Abstract
Many studies have shown that stress is associated with gut microbiota. Environmental enrichment (EE) could reduce stress in farm animals; however, limited information is available on the microbial community composition in rabbits raised with or without EE. This study aimed to identify EE influences on the behavior, serum hormonal levels, and cecal microbiota of rabbits. Two hundred Rex rabbits were segregated randomly within four cohorts (n = 50); reared for 76 d within standardized enclosures (non-enriched) or within cages containing a willow-stick (WS), rubber-duck (RD), or a can of beans (CB). The rabbits’ ingestive, rest, locomotion, exploratory, grooming, and abnormal behavior were observed. The serum hormone levels for rabbits were measured, and cecal specimens were sequencedfrom the V3–V4 region using 16S rRNA amplicons. Environmental enrichment increased feeding and drinking time, promoted exploratory behavior, and reduced abnormal behavior in rabbits. Insulin-like growth factor 1(IGF-1) levels of the enriched cohorts were elevated in comparison to the control cohort. Serum cortisol level for CB cohort was markedly reduced in comparison to the control cohort (p < 0.05), while dopamine levels for CB cohort peaked. Further, we found that EE mainly affected the dominant microbiota. Several families, such as Erysipelotrichaceae, Tannerellaceae, Enterobacteriaceae, Burkholderiaceae, and Prevotellaceae were markedly reduced within the CB cohort. Bacteria such as Alloprevotella, Bifidobacterium, Enterobacteriaceae, Parabacteroides, and Erysipelatoclostridium were identified as having negative associations with the presence of serum cortisol. EE influenced rabbit behavior and serum hormonal levels, and CB enrichment was the most suitable for rabbits. Further, cecal microbiota composition and diversity were affected by CB enrichment. These findings suggested that CB could be considered for use in rabbit husbandry.
Keywords: Environmental enrichment, Cecal microbiota, Rabbit husbandry, Rabbit behavior, Rex rabbits
Introduction
Modern animal husbandry typically involves raising animals under high-density conditions, which can cause environmental stress in animals. Stress can in turn lead to reduced productivity, physical and emotional suffering, and even death (Cheng et al., 2001). Environmental enrichment (EE) improves the environment of captive animals and enhances their physical and psychological well-being by addressing species-specific needs (Vera, 2005). It is important in animal production, because it can relieve environmental pressure, reduce abnormal behavior, and improve animal welfare (Bozicovich et al., 2016). Regarding rabbit production, Princz found that in growing rabbits, gnawing sticks reduced stereotypical behavior, such as cage bar biting or chewing (Princz et al., 2007). Trocino reported that elevated platforms were a useful structural enrichment for improving rabbit behavior (Trocino et al. , 2019). Further, Mohammed & Nasr (2017) found that a wooden stick promoted finalized body-weight, improved several carcass traits, reduced abnormal behavior, while possibly promoting the well-being in rabbits during intensive breeding. However, in previous studies, researchers often used only one type of EE, or solely performed comparative analyses over effects from similar EE resources (e.g., apple-sticks against willow-sticks) (Bozicovich et al., 2016; Princz et al., 2007; Mohammed & Nasr, 2017). Few studies have compared the influences of dissimilar EEs on rabbit behavior. Consequently, identifying the ideal EE resources having peak effectiveness within rabbits can be challenging.
In addition to behavior, hormonal levels are also potential indicators of animal stress. Stress reactions in animals are controlled by their neuroendocrine systems, particularly by the adrenal cortex for the hypothalamic-pituitary-adrenal (HPA) axis and the sympathetic-medullary-adrenal (SMA) axis (Hennessy, 1997). Dopamine (DA) and cortisol are released via these two systems, respectively. These hormones are often associated with stress and are functionally involved in controlling animals’ behavior and metabolic, endocrinal, and immune functions to ensure adequate coping strategies and well-being (Pani, Porcella & Gessa, 2000). Therefore, DA and cortisol levels could reflect the degree of stress in animals.
In recent years, several studies have addressed the role of stress in animal production. Some studies have focused on intestinal—cerebral inter-communication, a pathway known as the brain-gut-microbiota axis (Dinan & Cryan, 2012), and it has been proven that this signalling pathway is bidirectional (Mayer, Savidge & Shulman, 2014). Previous studies have demonstrated that the gut and the central nervous system (CNS) are closely linked and play a role in maintaining gastrointestinal homeostasis, any changes that reduce the beneficial bacteria in the gastrointestinal tract can negatively impact the animals’ neuroendocrine and the immune systems (Taché et al., 2001; Cryan & O’Mahony, 2011). Stress can play a considerable role in dysregulating GIT microbiota constituent levels (Mayer, Savidge & Shulman, 2014). Several evidences suggest that psychological stress may have important effects on the intestinal microbiota of animals and humans (Dinan & Cryan, 2012; Tsilimigras et al., 2018; Partrick et al., 2018). Previous research on animal welfare reported that road transport and rearing-room size affected animals’ cecal microbiota (Ludvigsen, Svihus & Rudi, 2016; Perry et al., 2018). However, few studies have considered the impact of EE on animals’ gastrointestinal microbiota. As EE might reduce stress, we hypothesised that its application could affect animals’ gut microbiota. Due to the association across animals’ general well-being/GIT microbiome harmony, proper knowledge on the effect of EE on gut microbial communities is vital.
Depending upon the biological characteristics of rabbits, we selected three types of EE materials in this study: a willow-stick (typical EE in such animals); a rubber-duck, with multi-sensorial appeal, that could be suspended within the enclosure to satisfy rabbits’ need for exploration, and a can of beans (self-made: we put some mung beans in the tin can) that made a noise when rabbits played with it, which also satisfied rabbits’ curiosity. We investigated which of the three EE materials were most effective in reducing abnormal behavior and reducing stress by measuring the rabbits’ serum hormonal levels and cecal microbiota content. This study represents the first attempt to study the effect of EE on cecum microbiota. Our research findings will offer important guidance to practitioners of rabbit husbandry who seek to enrich rabbits’ environments and improve productivity outcomes.
Materials and Methods
Animal, feeding, and housing
The experiment was conducted on July 8–September 24, 2019, at the YuanFa Rex Rabbit Farm in Baiyin, Gansu Province, China (latitude 36°44′N–37°10′N, longitude 104°58′E–05°11′E, and altitude 2,040 m). Two hundred Rex rabbits (2 months old; body weight = 1917 ± 31.45 g) were segregated randomly within four cohorts (n = 50): control cohort (CO) and three EE treatment cohorts. From birth to 2 months old, all rabbits were raised under the same conditions, i.e., in a setup of two rabbits/enclosure (60 × 45 × 40 cm; bamboo-floor). They were housed under natural light conditions at a temperature of 12 °C to 24 °C, and relative humidity of 50%–55%. The rabbits were reared for 76 d from the age of 2 months, which represented the fattening stage. They were inspected daily; and allowed access to 4-mm diameter pelleted diet and water ad libitum. Diet was prepared according to the dietary nutritional requirements for rabbits given by the Nutritional Research Council. The composition and nutrient levels of the basal diet are shown in Table S1. This investigation was accepted through the Animal Science and Technology, Gansu Agricultural University Animal Care and Use Committee (Approval #2019-2-161). Rabbit care/handling was consistent with the Regulations for the Administration of Affairs Concerning Experimental Animals (The State Science and Technology Commission of P.R. China, 1988). No death or disease in the rabbits was observed during the experimental period.
Environmental enrichment
Three types of EE material were used in different treatment cohorts: a willow-stick (WS), rubber-duck (RD), and one can of beans (CB)/cage. The RD was hung at approximately 20 cm from the cage bottom, and the WS and CB were placed on the cage floor. The CB could generate sound when rabbits played with it (Fig. 1).
Figure 1. Environmental enrichment materials.
(A) Willow stick; (B) rubber duck; (C) can of beans.
Behavioral observations
Direct focal observations of rabbits in their home cages were conducted to record different behaviors for 15 consecutive days throughout the experimental period (Abdelfattah et al., 2013). After the preliminary experiment, the behavioral observations began on the 16th day of the experiment. Seventeen observers stood inside the animal enclosure for 10 min before recording their observations to allow the rabbits to acclimatize to their presence. Instantaneous and scan sampling methods were used. To avoid subjective errors, we trained the seventeen observers and assessed their reliability by making them to all record the behavior of one rabbit before the experiment began. The records by the different observers were found to be similar. To minimize the subjective error further, rabbits were randomly assigned to the observers for each observation period. Rabbits were observed twice daily for 40 min each time at noon (12:00) and night (21:40). During the observations, instances of rabbits demonstrating any of the behaviors listed in Table 1 (as defined by Mohammed and Trocino) were recorded (Trocino et al. , 2019; Mohammed & Nasr, 2017).
Table 1. Behaviors of Rex rabbits and their definition.
| Category | Behavior | Definition |
|---|---|---|
| Ingestive behavior | Feeding | Head in feeder |
| Drinking | Mouth in contact with drinking nipples | |
| Exploratory behavior | Rearing-up | Hind-leg-based sitting-posture and body in vertical-posture |
| Sniffing | Sniffing air / enclosure | |
| Interaction | Playing or gnawing with cage enrichment material | |
| Abnormal behavior | Circling | Moving in circles |
| Abnormal rest posture | Poses other than abdominal-lateral pose, abdominal pose, and lateral pose | |
| Biting/licking | Wire/feeder gnawing/biting | |
| Locomotion behavior | Walking | Displacing the whole body |
| Standing | Standing on the hind legs with front legs on the side for cage | |
| Rest behavior | Rest | Rabbits lying down without any activity (with eyes closed or almost closed) |
| Grooming behavior | Grooming | Licking/nibbling its fur, forelimbs used for facial cleansing |
Collection of blood specimens and IGF-1, dopamine, and cortisol assays
Rabbit blood specimens were collected on August 29 and 30, 2019. Twenty rabbits per cohort were randomly selected for examining the blood, obtained at 19:00 h before the rabbits received their last daily meal. Auricular arterial blood was obtained via venipuncture, and blood was collected as gently as possible to avoid stress. Blood specimens were placed within serum-separating tubes. To better assist serum separation, the specimens were placed in a water-bath (38 ° C) for 30 min, followed by centrifuging (10 min / 3000 × g) and immediate serum collection/analysis. The serum-hormonal levels were determined using a rabbit IGF-1, DA, and cortisol - stimulating hormone ELISA Kit (HePengBio, Shanghai, China).
Slaughter, collection of cecal contents, and 16S rRNA gene sequencing
Based on the reduction rule of the “3Rs” experimental animals’ rules, further analysis was conducted depending upon the behavioral and hormonal data. The CB cohort rabbits spent the most time playing with the EE, and their serum hormone levels were distinct in comparison to the CO cohort. Therefore, 16S rRNA genomic-sequencing was employed for characterizing the microbiota constituents within six cecal specimens from the CO and the CB cohorts each. Other rabbits continued to feed until their commercial sale. At the end of the experiment, six rabbits were randomly selected from the two cohorts each for euthanization. The humane endpoint for study was the death of 12 rabbits. They were stunned and exsanguinated via their carotid arteries and jugular veins. The caeca specimens were collected under sterile conditions approximately three cm from the ileocaecal junction and their contents were sampled for microbial DNA extraction. The caecal specimens were snap-frozen within liquid-nitrogen and placed in −80 ° C storage.
Total genomic DNA was collected from each specimen using the CTAB/SDS technique. DNA concentrations/purity were observed using 1% agarose-gels. The extracted DNA was standardized at 1 ng/ µL for polymerase chain reaction (PCR)-template use using bar-coded primers adjacent to the V3–V4 hypervariable-region for bacterial 16S rRNA genome. Primer sequences used were 341F (5′-CCTAYGGGRBGCASCAG-3′)/806R (5′-GGACTACNNGGGTATCTAAT-3′). All PCR runs were performed using 15 µL of Phusion® High-Fidelity PCR Master Mix (New England Biolabs). Sequencing libraries were developed through TruSeq® DNA PCR-Free Sample Preparation Kit (Illumina™, USA) according to the kit protocols, with addition of index-codes. The library-quality was evaluated through Qubit® 2.0 Fluorometer (Thermo Scientific) and Agilent™ Bioanalyser® 2100 platforms. Finally, library sequencing was conducted across the Illumina™ NovaSeq® platform, with the generation of 250 bp paired-end reads. Paired-end reads were merged through FLASH® (V1.2.7), and quality-filtering was performed upon raw-tags with bespoke filtering-conditions for obtaining clean, reliable tags consistent with QIIME (V1.9.1) procedures. All tags were matched to the Silva Database (https://www.arb-silva.de/) through the UCHIME algorithm for identifying the chimeric sequences for exclusion.
Statistical analysis
For operational taxonomic unit (OTU) production classification, sequence analysis was employed through Uparse v7.0.1001. OTU clustering was performed using UCLUST (97% similarity), and singletons were excluded during downstream evaluations. Representative sequences within individual OTUs were examined for additional annotating. Regarding individual reflecting sequence, the Silva Database was employed along with the Mothur algorithm for annotating taxonomy datasets, and multiple-sequence alignments were conducted using MUSCLE version 3.8.31. Additional evaluations for alpha-/ beta-diversity were conducted depending upon the normalized output datasets. The OTU abundance information was rarefied to the lowest number of reads observed in a single specimen. Beta-diversity was employed for assessing species-complexity-based variations. Beta-diversity analysis was calculated depending upon unweighted UniFrac distances using QIIME version 1.9.1. Metastat analyses were used to evaluate the differences between the two cohorts, while the Benjamini and Hochberg procedures were used for estimating the q-value. Cluster analysis was preceded by principal component analysis (PCA), employed for reducing the dimensions for original variables through FactoMine R/ggplot2 packages in R version 2.15.3. Un-weighted pair-cohort technique with arithmetic means (UPGMA) clustering, for hierarchical clustering, was performed for interpreting distance matrix base on mean linkage through QIIME software version 1.9.1.
Other datasets were assessed through SPSS® 20. The alpha diversity index was analyzed using an independent-sample t-test. Behavior data obtained repeatedly at multiple sampling times and data from the same cage were considered to be repeated measures and were analysed via repeated-measures analysis of variance (ANOVA). Different behaviors were analysed separately using a generalized linear mixed model that considered EE materials as random effects. Other data were analysed via one-way analysis of variance (ANOVA). Major variations across mean values were determined through Duncan’s test. p < 0.05 and p < 0.01 were deemed to confer statistical significance, and dataset outcomes were presented as means ± standard error of means (SEM).
Results
Behavior observations
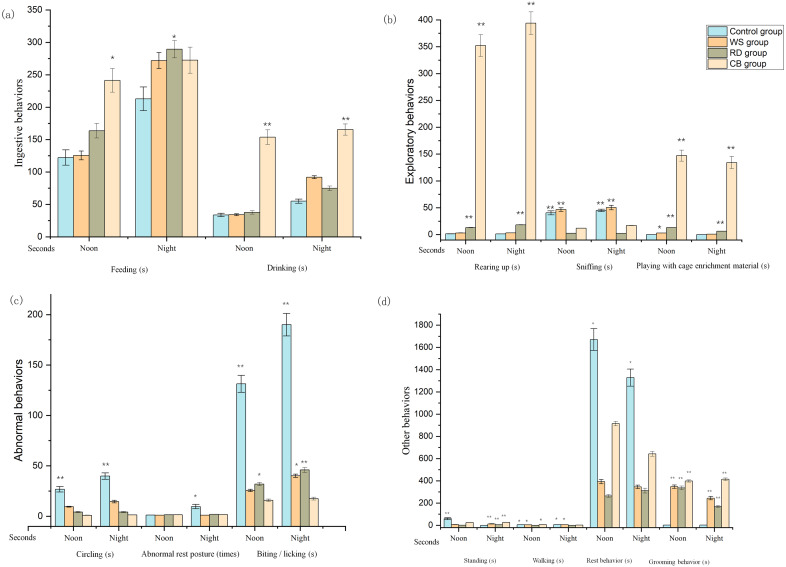
As shown in Fig. 2, the feeding time for enriched cohorts was more elevated than that for the CO cohort at noon and night. The feeding (noon) and drinking (noon and night) times of rabbits within CB cohort were markedly longer in comparison to the CO-cohort rabbits (p < 0.05 and p < 0.01 respectively). Regarding exploratory behavior, rearing-up behavior occurred more frequently in both the RD and CB cohorts than within the CO and WS cohorts at noon and night (p < 0.01). The sniffing time for RD- and CB- cohort rabbits was less than for those in the CO and WS cohorts (p < 0.01). Rabbits played with the CB longer than with the other forms of enrichment and spent the least amount of time playing with the WS. The circling time for the enriched cohort was significantly less in comparison to the CO cohort (p < 0.01). At night, fewer abnormal rest postures were observed within the EE cohorts than within the CO cohort (p < 0.05). Further, less incidences of biting were observed within the EE cohorts than within the CO cohort, and the CB cohort demonstrated the least number of biting instances, which was markedly less than that within the CO cohort (p < 0.01). Interestingly, the EE cohorts demonstrated significantly less standing behavior than the CO cohort at noon, while the opposite trend was observed at night (p < 0.01). The RD cohort spent the least amount of time walking (p < 0.05), and the walking time for the CB cohort was also significantly less in than for the CO cohort (p < 0.05). Rabbits within the enriched cohorts spent less time resting than those within CO cohort at noon and night (p < 0.05). Grooming behavior within the enriched cohorts was markedly more prevalent in comparison to within the CO cohort (p < 0.01).
Figure 2. Effect of different environmental enrichment (EE) materials on rabbits’ behavior (during the observation periods).
(A) Ingestive behavior; (B) Exploratory behavior; (C) Abnormal behavior; (D) Standing, walking, resting, and grooming behavior. CO, control; WS, willow stick; RD, rubber duck; CB, Can of beans. (s) Measured as duration; (times) Counted as instances of behavior. ∗ p < 0.05, ∗∗ p < 0.01; values not reported where p > 0.05.
IGF-1, dopamine, and cortisol levels
As shown in Table 2, the IGF-1 levels of rabbits within the enriched cages were markedly elevated in comparison to within the CO cohort (p < 0.05). The DA levels for the CB cohort were markedly elevated in comparison to other cohorts (p < 0.05). Serum cortisol levels for the CO cohort were markedly elevated in comparison to the WS/CB cohorts.
Table 2. IGF-1, dopamine, and cortisol levels in rabbits reared in enriched and conventional cages.
| Trait | CO cohort (n = 20) | WS cohort (n = 20) | RD cohort (n = 20) | CB cohort (n = 20) | p-value |
|---|---|---|---|---|---|
| IGF-1 ng/mL | 85.57 ± 29.26b | 119.47 ± 23.07a | 116.59 ± 17.07a | 105.21 ± 7.04a | 0.023 |
| Dopamine nmol/L | 31.22 ± 5.44b | 30.93 ± 7.13b | 33.41 ± 7.02b | 40.58 ± 14.22a | 0.039 |
| Cortisol ng/mL | 108.47 ± 34.46a | 80.42 ± 10.72b | 84.90 ± 14.19b | 76.58 ± 10.20b | 0.036 |
Notes.
a, b signify values that differ significantly (p <0.05).
- CO
- control
- WS
- willow stick
- RD
- rubber duck
- CB
- can of beans
Cecum microbiota
We acquired 1,156,501 high-quality paired-end sequences, with mean read length of 411 bp/specimen. Depending upon a 97% species-similarity-threshold, 1,105 OTUs were found from the specimens. Further, 13 phyla, 17 classes, 22 orders, 39 families, 73 genera, and 70 species were identified.
Relative presence for top-ranking ten phyla and microbial families present within CO- and CB- cohort rabbits are shown in Fig. 3. Firmicutes / Bacteroidetes predominated as phyla within both CO and CB cohorts. Firmicutes accounted for 74.8% for families within the CO cohort and 64.5% within the CB cohort (Fig. 3A). Ruminococcaceae and Lachnospiraceae were the most prevalent bacteria families within the CO and CB cohorts (Fig. 3B).
Figure 3. Comparison of cecal microbiota of rabbits in the CO and CB groups.
Mean relative abundance for the 10 best-ranking phyla (A)/top10 families (B) within the CB-and CO -cohort rabbits; (C) Box-plot of cecal microbial β-populations (Wilcoxon rank-sum test, p < 0.01); (D) PCA for rabbit cecal specimens depending upon unweighted UniFrac distances; (E) Similarity-cluster analyses of rabbit cecal specimens through UPGMA. (F) Bacterial taxa differences at the family level; (G) bacterial taxa differences at the genus level. Bacterial taxa having mean relative presence > 0.1% within a minimum of one cohort were encompassed; (h) Linear discriminant analysis (LDA) diagram of taxonomic differences between CO- and CB- cohort rabbits depending on LEfSe analysis. Species having major variations regarding presence with an LDA score > 3.0.Histogram bar reflects LDA scoring; (i) Cladogram demonstrates micro-organism-based populations that exhibited major variations across both cohorts. Red and green within phylogenetic-tree reflect micro-organism-based populations having pivotal parts within CB and CO cohorts respectively; (J) Heat map of Spearman correlation analysis results between bacterial genera and serum hormonal levels of rabbits. * p < 0.05; ∗∗ p < 0.01, CO, control; CB, Can of beans.
The observed_species, PD_whole_tree, and Ace indices were markedly elevated within the CO cohort than within the CB cohort. Alternative alpha diversity indices for cecal microbiota in rabbits raised with and without EE did not differ significantly (Table 3).
Table 3. Cecal bacterial alpha diversity in the CO- and CB- cohort rabbits.
| CO cohort (n = 6) | CB cohort (n = 6) | p-value | |
|---|---|---|---|
| Observed_species | 880.0 ± 14.95 | 787.17 ± 27.76 | 0.015 |
| Goods_coverage | 0.9981 ± 0.00013 | 0.99 ± 0.00017 | 0.554 |
| Shannon | 6.70 ± 0.20 | 6.99 ± 0.10 | 0.949 |
| PD_whole_tree | 62.91 ± 0.86 | 56.63 ± 1.65 | 0.007 |
| Simpson | 0.96 ± 0.01 | 0.98 ± 0.001 | 0.217 |
| Chao1 | 926.60 ± 16.42 | 846.05 ± 32.87 | 0.053 |
| Ace | 925.64 ± 15.00 | 841.66 ± 30.85 | 0.034 |
Notes.
- CO
- control
- CB
- Can of beans
A major variation was found within beta diversity for the OTU structures between the CO and CB cohorts (Wilcoxon rank-sum test: p < 0.01). Beta diversity was markedly reduced within the CB cohort than within CO cohort (Wilcoxon rank-sum test: p < 0.01; Fig. 3C, Table S2), indicating that CB enrichment caused low variance across cecal microbiota constituent make-up within rabbits. PCA trajectory plot also revealed distinctions between the microbiota communities within the CO and CB cohorts (Fig. 3D). Similarity-cluster analyses through UPGMA demonstrated adequate corroboration with the PCA analyses (Fig. 3E), suggesting that cecal micro-flora within rabbits changes with the progressive change in the environment caused by EE materials.
We performed a Linear discriminant analysis Effect Size (LEfSe) analysis to reveal differences within significance ranking of abundant bacterial taxa within the CB- and CO- cohort specimens (Fig. 3H). Fig. 3I demonstrates nine valuable microbial taxa. Within CB cohort, Clostridia and the order Clostridiales were important biomarkers. Biomarkers within the CO cohort included Bacteroidia (c), Erysipelotrichia (h), and Gammaproteobacteria (i). Variations were assessed within main bacterial taxa (mean relative presence >0.01% across both cohorts) for the CO/CB cohort rabbits using the t-test. For the two cohorts, five families (Fig. 3F) and three genera (Fig. 3G) showed significant differences between the CO and CB cohort rabbits. Similar microbial taxa were found through metastat analysis (Fig. S1) between the CO and CB cohort rabbits.
Spearman’s correlation was conducted for microbial genera/serum hormone levels (IGF-1, DA, and cortisol) in rabbits. As shown in Fig. 3J, a negative correlation was observed between Enterobacteriaceae and IGF-1 (p < 0.05). DA was positively correlated to Anaerostipes and Campylobacter (p < 0.05). Bifidobacterium, Parasutterella, Parabacteroides, and Enterobacteriaceae showed a strong positive correlation with cortisol levels (p < 0.01). Additionally, Erysipelatoclostridium and Alloprevotella showed a positive correlation with cortisol levels (p < 0.05).
FAPROTAX was employed in microbial functional assessment. Ecological roles for bacteria/archaea within gut specimens were classified through FAPROTAX. Some gut/nitrate functions for bacteria showed significant differences between the two cohorts (Fig. S2).
Discussion
Effects of environmental enrichment on behavior
Across all EE cohorts, CB was favoured by the rabbits, and they played the least with WS (Noon: 153.83 s vs. 2.97 s; Night: 134.17 s vs. 0.75 s for CB vs. WS). This may be attributable to the sound produced by CB when the rabbits played with it, which stimulated the animals’ desire for exploration. As expected, rabbits that spent more time playing with EE rested less during the daytime. The time that rabbits spent in comfortable resting (i.e., in a stretched position) within enriched cages was less than within the CO cohort. The expanded behavioral opportunities offered by the various forms of enrichment seemed to reduce the time rabbits spent lying down, as reported previously by Luzi et al. (2003). While feeding time at night and drinking time at noon and night were all longer among the EE-cohort rabbits than among the CO- cohort rabbits, the feeding and drinking time for the CB cohort was the longest. This indicated that EE could increase rabbits’ appetites and may promote weight gain in animals. The results of previous studies conducted by Luzi et al. (2003), Princz et al. (2005) and Mohammed & Nasr (2017) support this hypothesis and suggest that EE promotes weight gain in rabbits. In our trial, EE also had a positive effect on other behaviors. Exploratory behaviors (rearing-up and playing) were more common within the EE cohorts in comparison to within CO cohort. However, sniffing time was reduced within RD and CB cohorts, and we speculate that the increased time and attention devoted to playing with the EE shortened the rabbits’ sniffing time. At night, abnormal resting postures were increasingly observed within the CO-cohort rabbits than in the EE-cohort rabbits. The RD- cohort rabbits spent the least amount of time standing and walking but there was no obvious trend of standing and walking in other cohorts. This may reflect the fact that the RD that hung from the cage prevented the rabbits from standing and walking to some extent. Circling and biting are usually considered stereotypical behaviors of captive rabbits (Trocino et al. , 2019; Seidel, Beaton & Teague, 1979), and the decreased circling and biting behaviors among the EE-cohort rabbits may suggest that the EE re-directed the rabbits’ attention. These findings support those of previous studies, which showed that EE reduced abnormal behavior in growing rabbits (Mohammed & Nasr, 2017; Abdelfattah et al., 2013). We believe this is because EE relieved boredom and satisfied ethological needs. The increase in grooming behavior within present study diverges from who provided a wooden enrichment structure for rabbits and found that grooming behavior was reduced (Trocino et al., 2019). This might be attributable to the different types of EE and experimental methods used in the two studies.
Effect of environmental enrichment on serum hormone levels
Insulin-like growth factor 1 regulates cell proliferation and plays an important role in cell differentiation, proliferation, and individual growth development (Holzenberger et al., 2003). The high serum IGF-1 levels observed in EE-cohort rabbits indicate that EE could affect the production of serum IGF-1 and rabbit growth. Cortisol and DA are hormones associated with stress (Cheng et al., 2001; Barik et al., 2013). Animals’ stress reactions are controlled by their neuroendocrine systems. Cortisol is released from the adrenal cortex of the HPA axis, and DA is released from peripheral systems, including the medulliadrenal SMA axis (Hennessy, 1997). Both axes are important common pathways in controlling animals’ ability to cope with their environments and their responses to stressors (Negro et al., 2000; Ehlert, Gaab & Heinrichs, 2001). DA and cortisol are functionally involved in controlling an organism’s behavioral tendencies and metabolic, endocrine, and immune functions, and work to ensure adequate coping strategies and individual well-being (Cheng et al., 2001; Pani, Porcella & Gessa, 2000). Animals in stressful environments present high cortisol levels, Staay et al. (2010) and De Vry et al. (2012) found that the blood cortisol levels of pregnant sows raised in restrictive-breeding environments were markedly elevated in comparison to raised in cohort-breeding environments, Pavičić et al. (2003) and Leme et al. (2012) reported that transportation is a stressor that impacts plasmatic cortisol levels in lambs and pigs. In this study, the serum cortisol concentrations within enriched cohorts were reduced in comparison to within CO cohort (Table 3, p < 0.05), suggesting that EE decreased stress. Dopamine is also associated with stress, along with various comorbidities including insomnia, chronic pain, and depression (Finan & Smith, 2013). A moderate increase in dopamine within a certain range is beneficial for animals, but overly high DA is linked to increased aggressive behavior, cannibalism, and elevated mortality (Cheng et al., 2001; Craig & Muir, 1996). In our trial, aggressive behavior was not observed in EE-cohort rabbits. Moreover, CB-cohort rabbits, which had the highest DA levels, spent more time playing with EE, demonstrated less abnormal behavior (biting wire or abnormal rest postures), and displayed the most positive behavior among cohorts. This suggested that rabbits experienced less stress within the CB-enriched cage, and consequently, their DA and cortisol levels were the highest and lowest, respectively. Under the experimental conditions of this study, CB was found to be the most suitable EE for the rabbits.
Effect of environmental enrichment on cecal microbiota
Not many investigations have analysed associations across environmental enriched cages/gut microbiota within animals. To our knowledge, this is the first investigation to report influences of EE upon cecal microbiota within rabbits. The relationship between the mental health and gut microbial is more and more be accounted of researcher. The gut microbiota interacts with the host via neuroimmune, neuroendocrine and neural pathways. Gut -brain communication has been explored in many animal models. The brain-gut-microbiota axis and preclinical evidence suggests that the microbiota can recruit this bidirectional communication system to modulate brain development, function and behavior (Cryan & Dinan, 2012). A study in human have been provided that gut metabolite has effect the mental heath (Valles-Colomer et al., 2019). Some evidences indicated that gut microbiota may play a causal role in the development of features of depression (Kelly et al., 2016). Our data demonstrated that phyla such as Firmicutes and Bacteroidetes dominate the rabbit cecal ecosystem, representing more than 90% for the entire microbial composition of both CO- and CB- cohort rabbits. This was in accordance with the previous studies that have characterized the caecal microbiota in rabbits and reported that Firmicutes and Bacteroidetes are the predominant phyla in the New Zealand White and Rex rabbit cecal microbial communities (Chen et al., 2019; Zou et al., 2016). Conversely, other investigations mapped cecal microbiota within meat rabbits and showed elevated relative presence of Proteobacteria and Verrucomicrobia phyla (Kylie, Weese & Turner, 2018). These discrepancies among previous studies could be attributable to technical issues or biological reasons. Within the present study, the CB-cohort rabbits showed reduced relative abundances of Firmicutes and increased abundances of Bacteroidetes relative to those within other cohorts. Although no direct comparisons can be made between this study and others, elevated Firmicutes populations and reduced Bacteroidetes populations were observed among rabbits raised in more congenial environments (Velasco-Galilea et al., 2020). This may indicate that the addition of EE provided rabbits with healthier environments.
Regarding the alpha diversity assessment, the observed_species, Ace, and PD_whole_tree indices revealed significant differences between the CO and CB cohorts. Cecal specimens collected from the CO-cohort rabbits were more diverse in comparison to those from the CB-cohort rabbits. However, the Shannon diversity-index in the CB cohort was more elevated than in the CO cohort (not significant). Further, EE may favour the presence of only certain gut microbiota. Although no direct comparisons can be made between the present and previous studies, we found that our results were inconsistent with the results of other studies, which indicated that positive environments were associated with elevated alpha diversity in chickens and horses (Ludvigsen, Svihus & Rudi, 2016; Adhikari et al., 2020). Further research is needed to explain these differences.
Additionally, our study showed that EE affected several intestinal bacterial families within rabbits under identical diets/environmental conditions (except for the presence or absence of EE), namely Erysipelotrichaceae, Tannerellaceae, Enterobacteriaceae, Burkholderiaceae, and Prevotellaceae, all of which markedly increased within the CO cohort than within the CB cohort (Fig. 3). Within human and rodent models, Erysipelotrichaceae was linked to high-fat diets (Martinez et al., 2009; Fleissner et al., 2010) and several diseases, including inflammation-linked GIT conditions/metabolic disorders (Kaakoush, 2015; Bermingham et al., 2017). Similarly, Tannerellaceae and Enterobacteriaceae are often associated with gastrointestinal diseases (Winsen et al., 2002; Lopetuso et al., 2018). In this study, however, diarrhea and gastrointestinal diseases were not observed within the CO-cohort rabbits. Enterobacteriaceae, Burkholderiaceae, and Prevotellaceae were members for the Proteobacteria phylum. Increased Proteobacteria populations are associated with anxiety and further impact stress-disturbed gut microbiota compositions (Hyo-Min et al., 2018). These results may be attributable to reduced stress and anxiety under conditions with EE, and changes in rabbit well-being may further affect Proteobacteria populations. In the present study, considerable links across multiple genera and host serum cortisol levels were identified within rabbits. Presence of Alloprevotella, Bifidobacterium, Enterobacteriaceae, Parabacteroides, and Erysipelatoclostridium was strongly positively associated with host serum cortisol levels. The identical profile, recognized through elevated cortisol discharge, was found in horses exposed to stressors and in rhesus monkeys prenatally exposed to an acoustic stressor (Bailey, Lubach & Coe, 2004; Mach et al., 2017). Although still hypothetical, the mechanisms for cortisol discharge in driving gut microbiota constitutional dysregulations could be associated with stress-influenced shifts within the intestinal physiology that shifts bacterial colonies (e.g., alteration of gut permeability and barrier function or bile acid concentrations) to interbacterial signalling, growth, and virulence (Cryan & Dinan, 2012).
In general, our findings suggested that EE changed the rabbits’ behavior, stimulated their HPA and SMA axes, decreased the release of cortisol, and increased the release of DA and IGF-1. Further effects possibly attributable to EE included alterations in the cecal microbiota with decreased abundances of Erysipelotrichaceae, Tannerellaceae, Enterobacteriaceae, Burkholderiaceae, and Prevotellaceae. These bacteria communicate with the brain to reduce stress and decrease the anxiety in animals. Moreover, bacteria such as Alloprevotella, Bifidobacterium, Enterobacteriaceae, Parabacteroides, and Erysipelatoclostridium had negative links with serum cortisol levels. Most of such bacterial taxa displayed markedly different relative abundances between the CO and CB cohorts. These findings suggested a possible effect of EE on stress responses in hosts (Fig. 4).
Figure 4. Model of cecal microbiota variations and their effects on host physiology under enriched environments.
Conclusion
Rabbits’ behavior and serum hormonal levels were influenced by three different types of EE, among which, CB was found to be the most suitable. Furthermore, our results confirmed that rabbits raised in the CB enriched cages had more microbiota characteristic of healthy animals compared to rabbits in conventional cages. Psychological stress can alter the composition of animals’ intestinal microbiota, and the brain-gut-microbiota interactions involved in regulating the effects of stress on intestinal functions are now better understood. As animal husbandry practices often involve animals under stress, EE could represent a useful stress-reduction method. Although an extensive study is required to further explore these relationships, we suggest that the characteristics of enclosures should be given greater consideration in rabbit husbandry.
Supplemental Information
Acknowledgments
Thanks to the animals that contributed to this study. Yang Feng conceived and designed the experiments: Yang Feng, Huimei Fan, Xue Liang, Xiaofeng Wang, Guoyan Gao performed the experiment, Yang Feng, Xiaofeng Wang, Guoyan Gao, Shuangbao Gun analyzed the data
Funding Statement
This work was funded by the Youth Science and Technology Fund (20JR10RA547) and Discipline Construction Fund Project of Gansu Agricultural University (GAU-XKJS-2018-053). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Yang Feng conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Huimei Fan and Xue Liang performed the experiments, prepared figures and/or tables, and approved the final draft.
Xiaofeng Wang and Guoyan Gao performed the experiments, analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
Shuangbao Gun analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
Animal Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
Gansu Agricultural University Animal Care and Use Committee
Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
The Gansu Agricultural University granted Ethical approval to carry out the study within its facilities (Ethical Application Ref: GSAU513-a83).
Data Availability
The following information was supplied regarding data availability:
The raw measurements are available in the Supplementary Files.
References
- Abdelfattah et al. (2013).Abdelfattah E, Karousa M, Mahmoud E, ELLaithy S, ElGendi G, Eissa N. Effect of cage floor type on behavior and performance of growing rabbits. Journal of Veterinary Advances. 2013;3(2):34–42. doi: 10.5455/jva.20130219032609. [DOI] [Google Scholar]
- Adhikari et al. (2020).Adhikari B, Jun S-R, Kwon YM, Kiess AS, Adhikari P. Effects of housing types on cecal microbiota of two different strains of laying hens during the late production phase. Frontiers in Veterinary Science. 2020;7:331. doi: 10.3389/fvets.2020.00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey, Lubach & Coe (2004).Bailey MT, Lubach GR, Coe CL. Prenatal stress alters bacterial colonization of the gut in infant monkeys. Journal of Pediatric Gastroenterology Nutrition. 2004;38(4):414–421. doi: 10.1097/00005176-200404000-00009. [DOI] [PubMed] [Google Scholar]
- Barik et al. (2013).Barik J, Marti F, Morel C, Fernandez SP, Lanteri C, Godeheu G, Tassin J-P, Mombereau C, Faure P, Tronche F. Chronic stress triggers social aversion via glucocorticoid receptor in dopaminoceptive neurons. Science. 2013;339(6117):332–335. doi: 10.1126/science.1226767. [DOI] [PubMed] [Google Scholar]
- Bermingham et al. (2017).Bermingham EN, Maclean P, Thomas DG, Cave NL, Young W. Key bacterial families (Clostridiaceae, Erysipelotrichaceae and Bacteroidaceae) are related to the digestion of protein and energy in dogs. Peerj. 2017;5:e3019. doi: 10.7717/peerj.3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozicovich et al. (2016).Bozicovich TFM, Ana Silvia AMT, Moura, Fernandes S, Oliveira AA, Siqueira Siqueira ER. Effect of environmental enrichment and composition of the social group on the behavior, welfare, and relative brain weight of growing rabbits. Applied Animal Behaviour Science. 2016;182:72–79. doi: 10.1016/j.applanim.2016.05.025. [DOI] [Google Scholar]
- Chen et al. (2019).Chen SY, Deng F, Jia X, Liu H, Zhang GW, Lai SJ. Gut microbiota profiling with differential tolerance against the reduced dietary fibre level in rabbit. Scientific Reports. 2019;9:288. doi: 10.1038/s41598-018-36534-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng et al. (2001).Cheng HW, Eicher SD, Chen Y, Singleton P, Muirt WM. Effect of genetic selection for group productivity and longevity on immunological and hematological parameters of chickens. Poultry Science. 2001;80(8):1079–1086. doi: 10.1093/ps/80.8.1079. [DOI] [PubMed] [Google Scholar]
- Craig & Muir (1996).Craig JV, Muir WM. Group selection for adaptation to multiple-hen cages: beak-related mortality, feathering, and body weight responses. Poultry Science. 1996;75(3):294. doi: 10.3382/ps.0750294. [DOI] [PubMed] [Google Scholar]
- Cryan & Dinan (2012).Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nature Reviews Neuroscience. 2012;13(10):701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- Cryan & O’Mahony (2011).Cryan JF, O’Mahony SM. The microbiome-gut-brain axis: from bowel to behavior. Neurogastroenterology and Motility. 2011;23(3):187–192. doi: 10.1111/j.1365-2982.2010.01664.x. [DOI] [PubMed] [Google Scholar]
- De Vry et al. (2012).De Vry J, Prickaerts J, Jetten M, Hulst M, Steinbusch HWM, van den Hove DLA, Schuurman T, Van der Staay FJ. Recurrent long-lasting tethering reduces BDNF protein levels in the dorsal hippocampus and frontal cortex in pigs. Hormones Behavior. 2012;62(1):10–17. doi: 10.1016/j.yhbeh.2012.04.019. [DOI] [PubMed] [Google Scholar]
- Dinan & Cryan (2012).Dinan TG, Cryan JF. Regulation of the stress response by the gut microbiota: implications for psychoneuroendocrinology. Psychoneuroendocrinology. 2012;37(9):1369–1378. doi: 10.1016/j.psyneuen.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Ehlert, Gaab & Heinrichs (2001).Ehlert U, Gaab J, Heinrichs M. Psychoneuroendocrinological contributions to the etiology of depression, posttraumatic stress disorder, and stress-related bodily disorders: the role of the hypothalamus-pituitary-adrenal axis. Biological Psychology. 2001;57(1–3):141–152. doi: 10.1016/S0301-0511(01)00092-8. [DOI] [PubMed] [Google Scholar]
- Finan & Smith (2013).Finan PH, Smith MT. The comorbidity of insomnia, chronic pain, and depression: Dopamine as a putative mechanism. Sleep Medicine Reviews. 2013;17(3):173–183. doi: 10.1016/j.smrv.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleissner et al. (2010).Fleissner CK, Huebel N, Abd El-Bary MM, Loh G, Klaus S, Blaut M. Absence of intestinal microbiota does not protect mice from diet-induced obesity. British Journal of Nutrition. 2010;104(06):919–929. doi: 10.1017/S0007114510001303. [DOI] [PubMed] [Google Scholar]
- Hennessy (1997).Hennessy MB. Hypothalamic-pituitary-adrenal responses to brief social separation. Neuroscience Biobehavioral Reviews. 1997;21(1):11–29. doi: 10.1016/S0149-7634(96)00013-9. [DOI] [PubMed] [Google Scholar]
- Holzenberger et al. (2003).Holzenberger M, Dupont J, Ducos B, Leneuve P, Géloën A, Even PC, Cervera P, Bouc YL. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421(6919):182–187. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- Hyo-Min et al. (2018).Hyo-Min J, Kyung-Eon L, Hae-Ji L, Dong-Hyun K. Immobilization stress-induced Escherichia coli causes anxiety by inducing NF-κB activation through gut microbiota disturbance. Scientific Reports. 2018;8(1):13897. doi: 10.1038/s41598-018-31764-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaakoush (2015).Kaakoush NO. Insights into the role of erysipelotrichaceae in the human host. Frontiers in Cellular Infection Microbiology. 2015;5:84. doi: 10.3389/fcimb.2015.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly et al. (2016).Kelly JR, Borre Y, O’ Brien C, Patterson E, El Aidy S, Deane J, Kennedy PJ, Beers Sasja, Scott K, Moloney G, Hoban AE, Scott L, Fitzgerald P, Ross Paul, Stanton C, Clarke G, Cryan JF, Dinan TG. Transferring the blues: depression-associated gut microbiota induces neurobehavioural changes in the rat. Journal of Psychiatric Research. 2016;82:109–118. doi: 10.1016/j.jpsychires.2016.07.019. [DOI] [PubMed] [Google Scholar]
- Kylie, Weese & Turner (2018).Kylie J, Weese JS, Turner PV. Comparison of the fecal microbiota of domestic commercial meat, laboratory, companion, and shelter rabbits (Oryctolagus cuniculi) BMC Veterinary Research. 2018;14(1):143. doi: 10.1186/s12917-018-1464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leme et al. (2012).Leme TMDC, Titto EAL, Amadeu CCB, Neto PF, Vilela RA, Pereira AMF. Influence of transportation methods and pre-slaughter rest periods on cortisol level in lambs. Small Ruminant Research. 2012;107:8–11. doi: 10.1016/j.smallrumres.2012.05.010. [DOI] [Google Scholar]
- Lopetuso et al. (2018).Lopetuso LR, Petito V, Graziani C, Schiavoni E, Paroni Sterbini F, Poscia A, Gaetani E, Franceschi F, Cammarota G, Sanguinetti M, Masucci L, Scaldaferri F, Gasbarrini A. Gut microbiota in health, diverticular disease, irritable bowel syndrome, and inflammatory bowel diseases: time for microbial marker of gastrointestinal disorders? Digestive Diseases. 2018;36(1):56–65. doi: 10.1159/000477205. [DOI] [PubMed] [Google Scholar]
- Ludvigsen, Svihus & Rudi (2016).Ludvigsen J, Svihus B, Rudi K. Rearing room affects the non-dominant chicken cecum microbiota, while diet affects the dominant microbiota. Frontiers in Veterinary Science. 2016;3:16. doi: 10.3389/fvets.2016.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzi et al. (2003).Luzi F, Ferrante V, Heinzl E, Verga M. Effect of environmental enrichment on productive performance and welfare aspects in fattening rabbits. Italian Journal of Animal Science. 2003;2(1S):438–440. [Google Scholar]
- Mach et al. (2017).Mach N. The effects of weaning methods on gut microbiota composition and horse physiology. Frontiers in Physiology. 2017;8:535–535. doi: 10.3389/fphys.2017.00535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez et al. (2009).Martinez I, Wallace G, Zhang C, Legge R, Benson AK, Carr TP, Moriyama EN, Walter J. Diet-induced metabolic improvements in a hamster model of hypercholesterolemia are strongly linked to alterations of the gut microbiota. Applied Environmental Microbiology. 2009;75(12):4175–4184. doi: 10.1128/AEM.00380-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer, Savidge & Shulman (2014).Mayer EA, Savidge T, Shulman RJ. Brain–gut microbiome interactions and functional bowel disorders. Gastroenterology. 2014;146(6):1500–1512. doi: 10.1053/j.gastro.2014.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed & Nasr (2017).Mohammed H, Nasr M. Growth performance, carcass traits, behaviour and welfare of New Zealand White rabbits housed in different enriched cages. Animal Production Science. 2017;57(8):1759–1766. doi: 10.1071/AN15865. [DOI] [Google Scholar]
- Negro et al. (2000).Negro AB, Deuster PA, Gold PW, Singh A, Chrousos P. Individual reactivity and physiology of the stress response. Biomedicine Pharmacotherapy. 2000;54(3):122–128. doi: 10.1016/S0753-3322(00)89044-7. [DOI] [PubMed] [Google Scholar]
- Pani, Porcella & Gessa (2000).Pani L, Porcella A, Gessa GL. The role of stress in the pathophysiology of the dopaminergic system. Molecular Psychiatry. 2000;5(1):14–21. doi: 10.1038/sj.mp.4000589. [DOI] [PubMed] [Google Scholar]
- Partrick et al. (2018).Partrick KA. Acute and repeated exposure to social stress reduces gut microbiota diversity in Syrian hamsters. Behavioural Brain Research. 2018;345:39–48. doi: 10.1016/j.bbr.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavičić et al. (2003).Pavičić, Chassainga B, Beacha LQ, McCanna KE, Gewirtz AT, Huhmana KL. Cortisol level in the blood plasma of pigs immediately before and after transport. XI International congress in animal hygiene, proceedings; Mexico: 2003. [Google Scholar]
- Perry et al. (2018).Perry E, Cross T-WL, Francis JM, Holscher HD, Clark SD, Swanson KS. Effect of road transport on the equine cecal microbiota. Journal of Equine Veterinary Science. 2018;68:12–20. doi: 10.1016/j.jevs.2018.04.004. [DOI] [PubMed] [Google Scholar]
- Princz et al. (2005).Princz Z, Szendr ZS, Dalle ZA, Radnai I, Orova Z. Effect of different housing on productive traits and on some behaviour patterns of growing rabbits. Proceeding 17th Hungarian conference on rabbit production; Kaposvár, Hungary: 2005. [Google Scholar]
- Princz et al. (2007).Princz Z, Orova Z, Nagy I, Jordan D, Štuhec I, Luzi F, Verga M, Szendrö Z. Application of gnawing sticks in rabbit housing. World Rabbit Science. 2007;15:29–36. doi: 10.4995/wrs.2007.607. [DOI] [Google Scholar]
- Seidel, Beaton & Teague (1979).Seidel ER, Beaton JM, Teague RS. The effects of cholinergic agents on morphine-induced circling behavior in the intact mouse. European Journal of Pharmacology. 1979;56(1-2):75–80. doi: 10.1016/0014-2999(79)90435-7. [DOI] [PubMed] [Google Scholar]
- Staay et al. (2010).Staay FJVD, Schuurman T, Hulst M, Smits M, Prickaerts J, Kenis G, Korte SM. Effects of chronic stress: a comparison between tethered and loose sows. Physiology Behavior. 2010;100(2):154–164. doi: 10.1016/j.physbeh.2010.02.020. [DOI] [PubMed] [Google Scholar]
- Taché et al. (2001).Taché Y, Martinez V, Million M, Wang L. Stress and the gastrointestinal tract III. Stress-related alterations of gut motor function: role of brain corticotropin-releasing factor receptors. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2001;280(2):G173–G177. doi: 10.1152/ajpgi.2001.280.2.G173. [DOI] [PubMed] [Google Scholar]
- Trocino et al. (2019).Trocino A, Zomeño C, Filiou E, Birolo M, White P, Xiccato G. The use of environmental enrichments affects performance and behavior of growing rabbits housed in collective pens. Animals. 2019;9(8):537. doi: 10.3390/ani9080537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsilimigras et al. (2018).Tsilimigras MCB, Gharaibeh RZ, Sioda M, Gray L, Fodor AA, Lyte Mark. Interactions between stress and sex in microbial responses within the microbiota-gut-brain axis in a mouse model. Psychosomatic Medicine. 2018;80(4):361–369. doi: 10.1097/PSY.0000000000000572. [DOI] [PubMed] [Google Scholar]
- Valles-Colomer et al. (2019).Valles-Colomer M, Falony G, Darzi Y, Tigchelaar EF, Wang J, Tito RY, Schiweck C, Kurilshikov A, Joossens M, Wijmenga C, Claes S, Van Oudenhove L, Zhernakova A, Vieira-Silva S, Raes J. The neuroactive potential of the human gut microbiota in quality of life and depression. Nature Microbiology. 2019;4(4):623. doi: 10.1038/s41564-018-0337-x. [DOI] [PubMed] [Google Scholar]
- Velasco-Galilea et al. (2020).Velasco-Galilea M, Guivernau M, Piles M, Vias M, Sánchez JP. Housing conditions, level of feeding and presence of antibiotics in the feed shape rabbit cecal microbiota. 2020. https://www.researchgate.net/publication/342431077_Housing_Conditions_Level_of_Feeding_and_Presence_of_Antibiotics_in_The_Feed_Shape_Rabbit_Cecal_Microbiota. [DOI] [PMC free article] [PubMed]
- Vera (2005).Vera B. Environmental enrichment for laboratory rodents and rabbits: requirements of rodents, rabbits, and research. Ilar Journal. 2005;46(2):162–170. doi: 10.1093/ilar.46.2.162. [DOI] [PubMed] [Google Scholar]
- Winsen et al. (2002).Winsen R, Keuzenkamp D, Urlings BAP, Lipman LJA, Snijders JAM, Verheijden JHM, Van Knapen F. Effect of fermented feed on shedding of Enterobacteriaceae by fattening pigs. Veterinary Microbiology. 2002;87(3):267–276. doi: 10.1016/S0378-1135(02)00066-4. [DOI] [PubMed] [Google Scholar]
- Zou et al. (2016).Zou F, Zeng D, Wen B, Sun H, Zhou Y, Yang M, Peng Z, Xu S, Wang H, Fu X, Du D, Zeng Y, Zhu H, Pan K, Jing B, Wang P, Ni X. Illumina Miseq platform analysis caecum bacterial communities of rex rabbits fed with different antibiotics. AMB Express. 2016;6(1):100–100. doi: 10.1186/s13568-016-0273-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
The raw measurements are available in the Supplementary Files.