Abstract
Glaucoma is the second leading cause of blindness in India. Despite advances in diagnosing and managing glaucoma, there is a lack of India-specific clinical guidelines on glaucoma. Ophthalmologists often refer to the European Glaucoma Society (EGS) and Asia-Pacific Glaucoma Society (APGS) guidelines. A group of glaucoma experts was convened to review the recently released EGS guideline (fifth edition) and the APGS guideline and explore their relevance to the Indian context. This review provides the salient features of EGS and APGS guidelines and their utility in Indian scenario. Glaucoma diagnosis should be based on visual acuity and refractive errors, slit-lamp examination, gonioscopy, tonometry, visual field (VF) testing, and clinical assessment of optic nerve head, retinal nerve fiber layer (RNFL), and macula. The intraocular pressure target must be individualized to the eye and revised at every visit. Prostaglandin analogues are the most effective medications and are recommended as the first choice in open-angle glaucoma (OAG). In patients with cataract and primary angle-closure glaucoma (PACG), phacoemulsification alone or combined phacoemulsification and glaucoma surgery are recommended. Trabeculectomy augmented with antifibrotic agents is recommended as the initial surgical treatment for OAG. Laser peripheral iridotomy and surgery in combination with medical treatment should be considered in high-risk individuals aged <50 years. In patients with phakic and PACG, phacoemulsification alone or combined phacoemulsification and glaucoma surgery are recommended. Visual acuity, VF testing, clinical assessment of the optic disc and RNFL, and tonometry are strongly recommended for monitoring glaucoma progression.
Keywords: APGS, EGS, glaucoma, guidelines, RNFL, tonometry
The term “glaucoma” is derived from the ancient Greek, glaukos, which nonspecifically refers to the blue, green, or light grey color of the pupil.[1] Glaucoma is a group of eye disorders characterized by progressive, irreversible damage to the optic nerve with gradual vision loss and intraocular pressure (IOP).[2] Depending on the etiology, glaucoma may be primary or secondary. Open-angle glaucoma (OAG) is the most common form of glaucoma, but angle-closure glaucoma (ACG) leads to serious vision loss than OAG.[3]
Glaucoma is the second leading cause of blindness worldwide as well as in India. In 2004, Quigley et al.[4] estimated the global prevalence of glaucoma in 2020 as 79.6 million, with three-fourth of the cases being OAG. India’s share of the burden was estimated to be 16.09 million, of which 68.85% (11.08 million) was due to OAG. In 2010, George et al.[5] estimated that India has approximately 11.2 million persons aged 40 years and above with 6.48 million and 2.54 million cases related to primary OAG (POAG) and ACG, respectively. An additional 28.1 million people were estimated to have ocular hypertension, primary angle-closure suspects (PACSs), or PAC. One in eight person aged 40 years or above have glaucoma or are at risk of glaucoma. Worldwide, the number of people with glaucoma is expected to increase to 111.8 million in 2040, especially among women and Asians.[6]
Amid this growing prevalence of glaucoma, advances in the understanding of glaucoma and technology have broadened the tools and options for better screening, diagnosis, monitoring, and management of glaucoma. However, in clinical practice, ophthalmologists look forward to evidence-based recommendations as a guide to patient management.
Several associations, including the European Glaucoma Society (EGS) and Asia-Pacific Glaucoma Society (APGS) have released guidelines to support ophthalmologists in managing people with or at risk of glaucoma and provide useful information to fellow ophthalmologists and trainees.[7,8] Ophthalmologists in India often refer to the EGS and APGS guidelines as a guide to their daily practice.
Purpose
In the milieu of the growing burden of glaucoma in India, a group of experts in glaucoma came together to review the recently released EGS guideline (fifth edition) and the APGS guideline and explore their relevance to practice in India.
This paper aims to capture the salient features of EGS and APGS guidelines and their utility in the Indian scenario.
Methods
A group of glaucoma experts reviewed the similarities and differences between EGS and APGS guidelines and voiced their opinion about their relevance to Indian practice.
Panel Opinion on APGS and EGS
The APGS and EGS guidelines have primarily covered the major clinical aspects of glaucoma management. There are subtle differences in the APGS and EGS.
The APGS advocates screening of glaucoma in at-risk patients, but EGS does not. The APGS prefers opportunistic screening over universal screening. The APGS emphasizes n reassessment of risk factors, IOP/target IOP, all basic investigations, adverse effects on medications, and quality of life (QoL) issues. It offers timings for follow-up depending on the extent of the damage. The APGS provides additional information on the calibration of Goldmann applanation tonometry (GAT). Compared to EGS, the panelists found that the APGS guideline was more robust in detailing the procedures and providing diagnostic tips for using different tools. The format of each of the diagnostic tests provided in APGS is practice-oriented. It provides notes on interpreting optical coherence tomography (OCT) and visual fields (VFs). It also has included the Glaucoma QoL-15 Questionnaire to assess of QoL of the patient.
In context to Indian practice, the experts’ opinion on several aspects of diagnosis and management within the purview of EGS and APGS is provided in this article. An overview of the expert’s opinion is summarized in Box 1.
Box 1.
Expert panel opinions
| Parameters | Expert opinion | |
|---|---|---|
| Risk and risk assessment | A CCT using either ultrasonic or optic is recommended for risk stratification | |
| There is no merit in using formulas or nomograms to convert IOP | ||
| In the absence of data on CCT and risk in Indian patients, the panellists did not suggest any range of CCT for risk profiling | ||
| Screening for glaucoma | Opportunistic glaucoma screening during cataract camps or a visit to an eye clinic is a possible method of screening glaucoma | |
| There is a lack of evidence on the cost effectiveness of screening, diagnosing, monitoring, and treating glaucoma in India. Hence, glaucoma screening may be done at the discretion of individual hospitals or ophthalmologists | ||
| Diagnosis | Despite a low level of evidence, the panelists agreed to strongly recommend using visual acuity and refractive errors, slit-lamp examination, gonioscopy, tonometry, visual field testing, and clinical assessment of ONH, RNFL, and macula | |
| The panelists do not recommend CCT adjusted IOP values because CCT-corrected algorithms based on IOP are not validated | ||
| Diagnosis of glaucoma should not be made on the OCT findings alone | ||
| Central corneal thickness can be considered in case of normal tension glaucoma or ocular hypertension | ||
| Goldmann applanation tonometry is the gold standard for diagnosing glaucoma, and hence it is recommended over other tonometers | ||
| The accuracy and precision of a tonometer should influence the choice for use in the clinic | ||
| Tonometer must be regularly calibrated. For more details, refer to the APGS guideline | ||
| Anterior chamber angle imaging cannot replace gonioscopy. | ||
| Gonioscopy should be performed in every patient being evaluated for glaucoma | ||
| Some form of photography or imaging of ONH and RNFL features is recommended as sequential photographs help to detect progression | ||
| If photos are unavailable, a disc drawing enumerating the disc is warranted | ||
| Diagnosis of glaucoma should not be made on the OCT findings alone | ||
| Do not rely only on the CDR to describe or document the disc | ||
| Setting target IOP | The IOP target must be individualized to the eye and revised at every visit | |
| Target IOP is the upper limit of IOP judged to be compatible with this treatment goal | ||
| Documentation of target IOP is up to the discretion of the ophthalmologist | ||
| In early glaucoma, an IOP of 18-20 mmHg with a reduction of at least 20% may be sufficient | ||
| In moderate glaucoma, an IOP of 15-17 mmHg with a reduction of at least 30% may be required | ||
| In advanced glaucoma, a reduction of at least 40% may be required | ||
|
|
||
| Glaucoma stages | Target IOP to be achieved | |
|
| ||
| Mild glaucoma | 18.20 mmHg | |
| Moderate glaucoma | 15.17 mmHg | |
| Advanced glaucoma | 10.12 mmHg | |
|
| ||
| Topical glaucoma therapy | Start with monotherapy (except in high IOP and severe disease) | |

|
||
| The order of IOP lowering medications based on their IOP lowering efficacy is as follows: | ||
| Prostaglandin analogues are the most effective medications and are usually recommended as the first choice in OAG, provided the cost is not a limiting factor | ||
| Laser iridotomy | Laser iridotomy should be preferred over surgical iridotomy | |
| Laser trabeculoplasty | Selective laser trabeculoplasty is available in India in many ophthalmology departments. It could be tried as a first-line treatment in mild-to-moderate glaucoma, but it is not a universal recommendation | |
| Thermal laser peripheral iridoplasty | Once-daily pilocarpine can be used as an alternative to thermal laser peripheral iridoplasty (TLPI) for plateau iris syndrome and patent peripheral iridotomy | |
| Cyclodestructive procedures | Transcleral cyclophotocoagulation is the most commonly used method in India | |
| Incisional surgery | The commonly preferred surgical technique for penetrating glaucoma surgery is the nonpenetrating glaucoma surgery is not useful in the Indian context | |
| Minimally invasive glaucoma surgery | Minimally invasive glaucoma surgery is not widely available in India and hence no recommendations are made | |
| Antifibrotic agents in glaucoma management | Mitomycin C is the choice of drug in glaucoma surgery | |
| Antifibrotics should be judiciously used | ||
| Intraoperative mitomycin can be used at 0.1-0.4 mg/mL for 1-3 min, depending on the condition of the disease | ||
| Postoperatively both 5-FU and mitomycin-C can be used | ||
| 5-FU concentration: 0.1 mL injection of 50 mg/mL undiluted solution. It has to be administered as subconjunctival injection adjacent to but not into bleb (pH 9), with a small-caliber needle (e.g., 30 G needle on insulin syringe) Mitomycin C concentration: 0.1 mL injection of 0.1-0.5 mg/mL solution. It must be administered adjacent to but not into bleb, with a small-caliber needle (e.g., 30 G needle on insulin syringe) |
||
| Cataract and glaucoma surgery | In patients with cataract and PACG, phacoemulsification alone or combined phacoemulsification+glaucoma surgery is recommended. However, the decision should be made based on the disc and field damage and the status of the angle | |
| Open-angle glaucoma | Trabeculectomy augmented with antifibrotic agents is recommended as initial surgical treatment for OAG, provided the ophthalmologist is familiar with the use of antifibrotics. | |
| Antifibrotics should be used with caution | ||
| Alternatives like OlogenÒ should not be a preferred option due to a lack of evidence on its equality of superiority over trabeculectomy | ||
| Angle-closure disease | Treatment of PACG depends on the spectrum of disease and presence of cataract | |
| Laser peripheral iridotomy and surgery is combined with medical treatment should be considered in high-risk individuals below the age of 50 years, e.g., high hyperopia, and patients requiring repeated pupil dilation for retinal disease | ||
| Primary angle-closure suspect: LPI in high-risk individuals such as those with very high hyperopia, family history, or those requiring pupil dilatation due to retinal disease | ||
| PAC or PACG: Laser peripheral iridotomy is the first line of treatment | ||
| Visually significant cataract and PAC: Laser peripheral iridotomy to manage PAC or PACG and lens | ||
| extraction should be considered based on level and extent of angle closure and IOP | ||
| There may be a risk of aqueous misdirection or surgical complications if cataract surgery is done without LPI in patients with cataract and PAC or PACG | ||
| Ophthalmologists should be proficient in handling patients with cataract and PAC or PACG | ||
| Prostaglandin analogues are the most effective medications and are usually recommended as the first choice in PACG | ||
| In patients with phakic and PACG, phacoemulsification alone or combined phacoemulsification + glaucoma surgery is recommended. However, the decision should be made based on the disc and field damage and the status of the angle | ||
| Monitoring glaucoma progression | Despite a very low level of direct evidence, the panelists endorsed the EGS recommendations | |
| Keeping in view the goal of preventing vision impairment, the visual acuity, VF testing, clinical assessment of the optic disc and RNFL, tonometry is strongly recommended for monitoring glaucoma progression. | ||
| However, OCT of disc/RNFL/macula and repeat gonioscopy carries a weak recommendation | ||
| In preperimetric glaucoma, OCT is used for monitoring the disease progression. Visual field is mandatory for diagnosing and monitoring the progression of glaucoma | ||
| OCT is always complementary to visual field testing but cannot replace visual field testing in monitoring glaucoma progression | ||
CCT, Central corneal thickness; CDR, cup-to-disc ratio; OAG, open-angle glaucoma; OCT, optical coherence tomography; IOP, intraocular pressure; LPI, laser peripheral iridotomy; ONH, optic nerve head; PGAs, prostaglandin analogues RNFL, retinal nerve fiber layer; PAC, primary angle closure; PACG, primary angle-closure glaucoma.
Risk factors
The panel observed and agreed that risk factors mentioned in both guidelines are more or less universal and relevant to India as well [Box 1]. Besides the risk factors stated in the EGS guideline, hyperopia included as a risk factor for PACG in APGS holds good for the Indian context too.[7,8]
Risk profiling
According to the Ocular Hypertension Treatment Study, central corneal thickness (CCT) is a significant predictor of future glaucoma (POAG) in patients with ocular hypertension.[9] The risk for glaucoma was threefold greater in eyes with CCT of 555 μm or less compared with eyes that had CCT of more than 588 μm.[10]
According to a cross-sectional study (n = 81,082) of a multiethnic population, CCT was used to clarify the findings of increased risk of glaucoma among Blacks and Hispanics. Variation in CCT accounted for nearly 30% of the increased risk of glaucoma among Blacks and Hispanics.[11] In the Chennai Glaucoma Study (n = 7774), the CCT positively correlated with IOP. The CCT among urban subjects was 18 μm thicker than the rural population. The average CCT in the study was 511.4 μm.[12]
Concerning the role of CCT in risk profiling, the panel agreed to the EGS recommendation that CCT may be a useful tool for profiling the risk at baseline. In agreement with both EGS and APGS, the panel also felt that IOP correction algorithms/nomograms based on CCT are not valid and must be avoided [Box 1]. The APGS has given a CCT range of ≈520 ± 30 and ≈505 ± 30 μm for urban and rural Indians, respectively.[8]
Screening for glaucoma
Unquestionably, glaucoma screening is important; however, population-based screening found that it is not cost effective.[13] Although assessing the cost effectiveness of the screening for glaucoma is more relevant to the European setting, the EGS could not find substantiating evidence to relate glaucoma screening to the disease progression, visual loss, IOP, or patient-reported outcomes.[7]
In accordance with the APGS guideline, the panellist also agreed that opportunistic glaucoma screening is a viable option in India and universal glaucoma screening for glaucoma only may not be feasible. The panellists also opined that the clinical and cost effectiveness of screening, detection, or monitoring tests for glaucoma is pertinent to the Indian setting [Box 1]; however, there is no evidence from the Indian context relating the cost effectiveness of the available tests to improvement in outcomes.
Diagnosis
Patients with acute ACG may show signs and symptoms of glaucoma as pain radiating from the eye, visual impairment, conjunctival hyperemia, and sometimes nausea and vomiting. On the contrary, OAG is asymptomatic until it has progressed to an advanced stage. Hence, nearly one-third of patients with OAG will be in an advanced or late stage in at least one eye at the time of diagnosis.[2] The available diagnostic methods include ophthalmoscopy, tonometry, imaging techniques, and perimetry. An overview of the panellist opinion relating to the diagnosis of glaucoma is described in Box 1.
History taking
The complete initial glaucoma evaluation begins with history taking and eye examination of the patient. In agreement with the APGS, the panellists suggested that patients be questioned on their past and current medical factors, social factors, past ophthalmic history, socioeconomic factors, and family history of glaucoma.[8]
To help ophthalmologists get the right picture of the clinical condition at baseline, the panellists reviewed both EGS and APGS guidelines on history taking and endorsed the questionnaire proposed in the EGS for glaucoma patients [Table 1].[7]
Table 1.
History taking: Questions to be proposed to patients at baseline
| History of current eye problem |
| Suggestive risk factors |
| Specific questions on: |
| Current and past medication use |
| Family history (general/ophthalmological/blindness) |
| Corticosteroid therapy (topical/systemic) |
| Ocular trauma or inflammation |
| Ocular surgeries=Refractive surgery |
| Chronic or severe disease related to cardiovascular disease or respiratory disease. |
| Vascular disorders |
| Drug allergies |
| Any questions or anything that patient would like to discuss? |
Initial optical examination
The panellist discussed the recommended tools for initial assessment of glaucoma stated in the EGS and APGS and felt that visual acuity and refractive error, slit-lamp examination, gonioscopy, tonometry, VF testing, and clinical assessment of the optic nerve head (ONH), retinal nerve fiber layer (RNFL) and macula should be carried out at the time of assessing glaucoma for the first time. They also collectively agreed that OCT and CCT are weakly recommended but cannot be used in isolation to diagnose glaucoma.[7]
Intraocular pressure and tonometry
The mean IOP in adult populations is estimated at 15–16 mmHg, with a standard deviation of nearly 3.0 mmHg. An IOP of ≥21 mmHg is considered elevated.[7] Patients with glaucoma tend to show diurnal variations; hence, IOP has to be evaluated at different times of the day in such patients. IOP diurnal variations are common and extensive in glaucoma patients than in healthy individuals. The IOP readings are to be taken at 3-h intervals between 7 am and 10 pm.[14] Patients with high-baseline IOP might benefit from diurnal IOP monitoring. The diurnal IOP fluctuation is also high in patients with pseudoexfoliative or exfoliative glaucoma.[7]
GAT is the gold standard and the preferred tonometry for measuring IOP.[15] Error due to the presence of high or irregular astigmatism warrants correction while taking with GAT. Besides GAT, alternative tonometers such as the self-tonometer, noncontact tonometry, rebound tonometer (ICare®), and hand-held tonometer (Tono-pen®) are also available.[7]
A meta-analysis of six studies showed no significant difference in the intraindividual IOP deviation between ICare® PRO and GAT.[16] Results from another meta-analysis of studies comparing tonometers (dynamic contour tonometer, noncontact tonometer (NCT), ocular response analyzer, Ocuton S, hand-held applanation tonometer (HAT), rebound tonometer, trans palpebral tonometer, and Tono-pen®) with the GAT was hampered by poor reporting from the studies. However, it concluded that NCT and HAT were comparable to GAT.[17]
The APGS exclusively described the risk factors that affect the IOP measurement [Table 2],[8] which the panellists found relevant to the Indian context.
Table 2.
Risk factors affecting intraocular pressure measurement[8]
| Age, Exercise |
| Lifestyle |
| Posture |
| Circadian rhythm |
| Central corneal thickness |
| Blood pressure |
| Intraabdominal pressure |
In agreement with EGS and APGS, the panellists unanimously agreed that GAT is the gold standard for measuring IOP. They also revealed that ophthalmologists in India use Tono-pen® or ICare®, especially in children and patients with the scarred cornea or edematous cornea. However, they opined that neither self-tonometry nor iCare® tonometry should replace GAT for clinical measurement. They also pointed out that despite the influence of CCT on GAT readings, CCT-adjusted IOP values should not be considered in the diagnosis of glaucoma. The panellists endorsed the instructions for calibrating tonometer described in detail in APGS.
Gonioscopy
Gonioscopy is used to inspect the anterior chamber angle and it forms an essential component in evaluating patients with or suspected of having glaucoma.[7] Gonioscopy offers information about the pathophysiology of glaucoma.[2] A wide range of instruments are available for ophthalmologists to explore anterior chamber angle configuration; however, none of these methods may be considered a reliable substitute of slit-lamp gonioscopy.[18]
The Van Herick technique is a helpful adjunct to gonioscopy in terms of grading depth of anterior chamber; nevertheless, it is not a substitute for gonioscopy. The Van Herick technique fails to provide information about the neovascularization, inflammation, or tumors in the angle.[19]
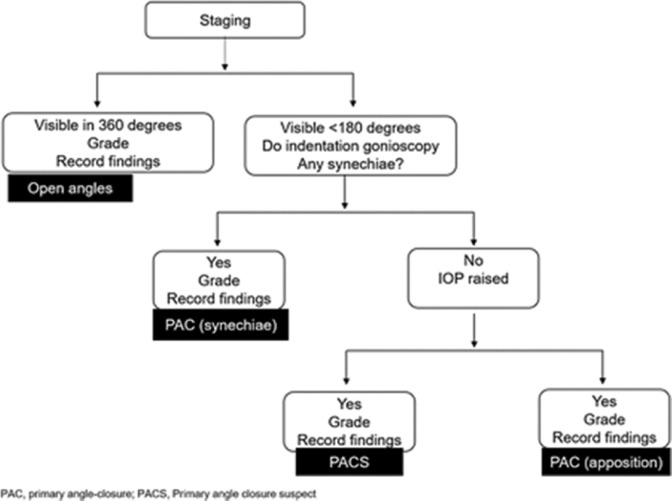
After reviewing the EGS and APGS, the panellists found that the APGS recommendation on gonioscopy is more practical to the Indian context [Fig. 1].[8]
Figure 1.
The APGS recommendation on gonioscopy for diagnosis of angle-closure glaucoma
Clinical evaluation of optic nerve head and retinal nerve fiber layer
Funduscopic examination of the optic disc and the RNFL is the key to glaucoma diagnosis.[2] OCT facilitates assessing the damage of the RNFL, while retinal tomography characterizes changes in the optic nerve topography. Photographic images are warranted to assess the static optic nerve damage and for detecting glaucoma progression. Confocal scanning laser ophthalmoscopy, OCT, and scanning laser polarimetry are available for quantitative imaging of the ONH, retinal nerve fiber layer, and inner macular layers.[20] Devices based on these technologies help in glaucoma diagnosis and detect glaucomatous progression during follow-up.[7,20]
OCT is a valuable clinical tool for glaucoma diagnosis and detection of progression. However, the quality of the diagnostic accuracy of the reviews on OCT for diagnosing glaucoma has not been encouraging.[21]
Quantifying the size and shape of the optic disc, cup, and neuroretinal rim enables one to detect the onset of glaucoma and follow-up on its progression. The damage to the disc can be assessed through cup-to-disc ratio (CDR) or rim-to-disc ratio.[22]
After reviewing the APGS and EGS, the panellist concluded that diagnosis of glaucoma should not made on the OCT findings alone. As suggested by EGS, the panellist also opined that ophthalmologists should focus on the neuroretinal rim—Inferior > Superior > Nasal > Temporal—the rule can be used but cautioned that the pattern might be less conspicuous in larger discs. The APGS has described the RNFL assessment technique in detail. The visibility of RNFL decreases with age and is more difficult to visualize in less pigmented fundi. Disc hemorrhage is a common finding but is often overlooked. So the panellist suggests that ophthalmologists should specifically look for hemorrhages, especially in patients at high risk of progression. As the vessel position changes, the panellists suggested that vessel positions should be assessed in sequential photography. Also, they reiterated EGS and APGS guidelines on the need for sequential photography or imaging of ONH and RNFL features for detecting the disease progression. As an alternative to photos unavailable, disc drawing, enumerating the disc, was strongly endorsed by the panellist. The EGS guideline recommends against the use of CDR to classify patients as glaucomatous and the panellist concurred. The panellists suggested that ophthalmologists could refer to APGS for the range of normal vertical CDRs for disc size for Indians. As per the guidelines, the panellist pointed out that ophthalmologist should be cautious while interpreting CDR in patients with different discs sizes.
Perimetry
VF testing using static automated perimetry is a vital tool for detecting and monitoring visual function loss associated with glaucoma. Further, it is also necessary for understanding visual loss relative to the level or the future risk of functional disability. Such an understanding will help ophthalmologists to make clinical decisions.[23]
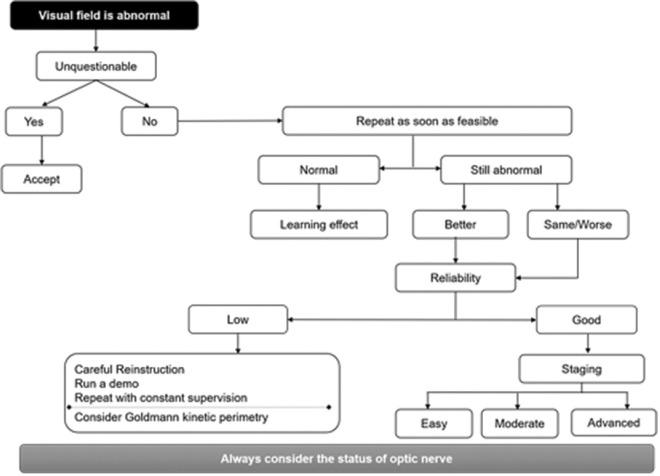
Glaucoma staging is based on the severity of VF damage. The panellist reviewed the EGS guideline and agreed that a simple system based on mean deviation (MD) alone is acceptable to the Indian context.[7] Glaucoma is defined as early glaucomatous loss, moderate glaucomatous loss, and advanced glaucomatous loss if MD is ≤6 dB, 6–12 dB and MD ≤12 dB, respectively. From a clinical perspective, the panellists found the EGS-based diagnostic strategy in case of the initial VF abnormality [Fig. 2] as a value added to Indian ophthalmologists.[7]
Figure 2.
Diagnostic strategy when initial visual field is abnormal (adapted from EGS guideline)
Management of glaucoma
Treatment of glaucoma involves medical therapy, laser, or surgery depending on the underlying cause and stage of the disease. The primary goal of glaucoma therapy is to slow or prevent disease progression by adequately lowering the IOP.[24]
Setting a target IOP
To achieve a targeted IOP, aggressive treatment and frequent change of therapy may be necessary. Setting a target IOP range is a dynamic concept. Setting a target IOP range is a dynamic concept. patient risk factors, life expectancy, and social circumstances.[24] Nevertheless, setting a target and applying it as a therapeutic guide remains a source of contention among ophthalmologists. A “target” IOP was set by percentage reduction or a threshold value in many randomized control trials and studies. Another method is the formula-based “target” IOP setting, which is more time-consuming, yet it is beneficial in addressing the risk factors in an individual patient.[25] Target IOP is the upper limit of IOP judged to be sufficient to slow the rate of VF deterioration to maintain the quality of life.[7]
Both the EGS and APGS guideline discusses setting an IOP target. The panelist agreed that a target IOP should be personalized and constantly reevaluated in the milieu of the stage of disease [Fig. 3].[7]
Figure 3.
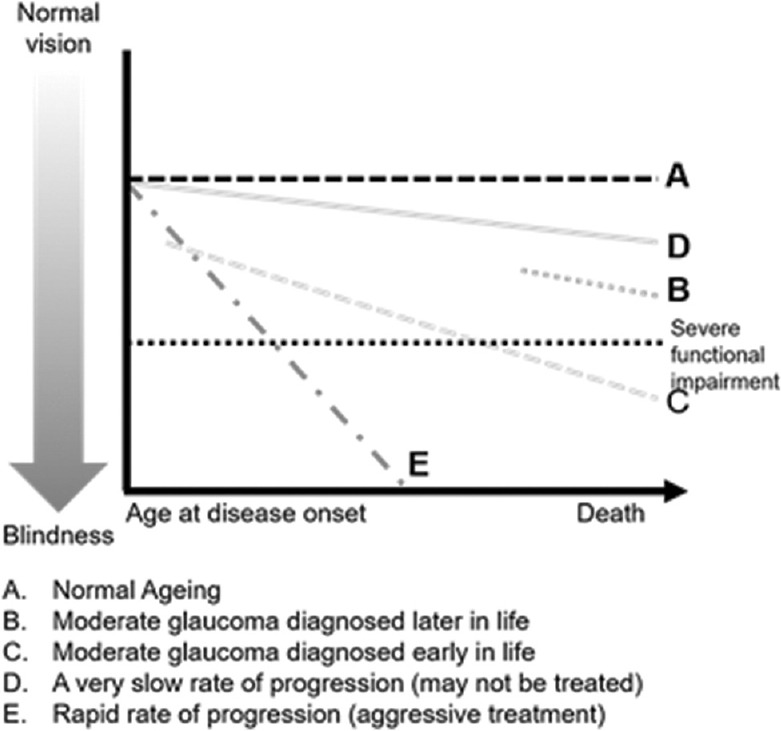
The whom to treat graph (adapted from EGS guideline)
Based on a detailed review and understanding of the APGS and EGS guidelines, the panellist made some observations that are relevant to the Indian settings [Box 1]. They suggested that the IOP target must be individualized to the eye and revised at every visit. The target IOP is the upper limit of IOP judged to be compatible with this treatment goal. Documentation of target IOP is up to the discretion of the ophthalmologist. In early glaucoma, an IOP of 18–20 mmHg with a reduction of at least 20% may be sufficient. In moderate glaucoma, an IOP of 15–17 mmHg with a decrease of at least 30% may be required. In advanced glaucoma, a reduction of at least 40% may be required.
Topical therapy
IOP-lowering topical therapy remains the mainstay of glaucoma management. As mentioned in the EGS and APGS, the panellists also found prostaglandin analogues (PGAs), β-blockers, α-adrenergic agonists, and carbonic anhydrase inhibitors (CAIs), and pilocarpine as the commonly used classes of topical therapies for glaucoma in India [Table 2].[2,7,24]
PGAs have been shown to have a more remarkable ability to reduce IOP than other prescribed therapeutic classes for patients with glaucoma. In addition, PGAs are associated with greater persistence than other classes of medications.[26]
After reviewing the EGS and APGS, the panelist agreed to start treatment with monotherapy and viewed PGAs as the most effective medication and the first choice in OAG, provided the cost is not a limiting factor. The panelists felt that PGAs should be the first choice followed by nonselective beta blockers, alpha agonists, Rho kinase inhibitors, selective beta blockers and topical CAIs.
Use of lasers in glaucoma
Lasers have revolutionized the treatment of glaucoma. Owing to the simplicity of the laser procedure, most ophthalmologists are routinely using the laser at a fundamental level. Neodymium: yttrium-aluminium-garnet (Nd: YAG) laser peripheral iridotomy (LPI) is the most common procedure for angle closure. Laser trabeculoplasty (LTP), gonioplasty/iridoplasty, diode laser cyclophotocoagulation and endocyclophotocoagulation, laser suturolysis, bleb remodelling, iridolenticular synechiolysis, and Nd: YAG laser hyaloidotomy are the other procedures that are currently used.[27]
Laser peripheral iridotomy
LPI is indicated for angle-closure disease (high-risk PACS, PAC, PACG), and treatment of AAC is done with suspected pupillary block or plateau iris mechanism.[7] LPI is contraindicated in neovascular glaucoma and eyes with angle closure due to the nonpupillary block mechanism.[27]
After reviewing the guidelines, the panellist opined that laser iridotomy is usually possible and surgical iridotomy is rarely required.
Laser trabeculoplasty
LTP is indicated for lowering IOP in POAG, pseudoexfoliative glaucoma (PXFG) and/or pigmentary dispersion glaucoma (PDG), high-risk Ocular Hypertension (OHT) either as initial treatment or as an add-on or replacement treatment.[7] LTP is contraindicated in the event of inadequate visualization of angle structures and glaucoma associated with uveitis, trauma, or angle dysgenesis. It is relatively contraindicated in eyes with normal-tension glaucoma, aphakia, and PACG with PAS.[27]
The Laser in Glaucoma and Ocular Hypertension trial supports SLT as a first-line treatment for OAG and ocular hypertension. Selective laser trabeculoplasty (SLT) as the first treatment was more cost effective than eye drops.[28]
The panelists reviewed the laser options provided in EGS and APGS and agreed that argon laser trabeculoplasty (ALT) and SLT have similar IOP-reducing effects. However, they stressed that SLT is commonly practiced in India. The panelists added that the success of SLT depends on the trabecular meshwork’s pigmentation.
Thermal laser peripheral iridoplasty
Thermal laser peripheral iridoplasty (TLPI) may be considered in those with plateau iris syndrome with remaining angle closure despite a patent peripheral iridotomy and elevated IOP. However, its efficacy in reducing IOP is limited.[7] TLPI is contraindicated in the event of nonvisibility of the iris due to corneal edema or opacity or a flat anterior chamber.[27]
The panellist reviewed EGS and APGS guidelines and opined that TLPI had limited IOP lowering efficacy. They also added that once daily, pilocarpine could be used as an alternative to TLPI for plateau iris syndrome and patent peripheral iridotomy.
Cyclodestructive procedures
Cyclodestructive procedures are considered for treating refractory glaucoma—uncontrolled glaucoma despite previous filtration surgery and/or laser treatment and/or with maximum tolerated medical treatment.[27] The available cyclodestructive procedures are lasers (endoscopic, transpupillary, transcleral cyclophotocoagulation), ultrasound, and cryoprobe.[7] Micropulse transcleral cyclophotocoagulation replaces continuous mode diode cyclophotocoagulation with fewer complications; however, there are concerns of unexplained visual loss in some eyes.[7]
After reviewing the EGS and APGS guidelines, the panellist concluded that transcleral cyclophotocoagulation is India’s most commonly used method. As ultrasound is not common in India, they did not make any recommendations.
Incisional surgery
Ophthalmologists resort to surgery when nonsurgical treatment options fail to lower the IOP to the target pressure or cause intolerable side effects.[2] However, it is also recommended in those whose glaucoma is relatively nonprogressive.[7] Primary congenital glaucoma is also treated surgically. Complicated glaucoma may require additional therapy (in addition to trabeculectomy). Cyclodestructive procedures and long-tube implants are more commonly used in case of repeat surgery. The outcome of the surgery can be evaluated in terms of IOP lowering in the absence of IOP lowering medications. The commonly preferred surgical technique for penetrating glaucoma surgery is trabeculectomy and trabeculotomy with goniotomy.[7]
The advantage of trabeculectomy is that it is associated with lower long-term postoperative IOP and requires fewer postoperative lOP-lowering medications. However, it also has certain disadvantages associated with a higher rate of cataract formation, postoperative bleb complications, and a higher risk of complications from postoperative hypotony (e.g., choroidal detachment).[7]
Long-tube glaucoma drainages are generally reserved for patients with risk factors for a poor result with trabeculectomy with antifibrotics. Recent trials have established their potential role as a primary surgical procedure in select cases.[7]
The ab-interno nonbleb forming procedures are defined as minimally invasive glaucoma surgery (MIGS). These procedures can be combined with phacoemulsification. MIGS surgeries are suitable for patients with mild to moderate glaucoma. Currently, there is not sufficient evidence to support the superiority or equivalence between these procedures versus trabeculectomy.[7]
The panelists discussed EGS and APGS guidelines and concluded that trabeculectomy in adults and trabeculotomy–trabeculectomy in congenital glaucoma was a commonly preferred surgical technique for penetrating glaucoma surgery. They added that nonpenetrating glaucoma surgery is not helpful in the Indian context. They also agreed that MIGS is not widely available in India.
Role of antifibrotic agents in glaucoma management
Antifibrotics such as 5-fluorouracil (5-FU) and mitomycin-C are generally used in patients undergoing glaucoma filtration surgery to reduce postoperative conjunctival scarring and improve drainage.[7] General precautions in using antifibrotic agents: antifibrotics are associated with a potential risk of postoperative infection. The use of antifibrotic requires careful surgical techniques to prevent complications. It should not enter the eye and contact with the cut edge of the conjunctival flap should be avoided. Precautions to the use and disposal of cytotoxic substances should be observed.[7]
After reviewing the use of adjunctive agents in glaucoma surgery in the guidelines, the panelist put forth a series of suggestions on adjunctive agents in intraoperative and postoperative [Box 1]. The felt mitomycin C is the choice of drug in glaucoma surgery and antifibrotics should be judiciously used. Intraoperative mitomycin can be used at 0.1–0.5 mg/mL for 1–3 min, depending on the condition of the disease. Postoperatively, both 5-FU and mitomycin-C can be used.
Cataract and glaucoma surgery
In the Indian setting, most often, glaucoma is detected in cataract screening camps. It is rather challenging to optimize the management of coexisting glaucoma and cataract. It is challenging because one has to achieve glaucoma control, accomplish visual improvement, and decrease complications due to surgery.[29]
Cataract and glaucoma surgery can be combined or performed sequentially. Cataract surgery alone is of limited benefit in lowering IOP in OAG and is not recommended. A clear lens extraction is an option in PACG and PAC with high IOP. Combined surgery allows, more significant IOP reduction. The success rate of combined phacoemulsification and filtration surgery is less than filtration surgery alone. However, the comparative evidence on outcomes of sequential versus combined cataract and glaucoma surgery is insufficient.[7]
In context to the guidelines, the panelists commented that in patients with phakic and PACG, phacoemulsification alone or combined phacoemulsification plus glaucoma surgery could be considered. However, the decision should be made based on the disc and field damage and the status of the angle.
Treatment algorithms
Topical therapy in glaucoma
Topical treatment for glaucoma is initiated with monotherapy to minimize side effects. After reviewing the EGS and APGS guidelines, the experts agreed that different classes of drugs have a different degree of lowering IOP [Table 3].[7] PGAs are first-line of therapy largely on the basis of their efficacy, once-daily dosing, and safety profile.
Table 3.
Commercially available intraocular pressure-lowering therapies
| Drug class | Drugs | Mechanism of action | Adverse effects |
|---|---|---|---|
| Prostaglandin analogue | Latanoprost; bimatoprost; travoprost; tafluprost | Increased uveoscleral outflow and trabecular mesh outflow | Conjunctival hyperaemia, lengthening and darkening of the eyelash, increased periocular and iris pigmentation, prostaglandin-associated periorbitopathy |
| β-Blocker | Timolol; betaxolol | Decreased aqueous humor production | Ocular irritation, bronchoconstriction, bradyarrhythmias, hypotension |
| Carbonic anhydrase inhibitor | Brinzolamide; dorzolamide; acetazolamide (per oral) | decreased aqueous humor production | Topical: ocular irritation, hyperaemia, dysgeusia. Per oral: polyuria, anorexia, sulphur reaction, metabolic acidosis, renal failure, renal calculi |
| α-2 Agonist | Brimonidine; apraclonidine | Decreased aqueous humor production and increased uveoscleral outflow | Conjunctival hyperaemia, allergic blepharoconjunctivitis, drowsiness, dry mouth |
| Cholinergic | Pilocarpine | Increased trabecular outflow | Blurred vision, dim vision, vitreous floaters, myopia, retinal tear or detachment, brow ache |
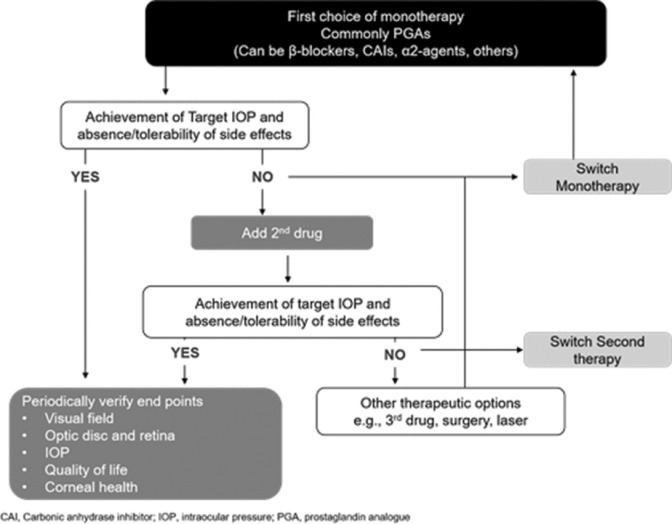
In accordance with the EGS and APGS guidelines, the panelists suggested that if the initial therapy failed to achieve the target IOP or is not tolerated, switch to another monotherapy or consider LTP. On the other hand, if monotherapy is well tolerated and effective but the desired IOP reduction is not achieved, then addition of a second drug of different class should be considered as per the APGS guideline [Fig. 4].[7,8]
Figure 4.
Algorithm for topical therapy in glaucoma (adapted from EGS guideline)
Open-angle glaucoma
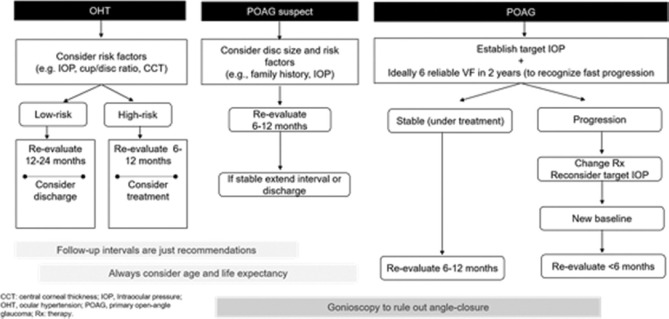
The panellists reviewed the diagnostic algorithm for POAG proposed in the EGS and endorsed it [Fig. 5].[7] Medical therapy (up to three drugs) or LTP can be considered initially in a patient with POAG or PXFG, or pigmentary glaucoma can be PDG. When the treatment response with laser or medical therapy is insufficient, then surgery can be an option. Treatment may not be required in POAG suspect as long as the IOP is not elevated. Table no 4. Class of drugs and their intraocular pressure reductions. Patients with OHT should be offered treatment only if they are at high risk of converting to glaucoma. The treatment principles of OHT are similar to POAG. Patients with POAG suspect or OHT can be followed up at intervals of 6–12 months, initially, or longer, if all parameters remain unchanged.[7] In the case of secondary OAG, the secondary cause should be evaluated and addressed.
Figure 5.
Algorithm for evaluating OHT, POAG, and POAG suspect (adapted from EGS guideline)
Table 4.
Class of drugs and their intraocular pressure reductions
| Class of drug | Reduction in IOP |
|---|---|
| Prostaglandin analogues (latanoprost, travoprost, tafluprost) | 25-35% |
| Prostamide (bimatoprost) | 25-35% |
| Nonselective beta antagonists (timolol, levobunalol, metipranol, and carteol) | 20-25% |
| Beta-1-selective antagonists (betaxolol) | ≈20% |
| Carbonic anhydrase inhibitors (brinzolamide and dorzolamide) | 20% |
| Systemic carbonic anhydrase inhibitors (azetazolamide) | 30-0% |
| Alpha-2-selective agonist (brimonidine) | 18-20% |
| Rho kinase inhibitors | |
| Netarsudil | 20-15% |
| Ripasudil | 20% |
The panel’s expert opinion in the management of OAG concerning EGS and APGS guidelines is summarized in Box 1.
Angle-closure glaucoma
Angle-closure glaucoma is defined by the presence of iridotrabecular contact (ITC > 180°). PACS is an angle in which 180–270° of the posterior trabecular meshwork cannot be seen gonioscopically.[8] Angle-closure glaucoma is diagnosed by gonioscopy, the gold standard. However, it is essential to rule out secondary causes. As provocative tests are of less diagnostic value, they can be avoided. Diagnostic mydriasis is generally safe and can be used to evaluate the retina as long as the angle is reasonably wide.[7]
Patients with chronic ACG have to be evaluated for the pathophysiological mechanisms. In the event of a pupillary block, medication along with LPI should be considered. In the case of plateau iris, medical therapy and LPI can be considered. Iridoplasty should be performed only if the angle remains closed even after LPI and the IOP remains high or medical management with pilocarpine can be considered. Eventually, trabeculectomy (filtration surgery) may also be considered in either pupillary block or plateau iris. Lens-induced blockage warrants lens extraction.[7]
In the case of acute primary angle-closure attack, the treatment is targeted at lowering the aqueous humor production, reopening the angle, and reducing inflammation. Topical therapy with beta-blockers and alpha 2 agonists or systemic treatment with acetazolamide/mannitol will help in reducing the aqueous humor production. In contrast, pilocarpine can help reopen the angle and steroids will take care of the inflammation.
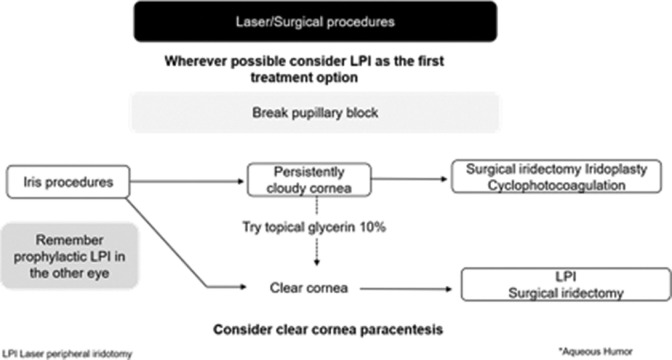
Experts endorsed the EGS guideline on laser or surgical approach to acute primary angle-closure attack Fig. 6.[7]
Figure 6.
Laser/surgical approach to acute primary angle-closure attack (adapted from EGS guideline)
The panel’s expert opinion in the management ACG in context to EGS and APGS guidelines is summarized in Box 1.
Monitoring glaucoma progression
The panelists endorsed the EGS guideline’s strong recommendation on monitoring glaucoma. Keeping in view the goal of preventing vision impairment, they agreed to use visual acuity, VF testing, clinical assessment of the optic disc, and RNFL and tonometry to monitor glaucoma progression. Repeat gonioscopy and OCT of disc/RNFL/macula may not be helpful as OCT analysis cannot replace VF analysis for assessing the progression.[7] The panelists also concurred that VF is mandatory for not only diagnosing but also for monitoring the progression of glaucoma. They opined that OCT should complement VF testing and cannot replace it in the progression of glaucoma. However, in preperimetric glaucoma, OCT is used for monitoring the disease progression.[7]
Summary and key expert opinions
After reviewing and discussing the EGS and APGS guidelines, the panelists issued recommendations that are practical from the Indian context [Box 1].
Conclusion
This review uses an expert-based assessment of the updated EGS and APGS guidelines from an Indian perspective. While the EGS guidelines are mainly applicable to the Indian context, the APGS guidelines are closer to Indian practice, especially for angle-closure disease. This highlights the impact of health care resources and disease prevalence on global glaucoma guidelines in the Indian context.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
All named authors for this manuscript meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship. All authors take full responsibility for the integrity of the work and have given final approval for the published version. The authors acknowledge Dr. Punit Srivastava from Mediception Science Pvt. Ltd (www.mediception.com), Gurgaon, India for providing writing and editing assistance for this project by Allergan/Abbvie India Pvt. Lt Allergan/Abbvie facilitated the experts group meeting and provided medical writing assistance but did not influence the content in any manner.
References
- 1.Leffler CT, Schwartz SG, Giliberti FM, Young MT, Bermudez D. What was glaucoma called before the 20th century? Ophthalmol Eye Dis. 2015;7:21–33. doi: 10.4137/OED.S32004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schuster AK, Erb C, Hoffmann EM, Dietlein T, Pfeiffer N. The diagnosis and treatment of glaucoma. Dtsch Arztebl Int. 2020;117:25–34. doi: 10.3238/arztebl.2020.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pan Y, Varma R. Natural history of glaucoma. Indian J Ophthalmol. 2011;59(Suppl 1):S19–23. doi: 10.4103/0301-4738.73682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–7. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.George R, Ve RS, Vijaya L. Glaucoma in India:Estimated burden of disease. J Glaucoma. 2010;19:391–7. doi: 10.1097/IJG.0b013e3181c4ac5b. [DOI] [PubMed] [Google Scholar]
- 6.Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040:A systematic review and meta-analysis. Ophthalmology. 2014;121:2081–90. doi: 10.1016/j.ophtha.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 7.2020 European Glaucoma Society. Terminology and Guidelines for Glaucoma. 5th ed. 2020. [Last accessed on 2021 Feb 16]. Available from: https://www.eugs.org/eng/egs_guidelines_reg.asp . [DOI] [PubMed]
- 8.Asia-Pacific Glaucoma Society (APGS) Amsterdam, The Netherlands: Kugler Publications; 2016. [Last accessed on 2021 Feb 16]. Available from: https://www.apglaucomasociety.org/Public/Public/Resources/APGG.aspx . [Google Scholar]
- 9.Gordon MO, Beiser JA, Brandt JD, Heuer DK, Higginbotham EJ, Johnson CA, et al. The ocular hypertension treatment study:Baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:714–20. doi: 10.1001/archopht.120.6.714. [DOI] [PubMed] [Google Scholar]
- 10.Medeiros FA, Weinreb RN. Is corneal thickness an independent risk factor for glaucoma? Ophthalmology. 2012;119:435–6. doi: 10.1016/j.ophtha.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang SY, Melles R, Lin SC. The impact of central corneal thickness on the risk for glaucoma in a large multiethnic population. J Glaucoma. 2014;239:606–12. doi: 10.1097/IJG.0000000000000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vijaya L, George R, Arvind H, Ve Ramesh S, Baskaran M, Raju P, et al. Central corneal thickness in adult South Indians:The Chennai Glaucoma Study. Ophthalmology. 2010;117:700–4. doi: 10.1016/j.ophtha.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 13.Khurana M, Kader M, Ramakrishnan R. Opportunistic Glaucoma Screening in Rural India:Role of Vision Centers ARVO Annual Meeting Abstract. Investigative Ophthalmology &Visual Science. 2013;54:4407. [Google Scholar]
- 14.Arora T, Bali SJ, Arora V, Wadhwani M, Panda A, Dada T. Diurnal versus office-hour intraocular pressure fluctuation in primary adult onset glaucoma. J Optom. 2015;8:239–43. doi: 10.1016/j.optom.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen M, Zhang L, Xu J, Chen X, Gu Y, Ren Y, et al. Comparability of three intraocular pressure measurement:iCare pro rebound, non-contact and Goldmann applanation tonometry in different IOP group. BMC Ophthalmol. 2019;14:225. doi: 10.1186/s12886-019-1236-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rödter TH, Knippschild S, Baulig C, Krummenauer F. Meta-analysis of the concordance of Icare® PRO-based rebound and Goldmann applanation tonometry in glaucoma patients. Eur J Ophthalmol. 2020;30:245–52. doi: 10.1177/1120672119866067. [DOI] [PubMed] [Google Scholar]
- 17.Cook JA, Botello AP, Elders A, Fathi Ali A, Azuara-Blanco A, Fraser C, et al. Systematic review of the agreement of tonometers with Goldmann applanation tonometry. Ophthalmology. 2012;119:1552–7. doi: 10.1016/j.ophtha.2012.02.030. [DOI] [PubMed] [Google Scholar]
- 18.Riva I, Micheletti E, Oddone F, Bruttini C, Montescani S, De Angelis G, et al. Anterior chamber angle assessment techniques:A review. J Clin Med. 2020;9:3814. doi: 10.3390/jcm9123814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alward WL, Longmuir RA. Gonioscopic Grading Systems. Color Atlas of Gonioscopy. 2017. [Last accessed on 2021 Feb 24]. Available from: https://www.aao.org/disease-review/gonioscopic-grading-systems .
- 20.Lin SC, Singh K, Jampel HD, Hodapp EA, Smith SD, Francis BA, et al. Optic Nerve Head and Retinal Nerve Fiber Layer Analysis. A Report by the American Academy of Ophthalmology. Ophthalmology. 2007;114:1937–49. doi: 10.1016/j.ophtha.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Michelessi M, Li T, Miele A, Azuara-Blanco A, Qureshi R, Virgili G. Accuracy of optical coherence tomography for diagnosing glaucoma:An overview of systematic reviews. Br J Ophthalmol. 2021;105:490–5. doi: 10.1136/bjophthalmol-2020-316152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar JR, Seelamantula C, Kamath YS, Jampala R. Rim-to-disc ratio outperforms cup-to-disc ratio for glaucoma prescreening. Sci Rep. 2019;9:7099. doi: 10.1038/s41598-019-43385-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu Z, Medeiros FA. Recent developments in visual field testing for glaucoma. Curr Opin Ophthalmol. 2018;29:141–6. doi: 10.1097/ICU.0000000000000461. [DOI] [PubMed] [Google Scholar]
- 24.Harasymowycz P, Birt C, Gooi P, Heckler L, Hutnik C, Jinapriya D, et al. Medical management of glaucoma in the 21st century from a Canadian perspective. J Ophthalmol. 2016;2016:6509809. doi: 10.1155/2016/6509809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sihota R, Angmo D, Ramaswamy D, Dada T. Simplifying “target“intraocular pressure for different stages of primary open-angle glaucoma and primary angle-closure glaucoma. Indian J Ophthalmol. 2018;66:495–505. doi: 10.4103/ijo.IJO_1130_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heo JH, Rascati KL, Wilson JP, Lawson KA, Richards KM, Nair R. Comparison of prostaglandin analog treatment patterns in glaucoma and ocular hypertension. J Manag Care Spec Pharm. 2019;25:1001–10. doi: 10.18553/jmcp.2019.25.9.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar H, Mansoori T, Warjri GB, Somarajan BI, Bandil S, Gupta V. Lasers in glaucoma. Indian J Ophthalmol. 2018;66:1539–53. doi: 10.4103/ijo.IJO_555_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gazzard G, Konstantakopoulou E, Garway-Heath D, Garg A, Vickerstaff V, Hunter R, et al. Selective laser trabeculoplasty versus eye drops for first-line treatment of ocular hypertension and glaucoma (LiGHT):A multicentre randomised controlled trial. Lancet. 2019;393:1505–16. doi: 10.1016/S0140-6736(18)32213-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saikumar SJ, Anup M, Nair A, Mathew NR. Coexistent cataract and glaucoma –Causes and management. TNOA J Ophthalmic Sci Res. 2019;57:132–8. [Google Scholar]