cooper 1993: first fracture and oral contraceptive use.
| Number | Ratea | Standardized rateb | Relative risk (95% CI)b | ||
| All fractures | |||||
| OC use |
Never | 512 | 2.60 | 2.54 | 1.00 |
| Ever | 853 | 2.99 | 3.04 | 1.20 (1.08 to 1.34) | |
| Duration of OC use (years) |
0 | 512 | 2.60 | 2.54 | ‐ |
| 1 to 4 | 497 | 2.82 | 3.10 | ‐ | |
| 5 to 9 | 259 | 3.19 | 3.01 | ‐ | |
| > 10 | 97 | 3.53 | 2.86 | ‐ | |
| Forearm fractures | |||||
| OC use |
Never | 132 | 0.67 | 0.64 | 1.00 |
| Ever | 187 | 0.66 | 0.68 | 1.06 (0.95 to 1.32) | |
aPer 1000 person‐years of observation bStandardized for age, parity at time of event, and smoking and social class at recruitment; not reported for 'Duration of OC use'
The case‐control study of Memon 2011 examined first‐time fracture versus no fracture. The investigators used data from a subset of women in the Royal College of General Practitioners Oral Contraception Study (UK). These women would have been older and followed longer than they had been for Cooper 1993. Cases and controls were age‐matched, and ORs were adjusted for some potential confounders. No association was apparent between risk of fracture and OC use ever nor for years of OC use (Table 6). For interval since OC use, the only increased risk noted was for those with 10 years or more since use (reported adjusted OR 1.55, 95% CI 1.03 to 2.33). The investigators also conducted analyses by age group and by fracture site, but showed no significant difference in risk between OC users and nonusers (data not shown here).
4. Memon 2011: first fracture and oral contraceptive use.
| Cases | Controls | OR (95% CI)a | ||
| All fractures | ||||
| OC use | Never | 227 | 472 | 1.00 |
| Ever | 420 | 823 | 1.05 (0.86 to 1.29) | |
| Fractures while under observation of general practitioner | ||||
| OC use | Never | 89 | 207 | 1.00 |
| Ever | 184 | 339 | 1.25 (0.90 to 1.72) | |
| Duration of OC use (years) | Never | 89 | 207 | 1.00 |
| < 5 | 116 | 212 | 1.25 (0.89 to 1.77) | |
| 5 to 9 | 53 | 97 | 1.25 (0.80 to 1.94) | |
| 10 to 14 | 13 | 26 | 1.16 (0.56 to 2.42) | |
| > 15 | 2 | 4 | 1.23 (0.22 to 7.02) | |
| Time since OC use and fracture diagnosis (years) | Never | 89 | 207 | 1.00 |
| < 5 | 49 | 100 | 1.06 (0.65 to 1.72) | |
| 5 to 9 | 35 | 75 | 1.01 (0.62 to 1.65) | |
| > 10 | 100 | 164 | 1.55 (1.03 to 2.33) | |
aAdjusted for smoking, social class, parity
Vessey 1998, a cohort study, used data from the Oxford‐Family Planning Association study in the UK. Women were OC users or nonusers. Few associations were apparent (Table 7). The relative risks (RRs) were adjusted for age. Increased fracture risk was noted for those who used OCs for longer periods, but the only notable increase was for those with use of 97 months or longer. For fractures of the radius and all fractures, the reported RRs were 1.5 (95% CI 1.1 to 2.1) and 1.2 (95% CI 1.1 to 1.4), respectively. When the interval since OC use was examined, increased risks for two groups were noted. For recent users (interval of 12 months or less), the reported RR for all fractures was 1.3 (95% CI 1.1 to 1.5). For an interval of 73 to 96 months, the RR for radius fracture was 2.5 (95% CI 1.5 to 4.0).
5. Vessey 1998: first fracture and oral contraceptive use.
| Relative risk (95% CI)a by fracture site | |||||
| Radius or ulna (lower end) | Ankle | Tarsals or metatarsals | All fractures | ||
| Duration of OC use (months)b | Nonuser | 1.0 | 1.0 | 1.0 | 1.0 |
| < 12 | 1.1 (0.3 to 2.8) | 0.7 (0.1 to 2.1) | 0.4 (0.0 to 1.5) | 0.8 (0.5 to 1.2) | |
| 13 to 24 | 1.8 (0.8 to 3.8) | 1.6 (0.7 to 3.2) | 0.9 (0.3 to 2.2) | 0.9 (0.6 to 1.3) | |
| 25 to 48 | 1.3 (0.7 to 2.2) | 0.9 (0.4 to 1.6) | 1.2 (0.7 to 2.0) | 1.2 (1.0 to 1.5) | |
| 49 to 72 | 1.1 (0.6 to 2.0) | 0.7 (0.3 to 1.3) | 1.2 (0.7 to 2.0) | 1.2 (0.9 to 1.4) | |
| 73 to 96 | 1.1 (0.6 to 2.1) | 1.3 (0.7 to 2.3) | 1.2 (0.6 to 2.0) | 1.2 (1.0 to 1.5) | |
| >= 97 | 1.5 (1.1 to 2.1) | 1.0 (0.7 to 1.5) | 0.8 (0.5 to 1.2) | 1.2 (1.1 to 1.4) | |
| Interval since OC use (months)b | Nonuser | 1.0 | 1.0 | 1.0 | 1.0 |
| Current user < 12 | 1.2 (0.7 to 1.8) | 1.2 (0.8 to 1.8) | 1.1 (0.7 to 1.6) | 1.3 (1.1 to 1.5) | |
| 13 to 24 | 1.2 (0.4 to 2.8) | 1.0 (0.4 to 2.3) | 1.1 (0.4 to 2.3) | 1.2 (0.9 to 1.6) | |
| 25 to 48 | 1.0 (0.4 to 1.9) | 1.0 (0.5 to 1.9) | 1.2 (0.6 to 2.2) | 1.0 (0.8 to 1.3) | |
| 49 to 72 | 1.7 (0.9 to 3.0) | 0.7 (0.3 to 1.5) | 0.8 (0.3 to 1.6) | 1.1 (0.8 to 1.4) | |
| 73 to 96 | 2.5 (1.5 to 4.0) | 0.9 (0.4 to 1.8) | 0.8 (0.4 to 1.8) | 1.2 (0.9 to 1.5) | |
| 97 to 120 | 1.6 (0.8 to 2.8) | 1.1 (0.5 to 2.1) | 0.8 (0.3 to 1.8) | 1.3 (1.0 to 1.6) | |
| > 121 | 1.1 (0.7 to 1.8) | 0.9 (0.5 to 1.4) | 0.7 (0.3 to 1.2) | 1.0 (0.8 to 1.2) | |
aAdjusted for age bSample sizes per cell not provided; recruited 17,032 women. OC ever‐use: 187,000 woman‐years; nonuse by age 45: 123,000 woman‐years
The cohort study of Barad 2005 used data from the observational study of the Women's Health Initiative in the USA. The women were postmenopausal. Hazard ratios were adjusted for a number of important potential confounders. The investigators examined any OC use, years of OC use, and years of OC use after excluding women with a prior fracture. Two associations were noted between OC use and fracture (Table 8). Small increased risks were found for any OC use (HR 1.07; 95% CI 1.01 to 1.15) and for OC use up to five years (HR 1.09; 95% CI 1.01 to 1.18). The latter was not evident after excluding women with prior fracture.
6. Barad 2005: first fracture and oral contraceptive use.
| Oral contraceptive usea | Hazard ratio (95% CI) | |
| Any OC useb | None | 1.00 |
| Any | 1.07 (1.01 to 1.15) | |
| Years of OC usec | None | 1.00 |
| < 5 years | 1.09 (1.01 to 1.18) | |
| 5 to 10 years | 1.07 (0.96 to 1.20) | |
| > 10 years | 1.02 (0.91 to 1.15) | |
| Excluding women with prior fractured | No OC use | 1.00 |
| < 5 years OC use | 1.08 (0.99 to 1.18) | |
| > 5 years OC use | 1.05 (0.96 to 1.16) | |
aSample sizes overall: OC users 33,025; OC nonusers 47,922 bAdjusted for baseline age (1‐year intervals), hormone therapy use and duration, follow‐up time, calcium intake (mg); use of corticosteroids, vitamin D, thiazide, thyroid hormone; age, race or ethnicity, smoking, alcohol use, exercise, body mass index, parity, irregular menses before menopause, hysterectomy, age at menopause, menopausal symptoms, prior fracture before age 55, length of OC use, age of last OC use, and age of first OC use. cModel adjusted as above, with duration of OC use in 5‐year intervals (excluding adjustment for duration of OC use as covariate) dBase model used excluding participants with prior fracture
The case‐control study of Meier 2010 examined use of combined oral contraceptives (COCs). The investigators used the UK‐based General Practice Research Database as did Lanza 2013. Cases were 20 to 44 years old and had a first‐time fracture diagnosis between 1995 and 2008. Controls were randomly selected from the base population and matched on several variables including age. The only subgroup with a significantly higher fracture risk than nonusers was current users with 10 or more prescriptions (reported OR 1.09, 95% CI 1.03 to 1.16) (Table 9).
7. Meier 2010: first fracture and use of combined oral contraceptives.
| Prescriptions (N) | Cases (N) | Controls (N) | OR (95% CI)a | Adjusted OR (95% CI)b | |
| Nonuse | ‐ | 6591 | 26,578 | reference | reference |
| Current use |
1 to 2 | 215 | 871 | 0.99 (0.85 to 1.16) | 1.01 (0.87 to 1.18) |
| 3 to 9 | 1136 | 4696 | 0.98 (0.91 to 1.05) | 1.01 (0.94 to 1.09) | |
| > 10 | 2327 | 9073 | 1.04 (0.98 to 1.10) | 1.09 (1.03 to 1.16) | |
| Past use |
1 to 2 | 1972 | 7820 | 1.02 (0.96 to 1.08) | 1.00 (0.95 to 1.07) |
| 3 to 9 | 3178 | 12,787 | 1.01 (0.96 to 1.06) | 0.99 (0.94 to 1.04) | |
| > 10 | 2108 | 8305 | 1.03 (0.97 to 1.10) | 1.03 (0.97 to 1.10) |
aCases and controls matched on age, general practice, calendar time, and history in database. bAdjusted for body mass index, smoking, asthma, epilepsy; use of progestin‐only preparations, medroxyprogesterone acetate low dose, β‐blockers, proton pump inhibitors, systemic corticosteroids, benzodiazepines, serotonin reuptake inhibitors, anticonvulsants, and contraceptive not under investigation.
Any fracture
For the case‐control study of Vestergaard 2006, cases were women with any fracture sustained in the year 2000. Fracture data were from the National Hospital Discharge Register of Denmark. Controls were from the general population database. Exposure was calculated as the average daily dose, i.e., the sum of redeemed prescriptions divided by time interval from first prescription to date of fracture or censoring; further details are in Characteristics of included studies. The 2006 paper compared OC use versus nonuse for all women. The crude ORs had indicated some association between OC use and fracture, but the reported adjusted ORs showed no association (Table 10). The investigators also examined the type of fracture; no association with OC use was apparent (data not shown here). A 2008 paper examined combined OC (COC) use rather than all OC use, and focused on very young women. Few significant differences were noted (Table 11):
8. Vestergaard 2006: fracture and oral contraceptive use.
| Age group (years) | Daily OC dosea | Cases (N) | Controls (N) | ORb (95% CI) |
| < 25 | ‐ | 16,219 | 48,659 | ‐‐‐ |
| < 0.3 | 331 | 795 | 0.97 (0.91 to 1.03) | |
| 0.3 to 0.99 | 1445 | 3872 | 0.96 (0.92 to 1.01) | |
| > 1 | 1156 | 3546 | 0.92 (0.86 to 0.98) | |
| 25 to 49 | ‐ | 10,545 | 31,631 | ‐ |
| < 0.3 | 1895 | 5491 | 0.91 (0.82 to 1.00) | |
| 0.3 to 0.99 | 2444 | 7445 | 0.90 (0.77 to 1.05) | |
| > 1 | 783 | 2546 | 0.87 (0.64 to 1.18) | |
| >50 | ‐ | 37,784 | 113,351 | ‐ |
| < 0.3 | 799 | 2820 | 0.92 (0.77 to 1.10) | |
| 0.3 to 0.99 | 253 | 977 | 0.69 (0.45 to 1.05) | |
| > 1 | 6 | 266 | 0.62 (0.27 to 1.41) | |
aExposure as average daily dose, i.e., redeemed prescriptions/time interval from first prescription to fracture or censoring (Characteristics of included studies); < 1 indicates < regular use; > 1 suggests lost prescription and obtained new one. bAdjusted for Charlson index (19 comorbid conditions), ever use of corticosteroids, alcoholism, working or not, number of bed days in 1999, contacts with physician in 1999, income, living with someone or living alone, prior fracture, education level, pregnancy; use of anti‐epileptic drugs, thyroid active drugs (levothyroxine or antithyroid drugs), or hormone replacement therapy.
9. Vestergaard 2006 (2008a): fracture and combined OC use in very young women.
| Age group (years) | Daily COC dosea | Cases (N) | Controls (N) | Adjusted OR (95% CI)b |
|
< 15 |
Never users | 12,192 | 36,652 | reference |
| < 0.3 | 19 | 71 | 0.58 (0.22 to 1.55) | |
| 0.3 to 0.99 | 63 | 146 | 1.50 (1.03 to 2.18) | |
| > 1 | 121 | 310 | 1.02 (0.77 to 1.35) | |
|
15.1 to 17 |
Never users | 495 | 1731 | reference |
| < 0.3 | 36 | 86 | 1.04 (0.68 to 1.60) | |
| 0.3 to 0.99 | 196 | 434 | 1.30 (1.05 to 1.61) | |
| > 1 | 254 | 704 | 1.05 (0.87 to 1.27) | |
|
17.1 to 19 |
Never users | 271 | 856 | reference |
| < 0.3 | 72 | 145 | 1.32 (0.94 to 1.85) | |
| 0.3 to 0.99 | 294 | 793 | 0.99 (0.80 to 1.22) | |
| > 1 | 265 | 908 | 0.83 (0.67 to 1.03) | |
|
> 19 |
Never users | 42,433 | 126,644 | reference |
| < 0.3 | 2888 | 8804 | 1.03 (0.98 to 1.08) | |
| 0.3 to 0.99 | 3589 | 10,921 | 1.04 (0.99 to 1.08) | |
| > 1 | 1360 | 4436 | 1.06 (0.99 to 1.13) | |
| Age group (years) | Ethinyl estradiol (EE) dose | Cases (N) | Controls (N) | Adjusted OR (95% CI)a |
|
< 15 |
20 µg | 100 | 264 | 1.23 (0.92 to 1.64) |
| > 30 µg | 82 | 225 | 0.97 (0.69 to 1.37) | |
| Changed between 20 µg and 30 µg | 32 | 59 | 1.34 (0.75 to 2.37) | |
| Other OCs | 13 | 45 | 0.20 (0.04 to 1.12) | |
|
15.1 to 17 |
20 µg | 205 | 550 | 1.07 (0.88 to 1.31) |
| > 30 µg | 181 | 478 | 1.08 (0.87 to 1.33) | |
| Changed between 20 µg and 30 µg | 113 | 236 | 1.42 (1.09 to 1.84) | |
| Other OCs | 11 | 35 | 0.95 (0.40 to 2.30) | |
|
17.1 to 19 |
20 µg | 209 | 645 | 0.96 (0.76 to 1.21) |
| > 30 µg | 286 | 787 | 0.98 (0.79 to 1.21) | |
| Changed between 20 µg and 30 µg | 157 | 451 | 0.95 (0.74 to 1.23) | |
| Other OCs | 13 | 55 | 0.78 (0.35 to 1.72) | |
|
> 19 |
20 µg | 785 | 2643 | 0.97 (0.89 to 1.06) |
| > 30 µg | 4718 | 14,498 | 1.03 (0.99 to 1.07) | |
| Changed between 20 µg and 30 µg | 1173 | 3195 | 1.13 (1.05 to 1.22) | |
| Other OCs | 1349 | 4396 | 1.03 (0.97 to 1.11) | |
| Analysis of young womenc | ||||
| Age group (years) | Daily COC dosea | Cases (N) | Controls (N) | ORb (95% CI) |
| < 19 years | 0.3 to 0.99 | 393 | 943 | 1.17 (1.01 to 1.37) |
| 19 to 25 | < 0.3 | 297 | 731 | 1.22 (1.02 to 1.47) |
| 0.3 to 0.99 | 1281 | 3573 | 1.14 (1.00 to 1.30) | |
| > 35 | > 1 | 367 | 1424 | 0.88 (0.78 to 0.99) |
aExposure as average daily dose, i.e., redeemed prescriptions/time interval from first prescription to fracture or censoring (Characteristics of included studies); < 1 indicates < regular use; > 1 suggests lost prescription and obtained new one bAdjusted for Charlson index (19 comorbid conditions), ever‐use of corticosteroids, alcoholism, working or not, bed days in 1999, contacts with physician in 1999, income, living with someone or living alone, prior fracture, education level, pregnancy; use of anti‐epileptic drugs, estrogen therapy, or estrogen–progestin therapy. cResults presented for reported significant differences.
For very young women: Reported adjusted ORs indicated increased risk in two age groups when the average dose was 0.3 to 0.99 per day. For those up to 15 years of age, the reported OR was 1.50 (95% CI 1.03 to 2.18). For those 15.1 to 17 years old, the reported OR was 1.30 (95% CI 1.05 to 1.61).
By dose of ethinyl estradiol: Risk was increased within the 15.1 to 17 year‐olds and those older than 19 years if they had changed between 20 µg and > 30 µg of ethinyl estradiol (EE). The reported adjusted ORs were 1.42 (95% CI 1.09 to 1.84) and 1.13 (95% CI 1.05 to 1.22), respectively.
By type of progestin for very young women: No association with fracture was apparent (data not shown here).
Young women: The report focused on 'very young women' but also analyzed 'young women.' Results indicated a difference in risk. Increased risk was noted for women less than 19 years old with an average dose of 0.3 to 0.99 (reported OR 1.17, 95% CI 1.01 to 1.37) and for women aged 19 to 25 years with an average dose less than 0.3 (reported OR 1.22, 95% CI 1.02 to 1.47) or dose of 0.3 to 0.99 (reported OR 1.14, 95% CI 1.00 to 1.30). Lower risk was noted for women older than 35 with an average dose of one or greater (reported OR 0.88, 95% CI 0.78 to 0.99). The investigators also analyzed forearm fractures within these age groups but showed no association with OC use.
From baseline data of cohort studies, two reports examined history of OC use and fracture history (Tuppurainen 1994; Wei 2011). Neither adjusted fracture risk for potential confounding factors. Tuppurainen 1994 analyzed fractures since age 15 (Analysis 1.1) and fractures sustained from 1980 to 1989 (Analysis 1.2). Analysis included OC use versus no use, as well as subgroups of use less than one year, one to five years, and more than six years versus no use. No relationship was shown between OC use and fractures. Wei 2011 analyzed history of any fracture. The investigators used baseline data from a cohort study in Tasmania. Unadjusted fracture rates did not differ significantly between OC users and nonusers (Analysis 1.3).
1.1. Analysis.
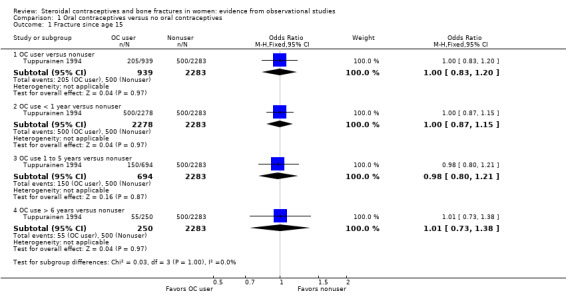
Comparison 1 Oral contraceptives versus no oral contraceptives, Outcome 1 Fracture since age 15.
1.2. Analysis.
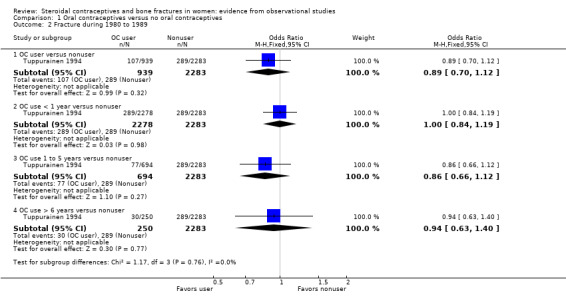
Comparison 1 Oral contraceptives versus no oral contraceptives, Outcome 2 Fracture during 1980 to 1989.
1.3. Analysis.
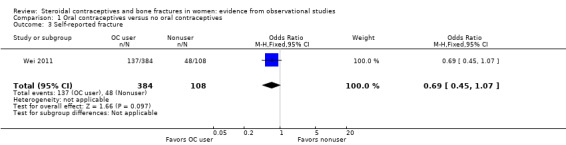
Comparison 1 Oral contraceptives versus no oral contraceptives, Outcome 3 Self‐reported fracture.
Specific fracture sites
Two case‐control studies examined hip fractures (La Vecchia 1999; Michaelsson 1999). The data for La Vecchia 1999 came from a case‐control study of hip fracture (versus no fracture) in Italy. Women were 25 to 74 years old. No association was noted between OC use ever and hip fracture nor for OC use for two years or more (Table 12). The brief report, in a letter to a journal editor, did not state the variables for which the ORs were adjusted. Michaelsson 1999 studied hip fractures among postmenopausal women in Sweden. Controls were obtained from the national population registry. The report provided age‐adjusted ORs as well as ORs adjusted for age and other potential confounders (Table 13). Decreased risk for fracture was noted for ever‐use of OCs (reported OR 0.75, 95% CI 0.59 to 0.96) and for ever‐use of high‐dose OCs (reported OR 0.56, 95% CI 0.42 to 0.75). Decreased risk was also noted for those who used OCs at age 40 or later (reported OR 0.69, 95% CI 0.51 to 0.94) and for those who used high‐dose OCs at age 40 or later (reported OR 0.61, 95% CI 0.42 to 0.89).
10. La Vecchia 1999: hip fracture and oral contraceptive use.
| ORa (95% CI) | ||
| OC useb | Ever | 0.98 (0.47 to 2.03) |
| > 2 years | 1.04 (0.42 to 2.55) | |
aOdds ratio for 'ever' identified as multivariate OR; variables not provided. For use > 2 years, did not specify whether OR adjusted or not. bSample sizes: cases 279; controls 1861. OC use ever: cases 10; controls 167
11. Michaelsson 1999: hip fracture and oral contraceptive use.
| Number | Age‐adjusted OR (95% CI) | Multivariate ORa (95% CI) | |||
| Cases | Controls | ||||
| OC use | Never | 994 | 2373 | 1.00 | 1.00 |
| Ever (any type) | 130 | 562 | 0.75 (0.60 to 0.95) | 0.75 (0.59 to 0.96) | |
| High dose ever | 77 | 456 | 0.54 (0.40 to 0.72) | 0.56 (0.42 to 0.75) | |
| Duration of OC use (per 2 years) | 116 | 526 | 1.06 (0.92 to 1.22) | 1.03 (0.89 to 1.19) | |
| Time since last OC use (per 2 years) | 117 | 529 | 1.01 (0.95 to 1.08) | 1.02 (0.96 to 1.09) | |
| Time since last OC use and menopause (per 2 years) | 117 | 529 | 0.95 (0.84 to 1.08) | 0.97 (0.85 to 1.11) | |
| Age at use of any OC | Never used | 994 | 2373 | 1.00 | 1.00 |
| < 30 years | 34 | 193 | 1.11 (0.68 to 1.82) | 1.26 (0.76 to 2.09) | |
| 30 to 39 years | 60 | 294 | 0.80 (0.57 to 1.12) | 0.82 (0.57 to 1.16) | |
| > 40 years | 64 | 271 | 0.72 (0.54 to 0.98) | 0.69 (0.51 to 0.94) | |
| Age at use of high‐dose OCb | Never used | 994 | 2373 | 1.00 | 1.00 |
| < 30 years | 27 | 183 | 0.97 (0.56 to 1.68) | 1.12 (0.64 to 1.97) | |
| 30 to 39 years | 46 | 264 | 0.74 (0.50 to 1.08) | 0.76 (0.51 to 1.13) | |
| > 40 years | 40 | 215 | 0.62 (0.43 to 0.90) | 0.61 (0.42 to 0.89) | |
1Adjusted for age (5‐year intervals), hormone replacement therapy, parity, body mass index (by quintiles). 2Containing > 50 µg ethinyl estradiol
Forearm fractures were examined in two case‐control studies (Mallmin 1994; O'Neill 1996). The Swedish study of Mallmin 1994 included women 40 to 80 years of age with fracture of distal forearm between April 1989 and March 1990. Controls were from the population registry. The investigators did not adjust fracture risk for potential confounding factors. They found no association between OC use and forearm fracture (Analysis 1.4). O'Neill 1996 was conducted in England. Cases were 45 years of age or older and had sustained a fracture of distal forearm between October 1991 and March 1993. Fracture cases were less likely to have used OCs than population controls (reported OR 0.3, 95% CI 0.1 to 0.9) (Table 14).
1.4. Analysis.
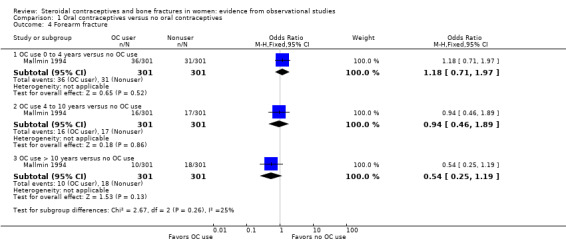
Comparison 1 Oral contraceptives versus no oral contraceptives, Outcome 4 Forearm fracture.
12. O'Neill 1996: forearm fracture and oral contraceptive use.
| Control group | ||
| Population OR (95% CI)a | Had fall OR (95% CI)a | |
| OC useb | 0.3 (0.1 to 0.9) | 0.7 (0.2 to 2.4) |
aAge adjusted bSample sizes: cases 62; fall control 50; population control 116
Progestin‐only contraceptives
Depot medroxyprogesterone acetate
In the cohort study of Kaunitz 2006, women were 25 to 35 years old at recruitment. New users of DMPA were compared with users of non‐hormonal contraceptive methods. The primary endpoint was change in bone mineral density. Fracture was recorded as an adverse event, and shown for the treatment phase and the post‐treatment follow‐up. Fracture risk was not adjusted for potential confounding factors; the study groups did not differ significantly for fracture risk (Analysis 2.1).
2.1. Analysis.
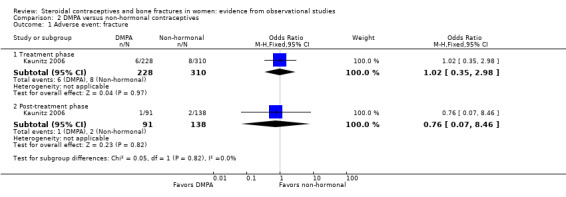
Comparison 2 DMPA versus non‐hormonal contraceptives, Outcome 1 Adverse event: fracture.
The case‐control study of Vestergaard 2006 also analyzed DMPA use versus nonuse in a 2008 paper. As noted earlier, cases were women with any fracture sustained in the year 2000. Exposure was calculated as the average daily dose, i.e., the sum of redeemed prescriptions divided by time interval from first prescription to date of fracture or censoring; further details are in Characteristics of included studies. ORs were adjusted (Table 15). DMPA use was associated with an increased risk of fracture compared with nonuse. The reported OR for ever‐using DMPA was 1.44 (95% CI 1.01 to 2.06). Increased risk was more apparent among women over 50 years of age (reported adjusted OR 2.25, 95% CI 1.14 to 4.42), those with regular use (reported OR 1.94, 95% CI 1.09 to 3.45), and those who used DMPA for more than four years (reported OR 2.16, 95% CI 1.32 to 3.53).
13. Vestergaard 2006 (2008b): fracture and DMPA use.
| Subgroup | Cases (N) | Controls (N) | ORa (95% CI) | |
| DMPAb use ever | ‐ | 58 | 105 | 1.44 (1.01 to 2.06) |
| Age of woman (years) |
< 25 | 15 | 29 | 1.20 (0.59 to 2.45) |
| 25 to 50 | 25 | 51 | 1.09 (0.64 to 1.85) | |
| > 50 | 18 | 25 | 2.25 (1.14 to 4.42) | |
| Average daily DMPA dosec |
< 0.25 | 21 | 32 | 1.73 (0.96 to 3.09) |
| 0.26 to 0.99 | 13 | 38 | 0.88 (0.45 to 1.74) | |
| > 1 | 24 | 35 | 1.94 (1.09 to 3.45) | |
| Duration of DMPA use (years) |
< 2.5 | 16 | 45 | 0.82 (0.43 to 1.56) |
| 2.6 to 4 | 9 | 20 | 1.51 (0.66 to 3.46) | |
| > 4 | 33 | 40 | 2.16 (1.32 to 3.53) | |
aAdjusted for prior fracture, Charlson index (comorbidities), income, working status, living with someone or not, pregnancy, IUD use, hysterectomy, alcoholism; use of OC, corticosteroid, hormonal replacement therapy, anti‐epileptic drugs, and strong (morphine and opioid agonists) and weak analgesics (acetaminophen, nonsteroidal anti‐inflammatory drugs, and acetylsalicylic acid). bDMPA = depot medroxyprogesterone acetate cExposure as average daily dose, i.e., redeemed prescriptions/time from first prescription to fracture or censoring (Characteristics of included studies); < 1 indicates < regular use; > 1 suggests lost prescription and obtained new one.
As noted above, the case‐control study of Meier 2010 used the UK‐based General Practice Research Database, as did Lanza 2013. Cases were 20 to 44 years old and had a first‐time fracture diagnosis between 1995 and 2008. Controls were randomly selected from the base population and matched on several variables including age. Current and past users of DMPA were generally more likely to have had a fracture than nonusers (Table 16). The odds increased slightly with the number of prescriptions. For current users with three to nine prescriptions, the reported adjusted OR was 1.36 (95% CI 1.15 to 1.60). For those with 10 or more prescriptions, the adjusted OR was reported as 1.54 (95% CI 1.33 to 1.78). Past DMPA use was also associated with increased risk regardless of the number of prescriptions (Table 16). The reported adjusted ORs were as follows: for one to two prescriptions, 1.17 (95% CI 1.07 to 1.29); for three to nine prescriptions, 1.23 (95% CI 1.11 to 1.36), and for 10 or more prescriptions, 1.30 (95% CI 1.09 to 1.55).
14. Meier 2010: first fracture and DMPA use.
| Prescriptions (N) | Cases (N) | Controls (N) | OR (95% CI)a | Adjusted OR (95% CI)b | |
| Nonuse | ‐ | 15,614 | 64,415 | reference | reference |
| Current use |
1 to 2 | 93 | 305 | 1.27 (1.01 to 1.61) | 1.18 (0.93 to 1.49) |
| 3 to 9 | 209 | 573 | 1.52 (1.30 to 1.79) | 1.36 (1.15 to 1.60) | |
| > 10 | 280 | 710 | 1.67 (1.45 to 1.92) | 1.54 (1.33 to 1.78) | |
| Past use |
1 to 2 | 620 | 1985 | 1.31 (1.19 to 1.44) | 1.17 (1.07 to 1.29) |
| 3 to 9 | 529 | 1609 | 1.38 (1.25 to 1.53) | 1.23 (1.11 to 1.36) | |
| > 10 | 182 | 533 | 1.45 (1.22 to 1.72) | 1.30 (1.09 to 1.55) |
aCases and controls matched on age, general practice, calendar time, and history in database bAdjusted for body mass index, smoking, asthma, epilepsy; use of progestin‐only preparations, MPA low dose, β‐blockers, proton pump inhibitors, systemic corticosteroids, benzodiazepines, serotonin reuptake inhibitors, anticonvulsants, and contraceptive not under investigation.
Lanza 2013 was a cohort study of DMPA users versus users of other hormonal contraceptives (mostly OCs). The investigators analyzed data from the UK‐based General Practice Research Database, as Meier 2010 did. Incident fractures were those assessed after the first DMPA injection or first OC prescription. DMPA users had an increased fracture risk compared with users of other hormonal contraceptives. The rate ratio for incident fractures was (RR 1.41, 95% CI 1.35 to 1.47) (Analysis 3.1). Compared with nonusers of DMPA, the increased risk was greater for those with one to seven DMPA injections (RR 1.47, 95% CI 1.40 to 1.54) than for those who had eight or more injections (RR 1.22, 95% CI 1.13 to 1.32) (Analysis 3.2).When fracture site was analyzed, the two groups did not differ significantly for axial fractures (Analysis 3.3). Compared with nonusers of DMPA, users had greater risk for fracture of the appendicular skeleton, i.e., arm, leg, wrist, ankle, hand, foot, clavicle, rib or sternum, and shoulder (RR 1.38, 95% CI 1.31 to 1.46). The risk for all other fractures (finger, toe, skull, face, multiple trauma, and unspecified) was also greater for DMPA users versus nonusers (RR 1.49, 95% CI 1.39 to 1.59) (Analysis 3.3).
3.2. Analysis.
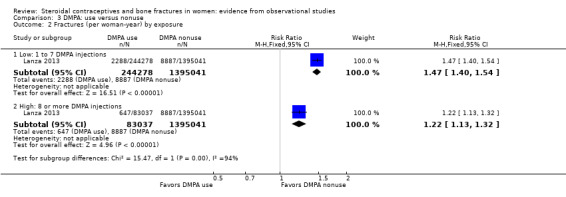
Comparison 3 DMPA: use versus nonuse, Outcome 2 Fractures (per woman‐year) by exposure.
3.3. Analysis.

Comparison 3 DMPA: use versus nonuse, Outcome 3 Fractures (per woman‐years) by site.
Levonorgestrel‐releasing intrauterine device
In the 2008 paper on DMPA use, Vestergaard 2006 also examined use of the hormonal IUD versus nonuse. Cases were women with any fracture sustained in the year 2000. All ORs were adjusted (Table 17). Hormonal IUD use was associated with reduced odds of fracture (reported OR 0.75, 95% CI 0.64 to 0.87). Fracture was also less likely for those who used the hormonal IUD for 1.6 to 4 years (reported OR 0.77, 95% CI 0.59 to 0.99).
15. Vestergaard 2006 (2008b): fracture and hormonal IUD use.
| Subgroup | Cases (N) | Controls (N) | OR (95% CI)a | |
| Hormonal IUDb use ever | ‐ | 219 | 791 | 0.75 (0.64 to 0.87) |
| Age of woman (years) |
< 25 | 3 | 5 | 0.82 (0.19 to 3.67) |
| 25 to 50 | 187 | 639 | 0.87 (0.73 to 1.04) | |
| > 50 | 29 | 147 | 0.72 (0.48 to 1.09) | |
| IUDs per year |
< 0.25 | 59 | 234 | 0.74 (0.55 to 1.00) |
| 0.26 to 0.6 | 84 | 280 | 0.84 (0.65 to 1.08) | |
| > 0.6 | 76 | 277 | 0.81 (0.62 to 1.05) | |
| Duration of hormonal IUD use (years) |
< 1.5 | 68 | 244 | 0.78 (0.59 to 1.03) |
| 1.6 to 4 | 83 | 289 | 0.77 (0.59 to 0.99) | |
| > 4 | 68 | 258 | 0.77 (0.58 to 1.01) | |
aAdjusted for prior fracture, Charlson index (comorbidities), income, working status, living with someone or not, pregnancy, DMPA use, hysterectomy, alcoholism; use of OC, corticosteroid, hormonal replacement therapy, anti‐epileptic drugs, and strong (morphine and opioid agonists) and weak analgesics (acetaminophen, nonsteroidal anti‐inflammatory drugs, and acetylsalicylic acid) bIUD = intrauterine device
Discussion
Summary of main results
Of 14 studies, six provided at least moderate quality evidence, according to our assessment with the Newcastle‐Ottawa Scale (Table 3; Table 4). The other eight studies provided lower quality evidence, in some cases due to reporting less information or to having a different focus. This section focuses on the sensitivity analysis from the six studies providing moderate or high quality evidence. Four of the six studies used data from the UK, one study was from the USA and one was from Denmark. Results of the lower quality studies are summarized separately below.
All six studies in our sensitivity analysis examined oral contraceptive use (Table 1); two also examined use of progestin‐only contraceptives (Table 2). Few associations were noted between oral contraceptive use and fracture risk. In Cooper 1993, OC ever‐users had some increased risk for all fractures. However, Memon 2011 used a subset of women from Cooper 1993 several years later, and found no association of OC use with fracture except for those with 10 years or more since use. Barad 2005 studied postmenopausal women and did not find increased risk for OC use after excluding women with prior fracture. Meier 2010 reported increased risk only for those who had 10 or more prescriptions. Vessey 1998 and Vestergaard 2006 found little evidence of association between OC use and fracture risk. Some increased risk was noted for subgroups, such as those with longer use or specific intervals since use (Vessey 1998) and young women with less than average use (Vestergaard 2006).
Two studies in the sensitivity analysis examined use of progestin‐only contraceptives (Vestergaard 2006; Meier 2010). Both studies reported increased fracture risk for longer current use of DMPA. In addition, one noted increased risk with ever using DMPA and the other noted increased risk for any past use. Vestergaard 2006 also examined use of the hormonal IUD and found reduced fracture risk for ever using the hormonal IUD and for longer use of that IUD.
Eight studies were not in the sensitivity analysis. Two studies of OC use indicated some association. O'Neill 1996 noted that forearm fracture cases were less likely to be OC users than population controls. Michaelsson 1999 found decreased hip fracture risk among postmenopausal women for ever‐use of OCs overall or high‐dose OCs. In contrast, La Vecchia 1999 showed no association of hip fracture with OC use. Mallmin 1994 indicated no association of OC use with forearm fracture, and Tuppurainen 1994 and Wei 2011 did not show any association between OC use and fracture risk. Two studies excluded from the sensitivity analysis examined DMPA use. Lanza 2013 noted increased risk for DMPA users versus users of other hormonal methods. The groups did not differ significantly for axial fractures (vertebrae, hip, and pelvis), but did differ in risk for fractures of the appendicular skeleton and for all other fractures. In Kaunitz 2006, fracture was provided as an adverse event. The incidence of fracture was not significantly different for DMPA users versus users of non‐hormonal methods.
Overall completeness and applicability of evidence
Data sources for the sensitivity analysis included country‐wide hospital discharges in Denmark and general practice in the UK. The others were a study of postmenopausal women in the USA and two long‐term studies of family planning methods in the UK. None of the studies in this review came from a less‐developed country.
Most studies did not report on the types of oral contraceptives used. However, Vestergaard 2006 examined risk by estrogen dose and progestin type. The investigators found no association with high‐dose estrogen use but some increased risk for OC users who changed between 20 µg and > 30 µg estrogen. Progestin type was apparently not associated with any increased risk. Of the DMPA studies, one had a small sample for DMPA users. The two large studies of DMPA used the same database, although one used population controls.
The timeframe for the studies affects exposure to the contraceptive method and years of follow‐up for outcome assessment. Since fragility fractures are rare in young people, fracture is not usually an outcome in studies of premenopausal bone health (Gourlay 2004). The two DMPA studies used national databases. Meier 2010 selected premenopausal women, while Vestergaard 2006 examined all fractures in a specific year regardless of the woman's age; DMPA users were few. Hormonal IUD use came from the same source. Of the OC studies, Vessey 1998 recruited premenopausal women and followed them until age 45 or for 20 years. Memon 2011 gathered fracture data 40 years after study enrollment. Barad 2005 enrolled women after menopause, so contraceptive use was assessed retroactively.
Quality of the evidence
We used the Newcastle‐Ottawa Scale (NOS) items to assess the quality of evidence (Wells 2011). Less than half of the evidence was considered to be moderate or high quality (from three case‐control and three cohort studies). In some cases, downgrading the evidence was due to study design; in others, it was due to lack of information in the report.
Most studies did not mention an a priori power analysis. The exceptions were two excluded from the sensitivity analysis. Mallmin 1994 was focused on HRT and fracture; O'Neill 1996 was exploratory and did not specify outcomes of interest.
Most of the cohort studies had large losses or differential losses between the exposed and non‐exposed cohorts. Large losses are not usual for contraceptive studies and some of these were long‐term studies. For the case‐control studies, the response rate was generally adequate or was not an issue due to gathering information from existing databases. One study in the sensitivity analysis had a limited length of follow‐up (Barad 2005).
Potential biases in the review process
Adjusted analysis addresses potential differences between study groups in observational studies. This can reduce confounding of fracture rates. Five of the six studies in the sensitivity analysis controlled for age and examined other important variables such as exercise, body mass index, hormone replacement therapy, and use of other relevant drugs. However, studies that used existing databases did not have access to some important variables, such as body mass index and exercise. Because studies conducted adjusted analyses, we could not analyze most of the data in this review. We would have needed individual participant data to do so. Consequently, we showed the results as given by the investigators, except for studies that provided unadjusted fracture incidence.
Maximum exposure time in Vestergaard 2006 was five years. Memon 2011 examined fractures after the study ended; OC use was obtained during the study. Nonusers of OCs during the study may have started using OCs later, which would not have been captured in the database.
Our criteria for the sensitivity analysis were determined post hoc, which could have biased the results. We used the findings from the Newcastle‐Ottawa Scale to determine the quality of the evidence. Of the eight studies excluded from the sensitivity analysis, five were published in the 1990s. The older studies did not meet some criteria due to information missing from the report. Newer studies tend to have better reporting due to standards for observational studies and clinical trials (Strobe 2007; Schulz 2010).
Agreements and disagreements with other studies or reviews
A review of RCTs examined the effect of hormonal contraceptives on the risk of fracture in women (Lopez 2014). Outcomes included fracture, bone mineral density (BMD) and biochemical markers of bone turnover. No trial had fracture as an outcome. Depot medroxyprogesterone acetate (DMPA) was associated with decreased bone mineral density. The placebo‐controlled trials showed BMD increases for DMPA plus an estrogen supplement and decreases for DMPA plus placebo (Cundy 2003; Cromer 2005). Combination contraceptives did not appear to negatively affect bone health, but those studies were not placebo‐controlled.
Other reviews were mentioned earlier (Description of the condition). Two systematic reviews included studies of various designs and considered bone mineral density as well as fractures. After reviewing the evidence for combined hormonal contraceptives, Martins 2006 noted that bone mineral density was affected by COC use in adolescent and young women but not in premenopausal or postmenopausal women. In our current review of fracture risk, we had more recent reports but no additional studies of younger women. Information from the more recent studies of older women was consistent with the conclusions of Martins 2006. For progestin‐only methods, Curtis 2006 noted an association between DMPA use and loss of bone mineral density but noted the clinical significance was not clear. More recently, Isley 2011 concluded that adolescent users of DMPA do have decreases in BMD, but stated the loss can be recovered one or two years after discontinuation. We did not have data specifically on adolescents, who are unlikely to have fragility fractures. We noted increased fracture risk for DMPA ever‐users, but the difference may not be due to fragility fractures.
Authors' conclusions
Implications for practice.
Evidence from observational studies does not indicate an overall association between oral contraceptive use and fracture risk. Some studies found increased risk for specific user subgroups. For depot medroxyprogesterone acetate (DMPA), users may have an increased fracture risk. Hormonal IUD use could be associated with decreased fracture risk.
Implications for research.
The majority of included studies provided low quality evidence due to design and implementation issues or insufficient reporting. Several did not account for potential confounding. Observational studies need adjusted analysis since the comparison groups are likely to differ. When reporting on multivariate analysis, investigators should be clear about the variables examined.
What's new
| Date | Event | Description |
|---|---|---|
| 7 July 2015 | New citation required but conclusions have not changed | Search updated |
| 30 June 2015 | Amended | Added Summary of findings tables in lieu of previous 'Sensitivity analysis summary' |
| 26 June 2015 | New search has been performed | Search updated; no new studies found Added publication (Lanza 2013) for previously included conference presentation |
Acknowledgements
Carol Manion of FHI 360 helped develop the search strategy and ran the searches for several databases.
Appendices
Appendix 1. Search 2015
PubMed (1 January 2012 to 7 July 2015)
(contraceptive agents, female OR ((steroid OR steroids OR steroidal) AND contracept*) OR ortho evra OR "ortho evra" OR "norelgestromin" OR (contraceptive devices, female and ring) OR NuvaRing OR cyclofem OR lunell* OR mesigyna OR cycloprovera OR (medroxyprogesterone 17‐acetate AND (contracept* OR inject* OR depo OR depot)) OR depot medroxyprogesterone OR depo medroxyprogesterone OR depot medroxyprogesterone OR depomedroxyprogesterone OR dmpa OR "net en" OR norethisterone enanthate OR norplant OR uniplant OR jadelle OR implanon OR ((levonorgestrel OR etonogestrel) AND implant) OR (levonorgestrel AND intrauterine device*) OR mirena OR ((progestational hormones OR progestin) AND contracept* AND (oral OR pill* OR tablet*))) AND (bone density OR fracture* OR osteoporosis OR "bone mass" OR "bone mineral density" OR "bone density" OR "bone turnover" OR "bone mineral content" OR "bone loss" OR "bone resorption") NOT hormone replacement therapy
CENTRAL (23 June 2015)
Search all text: contracept* AND Search all text: fracture* Publication year from 2012 to 2015
POPLINE (23 June 2015)
All fields: fracture Keyword: Contraceptive Methods OR Keyword: Contraceptive Agents Female Years: from 2012 to 2015
Web of Science (26 June 2015)
TOPIC: (contracept* AND fracture NOT (hormone substitut* OR hormone replac* OR estrogen therapy)) Timespan: 2012‐2015
LILACS (26 June 2015)
Title, abstract, subject: contracept* AND Title, abstract, subject: fracture* Limits: Female Year: 2012 to 2015
ClinicalTrials.gov (30 June 2015)
Search terms: (fracture OR fractures) AND (contraceptive OR contraception) Study type: Interventional studies First received: 1 March 2012 to 30 June 2015
ICTRP (30 June 2015)
1) Search terms: contracept* AND fracture*
2) Condition: fracture or fractures Intervention: contraception OR contraceptive Recruitment status: All
Appendix 2. Search 2012
PubMed (17 May 2012)
(contraceptive agents, female OR ((steroid OR steroids OR steroidal) AND contracept*) OR ortho evra OR "ortho evra" OR "norelgestromin" OR (contraceptive devices, female and ring) OR NuvaRing OR cyclofem OR lunell* OR mesigyna OR cycloprovera OR (medroxyprogesterone 17‐acetate AND (contracept* OR inject* OR depo OR depot)) OR depot medroxyprogesterone OR depo medroxyprogesterone OR depot medroxyprogesterone OR depomedroxyprogesterone OR dmpa OR "net en" OR norethisterone enanthate OR norplant OR uniplant OR jadelle OR implanon OR ((levonorgestrel OR etonogestrel) AND implant) OR (levonorgestrel AND intrauterine devices) OR mirena OR ((progestational hormones OR progestin) AND contracept* AND (oral OR pill* OR tablet*))) AND (fracture*)
CENTRAL (17 May 2012)
(contraceptive agents, female OR ((steroid OR steroids OR steroidal) AND contracept*) OR ortho evra OR "ortho evra" OR "norelgestromin" OR (contraceptive devices, female and ring) OR NuvaRing OR cyclofem OR lunell* OR mesigyna OR cycloprovera OR (medroxyprogesterone 17‐acetate AND (contracept* OR inject* OR depo OR depot)) OR depot medroxyprogesterone OR depo medroxyprogesterone OR depot medroxyprogesterone OR depomedroxyprogesterone OR dmpa OR "net en" OR norethisterone enanthate OR norplant OR uniplant OR jadelle OR implanon OR ((levonorgestrel OR etonogestrel) AND implant) OR (levonorgestrel AND intrauterine devices) OR mirena OR ((progestational hormones OR progestin) AND contracept* AND (oral OR pill* OR tablet*))) [search all text] AND fracture* [search all text]
POPLINE (17 May 2012)
(Contraceptive Agents Female/depo provera/dmpa/medroxyprogesterone/(steroid* & contracept*) /orthoevra/ortho evra /norelgestromin/(contraceptive devices, female and ring)/ NuvaRing /cyclofem /lunelle/ mesigyna/ cycloprovera/ (medroxyprogesterone 17‐acetate & (contracept* /inject*/depo/depot))/ depot medroxyprogesterone/ depo medroxyprogesterone/ depot medroxyprogesterone/depo medroxyprogesterone/dmpa/ net en/ norethisterone‐enantate/norplant/uniplant/jadelle/implanon/((levonorgestrel/ etonogestrel) & implant)/(levonorgestrel & intrauterine devices)/mirena /((progestational hormones/progestin) & contracept* & (oral/pill*/tablet*))) & (fracture*)
CINAHL (21 May 2012)
contracept* AND fracture
Web of Science (21 May 2012)
contracept* AND fracture NOT (hormone substitut* OR hormone replac* OR estrogen therapy) All terms were set to search the Topic field which includes title, abstract and keywords
EMBASE (08 February 2012)
s contraceptive agent s steroid? (w)contracept? s s1 or s2 s bone(w)fracture s fracture s s3 and s5 s hormone substitution s estrogen therapy s s7 or s8 s s6 not s9
LILACS (24 January 2012)
(contraceptive agents, female OR ((steroid OR steroids OR steroidal) AND contracept*) OR ortho evra OR "ortho evra" OR "norelgestromin" OR (contraceptive devices, female and ring) OR NuvaRing OR cyclofem OR lunell* OR mesigyna OR cycloprovera OR (medroxyprogesterone 17‐acetate AND (contracept* OR inject* OR depo OR depot)) OR depot medroxyprogesterone OR depo medroxyprogesterone OR depot medroxyprogesterone OR depomedroxyprogesterone OR dmpa OR "net en" OR norethisterone enanthate OR norplant OR uniplant OR jadelle OR implanon OR ((levonorgestrel OR etonogestrel) AND implant) OR (levonorgestrel AND intrauterine devices) OR mirena OR ((progestational hormones OR progestin) AND contracept* AND (oral OR pill* OR tablet*))) AND (fracture* or fractura or fracturas or fratura or fraturas or fractures, bone or Fracturas Óseas or Fraturas Ósseas)
ClinicalTrials.gov (21 May 2012)
fractures AND (contraceptive OR contraception)
ICTRP (21 May 2012)
contracept* AND fracture*
Appendix 3. Newcastle‐Ottawa Quality Assessment Scale for case control studies
Note: A study can be awarded a maximum of one star (✸) for each numbered item within the Selection and Exposure categories. A maximum of two stars can be given for Comparability.
Selection
1) Is the case definition adequate?
a) yes, with independent validation ✸ b) yes, eg record linkage or based on self reports c) no description
2) Representativeness of the cases
a) consecutive or obviously representative series of cases ✸ b) potential for selection biases or not stated
3) Selection of Controls
a) community controls ✸ b) hospital controls c) no description
4) Definition of Controls
a) no history of disease (endpoint) ✸ b) no description of source
Comparability
1) Comparability of cases and controls on the basis of the design or analysis
a) study controls for _______________ (Select the most important factor.) ✸ b) study controls for any additional factor ✸ (This criteria could be modified to indicate specific control for a second important factor.)
Exposure
1)Ascertainment of exposure
a) secure record (eg surgical records) ✸ b) structured interview where blind to case/control status ✸ c) interview not blinded to case/control status d) written self report or medical record only e) no description
2) Same method of ascertainment for cases and controls
a) yes ✸ b) no
3) Non‐Response rate
a) same rate for both groups ✸ b) non respondents described c) rate different and no designation
Appendix 4. Newcastle‐Ottawa Quality Assessment Scale for cohort studies
Note: A study can be awarded a maximum of one star (✸) for each numbered item within the Selection and Outcome categories. A maximum of two stars can be given for Comparability.
Selection
1) Representativeness of the exposed cohort
a) truly representative of the average _______________ (describe) in the community ✸ b) somewhat representative of the average ______________ in the community ✸ c) selected group of users eg nurses, volunteers d) no description of the derivation of the cohort
2) Selection of the non exposed cohort
a) drawn from the same community as the exposed cohort ✸ b) drawn from a different source c) no description of the derivation of the non exposed cohort
3) Ascertainment of exposure
a) secure record (eg surgical records) ✸ b) structured interview ✸ c) written self report d) no description
4) Demonstration that outcome of interest was not present at start of study
a) yes ✸ b) no
Comparability
1) Comparability of cohorts on the basis of the design or analysis
a) study controls for _____________ (select the most important factor) ✸ b) study controls for any additional factor ✸ (This criteria could be modified to indicate specific control for a second important factor.)
Outcome
1) Assessment of outcome
a) independent blind assessment ✸ b) record linkage ✸ c) self report d) no description
2) Was follow‐up long enough for outcomes to occur
a) yes (select an adequate follow up period for outcome of interest) ✸ b) no
3) Adequacy of follow up of cohorts
a) complete follow up ‐ all subjects accounted for ✸ b) subjects lost to follow up unlikely to introduce bias ‐ small number lost ‐ > ____ % (select an adequate %) follow up, or description provided of those lost) ✸ c) follow up rate < ____% (select an adequate %) and no description of those lost d) no statement
Data and analyses
Comparison 1. Oral contraceptives versus no oral contraceptives.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Fracture since age 15 | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 OC user versus nonuser | 1 | 3222 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.83, 1.20] |
| 1.2 OC use < 1 year versus nonuser | 1 | 4561 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.87, 1.15] |
| 1.3 OC use 1 to 5 years versus nonuser | 1 | 2977 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.80, 1.21] |
| 1.4 OC use > 6 years versus nonuser | 1 | 2533 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.73, 1.38] |
| 2 Fracture during 1980 to 1989 | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 OC user versus nonuser | 1 | 3222 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.70, 1.12] |
| 2.2 OC use < 1 year versus nonuser | 1 | 4561 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.84, 1.19] |
| 2.3 OC use 1 to 5 years versus nonuser | 1 | 2977 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.66, 1.12] |
| 2.4 OC use > 6 years versus nonuser | 1 | 2533 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.63, 1.40] |
| 3 Self‐reported fracture | 1 | 492 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.45, 1.07] |
| 4 Forearm fracture | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 OC use 0 to 4 years versus no OC use | 1 | 602 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.71, 1.97] |
| 4.2 OC use 4 to 10 years versus no OC use | 1 | 602 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.46, 1.89] |
| 4.3 OC use > 10 years versus no OC use | 1 | 602 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.25, 1.19] |
Comparison 2. DMPA versus non‐hormonal contraceptives.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adverse event: fracture | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Treatment phase | 1 | 538 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.35, 2.98] |
| 1.2 Post‐treatment phase | 1 | 229 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.07, 8.46] |
Comparison 3. DMPA: use versus nonuse.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Fractures (per woman‐years) | 1 | 1.722356E6 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [1.35, 1.47] |
| 2 Fractures (per woman‐year) by exposure | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Low: 1 to 7 DMPA injections | 1 | 1.639319E6 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [1.40, 1.54] |
| 2.2 High: 8 or more DMPA injections | 1 | 1.478078E6 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [1.13, 1.32] |
| 3 Fractures (per woman‐years) by site | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Axial | 1 | 1.722356E6 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.74, 1.23] |
| 3.2 Appendicular skeleton | 1 | 1.722356E6 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [1.31, 1.46] |
| 3.3 All other fractures | 1 | 1.722356E6 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [1.39, 1.59] |
3.1. Analysis.
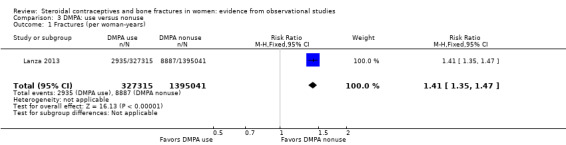
Comparison 3 DMPA: use versus nonuse, Outcome 1 Fractures (per woman‐years).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Barad 2005.
| Methods | Prospective observational cohort study; Women's Health Initiative (WHI) at 40 clinical centers in USA Enrollment in observational study (OS) between September 1994 and February 1997 |
|
| Participants | 80,947 women, aged 50 to 79 years, recruited to WHI, mostly through mass mailings to age‐eligible women. Women were directly recruited to OS or offered enrollment because ineligible for, or unwilling to participate in, clinical trial. Exclusions for OS: participation in a clinical trial; < 3 years predicted survival; alcohol or drug dependency; mental illness; dementia; or other inability to participate |
|
| Interventions | Oral contraceptive (OC) use; retrospective collection at baseline | |
| Outcomes | First fracture, self‐reported; prospective collection Insufficient data for analysis; results presented as reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Case definition and representativeness | Unclear risk | Not applicable |
| Exposed cohort: representativeness | Low risk | Exposed cohort: users of oral contraceptives (N = 33,025); short‐term (< 5 years) and long‐term (> 5 years) |
| Control selection and definition; non‐exposed cohort selection | Low risk | Non‐exposed cohort: nonusers of oral contraceptives (N = 47,922) |
| Exposure ascertainment, including same method for cases and controls | Low risk | Detailed interview at the baseline visit to assess past use of hormonal medications Past OC use measured by asking: Did you ever take birth control pills (oral contraceptives) for any reason? If yes, the timing and duration of OC use were determined by asking:
|
| Outcome assessment (cohort study): method and evidence outcome not present at study start | Low risk | First fracture incidence assessed annually after enrollment through 28 February 2000.
OS participants were asked: Since the date on the front of this form, has a doctor told you for the first time that you had new broken, fractured, or crushed bone? If yes: Which bones did you break, fracture or crush? (Mark all that apply) Hip, upper leg (not hip) pelvis, knee, lower leg or ankle, foot (not toe) tailbone (coccyx) spine or back (vertebra), lower arm or wrist, hand (not finger), elbow, upper area or shoulder or other Further questions documented hospitalization or diagnostic procedure associated with fracture and date of fracture. Hip fractures adjudicated; other fracture sites adjudicated for subset. |
| Comparability of groups on basis of design or analysis | Low risk | Exclusions from analysis: history of bone cancer N = 59 (7 fractures), biphosphonate use at baseline N = 2338 (162 fractures), missing information on key covariates N = 7953 (641 fractures) Cox proportional hazards model stratified for age at baseline (1‐year intervals), hormone therapy use (never, past, current) and duration (5‐year intervals) adjusted for race or ethnicity, smoking, and parity Retained covariates in model that may affect bone metabolism: calcium supplement, corticosteroid, thiazide diuretics, thyroid hormone, vitamin D supplementation, and alcohol use; reproductive factors such as irregular menses, hysterectomy, age at menopause, and history of menopausal symptoms. |
| Case‐control: non‐response rate | Unclear risk | Not applicable |
| Cohort: follow‐up length and adequacy (including loss to follow‐up) | Unclear risk | Mean follow‐up time 2.5 years No outcome data for 2.6% (2428/93,725) |
Cooper 1993.
| Methods | Prospective cohort study in UK; Royal College of General Practitioners (RCGP) Oral Contraception Study Study began May 1968; recruitment 14 months 1400 general practitioners (GP) recruited 23,000 OC users and "similar number" of never users; two groups were "matched for age." |
|
| Participants | All women had sustained first‐ever fracture by May 1990, and were married or living as married. Excluded: fractures of skull and ribs as well as multiple fractures |
|
| Interventions | Oral contraceptive use | |
| Outcomes | First‐ever fractures sustained during study Insufficient data for analysis; results presented as reported |
|
| Notes | Case‐control study of Memon 2011 (same research group) examined a subset at later date. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Case definition and representativeness | Unclear risk | Not applicable |
| Exposed cohort: representativeness | Low risk | Exposed cohort: women who ever used oral contraceptives (N = 853); if woman stopped OC use, subsequent observation was still included in 'ever user' group. |
| Control selection and definition; non‐exposed cohort selection | Low risk | Non‐exposed cohort: women who never used OCs (N = 512) If woman began to use OCs, pill experience was in 'ever user' group. |
| Exposure ascertainment, including same method for cases and controls | Low risk | Oral contraceptive prescriptions from physician reports on 6‐month basis |
| Outcome assessment (cohort study): method and evidence outcome not present at study start | Low risk | First‐ever fractures sustained during study from physician reports on 6‐month basis; confirmed by X‐ray Incidence rates for all fractures and forearm fractures Each event categorized by woman's contraceptive status at time of event. Incidence rates for all fractures and forearm fractures calculated per person‐years of observation. Separate analysis of fracture for women > 50 years, except if all OC use occurred before age 35 |
| Comparability of groups on basis of design or analysis | Low risk | Fracture incidence rates "indirectly standardized" for OC status, age, parity at time of event, and smoking and social class at recruitment. Standardization by applying stratum‐specific rates from total cohort to person‐years of observation in relevant strata of fracture group Expected numbers used to weight observed incidence rates. |
| Case‐control: non‐response rate | Unclear risk | Not applicable |
| Cohort: follow‐up length and adequacy (including loss to follow‐up) | High risk | Follow‐up from 1968 to 1990 Loss to follow‐up by 1990: 55% of total woman‐years; average annual loss was 6% pill users and 6% nonusers |
Kaunitz 2006.
| Methods | Prospective, open‐label, matched‐cohort post‐marketing study in USA (multisite); 7‐year study to examine changes in bone mineral density (BMD), body weight, lipid profiles, and biochemical markers of bone metabolism | |
| Participants | Women age 25 to 35 years with regular menses or postpartum (not breast‐feeding and resumed menses or breast‐feeding and 6 weeks post‐delivery); negative pregnancy test; reasonably capable of completing 7‐year study (i.e., not planning a family within 5 years) Exclusion criteria: previously used DMPA‐IM, lumbar spine or hip BMD more than 2 standard deviations below normal for age, history of pathologic or compression fracture, or > 30% over ideal body weight; known or suspected pregnancy; undiagnosed vaginal bleeding; active thrombophlebitis, current or past thromboembolic disorders, or cerebrovascular disease; history of cancer, known or suspected breast cancer, or abnormal cervical cytology; liver or renal disease; moderate hypertension, abnormal fasting serum glucose or hyperthyroidism; and present or past alcoholism or drug abuse |
|
| Interventions | DMPA (new users) versus non‐hormonal contraception Treatment duration 240 weeks plus post‐treatment phase of 96 weeks |
|
| Outcomes | Primary: bone mineral density Secondary: body weight, lipid profiles and biochemical markers of bone metabolism Fracture recorded as adverse event |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Case definition and representativeness | Unclear risk | Not applicable |
| Exposed cohort: representativeness | Low risk | Exposed cohort: new users of depot medroxyprogesterone acetate (N = 248) |
| Control selection and definition; non‐exposed cohort selection | Low risk | Non‐exposed cohort: users of non‐hormonal contraception (N = 360): women with tubal sterilization, women using an intrauterine device or barrier contraception, or women in a monogamous relationship with a vasectomized partner |
| Exposure ascertainment, including same method for cases and controls | Unclear risk | Most likely from participant responses during screening and enrollment |
| Outcome assessment (cohort study): method and evidence outcome not present at study start | Low risk | Fracture not a defined outcome; provided as adverse event during treatment or post‐treatment period. Type of fracture not provided. Excluded women with fracture history. |
| Comparability of groups on basis of design or analysis | Unclear risk | Matched on basis of race and current smoking status. Not matched on baseline BMD, calcium intake, body size, parity, exercise, family history of osteoporosis or alcohol use. Fractures not adjusted for potential confounders; outcomes of interest for study had adjusted analyses using analysis of covariance. |
| Case‐control: non‐response rate | Unclear risk | Not applicable |
| Cohort: follow‐up length and adequacy (including loss to follow‐up) | High risk | Completed 5‐year (240‐week) treatment period: DMPA, 42/248 (17%); non‐hormonal, 118/360 (33%) Completed 96‐week post‐treatment phase: DMPA, 44/248 (18%); non‐hormonal, 87/360 (24%) |
La Vecchia 1999.
| Methods | Case‐control study of hip fractures conducted at Ospedale Maggiore (included 4 largest teaching and general hospitals in Milan, Italy) between 1983 and 1992 | |
| Participants | Participants from control group within case‐control studies of breast and genital cancers (earlier reports: La Vecchia 1991; Parazzini 1996) | |
| Interventions | Oral contraceptive use | |
| Outcomes | Hip fracture Insufficient data for analysis; results presented as reported |
|
| Notes | Report was in a letter to journal editor. Earlier publications (cited in letter) provided study design information but time periods differed and analysis may have differed. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Case definition and representativeness | Low risk | Cases: 279 women, 29 to 74 years old, admitted for fracture of hip or proximal femur; median age 62 years |
| Exposed cohort: representativeness | Unclear risk | Not applicable |
| Control selection and definition; non‐exposed cohort selection | Low risk | Controls: 1861 women, 25 to 74 years old, admitted for acute condition (other than trauma) to same network of hospitals as cases; median age 55 years |
| Exposure ascertainment, including same method for cases and controls | Low risk | History of lifetime use of oral contraceptives (structured questionnaire); analyzed as 'ever use' and use for 2 years or longer. Ever use of OCs: cases (N = 10); controls (N = 167) Data collected for cancer studies as noted in 'Participants.' |
| Outcome assessment (cohort study): method and evidence outcome not present at study start | Unclear risk | Not applicable |
| Comparability of groups on basis of design or analysis | Low risk | OR from multivariate analysis; no detail in this brief report Earlier publications focused on different exposures; identified relevant variables (e.g., demographics, alcohol and tobacco use, body mass index, use of hormone replacement therapy, and menopause) included in logistic regression models. |
| Case‐control: non‐response rate | Unclear risk | Earlier report (La Vecchia 1991), with data through June 1989, noted < 3% refused to participate (8 cases, 38 controls) |
| Cohort: follow‐up length and adequacy (including loss to follow‐up) | Unclear risk | Not applicable |
Lanza 2013.
| Methods | Cohort study; UK‐based General Practice Research Database (GPRD) Analysis limited to sub cohort with > 6 months of baseline data before contraceptive use |
|
| Participants | 312,385 women with first hormonal contraceptive prescription before age 50 Inclusion criteria: known year of birth; at least 1 prescription contraceptive record including DMPA, oral contraceptives, intrauterine device, cervical cap, or diaphragm before age 50 years and before any bilateral oophorectomy between 1 January 1987 and 31 December 2005 |
|
| Interventions | DMPA versus other hormonal contraceptives (mainly OC) | |
| Outcomes | Incident fracture | |
| Notes |
Lanza 2013 is a published report of previously included conference presentation, which now listed as secondary (Kaunitz 2010) Investigator noted in Isley 2011 that analysis used same database as Meier 2010. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Case definition and representativeness | Unclear risk | Not applicable |
| Exposed cohort: representativeness | Unclear risk | Exposed cohort: 79,065 DMPA users; unknown contraception use prior to index date (or 6 months of baseline) |
| Control selection and definition; non‐exposed cohort selection | Unclear risk | Non‐exposed cohort: 233,330 who used hormonal contraceptives other than DMPA; unknown contraception use prior to index date (or 6 months of baseline) |
| Exposure ascertainment, including same method for cases and controls | Low risk | Index date = first DMPA injection or first OC prescription Prescription data generated by general practitioners (Meier 2010). |
| Outcome assessment (cohort study): method and evidence outcome not present at study start | Low risk | Incident fracture assessed after Index date: fracture overall; axial (vertebrae, hip, pelvis), appendicular skeleton (arm, leg, wrist, ankle, hand, foot, clavicle, rib or sternum, and shoulder), all other (finger, toe, skull, face, multiple trauma, and unspecified) |
| Comparability of groups on basis of design or analysis | Unclear risk | Reportedly, age was only confounding variable. Adjustment for other potential confounders (unspecified) did not result in meaningful difference after age. Crude incident rates in published report; age‐standardized rates did not differ substantially. |
| Case‐control: non‐response rate | Unclear risk | Not applicable |
| Cohort: follow‐up length and adequacy (including loss to follow‐up) | Unclear risk | Median follow‐up 5.5 years after first prescription; 42,204 followed for 10 to < 15 years, and 14,253 followed for > 15 years |
Mallmin 1994.
| Methods | Case‐control study in County of Uppsala, Sweden Mail survey; "explorative" study of risk factors for osteoporosis in younger people with fractures (distal forearm) |
|
| Participants | See criteria for cases and controls below. Exclusion criteria: previous fragility fracture after age 40 (hip, vertebral, or proximal humerus) |
|
| Interventions | Previous or current use of oral contraceptives | |
| Outcomes | Fracture of distal forearm | |
| Notes | HRT also assessed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Case definition and representativeness | Low risk | Cases: 302 women, age 40 to 80 years with fracture of distal forearm; mean age 62.8 years Fractures of upper extremity occurred 01 April 1989 to 31 March 1990 Participants registered through special casualty reports and positive X‐ray reports for all diagnostic radiology departments in county. |
| Exposed cohort: representativeness | Unclear risk | Not applicable |
| Control selection and definition; non‐exposed cohort selection | Low risk | Controls from population registry in County of Uppsala One control chosen for each case (N = 302). Not eligible if fracture of distal forearm, hip, vertebrae or proximal part of humerus after age 40 |
| Exposure ascertainment, including same method for cases and controls | Unclear risk | Mailed questionnaire included use of OCs; no information on item wording. Missing observations completed by telephone. OC users: cases, N = 62; controls, N = 66 |
| Outcome assessment (cohort study): method and evidence outcome not present at study start | Unclear risk | Not applicable |
| Comparability of groups on basis of design or analysis | Unclear risk | Cases and controls were matched for gender, birth date (+ 1 year), and current residency in County. The relationship between OC and fractures was not statistically significant in univariate analysis and was not examined further in multivariate analysis of risk factors. Questionnaire had 102 items including accident (cases only), heredity, chronic diseases, medications, operations, smoking; reproductive factors including age at menarche and menopause, parity, use of OCs or HRT; general factors such as education, sight, and hearing. |
| Case‐control: non‐response rate | Low risk | Of 427 men and women 40 to 80 years old with forearm fracture, 90% replied. Data for women alone not provided. Of controls initially selected, 85% included after non‐responders and those with fracture history. |
| Cohort: follow‐up length and adequacy (including loss to follow‐up) | Unclear risk | Not applicable |
Meier 2010.
| Methods | Case‐control study; UK‐based General Practice Research Database (GPRD) | |
| Participants | 17,527 female case patients with incident fracture diagnosis and 70,130 matched control women; definitions for cases and controls below | |
| Interventions | Depot medroxyprogesterone acetate (DMPA) versus nonusers; use of combined oral contraceptives versus nonusers Examined low‐dose medroxyprogesterone acetate (MPA) but no data in report (no association with fracture risk) |
|
| Outcomes | Incident first‐time fracture Insufficient data for analysis; results presented as reported |
|
| Notes | Lanza 2013 also used UK‐based General Practice Research Database. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Case definition and representativeness | Low risk | Cases (N = 17,527): females aged 20 to 44 years with first‐time fracture diagnosis between 1995 and 2008 Exclusion criteria: < 3 years active history in database before first‐time diagnosis of fracture (index date); diagnosis of cancer, Paget’s disease, osteoporosis, osteomalacia, alcoholism, HIV, or use of anti‐osteoporotic drugs (i.e., bisphosphonates, teriparatide, calcitriol, and raloxifen) before index date First‐time diagnosis of fracture (index date); identified via Oxford Medical Information System and Read codes; included vertebral and non‐vertebral fractures. All fractures were "clinically diagnosed." Mean years before first fracture = 9 Diagnosis of fracture in GPRD accurate; confirmed proportion of at least 90% after comparing computer‐recorded diagnoses with hospital discharge letters or questionnaire information from general practitioners |
| Exposed cohort: representativeness | Unclear risk | Not applicable |
| Control selection and definition; non‐exposed cohort selection | Low risk | Controls (N = 70,130): 4 per fracture case, randomly selected from base population. Same exclusion criteria as for cases |
| Exposure ascertainment, including same method for cases and controls | Low risk | DMPA: users (N = 7628) and nonusers (N = 80,029) Combined OCs: users (N = 54,488) and nonusers (N = 33,169) Current user if prescription < 180 days before index date; past user if prescription > 180 days before index date Duration based on number of prescriptions, as appropriate for specific contraceptive Physicians generated prescriptions via computer, and information automatically transcribed into computer record. Contains name of preparation, route of administration, dose, and number of tablets for prescription. Recorded information on drug exposure and diagnoses validated and shown to be high quality. |
| Outcome assessment (cohort study): method and evidence outcome not present at study start | Unclear risk | Not applicable |
| Comparability of groups on basis of design or analysis | Low risk | Controls selected randomly to match cases on calendar time (index date), age (year of birth), sex, general practice, and years of history in GPRD. Multivariate analysis using a conditional logistic regression model for estimating OR included potential confounders: age, sex, general practice, calendar time, and years of recorded history in database by matching, and smoking status (non, current, ex, or unknown) and body mass index (BMI) (< 18.5, 18.5 to 24.9, 25.0 to 29.9, > 30 kg/m2, unknown) Risk estimates further adjusted for recorded history of asthma, epilepsy, use of β‐blockers, proton pump inhibitors, and anticonvulsants; systemic corticosteroids, benzodiazepines, and serotonin reuptake inhibitors; and progestins (same classification as for DMPA). Potential confounding by other variables tested in univariate analyses, but not included in final model due to limited change in main effect measure. |
| Case‐control: non‐response rate | Low risk | Database used for exposure and outcome data. Missing information in database regarding DMPA use examined in sensitivity analysis with similar results. No information on amount of missing data |
| Cohort: follow‐up length and adequacy (including loss to follow‐up) | Unclear risk | Not applicable |
Memon 2011.
| Methods | Nested case‐control study in UK, data collected prospectively; Royal College of General Practitioners Oral Contraception Study. Study began May 1968; follow‐up ceased in 1996. 1978 to 1979, women remaining in study were "flagged" at National Health Service (NHS) Central Registry, so study could be notified of cancer or death. | |
| Participants | Women in Scotland, remaining in study in 1978 to 1979, linked to Scottish Morbidity Record in 2009 Data on women living in England had not yet been obtained. |
|
| Interventions | Oral contraceptive use | |
| Outcomes | First‐ever diagnosis of fracture or operation for fracture (index date for cases) Insufficient data for analysis; results presented as reported |
|
| Notes | From cohort examined in Cooper 1993. Same research group conducted this study; limited to women with records linked to Scottish Morbidity Record in 2009. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Case definition and representativeness | Low risk | Cases: first‐ever diagnosis of fracture or operation for fracture (N = 651) Excluded multiple fractures, fractures of skull or ribs, and fractures in women with history of cancer or previous fracture. Fractures sustained during study from physician reports on 6‐month basis and those recorded by NHS when no longer under physician observation |
| Exposed cohort: representativeness | Unclear risk | Not applicable |
| Control selection and definition; non‐exposed cohort selection | Low risk | Controls: 2 per case, randomly selected, age‐matched (N = 1302) No history of fracture or cancer at time of fracture for matched‐case Had different recruiting physician from case. |
| Exposure ascertainment, including same method for cases and controls | Low risk | OC prescriptions came from physician reports on 6‐month basis during study. Pill use ascertained at index date. Women classed as 'unknown' OC status if they left observation before age 38. OC users (N = 1243); never users of OCs (N = 699) |
| Outcome assessment (cohort study): method and evidence outcome not present at study start | Unclear risk | Not applicable |
| Comparability of groups on basis of design or analysis | Unclear risk | Matching by age (within one year) Calculated unadjusted and adjusted ORs using conditional logistic regression. Main analysis examined univariate associations between fracture and smoking, social class, parity and OC use before adjusting for same factors in multivariate analysis (except where variable itself was examined). In subgroup analysis of fractures occurring under general practitioner (GP) observation, additional univariate associations calculated between fracture and duration of OC use, time since last OC use and HRT use, and HRT used as additional variable in multivariate analysis. Age‐stratified analyses conducted (10‐year intervals). Investigators did not have data on other relevant variables, e.g., use of calcium supplements, bisphosphonates or corticosteroids; body mass index, physical activity or alcohol intake. |
| Case‐control: non‐response rate | Low risk | About 75% of original cohort study were still in study in 1978 to 1979, and therefore "flagged" and followed after they left GP observation. |
| Cohort: follow‐up length and adequacy (including loss to follow‐up) | Unclear risk | Not applicable |
Michaelsson 1999.
| Methods | Case‐control study; 6 counties in Sweden | |
| Participants | Postmenopausal women, age 50 to 81 years; descriptions of cases and controls below | |
| Interventions | Oral contraceptive use | |
| Outcomes | Hip fracture Insufficient data for analysis; results presented as reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Case definition and representativeness | Low risk | Cases: 1644 women with fractures of proximal femur that occurred between October 1993 and February 1995 Used clinical records or operation registers in all 24 hospitals in study area; residents in study area, born after 1913 Index date for cases: date of fracture Fracture data from clinical records or operation registers in 24 hospitals Excluded: women with fracture due to malignant disorder (26), high‐energy trauma (4), incorrect diagnosis (51), blindness (5), birth outside Sweden (202), severe alcohol abuse, psychosis, or dementia (576), or death within 3 months of fracture (123) |
| Exposed cohort: representativeness | Unclear risk | Not applicable |
| Control selection and definition; non‐exposed cohort selection | Low risk | Controls: 4059 Swedish‐born women, randomly selected from national population register the month before study. Index date for controls: 95 days before mailing of first questionnaire. 70 to 80 years old, frequency‐matched (2 per case) to age distribution of hip fracture cases in county of residence 50 to 69 years old, frequency‐matched to expected number of breast cancer cancers to be used in breast cancer study (same questionnaire); yielded 2 to 4 controls per fracture case in each 5‐year age group and county of residence |
| Exposure ascertainment, including same method for cases and controls | Low risk | OC use via mailed questionnaire; for cases, sent after fracture, mean 95 + 23 days; for controls, sent on 6 occasions during study
Questionnaire focused on reproductive history and use of exogenous sex
hormones, including OCs and HRT. Included anthropometry, education, profession, dietary habits, alcohol consumption, cigarette smoking, physical activity (at childhood, ages of 18 and 30, and recent years), and medical history (stroke, diabetes mellitus, cardiovascular diseases, and inflammatory bowel disease). Half of participants were contacted by telephone for missing information. Questions: dose and type of OCs used and dates of use Recall aided by picture charts of all preparations commonly used in Sweden during 1950 to 1995. Picture chart, with more detailed questionnaire, sent to women who indicated OC use in first questionnaire. All women responded to second questionnaire. |
| Outcome assessment (cohort study): method and evidence outcome not present at study start | Unclear risk | Not applicable |
| Comparability of groups on basis of design or analysis | Low risk | Multivariate analysis using logistic regression adjusted for covariates that "slightly affected" OR (age, body mass index, HRT, parity) Previous hip fracture examined; did not affect OR. Excluded from analysis those who reported natural menses (premenopausal): 1 case and 50 controls. |
| Case‐control: non‐response rate | Low risk | Responses: cases, 1328/1644 (81%); controls, 3312/4059 (82%) Of responses, those solely by phone (less extensive interview): cases, 202/1328 (15%); controls, 497/3312 (15%) |
| Cohort: follow‐up length and adequacy (including loss to follow‐up) | Unclear risk | Not applicable |
O'Neill 1996.
| Methods | Case‐control study conducted in Manchester, England; October 1991 to March 1993 | |
| Participants | See below for descriptions of cases and controls. Did not exclude those with previous fracture. | |
| Interventions | Oral contraceptive use | |
| Outcomes | Distal forearm fracture Insufficient data for analysis; results presented as reported |
|
| Notes | Aimed to study 65 subjects with wrist fracture; number to detect association with OR > 2.5 at 5% significance level and 80% power (assuming 2 controls per case and 30% exposure in non‐fracture group). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Case definition and representativeness | Low risk | Cases: 62 white women aged > 45 years who had fracture of distal forearm between October 1991 and March 1993 Fractures of distal forearm identified from accident and emergency records of hospitals in south Manchester. Diagnosis of distal forearm fracture confirmed by radiograph. |
| Exposed cohort: representativeness | Unclear risk | Not applicable |
| Control selection and definition; non‐exposed cohort selection | Low risk | Controls 1) 'Fall' control: 50 women of similar age who attended same accident and emergency departments with fall on hand during same period but no fracture 2) Population control: 116 women randomly selected from registers of two large general practices in hospital catchment area |
| Exposure ascertainment, including same method for cases and controls | Unclear risk | OC use via questionnaire; details provided on some variables but not OC use. Interviewed at home. Questionnaire included personal and medical history, reproductive and hormonal characteristics, smoking habits, alcohol consumption, dietary calcium intake, and physical activity. Included treatment with steroids for 3 months or more, such as HRT. OC use analyzed as 'yes' versus 'no'. |
| Outcome assessment (cohort study): method and evidence outcome not present at study start | Unclear risk | Not applicable |
| Comparability of groups on basis of design or analysis | Unclear risk | Analysis of OC use and forearm fracture was age‐adjusted. Examined factors potentially related to forearm fracture and found no association (e.g., HRT, parity, smoking and alcohol use, calcium intake, BMI). |
| Case‐control: non‐response rate | High risk | Response rate for participation in population control group was 41%. No information on cases or "fall" control group. |
| Cohort: follow‐up length and adequacy (including loss to follow‐up) | Unclear risk | Not applicable |
Tuppurainen 1994.
| Methods | Kuopio Osteoporosis Risk Factor and Prevention Study; cohort study began in 1989 Postal questionnaires to all women born 1932 to 1941 and living in Kuopio, Finland (N = 14,220; response N = 13,100) Questions included health‐related factors, comorbidity, medications, and anthropometrics. |
|
| Participants | Random sample (from baseline survey respondents) of women willing to undergo bone densitometry (dual‐energy X‐ray absorptiometry (DXA); 11,055 women willing (of 13,100 women who responded to initial questionnaire) Stratified random sample selected to participate in bone densitometry (N = 3686); 3222 women had bone mineral density (BMD) assessed in 1990 to 1991; 2942 women had valid spine measurements and 3203 women had valid hip measurements. Exclusion criteria: hip deformity (N = 19), spine osteophytes or deformities (N = 280) |
|
| Interventions | Oral contraceptive use (sum of progestin‐only and combination OC use) | |
| Outcomes | Fracture history | |
| Notes | Additional study methodology was obtained from later article (Sirola 2012). Website had basic information on study design. Unable to obtain further information from investigator regarding questionnaire items for OC use and fractures. Investigator communicated that women were followed for 20 years and additional data might be provided. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Case definition and representativeness | Unclear risk | Not applicable |
| Exposed cohort: representativeness | Low risk | Exposed cohort: (N = 939) women who had ever used OCs (perimenopausal women willing to have bone density assessed) |
| Control selection and definition; non‐exposed cohort selection | Low risk | Non‐exposed cohort: (N = 2283) women who had never used OCs (perimenopausal women willing to have bone density assessed) |
| Exposure ascertainment, including same method for cases and controls | Unclear risk | Duration and purpose of lifetime OC use; sum of progestin‐only and COC use Data from initial mail survey; no follow‐up questions when DXA done |
| Outcome assessment (cohort study): method and evidence outcome not present at study start | High risk | Fracture history came from initial survey (retrospective data); no follow‐up questions when DXA done and no external validation No distinction among types of fractures in report Fractures calculated as (1) all fractures since age 15 until BMD measurement and (2) all fractures during 1980s |
| Comparability of groups on basis of design or analysis | High risk | Differences between groups examined in univariate analyses. OC group differed from non‐OC group in age, time since menopause, parity, smoking, marital status, education, urban‐rural living. Fracture rates not adjusted for confounding Earlier paper (1993) examined fractures in 1985 to 1989; multivariate logistic regression included OC use > 6 years plus potential confounders such as age, weight and height, and parity. No detail on model development |
| Case‐control: non‐response rate | Unclear risk | Not applicable |
| Cohort: follow‐up length and adequacy (including loss to follow‐up) | High risk | Response for survey 92% (13,100/14,220); for DXA 87% (3222/3686). Study apparently conducted with data from first contact; no follow‐up; losses not applicable. Osteoporosis study continued at least 15 years, according to later articles. |
Vessey 1998.
| Methods | Cohort study in UK; Oxford‐Family Planning Association study Study began 1968; women followed until age 45 or 1994 |
|
| Participants | 17,032 women recruited at 17 large family clinics in England and Scotland, 1968 to 1974. Inclusion criteria: age 25 to 39 years; married; white and British; willing to cooperate; and either current OC user > 5 months or current user of diaphragm or intrauterine device of > 5 months without previous OC exposure |
|
| Interventions | Oral contraceptive use versus no OC use by age 45 | |
| Outcomes | First fracture Insufficient data for analysis; results presented as reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Case definition and representativeness | Unclear risk | Not applicable |
| Exposed cohort: representativeness | Low risk | Exposed cohort: OC used > 8 years or other duration by age 45 years (187,000 woman‐years for ever‐users; N not provided) |
| Control selection and definition; non‐exposed cohort selection | Low risk | Non‐exposed cohort: OC never used by age 45 years (123,000 woman‐years; N not provided) |
| Exposure ascertainment, including same method for cases and controls | Low risk | Interview by physician or nurse at clinic visits; included changes in contraceptive practices and reasons for changes Type of OC gathered, e.g., progestin‐only, or high or low estrogen content OC use analyzed by duration of use (months) and interval since last use (months); < 12, 13 to 24, 25 to 48, 49 to 72, 73 to 96, 97 to 120, > 121). |
| Outcome assessment (cohort study): method and evidence outcome not present at study start | Low risk | First referral to hospital for fracture (inpatient and outpatient), obtained in interview by physician or nurse at clinic follow‐up visits. Diagnoses on discharge confirmed by discharge letters, summaries, and pathology reports. Fractures presented for 3 most common types (radius or ulna, ankle, tarsals or metatarsals) and all fractures. |
| Comparability of groups on basis of design or analysis | Unclear risk | At age 45, OC use defined as (1) OC never used, (2) OC used > 8 years, and 3) other duration. First 2 groups followed annually until 1994. Group 3 excluded from study analysis from age 45 onward. Potential confounders examined in univariate analysis by type of fracture: age, parity, social class, smoking, height, weight, BMI. Association between fracture and age was significant (related to nearly all fracture types). For fractures of ankle and tarsals or metatarsals, weight and body mass index also significant. Analyses of association between fracture and OC use adjusted by age only. |
| Case‐control: non‐response rate | Unclear risk | Not applicable |
| Cohort: follow‐up length and adequacy (including loss to follow‐up) | Low risk | Women who stopped attending clinic were sent a postal version of follow‐up form; if not returned, women were interviewed by telephone or at home visit. Women followed annually until age 45 or 1994. Loss to follow‐up: 0.4% per year |
Vestergaard 2006.
| Methods | Case‐control study in Denmark; National Hospital Discharge Register (NHDR) | |
| Participants | See below for descriptions of cases and controls | |
| Interventions | 3 papers: a) 2006 on oral contraceptive use (ever use of OC, N = 37,969; nonusers of OCs N = 220,220); b) 2008a on combined OC use with emphasis on young women (ever use of OCs, N = 37,969; nonusers of OCs N = 220,220); c) 2008b on use of depot medroxyprogesterone acetate (ever use N = 163; nonuse N = 258,026); use of levonorgestrel‐releasing IUD (ever use N = 1010; nonuse N = 257,179) |
|
| Outcomes | Fractures sustained in year 2000 | |
| Notes | Same case‐control definitions in 3 papers; same methodology except slight variations for concomitant drug use in multivariate models | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Case definition and representativeness | Low risk | Cases: all women with fracture sustained in year 2000 in Denmark (N = 64,548) Fractures obtained from National Hospital Discharge Register (NHDR); analyzed as any fracture and fractures of hip, colles (radius), or spine |
| Exposed cohort: representativeness | Unclear risk | Not applicable |
| Control selection and definition; non‐exposed cohort selection | Low risk | Controls: 3 per case; age‐matched (year of birth), alive and at risk for fracture diagnosis at time of corresponding case diagnosis, randomly selected women from general population (Civil Registration System) (N = 193,641) |
| Exposure ascertainment, including same method for cases and controls | Low risk | OC use from 01 January 1996 to date of fracture (maximum 5 years, from 01 January 1996 to 31 December 2000); from Register of Medicinal Product Statistics (prescription database for pharmacies in Denmark). Exposure calculated as average daily dose [number of defined daily dosages (DDD) per day]; sum of redeemed prescriptions from 01 January 1996 to fracture date or censoring date among controls, divided by time interval from first prescription to date of fracture or censoring. |
| Outcome assessment (cohort study): method and evidence outcome not present at study start | Unclear risk | Not applicable |
| Comparability of groups on basis of design or analysis | Low risk | Cases and controls matched by age. Analysis adjusted for potential confounding using conditional logistic regression: pregnancy (medical codes), comorbidity (NHDR); prior fracture from 1997 to 2000; income, social status, working status, educational status (National Bureau Statistics); contacts to general practitioners and specialists from 1996 to 2000 (National Health Organization Register); alcoholism as diagnosis (NHDR or Psychiatric Central Register) or prescription for disulfiram in prescription database; concomitant drug use (any corticosteroid, HRT use, anti‐epileptic drugs, thyroid medication). Analyses of DMPA and non‐hormonal IUD use were adjusted for OC use. |
| Case‐control: non‐response rate | Unclear risk | Database used; missing data rate not provided. |
| Cohort: follow‐up length and adequacy (including loss to follow‐up) | Unclear risk | Not applicable |
Wei 2011.
| Methods | Data from prospective Tasmanian Older Adult Cohort (TASOAC) study (Australia); baseline April 2002 to September 2004. Study focuses on determinants of osteoarthritis and osteoporosis. 1100 men and women selected randomly from roll of electors in southern Tasmania. Exclusion criteria: institutionalized or contraindication to magnetic resonance imaging (including metal sutures, presence of shrapnel, iron fillings in eye and claustrophobia) |
|
| Participants | 491 women, age 50 to 80 years, who completed questionnaires and had bone mass measured | |
| Interventions | Oral contraceptive use | |
| Outcomes | Non‐vertebral fractures | |
| Notes | Contacted investigator about follow‐up data on fractures; had no further data at the time. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Case definition and representativeness | Unclear risk | Not applicable |
| Exposed cohort: representativeness | Low risk | Exposed cohort: contraceptive status categorized as OC ever used (N = 384) and duration of use for 'ever users' (< 5 years, 5 to 10 years and > 10 years use). |
| Control selection and definition; non‐exposed cohort selection | Low risk | Non‐exposed cohort: contraceptive status categorized as never used (N = 108) |
| Exposure ascertainment, including same method for cases and controls | Low risk | OC use by self‐administered questionnaire; questions: “Have you ever used the oral contraceptive pill?” and “How many years in total have you ever taken the oral contraceptive pill?” |
| Outcome assessment (cohort study): method and evidence outcome not present at study start | Unclear risk | Non‐vertebral fractures, self‐reported via questionnaire Vertebral deformities assessed by dual energy x‐ray absorptiometry but not used in this review. |
| Comparability of groups on basis of design or analysis | High risk | Fracture rates not adjusted for potential confounders. Bone mineral density (BMD) was outcome of focus; analysis was adjusted. OC users were younger, taller and leaner and were more physically active, more likely to smoke and to drink alcohol than nonusers. OC users were more likely to have used HRT and less likely to be postmenopausal. |
| Case‐control: non‐response rate | Unclear risk | Not applicable |
| Cohort: follow‐up length and adequacy (including loss to follow‐up) | High risk | Cross‐sectional study within cohort study; 1100 men and women selected in equal numbers, which would indicate response rate about 89% (491/550). No follow‐up |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Albertazzi 2006 | Not comparative Outcome was bone mineral density; history included fracture. |
| Johansson 1996 | Study focused on whether previous fracture was risk factor for fracture later in life. Birth cohorts 1900 to 1940. For OC use or not, repeated fracture data for women born 1930 to 1940 |
| Kruger 2011 | Cross‐sectional study; bone health markers as outcomes, past fracture as risk factor for bone health |
| Lappe 2001 | Special population of female Army recruits (USA); rigorous exercise in basic training described in article Stress fractures assessed during 8‐week basic training. |
| McGough 2007 | Outcome was bone mineral density. Family history of fracture |
| O'Neill 1997 | Vertebral deformities (compression fractures) assessed by spinal X‐rays for the study. No information on when deformity may have occurred. |
| Parisi Júnior 2007 | Analysis of reported contraceptive use and of reported fracture by bone mineral density: normal, osteopenia, osteoporosis |
| Pitts 2012 | Not comparative; all were DMPA users. |
| Ruffing 2007 | Fracture history as predictor of bone mineral density |
| Yang 2006 | Cross‐sectional study |
| Yazdani 2011 | Fracture history as predictor of vertebral osteoporosis |
Differences between protocol and review
We changed the wording in the first sentence of Types of studies. The protocol noted: Studies can be prospective observational studies of contraceptive users as well as case‐control studies. This review states: We considered cohort studies of contraceptive users as well as case‐control studies. In the protocol, we stated our intent to use cohort studies, which may be prospective in design yet collect some retrospective data. The latter includes information on exposure, e.g., contraceptive use. Case‐control studies, also in our plan, routinely collect retrospective data on exposure and outcomes.
Contributions of authors
LM Lopez reviewed search results, extracted data, and drafted the review. In 2012, S Mullins reviewed search results and checked data entry of tables and text. FM Helmerhorst initiated the idea, M Chen reviewed the quality assessment data and statistical analyses, and KM Curtis helped with the criteria for inclusion and identified additional studies. All authors reviewed and commented on the manuscript.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute of Child Health and Human Development, USA.
Funding to FHI 360 for conducting the review (LML, SM, MC)
-
United States Agency for International Development, USA.
Funding to FHI 360 for conducting the review (LML, SM, MC)
Declarations of interest
The authors have no conflicts of interest to declare.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Barad 2005 {published data only}
- Barad D, Kooperberg C, Wactawski‐Wende J, Liu J, Hendrix SL, Watts NB. Prior oral contraception and postmenopausal fracture: a Womens' Health Initiative observational cohort study. Fertility and Sterility 2005;84:374‐83. [DOI] [PubMed] [Google Scholar]
- Women's Health Initiative Study Group. Design of the Women's Health Initiative clinical trial and observational study. Controlled Clinical Trials. 1998/03/11 1998; Vol. 19, issue 1:61‐109. [DOI] [PubMed]
Cooper 1993 {published data only}
- Cooper C, Hannaford P, Croft P, Kay CR. Oral contraceptive pill use and fractures in women: a prospective study. Bone 1993;14(1):41‐5. [DOI] [PubMed] [Google Scholar]
Kaunitz 2006 {published data only}
- Kaunitz AM, Miller PD, Rice VM, Ross D, McClung MR. Bone mineral density in women aged 25‐35 years receiving depot medroxyprogesterone acetate: recovery following discontinuation. Contraception 2006;74(2):90‐9. [DOI] [PubMed] [Google Scholar]
Lanza 2013 {unpublished data only}
- Kaunitz A, Harel Z, Bone H, Ataher Q, Ross D, Arena P, et al. Retrospective cohort study of DMPA and fractures in reproductive age women. http://www.arhp.org/Professional‐Education/Annual‐Meetings/RH2010/2010‐Presentations (accessed 27 January 2012).
- Lanza LL, McQuay LJ, Rothman KJ, Bone HG, Kaunitz AM, Harel Z, et al. Use of depot medroxyprogesterone acetate contraception and incidence of bone fracture. Obstetrics and Gynecology 2013;121(3):593‐600. [DOI] [PubMed] [Google Scholar]
La Vecchia 1999 {published data only}
- Vecchia C, Negri E, Levi F, Baroni JA. Cigarette smoking, body mass and other risk factors for fractures of the hip in women. International Journal of Epidemiology 1991;20(3):671‐7. [DOI] [PubMed] [Google Scholar]
- Vecchia C, Tavani A, Gallus S. Oral contraceptives and risk of hip fractures. Lancet 1999;354(9175):335‐6. [DOI] [PubMed] [Google Scholar]
- Parazzini G, Tavani A, Ricci E, Vecchia C. Menstrual and reproductive factors and hip fractures in post menopausal women. Maturitas 1996;24:191‐6. [DOI] [PubMed] [Google Scholar]
Mallmin 1994 {published data only}
- Mallmin H, Ljunghall S, Persson I, Bergstrom R. Risk factors for fractures of the distal forearm: a population‐based case‐control study. Osteoporosis International 1994;4(6):298‐304. [DOI] [PubMed] [Google Scholar]
Meier 2010 {published data only}
- Meier C, Brauchli YB, Jick SS, Kraenzlin ME, Meier CR. Use of depot medroxyprogesterone acetate and fracture risk. Journal of Clinical Endocrinology and Metabolism 2010;95(11):4909‐16. [DOI] [PubMed] [Google Scholar]
Memon 2011 {published data only}
- Memon S, Iversen L, Hannaford PC. Is the oral contraceptive pill associated with fracture in later life? New evidence from the Royal College of General Practitioners Oral Contraception Study. Contraception 2011;84(1):40‐7. [DOI] [PubMed] [Google Scholar]
Michaelsson 1999 {published data only}
- Michaelsson K, Baron JA, Farahmand BY, Ljunghall S. Influence of parity and lactation on hip fracture risk. American Journal of Epidemiology 2001;153(12):1166‐72. [DOI] [PubMed] [Google Scholar]
- Michaelsson K, Baron JA, Farahmand BY, Persson I, Ljunghall S. Oral‐contraceptive use and risk of hip fracture: a case‐control study. Lancet 1999;353(9163):1481‐4. [DOI] [PubMed] [Google Scholar]
O'Neill 1996 {published data only}
- O'Neill TW, Marsden D, Adams JE, Silman AJ. Risk factors, falls, and fracture of the distal forearm in Manchester, UK. Journal of Epidemiology and Community Health 1996;50(3):288‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tuppurainen 1994 {published data only}
- Kuopio Osteoporosis Risk Factor and Prevention Study. Kuopio Osteoporosis Risk Factor and Prevention Study. Study Design. http://www.uku.fi/˜due2/indexeng.htm (accessed 27 February 2012).
- Sirola J, Rikkonen T, Tuppurainen M, Honkanen R, Kroger H. Should risk of bone fragility restrict weight control for other health reasons in postmenopausal women?‐‐A ten year prospective study. Maturitas. 2011/12/20 2012; Vol. 71, issue 2:162‐8. [DOI] [PubMed]
- Tuppurainen M, Honkanen R, Kröger H, Saarikoski S, Alhava E. Osteoporosis risk factors, gynaecological history and fractures in perimenopausal women‐‐the results of the baseline postal enquiry of the Kuopio Osteoporosis Risk Factor and Prevention Study. Mauritas 1993;17(2):89‐100. [DOI] [PubMed] [Google Scholar]
- Tuppurainen M, Kroger H, Saarikoski S, Honkanen R, Alhava E. The effect of previous oral contraceptive use on bone mineral density in perimenopausal women. Osteoporosis International 1994;4(2):93‐8. [DOI] [PubMed] [Google Scholar]
Vessey 1998 {published data only}
- Vessey M, Mant J, Painter R. Oral contraception and other factors in relation to hospital referral for fracture. Findings in a large cohort study. Contraception 1998;57(4):231‐5. [DOI] [PubMed] [Google Scholar]
- Vessey MP, Lawless M. The Oxford‐Family Planning Association contraceptive study. Clinical Obstetrics and Gynaecology 1984;11(3):743‐57. [PubMed] [Google Scholar]
Vestergaard 2006 {published data only}
- Vestergaard P, Rejnmark L, Mosekilde L. Fracture risk in very young women using combined oral contraceptives. Contraception 2008a;78(5):358‐64. [DOI] [PubMed] [Google Scholar]
- Vestergaard P, Rejnmark L, Mosekilde L. Oral contraceptive use and risk of fractures. Contraception 2006;73(6):571‐6. [DOI] [PubMed] [Google Scholar]
- Vestergaard P, Rejnmark L, Mosekilde L. The effects of depot medroxyprogesterone acetate and intrauterine device use on fracture risk in Danish women. Contraception 2008b;78(6):459‐64. [DOI] [PubMed] [Google Scholar]
Wei 2011 {published data only (unpublished sought but not used)}
- Wei S, Venn A, Ding C, Foley S, Laslett L, Jones G. The association between oral contraceptive use, bone mineral density and fractures in women aged 50‐80 years. Contraception 2011;84(4):357‐62. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Albertazzi 2006 {published data only}
- Albertazzi P, Bottazzi M, Steel SA. Bone mineral density and depot medroxyprogesterone acetate. Contraception 2006;73(6):577‐83. [DOI] [PubMed] [Google Scholar]
Johansson 1996 {published data only}
- Johansson C, Mellstrom D. An earlier fracture as a risk factor for new fracture and its association with smoking and menopausal age in women. Maturitas 1996;24(1‐2):97‐106. [DOI] [PubMed] [Google Scholar]
Kruger 2011 {published data only}
- Kruger MC, Kruger IM, Wentzel‐Viljoen E, Kruger A. Urbanization of black South African women may increase risk of low bone mass due to low vitamin D status, low calcium intake, and high bone turnover. Nutrition Research 2011;31(10):748‐58. [DOI] [PubMed] [Google Scholar]
Lappe 2001 {published data only}
- Lappe JM, Stegman MR, Recker RR. The impact of lifestyle factors on stress fractures in female Army recruits. Osteoporosis International 2001;12(1):35‐42. [DOI] [PubMed] [Google Scholar]
McGough 2007 {published data only}
- McGough P, Bigrigg A. Effect of depot medroxyprogesterone acetate on bone density in a Scottish industrial city. European Journal of Contraception and Reproductive Health Care 2007;12(3):253‐9. [DOI] [PubMed] [Google Scholar]
O'Neill 1997 {published data only}
- O'Neill TW, Silman AJ, Naves Diaz M, Cooper C, Kanis J, Felsenberg D. Influence of hormonal and reproductive factors on the risk of vertebral deformity in European women. European Vertebral Osteoporosis Study Group. Osteoporosis International 1997;7(1):72‐8. [DOI] [PubMed] [Google Scholar]
Parisi Júnior 2007 {published data only}
- Parisi Júnior PD, Chahade WH. Fatores de risco associados à osteoporose em uma população de mulheres brasileiras residentes em São José do Rio Pardo, estado de São Paulo. Revista Brasileira de Reumatologia 2007;47(1):16‐24. [Google Scholar]
Pitts 2012 {published data only}
- Pitts SA, Feldman HA, Dorale A, Gordon CM. Bone mineral density, fracture, and vitamin d in adolescents and young women using depot medroxyprogesterone acetate. Journal of Pediatric and Adolescent Gynecology 2012;25(1):23‐6. [DOI] [PubMed] [Google Scholar]
Ruffing 2007 {published data only}
- Ruffing JA, Nieves JW, Zion M, Tendy S, Garrett P, Lindsay R, et al. The influence of lifestyle, menstrual function and oral contraceptive use on bone mass and size in female military cadets. Nutrition & Metabolism 2007;4:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yang 2006 {published data only}
- Yang K, McElmurry BJ, Park CG. Decreased bone mineral density and fractures in low‐income Korean women. Health Care for Women International 2006;27(3):254‐67. [DOI] [PubMed] [Google Scholar]
Yazdani 2011 {published data only}
- Yazdani S, Asli AI, Salemi A, Heidarnia MA, Sarbakhsh P. Determination of Clinical Decision Rule for Estimation of Bone Mineral Density in Women. Medical Principles and Practice 2011;20(5):416‐21. [DOI] [PubMed] [Google Scholar]
Additional references
ACOG 2014
- Committee on Adolescent Health, Committee on Gynecologic Practice. Depot Medroxyprogesterone Acetate and Bone effects. Obstetrics and Gynecology 2014;123:727‐30. [Google Scholar]
Balshem 2011
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. [DOI] [PubMed] [Google Scholar]
CDC 2010
- Centers for Disease Control and Prevention. U.S. Medical Eligibility Criteria for Contraceptive Use, 2010. http://www.cdc.gov/mmwr/pdf/rr/rr59e0528.pdf (accessed 23 January 2012):34.
Cleland 2004
- Cleland J, Ali MM. Reproductive consequences of contraceptive failure in 19 developing countries. Obstetrics and Gynecology 2004;104:314‐20. [DOI] [PubMed] [Google Scholar]
Cromer 2005
- Cromer BA, Lazebnik R, Rome E, Stager M, Bonny A, Ziegler J, et al. Double‐blinded randomized controlled trial of estrogen supplementation in adolescent girls who receive depot medroxyprogesterone acetate for contraception. American Journal of Obstetrics and Gynecology 2005;192(1):42‐7. [DOI] [PubMed] [Google Scholar]
Cundy 2003
- Cundy T, Ames R, Horne A, Clearwater J, Roberts H, Gamble G, et al. A randomized controlled trial of estrogen replacement therapy in long‐term users of depot medroxyprogesterone acetate. Journal of Clinical Endocrinology and Metabolism 2003;88(1):78‐81. [DOI] [PubMed] [Google Scholar]
Curtis 2006
- Curtis KM, Martins SL. Progestin‐only contraception and bone mineral density. Contraception 2006;73:470‐87. [DOI] [PubMed] [Google Scholar]
FDA 2004
- U.S. Food, Drug Administration. FDA Talk Paper. Black box Warning Added Concerning Long‐term Use of Depo‐Provera Contraceptive Injection. http://web.archive.org/web/20070809090332/http://www.fda.gov/bbs/topics/ANSWERS/2004/ANS01325.html (accessed 24 January 2012).
Goldberg 2011
- Bartz D, Goldberg AB. Injectable contraceptives. In: Hatcher RA, Trussell J, Nelson AL, Cates W, Kowal D, Policar MS editor(s). Contraceptive Technology. 20th Edition. New York: Ardent Media, Inc., 2011:209‐36. [Google Scholar]
Golder 2011
- Golder S, Loke YK, Bland M. Meta‐analyses of adverse effects data derived from randomised controlled trials as compared to observational studies: methodological overview. PLoS Medicine 2011;8(5):e1001026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gourlay 2004
- Gourlay ML, Brown SA. Clinical considerations in premenopausal osteoporosis. Archives of Internal Medicine 2004;164:603‐14. [DOI] [PubMed] [Google Scholar]
Guilbert 2009
- Guilbert ER, Brown JP, Kaunitz AM, Wagner MS, Bérubé J, Charbonneau L, et al. The use of depot‐medroxyprogesterone acetate in contraception and its potential impact on skeletal health. Contraception 2009;79:167‐77. [DOI] [PubMed] [Google Scholar]
Herrmann 2010
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. John Wiley & Sons, Ltd, (accessed 30 March 2011).
Howe 2011
- Howe TE, Shea B, Dawson LJ, Downie F, Murray A, Ross C, et al. Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database of Systematic Reviews 2011, Issue 7. [DOI: 10.1002/14651858.CD000333.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Isley 2011
- Isley MM, Kaunitz AM. Update on hormonal contraception and bone density. Reviews in Endocrine & Metabolic Disorders 2011;12(2):93‐106. [DOI] [PubMed] [Google Scholar]
Lopez 2014
- Lopez LM, Grimes DA, Schulz KF, Curtis KM, Chen M. Steroidal contraceptives: effect on bone fractures in women. Cochrane Database of Systematic Reviews 2014, Issue 6. [DOI: 10.1002/14651858.CD006033.pub5] [DOI] [Google Scholar]
Martins 2006
- Martins SL, Curtis KM, Glasier AF. Combined hormonal contraception and bone health: a systematic review. Contraception 2006;73:445‐69. [DOI] [PubMed] [Google Scholar]
Rachner 2011
- Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet 2011;377(9773):1276‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sanchez‐Riera 2010
- Sanchez‐Riera L, Wilson N, Kamalaraj N, Nolla JM, Kok C, Li Y, et al. Osteoporosis and fragility fractures. Best Practices & Research in Clinical Rheumatology 2010;24(6):793‐810. [DOI] [PubMed] [Google Scholar]
Schulz 2010
- Schulz KF, Altman DG, Moher D, for the CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
Strobe 2007
- STROBE group. STROBE Statement: Strengthening the reporting of Observational studies in Epidemiology. http://www.strobe‐statement.org/ (accessed 24 April 2012).
Trussell 2011
- Trussell J. Contraceptive failure in the United States. Contraception 2011;83:397‐404. [DOI] [PMC free article] [PubMed] [Google Scholar]
UN 2013
- United Nations, Department of Economic and Social Affairs, Population Division. World Contraceptive Patterns 2013. http://www.un.org/en/development/desa/population/publications/family/contraceptive‐wallchart‐2013.shtml (accessed 6 July 2015).
Wells 2011
- GA Wells, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed 29 November 2011).
WHO 2006
- World Health Organization. WHO statement on hormonal contraception and bone health. Contraception 2006;73:443‐4. [DOI] [PubMed] [Google Scholar]
WHO 2015a
- World Health Organization, Department of Reproductive Healh. Medical eligibility criteria for contraceptive use. Fifth edition ‐ Executive Summary, 2015. http://www.who.int/reproductivehealth/publications/family_planning/Ex‐Summ‐MEC‐5/en/ (accessed 2 July 2015).
WHO 2015b
- World Health Organization, Department of Reproductive Health. Medical eligibility criteria wheel for contraceptive use. 2015. http://www.who.int/reproductivehealth/publications/family_planning/Ex‐Summ‐MEC‐5/en/ (accessed 2 July 2015).


