Abstract
Purpose:
The study aimed to estimate the prevalence of subnormal stereoacuity in school children and to assess the factors associated with it.
Methods:
In this prospective cross-sectional study, a total of 2,376 school children without amblyopia and manifest squint were screened by the titmus fly test, Snellen chart, tests for heterophoria, anterior segment examination, and fundoscopy. Children with a manifest squint, amblyopia (best-corrected visual acuity [BCVA] <6/18), and history of ocular trauma or surgery, and one-eyed children were excluded. Cycloplegic refraction was done in children with uncorrected or undercorrected refractive errors, and stereoacuity was assessed again with spectacle correction.
Results:
The prevalence of normal stereoacuity by titmus fly test was 93.18% with correction of refractive errors. Girls had slightly better stereopsis compared with boys. The subnormal stereoacuity was significantly associated with refractive error (P < 0.00001, significant at P < 0.05), unilateral refractive error (P < 0.00001, significant at P < 0.05), bilateral refractive error (P < 0.00001, significant at P < 0.05), anisometropia (P < 0.00001, significant at P < 0.05), ametropia (P < 0.00001, significant at P < 0.05), lower BCVA (P < 0.00001, significant at P < 0.05), hyperopia (P < 0.05, significant at P < 0.05), and heterophoria (P = 0.014, significant at P < 0.05). The subnormal stereoacuity was positively correlated with the magnitude of refractive error of the eyes.
Conclusion:
This study underlines the significant impact of identification and correction of refractive errors and squints in school children. The measurement of stereoacuity will be of immense importance and must be included in the screening programs for children.
Keywords: Heterophoria, refractive error, school children, stereoacuity, titmus fly test
Binocular vision is the ability of both eyes to work to maintain focus on one point or object at the same time to create a single visual image. Binocular vision helps us see in three dimensions, judge distance and spatial relationship, and refocus our eyes from far to near. Physiologically, a binocular vision has three components: fusion, depth perception, and stereopsis. Of these, stereopsis is considered the highest form of binocular vision.[1,2]
Stereopsis is clinically measured by tests for stereoacuity. Stereoacuity requires fine synchronization of optical, neural, and motor components of both eyes to achieve normal stereoacuity thresholds. It is important for children in learning fine motor tasks, in taking part in sports, and in the training of certain occupational courses.[3,4,5] It is also important in assessing the quality of life.[6] Stereoacuity tests are mandatory in screening, assessment, and monitoring treatment outcomes of amblyopia and its causes, including strabismus and refractive errors.[7,8] Stereoacuity is measured in seconds of arc (1° =60 minutes of arc, 1 minute = 60 seconds of arc). The lower the value of stereoacuity, the better is the stereopsis. According to the study by Cho et al.,[8] normal stereoacuity is 40 to 60 seconds of arc by the titmus fly test. Recently, studies testing both near and distance stereoacuity have also reported normative values.[7]
Random dot tests (TNO, Frisby) provide the most definitive evidence of high-grade binocular single vision as they use random element patterns. Where this is not feasible, contour-based tests (e.g., titmus fly test) may provide more reliable information.[2,8,9] Stereoacuity is subnormal in any condition with impaired vision in both eyes to a level less than 6/18 and impaired binocular vision such as strabismus and suppression.[10] Stereoacuity measurements are seldom used in the routine screening of school children, the main focus being on visual acuity. There is paucity of data on the prevalence of normal stereoacuity and the factors associated with subnormal stereoacuity among school children in India. This study has been undertaken with the objective of estimating the prevalence of subnormal stereoacuity in school children and assessing the factors associated with it.
Methods
In this prospective, cross-sectional study, 2,376 children in the age group of 7 to 14 years from five schools (one urban, two semiurban, and two rural schools) were screened. The mean age of the children was 11.06 years. The study included 1,414 boys and 962 girls with mean ages of 11.35 years and 10.63 years, respectively. The exclusion criteria were children with a manifest squint, amblyopia, or history of ocular trauma or surgery, and one-eyed children. The children with the best-corrected visual acuity (BCVA) less than 6/18 were considered as having amblyopia and were excluded from the study. The study was approved by the Institutional Ethics Committee and registered with the Clinical Trials Registry – India.
After registration of the study, the enrollment of the students was started on February 1, 2019. The permission of the school administration and written informed consent from the parents or guardians of all students were taken prior to enrollment of the students for the study. The research procedure and data collection were done in compliance with the legal requirements and the tenets of the Declaration of Helsinki were adhered to. The enrollment of 2,376 eligible students was done until January 31, 2020. The target sample size was 2,501, calculated at 7% prevalence of subnormal stereoacuity by a pilot study done by us. The enrollment was stopped after the first wave of the COVID-19 (coronavirus disease 2019) pandemic started and could not be reinitiated after the schools opened during the pandemic as it was considered unethical to screen the children during the pandemic. So the target sample size was reduced to 2,376 and the duration of the trial was extended up to April 2021after getting permission from the Institutional Ethics Committee.
All the enrolled students were assessed for history of previous ocular ailments, injuries, surgeries, and use of spectacles. Visual acuity was assessed using Snellen charts. Assessment of heterophoria was done by the Hirschberg test, cover test, cover–uncover test, alternate cover test, and Worth’s four dot test. Stereoacuity was measured using titmus fly test at a distance of 40 cm. After wearing polarized glasses, the students were asked to grasp the wings of the fly. If the student was able to grasp the wings of the fly, they were asked to identify the picture that seemed elevated than the rest of the pictures in each row. Then they were asked to press the button that was elevated out of the four buttons in each picture. For the students who could not identify a target, the test was repeated to assess correct stereoacuity. The students with spectacles wore polarized glasses over their spectacles. The students with uncorrected or undercorrected refractive errors and subnormal stereoacuity (>60 seconds of arc) were reassessed after cycloplegic refraction with 1% cyclopentolate eye drops and refractive correction. Anterior segment examination was done with a torchlight. Direct ophthalmoscopy was done through undilated pupils in all students except those dilated for refraction. The cutoff value chosen for myopia was −0.5 D, hyperopia was +2.00 D, and astigmatism was ± 1.00 D. These values were arbitrarily chosen. The refractive power of students with a BCVA of 6/6 was assessed by neutralization of the spectacles worn by them.
Results
Out of the 2,391 students enrolled in the study, 15 students were excluded. Manifest squint was seen in six students, and nine students had amblyopia with a BCVA less than 6/18. There were no one-eyed children or children with a history of trauma or surgery to the eyes. In the present study, 2,376 students were enrolled. All the calculations on stereoacuity were done using logarithms as the stereoacuity levels were expressed in geometrical progression and not as arithmetic progression.[11] Statistical analysis was done using the Chi-square test, independent t-test (standard error of the difference of means), and Pearson correlation coefficient [Tables 1, 2 and Fig. 1]. A P value of <0.05 was considered significant.
Table 1.
Age and gender-wise distribution of stereoacuity
| Age in Years | Stereoacuity | 40 | 50 | 60 | 80 | 100 | 140 | 200 | 400 | 800 | 3,000 | Total no. of Students | Mean (seconds of Arc) | SD | P Independent t-test |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 7 | Boys | 27 | - | 2 | 1 | 1 | - | - | - | - | - | 31 | 43.25 | 5.12 | 0.0075 |
| Girls | 40 | 2 | - | - | - | 1 | - | - | - | - | 43 | 40.80 | 2.40 | ||
| 8 | Boys | 87 | 7 | 3 | 1 | - | - | 4 | - | - | - | 102 | 44.08 | 6.07 | 0.5288 |
| Girls | 88 | 8 | 6 | 3 | - | 1 | 1 | - | - | - | 107 | 43.58 | 5.38 | ||
| 9 | Boys | 148 | 12 | 10 | 4 | 5 | - | 4 | - | - | - | 183 | 44.75 | 7.07 | 0.0042 |
| Girls | 146 | 8 | 6 | 5 | 1 | - | 2 | - | - | - | 168 | 42.91 | 4.49 | ||
| 10 | Boys | 151 | 10 | 5 | 6 | 7 | 1 | 2 | 3 | 1 | - | 186 | 46.80 | 9.01 | 0.5406 |
| Girls | 104 | 17 | 3 | 8 | 1 | 1 | 3 | 1 | - | - | 138 | 46.19 | 8.66 | ||
| 11 | Boys | 176 | 7 | 4 | 5 | 3 | 2 | 1 | 1 | - | - | 199 | 43.31 | 5.07 | 0.0492 |
| Girls | 154 | 3 | 4 | 4 | 2 | 1 | 1 | - | - | - | 169 | 42.38 | 3.73 | ||
| 12 | Boys | 194 | 9 | 4 | 7 | 5 | 1 | 6 | 1 | - | - | 227 | 44.91 | 7.50 | <0.0001 |
| Girls | 131 | 2 | 3 | 2 | 1 | 1 | 1 | - | - | - | 141 | 41.98 | 3.19 | ||
| 13 | Boys | 180 | 5 | 4 | 7 | 8 | 1 | 2 | - | - | - | 207 | 43.93 | 6.09 | 0.9107 |
| Girls | 99 | 2 | 5 | 5 | 3 | - | - | 1 | - | - | 115 | 44.01 | 6.20 | ||
| 14 | Boys | 248 | 4 | 10 | 8 | 2 | 2 | 2 | 2 | - | 1 | 279 | 44.06 | 6.25 | 0.3798 |
| Girls | 70 | 3 | 3 | 1 | 3 | 1 | - | - | - | - | 81 | 43.39 | 5.23 | ||
| All Ages | Boys | 1211 | 54 | 42 | 39 | 31 | 7 | 21 | 7 | 1 | 1 | 1414 | 44.49 | 6.84 | <0.0001 |
| Girls | 832 | 45 | 30 | 28 | 11 | 6 | 8 | 2 | - | - | 962 | 43.32 | 5.08 | ||
| Total | 2043 | 99 | 72 | 67 | 42 | 13 | 29 | 9 | 1 | 1 | 2376 | 44.01 | 6.11 |
SD=standard deviation
Table 2.
Association between refractive error, heterophoria, and stereoacuity
| Normal Stereoacuity | Subnormal Stereoacuity | P value of Chi-Square Test | |
|---|---|---|---|
| No Refractive Error | 1704 | 72 | |
| Refractive Error | 511 | 89 | <0.00001 |
| Unilateral Refractive Error | 119 | 27 | <0.00001 |
| Bilateral Refractive Error | 392 | 62 | <0.00001 |
| Ametropia | 213 | 29 | <0.00001 |
| Anisometropia | 298 | 60 | <0.00001 |
| Orthophoria | 2126 | 148 | P=0.014 |
| Heterophoria | 89 | 13 | |
| Myopia | 379 | 57 | <0.05 |
| Hyperopia | 132 | 32 |
Figure 1.
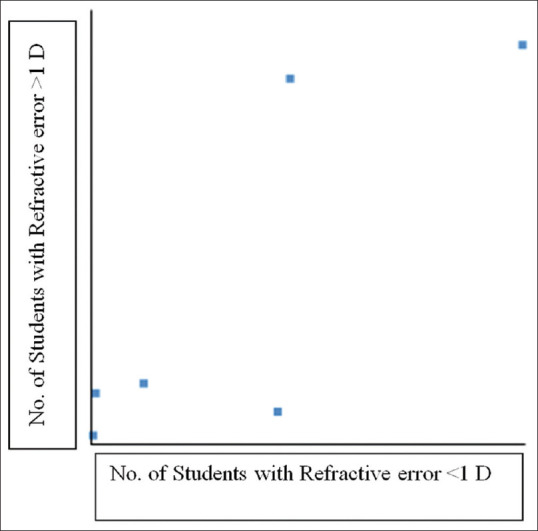
Pearson correlation coefficient depicting positive correlation between number of students with refractive error <1 D and >1 D. r(4) =0.82, significant at P < 0.05
The mean stereoacuity of all students was 44.01 ± 6.11 seconds of arc. The prevalence of normal stereoacuity by titmus fly test was 93.18% with correction of refractive errors [Table 3]. The means of stereoacuity in all age groups and genders were calculated [Table 1]. The difference in means of boys and girls was statistically significant. The difference in means of boys and girls was significant at ages of 7, 9, 11, and 12 years of age. Furthermore, all the means of stereoacuity at all age groups showed positive correlation between the boys and girls by Pearson correlation coefficient (R = 0.7499, n = 6, significant at P < 0.05). Overall, girls had slightly better stereoacuity than boys (43.32 ± 5.08 seconds in girls and 44.49 ± 6.84 seconds in boys).
Table 3.
Distribution of stereoacuity
| Stereoacuity (Seconds of Arc) | No. of students | Percentage | Cumulative Percentage |
|---|---|---|---|
| 40 | 2,043 | 85.98 | 85.98 |
| 50 | 99 | 4.17 | 90.15 |
| 60 | 72 | 3.03 | 93.18 |
| 80 | 67 | 2.82 | 96.00 |
| 100 | 42 | 1.77 | 97.77 |
| 140 | 13 | 0.55 | 98.32 |
| 200 | 29 | 1.22 | 99.54 |
| 400 | 9 | 0.38 | 99.92 |
| 800 | 1 | 0.04 | 99.96 |
| 3,000 (Fly test positive) | 1 | 0.04 | 100 |
| Total no. of students | 2,376 |
The mean BCVA of all the children was 6/6.18. There were 2,292 students with a BCVA of 6/6. The mean stereoacuity of students with a BCVA of 6/6 was 43.67 ± 5.6 seconds of arc. There were 84 students who had BCVA from 6/9 to 6/18. All these 84 students had refractive errors. The mean stereoacuity of these 84 students was 55.72 ± 22.22 seconds of arc. There was a statistically significant difference in means of stereoacuity among students with a BCVA of 6/6 and a BCVA of 6/9 to 6/18 (95% confidence interval [CI] =10.55–13.55, degrees of freedom [df] =2,374, P < 0.0001 significant at P < 0.05). There was no difference in the BCVA among students without refractive error, with or without normal stereoacuity. There were 72 students with normal visual acuity, subnormal stereoacuity, and without any refractive error [Table 2].
There were 600 students with refractive errors. Among these, 332 were boys with a 23.48% prevalence of refractive errors. Out of 332 boys, 276 boys had normal stereoacuity and 56 boys had subnormal stereoacuity. There were 268 girls with refractive errors with a 27.86% prevalence of refractive errors. Out of 268 girls, 235 girls had normal stereoacuity and 33 girls had subnormal stereoacuity. There was no statistically significant difference among boys and girls with refractive errors having either normal or subnormal stereoacuity by Chi-square test (P = 0.12,not significant at P < 0.05). There were 516 students with refractive errors who had a BCVA of 6/6 and a mean stereoacuity of 47.66 ± 11.36 seconds of arc. There was a statistically significant difference between means of stereoacuity in students with refractive error having a BCVA of 6/6 and a BCVA of 6/9 to 6/18 (95% CI = 4.96–11.16; df = 598; P < 0.0001, significant at P < 0.05). The Pearson correlation coefficient analysis showed statistically significant positive correlation between students with refractive error <1 D and >1 D; r(4) =0.82, significant at P < 0.05 [Fig. 1].
Furthermore, the spherical equivalent was used to categorize the students into myopia and hyperopia. The prevalence of subnormal stereoacuity in students with myopia was 13.07% and that of students with hyperopia was 19.51%. There was a statistically significant difference among students with myopia and hyperopia, with subnormal stereoacuity more prevalent in hyperopes (significant at P < 0.05) [Table 2].
All the students were stratified into groups based on the presence or absence of refractive error, whether their refractive error was unilateral or bilateral, and whether their stereoacuity was normal or subnormal. This stratification was arbitrarily chosen after data collection, as stereoacuity is a binocular function. In the Chi-square analysis, stereoacuity was significantly associated with the presence or absence of refractive errors (P < 0.00001, significant at P < 0.05). On further statistical analysis using the Chi-square test, the subnormal stereoacuity was significantly associated with unilateral refractive error (P < 0.00001, significant at P < 0.05), bilateral refractive error (P < 0.00001, significant at P < 0.05), anisometropia (P < 0.00001, significant at P < 0.05), ametropia (P < 0.00001, significant at P < 0.05), hyperopia (P < 0.05, significant at P < 0.05), and heterophoria (P = 0.014, significant at P < 0.05). In correlation analysis using Pearson correlation coefficient, the magnitude of refractive error of the eyes (<1 D and >1 D) was positively correlated with subnormal stereoacuity in children with refractive errors, unilateral and bilateral refractive errors (R = 0.8184, n = 6, significant at P < 0.05) [Table 4 and Fig. 1].
Table 4.
Number of students, magnitude of refractive error, and stereoacuity
| <1 D | >1 D | |
|---|---|---|
| Refractive Error + Normal Stereoacuity | 189 | 322 |
| Refractive Error + Subnormal Stereoacuity | 39 | 50 |
| Unilateral Refractive Error + Normal Stereoacuity | 92 | 27 |
| Unilateral Refractive Error + Subnormal Stereoacuity | 19 | 8 |
| Bilateral Refractive Error + Normal Stereoacuity | 97 | 295 |
| Bilateral Refractive Error + Subnormal Stereoacuity | 20 | 42 |
The analysis among urban, semiurban, and rural areas was not done as the place of residence was not noted. Heterophoria was observed in 102 children, with exophoria in 89 children and esophoria in 13 children. Heterophoria was significantly associated with subnormal stereoacuity (P = 0.014, significant at P < 0.05).
Discussion
In our study, the prevalence of normal stereoacuity in school children aged 7 to 14 years by titmus fly test was 93.18% with correction of refractive errors. Girls had slightly better stereopsis compared with boys. However, the means of stereoacuities among both genders were not consistently different at all ages. The presence of refractive errors whether unilateral or bilateral, ametropia, anisometropia, hyperopia, and heterophoria were the important factors significantly associated with subnormal stereoacuity as both Chi-square test and independent t-test results were similar in our study. The subnormal stereoacuity was positively correlated with the magnitude of refractive error of the eyes (<1 D and >1 D) when children were wearing corrective glasses. This has a significant value as the pharmacological methods to control the progression of myopia are gaining more validity and acceptance.
The presence of subnormal stereoacuity among students without refractive errors having normal visual acuity was noted In our study (Table 2: 72 students). It is comparable with the Shandong Children Eye Study[9] in which 3.9% (245 students) did not either complete or give reliable stereoacuity examination by titmus fly test. Se-Youp Lee and Nam-Kyun Koo,[12] in a study on 80 normal individuals aged between 7 and 76 years without eye diseases except refractive errors, suggested that the reduction of stereopsis was not associated with the decrease of visual acuity. However, there was a significant subnormal stereoacuity observed in students with refractive errors and a BCVA of 6/9 to 6/18. These two observations underline the importance of stereoacuity assessment in all children, irrespective of their refractive condition.
The prevalence of normal stereoacuity in school children was 93.18% by titmus fly test with correction of refractive errors in our study. This was comparable with 94.6% in the Shandong Children Eye Study[9] in which 5,780 children were screened and with 87.7% in the study by Joo Yean Lee et al.[13] in which 162 nonamblyopic children were enrolled. The distribution of stereoacuity was also comparable with that of the Shandong Children Eye Study.[9] The importance of regular vision assessment, including stereoacuity, cycloplegic refraction, and prompt correction of refractive errors cannot be overemphasized.
Although girls had a higher prevalence of refractive error, there was no statistically significant difference of mean stereoacuities when we considered both boys and girls with refractive errors. So refractive error might not be the factor responsible for the difference in stereoacuity among boys and girls. This requires further research to evaluate the factors contributing to this difference.
The subnormal stereoacuity in children with refractive errors was significantly associated with a BCVA of 6/9 to 6/18. This is important in prescribing spectacles for children, as better BCVA is associated with better stereopsis, which in turn influences performance in academics and certain sports activities that require good stereopsis.
The subnormal stereoacuity was associated with ametropia, anisometropia, unilateral or bilateral refractive errors, lower BCVA, hyperopia, and heterophoria. This was similar to the Shandong Children Eye Study[9] (5,780 children) and the studies by Weakley (411 children),[14] Ju-Wen Yang et al. (166 myopic and astigmatic children),[15] and Ju-Wen Yang et al. (117 hyperopic children).[16] In a study on 117 children with hyperopia and astigmatism, Ju-Wen Yang et al.[16] reported reduced stereoacuity in the hyperopia group and no significant reduced stereoacuity in anisometropia with spherical or astigmatic refractive errors. In a study on 166 children with myopic ametropia that included astigmatism, Ju-Wen Yang et al.[15] reported reduced stereoacuity in spherical anisometropia. However, the children in their study were tested for stereoacuity without spectacle correction. In our study, there was a significant positive correlation between the refractive power and stereoacuity. This was similar to the findings of multiple studies.[13,14,15,16,17,18] The higher prevalence of subnormal stereoacuity in students with refractive errors highlights the importance of regular periodic refractive assessment and prompt correction with subjective acceptance guided by stereoacuity assessment as an additional tool. Furthermore, the stereoacuity assessment by titmus fly test is a simple tool that can pick up the presence of latent squint.
The sample was collected from urban, semiurban, and rural schools. This is representative of school-going children. The statistical analysis was performed on logarithmically calculated means of stereoacuity.[11,15] The grouping of students based on unilateral or bilateral refractive errors, ametropia, anisometropia, refractive correction magnitude, and heterophoria eased the analysis and understanding of the factors associated with subnormal stereoacuity of school children. The present study was done with school children in the critical age group of onset and progression of refractive errors. It highlights the importance of regular assessment of children’s vision and timely correction of refractive errors and heterophoria through mandatory screening prior to admission in each schooling year by qualified ophthalmologists and optometrists.
Most of the studies on the stereoacuity in children include a selected group of students with a particular eye condition such as ametropia, anisometropia, astigmatism, heterophoria, or amblyopia.[13,14,15,16,17,18,19,20] Some more studies used experimentally induced refractive conditions to study the relation between the refractive errors and stereoacuity.[17,18] Our study was a cross-sectional study of normal school children that included all children without amblyopia and manifest squint. The refractive errors were corrected and stereoacuity was assessed again so that all the children were tested for stereoacuity with their BCVA for distance.
Our study has potential limitations. First, our study could not recruit the number of target samples. However, the sample size of 2,376 school children was adequate enough for assessing the prevalence of normal stereoacuity and to analyze the factors associated with subnormal stereoacuity. The titmus fly test has monocular clues that might enable the participants to identify the correct responses.[21] Despite this limitation, the test is easy to perform in a large group of subjects. The cooperation of students might vary with the examiners’ ability to explain the test. The analysis of individual refractive conditions was not done as the spectacle power was not assessed by the lensometer. Finally, this was a cross-sectional study and not a longitudinal study.
The presence of subnormal stereoacuity in students with normal visual acuity in the absence of any refractive errors warrants further investigation and research to elucidate its cause. The examination of further tests for accommodative-convergence over accommodation ratio, near point of accommodation, and near point of convergence might aid in such research.
Conclusion
This study underlines the significant impact of identification and correction of refractive errors and squints in school children. The measurement of stereoacuity will be of immense importance and must be included in the screening programs for children.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
The authors would like to acknowledge the help by Ophthalmologists Dr.Zareena Vahab, Dr. Anila Sarwani, Dr.Shravya,Dr.Sindhuri and Optometrists Krishna Veni, Karuna Prabha, Kiran Kumar and Asia, for their contribution in Pilot Project as well as the main Project. The management of Siddhartha Academy of General and Technical Education, the management, staff, students, and parents of all the 5 schools for their invaluable cooperation. We thank Dr.Amarnadh, Professor of Community Medicine and Mrs. Savithri, Statistician for sample size calculation and statistical analysis. We thank our Principal, Dr.P.S.N.Murthy, for all the encouragement and Dr.U.Satyanarayana, Research Director for the guidance at every level.
References
- 1.Iwata Y, Fujimura F, Handa T, Shoji N, Ishikawa H. Effects of target size and test distance on stereoacuity. J Ophthalmol. 2016;2016:7950690. doi: 10.1155/2016/7950690. doi:10.1155/2016/7950690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Read JC. Stereo vision and strabismus. Eye. 2015;29:214–24. doi: 10.1038/eye.2014.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bloch E, Uddin N, Gannon L, Rantell K, Jain S. The effects of absence of stereopsis on performance of a simulated surgical task in two-dimensional and three-dimensional viewing conditions. Br J Ophthalmol. 2015;99:240–5. doi: 10.1136/bjophthalmol-2013-304517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ponsonby AL, Williamson E, Smith K, Bridge D, Carmichael A, Jacobs A, et al. Children with low literacy and poor stereoacuity:An evaluation of complex interventions in a community-based randomized trial. Ophthalmic Epidemiol. 2009;16:311–21. [PubMed] [Google Scholar]
- 5.O'Connor AR, Tidbury LP. Stereopsis:Are we assessing it in enough depth? Clin Exp Optom. 2018;101:485–94. doi: 10.1111/cxo.12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuang TM, Hsu WM, Chou CK, Tsai SY, Chou P. Impact of stereopsis on quality of life. Eye (Lond) 2005;19:540–5. doi: 10.1038/sj.eye.6701538. [DOI] [PubMed] [Google Scholar]
- 7.Piano ME, Tidbury LP, O'Connor AR. Normative values for near and distance clinical tests of stereoacuity. Strabismus. 2016;24:169–72. doi: 10.1080/09273972.2016.1242636. [DOI] [PubMed] [Google Scholar]
- 8.Cho YA, Cho SW, Roh GH. Evaluation of criteria of stereoacuity for titmus, randot and TNO stereotests. J Korean Ophthalmol Soc. 1999;40:532–7. [Google Scholar]
- 9.Guo DD, Wu JF, Hu YY, Sun W, Lv TL, Jiang WJ, et al. Stereoacuity and related factors:The Shandong children eye study. PLoS One. 2016;11:e0157829. doi: 10.1371/journal.pone.0157829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams S, Simpson A, Silva PA. Stereoacuity levels and vision problems in children from 7 to 11 years. Ophthalmic Physiol Opt. 1988;8:386–9. doi: 10.1111/j.1475-1313.1988.tb01173.x. [DOI] [PubMed] [Google Scholar]
- 11.Peyman A, Peyman M, Akhlaghi M. Correct method for statistical analysis of stereopsis in ophthalmology research. Graefes Arch Clin Exp Ophthalmol. 2012;250:781. doi: 10.1007/s00417-011-1713-x. [DOI] [PubMed] [Google Scholar]
- 12.Lee SY, Koo NK. Change of stereoacuity with aging in normal eyes. Korean J Ophthalmol. 2005;19:136–9. doi: 10.3341/kjo.2005.19.2.136. [DOI] [PubMed] [Google Scholar]
- 13.Lee JY, Seo JY, Baek SU. The effects of glasses for anisometropia on stereopsis. Am J Ophthalmol. 2013;156:1261–66. doi: 10.1016/j.ajo.2013.07.016. e1. [DOI] [PubMed] [Google Scholar]
- 14.Weakley DR., Jr The association between nonstrabismic anisometropia, amblyopia, and subnormal binocularity. Ophthalmology. 2001;108:163–71. doi: 10.1016/s0161-6420(00)00425-5. [DOI] [PubMed] [Google Scholar]
- 15.Yang JW, Huang TY, Lee JS, Yeung L, Lin YF, Sun CC. Correlation between myopic ametropia and stereoacuity in school-aged children in Taiwan. Jpn J Ophthalmol. 2013;57:316–9. doi: 10.1007/s10384-013-0231-2. [DOI] [PubMed] [Google Scholar]
- 16.Yang JW, Huang TY, Yang KJ, Lee JS, Ku WC, Yeung L, et al. The effects of hyperopic and astigmatic ametropia on stereoacuity by Titmus stereo test. Taiwan J Ophthalmol. 2012;2:22–4. [Google Scholar]
- 17.Brooks SE, Johnson D, Fischer N. Anisometropia and binocularity. Ophthalmology. 1996;103:1139–43. doi: 10.1016/s0161-6420(96)30555-1. [DOI] [PubMed] [Google Scholar]
- 18.Katsumi O, Tanino T, Hirose T. Effect of aniseikonia on binocular function. Invest Ophthalmol Vis Sci. 1986;27:601–4. [PubMed] [Google Scholar]
- 19.Lee SY, Isenberg SJ. The relationship between stereopsis and visual acuity after occlusion therapy for amblyopia. Ophthalmology. 2003;110:2088–92. doi: 10.1016/S0161-6420(03)00865-0. [DOI] [PubMed] [Google Scholar]
- 20.Saladin JJ. Effects of heterophoria on stereopsis. Optom Vis Sci. 1995;72:487–92. [PubMed] [Google Scholar]
- 21.Kulp MT, Mitchell GL. Randot stereoacuity testing in young children. J Pediatr Ophthalmol Strabismus. 2005;42:360–4. doi: 10.3928/01913913-20051101-05. [DOI] [PubMed] [Google Scholar]


