Abstract
Background
Glioblastoma (GBM) is an incurable disease with few approved therapeutic interventions. Radiation therapy (RT) and temozolomide (TMZ) remain the standards of care. The efficacy and optimal deployment schedule of the orally bioavailable small-molecule tumor checkpoint controller lisavanbulin alone, and in combination with, standards of care were assessed using a panel of IDH-wildtype GBM patient-derived xenografts.
Methods
Mice bearing intracranial tumors received lisavanbulin +/−RT +/−TMZ and followed for survival. Lisavanbulin concentrations in plasma and brain were determined by liquid chromatography with tandem mass spectrometry, while flow cytometry was used for cell cycle analysis.
Results
Lisavanbulin monotherapy showed significant benefit (P < .01) in 9 of 14 PDXs tested (median survival extension 9%-84%) and brain-to-plasma ratios of 1.3 and 1.6 at 2- and 6-hours postdose, respectively, validating previous data suggesting significant exposure in the brain. Prolonged lisavanbulin dosing from RT start until moribund was required for maximal benefit (GBM6: median survival lisavanbulin/RT 90 vs. RT alone 69 days, P = .0001; GBM150: lisavanbulin/RT 143 days vs. RT alone 73 days, P = .06). Similar observations were seen with RT/TMZ combinations (GBM39: RT/TMZ/lisavanbulin 502 days vs. RT/TMZ 249 days, P = .0001; GBM26: RT/TMZ/lisavanbulin 172 days vs. RT/TMZ 121 days, P = .04). Immunohistochemical analyses showed a significant increase in phospho-histone H3 with lisavanbulin treatment (P = .01).
Conclusions
Lisavanbulin demonstrated excellent brain penetration, significant extension of survival alone or in RT or RT/TMZ combinations, and was associated with mitotic arrest. These data provide a strong clinical rationale for testing lisavanbulin in combination with RT or RT/TMZ in GBM patients.
Keywords: drug efficacy, glioblastoma, microtubule-targeting agents, patient-derived xenografts
Key Points
Lisavanbulin demonstrates benefit in GBM PDX models alone and in standards of care combinations.
Dosing schedule and duration are critical factors for optimal lisavanbulin efficacy.
Importance of the Study.
IDH-wildtype glioblastoma (GBM) represents one of the most aggressive cancer types with the vast majority of patients succumbing to the disease within 5 years of diagnosis. This partly reflects the limited efficacy of frontline therapies that include radiation therapy (RT) and the alkylating chemotherapeutic temozolomide (TMZ). The development of investigational agents and subsequent testing in clinically relevant disease models is required to identify agents likely to provide meaningful treatment benefits. The Mayo collection of GBM PDX models recapitulates the genetic diversity of GBM and fills an existing unmet need in the research community.1 This study describes how this PDX panel was used to define how a promising anticancer agent (lisavanbulin) could be optimally deployed in conjunction with standard-of-care therapy for GBM. Establishing experimental frameworks such as this may better position future studies to accurately reflect the clinical promise of a novel agent.
Introduction
Microtubules are highly dynamic cytoskeletal fibers composed of tubulin subunits that are involved in a diverse range of cellular functions including cell shape maintenance, intracellular transport, and the execution of mitosis.2–4 Cells with elevated mitotic rates, such as those found in human tumors, are highly sensitive to microtubule-targeting strategies, since dysregulation of microtubule dynamics can trigger cell cycle arrest and apoptosis induction.5,6 Consistent with a critical role in tumor cell biology, microtubule-targeting agents (MTAs) continue to be one of the most successful cancer targets more than 60 years after their discovery.
MTAs are typically classified into two main groups (microtubule stabilizers and destabilizers) based on their ability to affect polymer mass. Destabilizing agents, such as the vinca alkaloids, prevent microtubule polymerization, while stabilizing agents, including taxanes, enhance polymerization.7,8 These changes in polymer mass disrupt microtubule dynamics, and in proliferating cells, can trigger mitotic arrest. This mechanism of action may confer MTAs with the ability to elicit radiosensitizing effects if used in conjunction with radiation therapy (RT), since cells arrested in mitosis are highly sensitive to radiation-induced DNA damage. Unfortunately, inherent toxicities to the nervous system and bone marrow, in conjunction with poor distribution across the blood-brain barrier (BBB) and the emergence of resistance mechanisms, have limited the utility of FDA-approved MTAs for the treatment of brain tumors.2,4,9–11
Lisavanbulin (BAL101553) is a novel orally bioavailable, small-molecule tumor checkpoint controller, that is the soluble prodrug of the active moiety avanbulin (BAL27862). Avanbulin binds tubulin at a site not targeted by other approved MTAs, and has demonstrated broad in vitro activity across a number of models refractory to standard MTAs.3,12 In addition, avanbulin has shown good brain penetration achieving therapeutically relevant concentrations in the brain. Lisavanbulin is currently under clinical investigation in glioblastoma (GBM). To determine how best to maximize the clinical benefit for lisavanbulin therapy, the Mayo Clinic panel of GBM patient-derived xenograft (PDX) models was used to define the spectrum of sensitivity of lisavanbulin alone and in combination with current standards of care including RT and temozolomide (TMZ) chemotherapy. These studies provide the foundation for ongoing and planned clinical trials, which include evaluation of the efficacy of RT/lisavanbulin combinations in newly diagnosed, IDH-wildtype GBM.
Materials and Methods
Animal Studies
All animal studies were conducted in accordance with Mayo Clinic Institutional Animal Care and Use Committee policies. Female athymic nude mice (strain code 553, aged 6-7 weeks from Charles River, Wilmington, MA) were used for all studies. PDX maintenance and intracranial injections were performed as previously described.13 Mice with established orthotopic tumors were dosed as indicated, observed daily, and euthanized at moribund. A complete summary of study arms is included in Supplementary Table S1.
In Vitro Cell Cycle Analysis
Media were formulated and short-term explant cultures maintained as previously described.13 Cells were plated at a density of 1-3 million cells per 10 cm dish in DMEM or StemPro (GBM12 only) media. Cells were treated as indicated and harvested at 72 h for flow cytometry.
Drugs and Radiation
Lisavanbulin (BAL101553, Basilea Pharmaceutica International Ltd, Basel, Switzerland) was formulated in 0.9% sodium chloride (NaCl) in 10% water for injection and pH adjusted to 5.0 using sodium acetate. Avanbulin (BAL27862, Basilea Pharmaceutica International Ltd, Basel, Switzerland) was prepared for in vitro use in 100% DMSO. TMZ (Developmental Therapeutics Program, NCI, Bethesda, MD) was suspended in Ora-Plus. Radiation was delivered to the entire head of unanesthetized mice, immobilized in a plastic restraint, through a single right lateral beam from a 137Cs source. The remainder of the body was shielded with a lead block. Radiation dose and schedule varied by study and can be found in Supplementary Table S1.
Pharmacokinetics of Avanbulin
A single dose of 30 mg/kg lisavanbulin was administered in 10 mL/kg volume by oral gavage as a solution to FVB wild-type and triple knockout (TKO; Mdr1a/b−/−Bcrp1−/−) mice. Conversely, 8 mg/kg was delivered in 4 mL/kg volume intravenously to athymic nude mice bearing SW480 subcutaneous tumors. TKO mice were euthanized in a CO2 chamber at 2- and 6-hours postdose (N = 4 per timepoint) while athymic nude mice were euthanized at 5 min, 15 min, 30 min, 45 min, 1 h, 1.5 h, 2 h, 4 h, 6 h, and 24 h postdose (N = 3 per timepoint). Blood was collected via cardiac puncture in heparinized (TKO) or EDTA (nude) tubes. Brains were surgically collected and placed in preweighed tubes. Plasma was separated by centrifugation at 3500 rpm at 4°C for 15 min. Both plasma and brain samples were stored at −20/−80°C until LC-MS/MS analysis.
LC-MS/MS Analysis
Brain and plasma concentrations of avanbulin were determined using an LC-MS/MS assay similar to those previously published.14 Briefly, all samples were spiked with 5 ng palbociclib as internal standard. After extraction using 1 volume of pH 11 buffer and 5 volumes of ice-cold ethyl acetate, organic supernatant was dried under nitrogen, reconstituted in the mobile phase (70:30 distilled water with 0.1% formic acid:acetonitrile with 0.1% formic acid), and injected onto a Phenomenex Synergi Polar-RP column (75 × 2 mm, 4 μm). The m/z transitions were 388.18 > 276.96 for avanbulin and 448.24 > 380.04 for palbociclib (positive-ionization mode).
For PK assessments in tumor bearing athymic nude mice, samples were homogenized with equivalents of water (brain—nine, tumor—variable) prior to analysis. Individual dilutions were used for the back calculation of analyte concentrations in sample homogenates. 50 μL mouse K3-EDTA plasma, brain homogenate, or tumor homogenate were mixed with 150 μL methanol containing 0.5 μg/mL avanbulin-D5 and lisavanbulin-D4 as internal standards. Samples were vortexed and centrifuged and 20 μL of the supernatant was injected into the HPLC. For quantification of each analyte, a standard curve was prepared with a range from 5 to 10 000 ng/mL, 10 to 10 000 ng/mL respectively, in mouse K3-EDTA plasma, brain/water homogenate, and tumor/water homogenate. The different matrices were spiked (5 μL DMSO solution in 500 μL plasma, brain/water homogenate, and tumor/water homogenate) and prepared like the study samples.
Genomic Analyses
Whole exome sequencing was conducted as previously described.1
Pharmacodynamic Studies
Mice bearing orthotopic GBM39 tumors were dosed orally with 30 mg/kg lisavanbulin for 8 days and sacrificed 2 h after the final dose. Brains were harvested and fixed in formalin for paraffin embedding. Mitotic events and proliferation were assessed by phospho-histone H3 and Ki67 immunohistochemistry, respectively. Apoptosis was detected by TUNEL staining using the ApopTag In Situ Apoptosis kit (EMD Millipore, Burlington, MA). Quantification of Ki67 IHC was performed using Aperio ImageScope, while other stains were quantified manually by a board-certified neuropathologist.
Statistical Analysis
In vitro experiments were analyzed by two-sample t-tests. Survival was defined as the time from tumor implantation to reach a moribund state. Differences in survival across groups were assessed using the log-rank test. Two-sided P values of less than .05 were considered statistically significant. Animals euthanized for reasons other than tumor burden were censored.
Results
Avanbulin Distribution Across the Blood-Brain Barrier
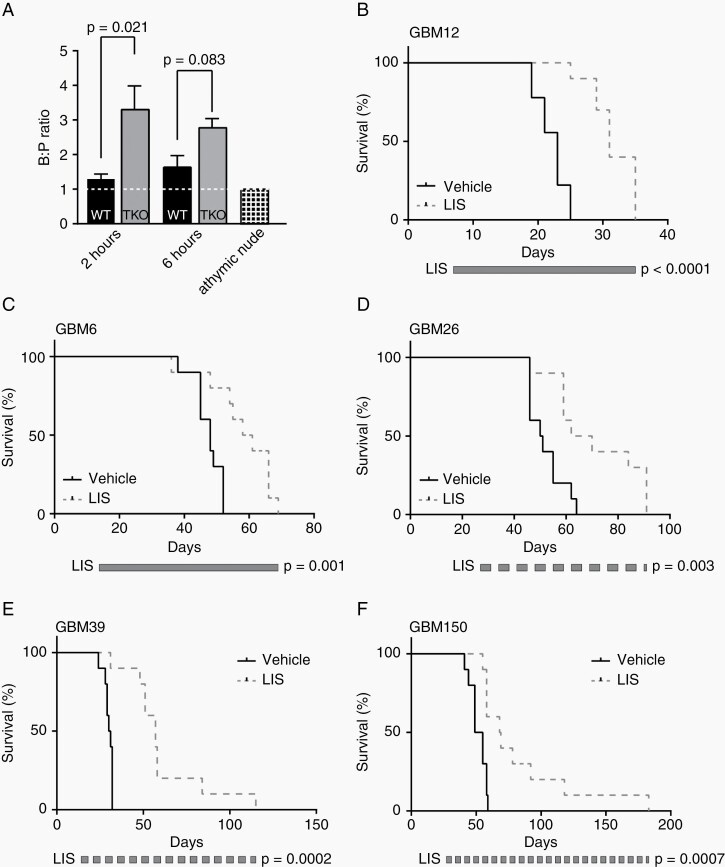
Distribution of avanbulin across the blood-brain barrier was evaluated in FVB wild-type (WT) and Mdr1a/b−/− Bcrp1−/− (TKO) mice dosed once orally with 30 mg/kg lisavanbulin prodrug and sacrificed 2 and 6 h later. Plasma and whole brains were harvested, and concentrations of the avanbulin active metabolite were measured by LC-MS/MS (Supplementary Table S2, Figure 1A). Consistent with previous data in CD1 Nu/Nu female mice, which showed an avanbulin brain/plasma ratio of around 1.0 and a brain/flank tumor or brain/normal tissue ratio of ≥0.8, similar avanbulin concentrations were detected in the brain (B) and plasma (P) of WT mice at 2 h (540.0 vs. 452.5 ng/mL, respectively; B:P ratio = 1.29) and 6 h (190.9 vs. 118.6 ng/mL, respectively; B:P ratio = 1.64).15 Higher avanbulin levels in the brain were also observed in TKO mice at both 2 h (1047.3 ng/mL, B:P ratio = 3.30) and 6 h (455.1, B:P ratio = 2.78). Similar observations were observed in an independent experiment using athymic nude mice bearing subcutaneous SW480 xenografts (B:P ratio = 0.98). Importantly, this experiment also revealed a brain-to-tumor ratio of 0.8 (AUC 38.8 brain vs. 45.5 tumor). These observations suggest that, while avanbulin is subject to drug efflux, sufficient concentrations of avanbulin were observed in the brain for several hours to support the use of lisavanbulin for intracranial models.
Fig. 1.
Lisavanbulin monotherapy significantly extends survival in orthotopic PDX models. (A) FVB wild-type and triple knockout mice were given a single oral dose of lisavanbulin (LIS). Animals were sacrificed postdose for brain and plasma collection. Brain and plasma levels were compared to that of athymic nude mice bearing SW480 subcutaneous xenografts dosed IV with LIS and collected 5 min later. Pharmacokinetic profiles of the active moiety BAL27862 were established by LC-MS/MS analysis. (B-F) Mice with the indicated orthotopic PDX tumors were randomized and treated with vehicle or 30 mg/kg LIS once daily until moribund. Time to reach a moribund state from tumor implantation is plotted.
Lisavanbulin Monotherapy in Orthotopic IDH-wildtype GBM PDXs
The efficacy of lisavanbulin monotherapy was studied in 14 IDH-wildtype GBM PDX lines established as orthotopic xenografts. For each experiment, mice were randomized to therapy with vehicle or 30 mg/kg lisavanbulin daily. In initial studies with GBM12 (Figure 1B) and GBM6 (Figure 1C), mice were dosed orally with 30 mg/kg lisavanbulin once daily (Monday through Sunday). Significant extensions in survival were observed relative to vehicle controls (GBM12: median lisavanbulin 31 days vs. vehicle 23 days, P < .01; GBM6: median lisavanbulin 60 days vs. vehicle 48 days, P < .01). Additional studies were performed with lisavanbulin dosing limited to 5 days per week with (GBM26, GBM39, and GBM150) exhibiting biologically significant enhancement in survival (Figure 1D–F). Across all 14 PDXs tested, nine demonstrated significant prolongation in survival (P < .05) with a range in median survival extension of 9%-84% (Table 1). Five of these PDX models (GBM6, GBM12, GBM26, GBM39, GBM150) were selected for further evaluation.
Table 1.
Lisavanbulin Monotherapy in Orthotopic PDX Models. Fourteen Orthotopic PDXs were Orally Dosed with Vehicle or 30 mg/kg Lisavanbulin (LIS) Once Daily Until Moribund. Bold italics rows were selected for Follow-up Studies
| Line | MGMT Promoter Status | N per Group | Median Survival (Days) | P Value | % Change | |
|---|---|---|---|---|---|---|
| Vehicle | LIS | |||||
| GBM6 | Unmethylated | 10 | 48 | 60 | <.01 | 25% |
| GBM8 | Methylated | 10a | 47 | 64 | <.01 | 36% |
| GBM10 | Unmethylated | 10 | 35 | 38 | .04 | 9% |
| GBM12 | Methylated | 10 a | 23 | 31 | <.01 | 35% |
| GBM15 | Methylated | 10 | 71 | 87 | .41 | 22% |
| GBM22TMZ | Methylated | 10 | 27 | 26 | .55 | −4% |
| GBM26 | Unmethylated | 10 | 51 | 66 | <.01 | 29% |
| GBM39 | Methylated | 10 | 31 | 57 | <.01 | 84% |
| GBM59 | Methylated | 10 | 45 | 56 | .02 | 24% |
| GBM84 | Methylated | 10 | 56 | 73 | <.01 | 30% |
| GBM108 | Unmethylated | 10 | 40 | 43 | .11 | 8% |
| GBM115 | Unmethylated | 10a | 139 | 183 | .07 | 32% |
| GBM122 | Unmethylated | 10a | 80 | 84 | .28 | 5% |
| GBM150 | Unmethylated | 10 | 52 | 69 | <.01 | 33% |
aVehicle group contained n = 9.
Lisavanbulin Administration During Radiation
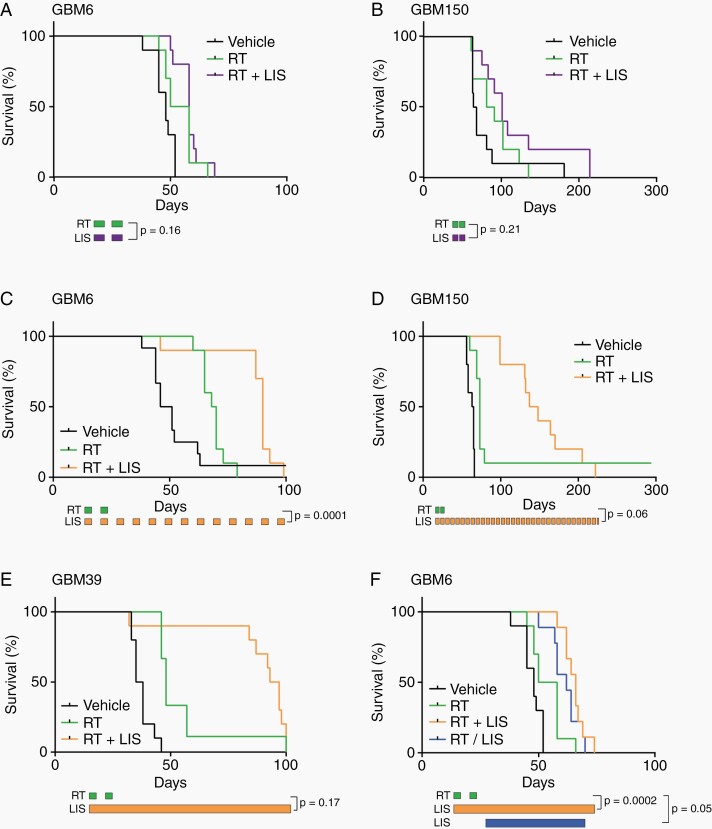
Mice bearing GBM6 and GBM150 intracranial tumors were randomized and treated with vehicle, 2 weeks of radiation, or 2 weeks of radiation (2 Gy × 10 fractions) with daily lisavanbulin limited to RT treatment time (Figure 2A and B). In both PDXs, concurrent lisavanbulin did not significantly improve survival compared to radiation only (GBM6: median survival RT+ lisavanbulin 58 days vs. RT 54 days, P = .16; GBM150: median survival RT+ lisavanbulin 101 days vs. RT 86 days, P = .21). However, prolonged lisavanbulin dosing from the initiation of radiation until reaching a moribund state significantly improved survival across two different radiation schedules (Figures 2 and 3). In a GBM6 intracranial experiment, 2 weeks of radiation (2 Gy × 10) conferred median survival of 69 days; the addition of long-term lisavanbulin dosing extended median survival to 90 days (P = .0001, Figure 2C). Moreover, a doubling in survival was observed in GBM150 and GBM39 intracranial studies (GBM150: median RT+ lisavanbulin 143 days vs. RT 73 days, P = .06, Figure 2D; GBM39: median RT+ lisavanbulin 95 days vs. RT 48 days, P = .17, Figure 2E). Lisavanbulin deployment during this two-week radiation schedule appears critical for optimal survival benefit as lisavanbulin treatment following radiation demonstrated shorter survival (Figure 2F). Overall, lisavanbulin dosing was well tolerated.
Fig. 2.
Prolonged lisavanbulin dosing is required to maximize survival gains in combination with two-week radiation course. (A-B) Mice with the indicated orthotopic PDXs were randomized and treated 13 days (GBM6) or 32 days (GBM150) after tumor implantation. Mice were dosed with vehicle, 2 Gy radiation (RT), or 2 Gy RT + once daily lisavanbulin (LIS) Monday through Friday for 2 weeks at which point treatment was ended, and mice were followed until moribund. (C-F) Mice with the indicated orthotopic PDXs were randomized and treated 14 days after tumor implantation. Mice were dosed with vehicle, single agent LIS, radiation (RT), or RT + LIS combination. Radiation was given in 2 Gy fractions Monday through Friday for 2 weeks while LIS dosing was continued until moribund. An additional GBM6 experiment highlighted the importance of LIS timing during RT schedules.
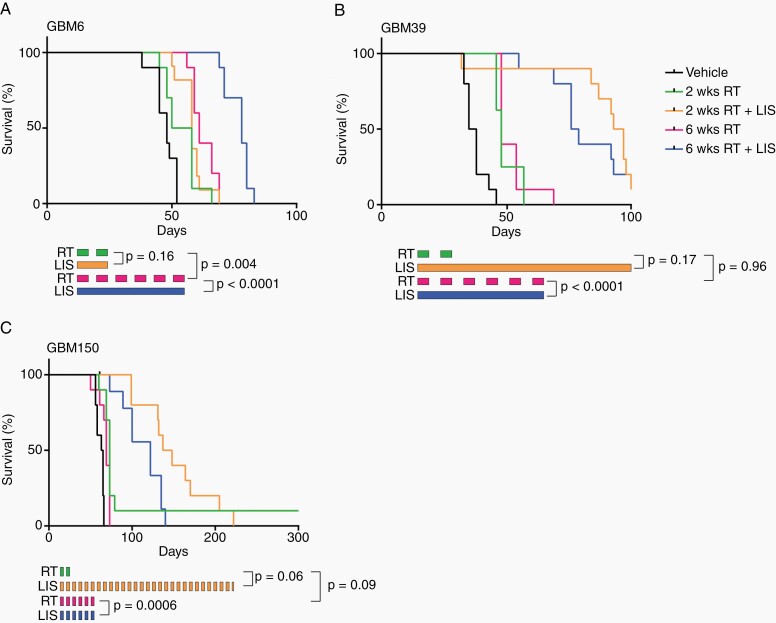
Fig. 3.
Lisavanbulin combined with six-week radiation regimens significantly improves overall survival (A-C). Mice with the indicated orthotopic PDXs were randomized and treated 14 days after tumor implantation. Mice were dosed with vehicle, RT, or RT + lisavanbulin (LIS). RT was given in 2 Gy fractions either Monday through Friday for 2 weeks or Monday, Wednesday, and Friday for 6 weeks. LIS dosing was limited to the RT window.
The radiation schedule used in these initial studies was limited to 5 days a week for 2 weeks because of the toxicity associated with whole head irradiation in mice. To evaluate a schedule more reminiscent of a typical 6-week clinical regimen, we also tested lisavanbulin in combination with radiation given 3 days a week for 6 weeks (2 Gy × 18 fractions) (Figure 3). The difference in overall treatment duration is significant in the context that tumor cells can continue to proliferate and repopulate the tumor during a six-week course of radiation, and if lisavanbulin significantly suppresses tumor repopulation, then we might anticipate a greater benefit for concurrent lisavanbulin when combined in this protracted RT dosing regimen. Consistent with this concept, when limited to the duration of RT, only lisavanbulin combined with the six-week RT regimen provided significant survival extension in GBM6 (78 days vs. 61 days, P < .0001), as compared to a two-week regimen (58 days vs. 54 days, P = .16). Similarly in GBM39 and GBM150, concurrent lisavanbulin and RT for 6 weeks significantly extended survival more than RT alone (P < .0001 and P = .0006, respectively), and provided a survival benefit that approximated the two-week RT schedule with extended lisavanbulin dosing (Figure 3B and C). These data support the concept that RT/lisavanbulin combinations can provide significant therapeutic benefit.
Lisavanbulin Addition to Chemoradiation and Adjuvant TMZ Regimens
GBM6 and GBM12 were treated with a two-week regimen of RT/TMZ alone or RT/TMZ with extended lisavanbulin dosing to test the potential value of lisavanbulin use in chemoradiation regimens (Supplementary Figure S1). GBM6 had the longest median survival (vehicle 46 days) and the RT/TMZ/lisavanbulin combination significantly extended survival over RT/TMZ alone (median 101 days vs. 66 days, P < .0001, Supplementary Figure S1a). Single agent lisavanbulin and RT/TMZ achieved intermediate rates of survival (lisavanbulin median 63 days vs. RT/TMZ median 66 days, P = .61). The RT/TMZ/lisavanbulin combination was not significantly different from RT/TMZ in the more aggressive GBM12 PDX (P = .56, Supplementary Figure S1b). Lisavanbulin alone extended survival 8 days over vehicle alone (P < .0001). This GBM12 study also contained two arms that evaluated continuous lisavanbulin combined with intermittent TMZ dosing on Days 1-5 of a 28-day cycle, which is similar to how patients are treated following the completion of combined RT/TMZ. In this regimen of ‘adjuvant’ TMZ, no significant difference in survival was observed between TMZ alone and TMZ+lisavanbulin (median TMZ 84 days vs. median adj TMZ+ lisavanbulin 98 days respectively, P = .79, Supplementary Figure S2). Thus, at least in these two PDXs, combination of lisavanbulin with RT/TMZ provides variable benefit in extending survival.
Lisavanbulin Combined with a Complete Chemo-radiotherapy Regimen
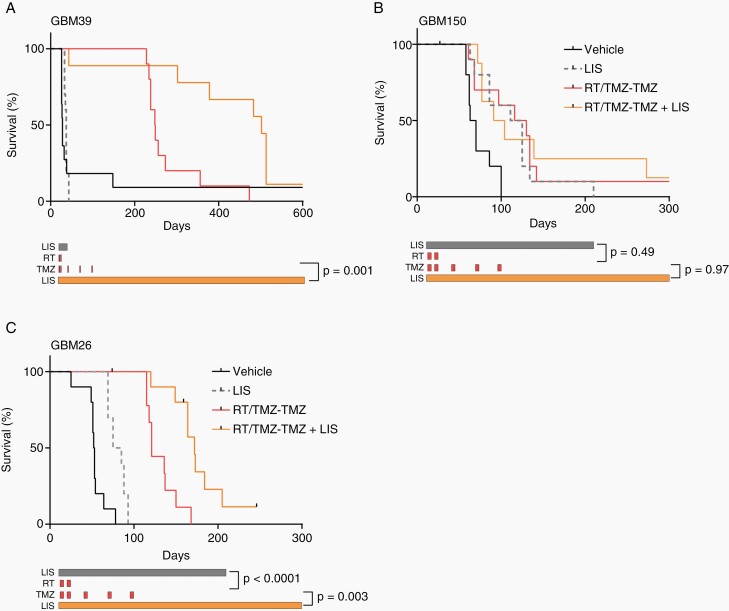
A combination of 6 weeks of RT (2 Gy × 30 fractions) and concurrent TMZ followed by 6 cycles of adjuvant TMZ (Days 1-5 every 28 days) is the standard of care for the treatment of newly diagnosed IDH-wildtype GBM. To approximate this regimen and test the potential additional benefit of lisavanbulin, mice were treated with 2 Gy × 10 fractions RT with concurrent TMZ followed by three cycles of TMZ (Days 1-5 every 28 days; RT/TMZ-TMZ), with or without continuous lisavanbulin therapy. Mice bearing GBM39, GBM150, and GBM26 intracranial tumors were treated with vehicle, lisavanbulin, RT/TMZ-TMZ, or lisavanbulin + RT/TMZ-TMZ (Figure 4). While lisavanbulin alone did not significantly extend survival in GBM39 (median vehicle 28 days vs. lisavanbulin 37 days, P = .54), lisavanbulin combined with RT/TMZ-TMZ doubled median survival rates (median RT/TMZ-TMZ 249 days vs. RT/TMZ-TMZ +lisavanbulin 502 days, P = .0001, Figure 4A). Of note, some variability in monotherapy performance was observed across GBM39 experiments which may be attributed to differences in dosing start time and number of cells implanted intracranially. In the TMZ resistant GBM150 PDX, no significant survival differences were observed between lisavanbulin monotherapy and RT/TMZ-TMZ treatment (median lisavanbulin 118 days vs. median RT/TMZ-TMZ 123 days, P = .49, Figure 4B), and lisavanbulin combined with RT/TMZ-TMZ did not significantly extend survival (median 98 days, P = .97). While these lisavanbulin dosing strategies were well tolerated in GBM39 and GBM150, substantial toxicity was observed in GBM26 (Figure 4C). In the latter model, single agent lisavanbulin extended survival from 53 days (median vehicle) to 80 days (P = .0001) with no documented toxicity. However, combination with RT alone or RT/TMZ resulted in dose-limiting toxicity that required treatment interruption and a 50% lisavanbulin dose-reduction in combinations with RT/TMZ (Supplementary Figure S3A). Despite treatment interruptions during RT/TMZ, the combination of lisavanbulin with RT/TMZ-TMZ resulted in significant survival gains compared to RT/TMZ-TMZ (median survival 172 days vs. 121 days, respectively; P = .003). Review of H&E slides at the study endpoint confirmed that both RT/TMZ-TMZ and lisavanbulin + RT/TMZ-TMZ treated animals succumbed to tumor burden (Supplementary Figure S3). These data demonstrate the potential for significant enhancement in survival in some GBM PDX models with the combination of lisavanbulin and concurrent RT/TMZ-TMZ.
Fig.4.
Lisavanbulin addition to RT/TMZ-TMZ significantly extends survival in responsive PDX model (A-C). Mice with the indicated orthotopic PDXs were randomized and treated approximately 14 days after tumor implantation. Mice were dosed with vehicle, lisavanbulin (LIS), RT/TMZ-TMZ, or RT/TMZ-TMZ + LIS. RT was given in 2 Gy fractions Monday through Friday for 2 weeks concurrently with 20 mg/kg TMZ. Adjuvant TMZ (50 mg/kg) was given days 1-5 every 28 days for three cycles while LIS dosing was continued Monday through Sunday until moribund.
Lisavanbulin Mechanism of Action
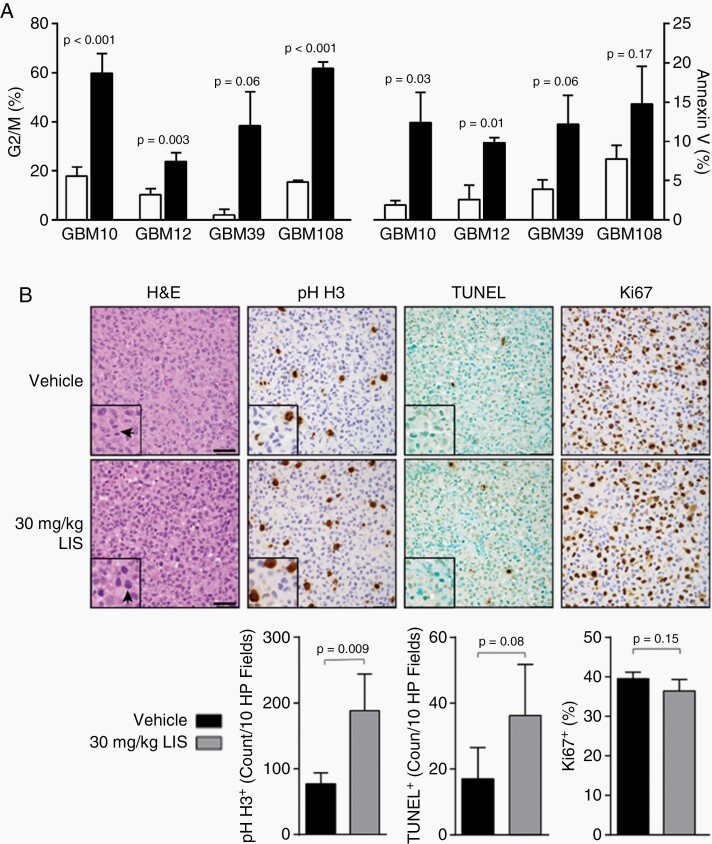
Cell cycle and annexin V analyses were performed on GBM10, GBM12, GBM39, and GBM108 short-term explant cultures using the active metabolite, avanbulin, to understand potential mechanisms underpinning the antitumor effects of lisavanbulin. Cells were exposed to 30 nM avanbulin for 72 h and harvested for analysis (Figure 5A). Collectively, increased G2/M arrest with LIS treatment was observed when compared to vehicle-treated controls (average 42.4% vs. 11.7%, P = .016). In parallel, increased apoptosis was also observed under these conditions (average 5.5% vehicle vs. 14.3% LIS, P = .001). These results are consistent with lisavanbulin effects on disrupting microtubule function and increased apoptosis.
Fig. 5.
Lisavanbulin mechanism of action. (A) GBM10, GBM12, GBM39, and GBM108 cells were treated with 30 nM avanbulin (BAL27862) for 72 h at which point cells were collected and analyzed for G2/M arrest (FACS) and apoptosis (annexin V). Comparisons were made across lines using two-sample t-tests. (B) Mice with orthotopic GBM39 tumors were dosed with 30 mg/kg lisavanbulin (LIS) for 8 days before being sacrificed 2 h after the final dose. Brains were harvested and fixed in formalin for paraffin embedding. Mitotic events (phospho-S10 H3 IHC) and apoptosis (TUNEL IHC) were quantified by our neuropathologist. Automated proliferation (Ki67 IHC) quantification was performed using Aperio ImageScope. Arrows in the H&E insets highlight the appearance of mitotic figures in vehicle and LIS-treated animals. Scale bars indicate 50 µm.
A subsequent pharmacodynamic experiment was conducted in orthotopic GBM39 xenografts to quantify mitotic events, apoptosis, and proliferation following lisavanbulin treatment (Figure 5B). H&E sections revealed hyper-condensed chromatin in a subset of cells. These cells were positive for the mitotic marker phospho-histone H3 (pH-H3) and quantification demonstrated a significantly higher percentage of pH-H3 positive (+) cells relative to vehicle-treated controls (P = .009). Follow-up TUNEL stain was largely negative in these pH-H3+ areas and was not significantly different across groups (P = .08). As no significant difference in Ki67 positivity was observed with lisavanbulin treatment (P = .15), these results are consistent with lisavanbulin-induced mitotic arrest as a predominant mechanism of antitumor effect. Whole exome sequencing data from these same cell lines grown as intracranial tumors was analyzed for alterations in apoptosis, cell cycle, DNA repair, and p53 signaling pathways. No features consistently associated with lisavanbulin response (Supplementary Table S3).
Discussion
The prognosis for GBM remains grim despite decades of intensive basic, translational, and clinical research efforts. Unlike most other solid malignancies, there have been no new FDA-approved drugs for GBM treatment in over a decade. While the reasons for this failure are multifactorial, the limited availability and utilization of clinically relevant, patient-derived, orthotopic tumor models may contribute to the lack of progress in developing curative therapies for this devastating disease. To address this limitation, the Mayo Clinic has developed a large panel of GBM PDX models that maintain the genomic and phenotypic characteristics of the original patient tumor samples.1 Here, we used a subset of these models to investigate the novel highly brain penetrant small-molecule tumor checkpoint controller, lisavanbulin, which binds to microtubules and promotes tumor cell death by activation of the spindle assembly checkpoint. With a focus on translating these studies into an optimal clinical therapeutic regimen, the studies evaluating the integration of lisavanbulin with conventional chemotherapy and fractionated radiation therapy were performed exclusively using orthotopic PDX tumors and dosing regimens that closely approximate those commonly used in patients. While the benefit of lisavanbulin monotherapy in most PDXs was relatively modest (average survival extension 29% in sensitive models), the combination of lisavanbulin with radiation alone or radio-chemotherapy resulted in more profound survival benefits in several models. The concept of multi-agent chemotherapy for solid tumors is based on the idea that each drug may preferentially kill a different sub-population of tumor clones to prevent or delay emergence of therapy resistance. We hypothesize that models which derive greater benefit from these combinatorial regimens have distinct sub-populations of tumor clones, one which is sensitive to LIS and another which is sensitive to RT/TMZ. Conversely, models less impacted by these combinations may be composed of tumor clone sub-populations with sensitivity to either LIS or RT/TMZ but not both. Not only does this study provide a strong rationale to pursue development of novel lisavanbulin combination therapies for GBM, but also provides an example of how orthotopic PDX models can be leveraged to clearly inform optimal clinical development of novel therapeutic strategies for GBM.
MTAs have been a foundation for cancer chemotherapy for over 60 years. Taxanes (paclitaxel, docetaxel, cabazitaxel) are one of the most commonly prescribed MTAs and function by stabilizing microtubules to prevent depolymerization. In contrast, vinca alkaloids, such as vincristine, and lisavanbulin bind to and block tubulin polymerization to prevent microtubule assembly. By deregulating microtubule dynamics, MTAs disrupt mitotic spindle assembly, which can result in mitotic arrest and subsequent cell death by apoptosis. Consistent with this mechanism of action, lisavanbulin's anticancer activity has previously been shown to be dependent on the activation of the spindle assembly checkpoint.16 Additionally, we have shown that lisavanbulin treatment of orthotopic GBM39 tumors resulted in a doubling in the number of cells arrested in mitosis and a trend towards increased apoptosis with drug treatment. These results are similar to studies with vincristine in GBM.17,18 Collectively, these data are all consistent with microtubule destabilization as an important mechanism of lisavanbulin action in GBM tumors.
Mitotic cells are exceptionally sensitive to radiation therapy, and the induction of a mitotic arrest likely is an important mechanism of radiosensitization for MTAs. The highly compact chromatin structure of mitotic chromosomes precludes access of DNA repair machinery to repair DNA double-strand breaks, and in classic cell synchronization studies, mitotic cells are 2- to 3-fold more sensitive to radiation-induced killing than cells in any other phase of the cell cycle.19 Although the fraction of cells arrested in mitosis at any point in time may be limited (see Figure 5B), the greater benefit of lisavanbulin combined with a six-week course of radiation (18 radiation fractions), as compared to a two-week regimen (10 fractions), could be related to the additional opportunities for a radiation-induced killing of arrested mitotic cells. Moreover, the 48-hour intervals between radiation during the 6-week course of radiation could have allowed time for a greater proportion of cycling cells to arrest in mitosis prior to the next radiation dose. This ‘reassortment’ following radiation exposure of surviving cells from radio-resistant to radio-sensitive phases of the cell cycle is a well-known phenomenon that governs response of tumors to radiation.19 Although combined treatment limited to just 2 weeks of lisavanbulin and radiation alone did not significantly enhance survival, the inclusion of concurrent lisavanbulin with RT/TMZ was significantly more effective than if lisavanbulin treatment was delayed until concurrent RT/TMZ treatment was completed. Collectively, these data strongly support the integration of lisavanbulin with concurrent RT/TMZ in clinical testing in GBM.
All GBM have regions of tumor protected by a relatively intact BBB, and consequently, distribution of drugs across the BBB is an important factor that can influence the efficacy of a given therapeutic strategy.20 Continuous tight junctions between brain capillary endothelial cells and activity of drug efflux pumps within the luminal membrane of these cells present both physical and biochemical barriers to drug distribution across the BBB. Most MTAs, including taxanes and vinca alkaloids, are subject to efflux at the BBB that limits their accumulation within the normal brain.
Taxanes, like paclitaxel and docetaxel, are substrates for both P-gp and MRP-1, two dominant efflux transporters within the BBB, and achievable levels of paclitaxel and docetaxel into normal mouse brain are 50% and 29% of plasma levels with 11 fold and 6.2 fold increased brain penetration respectively in P-gp knockout mice indicating limited delivery to the brain resulting from P-gp efflux.21–23 In addition, both these drugs are highly bound to plasma proteins (~98% bound for both paclitaxel and docetaxel) and have an even higher binding in the brain (>99.5% for both paclitaxel and docetaxel) indicating an extremely low free brain partitioning.24–26 Consistent with poor distribution across the BBB, these drugs have limited clinical activity in GBM.27,28 Vincristine also is an efflux substrate of P-gp and MRP-1 with restricted brain distribution having brain partition coefficients in P-gp and MRP-1 knockout mice greater than the wild-type mice by 1.3- to 3.6-fold. In addition to poor brain penetration, vincristine also has a plasma protein binding of 60.6% and displays dose-limiting toxicities such as peripheral neuropathies.29–31
While this drug has limited activity in GBM, vincristine is a component of the standard ‘PCV’ (procarbazine, CCNU, vincristine) chemotherapy regimen used for patients with relatively more indolent oligodendrogliomas, although the contribution of vincristine to PCV efficacy is controversial.32–35 In comparison to these FDA-approved MTAs, lisavanbulin has far superior brain distribution, with a brain-to-plasma ratio of 1.29 in wild-type mice and 0.98 in athymic nude mice bearing subcutaneous xenografts, and a relatively limited difference in brain distribution in mice lacking BCRP and P-gp. Interestingly, the initial Phase I clinical trial with lisavanbulin, two patients with recurrent GBM have had a sustained partial response to therapy.36 Coupled with the moderate to robust single agent activity of lisavanbulin across the majority of orthotopic PDXs tested, these data suggest that lisavanbulin, or other next generation, brain penetrant MTAs, may provide significant therapeutic benefits for a subset of patients with GBM.
Several salient results from these studies may directly inform the design of planned future clinical trials for lisavanbulin in brain tumors. First, in a subset of GBM models, the combination of lisavanbulin with either RT or RT/TMZ provided significantly greater benefit than lisavanbulin monotherapy and the extent of benefit was greatest with extended lisavanbulin dosing beyond the end of RT. Second, significant survival benefit was observed in PDXs with or without MGMT promoter hypermethylation (Table 1), which is a key prognostic biomarker in GBM related to tumor sensitivity to TMZ chemotherapy. Third, the triple combination of lisavanbulin, RT, and TMZ generally was well tolerated in most PDX, except in GBM26. In this PDX, excessive weight loss required treatment interruptions for both RT/TMZ and lisavanbulin, but despite the loss in treatment intensity, there was a substantial survival extension associated with combination therapy. The ongoing Adult Brain Tumor Consortium Phase I clinical trial is testing lisavanbulin in combination with RT only in newly diagnosed GBM lacking MGMT hypermethylation (ClinicalTrials.gov identifier: NCT03250299). Building on this trial, our data supports the concept of dosing lisavanbulin during and after RT/TMZ and adjuvant TMZ in all patients, regardless of MGMT promoter methylation status. Additional translational studies are necessary to define precision predictive biomarkers that can identify those GBM patients who are most likely to respond to lisavanbulin combination therapy.
Supplementary Material
Conflict of interest statement. The authors declare no potential conflicts of interest. H.L., F.B. and P.M. are employees and own stock options of Basilea Pharmaceutica International Ltd.
Authorship statement. The original idea was developed by J.N.S., H.L., F.B., and P.M. Intracranial survival experiments were done by K.K.B., B.L.C., M.A.S., J.L.P., L.H., and Z.H. Pharmacokinetic experiments and analyses were done by S.T., A.S-H, G.G., and W.F.E. Immunohistochemistry was completed by G.J.K. and D.M.B. In vitro experiments were performed by A.C.M. Data analysis was performed by D.M.B., P.A.D., M.L.K., and R.A.V. Tables and figures were created by D.M.B. and R.A.V. The manuscript was drafted by D.M.B. and J.N.S. All authors read and approved the final manuscript.
Funding
This study was supported by Basilea Pharmaceutica International Ltd.
References
- 1. Vaubel RA, Tian S, Remonde D, et al. Genomic and phenotypic characterization of a broad panel of patient-derived xenografts reflects the diversity of glioblastoma. Clin Cancer Res. 2020;26(5):1094–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dumontet C, Jordan MA. Microtubule-binding agents: a dynamic field of cancer therapeutics. Nat Rev Drug Discov. 2010;9(10):790–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Prota AE, Danel F, Bachmann F, et al. The novel microtubule-destabilizing drug BAL27862 binds to the colchicine site of tubulin with distinct effects on microtubule organization. J Mol Biol. 2014;426(8):1848–1860. [DOI] [PubMed] [Google Scholar]
- 4. Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer. 2004;4(4):253–265. [DOI] [PubMed] [Google Scholar]
- 5. Jordan MA, Wendell K, Gardiner S, Derry WB, Copp H, Wilson L. Mitotic block induced in HeLa cells by low concentrations of paclitaxel (Taxol) results in abnormal mitotic exit and apoptotic cell death. Cancer Res. 1996;56(4):816–825. [PubMed] [Google Scholar]
- 6. Yvon AM, Wadsworth P, Jordan MA. Taxol suppresses dynamics of individual microtubules in living human tumor cells. Mol Biol Cell. 1999;10(4):947–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schiff PB, Fant J, Horwitz SB. Promotion of microtubule assembly in vitro by taxol. Nature. 1979;277(5698):665–667. [DOI] [PubMed] [Google Scholar]
- 8. Jordan MA, Thrower D, Wilson L. Mechanism of inhibition of cell proliferation by Vinca alkaloids. Cancer Res. 1991;51(8):2212–2222. [PubMed] [Google Scholar]
- 9. Canta A, Chiorazzi A, Cavaletti G. Tubulin: a target for antineoplastic drugs into the cancer cells but also in the peripheral nervous system. Curr Med Chem. 2009;16(11):1315–1324. [DOI] [PubMed] [Google Scholar]
- 10. McCarroll JA, Gan PP, Liu M, Kavallaris M. betaIII-tubulin is a multifunctional protein involved in drug sensitivity and tumorigenesis in non-small cell lung cancer. Cancer Res. 2010;70(12):4995–5003. [DOI] [PubMed] [Google Scholar]
- 11. Goldstein LJ, Galski H, Fojo A, et al. Expression of a multidrug resistance gene in human cancers. J Natl Cancer Inst. 1989;81(2):116–124. [DOI] [PubMed] [Google Scholar]
- 12. Sharma A, Broggini-Tenzer A, Vuong V, et al. The novel microtubule targeting agent BAL101553 in combination with radiotherapy in treatment-refractory tumor models. Radiother Oncol. 2017;124(3):433–438. [DOI] [PubMed] [Google Scholar]
- 13. Carlson BL, Pokorny JL, Schroeder MA, Sarkaria JN. Establishment, maintenance and in vitro and in vivo applications of primary human glioblastoma multiforme (GBM) xenograft models for translational biology studies and drug discovery. Curr Protoc Pharmacol. 2011; Chapter 14:Unit 14 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gampa G, Kenchappa RS, Mohammad AS, et al. Enhancing brain retention of a KIF11 inhibitor significantly improves its efficacy in a mouse model of Glioblastoma. Sci Rep. 2020;10(1):6524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schmitt-Hoffman A, Klauer D, Gebhardt K, et al. BAL27862: A Unique Microtubule-targeted Agent with a Potential for the Treatment of Human Brain Tumors. Paper presented at: EORTC NCI AACR2009; Boston, MA. [Google Scholar]
- 16. Bachmann FBK, Lane HA. BAL101553 (prodrug of BAL27862): the spindle assembly checkpoint is required for anticancer activity. 2015. https://cancerres.aacrjournals.org/content/75/15_Supplement/3789
- 17. Tivnan A, Zakaria Z, O’Leary C, et al. Inhibition of multidrug resistance protein 1 (MRP1) improves chemotherapy drug response in primary and recurrent glioblastoma multiforme. Front Neurosci. 2015;9:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ryu J, Pyo J, Lee CW, Kim JE. An Aurora kinase inhibitor, AMG900, inhibits glioblastoma cell proliferation by disrupting mitotic progression. Cancer Med. 2018;7(11):5589–5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hall EJGA. Radiobiology for the Radiologist. 7th ed. Lippincott Williams & Wilkins; 2012. [Google Scholar]
- 20. Sarkaria JN, Hu LS, Parney IF, et al. Is the blood-brain barrier really disrupted in all glioblastomas? A critical assessment of existing clinical data. Neuro Oncol. 2018;20(2):184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kemper EM, van Zandbergen AE, Cleypool C, et al. Increased penetration of paclitaxel into the brain by inhibition of P-glycoprotein. Clin Cancer Res. 2003;9(7):2849–2855. [PubMed] [Google Scholar]
- 22. Kemper EM, Verheij M, Boogerd W, Beijnen JH, van Tellingen O. Improved penetration of docetaxel into the brain by co-administration of inhibitors of P-glycoprotein. Eur J Cancer. 2004;40(8):1269–1274. [DOI] [PubMed] [Google Scholar]
- 23. Brooks TA, Minderman H, O’Loughlin KL, et al. Taxane-based reversal agents modulate drug resistance mediated by P-glycoprotein, multidrug resistance protein, and breast cancer resistance protein. Mol Cancer Ther. 2003;2(11):1195–1205. [PubMed] [Google Scholar]
- 24. Uchida Y, Ohtsuki S, Kamiie J, Terasaki T. Blood-brain barrier (BBB) pharmacoproteomics: reconstruction of in vivo brain distribution of 11 P-glycoprotein substrates based on the BBB transporter protein concentration, in vitro intrinsic transport activity, and unbound fraction in plasma and brain in mice. J Pharmacol Exp Ther. 2011;339(2):579–588. [DOI] [PubMed] [Google Scholar]
- 25. Urien S, Barré J, Morin C, Paccaly A, Montay G, Tillement JP. Docetaxel serum protein binding with high affinity to alpha 1-acid glycoprotein. Invest New Drugs. 1996;14(2):147–151. [DOI] [PubMed] [Google Scholar]
- 26. Loryan I, Fridén M, Hammarlund-Udenaes M. The brain slice method for studying drug distribution in the CNS. Fluids Barriers CNS. 2013;10(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rice A, Michaelis ML, Georg G, Liu Y, Turunen B, Audus KL. Overcoming the blood-brain barrier to taxane delivery for neurodegenerative diseases and brain tumors. J Mol Neurosci. 2003;20(3):339–343. [DOI] [PubMed] [Google Scholar]
- 28. Heimans JJ, Vermorken JB, Wolbers JG, et al. Paclitaxel (Taxol) concentrations in brain tumor tissue. Ann Oncol. 1994;5(10):951–953. [DOI] [PubMed] [Google Scholar]
- 29. Greig NH, Soncrant TT, Shetty HU, Momma S, Smith QR, Rapoport SI. Brain uptake and anticancer activities of vincristine and vinblastine are restricted by their low cerebrovascular permeability and binding to plasma constituents in rat. Cancer Chemother Pharmacol. 1990;26(4):263–268. [DOI] [PubMed] [Google Scholar]
- 30. Wang F, Zhou F, Kruh GD, Gallo JM. Influence of blood-brain barrier efflux pumps on the distribution of vincristine in brain and brain tumors. Neuro Oncol. 2010;12(10):1043–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. De Witt M, Gamble A, Hanson D, et al. Repurposing mebendazole as a replacement for vincristine for the treatment of brain tumors. Mol Med. 2017;23:50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kellie SJ, Barbaric D, Koopmans P, Earl J, Carr DJ, de Graaf SS. Cerebrospinal fluid concentrations of vincristine after bolus intravenous dosing: a surrogate marker of brain penetration. Cancer. 2002;94(6):1815–1820. [DOI] [PubMed] [Google Scholar]
- 33. Stewart DJ. A critique of the role of the blood-brain barrier in the chemotherapy of human brain tumors. J Neurooncol. 1994;20(2):121–139. [DOI] [PubMed] [Google Scholar]
- 34. Chen W, Wang D, Du X, et al. Glioma cells escaped from cytotoxicity of temozolomide and vincristine by communicating with human astrocytes. Med Oncol. 2015;32(3):43. [DOI] [PubMed] [Google Scholar]
- 35. Xi G, Rajaram V, Mania-Farnell B, et al. Efficacy of vincristine administered via convection-enhanced delivery in a rodent brainstem tumor model documented by bioluminescence imaging. Childs Nerv Syst. 2012;28(4):565–574. [DOI] [PubMed] [Google Scholar]
- 36. Lopez JSKRS, Rulach R, et al. Phase 1/2a study of once daily oral BAL101553, a novel tumor checkpoint controller (TCC), in adult patients with progressive or recurrent glioblastoma (GBM) or high-grade glioma. Paper presented at: American Society of Clinical Oncology (ASCO) Annual Meeting 2019; Chicago, IL. https://ascopubs.org/doi/abs/10.1200/JCO.2019.37.15_suppl.2025
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.