Abstract
Bowen's disease (BD) is an in-situ squamous cell carcinoma of epidermis. The etiology of BD is multifactorial with high incidence among Caucasians. BD is common in photo-exposed areas of skin, but other sites can also be involved. Lesions are usually solitary. The morphology of BD differs based on age of the lesion, site of origin, and the degree of keratinization. BD is considered as the “lull before the storm,” which precedes an overt squamous cell carcinoma. Histopathology is the gold standard diagnostic modality to confirm the diagnosis. Immunohistochemistry, dermoscopy, and reflectance confocal microscopy are the adjuvant modalities used in the diagnosis of BD. The treatment depends on various factors like site, size, immune status, patient's age, esthetic outcome, etc. The available therapeutic modalities include topical chemotherapy, surgical modalities, light-based modalities, and destructive therapies. It requires a combined effort of dermatologist, oncosurgeon, and plastic surgeon to plan and execute the management in various presentations of BD.
Keywords: Bowen's disease, erythroplasia of Queyrat, squamous cell carcinoma
Introduction
Bowen's disease (BD) is an in-situ squamous cell carcinoma (SCC) of epidermis.[1] The major etiological factors of BD include ultraviolet light exposure, immunosuppression, and Human Papilloma Virus (HPV) infections. BD is common in photo-exposed areas of skin, but other sites can also be involved. The natural course of BD is usually prolonged, needing appropriate treatment.[2] In this review, various clinical presentations, differential diagnosis, and therapeutic options of BD are discussed.
History of Bowen's Disease
John Templeton Bowen (1857–1940), Professor from Boston, initially reported two unusual cases with multiple erythematous plaques over photo-protected areas under a descriptive title “chronic atypical epithelial proliferation” in 1912.[3] Jean Darier, the French Dermatologist also recognized two more similar cases and named it as “precancerous dermatosis of Bowen or dyskeratosis lenticularis et discoides” in his book. This disease has been called as Bowen's disease over a period of time.[4,5]
Etiopathogenesis of Bowen's Disease
The etiology of BD is multifactorial. The risk factors include Caucasian race, fair skin, photo-sensitive individuals, and an increase in total occupational and recreational sun exposure.[2] The cumulative exposure to ultraviolet light radiation produces DNA damage and immunosuppression facilitating the clonal expansion of underlying p53 mutation.[6,7]
The patients with allogeneic organ transplantation on immunosuppressive drugs such as systemic corticosteroids, azathioprine, and cyclosporine may activate different pathways resulting in induction and promotion of skin malignancies.[2] In immunosuppressed patients, Merkel cell polyomavirus has been associated.[8]
The BD arising secondary to arsenic exposure is known to occur after several decades.[9] Arsenic exposures produce oxidative stress and deplete antioxidants. It causes immune dysregulation, impaired DNA repair, genotoxicity, and disorganized signal transduction.[10]
In BD, several subtypes of Human Papilloma Virus such as HPV 16, 18, 31, 33, 35, 54, 58, 61, 62, and 73 have been detected.[1] A strong association with HPV 16 had been reported with vulvar BD.[11] HPV DNA is observed in 31% of the extragenital BD.[2] HPV 16, 31/33, 56, and 71 have been detected by in-situ hybridization in nail BD.[12]
BD is also known to arise following ionizing radiation, thermal skin injury, inflammatory dermatoses such as chronic lupus erythematosus, lupus vulgaris, and following Psoralens and Ultraviolet-A radiation.[2,9,13] BD has been reported in a person who was involved in arc welding.[14] The incidence of aneuploidy and DNA instability is high in lesional skin of BD.[15,16] [Figure 1] summarize the etiopathogenesis model of BD.
Figure 1.
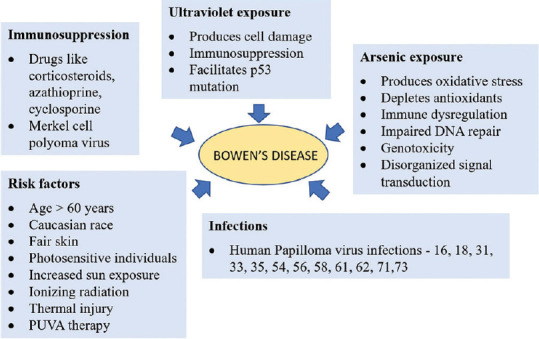
Etiopathogenesis model of Bowen's disease
Erythroplasia of Queyrat (EQ), also known as the squamous cell carcinoma in-situ of penis, has risk factors such as uncircumcised penis, phimosis, poor hygiene, chronic inflammation, tobacco usage, and PUVA therapy. HPV 8, 16, 39, and 51 have been isolated from the lesions of EQ. Around 6% of lichen sclerosus of penis is known to develop EQ.[2] Penile EQ is increasingly reported with the use of biologics in dermatology and rheumatology.[17]
Demographics
BD typically occurs in individuals above 60 years of age.[2] It is rare in individuals below 30 years of age.[18] Immunocompromised individuals are at risk of developing BD at younger age.[1] Most studies have revealed a slight female preponderance.[19] The incidence is high in Caucasians (1.42/1000).[20] However, the true incidence of BD in Indian population is not known.[21] BD is a rare disease among blacks.[22] In Caucasians, sun-exposed sites are commonly affected, whereas in Japanese population, truncal lesions are observed in 53% of individuals.[2]
Kossard and Rosen studied 1001 patients with BD and found head and neck (44%) as the most common site followed by lower extremity (29.8%), upper extremity (19.8%), and trunk (6.5%).[23] In men, BD is common in bald scalp, neck, and anterior trunk. However, cheeks and lower legs are more likely to be affected in females.[1] Rarely lips, palms, soles, nipple, nail bed, umblicus, eyelid, conjunctiva, and external auditory canal are affected.[24,25] Fu P et al. described two cases of familial BD indicating a probable genetic basis.[26]
Clinical features
The morphology of BD differs based on age of the lesion, site of origin, and degree of keratinization.[2] In places where keratinization is absent, lesions are erythematous and velvety in nature. This erythema is masked by scaling in lesions occurring over keratinized epithelium.[27] The clinical presentation is also altered when it occurs in intertriginous, moist or hyperkeratotic surfaces.[2] Lesions are usually solitary, whereas multiple lesions are seen in 10%–20% of the affected individuals. In case of multicentric BD, immunosuppression and arsenicosis should be considered.[18,28]
Classical BD
The classical lesions of BD are asymptomatic, while larger lesions can be pruritic. Most commonly BD presents as a slow-growing, well-demarcated, erythematous, scaly patch or plaque [Figures 2 and 3]. Classical BD is associated with some degree of redness, ranging from pink to bright salmon-red erythema. The overlying scale may be white or yellow in color and rupial in nature, which can be easily removed or adherent. The removal of scale does not produce any bleeding and reveals a erythematous wet surface.[2] The scale may be very well pronounced mimicking psoriasis.[29]
Figure 2.
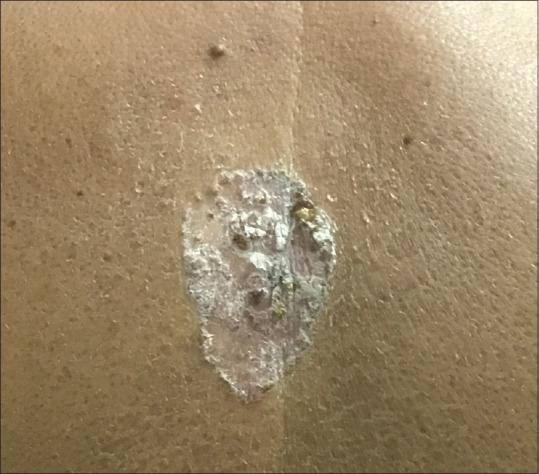
A single well-defined erythematous scaly crusted plaque
Figure 3.
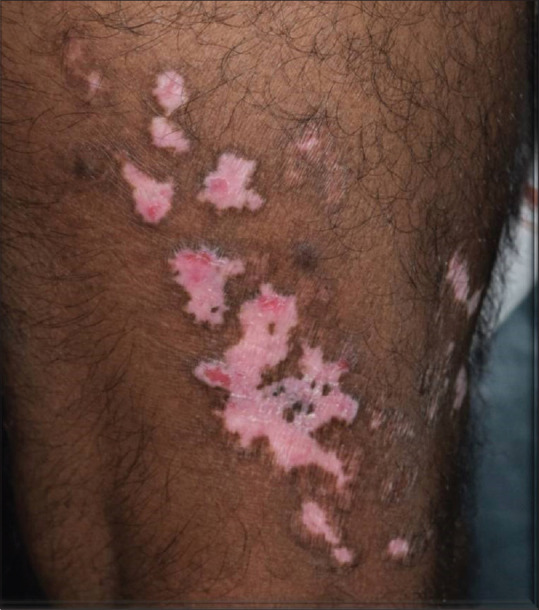
Multifocal Bowen's disease - Focal erythematous scaly infiltrated plaques localized to vitiligo macules
The lesions usually have a leveled surface, which sometimes become crusted, hyperkeratotic or fissured.[18,24] As the lesion evolves, spontaneous cicatrization may develop in a part of the lesion. Occasionally the plaque may have a stuck-on appearance and can easily mimic sessile seborrheic keratosis.[29] The size of the tumor varies from a few millimeters to several centimeters, based on the duration of disease.[18] The time taken for full expression of this premalignant condition varies from 2 to 40 years, favoring the slow and lateral spread of the condition in an erratic manner.[18,24] Morton et al.[30] defined “large Bowen's Disease” when the lesion dimension exceeded more than 2 cm. Lopez et al.[31] termed lesions with dimensions more than 3 cm as “extensive Bowen's Disease” [Figure 4].
Figure 4.
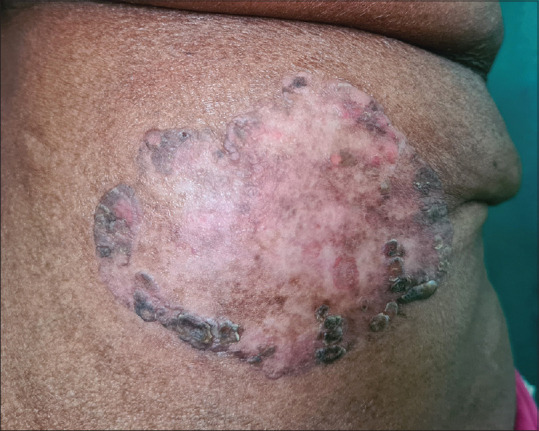
Giant Bowen's disease - A single, large, well-defined erythematous plaque with peripheral crusting
Arsenic exposure should be considered when lesions are multiple, recurrent, primarily occurring in sun-protected regions such as palms and soles.[32,33] HPV associated BD is also more common in sun-protected areas.[1]
BD of genitalia
The SCC in-situ of penis is eponymously termed as Erythroplasia of Queyrat and Bowen's disease. Though both are pathologically same process, the term EQ is used for lesions over mucosal surfaces such as inner prepuce, glans, and urethra. The term BD is used when the skin of shaft of penis is affected. BD of penis presents as well-defined, single, dull-red scaly plaques often with crusting and pigmentary changes. BD has also been reported to arise from inguinal and suprapubic area. EQ is painless, very well-demarcated, bright-red, velvety, shiny, plaque-like appearance[34,35] [Figure 5]. In circumcised individuals, the lesions are erythematous, crusted, or lichenoid without any velvety changes.[2] EQ have been reported to arise along with Zoon's balanitis.[36] Sexual partner (s) of men with EQ should be evaluated as they are prone to develop preinvasive and invasive malignancy of cervix or anus.[29] The vulvar BD presents with a heaped-up, leukotic, macerated surface over a dull erythematous background.[2]
Figure 5.
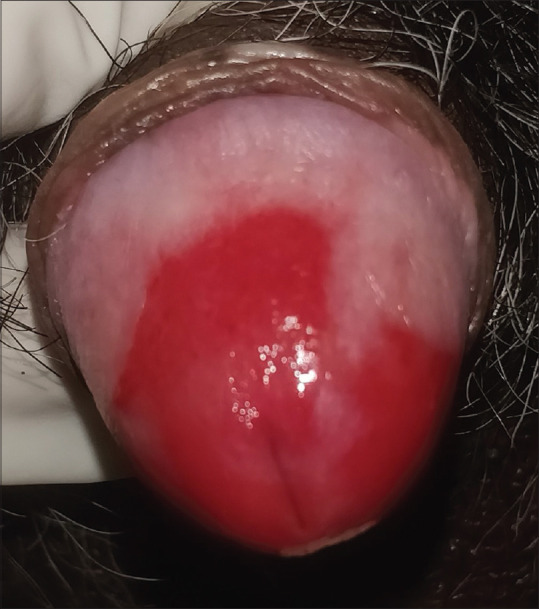
Erythroplasia of Queyrat - Sharply demarcated shiny, erythematous plaque over glans penis
Perianal BD
The perianal BD is more common among women.[25] Perianal BD have minor symptoms such as itching or burning sensation. Around one-third of the patients complain of a bleeding lesion or mass.[37] In anogenital BD, various morphological forms such as nodular, pigmented, verrucous, ulcerated, leukoplakic, and polypoidal have been described in literature. In the anogenital region, the mean size at the time of biopsy is around 1.3 cm2.[38] The risk of invasive carcinoma is 2%–6% in this entity.[25]
Periungual BD
Periungual BD is more common in men, usually observed in the first three digits of left hand. It clinically manifests as flat, erythematous patches with slight scaling to crusted verrucoid lesions.[2] It can also manifest as hyperkeratotic, papillomatous or warty proliferations, scaling or erosions of nail fold, whitish cuticle, periungual swelling, paronychia, fissuring or ulceration of the lateral nail groove, in-grown nails, granulation-like tissue beneath, partial loss of the nail plate, onycholysis, and nail dystrophy. Pseudo-Hutchinson sign, longitudinal melanonychia, or erythronychia have been observed in nail BD.[12,39,40,41] The pseudo-fibrokeratoma associated with melanocytic pigmentation serves as a diagnostic clue in nail BD.[42] Polydactylous BD is a rare nail BD subtype.[43] In a case of Fanconi's anemia, multiple fingers with multicentricity has been reported.[44] The HPV-associated periungual BD has put forward the genital digital spread as a probable mechanism of tumor induction.[40]
Palmar BD
BD of palms are often associated with aresenicosis and present with expanding diameter, fissuring, and frictional hypertrophy.[2,45] Ulceration can occur secondary to repeated friction.[24]
Facial BD
BD of face manifests as broad patches of faint erythema with minimal scaling or with a typical appearance of solar keratosis with bright red erythema and an adherent, yellow, keratotic scale.[2] The involvement of eccrine gland in BD is not common, usually confined to temple region.[14]
Mucosal BD
Grossly mucosal lesions have one of these three appearances: i) erythroplasia-like, ii) nodular or papillomatous, and iii) ulcerative. These morphological lesions correlate with three pathological stages proportionate to the age of tumor, severity of infiltration, and degeneration.[26] It can also present as a velvety reddish plaque.[1]
Nipple BD
Eventhough BD is intraepidermal, the uniqueness of nipple anatomy paves way for the tumor to spread via lactiferous ducts as it is in continuation with nipple epidermal layer. The nipple BD can present either as a raised, pruritic, scaly plaque or as eczematous lesion with crusting, bleeding, or ulceration.[46]
Pigmented BD
Pigmented BD is a rare subtype constituting less than 2% of BD. It presents clinically as a well-defined, hyperpigmented, flat or verrucous plaque with velvety surface.[47] It is more commonly seen in genital and interdigital areas, but also reported in sites such as lips, digits, and umblicus.[48] The cause for pigmentation in this variant is due to increased melanocyte hyperplasia with hypertrophic dendritic processes, the presence of well-differentiated atypical keratinocytes, or due to specific cytokines and growth factors produced by tumor cells.[49] The verrucous BD is a rare subtype, which often incites the suspicion of invasive malignancy.[9]
In HIV patients, extensive lesions involving penis, scrotum, and inguinal canal have been reported.[50] In a patient with chronic lymphocytic leukemia, symmetrical bilateral BD has been reported.[51] The differential diagnoses of BD based on the site and morphological variants are enlisted in Table 1.[1,2,9,12,32,46,47,52,53,54,55]
Table 1.
Clinical differential diagnoses of Bowen’s disease
| Cutaneous erythematous BD |
| Actinic keratosis |
| Amelanotic melanoma |
| Basal cell carcinoma |
| Clear cell acanthoma |
| Discoid lupus erythematosus |
| Irritated or inflamed seborrheic keratosis |
| Lichen simplex chronicus |
| Lichen planus |
| Nummular eczema |
| Psoriasis |
| Seborrheic eczema |
| Squamous cell carcinoma |
| Warts |
| Pigmented BD |
| Blue naevi |
| Bowenoid papulosis |
| Lichen planus-like keratosis |
| Melanocytic Naevi |
| Melanoma |
| Pigmented actinic keratosis |
| Pigmented basal cell carcinoma |
| Seborrheic keratosis |
| Solar lentigo |
| Verrucous BD |
| Hypertrophic lichen planus |
| Seborrheic keratosis |
| Verruca vulgaris |
| Verrucous carcinoma |
| Nail BD |
| Amelanotic malignant melanoma |
| Finger eczema |
| Glomus tumor |
| Nail dystrophy |
| Nail lichen planus |
| Onychomycosis |
| Paronychia |
| Periungual wart |
| Psoriasis |
| Pyogenic granuloma |
| Subungual exostosis |
| Subungual keratoacanthoma |
| Squamous cell carcinoma |
| Verrucous tuberculosis |
| Nipple BD |
| Paget’s disease |
| Erythroplasia of Queyrat |
| Candidiasis |
| Erosive lichen planus |
| Extramammary Paget’s disease |
| Fixed drug eruption |
| Lichen sclerosus |
| Penile psoriasis |
| Zoon’s balanitis |
| Perianal BD |
| Condyloma acuminata |
| External hemorrhoids |
| Monilial infections |
| Papillomas |
| Skin tags |
Disease course and prognosis
BD usually have an excellent prognosis because it is a slow-growing premalignant lesion.[21] Even spontaneous regression of BD has been reported, probably due to Fas-mediated apoptosis.[56] In BD, recurrence is relatively rare and is approximately 6% within 5 years of taking sufficient treatment. The recurrence is more common among immunosuppressed individuals.[1] BD is a marker of solar damage; hence, patients should be followed up as they are more prone to develop other UV-induced cutaneous malignancies.[9]
Progression to Squamous cell carcinoma
The development of invasive SCC is due to destruction of basement membrane mediated by metalloproteinases. The development of invasive carcinomas is more common among elderly people and immunocompromised individuals.[14] The HIV infection, by producing Tat protein and decreasing the expression of proteins such as HPV E6 and E7, directly promotes invasive SCC in-situ transformation.[50] The appearance of a large group of flat epithelial cells without keratin production in the dermis favors the progression into invasive SCC.[24]
The clinical signs suggestive of malignant transformation are ulceration, bleeding, and nodule formation.[19] According to few retrospective studies, the risk of malignant transformation is around 3% in case of extra-genital BD and 10% in EQ.[2] The invasive SCC of genitals tends to be more aggressive and metastatic.[29] Around 16% of the invasive SCC of penis arises from EQ, while almost 100% of the invasive SCCs arising from photo-exposed areas are from underlying BD.[2] When confined to the epidermis, BD does not carry any risk of metastasis. However, one-third of the BD which progressed to invasive SCC can metastasize.[18]
Bowen's disease vs Bowenoid papulosis
Bowenoid papulosis (BP) is a histopathological mimicker of BD with a lesser degree of cytological atypia. It is clinically characterized by the presence of reddish-brown pigmented verrucous papules and plaque on genitalia thereby mimicking genital warts. It usually affects glans penis and shaft of penis in men and vulva and perineal regions in women. BP can be differentiated from BD by earlier onset of age, smaller number of lesions, multiplicity of lesions, verrucoid appearance of few lesions, and tendency of spontaneous regression.[57,58]
Associations of BD
BD have been reported to arise from seborrheic keratosis, porokeratosis, Becker's nevus, erythema ab igne, smallpox vaccine scar, and outer sheet of epidermoid and follicular cyst.[59,60,61,62,63,64] There have been reports of a few cases of invasive adenxal carcinomas arising from BD. This includes phenotypes such as sebaceous, trichilemmal, poroid and in apocrine/mucinous glandular differentiation.[65] The various conditions associated with BD are multiple clear cell acanthoma, eccrine poroma, porocarcinoma, porokeratotic-eccrine ostial dermal ductal nevus, extramammary Paget's disease, Merkel cell carcinoma, sebaceous carcinoma, and trichilemmal carcinoma.[66,67,68,69,70,71,72,73]
Around 30%–50% of individuals with BD may have subsequent or previous history of nonmelanoma skin cancer.[33] Though previously BD have been reported to be associated with internal malignancies such as liver and lung carcinomas, recent studies failed to confirm those findings.[24,74,75]
Investigations
Histopathology
Histopathology is the gold standard diagnostic modality to confirm the diagnosis.[18] The epidermis shows hyperkeratosis and parakeratosis, marked acanthosis with elongation and thickening of rete ridges. The keratinocytes show atypia, which spans the entire epidermis, not breaching the dermo-epidermal junction. The keratinocytes in BD demonstrate intense mitotic activity, pleomorphism, and very large nuclei [Figure 6]. The accompanying loss of maturity and polarity gives the epidermis a “windblown” appearance.[76]
Figure 6.
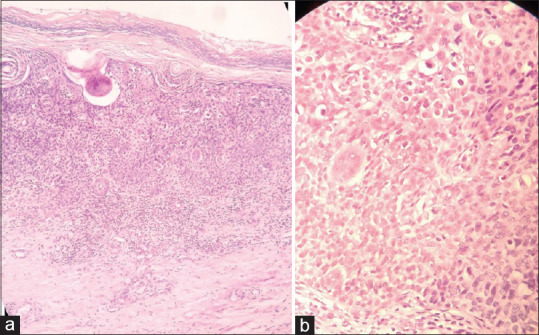
(a) Staining showing full thickness atypia/dysplasia (H and E 10x). (b) Staining showing areas of hyperchromasia, pleomorphism, and atypical mitosis (H and E 40x)
In BD, two types of giant cells are recognized in the epidermis. In one type, a keratinocyte “cannibalizes” an entire dyskeratotic cell and is seen within the cytoplasm of the phagocytosing cell. In another type, the giant cell contains multiple nuclei in the center, which are surrounded by dyskeratotic tonofilaments. There may be moderate lymphocytic infiltrates in the dermis.[77] The cells of the upper dermis occasionally undergo vacuolization with abundant and strong eosinophilic cytoplasm.[76] The secondary amyloid deposition is a known fact in BD and it might herald the regression of tumor. The BD have been described to be associated with sebaceous or mucinous metaplasia.[25] The histopathological variants of BD are tabulated in Table 2.[14,25,58,76,79]
Table 2.
Histopathological variants of Bowen’s disease
| Histopathological variant | Diagnostic histopathological clues |
|---|---|
| Acantholytic | Intraepidermal bulla/cleft in suprabasal location |
| Acantholytic anaplastic keratinocytes within bulla | |
| Atrophic BD | Thinning of epidermis |
| Full thickness atypia and disorganization | |
| Clear cell BD | Clear cell changes exceed 80% of total tumor population |
| Represents outer root sheath differentiation | |
| Express CK 13, CK 15, CK 16 | |
| Epidermolytic | Incidental findings of epidermolytic hyperkeratosis |
| Irregular BD | Highly pleomorphic |
| Absence of either hyperkeratosis or parakeratosis | |
| Irregular acanthosis | |
| Extensive chronic inflammation in dermis | |
| Orthokeratotic | Predominant orthokeratosis |
| Paucity of parakeratosis | |
| Preservation of granular layer | |
| Psoriasiform | Parakeratosis |
| Regular, marked acanthosis with thickening of rete ridges | |
| Pigmented | Melanin pigments in cytoplasm of atypical keratinocytes |
| Increased melanin in melanophages of dermis | |
| Papillomated BD | Exophytic/endophytic growth pattern |
| Prominent koilocytosis | |
| Pagetoid BD | Nests of cells with pale cytoplasm |
| Intervening thin strands of relatively normal keratinocytes | |
| May spare the basal layer | |
| Express cytokeratin 7 | |
| Verrucous- | Marked hyperkeratosis and church-spire papillomatosis |
| Hyperkeratotic | |
| Intervening pit-like invaginations |
The histopathology of mucous membrane BD lesions are less definite than skin BD.[26] In EQ, there are fewer dyskeratotic cells and multinucleate cells than in BD, with rich plasma cells in the dermis.[14] The EQ associated with HPV infections are undifferentiated, while those related to lichen sclerosus are differentiated.[19] The histopathological differential diagnoses of BD are mentioned in Table 3.[1,2,14,58,72,76,75,78,80,81,82,83,84]
Table 3.
Histopathological differential diagnosis of Bowen’s disease
| Disease | Differentiating feature |
|---|---|
| Actinic keratosis | Alternating pattern of parakeratosis and orthokeratosis |
| Basal layer always involved | |
| Sparing of acrosyringia and acrotrichia | |
| No clear border - Diffuse transition into surrounding epidermis | |
| Less prominent mitotic figures | |
| Bowenoid papulosis | Numerous mitosis in metaphase |
| Small basophilic inclusions in cytoplasm of granular layer | |
| Koilocyte-like cells | |
| Clonal seborrheic keratosis | Well-defined nests of cells in epidermis |
| Nuclei appear small and darkly stained | |
| Intercellular bridges seen in only few areas | |
| Hidracanthoma simplex | Exhibits “Jadassohn phenomenon” composed of bland basaloid cells |
| Characteristic intracytoplasmic glycogen and occasional ductal structures | |
| Invasive squamous cell carcinoma | Large islands of nests or islands of tumor cells expanding to deep dermis from overlying epidermis |
| Tumor have pushing and expansile border | |
| Intraepidermal Merkel cell carcinoma | Intraepidermal nests of merkel cells |
| Stain positive for CK20, synaptophysin, chromogranin | |
| Intraepidermal sebaceous carcinoma | Tumor composed of germinative, transitional, and mature sebaceous cells |
| Paget’s disease | Nest-like or glandular-like patterns with central-lumen abundant in basal layer |
| No dyskeratosis | |
| Stain positive for carcinoembryonic antigen, mucin, Alcian blue, aldehyde fuchsin | |
| Periodic acid-Schiff positive and diastase resistant | |
| Overexpress cytokeratin 7 and gross cystic disease fluid protein 15 (GCDFP-15). | |
| Pagetoid dyskeratosis | Scattered pale cells with small pyknotic nucleus |
| Pagetoid melanoma in-situ | Stains positive for S100 and/or Melan-A/MART 1 |
| Do not stain for cytokeratins | |
| Podophyllin-induced wart changes | Absence of atypical cells and multinucleate giant cells |
| Trichilemmal carcinoma | Lobular aggregations of tumor cells |
| Trichilemmal-type of keratinization | |
| Peripheral cell palisading of basaloid cells | |
| Glycogen deposition in cytoplasm of pale/clear cells |
Immunohistochemistry
In BD, the keratinocyte nuclei for proliferating cell nuclear antigen (PCNA) have a diffuse pattern of staining. The CK10 is widely expressed in all cases of BD. The p16 staining is helpful in differentiating different histological variants of BD. The p16 overexpression reflects the disrupted nature of G1/S checkpoint leading to uncontrolled cell cycle progression.[14,24] Ki-67 expression shows diffuse pattern of staining of atypical keratinocytes in BD differentiating it from actinic keratosis.[84] The BD histopathological specimen shows positive immunostaining for lumican.[85] Pagetoid BD express CK7, the specific marker of Paget's disease. The CK14 expression may be a marker of tumor progression.[14] BD of nipple clinicopathologically mimics Paget's disease, but stains positive for cytokeratin 5/6 and negative for cytokeratin 7.[46]
Dermoscopy
There are no standard dermoscopic criteria mentioned in the literature for the diagnosis of BD.[86] Zalaudek et al.[53] first described dermoscopic features such as glomerular vessels and scaly plaques in BD, glomerular vessels correlate with dilated papillary dermal vasculature histopathologically. The commonly observed features are scaly surface, small brown globules with patchy distribution, homogenous grey brownish and reticular pigmentation.[86]
Payapvipapong and Tanaka published a classification regarding three different types of BD. They include 1) Classic BD - Atypical vascular pattern, whitish scale and a pinkish network; 2) Pigmented BD - pigmentation without structure, pigmented stripes and crusts; 3) Partially pigmented BD - Combination of above two.[87] The dermoscopic differential diagnoses are actinic keratosis, seborrheic keratosis, basal cell carcinoma, lichen planus-like keratosis, mammary Paget's disease, and lentigo malignant melanoma.[86]
Reflectance confocal microscopy
Reflectance confocal microscopy is an ancillary tool, which enables in differentiating pigmented BD from other pigmented lesions. The findings suggestive of pigmented BD are polygonal, refractile structures in upper layers, atypical honeycomb pattern, targetoid cells, different size and shaped atypical keratinocytes, and “button-hole signs” homogenous with tortuous blood vessels.[84,87]
The electron microscopic examination of BD demonstrates many dyskeratotic cells surrounded by perinuclear aggregation and condensation of tonofilaments.[74] Phasor fluorescence lifetime imaging microscopy (FLIM) is a newer, simple tool which provides rapid confirmation of BD.[88]
Management
The treatment modality depends on factors such as the tumor size, location, thickness, number of lesions, patient's age, immune status, comorbidities, concomitant medication intake, compliance, esthetic outcome, equipment availability, and preference of the patient along with clinician's expertise.[18,19,89] Each therapeutic modality has its own place in the treatment armamentarium; hence, the treating physician should weigh its merits and demerits.[2] Because BD commonly occurs in old individuals, frequently located in regions with poor wound healing, noninvasive treatments are preferred.[90]
The available therapeutic options are
Topical chemotherapy
Light-based procedures
Surgical modality
Destructive modality
Topical chemotherapy
Imiquimod
This immune response modifier has antitumor activity by stimulating local production of cytokines.[2] Imiquimod is a good treatment option for BD lesions of difficult-to-treat areas like lower leg, shaft, glans penis, and in large facial lesions. The clinical efficacy varies between 57% and 86% after 6 weeks in various trials. Imiquimod has been reported to be used in conjunction with 5-fluorouracil and sulindac in immunosuppressed individuals.[2,19,24] Sotiriou et al.[91] treated a large BD measuring 100 cm2 with a single session of MAL-PDT along with daily topical imiquimod for a duration of 6 weeks. The associated inflammatory reaction, erythema, and pigmentation are the common reported adverse effects. It has limited efficacy in hyperkeratotic BD.[2,19] In a study, 38% (6/16) of the patients discontinued the imiquimod use earlier due to its side effects.[92]
Fluorouracil
This topical cytotoxic agent is used in the treatment of BD of skin, shaft of penis as once or twice daily application for a duration of 3–4 weeks, to be repeated if needed.[9,19] Clinical evidence showed a complete clinical response in 48% to 83% of individuals with daily use for 3–4 weeks.[24] The efficacy of 5-FU can be increased by application under occlusion, pretreatment with laser, iontophoresis, and with the use of dinitrochlorobenzene as a vehicle.[89] The pain, erythema, burning sensation, and ulceration at the applied site are the common adverse effects associated with its use.[9,24]
Light-based procedures
Photodynamic therapy
Photodynamic therapy (PDT) for BD involves topical application of Methyl Amino-levulinate (MAL) under occlusion for three hours followed by illumination using red light from a narrowband light-emitting diode source. It is repeated once after 7 days and again 3 months later if needed. The use of fluorescence helps in lesion delineation, recurrence detection with 100% sensitivity and 85% specificity.[19,93,94,95] It is of immense benefit in large lesions (>3 cm2), leg BD, and in difficult-to-treat sites. Topical PDT is noninvasive, tissue sparing, highly efficacious, and good esthetic therapeutic modality.[2,19,89] There are case reports of successful treatment of BD with PDT in the setting of radiodermatitis and epidermolysis bullosa.[19]
Radiotherapy
The various radiotherapy techniques used in the treatment of BD are external beam radiotherapy, radioactive skin patch, and Grenz rays.[96] In BD, both high and low dose regimens of radiotherapy are equally efficacious in the treatment.[19] The advantages of this technique include utilization in difficult-to-treat areas such as scalp, penile, and perianal BD. The disadvantages include high cost, patient inconvenience, and poor wound healing in legs.[19,24,96]
Lasers
LASERS are considered for genital and nail lesions.[89] In a large retrospective study, 44 BD patients were treated with CO2 laser achieving clearance in 86% of patients after one treatment.[96] Because CO2 laser spares the deeper follicular epithelium, chances of recurrence and treatment failure are high. To overcome this, diode laser can be used as a final pass following the three passes of CO2 laser.[19]
Surgical modalities
Excision
Simple wide excision of the lesion and primary closure is an acceptable method of treating BD of small size, single lesion, and perianal BD. It is helpful by histopathologically ruling out the invasive disease.[9,24,89] The BD is excised with a minimum of 4 mm margin in well-defined tumors of <2 cm in diameter and at least 6 mm margin for larger lesion or less-differentiated tumors or lesions in high-risk locations (e.g., scalp, eyelids, ears, nose, and lips).[21] Excision with a narrow lateral margin may lead to subclinical lateral spread, which is associated with higher chance of recurrence.[1] The recurrence rate varies from 2.8% to 19.4% in various studies. The limitations are prolonged wound healing in certain regions, functional and cosmetic outcome.[19,24,89]
Moh's micrographic surgery
Moh's micrographic surgery is very effective in difficult-to-treat areas like periorificial, periungal regions and genital lesions where tissue sparing is the main objective.[2] It is also useful in incompletely excised and recurrent BD. In a retrospective analysis of 270 cases of BD of head and neck region treated with Moh's micrographic surgery, 128 cases were previously treated with modalities such as cryotherapy, curettage and cautery, excision, and radiotherapy.[97] The recurrence after Moh's surgery is around 6.3%.[19]
Destructive modalities
The involvement of pilosebaceous units by atypical epithelium can lead to failure of treatment when superficial destructive modalities are used.[14]
Curettage with cautery
It is a simple, cheap, safe, and one of the most effective treatment modalities suitable in single, small BD.[89,98] It is preferred in patients who have large hyperkeratotic lesions, intolerant to cryotherapy, and those who cannot apply topical agents for prolonged period of time.[9] However, the results depend on the equipment used and skill of the operator. The cure rate ranges from 81% for curettage and up to 93% to 98% in case of curettage and cautery.[19,89]
Cryotherapy
Cryotherapy is a simple, inexpensive, out-patient modality of treatment of BD. It is used as a treatment modality in case of single, small BD located at well healing sites.[2] The efficacy of cryotherapy differs depending on technique and regimens used. It is avoided in poorly vascularized areas and legs where wound healing is prolonged. The failure rate is around 5%–10%.[2,19,24,89] Gaitanis et al.[99] successfully treated four cases of BD in renal transplant recipients with cryotherapy and topical imiquimod for 2 weeks with no recurrence. Various conventional treatment modalities are summarized in Table 4.
Table 4.
Summary of various treatment modalities of Bowen’s disease
| Drug | Application | Preferred | Limitations and adverse effects | Outcome |
|---|---|---|---|---|
| Imiquimod 5% cream | Once daily application for 16 weeks | Large lesions, face, lower leg, shaft of penis, glans penis | Limited response in hyperkeratotic lesions, erythema, inflammation, crusting, pigmentation | 57%-86% clearance |
| 5-Flourouracil cream | Once- or twice-daily application for 3-4 weeks, repeated if required | Large lesions, poor healing sites | Cannot be used in immunocompromised patients, pain, erythema, burning sensation, ulceration | 48%-83% clearance |
| Cryotherapy | Freeze of 30 s at least once or 20 s at least twice for one to three sittings | Good healing sites Multiple lesions | Cannot be used in poor wound healing sites, Hypopigmented scarring | 68%-100% clearance 5%-10% failure rate |
| Curettage with cautery | Simple, single-time, safe method | Small/single lesion, facial lesions | Cannot be performed for larger lesions, Success depends on skills of the operator | 93%-98% cure rate 2%-20% recurrence |
| Excision | Simple, wide excision of the lesion | Small/single lesion with poor healing | Prolonged wound healing, poor functional and cosmetic outcomes | 2.8% to 19.4% recurrence |
| Moh’s micrographic surgery | Individual layers of tissue are removed and examined under microscope | Tissue sparing sites such as periorificial, genital, and periungual regions | Expensive, needs skilled operator | Recurrence is around 6.3% |
| Photodynamic therapy | Day 0, 7, and repeated after 1 month | For larger lesions and difficult-to-treat areas | Pain | 88%-100% clearance 3 months after one cycle of MAL-PDT |
| Radiotherapy | Both high- and low dose regimens are equally efficacious | Difficult-to-treat sites such as digits and penis | High-cost, patient inconvenience, poor healing, erythema, edema | Failure to heal in 33% of individuals |
| LASERS | CO2 LASER | Difficult-to-treat sits such as digits and penis | Spares deeper follicular epithelium | Clearance of 86% after one treatment |
Miscellaneous non-conventional modalities
The nonconventional therapies such as a combination of oral cyclooxygenase enzyme inhibitor and topical imiquimod, topical tazarotene, topical diclofenac, topical maxacalcitol, phenol peel, topical and intralesional bleomycin, oral acitretin, oral isoretinoin with subcutaneous interferon-alpha and ultrasonic surgical aspiration have been useful.[2,9,19,24,89,100]
A case of extensive BD involving vulva, perineum, and anus has been treated with methotrexate and 5-FU.[24] A 63-year-old man with a biopsy-proven BD had clinical resolution of tumor when he applied a homemade paste of turmeric, milk, and black pepper for a month. He remained disease-free during the follow-up for 2 years.[101]
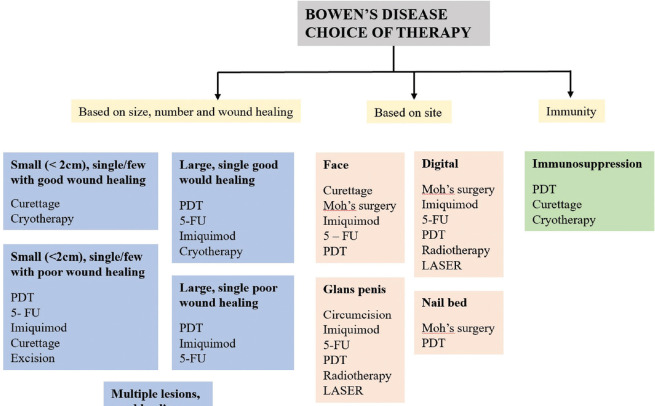
Choice of treatment
The treatment varies from topical agents to surgical methods. The first choice is often decided by the physician based on the site, size, immunity, and available facilities. In BD of less than 2 cm diameter with proper wound healing, curettage is the best option available followed by cryotherapy. In case of poor wound healing, PDT is preferred followed by 5-FU and imiquimod. Cryotherapy is the first choice of treatment modality in case of multiple BD. In facial BD, curettage is the initial choice followed by topical therapy and PDT.[24] The management of EQ starts with circumcision.[19] Penile BD can be treated with topical therapy and PDT.[24] The periungual BD is usually treated by local excision, Moh's micrographic surgery, or amputation of distal phalanx.[19] In organ transplant recipients and immunocompromised individuals, PDT, curettage, and cryotherapy are the treatment options available.[24]
Surgical treatment is preferred in case of smaller lesions. However, in view of esthetics and preservation of function, surgical options are not considered in case of large, multiple, facial, and penile lesions.[19,24] In elderly patients with thin and slowly progressive BD, the best approach is to wait and watch. Patient can be advised to use emollients containing keratolytics like urea to reduce scaling, making the lesion less obvious.[19] The choice of therapy in BD is depicted in [Figure 7].
Figure 7.
Choice of therapy in Bowen's disease
Conclusion
Theoretically BD can occur on any keratinizing surface. The asymptomatic nature, early subtle nonspecific presentation, and varied presentations of BD make the clinical diagnosis challenging even for experienced dermatologists. Considering the risk of malignant transformation, early diagnosis and treatment should be done. It requires a combined effort of dermatologist, oncosurgeon, and plastic surgeon to plan and execute the management in various presentations of BD.
Financial support andsponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Heppt MV, Schlager G, Berking C. Epithelial precancerous lesions. In: Kang S, Amagai M, Bruckner AL, Enk AH, Margolis DJ, McMichael AJ, et al., editors. Fitzpatrick's Dermatology in General Medicine. 9th ed. New York: Mcgraw-Hill; 2019. pp. 1857–83. [Google Scholar]
- 2.Arlette JP, Trotter MJ. Squamous cell carcinoma in situ of the skin: History, presentation, biology and treatment. Australas J Dermatol. 2004;45:1–9. doi: 10.1111/j.1440-0960.2004.00025.x. [DOI] [PubMed] [Google Scholar]
- 3.Bowen JT. Precancerous dermatosis. J Cutan Dis. 1912;30:241. [PubMed] [Google Scholar]
- 4.Bernhard JD, Elliot AD. A letter from darier to bowen on the naming of Bowen's disease. Arch Dermatol. 1983;119:261–2. [PubMed] [Google Scholar]
- 5.Darier J. La dermatose precancereuse de Bowen-dyskeratose lentiularie et en disques. Ann Dermatologie. 1914;5:449–71. [Google Scholar]
- 6.Grossman D, Leffell DJ. The molecular basis of nonmelanoma skin cancer.New understanding. Arch Dermatol. 1997;133:1263–70. [PubMed] [Google Scholar]
- 7.Brash DE, Ponten J. Skin precancer. Cancer Surv. 1998;32:69–113. [PubMed] [Google Scholar]
- 8.Kassem A, Technau K, Kurz AK, Pantulu D, Löning M, Kayser G, et al. Merkel cell polyomavirus sequences are frequently detected in nonmelanoma skin cancer of immunosuppressed patients. Int J Cancer. 2009;125:356–61. doi: 10.1002/ijc.24323. [DOI] [PubMed] [Google Scholar]
- 9.Gupta G, Madan V, Lear JT. Squamous cell carcinoma and its precursors. In: Griffiths C, Barker J, Bleiker T, Chalmer R, Creamer D, editors. Rook's Textbook of Dermatology. 9th ed. United Kingdom: Wiley Blackwell; 2016. pp. 3931–53. [Google Scholar]
- 10.Huang HW, Lee CH, Yu HS. Arsenic-Induced carcinogenesis and immune dysregulation. Int J Environ Res Public Health. 2019;16:2746. doi: 10.3390/ijerph16152746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mishra J, Pandia A, Padhy AK, Mahapatra M, Mohapatra J, Nayak B, et al. Bowen's disease of vulva: A rare case of vulvar premalignant disorder. Clin Cancer Investig J. 2020;9:210–1. [Google Scholar]
- 12.Wollina U. Bowen's disease of the nail apparatus: A series of 8 patients and a literature review. Wien Med Wochenschr. 2015;165:401–5. doi: 10.1007/s10354-015-0383-4. [DOI] [PubMed] [Google Scholar]
- 13.Tam DW, Van Scott EJ, Urbach F. Bowen's disease and squamous cell carcinoma.Occurrence in a patient with psoriasis after topical, systemic, and PUVA therapy. Arch Dermatol. 1979;115:203–4. doi: 10.1001/archderm.115.2.203. [DOI] [PubMed] [Google Scholar]
- 14.Patterson JW. Weedon's Skin Pathology. 4th ed. China: Elsevier; 2016. [Google Scholar]
- 15.Ishida H, Kumakiri M, Ueda K, Lao LM, Yanagihara M, Asamoto K, et al. Comparative histochemical study of Bowen's disease and actinic keratosis: Preserved normal basal cells in Bowen's disease. Eur J Histochem. 2001;45:177–90. doi: 10.4081/1628. [DOI] [PubMed] [Google Scholar]
- 16.Newton JA, Camplejohn RS, McGibbon DH. Aneuploidy in bowen's disease. Br J Dermatol. 1986;114:691–4. doi: 10.1111/j.1365-2133.1986.tb04877.x. [DOI] [PubMed] [Google Scholar]
- 17.Kreuter A, Meyer MF, Wieland U. Occurrence of penile intraepithelial neoplasia following adalimumab treatment for psoriatic arthritis. Arch Dermatol. 2011;147:1001–2. doi: 10.1001/archdermatol.2011.218. [DOI] [PubMed] [Google Scholar]
- 18.Nagakeerthana S, Rajesh G, Madhavi S, Karthikeyan K. Bowen's disease: Two case reports of a giant and dwarf lesions. J Can Res Ther. 2017;13:371–3. doi: 10.4103/0973-1482.187237. [DOI] [PubMed] [Google Scholar]
- 19.Morton CA, Birnie AJ, Eedy DJ. British association of dermatologists' guidelines for the management of squamous cell carcinoma in situ (Bowen's disease) 2014. Br J Dermatol. 2014;170:245–60. doi: 10.1111/bjd.12766. [DOI] [PubMed] [Google Scholar]
- 20.Reizner GT, Chuang TY, Elpern DJ, Stone JL, Farmer ER. Bowen's disease (squamous cell carcinoma in situ) in Kauai, Hawaii. A population-based incidence report. J Am Acad Dermatol. 1994;31:596–600. doi: 10.1016/s0190-9622(94)70222-5. [DOI] [PubMed] [Google Scholar]
- 21.Barad P, Fernandes J, Shukla P. Bowen's disease: A favorable response to imiquimod. Indian Dermatol Online J. 2014;5:546–7. doi: 10.4103/2229-5178.142570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosen T, Tucker SB, Tschen J. Bowen's disease in blacks. J Am Acad Dermatol. 1982;7:364–8. doi: 10.1016/s0190-9622(82)80316-2. [DOI] [PubMed] [Google Scholar]
- 23.Kossard S, Rosen R. Cutaneous Bowen's disease.An analysis of 1001 cases according to age, sex, and site. J Am Acad Dermatol. 1992;27:406–10. doi: 10.1016/0190-9622(92)70208-w. [DOI] [PubMed] [Google Scholar]
- 24.Neagu TP, Ţigliş M, Botezatu D, Enache V, Cobilinschi CO, Vâlcea-Precup MS, et al. Clinical, histological and therapeutic features of Bowen's disease. Rom J Morphol Embryol. 2017;58:33–40. [PubMed] [Google Scholar]
- 25.Calonje JE, Brenn T, Lazar A, Billings S. 5th ed. Elsevier; 2019. McKee's Pathology of the Skin: With Clinical Correlation. [Google Scholar]
- 26.Fu P, Liu XH, Liu R, Suo GY. Familial Bowen's disease. Indian J Dermatol Venereol Leprol. 2013;79:717–9. doi: 10.4103/0378-6323.116752. [DOI] [PubMed] [Google Scholar]
- 27.Gorlin RJ. Bowen's disease of the mucous membrane of the mouth; A review of the literature and a presentation of six cases. Oral Surg Oral Med Oral Pathol. 1950;3:35–51. doi: 10.1016/0030-4220(50)90081-8. [DOI] [PubMed] [Google Scholar]
- 28.Zawar VP, Tolat SN, Patil DJ, Kotkar DB. Bowen's disease of multicentric origin. Indian J Dermatol Venereol Leprol. 1993;59:266–8. [Google Scholar]
- 29.James WD, Elston DM, Treat JR, Rosenbach MA, Neuhaus IM. Andrews' Diseases of the Skin. 13th ed. London: Elsevier; 2020. [Google Scholar]
- 30.Morton CA, Whithurst C, McColl JH, Moore JV, MacKie RM. Photodynamic therapy for large or multiple patches of Bowen's disease and basal cell carcinoma. Arch Dermatol. 2001;137:319–24. [PubMed] [Google Scholar]
- 31.Lopez N, Meyer-Gonzales T, Herrera-Acosta E, Bosch R, Castillo R, Herrera E. Photodynamic therapy in the treatment of extensive Bowen's disease. J Dermatol Treat. 2012;23:428–30. doi: 10.3109/09546634.2011.590789. [DOI] [PubMed] [Google Scholar]
- 32.Verma SB. Hyperkeratotic Bowen disease--A case report. Dermatol Online J. 2008;14:24. [PubMed] [Google Scholar]
- 33.Saini R, Sharma N, Pandey K, Puri K. Multiple skin cancers in a single patient: Multiple pigmented Bowen's disease, giant basal cell carcinoma, squamous cell carcinoma. J Can Res Ther. 2015;11:669. doi: 10.4103/0973-1482.140803. [DOI] [PubMed] [Google Scholar]
- 34.Shabbir M, Minhas S, Muneer A. Diagnosis and management of premalignant penile lesions. Ther Adv Urol. 2011;3:151–8. doi: 10.1177/1756287211412657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fanning DM, Flood H. Erythroplasia of queyrat. Clin Pract. 2012;2:e63. doi: 10.4081/cp.2012.e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Divakaruni AK, Rao AV, Mahabir B. Erythroplasia of Queyrat with Zoon's balanitis: A diagnostic dilemma. Int J STD AIDS. 2008;19:861–3. doi: 10.1258/ijsa.2007.007171. [DOI] [PubMed] [Google Scholar]
- 37.Leonard D, Beddy D, Dozois EJ. Neoplasms of anal canal and perianal skin. Clin Colon Rectal Surg. 2011;24:54–63. doi: 10.1055/s-0031-1272824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaye V, Zhang G, Dehner LP, Fraley EE. Carcinoma in situ of penis.Is distinction between erythroplasia of Queyrat and Bowen's disease relevant? Urology. 1990;36:479–82. doi: 10.1016/0090-4295(90)80181-l. [DOI] [PubMed] [Google Scholar]
- 39.Guitart J, Bergfeld WF, Tuthill RJ, Tubbs RR, Zienowicz R, Fleegler EJ. Squamous cell carcinoma of the nail bed: A clinicopathological study of 12 cases. Br J Dermatol. 1990;123:215–22. doi: 10.1111/j.1365-2133.1990.tb01849.x. [DOI] [PubMed] [Google Scholar]
- 40.Barans R, Richert B. Common nail tumors. Dermatol Clin. 2006;24:297–311. doi: 10.1016/j.det.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 41.Singh S, Khaitan BK, Sharma MC, Seenu V, Kumawat M, Chatterjee P. Bowen's disease on finger: A diagnostic and therapeutic challenge. Indian J Dermatol Venereol Leprol. 2013;79:227–30. doi: 10.4103/0378-6323.107643. [DOI] [PubMed] [Google Scholar]
- 42.Baran R, Perrin C. Pseudo-fibrokeratoma of the nail apparatus with melanocytic pigmentation: A clue for diagnosing Bowen's disease. Acta Derm Venereol. 1994;74:449–50. doi: 10.2340/0001555574449450. [DOI] [PubMed] [Google Scholar]
- 43.Koch A, Schönlebe J, Haroske G, Köstler E, Wollina U. Polydactylous Bowen's disease. J Eur Acad Dermatol Venereol. 2003;17:213–5. doi: 10.1046/j.1468-3083.2003.00657.x. [DOI] [PubMed] [Google Scholar]
- 44.Lebbé C, Pinquier L, Rybojad M, Chomienne C, Ochonisky S, Miclea JM, et al. Fanconi's anaemia associated with multicentric Bowen's disease and decreased NK cytotoxicity. Br J Dermatol. 1993;129:615–8. doi: 10.1111/j.1365-2133.1993.tb00497.x. [DOI] [PubMed] [Google Scholar]
- 45.Harnalikar M, Dongre A, Khopkar U. Bowen's disease on palm: A rare presentation. Indian J Dermatol. 2011;56:353–4. doi: 10.4103/0019-5154.82497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liang DG, Soliman B, Cha J. A rare case of Bowen's disease of the nipple: Literature review and management pathway. Breast J. 2020;26:1234–8. doi: 10.1111/tbj.13824. [DOI] [PubMed] [Google Scholar]
- 47.Vivan MM, Hirata SH, Nascimento LS, Enokihara MM. A case of pigmented Bowen's disease. An Bras Dermatol. 2017;92:124–5. doi: 10.1590/abd1806-4841.20175381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ragi G, Turner MS, Klein LE, Stoll HL., Jr Pigmented Bowen's disease and review of 420 Bowen's disease lesions. J Dermatol Surg Oncol. 1988;14:765–9. doi: 10.1111/j.1524-4725.1988.tb01161.x. [DOI] [PubMed] [Google Scholar]
- 49.Rodrigues MM, Wiziack NC, Chacha JJ, Takita LC. Pigmented Bowen's disease: A case report of an unusual variant. J Bras Patol Med Lab. 2015;51:265–7. [Google Scholar]
- 50.Schmitz MW, Goldberg LJ, Adler AJ. An extensive case of Bowen's disease in an HIV-positive male. AIDS Patient Care STDS. 2007;21:78–80. doi: 10.1089/apc.2006.0059. [DOI] [PubMed] [Google Scholar]
- 51.Nishimura Y, Kishigawa T, Tanaka T. Bilateral Bowen's disease. Br J Dermatol. 2004;151:227–8. doi: 10.1111/j.1365-2133.2004.06039.x. [DOI] [PubMed] [Google Scholar]
- 52.Dandale A, Mantri MD, Thakkar V, Dhurat RS, Ghate S. Bowen's disease: An unusual clinical presentation. Indian Dermatol Online J. 2014;5:526–8. doi: 10.4103/2229-5178.142546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zalaudek I, Argenziano G, Leinweber B, Citarella L, Hofmann-Wellenhof R, Malvehy J, et al. Dermoscopy of Bowen's disease. Br J Dermatol. 2004;150:1112–6. doi: 10.1111/j.1365-2133.2004.05924.x. [DOI] [PubMed] [Google Scholar]
- 54.Grewal RS, Chatterjee M, Das AL. Non-Venereal disorders of genitalia. In: Sacchidanand S, Oberai S, Inamadar AC, editors. IADVL Textbook of Dermatology. 4th ed. Bhalani: 2016. pp. 2101–25. [Google Scholar]
- 55.Strauss RJ, Fazio VW. Bowen's disease of the anal and perianal area.A report and analysis of twelve cases. Am J Surg. 1979;137:231–4. doi: 10.1016/0002-9610(79)90152-1. [DOI] [PubMed] [Google Scholar]
- 56.Masuda T, Hara H, Shimojima H, Suzuki H. Spontaneous complete regression of multiple Bowen's disease in the webspaces of the feet. Int J Dermatol. 2006;45:783–5. doi: 10.1111/j.1365-4632.2006.02698.x. [DOI] [PubMed] [Google Scholar]
- 57.Rajabi P, Adibi N, Nematollahi P, Heidarpour M, Eftekhari M, Siadat AH. Bowenoid transformation in seborrheic keratosis: A retrospective analysis of 429 patients. J Res Med Sci. 2012;17:217–21. [PMC free article] [PubMed] [Google Scholar]
- 58.Coskey RJ, Mehregan A. Bowen disease associated with porokeratosis of Mibelli. Arch Dermatol. 1975;111:1480–1. [PubMed] [Google Scholar]
- 59.Honda M, Suzuki T, Kudoh K, Tagami H. Bowen's disease developing within a Becker's melanosis (Becker's naevus) Br J Dermatol. 1997;137:659–61. doi: 10.1111/j.1365-2133.1997.tb03817.x. [DOI] [PubMed] [Google Scholar]
- 60.Arrington JH, 3rd, Lockman DS. Thermal keratoses and squamous cell carcinoma in situ associated with erythema ab igne. Arch Dermatol. 1979;115:1226–8. [PubMed] [Google Scholar]
- 61.Kanitakis J, Euvrard S, Claudy A. Bowen's disease masquerading clinically as a follicular cyst in a renal transplant recipient. Am J Dermatopathol. 2007;29:412–3. doi: 10.1097/DAD.0b013e3180dddea0. [DOI] [PubMed] [Google Scholar]
- 62.Walling HW, Persichetti GB, Scupham RK. Squamous cell carcinoma in situ arising in a smallpox vaccination scar. Int J Dermatol. 2008;47:599–600. doi: 10.1111/j.1365-4632.2008.03537.x. [DOI] [PubMed] [Google Scholar]
- 63.DeCoste R, Moss P, Boutilier R, Walsh NM. Bowen disease with invasive mucin-secreting sweat gland differentiation: Report of a case and review of the literature. J Cutan Pathol. 2019;46:425–30. doi: 10.1111/cup.13437. [DOI] [PubMed] [Google Scholar]
- 64.Shirai A, Saeki H, Matsuzaki H, Ito K, Nakagawa H. Multiple clear cell acanthoma associated with multiple Bowen′s disease. Int J Dermatol. 2014;53:386–8. doi: 10.1111/ijd.12406. [DOI] [PubMed] [Google Scholar]
- 65.Watanabe T, Murakami T, Okochi H, Kikuchi K, Furue M. Eccrine poroma associated with Bowen's disease. Int J Dermatol. 2004;43:472–3. doi: 10.1111/j.1365-4632.2004.02367.x. [DOI] [PubMed] [Google Scholar]
- 66.Zheng LQ, Han XC, Huang Y, Li HW, Niu XD, Li J. Porocarcinoma coexisting at a site of Bowen disease in a 63-year-old woman. Clin Exp Dermatol. 2015;40:293–7. doi: 10.1111/ced.12534. [DOI] [PubMed] [Google Scholar]
- 67.Coras B, Vogt T, Roesch A, Landthaler M, Hohenleutner U. Bowen's disease on porokeratotic eccrine ostial and dermal duct nevus. Dermatol Surg. 2007;33:496–9. doi: 10.1111/j.1524-4725.2007.33099.x. [DOI] [PubMed] [Google Scholar]
- 68.Goyal T, Varshney A, Solanki R. Co-existence of extramammary Paget's disease and Bowen's disease of vulva. Indian J Dermatol Venereol Leprol. 2014;80:530–3. doi: 10.4103/0378-6323.144170. [DOI] [PubMed] [Google Scholar]
- 69.Ishida M, Okabe H. Merkel cell carcinoma concurrent with Bowen's disease: Two cases, one with an unusual immunophenotype. J Cutan Pathol. 2013;40:839–43. doi: 10.1111/cup.12176. [DOI] [PubMed] [Google Scholar]
- 70.Ishida M, Iwai M, Yoshida K, Kagotani A, Okabe H. Sebaceous carcinoma associated with Bowen's disease: A case report with emphasis on the pathogenesis of sebaceous carcinoma. Int J Clin Exp Pathol. 2013;6:3029–32. [PMC free article] [PubMed] [Google Scholar]
- 71.Misago N, Toda S, Nakao T. Focus of tricholemmal differentiation (tricholemmal carcinoma) within Bowen's disease/carcinoma. J Dermatol. 2016;43:439–42. doi: 10.1111/1346-8138.13098. [DOI] [PubMed] [Google Scholar]
- 72.Chuang TY, Tse J, Reizner GT. Bowen's disease (squamous cell carcinoma in situ) as a skin marker for internal malignancy: A case-control study. Am J Prev Med. 1990;6:238–43. [PubMed] [Google Scholar]
- 73.Lycka BA. Bowen's disease and internal malignancy.A meta-analysis. Int J Dermatol. 1989;28:531–3. doi: 10.1111/j.1365-4362.1989.tb04607.x. [DOI] [PubMed] [Google Scholar]
- 74.Yanofsky VR, Mercer SE, Phelps RG. Histopathological variants of cutaneous squamous cell carcinoma: A review? J Skin Cancer. 2011;2011:210813. doi: 10.1155/2011/210813. doi: 10.1155/2011/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kirkham N. Tumors and cysts of the epidermis. In: Elder D, editor. Lever's Histopathology of the Skin. 10th ed. Philadelphia: Lippincott Williams & Wilkins; 2008. pp. 791–849. [Google Scholar]
- 76.Pai K, Shetty S, Padmapriya J, Pai S, Rao L. Acantholytic variant of bowen's disease with micro-invasive squamous cell carcinoma: A case report of a unique variant. Indian J Dermatol. 2014;59:635. doi: 10.4103/0019-5154.143592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Idriss MH, Misri R, Böer-Auer A. Orthokeratotic Bowen disease: A histopathologic, immunohistochemical and molecular study. J Cutan Pathol. 2016;43:24–31. doi: 10.1111/cup.12610. [DOI] [PubMed] [Google Scholar]
- 78.Jour G, Aung PP, Rozas-Muñoz E, Curry JL, Prieto V, Ivan D. Intraepidermal Merkel cell carcinoma: A case series of a rare entity with clinical follow up. J Cutan Pathol. 2017;44:684–91. doi: 10.1111/cup.12966. [DOI] [PubMed] [Google Scholar]
- 79.Oka K, Katsumata M. Intraepidermal sebaceous carcinoma: Case report. Dermatologica. 1990;180:181–5. doi: 10.1159/000248026. [DOI] [PubMed] [Google Scholar]
- 80.Lu X, Wu M, Chen J, Wu J, Gu Y, Zhao L. A case of hidroacanthoma simplex. Indian J Dermatol. 2013;58:245. doi: 10.4103/0019-5154.110884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sundharam JA. Podophyllin and its use in the treatment of condylomata acuminata. Indian J Dermatol Venereol Leprol. 1990;56:10–14. [Google Scholar]
- 82.Oh CW, Penneys N. P27 and mib1 expression in actinic keratosis, Bowen disease, and squamous cell carcinoma. Am J Dermatopathol. 2004;26:22–6. doi: 10.1097/00000372-200402000-00004. [DOI] [PubMed] [Google Scholar]
- 83.Takayama R, Ishiwata T, Ansai S, Yamamoto T, Matsuda Y, Naito Z, et al. Lumican as a novel marker for differential diagnosis of Bowen disease and actinic keratosis. Am J Dermatopathol. 2013;35:827–32. doi: 10.1097/DAD.0b013e31827c7f31. [DOI] [PubMed] [Google Scholar]
- 84.Ianosi SL, Batani A, Ilie MA, Tampa M, Georgescu SR, Zurac S, et al. Non-invasive imaging techniques for the in vivo diagnosis of Bowen's disease: Three case reports. Oncol Lett. 2019;17:4094–101. doi: 10.3892/ol.2019.10079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Payapvipapong K, Tanaka M. Dermoscopic classification of Bowen's disease. Australas J Dermatol. 2015;56:32–5. doi: 10.1111/ajd.12200. [DOI] [PubMed] [Google Scholar]
- 86.Yang Y, Lin J, Fang S, Han S, Song Z. What's new in dermoscopy of Bowen's disease: Two new dermoscopic signs and its differential diagnosis. Int J Dermatol. 2017;56:1022–5. doi: 10.1111/ijd.13734. [DOI] [PubMed] [Google Scholar]
- 87.Mazilli S, Gamo-Villegas R, Pampin-Franco A, Lopez Estebaran JL, Pinedo F, Vollono L, et al. Reflectance confocal microscopy of pigmented bowen's disease: A Case series of difficult to diagnose lesions. Case Rep Dermatol. 2020;12:98–106. doi: 10.1159/000507916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Luo T, Lu Y, Liu S, Lin D, Qu J. Phasor-FLIM as a screening tool for the differential diagnosis of actinic keratosis, bowen's disease, and basal cell carcinoma. Anal Chem. 2017;89:8104–11. doi: 10.1021/acs.analchem.7b01681. [DOI] [PubMed] [Google Scholar]
- 89.Neubert T, Lehmann P. Bowen's disease-A review of newer treatment options. Ther Clin Risk Manag. 2008;4:1085–95. [PMC free article] [PubMed] [Google Scholar]
- 90.Chang YC, Anolik RB, Cabral H, Bhawan J. Frequency of squamous cell carcinoma in situ (SCCIS) and SCC in re-excisions of biopsy-proven cutaneous SCCIS. Br J Dermatol. 2017;177:1747–8. doi: 10.1111/bjd.15230. [DOI] [PubMed] [Google Scholar]
- 91.Sotiriou E, Lallas A, Apalla Z, Ioannides D. Treatment of giant Bowen's disease with sequential use of photodynamic therapy and imiquimod cream. Photodermatol Photoimmunol Photomed. 2011;27:164–6. doi: 10.1111/j.1600-0781.2011.00586.x. [DOI] [PubMed] [Google Scholar]
- 92.Mackenzie-Wood A, Kossard S, de Launey J, Owens ML. Imiquimod 5% cream in the treatment of Bowen's disease. J Am Acad Dermatol. 2001;44:462–70. doi: 10.1067/mjd.2001.111335. [DOI] [PubMed] [Google Scholar]
- 93.Zhong S, Zhang R, Mei X, Wang L. Efficacy of photodynamic therapy for the treatment of Bowen's disease: An updated systematic review and meta-analysis of randomized controlled trials. Photodiagnosis Photodyn Ther. 2020;32:102037. doi: 10.1016/j.pdpdt.2020.102037. [DOI] [PubMed] [Google Scholar]
- 94.Morton CA, Szeimies RM, Basset-Seguin N, Calzavara-Pinton P, Gilaberte Y, Haedersdal M, et al. European Dermatology Forum guidelines on topical photodynamic therapy 2019 Part 1: Treatment delivery and established indications-Actinic keratoses, Bowen's disease and basal cell carcinomas. J Eur Acad Dermatol Venereol. 2019;33:2225–38. doi: 10.1111/jdv.16017. [DOI] [PubMed] [Google Scholar]
- 95.Bath-Hextall FJ, Matin RN, Wilkinson D, Leonardi-Bee J. Interventions for cutaneous Bowen's disease. Cochrane Database Syst Rev. 2013;2013:CD007281. doi: 10.1002/14651858.CD007281.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Covadonga Martínez-González M, del Pozo J, Paradela S, Fernández-Jorge B, Fernández-Torres R, Fonseca E. Bowen's disease treated by carbon dioxide laser.A series of 44 patients. J Dermatolog Treat. 2008;19:293–9. doi: 10.1080/09546630701870772. [DOI] [PubMed] [Google Scholar]
- 97.Leibovitch I, Huilgol SC, Selva D, Richards S, Paver R. Cutaneous squamous carcinoma in situ (Bowen's disease): Treatment with Mohs micrographic surgery. J Am Acad Dermatol. 2005;52:997–1002. doi: 10.1016/j.jaad.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 98.Ramrakha-Jones VS, Herd RM. Treating Bowen's disease: A cost minimization study. Br J Dermatol. 2003;148:1167–72. doi: 10.1046/j.1365-2133.2003.54013.x. [DOI] [PubMed] [Google Scholar]
- 99.Gaitanis G, Bassukas ID. Immunocryosurgery – An effective combinational modality for Bowen's disease. Dermatol Ther. 2016;29:334–7. doi: 10.1111/dth.12371. [DOI] [PubMed] [Google Scholar]
- 100.Karashima T, Hashikawa K, Ono F, Eguchi H, Hamada T, Ishii N, et al. Successful treatment of Bowen's disease with topical maxacalcitol. Acta Derm Venereol. 2012;92:660–1. doi: 10.2340/00015555-1294. [DOI] [PubMed] [Google Scholar]
- 101.Harper N, Saigal H, Kaur MR, Orpin S. The 'golden goddess' of spices: A potentially effective topical therapy for Bowen disease? Clin Exp Dermatol. 2017;42:545–7. doi: 10.1111/ced.13099. [DOI] [PubMed] [Google Scholar]