Abstract
Background:
Breast cancer survival continues to improve, with women living longer after treatment. It is not well understood how long-term satisfaction and well-being differ following treatment or how types of reconstruction differ when compared to the norm.
Methods:
In a propensity-matched sample, we compared patient-reported outcomes (PROs) in breast cancer patients at various time intervals from surgery with normative BREAST-Q data. All data were obtained using the Army of Women, an online community fostering breast cancer research. Breast cancer patients were stratified by surgical treatment and reconstruction type. Regression lines were estimated and differences in slope tested between cancer patients and non-cancer controls.
Results:
We compared normative (n=922) and breast cancer (n=4,343) cohorts in a propensity-matched analysis. Among the breast cancer patients, 49.4% underwent lumpectomy, 17.0% mastectomy, 21.7% implant reconstruction, and 11.9% autologous reconstruction. Median time since surgery was 4.7 years, with 21.1% >10 years post-surgery. At the time of survey, breast cancer patients reported higher Breast Satisfaction and Psychosocial Well-being scores compared to non-cancer controls (p<0.01), with the cohorts undergoing lumpectomy and autologous reconstruction both reporting higher scores than the normative controls. After mastectomy, scores averaged lower than the non-cancer controls, but improved over time. However, all breast cancer groups reported significantly lower Physical Well-being than the non-cancer cohort (all p<0.01).
Conclusions:
Breast cancer patients undergoing lumpectomy or autologous reconstruction reported higher psychosocial well-being compared to non-cancer controls. These differences were influenced both by time since treatment and choice of surgical procedure.
INTRODUCTION
Breast cancer is the most commonly diagnosed cancer among women in the United States, with an estimated 3.1 million living survivors (1-3). It is projected that in 2018, there will be 270,000 new invasive cancer diagnoses, with an additional 64,000 women diagnosed with carcinoma in situ (4). Following diagnosis, women face complex surgical decisions regarding oncologic resection and reconstructive options. Surgical options include breast conserving surgery (BCS) typically followed by radiation, or mastectomy with or without implant or autologous-based reconstruction. These choices are associated with equivalent disease-specific survival rates, and thus decision-making is largely based on personal perspectives and motivations regarding a patient’s breasts. As a result, patient-reported outcomes (PROs) describing quality of life and patient satisfaction are particularly important to consider when counseling patients about surgical options.
The majority of studies evaluating breast cancer treatment PROs focus on short-term differences in outcomes, and compare different methods of reconstruction over several months to a few years (5). However in recent decades, long-term survival has reached 90% at 5-years and a woman’s initial surgical decision making will likely be relevant for decades into the future (6). It is therefore imperative to consider PROs over 10-20 years to help guide surgical decision-making. In addition, most published data compare pre- and post-operative PROs, allowing the pre-operative data points to serve as the baseline. However, as pre-operative data is collected after the patient is diagnosed with breast cancer, this “baseline” data incorporates not only the psychosocial alterations of a new cancer diagnosis, but also the physical trauma of core needle and/or excisional biopsies. The use of data from non-cancer controls (“normative sample”) allows for a population based baseline comparison, without the influence of emotional, psychosocial, and physical aspects of a new cancer diagnosis.
There are few studies evaluating the impact of various surgical procedures on changes in women’s breast satisfaction compared to women without a diagnosis of breast cancer, and over the long-term. Thus, the aims of the study were to compare self-reported perspectives of breast satisfaction and well-being between women diagnosed with breast cancer to a normative sample, both at baseline and over time. We hypothesized that BREAST-Q scores in breast cancer patients will differ at different points in time in comparison to the norm.
METHODS
BREAST-Q
In this study, previously published BREAST-Q data from normative (women with no history of breast surgery and/or cancer) and breast cancer populations were compared to evaluate differences from baseline and potential changes over time. The BREAST-Q is a rigorously designed, well-validated, PRO instrument designed specifically for use in evaluating PROs in breast cancer treatment and reconstruction patients (7-11). The BREAST-Q was first published in 2009, following international guidelines for development (12), and has modules specific to breast cancer treatment and reconstruction. The breast cancer modules of the BREAST-Q have Cronbach’s alpha scores of 0.88 to 0.96, item total correlations of 0.56 to 0.86, and test-retest reliability with intraclass correlation coefficients of 0.93 to 0.96 (11). The breast cancer scales used in this analysis include Satisfaction with Breasts, Physical Well-being, Psychosocial Well-being, and Sexual Well-being. Responses on each scale are summed and then transformed using Q-Score (New York, NY; https://webcore.mskcc.org/breastq/scoring.html) to a scale from 0 (worst) to 100 (best). The BREAST-Q has been used in over 22,000 patients to demonstrate differences in patient satisfaction and quality of life outcomes (5).
Study Population and Recruitment
Participants were recruited through the Army of Women (AOW), an online community of volunteers with and without breast cancer, fostering breast cancer research. The AOW was founded in 2008 by the Dr. Susan Love Research Foundation, and has been used to recruit patients for numerous basic science and clinical research projects (13). Details regarding study design and data collection have previously been published for both normative (14), and breast cancer populations (15, 16).
Participants were recruited into both cohorts after an e-blast was sent to AOW members (normative group 8/2015 and 11/2015, breast cancer group 5/2012), with women self-screening for inclusion criteria and then self-selecting for participation. IRB approval was obtained at Dartmouth University for normative data and Duke University for cancer data. Normative participants included adult women with no prior history of breast cancer or breast surgery, with BREAST-Q breast cancer pre-operative modules, demographic information, body mass index, and bra cup size collected (14). Breast cancer participants were females with a history of cancer treatment in the United States, with data collection including the BREAST-Q, demographics, post-operative modules, additional quality of life instruments, and details regarding cancer diagnosis, treatment, and any associated reconstruction (15, 16).
Variables
Income was collected with different cutoff points between datasets and required recoding to create common classifications. The samples were grouped by income as <$50,000 or <$60,000, $50,000 or $60,000 - $100,000, and > $100,000. Normative participants in the $40,000 - $59,999 group and cancer patients in the $35,000 - $49,999 group served as cutoffs for the above stated groupings. Similarly, race demographics were collected variably between samples. Recoding using common demographic categories resulted in the following combined groups: Asian or Pacific Islander (South Asian/East Indian, Asian/Pacific Islander), Black or African American (Black Non-Hispanic, Black Hispanic), White (White Non-Hispanic, White Hispanic), American Indian or Alaskan Native (Native Canadian/American), Other/Multiracial (other). The study had a cross-sectional design consisting of a one-time survey for each group, with cancer patients reporting the surgery date to calculate the time since surgery.
Propensity Matching
We used the normative sample BREAST-Q scores as representative of the scores of breast cancer patients prior to any testing or concerns about a cancer diagnosis. To reduce the potential impact of the differences between the two study samples, propensity matching was performed. Starting with 6,840 women with breast cancer and 1,200 women without cancer, logistic regression was used to calculate the probability of being a normative participant according to the combination of age, BMI at time of survey, race, marital status, income group, highest educational attainment, employment status, and indicator variables for each of the quality of life modules to capture missing module scores. Up to five cancer volunteers were matched to each normative volunteer using a greedy nearest neighbor algorithm and a caliper of 10% of the standard deviation of the pooled propensity scores (17). There were 4,343 cancer participants and 922 normative participants selected into the matched sample for further analysis.
Analyses
Differences in patient demographics among the cancer cohort were examined using rank sum tests for continuous variables and chi-square tests for categorical variables. Mean differences in quality of life scores between the normative and cancer participants were tested overall using ANOVA. Regression analysis was used to test differences in scores between the normative sample and specific surgery types. To estimate the changes in scores associated with time since surgery, additional regression models excluded normative patients while controlling for surgery type, time since surgery, and an interaction term of the two variables.
All statistical analyses were performed using SAS 9.4 (Cary, NC). A significance threshold of 0.05 was utilized.
RESULTS
Complete detailed results of the cross-sectional normative data (14) and the cross-sectional breast cancer data (15, 16) have been published previously. For comparison, 1,201 women completed the BREAST-Q in the normative population and 7,619 women in the breast cancer population. Patients with a complex cancer history, stage 4 disease, atypical ductal hyperplasia, or inflammatory breast cancer were excluded, resulting in 6,840 breast cancer participants and 1,201 normative participants. One patient was excluded from the normative sample for missing data. There were 4,343 breast cancer patients and 922 normative patients included in the propensity-matched sample (See Table, Supplemental Digital Content 1, which demonstrates the result of the propensity matching process and how successful it was at creating two groups that were more similar on their demographics than the original two groups, (http://links.lww.com/PRS/D929). The mean patient age for the matched normative and breast cancer samples was 56.2 ±10.9 and 56.4 ±9.7 years, respectively. Women from both samples (matched normative vs. breast cancer cohorts) were mostly white (97.0% vs. 95.9%), married (73.1% vs. 72.1%), college educated (82.7% vs. 80.7%), employed or retired (82.1% vs. 80.9%), and earned ≥$50,000/$60,000 (74.6% vs. 73.5%).
After matching, differences between the normative and overall breast cancer sample were minimized while maintaining some heterogeneity between the surgery types. Demographic and cancer specific details for the matched cancer cohort are listed in Table 1. Stage III disease was reported in 9.6% with the remaining cohort evenly distributed between Stages 0, I, and II. The majority of breast cancer patients in the matched analysis underwent chemotherapy (58.8%). Surgical treatment was lumpectomy in 49.4%, mastectomy alone in 17.0%, implant-based reconstruction in 21.7%, and autologous reconstruction in 11.9%. Time since surgery was reported to be a median of 4.7 years, with 21.1% of patients reporting that they were more than 10 years from surgery at the time of survey participation. There were few substantial demographic differences between the surgical groups, however, women who underwent lumpectomy or mastectomy without reconstruction were older, less likely to be married, and more likely to be widowed. The mastectomy alone group also had a lower income compared to the rest of the breast cancer sample.
Table 1.
Selected Matched Breast Cancer Patient Treatment Variables, Overall and by Surgery Type
| Overall | Lumpectomy | Mastectomy | Implant | Autologous | ||
|---|---|---|---|---|---|---|
| N (%)* | N (%)* | N (%)* | N (%)* | N (%)* | p-value | |
| Total | 4343 (100%) | 2144 (49.4%) | 740 (17.0%) | 942 (21.7%) | 517 (11.9%) | |
| Age, Median (Range) | 56.6 (25.3 – 86.7) | 57.7 (25.3 – 83.6) | 60.2 (32.4 – 86.7) | 51.9 (27.2 – 83.4) | 55.3 (31.3 - 81.6) | <0.01 |
| BMI, Median (Range) | 25.2 (14.8 – 65.4) | 25.6 (16.3 – 58.1) | 25.8 (15.8 – 65.4) | 23.8 (14.8 – 48.3) | 26.1 (16.4 – 45.4) | <0.01 |
| Race | 0.07 | |||||
| White | 4166 (95.9%) | 2062 (96.2%) | 708 (95.7%) | 904 (96.0%) | 492 (95.2%) | |
| Black or African American | 55 (1.3%) | 21 (1.0%) | 11 (1.5%) | 9 (1.0%) | 14 (2.7%) | |
| Asian or Pacific Islander | 44 (1.0%) | 20 (0.9%) | 8 (1.1%) | 10 (1.1%) | 6 (1.2%) | |
| American Indian or Alaskan Native | 7 (0.2%) | 1 (<0.1%) | 3 (0.4%) | 2 (0.2%) | 1 (0.2%) | |
| Other (includes Biracial/Multiracial) | 58 (1.3%) | 34 (1.6%) | 7 (0.9%) | 14 (1.5%) | 3 (0.6%) | |
| Marital Status | <0.01 | |||||
| Married | 3130 (72.1%) | 1506 (70.2%) | 515 (69.6%) | 724 (76.9%) | 385 (74.5%) | |
| Single, never married | 331 (7.6%) | 173 (8.1%) | 63 (8.5%) | 61 (6.5%) | 34 (6.6%) | |
| Living with significant other | 241 (5.5%) | 119 (5.6%) | 45 (6.1%) | 49 (5.2%) | 28 (5.4%) | |
| Divorced | 402 (9.3%) | 215 (10.0%) | 62 (8.4%) | 74 (7.9%) | 51 (9.9%) | |
| Separated | 54 (1.2%) | 29 (1.4%) | 10 (1.4%) | 13 (1.4%) | 2 (0.4%) | |
| Widowed | 185 (4.3%) | 102 (4.8%) | 45 (6.1%) | 21 (2.2%) | 17 (3.3%) | |
| Income Group | <0.01 | |||||
| < $50,000/$60,000 | 960 (22.1%) | 463 (21.6%) | 215 (29.1%) | 172 (18.3%) | 110 (21.3%) | |
| $50,000/$60,000 - $99,999 | 1378 (31.7%) | 664 (31.0%) | 292 (39.5%) | 257 (27.3%) | 165 (31.9%) | |
| $100,000+ | 1817 (41.8%) | 908 (42.4%) | 204 (27.6%) | 478 (50.7%) | 227 (43.9%) | |
| Ethnicity | 0.67 | |||||
| Hispanic or Latino | 71 (1.6%) | 35 (1.6%) | 11 (1.5%) | 16 (1.7%) | 9 (1.7%) | |
| Not Hispanic or Latino | 4209 (96.9%) | 2085 (97.2%) | 718 (97.0%) | 908 (96.4%) | 498 (96.3%) | |
| Stage | <0.01 | |||||
| Stage 0 | 1191 (27.4%) | 630 (29.4%) | 135 (18.2%) | 283 (30.0%) | 143 (27.7%) | |
| Stage 1 | 1269 (29.2%) | 742 (34.6%) | 149 (20.1%) | 263 (27.9%) | 115 (22.2%) | |
| Stage 2 | 1272 (29.3%) | 587 (27.4%) | 248 (33.5%) | 289 (30.7%) | 148 (28.6%) | |
| Stage 3 | 416 (9.6%) | 97 (4.5%) | 157 (21.2%) | 70 (7.4%) | 92 (17.8%) | |
| Treatment Facility | <0.01 | |||||
| Community Hospital or Community Hospital Affiliated Cancer Center | 2183 (50.3%) | 1103 (51.4%) | 346 (46.8%) | 497 (52.8%) | 237 (45.8%) | |
| Managed Care Organizations | 219 (5.0%) | 105 (4.9%) | 57 (7.7%) | 38 (4.0%) | 19 (3.7%) | |
| Stand Alone Cancer Center (not affiliated with a community or university hospital) | 630 (14.5%) | 294 (13.7%) | 126 (17.0%) | 140 (14.9%) | 70 (13.5%) | |
| University Hospital or University Affiliated Cancer Center | 888 (20.4%) | 435 (20.3%) | 130 (17.6%) | 184 (19.5%) | 139 (26.9%) | |
| Other | 317 (7.3%) | 149 (6.9%) | 65 (8.8%) | 64 (6.8%) | 39 (7.5%) | |
| Time Since Surgery in years, Median (Range) | 4.7 (<0.1 – 45.7) | 5.0 (<0.1 – 32.2) | 5.5 (<0.1 – 45.7) | 3.5 (<0.1 – 33.1) | 5.2 (<0.1 – 32.3) | <0.01 |
| Time Since Surgery (Categorical) | <0.01 | |||||
| < 1 year | 472 (10.9%) | 227 (10.6%) | 63 (8.5%) | 135 (14.3%) | 47 (9.1%) | |
| [1, 5] years | 1806 (41.6%) | 844 (39.4%) | 264 (35.7%) | 495 (52.5%) | 203 (39.3%) | |
| (5,10] years | 1144 (26.3%) | 625 (29.2%) | 201 (27.2%) | 180 (19.1%) | 138 (26.7%) | |
| (10,15] years | 547 (12.6%) | 292 (13.6%) | 106 (14.3%) | 64 (6.8%) | 85 (16.4%) | |
| (15,20] years | 223 (5.1%) | 105 (4.9%) | 53 (7.2%) | 33 (3.5%) | 32 (6.2%) | |
| > 20 years | 149 (3.4%) | 50 (2.3%) | 52 (7.0%) | 35 (3.7%) | 12 (2.3%) | |
Percentages may not add up to 100 due to rounding or missing values; Overall = all breast cancer patients; p-values compare the surgery groups. With the exception of race (missing n=13), tests for categorical variables include the patients coded as missing or unknown as a “missing” category.
The BREAST-Q scores for the propensity-matched analysis sample are presented in Table 2a. Comparisons of the breast cancer participants to the normative sample are in Table 2b. The comparisons list the differences between groups and the 95% confidence interval. Notably, breast cancer patients had lower Physical and Sexual Well-being scores, but higher Satisfaction with Breasts and Psychosocial scores compared to the norm at the time of survey participation. The higher Psychosocial Well-being score was primarily driven by the lumpectomy and autologous reconstruction patients, and the lower Sexual Well-being score was primarily driven by implant reconstruction and mastectomy without reconstruction patients. The only surgery group with an average Satisfaction with Breasts scores lower than the normative sample was the Mastectomy alone group (difference of −2.38 versus normative participants, 95% CI −4.33 to −0.43). That estimated difference was reduced and no longer statistically significant upon adjustment (See Table, Supplemental Digital Content 2, which shows the differences among the surgery types, (http://links.lww.com/PRS/D930).
Table 2a.
BREAST-Q Scores Among Propensity-Matched Participants, Mean and Standard Deviation
| Normative | Overall | Lumpectomy | Mastectomy | Implant | Autologous | ||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | p | |
| Breast Satisfaction | 57.7 (19.0) | 63.1 (20.9) | 65.8 (21.8) | 55.3 (20.9) | 60.2 (17.2) | 68.7 (19.5) | <0.01 |
| Psychosocial Well-being | 71.2 (17.6) | 76.4 (20.0) | 80.2 (19.6) | 69.8 (19.3) | 71.8 (19.5) | 78.4 (19.7) | <0.01 |
| Physical Well-being | 92.7 (10.7) | 75.2 (15.8) | 73.4 (15.2) | 76.8 (16.9) | 76.3 (16.0) | 78.3 (15.4) | <0.01 |
| Sexual Well-being | 56.0 (18.5) | 51.3 (22.0) | 54.7 (20.7) | 42.7 (23.3) | 48.8 (21.8) | 54.1 (21.8) | <0.01 |
SD = standard deviation; Overall = all breast cancer patients, p = p-value for comparison of Normative and Surgery groups (columns 1 and 3 to 6, ANOVA)
Table 2b.
Tests of BREAST-Q Score Differences Among Propensity-Matched Participants, Unadjusted
| Satisfaction with Breasts | Psychosocial Well-being | Physical Well-being | Sexual Well-being | |||||
|---|---|---|---|---|---|---|---|---|
| Variable | Diff. in Mean (lower to upper 95% CI) |
p-value | Diff. in Mean (lower to upper 95% CI) |
p-value | Diff. in Mean (lower to upper 95% CI) |
p-value | Diff. in Mean (lower to upper 95% CI) |
p-value |
| General | ||||||||
| Cancer Pt vs. Normative | 5.47 (4.01 to 6.93) | <.01 | 5.23 (3.83 to 6.62) | <.01 | −17.55 (−18.61 to −16.48) | <.01 | −4.77 (−6.34 to −3.21) | <.01 |
| Surgery Types | ||||||||
| BCS vs. Normative | 8.15 (6.59 to 9.71) | <.01 | 9.02 (7.53 to 10.50) | <.01 | −19.32 (−20.48 to −18.17) | <.01 | −1.38 (−3.05 to 0.30) | 0.11 |
| Mastectomy vs. Normative | −2.38 (−4.33 to −0.43 | 0.02 | −1.36 (−3.22 to 0.50) | 0.15 | −15.92 (−17.36 to −14.47) | <.01 | −13.35 (−15.46 to −11.24) | <.01 |
| Implants vs. Normative | 2.50 (0.67 to 4.33) | <.01 | 0.67 (−1.08 to 2.41) | 0.45 | −16.48 (−17.83 to −15.12) | <.01 | −7.27 (−9.22 to −5.31) | <.01 |
| Autologous vs. Normative | 11.04 (8.86 to 13.21) | <.01 | 7.29 (5.22 to 9.36) | <.01 | −14.48 (−16.09 to −12.88) | <.01 | −1.97 (−4.30 to 0.36) | 0.10 |
Diff. = difference; CI = confidence interval; Pt = patient; BCS = breast conserving surgery; Difference in Mean: positive number indicates higher scores in the cancer group
Table 3 demonstrates an evaluation of differences in the relationship between time and BREAST-Q score by surgical type in patients matched to a normative sample. The interaction of time since surgery and surgery type in the regression model tests if the relationship between the former and the BREAST-Q domain depends on the latter. Slope differences were found among the surgery types for Satisfaction with Breasts (p < 0.0001). Cancer patients with breast conservation or an implant-reconstructed breast had decreasing Satisfaction with Breasts over time, but the mastectomy-only cohort had increasing Satisfaction with Breasts over time. There were no other differences in slopes between the surgery types. For Psychosocial, Physical, and Sexual Well-being, scores generally increased over time at comparable rates for all groups, with the exception of stagnant Sexual Well-being scores in implant-based reconstruction patients. After adjustment for age, BMI, marital status, income, and stage, the breast conservation and mastectomy-only cohorts joined the implant cohort in unchanging Sexual Well-being scores over time (See Table, Supplemental Digital Content 3, which shows the BREAST-Q Scores Over Time Among Normative-Matched Breast Cancer Patients, (http://links.lww.com/PRS/D931). Results consistent with those reported were obtained using quantile regression for sensitivity analyses.
Table 3a.
BREAST-Q Scores Over Time Among Normative-Matched Breast Cancer Patients, Slope (change in mean per year)
| Lumpectomy | Mastectomy | Implant | Autologous | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Module | Interaction Term p |
Estimate | 95%CI | Estimate | 95 % CI | Estimate | 95 % CI | Estimate | 95 % CI |
| Satisfaction with Breasts | <.01 | −0.43 | (−0.600, −0.257) | 0.45 | (0.239, 0.664) | −0.34 | (−0.572, −0.102) | −0.11 | (−0.437, 0.223) |
| Psychosocial Well-being | 0.07 | 0.25 | (0.091, 0.418) | 0.61 | (0.406, 0.811) | 0.37 | (0.143, 0.592) | 0.43 | (0.117, 0.747) |
| Physical Well-being | 0.07 | 0.51 | (0.380, 0.639) | 0.77 | (0.611, 0.932) | 0.52 | (0.341, 0.696) | 0.54 | (0.292, 0.790) |
| Sexual Well-being | 0.13 | 0.20 | (0.012, 0.394) | 0.50 | (0.268, 0.738) | 0.13 | (−0.126, 0.381) | 0.36 | (0.002, 0.721) |
CI = confidence interval
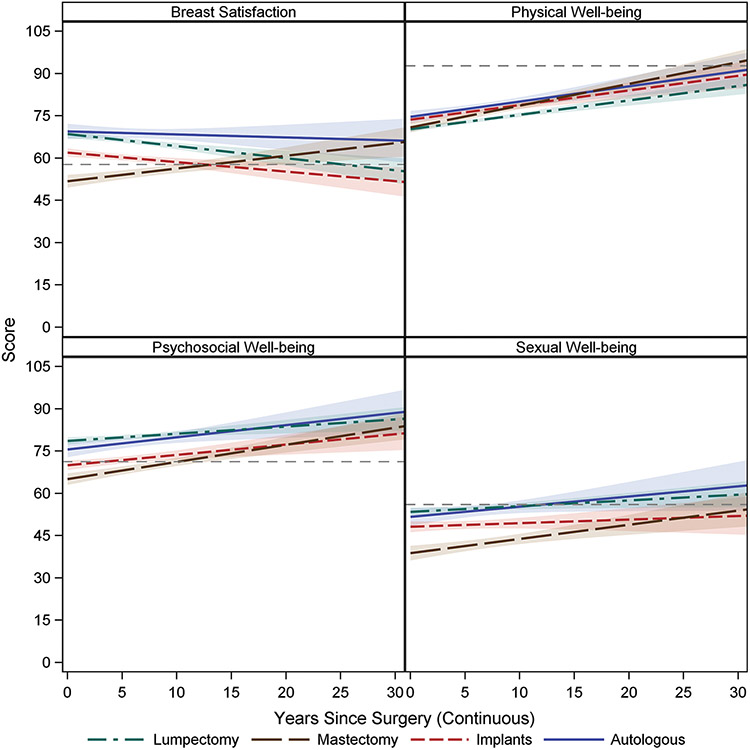
The normative data was used as a reference point to visually compare differences in BREAST-Q scores of the breast cancer patients, by surgical groups, over time, see Figure 1. The mean of the normative data for each BREAST-Q domain is represented by the horizontal reference line (dashed) within the plot of the estimated regression lines for each surgery type in the absence of covariates. For breast cancer patients undergoing mastectomy without reconstruction, scores averaged lower than the normative sample, but improved over time. Patients undergoing mastectomy with autologous reconstruction had higher BREAST-Q scores in comparison to implant reconstruction in the Satisfaction with Breasts, Psychosocial and Sexual Well-being domains.
Figure 1.
BREAST-Q Scores in Breast Cancer Patients in Comparison to the Norm Over Time with 95% Confidence Bands
DISCUSSION
Decision-making for breast cancer surgery is unique in that there are various patient-selected surgical options, with near equivalent oncologic outcomes. Surgical decision-making is often based on a woman’s own goals and perspectives. As a result, PROs serve an important role in framing the discussion between patient and providers when choosing between highly preference sensitive options. One of the most frequently used instruments to better understand patients’ perspectives in this population, and direct these conversations, has been the BREAST-Q. The BREAST-Q has been used to demonstrate findings including increased patient satisfaction in autologous versus implant-based reconstruction (18-20) and reconstruction versus mastectomy alone.
An area with a paucity of data in the literature has been that of long-term PROs. The majority of studies evaluating PROs in breast cancer patients have reported outcomes with follow-up intervals less than 2-3 years (5). Some of the longest follow-up was reported in a recent study by Thorarinsson et al, where PROs were evaluated at 5-6 years following autologous versus implant reconstruction (21). The authors found the highest BREAST-Q scores in patients who underwent deep inferior epigastric perforator flap reconstruction at the time of the study, in comparison to other types of autologous reconstruction or implant reconstruction. However, with 5-year survival rates of 90%, and many women living decades beyond their cancer diagnosis, there is significant need for PRO data with substantially longer follow up.
Our aim was to evaluate not only the differences in PROs across various surgical procedures over a longer period of time from surgery, but to compare these results to a normative population without a breast cancer diagnosis. In our analysis, we identified significant variability in BREAST-Q outcomes in patients across several decades from surgery, with important insights from the normative comparison, highlighting the importance of this long-term evaluation.
First, we were surprised to discover that at the time of survey participation, breast cancer patients reported significantly higher Satisfaction with Breasts and Psychosocial scores, although lower Physical and Sexual Well-being scores, compared to the norm. This finding was largely driven by the lumpectomy group, although the autologous reconstruction group also reported higher scores in the Satisfaction with Breasts and Psychosocial domains. This observation underscores that current surgical treatment with breast conservation is highly effective not only for treatment of cancer, but in preserving psychosocial health.
Our findings also support that all groups undergoing surgery for breast cancer experience a significant reduction in Physical Well-being and Sexual Well-being compared to the normative cohort, even in the lumpectomy group. These results indicate that although lumpectomy and radiation are considered to have relatively low morbidity compared to mastectomy, they may nevertheless contribute to long term physical symptoms that can significantly diminish quality of life (22). Ongoing surveillance for post-surgical symptoms is thus important in order to identify opportunities to improve physical and sexual function throughout survivorship.
We and others have previously reported superior PROs in patients undergoing autologous reconstruction compared to implant-based reconstruction (18-20). The findings here support the recommendation for autologous reconstruction when feasible. In our analysis, we found consistently higher BREAST-Q scores after autologous reconstruction compared to implant-based reconstruction especially over the long-term. In addition, with the exception of Physical Well-being, patients who had autologous reconstruction were either near or above the normative data scores across time. These findings highlight the importance of providing breast cancer patients the opportunity to discuss surgical treatment options with a plastic surgeon, and even more so, a plastic surgeon that is capable of providing autologous-based reconstruction prior to final surgical decision making.
The positive impact of breast reconstruction, as compared to mastectomy alone, has been another common theme well established in the literature (23-27). However, the majority of these studies have focused on short-term outcomes without a normative comparison. In patients with the shortest follow-up time in our analysis, BREAST-Q scores are lowest in patients after mastectomy without reconstruction, both in comparison to other surgical groups and the norm. In a recent case-controlled cross-sectional study by Howes, comparing 400 women with and without breast cancer, the authors reported significantly lower BREAST-Q scores for Satisfaction with Breasts and Sexual Well-being in mastectomy patients without reconstruction compared to normative data and all other breast surgery cohorts (23). This is similar to our findings in the early time period following surgery. However, Howes et al. only provided data at a single point in time, with a mean 4 years follow-up. In our study, we found that scores for mastectomy patients continue to increase with time from surgery and by approximately 10 years post-treatment, PROs in the mastectomy-alone cohort rose above the norm in the Psychosocial Well-being and Satisfaction with Breasts domains. These findings suggest that the majority of the associated negative PROs in patients foregoing breast reconstruction are most relevant in the first few years after treatment, and conversely, a large portion of the value of breast reconstruction is recognized early rather than over the long term.
There are several strengths to our analysis. This is the largest known study to date comparing long-term BREAST-Q outcomes in patients with and without breast cancer. There were over 900 patients in the normative cohort, and over 4,000 breast cancer patients in the propensity-matched analysis, with a wide variety of cancer characteristics and surgical-based oncologic treatments and associated reconstructions. Although survey data were collected at a single time point, the cancer cohort sampled patients from a large range of follow-up, from less than 1 year to 45 years post-surgery. Additionally, we evaluated PROs as a function of time, allowing for an understanding of both early and late outcomes, and trends over time.
Use of a normative population in our analysis is of particular strength. The majority of current breast cancer PRO literature compares post-operative outcomes between treatment groups, or pre- and post-operative data. By using a normative population as our control, we have eliminated the bias a breast cancer diagnosis potentially introduces to “baseline” PRO scores. The data presented here provides women with unique information about PROs associated with different treatment options, as compared to women who have never experienced a breast cancer diagnosis. This comparison of PRO data to a normative population is additionally an important component of the discussion regarding resource allocation and health care policy, as restoring PROs to the level of the normative population after breast cancer treatment is an important benchmark.
The limitations of this analysis should be acknowledged. We performed an analysis comparing two cross-sectional datasets; therefore our results are subject to biases associated with the nature of the study design. Given the otherwise complexity of our analysis and self-reported data, we did not account for unilateral versus bilateral reconstruction, mastectomy skin incision pattern, pre- versus post-operative radiation, mastectomy specimen weight, or bra cub size. The single timepoint for data collection precluded a within-patient longitudinal analysis, including comparisons of pre- and post-operative BREAST-Q data. Additionally, while a longitudinal, prospective study would produce a more robust dataset, it would require 20-30 years to generate long-term outcomes, therefore we believe our analysis provides value in the immediate setting. Further, there was a higher rate of missing data in the Sexual Well-being module for the normative sample (15% vs. 5%) which was partly overcome by use of propensity matching. Moreover, we made comparisons between patients that were treated over a long time span which may have exposed the study results to recall bias. In addition, surgical techniques, implant quality and overall patient management has evolved over the past 30 years. It is presumed that the care patients received 30 years ago is not the same as it is today, however this is an inherent limitation to long-term outcomes research in medicine. Lastly, the AOW represents a skewed demographic, with higher rates of wealthy, educated, and white females in comparison to population norms, limiting the generalizability of this data. This is an important limitation that future studies must be designed to address when collecting PRO data that captures the patient perspective.
CONCLUSION
This study found that patients who undergo breast surgery for breast cancer have higher Satisfaction with Breasts and Psychosocial scores compared to the norm at the time of survey participation, and that differences between breast cancer and normative groups varied by PRO module and surgical procedure. Overall, our findings are highly encouraging. While ongoing monitoring for treatment-related symptoms are clearly needed, many patients undergoing surgery for breast cancer can expect to regain equivalent or higher quality of life compared to those women without a history of breast cancer.
Supplementary Material
See Table, which demonstrates the result of the propensity matching process and how successful it was at creating two groups that were more similar on their demographics than the original two groups, http://links.lww.com/PRS/D929.
See Table, which shows the differences among the surgery types, http://links.lww.com/PRS/D930.
See Table, which shows the BREAST-Q Scores Over Time Among Normative-Matched Breast Cancer Patients, http://links.lww.com/PRS/D931.
Acknowledgements:
The authors would like to thank Carolyn Kerrigan, MD, for contributing to the generation of the normative data sample.
Financial Disclosure Statement:
The BREAST-Q is owned by Memorial Sloan-Kettering Cancer Center. Dr. Pusic is a co-developer. She receives a portion of licensing fees when the BREAST- Q is used in industry sponsored clinical trials. The remaining authors have no disclosures. Dr. Pusic received support through the NIH/NCI Cancer Center Support Grant P30 CA008748. Funding for the collection of the normative data was provided a discretionary account of Dr. Carolyn Kerrigan’s held by The Dartmouth Institute, and the breast cancer data was supported in part by Grant No. 235066 from the Plastic Surgery Foundation.
REFERENCES
- 1.Centers for Disease Control and Prevention. Cancer Prevention and Control: Data and Statistics, Cancer Amoung Women. Available at: http://www.cdc.gov/cancer/dcpc/data/women.htm. Accessed November 2017. [Google Scholar]
- 2.American Cancer Society. Cancer Facts and Figures 2016. Atlanta, GA: American Cancer Society, 2016. [Google Scholar]
- 3.Hanspal RS, Fisher K Assessment of cognitive and psychomotor function and rehabilitation of elderly people with prostheses.[Erratum appears in BMJ 1991 May 18;302(6786):1182]. Bmj 1991;302:940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.BREASTCANCER.ORG: U.S. Breast Cancer Statistics. Available at: http://www.breastcancer.org/symptoms/understand_bc/statistics. Accessed January 2018. [Google Scholar]
- 5.Cohen WA, Mundy LR, Ballard TN, et al. The BREAST-Q in surgical research: A review of the literature 2009-2015. Journal of plastic, reconstructive & aesthetic surgery : JPRAS 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.NIH: National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Female Breast Cancer. Available at: https://seer.cancer.gov/statfacts/html/breast.html. Accessed January 2018. [Google Scholar]
- 7.Cano S, Klassen AF, Scott A, Thoma A, Feeny D, Pusic A Health outcome and economic measurement in breast cancer surgery: challenges and opportunities. Expert review of pharmacoeconomics & outcomes research 2010;10:583–594. [DOI] [PubMed] [Google Scholar]
- 8.Cano SJ, Klassen AF, Scott AM, Cordeiro PG, Pusic AL The BREAST-Q: further validation in independent clinical samples. Plastic and reconstructive surgery 2012;129:293–302. [DOI] [PubMed] [Google Scholar]
- 9.Pusic AL, Klassen AF, Cano SJ Use of the BREAST-Q in clinical outcomes research. Plastic and reconstructive surgery 2012;129:166e–167e; author reply 167e. [DOI] [PubMed] [Google Scholar]
- 10.Cano SJ, Klassen AF, Scott AM, Pusic AL A closer look at the BREAST-Q((c)). Clinics in plastic surgery 2013;40:287–296. [DOI] [PubMed] [Google Scholar]
- 11.Pusic AL, Klassen AF, Scott AM, Klok JA, Cordeiro PG, Cano SJ Development of a new patient-reported outcome measure for breast surgery: the BREAST-Q. Plastic and reconstructive surgery 2009;124:345–353. [DOI] [PubMed] [Google Scholar]
- 12.Aaronson N, Alonso J, Burnam A, et al. Assessing health status and quality-of-life instruments: attributes and review criteria. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation 2002;11:193–205. [DOI] [PubMed] [Google Scholar]
- 13.Army of Women, Dr. Susan Love Research Foundation: Closed Studies. Available at: https://www.armyofwomen.org/studies/closed. Accessed November 2017. [Google Scholar]
- 14.Mundy LR, Homa K, Klassen AF, Pusic AL, Kerrigan CL Breast Cancer and Reconstruction: Normative Data for Interpreting the BREAST-Q. Plastic and reconstructive surgery 2017;139:1046e–1055e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atisha DM, Rushing CN, Samsa GP, et al. A national snapshot of satisfaction with breast cancer procedures. Annals of surgical oncology 2015;22:361–369. [DOI] [PubMed] [Google Scholar]
- 16.Hwang ES, Locklear TD, Rushing CN, et al. Patient-Reported Outcomes After Choice for Contralateral Prophylactic Mastectomy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2016;34:1518–1527. [DOI] [PubMed] [Google Scholar]
- 17.Austin PC A comparison of 12 algorithms for matching on the propensity score. Statistics in medicine 2014;33:1057–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu C, Zhuang Y, Momeni A, et al. Quality of life and patient satisfaction after microsurgical abdominal flap versus staged expander/implant breast reconstruction: a critical study of unilateral immediate breast reconstruction using patient-reported outcomes instrument BREAST-Q. Breast cancer research and treatment 2014;146:117–126. [DOI] [PubMed] [Google Scholar]
- 19.McCarthy CM, Mehrara BJ, Long T, et al. Chest and upper body morbidity following immediate postmastectomy breast reconstruction. Annals of surgical oncology 2014;21:107–112. [DOI] [PubMed] [Google Scholar]
- 20.Eltahir Y, Werners LL, Dreise MM, Zeijlmans van Emmichoven IA, Werker PM, de Bock GH Which Breast Is the Best? Successful Autologous or Alloplastic Breast Reconstruction: Patient-Reported Quality-of-Life Outcomes. Plastic and reconstructive surgery 2015;135:43–50. [DOI] [PubMed] [Google Scholar]
- 21.Thorarinsson A, Frojd V, Kolby L, Ljungdal J, Taft C, Mark H Long-Term Health-Related Quality of Life after Breast Reconstruction: Comparing 4 Different Methods of Reconstruction. Plastic and reconstructive surgery Global open 2017;5:e1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gartner R, Jensen MB, Nielsen J, Ewertz M, Kroman N, Kehlet H Prevalence of and factors associated with persistent pain following breast cancer surgery. Jama 2009;302:1985–1992. [DOI] [PubMed] [Google Scholar]
- 23.Howes BH, Watson DI, Xu C, Fosh B, Canepa M, Dean NR Quality of life following total mastectomy with and without reconstruction versus breast-conserving surgery for breast cancer: A case-controlled cohort study. Journal of plastic, reconstructive & aesthetic surgery : JPRAS 2016;69:1184–1191. [DOI] [PubMed] [Google Scholar]
- 24.Eltahir Y, Werners LL, Dreise MM, et al. Quality-of-life outcomes between mastectomy alone and breast reconstruction: comparison of patient-reported BREAST-Q and other health-related quality-of-life measures. Plastic and reconstructive surgery 2013;132:201e–209e. [DOI] [PubMed] [Google Scholar]
- 25.Jeevan R, Cromwell DA, Browne JP, et al. Findings of a national comparative audit of mastectomy and breast reconstruction surgery in England. Journal of plastic, reconstructive & aesthetic surgery : JPRAS 2014;67:1333–1344. [DOI] [PubMed] [Google Scholar]
- 26.Ng SK, Hare RM, Kuang RJ, Smith KM, Brown BJ, Hunter-Smith DJ Breast Reconstruction Post Mastectomy: Patient Satisfaction and Decision Making. Annals of plastic surgery 2014. [DOI] [PubMed] [Google Scholar]
- 27.Sisco M, Johnson DB, Wang C, Rasinski K, Rundell VL, Yao KA The quality-of-life benefits of breast reconstruction do not diminish with age. Journal of surgical oncology 2015;111:663–668. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See Table, which demonstrates the result of the propensity matching process and how successful it was at creating two groups that were more similar on their demographics than the original two groups, http://links.lww.com/PRS/D929.
See Table, which shows the differences among the surgery types, http://links.lww.com/PRS/D930.
See Table, which shows the BREAST-Q Scores Over Time Among Normative-Matched Breast Cancer Patients, http://links.lww.com/PRS/D931.