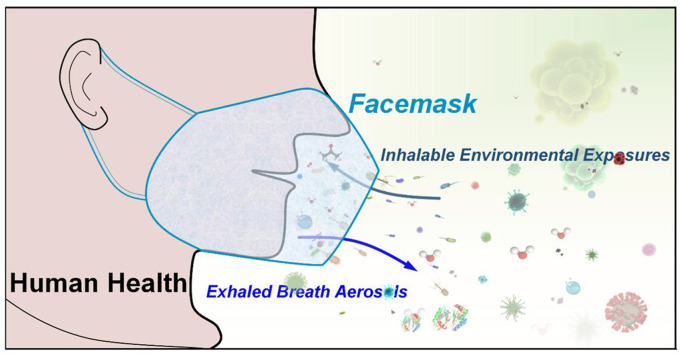
Abstract
Since the COVID-19 pandemic, the unprecedented use of facemasks has been requiring for wearing in daily life. By wearing facemask, human exhaled breath aerosols and inhaled environmental exposures can be efficiently filtered and thus various filtration residues can be deposited in facemask. Therefore, facemask could be a simple, wearable, in vivo, onsite and noninvasive sampler for collecting exhaled and inhalable compositions, and gain new insights into human health and environmental exposure. In this review, the recent advances in developments and applications of in vivo facemask sampling of human exhaled bacteria, viruses, proteins, and metabolites, and inhalable facemask contaminants and air pollutants, are reviewed. New features of facemask sampling are highlighted. The perspectives and challenges on further development and potential applications of facemask devices are also discussed.
Keywords: Facemask, Environmental exposures, Breath analysis, COVID-19, SARS-CoV-2, Breath sampling, In vivo sampling, Exhaled breath, Inhaled air, Inhalable exposures
Graphical abstract
Abbreviations
- AMR
Antimicrobial resistance
- CFU
Colony-forming unit
- COPD
Chronic obstructive pulmonary disease
- COVID-19
Coronavirus disease 2019
- CPAP
Continuous positive airway pressure
- Ct
Cycle threshold
- DART
Direct analysis in real time
- FFP
Filtering facepiece
- FID
Flame ionization detector
- FLD
Fluorescence detection
- FPSM
Fabric-phase sorptive membrane
- FT-IR
Fourier transform infrared spectroscopy
- FWAs
Fluorescent whitening agents
- HR-GC
High resolution gas chromatography
- ICA
Immunochemistry analysis
- ICM
Indirect calorimeter
- LC
Liquid chromatography
- MALDI
Matrix-assisted laser desorption/ionization
- MS
Mass spectrometry
- MSD
Mass selective detector
- Mtb
Mycobacterium tuberculosis
- NA
Not available
- RT-qPCR
Reverse transcription quantitative real-time polymerase chain reaction
- PAHs
Polynuclear aromatic hydrocarbons
- PVA
Polyvinyl alcohol
- PS
Paper spray
- PTR
Proton transfer reaction
- SARS
Severe acute respiratory syndrome
- SFP
Sodium flame photometry
- SPME
Solid-phase microextraction
- THS
Temperature and humidity sensor
- VOCs
Volatile organic compounds
- XRD
X-ray diffraction
1. Introduction
Nowadays, facemasks have become a necessity and new normal in daily use since the Coronavirus disease (COVID-19) pandemic [1]. Much evidence indicates that facemask wearing can reduce transmissibility of COVID-19 [[2], [3], [4]], because human exhaled/inhaled air can be efficiently filtered by facemask [[5], [6], [7]]. The literature on facemask has shown an explosive growth since 2019 (Fig. 1). The use of facemask can be traced back to the Middle Ages for wearing beak-like mask to prevent bubonic plague [8]. At the end of the 19th century and the early 20th century, the surgical mask was used for reducing the transmission of bacterial infection [9,10]. Since the SARS outbreak in 2003, great attention has been paid to develop highly-efficient facemasks to protection of healthcare workers [11,12].
Fig. 2.
Relative size chart of human exhaled aerosols and inhalable contaminants and the pore size of typical facemasks (diagrams not to scale).
To date, there are commercially available for different types of facemasks in daily use, e.g., surgical facemask, filtering facepiece (FFP)-type facemask, N/KN-type facemasks, cloth facemasks, and others [[13], [14], [15]]. Most facemasks are made by synthetic nonwoven polypropylene fiber. However, the filtering efficiency of facemask is attributed to densely packed and multi-layer web-like structure of filament microfibers with different designs and materials. FFP-type facemasks (e.g., FFP1, FFP2, and FFP3), N/KN-type facemasks (e.g., N90/KN90, N95/KN95, N99/KN99, N100/KN100), and other similar equivalent facemasks are made by four or more layers of filtering medias and thus have high efficiency for filtering particles (e.g., size of ≥0.3 μm, N90/KN90: ≥90%; N100/KN100: ≥99.97%). Surgical facemasks are made by three-layer filtering medias and are considered used for filtering larger particles (size ≥1.0 μm) [[14], [15], [16], [17]]. The costs of surgical facemasks are usually much lower than FFP/N/KN-type facemasks. Cloth facemasks are typically made by two or three cotton and plastic fabric layers, which create a more comfortable, washable, and reusable device than surgical and FFP/N/KN type facemask for daily use. However, it is reported that cloth facemasks show minimum filtration efficacy than surgical and FFP/N/KN facemasks [18].
In general, the efficacy of facemask filtration varies and depends on the type of material used, number of layers, and fitting of facemask on face. Fig. 2 shows the pore sizes of some typical facemasks against the sizes of contaminants in the air and human exhaled breath aerosols [[14], [15], [16], [17], [18], [19], [20]]. Therefore, different facemasks offer different levels for filtering human exhaled breath aerosols and inhalable contaminants, ranging from sneezing droplets to gaseous molecules [[13], [14], [15]]. Wearing facemask can also prevent exhaling hazardous aerosols and inhaling airborne contaminants [[21], [22], [23], [24]]. Therefore, facemask device could be a safe, noninvasive, and cost-effective wearable tool for direct sampling of various exhaled and inhaled analytes and enables new insights into the human health and environmental exposure, and provides many new possibilities in many fields such as life science, medical science, and environmental health.
Fig. 1.
Number of Publications per year on facemask for nearly a century. Number of publications obtained on Science Citation Index (Web of Science) with the following query: “face mask” or “facemask” from 1920 to 2021 (access on Mar 1, 2022).
In the past two years, unprecedented research efforts have been focused on facemask devices in the battle against COVID-19. Various excellent review articles regarding the development history, types, manufacturing methods, filtration effectiveness and mechanisms, and the impact on environment of facemask devices have been published [8,[13], [14], [15]]. However, there are very few comprehensive reviews focusing on facemask sampling of exhaled human breath and inhalable environmental exposures. Thus, this review attempts to fill this gap by carrying out a comprehensive review of facemask sampling in different dimensions, including human exhaled bacteria, viruses, proteins, and metabolites, and inhalable facemask contaminants and air pollutants, emphasizing the fabrication of facemask devices and analytical strategies. Prospects and challenges on further developments and applications of facemask sampling are also discussed.
2. Facemask sampling of human exhaled breath aerosols
By wearing a facemask, human exhaled endogenous and exogenous substances can be collected onto the inside surface of facemask. Target analytes from human exhaled breath aerosols can be classified into bacteria, viruses, proteins, and metabolites based on their particle sizes (Fig. 2). The target analytes, facemask types, threshold values, detection limits, and analytical approaches are summarized in Table 1 . Flowcharts outline the typical workflow of facemask sampling, sample processes and analytical methods in Fig. 3 .
Table 1.
Typical human exhaled breath aerosols by facemask sampling.
| Exhaled aerosols | Analytes | Types of facemasks | Detection limits/thresold values | Analytical approaches | Ref. |
|---|---|---|---|---|---|
| Bacteria | Mycobacterium tuberculosis | FFP3 facemask | 102-3 CFU | qPCR | [28] |
| Mycobacterium tuberculosis | N95 facemask | Ct value: 29 | qPCR | [31] | |
| Antimicrobial resistance genes | Modified FFP1 facemask with gelatin filter | Ct value:100 | qPCR | [32] | |
| Unspecified Bacteria | Surgical facemask loaded with 3D-PVA | NA | Unspecified | [33] | |
| Viruses | Rhinovirus, Influenza A virus, and Parainfluenza virus | Homemade facemask by PVA | NA | PCR | [41] |
| Bacillus atrophaeus, Bacteriophage MS2 | Homemade by cotton t-shirt fabric and surgical mask | NA | Henderson apparatus | [42] | |
| SARS-CoV-2 | Modified cup-type N95 mask with a gelatin membrane | Ct value: 27.29 | RT-PCR | [48] | |
| SARS-CoV-2 | Modified duckbilled surgical facemask containing 3D-PVA | ≤999 copies/strip | RT-qPCR | [49] | |
| SARS-CoV-2 | Surgical facemasks with three Steri-Strips | Ct value: 25.2 | RT-qPCR | [50] | |
| SARS-CoV-2, HCov-NL63 | CPAP-type | 10 to 100 copies/flte | RT-qPCR | [51] | |
| Proteins | Cytokines | Hard-surface plastic masks, disposable hospital paper masks | NA | ICA | [60] |
| α-amylase | Neck gaiter, cloth mask, surgical mask, N95 | ≤200 U/L | Sensor | [61] | |
| β-casein | Gold foil sheets in KN95 | NA | MALDI-MS | [62] | |
| Metabolites | Preterm Infants' exhaled O2/CO2 | Plastic face mask with head hood, canopy | NA | ICM | [66] |
| Healthy volunteer exhaled oral and nasal VOCs | Silicone face masks | ≤0.0058 mol/L | PTR-MS | [67] | |
| Healthy volunteers exhaled VOCs and non-VOCs | Hard-surface plastic and paper facemask | NA | LC-MS | [60] | |
| Mechanically ventilated patients or volunteers exhaled VOCs | Face mask with a turbine-driven portable ventilator | 0.40–0.90 ppbV | PTR-MS | [71] | |
| Lung inflammation-related exhaled water vapor | FFP with an iButton sensor | NA | THS | [72] | |
| Childhood asthma-related exhaled VOCs | Full-face air-tight mask with a breath-sampler | 5–50 pg | GC-MS | [73] | |
| Stress-intervention related exhaled VOCs | Nonvented full facemask with a silicone pillow | 0.5–1.4 dm3/min | GC-MS | [74] | |
| Food/drug/smoke-related exhaled breath | SPME fibers in KN95 and surgical facemask | NA | DART-MS, GC-MS | [46] | |
| Food/drug/smoke- related exhaled breath | Paper strips in KN95 facemask | NA | PS-MS | [77] | |
| Food/drug-related exhaled breath | FPSM array in FFP2 facemask | ≤300 ng/mL | LC-MS | [80] |
Fig. 3.
Workflow of analytical procedures of human exhaled and inhalable substances with facemask sampling. Solid lines show the conventional processes for chemical and biological analysis, and dotted lines show an optional process for direct sampling (e.g., extracting analyte with adsorbent materials that was fixed in the facemask) and direct sample analysis (e.g., direct MS and sensors).
2.1. Human exhaled breath bacteria
Probably the earliest study on collection of human exhaled bacteria using a facemask-like device can be traced back to Boston's design in 1901 [25]. A reusable anesthetic mask was introduced for sampling exhaled bacteria by Livingstone in 1941 [26]. Based on modern facemask, Williams et al. [27,28] developed a novel FFP3 facemask sampling method for 10 min to 5 h to continuously capture exhaled droplets and aerosols from pulmonary tuberculosis patients. The feature of this facemask device is that there is a square gelatin membrane (5.5 × 5.5 cm2) in the center of facemask for adsorbing Mycobacterium tuberculosis (Mtb) (Fig. 4 a). After sampling, the facemask was then subjected to further identification of Mtb by DNA analysis using quantitative polymerase chain reaction (qPCR). The detection limits of Mtb were found to be 102−3 colony per membrane. This work for the first time demonstrated that modern facemask can be a simple, efficient, and noninvasive sampling method used for sampling Mtb in human exhaled aerosols. In 2020, Williams et al. [29] further investigated the Mtb in human exhaled droplets using facemask sampling and found the discordance between Mtb output in human breath and sputum samples. Compared to traditional diagnostic methods, this work revealed that facemask sampling represents an innovative approach for diagnosing tuberculosis [30]. Shaikh et al. [31] applied facemask sampling coupled with PCR analysis for screening Mtb RNA in human exhaled aerosols from pulmonary tuberculosis patients. This work also revealed that cellulose acetate N95 facemask had better bacterial recovery and shorter sampling time than other facemasks.
Fig. 4.
Representative facemask devices for in vivo sampling of human exhaled breath aerosol: a) FFP3 facemask with a filter, reproduced from Ref. [28] with permission, b) FFP1 facemask with gelatin filter, reproduced from Ref. [32] with permission; c) surgical facemask with dog-bone specimen, reproduced from Ref. [33] with permission; d) Duckbill face mask fitted with PVA matrices, reproduced from Ref. [49] with permission; e) A filter held by a CPAP-type face mask, reproduced from Ref. [51] with permission, f) Wearable collector with facemask, reproduced from Ref. [53] with permission. g) Paper facemask, reproduced from Ref. [60] with permission h) hard-surface plastic facemask, reproduced from Ref. [60] with permission; i) SPME fibers in KN95 facemask, reproduced from Ref. [46] with permission; j) paper strips in KN95 facemask, reproduced from Ref. [62] with permission.
Kennedy et al. [32] modified FFP1 facemask with a discal gelatin filter (Fig. 4b) to collect human exhaled bacteria from patients with chronic obstructive pulmonary disease (COPD) and healthy volunteers for 15 min. Collected bacteria were analyzed by PCR for screening 10 antimicrobial resistance (AMR) encoding genes. This work showed that four bacterial AMR encoding genes in exhaled aerosols generated by COPD patients and healthy volunteers. This work was the first direct recognition that human exhaled aerosol is a potential source for the dissemination of AMR genes. It should be noted that the gelatin used in this work was from animal collagen which can be contaminated with microbes during husbandry and in abattoirs. Al-Taie et al. [33] investigated surgical facemask that was loaded with 3D-printed polyvinyl alcohol (PVA) matrices (Fig. 4c) for breath sampling. The different mechanical properties of 3D-PVA matrices were systematically studied, indicating that there is a sophisticated relation between printing parameters and the mechanical properties of the printed polyvinyl alcohol.
Many bacteria can be transmitted through the air when an individual patient exhales breath aerosol. Therefore, human exhaled breath aerosols could provide new insight into bacterial infection and transmission risk. Identifying bacteria in human exhaled breath aerosols is the key to understanding and preventing bacterial transmission. Several studies involving bacterial transmission have attempted sampling, evaluating, and characterizing human exhaled breath aerosols from patients using complicated devices [[34], [35], [36]].
Facemask devices have been proved to be useful tools for collecting exhaled bacteria. In routine clinical practice, facemask wearing is convenient, safe, and mandatory in the most modern hospitals, and thus can play a critical role in clinical sources and diagnosis methods of bacteria-induced respiratory diseases. It should be noted that after sampling, the waste facemask should be handled in right way, considering the infectious bacteria in the air, while the collected bacteria from human exhaled breath should also be appropriately stored in the container (normally at – 70°C) until DNA/RNA extraction and PCR analysis. The limitation of facemask sampling is that many bacteria can hang in the air and may be deposited onto facemask, reducing background signals and improving bacterial identification should be considered in the future.
2.2. Human exhaled breath viruses
Viruses are much smaller than bacteria (Fig. 2). For example, influenza viruses have a size from 80 to 100 nm [37], while SARS-CoV-2 have sizes between 60 and 160 nm [38]. Coughing, sneezing, talking, and breathing possibly generate a cloud of airborne viral particles in microdroplets that can be smaller than 1.0 μm [39]. Therefore, fine virus particles can hang in the air for several hours and thus can be inhaled by people who breath through the air. These virus amounts in the air are generally not enough to cause a disease in people due to human immune systems [40]. However, the risk of human infection can be increased dramatically when highly contagious airborne viruses are in a high concentration in the air [39,40]. Facemask wearing for filtering contaminated air is highly recommended, and thus can also be used for collecting exhaled viruses.
Huynh et al. [41] have developed a homemade facemask-based sampler for collecting exhaled respiratory viruses generated by upper respiratory events, including coughing, talking, and breathing. The modified facemask sampler consisted of a half-facemask and an electret filter material that can be removed for PCR analysis after sampling. In this work, different viruses, including rhinovirus, influenza A virus, and parainfluenza virus were detected from patients with cold symptoms by facemask sampling. This result revealed that a facemask device can be used for sampling exhaled viruses, providing a new method for screening populations for the presence of bioaerosols from the respiratory tract and allowing new investigation of microbial pathogenesis and transmission. Because the special homemade facemask used in this work, there are still some unanswered questions on the efficacy of virus sampling. Davies et al. [42] examined filtration efficiency of the influenza virus by wearing different facemasks, showing that the lower collection capabilities were obtained by homemade facemasks than commercial surgical masks. Because of the size of virus particles at the nanometer level, it is widely recommended that KN95/N95 or better facemasks have excellent efficiency for preventing exhaled and inhaled viral aerosols [43].
Since the outbreak of COVID-19, researchers have been exploring to develop an alternative diagnosis method based on human breath analysis, which is expected to be safe, simple, non-invasive, and easy-to-operation for covering a wide range of age groups [[44], [45], [46]]. Many human breath analysis strategies have been proposed to capture exhaled SARS-Cov-2 and related specific biomarkers for diagnosing COVID-19 [7]. In 2020, Kanaujia et al. [47] proposed a COVID-19 diagnostic strategy by facemask sampling and PCR analysis. The facemask sampling is considered easier and less bio-hazardous waste than conventional nasopharyngeal swabs in diagnosing COVID-19. The detectability of exhaled SARS-Cov-2 in facemask sampling was rapidly confirmed by Sriraman and co-workers [48], showing that low detection level can reach to ≤ 999 copies/strip. Williams et al. [49] collected exhaled SARS-Cov-2 by wearing facemask (Fig. 4d) and found a potentially significant relationship between facemask sampling and nasopharyngeal swabs viral load which demonstrated a pick-up rate of 65–70% in positive individuals. Ng et al. [50] systematically evaluated the feasibility of facemask for detecting exhaled SARS-CoV-2, showing that facemask sampling had a pick-up rate of 62.6–87.5% of patients who were tested within the first 5 days of symptom onset. Moreover, Smolinska et al. [51] developed filter-modified CPAP-type face mask to capture exhaled virus particles. The face mask, holder and head strap shown on a glass head in profile (Fig. 4e). This work reported that the SARS-CoV-2 viral load in facemasks is lower than those in nasopharyngeal swabs from COVID-19 patients.
Given the widespread use of facemasks, facemask sampling can be potentially used for population screening of febrile patients during the early phase of illness, and thus facilitating timely isolation and treatment decisions. After sampling, viral specimen should be stored immediately in buffer solution for subsequent transportation and identification. Like bacteria sampling, extracting viruses from facemask specimen should also be carefully processed to avoid polluting the environment. However, the poor concordance of SARS-CoV-2 detection between facemask sampling and nasopharyngeal swabs indicated that facemask sampling was not as sensitive as routine nasopharyngeal swabs diagnosis of COVID-19 patients. The limitation of facemask sampling is that intrinsic viral load in human exhaled breath aerosols is very low, which requires extremely sensitive detection method. Therefore, at this stage, facemask sampling methods are far from being a clinical tool for COVID-19 diagnosis. Further studies on improving the facemask viral breath sampling are still highly needed to facilitate facemask as a practical clinical tool. Purposeful open-mouth breathing or coughing [50], long time facemask wearing [52], and a designed collector in facemask (Fig. 4f) [53] might help to increase sampling efficiency and sensitivity.
2.3. Human exhaled breath proteins
Human exhaled breath aerosol contains a variety of biomacromolecules such as proteins and cytokines from oral cavity, respiratory tract, and lung epithelial lining fluid [54], which are potential clinical sources for providing valuable biochemical information on human biology. However, there is an extremely low concentration of exhaled endogenous proteins [55]. Many exhaled breath condenser-based methods were usually used to collect human exhaled proteins into a cold collector under a low temperature [[56], [57], [58]]. Moreover, exhaled breath condensates from different donors were pooled to improve the enrichment and identification of exhaled proteins [55,59]. There is a limitation to individual studies by pool testing. Wallace et al. [60] compared the sampling of cytokines from exhaled breath aerosols by disposable paper facemask (Fig. 4g) and hard-surface plastic facemasks (Fig. 4h). In this work, three cytokines (IL-1β, IL-2, and IL-8) were collected by wearing both facemasks. The detection of cytokines confirmed the presence of human material in exhaled breath samples. Jin et al. [61] determined the concentration and distribution of exhaled aerosolized saliva by detecting the α-amylase levels captured on various facemasks. The results showed that α-amylase accumulated on face coverings in a time-dependent way albeit at different levels. It is also found that α-amylase was primarily detected on the inner layer of multilayered face coverings while the distribution of α-amylase on the center of facemask correlated with the morphologies of face coverings and their coherence to the face curvature. The upper detection limit of this method is Absmax ∼17 (enzymatic actives were ≥4000 U/L), while the blank facemask was found no amylase activity (≤200 U/L). In addition, it is reported that β-casein can be detected from exhaled breath aerosol after drinking milk [62]. The exhaled β-casein should attribute aerosolized saliva that contained milk residues.
These works demonstrated possibility of facemask devices for collecing human exhaled proteins. However, although the facemask devices for collecting exhaled breath proteins is quite simple, breath proteomics analysis is still difficult to investigate because of the extremely low concentration and complex biometrics [55,63]. Further improvements are needed to facilitate facemask sampling strategy to be a powerful tool for investigating exhaled breath protein. Small and sensitive biosensors in the facemask would be beneficial for developing wearable tools for monitoring target proteins. Direct MS methods might give some empirical hints to protein analysis [64,65]. A long-time and comfortable breath sampling for several hours would be beneficial for continuously collecting more exhaled breath proteins. Another challenge is that many exhaled proteins can be mixed with saliva and food residues, giving an additional difficulty to support protein biomarker discovery and provide new biomedical knowledge of human biology.
2.4. Human exhaled breath metabolites
A lot of human exhaled volatiles and nonvolatile metabolites that dissolved in microdroplets could be congregated in a small space between mouth and facemask. Therefore, facemask wearing is a convenient method for in vivo sampling of human exhaled breath metabolites, including endogenous basic metabolites, disease-related metabolites, and exogenous food/drug-related metabolites. Bauer et al. [66] investigated the measurement of O2 consumption(CO2 production) in expired air of preterm infants <1500 g using facemask sampling and O2/CO2 sensor, showing that a facemask is suitable for breath sampling of infants, because it is accurate at low flows and does not change body temperature of infants without any further sample pretreatment in analytical procedures (Fig. 3). Sukul et al. [67] investigated concentrations of exhaled VOCs by switching oral and nasal breathing via facemask sampling. This work demonstrated that a sterile, inert, non-rebreathing and transparent silicone facemask was used to introduce exhaled breath via transfer line for subsequently MS analysis. Wallace et al. [60] have identified many human exhaled endogenous compounds by high resolution-liquid chromatography-mass spectrometry (HR-LC-MS) analysis via paper and plastic facemask sampling. Most human exhaled endogenous metabolites were identified as fatty acids, esters, sterols, ribonucleosides, and others.
In human disease processes, many diseases-related metabolites can be exhaled [[68], [69], [70]], some of which are collectable by wearing facemask devices for diagnosis and research of human diseases. Trefz et al. [71] demonstrated that exhaled breath metabolites were sampled from mechanically ventilated patients and healthy volunteers by wearing a facemask with a turbine-driven portable ventilator system. This work also found that the breath profiles can be impacted by clinical environmental exposures. Cherrie et al. [72] described the novel use of a data-logging temperature and humidity sensor located inside a facemask to evaluate the lung health status. This study demonstrated that water vapor and temperature in facemask could offer a simple way for characterizing lung inflammation and showed new insight into exhaled water associated with lung hearth status. Thomas research group demonstrated a metabolomics study to identify eight candidate markers from exhaled VOCs of childhood asthma using GC system via full-facemask sampling [73], showing that this approach had a detection limit for on-column masses in the range between 5 and 50 pg for individual components; and further established a workflow for investigating human exhaled breath metabolites using GC-MS approach via the facemask-based breath sampler (Fig. 3), which was made by a nonvented full facemask with a silicone pillow [74]. This workflow also successfully demonstrated in a case study of breath sampling and analysis from a stress-intervention experiment.
Cigarette smoking, daily diet, medication intake, and other behaviors may generate various specific exhaled exogenous metabolites that will help for understanding of human health and behavior [60]. Yuan et al. [46] developed a facemask microextraction device by fixing solid-phase microextraction (SPME, a simple, versatile, non-exhaustive sample preparation tool [75,76]) fibers into a facemask (SPME-in-facemask) to extract human exhaled metabolites that induced by intaking of food, drink, medicines, and cigarettes (Fig. 4i), the extracted analytes on SPME fiber can be identified by DART-MS without separation or conventional MS via GC separation (Fig. 3). The feature of SPME-in-facemask for breath sampling is that exhaled ultra-trace VOCs can be enriched. Considering that non-volatiles in microdroplets can adhere to SPME fiber, Cai et al. [77] developed a facemask device to adsorb non-volatile exhaled metabolites by fixing porous paper strip in the inner surface of facemask (paper-in-facemask), as shown in Fig. 4j. Various oral residues and metabolites in exhaled breath can be adsorbed onto paper strip, and the paper strip can be then directly analyzed by paper spray mass spectrometry (PSMS), which is a direct MS method without any sample pretreatment (Fig. 3) [78,79]. The feature of paper-in-facemask sampling is that adsorbed analytes can be directly analyzed for rapid screening. Locatelli et al. [80] further developed a fabric-phase sorptive membrane (FPSM) array in facemask (FPSM array-in-facemask) to collect human exhaled breath metabolites for subsequently LC-MS analysis. The FPSM array-in-facemask showed that different sorbent coatings, including polar, nonpolar, and mixed mode, and zwitterionic phases, can be composed the powerful array to capturing a wide range of analytes regarding the metabolites and consumption of drinks, food, or drugs. The detection limit of this method would be lower than the cutoff concentration (∼300 ng/mL for illicit drugs).
The exhaled endogenous and exogenous compounds identified from facemasks support the concept of facemask breath sampling. In these MS-based methods, direct MS could rapidly provide detection results within minutes, while conventional GC/LC-MS approaches could provide comprehensive analytical results (Fig. 3). It is important to note that there is an overlap between some compounds from exhaled breath aerosol and aerosolized saliva. In addition, the presence of environmental constituents during sampling would be a new problem for determining metabolite markers. Moreover, there is still a challenging task in quantitative analysis of a wide range of breath metabolites by facemask sampling. Deciphering the metabolic profile with machine learning and intelligent algorithms [81,82] are highly recommended research interests that may give a better understanding of human exhaled metabolomics that is associated with diseases, behaviors, and environmental exposures.
3. Facemask sampling of human inhalable environmental exposures
The facemask is not only used to effectively filter exhaled biohazardous particles such as bacteria and viruses, but also expected to prevent inhalable environmental exposures such as dust, particles, and aerosols in ambient air. However, various chemical residues such as VOCs, impurities, additives, even plastic fibers or particles, many of which are inhalable, can also be found from various facemasks that depending on the materials used and the engineering design, because facemasks are manufactured from fossil fuel-based petrochemicals. On the other hand, there are fiber structures at outer layer of facemask, many environmental exposures from ambient air can be adsorbed onto the outside surface of facemask. Therefore, many detectable facemask contaminants and environmental exposures can be inhaled by wearers during facemask wearing and thus impact to human health in some ways (Table 2 ).
Table 2.
Typical inhalable environmental exposures by facemask sampling.
| Environmental exposures | Analytes | Types of facemasks | limits of detection | Analytical approaches | Ref. |
|---|---|---|---|---|---|
| Chemicals in facemask | 16 organophosphate esters | Surgical facemasks, self-filtering masks, re-useable facemasks | 0.005–0.644 ng/mask | LC-MS | [86] |
| Phthalate esters | 12 surgical facemask and four N95/P1/P2 facemasks | 0.016–10 ng/sample | GC-MS | [89] | |
| Alkanes, polycyclic aromatic hydrocarbons, phthalate esters, and reactive carbonyls | 60 commercial facemasks | 0.05–24.9 ng | GC/MSD, LC-FID, LC-MS | [91] | |
| 31 fluorescent whitening agents | 18 brands of children facemasks | 0.002–10 mg/kg | LC-MS | [92] | |
| volatile chemicals | KN95 facemask | NA | GC-MS | [95] | |
| 12 high-risk volatile chemicals | 60 mask samples of different brands | 0.23 mg/kg | GC-MS | [96] | |
| Pollutants in ambient air | CO, CO2, benzene, HCN, HCl, H2SO4, HF, acrolein, CH4, formaldehyde and PNAs | Self-contained breathing apparatus facepiece | 5 μg m−3 | FT-IR | [98] |
| ∼1 ppm | |||||
| Dust and crystalline silica | FFP3 facemasks with a miniature sampler | 30–500 μg | XRD | [99] | |
| Sodium chloride aerosol | 3 FFP3 facemasks and one half-mask fitted with P3 filters | 0.3 μg | SFP | [100] | |
| 9 PAHs | Fabric facemasks | 0.06–0.8 ng | LC-FLD | [101] | |
| Tobacco smoke | Polyurethane foam facemasks | 0.03 μg/m3 | LC-MS | [102] | |
| Volatile chemicals, aerosols, and particles | KN95 facemasks with paper strips | NA | PS-MS | [77] |
3.1. Inhalable chemical contaminants from facemask
Early studies have noted the formaldehyde in certain facemasks, and such formaldehyde has been reported to cause various allergic contact dermatitis [[83], [84], [85]]. The chemical contaminants in facemask have been paid more attention, since the outbreak of COVID-19 pandemic. Fernández-Arribas et al. [86] for the first time found 12 organophosphate esters (which are widely used as plasticizers and flame retardants and has been raising increasing concern due to toxic effects [87,88]) in facemasks in indoor (Fig. 5 a) and outdoors (Fig. 5b), showing that detection limits were ranged between 0.005 and 0.644 ng/mask, and between 0.002 and 0.114 ng/m3. This work indicated that 10% of organophosphate esters content in facemasks can be inhaled during facemask wearing process by dummy human heads. Nonetheless, the non-carcinogenic and carcinogenic risks were much lower than the threshold risk values. Wang et al. [89] quantified the concentration of phthalate esters (which have reproductive toxicity [90]) in facemasks and found that di-n-butyl phthalate (DnBP) and di(2-ethylhexyl) phthalate (DEHP) were the greatest contributors to the summed phthalates. This work showed that detection limits ranged from 0.016 to 10 ng/sample, also indicating that the inhalation risk associated with the exposure of phthalate esters is low. Jin et al. [91] conducted a survey of semi-VOCs and VOCs, including alkanes, polycyclic aromatic hydrocarbons (PAHs), phthalate esters, and reactive carbonyls in 60 commercial facemasks. Luo et al. [92] established a LC-MS-based non-target screening method for identifying 31 fluorescent whitening agents (FWAs) in children's facemasks, and found 4 FWAs from 3 out of 18 brands of children facemasks. There are potential health risks to children when they are exposing to inhalable FWAs by wearing such facemasks, due to the toxicity of FWAs [93,94]. Chen et al. reported that many unknown background VOCs of KN95 facemask can be detected along with exhaled breath VOCs by coupling SPME with GC × GC-MS [95]. Liu et al. [96] reported that a total of 69 volatile substances were found in medical facemasks by headspace HR-GC-MS.
Fig. 5.
Representative facemask devices for in vivo sampling of human inhalable aerosols: a) Inhalation experiments in indoors with facemasks M7 and M10, reproduce from Ref. [86] with permission, b) inhalation experiments at outdoors with facemasks M12 and M18, reproduced from Ref. [86] with permission; c) a miniature sampler in a FFP3 facemask, reproduced from Ref. [99] with permission; d) two miniature samplers in FFP3 facemask, reproduced from Ref. [100] with permission; e) facemask expose to airborne polycyclic aromatic hydrocarbons, reproduced from Ref. [101] with permission; f) facemask expose to tobacco smoke, reproduced from Ref. [102] with permission; g) paper strip outside in facemask to expose ambient air, reproduced from Ref. [77] with permission.
Although many studies indicated the low exposure risks and low concentrations of some target chemicals in facemasks, there is an increasing interest focusing on the inhalation risks from facemasks, because such unprecedented use of facemasks worldwide means long-term dermal contact or inhalation exposure at the population level [97]. It is also important to note that more unknown chemicals in facemask than reported studies. Therefore, improving sensitivity and accuracy are required for identifying more contaminants. Another limitation is that there is a lack of efficient analytical strategy to quantify the inhaled chemicals from facemask during human wearing facemask rather than dummy human head. Therefore, it is still difficult to understand the impact of inhalable contaminants to human health by wearing a facemask for a long time, e.g., from several months to years. Various toxic chemicals in facemask may lead to significant exposure levels and associated health risks to the wearer. This needs particular attention for frontline health care workers who may wear a facemask for a long time in regular work, and the facemask production should also be improved to reduce the chemical residues, which should warrant the attention of the public and regulatory agencies.
3.2. Inhalable pollutants from ambient air
Jankovic et al. [98] conducted a study that firefighter exposed to firefighting activities by wearing a facemask-based sampler. This work demonstrated that both inside and outside surfaces of facemask can be simultaneously served to characterize the ambient environment and firefighter exposure by FT-IR analysis. Stacey et al. [99] developed a facemask-based air sampler (Fig. 5c) to measure respirable dust and crystalline silica mass concentrations. Mogridge et al. [100] further assessed the comparability of facemask-based air sampler (Fig. 5d) with the traditional inward leakage measurement technique. Facemask was applied as a personal dosimeter for quantifying environmental exposure to airborne PAHs (Fig. 5e) and tobacco smoke (Fig. 5f) in the Chan research group [101,102]. These studies showed that facemask could be a simple, convenient, and inexpensive sampler to collect inhalable air pollutants compared with traditional filter/sorbent-based approaches, which usually require a sampling pump. A versatile facemask-based sampler is that porous paper strip was fixed at the outside surface of facemask (Fig. 5g) to serve as sorbent for adsorbing inhalable volatiles, aerosols, and particles from ambient air [77]. This work also proposed that facemask sampling mechanism involves wearable continuous-flow adsorptive/microextraction processes of air flow.
Inhalable environmental exposures including VOCs, aerosol, and particles in the outside surface of facemask support the effectiveness of wearing facemasks during periods of air pollution. These results indicated that the facemask device is a good sampler to prove a viable option for in-mask measurements of environmental exposures and thus gain a better understanding of the exposure of wearers to hazardous substances. More importantly, facemask can be closely applied for tracking human exposures at individual-level analysis, thus the used facemask has a great potential to be sample sources for monitoring routine occupational and environmental exposures. However, there are obvious limitations with interferences of facemask contaminants, and it should be removed before air sampling.
4. Conclusions and prospects
In summary, facemask device has been attracting much research attention in the past two years than ever before and has gained increasing applications. Facemask sampling also greatly extends the scope and applications of human breath. The unique features of technical aspects in facemask sampling can be summarized: 1) facemask is a wearable device, which has a great convenience for a long-time sampling for many hours; 2) wearing a facemask is easy operation that it is comfortable and quite simple for a large range people, including adults, children, even infants and seriously-ill patients; 3) facemask and modified facemask devices are highly effective for adsorbing/extracting various analytes for both human exhaled breath aerosols and inhalable environmental exposures; 4) facemasks are commercially available at low-cost; and most facemasks can be re-used after right processing, considering the environmental issues; 5) facemask device is a safety and powerful platform for loading special adsorbent materials, because facemask sampling separates from analyte detection process in time and space, which is an especial feature in cases where the clinical breath sample is not easily collected because of biohazardous conditions such as bacteria and viruses.
Although facemask sampling method has good potential for investigation of human exhaled breath aerosols and inhalable environmental exposures, it should be noted that there are some drawbacks and challenges in practical applications. First, as a new sampling method by wearing facemask, there is a challenging task for directly quantification of exhaled and inhaled analytes; therefore, further mechanistic study on the extraction and adsorption of analytes from exhaled breath aerosol and inhaled air to facemask is needed for a better understanding and wider applications of facemask sampling in the future. Second, there is low facemask sampling efficiency that led to poor detection limits of target analytes (Table 1, Table 2); more effective and profound developments of facemask materials are highly expected; e.g., functionalizing facemask by modifying facemask materials or loading extract phase are beneficial for improving the detectability, selectivity, and sensitivity, and reducing sampling time of target analytes. Third, there is a complicated analytical procedure that greatly limited onsite applications; therefore, direct coupling facemask sampling with portable/miniature analytical approaches or sensors would be a good way toward onsite investigation. Finally, facemask cotaminatants might be considered a new kind of inhalable exposures, the characteristic is still unclear how facemask contaminants impact to human health for long-time wearing facemask; more human experimentations and elaborate designs by wearing different facemasks under different real environments would provide an unbiased insight into the role of facemask devices.
Declaration of competing interest
The author declares that he has no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos. 22127810 and 21804053) and the Foundation for New Faculty Start-up Program of Jinan University.
Footnotes
Prepared for the Special Issue of “On-site and In-vivo Instrumentation and Applications” in Trends in Analytical Chemistry.
References
- 1.Rab S., Javaid M., Haleem A., Vaishya R. Face masks are new normal after COVID-19 pandemic. Diabetes Metab. Syndr. 2020;14:1617–1619. doi: 10.1016/j.dsx.2020.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leung N.H.L., Chu D.K.W., Shiu E.Y.C., Chan K.-H., McDevitt J.J., Hau B.J.P., Yen H.-L., Li Y., Ip D.K.M., Peiris J.S.M., Seto W.-H., Leung G.M., Milton D.K., Cowling B.J. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat. Med. 2020;26:676–680. doi: 10.1038/s41591-020-0843-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng Y., Ma N., Witt C., Rapp S., Wild P.S., Andreae M.O., Pöschl U., Su H. Face masks effectively limit the probability of SARS-CoV-2 transmission. Science. 2021;372:1439–1443. doi: 10.1126/science.abg6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scheuch G. Breathing is enough: for the spread of influenza virus and SARS-CoV-2 by breathing only. J. Aerosol Med. Pulm. Drug Deliv. 2020;33:230–234. doi: 10.1089/jamp.2020.1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Papineni R.S., Rosenthal F.S. The size distribution of droplets in the exhaled breath of healthy human subjects. J. Aerosol Med. 1997;10:105–116. doi: 10.1089/jam.1997.10.105. [DOI] [PubMed] [Google Scholar]
- 6.Johnson G.R., Morawska L. The mechanism of breath aerosol formation. J. Aerosol Med. Pulm. Drug Deliv. 2009;22:229–237. doi: 10.1089/jamp.2008.0720. [DOI] [PubMed] [Google Scholar]
- 7.Yuan Z.-C., Hu B. Mass spectrometry-based human breath analysis: towards COVID-19 diagnosis and research. J. Anal. Test. 2021;5:287–297. doi: 10.1007/s41664-021-00194-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matuschek C., Moll F., Fangerau H., Fischer J.C., Zänker K., van Griensven M., Schneider M., Kindgen-Milles D., Knoefel W.T., Lichtenberg A., Tamaskovics B., Djiepmo-Njanang F.J., Budach W., Corradini S., Häussinger D., Feldt T., Jensen B., Pelka R., Orth K., Peiper M., Grebe O., Maas K., Bölke E., Haussmann J. The history and value of face masks. Eur. J. Med. Res. 2020;25:23. doi: 10.1186/s40001-020-00423-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belkin N.L. The evolution of the surgical mask: filtering efficiency versus effectiveness. Infect. Control Hosp. 1997;18:49–57. doi: 10.2307/30141964. [DOI] [PubMed] [Google Scholar]
- 10.Romney M.G. Surgical face masks in the operating theatre: re-examining the evidence. J. Hosp. Infect. 2001;47:251–256. doi: 10.1053/jhin.2000.0912. [DOI] [PubMed] [Google Scholar]
- 11.Li Y., Guo Y.P., Wong K.C.T., Chung W.Y.J., Gohel M.D.I., Leung H.M.P. Transmission of communicable respiratory infections and facemasks. J. Multidiscip. Healthc. 2008;1:17–27. doi: 10.2147/jmdh.s3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y., Wong T., Chung J., Guo Y.P., Hu J.Y., Guan Y.T., Yao L., Song Q.W., Newton E. In vivo protective performance of N95 respirator and surgical facemask. Am. J. Ind. Med. 2006;49:1056–1065. doi: 10.1002/ajim.20395. [DOI] [PubMed] [Google Scholar]
- 13.Karmacharya M., Kumar S., Gulenko O., Cho Y.-K. Advances in facemasks during the COVID-19 pandemic era. ACS Appl. Bio Mater. 2021;4:3891–3908. doi: 10.1021/acsabm.0c01329. [DOI] [PubMed] [Google Scholar]
- 14.Tuñón-Molina A., Takayama K., Redwan E.M., Uversky V.N., Andrés J., Serrano-Aroca Á. Protective face masks: current status and future trends. ACS Appl. Mater. Interfaces. 2021;13:5625–56751. doi: 10.1021/acsami.1c12227. [DOI] [PubMed] [Google Scholar]
- 15.Chua M.H., Cheng W., Goh S.S., Kong J., Li B., Lim J.Y.C., Mao L., Wang S., Xue K., Yang L., Ye E., Zhang K., Cheong W.C.D., Tan B.H., Li Z., Tan B.H., Loh X.J. Face masks in the new COVID-19 normal: materials, testing, and perspectives. Research. 2020;2020:7286735. doi: 10.34133/2020/7286735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klimek L., Huppertz T., Alali A., Spielhaupter M., Hörmann K., Matthias C., Hagemann J. A new form of irritant rhinitis to filtering facepiece particle (FFP) masks (FFP2/N95/KN95 respirators) during COVID-19 pandemic. World Allergy Organ. J. 2020;13:100474. doi: 10.1016/j.waojou.2020.100474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eninger R.M., Honda T., Adhikari A., Heinonen-Tanski H., Reponen T., Grinshpun S.A. Filter performance of N99 and N95 facepiece respirators against viruses and ultrafine particles. Ann. Occup. Hyg. 2008;52:385–396. doi: 10.1093/annhyg/men019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma S.K., Mishra M., Mudgal S.K. Efficacy of cloth face mask in prevention of novel coronavirus infection transmission: a systematic review and meta-analysis. J. Educ. Health Promot. 2020;9 doi: 10.4103/jehp.jehp_533_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neupane B.B., Mainali S., Sharma A., Giri B. Optical microscopic study of surface morphology and filtering efficiency of face masks. PeerJ. 2019;7:e7142. doi: 10.7717/peerj.7142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Y., Leachman S.A., Bar A. Proposed approach for reusing surgical masks in COVID-19 pandemic. J. Am. Acad. Dermatol. 2020;83:e53–e54. doi: 10.1016/j.jaad.2020.04.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jayaweera M., Perera H., Gunawardana B., Manatunge J. Transmission of COVID-19 virus by droplets and aerosols: a critical review on the unresolved dichotomy. Environ. Res. 2020;188:109819. doi: 10.1016/j.envres.2020.109819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pendar M.-R., Páscoa J.C. Numerical modeling of the distribution of virus carrying saliva droplets during sneeze and cough. Phys. Fluids. 2020;32:83305. doi: 10.1063/5.0018432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shafaghi A.H., Rokhsar Talabazar F., Koşar A., Ghorbani M. On the effect of the respiratory droplet generation condition on COVID-19 transmission. Fluids. 2020;5:113. [Google Scholar]
- 24.Loeb M., Dafoe N., Mahony J., John M., Sarabia A., Glavin V., Webby R., Smieja M., Earn D.J.D., Chong S., Webb A., Walter S.D. Surgical mask vs N95 respirator for preventing influenza among health care workers: a randomized trial. JAMA. 2009;302:1865–1871. doi: 10.1001/jama.2009.1466. [DOI] [PubMed] [Google Scholar]
- 25.Boston L.N. The spread of tuberculosis by coughing. JAMA. 1901;37:685–688. [Google Scholar]
- 26.Livingstone H., Heidrick F., Holicky I., Dack G.M. Cross-infections from anesthetic face masks. Surgery. 1941;3:433–435. [Google Scholar]
- 27.Williams C., Haldar P., Barer M. P33 Mask sampling in pulmonary tuberculosis. Thorax. 2013;68 [Google Scholar]
- 28.Williams C.M.L., Cheah E.S.G., Malkin J., Patel H., Otu J., Mlaga K., Sutherland J.S., Antonio M., Perera N., Woltmann G., Haldar P., Garton N.J., Barer M.R. Face mask sampling for the detection of Mycobacterium tuberculosis in expelled aerosols. PLoS One. 2014;9 doi: 10.1371/journal.pone.0104921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams C.M., Abdulwhhab M., Birring S.S., De Kock E., Garton N.J., Townsend E., Pareek M., Al-Taie A., Pan J., Ganatra R., Stoltz A.C., Haldar P., Barer M.R. Exhaled Mycobacterium tuberculosis output and detection of subclinical disease by face-mask sampling: prospective observational studies. Lancet Infect. Dis. 2020;20:607–617. doi: 10.1016/S1473-3099(19)30707-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bothamley G.H., Spong V. Face-mask sampling or sputum to diagnose lung tuberculosis? Lancet Infect. Dis. 2020;20:520–521. doi: 10.1016/S1473-3099(19)30680-2. [DOI] [PubMed] [Google Scholar]
- 31.Shaikh A., Sriraman K., Vaswani S., Oswal V., Mistry N. Detection of Mycobacterium tuberculosis RNA in bioaerosols from pulmonary tuberculosis patients. Int. J. Infect. Dis. 2019;86:5–11. doi: 10.1016/j.ijid.2019.06.006. [DOI] [PubMed] [Google Scholar]
- 32.Kennedy M., Ramsheh M.Y., Williams C.M.L., Auty J., Haldar K., Abdulwhhab M., Brightling C.E., Barer M.R. Face mask sampling reveals antimicrobial resistance genes in exhaled aerosols from patients with chronic obstructive pulmonary disease and healthy volunteers. BMJ Open Respir. Res. 2018;5 doi: 10.1136/bmjresp-2018-000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Al-Taie A., Pan J., Polak P., Barer M.R., Han X., Abbott A.P. Mechanical properties of 3-D printed polyvinyl alcohol matrix for detection of respiratory pathogens. J. Mech. Behav. Biomed. Mater. 2020;112:104066. doi: 10.1016/j.jmbbm.2020.104066. [DOI] [PubMed] [Google Scholar]
- 34.Tellier R., Li Y., Cowling B.J., Tang J.W. Recognition of aerosol transmission of infectious agents: a commentary. BMC Infect. Dis. 2019;19:101. doi: 10.1186/s12879-019-3707-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pan M., Lednicky J.A., Wu C.-Y. Collection, particle sizing and detection of airborne viruses. J. Appl. Microbiol. 2019;127:1596–1611. doi: 10.1111/jam.14278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verreault D., Moineau S., Duchaine C. Methods for sampling of airborne viruses. Microbiol. Mol. Biol. Rev. 2008;72:413–444. doi: 10.1128/MMBR.00002-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mosley V., Wyckoff R.W. Electron micrography of the virus of influenza. Nature. 1946;157 doi: 10.1038/157263a0. 263–263. [DOI] [PubMed] [Google Scholar]
- 38.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang C.C., Prather K.A., Sznitman J., Jimenez J.L., Lakdawala S.S., Tufekci Z., Marr L.C. Airborne transmission of respiratory viruses. Science. 2021;373:eabd9149. doi: 10.1126/science.abd9149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nikitin N., Petrova E., Trifonova E., Karpova O. Influenza virus aerosols in the air and their infectiousness. Adv. Virol. 2014;2014:859090. doi: 10.1155/2014/859090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huynh K.N., Oliver B.G., Stelzer S., Rawlinson W.D., Tovey E.R. A new method for sampling and detection of exhaled respiratory virus aerosols. Clin. Infect. Dis. 2008;46:93–95. doi: 10.1086/523000. [DOI] [PubMed] [Google Scholar]
- 42.Davies A., Thompson K.-A., Giri K., Kafatos G., Walker J., Bennett A. Testing the efficacy of homemade masks: would they protect in an influenza pandemic? Disaster Med. Public Health Prep. 2013;7:413–418. doi: 10.1017/dmp.2013.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bałazy A., Toivola M., Reponen T., Podg rski A., Zimmer A., Grinshpun S.A. Manikin-based performance evaluation of N95 filtering-facepiece respirators challenged with nanoparticles. Ann. Occup. Hyg. 2006;50:259–269. doi: 10.1093/annhyg/mei058. [DOI] [PubMed] [Google Scholar]
- 44.Subali A.D., Wiyono L., Yusuf M., Zaky M.F.A. The potential of volatile organic compounds-based breath analysis for COVID-19 screening: a systematic review & meta-analysis. Diagn. Microbiol. Infect. Dis. 2022;102:115589. doi: 10.1016/j.diagmicrobio.2021.115589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khoubnasabjafari M., Jouyban-Gharamaleki V., Ghanbari R., Jouyban A. Exhaled breath condensate as a potential specimen for diagnosing COVID-19. Bioanalysis. 2020;12:1195–1197. doi: 10.4155/bio-2020-0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yuan Z.C., Li W., Wu L., Huang D., Wu M.M., Hu B. Solid-phase microextraction fiber in face mask for in vivo sampling and direct mass spectrometry analysis of exhaled breath aerosol. Anal. Chem. 2020;92:11543–11547. doi: 10.1021/acs.analchem.0c02118. [DOI] [PubMed] [Google Scholar]
- 47.Kanaujia R., Biswal M., Angrup A., Ray P. Inhale, then exhale: start afresh to diagnose Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) by non-invasive face-mask sampling technique. Clin. Microbiol. Infect. 2020;26:1701–1702. doi: 10.1016/j.cmi.2020.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sriraman K., Shaikh A., Parikh S., Udupa S., Chatterjee N., Shastri J., Mistry N. Non-invasive adapted N-95 mask sampling captures variation in viral particles expelled by COVID-19 patients: implications in understanding SARS-CoV2 transmission. PLoS One. 2021;16 doi: 10.1371/journal.pone.0249525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Williams C.M., Pan D., Decker J., Wisniewska A., Fletcher E., Sze S., Assadi S., Haigh R., Abdulwhhab M., Bird P., Holmes C.W., Al-Taie A., Saleem B., Pan J., Garton N.J., Pareek M., Barer M.R. Exhaled SARS-CoV-2 quantified by face-mask sampling in hospitalised patients with COVID-19. J. Infect. 2021;82:253–259. doi: 10.1016/j.jinf.2021.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ng D.H.L., Sim M.Y., Huang H.H., Sim J.X.Y., Low J.G.H., Lim J.K.S. Feasibility and utility of facemask sampling in the detection of SARS-CoV-2 during an ongoing pandemic. Eur. J. Clin. Microbiol. Infect. Dis. 2021;40:2489–2496. doi: 10.1007/s10096-021-04302-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smolinska A., Jessop D.S., Pappan K.L., De Saedeleer A., Kang A., Martin A.L., Allsworth M., Tyson C., Bos M.P., Clancy M., Morel M., Cooke T., Dymond T., Harris C., Galloway J., Bresser P., Dijkstra N., Jagesar V., Savelkoul P.H.M., Beuken E.V.H., Nix W.H.V., Louis R., Delvaux M., Calmes D., Ernst B., Pollini S., Peired A., Guiot J., Tomassetti S., Budding A.E., McCaughan F., Marciniak S.J., van der Schee M.P. The SARS-CoV-2 viral load in COVID-19 patients is lower on face mask filters than on nasopharyngeal swabs. Sci. Rep. 2021;11:13476. doi: 10.1038/s41598-021-92665-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rahmani A.R., Leili M., Azarian G., Poormohammadi A. Sampling and detection of corona viruses in air: a mini review. Sci. Total Environ. 2020;740:140207. doi: 10.1016/j.scitotenv.2020.140207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Soto F., Ozen M.O., Guimarães C.F., Wang J., Hokanson K., Ahmed R., Reis R.L., Paulmurugan R., Demirci U. Wearable collector for noninvasive sampling of SARS-CoV-2 from exhaled breath for rapid detection. ACS Appl. Mater. Interfaces. 2021;13:41445–41453. doi: 10.1021/acsami.1c09309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kubáň P., Foret F. Exhaled breath condensate: determination of non-volatile compounds and their potential for clinical diagnosis and monitoring. A review, Anal. Chim. Acta. 2013;805:1–18. doi: 10.1016/j.aca.2013.07.049. [DOI] [PubMed] [Google Scholar]
- 55.Lacombe M., Marie-Desvergne C., Combes F., Kraut A., Bruley C., Vandenbrouck Y., Chamel Mossuz V., Couté Y., Brun V. Proteomic characterization of human exhaled breath condensate. J. Breath Res. 2018;12:21001. doi: 10.1088/1752-7163/aa9e71. [DOI] [PubMed] [Google Scholar]
- 56.Hayes S.A., Haefliger S., Harris B., Pavlakis N., Clarke S.J., Molloy M.P., Howell V.M. Exhaled breath condensate for lung cancer protein analysis: a review of methods and biomarkers. J. Breath Res. 2016;10:34001. doi: 10.1088/1752-7155/10/3/034001. [DOI] [PubMed] [Google Scholar]
- 57.Griese M., Noss J., von Bredow C. Protein pattern of exhaled breath condensate and saliva. Proteomics. 2002;2:690–696. doi: 10.1002/1615-9861(200206)2:6<690::AID-PROT690>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 58.Rosias P. Methodological aspects of exhaled breath condensate collection and analysis. J. Breath Res. 2012;6:27102. doi: 10.1088/1752-7155/6/2/027102. [DOI] [PubMed] [Google Scholar]
- 59.Bredberg A., Gobom J., Almstrand A.-C., Larsson P., Blennow K., Olin A.-C., Mirgorodskaya E. Exhaled endogenous particles contain lung proteins. Clin. Chem. 2012;58:431–440. doi: 10.1373/clinchem.2011.169235. [DOI] [PubMed] [Google Scholar]
- 60.Wallace M.A.G., Pleil J.D., Madden M.C. Identifying organic compounds in exhaled breath aerosol: non-invasive sampling from respirator surfaces and disposable hospital masks. J. Aerosol Sci. 2019;137:105444. doi: 10.1016/j.jaerosci.2019.105444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jin Z., Jorns A., Yim W., Wing R., Mantri Y., Zhou J., Zhou J., Wu Z., Moore C., Penny W.F., Jokerst J.V. Mapping aerosolized saliva on face coverings for biosensing applications. Anal. Chem. 2021;93:11025–11032. doi: 10.1021/acs.analchem.1c02399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hu B. CCS; 2021. Face Mask Microextraction Sampling of Human Exhaled Breath Aerosol for Multidimensional Mass Spectrometry Analysis, the 32 CCS Congress, Zhuhai, China. [Google Scholar]
- 63.Kurova V.S., Anaev E.C., Kononikhin A.S., Fedorchenko K.Y., Popov I.A., Kalupov T.L., Bratanov D.O., Nikolaev E.N., Varfolomeev S.D. Proteomics of exhaled breath: methodological nuances and pitfalls. Clin. Chem. Lab. Med. 2009;47:706–712. doi: 10.1515/CCLM.2009.166. [DOI] [PubMed] [Google Scholar]
- 64.Hu B., Yao Z.-P. Mobility of proteins in porous substrates under electrospray ionization conditions. Anal. Chem. 2016;88:5585–5589. doi: 10.1021/acs.analchem.6b00894. [DOI] [PubMed] [Google Scholar]
- 65.Yao Z.P. Characterization of proteins by ambient mass spectrometry. Mass Spectrom. Rev. 2012;31:437–447. doi: 10.1002/mas.20346. [DOI] [PubMed] [Google Scholar]
- 66.Bauer K., Pasel K., Uhrig C., Sperling P., Versmold H. Comparison of face mask, head hood, and canopy for breath sampling in flow-through indirect calorimetry to measure oxygen consumption and carbon dioxide production of preterm infants <1500 grams. Pediatr. Res. 1997;41:139–144. doi: 10.1203/00006450-199701000-00022. [DOI] [PubMed] [Google Scholar]
- 67.Sukul P., Oertel P., Kamysek S., Trefz P. Oral or nasal breathing? Real-time effects of switching sampling route onto exhaled VOC concentrations. J. Breath Res. 2017;11:27101. doi: 10.1088/1752-7163/aa6368. [DOI] [PubMed] [Google Scholar]
- 68.Miekisch W., Schubert J.K. From highly sophisticated analytical techniques to life-saving diagnostics: technical developments in breath analysis. Trac. Trends Anal. Chem. 2006;25:665–673. [Google Scholar]
- 69.Berna A.Z., Odom John A.R. Breath metabolites to diagnose infection. Clin. Chem. 2022;68:43–51. doi: 10.1093/clinchem/hvab218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wilson A.D. Advances in electronic-nose technologies for the detection of volatile biomarker metabolites in the human breath. Metabolites. 2015;5:140–163. doi: 10.3390/metabo5010140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Trefz P., Schmidt M., Oertel P., Obermeier J., Brock B., Kamysek S., Dunkl J., Zimmermann R., Schubert J.K., Miekisch W. Continuous real time breath gas monitoring in the clinical environment by proton-transfer-reaction-time-of-flight-mass spectrometry. Anal. Chem. 2013;85:10321–10329. doi: 10.1021/ac402298v. [DOI] [PubMed] [Google Scholar]
- 72.Cherrie J.W., Wang S., Mueller W., Wendelboe-Nelson C., Loh M. In-mask temperature and humidity can validate respirator wear-time and indicate lung health status. J. Expo. Sci. Environ. Epidemiol. 2019;29:578–583. doi: 10.1038/s41370-018-0089-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gahleitner F., Guallar-Hoyas C., Beardsmore C.S., Pandya H.C., Thomas C.P. Metabolomics pilot study to identify volatile organic compound markers of childhood asthma in exhaled breath. Bioanalysis. 2013;5:2239–2247. doi: 10.4155/bio.13.184. [DOI] [PubMed] [Google Scholar]
- 74.Guallar-Hoyas C., Turner M.A., Blackburn G.J., Wilson I.D., Thomas C.P. A workflow for the metabolomic/metabonomic investigation of exhaled breath using thermal desorption GC–MS. Bioanalysis. 2012;4:2227–2237. doi: 10.4155/bio.12.193. [DOI] [PubMed] [Google Scholar]
- 75.Reyes-Garcés N., Gionfriddo E., Gómez-Ríos G.A., Alam M.N., Boyacı E., Bojko B., Singh V., Grandy J., Pawliszyn J. Advances in solid phase microextraction and perspective on future directions. Anal. Chem. 2018;90:302–360. doi: 10.1021/acs.analchem.7b04502. [DOI] [PubMed] [Google Scholar]
- 76.Hu B., Ouyang G. In situ solid phase microextraction sampling of analytes from living human objects for mass spectrometry analysis. Trac. Trends Anal. Chem. 2021;143:116368. [Google Scholar]
- 77.Cai S.-H., Di D., Yuan Z.-C., Chen W., Hu B. Paper-in-Facemask device for direct mass spectrometry analysis of human respiratory aerosols and environmental exposures via wearable continuous-flow adsorptive sampling: a proof-of-concept study. Anal. Chem. 2021;93:13743–13748. doi: 10.1021/acs.analchem.1c03406. [DOI] [PubMed] [Google Scholar]
- 78.Frey B.S., Damon D.E., Badu-Tawiah A.K. Emerging trends in paper spray mass spectrometry: microsampling, storage, direct analysis, and applications. Mass Spectrom. Rev. 2020;39:336–370. doi: 10.1002/mas.21601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yao Y.-N., Di D., Yuan Z.-C., Wu L., Hu B. Schirmer paper noninvasive microsampling for direct mass spectrometry analysis of human tears. Anal. Chem. 2020;92:6207–6212. doi: 10.1021/acs.analchem.9b05078. [DOI] [PubMed] [Google Scholar]
- 80.Locatelli M., Tartaglia A., Ulusoy H.I., Ulusoy S., Savini F., Rossi S., Santavenere F., Merone G.M., Bassotti E., D'Ovidio C., Rosato E., Furton K.G., Kabir A. Fabric-phase sorptive membrane array as a noninvasive in vivo sampling device for human exposure to different compounds. Anal. Chem. 2021;93:1957–1961. doi: 10.1021/acs.analchem.0c04663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lawson C.E., Martí J.M., Radivojevic T., Jonnalagadda S.V.R., Gentz R., Hillson N.J., Peisert S., Kim J., Simmons B.A., Petzold C.J., Singer S.W., Mukhopadhyay A., Tanjore D., Dunn J.G., Garcia Martin H. Machine learning for metabolic engineering: a review. Metab. Eng. 2021;63:34–60. doi: 10.1016/j.ymben.2020.10.005. [DOI] [PubMed] [Google Scholar]
- 82.Gubbi S., Hamet P., Tremblay J., Koch C.A., Hannah-Shmouni F. Artificial intelligence and machine learning in endocrinology and metabolism: the dawn of a new era. Front. Endocrinol. 2019;10 doi: 10.3389/fendo.2019.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Donovan J., Skotnicki-Grant S. Allergic contact dermatitis from formaldehyde textile resins in surgical uniforms and nonwoven textile masks. Dermatitis. 2007;18:40–44. doi: 10.2310/6620.2007.05003. [DOI] [PubMed] [Google Scholar]
- 84.Foo C.C., Goon A.T., Leow Y.H., Goh C.L. Adverse skin reactions to personal protective equipment against severe acute respiratory syndrome--a descriptive study in Singapore. Contact Derm. 2006;55:291–294. doi: 10.1111/j.1600-0536.2006.00953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Donovan J., Kudla I., Holness L.D., Skotnicki-Grant S., Nethercott J.R. Skin reactions following use of N95 facial masks. Dermatitis. 2007;18:104. [Google Scholar]
- 86.Fernández-Arribas J., Moreno T., Bartrolí R., Eljarrat E. COVID-19 face masks: a new source of human and environmental exposure to organophosphate esters. Environ. Int. 2021;154:106654. doi: 10.1016/j.envint.2021.106654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tran C.M., Lee H., Lee B., Ra J.-S., Kim K.-T. Effects of the chorion on the developmental toxicity of organophosphate esters in zebrafish embryos. J. Hazard Mater. 2021;401:123389. doi: 10.1016/j.jhazmat.2020.123389. [DOI] [PubMed] [Google Scholar]
- 88.He C., Lin C.-Y., Mueller J.F. Organophosphate flame retardants in the environment: source, occurrence, and human exposure. Compr. Anal. Chem. 2020;88:341–365. [Google Scholar]
- 89.Wang X., Okoffo E.D., Banks A.P.W., Li Y., Thomas K.V., Rauert C., Aylward L.L., Mueller J.F. Phthalate esters in face masks and associated inhalation exposure risk. J. Hazard Mater. 2022;423:127001. doi: 10.1016/j.jhazmat.2021.127001. [DOI] [PubMed] [Google Scholar]
- 90.Martino-Andrade A.J., Chahoud I. Reproductive toxicity of phthalate esters. Mol. Nutr. Food Res. 2010;54:148–157. doi: 10.1002/mnfr.200800312. [DOI] [PubMed] [Google Scholar]
- 91.Jin L., Griffith S.M., Sun Z., Yu J.Z., Chan W. On the flip side of mask wearing: increased exposure to volatile organic compounds and a risk-reducing solution. Environ. Sci. Technol. 2021;55:14095–14104. doi: 10.1021/acs.est.1c04591. [DOI] [PubMed] [Google Scholar]
- 92.Luo X., Wang Z.-J., Xie T.-T., Ding Y.-C., Tang Z.-X., Ye X.-W., Niu Z.-Y. Non-target screening and identification of fluorescent whitening agents in children masks based on high resolution mass spectrometry. Chin. J. Anal. Chem. 2021;49:1926–1936. [Google Scholar]
- 93.Keplinger M.L., Fancher O.E., Lyman F.L., Calandra J.C. Toxicologic studies of four fluorescent whitening agents. Toxicol. Appl. Pharmacol. 1974;27:494–506. doi: 10.1016/0041-008x(74)90029-5. [DOI] [PubMed] [Google Scholar]
- 94.Guo X., Xian Y., Luo H., Wu Y., Luo D., Chen Y., Lu Y., Xu D. Quantitative determinations of seven fluorescent whitening agents in polystyrene and polyvinyl chloride plastics by ultrahigh performance liquid chromatography–tandem mass spectrometry. Anal. Methods. 2013;5:6086–6093. [Google Scholar]
- 95.Chen W., Yuan Z.-C., Cai S.-H., Zou Y., Xin G., Di D., Song X., Zhao P., Wu M., Hu B. Gas chromatography-mass spectrometry analysis of human exhaled volatile organic compounds via wearable facemask microextraction sampling. Chin. J. Anal. Chem. 2022;50:445–453. [Google Scholar]
- 96.Liu Y., Wang Z., Wang W., Xing J., Zhang Q., Ma Q., Lv Q. Non-targeted analysis of unknown volatile chemicals in medical masks. Environ. Int. 2022;161:107122. doi: 10.1016/j.envint.2022.107122. [DOI] [PubMed] [Google Scholar]
- 97.Raval M., Sangani H. Certain face masks contain toxic chemicals, inhalation of which has the potential to affect the upper respiratory system. J. Trop. Dis. 2021;9:295. [Google Scholar]
- 98.Jankovic J., Jones W., Burkhart J., Noonan G. Environmental study of firefighters. Ann. Occup. Hyg. 1991;35:581–602. doi: 10.1093/annhyg/35.6.581. [DOI] [PubMed] [Google Scholar]
- 99.Stacey P., Thorpe A., Mogridge R., Lee T., Harper M. A new miniature respirable sampler for in-mask sampling: Part 1-particle size selection performance. Ann. Occup. Hyg. 2016;60:1072–1083. doi: 10.1093/annhyg/mew053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mogridge R., Stacey P., Forder J. A new miniature respirable sampler for in-mask sampling: Part 2-tests performed inside the mask. Ann. Occup. Hyg. 2016;60:1084–1091. doi: 10.1093/annhyg/mew051. [DOI] [PubMed] [Google Scholar]
- 101.Chan W., Jin L., Sun Z., Griffith S.M., Yu J.Z. Fabric masks as a personal dosimeter for quantifying exposure to airborne polycyclic aromatic hydrocarbons. Environ. Sci. Technol. 2021;55:5128–5135. doi: 10.1021/acs.est.0c08327. [DOI] [PubMed] [Google Scholar]
- 102.Chan W., Guo W., Yu J.Z. Polyurethane-based face mask as a sampling device for environmental tobacco smoke. Anal. Chem. 2021;93:13912–13918. doi: 10.1021/acs.analchem.1c02906. [DOI] [PubMed] [Google Scholar]