Abstract
Despite therapeutic advances and improvement in outcomes of patients with acute lymphoblastic leukemia, as reported by specialized centers, healthcare disparities exist. This is a retrospective cohort analysis of the clinical outcomes of 146 patients with newly diagnosed acute lymphoblastic leukemia treated in the safety-net hospital system of the third most populous county in the US. Addressing social factors which may limit access to health care, adopting effective and less costly therapies and improving patient diversification in clinical trials may all improve the outcomes of underserved patient populations.
Background:
Major advances in the treatment of acute lymphoblastic leukemia (ALL) over the past decade have resulted in 5-year overall survival (OS) rates of 80% in mature B cell ALL, 50% in precursor B cell ALL, 50%–60% in T cell ALL, and 60%–70% in Philadelphia chromosome–positive (Ph+) ALL, as reported in studies from large, specialized centers. However, many patients treated in the community have limited access to novel therapies and stem cell transplantation (HSCT).
Patients and Methods:
The purpose of this retrospective cohort analysis was to evaluate the clinical outcomes of patients ≥ 16 years with newly diagnosed ALL treated from October 2007 to June 2019 in the Harris County Health System, Houston, TX.
Results:
One hundred forty-six patients were included, with newly diagnosed pre-B-ALL (n=127), T-ALL (n=18), and chronic myeloid leukemia / lymphoid blast crisis (n=1). Median age was 35 years (16–82) at diagnosis, and 81(55%) were male. The majority of patients with pre-B ALL identified as Hispanic (n=118, or 92%). Ninety-eight (67%) of patients were uninsured or indigent, receiving care under the county’s financial assistance programs. Hyper-CVAD-based induction chemotherapy was administered in 134(92%) of patients, while 9 (6%) were treated on different protocols, and 3 (2%) were not treated due to early death, or patient refusal. Imatinib was the most common TKI used in 17/30 or 57% of patients with Ph+ disease. Out of 137 evaluable for response patients, 117 (85%) achieved complete remission (CR+CRi), 19 (14%) had refractory disease, and 1 (1%) died within 4 weeks of diagnosis. Median follow-up time was 50 months (1.5–135). For the entire study cohort, the median duration of CR/CRi was 15.4 months. Out of 62 patients who were eligible for consolidative HSCT at first CR, 52 (89%) did not receive it, with lack of insurance being the most common reason (n=29, or 56%). Barriers to utilization of novel therapies such as blinatumomab or CAR-T were also observed. Patient-caused delays in administration of chemotherapy and treatment interruptions of at least 30 days were seen in 31(23%) patients. At 1, 2, and 5 years, relapse rates were 37%, 56%, and 70%. Recurrent/refractory disease was the cause of death in most patients (n=69 [85%]). Five-year EFS and OS rates were 22% and 38% for patients with pre-B ALL, 24% and 44% for patients with T ALL, and 13% and 27% for patients with Ph+ ALL. Median OS was significantly increased (not reached [NR] vs. 24 months; p=0.00088) in patients with an indication for HSCT in first CR due to high-risk features who underwent HSCT, versus those who did not.
Conclusion:
Addressing barriers raised by socioeconomic disparities, increasing access to effective therapies, and including patients with ALL treated in the community in clinical trials may improve survival for underserved populations.
Introduction
Acute lymphoblastic leukemia (ALL) is a hematologic malignancy characterized by blocked differentiation and clonal expansion of lymphoid progenitor cells within the bone marrow and extra-medullary sites. ALL constitutes about 20% of leukemia cases in adults, with a peak incidence around 50 years of age. B cell ALL is the most commonly identified immunophenotype, accounting for approximately 75% of cases. Traditionally, multi-agent chemotherapy, along with central nervous system prophylaxis, has been the mainstay of treatment. In patients whose disease has adverse features or relapses, consolidative allogeneic stem cell transplantation (HSCT) is an important modality for sustaining long-term remissions.
In 2004, Kantarjian et al (1) reported on the clinical outcomes of a cohort of 288 patients with ALL with a median age of 40 years who were treated with the hyper-CVAD regimen at the MD Anderson Cancer Center from 1992 until 2000. After a median follow-up of 63 months, 38% of patients remained alive and in complete remission (CR). Since these original reports, an improved understanding of the biology of ALL has resulted in major therapeutic advances that have altered the natural history of the disease. These advances have resulted in 5-year survival rates of 80% in mature B cell ALL, 50% in precursor B cell ALL (2, 3), 50%–60% in T cell ALL (4,5,6), and 60%–70% in Philadelphia chromosome–positive ALL (7,8,9, 10), as reported in studies from large specialized centers (11).
Improved hematopoietic stem cell transplantation (HSCT) conditioning regimens, graft-versus-host disease prophylaxis, expansion of donor banks; utilization of advanced-generation tyrosine kinase inhibitors (TKIs), targeted therapies, monoclonal antibodies such as blinatumomab (12,13,14) and inotuzumab ozogamycin (15), and use of chimeric antigen receptor T cell therapy (CAR-T) (16); and improvements in supportive care of leukemia patients have all contributed to significantly improved outcomes in ALL.
Despite scientific and therapeutic advances, significant healthcare disparities exist (17–21). In a retrospective, population-based study using the California Cancer Registry from 2003 through 2012, race/ethnicity (Hispanic and non-Hispanic Blacks) as well as lower socioeconomic status portended significantly lower utilization of chemotherapy followed by HCT (22). A 2018 report from the National Cancer Database demonstrated Hispanic and Black patients were more likely to be uninsured (18% and 10%, respectively) compared to non-Hispanic Whites (5%) (23). Evaluations of healthcare disparities including access to HSCT and specialized healthcare for disadvantaged populations and minorities identified age, gender and insurance status as factors influencing utilization of transplant services (24), difficulties securing a suitable donor (25, 26), and influencing physicians’ referral patterns (27).
There is limited data regarding the impact of healthcare disparities on treatment approaches and outcomes of patients with ALL who receive care outside specialized centers and clinical trials. Harris County is the third largest county in the United States and the largest county in Texas, with a population of 4.6 million. Harris Health is a fully integrated, community-focused, academic health care system that serves a predominantly indigent patient population. Indeed, 54% of its patients are uninsured, 34% are covered under Medicaid or Medicare systems, and only 12% have private insurance or other funding. Most of Harris Health’s non-operating revenues come from ad valorem taxation. Financial assistance programs are offered to patients who reside in Harris County, with a household income that does not exceed 150% of the federal poverty level. Given the wide socioeconomic diversity across the region, this analysis aimed to evaluate outcome disparities in a cohort of adult patients with newly diagnosed ALL treated within the Harris County healthcare system.
Patients and Methods
Patients ≥16 years old with newly diagnosed ALL and treated in the Harris County Hospital System between October 2007 and June 2019 were included in this analysis. The protocol was approved by the Institutional Review Boards of The University of Texas Health Science Center at Houston and Harris Health System. The patients’ electronic medical records were reviewed, and the integrated Social Security Death Index was used for information on survival data, when applicable. The diagnosis of ALL required the presence of ≥20% lymphoblasts in peripheral blood or bone marrow aspirate and biopsy specimens. Comprehensive immunophenotyping using multiparameter flow cytometry (MFC) was performed in a CLIA-certified laboratory for immunophenotypic characterization of ALL subtype as well as for monitoring for minimal residual disease (MRD). Karyotypic analysis was performed using conventional metaphase cytogenetics. Identification of t(9;22)(BCR-ABL fusions) indicative of Philadelphia chromosome–positive (Ph+) ALL were assessed using interphase fluorescence in situ hybridization (FISH) or polymerase chain reaction (PCR) testing, with PCR utilized in addition to MFC for MRD monitoring in Ph+ ALL. Patients were risk stratified according to NCCN cytogenetic risk guidelines. Additionally, clinical risk was determined based on response to induction therapy as assessed by MRD measurement after induction therapy, with MRD positivity measured as BCR-ABL transcripts >0.1% in Ph+ ALL or positive MFC.
Treatment Regimens
Treatment regimens included the hyper-CVAD and augmented hyper-CVAD regimens and central nervous system (CNS) prophylaxis. Hyper-CVAD consisted of 8 courses of fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (during courses 1, 3, 5, and 7) alternating with high-dose methotrexate and cytarabine (during courses 2, 4, 6, and 8)(1). The augmented hyper-CVAD regimen incorporated intensified doses of vincristine, dexamethasone, and pegylated asparaginase (28). Other therapies used were the augmented Berlin-Frankfurt-Münster (BFM) regimen (29); and the Linker protocol (30). Risk-adapted CNS prophylaxis consisted of intrathecal administration of 12 mg of methotrexate (6 mg if via the Ommaya reservoir) on day 2 and 100 mg of intrathecal cytarabine on day 8 of each even-numbered cycle. High-risk patients (initial WBC ≥30,000, LDH ≥600 U/L) received a total of 16 CNS prophylaxis treatments, patients with Ph+ ALL received 12 treatments, and, patients with standard-risk ALL received 8. Additional therapies included anti-CD20 therapy with rituximab for patients with ≥20% of CD-20–expressing lymphoblasts (3); and TKIS added to hyper-CVAD for patients with Ph+ ALL (31).
Antibiotic prophylaxis during the dose-intensive phase consisted of ciprofloxacin 500 mg orally twice daily, fluconazole 200 mg orally daily, and acyclovir 400 mg orally twice daily. Supportive care with granulocyte-colony-stimulating factor (peg filgrastim) was given at 6 mg subcutaneously 24–72 hours following the administration of chemotherapy.
Patients receiving hyper-CVAD who achieved CR received maintenance chemotherapy with mercaptopurine (6-MP), methotrexate, vincristine, and prednisone (POMP) for 2 years.
Statistical Analysis
The primary study objective was to assess response based on NCCN criteria as measured by overall response (ORR; CR + CR with incomplete count recovery [CRi]). Time-to-event endpoints included measurement of event-free survival (EFS; defined as the time from cycle 1 day 1 of induction to death or relapse) and overall survival (OS; time from diagnosis to death or last follow-up) of patients with pre-B ALL treated in the Harris Health System.
The distribution of each continuous variable was summarized by its median, standard deviation, and interquartile range. The distribution of each categorical variable was summarized in terms of its frequencies and percentages. Time-to-event analysis was analyzed using the Kaplan-Meier method with the log-rank test. Cox multivariate regression analysis was used to determine factors associated with OS and EFS. Computations were carried out in SAS version 9.4 and TIBCO Spotfire S+ 8.2.
Results
Demographic characteristics of the 146 patients are summarized in Table 1. Median patient age was 35 years (range, 16–82 years) at diagnosis; 26 patients (18%) were ≥50 years old, and 10 (7%) patients were ≥60 years old. The majority of the pre-B ALL patients were of Hispanic ethnicity (n=118, [92%]), whereas those with T cell ALL (n=18) were more racially and ethnically diverse: 7 (39%) were Hispanic, 6 (33%) were Black, and 5 (28%) were White/Middle Eastern.
Table 1:
Patient Characteristics (N=146)
| Age, median ± SD | 35 | 16–82 |
| Male, n (%) | 81 | 55% |
| Race/Ethnicity, n (%) | ||
| White | 9 | 6% |
| Black | 11 | 7% |
| Asian | 1 | 1% |
| Hispanic | 125 | 86% |
| Health Insurance | ||
| Uninsured/Harris County Funds | 79 | 54% |
| Managed Medicaid/Commercial | 30 | 21% |
| Indigent | 19 | 13% |
| Traditional Medicaid/Medicare | 18 | 12% |
Disease subtypes and treatments are shown in Table 2. Hyper-CVAD–based induction was administered in 134 (92%) patients (hyper-CVAD ± rituximab, n=87 [59%]; augmented hyper-CVAD, n=17 [12%]; hyper-CVAD + TKI, n=30 [21%]). A minority of patients (n=9 [6%]) received alternative regimens (augmented BFM, n=2; Linker protocol, n=2; dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin (DA-EPOCH), n=1; vincristine, doxorubicin, and dexamethasone (VAD), n=3; pediatric CALGB, n=1) (32). Three patients (2%) did not receive treatment due to early death, patient refusal, or relocation. All patients with Ph+ ALL received frontline therapy incorporating a TKI. Most patients (n=17 [57%]) were initially treated with imatinib, but 35% (n=6) of them transitioned to a second- or third-generation TKI due to lack of response. The majority (n=12 [92%]) of the remaining patients (n=13 [43%]) received the second-generation TKI dasatinib during induction therapy, and 1 patient received the third-generation TKI ponatinib.
Table 2:
ALL Diagnosis and Initial Treatment
| Disease Subtype, n (%) | ||
| Pre-B | 127 | 87% |
| T cell | 18 | 12% |
| CML/Lymphoid BC | 1 | 1% |
| Ph+ Karyotype | 30 | 24% |
| CD20+ Immunophenotype | 68 | 54% |
| Induction Chemotherapy | ||
| Hyper-CVAD ± Rituximab | 87 | 59% |
| Augmented hyper-CVAD | 17 | 12% |
| Hyper-CVAD + TKI | 30 | 21% |
| Augmented BFM, Linker, other | 9 | 6% |
| None | 3 | 2% |
Abbreviations: ALL = acute lymphoblastic leukemia; Pre-B = precursor B; CML = chronic myeloid leukemia; BC = blast crisis; Ph = Philadelphia chromosome; hyper-CVAD = hyperfractionated cyclophosphamide, doxorubicin, vincristine and dexamethasone alternating with methotrexate and high-dose cytarabine; BFM = Berlin-Frankfurt-Munich.
Of the 146 patients initially included, 137 (94%) were evaluable for response. Nine patients were unevaluable due to inadequate follow-up data or no available bone marrow examination documenting disease response following induction. Of evaluable patients, 85% (n=117) achieved a CR (including 21 with CRi), 14% (n=19) were refractory to induction, and 1 (1%) died within 4 weeks of diagnosis from disease complications without having received induction. Among patients achieving a CR in whom MRD status could be obtained (n=114 [97%]), 61% (n=69) attained MRD negativity versus 39% (n=45) with positive MRD. MRD status was unknown in 3 patients who achieved a CR (Tables 3 and 4). Of those who achieved a CR, 77 (66%) went on to receive POMP maintenance therapy (n=63) or vincristine/prednisone ± TKI (n=8), mercaptopurine and methotrexate (n=2), TKI (n=3), or as part of pediatric protocols (n=1). The remaining patients did not receive maintenance due to relapse (n=18), disease complications or death (n=7), loss of follow-up or transfer to another institution (n=13), or HSCT (n=2).
Table 3:
Therapy Results
| Responses | N=137 | % |
|---|---|---|
|
| ||
| Complete Remission (CR) | 96 | 70 |
| CR / Incomplete count recovery (CRi) | 21 | 15 |
| Minimal Residual Disease Negative at CR | 69 | 59 |
| Primary Refractory | 19 | 14 |
| Early Death (< 4 weeks) | 1 | 1 |
Table 4:
Complete Remission Rates by Disease Subtype
| Responses | N=137 | % |
|---|---|---|
|
| ||
| Complete Remission (CR+CRi) | 117 | 85 |
| Pre-B ALL | 106 | 89 |
| Ph+ ALL | 23 | 82 |
| T-cell | 11 | 65 |
| Primary Refractory | 19 | 14 |
| Early Death (< 4 weeks) | 1 | 1 |
Abbreviations: Pre-B = Precursor B; Ph = Philadelphia chromosome
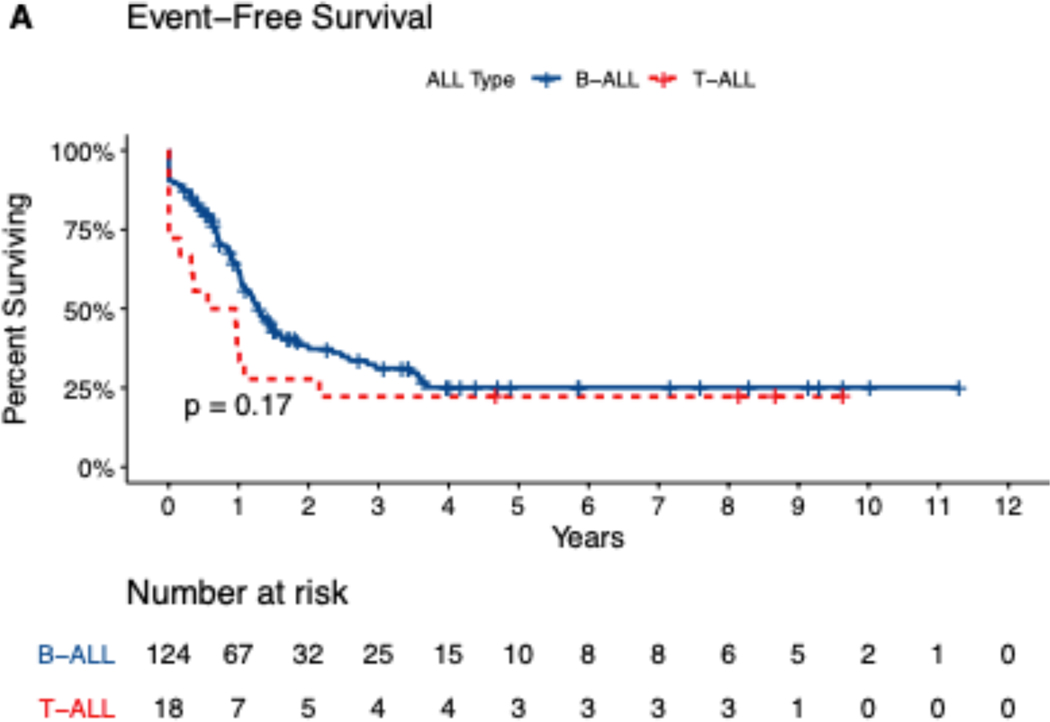
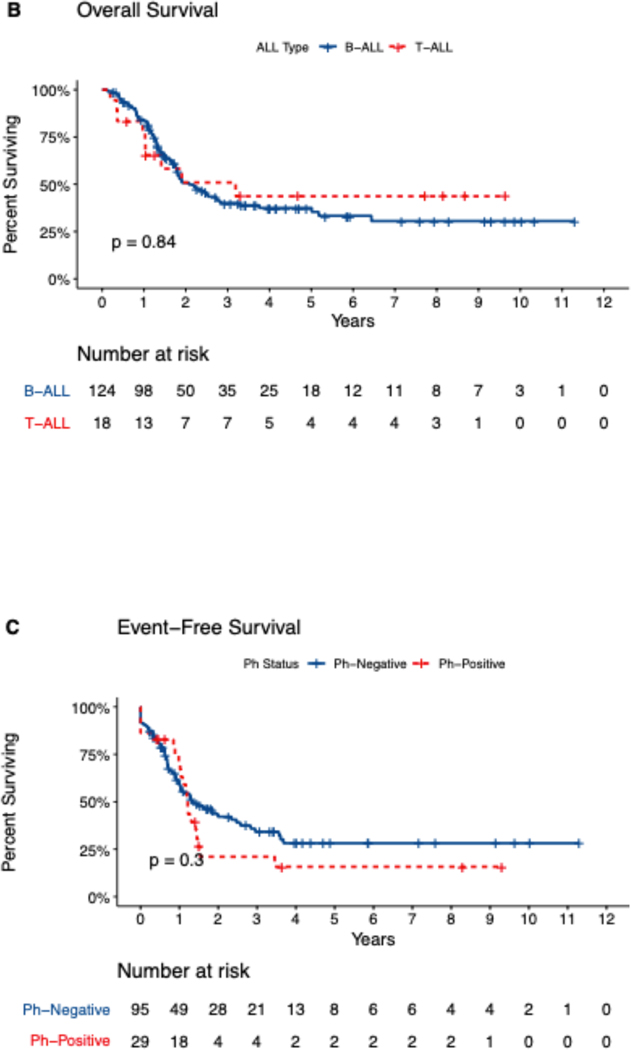
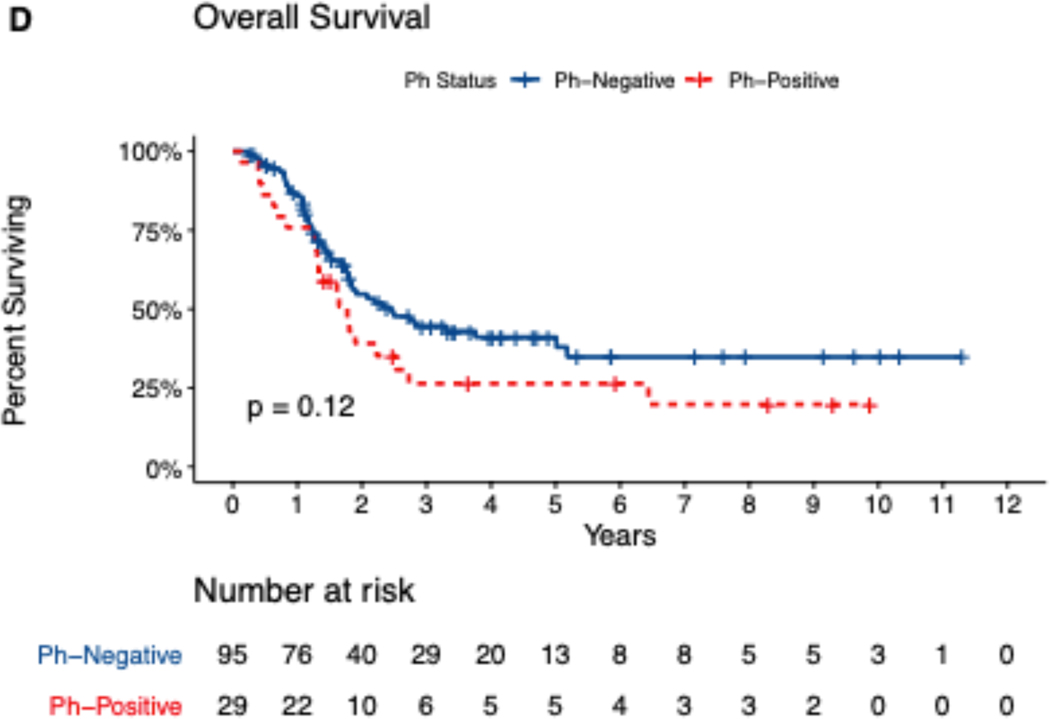
Median follow-up time for the study cohort was 50 months (range, 1.5–135 months). For the entire study cohort, the median duration of CR/CRi was 15.4 months. At 1, 2, and 5 years, relapse rates were 37%, 56%, and 70% and OS rates were 82%, 51%, and 38%. Median OS was 25 months for patients with pre-B ALL, 38 months for patients with T ALL, and 20 months for patients with Ph+ ALL. Five-year EFS and OS rates were 22% and 38% for patients with pre-B ALL, 24% and 44% for patients with T ALL, and 13% and 27% for patients with Ph+ ALL (Figure 1[A–D]). Analyzing OS longitudinally for the whole cohort and subtypes, we did not observe statistically significant differences (i.e. median OS for all patients was 1.68 years in 2011–2015 vs. 1.99 years in 2016–2019, p=0.289, for pre-B ALL 1.68 vs 2.23 (p=0.2), for T-ALL 3.19 vs 2.14 (p=0.3) and for Ph+ALL 1.3 vs 1.8 years (p=0.13)
Figure 1.
Kaplan-Meir Survival Curves showing (A) Event-free survival and (B) Overall survival, by Precursor-B or T cell ALL (n=142), and (C) event-free survival, (D) overall survival by Philadelphia negative or positive status (n=124). Data missing for 4 patients.
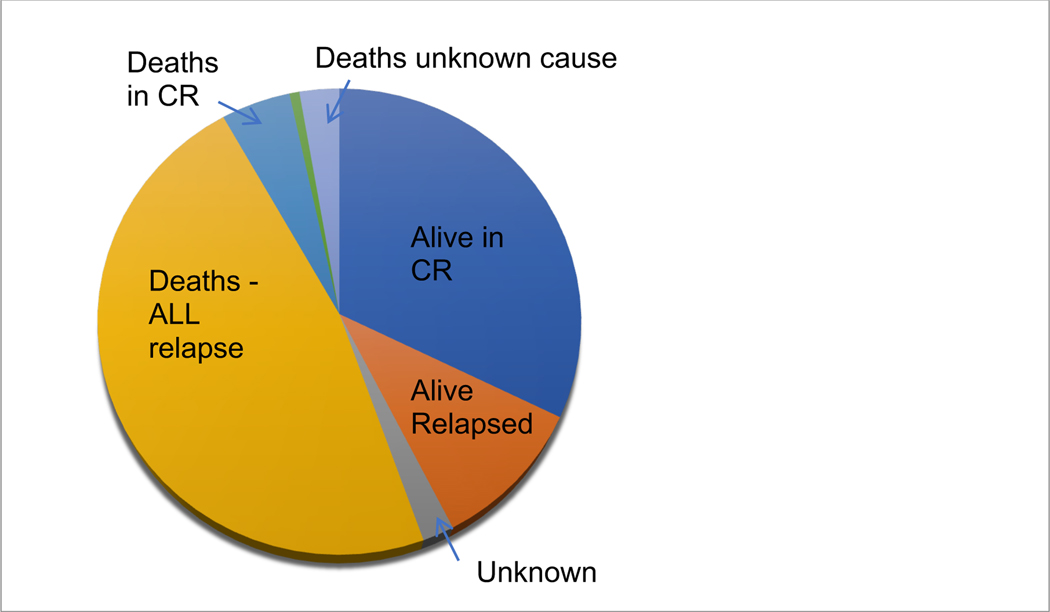
At the time of data analysis, 32% (n=47) of patients were alive in CR, 10% (n=15) patients had refractory or relapsed disease, 55% (n=81) of patients had died, 2 had unknown disease status, and 1 had developed secondary MDS. Cause of death was secondary to recurrent/refractory disease in the majority of patients (n=69 [85%]). Other causes of death included deaths in CR (n=7 [9%]: 5 from infections/sepsis, 1 from intracranial hemorrhage, and 1 from post-HSCT complications), secondary to other diseases (n=1 [1%]), and deaths of unknown cause due to loss to follow-up (n=4 [5%]) (Figure 2).
Figure 2.
Status of the entire patient cohort at time of data analysis. Abbreviations: ALL = acute lymphoblastic leukemia; CR = complete remission.
Targeted therapy with the bispecific T cell engager blinatumomab was used in 7 patients, including 6 with relapsed disease as a bridge to transplant and 1 with MRD+ disease after induction and consolidation. Blinatumomab could not be administered at 1 of the 2 Harris Health hospitals due to logistical issues associated with its prolonged continuous infusion. Inotuzumab ozogamicin or another anti-CD22 blocking antibody was utilized in 18 patients, including 17 with relapsed ALL and 1 patient for MRD+ ALL who was bridged to HSCT.
Delays of at least 30 days in administration of chemotherapy or targeted therapies, excluding TKIs, were noted in 31 (23%) patients. Causes of such delays were patient non-compliance (n=12 [39%]), lack of healthcare coverage (n=10 [32%]), inter-institutional transfers (n=6 [19%]) and patient relocation (n=3 [10%]).
Hematopoietic Stem cell transplantation
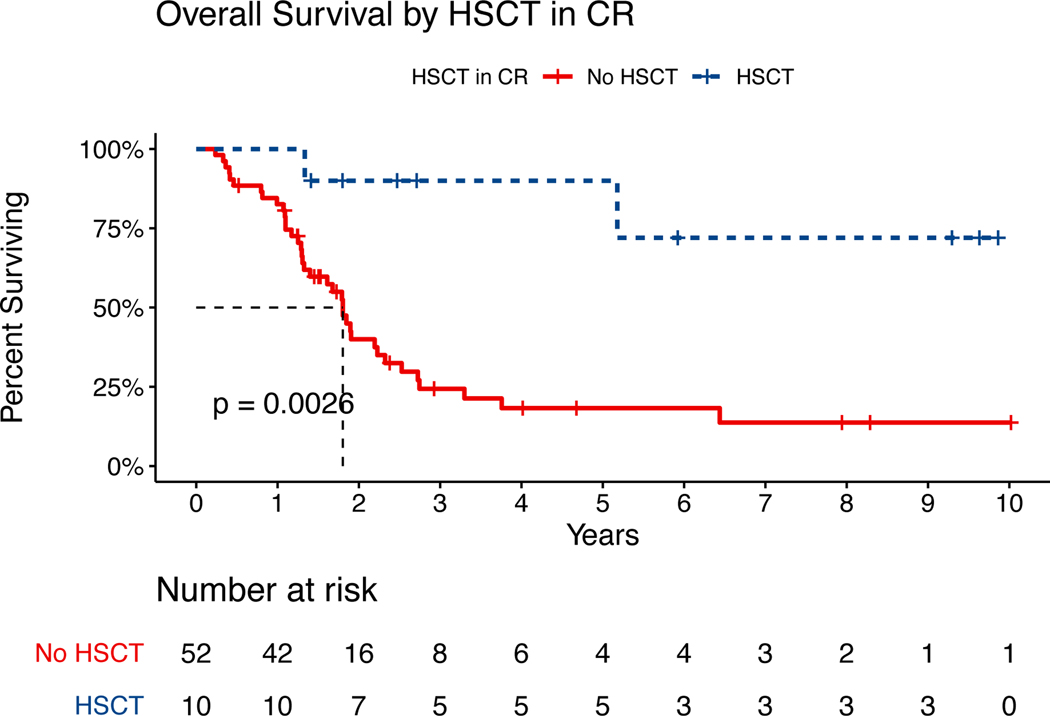
Fourteen patients (10%) received HSCT (matched related/unrelated donor [n=7], haploidentical [n=4], or cord blood [n=3]), including 8 patients with relapsed disease prior to transplant. Out of 62 patients who were candidates for consolidative HSCT in first CR (high-risk features, MRD positivity following induction, Ph+ ALL, T cell precursor ALL), 52 (89%) did not proceed with transplantation. Reasons for not proceeding with HSCT included lack of health insurance (n=29 [56%]), fragmented follow-up with interruptions in healthcare coverage (n=7 [13%]), death in CR (n=4 [8%]), or other reasons (n=12 [23%]), including no donor, no referral to transplant, or continued toxicity from chemotherapy. Median OS was significantly increased (not reached [NR] vs. 24 months; p=0.00088) in patients with an indication for HSCT in first CR who underwent HSCT versus those who did not (Figure 3).
Figure 3.
Overall Survival by HSCT in CR. Abbreviations: HSCT = hematopoietic stem cell transplant; CR = complete remission
Discussion
To our knowledge, this analysis is the first report of outcomes in patients with ALL treated within a County health system that serves the third most populous county in the United States, and predominantly provides care to an underserved patient population. As expected with multi-agent cytotoxic regimens, most of our patients achieved CR following induction chemotherapy at rates similar to those observed at large, specialized centers. However, the majority of them relapsed within 5 years and eventually died due to disease complications. Our patients’ 5-year EFS and OS were lower than those of historical adult ALL cohorts from clinical trials during the past decade: such disparities were more pronounced for the pre-B immunophenotypic subgroups: the 5-year OS was 27% in our group for Ph+ ALL versus 60%–70% reported elsewhere (7)(9); for Ph- ALL, 5-year OS was 38% in our group and 50%–60% elsewhere (33) (34). For T cell–derived ALL, the difference was less dramatic: the 5-year OS was 44% in our group versus 40%–50% elsewhere, owing to the inapplicability of novel therapies such as monoclonal antibodies and CAR-T (35). However, it is important to emphasize that our patients’ early (4-week) mortality rate was low (1%) and death in CR rate (5%) was comparable to that observed in highly specialized centers, advocating for the presence of robust supportive care in our hospital system.
The above comparisons must be interpreted with caution, given the uniqueness of our patient cohort in terms of both biological and sociodemographic characteristics. Most of our patients were of Hispanic origin. Both Hispanic children and adults with ALL have higher incidence and significantly higher mortality rates than all other racial and ethnic groups, based on population studies (Jamie M Shoag, acute lymphoblastic leukemia,Leukemia and Lymphoma, 2020)(47). The discovery of risk alleles and susceptibility loci in the CDKN2A, PIP4K2A, ARID5B, CEBPE and ERG genes, by genome-wide association studies (GWAS), suggests a genetic basis for these disparities (Yun J Yang, ancestry and pharmacogenomics, Nature Genetics 2011) (39)(Qian M, novel susceptibility variants at the ERG locus, Blood 2019)(48). Furthermore, it is possible that many of our Hispanic pre-B ALL patients harbored an inherently poor-risk disease and carried a Ph-like gene expression profile. Such a subtype portends high relapse rates and shortened survival. However, this was not testable in our health system during the assessment period (36–39).
Beyond genomic signatures, an array of factors, known as social determinants of health, are known to influence health metrics, such as longevity, access to health care, and patient health-related behaviors as well, especially in safety-net hospital systems like ours. Factors such as employment and economic stability, housing and transportation, health literacy, community and social support, and health insurance and expense coverage are often impaired in patients of low socioeconomic status. Most of our patients received financial assistance status due to their low income. In addition, decreased access to HSCT and advanced therapies, appear to have negatively influenced outcomes among them. It is well established that in patients with high-risk cytogenetic, molecular, or immunophenotypic features (such as Ph+ or early T ALL) or who have MRD positivity in CR, consolidative HSCT may offer a chance for cure (40,41). However, this procedure is associated with significant costs. Although the Harris County healthcare system offers financial assistance for low-income residents, rendering inpatient stay, medications and clinic visits affordable, transplant services are not included. When indicated, referrals to specialized transplant centers in our area were pursued. Nevertheless, almost 90% of our patients eligible for transplant did not proceed with this treatment modality. In at least half of them (n=29 [56%]), the reason was lack of insurance or US citizenship. Given that these procedures are associated with significant costs, pro-bono transplantation as part of a clinical trial, as offered by the National Institutes of Health, was utilized in a few of our patients. Most patients were unable to accommodate the need for relocation to another state and rigorous follow-up schedule. Several patients from Latin America declined pursuing transplantation in their home country due to concerns of no re-entry and risk of permanent abandonment of their families.
In addition to HSCT, barriers to access for other advanced therapies were identified. Blinatumomab, which is important for eradication of MRD in pre-B ALL, was not available in one of our hospitals due to logistical challenges relating to the need for prolonged continuous infusion of this medication. Similarly, CAR-T cell treatments were not provided due to prohibitive costs and lack of expertise and necessary infrastructure.
Imatinib is the only TKI available in our system’s formulary, accounting for its higher utilization compared to the advanced generation TKIs. Dasatinib and ponatinib are provided via pharmaceutical company assistance programs. The need for approval processes to obtain drug occasionally resulted in a delay of TKI initiation until several days after induction chemotherapy had commenced, causing deviations from standard protocol-designated care. This factor, along with decreased use of HSCT and possibly patient non-compliance, have all co-driven the inferior outcomes in our patients with Ph+ ALL (3-year OS of 27%) compared to historical reports from clinical trials in specialized centers during the same period (3-year OS of 83% for HCVAD/ponatinib vs. 56% for HCVAD/dasatinib) (7,42).
Patient-caused delays in administration of intravenous chemotherapy and treatment interruptions of at least 30 days accounted for a non-compliance rate of approximately 23% in our cohort (compliance to oral drugs not assessed). Although healthcare coverage interruptions and socioeconomic or familial challenges intermittently reduced patient adherence to treatment timelines, on infrequent occasions, patients refused further treatment. While the contribution of this fragmented care as it pertains to clinical outcomes cannot be precisely calculated, it would be a misconception to attribute compromised outcomes primarily to patient non-compliance. Factors external to the patient were much greater contributors.
The Unites States healthcare system is complex, spanning the private sector as well as state and federal government. Irrespective of pioneering research and new treatment discoveries, life expectancy in the US has risen only by 3 years since 1990 (data: World Bank; https://data.worldbank.org/indicator/SP.DYN.LE00.IN?locations=US). Health care inequalities (a reflection – in part – of economic inequality) contribute to this lack of improvement in life-related metrics. Texas continues to have the highest percentage of uninsured population under the age of 65 in the country: 18.4%, compared to a median of 9.5% nationally (2020 US Census Bureau; https://www2.census.gov/programs-surveys/demo/tables/p60/271/tableA3.pdf). For many poor and uninsured patients such as ours, newer, more sophisticated and advanced therapies are not readily available. In this regard, our patients’ clinical outcomes as well as access to HSCT appear very similar to those from a primarily Hispanic ALL patient cohort treated in a public safety-net hospital in the State of California during the same period (43). The percentage of residents below the poverty line was the most significant sociodemographic factor associated with low alloHCT utilization in patients with hematologic malignancies per Surveillance, Epidemiology and End Results Program (SEER) and the Center for International Blood and Marrow Transplant Research (CIBMTR) (n=30468 cases, 612 countries) (44).
Identifying the factors that lead to poor outcomes is critical for adjusting strategies and implementing change. Regarding the non-health care sector, initiatives may be undertaken to address issues pertaining to neighborhood safety and physical environment, housing and transportation, early childhood education, and community engagement, all of which may influence health literacy and related behaviors as well as access to care.
In the health care sector, factors are equally multidimensional and complex, yet targetable. The state of Texas has not expanded Medicaid, resulting in a gap in health care coverage for adults whose annual income falls between 41% and 100% of the annual federal poverty level (45). Hence, Medicaid expansion would diminish the percentage of underprivileged persons without coverage.
HCST is a resource-intense and highly expensive procedure. Introducing such costly therapies may not be realized within the present system, as it may jeopardize the system’s financial stability. Instead, the use of advanced generation TKIs or combinations with targeted agents such as blinatumomab (46) may constitute attractive alternatives and eventually obviate the need for HSCT for subsets of patients with ALL.
Provision of costly therapeutic agents at no cost to our patients via pharmaceutical industry financial assistance programs has been an important tool for sustaining care and should continue to take place and even expand. Given the high medical acuity of patients with hematologic malignancies, hospital operational and organizational issues such as lack of bed availability should be addressed by allocating specific units to ensure the timely administration of potentially curative chemotherapy. Utilization of patient navigators may be paramount for safe inpatient-outpatient transitions and must be employed. Methods to measure compliance with oral medications should be undertaken as well as education to enhance this compliance. Socioeconomic issues such as lack of transportation or child care for our patients are barriers to their ability to comply with necessary follow-ups and should continue to be addressed.
The adoption of telemedicine, the use of which has been recently accelerated in the setting of novel coronavirus disease, can be applied to populations like ours even in the post-pandemic era to facilitate care in home environments and secure continuity of care. Additionally, and of particular importance, inclusion of underserved populations in clinical trials, in collaboration with large leukemia centers, will further characterize the biological, environmental, social, and institutional factors resulting in healthcare disparities in patients with acute leukemia and hopefully improve outcomes.
References
- 1.Kantarjian H, Thomas D, O’Brien S, et al. Long-term follow-up results of hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (Hyper-CVAD), a dose-intensive regimen, in adult acute lymphocytic leukemia. Cancer. 2004;101(12):2788–2801. doi: 10.1002/cncr.20668 [DOI] [PubMed] [Google Scholar]
- 2.Maury S, Chevret S, Thomas X, et al. Rituximab in B-lineage adult acute lymphoblastic leukemia. N Engl J Med. 2016; 375:1044–1053 DOI: 10.1056/NEJMoa1605085 [DOI] [PubMed] [Google Scholar]
- 3.Thomas DA, O’Brien S, Faderl S, et al. Chemoimmunotherapy with a modified hyper-CVAD and rituximab regimen improves outcome in de novo Philadelphia chromosome-negative precursor B-lineage acute lymphoblastic leukemia. J Clin Oncol. 2010;28(24):3880–3889. doi: 10.1200/JCO.2009.26.9456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamilton BK, Rybicki L, Abounader D, et al. Allogeneic hematopoietic cell transplantation for adult T-cell acute lymphoblastic leukemia. Biol Blood Marrow Transplant. 2017;23(7):1117–1121. doi: 10.1016/j.bbmt.2017.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Litzow MR, Ferrando AA. How I treat T-cell acute lymphoblastic leukemia in adults. Blood 2015; 126 (7): 833–841. doi: 10.1182/blood-2014-10-551895 [DOI] [PubMed] [Google Scholar]
- 6.Abaza Y, M Kantarjian H, Faderl S, et al. Hyper-CVAD plus nelarabine in newly diagnosed adult T-cell acute lymphoblastic leukemia and T-lymphoblastic lymphoma. Am J Hematol. 2018;93(1):91–99. doi: 10.1002/ajh.24947 [DOI] [PubMed] [Google Scholar]
- 7.Ravandi F, O’Brien S, Thomas D, et al. First report of phase 2 study of dasatinib with hyper-CVAD for the frontline treatment of patients with Philadelphia chromosome–positive (Ph+) acute lymphoblastic leukemia. Blood. 2010; 116 (12): 2070–2077. doi: 10.1182/blood-2009-12-261586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ravandi F, O’Brien SM, Cortes JE, et al. Long-term follow-up of a phase 2 study of chemotherapy plus dasatinib for the initial treatment of patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Cancer. 2015;121(23):4158–4164. doi: 10.1002/cncr.29646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jabbour E, Short NJ, Ravandi F, et al. Combination of hyper-CVAD with ponatinib as first-line therapy for patients with Philadelphia chromosome-positive acute lymphoblastic leukaemia: long-term follow-up of a single-centre, phase 2 study. Lancet Haematol. 2018;5(12):e618–e627. doi: 10.1016/S2352-3026(18)30176-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fielding AK, Rowe JM, Buck G, et al. UKALLXII/ECOG2993: addition of imatinib to a standard treatment regimen enhances long-term outcomes in Philadelphia positive acute lymphoblastic leukemia. Blood. 2014;123(6):843–850. doi: 10.1182/blood-2013-09-529008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoelzer D, Walewski J, Döhner H, et al. Improved outcome of adult Burkitt lymphoma/leukemia with rituximab and chemotherapy: report of a large prospective multicenter trial. Blood. 2014;124(26):3870–3879. doi: 10.1182/blood-2014-03-563627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Topp MS, Kufer P, Gökbuget N, et al. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol 2011;29(18):2493–8. doi: 10.1200/JCO.2010.32.7270 [DOI] [PubMed] [Google Scholar]
- 13.Kantarjian H, Stein A, Gökbuget N, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med 2017;376(9):836–847. doi: 10.1056/NEJMoa1609783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinelli G, Boissel N, Chevallier P, et al. Complete hematologic and molecular response in adult patients with relapsed/refractory philadelphia chromosome-positive B-precursor acute lymphoblastic leukemia following treatment with blinatumomab: Results from a phase II, single-arm, multicenter study. J Clin Oncol. 2017;35(16):1795–1802. doi: 10.1200/JCO.2016.69.3531 [DOI] [PubMed] [Google Scholar]
- 15.Kantarjian HM, DeAngelo DJ, Stelljes M, et al. Inotuzumab ozogamicin versus standard of care in relapsed or refractory acute lymphoblastic leukemia: Final report and long-term survival follow-up from the randomized, phase 3 INO-VATE study. Cancer 2019;125(14):2474–2487. doi: 10.1002/cncr.32116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with b-cell lymphoblastic leukemia. N Engl J Med 2018;378(5):439–448. doi: 10.1056/NEJMoa1709866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joshua TV, Rizzo JD, Zhang MJ, et al. Access to hematopoietic stem cell transplantation: effect of race and sex. Cancer. 2010;116(14):3469–3476. doi: 10.1002/cncr.25297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Majhail NS, Nayyar S, Santibañez ME, Murphy EA, Denzen EM. Racial disparities in hematopoietic cell transplantation in the United States. Bone Marrow Transplant. 2012;47(11):1385–1390. doi: 10.1038/bmt.2011.214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bierenbaum J, Davidoff AJ, Ning Y, Tidwell ML, Gojo I, Baer MR, et al. Racial differences in presentation, referral and treatment patterns and survival in adult patients with acute myeloid leukemia: a single-institution experience. Leuk Res. 2012;36(2):140–145. doi: 10.1016/j.leukres.2011.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Medeiros BC. “It doesn’t matter if you’re black or white”, does it? Leuk Res. 2012 Feb;36(2):127. doi: 10.1016/j.leukres.2011.10.028. Epub 2011 Nov 21. PMID: 22112975. [DOI] [PubMed] [Google Scholar]
- 21.Ritter AJ, Goldstein JS, Ayers AA, et al. Rural and urban patients with diffuse large B-cell and follicular lymphoma experience reduced overall survival: a National Cancer DataBase study. Leuk Lymphoma. 2019;60(7):1656–1667. doi: 10.1080/10428194.2018.154685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jabo B, Morgan JW, Martinez ME, et al. Sociodemographic disparities in chemotherapy and hematopoietic cell transplantation utilization among adult acute lymphoblastic and acute myeloid leukemia patients. PLoS One. 2017;12(4):e0174760. Published 2017 Apr 6. doi: 10.1371/journal.pone.0174760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han X, Jemal A, Flowers CR, et al. Insurance status is related to diffuse large B-cell lymphoma survival. Cancer. 2014;120(8):1220–1227. doi: 10.1002/cncr.28549 [DOI] [PubMed] [Google Scholar]
- 24.Majhail NS, Omondi NA, Denzen E, Murphy EA, Rizzo JD. Access to hematopoietic cell transplantation in the United States. Biol Blood Marrow Transplant. 2010;16(8):1070–1075. doi: 10.1016/j.bbmt.2009.12.529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pidala J, Kim J, Schell M. et al. Race/ethnicity affects the probability of finding an HLA-A, -B, -C and -DRB1 allele-matched unrelated donor and likelihood of subsequent transplant utilization. Bone Marrow Transplant 48, 346–350 (2013). 10.1038/bmt.2012.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murphy EA, Ferguson SS, Omondi NA, et al. The National Marrow Donor Program’s symposium on patient advocacy in cellular transplantation therapy: addressing barriers to hematopoietic cell transplantation [published correction appears in Biol Blood Marrow Transplant. 2010 Dec;16(12):1752. Biol Blood Marrow Transplant. 2010;16(2):147–156. doi: 10.1016/j.bbmt.2009.08.004 [DOI] [PubMed] [Google Scholar]
- 27.Pidala J, Craig BM, Lee SJ, et al. Practice variation in physician referral for allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2013;48(1):63–67. doi: 10.1038/bmt.2012.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Faderl S, Thomas DA, O’Brien S, et al. Augmented hyper-CVAD based on dose-intensified vincristine, dexamethasone, and asparaginase in adult acute lymphoblastic leukemia salvage therapy. Clin Lymphoma Myeloma Leuk. 2011;11(1):54–59. doi: 10.3816/CLML.2011.n.007 [DOI] [PubMed] [Google Scholar]
- 29.Nachman JB, Sather HN, Sensel MG, et al. Augmented post-induction therapy for children with high-risk acute lymphoblastic leukemia and a slow response to initial therapy. N Engl J Med. 1998; 338:1663–1671. doi: 10.1056/NEJM199806043382304 [DOI] [PubMed] [Google Scholar]
- 30.Linker C, Damon L, Ries C, Navarro W. Intensified and shortened cyclical chemotherapy for adult acute lymphoblastic leukemia. J Clin Oncol. 2002;20(10):2464–2471. doi: 10.1200/JCO.2002.07.116 [DOI] [PubMed] [Google Scholar]
- 31.Thomas DA, Faderl S, Cortes J, et al. Treatment of Philadelphia chromosome-positive acute lymphocytic leukemia with hyper-CVAD and imatinib mesylate. Blood. 2004;103(12):4396–4407. doi: 10.1182/blood-2003-08-2958 [DOI] [PubMed] [Google Scholar]
- 32.Stock W, Luger SM, Advani AS, et al. A pediatric regimen for older adolescents and young adults with acute lymphoblastic leukemia: results of CALGB 10403. Blood. 2019;133(14):1548–1559. doi: 10.1182/blood.2019002613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gökbuget N, Zugmaier G, Klinger M, et al. Long-term relapse-free survival in a phase 2 study of blinatumomab for the treatment of patients with minimal residual disease in B-lineage acute lymphoblastic leukemia. Haematologica. 2017;102(4):e132–e135. doi: 10.3324/haematol.2016.153957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richard-Carpentier G, Kantarjian H, Short NJ, et al. Updated results from the Phase II study of hyper-CVAD in sequential combination with blinatumomab in newly diagnosed adults with B-Cell Acute Lymphoblastic Leukemia (B-ALL). Blood. 2019;134(Suppl 1):3807. doi: 10.1182/blood-2019-129657 [DOI] [Google Scholar]
- 35.Jain P, Kantarjian H, Jain N, et al. Clinical characteristics and outcomes of previously untreated patients with adult onset T-acute lymphoblastic leukemia and T- lymphoblastic lymphoma with hyper-CVAD based regimens. Am J Hematol. 2017;92(10):E595–E597. doi: 10.1002/ajh.24833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jain N, Roberts KG, Jabbour E, et al. Ph-like acute lymphoblastic leukemia: a high-risk subtype in adults. Blood. 2017;129(5):572–581. doi: 10.1182/blood-2016-07-726588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harvey RC, Mullighan CG, Chen IM, et al. Rearrangement of CRLF2 is associated with mutation of JAK kinases, alteration of IKZF1, Hispanic/Latino ethnicity, and a poor outcome in pediatric B-progenitor acute lymphoblastic leukemia. Blood. 2010;115(26):5312–5321. doi: 10.1182/blood-2009-09-245944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perez-Andreu V, Roberts KG, Harvey RC, et al. Inherited GATA3 variants are associated with Ph-like childhood acute lymphoblastic leukemia and risk of relapse. Nat Genet. 2013;45(12):1494–1498. doi: 10.1038/ng.2803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang JJ, Cheng C, Devidas M, et al. Ancestry and pharmacogenomics of relapse in acute lymphoblastic leukemia. Nat Genet. 2011;43(3):237–241. doi: 10.1038/ng.763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berry DA, Zhou S, Higley H, et al. Association of minimal residual disease with clinical outcome in pediatric and adult acute lymphoblastic leukemia: A meta-analysis. JAMA Oncol. 2017;3(7):e170580. doi: 10.1001/jamaoncol.2017.0580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giebel S, Marks DI, Boissel N, et al. Hematopoietic stem cell transplantation for adults with philadelphia chromosome-negative acute lymphoblastic leukemia in first remission: a position statement of the European Working Group for Adult Acute Lymphoblastic Leukemia (EWALL) and the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation (EBMT). Bone Marrow Transplant. 2019;54(6):798–809. doi: 10.1038/s41409-018-0373-4 [DOI] [PubMed] [Google Scholar]
- 42.Sasaki K, Jabbour EJ, Ravandi F, et al. Hyper-CVAD plus ponatinib versus hyper-CVAD plus dasatinib as frontline therapy for patients with Philadelphia chromosome-positive acute lymphoblastic leukemia: A propensity score analysis. Cancer. 2016;122(23):3650–3656. doi: 10.1002/cncr.30231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gupta R, Othman T, Uche A, et al. Characteristics and trends of adult acute lymphoblastic leukemia in a large, public safety-net hospital. Clin Lymphoma Myeloma Leuk 2020;20(6):e320–e327. doi: 10.1016/j.clml.2019.11.003 [DOI] [PubMed] [Google Scholar]
- 44.Paulson K, Brazauskas R, Khera N, et al. Inferior access to allogeneic transplant in disadvantaged populations: a center for international blood and marrow transplant research analysis. Biol Blood Marrow Transplant 2019;25(10):2086–2090. doi: 10.1016/j.bbmt.2019.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaiser Family Foundation (KFF): The cost and coverage implications of the ACA Medicaid expansion: National and State-by-State Analysis. Available at: https://www.kff.org/health-reform/report/the-cost-and-coverage-implications-of-the/
- 46.Foà R, Bassan R, Vitale A, et al. ; GIMEMA Investigators. Dasatinib-blinatumomab for ph-positive acute lymphoblastic leukemia in adults. N Engl J Med 2020;383(17):1613–1623. doi: 10.1056/NEJMoa2016272 [DOI] [PubMed] [Google Scholar]
- 47.Shoag JM, Barredo JC, Lossos IS, Pinheiro PS. Acute lymphoblastic leukemia mortality in Hispanic Americans. Leuk Lymphoma 2020;61(11):2674–2681. doi: 10.1080/10428194.2020.1779260. Epub 2020 Jun 22. PMID: 32568614. [DOI] [PubMed] [Google Scholar]
- 48.Qian M, Xu H, Perez-Andreu V, et al. Novel susceptibility variants at the ERG locus for childhood acute lymphoblastic leukemia in Hispanics. Blood 2019;133(7): 724–729. doi: 10.1182/blood-2018-07-0862946. [DOI] [PMC free article] [PubMed] [Google Scholar]