Abstract
Background
RAD51 proteins, which are conserved in all eukaryotes, repair DNA double-strand breaks. This is critical to homologous chromosome pairing and recombination enabling successful reproduction. Work in Arabidopsis suggests that RAD51 also plays a role in plant defense; the Arabidopsis rad51 mutant is more susceptible to Pseudomonas syringae. However, the defense functions of RAD51 and the proteins interacting with RAD51 have not been thoroughly investigated in maize. Uncovering ligands of RAD51 would help to understand meiotic recombination and possibly the role of RAD51 in defense. This study used phage display, a tool for discovery of protein-protein interactions, to search for proteins interacting with maize RAD51A1.
Results
Maize RAD51A1 was screened against a random phage library. Eleven short peptide sequences were recovered from 15 phages which bound ZmRAD51A1 in vitro; three sequences were found in multiple successfully binding phages. Nine of these phage interactions were verified in vitro through ELISA and/or dot blotting.
BLAST searches did not reveal any maize proteins which contained the exact sequence of any of the selected phage peptides, although one of the selected phages had a strong alignment (E-value = 0.079) to a binding domain of maize BRCA2. Therefore, we designed 32 additional short peptides using amino acid sequences found in the predicted maize proteome. These peptides were not contained within phages. Of these synthesized peptides, 14 bound to ZmRAD51A1 in a dot blot experiment. These 14 sequences are found in known maize proteins including transcription factors putatively involved in defense.
Conclusions
These results reveal several peptides which bind ZmRAD51A1 and support a potential role for ZmRAD51A1 in transcriptional regulation and plant defense. This study also demonstrates the applicability of phage display to basic science questions, such as the search for binding partners of a known protein, and raises the possibility of an iterated approach to test peptide sequences that closely but imperfectly align with the selected phages.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12864-022-08419-6.
Keywords: RAD51, Phage Display, Peptide, BRCA2, Transcription factor, Maize, Zea mays
Background
The DNA double-strand break repair protein RAD51 is conserved in all eukaryotes but was initially identified in Saccharomyces cerevisiae [1]. It enables double-strand break repair, specifically catalyzing the alignment of the broken DNA ends to a double-stranded template, a process known as strand invasion. This homology-directed repair occasionally results in homologous recombination [1, 2].
In many eukaryotes including humans and Arabidopsis, RAD51-mediated DNA repair requires the DNA repair protein BRCA2. Mutations in the BRCA2 gene are risk factors for breast and ovarian cancer in humans. BRCA2 proteins are large, containing several 39-amino-acid repeats, known as BRC repeats. The number and composition of these repeats varies between species. For example, both of the Arabidopsis BRCA2 proteins contain four BRC repeats while rice BRCA2 contains six BRC repeats [3–6]. Annotations in GenBank and pFAM suggest that the maize BRCA2 protein contains two BRC repeats ([7, 8] GenBank ID# AQK42450.1). In many species including humans and Arabidopsis, RAD51 binds to the BRC repeat region of the BRCA2 protein. The multiple BRC repeats allow multiple RAD51 molecules bind to a single BRCA2 molecule [3, 9, 10].
The crossing-over process in meiosis is a specialized form of homology-directed DSB repair [11]. This process in meiosis I allows DNA to be exchanged between chromosomes, leading to increased diversity and ensuring faithful chromosome pairing [12]. Therefore, mutations in RAD51 genes cause meiotic and reproductive defects in many species [13–16]. Because maize is grown for its edible seeds, its reproductive processes, including meiosis, are of special importance. The function of RAD51 is less well characterized in maize than in Arabidopsis, and a single BRCA2 protein in maize has only been tentatively identified [8, 16]. Our understanding of the interactions of RAD51 proteins in maize is still at an early stage.
In maize, as in rice, there are five RAD51 genes: RAD51A1, RAD51A2, RAD51B, RAD51C, and RAD51D [17, 18]. The two maize RAD51A genes, RAD51A1 and RAD51A2, appear functionally redundant--a mutation in RAD51A1 alone does not cause a macroscopically observable reproductive phenotype, but rad51a1/rad51a2 double mutants are male sterile with very low female fertility [2]. The RAD51A1/RAD51A2 split appears to be basal to all grasses and is present in both maize and rice [19]. In maize and Arabidopsis, RAD51 plays an additional role in pathogen resistance [20, 21]. The relationship between the defense function of RAD51 and DNA repair is unclear. There are numerous examples of plant cells having an increased rate of DSBs in response to pathogen infection since Mittler and Lam first identified this phenomenon in 1997 [22, 23]. Arabidopsis with loss-of-function mutations in either RAD51 or BRCA2A had increased susceptibility to infection by Pseudomonas syringae, and when wild-type Arabidopsis plants were treated with the defense hormone INA, AtRAD51 expression increased [20]. In Arabidopsis, RAD51 and RAD51D appear to play a direct role in inducing defense gene transcription and interact physically with transcription start sites. Additionally, RAD51D interacts physically with the defense gene suppressor SNI1 [20, 24–26]. SNI1 itself, although first discovered as a negative regulator of defense, is also involved in meiotic recombination; sni1 mutant Arabidopsis displays increased rates of crossover formation [27]. Adding a maize RAD51A1 transgene to Arabidopsis, rice, or tobacco increases resistance to selected pathogens [21].
Neither the overall binding affinity of maize RAD51A proteins nor the possible relationship between maize RAD51 proteins and transcription regulation has been investigated in the published literature. Therefore, this study aimed to identify proteins which may bind to RAD51A1. The phage display method, which casts a wide net for potential binding motifs, is well suited to this initial stage of investigation.
Phage display is a discovery-driven technique that screens for short peptides with high affinity for a given bait protein, in this case RAD51A1. When phage display is used in monoclonal antibody development, an antigen of interest is used as a bait protein while phages containing small fragments of the antibody protein on their surface are screened [28]. However, other experiments use a phage library composed of random amino acid sequences. Phage display harnesses the power of natural selection, since phages with phage coat additions that enable higher-affinity binding to the bait protein are more likely to be retained, grown in E. coli, and sequenced [29]. Phage display was chosen over a yeast-2-hybrid method for this study because of the possibility of using a large random library. Additionally, targeting specific short peptide sequences suggests future possible cytological experiments. Fluorescent-labeled peptides could be used as an alternative to fluorescent-labeled antibodies in protein detection [30–32].
Results
Properties of synthesized RAD51A1
Two separate blots with different antibodies were used to verify the purity of the RAD51A1 grown in E. coli: one blot used an HRP-conjugated α-rabbit IgG secondary antibody from goat to bind to the α-ZmRAD51A antibody from rabbit; the other used an HRP-conjugated α-His-tag monoclonal antibody from mouse to bind to the His-tag of the synthesized RAD51A1. Both western blots revealed the presence of RAD51A1 dimers. The molecular weight of monomeric maize RAD51A1 is 46KDa, and there was a clear band of that size in the SDS-PAGE gel. However, in the western blot analyses, there was no signal at 46KDa but there was an apparent dimer band of about twice that size (Fig. 1). A separate blot contained also an apparent RAD51A1 trimer band. Furthermore, previous mass spectrometry analysis did confirm that the construct expressed full length anti-RAD51A1 before being used to generate anti-RAD51A1 antibody.
Fig. 1.
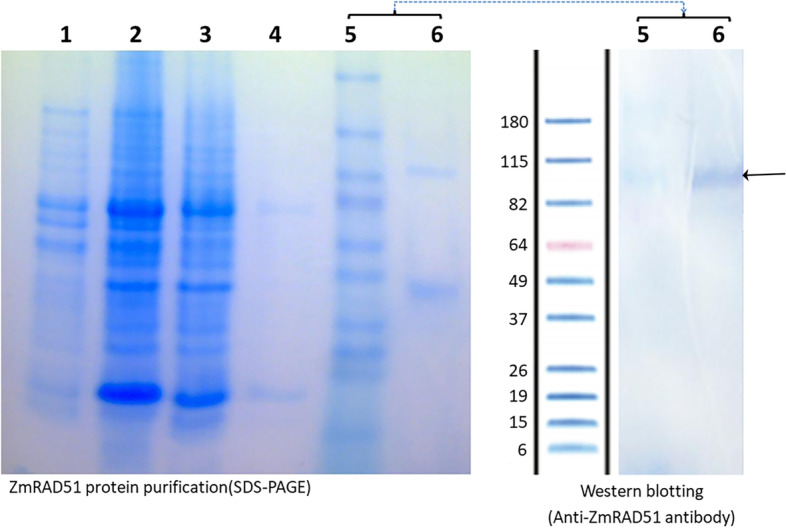
SDS-PAGE gel and western blot showing purification of RAD51A1. An SDS-PAGE gel is shown on the left, and a western blot probed with an α-ZmRAD51 antibody from rabbit is shown on the right. Gel lanes represent (1) supernatant, (2) pellet, (3) flow through, (4) wash, (5) marker, and (6) eluted protein. In the western blot on the right, lane (6) contains the eluted RAD51 protein probed with an α-ZmRAD51 antibody from rabbit. The RAD51A1 dimer band (92 KDa), indicated by an arrow, can be seen more clearly than the monomer band (46 KDa)
Phage peptides selected by phage display
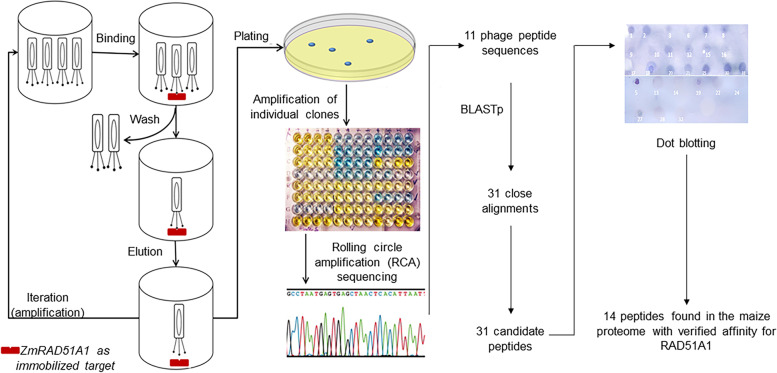
Phages with coat proteins that enabled binding to maize RAD51A1 were grown in E. coli and the selected phages were sequenced (Fig. 2A). BLASTp and pFAM were used to analyze the phage sequences and search for either candidate maize proteins or conserved domains containing these amino acid sequences. When no such matches were found, 32 short (13–20 amino acids) peptides were designed from the primary sequences of maize proteins that imperfectly aligned with the selected peptides. For clarity, these peptides are referred to as “synthesized peptides” in contrast to the “phage peptides” selected in the phage display experiment. These synthesized peptides, whose sequences were found in the predicted maize proteome, were dot blotted to test their affinity for RAD51A1 (Fig. 2B).
Fig. 2.
An outline of the methods used in the phage display and subsequent experiments. A A random phage display library was allowed to hybridize with RAD51A1, then washed to remove non-binding phages, and then eluted. Three rounds of selection were performed for a more rigorous screening of interactions. Following plating, phages selected for binding affinity for RAD51A1 were amplified and sequenced. B Maize peptides were selected for synthesis based on phage display results. These synthesized peptides were dot blotted for affinity with RAD51A1
The sequencing reaction only succeeded for 15 of the 24 single-plaque phages selected for affinity with RAD51A1; the remaining 9 reactions yielded low-quality results, possibly due to low concentrations of DNA. These 15 sequenced phages were found to contain 11 distinct peptide sequences, some of which were selected multiple times (Table 1). This included eight sequences of 16 amino acids. Sequencing also revealed three peptide sequences longer than the expected 16 amino acids: two 17-amino-acid peptides and one 18-amino-acid peptide. Analysis of these peptide sequences revealed three apparent recurring motifs: an isoleucine-tryptophan motif, a glutamate-tryptophan motif, and a more variable motif consisting of tryptophan in conjunction with glutamine, proline and/or serine. At least one of these motifs was present in each of the selected phage peptides. These motifs may be important to the ability to bind to maize RAD51A1. Tryptophan is present in all selected sequences except for phage peptide #24, suspected to be a false positive because it perfectly matches the sequence of a coat protein of the M13 bacteriophage used in the phage display experiment. The full list of peptides, along with ELISA and dot blot results, is shown (Table 1).
Table 1.
Phage peptides identified after selection and amplification
| Phage # | Peptide sequence | Phage clone(s) | ELISA affinity (native/denatured) | Dot blot |
|---|---|---|---|---|
| 1 | HLEYNAGYHSPATHQWS | 1 | −/− | – |
| 2 | TWHDTFHAKGTGQSWS | 5, 6 | +/+ | + |
| 3 | LSISIWFFPXESSHKS | 8 | −/− | + |
| 4 | HQTPMHIWPEHKLGHR | 9 | −/+ | – |
| 5 | SSLGQPWIGAPRAYPW | 10 | −/+ | – |
| 6 | HYSQSLTYTWPKFGEI | 13 | −/+ | + |
| 7 | TSNTTPWQTSWELMYA | 14, 15, 21 | +/+ | + |
| 8 | HHTHWHTSQDWEPQPHAS | 16 | +/+ | – |
| 9 | YTGLHYQPWWPDVVQG | 20 | +/+ | + |
| 10 | DEWNEMGNIPSQLIMA | 22, 23 | +/+ | – |
| 11 | AAEGDDPAKAAFNSLQA | 24 | −/− | – |
Phages containing these peptides showed the ability to bind to maize RAD51A1 in vitro. Some sequences were selected multiple times. Recurring motifs are underlined and bolded. ELISA affinity and Dot blot refer to the results of experiments to verify the affinity of the phages for RAD51A1. ELISA affinity refers to affinity for either native or denatured RAD51A1. “+/+” signifies that the phage had affinity for native and denatured RAD51A1; “−/+” signifies affinity only for denatured RAD51A1 and so on. Dot blot result refers to binding affinity for native RAD51A1 as determined by dot blot. ELISA and dot blots verified RAD51A1 affinity of most selected phages
Binding affinity for RAD51A1 was verified for all the selected phages whose additional peptide sequences had been sequenced at 16 amino acids in length; only phages 1, 16, and 24 did not demonstrate in vitro affinity with RAD51A1 (Table 1). In the ELISA, phages’ affinity for native and denatured RAD51 was compared to their affinity for a BSA negative control. Wells with 1.5-fold or greater increase in optical density above control were coded as positive. Phages 4, 5, 6, 7, 14, 15, 16, 20, 21, 22, and 23 were positive against both native and denatured RAD51A1 protein; four additional phage strains [9, 10, 13, 17] were positive against denatured protein only (Fig. S1). Peptide 3 did not clear the 1.5-fold threshold in the ELISA, possibly due to high affinity for the BSA control, but demonstrated affinity in the dot blot and in the western blot (Figs. S1, S2). Each phage/protein combination was repeated two or three times, and phages found to contain the same peptide sequences as other phages were still tested separately.
Phages were also incubated in dot blots to further confirm affinity for RAD51A1. To investigate possible affinity with other proteins produced alongside RAD51A1, phages were incubated with the supernatant, pellet, flow through, and two subsequent elutions of native RAD51A1. Phages 5, 8, 13, 14 and 20 bound to the protein supernatant, protein in pellet and flow through and elution 1. This unexpected binding to earlier steps in the extraction of the RAD51A1 protein may be due to presence of RAD51A1 in these stages (see the possible RAD51A1 dimer bands in the supernatant, pellet, and flow-through in Fig. 1), or since these are M13 bacteriophages, liable to also interact with some proteins from their natural host, E. coli. The second eluted fraction did not bind in vitro to any phages, possibly due to a high concentration of the base imidazole in the elution buffer raising the pH and causing the protein to fail to immobilize (Fig. S2).
Bioinformatic analysis of phage peptide sequences
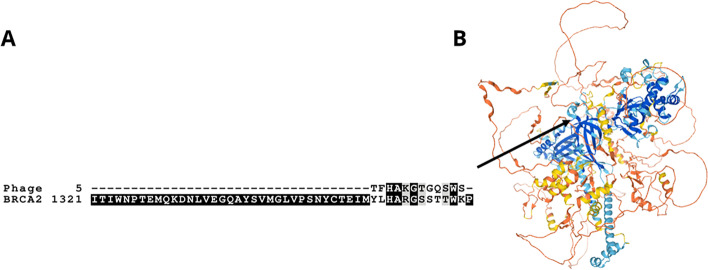
BLASTp searches for the peptides selected in the phage display did not reveal any known or predicted maize proteins aligning perfectly to the full peptide sequence of any of the selected peptides, but several alignments to maize proteins, with E-values between 0.26 and 8.9, were identified (Table 2). Several of the closest alignments were to DNA-associated proteins such as transcription factors, proteins related to defense, and uncharacterized proteins. Additionally, a MEGA7 muscle alignment and a BLAST pairwise alignment to the BRCA2 revealed that a portion of phage peptide #2, selected twice, aligns imperfectly to a region of the BRCA2 protein with 44% identity and an E- value = 0.5 when aligned with the full-length Zm00001d024953_P024 sequence, or an E-value of 0.079 when aligned to the RPA_2b-aaRSs_OBF_like domain specifically, see Fig. 3.
Table 2.
Maize proteins aligning to the selected phage peptide sequences shown in Table 1
| Phage peptide # | Aligned protein annotation | E-value | Query coverage | Percent identity | NCBI accession |
|---|---|---|---|---|---|
| 1 | Uncharacterized protein LOC100381696 isoform X1 | 2.4 | 47% | 87.5% | XP_008673086.1 |
| 1 | Integrator complex subunit 9 | 2.4 | 47% | 87.5% | PWZ34425.1 |
| 1 | Mitogen-activated protein kinase kinase kinase A | 4.9 | 47% | 87.5% | PWZ31635.1 |
| 4 | Boron transporter 4 | 2.9 | 43% | 85.71% | ONM62448.1 |
| 7 | Putative ribosomal protein S4 (RPS4A) family protein | 1.4 | 43% | 85.71% | AQK78541.1 |
| 7 | Disease resistance gene analog PIC21 | 4.1 | 50% | 75% | AAC83569.1 |
| 7 | Disease resistance protein RPS2 | 4.2 | 50% | 75% | PWZ33587.1 |
| 7 | Uncharacterized protein LOC109944554 | 5.9 | 43% | 88% | XP_020404914.1 |
| 7 | Glucuronoxylan 4-O-methyltransferase 2 | 5.9 | 62% | 90% | ONL96892.1 |
| 7 | Pectate lyase 12 | 5.9 | 50% | 87.5% | ONM06095.1 |
| 8 | Heat stress transcription factor B-4 | 8 | 88% | 50% | PWZ13703.1 |
| 9 | Phosphoinositide phosphatase SAC6 | 2.1 | 56% | 71.43% | AQK71706.1 |
| 9 | Prolyl endopeptidase isoform X2 | 4.2 | 43% | 85.71% | XP_008669028.1 |
| 9 | Prolyl oligopeptidase family protein | 4.2 | 43% | 85.71% | ONM15068.1 |
| 10 | Citrate synthase 1 | 0.26 | 62% | 70% | AQK69670.1 |
| 10 | Transcription regulator | 4.2 | 50% | 87.5% | ONM03717.1 |
| 10 | CCR4-NOT transcription complex subunit 1 | 4.2 | 75% | 87.5% | PWZ58289.1 |
| 10 | Uncharacterized protein LOC100502409 isoform X10 | 4.2 | 75% | 87.5% | XP_020399402.1 |
| 10 | Uncharacterized LOC100383837 | 4.2 | 75% | 87.5% | NP_001349390.1 |
A cutoff E-value of 8 was used. Hypothetical proteins not supported by EST or cDNA, suspected false positives (phage peptide #11), and cases where multiple proteins with similar or identical annotation were listed are not included. This table is based on maize genome sequence draft v5
Fig. 3.
Maize BRCA2 residues 1358–1367 align with a portion of phage peptide #2. Peptide #2 was selected twice in the phage display experiment. The aligning region of maize BRCA2 is annotated as RPA_2b-aaRSs_OBF_like Superfamily in GenBank. A The amino acid sequence at the site of alignment. Black represents an alignment between two identical amino acids and grey represents a positive alignment. The E-value when this specific alignment is BLASTed is 0.5, with 44% identity. Amino acids aligned using ClustalW. Diagram produced using Boxshade (http://www.ch.embnet.org/software/BOX_form.html) B Location within the structure of maize BRCA2, as predicted by Alphafold [33]. See Uniprot s# A0A1D6J355. Dark blue represents very high confidence (pLDDT >90) and orange represents very low confidence (pLDDT <50). This region’s structure is predicted at “confident” to “very high” confidence
BLASTing the selected phage peptides against all non-redundant proteins from all species in the NCBI database resulted in only one perfect match, between the 17-mer peptide #11 from phage clone #24 and the coat protein of the E. coli bacteriophage virus M13 (Table S1). This perfect match to the bacteriophage used in the phage display experiment suggests that phage peptide #11 is a false positive selected due to off-target amplification of viral DNA. Additionally, this phage did not bind to the RAD51A1 protein in either ELISA or dot blot assays (Table 1). Several of the selected sequences revealed weak alignments (E-values of 1.6–8.9) with DNA-associated proteins in other organisms. No putative conserved domains were detected by either BLAST or pFAM analysis; this may be because these tools were not designed for such short input sequences.
Synthesis and binding affinity of selected maize peptides
An initial search, performed in 2015 using maize genome version 3, revealed several imperfect alignments between selected peptides and the maize proteome. These close alignments led to the selection of 32 peptides to be synthesized by GenScript (genscript.com; GenScript USA Inc., Piscataway, NJ 08854, USA) (Table 3). Dot blots showed that 14 of these peptides had in vitro affinity for RAD51A1 (Fig. S3). A list of the maize proteins which contains these positive synthesized peptide sequences can be found in Table 4.
Table 3.
Synthesized peptides used in the final dot-blotting experiments
| Synthesized peptide sequence | Derived from phage peptide # | Dot blot | |
|---|---|---|---|
| 1 | EALYHSPNVATHS | 1 | + |
| 2 | RGGYNGGYRGPAA | 1 | + |
| 3 | RTWLASYHLRGTAQTW | 2 | + |
| 4 | KISWNETFHSVVEA | 2 | – |
| 5 | HSLTFTWHDAFKG | 2 | – |
| 6 | SMISVFFPNESVQKS | 3 | – |
| 7 | AGARAWRWPEHELGA | 3 | + |
| 8 | SKLGHRLSVETGPKPSRGK | 3 | + |
| 9 | RPMRKWSESKLGTEL | 4 | – |
| 10 | GHRSEETHQEGATER | 4 | – |
| 11 | RIGEPWIGVPSSG | 5 | + |
| 12 | RPGPSPRLGQPWRLG | 5 | + |
| 13 | GTPWISAPKNTLR | 5 | – |
| 14 | ANIYSRGVTYEWHKF | 6 | – |
| 15 | QTLMYTATWPKEVRK | 6 | + |
| 16 | PWEPSWELFREKVGD | 7 | – |
| 17 | DRGWSIRAWELMY | 7 | – |
| 18 | EHVWQTSWAISTRFVG | 7 | + |
| 19 | SHTSTSNATTPRSFD | 8 | + |
| 20 | KGGGVPQPHLSAEAR | 8 | + |
| 21 | EPQPHSNGTASPAPPA | 8 | – |
| 22 | ANHTSQDEPHGGAL | 8 | – |
| 23 | SAAPPQPHANAMNL | 8 | – |
| 24 | EQFVVIGSAETVPPRHSL | 8 | – |
| 25 | RPRWPDVQGRSYASR | 9 | + |
| 26 | GGAEMGLQTQLLMANA | 10 | – |
| 27 | DDWTNMAMVGISPQILMKSA | 10 | – |
| 28 | AAEEDAAAPARAASLQA | 11 | – |
| 29 | TFAFNSLQVSFSFE | 11 | – |
| 30 | GDQEDSAAAFPSLQA | 11 | + |
| 31 | SKDDPAKEAASFTS | 11 | + |
| 32 | SSLGQPWRGALLLPAPRA | 11 | – |
These 32 peptides were derived from close alignments between the selected phage peptides shown in Table 1 and known or predicted proteins found in the maize proteome. Dot blot refers to binding affinity for native RAD51A1. Interactions demonstrating affinity are bolded. More information on the peptides found to have affinity with RAD51A1 in the dot blot assay can be found in Table 4
Table 4.
Maize proteins containing the synthesized peptides which bound RAD51A1 in vitro
| Synthesized peptide # | Derived from phage peptide # | Matching protein(s) |
|---|---|---|
| 1 | 1 | Probable WRKY transcription factor 63XM_008671716 |
| 2 | 1 | Plasminogen activator inhibitor RNA-binding protein, NM_001371832 |
| 11 | 5 | Two heat stress transcription factors: XP_008670235, heat stress transcription factor B-1, and ACG38635, heat shock factor protein 4 |
| 15 | 6 | Three DEAD-like helicases: putative DEAD-box ATP-dependent RNA helicase family protein XP_020405421, putative DEAD-box ATP-dependent RNA helicase family protein AQK96024, and DEAD-box ATP-dependent RNA helicase 40-like NP_001348908 |
| 18 | 7 | Prolyl-tRNA synthetase, NP_001149927 |
| 19 | 8 | LRP1 - lateral root primordia 1, XP_008656860 |
| 20 | 8 | DUF455 family protein, ONM28467 |
| 25 | 9 | Suspected annotation error |
| 30 | 11 | Two bHLH transcription factors: transcription factor MYC2 XP_020402137 and uncharacterized protein NP_001168647 |
| 31 | 11 | Ten Elongation factor 1-alpha-like proteins: AQK85106, NP_001152668, XP_008656156, XP_008656153, NP_001338678, NP_001105587, NP_001105933, XP_035815740, AQK85098, Q41803 |
These peptides were designed based on regions of known or predicted maize proteins with close alignment to selected phage peptides from Table 1. Of these 32 synthesized peptides, 14 successfully bound RAD51A1. Putative transcription factors are bolded. Annotations are based on maize B73 reference genome v5. Synthesized peptides 3, 7, 8, and 12 bound RAD51A1 but were derived from putative peptide sequences now thought to be pseudogenes based on more recent annotations; these are not shown
Discussion
RAD51A1 dimerization may influence peptide binding affinity
RAD51 proteins are known to polymerize in many species including Saccharomyces cerevisiae and the archaeon Pyrococcus furiosus [9, 34]. Previous Western blot analysis has shown a signal from the maize RAD51A1 dimer using anti-HsRAD51 antibody [35]. Both western blots revealed a RAD51A1 dimer band at 92 KDa with a stronger signal than the expected monomer band at 46 KDa. This stronger signal could be due to the higher rate of formation of dimers, increased solubility of dimers, or increased affinity of the antibodies for the dimer form— although the dimer signal was stronger regardless of whether the α-ZmRAD51 antibody or an α-His-tag antibody was used, suggesting that the specific antibody is not the cause of the bias towards the dimer signal. This result is in line with previous studies using anti-human RAD51 antibody to detect maize RAD51A1, which were successful but showed multiple bands, indicating RAD51 dimerization [35]. The construct pET28b to express the full length ZmRAD51A1 used in this study was built to generate the anti-ZmRAD51A1 antibody. The product of pET28b was confirmed using mass spectrometry analysis as the full length of ZmRAD51A1 protein.
Properties of peptides found to confer phage affinity for maize RAD51A1
Of the 11 distinct phage peptides, three (#2, #7, and #10) were selected multiple times. Given the number of single-plaque phages sequenced (24) and the size of the phage library (2.6E10 peptides), repeated selection of a given peptide would be unlikely due to chance. Furthermore, the three phages that were selected multiple times all displayed affinity for both native and denatured RAD51A1 in ELISA assays; phages #2 and #7 also bound to native RAD51A1 in dot blots. Phage peptide #2, selected twice, aligns to a binding region of maize BRCA2 (Fig. 3). This alignment between a binding domain of a putative RAD51A1-interacting protein (BRCA2) and a phage peptide selected twice for affinity with RAD51A1 may help elucidate the in vivo interaction between maize RAD51A1 and BRCA2.
The presence of both DNA-associated and plant defense related proteins in both the maize-specific BLASTp hits (Table 2) and the general BLASTp hits (Table S1) supports the idea of a dual function for RAD51 in DNA repair and plant defense. However, the E-values of these alignments (0.26–8.9) indicate that alignments of this quality might be expected by chance.
Some recurring short motifs were initially noted among the peptides generated by phage display (Table 1), but this may have been due to chance. In a later experiment, the majority of the synthesized peptides which bound RAD51A1 did not contain these short motifs. Similarly, the initial observation that tryptophan was present in every selected phage peptide (with the exception of the suspected false positive, phage peptide #11) did not hold true in the synthesized peptides, many of which bound RAD51A1 in a dot blot despite lacking tryptophan. However, despite this difficulty uncovering recurring motifs, the phage display results did identify interactions for further investigation, including those selected for the synthesized peptide experiment.
Maize peptides show affinity for RAD51A1
Since there were no perfect matches between the phage peptides and the primary sequence of any known maize proteins, this study included an additional in vitro experiment with non-phage synthesized peptides. Peptide sequences were selected from maize proteins that aligned with, but did not perfectly match, the phage peptides (Tables 3 and 4). Selection of peptides for synthesis occurred before the release of the fourth and fifth versions of the maize genome, and the results were slightly different from what was found in later analyses; the fact that these annotations were later removed suggests that some of the proteins initially identified may have been pseudogenes (Table 3).
Sequences from two transcription factors possibly involved in defense bound to RAD51A1 in dot blot experiments. Synthesized peptides 1 and 2 successfully bound to RAD51A1 in vitro (Tables 3 and 4), unlike phage peptide #1 (Table 1), from which they were derived. Synthesized peptide #1 was designed to match part of the amino acid sequence of a WRKY transcription factor. These transcription factors (aligning with peptides 1 and 2), which contain a conserved amino acid motif beginning with WRKY, can be involved in regulating response to a range of biotic and abiotic stresses in plants, including pathogens [36]. The negative recombination regulator SNI1, which also serves as a defense gene regulator, appears to interact physically with several WRKY transcription factors in Arabidopsis [25, 27, 36, 37]. Synthesized peptide #30, which was developed from the transcription factor MYC2, also binds RAD51A1 in vitro. In Arabidopsis, MYC2 serves as a negative regulator of plant defense in the salicylic acid pathway and a positive regulator of the jasmonic acid pathway [38]. In both proteins (transcription factors WRKY and MYC2), the region containing the selected peptide is not a known conserved domain, nucleotide-binding or otherwise.
Sequences from two other proteins thought to be involved in transcription regulation bound to RAD51A1 in dot blots. Synthesized peptide #20 was derived from ONM28467, a Domain of Unknown Function (DUF) 455 protein with a Bromodomain extra-terminal (BET) domain. BET domains are associated with transcription factors which may also play a role in remodeling acetylated chromatin to enable transcription [39, 40]. Previous annotations noted similarity between this protein and the Arabidopsis transcription factor GTE4. While this locus is not the most similar maize gene to Arabidopsis GTE4, a BLAST search of the Arabidopsis proteome revealed that it does align closely to an Arabidopsis GTE4 isoform (E-value = 5e-80, 58% identities, 68% positives). In Arabidopsis, GTE4 is involved in controlling the mitotic cell cycle; gte4 mutants have many aneuploid somatic cells due to endoreduplication taking the place of mitosis [41]. In mice BET domain containing proteins are required for both mitosis and spermatogenesis [40]. Synthesized peptide #11 is derived from a sequence in a protein annotated as Heat Stress Transcription Factor B-1 and also matches the sequence of another transcription factor annotated as Heat Shock Factor Protein 4.
Strengths and weaknesses of the phage display method
This investigation into specific sequences that enable affinity for RAD51A1 involved short peptide sequences as opposed to full-length proteins which fold themselves into higher-order structures. Therefore, the short peptide sequences selected in the phage display may not match the primary amino acid sequence of the proteins that interact in vivo with the protein of interest. BLAST and pFAM algorithms were not designed for such short input sequences, and there is a potential for false positive BLASTp hits due to chance. The second experiment, investigating the binding affinity of short, synthesized peptides which perfectly matched portions of the primary sequences of maize proteins (Tables 3 and 4), was intended to validate some of these possible interactions.
The strength of phage display compared to other methods—the ability to screen a large factorial library of 2.6E10 sequences—is also a weakness since this increases the likelihood of selecting proteins not produced in maize. Other methods, such as a competitive yeast-2-hybrid assay with a specific cDNA library, avoid this problem but do not screen as many possible prey sequences.
Finally, these peptides could be used as alternatives to antibodies to verify the presence of RAD51 in vitro or even to localize it in vivo. Discovery of the 14 synthesized peptides with binding affinity to RAD51A1 provides a great opportunity for further screening of peptides that can be synthesized and conjugated with fluorescent markers. These modified peptides could be used to directly localize RAD51A1 without an α-ZmRAD51 antibody. Various systems using small fluorescent-labeled peptides as an alternative to antibodies have shown promising results in vitro and in vivo [30–32]. Antibody development is costly, and immunolocalization often relies on secondary antibodies, which limit the combinations of primary antibodies that can be co-localized together. Furthermore, the cell wall can interfere with large molecules such as antibodies entering the cell. Short fluorescent-labeled peptides could therefore provide an alternative to antibodies for immunolocalization.
Conclusion
The phage display results together with the experiments with synthesized peptides show that the maize RAD51A1 protein has the capacity to bind to a variety of different small peptides. The alignment of phage peptide #2 with a putative binding domain of BRCA2 suggests that this may be the region of BRCA2 to which RAD51A1 binds. DNA-binding and plant defense related proteins were found among the set of proteins closely aligning with the phage peptides and the set of proteins containing the synthesized peptides; this supports the idea of a dual function for RAD51 in DNA repair and plant defense.
The most intriguing finding of this series of experiments was that a synthesized peptide sequence found in the defense-related transcription factor MYC2, as well as a second bHLH transcription factor of uncharacterized function, binds to RAD51A1 in a dot blot experiment. A synthesized peptide sequence found in a WRKY transcription factor, from a family of transcription factors often involved in defense, also bound to RAD51A1. This is congruous with the fact that in Arabidopsis, RAD51 appears to function as a transcription factor during defense; interactions with defense-related transcription factors would help to explain this function. The synthesized peptides have an apparent pattern of aligning to regions of transcription factors that are not conserved domains; RAD51A1 may bind to the non-conserved portions of these proteins, leaving their conserved domains free to bind other ligands including DNA. In contrast, phage peptide #2 aligns with a conserved binding domain of maize BRCA2.
At present, the most common uses of phage display are in medicine and immunology, including developing antibodies [29, 42, 43]. However, phage display is also a powerful tool for basic science research in other organisms, including plants [44]. Phage display can help to discover novel protein-protein interactions in vitro, as well as the motifs or domains that may drive known interactions. For example, these phage display findings suggest a possible RAD51 binding site in a region of maize BRCA2 that aligns closely with a phage peptide sequence that was selected twice for affinity with RAD51. The ability to screen a large random library is especially promising for understanding and predicting the binding affinities of highly polymorphic proteins such as defense genes that are in flux due to an evolutionary arms race.
Methods
Key reagents
The E. coli strain BL21(DE3) was purchased from Novagen (Gibbstown, NJ). The plasmid pET28b carrying the maize RAD51A1 gene with the addition of a C-terminal His-tag, the RAD51A1 protein purified from E. coli and denatured and stored in a mixture of SDS and β-mercaptoethanol. The product of pET28b was previously verified to be RAD51A1 using mass spectrometry. The α -ZmRAD51 antibody from rabbit and the a-rabbit secondary antibody were provided by Dr. Wojciech Pawlowski at Cornell University. The α -ZmRAD51 antibody was generated using the full-length maize RAD51A1 protein from the pET28b plasmid and has been successfully used in both maize RAD51A1 immunolocalization and ZmRAD51A1-ChIP sequencing [18, 45]. Ni-nitrilotriacetic acid (Ni-NTA) agarose was purchased from Qiagen.
The horseradish peroxidase (HRP) conjugated α-His-tag monoclonal antibody from mouse and the Ultra TMB-Blotting Solution were purchased from Thermo Fisher (Waltham, MA). The HRP-conjugated α-rabbit antibody from goat was purchased from Abcam.
A library of M13 bacteriophages expressing random additional 16-amino acid surface peptides was purchased from Creative Biolabs (Piscataway NJ). E. coli strain ER 2738 was obtained from New England Biolabs (Ipswich, MA). The LB medium, isopropyl-β-D-thiogalactoside (IPTG), 5-bromo-4-chloro-3-indolyl-β-D-galactoside (X-gal), polyethylene glycol 8000, tris-HCl, and tetracycline were obtained from Sigma-Aldrich (St. Louis, MO). Synthesized peptides were synthesized by Genscript (Piscataway, NJ, USA).
Expression and purification of His-tagged RAD51A1
The plasmid pET28b carrying the RAD51A1 gene containing a C-terminal His-tag was transformed into BL21(DE3) E. coli. The BL21(DE3) cells were grown at 25 °C in 2YT medium supplemented with 50 μg/mL kanamycin to select for the pET28b plasmid. The presence of the RAD51A1 sequence in the BL21(DE3) cells carrying pET28b was validated by sequencing the ORF of the plasmid. When the OD600 of the culture reached a value of 0.6, IPTG was added to a final concentration of 1 mM. The induced cell culture was grown for three days at 25 °C. The cell cultures were then incubated on ice for 15 min and harvested by centrifugation (4 °C; 30 min; 4000 rpm). The cell pellets were frozen at −20 °C.
The RAD51A1 protein was extracted from the E. coli pellet. The cell pellets were thawed at 4 °C and re-suspended in lysis buffer A (50 mM NaH2PO4, 300 mM NaCl, 10 mM imidazole, 10% glycerol, pH 8.0) with 1 mg/l lysozyme. The cells were then disrupted by sonication in lysis buffer A. Sonication consisted of 10 short bursts of 10 s, each followed by a 30-s cooling interval. The cell lysate was centrifuged (4 °C; 90 min; 4300 × g), and the supernatant was applied to a Ni-NTA agarose column (Qiagen.com, Catalog #30210) equilibrated with lysis buffer A. After the column was washed with 25 column volumes of buffer B (20 mM imidazole, 50 mM NaH2PO4, 300 mM NaCl, pH 8.0), the RAD51A1 protein was eluted with buffer C (300 mM imidazole, 50 mM NaH2PO4, 300 mM NaCl, pH 8.0). This yielded six 1.5 mL aliquots of eluted RAD51A1. Purity of the eluted RAD51A1 was verified by Western Blot. The remaining eluted RAD51A1 was stored at −20 °C with 20% glycerol.
Western blotting
The eluted proteins were separated by 4–12% SDS-PAGE. Samples of native RAD51A1 from E. coli, eluted in buffer C were loaded in one 12-well NuPAGE gel (Life Technologies). BenchMark™ Pre-stained Protein Ladder (Life Technologies) was used as marker. Proteins were transferred onto a nitrocellulose membrane using the iBlot® 2 Gel Transfer Device. The resulting blots were blocked with 5% BSA in PBS. One blot was probed with a 1:1000 α-ZmRAD51 antibody for one hour, and subsequently incubated with an HRP-conjugated α-rabbit IgG secondary antibody for one hour. A second western blot was performed using an HRP-conjugated α-HisTag monoclonal antibody. The membranes were washed six times with phosphate-buffered saline with Tween® detergent (PBST). Each PBST wash consisted of a five-minute incubation at room temperature on a shaker at 150 rpm. The Ultra TMB blotting substrate was used for color development.
M13 phage display
The library of 2.6E10 M13 bacteriophages were tested for affinity with RAD51A1. This experiment used RAD51A1 produced in E. coli. Three rounds of bio-panning were performed for the selection of RAD51A1-binding peptides (Fig. 2, Fig. S2).
For the first round of selection, 10 μg/mL RAD51A1 protein, previously denatured in SDS and β -mercaptoethanol and suspended in a pH 9.4 coating buffer, was added to each well of a 96-well ELISA plate and incubated overnight at 4 °C. A blocking solution of 3% BSA in PBST, and plates were incubated at room temperature for two hours to prevent non-specific binding. 2E-12 mol of the phage particle solution were added to each well.
The plate was incubated for one hour at room temperature in 2% BSA. Plates were washed three times with PBST for five minutes on a shaker at 150 rpm. Phages were eluted by a 10-min incubation at room temperature with 10 mM acetic acid (pH 2.2) and pH-neutralized with 1 M Tris-HCl (pH 8.0).
For the second round of selection, the eluted phages from the first round of selection were screened for affinity a second time in a similar 96-well plate trial. This was similar to the first round of selection but with the concentration of denatured RAD51A1 was decreased to 1 μg/mL. The plates were washed six times with PBST as described above.
For the final round of selection, the eluted phages from the second round of selection were screened for affinity with 1 μg/mL denatured RAD51A1 immobilized on a small PVDF membrane disc to prevent selection of phages with affinity for the plastic material of the ELISA plate. The membrane was placed in a flask and incubated for one hour in 2% BSA and washed three times with PBST for five minutes on a shaker at 150 rpm. Phages were eluted in acetic acid and Tris-HCl as in the previous rounds of selection.
Phage amplification, purification, and titration
E. coli strain ER2738 cells were cultured in LB broth medium with 20 μg/mL of tetracycline. The cells were grown until the early-log phase (OD600 = 0.5), then used as host cells. The phages from the phage display, suspended in acetic acid and Tris-HCl, were added to the culture and co-cultured in a shaking incubator for four hours at 37 °C at 200 rpm.
The culture was centrifuged at 8000 rpm for 10 min to remove E. coli ER2738 cells. The supernatant solution was added to a PEG/NaCl solution at a 5:1 ratio and incubated at 4 °C for two hours. The resulting phage precipitate was recovered by centrifuging the sample at 4000 rpm for 30 min, removing the supernatant. The pellet was re-suspended in 2 mL of Tris-buffered saline (pH 8.0). The amplified phage product was stored at 4 °C.
LB broth medium with E. coli ER2738 was dispensed into a 96 well plate for dilution. Serial dilutions (1:10) of the suspended phage products from each selected colony were inoculated on pre-warmed (37 °C) LB agar plates with 3 μL each of IPTG, X-gal, and tetracycline. Phage titer was calculated according to the plaque count on each plate.
Serial dilutions (1:10) of the suspended phage products from each selected colony were mixed into tubes of soft 7.5 g/l agar maintained at 45 °C. Each culture tube was then vortexed, and the soft agar was poured onto LB agar plates. The plates were cooled for five minutes, inverted, and then incubated overnight at 37 °C. Single plaque phages on the plates were counted on the next day.
DNA Sequencing of Phages
The DNA sequencing was conducted at Molecular Cloning Laboratories (MCLAB, South San Francisco, CA) using rolling circle amplification followed by chain-termination sequencing. The 96 gIII primer was used for sequencing [29].
ELISA screening to verify phage affinity
ELISA with an HRP-conjugated α-M13 antibody was conducted to verify in vitro interaction between selected M13 phages and both native and denatured RAD51A1. The negative control wells were incubated with BSA. The 96-well ELISA plates were coated with 100 μg/mL of either native or denatured RAD51A1 in 0.2 M Na2CO3 buffer (pH 9.5) and incubated overnight at 4 °C. All wells were subsequently washed with PBST and blocked with 3% BSA for one hour at room temperature. After four washes with PBST, suspended phage solutions from the selected colonies were added and the plates were incubated for one hour at room temperature to allow binding. Four more PBST washes were used to remove unbound phages. To detect bound phages, plates were incubated for two hours with a 1:5000 dilution of HRP-conjugated α-M13 antibody (GE Healthcare) and washed four times with PBST. Negative control wells were incubated with BSA. The Ultra TMB-Blotting Solution was applied to all wells for color development.
Dot blotting to verify phage affinity
Affinity of the RAD51A1 protein for the selected peptides was verified by dot blotting on nitrocellulose. A nitrocellulose membrane (Thermo Scientific) was prepared with 2 μl of each suspended phage solution and dried at room temperature for 30 min. The membrane was then incubated in 10 mL of blocking buffer (3% BSA in PBS) for one hour at room temperature, followed by three washes of 15 min each with 15 mL of PBST The next incubation was with a 1:1000 dilution of the α-ZmRAD51 antibody from rabbit in PBS with 3% BSA for one hour with gentle agitation. Three washes of 15 min each with 15 mL of PBST followed. Finally, the membrane was incubated with 1:2000 diluted HRP-conjugated goat α-rabbit antibody, washed three times in PBST as previously described, and incubated with 5 mL TMB-Blotting substrate solution with gentle agitation. When color appeared on the dots, the reaction was stopped by adding water.
Bioinformatic analysis of phage peptide sequences
The phage peptide sequences were used as BLASTp queries in a search of the maize proteome as well as against the set of non-redundant peptides from all available organisms. Uniprot and tBLASTn were also used to screen the selected peptides against known and predicted maize peptides. The pFam database was used to determine whether any of the selected peptides represented known conserved protein domains [7]. BLASTp and ClustalW were both used to align the resulting peptides to the newly confirmed single maize BRCA2 gene Zm00001d024953_P024, and to RAD51A1.
Design, synthesis, and binding affinity of synthesized maize peptides
Initial BLASTp results revealed several maize proteins that closely but not identically aligned with the selected phage sequences. The most closely aligned subject sequences from the potentially interacting proteins were identified. These 32 short (13–20 amino acids) peptides were synthesized by Genscript (Piscataway, NJ, USA). The N-terminal was modified by acetylation and C-terminal was modified by amidation. Genscript guarantees purity of at least 85% for each peptide.
Synthesized peptides were dot blotted with RAD51A1 to determine if these synthesized peptide sequences, thought to be present in proteins actually produced by the maize plant, have in vitro binding affinity for maize RAD51A1, using the protocol described above.
Supplementary Information
Additional file 1: Figure S1. ELISA screening of phages with native and denatured RAD51A1 proteins. Wells with an OD450 1.5-fold or more above the BSA control were coded as positive. Phages 4, 5, 6, 7, 14, 15, 16, 19, 20, 21, 22 and 23 were positive against both native and denatured protein. Phages 9, 10, 13, and 17 were positive against denatured protein only. Figure S2. Phage dot blotting with native RAD51A1. (1) supernatant (2) pellet (3) flow-through (4) first elution and (5) second elution. Phages 5,8,13,14, and 20 bound to the protein supernatant, protein pellet, flow-through, as well as the first elution. This could be due to RAD51A1 present in these stages of the purification process, or due to the fact that the earlier stages of the purification process would contain E. coli host proteins and these phages which live in E. coli may interact with E. coli proteins. High imidazole concentration may have inhibited localization of the protein on the membrane in the second elution, resulting in false negatives. Table S1. Proteins from non-maize species with alignments to selected phage peptides in Table 1. Figure S3. Dot blotting of 32 synthesized peptides with RAD51A1 on nitrocellulose. Amino acid sequences of all 32 peptides are listed in Table 3. Some peptides were blotted twice. Peptides 1, 2, 3, 7, 8, 11, 12, 15, 18, 19, 20, 25, 30, and 31 bound to RAD51A1. These 14 peptides are listed in Table 4. Note: Two dot blotting experiments were conducted due to the availability of synthesized peptides, GenScript provided two batches of peptide products
Acknowledgements
Dr. Nathan Springer and Dr. Deborah Samac of University of Minnesota provided valuable guidance on analysis and writing. We would like to thank our lab-mates and other colleagues with whom we discussed this work. This research has been sponsored by the National Science Foundation (IOS-1025881 & IOS-1546792). CM was also supported by the Plant and Microbial Biology Graduate Program at the University of Minnesota.
Abbreviations
- DSB
Double-strand break
- HRP
Horseradish peroxidase
- PBST
Phosphate-buffered saline with Tween® detergent
Authors’ contributions
CC, BD designed this study. YH and WP provided necessary materials. BD and CC performed the bench work and initial data analysis. CM performed the analysis and drafted most of the manuscript with assistance from BD on the methods section. CC, WP, LY helped writing the manuscript. All authors reviewed the manuscript. The authors read and approved the final manuscript.
Funding
This work was supported by the National Science Foundation (IOS-1025881 & IOS-1546792).
Availability of data and materials
All data generated or analyzed during this study are included in this article and its supplementary information files.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Claire Milsted and Bo Dai contributed equally to this work.
References
- 1.Sung P. Catalysis of ATP-Dependent Homologous DNA Pairing and Strand Exchange by Yeast RAD51. 1994. [DOI] [PubMed] [Google Scholar]
- 2.Li J, Harper LC, Golubovskaya I, Wang CR, Weber D, Meeley RB, et al. Functional analysis of maize RAD51 in meiosis and double-strand break repair. Genetics. 2007;176:1469–1482. doi: 10.1534/genetics.106.062604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dray E, Siaud N, Dubois E, Doutriaux M-P. Interaction between Arabidopsis Brca2 and its partners Rad51, Dmc1, and Dss1. Plant Physiol. 2006;140:1059–1069. doi: 10.1104/pp.105.075838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siaud N, Dray E, Gy I, Gérard E, Takvorian N, Doutriaux M-P. Brca2 is involved in meiosis in Arabidopsis thaliana as suggested by its interaction with Dmc1. EMBO J. 2004;23:1392–1401. doi: 10.1038/sj.emboj.7600146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fu R, Wang C, Shen H, Zhang J, Higgins JD, Liang W. Rice OsBRCA2 Is Required for DNA Double-Strand Break Repair in Meiotic Cells. Front Plant Sci. 2020;11:600820. doi: 10.3389/fpls.2020.600820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bork P, Blomberg N, Nilges M. Internal repeats in the BRCA2 protein sequence. Nat Genet. 1996;13:22–23. doi: 10.1038/ng0596-22. [DOI] [PubMed] [Google Scholar]
- 7.Mistry J, Chuguransky S, Williams L, Qureshi M, Salazar GA, Sonnhammer ELL, et al. Pfam: The protein families database in 2021. Nucleic Acids Res. 2021;49:D412–D419. doi: 10.1093/nar/gkaa913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coordinators NR. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2018;46:Database issue:D8. doi: 10.1093/NAR/GKX1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shin DS, Pellegrini L, Daniels DS, Yelent B, Craig L, Bates D, et al. Full-length archaeal Rad51 structure and mutants: Mechanisms for RAD51 assembly and control by BRCA2. EMBO J. 2003;22:4566–4576. doi: 10.1093/emboj/cdg429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong AK, Pero R, Ormonde PA, Tavtigian SV, Bartel PL. RAD51 interacts with the evolutionarily conserved BRC motifs in the human breast cancer susceptibility gene brca2. J Biol Chem. 1997;272:31941–31944. doi: 10.1074/JBC.272.51.31941. [DOI] [PubMed] [Google Scholar]
- 11.Stahl F. Meiotic recombination in yeast: Coronation of the double-strand-break repair model. Cell. 1996;87:965–968. doi: 10.1016/S0092-8674(00)81791-2. [DOI] [PubMed] [Google Scholar]
- 12.Mercier R, Mézard C, Jenczewski E, Macaisne N, Grelon M. The Molecular Biology of Meiosis in Plants. Annu Rev Plant Biol. 2015;66:297–327. doi: 10.1146/annurev-arplant-050213-035923. [DOI] [PubMed] [Google Scholar]
- 13.Bleuyard JY, Gallego ME, Savigny F, White CI. Differing requirements for the Arabidopsis Rad51 paralogs in meiosis and DNA repair. Plant J. 2005;41:533–545. doi: 10.1111/j.1365-313X.2004.02318.x. [DOI] [PubMed] [Google Scholar]
- 14.Shinohara A, Ogawa H, Ogawa T. Rad51 protein involved in repair and recombination in S. cerevisiae is a RecA-like protein. Cell. 1992;69:457–470. doi: 10.1016/0092-8674(92)90447-K. [DOI] [PubMed] [Google Scholar]
- 15.Li W, Chen C, Markmann-Mulisch U, Timofejeva L, Schmelzer E, Ma H, et al. The Arabidopsis AtRAD51 gene is dispensable for vegetative development but required for meiosis. Proc Natl Acad Sci U S A. 2004;101:10596–10601. doi: 10.1073/pnas.0404110101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Milsted C, Chen C. Reproductive and somatic functions of RAD51A and BRCA2 genes in maize. 2021. [Google Scholar]
- 17.Lin Z, Kong H, Nei M, Ma H. Origins and evolution of the recA/RAD51 gene family: Evidence for ancient gene duplication and endosymbiotic gene transfer. Proc Natl Acad Sci U S A. 2006;103:10328–33. 10.1073/pnas.0604232103. [DOI] [PMC free article] [PubMed]
- 18.Jing J, Zhang T, Wang Y, Cui Z, He Y. Zmrad51c is essential for double-strand break repair and homologous recombination in maize meiosis. Int J Mol Sci. 2019;20. 10.3390/ijms20215513. [DOI] [PMC free article] [PubMed]
- 19.Sidhu GK, Warzecha T, Pawlowski WP. Evolution of meiotic recombination genes in maize and teosinte. BMC Genomics. 2017;18:1–17. doi: 10.1186/s12864-017-3486-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang S, Durrant WE, Song J, Spivey NW, Dong X. Arabidopsis BRCA2 and RAD51 proteins are specifically involved in defense gene transcription during plant immune responses. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu F, Xu Y, Zhou L, Ali A, Jiang H, Zhu S, et al. DNA repair gene ZmRAd51A improves rice and arabidopsis resistance to disease. Int J Mol Sci. 2019;20. 10.3390/ijms20040807. [DOI] [PMC free article] [PubMed]
- 22.Lucht JM, Mauch-Mani B, Steiner HY, Metraux JP, Ryals J, Hohn B. Pathogen stress increases somatic recombination frequency in Arabidopsis. Nat Genet. 2002;30:311–314. doi: 10.1038/ng846. [DOI] [PubMed] [Google Scholar]
- 23.Mittler R, Lam E. Characterization of nuclease activities and DNA fragmentation induced upon hypersensitive response cell death and mechanical stress. Kluwer Academic Publishers; 1997. [DOI] [PubMed] [Google Scholar]
- 24.Durrant WE, Wang S, Dong X. Arabidopsis SNI1 and RAD51D regulate both gene transcription and DNA recombination during the defense response. Proc Natl Acad Sci U S A. 2007;104:4223–4227. doi: 10.1073/pnas.0609357104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song J, Durrant WE, Wang S, Yan S, Tan EH, Dong X. DNA repair proteins are directly involved in regulation of gene expression during plant immune response. Cell Host Microbe. 2011;9:115–124. doi: 10.1016/j.chom.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 26.Chen H, He C, Wang C, Wang X, Ruan F, Yan J, et al. RAD51 supports DMC1 by inhibiting the SMC5/6 complex during meiosis. Plant Cell. 2021;33:2869–2882. doi: 10.1093/plcell/koab136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu L, Fernández-Jiménez N, Szymanska-Lejman M, Pelé A, Underwood CJ, Serra H, et al. Natural variation identifies SNI1, the SMC5/6 component, as a modifier of meiotic crossover in Arabidopsis. Proc Natl Acad Sci. 2021;118:2021. doi: 10.1073/PNAS.2021970118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alfaleh MA, Alsaab HO, Mahmoud AB, Alkayyal AA, Jones ML, Mahler SM, et al. Phage Display Derived Monoclonal Antibodies: From Bench to Bedside. Front Immunol. 2020;11:1986. doi: 10.3389/fimmu.2020.01986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee JH, Warner CM, Jin HE, Barnes E, Poda AR, Perkins EJ, et al. Production of tunable nanomaterials using hierarchically assembled bacteriophages. Nat Protoc. 2017;12:1999–2013. doi: 10.1038/nprot.2017.085. [DOI] [PubMed] [Google Scholar]
- 30.Mendive-Tapia L, Wang J, Vendrell M. Fluorescent cyclic peptides for cell imaging. Pept Sci. 2021;113:e24181. doi: 10.1002/PEP2.24181. [DOI] [Google Scholar]
- 31.Baechle D, Cansier A, Fischer R, Brandenburg J, Burster T, Driessen C, et al. Biotinylated fluorescent peptide substrates for the sensitive and specific determination of cathespin D activity. J Pept Sci. 2005;11:166–174. doi: 10.1002/psc.607. [DOI] [PubMed] [Google Scholar]
- 32.Takahashi M, Nokihara K, Mihara H. Construction of a Protein-Detection System Using a Loop Peptide Library with a Fluorescence Label. Chem Biol. 2003;10:53–60. doi: 10.1016/S1074-5521(02)00308-3. [DOI] [PubMed] [Google Scholar]
- 33.Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, et al. Highly accurate protein structure prediction with AlphaFold. Nat. 2021;596:583–9. doi: 10.1038/s41586-021-03819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sasanuma H, Tawaramoto MS, Lao JP, Hosaka H, Sanda E, Suzuki M, et al. A new protein complex promoting the assembly of Rad51 filaments. Nat Commun. 2013;4:1–13. doi: 10.1038/ncomms2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Franklin AE, McElver J, Sunjevaric I, Rothstein R, Bowen B, Zacheus Cande W. Three-dimensional microscopy of the Rad51 recombination protein during meiotic prophase. Plant Cell. 1999;11:809–24. 10.1105/tpc.11.5.809. [DOI] [PMC free article] [PubMed]
- 36.Chen X, Li C, Wang H, Guo Z. WRKY transcription factors: evolution, binding, and action. Phytopathol Res. 2019;1:13. doi: 10.1186/s42483-019-0022-x. [DOI] [Google Scholar]
- 37.Seo E, Choi D, Choi. Functional studies of transcription factors involved in plant defenses in the genomics era. Brief Funct. Genomics. 2015;14:260–267. doi: 10.1093/bfgp/elv011. [DOI] [PubMed] [Google Scholar]
- 38.Cui H, Qiu J, Zhou Y, Bhandari DD, Zhao C, Bautor J, et al. Antagonism of Transcription Factor MYC2 by EDS1/PAD4 Complexes Bolsters Salicylic Acid Defense in Arabidopsis Effector-Triggered Immunity. Mol Plant. 2018;11:1053–1066. doi: 10.1016/j.molp.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 39.Florence B, Faller DV. You BET-cha: A Novel Family Of Transcriptional Regulators. Front Biosci. 2001;6:1008. doi: 10.2741/florence. [DOI] [PubMed] [Google Scholar]
- 40.Taniguchi Y. The bromodomain and extra-terminal domain (BET) family: Functional anatomy of BET paralogous proteins. International Journal of Molecular Sciences. 2016;17. 10.3390/ijms17111849. [DOI] [PMC free article] [PubMed]
- 41.Airoldi CA, Della Rovere F, Falasca G, Marino G, Kooiker M, Altamura MM, et al. The arabidopsis BET bromodomain factor GTE4 is involved in maintenance of the mitotic cell cycle during plant development. Plant Physiol. 2010;152:1320–1334. doi: 10.1104/pp.109.150631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bazan J, Całkosiński I, Gamian A. Phage display--a powerful technique for immunotherapy: 1. Introduction and potential of therapeutic applications. Hum Vaccin Immunother. 2012;8:1817–1828. doi: 10.4161/hv.21703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clackson T, Hoogenboom HR, Griffiths AD, Winter G. Making antibody fragments using phage display libraries. Nature. 1991;352:624–628. doi: 10.1038/352624a0. [DOI] [PubMed] [Google Scholar]
- 44.Bouché N, Yellin A, Snedden WA, Fromm H. Plant-specific calmodulin-binding proteins. Annual Review of Plant Biology. 2005;56:435–66. 10.1146/annurev.arplant.56.032604.144224. [DOI] [PubMed]
- 45.He Y, Wang M, Dukowic-Schulze S, Zhou A, Tiang CL, Shilo S, et al. Genomic features shaping the landscape of meiotic double-strand-break hotspots in maize. Proc Natl Acad Sci U S A. 2017;114:12231–12236. doi: 10.1073/pnas.1713225114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. ELISA screening of phages with native and denatured RAD51A1 proteins. Wells with an OD450 1.5-fold or more above the BSA control were coded as positive. Phages 4, 5, 6, 7, 14, 15, 16, 19, 20, 21, 22 and 23 were positive against both native and denatured protein. Phages 9, 10, 13, and 17 were positive against denatured protein only. Figure S2. Phage dot blotting with native RAD51A1. (1) supernatant (2) pellet (3) flow-through (4) first elution and (5) second elution. Phages 5,8,13,14, and 20 bound to the protein supernatant, protein pellet, flow-through, as well as the first elution. This could be due to RAD51A1 present in these stages of the purification process, or due to the fact that the earlier stages of the purification process would contain E. coli host proteins and these phages which live in E. coli may interact with E. coli proteins. High imidazole concentration may have inhibited localization of the protein on the membrane in the second elution, resulting in false negatives. Table S1. Proteins from non-maize species with alignments to selected phage peptides in Table 1. Figure S3. Dot blotting of 32 synthesized peptides with RAD51A1 on nitrocellulose. Amino acid sequences of all 32 peptides are listed in Table 3. Some peptides were blotted twice. Peptides 1, 2, 3, 7, 8, 11, 12, 15, 18, 19, 20, 25, 30, and 31 bound to RAD51A1. These 14 peptides are listed in Table 4. Note: Two dot blotting experiments were conducted due to the availability of synthesized peptides, GenScript provided two batches of peptide products
Data Availability Statement
All data generated or analyzed during this study are included in this article and its supplementary information files.