Abstract
A family with congenital hypothyroidism was identified with two novel deleterious compound heterozygous thyroid peroxidase (TPO) mutations (c.962C>A, and c.1577C>T). Serum thyroid tests showed higher-than-expected serum-free thyroxine (T4) relative to TT3, while reverse triiodothyronine (rT3) was also elevated. Two siblings manifested a more severe phenotype of developmental delay compared with another sibling and were found to harbor an additional novel heterozygous deleterious iodothyronine deiodinase 1 (DIO1) mutation (c.395G>A). In the context of L-T4 replacement, the decreased D1 activity results in abnormal thyroid hormone metabolism with decreased triiodothyronine (T3) generation from L-T4 and may result in decreased T3 bioavailability during critical stages of development.
Keywords: congenital hypothyroidism, deiodinase, reverse T3, thyroperoxidase
Introduction
The iodothyronine deiodinase-type 1 (D1) is a selenoenzyme that regulates the level of serum triiodothyronine (T3) through outer ring deiodination of thyroxine (T4) to bioactive T3 and the bioinactive reverse triiodothyronine (rT3) to 3,3′-diiodothyronine (T2) (1). Previous studies have identified variants in the deiodinase 1 gene that result in alteration of the rT3 and rT3/T3 levels in mice (2) and recently a first report in humans (1). We describe the first family with an iodothyronine deiodinase 1 (DIO1) mutation acting as a modulator for deleterious compound heterozygous mutations in the thyroid peroxidase (TPO) gene causing congenital hypothyroidism (CH) and developmental delay.
Case Report
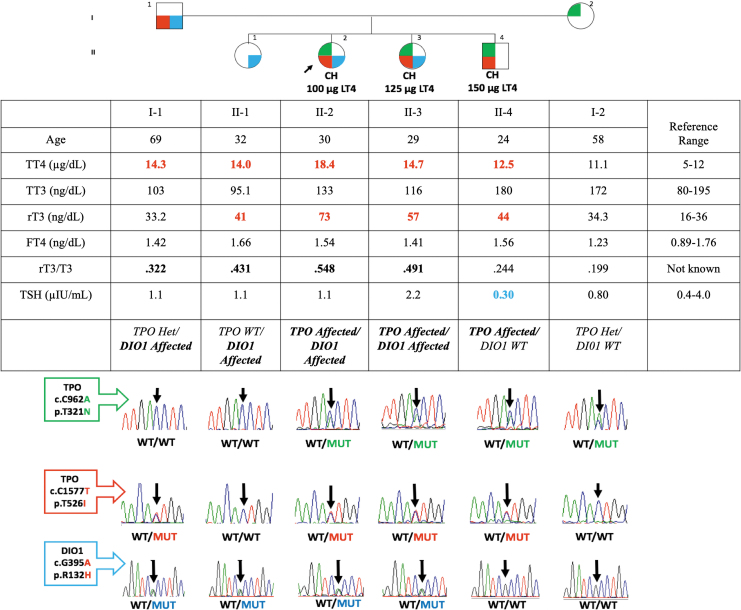
The proposita (II-2) (Fig. 1) born to nonconsanguineous parents of Latin origin presented with CH and was referred at age 30 years for evaluation of thyroid hormone replacement after thyroidectomy due to a goiter. She was on 100 μg levothyroxine (L-T4) (body weight [BW] 60 kg). Her sister, II-3, was on L-T4 125 μg (BW 77 kg), after thyroidectomy and her brother II-4 is on 150 μg L-T4 (BW 74 kg) due to goiter and CH. II-2 and II-3 had similar developmental delay, both graduated high school with a “special degree” and are dependent on the mother for instrumental activities of daily life (iADLs) and some ADLs. II-4 has a milder developmental delay phenotype, graduated high school with a regular diploma, and entered, but did not complete, college and now is employed full time.
FIG. 1.
Pedigree of the family, TFTs and genotyping results for TPO and DIO1 mutations. In the pedigree, generations are indicated with Roman numerals and individuals with Arabic numbers above each symbol. The propositus is indicated by the arrow. TFTs are aligned below each symbol, high values in red and low values in blue. Sanger sequencing results are aligned below each symbol. The electropherograms depict the heterozygous TPO mutations c.962C>A (p.T321N) and c.1577C>T (p.T526I) as well as the heterozygous DIO1 mutation c.395G>A (p.R132H). Five different genotypes are present: (a) proposita II-2 and sister II-3 harbor compound heterozygous TPO defects and a heterozygous DIO1 defect (TPO Affected/DIO1 Affected); (b) sister II-1 is unaffected for TPO and harbors a heterozygous DIO1 defect (TPO WT/DIO1 Affected); (c) brother II-4 harbors compound heterozygous TPO defects and is unaffected for DIO1 (TPO Affected/DIO1 WT); (d) father I-1 harbors a heterozygous TPO defect and a heterozygous DIO1 defect (TPO Het/DIO1 Affected); and (e) mother I-2 harbors only a heterozygous TPO defect and is unaffected for DIO1 (TPO Het/DIO1 WT). DIO1, iodothyronine deiodinase 1; TFTs, thyroid function tests; TPO, thyroid peroxidase.
Methods
Thyroid function tests (TFTs) and genomic DNA (gDNA) samples were obtained from family members using Qiagen QIAamp DNA Blood Mini Kit and analyzed as previously described (1). The DIO1 and TPO variants were confirmed by Sanger sequencing in all subjects.
The study was approved by University of Miami institutional review board and written informed consent was obtained.
Results
TFTs (Fig. 1) showed high free T4 in 5 of 6 family members. While 3 of them were on full L-T4 replacement for CH (II-2, II-3, II-4), 2 of them (I-1 and II-1) were not. Elevated rT3 was also noted in some family members. TSH was low in II-4 due to slight over-replacement with L-T4.
Whole-exome sequencing of dDNA from II-2 and II-3 revealed the presence of two novel TPO gene variants (c.962C>A, p.T321N and c.1577C>T, p.T526I) and a rare heterozygous pathogenic variant in the DIO1 gene (NM_001039715.3_c.395G>A rs1450006861, p.R132H) that are predicted deleterious by most in silico algorithms (Supplementary Table S1) and conservation of the sequence in various species (Supplementary Fig. S1). It was confirmed that the proposita II-2 and her affected sister II-3 harbor compound heterozygous deleterious TPO defects and the deleterious DIO1 heterozygous defect (TPO Affected/DIO1 Affected) (Fig. 1). Their brother II-4 harbors the same compound heterozygous TPO defects, however, he is WT for DIO1 (TPO Affected/DIO1 WT). Parents were each heterozygous for a TPO mutation and both were transmitted to II-2, II-3, and II-4 resulting in CH. In addition, the father harbored the deleterious DIO1 heterozygous defect, which was also transmitted to the daughter II-1 who was WT for the TPO gene mutations. All sequences were confirmed by Sanger sequencing (Fig. 1).
Discussion
Mutant TPO proteins are known to result in defective thyroidal iodide organification and cause CH (3). Mouse models of D1 deficiency (2) and heterozygous individuals from two families harboring D1 mutant proteins [p.N94K, p.M201I, (1)] show higher rT3/T3 ratios than unaffected controls. This occurs as the mutant D1 selenoenzymes have reduced affinity for T4 and rT3. With D1 modulating serum T3 and rT3 levels, T3 generation is decreased and clearance of rT3 is decreased in D1 defects.
Among the three individuals with CH on L-T4 replacement, the affected sisters (II-2, II-3) harboring the DIO1 defect have higher rT3/T3 ratios of 0.55 and 0.50, respectively, than the brother whose rT3/T3 ratio was 0.24. Similarly, among the other three individuals without L-T4 treatment, the father I-1 and daughter II-1 who harbor the deleterious heterozygous DIO1 defect had higher rT3/T3 ratios of 0.32 and 0.43, respectively, than the mother I-2 with rT3/T3 ratio of 0.20.
The deleterious compound heterozygous TPO mutations are the cause of the CH in this family. However, the more severe developmental delay in the two affected sisters (II-2, II-3) may be due to the additional deleterious DIO1 defect, R132H. The brother (II-4) without the DIO1 defect raises the possibility of a modulating effect of the D1 mutation, decreasing thyroid hormone bioavailability by reducing the generation of T3 from exogenous L-T4. Aligned with this finding, other studies have demonstrated that in oligogenic defects, the CH may be more severe than in monogenic cases, suggesting a gene dosage effect (4).
Conclusion
We report novel pathogenic compound heterozygous TPO mutations that may be modulated by a missense DIO1 pathogenic variant in a family with CH. This is the third family reported with inherited D1 deficiency in humans.
Supplementary Material
Acknowledgments
We thank the patients for participation in the research and Xiaohui Liao for performance of reverse T3 assays.
Authors' Contributions
All authors met authorship criteria and participated sufficiently in the study. All authors certify that this material has not been submitted or published before. All authors confirm that all of the research meets the ethics guidelines. A.F. performed the serum and genetic analyses, discussed the results, reviewed and participated in the writing of the article, and gave final approval to the article. Z.H. identified the family in Miami, obtained the samples, discussed the results, and participated in the writing of the article and gave final approval to the article. F.B.E. obtained samples from the family in Ecuador, discussed the results, and gave final approval to the article. A.D. was involved in the study design, discussed the results, participated in the writing of the article, and gave final approval to the article. S.R. was involved in the study design, discussed the results, participated in the writing of the article, and gave final approval to the article. R.W. participated in the performance of the analysis, designed the study, discussed the results, participated in the writing of the article, and gave final approval to the article.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This study was supported by grants from the National Institutes of Health, USA, DK15070 to S.R. and DK110322 to A.M.D., and MD010722 to R.E.W. and by funds from the Esformes Thyroid Research Fund.
Supplementary Material
References
- 1. Franca MM, German A, Fernandes GW, Liao XH, Bianco AC, Refetoff S, Dumitrescu AM. 2021. Human type 1 iodothyronine deiodinase (DIO1) mutations cause abnormal thyroid hormone metabolism. Thyroid 31:202–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liao XH, Di Cosmo C, Dumitrescu AM, Hernandez A, Van Sande J, St Germain DL, Weiss RE, Galton VA, Refetoff S. 2011. Distinct roles of deiodinases on the phenotype of Mct8 defect: a comparison of eight different mouse genotypes. Endocrinology 152:1180–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pannain S, Weiss RE, Jackson CE, Dian D, Beck JC, Sheffield VC, Cox N, Refetoff S. 1999. Two different mutations in the thyroid peroxidase gene of a large inbred Amish kindred: power and limits of homozygosity mapping. J Clin Endocrinol Metab 84:1061–1071. [DOI] [PubMed] [Google Scholar]
- 4. Yamaguchi T, Nakamura A, Nakayama K, Hishimura N, Morikawa S, Ishizu K, Tajima T. 2020. Targeted next-generation sequencing for congenital hypothyroidism with positive neonatal TSH screening. J Clin Endocrinol Metab 105:e2825–e2833. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.