Abstract
Introduction
The COVID-19 pandemic has presented population-wide novel stressors. Acceptance and Commitment Therapy (ACT) may be potent for coping with novel, unpredictable stressors, but it is unknown whether pre-pandemic ACT treatment conferred protective benefit during the COVID-19 pandemic.
Methods
Participants (N = 73) from a previous randomized controlled trial of ACT (seven 2-h group sessions) versus minimally-enhanced usual care (MEUC) for anxious cancer survivors completed measures of anxiety symptoms, fear of cancer recurrence, and emotional approach coping during the trial and again during the pandemic in May, June/July, and November 2020, an average of 2.71 years after completing ACT or MEUC. We estimated hierarchical linear models to test overall and conditional differences over the trial timepoints, in the interval between the trial and May 2020, and between the pandemic timepoints.
Results
Compared to MEUC, ACT led to greater improvement on the outcomes during the 8-month trial follow-up, consistent with the main trial findings. Across the entire sample, anxiety symptoms and emotional approach coping worsened from the final trial assessment timepoint to May 2020 (ps < .001). During this period, ACT participants worsened significantly more on emotional approach coping (p = .035) than MEUC participants. No significant condition differences emerged at later pandemic timepoints.
Conclusions
Treatment with ACT several years earlier did not provide protective benefit to anxious cancer survivors during the pandemic, relative to MEUC. ACT interventions may need to be targeted to pandemic-specific stressors, or booster sessions may be required for prior ACT treatment completers when faced with novel stressors.
Keywords: Acceptance and commitment therapy, COVID-19, Pandemic, Psychotherapy, Anxiety, Fear of recurrence
1. Introduction
The COVID-19 pandemic has caused increased psychological distress across virtually all segments of society (Daly & Robinson, 2021; Taylor et al., 2020). Cancer survivors, due to their previous medical conditions, being older on average, and financial and employment-related impacts of cancer, may be experiencing especially heightened distress during the pandemic (Al-Shamsi et al., 2020; Chen et al., 2021). Psychological interventions for pandemic-related distress remain needed for cancer populations (e.g., Agyapong et al., 2020).
Acceptance and Commitment Therapy (ACT) is a promising intervention for pandemic-related distress because it promotes psychological flexibility in the face of unpredictable, uncontrollable stressors (e.g., Kroska et al., 2020). Many people received ACT or a related behavioral intervention before the pandemic. In developing and disseminating interventions for COVID-19-related distress, a critical question is whether prior treatment with ACT or similar evidence-based interventions provides enduring protective benefit towards new stressors, or whether additional treatment may be needed. Answering this question would also elucidate whether ACT completers can successfully generalize learning from their presenting problem during the ACT intervention to new, post-intervention stressors and contexts. However, to our knowledge, no studies have evaluated how individuals treated with ACT or other evidence-based psychotherapies prior to the COVID-19 pandemic fared during the pandemic, relative to comparable individuals who did not receive the intervention.
The current study is a follow-up to a randomized controlled trial (RCT) of ACT versus minimally enhanced usual care (MEUC) for anxious cancer survivors (Arch et al., 2021). In May, June/July, and November 2020, the follow-up study administered measures of anxiety, fear of cancer recurrence, and coping skill use that showed prior treatment effects during the trial. We evaluated two exploratory research questions: first, consistent with extant findings in cancer populations (Yildirim et al., 2021; Zomerdijk et al., 2021), did participants across conditions experience worsened anxiety, fear of recurrence, and coping skill use during the pandemic? Second, did participants who were randomized to ACT in the original trial experience less deterioration on these outcome measures during the pandemic than those randomized to MEUC?
2. Methods
2.1. Participants and RCT conditions
Participants in the current study had previously participated in the Valued Living RCT (henceforth, the original trial; Arch et al., 2021). In the original trial, participants were randomized to either a 7-week (one 2-h session per week) group ACT intervention delivered by clinical social workers in community oncology clinics or to MEUC.1 Participants completed the ACT intervention or parallel timing in MEUC an average of 2.71 (range 1.43–4.30) years prior to the beginning of the major societal impacts of COVID-19's effects in Colorado, approximated as March 15, 2020.
To be eligible for the original trial, participants must have met the following criteria: 1) completed primary treatment for cancer 1.5–24 months earlier; 2) evidenced no current disease for solid tumor cancers, or for hematologic malignancies, cancer that was asymptomatic or in remission after initial treatment; 3) screened positive for moderate to high cancer-related anxiety and general anxiety or depression symptoms in daily life; 4) proficient in English; and 5) had not started a new antidepressant or anxiolytic medication in the two months prior to enrollment. One hundred thirty-four participants enrolled in the original trial, completed baseline questionnaires, and were randomized. As part of the original trial, participants’ cancer diagnosis and staging was assessed via medical record review.
In May 2020, we invited 122 of the original trial participants to participate in the current study. We did not invite the other twelve participants because we lacked contact information for them, because they moved out of state, they died prior to May 2020, or because they did not wish to be recontacted. Eighty participants (65.57% of those invited) consented and completed the survey. Of those, seven had been randomized to MEUC but took part in the ACT intervention after completing the original trial (as offered to all MEUC participants), and were thus excluded from the current analytic sample (N = 73). The original trial and the current follow-up study were approved by the University of Colorado Boulder institutional review board.
Table 1 presents the sociodemographic and medical data from the current study sample, which did not differ between conditions (ACT n = 38; MEUC n = 35).2 Table 1 also compares original trial participants who consented to the current follow-up study versus those who did not; the only significant difference is that current study participants were more likely to have been initially diagnosed with Stage 0 or I cancer (p =.007) than later-stage cancer.
Table 1.
Baseline characteristics.
| Original Trial Participants Who Consented or Not to the COVID Surveys |
Current Sample by RCT Condition |
|||||
|---|---|---|---|---|---|---|
| Category | Yesa | No | p | ACT | MEUC | p |
| N | 73 | 54 | 38 | 35 | ||
| Years between end of ACT treatment or parallel observational window and beginning of COVID-19 Pandemic – M (SD) | 2.71 (1.04) | 2.77 (0.97) | .754 | 2.81 (1.01) | 2.60 (1.07) | .398 |
| Demographic Variables | ||||||
| Age at Original Trial Enrollment – M (SD) | 55.36 (11.09) | 57.52 (12.42) | .304 | 55.95 (10.50) | 54.71 (11.83) | .638 |
| Female - % (N) | 90.41 (66) | 85.19 (46) | .533 | 89.47 (34) | 91.43 (32) | >.999 |
| White, Non-Latinx Race/Ethnicity - % (N) | 84.93 (62) | 90.38 (47) | .530 | 92.11 (35) | 77.14 (27) | .145 |
| Annual Income at VL enrollment - % (N) | ||||||
| $0–40,00 | 24.66 (18) | 42.59 (23) | 21.05 (8) | 28.57 (10) | ||
| $41–60,000 | 15.07 (11) | 14.81 (8) | 15.79 (6) | 14.29 (5) | ||
| $61–80,000 | 16.44 (12) | 14.81 (8) | 23.68 (9) | 8.57 (3) | ||
| $81,000+ | 43.84 (32) | 27.78 (15) | 39.47 (15) | 48.57 (17) | ||
| Annual Income at least $61,000 at VL enrollment - % (N) | 60.27 (44) | 42.59 (23) | .073 | 63.16 (24) | 57.14 (20) | .775 |
| Education at VL Enrollment - % (N) | ||||||
| Less than Bachelor's Degree | 32.88 (24) | 50.00 (27) | 31.58 (12) | 34.29 (12) | ||
| Bachelor's Degree | 32.88 (24) | 29.63 (16) | 36.84 (14) | 28.57 (10) | ||
| Graduate or Professional Degree | 34.25 (25) | 20.37 (11) | 31.58 (12) | 37.14 (13) | ||
| At Least Bachelor's Degree - % (N) | 67.12 (49) | 50.00 (27) | .078 | 68.42 (26) | 65.71 (23) | >.999 |
| Initial Cancer Diagnostics | ||||||
| Initial diagnosis Stage 0-I - % (N)b | 53.97 (34) | 26.09 (12) | .007 | 57.58 (19) | 50.00 (15) | .727 |
| Breast Cancer (vs other primary) - % (N) | 64.38 (47) | 50.94 (27) | .184 | 63.16 (24) | 65.71 (23) | >.999 |
| Outcome Variables Scores at Original Trial Pre-Intervention | ||||||
| GAD-7 – M (SD) | 9.47 (5.01) | 8.15 (4.40) | .125 | 10.26 (5.32) | 8.59 (4.55) | .158 |
| CARS – M (SD) | 4.15 (1.07) | 4.13 (1.19) | .927 | 4.37 (0.88) | 3.92 (1.22) | .075 |
| EAC – M (SD) | 2.42 (0.74) | 2.49 (0.74) | .608 | 2.31 (0.66) | 2.55 (0.82) | .182 |
Note. Demographic data displayed reflect participant responses upon their enrollment in the Valued Living Study. The post-intervention assessment occurred 1 week after completion of the ACT intervention, with parallel timing in the MEUC group. The beginning of the COVID-19 pandemic is defined as March 15, 2020, since that date was approximately when local virus-related restrictions on businesses and movement outside the home were enacted in [US state omitted for blind review]. Between-group dichotomous variable differences were examined with χ2 tests and continuous variable differences were examined with independent t-tests.
CARS = Concerns About Recurrence Scale; EAC = Emotional Approach Coping scale; GAD-7 = Generalized Anxiety Disorder-7 scale.
Seven additional participants enrolled in the follow-up study but, after completing MEUC, subsequently participated in the Valued Living intervention. Thus, data from these seven are not analyzed in the current COVID-19 pandemic follow-up study.
Cancer staging data could not be obtained from the medical chart for ten participants in the current sample, and eight participants enrolled in the Valued Living RCT but not in the current study.
To establish whether the current study sample was broadly reflective of the full original RCT sample, we examined response to the intervention in both the original RCT sample and the current study sample (see Supplemental Methods and Results). Estimates and bootstrapped confidence intervals (Supplemental Table 1) of overall slopes and conditional slope differences yielded the same patterns of intervention effects in the full RCT sample and the current study sample, with the exception that the intervention effect on anxiety symptoms was significant in the current sample (p = .009) but marginal in the full RCT sample (p = .075). Thus, the intervention effect on general anxiety symptoms appears to have been somewhat stronger in the current study sample than the full RCT sample; otherwise, findings were very similar.
2.2. Measures
To examine within-person longitudinal change spanning the trial and pandemic periods, the current study analyzed data from three measures that were administered both during the original trial and in the current COVID-19 pandemic follow-up study. Current study participants also completed other measures at the pandemic assessment timepoints as part of data collection for unrelated studies, but we did not analyze data from those unrelated studies in the current study.
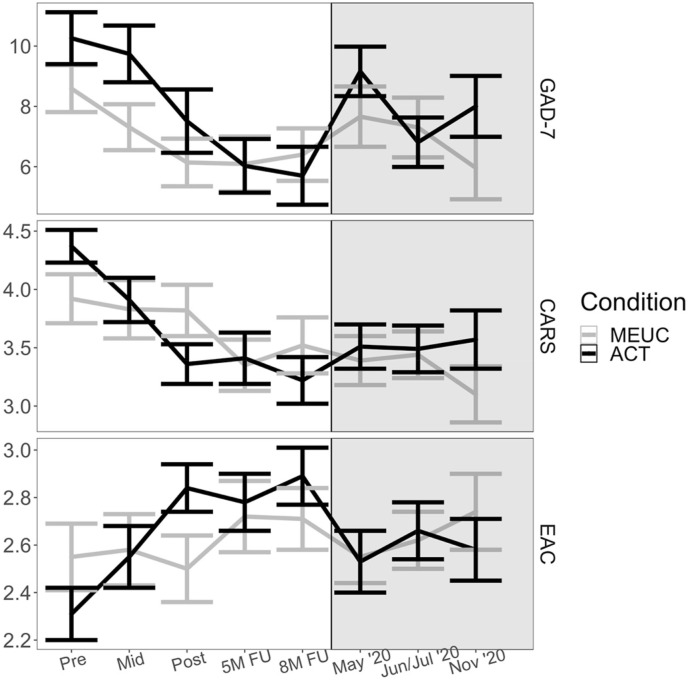
Each of the three measures analyzed in the current study was administered at five timepoints during the original trial: Pre-intervention, Mid-intervention (four weeks after Pre), Post-intervention (one week after ACT, with parallel timing in MEUC), 5-month post-randomization follow-up, and 8-month post-randomization follow-up. During the COVID-19 pandemic follow-up study, the measures were administered in May, June or July (henceforth June/July), and November 2020,3 per Fig. 1 . We assessed reliability at the trial pre-intervention timepoint using ωTotal, which is interpreted similarly to Cronbach's α but is widely considered to be psychometrically superior (e.g., McNeish, 2018).
Fig. 1.
Outcome Variable Means and Standard Errors During the Original Trial and the COVID-19 Pandemic. Note. Pre, Mid, Post, 5M FU, and 8M FU data were collected during the original trial (i.e., the Valued Living RCT; white background), and data from May 2020, June/July 2020, and November 2020 were collected during the COVID-19 pandemic (grey background). Bars = ±1 standard error. 5M FU = RCT five-month post-randomization follow-up; 8M FU = RCT eight-month post-randomization follow-up; ACT = Acceptance and Commitment Therapy; CARS = Concerns About Recurrence Scale; EAC = Emotional Approach Coping scale; GAD-7 = Generalized Anxiety Disorder-7 scale; MEUC = Minimally-enhanced usual care; Mid = Mid-intervention (four weeks after Pre); Pre = Pre-intervention; Post = Post-intervention (eight weeks after Pre).
The examined outcomes include two measures of anxiety: the Generalized Anxiety Disorder-7 scale (GAD-7, ωTotal = 0.90; Spitzer et al., 2006), which measures symptoms of GAD and other anxiety disorders (Beard & Björgvinsson, 2014),4 and the four-item Overall Fear Scale from the Concerns About Recurrence Scale (CARS, ωTotal = 0.84; Vickberg, 2003), which measures cancer recurrence fears. Higher scores indicate greater symptoms or fear on these measures. The third examined outcome is use of emotional approach coping (EAC), a strategy wherein stressors are managed by processing and expressing emotions about them, with the 8-item EAC scale (ωTotal = 0.93; Stanton et al., 2000). Higher scores on this measure indicate greater EAC use. Improvement in EAC marginally or significantly mediated intervention effects in the original trial, indicating that it is mechanistically linked to anxiety maintenance in the current sample (Fishbein et al., 2021).
2.3. Analytic approach
We examined change in each outcome over the eight assessment timepoints using hierarchical linear models (HLMs), estimated in R (R Core Team, 2020). The modeling syntax is available at https://osf.io/exuyb. In all analyses, we used a two-tailed level of 0.05 to identify statistically significant effects.
Change over time was captured using four coded slope predictors (Table 2 ). The first slope code captured linear change during the original trial, following the approach used to analyze that trial's main outcomes (Arch et al., 2021). The three other slope codes captured changes from the last timepoint in the original trial to May 2020 (Δ May 2020), from May to June/July 2020 (Δ June/July 2020), and from June/July to November 2020 (Δ Nov 2020).5
Table 2.
Values of coded predictors at each timepoint.
|
Predictor |
Original Trial |
COVID-19 Follow-up Study |
||||||
|---|---|---|---|---|---|---|---|---|
| Pre | Mid | Post | 5mFU | 8mFU | May 2020 | June/July 2020 | November 2020 | |
| RCT Slope | −4 | −3 | −2 | −1 | 0 | 0 | 0 | 0 |
| Δ May 2020 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 |
| Δ June/July 2020 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Δ Nov 2020 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Note. Four coded predictors were entered into hierarchical linear models (see Table 3) to model data from the eight assessment timepoints.
5mFU = RCT five-month post-randomization follow-up; 8mFU = RCT eight-month post-randomization follow-up; Mid = Mid-intervention = Pre-intervention; Post = Post-intervention, see Methods for timing.
Two-level HLMs6 with random effects included for all coded slope predictors failed to converge; we thus sequentially dropped random effects until the models converged (e.g., Grimm et al., 2016). The GAD-7 model converged when only the random effect for the intercept and ΔMay 2020 was included. The CARS and EAC models converged with random effects for models with additional (but not all of) the slope predictors, but the findings were similar to models with random effects for only the intercept and ΔMay 2020. Thus, for ease of comparison, the results described below reflect models with random effects included for the intercept and ΔMay 2020.
Because participants were randomized to ACT or MEUC at different times depending on their original trial enrollment date, the models included a moderating variable capturing the time interval in years, grand mean-centered, between the participant's original trial Post-intervention assessment and March 15, 2020, the approximate start date for the COVID-19 pandemic's major societal impact in Colorado. We modeled two- and three-way interactions between the moderating variable and the COVID-19 coded slope predictors, though observed no significant three-way interactions. As a sensitivity analysis, we also ran unmoderated versions of the models. We computed 95% confidence intervals from 5000 nonparametric bootstraps of each model to ascertain the range of plausible values of model parameters (Efron and Tibshirani, 1993, p. 157).
Results of the moderated (Table 3 ) and unmoderated (Supplemental Table 2) models yielded the same patterns regarding overall and conditional change over time. Thus, for brevity, the results of the moderated models are described below.
Table 3.
Fixed effects estimates of moderated two-level models.
| Parameter | Outcome Variable |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GAD7 |
CARS |
EAC |
||||||||||
| Estimate | SE | 95% CI | P | Estimate | SE | 95% CI | P | Estimate | SE | 95% CI | p | |
| Intercept | 5.51 | 0.62 | [4.28, 6.60] | <.001 | 3.27 | 0.15 | [3.00, 3.54] | <.001 | 2.83 | 0.08 | [2.67, 2.98] | <.001 |
| Condition | −0.78 | 1.24 | [-3.18, 1.70] | .530 | −0.41 | 0.29 | [-0.95, 0.14] | .159 | 0.25 | 0.17 | [-0.07, 0.56] | .152 |
| Moderator | 0.23 | 0.48 | [-0.61, 1.17] | .633 | 0.10 | 0.11 | [-0.10, 0.31] | .371 | 0.09 | 0.08 | [-0.04, 0.21] | .251 |
| Condition x Moderator |
−0.76 | 0.95 | [-2.30, 1.03] | .425 | −0.01 | 0.22 | [-0.40, 0.40] | .965 | 0.16 | 0.16 | [-0.09, 0.42] | .304 |
| RCT Slope | −0.94 | 0.16 | [-1.32, −0.58] | <.001 | −0.20 | 0.04 | [-0.28, −0.12] | <.001 | 0.10 | 0.01 | [0.06, 0.14] | <.001 |
| RCT Slope x Condition |
−0.77 | 0.32 | [-1.56, −0.01] | .020 | −0.17 | 0.08 | [-0.34, 0.01] | .027 | 0.10 | 0.03 | [0.02, 0.18] | <.001 |
| Δ May 2020 | 3.03 | 0.59 | [1.55, 4.66] | <.001 | 0.18 | 0.14 | [-0.11, 0.47] | .198 | −0.29 | 0.07 | [-0.46, -0.13] | <.001 |
|
Δ May 2020 x Condition |
2.00 | 1.18 | [-1.21, 5.03] | .093 | 0.50 | 0.27 | [-0.08, 1.11] | .066 | −0.29 | 0.14 | [-0.62, 0.03] | .035 |
| Δ May 2020 x Moderator |
0.91 | 0.51 | [-0.41, 2.27] | .078 | 0.02 | 0.11 | [-0.21, 0.23] | .834 | 0.03 | 0.06 | [-0.10, 0.16] | .605 |
| Δ May 2020 x Condition x Moderator |
−1.13 | 1.02 | [-4.00, 1.31] | .270 | 0.06 | 0.22 | [-0.45, 0.46] | .774 | −0.06 | 0.12 | [-0.32, 0.21] | .624 |
| Δ June/July 2020 | −1.28 | 0.57 | [-2.90, 0.26] | .027 | 0.04 | 0.12 | [-0.19, 0.27] | .734 | 0.10 | 0.06 | [-0.05, 0.26] | .101 |
|
Δ June/July 2020 x Condition |
−1.60 | 1.15 | [-4.85, 1.82] | .164 | −0.07 | 0.23 | [-0.53, 0.39] | .747 | 0.08 | 0.12 | [-0.20, 0.41] | .508 |
| Δ June/July 2020 x Moderator |
0.05 | 0.56 | [-1.74, 1.65] | .930 | −0.08 | 0.11 | [-0.27, 0.13] | .487 | −0.02 | 0.06 | [-0.16, 0.13] | .727 |
| Δ June/July 2020 x Condition x Moderator |
0.03 | 1.11 | [-2.89, 3.74] | .975 | −0.17 | 0.22 | [-0.54, 0.27] | .445 | −0.07 | 0.12 | [-0.38, 0.21] | .557 |
| Δ November 2020 | 0.06 | 0.62 | [-1.69, 1.87] | .924 | 0.02 | 0.13 | [-0.25, 0.34] | .865 | −0.02 | 0.07 | [-0.19, 0.18] | .806 |
|
Δ November 2020 x Condition |
2.13 | 1.25 | [-1.42, 5.65] | .089 | 0.25 | 0.25 | [-0.33, 0.88] | .329 | −0.05 | 0.13 | [-0.44, 0.30] | .723 |
| Δ November 2020 x Moderator |
0.45 | 0.60 | [-1.50, 2.52] | .451 | 0.15 | 0.12 | [-0.11, 0.44] | .223 | −0.02 | 0.06 | [-0.20, 0.19] | .789 |
| Δ November 2020 x Condition x Moderator |
0.65 | 1.20 | [-3.45, 4.37] | .586 | 0.11 | 0.24 | [-0.48, 0.63] | .661 | 0.11 | 0.13 | [-0.29, 0.51] | .406 |
Note. Parameter estimates reflect two-level hierarchical linear models with random effects included for the intercept and the Δ May 2020 terms. Separate models were estimated for each outcome variable. The moderating variable was the interval in years/months between a participant's completion of the Post-intervention assessment and March 15, 2020, grand mean-centered. Bolding highlights the tests of primary interest. 95% CIs were computed based on percentile bootstrap estimates (5000 replicates).
CARS = Concerns About Recurrence Scale; EAC = Emotional Approach Coping scale; GAD-7 = Generalized Anxiety Disorder-7 scale.
3. Results
3.1. Anxiety symptoms
Over the five original trial timepoints, participants across conditions displayed significant declines in GAD-7 scores (p < .001; Table 3), with greater improvement in the ACT condition than the MEUC condition (p = .020). In contrast, from the 8-month trial follow-up to May 2020, participants across conditions evidenced increased GAD-7 scores (p <.001). ACT and MEUC did not differ in GAD-7 score change from the 8-month original trial follow-up to May 2020 (p = .093). Overall sample GAD-7 scores subsequently decreased from May to June/July 2020 (p = .027) but not from June/July to November 2020 (p =.924). There were no significant condition differences in change between pandemic timepoints (ps ≥ .089), and the three-way interactions of time interval predictors with condition and time since trial participation were non-significant (ps ≥ .270).
3.2. Fear of cancer recurrence
Over the five original trial timepoints, participants’ CARS (fear of cancer recurrence) scores improved overall (p < .001), and more so in the ACT condition (p = .027; Table 3). Across conditions, CARS scores did not significantly change from the 8-month trial follow-up to May 2020 (p = .198), and conditions did not significantly differ in CARS score change from the trial 8-month follow-up to May 2020 (p = .066). No further overall change or change by condition was evident during the COVID-19 pandemic (ps ≥ .329), and three-way interactions with time since trial participation were likewise non-significant (ps ≥ .445).
3.3. Emotional approach coping
Over the five original trial timepoints, participants showed overall increases on EAC (p < .001), and ACT participants increased significantly more than MEUC participants (p < .001). From the original trial 8-month follow-up to May 2020, EAC scores declined across the sample as a whole (p < .001), and declined significantly more among ACT participants (p = .035). No additional overall changes or condition differences emerged at later COVID-19 timepoints (ps ≥ .101), and three-way interactions with time since trial participation were non-significant (ps ≥ .406).
4. Discussion
The current study evaluated anxiety, fear of cancer recurrence, and emotional approach coping usage in a sample of participants who previously participated in a randomized controlled trial of ACT versus minimally enhanced usual care for anxious cancer survivors.
Regarding overall trends during the pandemic evident in the current study sample, we observed initial deterioration for anxiety and emotional approach coping, followed by either slight improvement or stabilization. By contrast, fear of cancer recurrence did not change during the pandemic relative to before. Next, regarding condition differences, ACT participants experienced similar or more deterioration than MEUC participants. Time since ACT treatment did not significantly moderate condition effects on distress during the pandemic. Thus, though ACT led to greater improvement across the three outcomes during the original trial, it did not appear to protect from deterioration during the pandemic.
It is useful to contextualize the deterioration observed during the pandemic, especially at the first timepoint, in light of the change observed during the original trial period. Across the current study sample, participants improved an average of about 5 points on the GAD-7, a widely-used measure of anxiety symptoms, during the trial period. However, from the last timepoint of the trial period to May 2020, participants’ scores worsened by 3 points on average, meaning that the average participant (irrespective of condition) deteriorated by over half the improvement they had experienced during the original trial. A similar pattern emerged for emotional approach coping scores. Further, GAD-7 scores improved by only about one point on average from May to June/July 2020, and no further significant change occurred in emotional approach coping during the pandemic period. Thus overall, participants appeared to lose, and not gain back, a considerable amount of the clinical improvement they had experienced during the original trial.
Aligning with our sample-wide findings, studies of general North American samples (e.g., Asmundson & Taylor, 2020; Shuster et al., 2021) found higher anxiety scores in May 2020 relative to subsequent pandemic assessment timepoints, and previous research by Yildirim et al. (2021) demonstrated increased anxiety symptoms in cancer patients during the pandemic relative to before. Fear of cancer recurrence may not have increased overall in the present sample because current participants had completed primary cancer treatment several years prior to the pandemic, reducing the salience of recurrence risk, and the pandemic did not present new cancer recurrence risks.
This study is the first to our knowledge that evaluates the effects of a pre-pandemic ACT or related intervention on psychological functioning during the pandemic, compared to an inactive control group. Pre-pandemic ACT (received several years earlier in the original trial) did not confer a protective benefit during the pandemic on examined outcomes. We offer three potential explanations for this finding. First, cancer survivors in the current study participated in the group ACT intervention an average of 2.7 years prior to the beginning of the pandemic (range 1–4 years); thus, ACT's treatment effect may have decayed, particularly in the absence of booster sessions. Second, the ACT intervention was only seven weeks in duration, delivered in group format, and focused largely on cancer survivorship-related concerns. If the intervention had been longer, more individually tailored, or had focused on coping with novel stressors, its effect during the COVID-19 pandemic might have been greater. Finally, ACT participants declined more on emotional approach coping, a variable that was shown to mediate treatment effects in the full trial sample (Fishbein et al., 2021). Following the logic of those mediation findings, it may be that ACT conferred no protection from anxiety outcome deterioration at least in part because ACT participants did not maintain their original trial treatment gains in emotional approach coping. However, we have refrained from conducting follow-up mediation analyses given that the current sample size would likely provide insufficient power for such analyses (Fritz & MacKinnon, 2007).
This study has several unique strengths. Unlike many psychological studies on either general or cancer survivor samples conducted during the pandemic, this study used participants’ pre-pandemic data along with new data collecting during the pandemic, enabling direct estimation of how functioning changed pre-to post-pandemic. Furthermore, the recruitment of previous trial participants enabled causal claims about the effects of being randomized to ACT versus minimally enhanced usual care. Finally, participants completed the first pandemic assessment just six to ten weeks into the pandemic, and again when COVID-19 case counts in Colorado were their highest to that point in the pandemic (November 2020; Colorado Department of Public Health and Environment, 2021). Thus, the data reflect multiple, distinct periods when distress was likely to be elevated during the pandemic.
A key limitation of the current study is that only 60% of the original trial sample completed this follow-up study. The current sample has a greater proportion of early-stage cancer survivors than the overall trial sample; this difference could indicate that current sample participants were physically healthier and had lower likelihood of cancer recurrence. Findings may have differed if additional later-stage cancer survivors had participated. Additionally, although bootstrapped estimates indicated that ACT was unlikely to have outperformed MEUC early in the pandemic (May 2020), plausible values for conditional differences in change later in the pandemic (June/July 2020 and November 2020 timepoints) indicated both the possibility of ACT outperforming MEUC, or vice versa, in terms of clinical change; a larger sample size might have helped to increase precision in these estimates.
The present findings should not be interpreted as implying that ACT is an ineffective treatment, or that it is ineffective for treating pandemic-related distress. Rather, empirically, these findings suggest that treated individuals may not generalize learning in treatment to new contexts (such as a global pandemic) in the absence of explicit practice (e.g. Mystkowski et al., 2002), or that treatment effects may wane. Relatively few anxiety psychotherapy clinical trials report outcomes beyond one-year follow-up (van Dis et al., 2020), though our findings align with Forman and colleagues' (2012) observation of decay in ACT's effects on anxiety at 18 months. Additional research on long-term treatment effects of ACT, and of relatively short, group-based interventions, is needed, and could inform future research on ‘booster’ interventions.
5. Conclusion
In a sample of participants randomized to ACT or minimally enhanced usual care several years prior to the COVID-19 pandemic, anxiety symptoms and use of emotional approach coping worsened when the pandemic began, and prior ACT did not buffer against worsening on these outcomes. Though many cancer survivors receive some form of supportive intervention during their cancer care, the current results suggest that such prior interventions (completed several years earlier) may not be enough to shield them from worsened psychological functioning when encountering novel stressors such as the pandemic. Individuals previously or currently being treated with ACT may need additional COVID-19 pandemic-specific intervention content to buffer against the negative mental health effects of the pandemic.
Funding
The Valued Living study was supported by an American Cancer Society Research Scholar Grant (PI: Arch, RSG-15-020-01–CPPB). Current study data collection was also undertaken as part of National Cancer Institute/NIH-funded project (PI: Arch, R21CA218723).
Declaration of competing interest
Mr. Fishbein declares no conflict of interest. Dr. Arch receives unrelated research funding from AstraZeneca. Dr. Arch also declares that she is a Guest Editor for an upcoming JCBS special issue. This manuscript is not being submitted for consideration in that special issue. Dr. Arch had no involvement in the peer-review of this manuscript, and had no access to information regarding its peer review.
Footnotes
Usual care was minimally enhanced by provision of a list of local oncology support groups and related resources, and encouragement to contact the cancer clinic's social workers for support as needed.
To reduce burden on participants, demographic data were not re-collected in the follow-up study; thus, we present findings from the Valued Living RCT demographic data (Arch et al., 2021).
Participants responded to the first questionnaire between May 6–29, 2020. They were invited to complete the second questionnaire six weeks after their completion date of the first questionnaire, resulting in completion dates ranging between June 17-July 15, 2020. We sent all participants invitations to complete the third questionnaire on the same date, and they completed that questionnaire between November 19-December 3, 2020.
The Valued Living RCT main outcome was the Hospital Anxiety and Depression Scale-Anxiety subscale (HADS-A; Zigmond and Snaith, 1983), a brief scale for anxiety in medical populations. Because the HADS-A was not administered in the COVID-19 pandemic follow-up study, we instead analyzed data from the GAD-7, the only broad anxiety measure administered during both the RCT and the follow-up study. The HADS-A and GAD-7 perform similarly in medical populations (Baker et al., 2018; Esser et al., 2018).
We refrained from fitting a higher-order time function across the final three timepoints because we had no a priori assumptions about how participants' scores would change during the pandemic.
We initially estimated three-level HLMs (observations within participants within cohorts) because participants were treated in in twelve group cohorts in the original trial (Arch et al., 2021). However, the three-level models failed to consistently converge, and when they did converge, had low intraclass correlation coefficients for cohort (ICCs = .01-.03); thus, we proceeded with the better-fitting and more parsimonious 2-level models.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jcbs.2022.03.006.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Agyapong V.I.O., Hrabok M., Shalaby R., Mrklas K., Vuong W., Gusnowski A., Surood S., Greenshaw A.J., Nkire N. Closing the COVID-19 psychological treatment gap for cancer patients in Alberta: Protocol for the implementation and evaluation of Text4Hope-Cancer Care. JMIR Research Protocols. 2020;9(8) doi: 10.2196/20240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al‐Shamsi H.O., Alhazzani W., Alhuraiji A., Coomes E.A., Chemaly R.F., Almuhanna M., Wolff R.A., Ibrahim N.K., Chua M.L.K., Hotte S.J., Meyers B.M., Elfiki T., Curigliano G., Eng C., Grothey A., Xie C. A practical approach to the management of cancer patients during the novel coronavirus disease 2019 (COVID-19) pandemic: An international collaborative group. The Oncologist. 2020;25(6):e936–e945. doi: 10.1634/theoncologist.2020-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arch J.J, Mitchell J.L., Genung S.R., Judd C.M., Andorsky D.J., Bricker J.B., Stanton A.L. Randomized trial of acceptance and commitment therapy for anxious cancer survivors in community clinics: Outcomes and moderators. Journal of Consulting and Clinical Psychology. 2021;89(4):327–340. doi: 10.1037/ccp0000630. [DOI] [PubMed] [Google Scholar]
- Asmundson G.J.G., Taylor S. Coronaphobia revisted: A state-of-the-art on pandemic-related fear, anxiety, and stress. Journal of Anxiety Disorders. 2020;76:102326. doi: 10.1016/j.janxdis.2020.102326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker A.M., Holbrook J.T., Yohannes A.M., Eakin M.N., Sugar E.A., Henderson R.J., Casper A.S., Kaminsky D.A., Rea A.L., Mathews A.M., Que L.G., Ramsdell J.W., Gerald L.B., Wise R.A., Hanania N.A., Hanania N., Sockrider M., Bertrand L., Atik M.…Lancet E. Test performance characteristics of the AIR, GAD-7, and HADS-Anxiety screening questionnaires for anxiety in chronic obstructive pulmonary disease. Annals of the American Thoracic Society. 2018;15(8):926–934. doi: 10.1513/AnnalsATS.201708-631OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard C., Björgvinsson T. Beyond generalized anxiety disorder: Psychometric properties of the GAD-7 in a heterogeneous psychiatric sample. Journal of Anxiety Disorders. 2014;28(6):547–552. doi: 10.1016/j.janxdis.2014.06.002. [DOI] [PubMed] [Google Scholar]
- Chen Y.S., Zhou Z.N., Glynn S.M., Frey M.K., Balogun O.D., Kanis M., Holcomb K., Gorelick C., Thomas C., Christos P.J., Chapman-Davis E. Financial toxicity, mental health, and gynecologic cancer treatment: The effect of the COVID-19 pandemic among low-income women in New York City. Cancer. 2021;127(14):2399–2408. doi: 10.1002/cncr.33537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly M., Robinson E. Psychological distress and adaptation to the COVID-19 crisis in the United States. Journal of Psychiatric Research. 2021;136:603–609. doi: 10.1016/j.jpsychires.2020.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dis E.A.M., van Veen S.C., Hagenaars M.A., Batelaan N.M., Bockting C.L.H., van den Heuvel R.M., Cuijpers P., Engelhard I.M. Long-term outcomes of cognitive behavioral therapy for anxiety-related disorders: A systematic review and meta-analysis. JAMA Psychiatry. 2020;77(3):265. doi: 10.1001/jamapsychiatry.2019.3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efron B., Tibshirani R. Chapman & Hall/CRC; 1993. An introduction to the bootstrap. [Google Scholar]
- Esser P., Hartung T.J., Friedrich M., Johansen C., Wittchen H.-U., Faller H., Koch U., Härter M., Keller M., Schulz H., Wegscheider K., Weis J., Mehnert A. The Generalized Anxiety Disorder Screener (GAD-7) and the anxiety module of the Hospital and Depression Scale (HADS-A) as screening tools for generalized anxiety disorder among cancer patients. Psycho-Oncology. 2018;27(6):1509–1516. doi: 10.1002/pon.4681. [DOI] [PubMed] [Google Scholar]
- Fishbein J.N., Judd C.M., Genung S., Stanton A.L., Arch J.J. Intervention and mediation effects of target processes in a community oncology clinic-based randomized controlled trial of Acceptance and Commitment Therapy [Preprint] 2021. [DOI] [PubMed] [Google Scholar]
- Forman E.M., Shaw J.A., Goetter E.M., Herbert J.D., Park J.A., Yuen E.K. Long-term follow-up of a randomized controlled trial comparing Acceptance and Commitment Therapy and standard cognitive behavior therapy for anxiety and depression. Behavior Therapy. 2012;43(4):801–811. doi: 10.1016/j.beth.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Fritz M.S., MacKinnon D.P. Required sample size to detect the mediated effect. Psychological Science. 2007;18(3):233–239. doi: 10.1111/j.1467-9280.2007.01882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm K.J., Ram N., Estabrook R. Guilford Press; 2016. Growth modeling. [Google Scholar]
- Kroska E.B., Roche A.I., Adamowicz J.L., Stegall M.S. Psychological flexibility in the context of COVID-19 adversity: Associations with distress. Journal of Contextual Behavioral Science. 2020;18:28–33. doi: 10.1016/j.jcbs.2020.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeish D. Thanks coefficient alpha, we’ll take it from here. Psychological Methods. 2018;23(3):412–433. doi: 10.1037/met0000144. [DOI] [PubMed] [Google Scholar]
- Mystkowski J.L., Craske M.G., Echiverri A.M. Treatment context and return of fear in spider phobia. Behavior Therapy. 2002;33(3):399–416. doi: 10.1016/S0005-7894(02)80035-1. [DOI] [PubMed] [Google Scholar]
- Shuster A., O'Brien M., Luo Y., Berner L.A., Perl O., Heflin M., Kulkarni K., Chung D., Na S., Fiore V.G., Gu X. Emotional adaptation during a crisis: Decline in anxiety and depression after the initial weeks of COVID-19 in the United States. Translational Psychiatry. 2021;11(1):1–7. doi: 10.1038/s41398-021-01552-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer R.L., Kroenke K., Williams J.B.W., Löwe B. A brief measure for assessing Generalized Anxiety Disorder: The GAD-7. Archives of Internal Medicine. 2006;166(10):1092. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- Stanton A.L., Kirk S.B., Cameron C.L., Danoff-Burg S. Coping through emotional approach: Scale construction and validation. Journal of Personality and Social Psychology. 2000;78(6):1150–1169. doi: 10.1037/0022-3514.78.6.1150. [DOI] [PubMed] [Google Scholar]
- Taylor S., Landry C.A., Paluszek M.M., Fergus T.A., McKay D., Asmundson G.J.G. COVID stress syndrome: Concept, structure, and correlates. Depression and Anxiety. 2020;37(8):706–714. doi: 10.1002/da.23071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickberg S.M.J. The concerns about recurrence scale (CARS): A systematic measure of women's fears about the possibility of breast cancer recurrence. Annals of Behavioral Medicine. 2003;25(1):16–24. doi: 10.1207/S15324796ABM2501_03. [DOI] [PubMed] [Google Scholar]
- Yildirim O.A., Poyraz K., Erdur E. Depression and anxiety in cancer patients before and during the SARS-CoV-2 pandemic: Association with treatment delays. Quality of Life Research. 2021;30(7):1903–1912. doi: 10.1007/s11136-021-02795-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond A.S., Snaith R.P. The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- Zomerdijk N., Jongenelis M., Short C.E., Smith A., Turner J., Huntley K. Prevalence and correlates of psychological distress, unmet supportive care needs, and fear of cancer recurrence among haematological cancer patients during the COVID-19 pandemic. Supportive Care in Cancer. 2021 doi: 10.1007/s00520-021-06369-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colorado Department of Public Health and Environment. (2021). Cases of COVID-19 in Colorado by Date Reported to the State. Retrieved September 2, 2021, from https://covid19.colorado.gov/data.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.