Abstract
Since the introduction of the first toric intraocular lens (IOLs) in the early 1990s, these lenses have become the preferred choice for surgeons across the globe to correct corneal astigmatism during cataract surgery. These lenses allow patients to enjoy distortion-free distance vision with excellent outcomes. They also have their own set of challenges. Inappropriate keratometry measurement, underestimating the posterior corneal astigmatism, intraoperative IOL misalignment, postoperative rotation of these lenses, and IOL decentration after YAG-laser capsulotomy may result in residual cylindrical errors and poor uncorrected visual acuity resulting in patient dissatisfaction. This review provides a broad overview of a few important considerations, which include appropriate patient selection, precise biometry, understanding the design and science behind these lenses, knowledge of intraoperative surgical technique with emphasis on how to achieve proper alignment manually and with image-recognition devices, and successful management of postoperative complications.
Keywords: Indications of toric IOLs, post cataract surgery astigmatism, posterior corneal astigmatism, Toric IOLs, Toric IOL marking
While the overall prevalence of corneal astigmatism in patients undergoing cataract surgery ranges from 30%–39% for > 1D, about 3%–4% patients have high astigmatism (>3D) at the time of surgery.[1,2] Significant astigmatism is associated with poor uncorrected distance visual acuity, increases spectacle dependence, and decreases the overall quality of vision by distortion and smearing of the images. As the prevalence is not uncommon, it becomes important to identify and treat astigmatism effectively. This review focuses primarily on the indications of toric intraocular lenses (IOL), both conventional and expanding indications, and the pre, intra, and postoperative methods to optimize the outcomes, and discuss the management of complications specific to these lenses.
Management of Astigmatism in cataract surgery
While lenticular contribution to astigmatism is eliminated by the surgery itself, corneal astigmatism (anterior and posterior) decides the postoperative residual astigmatic error. Therefore, precise measurement is a prerequisite to surgical planning. Various intraoperative modalities to correct corneal astigmatism include incision on the steep axis, limbal relaxing incisions (LRIs) or peripheral corneal incisions (PCIs), opposite clear corneal incisions (OCCI), and toric IOLs. These techniques can be used as stand-alone or combined, based on the amount of astigmatism. Usually, a step ladder approach is preferred for astigmatism management which involves the use of a single treatment modality for astigmatism of lesser magnitude (<1D) and the use of two or more modalities when the astigmatism is higher (>1D).[3] A clear corneal phacoemulsification incision over the steep axis flattens the meridian by about 0.25–0.75 D depending on the incision site.[4] At the same time, an LRI can be used to manage about 1 to 4 D of astigmatism.[5,6,7,8] However, LRI is less predictable, is more prone to overcorrections, and carries an inherent risk of iatrogenic perforation during the surgery and infection postoperatively. The risk of perforation can be eliminated using femtosecond laser astigmatic keratotomy, but the cost is the limiting factor.[9]
Historical perspective of toric IOLs
Shimizu from Japan, in the year 1992, devised the first toric IOL, which was a three-piece (PMMA optics and polypropylene haptics), open-loop design.[10] The first US Food and Drug Association-approved foldable toric IOL was by Staar surgical (Monrovia, CA, USA). It was a silicone IOL with a 10.8 mm plate haptic design having fenestrations to provide better rotational stability.[11] However, severe (>30°) rotation was noticed during the early days of surgery in 24% of the patients.[11] Therefore, the next generation of IOLs were a little larger and had larger fenestrations to promote fibrotic capsular fixation. These were widely used until 2006 when Alcon (Alcon Laboratories, Inc., Fort Worth, TX, USA) introduced the single-piece open loop, hydrophobic acrylic foldable IOL. The advantage of these lenses is the hydrophobic nature that provides excellent rotational stability in the bag and a square-edge design that reduces the posterior capsular opacification (PCO), therefore decreasing the need for YAG-laser capsulotomy and further risk of rotation of the IOL. These lenses are aspheric and can correct up to - 4.11 D of corneal astigmatism.[12] These lenses have 3 dots on either side of the optic edge near the optic-haptic junction that helps the surgeon align the IOL during the surgery. Another widely used single-piece aspheric hydrophobic acrylic IOL (AMO Tecnis toric IOL) by Abbott Medical Optics, Inc, Santa Ana, CA, was approved for use by the FDA in 2013. The range of correction offered by these lenses are the same as Alcon IOLs. Toric multifocal, toric extended depth of focus IOLs (EDOF), and phakic toric IOLs are also available.[12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37] Tables 1 and 2 lists the various monofocal, multifocal, and EDOF toric IOLs.
Table 1.
List of monofocal toric intraocular lenses
| IOL | Material | Design | Spherical power | Cylinder Power | Literature review | |
|---|---|---|---|---|---|---|
|
| ||||||
| Post op residual astigmatism | IOL rotation after surgery | |||||
| Acriol EC Toric (Care group) | Hydrophobic acrylic | Single piece aspheric with modified C-loop haptic | +0.0D-+30.0D (0.5 D steps) | 1.0D to 6.00D (0.5 D Steps) | NA | NA |
| AcrySof (Alcon) | Hydrophobic acrylic | Single piece aspheric C-loop haptic | +6.0 to+34.0 | 1.0 to 6.0 (0.75 steps) | Lane et al.[12] < 0.50 D in 60% < 1.0 D in 95% Ahmed et al.[13] < 0.50 D in 71% < 1.0 D in 90% Holland et al.[14] < 0.50 D in 53% < 1.0 D in 88% |
Seth et al.[15] 2.15 +/−2.58 Holland et al.[14] 4° |
| Ankoris (PhysIOL) | Hydrophilic acrylic | Single piece acrylic with anterior aspheric surface and double C-loop haptics | +6.0 to+30.0D | 1.5 to 6 (0.75 D steps) | Biana Dubinsky-Pertzov et al.[16] < 0.5 D in 84% | Biana Dubinsky-Pertzov et al. < 5° in 82% |
| AT TORBI (Carl Zeiss Meditec) | Hydrophilic acrylic with hydrophobic surface | Plate haptic, Bitoric | −4.0 to+32.0 | 1.0 to 12.0 (0.5 D steps) | Seth et al.[15] < 0.50 D in 62% < 1.0 D in 100% Bascaran et al.[17] < 0.50 D in 95% < 1.0 D in 100% |
Seth et al.[15] 3.52±3.84° Bascaran et al.[17] 4.42° +/− 4.31° |
| Auroflex Toric (Aurolab) | Hydrophilic acrylic | Single piece with anterior toric | +10.0 to 30.0 D (<+15 and > +25.0 D in 1.0 D steps, rest 0.5 D steps) | 1.5 to 6.0 (0.5 D steps) | NA | NA |
| Aurvue EV Toric (Aurolab) | Hydrophobic acrylic | Single piece negative aspheric and anterior toricity | +10.0 to+15.0 D in 1.0 D steps, +15.0 to+25.0 D in 0.5 D steps | 1.5 to 6.0 (0.5 D steps) | NA | NA |
| LENTIS Tplus (Oculentis) | Hydrophilic acrylic with hydrophobic surface | C-loop/Plate haptic with aspheric optic | −10.0 to+35.0 | 0.25-12.0 (0.75-1.0 steps) | 0.16±0.24 Gerding et al.[18] 0.63±0.56 |
NA |
| Light-adjustable lens (Calhoun Vision) | Silicone with PMMA haptics | A three piece IOL with modified C-loop | +17.0 to+24.0 | 0.75-2.0 | Chayet et al.[19] −0.5 D in 100% |
NA |
| Microsil (HumanOptics) | Silicone with PMMA haptics | A three piece IOL with C-loop haptic | −10.0 to+35.0 | 1.0-15.0 (1.0 steps) | De Silva et al.[20] 1.23 +/− 0.90 D |
De Silva et al.[20] Rotation<5°for all |
| Morcher 89A, 92S (Morcher GmbH) | Hydrophilic acrylic | Bag-in-the-lens | +10.0 to+30.0 D | 0.5-8.0 (0.25 steps) | Rozema et al. | Rozema et al.[21] 0.36° +/− 1.39° |
| Precizon toric IOL (OPHTEC) | Hydrophilic acrylic | Biconvex transitional conic toric design offset-shaped haptic | +1.0 to+34.0 | 1.0-10.0 (0.5 steps) | Jung et al.[22] − 0.31±0.29 D | Jung et al.[22] 1.50° ± 0.84° |
| STAAR (STAAR Surgical Company) | Silicone | Plate haptic | +9.5 to+28.5 | 2.0 or 3.5 | Till et al.[23] < 0.50 D in 48% < 1.0 D in 75% |
NA |
| Sulcoflex toric (Rayner) | Hydrophilic acrylic | Single piece with posterior toric surface and undulating and rounded C- loop haptic | −7.0 to+7.0 (0.5D steps) | 1.0 to 6.0 (0.5D steps) | NA | NA |
| Supraphob Toric (Appasamy) | Hydrophobic acrylic | Single piece | +10.0 to+30.0 D (0.5 D steps) | 1.50 to 6.0 D (0.75 D steps) | NA | NA |
| TECNIS Toric IOL (Abbott Medical Optics) | Hydrophobic acrylic | Single piece with anterior toric aspheric surface with modified C - loop haptic | +5.0 to+34.0 | 1.5-6 (0.5-1.0 steps) | Ferreira et al.[24] < 0.50 D in 75% < 1.0 D in 100% Jung et al.[22] 0.41±0.33 D |
Ferreira et al.[24] 3.25 +/−2.04 Jung et al.[22] 2.56° ± 0.68° |
| T-flex/RayOne (Rayner) | Hydrophilic acrylic | Single piece with anterior aspheric surface and C-loop haptic with antivault haptic technology | −10.0 to+35.0 (−9.5 to+34.5 for RayOne) | 1.0-11.0 (0.5 steps) | Alberdi et al.[25] < 0.50 D in 85% < 1.0 D in 100% |
Alberdi et al.[25] 92.6% of eyes had IOL rotation<10° |
| TORICA (HumanOptics) | Hydrophilic acrylic | Single piece IOL with anterior toric aspheric surface with C-loop | −20.00 to+60.0 D | 1.0 to 30.0 D (0.5D steps) | Gyöngyössy et al.[26] −0.60±0.40 D |
Gyöngyössy et al.[26] 1.81°± 1.87° |
| Ultima smart toric (Care group) | Hydrophilic acrylic | Plate haptic aspheric with antirotational haptic pads | −10.0 to+40.0 D (0.5 D steps) | 0.5 to 20.0 (0.5 D steps- customized range) | NA | NA |
| Vivinex XY1A Toric (Hoya) | Hydrophobic acrylic | Single piece acrylic with anterior aspheric and posterior toric surface | +10.0 to+30.0 | 1.0-6.0 (0.5/0.75 steps) | Razmjoo et al.[27] 0.87±0.66 D Post-op 6 months |
Schartmüller et al.[27] < 5° in 100% |
*NA - No major data available, IOL- Intraocular lense
Table 2.
List of multifocal and extended depth of focus toric intraocular lenses
| IOL | Material | Design | Spherical power | Cylinder power | Literature review | |
|---|---|---|---|---|---|---|
|
| ||||||
| Post-op residual astigmatism | IOL rotation after surgery | |||||
| Acriol toric multifocal (Care group) | Hydrophobic acrylic | Single piece with anterior diffractive aspheric and posterior toric with modified C-loop haptic | +6.00 to+ 30.00 (0.5 D steps) | to 4.0 (0.5 D steps) | NA* | NA |
| Acrysof IQ Restor toric (Alcon) | Hydrophobic acrylic | Single piece with anterior diffractive aspheric and posterior toric surface with C-loop haptic | +6.00 to+30.00 | 1.5 to 3.75 (0.75 D steps) | Garzón et al.[28] 0.64±0.53 |
Garzón et al.[28] 67.9% < 5° 2.97±2.33 |
| AT Lara Toric (Carl Zeiss Meditec) | Hydrophilic acrylic with hydrophobic surface | Plate haptic aspheric diffractive bitoric extended depth of focus IOL | −4.00 to+ 32.00 | 1.0 to 12.0 (0.5 steps) | NA | NA |
| AT Lisa Toric (Carl Zeiss Meditec) | Hydrophilic acrylic with hydrophobic surface | Plate haptic aspheric diffractive bitoric | −5.00 to+ 35.00 | 1.0 to 12.0 (0.5 steps) | Piovella et al.[29] < 0.5 D- 79.7% Mojzis et al. 0.06±0.30 D |
NA |
| enVista (Bausch and Lomb) | Hydrophobic acrylic | Single piece aspheric optic with modified C loop with fenestrations | +6 to+30.0D | 1.25 to 5.75 (0.75 D steps) | Garzón et al.[28] 0.41±0.51 |
Garzón et al.[28] 69.6% < 5° |
| Lentis Mplus Toric (Oculentis) | Hydrophilic acrylic with hydrophobic surface | Single piece with C-loop/Plate haptic with aspheric optic | 0.00 to+36.00 | 0.25 to 12.0 (0.75/0.01 D steps) | Chiam et al.[31] 0.00-1.46 D |
NA |
| M-flex T (Rayner) | Hydrophilic acrylic with hydrophobic surfaces | Single-piece acrylic with the closed-loop anti-vaulting haptic design | +14.00 to+32.00 | 1.0 to 6.0 (0.5 steps) | Shimoda et al.[32] Mean -0.44 D after 3 months |
NA |
| Panoptix toric (Alcon) | Hydrophobic acrylic | Single piece aspheric with diffractive-refractive optics and loop haptic | +6.0 to+34.0 D | 1.0 to 3.75 D | Ribeiro et al.[33] - 0.09 D Kohnen et al.[34] 98% - < 0.75 D |
Ribeiro et al.[33] 1.59° ± 2.15° |
| FineVision toric (PhysIOL) | Hydrophilic acrylic | Single piece aspheric with diffractive optics and double C-loop haptics | +6.00 to+ 35.00 | 1.0, 1.5 to 6.0 D (0.75 D steps) | Ribeiro et al. - 0.11 D |
Ribeiro et al.[33] 1.89° ± 3.31° |
| Sulcoflex multifocal toric (Rayner) | Hydrophilic acrylic | Single piece with posterior toric surface and undulating and rounded C- loop haptic | −7.0 to+7.0 (0.5D steps) | 1.0 to 6.0 (0.5D steps) | NA | NA |
| TECNIS multifocal Toric (Abbot Medical Optics) | Hydrophobic acrylic | Single piece anterior aspheric with posterior diffractive optics | +5.00 to+ 34.00 | 1.5, 2.25, 3.0, 4.0D | Marques et al.[35] −0.44±0.49 D (range: −1.25 to 0.00) at 6 months |
Marques et al.[35] 3.18° ± 3.28° |
| TECNIS Symfony Toric (Abbot Medical Optics) | Hydrophobic acrylic | Single piece with anterior aspheric toric and posterior diffractive optics for extended depth of focus | +5.00 to+ 34.00 | 1.0, 1.5 to 6.0 D (0.75 D steps) | Gundersen et al.[36] < 0.5 D-88% < 1.0 D-97% |
Gundersen et al.[35] < 5-87% <10°-96% |
| Trulign Toric (Bausch and Lomb) | Silicone with Silicone and Polyimide haptics | Modified plate haptic with hinges across the plate close to the optics, anterior and posterior aspheric surface with posterior toricity | +4.00 to+33.00 | 1.25, 2.00, 2.75 | Epitropoulos[37] ≤0.50 D in 97.5% of eyes (≤1.00 D in 100%) |
Epitropoulos[37] <5°-100% |
*NA - No major data available, IOL - Intraocular lense
Indications and contraindications
Since there is a rather large repertoire of toric IOLs available, it can be confusing to know when to use them and when to refrain.
Indications
Senile cataract with regular astigmatism: the best indication for this IOL is cataract with mild to moderate corneal astigmatism. Patients with visually significant cataracts, regular astigmatism of usually > 1D, and having realistic expectations from the surgery are the ideal candidates for toric IOL implantation.
Ectatic disorders: mild to moderate non-progressive keratoconus and pellucid marginal degeneration patients with fairly regular astigmatism are another indication to use these lenses. However, sometimes patients with irregular astigmatism (this is an off-label use) also benefit from these lenses.[38,39] Since these patients often have high astigmatism, use of customized IOLs have resulted in a significant reduction of astigmatism with good outcomes.[40]
Post-penetrating keratoplasty: post-keratoplasty patients often have early cataract formation due to prolonged use of steroids and also have high astigmatism due to irregular healing of the graft host junction. Toric IOLs both conventional and customized have been used with success in these cases. The dictum here is to ensure all sutures are removed and the keratometry has stabilized before going in for cataract surgery.[41,42]
Stable, non-progressive peripheral corneal scars following etiology such as post-microbial keratitis, post-corneal laceration repair (sparing the central visual axis), post-pterygium excision are other indications where these IOLs have been used.[43]
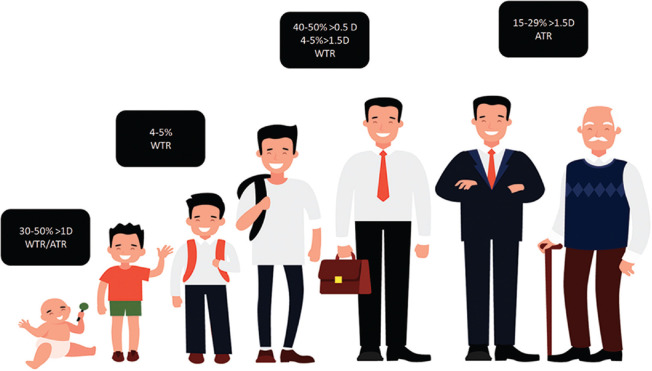
Pediatric cataract surgery: the use in pediatric cataracts is more an exception rather than a routine recommendation. Phakic toric lenses have been tried in children with high astigmatism with acceptable outcomes to reduce the risk of anisometropic amblyopia.[44] Toric IOLs in older children with developmental cataracts have also significantly reduced the preoperative astigmatism and resulted in better postoperative visual recovery.[45] However, its use in younger children (less than 2 years) is not recommended due to following reasons: one, the corneal astigmatism may change in axis and magnitude as the child grows older [Fig. 1].[46,47,48,49,50,51,52,53] Second, postoperative misalignment may warrant additional procedure which predisposes these children to harmful effects of anesthesia; and lastly, a requirement of YAG- laser capsulotomy may result in IOL decentration.
Fuch’s uveitis syndrome (FUS): astigmatism in patients with FUS undergoing cataract surgery is relatively common. Faramarzi et al.[54] reported astigmatism of > 1D in 67% eyes with FUS as compared to 30% normal fellow eye. The lack of posterior synechiae ensures that the IOL does not get decentered. The only disadvantage is the requirement of YAG-laser capsulotomy for all these patients, which needs to be done with care.
Figure 1.
Trend of change of steep axis of astigmatism with age. The axis and power of astigmatism changes with age. 30% to 50% of newborns and infants have astigmatism of more than 1D. The most common type of astigmatism in this age group is ATR. As the child enters the preschool age, the magnitude of astigmatism decreases to less than 1D, and the axis changes from ATR to WTR. In adolescence and till early adulthood, the vertical meridian remains steeper. There are two sources of astigmatism in the eye, corneal and lenticular. The lens contributes to the lenticular myopic astigmatism; however, its effects are negated by the steeper vertical meridian of the cornea. As the person ages (40 years and beyond), the tone of the orbicularis decreases, thereby decreasing the pressure exerted by the upper eyelid on the cornea. As a result, the vertical meridian of the cornea is no more the steeper meridian, the canceling effect of corneal astigmatism on the lens astigmatism decreases, and the ATR astigmatism from the lens begins to manifest
Contraindications
Patients with a history of trauma or any developmental abnormality where the capsular bag support is compromised are far from ideal candidates for these IOLs. These lenses should also be avoided in patients with anterior or posterior uveitis in the presence of synechia or poorly controlled inflammation, cases with zonular instability due to any cause, uncontrolled glaucoma, corneal dystrophies, poor endothelial cell counts, and complicated cataract surgeries where intraoperative complications are expected.
Large angle alpha: another relevant factor that is often neglected during toric IOL planning is the angle alpha. Angle alpha is the angle between the limbal center and the visual axis. When angle alpha is more than 0.5 mm, the capsular bag center may not correspond with the patient’s visual axis and may lead to unwanted refractive surprises postoperatively.[55,56]
Pre-operative planning
Three steps should be followed for maximizing outcomes with toric IOL implantation.
Step 1: Calculation of the total corneal astigmatism:
-
Understanding posterior corneal astigmatism
For a long time, it was believed that the contribution of the posterior corneal astigmatism (PCA) would be negligible.[57] With improved understanding, various researchers studied the PCA and reported the mean magnitude to range from − 0.26 D to − 0.78 D.[58,59,60,61,62] Koch and colleagues in 2012 noticed that the mean magnitude of PCA was 0.30 D in 435 patients involved in the study.[63] They also noticed a mismatch in the progression of the anterior and posterior corneal surfaces with advancing age. While the steep meridian changed from WTR to ATR in 52% of the patients’ anterior corneal surface, on the posterior corneal surface, the vertical meridian continued to remain steep in 87% of patients. In another study by Reitblat et al.[64] in 2015, it was concluded that the mean residual astigmatism was lower when the mean vector of anterior and posterior astigmatism was considered rather than anterior astigmatism alone.
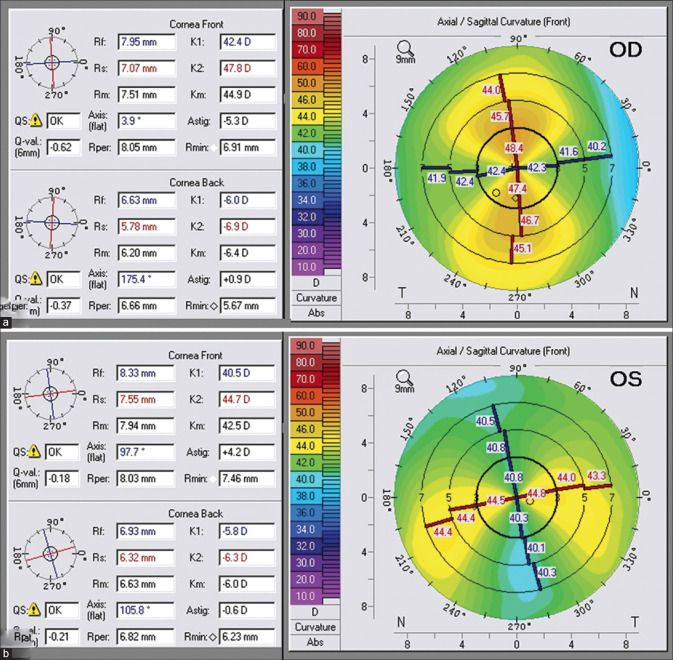
To further understand the concept and importance of PCA, let us go through this example. Fig. 2a is a patient with − 5.3D anterior WTR astigmatism and − 0.9D of WTR posterior corneal astigmatism. As the posterior corneal surface always acts as a negative lens, −0.9D will transcribe into a plus lens in the horizontal meridian. Thus, the effective total corneal astigmatism would be − 4.4D instead of − 6.2D. If we were to select − 5.3 D for calculating the toric IOL, we would have overcorrected the patient by − 0.9 D [Fig. 2a]. Similarly, in a patient with ATR astigmatism [Fig. 2b], the total corneal astigmatism would be − 4.8 D instead of − 4.2 D.
-
Measurement of PCA: Devices such as manual and automated keratometers and Placido-based corneal topographers consider the refractive index of 1.3375 to calculate power from the anterior curvature alone and cannot calculate the PCA. Devices utilizing a scanning-slit (Eg. Orbscan II; Bausch and Lomb, Rochester, New York, USA), or Scheimpflug imaging devices [Eg. Pentacam (Oculus Optikgeräte GmbH), Galilei (Ziemer USA, Wood River, IL)], ray tracing devices [Cassini, OPHTEC], and the anterior segment optical coherence tomographers (ASOCT) can measure the total corneal astigmatism (anterior and posterior). However, none of the devices are entirely reliable and the prediction error can range from 0.5 to 0.6 D for WTR astigmatism, and 0.2 to 0.3D for ATR astigmatism.[65,66,67]
Koch et al.,[67] in their study, came up with Baylor’s toric nomogram for estimation of astigmatism at the corneal plane by compensating for the PCA [Table 3]. It can also be used as a reference guide for toric IOL implantation. As per the nomogram, if the patient has WTR astigmatism, the threshold for toric IOL implantation is shifted up by 0.7 D. Similarly, the threshold decreases by 0.7 D in ATR astigmatism. To understand this better, a surgeon will use a T3 lens in WTR astigmatism only if the anterior corneal astigmatism is 1.7D (below which an LRI would be sufficient), and the threshold decreases to as low as 0.4 D in ATR astigmatism.
Surgically induced astigmatism and its role: the second parameter to be considered is the surgically induced astigmatism (SIA) while planning for surgery. As we have advanced from small incision cataract surgery (SICS) to phacoemulsification with incisions as small as 2.2 mm, the magnitude of SIA is very low. Visser et al.[68] reported an SIA of zero for incisions smaller than 2.2 mm, 0.3 D for an incision of 3.4 mm, and an SIA of 0.5 D for an incision size of 5.4 mm. This, however, is variable from patient to patient and differs for various surgeons as multiple factors like shape and location of the incision, use of sutures, and the postoperative corneal wound healing response play an important role in deciding the SIA. Moreover, SIA is a vector as it has both magnitudes as well as a direction. Calculating just the mean or the median, thus, would be inappropriate. Therefore, all the surgeons must calculate their vector SIA also termed as Centroid vector. This can be done using the SIA calculator developed by Dr. Warren Hill and his group and is readily available online (www.doctor-hill.com).
Figure 2.
Scheimpflug imaging showing the impact of posterior corneal astigmatism (PCA) on the total cornea astigmatism. (a) A patient with − 5.3D anterior WTR astigmatism and − 0.9 of WTR posterior corneal astigmatism. As the posterior corneal surface always acts as a negative lens, this − 0.9D will transcribe into a plus lens in the horizontal meridian. Thus, the effective total corneal astigmatism would be − 4.4D instead of − 6.2D. Similarly, in a patient with ATR astigmatism (b), the total corneal astigmatism would be − 4.8D instead of − 4.2D
Table 3.
Baylor’s toric IOL nomogram
| Baylor’s Toric IOL Nomogram Target range 0.25D 0.50D WTR | ||||
|---|---|---|---|---|
| WTR Astigmatism (D) | ATR Astigmatism (D) | Toric IOL to be implanted | IOL cylinder power at IOL plane (D) | Effective IOL cylinder power at corneal plane (D) |
| Alcon toric IOLs | ||||
| ≤1.69 (PCRI if >1.00) | <0.39 | None | NA | NA |
| 1.70-2.19 | 0.40-0.79 | T3 | 1.50 | 1.03 |
| 2.20-2.69 | 0.80-1.29 | T4 | 2.25 | 1.55 |
| 2.70-3.19 | 1.30-1.79 | T5 | 3.00 | 2.06 |
| 3.20-3.69 | 1.80-2.29 | T6 | 3.75 | 2.57 |
| 3.70-4.19 | 2.30-2.79 | T7 | 4.50 | 3.08 |
| 4.20-4.69 | 2.80-3.29 | T8 | 5.25 | 3.60 |
| 4.70-5.19 | 3.30-3.79 | T9 | 6.00 | 4.11 |
| AMO Tecnis toric IOLs | ||||
| ≤1.69 (PCRI if >1.00) | <0.39 | None | NA | NA |
| 1.70-2.19 | 0.40-0.79 | ZCT150 | 1.50 | 1.03 |
| 2.20-2.69 | 0.80-1.29 | ZCT225 | 2.25 | 1.54 |
| 2.70-3.24 | 1.30-1.79 | ZCT300 | 3.00 | 2.06 |
| 3.20-3.69 | 1.80-2.29 | ZCT400 | 4.00 | 2.74 |
WTR=With-the-rule astigmatism; ATR=Against-the-rule astigmatism; D=Diopter; PCRI=Peripheral corneal relaxing incision; IOL=Intra-ocular lens. All astigmatism values are the vector sum of the anterior corneal and surgically induced astigmatism. The nomogram takes into consideration the type of astigmatism (WTR or ATR) that the patient has and suggests the surgeon the series of toric IOL to be implanted for correcting corneal astigmatism during cataract surgery. In the table above, toric IOLs from Alcon (Alcon laboratories, Inc., Fort Worth, Texas, USA) and Tecnis (Abbott Medical Optics, Inc, Santa Ana, CA) are listed with the series of IOL, the cylindrical power available for the series, and the cylindrical error it corrects at the corneal plane. Tecnis models ZCT450, ZCT525, and ZCT600 are also available and correct cylinder up to 4.1 D
Step 2: Perform the spherical IOL power calculation:
The spherical power calculation can be performed routinely using optical biometers like IOLMaster (Carl Zeiss Meditec) and the Lenstar (Haag-Streit). Over the years, several studies have been performed quoting the advantages of one over the other. Most studies have shown no significant difference in the outcome using either device.[69,70,71] Once the keratometry and the axial length are derived from these devices, corneal topography should be obtained, PCA should be derived, and the axis and magnitude of astigmatism should be confirmed.
Step 3: Use the toric IOL calculators to make a surgical plan
All these values are then fed into the online toric IOL calculators. The commonly used online calculators, the Barrett online calculator [Fig. 3], the Alcon, and the AMO toric IOL calculators incorporate the PCA, SIA, and Baylor’s nomogram. Alternatively, various IOL formulas can be combined with Baylor’s nomogram to plan for the IOL power calculations. As per the study by Melles et al.,[72] the prediction error was minimal with Barrett universal II formula followed by Olsen, Haigis, Holladay 2, Holladay 1, SRK/T, and Hoffer Q in the sequence. Additionally, as astigmatism changes from WTR to ATR with the advancing age [Fig. 1], keeping the patient with some amount of WTR astigmatism is desirable at times, especially if the patients are younger.[73]
Figure 3.
Barrett toric calculator available online at ascrs.org. Most of the toric calculators incorporate the posterior corneal astigmatism (PCA), the surgically induced astigmatism (SIA), and Baylor’s nomogram
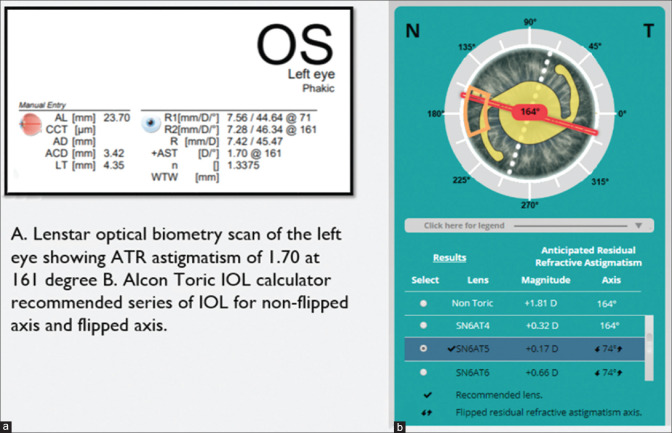
The choice of the toric IOL series should be the one with the least amount of residual astigmatism. If a particular toric IOL series is overcorrecting corneal astigmatism, the remaining postoperative cylinder will act in an axis 90° to the preoperative measured corneal axis. This is called an axis flip. Flipping of the axis is a subject of debate among surgeons, with most surgeons opting against it. However, there are reports that minimal overcorrection does not lead to any optical discomfort to the patient and, at times, can be beneficial.[74,75] For example, a 62-year-old female patient was being planned for left eye phacoemulsification and toric IOL. Preoperative astigmatism in the left eye, as seen in Fig. 4a, was 1.70 D at 161°. Alcon calculator was used in calculating the alignment axis of IOL. The final calculation sheet [Fig. 4b] of IOL with the flipped axis leading to WTR astigmatism was preferred. The postoperative outcome was good, and the patient’s uncorrected visual acuity was 20/20.
Figure 4.
An example for a toric IOL flip. The scan belongs to a 62-year-old female patient planned for left eye phacoemulsification and toric IOL. (a) Preoperative astigmatism in the left eye was 1.70D at 161°. Alcon calculator was used for calculating the alignment axis of IOL. (b) Final calculation sheet of IOL with the flipped axis leading to WTR astigmatism was preferred. Postoperatively patient had an uncorrected visual acuity of 20/20
Preoperative marking
The most important step in any successful toric IOL surgery is the toric IOL axis alignment to the steep axis of astigmatism. The toricity of the IOL is on the posterior surface. It is denoted by dots or a line present near the optic-haptic junction. The axis along this line is the flatter axis of the toric IOL which has to be aligned with the preoperative marking. The alignment is essential as a 10° rotation or misalignment decreases the toricity of the IOL by 33%.[76] Whereas if a toric IOL rotates by 30° or more, there is a cancellation of the toricity of the IOL. On the contrary, it induces a cylinder in another meridian, which can visually disturb the patients.
-
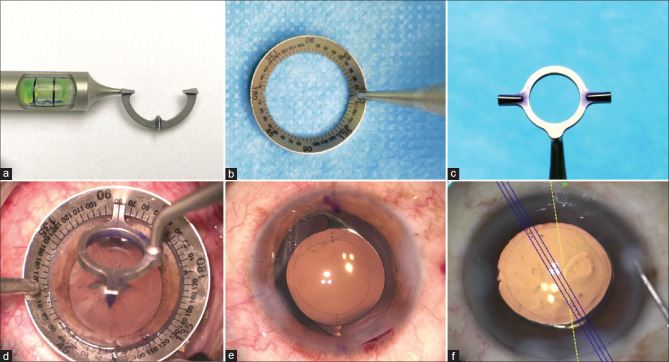
Manual marking: Preoperative marking can be done either manually or it can be image-guided. The manual method conventionally described is the three-step technique. It consists of marking the horizontal axis (reference marking) in a seated position on a slit lamp followed by a graduation marker such as Mendez gauge intraoperatively to align the horizontal axis. The third step is to mark the desired axis of alignment (axis marking) about the horizontal axis. While marking the reference axis, the patient should be in a sitting position, preferably chin lying on the chin-rest and forehead supported by the headrest of the slit lamp. This is important as the change of posture from sitting to lying down position can result in cyclotorsion of 2°–3° (maximum up to 16°).[77,78,79,80] The reference marking can be done either free-hand by marking at the limbal area 180° apart with the help of a marker pen, or it can also be done on a slit lamp by making a thin horizontal slit and then using the slit as a guide to mark at the limbal region.[81] Alternatively, a bubble marker, a pendular marker, or a tonometer marker can be used to mark the horizontal axis [Fig. 5]. These devices are easy to use, and the alignment error noted with these instruments ranges from 2° to 5°.[82,83,84,85,86]
While using the bubble marker, the handle of the marker should always be parallel to the lateral canthus. However, due to their limited field of view, the oculars of the microscope do not allow the surgeon to ensure this. Alternatively, marking is done by sitting in front of the patient at the same level and asking the patient to fix it at a distant object. Occasionally, patients cannot open their eyes wide enough due to a lack of muscle tone or senile ptosis. A speculum can be used in such situations after instilling a topical proparacaine 0.5%. The possible errors that can result during manual marking should also be considered. The head of the patient during marking might not be straight, resulting in parallax error. Also, the marks might fade away or get smudged during painting and draping or due to the irrigation fluid used during surgery. To avoid this, the conjunctiva should always be dried with the help of a cotton bud before marking. Also, the marking pen should be applied in twisting motion so that capillary action results in the tattooing of the ink. A scratch mark can also be made with a 26 gauge needle on the cornea so that even if the ink fades, the abrasion persists and can be visualized during the surgery. Another source of error is the thickness of the marking pen. Too thick a marking pen itself can result in alignment errors. A thick mark can correspond to up to 10° on the graduation scale, leading to a decrease in the toricity of the IOL by 33%. A pen with thin marks should be used instead. Every operating room staff should be made aware of the patient scheduled for the surgery preoperatively so that any accidental local anesthesia block/sedation/accidental shifting without marking the patient is avoided.
Other marking methods: the mapping method and femtosecond laser-assisted method.[87,88] Laser systems like Catalys Precision Laser System (Abbott Medical Optics, Inc, Santa Ana, CA) and LENSAR (LENSAR, Orlando, FL, USA) can create intrastromal incisions along the steep meridian to guide the alignment of the toric IOL axis intraoperatively.[88,89,90] IntelliAxis, now combined with the LENSAR laser delivery system, is a recent development. It helps the precision of LENSAR by marking the steep axis at the capsular plane and thereby creates two small tabs 180° apart to guide the alignment of the IOL (tabs measure approximately 300 μm in height and arc length of 5° at their base). Kaur et al.[89] noticed a postoperative misalignment of 2.07° ±1.49 with the intended axis of toric IOL using the LENSAR system. Cao et al.,[91] in their study, noted significantly lesser misalignment of the IOL from the intended axis with the femtosecond created capsular marks as compared to the manual markings; however, there was no significant difference in the postoperative residual astigmatism between the two groups. Another noted advantage of femtosecond laser-assisted capsular marks is eliminating the parallax error as the anterior capsule of the lens is much closer to the IOL plane than the corneal markings. Femtosecond laser-assisted cataract surgery additionally also helps in arcuate incision planning. However, the clear advantage of femtosecond-assisted methods of marking over other methods has not been established.
Image-guided systems: The common ones are the Callisto and Z aligns (Carl Zeiss), Verion (Alcon), OTAS (Haag- Streit), iTrace (Tracey Technologies), TrueGuide (TrueVision 3D Surgical system), ORA (Alcon), and LENSAR- IntelliAxis. During preoperative biometry, high-resolution digital images of the iris architecture, limbal vasculature, and scleral vessels are obtained and configured with the Callisto. Verion again is a noncontact device that gives information about the visual axis and pupillometry, and takes several high-definition images of the iris, limbal, and scleral vessels. Both Callisto and Verion are integrated with the microscope unit, and using the digital images, both devices give the surgeon the incision guide, capsulorrhexis guide, centration, and toric IOL guide for precise alignment of the axis. In a study comparing the two sophisticated devices, they were found to be nonsuperior to each other, and the alignment error was found to be <3° in 53% of the patients.[92]
Figure 5.
(a) Nuijts-Solomon pre-op toric bubble marker, (b) bevelled degree gauge, and (c) Nuijts-Solomon toric axis marker (Asico, Westmont, IL, USA). (d) Intraoperative marking of the desired axis using the toric axis marker and (e) final alignment of the IOL with the marked axis. (f) Alignment of toric IOL to the desired axis with the help of Callisto, and Z-align image-guided system (Carl Zeiss)
Osher Toric Alignment System (OTAS) is an imaging system wherein a 360° protractor is layered and superimposed over the high-resolution image of the patient’s eye. The desired axis of incision and toric IOL alignment can be marked over this picture and then carried to the OR in a USB drive or a printout used by the surgeon as a reference. iTRACE ray-tracing aberrometer has an additional integrated toric planner; apart from the critical information, it gives about the magnitude of angle alpha, Kappa, and the higher-order aberrations. TrueGuide is one of the latest innovations in this group of gadgets. It allows the surgeon to perform stereoscopic surgery by looking at a TV screen and wearing 3D glasses. In their study, Montes De Oca et al.[84] showed that the mean error induced by TrueGuide was 0.5 D to 4.0 D and was comparable to the manual marking system.
Optiwave Refractive Analysis (ORA) measures the refractive state of the eye intraoperatively and guides the surgeon regarding the IOL power and axis of alignment. It is one of the most revolutionary technologies available today. Various android and iOS toric axis markers and calculator applications are easily and freely available on the phone. One such novel phone application significantly reduced the alignment error compared to the manual marking technique used alone.[93]
The image-guided systems have been shown to incur a lesser degree of postoperative alignment errors than the manual marking techniques. There is no significant difference in the final visual acuity outcomes between the two groups; however, the visual quality was better in the surgeries planned with the image-guided systems.[94,95]
Intraoperative care, complications, and management
While the general complications are similar to the other cataract surgeries, those specific for toric IOL include misalignment which is a significant concern as it can lead to a significant amount of residual error. Patients can tolerate up to 0.5 D of astigmatism and still enjoy good visual quality with glasses. Beyond 0.75 D, patients complain of distorted vision and a decrease in contrast sensitivity. Dick et al.[96] reported their results of toric IOLs and reported a reduction of total astigmatism from preoperative mean astigmatism of 4.6 D to postoperative mean astigmatism of 1.12 D, and 85% of their patients had an IOL rotation of fewer than 5°. In another study, Visser et al.[68] noticed that the mean residual astigmatism in 35 patients was less than 0.5 D. These errors can occur during various stages of surgery, starting from the incorrect estimation of the astigmatic axis to inappropriate alignment of the toric IOL axis with the desired axis and finally, postoperative rotation of the IOL.
Surgical tips
Intraoperatively, there are certain dos and don’ts that one must remember.
Capsulorhexis: to start with, the capsulorrhexis has to be round, central, and of adequate size. The capsulorrhexis margin should be just smaller than the optics of the IOL chosen to ensure adequate overlap between the two. A larger capsulorrhexis will result in IOL instability, IOL rise above the capsulorrhexis, and postoperative myopic refractive surprise.
Cortical removal: ensure that cortical removal is done adequately and the anterior margin of the capsulorrhexis is polished. This helps in reducing the volume of the proliferating cells and subsequent formation of posterior capsular opacification (PCO). YAG capsulotomy for PCO is known to result in gross IOL tilt and astigmatic error.[97]
IOL insertion and dialing: once the cortical removal is done, cohesive viscoelastic substances (OVDs) are injected to inflate the bag for IOL implantation. A dispersive viscoelastic substance is difficult to remove and tends to stay behind the IOL resulting in early rotation of the IOL. Once the OVD underneath the IOL is removed, gross IOL dialing (15° to 30° from the desired axis) is performed to align the axis, followed by removing the remaining OVD [Fig. 6]. The last part of the IOL dialing is then performed once complete removal of OVD is ensured and can be done under irrigation fluid. Intraoperative aberrometer like ORA can give a live update about the eye’s refractive state and the axis and magnitude of the cylinder. It can guide us intraoperatively to make certain amends to the surgery.
Figure 6.
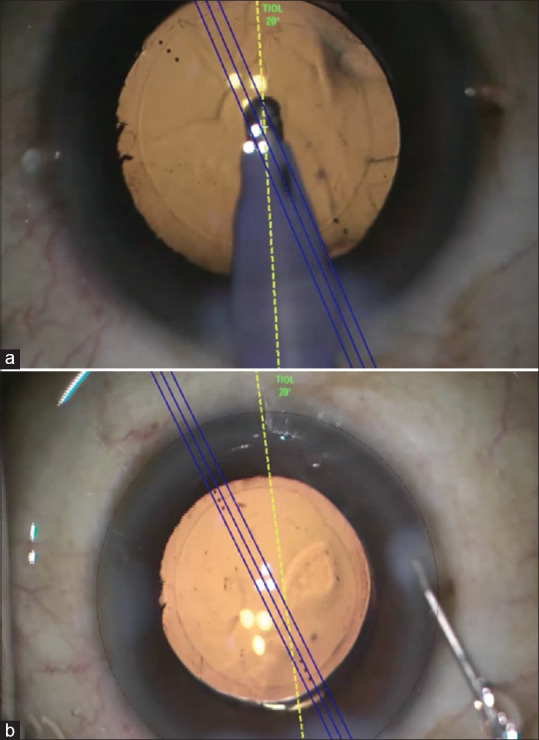
(a) Surgeon performs visco-expression using a co-axial irrigation-aspiration cannula. Blue color mark is the intended axis of alignment. The final 20°–30° of toric IOL alignment are done after the complete evacuation of viscoelastic devices; (b) final alignment after complete viscoelastic removal
Postoperative assessment: the residual error can be noted by the keratometry and the refraction. The misalignment can be confirmed on the slit lamp after dilating the eye, checking for the marks, and correlating with the desired axis. Alternatively, iTrace ray-tracing aberrometer gives us a good idea about the position of the IOL, the amount of misalignment from the desired axis, and how much re-rotation is required [Fig. 7]. Another way of calculating the amount of re-rotation required is by calculating the vector analysis of the misalignment. This can be done by Berdahl and Hardten toric IOL calculator available online (astigmatismfix.com).
Figure 7.
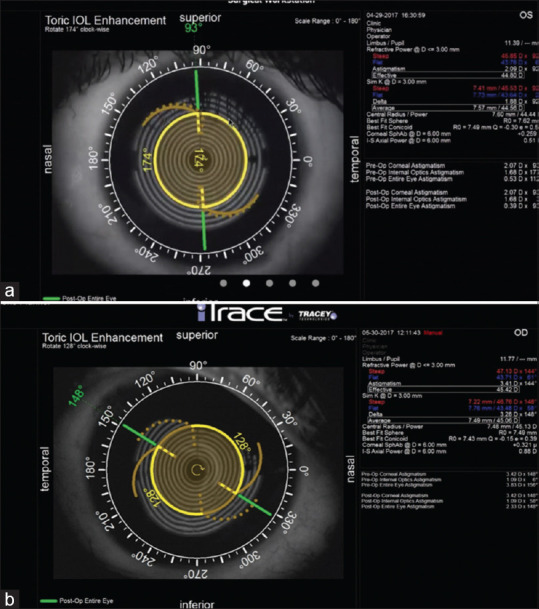
(a) iTrace (Tracey technology, Houston, Texas, USA), appropriate alignment of toric IOL in postoperative period; (b) The postoperative misalignment of the IOL
Causes of IOL rotation: There are numerous causes of IOL instability. Usually, the lens instability results during the first week of the surgery. The rotation stability depends on the material and the design of the toric IOL. Hydrophobic IOLs, due to their adhesive nature, are found to be the most stable lenses, followed by hydrophilic, PMMA and silicone IOLs in that sequence.[98] Hydrophobic plate haptic IOLs are noted to have similar rotational stability as the open-loop IOLs, but with the silicone lenses, open-loop IOLs are found to have a better IOL stability than the plate haptic models.[99,100] IOL misalignment should be diagnosed as early as possible as late surgical intervention and re-rotation of the IOL becomes difficult due to the adhesions formed between the bag and IOL.
Conclusion
Toric IOLs are a safe and effective surgical strategy for accurately correcting astigmatism. Adequate knowledge of the science behind using these lenses, appropriate case selection, meticulous preoperative measurements and planning, robust intraoperative surgical steps, and early postoperative recognition IOL misalignment should be followed rigorously for successful postoperative outcomes.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Dr. Vivek M Singh, Dr. Muralidhar Ramappa, and Dr. Somasheila I Murthy have no financial disclosures.
Dr. Audrey Rostov is a consultant for Johnson and Johnson, Alcon, and Bausch and Lomb.
Conflicts of interest
There are no conflicts of interest.
Funding: Hyderabad Eye Research Foundation Hyderabad, Telangana, India for Dr. Vivek M Singh, Dr. Muralidhar Ramappa, and Dr. Somasheila I Murthy.
References
- 1.Michelitsch M, Ardjomand N, Vidic B, Wedrich A, Steinwender G. Prevalence and age-related changes of corneal astigmatism in patients before cataract surgery. Ophthalmologe. 2017;114:247–51. doi: 10.1007/s00347-016-0323-8. [DOI] [PubMed] [Google Scholar]
- 2.Prasher P, Sandhu JS. Prevalence of corneal astigmatism before cataract surgery in Indian population. Int Ophthalmol. 2017;37:683–9. doi: 10.1007/s10792-016-0327-z. [DOI] [PubMed] [Google Scholar]
- 3.Chang DF. Mastering Refractive IOLs:The art and Science. SLACK Incorporated; 2008 [Google Scholar]
- 4.Gross RH, Miller KM. Corneal astigmatism after phacoemulsification and lens implantation through unsutured scleral and corneal tunnel incisions. Am J Ophthalmol. 1996;121:57–64. doi: 10.1016/s0002-9394(14)70534-3. [DOI] [PubMed] [Google Scholar]
- 5.Budak K, Friedman NJ, Koch DD. Limbal relaxing incisions with cataract surgery. J Cataract Refract Surg. 1998;24:503–8. doi: 10.1016/s0886-3350(98)80292-7. [DOI] [PubMed] [Google Scholar]
- 6.Gills JP. Treating astigmatism at the time of cataract surgery. Curr Opin Ophthalmol. 2002;13:2–6. doi: 10.1097/00055735-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Goggin M. Reply:Nomogram for limbal relaxing incisions. J Cataract Refract Surg. 2006;32:1408. doi: 10.1016/j.jcrs.2006.03.046. [DOI] [PubMed] [Google Scholar]
- 8.Nichamin LD. Nomogram for limbal relaxing incisions. J Cataract Refract Surg. 2006;32:1408. doi: 10.1016/j.jcrs.2006.03.046. [DOI] [PubMed] [Google Scholar]
- 9.Vickers LA, Gupta PK. Femtosecond laser-assisted keratotomy. Curr Opin Ophthalmol. 2016;27:277–84. doi: 10.1097/ICU.0000000000000267. [DOI] [PubMed] [Google Scholar]
- 10.Shimizu K, Misawa A, editors. San Diego, USA: 1992. Effects of toric IOL 2nd American-International Congress on Cataract, IOL and Refractive Surgery and Congress on Ophthalmic Practice Management. [Google Scholar]
- 11.Patel C, Ormonde S, Rosen PH, Bron AJ. Postoperative intraocular lens rotation:A randomized comparison of plate and loop haptic implants. Ophthalmology. 1999;106:2190–6. doi: 10.1016/S0161-6420(99)90504-3. [DOI] [PubMed] [Google Scholar]
- 12.Lane SS, Ernest P, Miller KM, Hileman KS, Harris B, Waycaster CR. Comparison of clinical and patient-reported outcomes with bilateral AcrySof toric or spherical control intraocular lenses. J Refract Surg. 2009;25:899–901. doi: 10.3928/1081597X-20090617-05. [DOI] [PubMed] [Google Scholar]
- 13.Ahmed, II, Rocha G, Slomovic AR, Climenhaga H, Gohill J, Grégoire A, et al. Visual function and patient experience after bilateral implantation of toric intraocular lenses. J Cataract Refract Surg. 2010;36:609–16. doi: 10.1016/j.jcrs.2009.10.044. [DOI] [PubMed] [Google Scholar]
- 14.Holland E, Lane S, Horn JD, Ernest P, Arleo R, Miller KM. The AcrySof Toric intraocular lens in subjects with cataracts and corneal astigmatism:A randomized, subject-masked, parallel-group, 1-year study. Ophthalmology. 2010;117:2104–11. doi: 10.1016/j.ophtha.2010.07.033. [DOI] [PubMed] [Google Scholar]
- 15.Seth SA, Bansal RK, Ichhpujani P, Seth NG. Comparative evaluation of two toric intraocular lenses for correcting astigmatism in patients undergoing phacoemulsification. Indian J Ophthalmol. 2018;66:1423–8. doi: 10.4103/ijo.IJO_73_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dubinsky-Pertzov B, Hecht I, Gazit I, Or L, Mahler O, Rotman S, et al. Clinical outcomes of Ankoris toric intraocular lens implantation using a computer-assisted marker system. Int Ophthalmol. 2020;40:3259–67. doi: 10.1007/s10792-020-01511-4. [DOI] [PubMed] [Google Scholar]
- 17.Bascaran L, Mendicute J, Macias-Murelaga B, Arbelaitz N, Martinez-Soroa I. Efficacy and stability of AT TORBI 709 M toric IOL. J Refract Surg (Thorofare, NJ: 1995) 2013;29:194–9. doi: 10.3928/1081597X-20130129-02. [DOI] [PubMed] [Google Scholar]
- 18.Gerding H, Somfai GM, Langenegger M. A simple, inexpensive, and precise photographic method for intraoperative toric IOL alignment. Klin Monbl Augenheilkd. 2019;236:391–7. doi: 10.1055/a-0861-9601. [DOI] [PubMed] [Google Scholar]
- 19.Chayet A, Sandstedt C, Chang S, Rhee P, Tsuchiyama B, Grubbs R, et al. Use of the light-adjustable lens to correct astigmatism after cataract surgery. Br J Ophthalmol. 2010;94:690–2. doi: 10.1136/bjo.2009.164616. [DOI] [PubMed] [Google Scholar]
- 20.De Silva DJ, Ramkissoon YD, Bloom PA. Evaluation of a toric intraocular lens with a Z-haptic. J Cataract Refract Surg. 2006;32:1492–8. doi: 10.1016/j.jcrs.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 21.Rozema JJ, Gobin L, Verbruggen K, Tassignon MJ. Changes in rotation after implantation of a bag-in-the-lens intraocular lens. J Cataract Refract Surg. 2009;35:1385–8. doi: 10.1016/j.jcrs.2009.03.037. [DOI] [PubMed] [Google Scholar]
- 22.Jung NY, Lim DH, Hwang SS, Hyun J, Chung TY. Comparison of clinical outcomes of toric intraocular lens, Precizon vs Tecnis:A single center randomized controlled trial. BMC Ophthalmol. 2018;18:292. doi: 10.1186/s12886-018-0955-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Till JS, Yoder PR, Jr, Wilcox TK, Spielman JL. Toric intraocular lens implantation:100 consecutive cases. J Cataract Refract Surg. 2002;28:295–301. doi: 10.1016/s0886-3350(01)01035-5. [DOI] [PubMed] [Google Scholar]
- 24.Ferreira TB, Almeida A. Comparison of the visual outcomes and OPD-scan results of AMO Tecnis toric and Alcon Acrysof IQ toric intraocular lenses. J Refract Surg (Thorofare, NJ: 1995) 2012;28:551–5. doi: 10.3928/1081597X-20120703-03. [DOI] [PubMed] [Google Scholar]
- 25.Alberdi T, Macías-Murelaga B, Bascarán L, Goñi N, de Arregui SS, Mendicute J. Rotational stability and visual quality in eyes with Rayner toric intraocular lens implantation. J Refract Surg (Thorofare, NJ: 1995) 2012;28:696–701. doi: 10.3928/1081597X-20120921-04. [DOI] [PubMed] [Google Scholar]
- 26.Gyöngyössy B, Jirak P, Schönherr U. Long-term rotational stability and visual outcomes of a single-piece hydrophilic acrylic toric IOL:A 1.5-year follow-up. Int J Ophthalmol. 2017;10:573–8. doi: 10.18240/ijo.2017.04.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Razmjoo H, Ghoreishi M, Milasi AM, Peyman A, Jafarzadeh Z, Mohammadinia M, et al. Toric intraocular lens for astigmatism correction in cataract patients. Adv Biomed Res. 2017;6:123. doi: 10.4103/2277-9175.216777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garzón N, Poyales F, de Zárate BO, Ruiz-García JL, Quiroga JA. Evaluation of rotation and visual outcomes after implantation of monofocal and multifocal toric intraocular lenses. J Refract Surg (Thorofare, NJ: 1995) 2015;31:90–7. doi: 10.3928/1081597X-20150122-03. [DOI] [PubMed] [Google Scholar]
- 29.Piovella M, Colonval S, Kapp A, Reiter J, Van Cauwenberge F, Alfonso J. Patient outcomes following implantation with a trifocal toric IOL:Twelve-month prospective multicentre study. Eye (London, England) 2019;33:144–53. doi: 10.1038/s41433-018-0076-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mojzis P, Majerova K, Plaza-Puche AB, Hrckova L, Alio JL. Visual outcomes of a new toric trifocal diffractive intraocular lens. J Cataract Refract Surg. 2015;41:2695–706. doi: 10.1016/j.jcrs.2015.07.033. [DOI] [PubMed] [Google Scholar]
- 31.Chiam PJ, Quah SA. The refractive outcome of Toric Lentis Mplus implant in cataract surgery. Int J Ophthalmol. 2016;9:699–702. doi: 10.18240/ijo.2016.05.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shimoda T, Shimoda G, Hida WT, Nakano CT, Motta AF, Guimarães AS, et al. Visual outcomes after implantation of a novel refractive toric multifocal intraocular lens. Arq Brasil Oftalmol. 2014;77:71–5. doi: 10.5935/0004-2749.20140018. [DOI] [PubMed] [Google Scholar]
- 33.Ribeiro FJ, Ferreira TB. Comparison of visual and refractive outcomes of 2 trifocal intraocular lenses. J Cataract Refract Surg. 2020;46:694–9. doi: 10.1097/j.jcrs.0000000000000118. [DOI] [PubMed] [Google Scholar]
- 34.Kohnen T, Lwowski C, Hinzelmann L, Ahmad W, Petermann K, Hemkeppler E, et al. Presbyopia correction in astigmatic eyes using a toric trifocal intraocular lens with quadrifocal technology. J Refract Surg (Thorofare, NJ: 1995) 2020;36:638–44. doi: 10.3928/1081597X-20200729-04. [DOI] [PubMed] [Google Scholar]
- 35.Marques EF, Ferreira TB, Simões P. Visual performance and rotational stability of a multifocal toric intraocular lens. J Refract Surg (Thorofare, NJ: 1995) 2016;32:444–50. doi: 10.3928/1081597X-20160502-01. [DOI] [PubMed] [Google Scholar]
- 36.Gundersen KG. Rotational stability and visual performance 3 months after bilateral implantation of a new toric extended range of vision intraocular lens. Clin Ophthalmol (Auckland, NZ) 2018;12:1269–78. doi: 10.2147/OPTH.S173120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Epitropoulos AT. Visual and refractive outcomes of a toric presbyopia-correcting intraocular lens. J Ophthalmol. 2016;2016:7458210. doi: 10.1155/2016/7458210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nanavaty MA, Lake DB, Daya SM. Outcomes of pseudophakic toric intraocular lens implantation in keratoconic eyes with cataract. J Refract Surg. 2012;28:884–90. doi: 10.3928/1081597X-20121106-02. [DOI] [PubMed] [Google Scholar]
- 39.Gao Y, Ye Z, Chen W, Li J, Yan X, Li Z. Management of cataract in patients with irregular astigmatism with regular central component by phacoemulsification combined with toric intraocular lens implantation. J Ophthalmol. 2020;2020:3520856. doi: 10.1155/2020/3520856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matalia H, Nandini C, Matalia J. Long-term outcome of custom toric intraocular lens for treating high astigmatism in case of cataract associated with pellucid marginal corneal degeneration. Indian J Ophthalmol. 2020;68:3082–4. doi: 10.4103/ijo.IJO_2943_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stewart CM, McAlister JC. Comparison of grafted and non-grafted patients with corneal astigmatism undergoing cataract extraction with a toric intraocular lens implant. Clin Exp Ophthalmol. 2010;38:747–57. doi: 10.1111/j.1442-9071.2010.02336.x. [DOI] [PubMed] [Google Scholar]
- 42.Srinivasan S, Ting DS, Lyall DA. Implantation of a customized toric intraocular lens for correction of post-keratoplasty astigmatism. Eye (London, England) 2013;27:531–7. doi: 10.1038/eye.2012.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ra H, Hwang HS, Kim HS, Kim MS, Kim EC. Toric intraocular lens implantation in cataract patients with corneal opacity. BMC Ophthalmol. 2020;20:98. doi: 10.1186/s12886-020-01352-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Althomali TA. Posterior chamber toric phakic IOL implantation for the management of pediatric anisometropic amblyopia. J Refract Surg. 2013;29:396–400. doi: 10.3928/1081597X-20130410-01. [DOI] [PubMed] [Google Scholar]
- 45.Ram J, Singh R, Gupta R, Bhutani G, Gupta PC, Sukhija J. Toric intraocular lens implantation in children with developmental cataract and preexisting corneal astigmatism. Acta Ophthalmol. 2017;95:e95–100. doi: 10.1111/aos.13220. [DOI] [PubMed] [Google Scholar]
- 46.Gwiazda J, Scheiman M, Mohindra I, Held R. Astigmatism in children:Changes in axis and amount from birth to six years. Invest Ophthalmol Vis Sci. 1984;25:88–92. [PubMed] [Google Scholar]
- 47.Abrahamsson M, Fabian G, Sjöstrand J. Changes in astigmatism between the ages of 1 and 4 years:A longitudinal study. Br J Ophthalmol. 1988;72:145–9. doi: 10.1136/bjo.72.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baldwin WR, Mills D. A longitudinal study of corneal astigmatism and total astigmatism. Am J Optom Physiol Opt. 1981;58:206–11. doi: 10.1097/00006324-198103000-00004. [DOI] [PubMed] [Google Scholar]
- 49.Ehrlich DL, Braddick OJ, Atkinson J, Anker S, Weeks F, Hartley T, et al. Infant emmetropization:Longitudinal changes in refraction components from nine to twenty months of age. Optom Vis Sci. 1997;74:822–43. doi: 10.1097/00006324-199710000-00022. [DOI] [PubMed] [Google Scholar]
- 50.Varghese RM, Sreenivas V, Puliyel JM, Varughese S. Refractive status at birth:Its relation to newborn physical parameters at birth and gestational age. PLoS One. 2009;4:e4469. doi: 10.1371/journal.pone.0004469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.MARIN-AMART M. Physiological Variations of cornea curvature during life time;their importance and transcendence into ocular refraction. Bull Soc Belge Ophthalmol. 1956;113:251–93. [PubMed] [Google Scholar]
- 52.Grosvenor TP, Flom MC. Refractive Anomalies:Research and Clinical Applications. Butterworth-Heinemann Medical. 1991 [Google Scholar]
- 53.Artal P, Guirao A, Berrio E, Williams DR. Compensation of corneal aberrations by the internal optics in the human eye. J Vis. 2001;1:1–8. doi: 10.1167/1.1.1. [DOI] [PubMed] [Google Scholar]
- 54.Faramarzi A, Soheilian M, Jabbarpoor Bonyadi MH, Yaseri M. Corneal astigmatism in unilateral Fuchs heterochromic iridocyclitis. Ocul Immunol Inflamm. 2011;19:151–5. doi: 10.3109/09273948.2011.555054. [DOI] [PubMed] [Google Scholar]
- 55.Park CY, Oh SY, Chuck RS. Measurement of angle kappa and centration in refractive surgery. Curr Opin Ophthalmol. 2012;23:269–75. doi: 10.1097/ICU.0b013e3283543c41. [DOI] [PubMed] [Google Scholar]
- 56.Piracha AR. Using angle alpha in premium IOL screening. Cataract Refract Surg Today. 2016;3:24–5. [Google Scholar]
- 57.Cheng LS, Tsai CY, Tsai RJ, Liou SW, Ho JD. Estimation accuracy of surgically induced astigmatism on the cornea when neglecting the posterior corneal surface measurement. Acta Ophthalmol. 2011;89:417–22. doi: 10.1111/j.1755-3768.2009.01732.x. [DOI] [PubMed] [Google Scholar]
- 58.Royston J, Dunne M, Barnes D. Measurement of posterior corneal surface toricity. Optom Vis Sci. 1990;67:757–63. doi: 10.1097/00006324-199010000-00002. [DOI] [PubMed] [Google Scholar]
- 59.Dunne M, Royston JM, Barnes DA. Posterior corneal surface toricity and total corneal astigmatism. Optom Vis Sci. 1991;68:708–10. doi: 10.1097/00006324-199109000-00006. [DOI] [PubMed] [Google Scholar]
- 60.Dubbelman M, Sicam V, Van der Heijde G. The shape of the anterior and posterior surface of the aging human cornea. Vis Res. 2006;4:993–1001. doi: 10.1016/j.visres.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 61.Prisant O, Hoang-Xuan T, Proano C, Hernandez E, Awad S, Azar DT. Vector summation of anterior and posterior corneal topographical astigmatism. J Cataract Refract Surg. 2002;28:1636–43. doi: 10.1016/s0886-3350(01)01258-5. [DOI] [PubMed] [Google Scholar]
- 62.Módis L, Jr, Langenbucher A, Seitz B. Evaluation of normal corneas using the scanning-slit topography/pachymetry system. Cornea. 2004;23:689–94. doi: 10.1097/01.ico.0000126315.05519.0b. [DOI] [PubMed] [Google Scholar]
- 63.Koch DD, Ali SF, Weikert MP, Shirayama M, Jenkins R, Wang L. Contribution of posterior corneal astigmatism to total corneal astigmatism. J Cataract Refract Surg. 2012;38:2080–7. doi: 10.1016/j.jcrs.2012.08.036. [DOI] [PubMed] [Google Scholar]
- 64.Reitblat O, Levy A, Kleinmann G, Abulafia A, Assia EI. Effect of posterior corneal astigmatism on power calculation and alignment of toric intraocular lenses:Comparison of methodologies. J Cataract Refract Surg. 2016;42:217–25. doi: 10.1016/j.jcrs.2015.11.036. [DOI] [PubMed] [Google Scholar]
- 65.Srivannaboon S, Soeharnila, Chirapapaisan C, Chonpimai P. Comparison of corneal astigmatism and axis location in cataract patients measured by total corneal power, automated keratometry, and simulated keratometry. J Cataract Refract Surg. 2012;38:2088–93. doi: 10.1016/j.jcrs.2012.07.024. [DOI] [PubMed] [Google Scholar]
- 66.Visser N, Berendschot TT, Verbakel F, de Brabander J, Nuijts RM. Comparability and repeatability of corneal astigmatism measurements using different measurement technologies. J Cataract Refract Surg. 2012;38:1764–70. doi: 10.1016/j.jcrs.2012.05.036. [DOI] [PubMed] [Google Scholar]
- 67.Koch DD, Jenkins RB, Weikert MP, Yeu E, Wang L. Correcting astigmatism with toric intraocular lenses:Effect of posterior corneal astigmatism. J Cataract Refract Surg. 2013;39:1803–9. doi: 10.1016/j.jcrs.2013.06.027. [DOI] [PubMed] [Google Scholar]
- 68.Visser N, Berendschot TT, Bauer NJ, Nuijts RM. Vector analysis of corneal and refractive astigmatism changes following toric pseudophakic and toric phakic IOL implantation. Invest Ophthalmol Vis Sci. 2012;53:1865–73. doi: 10.1167/iovs.11-8868. [DOI] [PubMed] [Google Scholar]
- 69.Uckmann MS, Stattin M, Zehetner C, Neururer S, Speicher L. [Comparison of two optical biometric devices for intraocular lens calculation] Ophthalmologe. 2019;116:253–60. doi: 10.1007/s00347-018-0655-7. [DOI] [PubMed] [Google Scholar]
- 70.Ortiz A, Galvis V, Tello A, Viaña V, Corrales MI, Ochoa M, et al. Comparison of three optical biometers:IOLMaster 500, Lenstar LS 900 and Aladdin. Int Ophthalmol. 2019;39:1809–18. doi: 10.1007/s10792-018-1006-z. [DOI] [PubMed] [Google Scholar]
- 71.Hui S, Yi L. Comparison of two optical biometers in intraocular lens power calculation. Indian J Ophthalmol. 2014;62:931–4. doi: 10.4103/0301-4738.143930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Melles RB, Holladay JT, Chang WJ. Accuracy of intraocular lens calculation formulas. Ophthalmology. 2018;125:169–78. doi: 10.1016/j.ophtha.2017.08.027. [DOI] [PubMed] [Google Scholar]
- 73.Hayashi K, Hirata A, Manabe S, Hayashi H. Long-term change in corneal astigmatism after sutureless cataract surgery. Am J Ophthalmol. 2011;151:858–65. doi: 10.1016/j.ajo.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 74.Beheregaray S, Goggin M, LaHood B. Astigmatic overcorrection and axis flip for targeting minimal remaining refractive astigmatism with toric intraocular lenses. J Cataract Refract Surg. 2018;44:109–10. doi: 10.1016/j.jcrs.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 75.Hoffmann PC, Auel S, Hütz WW. Results of higher power toric intraocular lens implantation. J Cataract Refract Surg. 2011;37:1411–8. doi: 10.1016/j.jcrs.2011.02.028. [DOI] [PubMed] [Google Scholar]
- 76.Novis C. Astigmatism and toric intraocular lenses. Curr Opin Ophthalmol. 2000;11:47–50. doi: 10.1097/00055735-200002000-00007. [DOI] [PubMed] [Google Scholar]
- 77.Chang J. Cyclotorsion during laser in situ keratomileusis. J Cataract Refract Surg. 2008;34:1720–6. doi: 10.1016/j.jcrs.2008.06.027. [DOI] [PubMed] [Google Scholar]
- 78.Mendicute J, Irigoyen C, Aramberri J, Ondarra A, Montés-Micó R. Foldable toric intraocular lens for astigmatism correction in cataract patients. J Cataract Refract Surg. 2008;34:601–7. doi: 10.1016/j.jcrs.2007.11.033. [DOI] [PubMed] [Google Scholar]
- 79.Ciccio AE, Durrie DS, Stahl JE, Schwendeman F. Ocular cyclotorsion during customized laser ablation. J Refract Surg (Thorofare, NJ: 1995) 2005;21:S772–4. doi: 10.3928/1081-597X-20051101-25. [DOI] [PubMed] [Google Scholar]
- 80.Smith EM, Jr, Talamo JH, Assil KK, Petashnick DE. Comparison of astigmatic axis in the seated and supine positions. J Refract Corneal Surg. 1994;10:615–20. [PubMed] [Google Scholar]
- 81.Ma JJ, Tseng SS. Simple method for accurate alignment in toric phakic and aphakic intraocular lens implantation. J Cataract Refract Surg. 2008;34:1631–6. doi: 10.1016/j.jcrs.2008.04.041. [DOI] [PubMed] [Google Scholar]
- 82.Farooqui JH, Koul A, Dutta R, Shroff NM. Comparison of two different methods of preoperative marking for toric intraocular lens implantation:Bubble marker versus pendulum marker. Int J Ophthalmol. 2016;9:703–6. doi: 10.18240/ijo.2016.05.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bayramlar H, Dag Y, Karadag R, Cakici O. An easy and practical method for toric intraocular lens implantation:Marking corneal astigmatic axis at slit-lamp. Int Ophthalmol. 2017;37:179–84. doi: 10.1007/s10792-016-0250-3. [DOI] [PubMed] [Google Scholar]
- 84.Montes de Oca I, Kim EJ, Wang L, Weikert MP, Khandelwal SS, Al-Mohtaseb Z, et al. Accuracy of toric intraocular lens axis alignment using a 3-dimensional computer-guided visualization system. J Cataract Refract Surg. 2016;42:550–5. doi: 10.1016/j.jcrs.2015.12.052. [DOI] [PubMed] [Google Scholar]
- 85.Woo YJ, Lee H, Kim HS, Kim EK, Seo KY, Kim TI. Comparison of 3 marking techniques in preoperative assessment of toric intraocular lenses using a wavefront aberrometer. J Cataract Refract Surg. 2015;41:1232–40. doi: 10.1016/j.jcrs.2014.09.045. [DOI] [PubMed] [Google Scholar]
- 86.Popp N, Hirnschall N, Maedel S, Findl O. Evaluation of 4 corneal astigmatic marking methods. J Cataract Refract Surg. 2012;38:2094–9. doi: 10.1016/j.jcrs.2012.07.039. [DOI] [PubMed] [Google Scholar]
- 87.Cha D, Kang SY, Kim SH, Song JS, Kim HM. New axis-marking method for a toric intraocular lens:Mapping method. J Refract Surg (Thorofare, NJ: 1995) 2011;27:375–9. doi: 10.3928/1081597X-20101005-01. [DOI] [PubMed] [Google Scholar]
- 88.Dick HB, Schultz T. Laser-assisted marking for toric intraocular lens alignment. J Cataract Refract Surg. 2016;42:7–10. doi: 10.1016/j.jcrs.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 89.Kaur M, Titiyal JS, Shaikh F, Rani D. Femtosecond laser-assisted refractive capsulorhexis-Precise capsulotomy with accurate toric intraocular lens alignment. Indian J Ophthalmol. 2020;68:2562–4. doi: 10.4103/ijo.IJO_1677_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Diakonis VF, Swann BF, Weinstock RJ. Femtosecond laser-assisted capsulotomy markings for the alignment of toric IOLs:A new technique. J Refract Surg (Thorofare, NJ: 1995) 2018;34:711–2. doi: 10.3928/1081597X-20180820-01. [DOI] [PubMed] [Google Scholar]
- 91.Cao D, Xu Y, Wang Y. Comparison of toric intraocular lens alignment between femtosecond laser-assisted capsular marking and manual corneal marking. J Refract Surg (Thorofare, NJ: 1995) 2020;36:536–42. doi: 10.3928/1081597X-20200602-01. [DOI] [PubMed] [Google Scholar]
- 92.Hura AS, Osher RH. Comparing the Zeiss Callisto eye and the Alcon Verion image guided system toric lens alignment technologies. J Refract Surg (Thorofare, NJ: 1995) 2017;33:482–7. doi: 10.3928/1081597X-20170504-02. [DOI] [PubMed] [Google Scholar]
- 93.Pallas A, Yeo TK, Trevenen M, Barrett G. Evaluation of the accuracy of two marking methods and the Novel toriCAM application for toric intraocular lens alignment. J Refract Surg (Thorofare, NJ: 1995) 2018;34:150–5. doi: 10.3928/1081597X-20180115-03. [DOI] [PubMed] [Google Scholar]
- 94.Titiyal JS, Kaur M, Jose CP, Falera R, Kinkar A, Bageshwar LM. Comparative evaluation of toric intraocular lens alignment and visual quality with image-guided surgery and conventional three-step manual marking. Clin Ophthalmol (Auckland, NZ) 2018;12:747–53. doi: 10.2147/OPTH.S164175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Webers VS, Bauer NJ, Visser N, Berendschot T, van den Biggelaar F, Nuijts R. Image-guided system versus manual marking for toric intraocular lens alignment in cataract surgery. J Cataract Refract Surg. 2017;43:781–8. doi: 10.1016/j.jcrs.2017.03.041. [DOI] [PubMed] [Google Scholar]
- 96.Dick H, Krummenauer F, Tröber L. Compensation of corneal astigmatism with toric intraocular lens:Results of a multicentre study. Klin Monbl Augenheilkd. 2006;223:593–608. doi: 10.1055/s-2006-926652. [DOI] [PubMed] [Google Scholar]
- 97.Kaindlstorfer C, Kneifl M, Reinelt P, Schönherr U. Rotation of a toric intraocular lens from neodymium:YAG laser posterior capsulotomy. J Cataract Refract Surg. 2018;44:510–1. doi: 10.1016/j.jcrs.2018.02.018. [DOI] [PubMed] [Google Scholar]
- 98.Lombardo M, Carbone G, Lombardo G, De Santo MP, Barberi R. Analysis of intraocular lens surface adhesiveness by atomic force microscopy. J Cataract Refract Surg. 2009;35:1266–72. doi: 10.1016/j.jcrs.2009.02.029. [DOI] [PubMed] [Google Scholar]
- 99.Chang DF. Comparative rotational stability of single-piece open-loop acrylic and plate-haptic silicone toric intraocular lenses. J Cataract Refract Surg. 2008;34:1842–7. doi: 10.1016/j.jcrs.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 100.Prinz A, Neumayer T, Buehl W, Vock L, Menapace R, Findl O, et al. Rotational stability and posterior capsule opacification of a plate-haptic and an open-loop-haptic intraocular lens. J Cataract Refract Surg. 2011;37:251–7. doi: 10.1016/j.jcrs.2010.08.049. [DOI] [PubMed] [Google Scholar]