Abstract
Background
To summarize the characteristics of local invasion and distribution of metastatic lymph nodes in unilateral nasopharyngeal carcinoma (NPC) by magnetic resonance imaging (MRI) to provide references for the optimization of clinical target volume.
Methods
MRI and clinical data of 176 cases of unilateral NPC admitted to the Hunan Cancer Hospital from January 2019 to December 2019 were collected. Unilateral NPC was defined as a lesion confined to the one side of the nasopharynx and had not exceeded the midline as judged by MRI.
Results
Ipsilateral levator veli muscle (63.1%, 111/176), tensor veli palatini muscle (55.7%, 98/176), parapharyngeal space (50.0%, 88/176), and prevertebral muscle (43.7%, 77/176) were more likely to be invaded. Contralateral parapharyngeal space and skull base foramina were not invaded. All local invasions presented as continuous invasion from gross lesions and discontinuous invasions were not observed. The overall lymph node metastatic rate was 89.8% (158/176), of which bilateral metastasis accounted for 56.3% (89/158), and ipsilateral metastasis accounted for 88.1% (155/176), which was higher than the contralateral metastatic rate (55.4%, 94/176) (P < 0.001). The most common regions of lymph node metastasis were level IIb (82.4%), VIIa (69.9%), IIa (54.0%), and III (54.0%). Only one patient had skipping lymph node metastasis (0.6%).
Conclusion
Local invasion of unilateral NPC was characterized by continuous invasion from proximal to distal sites, and lymph node metastasis occurred from the upper to lower neck. Contralateral parapharyngeal space and skull base foramina had a very low probability of invasion, and routine prophylactic radiation may not be necessary.
Keywords: Nasopharyngeal carcinoma, Magnetic resonance imaging, Local invasion, Lymph node spread, Clinical target volume
Background
Nasopharyngeal carcinoma (NPC) is a malignant tumor derived from nasopharyngeal epithelial cells. It is more common in South China and Southeast Asia, and radiotherapy is the most important radical cure for NPC. With the application of intensity-modulated radiotherapy, the prognosis of NPC has been significantly improved. The 5-year locoregional recurrence-free survival has reached 85–90% [1–3]. The incidence and severity of radiotherapy-induced xerostomia has also decreased significantly. However, patients who have been cured still have some sequelae of radiotherapy, such as hearing loss, temporal lobe injury, and endocrine dysfunction, which seriously affect quality of life [4, 5]. Therefore, further reducing the toxic and late side effects of radiotherapy is a key issue in NPC treatment.
In recent years, researchers have optimized the clinical target volume (CTV) of radiotherapy for NPC to reduce the side effects [6, 7]. Sanford et al. used individualized target delineation for NPC radiotherapy based on the tumor invasion trend [6]. It was not required to include all traditional high risk structures of NPC. For unilateral NPC, the contralateral parapharyngeal space and skull base foramina could not be included. Long-term follow-up showed that the overall 5-year local control rate reached 96%, and there was no recurrence of contralateral tissue structures. According to the current CTV delineation guidelines, parapharyngeal space and skull base foramina on both sides belong to high-risk regions, and require prophylactic radiation regardless of the location and stage of the tumor [8, 9]. However, as the primary lesion of unilateral NPC is relatively far from the contralateral parapharyngeal space and skull base foramina, and skull base fascia can act as a barrier, it is worth discussing the necessity of prophylactic radiation of contralateral tissues.
Unilateral NPCs constitute about 10% of all NPC cases [10, 11]. At present, data are limited regarding the characteristics of locoregional extension of this subtype of NPC. In the present study, we analyzed the characteristics of local invasion, as well as the distribution of lymph node metastasis in unilateral NPCs, and explored the feasibility of omitting prophylactic radiation of contralateral parapharyngeal space and the skull base foramina, and provided references for CTV optimization for unilateral NPCs.
Materials and methods
Patients
Patients who were newly diagnosed with unilateral NPC and treated in the Department of Radiation Oncology of Hunan Cancer Hospital from January 2019 to December 2019 were enrolled. Unilateral NPC was defined as a lesion that was confined to the one side of the nasopharynx and had not exceeded the midline as determined by magnetic resonance imaging (MRI) (Fig. 1). Inclusion criteria were as follows: pathologically diagnosed as nasopharyngeal carcinoma; pretreatment nasopharynx and neck MRI imaging; pretreatment nasopharyngoscopy data; meeting the definition of unilateral NPC; and not received prior anti-tumor treatment. Patients with poor MRI imaging or undergoing nasopharyngeal mass resection or neck lymph node mass resection were excluded. All patients were staged using the 8th edition UICC/AJCC NPC staging system [12].
Fig. 1.
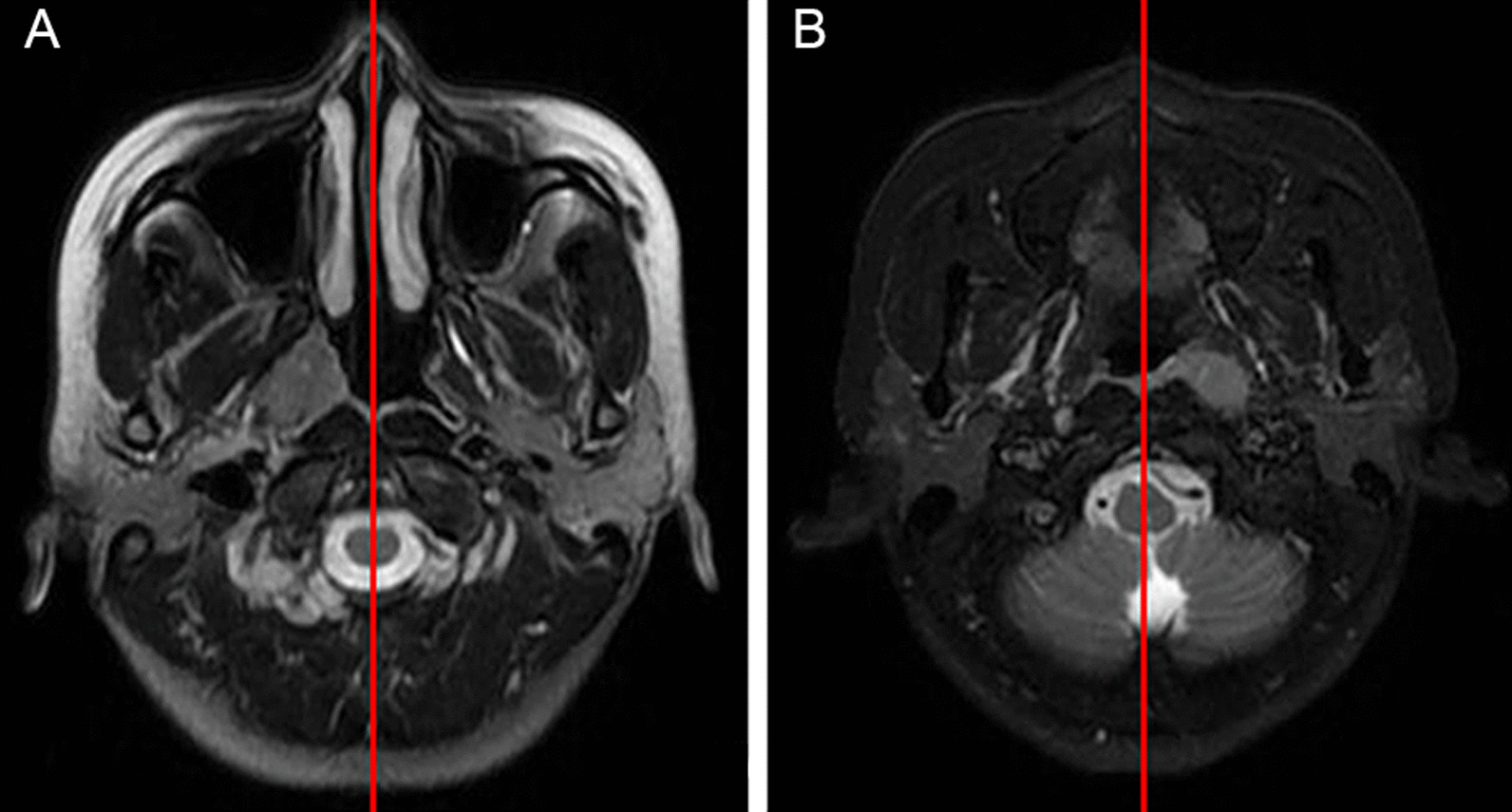
Criteria for diagnosis of unilateral nasopharyngeal carcinoma: A MRI of a lesion that did not exceed the midline of the nasopharynx; B MRI of a lesion that reached the midline but did not exceed the midline
MRI scan
All patients received an MRI scan of the nasopharynx and neck with a scan range from the middle of the temporal lobe to the thoracic entrance. The scanning plane was parallel to the third cervical vertebra in the coronal position, and perpendicular to the third cervical vertebra in the axial position. Slice thickness was 5 mm and slice gab was 0.5 mm. The scanning sequence included axial T1WI, T2WI, and diffusion-weighted imaging. After injection of the contrast agent gadopentetate meglumine (Gd-DTPA), the axial, coronal, and sagittal T1WI fat suppression sequence scans were performed.
Diagnostic criteria
MRI images were reviewed by a senior imaging physician and a senior radiation oncologist independently, and disagreements were resolved by discussion. Tumor invasion was defined as low signal in T1WI, high signal in T2WI in MRI plain scan, and uneven enhancement in contrast scan [13]. Extra-nasopharynx cavity invasion was defined as invasion beyond the fascia of the pharyngeal skull base. Bone invasion was defined as disappearance of the high signal of fat in T1WI in the skull base bone, and obvious enhancement in contrast scan. Criteria of lymph node metastasis [14, 15]: ≥ 10 mm minimum diameter of lymph node in the largest cross-sectional image; ≥ 3 lymph nodes in the same high-risk region, and at least one lymph node having ≥ 8 mm minimum diameter in the largest cross-sectional image; ≥ 5 mm minimum diameter of posterior pharyngeal lymph nodes in the largest cross-sectional image; central necrosis or edge ring enhancement in any lymph nodes; extracapsular invasion of lymph nodes; significant shrinkage or disappear of lymph nodes in MRI image after chemotherapy or radiotherapy. Lymph node drainage levels were defined as previously described [16].
Statistic analysis
Statistical analysis was performed using Statistical Package for the Social Sciences (SPSS) 22.0 (Chicago, IL, USA). The chisquare test was used to examine differences between categorical variables, and twotailed values < 0.05 were considered significant.
Results
Patient characteristics
A total of 176 patients who met the definition of unilateral NPC and the enrollment criteria were included in the study. There were 124 males and 52 females with median age of 51.5 years old (24–74-years-old). There were 174 cases of non-keratinizing carcinoma and two cases of keratinizing squamous cell carcinoma. There were 82 cases with left-side lesions and 94 cases with right-side lesions. Patients’ characteristics are shown in Table 1.
Table 1.
Patient characteristics
| Characteristic | No. of patients (%) |
|---|---|
| Sex | |
| Male | 124 (70.5) |
| Female | 52 (29.5) |
| Median age (years old) | 51.5 (24–74) |
| Pathology | |
| Non-keratinized carcinoma | 174 (98.9) |
| Keratinized carcinoma | 2 (1.1) |
| Site | |
| Right | 94 (53.4) |
| Left | 82 (46.6) |
| T classification | |
| T1 | 53 (30.1) |
| T2 | 50 (28.4) |
| T3 | 54 (30.7) |
| T4 | 19 (10.8) |
| N classification | |
| N0 | 18 (10.3) |
| N1 | 66 (37.5) |
| N2 | 52 (29.5) |
| N3 | 40 (22.7) |
| M | |
| M0 | 173 (98.3) |
| M1 | 3 (1.7) |
| Clinic stage | |
| I | 9 (5.1) |
| II | 46 (26.1) |
| III | 67 (38.1) |
| IVa | 51 (29.0) |
| IVb | 3 (1.7) |
Characteristics of local invasion
In this cohort, tissue structures adjacent to the nasopharynx were most often invaded, including the ipsilateral levator veli muscle (63.1%, 111/176), tensor veli palatini muscle (55.7%, 98/176), parapharyngeal space (50.0%, 88/176), and prevertebral muscle (43.7%, 77/176). The orbit, hypopharynx, frontal sinus, contralateral parapharyngeal space, and skull base foramina were not invaded. Invasions showed continuity in all patients and discontinuous invasions were not observed. The frequency of invasion of various structures around the nasopharynx is shown in Table 2.
Table 2.
Incidence of tumor invasion into anatomic sites surrounding nasopharynx
| Anatomic site | No. of patients (%) | Anatomic site | No. of patients (%) |
|---|---|---|---|
| Levator veli muscle | 111 (63.1) | Cavernous sinus | 11 (6.3) |
| Tensor veli palatini muscle | 98 (55.7) | Meninges | 10 (5.7) |
| Parapharyngeal space | 88 (50.0) | Nasal cavity | 10 (5.7) |
| Prevertebral muscle | 77 (43.7) | Sphenoidal sinus | 9 (5.1) |
| Foramen lacerum | 55 (31.2) | Inferior orbital fissure | 8 (4.5) |
| Petrous apex | 51 (29.0) | Parotid gland | 5 (2.8) |
| Basis of sphenoid bone | 43 (24.4) | Hypoglossal canal | 3 (1.7) |
| Clivus | 45 (25.6) | Maxillary sinus | 2 (1.1) |
| Pterygoid process | 34 (19.3) | Infratemporal fossa | 2 (1.1) |
| Medial pterygoid muscle | 32 (18.2) | Orbital apex | 2 (1.1) |
| Pterygopalatine fossa | 20 (11.4) | Jugular foramen | 1 (0.6) |
| Foramen ovale | 18 (10.2) | Cervical vertebrae | 1 (0.6) |
| Oropharynx | 16 (9.1) | Ethmoid sinus | 1 (0.6) |
| Lateral pterygoid muscle | 15 (8.5) |
Distribution of metastatic lymph nodes
The overall lymph node metastatic rate was 89.8% (158/176), of which bilateral metastases accounted for 56.3% (89/158). The levels with the highest metastatic rate were IIb (82.4%), VIIa (69.9%), IIa (54.0%), and III (54.0%). Lymph node metastasis was not observed with levels Ia, IX, VI, and Xa. The ipsilateral lymph node metastatic rate (88.1%, 155/176) was significantly higher than contralateral metastatic rate (53.4%, 94/176) (χ2 = 12.937, P < 0.001). The distribution of metastatic lymph nodes is shown in Table 3.
Table 3.
Distribution of metastatic lymph nodes
| Level | Ipsilateral lymph node metastasis only | Contralateral lymph node metastasis only | Bilateral lymph node metastasis | Total |
|---|---|---|---|---|
| No. of patients (%) | No. of patients (%) | No. of patients (%) | No. of patients (%) | |
| Ia | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Ib | 8 (4.5) | 0 (0) | 3 (1.7) | 11 (6.2) |
| IIa | 56 (31.8) | 9 (5.1) | 30 (17.0) | 95 (54.0) |
| IIb | 70 (39.8) | 7 (4.0) | 68 (38.6) | 145 (82.4) |
| III | 68 (38.6) | 5 (2.8) | 22 (12.5) | 95 (54.0) |
| IVa | 29 (16.5) | 3 (1.7) | 3 (1.7) | 35 (19.9) |
| IVb | 8 (4.5) | 2 (1.1) | 0 (0) | 10 (5.7) |
| Va | 44 (25.0) | 6 (3.4) | 6 (3.4) | 56 (31.8) |
| Vb | 17 (9.7) | 2 (1.1) | 1 (0.6) | 20 (11.4) |
| Vc | 2 (1.1) | 0 (0) | 1 (0.6) | 3 (1.7) |
| VI | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| VIIa | 82 (46.6) | 6 (3.4) | 35 (19.9) | 123 (69.9) |
| VIIb | 3 (1.7) | 0 (0) | 0 (0) | 3 (1.7) |
| VIII | 4 (2.3) | 0 (0) | 0 (0) | 4 (2.3) |
| IX | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Xa | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Xb | 1 (0.6) | 0 (0) | 0 (0) | 1 (0.6) |
Skipping lymph node metastasis and extra-regional lymph node metastasis
Only one patient had III and IVa metastases without ipsilateral IIa, IIb, and VIIa metastases, which was considered as skipping metastasis (0.6%, 1/176). All of the patients with IB, XIII, and Xb metastases had extensive ipsilateral lymph node metastases (more than three drainage levels were metastasized or the diameter of metastatic lymph nodes in the adjacent area was greater than 3 cm).
Relationship between local invasion and lymph node metastasis
In the cohort, the lymph node metastatic rate was 83.0% (44/53) in T1-stage patients, 88.0% (44/50) in T2-stage patients, 96.3% (52/54) in T3-stage patients, and 94.7% (18/19) in T4-stage patients. There was no significant difference in lymph node metastatic rate among various T stages (χ2 = 6.249, P = 0.100).
There were 126 cases whose lesions were close to (≤ 0.5 cm) or arrived at the midline. The contralateral cervical lymph node metastatic rate was 56.3% (71/126) in these patients. Lesions in another 50 cases exceeded the 0.5 cm from the midline, and contralateral cervical lymph node metastatic rate was 46.0% (23/50) in these patients, which was not significantly different from the former (χ2 = 1.153, P = 0.283). In addition, there was no significant difference in the extent of local invasion between gross tumors with a distance of more than 0.5 cm and gross tumors with a distance of less than 0.5 cm from the midline (P > 0.05, all).
Discussion
To our knowledge, this study is the first to focus on the characteristics of local invasion and lymph node metastasis of unilateral NPC. Our results showed that unilateral NPC tended to invade ipsilateral adjacent tissues. Ipsilateral muscles, parapharyngeal fat space, and skull base bones had a high chance of invasion. Ipsilateral intracranial structures such as meninges and cavernous sinuses could also be involved; this may occur through skull base foramina including ipsilateral foramen ovale cranium and foramen lacerum, or may be secondary to the invasion of surrounding tissues. Tissues far from primary lesions, e.g., hypopharynx, frontal sinus, and orbit, had only a low chance of invasion. Local invasions were all continuous in our cohort and discontinuous invasions were not observed. In summary, the local invasion in unilateral NPC showed characteristics of progressive involvement from proximal to distal sites.
The nasopharynx is the middle structure; thus, bilateral tissues are likely to be invaded. Previous studies have used MRI to study the characteristics of local invasion of NPC, which was found to follow certain pathways [13, 17, 18]. The surrounding structures have been classified as high, intermediate, or low risk to guide CTV delineation. In these studies, it was recommended that high risk structures on both sides be given prophylactic radiation [13, 18]. However, the studies also found that the probability of invasion of both sides was less than 10%, and the majority of NPCs showed an eccentric distribution. Furthermore, these studies did not compare local invasion of ipsilateral and contralateral tissues, especially for unilateral NPC. Our study indicated that the adjacent ipsilateral parapharyngeal space and skull base foramina were more likely to be affected, which is consistent with previous studies [13, 17, 18]. However, contralateral parapharyngeal space and other contralateral structures were not invaded, and skipping local invasion was also not observed. In light of this, we suggest equal prophylactic radiation on both side structures is not necessary for CTV delineation in unilateral NPC. Sanford et al. preliminarily reported the feasibility of omitting prophylactic radiation of the contralateral parapharyngeal space for unilateral NPC [6].
Another reason to include both sides of the parapharyngeal space and skull base foramina in the scope of prophylactic radiation was the concern about multicentric origin of NPC lesions. Mo et al. performed biopsies on contralateral nasopharyngeal tissues in 50 cases of unilateral NPC and found that the rate of contralateral pharyngeal crypt reached 18% [19]. However, it should be noted that they used plain CT and an electronic nasopharyngoscopy to diagnose unilateral NPC. Li et al. performed biopsies of the posterior wall of the contralateral pharyngeal roof as well as the contralateral pharyngeal crypt in 20 unilateral NPC cases judged by MRI [11]. Their result showed that five patients had subclinical lesions on the posterior wall of the contralateral pharyngeal roof and pharyngeal crypt biopsies were also positive for two patients. However, the primary lesions in four of the above five patients had invaded to the midline of the posterior wall. The researchers thereby ascribed the contralateral positive lesions to continuous subclinical infiltration of primary lesions instead of multicentric lesions, and suggested for patients with unilateral NPC but without contralateral LN metastasis or positive EBV-DNA level, CTV expansion to the contralateral normal mucosa of the nasopharynx probably could be reduced.
There is a rich lymphatic vascular network in nasopharynx mucosal tissue and lymphatic drainage on both sides of the neck. Previous studies have rarely discussed the characteristics of lymph node metastasis in unilateral NPC. Sun et al. summarized the distribution of metastatic lymph nodes in 112 patients with unilateral NPC, and found that the metastatic rate of contralateral cervical lymph nodes was only 8% [10]. Our study showed that the overall metastatic rate of lymph nodes was 89.8%. Although ipsilateral metastasis was more common (88.1%), contralateral metastasis was also high (53.4%). In addition, even with primary lesions not close to the midline, the contralateral lymph node metastatic rate was still 46.0%. Lymph node metastasis was not significantly associated with T stage, suggesting lymph node metastasis can occur in the early stage of NPC. Therefore, for unilateral NPC, early prophylactic radiation of both sides of the neck is still necessary.
Our results regarding the distribution of metastatic lymph nodes in lymphatic drainage regions were consistent with previous findings [18, 20, 21]. Levels IIa, IIb, and VIIa, which are considered the first station nodes, more commonly showed metastasis (metastatic rate exceeded 50%), followed by levels III, V, and IV, which had a > 20% metastatic rate. Extra-regional lymph nodes such as levels VI, VIII, IX, and X had the lowest metastatic rate (< 5%). Skipping lymph node metastasis happened in only one patient (0.6%), and metastases in other patients followed the rule of from the upper neck to the lower neck and station by station. All patients with metastases of extra-regional lymph nodes also had extensive ipsilateral cervical lymph node metastases, possibly due to obstruction of the lymphatic ducts and retrograde lymphatic drainage.
Our study suggested, for prophylactic radiation in unilateral NPC, the ipsilateral parapharyngeal space and skull base foramina were still high risk regions and should be included in CTV. The contralateral parapharyngeal space and skull base foramina were low risk regions, and routine prophylactic radiation may not be necessary. For prophylactic radiation of lymphatic drainage regions, the radiation scope and indications are consistent with the current guidelines [9, 22]. For N0 patients, the bilateral upper neck lymph nodes (levels II, III, Va, and VII) required prophylactic radiation; for N + patients, both bilateral upper and lower neck lymph nodes (levels II, III, IV, V, and VII) required prophylactic radiation. For extra-regional lymph nodes, routine prophylactic radiation was not indispensable; however, if there was extensive ipsilateral lymph node metastasis, it was necessary to pay close attention to extra-regional lymph nodes. A large-scale clinical study is needed to determine whether the contralateral parapharyngeal space and skull base foramina can be omitted from prophylactic radiation.
Our study had some limitations. Although MRI was the first choice for judging the extent of NPC invasion, false positive or false negative results were possible. Because contralateral nasopharyngeal mucosa biopsies were not performed, we were unable to adequately judge invasion of the contralateral nasopharyngeal mucosa. Therefore, we also reviewed the electronic nasopharyngoscope data of all patients to ensure accuracy of the diagnosis. In addition, the sample size of this study was relatively small, and the results may be biased. We will collect more data to verify our findings.
Conclusions
Local invasion of unilateral NPC was characterized by continuous spread from proximal to distal sites. Adjacent ipsilateral tissues were invaded more easily, and the contralateral parapharyngeal space and skull base foramina were at low risk of invasion. Lymph node metastasis followed the rule of from the upper neck to the lower neck, and bilateral cervical lymph node metastasis was common. In the setting of CTV for unilateral NPC, the ipsilateral parapharyngeal space and cranial foramina still needed to be included in the scope of prophylactic radiation, while routine prophylactic radiation of contralateral structures such as the parapharyngeal space may not be necessary. Even for unilateral NPC, both sides of the neck required prophylactic radiation.
Acknowledgements
Not applicable.
Abbreviations
- NPC
Nasopharyngeal carcinoma
- CTV
Clinical target volume
- MRI
Magnetic resonance imaging
Authors' contributions
YH: conception and design; ZW and FL: data collection; ZW, LZ and QH: statistical analysis; ZW, LZ and QH: manuscript preparation; HM, YZ and HW: manuscript editing; YH: quality control of data and manuscript review. All authors read and approved the final manuscript.
Funding
This work was supported by Hunan Provincial Health Commission Scientific Research Project (202109031170), Hunan Cancer Hospital Climb Plan (2020QH006), Hunan Province Key R & D Fund (2022SK2051), Changsha Science and Technology Planning Project (KQ1901082).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
This study was approved by the institutional review board of Hunan Cancer Hospital and was performed in accordance with the Declaration of Helsinki.
Consent for publication
Agreed.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zheng Wu, Lin Zhang and Qian He contributed equally to this work and should be considered co-first authors.
References
- 1.Sun X, Su S, Chen C, Han F, Zhao C, Xiao W, et al. Long-term outcomes of intensity-modulated radiotherapy for 868 patients with nasopharyngeal carcinoma: an analysis of survival and treatment toxicities. Radiother Oncol. 2014;110(3):398–403. doi: 10.1016/j.radonc.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 2.Au KH, Ngan RKC, Ng AWY, Poon DMC, Ng WT, Yuen KT, et al. Treatment outcomes of nasopharyngeal carcinoma in modern era after intensity modulated radiotherapy (IMRT) in Hong Kong: A report of 3328 patients (HKNPCSG 1301 study) Oral Oncol. 2018;77:16–21. doi: 10.1016/j.oraloncology.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Wu LR, Liu YT, Jiang N, Fan YX, Wen J, Huang SF, et al. Ten-year survival outcomes for patients with nasopharyngeal carcinoma receiving intensity-modulated radiotherapy: An analysis of 614 patients from a single center. Oral Oncol. 2017;69:26–32. doi: 10.1016/j.oraloncology.2017.03.015. [DOI] [PubMed] [Google Scholar]
- 4.McDowell LJ, Rock K, Xu W, Chan B, Waldron J, Lu L, et al. Long-term late toxicity, quality of life, and emotional distress in patients with nasopharyngeal carcinoma treated with intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2018;102(2):340–352. doi: 10.1016/j.ijrobp.2018.05.060. [DOI] [PubMed] [Google Scholar]
- 5.Zeng L, Tian YM, Sun XM, Chen CY, Han F, Xiao WW, et al. Late toxicities after intensity-modulated radiotherapy for nasopharyngeal carcinoma: patient and treatment-related risk factors. Br J Cancer. 2014;110(1):49–54. doi: 10.1038/bjc.2013.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanford NN, Lau J, Lam MB, Juliano AF, Adams JA, Goldberg SI, et al. Individualization of clinical target volume delineation based on stepwise spread of nasopharyngeal carcinoma: Outcome of more than a decade of clinical experience. Int J Radiat Oncol Biol Phys. 2019;103(3):654–668. doi: 10.1016/j.ijrobp.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Guo Q, Zheng Y, Lin J, Xu Y, Hu C, Zong J, et al. Modified reduced-volume intensity-modulated radiation therapy in non-metastatic nasopharyngeal carcinoma: A prospective observation series. Radiother Oncol. 2021;156:251–257. doi: 10.1016/j.radonc.2020.12.035. [DOI] [PubMed] [Google Scholar]
- 8.Lee AW, Ng WT, Pan JJ, Poh SS, Ahn YC, AlHussain H, et al. International guideline for the delineation of the clinical target volumes (CTV) for nasopharyngeal carcinoma. Radiother Oncol. 2018;126(1):25–36. doi: 10.1016/j.radonc.2017.10.032. [DOI] [PubMed] [Google Scholar]
- 9.Tang LL, Chen YP, Chen CB, Chen MY, Chen NY, Chen XZ, et al. The Chinese Society of Clinical Oncology (CSCO) clinical guidelines for the diagnosis and treatment of nasopharyngeal carcinoma. Cancer Commun (Lond) 2021;41(11):1195–1227. doi: 10.1002/cac2.12218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun Y, Yu XL, Zhang GS, Liu YM, Tao CJ, Guo R, et al. Reduction of clinical target volume in patients with lateralized cancer of the nasopharynx and without contralateral lymph node metastasis receiving intensity-modulated radiotherapy. Head Neck. 2016;38(1):E468–472. doi: 10.1002/hed.24020. [DOI] [PubMed] [Google Scholar]
- 11.Li AC, Zhang YY, Zhang C, Wang DS, Xu BH. Pathologic study of tumour extension for clinically localized unilateral nasopharyngeal carcinoma: Should the contralateral side be included in the clinical target volume. J Med Imaging Radiat Oncol. 2018;62(5):540–547. doi: 10.1111/1754-9485.12741. [DOI] [PubMed] [Google Scholar]
- 12.Amin MB, editor. American Joint Committee on Cancer Staging Manual. 8. New York: Springer; 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liang SB, Sun Y, Liu LZ, Chen Y, Chen L, Mao YP, et al. Extension of local disease in nasopharyngeal carcinoma detected by magnetic resonance imaging: improvement of clinical target volume delineation. Int J Radiat Oncol Biol Phys. 2009;75(3):742–750. doi: 10.1016/j.ijrobp.2008.11.053. [DOI] [PubMed] [Google Scholar]
- 14.Van den Brekel MW, Stel HV, Castelijns JA, Nauta JJ, Van der Waal I, Valk J, et al. Cervical lymph node metastasis: assessment of radiologic criteria. Radiology. 1990;177(2):379–384. doi: 10.1148/radiology.177.2.2217772. [DOI] [PubMed] [Google Scholar]
- 15.King AD, Ahuja AT, Leung SF, Lam WW, Teo P, Chan YL, et al. Neck node metastases from nasopharyngeal carcinoma: MRI of patterns of disease. Head Neck. 2000;22(3):275–281. doi: 10.1002/(SICI)1097-0347(200005)22:3<275::AID-HED10>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 16.Grégoire V, Ang K, Budach W, Grau C, Hamoir M, Langendijk JA, et al. Delineation of the neck node levels for head and neck tumors: A 2013 update. DAHANCA, EORTC, HKNPCSG, NCIC CTG, NCRI, RTOG, TROG consensus guidelines. Radiother Oncol. 2014;110(1):172–181. doi: 10.1016/j.radonc.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 17.Dubrulle F, Souillard R, Hermans R. Extension patterns of nasopharyngeal carcinoma. Eur Radiol. 2007;17(10):2622–2630. doi: 10.1007/s00330-007-0616-z. [DOI] [PubMed] [Google Scholar]
- 18.Li WF, Sun Y, Chen M, Tang LL, Liu LZ, Mao YP, et al. Locoregional extension patterns of nasopharyngeal carcinoma and suggestions for clinical target volume delineation. Chin J Cancer. 2012;31(12):579–587. doi: 10.5732/cjc.012.10095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mo HY, Chen DL, Hong MH, Min HQ. Study of local tumor involvement in nasopharyngeal carcinoma with fiberendoscope, CT scanning, and pathological evidence. Aizheng. 1998;17(4):293–295. [Google Scholar]
- 20.Wang X, Hu C, Ying H, He X, Zhu G, Kong L, et al. Patterns of lymph node metastasis from nasopharyngeal carcinoma based on the 2013 updated consensus guidelines for neck node levels. Radiother Oncol. 2015;115(1):41–45. doi: 10.1016/j.radonc.2015.02.017. [DOI] [PubMed] [Google Scholar]
- 21.Jiang C, Gong B, Gao H, Zhang T, Li Z, Wang J, et al. Correlation analysis of neck node levels in 960 cases of nasopharyngeal carcinoma. Radiother Oncol. 2021;161:23–28. doi: 10.1016/j.radonc.2021.05.020. [DOI] [PubMed] [Google Scholar]
- 22.Biau J, Lapeyre M, Troussier I, Budach W, Giralt J, Grau C, et al. Selection of lymph node target volumes for definitive head and neck radiation therapy: a 2019 Update. Radiother Oncol. 2019;134:1–9. doi: 10.1016/j.radonc.2019.01.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.


