Abstract
Recent evidence suggests that the malaria parasite Plasmodium falciparum utilizes a branched respiratory pathway including both a cytochrome chain and an alternative oxidase. This branched respiratory pathway model has been used as a basis for examining the mechanism of action of two antimalarial agents, atovaquone and proguanil. In polarographic assays, atovaquone immediately reduced the parasite oxygen consumption rate in a concentration-dependent manner. This is consistent with its previously described role as an inhibitor of the cytochrome bc1 complex. Atovaquone maximally inhibited the rate of P. falciparum oxygen consumption by 73% ± 10%. At all atovaquone concentrations tested, the addition of the alternative oxidase inhibitor, salicylhydroxamic acid, resulted in a further decrease in the rate of parasite oxygen consumption. At the highest concentrations of atovaquone tested, the activities of salicylhydroxamic acid and atovaquone appear to overlap, suggesting that at these concentrations, atovaquone partially inhibits the alternative oxidase as well as the cytochrome chain. Drug interaction studies with atovaquone and salicylhydroxamic acid indicate atovaquone’s activity against P. falciparum in vitro is potentiated by this alternative oxidase inhibitor, with a sum fractional inhibitory concentration of 0.6. Propyl gallate, another alternative oxidase inhibitor, also potentiated atovaquone’s activity, with a sum fractional inhibitory concentration of 0.7. Proguanil, which potentiates atovaquone activity in vitro and in vivo, had a small effect on parasite oxygen consumption in polarographic assays when used alone or in the presence of atovaquone or salicylhydroxamic acid. This suggests that proguanil does not potentiate atovaquone by direct inhibition of either branch of the parasite respiratory chain.
We recently presented evidence that the Plasmodium falciparum respiratory chain is branched and contains an alternative oxidase as well as a cytochrome chain (21). The alternative oxidases of plants, fungi, and trypanosomatids transfer electrons directly from ubiquinone to oxygen in a cyanide-insensitive reaction (19). In systems containing both an alternative oxidase and the cytochrome pathway, the alternative oxidase does not appear to contribute directly to the mitochondrial membrane potential or the energy balance of the cell. It can, however, contribute indirectly by accepting electrons from enzymes which donate electrons to ubiquinone. Alternative oxidase has been shown to contribute to the survival of plant cells under conditions in which the cytochrome chain is overloaded or blocked (25). The respiratory pathway of P. falciparum appears to be more important for pyrimidine biosynthesis than for energy generation (12, 22). Interestingly, the activity of P. falciparum dihydroorotate dehydrogenase, the enzymatic link between electron transport and pyrimidine biosynthesis, is inhibited by both alternative oxidase and cytochrome chain inhibitors (12, 14, 15).
Atovaquone, a hydroxynaphthoquinone, is a potent antimalarial agent which is known to inhibit dihydroorotate dehydrogenase activity (13, 14). At concentrations selective for P. falciparum, atovaquone has been shown to act specifically on the cytochrome bc1 complex (complex III of the cytochrome chain) (10). Clinical trials in which atovaquone was used alone to treat P. falciparum malaria resulted in an initial clearance of parasites from the blood followed by recrudescence in 25 to 75% of the patients (5, 18). The model of a branched respiratory pathway in P. falciparum suggests that an alternative oxidase in these parasites could enable the survival of some parasites in the presence of atovaquone. This could explain the high recrudescence rate seen when atovaquone is used singly to treat P. falciparum malaria in clinical trials.
Screening studies have demonstrated that several antimalarial agents potentiate atovaquone (4, 18, 28, 29). Of these, proguanil is of particular interest because its mechanism of potentiation of atovaquone is unknown. Originally, proguanil was thought to act through its metabolite, cycloguaunil, which specifically inhibits parasite dihydrofolate reductase (DHFR) and thus folate synthesis (9, 27). However, proguanil was shown to potentiate atovaquone’s activity in vitro under conditions in which cycloguanil would not be produced (4). Further evidence that proguanil can act via a mechanism distinct from that of cycloguanil was obtained by transforming P. falciparum with human DHFR (9). This study showed that the expression of human DHFR in P. falciparum decreased the parasite’s sensitivity to cycloguanil but had no effect on its sensitivity to proguanil (9).
Using the branched respiratory model for P. falciparum, we have determined the effects of interactions of alternative oxidase inhibitors, proguanil, and atovaquone on P. falciparum oxygen consumption. The results suggest that alternative oxidase inhibitors should potentiate the chemotherapeutic activity of atovaquone. In vitro growth inhibition assays confirm this prediction.
MATERIALS AND METHODS
Parasites.
P. falciparum FCR3F86 and 3D7 were cultured in RPMI medium as previously described (16).
Drugs and inhibitors.
Cyanide, salicylhydroxamic acid (SHAM), and propyl gallate were prepared immediately prior to use. A 25-mg/ml atovaquone stock was made in dimethyl sulfoxide (DMSO), aliquoted, and stored at −20°C. A 100 mM proguanil stock was prepared in 10% DMSO-RPMI and stored in a similar manner. Aliquots were used only once and then discarded. Atovaquone was a gift from the Wellcome Research Laboratories, Beckenham, Kent, United Kingdom. Other chemicals and their sources were as follows: cyanide, J. T. Baker, Inc. (Phillipsburg, N.J.); SHAM and propyl gallate, Sigma Chemical Co. (St. Louis, Mo.); and proguanil, Jacobus Pharmaceutical Co., Inc. (Princeton, N.J.).
Polarographic assays.
Polarographic assays were performed over a period of 15 to 20 min as described previously (21), with the following modifications. All experiments were performed at 35°C. Each atovaquone concentration was tested two to four times in the polarographic assay, using a stir rate of 450 to 500 rpm. Each atovaquone concentration to be tested was prepared from a 25-mg/ml stock at 100 times the final concentration such that the final concentration of DMSO in the assay was 0.1%. DMSO concentrations of less than 0.5% did not alter the rate of parasite oxygen consumption. Between 1 × 109 and 3 × 109 parasites were used per experiment.
Inhibition of P. falciparum growth in vitro.
The atovaquone IC50 and IC60 (concentrations which inhibited 50% and 60% of parasite growth) for the FCR3F86 strain were determined by measuring the incorporation of [3H]hypoxanthine in a 48-h assay as previously described (8). All assays were done in triplicate. The fractional inhibitory concentrations (FICs) were determined by using the same assay with the following modifications. The atovaquone IC50 was determined alone and at each of two fixed concentrations of SHAM (25 and 100 μM). Since the assay was done in triplicate, three IC50s were determined for each drug combination. The mean and standard deviation of the IC50 of atovaquone determined in the presence of SHAM was divided by the IC50 for atovaquone alone to generate the FIC for atovaquone. The FIC for SHAM in each experiment was determined by dividing the fixed concentration of SHAM used in the assay by the IC50 for SHAM (21). The FICs for atovaquone in the presence of three concentrations of propyl gallate (2 μM, 4 μM, and 8 μM) were determined by using the same method except that the IC60 values were used. Isobolograms were generated by plotting the FICs for each compound. As previously described by Tallarida and Jacob (23) and Canfield et al. (4), the curve shape of the isobologram is indicative of the type of drug interaction. A convex curve depicts an antagonistic relationship, a straight line an additive relationship, and a concave line a synergistic relationship between the inhibitors. Results were also expressed as the means of the sums of FICs for each pair of compounds, where the sum FIC equals the FIC of atovaquone plus the FIC of the alternative oxidase inhibitor. A sum FIC of one suggests an additive relationship, a sum FIC of greater than one suggests antagonism, and a sum FIC of less than one suggests synergism.
RESULTS
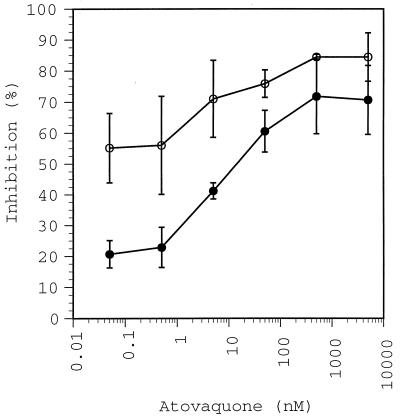
The effect of several concentrations of atovaquone on P. falciparum oxygen consumption was measured in a series of polarographic assays. Atovaquone caused an immediate reduction in the rate of P. falciparum oxygen consumption at all concentrations from 50 pM to 5 μM that were tested. The relationship of atovaquone concentration to parasite oxygen consumption rate is sigmoidal (Fig. 1). At the highest concentrations tested (0.5 to 5 μM), there was a 73% ± 10% inhibition of the rate of parasite oxygen consumption.
FIG. 1.
The effect of atovaquone concentration on oxygen consumption by P. falciparum. The percent inhibition of the overall rate of parasite oxygen consumption in the presence of atovaquone alone (closed circles) or of atovaquone plus 1 mM SHAM (open circles) is plotted versus the concentration of atovaquone. Error bars show standard deviations for 3 to 4 assays at concentrations of 50 pM to 50 nM and mean deviations for duplicate assays at concentrations of 0.5 to 5 μM of atovaquone.
Since previous studies demonstrated that cyanide-resistant oxygen consumption by P. falciparum is inhibited by the alternative oxidase inhibitors SHAM and propyl gallate (21), the effect of SHAM on atovaquone-resistant oxygen consumption was tested. At the highest concentrations of atovaquone tested (0.5 to 5 μM), SHAM inhibited an additional 10% ± 6% of the total rate of oxygen consumed by the parasite (Fig. 1). At lower concentrations of atovaquone, SHAM inhibited a greater proportion of the total rate of oxygen consumption. In fact, at the lowest atovaquone concentrations tested (50 to 500 pM), SHAM inhibited the rate of oxygen consumption by 34% ± 9%. This is equivalent to the maximum inhibition of the rate of parasite oxygen consumption seen when SHAM is used alone (30% ± 2%) (21). The remaining rate of parasite oxygen consumption that was not inhibited by the combination of atovaquone and 1 mM SHAM was inhibitable by 1 mM cyanide.
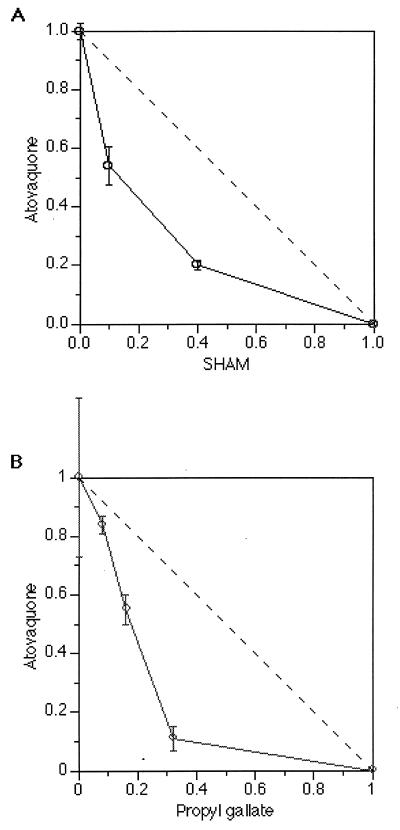
Since SHAM and atovaquone appear to inhibit complementary parts of parasite oxygen consumption, it seemed possible that they might also complement each other in their ability to inhibit parasite growth. Previous studies demonstrated that the IC50s of the alternative oxidase inhibitors SHAM and propyl gallate were 247 ± 6 μM and 24 ± 3 μM, respectively (21). The IC50 for atovaquone in various P. falciparum isolates is approximately 0.9 nM (2, 11). We determined a similar IC50 for our FCR3F86 strain (2.1 ± 0.2 nM). The IC50 for atovaquone in the presence of two fixed concentrations of SHAM was then determined. In the presence of 25 μM SHAM, the IC50 for atovaquone was 1.2 ± 0.1 nM and at 100 μM SHAM, the IC50 for atovaquone was 0.4 ± 0.03 nM. Based on these results, the sum FIC for SHAM and atovaquone was determined to be 0.6, suggesting that SHAM and atovaquone inhibit parasite growth synergistically. Graphically, synergy is denoted by the concave shape of the isobologram for these compounds (Fig. 2A).
FIG. 2.
Isobolograms of the interaction of atovaquone with SHAM (A) or propyl gallate (B). Axes indicate the FIC50s (A) or FIC60s (B) for each compound in a given combination. The dashed line depicts additive effect.
Similar assays were done with propyl gallate and atovaquone to investigate whether atovaquone was also potentiated by another alternative oxidase inhibitor. The IC60 for atovaquone alone was 2.3 ± 0.6 nM. The IC60 for atovaquone in the presence of 2 μM propyl gallate was 1.9 ± 0.08 nM, in the presence of 4 μM propyl gallate was 1.3 ± 0.1 nM, and in the presence of 8 μM propyl gallate was 0.2 ± 0.08 nM. The sum FIC for propyl gallate and atovaquone was 0.7. The synergy between propyl gallate and atovaquone is also indicated by the concave isobologram shown in Fig. 2B.
Proguanil also potentiates the activity of atovaquone in vitro (4) and in vivo (18), but the mechanism by which proguanil inhibits parasite growth is not understood (9). Therefore, this drug was tested for its effect on the rate of P. falciparum oxygen consumption by using the polarographic assay. At a concentration of 1 mM, proguanil alone inhibited parasite oxygen consumption by only 9% ± 6%. The rate of parasite oxygen consumption was further reduced by the addition of either 50 nM atovaquone or 1 mM SHAM, suggesting that the proguanil did not substantially inhibit either the cytochrome chain or the alternative oxidase (Table 1).
TABLE 1.
The effect of antimalarial drugs and inhibitors on the rate of P. falciparum oxygen consumption
| First inhibitorb | Second inhibitor | Rate of O2 consumption (% inhibition)a
|
||
|---|---|---|---|---|
| Uninhibited | First inhibitor | Both inhibitors | ||
| Atovaquone | Proguanil | 4.2 ± 0.8c | 1.6 ± 0.2c (61%) | 1.4 ± 0.2c (64%) |
| Proguanil | Atovaquone | 4.7 ± 0.5d | 4.4 ± 0.4d (6%) | 1.2 ± 0.7d (74%) |
| Proguanil | SHAM | 4.1 ± 0.7d | 3.6 ± 0.9d (10%) | 2.0 ± 0.6d (52%) |
The rate of O2 consumption is in nanomoles per minute. The percent inhibition of the total rate of parasite oxygen is indicated in parentheses.
The concentrations of the inhibitors used were 50 nM atovaquone, 1 mM proguanil, and 1 mM SHAM.
Results are the means of three experiments ± the standard deviation.
Results are the means of duplicate experiments ± the range.
DISCUSSION
The data presented here indicate that atovaquone inhibits up to 73% of the rate of P. falciparum oxygen consumption. This is consistent with atovaquone’s known activity on the cytochrome bc1 complex of the cytochrome chain (10) and with evidence that cyanide, an inhibitor of complex IV of the cytochrome chain, inhibits 70% of the rate of P. falciparum oxygen consumption (21).
Some of the parasite oxygen consumption that is resistant to atovaquone is sensitive to the alternative oxidase inhibitor SHAM. At the lowest concentrations of atovaquone, SHAM inhibited the total rate of oxygen consumption as much as it would in the absence of atovaquone (34% ± 9%) (21). However, at the highest concentrations of atovaquone, SHAM inhibited a smaller portion of the total rate of parasite oxygen consumption (10% ± 6%). In contrast, SHAM inhibits the rate of parasite oxygen consumption by essentially the same amount in the presence (26% ± 9%) or absence (30% ± 2%) of maximally inhibiting concentrations of cyanide (21).
It has been suggested that high concentrations of atovaquone generally inhibit ubiquinone-mediated reactions (10). Since alternative oxidase accepts electrons from ubiquinol (20), high levels of atovaquone might be expected to at least partially inhibit the alternative respiratory pathway as well as the cytochrome chain. The apparently reduced activity of SHAM at high concentrations of atovaquone might be due to the already reduced activity of the alternative pathway under these conditions.
At atovaquone concentrations that selectively inhibit the growth of P. falciparum in vitro (IC50 = 1 nM) and that selectively inhibit parasite cytochrome c reductase activity (IC50 = 1 nM) (10), atovaquone appears to have little if any effect on the SHAM-sensitive oxygen consumption of the parasite. SHAM inhibits parasite oxygen consumption as much in the presence of 0.5 to 5 nM atovaquone (29% ± 13%) as in its absence. This suggests that the target of SHAM is distinct from that of atovaquone and is therefore consistent with SHAM acting on an alternative oxidase. Thus, at clinically useful concentrations, atovaquone-treated parasites may maintain some electron transport through an alternative branch of the respiratory chain. This might enable the parasite to complete a round of replication in which an atovaquone-resistant mutant might be selected. There is clinical evidence that a single base change in the P. falciparum cytochrome b gene can dramatically increase atovaquone resistance (up to 30,000 fold) (17). If alternative respiration enables the parasite enough growth to permit such a mutation, this could explain the high recrudescence rates seen in patients suffering P. falciparum infections who have been treated with atovaquone alone (18).
Based on the results of the biochemical assays presented here, we predicted that atovaquone could be potentiated by alternative oxidase inhibitors. This prediction was confirmed for both SHAM and propyl gallate (Fig. 2). Of the two alternative oxidase inhibitors, propyl gallate would probably be more clinically useful because it has a lower IC50 for P. falciparum (24 μM) (21) and because it appears to have a relatively low mammalian toxicity. As an antioxidant, propyl gallate is commonly added to processed foods and cosmetics to prevent spoilage. For these uses, the acceptable daily intake is 0.2 mg/kg of body weight (24). Long-term studies indicated that propyl gallate is not carcinogenic in mice or rats (1, 7). Rat liver respiration is inhibited only 13% by 1.16 mM propyl gallate (6). This is about 50 times the IC50 for P. falciparum and more than 10 times the concentration of propyl gallate that has been shown to inhibit all cyanide-resistant oxygen consumption (21). Together, these data suggest that propyl gallate selectively inhibits the parasite as compared to mammalian cells.
The ability of alternative oxidase inhibitors to potentiate atovaquone activity suggested a new possible mechanism of action for proguanil, which also potentiates atovaquone in vitro and in vivo (4, 18). Polarographic assays were used to test the possibility that proguanil inhibits P. falciparum oxygen consumption alone or in combination with atovaquone. For these studies, proguanil was used at a concentration 100-fold higher than its IC50 for P. falciparum in vitro (26). Yet, this concentration of proguanil reduced parasite oxygen consumption by much less than the amount that would be expected if either the cytochrome chain or the alternative oxidase were fully inhibited. The remaining rate of parasite oxygen consumption retained susceptibility to either atovaquone or SHAM. This suggests that neither the cytochrome chain nor the alternative respiratory pathway was specifically inhibited. Instead, proguanil may have had a more generalized effect on the cell, which may have decreased the rate of electrons entering either pathway.
Previous studies have screened known antimalarial compounds to identify those that could potentiate atovaquone (4, 18, 28, 29). In this study, a model of the P. falciparum respiratory chain was used to assess a possible mechanism of action of one antimalarial agent which is known to potentiate atovaquone and to predict the potentiating activity of two previously untested compounds. The results of these studies support the branched respiratory chain model for P. falciparum and have identified a new class of compounds that may prove useful for the clinical augmentation of atovaquone.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grant RO1-AI38329 from the National Institute of Allergy and Infectious Diseases.
We thank Craig Wilson for his many contributions to this study.
REFERENCES
- 1.Abdo K M, Huff J E, Haseman J K, Alden C J. No evidence of carcinogenicity of d-mannitol and propyl gallate in F344 rats or B6C3F1 mice. Food Chem Toxicol. 1986;24:1091–1097. doi: 10.1016/0278-6915(86)90293-0. [DOI] [PubMed] [Google Scholar]
- 2.Basco L K, Ramiliarisoa O, Bras J L. In vitro activity of atovaquone against the African isolates and clones of Plasmodium falciparum. Am J Trop Med Hyg. 1995;53:388–391. doi: 10.4269/ajtmh.1995.53.388. [DOI] [PubMed] [Google Scholar]
- 3.Berenbaum M C. A method for testing synergy with any number of agents. J Infect Dis. 1978;137:122–120. doi: 10.1093/infdis/137.2.122. [DOI] [PubMed] [Google Scholar]
- 4.Canfield C J, Pudney M, Gutteridge W E. Interactions of atovaquone with other antimalarial drugs against Plasmodium falciparum in vitro. Exp Parasitol. 1995;80:373–381. doi: 10.1006/expr.1995.1049. [DOI] [PubMed] [Google Scholar]
- 5.Chiodini P L, Conlon C P, Hutchinson D B, Farquhar J A, Hall A P, Peto T E, Birley H, Warrell D A. Evaluation of atovaquone in the treatment of patients with uncomplicated Plasmodium falciparum malaria. J Antimicrob Chemother. 1995;36:1073–1078. doi: 10.1093/jac/36.6.1073. [DOI] [PubMed] [Google Scholar]
- 6.Clarkson A B, Bienen E J, Pollakis G, Grady R W. Trypanocidal CoQ analogues: their effect on other mitochondrial systems. Comp Biochem Physiol B. 1989;94:245–251. doi: 10.1016/0305-0491(89)90341-6. [DOI] [PubMed] [Google Scholar]
- 7.Dacre J C. Long-term toxicity study of n-propyl gallate in mice. Food Cosmet Toxicol. 1974;12:125–129. doi: 10.1016/0015-6264(74)90328-9. [DOI] [PubMed] [Google Scholar]
- 8.Desjardins R E, Canfield C J, Haynes J D, Chulay J D. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob Agents Chemother. 1979;16:710–718. doi: 10.1128/aac.16.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fidock D A, Wellems T E. Transformation with human dihydrofolate reductase renders malaria parasites insensitive to WR99210 but does not affect the intrinsic activity of proguanil. Proc Natl Acad Sci USA. 1997;94:10931–10936. doi: 10.1073/pnas.94.20.10931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fry M, Pudney M. Site of action of the antimalarial hydroxynapthoquinone, 2-[trans-4-(4′-chlorphenyl) cyclohexyl]-3-hydroxy-1,4-napthoquinone (566C80) Biochem Pharmacol. 1992;43:1545–1553. doi: 10.1016/0006-2952(92)90213-3. [DOI] [PubMed] [Google Scholar]
- 11.Gutteridge W E. 566C80, an antimalarial hydroxynapthoquinone with broad spectrum: experimental activity against opportunistic parasitic infections of AIDS patients. J Protozool. 1991;38:141S–143S. [PubMed] [Google Scholar]
- 12.Gutteridge W E, Dave D, Richards W H. Conversion of dihydroorotate to orotate in parasitic protozoa. Biochim Biophys Acta. 1979;582:390–401. doi: 10.1016/0304-4165(79)90131-4. [DOI] [PubMed] [Google Scholar]
- 13.Hudson A T, Dickins M, Ginger C D, Gutteridge W E, Holdich T, Hutchinson D S A, Pudney M, Randall A W, Latter V S. 566C80: a potent broad spectrum anti-infective agent with activity against malaria and opportunistic infection in AIDS patients. Drugs Exp Clin Res. 1991;17:427–435. [PubMed] [Google Scholar]
- 14.Ittarat I, Asawamahasakda W, Meshnick S R. The effects of antimalarials on the Plasmodium falciparum dihydroorotate dehydrogenase. Exp Parasitol. 1994;79:50–56. doi: 10.1006/expr.1994.1058. [DOI] [PubMed] [Google Scholar]
- 15.Ittarat I, Webster H K, Yuthavong Y. High-performance liquid chromatographic determination of dihydroorotate dehydrogenase of Plasmodium falciparum and effects of antimalarials on enzyme activity. J Chromatogr. 1992;582:57–64. doi: 10.1016/0378-4347(92)80302-7. [DOI] [PubMed] [Google Scholar]
- 16.Lang-Unnasch N. Purification and properties of Plasmodium falciparum malate dehydrogenase. Mol Biochem Parasitol. 1992;50:17–26. doi: 10.1016/0166-6851(92)90240-k. [DOI] [PubMed] [Google Scholar]
- 17.LeBlanc S B. Studies on pyrimidine biosynthesis and linkage with mitochondrial respiration in Plasmodium falciparum. Ph.D. thesis. University of Alabama at Birmingham; 1995. [Google Scholar]
- 18.Looareesuwan S, Viravan C, Webster H K, Kyle D E, Hutchinson D B, Canfield C J. Clinical studies of atovaquone, alone or in combination with other antimalarial drugs, for the treatment of acute uncomplicated malaria in Thailand. Am J Trop Med Hyg. 1996;54:62–66. doi: 10.4269/ajtmh.1996.54.62. [DOI] [PubMed] [Google Scholar]
- 19.McIntosh L. Molecular biology of the alternative oxidase. Plant Physiol. 1994;105:781–786. doi: 10.1104/pp.105.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moore A L, Siedow J N. The regulation and nature of the cyanide-resistant alternative oxidase of plant mitochondria. Biochim Biophys Acta. 1991;1059:121–140. doi: 10.1016/s0005-2728(05)80197-5. [DOI] [PubMed] [Google Scholar]
- 21.Murphy A D, Doeller J, Hearn B, Lang-Unnasch N. Plasmodium falciparum: cyanide-resistant oxygen consumption. Exp Parasitol. 1997;87:112–120. doi: 10.1006/expr.1997.4194. [DOI] [PubMed] [Google Scholar]
- 22.Scheibel L W. Plasmodium metabolism and related organellar functions during various stages of the life-cycle: carbohydrates. In: Wernsdorfer W H, McGregor S I, editors. Malaria principles and practice of malariology. New York, N.Y: Churchill Livingstone; 1988. pp. 171–217. [Google Scholar]
- 23.Tallarida R J, Jacob L S. The dose-response relation in pharmacology. New York, N.Y: Springer-Verlag; 1979. [Google Scholar]
- 24.van der Heijden C A, Janssen P J, Strik J J. Toxicology of gallates: a review and evaluation. Food Chem Toxicol. 1986;24:1067–1070. doi: 10.1016/0278-6915(86)90290-5. [DOI] [PubMed] [Google Scholar]
- 25.Vanlerberghe G C, Vanlerberghe A E, McIntosh L. Molecular genetic alteration of plant respiration: silencing and overexpression of alternative oxidase in transgenic tobacco. Plant Physiol. 1994;106:1503–1510. doi: 10.1104/pp.106.4.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watkins W M, Sixsmith D G, Chulay J D. The activity of proguanil and its metabolites, cycloguanil and p-chlorophenylbiguanide, against Plasmodium falciparum in vitro. Ann Trop Med Parasitol. 1984;78:273–278. doi: 10.1080/00034983.1984.11811816. [DOI] [PubMed] [Google Scholar]
- 27.Wooden J M, Hartwell L H, Vasquez B, Sibley C H. Analysis in yeast of antimalarial drugs that target the dihydrofolate reductase of Plasmodium falciparum. Mol Biochem Parasitol. 1997;85:25–40. doi: 10.1016/s0166-6851(96)02808-3. [DOI] [PubMed] [Google Scholar]
- 28.Yeo A E, Edstein M D, Rieckmann K H. Antimalarial activity of the triple combination of proguanil, atovaquone and dapsone. Acta Trop. 1997;67:207–214. doi: 10.1016/s0001-706x(97)00060-0. [DOI] [PubMed] [Google Scholar]
- 29.Yeo A E T, Edstein M D, Shanks G D, Rieckmann K H. Potentiation of the antimalarial activity of atovaquone by doxycycline against Plasmodium falciparum in vitro. Parasitol Res. 1997;83:489–491. doi: 10.1007/s004360050285. [DOI] [PubMed] [Google Scholar]