Abstract
Background
The triglyceride glucose (TyG) index serves as a surrogate indicator of insulin resistance. However, there is limited evidence on the association between the TyG index and carotid artery plaque (CAP) in patients with coronary heart disease (CHD).
Methods
The 10,535 CHD patients were divided according to TyG index quartiles (Q1: TyG index < 8.52; Q2: 8.52 ≤ TyG index < 8.93; Q3: 8.93 ≤ TyG index ≤ 9.40; Q4: TyG index > 9.40). The presence or absence of CAP was determined by carotid ultrasonography. Logistic regression was used to analyze the relationship between the TyG index and CAP in CHD patients. The relationship between the TyG index and CAP in according to sex, age groups, and glucose metabolism states were also assessed.
Results
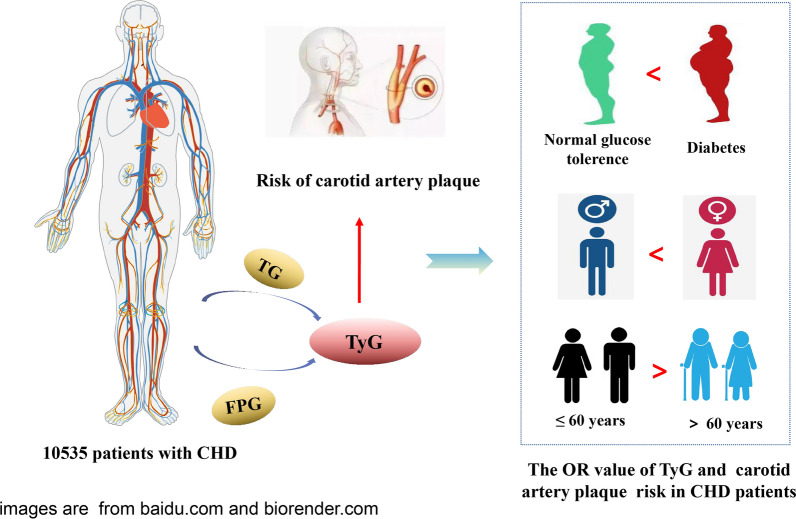
The baseline analysis showed that there were significant differences in related parameters among CHD patients divided into four groups according to the quartile of the TyG index. In the multi-adjusted modles, compared to Q1 of the TyG index, the odds ratios (OR) for Q4 of the TyG index for CAP were 1.37 (95% confidence interval [CI] 1.28–1.47) in CHD patients. The association between the TyG index and CAP in female (OR: 1.35; 95% CI 1.29–1.43) was higher than that in male (OR: 1.20; 95% CI 1.13–1.27). The OR value of middle-aged (≤ 60 years old) patients (OR: 1.34; 95% CI 1.26–1.42) was higher than that in elderly (> 60 years old) patients (OR: 1.16; 95% CI 1.11–1.22). In different glucose metabolism states, the TyG index of CHD patients was significantly related to the risk of CAP, with the highest OR value observed for diabetes (OR: 1.36; 95% CI 1.26–1.46).
Conclusions
The TyG index and CAP showed a significant association in CHD patients. This association between TyG index and CAP in CHD patients is higher in female than in male, and the association in middle-aged and elderly patients is higher than that in elderly patients. In the condition of DM, the association between TyG index and carotid artery plaque in CHD patients is higher.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s12933-022-01470-3.
Keywords: Triglyceride, Fasting plasma glucose, TyG index, Coronary heart disease, Carotid plaques
Background
Coronary heart disease (CHD) is a class of chronic noncommunicable diseases (NCD) with extremely high incidence and mortality rates. Diabetes mellitus (DM) usually coexists with arterial hypertension and dyslipidemia [1, 2]. Type 2 diabetes mellitus (T2DM) accounts for 95% of all the diseases diagnosed with DM. Which is one of the risk factors for coronary artery disease (CAD) and the progression and rupture of atherosclerotic plaques [3]. CAD is also a common comorbidity and major cause of death in DM patients. Studies have shown that in participants without CHD history, T2DM is associated with carotid artery plaques (CAP), which is a better predictor than high carotid artery intima-media thickness (CIMT) or recurrent cardiovascular events [4]. Pre-DM patients have a high propensity for developing DM [5]. Many studies have reported that CHD patients have a higher risk of adverse prognosis in Pre-DM and glucose metabolism disorders [6–8].
The triglyceride glucose (TyG) index is a valuable biomarker for the development of diabetes and is used as a marker of insulin resistance (IR), leading to the occurrence of NCD [9, 10]. The TyG index is related to the high prevalence of CAD, while the increased risk of major adverse cardiovascular and cerebrovascular events (MACEs) [11, 12], including ischemic stroke [13], increased arterial stiffness [14–16], hypertension [17], coronary artery stenosis [18], and carotid atherosclerosis (AS) [19] are related to the morbidity. However, no relevant studies have investigated the association between the TyG index and CAP in CHD patients according to their glucose metabolism statuses.
Therefore, this study aimed to clarify the association between the TyG index and CAP in the different glucose metabolic statuses of CHD patients, and to further investigate the association of TyG index and CAP in the different stratification of gender and age. In the clinical treatment of CHD, there is a need to identify simple biochemical indicators to prevent the risk of AS (such as CAP).
Methods
Patients
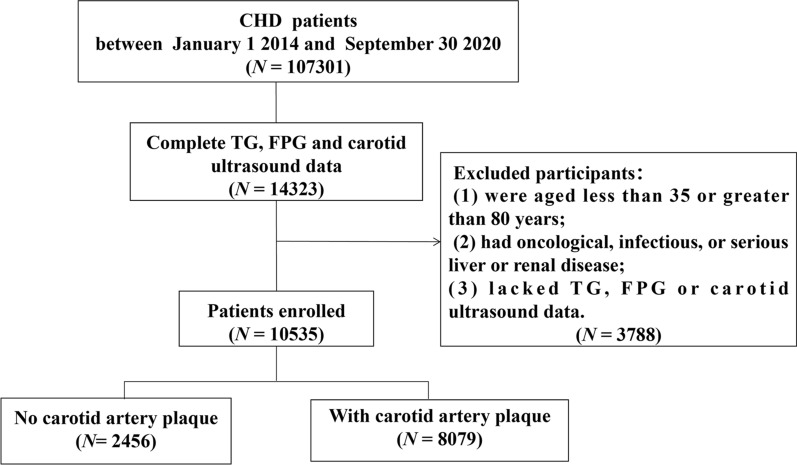
This large-scale, multi-center retrospective cohort study included 107,301 CHD inpatients who were admitted to six hospitals in Tianjin between January 1, 2014, and September 30, 2020. The root investigation study design excluded patients aged less than 35 years or older than 80 years, patients with tumor, infectious, or severe liver or kidney disease, and patients lacking data on triglyceride (TG), fasting plasma glucose (FPG), and carotid ultrasound measurements. A total of 10,535 participants were eventually included in the study. A flowchart of the patients recruitment was shown in Fig. 1. This study was approved by the ethics committee of Tianjin University of Traditional Chinese Medicine (TJUTCM-EC20190008) and registered with the Chinese Clinical Trial Registry (ChiCTR-1900024535) and ClinicalTrials.gov (NCT04026724).
Fig. 1.
Flow chart of patient recruitment
Data collection
In this study, age, sex, smoking, drinking, and medication history of patients were recorded using standard structured questionnaires [20, 21]. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured by experienced technicians at the heart level using automatic blood pressure monitors. SBP ≥ 130 mmHg or DBP ≥ 80 mmHg was defined as hypertension [22].
Fasting venous blood samples were collected early in the morning from all participants. FPG, total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), TG, low-density lipoprotein cholesterol (LDL-C), C-reactionprotein (CRP), and glycated haemoglobin (HbA1c) leves were measured using an automatic haematology analyzer. Standard laboratory procedure for quality control were strictly followed [23]. The TyG index was calculated as Ln[fasting triglycerides (mg/dL) × fasting glucose (mg/dL)/2] [24]. Hyperlipidemia was defined as TC ≥ 6.2 mmol/L (240 mg/dL), TG ≥ 2.3 mmol/L (200 mg/dL), LDL-C ≥ 4.1 mmol/L (160 mg/dL), or HDL-C ≤ 1.0 mmol/L (40 mg/dL) [25]. Normal glucose tolerance (NGT) was defined as FPG < 5.6 mmol/L or HbA1c < 5.7%, Pre-DM was defined as FPG 5.6–6.9 mmol/L or HbA1c of 5.7–6.4%, DM was defined as FPG ≥ 7.0 mmol/L or HbA1c ≥ 6.5% [26].
The carotid ultrasound examinations were performed by a certified professional technician using a diagnostic ultrasound system. In B-mode imaging, the common carotid artery, internal carotid artery, and carotid artery bifurcation were scanned and imaged. CIMT was defined as the average IMT value of the left and right common carotid arteries [27]. Professional doctors analyzed the color of the carotid artery based on Doppler ultrasound results and recorded the number of CAPs and echo characteristics. CAP cases were divided into single (n = 1) and multiple (n ≥ 2). The echogenic properties of the CAP were categorized as hypoechoic, isoechoic, hyperechoic, and mixed types. This study employed rigorous quality control procedures to maintain consistency in the monitoring and test image acquisition and analysis. The inter-laboratory quality was assessed by licensed experimenters.
Statistical analyses
The characteristics of the participants in the different groups were compared using χ2 tests and Kruskal–Wallis H tests. Odds ratios (ORs) and 95% confidence intervals (CIs) of CAP were estimated for the TyG index using logistic regression. Age, sex, SBP, DBP, CRP, HbA1c, TC, HDL-C, LDL-C, smoking, drinking, hypertension, hyperlipidemia, use of antihypertensives, and use of antilipidemic were considered as potential confounders in this association. The collinearity of the different models was tested before logistic regression. Missing values were imputed using the multiple imputation method. All statistical analyses were performed using SPSS 24.0 (IBM Corp, New York, NY, USA).
Results
Baseline characteristics
The basic characteristics of the study population of 10,535 cases are shown in Table 1. The median age of the participants was 64 years old, and the proportion of female (51.4%) was slightly higher than that of male (48.6%). Among them, 8079 (76.69%) patients had CAP, while the highest proportion of DM patients was 37.6%. The subjects were divided into four groups according to the quartile level of the TyG index. Generally speaking, DBP, SBP, FPG, TC, TG, HbA1c, smoking, drinking, hyperlipidemia, use of antihypertensives, and use of antilipidemic were positively associated with the quartile level of the TyG index, while HDL-C was negatively associated with the quartile level of the TyG index.
Table 1.
General characteristics of the study participants according to the TyG index
| Characteristic | Total (N = 10,535) | TyG index | P-value | |||
|---|---|---|---|---|---|---|
| Q1 (n = 2650) | Q2 (n = 2647) | Q3 (n = 2590) | Q4 (n = 2648) | |||
| Sex, n (%) | < 0.001 | |||||
| Male | 5121 (48.6) | 1375 (51.9) | 1256 (47.4) | 1183 (45.7) | 1307 (49.4) | |
| Female | 5414 (51.4) | 1275 (48.1) | 1391 (52.6) | 1407 (54.3) | 1341 (50.6) | |
| Age, years, median (IQR) | < 0.001 | |||||
| Total | 64.0 (59.0–70.0) | 65.0 (60.0–71.0) | 64.0 (59.0–69.0) | 64.0 (59.0–69.0) | 63.0 (57.0–68.0) | |
| ≤ 60 | 55.0 (51.0–58.0) | 55.0 (51.0–58.0) | 55.0 (52.0–58.0) | 55.0 (52.0–58.0) | 55.0 (51.0–57.0) | |
| > 60 | 67.0 (64.0–71.0) | 68.0 (64.0–72.0) | 67.0 (64.0–71.0) | 67.0 (63.0–71.0) | 67.0 (64.0–71.0) | |
| SBP, mmHg, median (IQR) | 140.0 (128.0–156.0) | 139.0 (124.0–152.0) | 140.0 (126.0–155.0) | 140.0 (130.0–159.0) | 141.0 (130.0–159.0) | < 0.001 |
| DBP, mmHg, median (IQR) | 83.0 (77.0–90.0) | 80.0 (75.0–90.0) | 82.0 (76.0–90.0) | 83.0 (78.0–90.0) | 84.0 (78.0–91.0) | < 0.001 |
| CRP, mg/L, median (IQR) | 4.2 (2.0–12.8) | 4.1 (1.9–14.9) | 4.3 (2.0–14.2) | 4.0 (2.0–11.3) | 4.5 (2.2–11.2) | < 0.001 |
| FPG, mmol/L, median (IQR) | 6.2 (5.3–8.1) | 5.3 (4.8–5.9) | 5.8 (5.1–6.8) | 6.54 (5.6–8.1) | 9.4 (7.0–12.6) | < 0.001 |
| LDL-C, mmol/L, median (IQR) | 2.8 (2.1–3.4) | 2.5 (1.9–3.1) | 2.8 (2.2–3.4) | 3.0 (2.3–3.6) | 2.8 (2.2–3.5) | < 0.001 |
| HDL-C, mmol/L, median (IQR) | 1.1 (0.9–1.3) | 1.2 (1.0–1.4) | 1.1 (0.9–1.3) | 1.1 (0.9–1.2) | 1.0 (0.8–1.1) | < 0.001 |
| TG, mmol/L, median (IQR) | 1.4 (1.0–2.1) | 0.9 (0.7–1.0) | 1.3 (1.1–1.5) | 1.8 (1.5–2.1) | 2.6 (2.0–3.5) | < 0.001 |
| TC, mmol/L, median (IQR) | 4.6 (3.8–5.4) | 4.1 (3.4–4.9) | 4.5 (3.8–5.2) | 4.7 (4.0–5.5) | 5.0 (4.2–5.7) | < 0.001 |
| HbA1c, mmol/L, median (IQR) | 6.0 (5.6–7.0) | 5.7 (5.4–6.2) | 5.9 (5.5–6.5) | 6.1 (5.6–6.9) | 7.1 (6.1–8.6) | < 0.001 |
| TyG index | 9.0 (8.5–9.4) | 8.2 (8.0–8.4) | 8.7 (8.6–8.8) | 9.1 (9.0–9.3) | 9.8 (9.6–10.2) | < 0.001 |
| Smoking, n (%) | 4590 (43.6) | 1173 (44.3) | 1121 (42.3) | 1101 (42.5) | 1195 (45.1) | < 0.001 |
| Drinking, n (%) | 5863 (55.7) | 1457 (55) | 1480 (55.9) | 1421 (54.9) | 1505 (56.8) | < 0.001 |
| Hypertension, n (%) | 8875 (84.2) | 2114 (23.8) | 2201 (24.8) | 2241(25.3) | 2319 (26.1) | < 0.001 |
| Hyperlipidemia, n (%) | 5770 (54.8) | 690 (12.0) | 961 (16.7) | 1326 (23.0) | 1981 (34.3) | < 0.001 |
| Use of antihypertensives, n (%) | 5239 (49.7) | 1160 (43.8) | 1240 (46.8) | 1363 (52.6) | 1476 (55.7) | < 0.001 |
| Use of antilipidemic, n (%) | 7787 (73.9) | 1703 (64.3) | 1947 (73.6) | 2026 (78.2) | 2111 (79.7) | < 0.001 |
| CIMT, mm, median (IQR) | 0.10 (0.09–0.12) | 0.10 (0.09–0.12) | 0.10 (0.09- 0.12) | 0.10 (0.09–0.12) | 0.10 (0.09–0.12) | 0.742 |
| Carotid artery plaque, n (%) | 8079 (76.69) | 1985 (74.91) | 1996 (75.41) | 2006 (77.45) | 2092 (79.00) | < 0.001 |
| Glucose regulation state, n (%) | < 0.001 | |||||
| Normal glucose regulation | 3756 (35.7) | 1735 (65.5) | 1160 (43.8) | 654 (25.3) | 207 (7.8) | |
| Prediabetes | 2813 (26.7) | 694 (26.2) | 861 (32.5) | 834 (32.2) | 424 (16.0) | |
| Diabetes | 3966 (37.6) | 221 (8.3) | 626 (23.7) | 1102 (42.5) | 2017 (76.2) | |
| No. of carotid artery plaque, n (%) | < 0.001 | |||||
| 0 | 2456 (23.3) | 665 (25.1) | 651 (24.6) | 584 (22.5) | 556 (21) | |
| 1 | 406 (3.9) | 96 (3.6) | 100 (3.8) | 100 (3.9) | 110 (4.2) | |
| ≥ 2 | 7673 (72.8) | 1889 (71.3) | 1896 (71.6) | 1906 (73.6) | 1982 (74.8) | |
| Carotid artery plaque echo property, n (%) | < 0.001 | |||||
| Hypoechoic plaque | 497 (4.7) | 114 (4.3) | 131 (4.9) | 130 (5) | 122 (4.6) | |
| Isoechoic plaque | 548 (5.2) | 137 (5.2) | 133 (5) | 136 (5.3) | 142 (5.4) | |
| Hyperechoic plaque | 4792 (45.5) | 1211 (45.7) | 1162 (43.9) | 1194 (46.1) | 1225 (46.3) | |
| Mixture plaque | 2242 (21.3) | 523 (19.7) | 570 (21.5) | 546 (21.1) | 603 (22.8) | |
Data are presented as median (interquartile) or number (proportion, %)
Q1: TyG index < 8.52, Q2: 8.52 ≤ TyG index < 8.93, Q2: 8.93 ≤ TyG index ≤ 9.40, Q4: TyG index > 9.40
TyG, triglyceride-glucose; SBP, systolic blood pressure; DBP, diastolic blood pressure; CRP, C-reactionprotein; FPG, fasting plasma glucose; TC, total cholesterol; TG, triglycerides; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; HbA1c, glycated haemoglobin; IQR, interquartile range
Association between the TyG index and the risk of carotid artery plaques
As shown in Table 2, in multivariate logistic regression analysis, when the TyG index was used as a continuous variable, it was significantly associated with the risk of CAP (OR: 1.29; 95% CI 1.24–1.34). The association between the TyG index and CAP was further explored using the TyG index as a categorical variable. Multivariate logistic regression analysis showed that the TyG index levels for Q3 and Q4 were associated with an increased OR for CAP when Q1 was used as a reference, with the highest association observed for Q4 (OR: 1.37; 95% CI 1.28–1.47). In both unadjusted and adjusted models, the TyG index (quartiles) was consistent with the P for trend of the CAP, when the TyG index was served as a continuous variable (P < 0.001). The association of TyG index with the number and echoproperties of carotid plaques were further evaluated. The results show that the association remained significant (Adittional file 1: Table S1–S2).
Table 2.
Association between the TyG index and the risk of carotid artery plaques
| Variables | Carotid artery plaques | |||||
|---|---|---|---|---|---|---|
| OR (95% CI)a | P-value | OR (95% CI)b | P-value | OR (95% CI)c | P-value | |
| TyG index | 1.14 (1.11–1.17) | < 0.001 | 1.28 (1.24–1.31) | < 0.001 | 1.29 (1.24–1.34) | < 0.001 |
| Q1 | Reference | Reference | Reference | |||
| Q2 | 1.00 (0.95–1.05) | 0.969 | 1.10 (1.04–1.16) | 0.537 | 1.01 (0.96–1.07) | 0.682 |
| Q3 | 1.09 (1.04–1.15) | 0.001 | 1.22 (1.16–1.29) | 0.013 | 1.07 (1.01–1.14) | 0.025 |
| Q4 | 1.22 (1.16–1.28) | < 0.001 | 1.51 (1.43–1.59) | < 0.001 | 1.37 (1.28–1.47) | < 0.001 |
| P-trend | < 0.001 | < 0.001 | < 0.001 | |||
aModel 1: unadjusted
bModel 2: adjusted for sex, age, SBP, DBP
cModel 3: adjusted for sex, age, SBP, DBP, CRP, TC, HDL-C, LDL-C, smoking, drinking, hypertension, hyperlipidemia, use of antihypertensives, and use of antilipidemic
Regardless of sex, this relationship remained statistically significant after adjusting for variables. As shown in Table 3, after multivariate adjustment, the association between the TyG index and CAP in female (OR: 1.35; 95% CI 1.29–1.43), which was higher than that in male (OR: 1.20; 95% CI 1.13–1.27). As shown in Table 4, after multivariate adjustment, the TyG index of CHD patients was significantly associated with CAP at different ages. The OR value of middle-aged (≤ 60 years old) patients (OR: 1.34; 95% CI 1.26–1.42) was higher than that of elderly (> 60 years old) patients (OR: 1.16; 95% CI 1.11–1.22). For both sexes and different ages, using Q1 as the reference, Q4 was significantly related to the increased risks of CAP, even after multivariate adjustment, this relationship remained significant.
Table 3.
Association between the TyG index and the risk of carotid artery plaques according to sex
| Sex | Variables | Carotid artery plaques | |||||
|---|---|---|---|---|---|---|---|
| OR (95% CI)a | P-value | OR (95% CI)b | P-value | OR (95% CI)c | P-value | ||
| Male | TyG index | 0.94 (0.91–0.98) | 0.003 | 1.14 (1.10–1.19) | < 0.001 | 1.20 (1.13–1.27) | < 0.001 |
| Q1 | Reference | Reference | Reference | ||||
| Q2 | 1.00 (0.91–1.07) | 0.789 | 1.13 (1.04–1.23) | 0.004 | 1.05 (0.96–1.15) | 0.268 | |
| Q3 | 0.95 (0.88–1.03) | 0.213 | 1.17 (1.07–1.28) | < 0.001 | 1.03 (0.94–1.13) | 0.548 | |
| Q4 | 0.87 (0.80–0.94) | < 0.001 | 1.23 (1.13–1.34) | < 0.001 | 1.18 (1.06–1.31) | 0.003 | |
| Female | TyG index | 1.34 (1.30–1.39) | < 0.001 | 1.38 (1.33–1.44) | < 0.001 | 1.35 (1.29–1.43) | < 0.001 |
| Q1 | Reference | Reference | Reference | ||||
| Q2 | 1.08 (1.01–1.15) | 0.019 | 1.08 (1.01–1.16) | 0.031 | 0.99 (0.92–1.07) | 0.875 | |
| Q3 | 1.31 (1.23–1.40) | < 0.001 | 1.25 (1.17–1.34) | < 0.001 | 1.10 (1.01–1.19) | 0.019 | |
| Q4 | 1.62 (1.52–1.74) | < 0.001 | 1.74 (1.61–1.87) | < 0.001 | 1.52 (1.38–1.66) | < 0.001 | |
aModel 1: unadjusted
bModel 2: adjusted for age, SBP, DBP
cModel 3: adjusted for age, SBP, DBP, CRP, TC, HDL-C, LDL-C, smoking, drinking, hypertension, hyperlipidemia, use of antihypertensives, and use of antilipidemic
Table 4.
Association between the TyG index and the risk of carotid artery plaques according to age
| Age | Variables | Carotid artery plaques | |||||
|---|---|---|---|---|---|---|---|
| OR (95% CI)a | P-value | OR (95% CI)b | P-value | OR (95% CI)c | P-value | ||
| ≤ 60 | TyG index | 1.37 (1.32–1.42) | < 0.001 | 1.27 (1.22–1.32) | < 0.001 | 1.34 (1.26–1.42) | < 0.001 |
| Q1 | Reference | Reference | Reference | ||||
| Q2 | 1.19 (1.09–1.29) | < 0.001 | 1.18 (1.08–1.30) | < 0.001 | 1.05(0.95–1.16) | 0.359 | |
| Q3 | 1.59 (1.45–1.73) | < 0.001 | 1.45 (1.32–1.59) | < 0.001 | 1.23 (1.11–1.37) | < 0.001 | |
| Q4 | 1.90 (1.75–2.06) | < 0.001 | 1.68 (1.54–1.83) | < 0.001 | 1.54 (1.37–1.72) | < 0.001 | |
| > 60 | TyG index | 1.16 (1.12–1.21) | < 0.001 | 1.21 (1.16–1.25) | < 0.001 | 1.16 (1.11–1.22) | < 0.001 |
| Q1 | Reference | Reference | Reference | ||||
| Q2 | 0.99 (0.93–1.06) | 0.840 | 1.05 (1.00–1.12) | 0.170 | 0.98 (0.91–1.05) | 0.563 | |
| Q3 | 0.99 (0.93–1.05) | 0.720 | 1.07 (1.00–1.15) | 0.040 | 0.95 (0.88–1.02) | 0.161 | |
| Q4 | 1.23 (1.15–1.32) | < 0.001 | 1.30 (1.21–1.39) | < 0.001 | 1.15 (1.06–1.26) | 0.002 | |
aModel 1: unadjusted
bModel 2: adjusted for sex, SBP, DBP
cModel 3: adjusted for sex, SBP, DBP, CRP, TC, HDL-C, LDL-C, smoking, drinking, hypertension, hyperlipidemia, use of antihypertensives, and use of antilipidemic
Association between the TyG index and CAP according to glucose regulation state
As shown in Table 5, after multivariate adjustment showed significant associations between the TyG index and the risk of CAP in CHD patients according to different glucose metabolism states, with the highest OR value observed for DM (OR: 1.36; 95% CI 1.26–1.46). Taking the Q1 as a reference, Q4 was significantly associated with an increased risk of CAP during the DM.
Table 5.
Association between TyG index and the risk of carotid artery plaques according to glucose regulation state
| Glucose regulation state | Variables | Carotid artery plaques | |||||
|---|---|---|---|---|---|---|---|
| OR (95% CI)a | P-value | OR (95% CI)b | P-value | OR (95% CI)c | P-value | ||
| Normal glucose regulation | TyG index | 0.95 (0.90–1.01) | 0.080 | 1.16 (1.09–1.24) | < 0.001 | 1.11 (1.02–1.22) | 0.017 |
| Q1 | Reference | Reference | Reference | ||||
| Q2 | 0.98 (0.92–1.05) | 0.526 | 1.11 (1.03–1.19) | 0.007 | 1.04 (0.95–1.13) | 0.412 | |
| Q3 | 0.94 (0.86–1.02) | 0.112 | 1.18 (1.08–1.29) | < 0.001 | 1.03 (0.93–1.15) | 0.563 | |
| Q4 | 0.81 (0.71–0.92) | 0.001 | 1.11 (0.97–1.28) | 0.129 | 1.11 (0.93–1.32) | 0.259 | |
| Prediabetes | TyG index | 0.87 (0.82–0.92) | < 0.001 | 1.06 (0.99–1.14) | 0.078 | 1.08 (0.97–1.19) | 0.182 |
| Q1 | Reference | Reference | Reference | ||||
| Q2 | 0.90 (0.83–0.99) | 0.032 | 1.06 (0.96–1.17) | 0.288 | 0.94 (0.85–1.05) | 0.295 | |
| Q3 | 1.02 (0.93–1.12) | 0.645 | 1.23 (1.11–1.37) | < 0.001 | 1.06 (0.94–1.20) | 0.330 | |
| Q4 | 0.69 (0.62–0.77) | < 0.001 | 0.99 (0.88–1.12) | 0.879 | 0.90 (0.76–1.06) | 0.202 | |
| Diabetes | TyG index | 1.16 (1.11–1.22) | < 0.001 | 1.36 (1.30–1.43) | < 0.001 | 1.36 (1.26–1.46) | < 0.001 |
| Q1 | Reference | Reference | Reference | ||||
| Q2 | 1.11 (0.97–1.28) | 0.142 | 1.26 (1.09–1.47) | 0.003 | 1.13 (0.96–1.33) | 0.147 | |
| Q3 | 1.18 (1.03–1.35) | 0.017 | 1.37 (1.19–1.58) | < 0.001 | 1.07 (0.91–1.25) | 0.408 | |
| Q4 | 1.39 (1.22–1.58) | < 0.001 | 1.87 (1.63–2.15) | < 0.001 | 1.42 (1.21–1.66) | < 0.001 | |
aModel 1: unadjusted
bModel 2: adjusted for age, sex, SBP, DBP
cModel 3: adjusted for age, sex, SBP, DBP, CRP, TC, HDL-C, LDL-C, smoking, drinking, hypertension, hyperlipidemia, use of antihypertensives, and use of antilipidemic
This study also observed a significant association between TyG index and CAP risk in male patients with NGT (OR: 1.21; 95% CI 1.06–1.39). The association in females (OR: 1.45; 95% CI 1.32–1.60) with DM status was higher than in males (OR: 1.23; 95% CI 1.10–1.37) (Table 6). The association with CAP in CHD patients with DM status aged > 60 years old (OR: 1.35; 95% CI 1.24–1.48) with DM status was higher than those aged ≤ 60 years old (OR: 1.21; 95% CI 1.08–1.35) (Table 7).
Table 6.
Association between the TyG index and the risk of carotid artery plaques according to different glucose regulation state and sex
| Sex | Glucose regulation state | Variables | Carotid artery plaques | |||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI)a | P-value | OR (95% CI)b | P-value | OR (95% CI)c | P-value | |||
| Male | Normal glucose regulation | TyG index | 0.88 (0.81–0.96) | 0.005 | 1.24 (1.13–1.36) | < 0.001 | 1.21 (1.06–1.39) | 0.005 |
| Q1 | Reference | Reference | Reference | |||||
| Q2 | 0.98 (0.88–1.10) | 0.751 | 1.23 (1.09–1.38) | 0.001 | 1.06 (0.93–1.21) | 0.398 | ||
| Q3 | 0.79 (0.69–0.90) | < 0.001 | 1.10 (0.96–1.27) | 0.182 | 0.83 (0.70–0.96) | 0.033 | ||
| Q4 | 0.57 (0.48–0.68) | < 0.001 | 1.00 (0.82–1.22) | 0.974 | 0.91 (0.69–1.19) | 0.494 | ||
| Prediabetes | TyG index | 0.67 (0.61–0.74) | < 0.001 | 0.97 (0.87–1.07) | 0.505 | 0.95 (0.82–1.12) | 0.557 | |
| Q1 | Reference | Reference | Reference | |||||
| Q2 | 0.94 (0.81–1.10) | 0.420 | 1.05 (0.89–1.24) | 0.552 | 0.94 (0.79–1.12) | 0.511 | ||
| Q3 | 0.85 (0.73–0.99) | 0.038 | 1.18 (1.00–1.39) | 0.055 | 1.02 (0.84–1.23) | 0.846 | ||
| Q4 | 0.49 (0.42–0.58) | < 0.001 | 0.83 (0.69–1.00) | 0.045 | 0.67 (0.52–0.86) | 0.002 | ||
| Diabetes | TyG index | 0.91 (0.84–0.97) | 0.005 | 1.11 (1.03–1.19) | 0.009 | 1.23 (1.10–1.37) | < 0.001 | |
| Q1 | Reference | Reference | Reference | |||||
| Q2 | 0.99 (0.78–1.24) | 0.904 | 1.19 (0.94–1.51) | 0.143 | 1.13 (0.88–1.45) | 0.331 | ||
| Q3 | 1.06 (0.85–1.32) | 0.592 | 1.34 (1.07–1.67) | 0.011 | 1.13 (0.90–1.44) | 0.284 | ||
| Q4 | 0.98 (0.79–1.20) | 0.821 | 1.48 (1.19–1.84) | < 0.001 | 1.37 (1.08–1.74) | 0.010 | ||
| Female | Normal glucose regulation | TyG index | 1.05 (0.97–1.14) | 0.198 | 1.09 (1.00–1.19) | 0.046 | 1.02 (0.90–1.15) | 0.794 |
| Q1 | Reference | Reference | Reference | |||||
| Q2 | 1.06 (0.97–1.15) | 0.223 | 1.04 (0.94–1.14) | 0.469 | 1.01 (0.91–1.23) | 0.825 | ||
| Q3 | 1.17 (1.06–1.30) | 0.003 | 1.22 (1.09–1.37) | 0.001 | 1.16 (1.01–1.33) | 0.043 | ||
| Q4 | 1.03 (0.86–1.24) | 0.739 | 1.23 (1.01–1.5) | 0.041 | 1.22 (0.95–1.56) | 0.115 | ||
| Prediabetes | TyG index | 1.05 (0.97–1.14) | 0.244 | 1.14 (1.04–1.24) | 0.005 | 1.17 (1.02–1.35) | 0.023 | |
| Q1 | Reference | Reference | Reference | |||||
| Q2 | 0.94 (0.84–1.06) | 0.327 | 1.05 (0.93–1.20) | 0.410 | 0.93 (0.81–1.07) | 0.336 | ||
| Q3 | 1.22 (1.08–1.38) | 0.001 | 1.26 (1.11–1.44) | < 0.001 | 1.10 (0.94–1.29) | 0.222 | ||
| Q4 | 0.89 (0.77–1.03) | 0.127 | 1.12 (0.96–1.31) | 0.145 | 1.12 (0.89–1.40) | 0.328 | ||
| Diabetes | TyG index | 1.44 (1.35–1.53) | < 0.001 | 1.58 (1.48–1.69) | < 0.001 | 1.45 (1.32–1.60) | < 0.001 | |
| Q1 | Reference | Reference | Reference | |||||
| Q2 | 1.31 (1.08–1.59) | 0.005 | 1.30 (1.06–1.59) | 0.012 | 1.14 (0.91–1.42) | 0.261 | ||
| Q3 | 1.44 (1.21–1.73) | < 0.001 | 1.38 (1.14–1.67) | 0.001 | 1.01 (0.82–1.25) | 0.930 | ||
| Q4 | 1.97 (1.65–2.34) | < 0.001 | 2.15 (1.78–2.58) | < 0.001 | 1.42 (1.14–1.77) | 0.002 | ||
aModel 1: unadjusted
bModel 2: adjusted for age, SBP, DBP
cModel 3: adjusted for age, SBP, DBP, CRP, TC, HDL-C, LDL-C, smoking, drinking, hypertension, hyperlipidemia, use of antihypertensives, and use of antilipidemic
Table 7.
Association between the TyG index and the risk of carotid artery plaques according to different glucose regulation state and age
| Age | Glucose regulation state | Variables | carotid artery plaques | |||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI)a | P-value | OR (95% CI)b | P-value | OR (95% CI)c | P-value | |||
| ≤ 60 | Normal glucose regulation | TyG index | 1.32 (1.22–1.44) | < 0.001 | 1.16 (1.06–1.28) | < 0.001 | 1.12 (0.98–1.28) | 0.099 |
| Q1 | Reference | Reference | Reference | |||||
| Q2 | 1.27 (1.14–1.43) | < 0.001 | 1.24 (1.10–1.40) | < 0.001 | 1.17 (1.02–1.33) | 0.026 | ||
| Q3 | 1.53 (1.34–1.74) | < 0.001 | 1.40 (1.22–1.60) | < 0.001 | 1.24 (1.04–1.46) | 0.015 | ||
| Q4 | 1.38 (1.15–1.64) | < 0.001 | 1.09 (0.91–1.32) | 0.360 | 1.11 (0.86–1.43) | 0.430 | ||
| Prediabetes | TyG index | 1.05 (0.96–1.15) | 0.290 | 0.99 (0.90–1.09) | 0.770 | 0.93 (0.80–1.09) | 0.367 | |
| Q1 | Reference | Reference | Reference | |||||
| Q2 | 0.86 (0.73–1.02) | 0.090 | 0.92 (0.77–1.09) | 0.320 | 0.74 (0.61–0.90) | 0.002 | ||
| Q3 | 1.21 (1.02–1.43) | 0.030 | 1.12 (0.94–1.34) | 0.190 | 0.86 (0.70–1.05) | 0.141 | ||
| Q4 | 1.08 (0.91–1.29) | 0.390 | 1.04 (0.87–1.26) | 0.650 | 0.89 (0.69–1.15) | 0.374 | ||
| Diabetes | TyG index | 1.21 (1.13–1.30) | < 0.001 | 1.16 (1.08–1.25) | < 0.001 | 1.21(1.08–1.35) | 0.001 | |
| Q1 | Reference | Reference | Reference | |||||
| Q2 | 1.32 (0.98–1.78) | 0.070 | 1.23 (0.91–1.67) | 0.190 | 0.90 (0.64–1.26) | 0.544 | ||
| Q3 | 1.71 (1.29–2.27) | < 0.001 | 1.49 (1.11–2.00) | 0.010 | 0.95 (0.68–1.31) | 0.738 | ||
| Q4 | 1.98 (1.51–2.60) | < 0.001 | 1.69 (1.27–2.24) | < 0.001 | 1.03 (0.74–1.43) | 0.866 | ||
| > 60 | Normal glucose regulation | TyG index | 0.94 (0.87–1.02) | 0.160 | 1.05 (0.96–1.14) | 0.300 | 0.99 (0.88–1.11) | 0.817 |
| Q1 | Reference | Reference | Reference | |||||
| Q2 | 0.94 (0.86–1.03) | 0.200 | 1.01 (0.92–1.11) | 0.780 | 0.94 (0.85–1.04) | 0.241 | ||
| Q3 | 0.85 (0.76–0.95) | < 0.001 | 0.99 (0.88–1.11) | 0.890 | 0.87 (0.76–1.00) | 0.048 | ||
| Q4 | 0.90 (0.74–1.10) | 0.320 | 0.95 (0.77–1.17) | 0.640 | 0.90 (0.70–1.16) | 0.420 | ||
| Prediabetes | TyG index | 0.98 (0.90–1.07) | 0.640 | 1.03 (0.94–1.13) | 0.510 | 0.96 (0.84–1.10) | 0.568 | |
| Q1 | Reference | Reference | Reference | |||||
| Q2 | 1.03 (0.91–1.15) | 0.680 | 1.08 (0.96–1.21) | 0.230 | 0.97 (0.85–1.11) | 0.686 | ||
| Q3 | 1.09 (0.97–1.23) | 0.150 | 1.19 (1.05–1.35) | 0.010 | 1.01 (0.87–1.17) | 0.916 | ||
| Q4 | 0.76 (0.66–0.88) | < 0.001 | 0.80 (0.69–0.94) | 0.010 | 0.65 (0.52–0.80) | < 0.001 | ||
| Diabetes | TyG index | 1.38 (1.29–1.47) | < 0.001 | 1.43 (1.34–1.53) | < 0.001 | 1.35 (1.24–1.48) | < 0.001 | |
| Q1 | Reference | Reference | Reference | |||||
| Q2 | 1.18 (0.99–1.40) | 0.060 | 1.25 (1.05–1.49) | 0.010 | 1.19 (0.99–1.43) | 0.059 | ||
| Q3 | 1.15 (0.98–1.35) | 0.080 | 1.25 (1.06–1.47) | 0.010 | 1.05 (0.88–1.25) | 0.618 | ||
| Q4 | 1.63 (1.39–1.90) | < 0.001 | 1.78 (1.52–2.09) | < 0.001 | 1.45 (1.21–1.74) | < 0.001 | ||
aModel 1: unadjusted
bModel 2: adjusted for sex, SBP, DBP
cModel 3: adjusted for sex, SBP, DBP, CRP, TC, HDL-C, LDL-C, smoking, drinking, hypertension, hyperlipidemia, use of antihypertensives, and use of antilipidemic
Discussion
The results of this study revealed a significant association between the TyG index and CAP in CHD patients. This is the first large-scale study to demonstrate this relationship between the TyG index and CAP in CHD patients, and assessed this relationship according to sex, ages and glucose metabolism states.
Studies in recent years have shown the close relationship between the TyG index and the homeostasis model assessment of insulin resistance (HOMA-IR). And the predictive value of the TyG index for IR was better than that for HOMA-IR [19]. Therefore, the TyG index reflect the indicator of peripheral IR. A cross-sectional study reported that the TyG index was positively associated with the prevalence of CAD and could be used as a marker of AS [28]. Compared to patients with the lowest the TyG index, those in the quartile with the highest TyG index have a higher risk of stroke and myocardial infarction (MI) [29]. The TyG index was also significantly associated with the progression of arterial stiffness in hypertensive people but not prehypertensive individuals [30]. The TyG index was also closely related to coronary artery calcification and carotid AS [31]. This finding is consistent with the results of the present study. The related mechanism of action may involve several aspects. Firstly, insulin can cause lipid hyaluronic degeneration by enhancing sympathetic nerve activity or acting as a growth factor. Lipohyaline deposition can block small arteries, leading to the development of CVD [32]. Secondly, the TyG index is associated with inflammation. IR can induce inflammation, oxidative stress, and metabolic changes, causing damage to the vascular endothelium due to inflammation [33]. Therefore, the TyG index in CHD patients was associated with the occurrence of CAP, in which a high level of TyG index was associated with the occurrence of higher CAP.
The close association between the TyG index and cerebral small vessel disease (cSVD) may be caused by other concomitant metabolic syndromes. IR patients usually have other comorbidities, including hypertension, DM and obesity [13, 34]. The TyG index has received attention in the field of DM and metabolism, and has a positive impact on the assessment and prediction of IR and metabolic syndrome in DM patients. A higher TyG index was associated with an increased risk of coronary artery stenosis in asymptomatic T2DM patients [35]. However, cardiometabolic heterogeneity in non-DM individuals has been reported [36, 37]. Consistent with the results of this study, this study observed a significant association between the TyG index in CHD patients and the risk of CAP according to glucose metabolism states and after adjusting for confounding variables, with the highest OR value observed for DM.
Recent studies have shown that female have a lower risk of cardiovascular disease compared to male, but that hyperglycemia and hyperinsulinemia caused by IR may reverse this sex-based protection. All insulin replacement markers showed that good association with HOMA-IR of both sexes, and the association between female and HOMA-IR was stronger than that of male [38]. However, some studies have found that there was no sex difference between the TyG index and MACEs in patients with hypertension [39]. There has been an investigation of the association between the TyG index and the early stages of subclinical atherosclerosis (SA) between the sexes. A high TyG index was independently associated with SA in non-diabetic female, but in NGT male. Regardless of sex, the TyG index is unrelated to the presence of SA in DM patients [40]. Higher TG and blood pressure had greater impact in both DM patients and those with NGT. Moreover, the number of CVD events and deaths was higher in female than that in male [41]. Consistent with the results of this study, the TyG index in female was more highly associated with CAP compared to the association in male. A high TyG index showed a higher association with CAP.
In the middle-aged and elderly populations, an increase in the TyG index was significantly associated with hypertension and isolated systolic hypertension [42]. This study showed that the OR value of middle-aged patients with CHD was higher than that of elderly patients with CHD. The TyG index was significantly related to the risk of CAP in middle-aged patients with NGT, whereas the TyG index was significantly related to the risk of CAP in elderly patients with DM. This may be because this study is a CHD population, with an average age of over 60 years old, belonging to the middle-aged and elderly population, and there is a certain bias for age. Therefore, future research should inclued people of different ages to determine the association between the TyG index and CAP according to ages.
To sum up, with an increasing number of studies on the influence of the TyG index on patients with cardiovascular diseases, the clinical significance of the TyG index is becoming increasingly clear. Evaluation of the TyG index may have important clinical significance for risk stratification and individualized treatment of CHD patients.
Strengths and limitations
This large-scale, multi-center cohort study had several limitations. First, this study was a multi-center study, thus, there may be bias in the measurement methods at different research centers. However, the practitioners conducted external quality assessments between clinical laboratories in each center. Second, this study was a cross-sectional study. Therefore, the results of this study cannot establish causality. The exact mechanism of the relationship between the TyG index and CAP requires further prospective large-scale research.
Conclusion
This study demonstrated a significant association between the TyG index and CAP in CHD patients. In addition, the association between the TyG index and CAP in CHD patients was higher in female than in male, and higher in middle-aged and elderly than in the elderly. In DM patients, the association between the TyG index and CAP in CHD patients was higher. As a marker of IR, the TyG index is easy to calculate and may reflect the risk of CAP in CHD patients. The results of this study may emphasize the need for a risk management strategy for specific sex and different age groups to prevent the occurrence of CAP in CHD patients.
Supplementary Information
Additional file 1: Table S1. Correlation between the TyG index and the number of carotid artery plaque. Table S2. Correlation between the TyG index and the echogenicity of carotid artery plaque.
Acknowledgements
We thank all the participants in the study and the members of the survey teams, and the groups providing financial support.
Abbreviations
- TyG
Triglyceride glucose
- CHD
Coronary heart disease
- DM
Diabetes mellitus
- NCD
Non-communicable diseases
- T2DM
Type 2 diabetes
- CAD
Coronary artery disease
- CAP
Carotid artery plaque
- CIMT
Carotid artery intima-media thickness
- Pre-DM
Prediabetes
- AS
Atherosclerosis
- SBP
Systolic blood pressure
- DBP
Diastolic blood pressure
- FPG
Fasting plasma glucose
- TC
Total cholesterol
- HDL-C
High-density lipoprotein cholesterol
- TG
Triglycerides
- LDL-C
Low-density lipoprotein cholesterol
- CRP
C-reactionprotein
- HbA1c
Glycated haemoglobin
- OR
Odds ratios
- CIs
Confidence intervals
- HOMA-IR
Homeostasis model assessment of insulin resistance
- SA
Subclinical atherosclerosis
Authors' contributions
CY, SG, HZ, and ZL participated in the study design and statistical analysis; ZL and YH analyzed the data together and drafted the manuscript; SW, LL, RY, YL, QC, LY, and YZ participated in data collection. All authors have read and approved the final manuscript.
Funding
This study was supported by the National Basic Research Program of China (973 project, Grant Number: 2014CB542902).
Availability of data and materials
The datasets used and/or analyzed in the current study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the ethics committee of Tianjin University of Traditional Chinese Medicine (TJUTCM-EC20190008) and registered in the Chinese Clinical Trial Registry (ChiCTR-1900024535) and in ClinicalTrials.gov (NCT04026724).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zhu Li and Yuanyuan He are co-first authors
Contributor Information
Hongmei Zheng, Email: zhenghm_1973@163.com.
Shan Gao, Email: bianjibugs@163.com.
Chunquan Yu, Email: ycqtjutcm@foxmail.com.
References
- 1.Wang AL, Zhang H, Zhang J, Zhang Y, Cao HJ, Liu JP, Xu H, Chen KJ. Adjuvant effects of health education of chinese medicine for chronic diseases: a systematic review and meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med. 2020;2020:3738753. doi: 10.1155/2020/3738753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang MY, Zhang JH, Chua HZ, Feng R, Lu MJ, Tian Y. Core outcome set for stable angina pectoris in traditional Chinese medicine (COS-SAP-TCM) Acupuncture Herb Med. 2021;1(1):39–48. doi: 10.1097/HM9.0000000000000007. [DOI] [Google Scholar]
- 3.Zhao Q, Cheng YJ, Xu YK, Zhao ZW, Liu C, Sun TN, Zhou YJ. Comparison of various insulin resistance surrogates on prognostic prediction and stratification following percutaneous coronary intervention in patients with and without type 2 diabetes mellitus. Cardiovasc Diabetol. 2021;20(1):190. doi: 10.1186/s12933-021-01383-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Juárez-Rojas JG, Posadas-Romero C, Martínez-Alvarado R, Jorge-Galarza E, Reyes-Barrera J, Sánchez-Lozada LG, Torres-Tamayo M, Medina-Urrutia AX. Type 2 diabetes mellitus is associated with carotid artery plaques in patients with premature coronary heart disease. Rev Invest Clin. 2018;70(6):301–309. doi: 10.24875/RIC.18002591. [DOI] [PubMed] [Google Scholar]
- 5.Wang L, Gao P, Zhang M, Huang Z, Zhang D, Deng Q, Li Y, Zhao Z, Qin X, Jin D, Zhou M, Tang X, Hu Y, Wang L. Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA. 2017;317(24):2515–2523. doi: 10.1001/jama.2017.7596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jin JL, Cao YX, Liu HH, Zhang HW, Guo YL, Wu NQ, Zhu CG, Xu RX, Gao Y, Sun J, Dong Q, Li JJ. Impact of free fatty acids on prognosis in coronary artery disease patients under different glucose metabolism status. Cardiovasc Diabetol. 2019;18(1):134. doi: 10.1186/s12933-019-0936-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu HH, Cao YX, Li S, Guo YL, Zhu CG, Wu NQ, Gao Y, Dong QT, Zhao X, Zhang Y, Sun D, Li JJ. Impacts of prediabetes mellitus alone or plus hypertension on the coronary severity and cardiovascular outcomes. Hypertension. 2018;71(6):1039–1046. doi: 10.1161/HYPERTENSIONAHA.118.11063. [DOI] [PubMed] [Google Scholar]
- 8.Jin JL, Cao YX, Zhang HW, Sun D, Hua Q, Li YF, Guo YL, Wu NQ, Zhu CG, Gao Y, Dong QT, Liu HH, Dong Q, Li JJ. Lipoprotein(a) and cardiovascular outcomes in patients with coronary artery disease and prediabetes or diabetes. Diabetes Care. 2019;42(7):1312–1318. doi: 10.2337/dc19-0274. [DOI] [PubMed] [Google Scholar]
- 9.Chamroonkiadtikun P, Ananchaisarp T, Wanichanon W. The triglyceride-glucose index, a predictor of type 2 diabetes development: a retrospective cohort study. Prim Care Diabetes. 2020;14(2):161–167. doi: 10.1016/j.pcd.2019.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Alizargar J, Bai CH, Hsieh NC, Wu SV. Use of the triglyceride-glucose index (TyG) in cardiovascular disease patients. Cardiovasc Diabetol. 2020;19(1):8. doi: 10.1186/s12933-019-0982-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.da Silva A, Caldas APS, Hermsdorff HHM, Bersch-Ferreira ÂC, Torreglosa CR, Weber B, Bressan J. Triglyceride-glucose index is associated with symptomatic coronary artery disease in patients in secondary care. Cardiovasc Diabetol. 2019;18(1):89. doi: 10.1186/s12933-019-0893-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo E, Wang D, Yan G, Qiao Y, Liu B, Hou J, Tang C. High triglyceride-glucose index is associated with poor prognosis in patients with acute ST-elevation myocardial infarction after percutaneous coronary intervention. Cardiovasc Diabetol. 2019;18(1):150. doi: 10.1186/s12933-019-0957-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi W, Xing L, Jing L, Tian Y, Yan H, Sun Q, Dai D, Shi L, Liu S. Value of triglyceride-glucose index for the estimation of ischemic stroke risk: insights from a general population. Nutr Metab Cardiovasc Dis. 2020;30(2):245–253. doi: 10.1016/j.numecd.2019.09.015. [DOI] [PubMed] [Google Scholar]
- 14.Zhao S, Yu S, Chi C, Fan X, Tang J, Ji H, Teliewubai J, Zhang Y, Xu Y. Association between macro- and microvascular damage and the triglyceride glucose index in community-dwelling elderly individuals: the Northern Shanghai Study. Cardiovasc Diabetol. 2019;18(1):95. doi: 10.1186/s12933-019-0898-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee SB, Ahn CW, Lee BK, Kang S, Nam JS, You JH, Kim MJ, Kim MK, Park JS. Association between triglyceride glucose index and arterial stiffness in Korean adults. Cardiovasc Diabetol. 2018;17(1):41. doi: 10.1186/s12933-018-0692-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim MK, Ahn CW, Kang S, Nam JS, Kim KR, Park JS. Relationship between the triglyceride glucose index and coronary artery calcification in Korean adults. Cardiovasc Diabetol. 2017;16(1):108. doi: 10.1186/s12933-017-0589-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jian S, Su-Mei N, Xue C, Jie Z, Xue-Sen W. Association and interaction between triglyceride-glucose index and obesity on risk of hypertension in middle-aged and elderly adults. Clin Exp Hypertens. 2017;39(8):732–739. doi: 10.1080/10641963.2017.1324477. [DOI] [PubMed] [Google Scholar]
- 18.Lee EY, Yang HK, Lee J, Kang B, Yang Y, Lee SH, Ko SH, Ahn YB, Cha BY, Yoon KH, Cho JH. Triglyceride glucose index, a marker of insulin resistance, is associated with coronary artery stenosis in asymptomatic subjects with type 2 diabetes. Lipids Health Dis. 2016;15(1):155. doi: 10.1186/s12944-016-0324-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Irace C, Carallo C, Scavelli FB, De Franceschi MS, Esposito T, Tripolino C, Gnasso A. Markers of insulin resistance and carotid atherosclerosis. A comparison of the homeostasis model assessment and triglyceride glucose index. Int J Clin Pract. 2013;67(7):665–72. doi: 10.1111/ijcp.12124. [DOI] [PubMed] [Google Scholar]
- 20.Barua RS, Rigotti NA, Benowitz NL, Cummings KM, Jazayeri MA, Morris PB, Ratchford EV, Sarna L, Stecker EC, Wiggins BS. 2018 ACC expert consensus decision pathway on tobacco cessation treatment: a report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2018;72(25):3332–3365. doi: 10.1016/j.jacc.2018.10.027. [DOI] [PubMed] [Google Scholar]
- 21.Fan AZ, Ruan WJ, Chou SP. Re-examining the relationship between alcohol consumption and coronary heart disease with a new lens. Prev Med. 2019;118:336–343. doi: 10.1016/j.ypmed.2018.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flack JM, Adekola B. Blood pressure and the new ACC/AHA hypertension guidelines. Trends Cardiovasc Med. 2020;30(3):160–164. doi: 10.1016/j.tcm.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Li Z, Cheng Q, Liu Y, Cheng X, Wang S, He Y, Wang X, Huang M, Li Y, Xue X, Xu Y, Li L, Zheng Y, Yang R, Gao S, Yu C. Low-/high-density lipoprotein cholesterol ratio and carotid plaques in patients with coronary heart disease: a Chinese cohort study. Lipids Health Dis. 2021;20(1):144. doi: 10.1186/s12944-021-01575-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu S, Xu L, Wu M, Chen S, Wang Y, Tian Y. Association between triglyceride-glucose index and risk of arterial stiffness: a cohort study. Cardiovasc Diabetol. 2021;20(1):146. doi: 10.1186/s12933-021-01342-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang L, Li Z, Song Y, Liu Y, Zhao H, Liu Y, Zhang T, Yuan Y, Cai X, Wang S, Wang P, Gao S, Li L, Li Y, Yu C. Study on urine metabolic profiling and pathogenesis of hyperlipidemia. Clin Chim Acta. 2019;495:365–373. doi: 10.1016/j.cca.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 26.American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44(Suppl 1):S15-S33. 10.2337/dc21-S002. [DOI] [PubMed]
- 27.Liu Y, Zhu Y, Jia W, Sun D, Zhao L, Zhang C, Wang C, Chen G, Fu S, Bo Y, Xing Y. Association between lipid profiles and presence of carotid plaque. Sci Rep. 2019;9(1):18011. doi: 10.1038/s41598-019-54285-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Won KB, Lee BK, Park HB, Heo R, Lee SE, Rizvi A, Lin FY, Kumar A, Hadamitzky M, Kim YJ, Sung JM, Conte E, Andreini D, Pontone G, Budoff MJ, Gottlieb I, Chun EJ, Cademartiri F, Maffei E, Marques H, de Araújo GP, Leipsic JA, Shin S, Choi JH, Virmani R, Samady H, Chinnaiyan K, Raff GL, Stone PH, Berman DS, Narula J, Shaw LJ, Bax JJ, Min JK, Chang HJ. Quantitative assessment of coronary plaque volume change related to triglyceride glucose index: the Progression of AtheRosclerotic PlAque DetermIned by Computed TomoGraphic Angiography IMaging (PARADIGM) registry. Cardiovasc Diabetol. 2020;19(1):113. doi: 10.1186/s12933-020-01081-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y, Ding X, Hua B, Liu Q, Gao H, Chen H, Zhao XQ, Li W, Li H. High triglyceride-glucose index is associated with adverse cardiovascular outcomes in patients with acute myocardial infarction. Nutr Metab Cardiovasc Dis. 2020;30(12):2351–2362. doi: 10.1016/j.numecd.2020.07.041. [DOI] [PubMed] [Google Scholar]
- 30.Wu Z, Zhou D, Liu Y, Li Z, Wang J, Han Z, Miao X, Liu X, Li X, Wang W, Guo X, Tao L. Association of TyG index and TG/HDL-C ratio with arterial stiffness progression in a non-normotensive population. Cardiovasc Diabetol. 2021;20(1):134. doi: 10.1186/s12933-021-01330-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park K, Ahn CW, Lee SB, Kang S, Nam JS, Lee BK, Kim JH, Park JS. Elevated TyG index predicts progression of coronary artery calcification. Diabetes Care. 2019;42(8):1569–1573. doi: 10.2337/dc18-1920. [DOI] [PubMed] [Google Scholar]
- 32.Ormazabal V, Nair S, Elfeky O, Aguayo C, Salomon C, Zuñiga FA. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol. 2018;17(1):122. doi: 10.1186/s12933-018-0762-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nam KW, Kwon HM, Jeong HY, Park JH, Kwon H, Jeong SM. High triglyceride-glucose index is associated with subclinical cerebral small vessel disease in a healthy population: a cross-sectional study. Cardiovasc Diabetol. 2020;19(1):53. doi: 10.1186/s12933-020-01031-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu J, Rutten-Jacobs L, Liu M, Markus HS, Traylor M. Causal impact of type 2 diabetes mellitus on cerebral small vessel disease: a mendelian randomization analysis. Stroke. 2018;49(6):1325–1331. doi: 10.1161/STROKEAHA.117.020536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.da Silva A, Caldas APS, Rocha DMUP, Bressan J. Triglyceride-glucose index predicts independently type 2 diabetes mellitus risk: a systematic review and meta-analysis of cohort studies. Prim Care Diabetes. 2020;14(6):584–593. doi: 10.1016/j.pcd.2020.09.001. [DOI] [PubMed] [Google Scholar]
- 36.Emerging Risk Factors Collaboration. Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, Ingelsson E, Lawlor DA, Selvin E, Stampfer M, Stehouwer CD, Lewington S, Pennells L, Thompson A, Sattar N, White IR, Ray KK, Danesh J. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375(9733):2215–22. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ariel D, Reaven G. Modulation of coronary heart disease risk by insulin resistance in subjects with normal glucose tolerance or prediabetes. Acta Diabetol. 2014;51(6):1033–1039. doi: 10.1007/s00592-014-0667-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakagomi A, Sunami Y, Kawasaki Y, Fujisawa T, Kobayashi Y. Sex difference in the association between surrogate markers of insulin resistance and arterial stiffness. J Diabetes Complications. 2020;34(6):107442. doi: 10.1016/j.jdiacomp.2019.107442. [DOI] [PubMed] [Google Scholar]
- 39.Yang K, Liu W. Triglyceride and glucose index and sex differences in relation to major adverse cardiovascular events in hypertensive patients without diabetes. Front Endocrinol (Lausanne) 2021;12:761397. doi: 10.3389/fendo.2021.761397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu YW, Chang CC, Chou RH, Tsai YL, Liu LK, Chen LK, Huang PH, Lin SJ. Gender difference in the association between TyG index and subclinical atherosclerosis: results from the I-Lan Longitudinal Aging Study. Cardiovasc Diabetol. 2021;20(1):206. doi: 10.1186/s12933-021-01391-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tramunt B, Smati S, Grandgeorge N, Lenfant F, Arnal JF, Montagner A, Gourdy P. Sex differences in metabolic regulation and diabetes susceptibility. Diabetologia. 2020;63(3):453–461. doi: 10.1007/s00125-019-05040-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zheng R, Mao Y. Triglyceride and glucose (TyG) index as a predictor of incident hypertension: a 9-year longitudinal population-based study. Lipids Health Dis. 2017;16(1):175. doi: 10.1186/s12944-017-0562-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Correlation between the TyG index and the number of carotid artery plaque. Table S2. Correlation between the TyG index and the echogenicity of carotid artery plaque.
Data Availability Statement
The datasets used and/or analyzed in the current study are available from the corresponding author upon reasonable request.