Abstract
Background:
Early low socioeconomic status (SES) is associated with poor outcomes in childhood, many of which endure into adulthood. It is critical to determine how early low SES relates to trajectories of brain development, and whether these mediate relationships to poor outcomes. We use data from a unique 17-year longitudinal study with five waves of structural brain imaging to prospectively examine relationships between preschool SES and cognitive, social, academic, and psychiatric outcomes in early adulthood.
Methods:
Children (n=216, 50% female, 47.2% non-white) were recruited from a study of early onset depression and followed approximately annually. Family income-to-needs ratios (SES) were assessed when children were ages 3 to 5. Volumes of cortical gray and white matter, and subcortical gray matter collected across five scan waves were processed using the Freesurfer longitudinal pipeline. When youth were ages 16+, cognitive function was assessed using the NIH Toolbox, and psychiatric diagnoses, high-risk behaviors, educational function, and social function were assessed using clinician administered and parent/youth report measures.
Results:
Lower preschool SES related to worse cognitive, high-risk, educational, and social outcomes (|Std.B|=.20-.31, ps<.003). Lower SES was associated with overall lower cortical (Std.B=.12, p<.0001) and subcortical gray matter (Std.B=.17, p<.0001) volumes, as well as a shallower slope of subcortical gray matter growth over time (Std.B=.04, p=.012). Subcortical gray matter mediated the relationship of preschool SES to cognition and high-risk behaviors.
Conclusions:
These novel longitudinal data underscore the key role of brain development in understanding the long lasting relations of early low SES to outcomes in children.
Keywords: Socioeconomic status, brain development, cognition, adaptive function, risk-taking, social function
Introduction
There is overwhelming evidence that early poverty is associated with a host of poor developmental outcomes in childhood(1–6) that can endure into adulthood (7, 8). Further, there is robust evidence that early poverty is associated with differences in brain structure and function (9–16). However, we know little about how early poverty relates to trajectories of brain development, and even less about whether variation in such trajectories is part of the pathway linking early socioeconomic status (SES) to adult outcomes. The current study used data from a unique longitudinal study with five waves of structural brain imaging across development to prospectively examine relationships of preschool SES to behavioral, social, academic, cognitive and psychiatric outcomes at the transition to adulthood.
A growing number of studies support that early low SES is associated with poor functional outcomes in adulthood (1, 17–23), but few have examined multiple outcomes simultaneously. There is also robust support for a relationship of early poverty to brain structure. The most consistent evidence is for reductions in hippocampal volume associated with poverty (9–15, 24), with some data also suggesting reduced amygdala volume (10, 11, 13). There is also evidence for variation in the volume, thickness and/or surface area of prefrontal regions associated with poverty (25), though findings have been mixed (15).
The majority of the literature has examined differences in indices of brain function and structure at a single time point in relationship to childhood SES. The majority of these studies do not address whether preschool SES relates to the trajectories of brain development within an individual, a question with high relevance for evaluating the timing and duration of SES relationships. However, Hanson and colleagues found that children from poor families had slower trajectories of growth in whole brain volume, and in regional frontal and parietal lobe volumes, with these effects related to the emergence of externalizing symptoms. Importantly, the patterns of growth suggested few differences as a function of poverty at the very earliest ages (e.g., 5–9 months old), but increasing differences as children grew older (26). Such findings are consistent with the idea that continued exposure to impoverished environments slows brain development. However, not all studies have found similar results (27–30). This literature is constrained by few studies having multiple waves of brain imaging, with only the Hanson study (26) to our knowledge having more than three timepoints. There is also some evidence for such mediation in the domains of mental health (31–34) and cognition (1, 35–37). This has led to call for more studies that assess the same individuals longitudinally(1) to address such critical questions about whether brain structure mediates the relationship of preschool SES to a broader array of adult outcomes. Such investigations have important developmental implications in understanding how early exposures may set trajectories for long-term outcomes.
To address this major gap in our understanding of the pathways from early socioeconomic status (SES) to poor adaptive outcomes as youth transition to adulthood, we analyzed data from the Preschool Depression Study, a 17 year longitudinal study that followed children from preschool through the transition to adulthood, with up to five waves of imaging in each youth. We asked if income-to-needs in preschool related to either or both: 1) cognitive function, high-risk behaviors, social function, educational function, and/ or psychiatric outcomes when youth were between the ages of 16 and 21; 2) trajectories of growth in cortical and subcortical gray matter, white matter, or specific cortical and subcortical regions; and 3) whether variation in structural brain development mediated the relationship between preschool SES and outcomes at the transition to adulthood.
Methods
Participants
The Preschool Depression Study (PDS) is a 17 year longitudinal study that includes 5 waves of brain scans across school age to early adulthood (Figure S1). At Time (T1), 306 children aged 3 to 5 years and their primary caregivers were recruited from the St. Louis area, using a checklist to oversample preschoolers with elevated symptoms of depression (38). At school age (~7 to 12 years), healthy children and those with a history of depression and/or anxiety were invited to participate in brain imaging; an additional 42 healthy children were also recruited (N=210 completed the first wave of imaging). See Supplement for exclusions. All methods were approved by the Institutional Review Board at Washington University (IRB #201502094). Written informed consent and assent was obtained from all participants.
Preschool SES
SES was operationalized as the income-to-needs ratio, defined as the total family income at T1 (Figure S1) divided by the federal poverty level based on family size (39).
Preschool Psychopathology and Life Events
Trained staff from the Early Emotional Development Program conducted up to ten in-person assessment sessions with participants and their primary caregivers over the course of the study (see Figure 1). The children were between the ages of 3.0–5.11 years at the time of their first interview (T1) and between the ages of 15.3–21.6 at the most recent assessment wave (T10/MRI5). The Preschool-Age Psychiatric Assessment (PAPA) (40, 41) was the diagnostic assessment when children were age 3.0–7.11. The PAPA is designed for diagnostic use with the caregivers of children ages 2.0–6.0 years (but has been used up to age 8.0) and has acceptable reliability (41). It consists of questions about developmentally appropriate symptom manifestations of DSM-IV criteria for all Axis I disorders, including major depression disorder (MDD), attention deficit hyperactivity disorder (ADHD), and anxiety disorders. We created the following dimensional T1 psychopathology scores by computing the number of core items from the PAPA endorsed by the parents (Figure 1) to estimate early psychiatric challenges in these children: 1) depression (major depressive disorder); 2) anxiety (separation anxiety disorder, generalized anxiety disorder, and post-traumatic stress disorder); and 3) externalizing (oppositional defiant disorder, attention deficit hyperactivity disorder, and conduct disorder). Each of these were significantly correlated with T1 Income-to-needs (rs −.20 to −.33, ps .011 to .0001), and were included as covariates in follow-up analyses to determine whether any relationships of SES to later outcomes were over and above early psychopathology. Youth’s life events were assessed at each annual assessment wave starting at T1, as part of the PAPA and then the Childhood-Age Psychiatric Assessment (see Supplement for description of all possible events) (42). Life events were measured as the total number of events through the first scan wave (Figure S1).
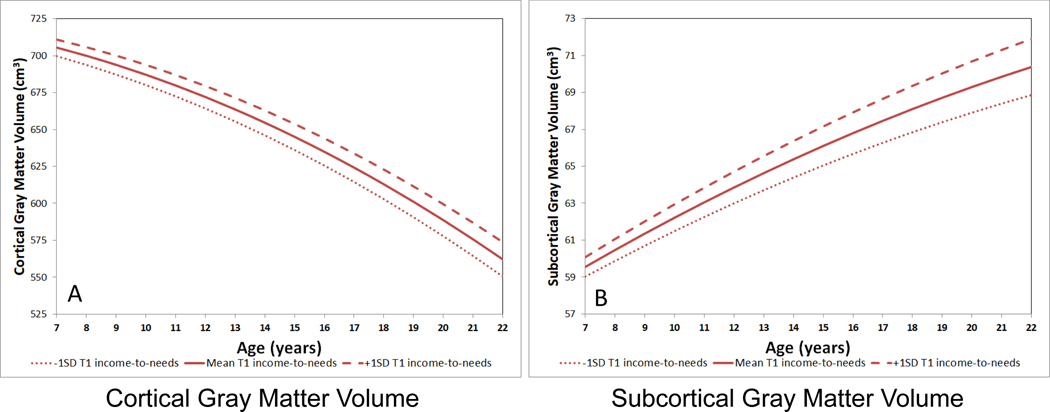
Figure 1: Trajectories of Cortical and Subcortical Gray Matter as a Function of T1 Income-to-needs Ratio.
A) Trajectory of cortical gray matter, with the estimated fit lines plotted separately for the mean T1 income-to-needs and +/− 1 standard deviation; B) Trajectory of subcortical gray matter, with the estimated fit lines plotted separately for the mean T1 income-to-needs and +/− 1 standard deviation. These graphs were generated using the original values for interpretation, though the analyses were run on standardized variables. Mean values of 0.5 (neutral) for sex and 1547.9 cm3 for intracranial volume were entered into the model equation.
Maternal Mental Health
Maternal history of mental illness (number of diagnoses) was assessed using the Family Interview for Genetic Studies (43). This measure were used as a covariate in analyses presented below to determine the degree to which SES associated with adaptative outcomes and brain measures over and above maternal mental health. We focused on maternal mental health as the vast majority of our participating parents were mothers, and we felt most confident in maternal mental health reports. See Supplement for details.
Late Adolescence/Early Adulthood Adaptive Outcomes
We modelled our outcome metrics after those used in the Great Smoky Mountains study (44–46), but did not include health as it was not well assessed in our study and added cognitive function. We used data from T9/MRI 4 and T10/MRI 5 (Figure S1), but only when the participant was age 16 years or older. We chose 16+ as a good balance of capturing late adolescence/early adulthood, without having too narrow of an age range for outcomes. We used T9 and T10 because age varied and some youth were 16+ at both T9 and T10, and some youth were not 16+ until T10. The measure of each of these outcomes is described in detail in the Supplement, with a brief overview provided below.
Cognitive Function:
Participants completed the following NIH Toolbox cognitive measures:(47) 1) Picture Sequence Memory episodic memory; 2) List Sorting working memory; 3) Flanker selective attention; 4) Pattern Comparison processing speed; and 5) Picture Vocabulary verbal IQ (see Supplement). We used age-corrected t-scores and averaged the five tasks to create a composite.
High-Risk Behavioral Outcomes:
We coded for eight behaviors, each coded ‘1’ for present at either T9 or T10, or ‘0’ for absent at both (see Supplement): (1) pathological lying; (2) initiating physical fights; (3) breaking and entering; (4) arrested, detained, cited, adjudicated, placed on probation, juvenile detention, court-ordered treatment, or incarcerated since last assessment; (5) being intoxicated or drunk at least 1–2 times per week in past year; (6) marijuana or other illicit drug use in past year; (7) consensual sex with someone known for less than 24 hours; and (8) being pregnant (female) or impregnating someone (male) prior to age 18 (not the result of rape).
Social Outcomes:
We coded for seven outcomes (Supplement): (1) poor peer relationships; (2) peer acceptance/rejection; (3) bullied; (4) relational victimization; and (5) social withdrawal; (6) social inhibition; and (7) prosocial behavior.
Education Outcomes:
We coded two outcomes (Supplement): (1) poor school engagement; and 2) poor academic function.
Psychiatric Outcomes:
We coded seven outcomes (Supplement): (1) anxiety disorder; (2) a unipolar depressive disorder; (3) conduct disorder; (4) alcohol, (5) marijuana or (6) substance use disorder; and (7) significant borderline personality symptoms. We also conducted secondary analyses with a variable that had only six outcomes, excluding borderline personality symptoms. We also examined each of the six disorder categories individually.
The final outcome score for the High-Risk, Social, Education, and Psychiatric Outcomes was the mean of the items within each domain.
Structural Imaging Acquisition
All five waves of MRI data collection acquired 3D T1-weighted scans used a 3.0T Siemen’s whole-body scanner (Trio or Prisma). See Supplement for details.
Structural Imaging Processing
The data from MRI waves 1–3 have been previously processed (48). Processing of structural data used the FreeSurfer Longitudinal processing stream v5.3 [http://surfer.nmr.mgh.harvard.edu] (49). See Supplement for details of processing, quality control, and harmonization across platforms. Volume of cortical gray, cortical white, subcortical gray, hippocampus, amygdala, caudate, putamen, and thalamus were obtained using FreeSurfer’s “aseg.stats” report, as was intracranial volume. Volumes of the dorsolateral prefrontal cortex and dorsal anterior cingulate were obtained from the Destrieux Atlas (50). We did not have hypotheses about asymmetries and thus averaged the left and right. We examined global measures (e.g., cortical gray, cortical white, subcortical gray) as well as regional measures because of the possibility that SES relations to brain development reflect mechanisms that could have broad effects across cortex or subcortex or both (e.g., toxins, nutrition, even stress, etc.).
Statistical Analysis
Continuous predictors and outcomes were standardized to facilitate comparison of effect sizes (“Std. B”; Supplement). Covariates for all analyses included sex and analyses of brain variables included intracranial volume. Significant results were followed-up by analyses adding T1 psychopathology (depression, anxiety, and externalizing), life events (see Supplement), and maternal mental health as covariates (Supplement). Sex did not interact significantly with (T1 Income-to-needs ratio; T1INR) or any brain variable to relate to any outcome and thus sex interactions were not included in final models. Because some of the outcome variables were counts, the results of the linear regressions were confirmed using zero-inflated Poisson regression.
Multiple regression was used to examine relationships of early T1INR to each outcome, with bootstrapped confidence intervals (1000), and false discovery rate (FDR) correction(51) across the five outcome measures. For the individual psychiatric disorder outcomes, we conducted logistic regressions. To examine whether T1INR relates to trajectories of brain volume across MR1-MR5, we used multilevel models (MLM’s) that included both random intercept and slope components in addition to fixed effects (see Supplement for details and code examples). Analyses were FDR corrected across both the main effects of T1INR (intercepts) and interactions with age (slopes) for cortical gray and white matter, and subcortical gray [i.e., 6 tests]. We conducted follow-up analyses on five subcortical regions (hippocampus, amygdala, putamen, caudate, thalamus) and two cortical regions (dorsolateral prefrontal cortex, dorsal anterior cingulate) previously associated with SES(15) to assess specificity. There were no FDR significant non-linear relations of T1INR, so T1INR by age squared interactions were not included in final models (see Supplement). As an additional exploratory analyses, we examined the relationship of T1INR to the additional 71 regions in the Destrieux Atlas (50).
We generated individual intercepts and slopes of each brain metric across MRI 1–5 for each youth, using the same type of MLM described above. For brain metrics significantly related to T1INR, linear regressions examined whether the brain metric related to any of the outcomes associated with T1INR with FDR correction.
For any brain metric related to both T1INR and an outcome measure also associated with T1INR, we conducted mediation analyses using the PROCESS procedure (model 4) in R (52).
Results
Participant Characteristics
Demographic characteristics and distributions of adaptive outcomes are shown in Table S1, with zero-order correlations among variables in Table S2. See Supplement for additional analyses incorporating race.
Early SES and Outcomes in the Transition to Adulthood
As shown in Table 1, lower early SES (i.e., lower T1INR) was associated with worse cognitive function, more high-risk behaviors, worse social, and worse educational outcomes, but not with psychiatric outcomes (Table 1). All of these relationships remained significant when controlling for preschool psychopathology, maternal mental health, and cumulative life events. For psychiatric outcomes, there was still no significant relationship to T1INR if we examined a variable that excluded borderline symptoms (p > 0.67) and no significant relationships in logistic regressions using T1INR and sex to predict each of the six categories of diagnostic outcomes (all ps > 0.10). As described above, some of the outcome variables represent counts for which we took the mean. Thus, we repeated all of the analyses with zero inflated Poisson regression with the counts themselves, with the same results.
Table 1:
T1 Income-to-needs Ratio Relating to Outcomes at the Transition to Adulthood
| T1 Income-to-needs as an independent variable, with sex as a covariate | T1 Income-to-needs as an independent variable, with sex, T1 depression, anxiety and externalizing severity, cumulative life events, and maternal mental health as covariates | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Outcome Variable | Std. B | Lower 95% CI | Upper 95% CI | t | p | Std. B | Lower 95% CI | Upper 95% CI | t | p |
| Cognitive Function Outcome | .308 | .165 | .452 | 4.25 | <.001* | .279 | .118 | .441 | 3.42 | <.001 |
| High Risk Behaviors Outcome | −.244 | −.400 | −.091 | −3.15 | .002* | −.232 | −.409 | −.055 | −2.56 | .01 |
| Poor Social Outcomes | −.202 | −.361 | −.044 | −2.52 | .01* | −.224 | −.403 | −.045 | −2.48 | .01 |
| Poor Education Outcomes | −.256 | −.425 | −.086 | −.298 | .003* | −.270 | −.461 | −.079 | −2.80 | .006 |
| Psychiatric Outcomes | −.076 | −.233 | .081 | −.96 | .340 | --- | --- | --- | --- | --- |
Survives FDR correction across all five outcomes, also indicated by light gray shading. Models controlling for T1 psychopathology were only run if the effect was significant in models not including T1 psychopathology.
Early SES and Trajectories of Brain Development
As can be seen in Table 2, Figure 1, and Figure S2, there were main effects of lower early SES (i.e., lower T1INR) relating to both overall reduced (i.e., lower intercept) cortical gray matter and subcortical gray matter volumes, but no significant effect for white matter. T1INR also related to the slope of subcortical gray matter (i.e., significant interaction with age). As shown in Figure 1, the difference in subcortical gray matter between children with greater versus lesser T1INR increased across the course of development. These effects remained significant when including T1 depression, anxiety, and externalizing severity, life events and maternal mental health (Table S3).
Table 2:
Multilevel Models of T1 Income-to-needs Ratio Relating To Cortical Gray, Cortical White, and Subcortical Gray Volumes
| Dependent Variable → Cortical Gray Volume | Std. B | SE | t | p | Semi-Partial R2 |
|---|---|---|---|---|---|
| Predictors | |||||
| Intercept | 0.0039 | 0.0286 | 0.14 | 0.89 | |
| Age | −0.4210 | 0.0117 | −36.09 | <0.001 | 0.372 |
| Age squared | −0.0548 | 0.0103 | −5.31 | <0.001 | 0.010 |
| Female Sex | −0.0577 | 0.0315 | −1.83 | 0.07 | 0.015 |
| Intracranial volume | 0.7647 | 0.0317 | 24.12 | <0.001 | 0.717 |
| T1 Income-to-needs ratio | 0.1229 | 0.0295 | 4.16 | <0.001* | 0.072 |
| T1 income-to-needs ratio X Age | 0.0193 | 0.0098 | 1.97 | 0.05 | 0.002 |
| Dependent Variable → Cortical White Matter Volume | Std. B | SE | t | P | Semi-Partial R2 |
| Predictors | |||||
| Intercept | −0.0084 | 0.0321 | −0.26 | 0.79 | |
| Age | 0.2066 | 0.0063 | 32.69 | <0.001 | 0.114 |
| Age squared | −0.0695 | 0.0045 | −15.39 | <0.001 | 0.014 |
| Female Sex | 0.0048 | 0.0352 | 0.14 | 0.89 | 0.000 |
| Intracranial volume | 0.8550 | 0.0353 | 24.20 | <0.001 | 0.740 |
| T1 Income-to-needs ratio | 0.0224 | 0.0331 | 0.68 | 0.50 | 0.002 |
| T1 income-to-needs ratio X Age | 0.0078 | 0.0057 | 1.3 | 0.17 | 0.000 |
| Dependent Variable → Subcortical Gray Matter Volume | Std. B | SE | t | p | Semi-Partial R2 |
| Predictors | |||||
| Intercept | −0.0009 | 0.0398 | −0.02 | 0.98 | |
| Age | 0.4176 | 0.0165 | 25.34 | <0.001 | 0.231 |
| Age squared | −0.0300 | 0.0137 | −2.19 | 0.03 | 0.002 |
| Female Sex | −0.0760 | 0.0403 | −1.88 | 0.06 | 0.013 |
| Intracranial volume | 0.6046 | 0.0412 | 14.69 | <0.001 | 0.496 |
| T1 Income-to-needs ratio | 0.1645 | 0.0410 | 4.01 | <0.001* | 0.067 |
| T1 income-to-needs ratio X Age | 0.0363 | 0.0142 | 2.55 | 0.01* | 0.003 |
Note: The multilevel models included both random intercept and random slope components in addition to fixed effects.
Survives FDR correction across both main effects and interactions with age (6 total tests), also indicated by light gray shading.
As shown in Table S4 and Figure S3, T1INR did not significantly relate to the volume of the dorsolateral prefrontal cortex or dorsal anterior cingulate (see Table S5 for exploratory analysis of the other 73 Destrieux regions, a few of which showed significant relations to T1INR after FDR correction). As shown in Table S6 and Figures S4 and S5, there were main effects of lower early SES relating to overall reduced (i.e., lower intercept) hippocampal, caudate, putamen, and thalamus volumes, but not amygdala volume or the slopes of any subcortical brain region. The relationships of T1INR to hippocampal, caudate, putamen, and thalamus volumes remained significant when controlling for T1 psychopathology, life events, and maternal psychopathology (Table S7).
Trajectories of Brain Development and Early Adult Outcomes
Early SES related to only the intercept of cortical gray matter but not to the slope of cortical gray matter (e.g., main effect of T1INR, but no interaction with age). However, early SES related significantly to both the intercept and slope of subcortical gray matter. Cognitive function and high-risk behaviors were significantly associated with intercepts of cortical gray and both intercepts and slopes of subcortical gray volumes (Table 3). Poor social outcomes were related to cortical gray intercepts and poor educational outcomes were related to subcortical gray slopes. Cognitive function and high-risk behaviors remained significantly related to subcortical intercepts and slopes when covarying for T1 psychopathology, life events and maternal psychopathology (Table 3). As noted above, some of the outcome variables represent counts. Thus, we repeated all of the analyses with zero inflated Poisson regression with the same pattern of results, other than education outcomes for subcortical gray slopes (p=.09 for Poisson regression), which did not survive correction for the full set of covariates (see Table 3).
Table 3:
Cortical and Subcortical Brain Volume Trajectories Relating to Late Adolescence/Early Adulthood Outcomes
| Independent Variable, with sex and intracranial volume as covariates | Independent Variable, with sex, intracranial volume, T1 depression, anxiety and externalizing severity, cumulative life events, and maternal mental health as covariates | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Outcome Variable | Std. B | Lower 95% CI | Upper 95% CI | t | p | Std. B | Lower 95% CI | Upper 95% CI | t | p |
| Independent Variable = Cortical Gray Matter Intercepts | ||||||||||
| Cognitive Function Outcome | .422 | .121 | .724 | 2.77 | .006* | .247 | −.100 | .594 | 1.41 | .16 |
| High Risk Behaviors Outcome | −.335 | −.637 | −.032 | −2.18 | .03* | −.290 | −.661 | .081 | −1.55 | .13 |
| Poor Social Outcomes | −.397 | −.680 | −.114 | −2.77 | .006* | −.335 | −.682 | .012 | −1.91 | .06 |
| Poor Education Outcomes | −.186 | −.511 | .140 | −1.13 | .26 | --- | --- | --- | --- | --- |
| Independent Variable = Subcortical Gray Matter Intercepts | ||||||||||
| Cognitive Function Outcome | .410 | .189 | .630 | 3.67 | <.001* | .308 | .072 | .544 | 2.58 | .01 |
| High Risk Behaviors Outcome | −.375 | −.593 | −.156 | −3.39 | <.001* | −.358 | −.613 | −.104 | −2.79 | .006 |
| Poor Social Outcomes | −.169 | −.378 | .041 | −1.59 | .11 | --- | --- | --- | --- | --- |
| Poor Education Outcomes | −.222 | −.453 | .009 | −1.90 | .06 | --- | --- | --- | --- | --- |
| Independent Variable = Subcortical Gray Matter Slopes | ||||||||||
| Cognitive Function Outcome | .341 | .171 | .510 | 3.97 | <.001* | .259 | .065 | .452 | 2.65 | .009^ |
| High Risk Behaviors Outcome | −.332 | −.502 | −.161 | −3.85 | <.001* | −.340 | −.543 | −.136 | −3.31 | .001^ |
| Poor Social Outcomes | −.073 | −.240 | .095 | −0.86 | .39 | --- | --- | --- | --- | --- |
| Poor Education Outcomes | −.236 | −.422 | −.049 | −2.50 | .01* | −.193 | −.414 | .028 | −1.73 | .09 |
Note: The multilevel models used to generate the intercepts and slopes including random intercept and random slope components in addition to fixed effects.
Survives FDR correction across all four outcomes. Note: Darker gray shading indicates relationships significant even when controlling for T1 psychopathology, while lighter gray shading indicates only significant when not controlling for T1 psychopathology. Models controlling for T1 psychopathology, life events, and maternal psychopathology were only run if the effect was significant in models not including these covariates.
Survives FDR correction for the number of follow-up models run for that independent variable.
To determine whether subcortical intercepts and slopes were accounting for dissociable variance in outcomes, we ran linear models that included both. For cognitive outcomes, subcortical intercepts were no longer significant (Std. B=.19, t=1.16, p=.25), but slopes remained trend level (Std. B=.23, t=1.85, p=.07). For high-risk outcomes, subcortical intercepts were not significant (Std. B=−.15, t=−0.95, p=.34), but slopes were (Std. B=−.25, t=−2.00, p=.047). Thus, for mediations below we focused on slopes and not intercepts.
Neither hippocampal nor caudate intercepts significantly related to any outcomes. Cognitive function related to both putamen and thalamic intercepts (Table S8), though only thalamus remained significant accounting for additional covariates. High-risk behaviors were significantly related to both putamen and thalamus intercepts, even controlling for additional covariates of preschool psychopathology, maternal mental health and life events.
Mediation
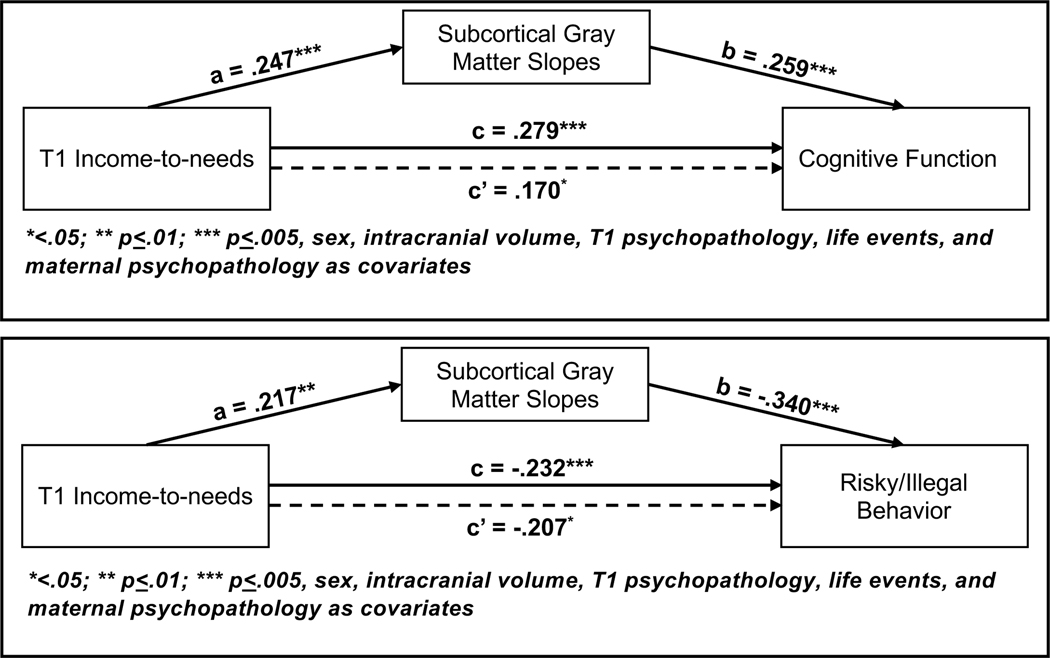
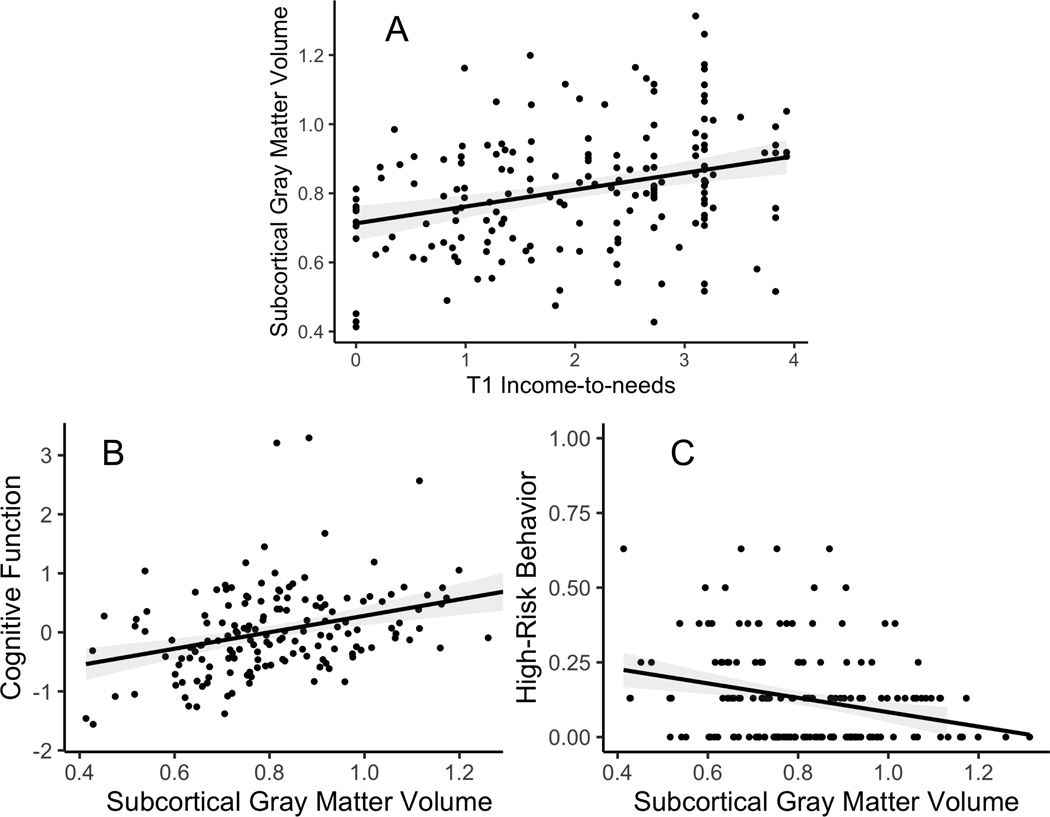
We only examined mediation for adaptive outcomes related to both T1INR (Table 2) and the brain metric (Table 3) with the full covariate set in the model. Slopes of subcortical gray matter significantly mediated the relationship between T1INR and both cognitive function and high-risk behavior even when controlling for additional covariates (Figure 2 and Table S9). The bivariate relationships between T1INR and slopes of subcortical gray matter, and of subcortical gray matter to both cognitive function and high-risk behavior are shown in Figure 3. The only significant mediation for individual subcortical volumes was thalamic volumes mediating the relationship between T1INR and cognitive function, but this did not survive inclusion of additional covariates (Table S10).
Figure 2: Mediation Models for Subcortical Gray Matter Volumes.
Mediation results were generated using the PROCESS 3.5 macro in R version 4.03, beta 0.5. The “a” values refer to the relationship between the predictor and the mediator, and the “b” values refer to the relationship the mediator and the outcome. The “c” values are the direct relationship between the predictor and the outcome with no mediator in the model, and the “c’” values are the relationships between the predictor and the outcome with the mediator in the model.
Figure 3: Graphs Illustrating Bivariate Relationships Subcortical Gray Matter Volumes.
A) Scatterplot depicting the relationship between T1 income-to-needs and the slope of subcortical gray matter volume, with the estimated linear fit and 95% confidence interval overlayed. B) Scatterplot depicting the relationship between the slope of subcortical gray matter volume and cognitive function, with the estimated linear fit and 95% confidence interval overlayed. C) Scatterplot depicting the relationship between the slope of subcortical gray matter volume, and high-risk behaviors with the estimated linear fit and 95% confidence interval overlayed.
Discussion
Preschool SES was associated with cognitive function, high-risk behaviors, social function and educational function over 13 years later, even when controlling for preschool psychopathology, life events, and maternal psychopathology, providing robust evidence for the enduring influence of early childhood SES. Early SES was associated with both cortical and subcortical gray matter development, with the relationship of low preschool SES to differences in subcortical gray matter development increasing with age. Further, subcortical gray matter trajectories mediated the relationship between preschool SES and cognition and high-risk behaviors, even when controlling for preschool psychopathology, life events, and maternal psychopathology. Together these novel longitudinal data underscore the key role of brain development in understanding the long lasting relations of early SES in children to later outcomes, and highlight the need to address this pressing public health concern.
An unresolved question is the pathway by which early childhood SES relates to higher risk of poor adult outcomes. One possibility is that low early SES indexes ongoing low SES and thus ongoing exposure to a range of chronic stressors, reduced access to health care and poor nutrition, exposures to environmental toxins, etc., that persists into adulthood. If so, it may not be the early low SES per se that relates to poor outcomes, but rather the fact that early low SES is a harbinger of ongoing low SES and chronic stressors that continue to be present through adulthood. The evidence that improving SES during childhood improves cognitive, educational and mental health outcomes in youth is indirectly consistent with this interpretation (53–55). Alternatively, it may be that early childhood is a particularly sensitive period for poverty, with relations that last even if a child moves out of poverty at a latter point. This interpretation is consistent with the evidence that early childhood poverty has lasting negative relationships into adulthood, even when the individual’s SES improves (56–63). We cannot arbitrate between these possibilities with the current data as there is little social mobility in our sample, and thus we could not examine the relationships between variation in SES and brain volume over time within a child, but this is a critical area for future research. It will be important in future research to examine populations where SES varies more over the course of a child’s life, or to examine interventions that improve SES. It will also be essential to better disentangle SES as one form of social disadvantage from other factors that may also confer social disadvantage, such as the effects of systemic racism. Further, it is important to acknowledge that there can be many factors that influence SES. We focused on family income-to-needs, but parental education and neighborhood income levels are also relevant.
It is relevant to note that we continued to see relationships of early SES to later adaptive outcomes and brain development even when we controlled for a variety of factors that often co-occur with low early SES and which could in theory be mediating mechanisms. This included preschool depression, anxiety, and externalizing symptoms, maternal psychopathology, and cumulative life events experienced by the child. The relationship between cortical gray matter intercepts and later outcomes were no longer significant when controlling for these factors, suggesting that they may play a role in linking cortical brain development to adult cognitive function, high risk behaviors, and social function. However, the relationships of subcortical intercepts and slopes to both cognitive function and high risk behaviors remained significant even when controlling for these factors. While efforts to further unpack the differential effects and causal pathways from SES to adaptive outcomes is of course worthwhile, the robust and powerful evidence here and in the literature repeatedly linking low childhood SES to a greater likelihood of poor adaptive outcomes suggests that directly addressing low SES itself may be the most tangible and effective intervention with greatest potential of broad impacts across many different mediating mechanisms. It also possible that some of the relationship of early SES to brain development and poor adaptive outcomes is accounted by shared genetics that contribute to poor adaptive function in both children and their parents (resulting in early low SES for their children), though there is evidence for causal effects of poverty and children’s outcomes (53–55).
We did not find a relationship between preschool SES and psychiatric outcomes at ages 16+. This result is not consistent with a body of research suggesting that poverty is associated with higher rates of psychopathology (1, 20, 21), and our own prior research in this sample indicating a relationship between early low SES and depression and externalizing behaviors prior to age 16 through MRI wave 3 (31, 64–66). There are two speculative explanations for this finding. One is that because this was a prospective sample followed over time with repeated assessments, youth may have been more likely to receive treatment given potentially greater parental awareness of psychopathology. A second is that the youth with greater mental health problems in childhood were less likely to stay in the study into the transition to adulthood. However we compared depression, anxiety and externalizing symptoms for those children who continued in the study at MRI wavs 4/5 and those who did not, and there were no significant differences in these variables (Table S11).
We found robust relationships of preschool SES to brain development, but with important evidence of specificity. Preschool SES related to cortical and subcortical gray matter, but not cortical white matter, relationships that reminded when controlling for early child mental health, cumulative life events, and maternal mental health. These results provide evidence that the relationship of early SES to subcortical brain development is not just reflecting greater levels of early psychopathology or some other risk factors that might also relate to brain development. Further, we found that subcortical gray matter volume, and more specifically reduced increases over development (slopes) served as a mediator of the relationship between early SES and cognitive and high-risk outcomes. The finding that early SES relates to slopes of subcortical gray matter has important developmental implications. It suggests that the relationship of SES to brain development is not static (e.g., present from the start with no change across development), but in the case of subcortical volumes, the difference between youth raised in low versus higher SES increases over development. We were somewhat surprised to find that cortical gray matter and white matter did not show similar relationships, but it may be that subcortical regions are uniquely susceptible to early and/or sustained low SES.
We found early SES related to reductions in hippocampal, caudate, putamen and thalamic volumes, but not amygdala, dorsolateral prefrontal or dorsal anterior cingulate volumes, consistent with a number of prior studies (10–12, 27, 67, 68). Reduced hippocampal volume in humans associated with early low SES has been interpreted as consistent with animal literature showing opposite effects of stress and environmental enrichment on hippocampal cell proliferation, and dendritic length and branching (69, 70). In turn, the hippocampus is important for both cognitive function and stress reactivity (71). Palacios-Barrios and colleagues have argued that impairments in self-regulation may be a final common pathway linking early adversity, alterations in hippocampal development and other brain regions, and poor physical and mental health outcomes (15). The striatum and the thalamus may also be critical components of such a pathway, given the role of striatum in reward processing (72), and the thalamus as a key pathway of communication with cortical regions (73). Early life stress in animal models alters the development of striatal regions involved in reward processing (74), as well as disrupting thalamic structure and function (75, 76). However, we did not have direct measures of these functions in the current work, and thus these hypotheses about functional relevance are speculative and need prospective confirmation. We may not have found relationships to amygdala given the possibility that amygdala structure may be more related to threat relevant experiences (77).
This study has several limitations. We did not start brain imaging until the children were school age, and thus do not know how early variation in brain structure related to SES manifest. Second, we experienced a scanner upgrade between scan waves 3 and 4, and cannot make general statements about normative patterns of brain development as overall shifts in volume across time could be confounded with the upgrade. However, we do not think the scanner change impairs our ability to look at individual differences in such trajectories, since all youth shifted scanner platforms at waves 4 and 5 and we used longitudinal harmonization methods. Third, it is possible that associations in mediation models may be bi-directional. Fourth, while a number of our findings fit with the extant literature on SES (e.g., relationships to hippocampal volume, gray matter volume, cognitive function, etc.), the specialized nature of this sample (overrecruited for early depression) may limit generalizability. Fifth, in order to create interpretable outcome measures that aggregated multiple indicators, we had to dichotomize measures in ways that may have resulted in some loss of information.
In summary, the current study provides highly novel longitudinal prospective data demonstrating that low childhood SES has long lasting relationships to a range of outcomes at the transition to adulthood that are mediated in part by brain development. These data add to the ongoing need for policy initiatives that address this crisis, as childhood poverty is one of the most critical factors relating to long term outcomes for far too many youth across the world.
Supplementary Material
Acknowledgments
This study was supported by grants R01MH064769 to Dr. Luby, R01 MH090786 and T32 MH100019 to Drs. Luby and Barch, and K23MH121792 and L30 MH120574 to Dr. Karcher. The funding organization for this work, the National Institute of Mental Health, had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Dr. Luby receives royalties from Guilford Press. All other authors report no biomedical financial interests or potential conflicts of interest.
Dr. Barch and Ms. Tillman (Washington University) had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dufford AJ, Kim P, Evans GW (2020): The impact of childhood poverty on brain health: Emerging evidence from neuroimaging across the lifespan. Int Rev Neurobiol. 150:77–105. [DOI] [PubMed] [Google Scholar]
- 2.Schwabe L, Bohbot VD, Wolf OT (2012): Prenatal stress changes learning strategies in adulthood. Hippocampus. 22:2136–2143. [DOI] [PubMed] [Google Scholar]
- 3.Freedman D, Woods GW (2013): Neighborhood Effects, Mental Illness and Criminal Behavior: A Review. Journal of politics and law. 6:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leung JT, Shek DT (2011): Poverty and adolescent developmental outcomes: a critical review. International journal of adolescent medicine and health. 23:109–114. [DOI] [PubMed] [Google Scholar]
- 5.Perkins SC, Finegood ED, Swain JE (2013): Poverty and language development: roles of parenting and stress. Innovations in clinical neuroscience. 10:10–19. [PMC free article] [PubMed] [Google Scholar]
- 6.Blair C, Raver CC (2016): Poverty, Stress, and Brain Development: New Directions for Prevention and Intervention. Acad Pediatr. 16:S30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Melchior M, Moffitt TE, Milne BJ, Poulton R, Caspi A (2007): Why do children from socioeconomically disadvantaged families suffer from poor health when they reach adulthood? A life-course study. Am J Epidemiol. 166:966–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poulton R, Caspi A, Milne BJ, Thomson WM, Taylor A, Sears MR, et al. (2002): Association between children’s experience of socioeconomic disadvantage and adult health: a life-course study. Lancet. 360:1640–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luby J, Belden A, Botteron K, Marrus N, Harms MP, Babb C, et al. (2013): The effects of poverty on childhood brain development: the mediating effect of caregiving and stressful life events. JAMA pediatrics. 167:1135–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanson JL, Nacewicz BM, Sutterer MJ, Cayo AA, Schaefer SM, Rudolph KD, et al. (2015): Behavioral problems after early life stress: contributions of the hippocampus and amygdala. Biol Psychiatry. 77:314–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jednorog K, Altarelli I, Monzalvo K, Fluss J, Dubois J, Billard C, et al. (2012): The influence of socioeconomic status on children’s brain structure. PLoS One. 7:e42486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanson JL, Chandra A, Wolfe BL, Pollak SD (2011): Association between income and the hippocampus. PLoS One. 6:e18712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noble KG, Grieve SM, Korgaonkar MS, Engelhardt LE, Griffith EY, Williams LM, et al. (2012): Hippocampal volume varies with educational attainment across the life-span. Front Hum Neurosci. 6:307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Butterworth P, Cherbuin N, Sachdev P, Anstey KJ (2012): The association between financial hardship and amygdala and hippocampal volumes: results from the PATH through life project. Soc Cogn Affect Neurosci. 7:548–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palacios-Barrios EE, Hanson JL (2019): Poverty and self-regulation: Connecting psychosocial processes, neurobiology, and the risk for psychopathology. Compr Psychiatry. 90:52–64. [DOI] [PubMed] [Google Scholar]
- 16.Farah MJ (2018): Socioeconomic status and the brain: prospects for neuroscience-informed policy. Nat Rev Neurosci. 19:428–438. [DOI] [PubMed] [Google Scholar]
- 17.Ziol-Guest KM, Duncan GJ, Kalil A, Boyce WT (2012): Early childhood poverty, immune-mediated disease processes, and adult productivity. Proc Natl Acad Sci U S A. 109 Suppl 2:17289–17293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beach SR, Lei MK, Brody GH, Kim S, Barton AW, Dogan MV, et al. (2016): Parenting, Socioeconomic Status Risk, and Later Young Adult Health: Exploration of Opposing Indirect Effects via DNA Methylation. Child Dev. 87:111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ehrlich KB, Ross KM, Chen E, Miller GE (2016): Testing the biological embedding hypothesis: Is early life adversity associated with a later proinflammatory phenotype? Dev Psychopathol. 28:1273–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evans GW, Cassells RC (2014): Childhood Poverty, Cumulative Risk Exposure, and Mental Health in Emerging Adults. Clin Psychol Sci. 2:287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shanahan L, Copeland WE, Costello EJ, Angold A (2011): Child-, adolescent- and young adult-onset depressions: differential risk factors in development? Psychol Med. 41:2265–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Evans GW, Fuller-Rowell TE (2013): Childhood poverty, chronic stress, and young adult working memory: the protective role of self-regulatory capacity. Dev Sci. 16:688–696. [DOI] [PubMed] [Google Scholar]
- 23.Duncan GJ, Ziol-Guest KM, Kalil A (2010): Early-childhood poverty and adult attainment, behavior, and health. Child Dev. 81:306–325. [DOI] [PubMed] [Google Scholar]
- 24.Barch D, Pagliaccio D, Belden A, Harms MP, Gaffrey M, Sylvester CM, et al. (2016): Effect of Hippocampal and Amygdala Connectivity on the Relationship Between Preschool Poverty and School-Age Depression. Am J Psychiatry.appiajp201515081014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson SB, Riis JL, Noble KG (2016): State of the Art Review: Poverty and the Developing Brain. Pediatrics. 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanson JL, Hair N, Shen DG, Shi F, Gilmore JH, Wolfe BL, et al. (2013): Family poverty affects the rate of human infant brain growth. PLoS One. 8:e80954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McDermott CL, Seidlitz J, Nadig A, Liu S, Clasen LS, Blumenthal JD, et al. (2019): Longitudinally Mapping Childhood Socioeconomic Status Associations with Cortical and Subcortical Morphology. J Neurosci. 39:1365–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raffington L, Czamara D, Mohn JJ, Falck J, Schmoll V, Heim C, et al. (2019): Stable longitudinal associations of family income with children’s hippocampal volume and memory persist after controlling for polygenic scores of educational attainment. Dev Cogn Neurosci. 40:100720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ellwood-Lowe ME, Humphreys KL, Ordaz SJ, Camacho MC, Sacchet MD, Gotlib IH (2018): Time-varying effects of income on hippocampal volume trajectories in adolescent girls. Dev Cogn Neurosci. 30:41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.King LS, Dennis EL, Humphreys KL, Thompson PM, Gotlib IH (2020): Cross-sectional and longitudinal associations of family income-to-needs ratio with cortical and subcortical brain volume in adolescent boys and girls. Dev Cogn Neurosci. 44:100796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barch DM, Shirtcliff EA, Elsayed NM, Whalen D, Gilbert K, Vogel AC, et al. (2020): Testosterone and hippocampal trajectories mediate relationship of poverty to emotion dysregulation and depression. Proc Natl Acad Sci U S A. 117:22015–22023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holz NE, Boecker R, Hohm E, Zohsel K, Buchmann AF, Blomeyer D, et al. (2015): The long-term impact of early life poverty on orbitofrontal cortex volume in adulthood: results from a prospective study over 25 years. Neuropsychopharmacology. 40:996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Machlin L, McLaughlin KA, Sheridan MA (2020): Brain structure mediates the association between socioeconomic status and attention-deficit/hyperactivity disorder. Dev Sci. 23:e12844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang J, Liu H, Wei D, Liu W, Meng J, Wang K, et al. (2016): Regional gray matter volume mediates the relationship between family socioeconomic status and depression-related trait in a young healthy sample. Cogn Affect Behav Neurosci. 16:51–62. [DOI] [PubMed] [Google Scholar]
- 35.Noble KG, Houston SM, Brito NH, Bartsch H, Kan E, Kuperman JM, et al. (2015): Family income, parental education and brain structure in children and adolescents. Nat Neurosci. 18:773–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hair NL, Hanson JL, Wolfe BL, Pollak SD (2015): Association of Child Poverty, Brain Development, and Academic Achievement. JAMA pediatrics. 169:822–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beck A, Franz CE, Xian H, Vuoksimaa E, Tu X, Reynolds CA, et al. (2018): Mediators of the Effect of Childhood Socioeconomic Status on Late Midlife Cognitive Abilities: A Four Decade Longitudinal Study. Innov Aging. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luby JL, Heffelfinger A, Koenig-McNaught AL, Brown K, Spitznagel E (2004): The Preschool Feelings Checklist: a brief and sensitive screening measure for depression in young children. J Am Acad Child Adolesc Psychiatry. 43:708–717. [DOI] [PubMed] [Google Scholar]
- 39.McLoyd VC (1998): Socioeconomic disadvantage and child development. Am Psychol. 53:185–204. [DOI] [PubMed] [Google Scholar]
- 40.Egger HL (2009): Psychiatric assessment of young children. Child Adolesc Psychiatr Clin N Am. 18:559–580. [DOI] [PubMed] [Google Scholar]
- 41.Egger HL, Erkanli A, Keeler G, Potts E, Walter BK, Angold A (2006): Test-Retest Reliability of the Preschool Age Psychiatric Assessment (PAPA). J Am Acad Child Adolesc Psychiatry. 45:538–549. [DOI] [PubMed] [Google Scholar]
- 42.Angold A, Costello EJ (1995): A test-retest reliability study of child-reported psychiatric symptoms and diagnoses using the Child and Adolescent Psychiatric Assessment (CAPA-C). Psychol Med. 25:755–762. [DOI] [PubMed] [Google Scholar]
- 43.Maxwell ME (1992): Family interview for genetic studies (FIGS). A manual for FIGS. Bethesda, Maryland: Clinical Neurogenetics Branch, Intramural Research Program, National Institute of Mental Health. [Google Scholar]
- 44.Gifford EJ, Kozecke LE, Golonka M, Hill SN, Costello EJ, Shanahan L, et al. (2019): Association of Parental Incarceration With Psychiatric and Functional Outcomes of Young Adults. Jama Network Open. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Copeland WE, Shanahan L, Hinesley J, Chan RF, Aberg KA, Fairbank JA, et al. (2018): Association of Childhood Trauma Exposure With Adult Psychiatric Disorders and Functional Outcomes. Jama Network Open. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Copeland WE, Alaie I, Jonsson U, Shanahan L (2020): Associations of Childhood and Adolescent Depression With Adult Psychiatric and Functional Outcomes. J Am Acad Child Adolesc Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gershon RC, Wagster MV, Hendrie HC, Fox NA, Cook KF, Nowinski CJ (2013): NIH toolbox for assessment of neurological and behavioral function. Neurology. 80:S2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luby JL, Belden AC, Jackson JJ, Lessov-Schlaggar CN, Harms MP, Tillman R, et al. (2016): Early Childhood Depression and Alterations in the Trajectory of Gray Matter Maturation in Middle Childhood and Early Adolescence. JAMA Psychiatry. 73:31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reuter M, Schmansky NJ, Rosas HD, Fischl B (2012): Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage. 61:1402–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Destrieux C, Fischl B, Dale A, Halgren E (2010): Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. NeuroImage. 53:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Benjamini Y, Yekutieli D (2001): The control of the false discovery rate: a practical and powerful approach to multiple testing. The Annals of Statistics. 29:1165–1188. [Google Scholar]
- 52.Hayes A F. (2013): Introduction to mediation, moderation, and conditional process analysis : A regression-based approach. New York, NY: The Guildford Press. [Google Scholar]
- 53.Costello EJ, Erkanli A, Copeland W, Angold A (2010): Association of family income supplements in adolescence with development of psychiatric and substance use disorders in adulthood among an American Indian population. JAMA. 303:1954–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Akee RK, Copeland WE, Keeler G, Angold A, Costello EJ (2010): Parents’ Incomes and Children’s Outcomes: A Quasi-Experiment. Am Econ J Appl Econ. 2:86–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Costello EJ, Compton SN, Keeler G, Angold A (2003): Relationships between poverty and psychopathology: a natural experiment. JAMA. 290:2023–2029. [DOI] [PubMed] [Google Scholar]
- 56.Austin MK, Chen E, Ross KM, McEwen LM, Maclsaac JL, Kobor MS, et al. (2018): Early-life socioeconomic disadvantage, not current, predicts accelerated epigenetic aging of monocytes. Psychoneuroendocrinology. 97:131–134. [DOI] [PubMed] [Google Scholar]
- 57.Power C, Hypponen E, Smith GD (2005): Socioeconomic position in childhood and early adult life and risk of mortality: a prospective study of the mothers of the 1958 British birth cohort. Am J Public Health. 95:1396–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pensola TH, Martikainen P (2003): Cumulative social class and mortality from various causes of adult men. J Epidemiol Community Health. 57:745–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kuh D, Hardy R, Langenberg C, Richards M, Wadsworth ME (2002): Mortality in adults aged 26–54 years related to socioeconomic conditions in childhood and adulthood: post war birth cohort study. BMJ. 325:1076–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hart CL, Hole DJ, Smith GD (2000): Influence of socioeconomic circumstances in early and later life on stroke risk among men in a Scottish cohort study. Stroke. 31:2093–2097. [DOI] [PubMed] [Google Scholar]
- 61.Smith GD, Hart C, Blane D, Hole D (1998): Adverse socioeconomic conditions in childhood and cause specific adult mortality: prospective observational study. BMJ. 316:1631–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hart CL, Smith GD, Blane D (1998): Social mobility and 21 year mortality in a cohort of Scottish men. Soc Sci Med. 47:1121–1130. [DOI] [PubMed] [Google Scholar]
- 63.Blane D, Hart CL, Smith GD, Gillis CR, Hole DJ, Hawthorne VM (1996): Association of cardiovascular disease risk factors with socioeconomic position during childhood and during adulthood. BMJ. 313:1434–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barch DM, Belden AC, Tillman R, Whalen D, Luby JL (2018): Early Childhood Adverse Experiences, Inferior Frontal Gyrus Connectivity, and the Trajectory of Externalizing Psychopathology. J Am Acad Child Adolesc Psychiatry. 57:183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Luby JL, Barch D, Whalen D, Tillman R, Belden A (2017): Association Between Early Life Adversity and Risk for Poor Emotional and Physical Health in Adolescence: A Putative Mechanistic Neurodevelopmental Pathway. JAMA pediatrics. 171:1168–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barch D, Pagliaccio D, Belden A, Harms MP, Gaffrey M, Sylvester CM, et al. (2016): Effect of Hippocampal and Amygdala Connectivity on the Relationship Between Preschool Poverty and School-Age Depression. Am J Psychiatry. 173:625–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jenkins LM, Chiang JJ, Vause K, Hoffer L, Alpert K, Parrish TB, et al. (2020): Subcortical structural variations associated with low socioeconomic status in adolescents. Hum Brain Mapp. 41:162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Noble KG, Houston SM, Kan E, Sowell ER (2012): Neural correlates of socioeconomic status in the developing human brain. Dev Sci. 15:516–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hirase H, Shinohara Y (2014): Transformation of cortical and hippocampal neural circuit by environmental enrichment. Neuroscience. 280:282–298. [DOI] [PubMed] [Google Scholar]
- 70.Eiland L, Ramroop J, Hill MN, Manley J, McEwen BS (2012): Chronic juvenile stress produces corticolimbic dendritic architectural remodeling and modulates emotional behavior in male and female rats. Psychoneuroendocrinology. 37:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.van Bodegom M, Homberg JR, Henckens M (2017): Modulation of the Hypothalamic-Pituitary-Adrenal Axis by Early Life Stress Exposure. Front Cell Neurosci. 11:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schultz W (2016): Reward functions of the basal ganglia. J Neural Transm (Vienna). 123:679–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Parnaudeau S, Bolkan SS, Kellendonk C (2018): The Mediodorsal Thalamus: An Essential Partner of the Prefrontal Cortex for Cognition. Biol Psychiatry. 83:648–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pena CJ, Smith M, Ramakrishnan A, Cates HM, Bagot RC, Kronman HG, et al. (2019): Early life stress alters transcriptomic patterning across reward circuitry in male and female mice. Nat Commun. 10:5098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Banqueri M, Gutierrez-Menendez A, Mendez M, Conejo NM, Arias JL (2020): Early life stress due to repeated maternal separation alters the working memory acquisition brain functional network. Stress.1–9. [DOI] [PubMed] [Google Scholar]
- 76.Skilbeck KJ, Johnston GAR, Hinton T (2018): Long-lasting effects of early-life intervention in mice on adulthood behaviour, GABAA receptor subunit expression and synaptic clustering. Pharmacol Res. 128:179–189. [DOI] [PubMed] [Google Scholar]
- 77.McLaughlin KA, Weissman D, Bitran D (in press): Childhood adversity and neural development: A systematic review. Annual Reviews in Developmental Psychology./References> [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.