Abstract
Background: Accurate assessment of parathyroid gland vascularity is important during thyroidectomy to preserve the function of parathyroid glands and to prevent postoperative hypocalcemia. Laser speckle contrast imaging (LSCI) has been shown to be accurate in detecting differences in parathyroid vascularity. In this surgeon-blinded prognostic study, we evaluate the relationship between intraoperative LSCI measurements and postoperative outcomes of total thyroidectomy patients.
Methods: Seventy-two thyroidectomy patients were included in this study. After thyroid resection, an LSCI device was used to image all parathyroid glands identified, and a speckle contrast value was calculated for each. An average value was calculated for each patient, and the data were grouped according to whether the patient had normal (16–77 pg/mL) or low levels of parathyroid hormone (PTH) measured on postoperative day 1 (POD1). The aim of this study was to establish a speckle contrast threshold for classifying a parathyroid gland as adequately perfused and to determine how many such glands are required for normal postoperative parathyroid function.
Results: A speckle contrast limit of 0.186 separated the normoparathyroid and hypoparathyroid groups with 87.5% sensitivity and 84.4% specificity: 7 of 8 patients with low PTH on POD1 had an average parathyroid speckle contrast above this limit, while 54 of 64 patients with normal postoperative PTH had an average parathyroid speckle contrast below this limit. Taking this value as the threshold for adequate parathyroid perfusion, it was determined that only one vascularized gland was needed for normal postoperative parathyroid function: 64 of 69 patients (92.8%) with at least one vascularized gland (determined by LSCI) had normal postoperative PTH, while all 3 patients (100%) with no vascularized glands had low postoperative PTH. Overall, the rates of temporary and permanent hypoparathyroidism in this study were 8.3% and 1.4%, respectively.
Conclusions: LSCI is a promising technique for assessing parathyroid gland vascularity. It has the potential to help reduce the incidence of hypocalcemia after thyroidectomy by providing surgeons with additional information during surgery to aid in the preservation of parathyroid function.
Keywords: clinical translation, hypocalcemia, hypoparathyroidism, parathyroid vascularity, surgical guidance, thyroidectomy
Introduction
Postsurgical hypoparathyroidism is a major complication following thyroid surgery due to inadvertent damage to parathyroid glands or their blood supply. Loss of parathyroid function (termed hypoparathyroidism) leads to hypocalcemia—below normal levels of serum calcium, which is needed for a variety of functions such as muscle contraction and neuronal excitability (1). Patients suffering from hypocalcemia must rely on calcium and vitamin D supplementation to avoid the associated negative effects, which include numbness, muscle spasms, tetany, and seizures.
Reports on the rates of hypoparathyroidism or hypocalcemia after thyroidectomy vary widely (2–9). The variation is due to differing definitions of postsurgical hypoparathyroidism and hypocalcemia (10), and differences in surgeon experience (11). However, one review found the medians of reported rates to be 27% for temporary cases and 1% for permanent cases (12).
Since the discovery that parathyroid glands fluoresce in the near-infrared (13), numerous studies have utilized this phenomenon in optical fiber-based (14–18) and imaging (19–26) approaches to help surgeons accurately identify the glands intraoperatively and avoid accidental excision. Two clinical devices employing near-infrared autofluorescence (NIRAF) have now received U.S. Food and Drug Administration (FDA) clearance and Conformitè Europëenne (CE) marking for parathyroid detection (27).
However, numerous studies have shown that identifying the glands alone is insufficient to improve hypocalcemia rates post-thyroidectomy (28–31), and it is more crucial to preserve the vascularity of glands to ensure normoparathyroid/normocalcemic status after thyroid surgery. Assessing the vascularity of intact parathyroid glands remains a challenge, and surgeons often rely on visual inspection and their experience in doing so. Accurate knowledge of the parathyroid glands' state of vascularity is also important as it guides decision-making on autotransplantation—when devascularized, autotransplantation of a parathyroid gland can help regain its function over time (32–34).
A few experimental techniques have been reported for assessing parathyroid vascularity including topical application of lidocaine (35), laser Doppler flowmetry (36), intraoperative parathyroid hormone (PTH) assay (37), and confocal endomicroscopy using the dye fluorescein (38). Recently, the use of indocyanine green (ICG) angiography has gained traction (39–45). While there have been promising reports, there are a few limitations.
First, while ICG is generally considered safe, a small percentage of patients can still suffer severe allergic reactions to the dye (46). Second, it cannot be performed simultaneously with NIRAF detection for parathyroid identification. This is because the fluorescence of ICG is in the same spectral range as the parathyroid NIRAF is much stronger in intensity, while also persisting for long periods. Once ICG has been injected, NIRAF detection can no longer be used to localize other parathyroid glands. This creates a challenge for the surgeon who might want to make immediate decisions on preserving a parathyroid gland before continuing with the procedure. Finally, current practice relies on qualitative scoring of ICG fluorescence intensity, which makes it difficult to standardize measurements, although efforts have been made to make it more quantitative (47).
An alternative technique that overcomes these limitations is laser speckle contrast imaging (LSCI), a real-time label-free imaging technique that is sensitive to superficial blood flow. The technique was first used to image blood flow in the human retina (48). It works by analyzing blurriness of the interference or speckle pattern produced when laser light illuminates a surface. To produce a flow map from an image of a speckle pattern, a quantity called the speckle contrast is calculated. In one form, it is calculated as the standard deviation of pixel intensities within local regions of the image divided by their corresponding mean intensities (49). Lower speckle contrast values indicate more blurring of the speckle pattern and hence greater blood flow, while higher contrast values indicate the converse.
In previous work (50), we showed that LSCI is able to differentiate with high accuracy between parathyroid glands classified as well vascularized or devascularized by an experienced endocrine surgeon. The purpose of this current work was to determine how intraoperative LSCI measurements relate to total thyroidectomy patient outcomes, namely postoperative PTH levels since it has been suggested that PTH within the first 24 hours after surgery is indicative of hypoparathyroidism (51).
Materials and Methods
LSCI system
Imaging was performed using a modified version of an LSCI system previously described (50). Modifications are detailed in the Supplementary Methods section in the Supplementary Data. Briefly, the device consists of a 785 nm laser to provide illumination, and a near-infrared-optimized camera to image the speckle pattern produced, which is then sent to a computer for real-time processing. The camera is mounted to an articulated arm with a detachable sterile handle that the surgeon uses in positioning the device above the tissue of interest.
Patient recruitment and study design
This study was approved by the Vanderbilt University Medical Center (VUMC) Institutional Review Board. Seventy-nine patients scheduled to undergo thyroidectomy were recruited by three surgeons, and informed written consent was obtained from each patient before participation. These included 58 patients who underwent total thyroidectomy alone, 10 who underwent total thyroidectomy with lymph node dissection, and 4 who underwent completion thyroidectomy.
After resection of the thyroid, all parathyroid glands identified during the course of the operation were imaged with the LSCI system. Imaging took up to 1 minute per gland, including time for the surgeon to position the camera above the parathyroid. Since LSCI is label-free and poses no risk to the patient, imaging can be repeated as often as desired. Overall, imaging added about 5 minutes to each case. For each gland, ten frames were averaged, and this averaged image was then segmented to obtain the average speckle contrast for that parathyroid. Only parathyroid glands identified with high confidence by the surgeon were included in analyses. No additional dissection was performed to look for missing parathyroid glands.
Any parathyroid gland that was inadvertently or unavoidably (e.g., intrathyroidal parathyroid) resected with the thyroid was autotransplanted. Since in situ LSCI measurements were unobtainable for these excised glands, they were assigned the highest parathyroid speckle contrast value that was measured throughout the study.
The surgeon's visual assessment of each parathyroid gland was also recorded into one of three categories: well vascularized, compromised (e.g., bruised but still perfused), and devascularized (these glands were subsequently autotransplanted). Parathyroid speckle contrast measurements were grouped according to these classifications, and a one-way analysis of variance was performed to look for significant differences. Throughout the study, the surgeons were blinded from LSCI data so as not to influence patient care. PTH and serum calcium levels on postoperative day 1 (POD1) were available for 72 patients, hence only these were included in further analyses. Patients with POD1 PTH below the accepted normal range at VUMC of 16–77 pg/mL were evaluated up to 6 months postoperatively to look for recovery within that period. All patients received calcium supplementation postoperatively.
Data analysis
The goal of this study was to establish a speckle contrast threshold for classifying a parathyroid gland as adequately vascularized and to determine how many vascularized glands are needed for a patient to have normal PTH levels postoperatively. This was achieved by first calculating the average parathyroid speckle contrast for each patient and grouping the data according to whether the patient had normal or low levels of PTH on POD1. Note that this is the average value of all parathyroid glands in each patient. An receiver operating characteristic (ROC) curve was generated using average parathyroid speckle contrast as a classifier to separate the two groups of patients. The optimum point on the curve was taken to be the speckle contrast threshold for adequate parathyroid vascularity.
The minimum number of vascularized parathyroid glands needed for normal postoperative function was then determined based on this value. This minimum required number of vascularized parathyroid glands is designated as nmin. Data analysis was conducted for two scenarios: nmin = 1 and nmin = 2. For each of these, the percentage of patients with less than nmin vascularized glands (as determined by the speckle contrast threshold) who then had low PTH levels on POD1 was calculated. This is also the positive predictive value of postoperative hypoparathyroidism.
Additionally, the percentage of patients with nmin vascularized parathyroid glands who then had normal PTH levels on POD1 was calculated. This is the negative predictive value. Due to insufficient data, calculations could not be performed for nmin = 3 or nmin = 4. However, we do not anticipate that three or more vascularized parathyroid glands are crucial to maintain a normoparathyroid state, as there is ample evidence to show that the human body is able to compensate when one or more parathyroid glands are injured (52).
The calculations were performed on the data set of 72 patients, as well as on groups determined by the number of parathyroid glands identified during the operation and left in the patient (including autotransplanted glands). These groups consist of cases where there were four glands (n = 25), three glands (n = 22), and one or two glands (n = 25).
Results
Patient demographics and clinical data for the 72 patients with postoperative PTH and calcium data are shown in Table 1. A multinomial logistic regression revealed that neither sex, race, age, nor body mass index significantly influenced whether or not a patient had low levels of PTH on POD1. Indication for surgery similarly had no significant impact on patient outcome (although patients with Hashimoto's thyroiditis had to be excluded from this analysis due to insufficient data). Surgical procedure could not be included in the regression analysis due to insufficient data.
Table 1.
Patient Demographics and Clinical Characteristics
| Total population (n = 72) | Low POD1 PTH (n = 8) | Normal POD1 PTH (n = 64) | |
|---|---|---|---|
| Sex, n (%) | |||
| Female | 56 (78%) | 7 (88%) | 49 (77%) |
| Male | 16 (22%) | 1 (13%) | 15 (23%) |
| Race/ethnicity, n (%) | |||
| White | 51 (71%) | 4 (50%) | 47 (73%) |
| Non-white | 21 (29%) | 4 (50%) | 17 (27%) |
| Indication for surgery, n (%) | |||
| Graves' disease | 21 (29%) | 2 (25%) | 19 (30%) |
| Papillary thyroid carcinoma | 13 (18%) | 2 (25%) | 11 (17%) |
| Other thyroid cancer | 15 (21%) | 1 (13%) | 14 (22%) |
| Toxic/nontoxic thyroid nodule | 20 (28%) | 2 (25%) | 18 (28%) |
| Hashimoto's thyroiditis | 3 (4%) | 1 (13%) | 2 (3%) |
| Surgical procedure, n (%) | |||
| Total thyroidectomy | 58 (81%) | 4 (50%) | 54 (84%) |
| Total thyroidectomy with lymph node dissection | 10 (14%) | 3 (38%) | 7 (11%) |
| Completion thyroidectomy | 4 (6%) | 1 (13%) | 3 (5%) |
| Age, mean ± SD, years | 48.8 ± 13.2 | 43.9 ± 11.6 | 49.4 ± 13.3 |
| Body mass index, mean ± SD, kg/m2 | 33.6 ± 8.4 | 31.9 ± 8.0 | 33.8 ± 8.5 |
| No. of PGs per patient, mean ± SD | 3 ± 0.9 | 3.4 ± 0.7 | 2.9 ± 0.9 |
PG, parathyroid gland; POD1, postoperative day 1, PTH, parathyroid hormone; SD, standard deviation.
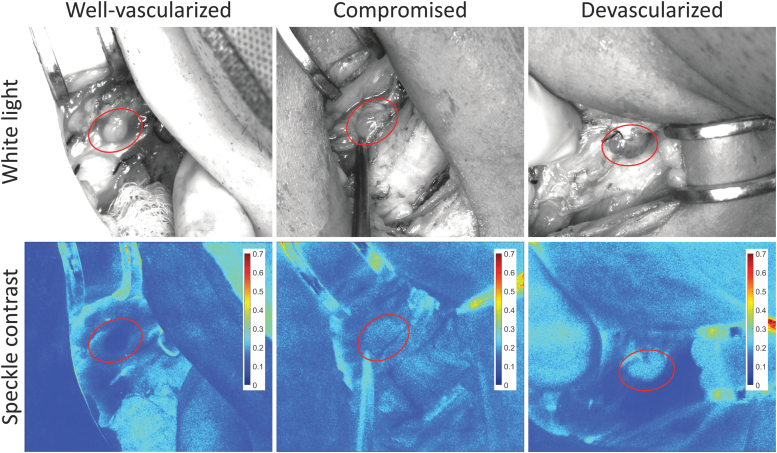
Representative images of a well-vascularized, a compromised, and a devascularized parathyroid gland are shown in Figure 1. Their average speckle contrast values were 0.11, 0.18, and 0.21, respectively. A total of 231 parathyroid glands were imaged in this study, yielding an average of ∼2.9 glands per patient. The distribution of speckle contrast for these glands, grouped according to the surgeons' assessment of vascularity, is shown in Supplementary Figure S1.
FIG. 1.
Representative white light and speckle contrast images of a well-vascularized, a compromised, and a devascularized parathyroid gland. Parathyroid glands are indicated with ellipses. Speckle contrast values were 0.11, 0.18, and 0.21, respectively. Color images are available online.
Speckle contrast was lowest for well-vascularized glands and highest for devascularized glands. The range of parathyroid speckle contrast values for this instrument was 0.09–0.27, with a mean value of 0.17. Statistical testing using a one-way analysis of variance showed that well-vascularized, compromised, and autotransplanted parathyroid glands all had significantly different speckle contrast from one another (p < 0.05). Despite this significance, there were a number of cases where LSCI greatly disagreed with the surgeon. A few examples are shown in Supplementary Figure S2, where glands that were considered well vascularized by the surgeon had high speckle contrast values (indicating reduced perfusion), and glands that were considered devascularized had low speckle contrast. This further motivates the need to evaluate patient outcomes against intraoperative LSCI.
There were 8 patients of 72 (11.1%) who had PTH levels below 16 pg/mL on POD1. Of these, four patients also had serum calcium levels below the normal range of 8.4–10.5 mg/dL. These four patients mostly had lower PTH levels than the other four patients. Data on all eight patients are summarized in Table 2. These patients underwent surgery for a variety of conditions including thyroid cancers, Graves' disease, Hashimoto's thyroiditis, and multinodular goiters.
Table 2.
Summary Information on Patients with Low Parathyroid Hormone on Postoperative Day 1
| Patient | Diagnosis | Procedure | Postoperative PTHa | Postoperative Cab | Average PG speckle contrastc | No. of PGs found (left) | Autotransplant? | PTH recovered? |
|---|---|---|---|---|---|---|---|---|
| 1 | Papillary thyroid carcinoma | Thyroidectomy with neck dissection | 13 | 9.3 | 0.21 (0.21, 0.17, 0.24, 0.23) | 4 (4) | Yes | Yes |
| 2 | Multinodular goiter | Completion thyroidectomy | 3 | 8.7 | 0.21 (0.19, 0.24, 0.20) | 3 (3) | No | Yes |
| 3 | Papillary thyroid carcinoma | Thyroidectomy with neck dissection | 3 | 8.1 | 0.19 (0.22, 0.12, 0.21) | 3 (3) | Yes | No |
| 4 | Graves' disease | Total thyroidectomy | 3 | 8.2 | 0.20 (0.24, 0.19, 0.16, 0.21) | 4 (4) | No | Yes |
| 5 | Toxic multinodular goiter | Total thyroidectomy | 7 | 8.2 | 0.23 (0.20, 0.27, 0.21) | 3 (3) | Yes | Yes |
| 6 | Hashimoto's thyroiditis | Total thyroidectomy | 6 | 8.6 | 0.20 (0.17, 0.19, 0.16, 0.27) | 4 (4) | Yes | — |
| 7 | Papillary thyroid carcinoma | Thyroidectomy with neck dissection | 3 | 7.5 | 0.23 (0.23, 0.22) | 2 (2) | No | — |
| 8 | Graves' disease | Total thyroidectomy | 13 | 9.1 | 0.15 (0.19, 0.11, 0.19, 0.13) | 4 (4) | No | Yes |
Values are expressed in pg/mL; normal range 16–77 pg/mL.
Values are expressed in mg/dL; normal range 8.4–10.5 mg/dL. Values below normal are given in bold.
Values in parentheses indicate speckle contrast of individual PGs.
Three of 10 patients who underwent lymph node dissection had low postoperative PTH, suggesting an increased likelihood of postoperative hypoparathyroidism with more extensive surgical procedures (compared with 4 of 58 total thyroidectomy patients). Four patients had parathyroid autotransplantation, and of these, in one case, PTH levels did not return to normal within the first 6 months postoperatively. For two patients, data on subsequent PTH measurements were unavailable. However, their calcium levels returned to normal within the first 6 months after surgery and the patients no longer required calcium supplementation.
The average parathyroid gland speckle contrast in most cases was at least 0.19, with the exception of Patient 8 who had a very transient drop in PTH to 13 pg/mL and rapid recovery to 31 pg/mL on POD4. For comparison, it took weeks to months for PTH to recover in the other patients, suggesting that this was not a case of permanent hypoparathyroidism. Altogether, the rate of temporary hypoparathyroidism in this study was 8.3%, and the rate of permanent hypoparathyroidism was 1.4%.
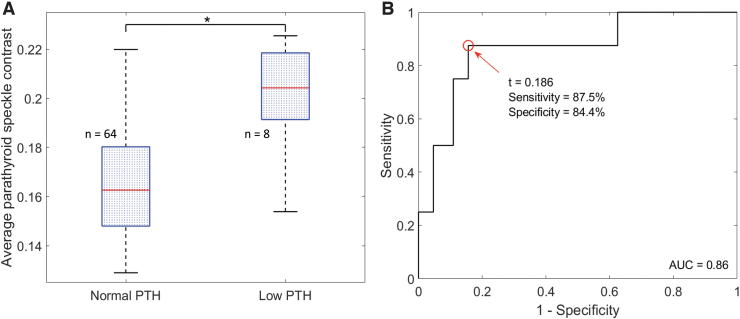
Average parathyroid speckle contrast was calculated for each of the 72 patients and grouped according to PTH status on POD1. The distribution of values is shown in Figure 2A. As expected, the average parathyroid speckle contrast values for patients with low postoperative PTH are higher than those for patients with normal postoperative PTH. This difference was statistically significant when evaluated with a two-sided two-sample t-test (p < 10−4). Using average parathyroid speckle contrast as a classifier to separate the two groups, the ROC curve in Figure 2B was generated.
FIG. 2.
(A) Distribution of average parathyroid speckle contrast values in patients with normal and low levels of PTH on POD1. Medians are indicated by horizontal red lines, and each box spans the 25th to 75th percentile of that group. Whiskers extend to the most extreme data points not considered outliers. (B) ROC curve resulting from using average speckle contrast as a classifier. The two groups were significantly different (*:p < 10−4), and the optimum speckle contrast value (t) separating them was 0.186. POD1, postoperative day 1; PTH, parathyroid hormone; ROC, receiver operating characteristic. Color images are available online.
The optimum point on this curve was at a speckle contrast of 0.186, which resulted in a sensitivity and specificity of 87.5% and 84.4%, respectively. In other words, 87.5% of the patients with low PTH on POD1 had average parathyroid speckle contrast greater than 0.186, and 84.4% of the patients with normal PTH on POD1 had average parathyroid speckle contrast less than or equal to 0.186. This value of 0.186 was identified as the optimal cutoff point for adequate parathyroid vascularity.
To determine how many vascularized glands are needed for normal postoperative PTH levels, positive and negative predictive values were calculated for nmin = 1 and nmin = 2. The results are shown in Table 3. Overall, requiring one vascularized gland for normal postoperative parathyroid function had the higher combined predictive values. All three patients with no vascularized parathyroid glands (i.e., no glands with speckle contrast below 0.186) had low PTH levels on POD1, corresponding to a positive predictive value of 100%. These were Patients 2, 5, and 7 in Table 2. However, 64 of 69 patients who had at least one vascularized parathyroid gland had normal PTH levels on POD1, corresponding to a negative predictive value of 92.8%. The exceptions were Patients 1, 3, 4, 6, and 8 in Table 2, who also generally had high average values. This trend largely held regardless of the number of parathyroid glands identified during the surgery. These data are presented in Supplementary Table S1.
Table 3.
Minimum Number of Vascularized Parathyroid Glands Needed for Normal Postoperative Function
| At least 1 vascularized PG required | ||
| 0 PG with speckle contrast below 0.185 | At least 1 PG with speckle contrast below 0.185 | |
| Patients with low POD1 PTH | 3 | 5 |
| Patients with normal POD1 PTH | 0 | 64 |
| PPV: 100% | NPV: 92.8% | |
| At least 2 vascularized PGs required | ||
| <2 PGs with speckle contrast below 0.185 | At least 2 PGs with speckle contrast below 0.185 | |
| Patients with low POD1 PTH | 5 | 3 |
| Patients with normal POD1 PTH | 19 | 45 |
| PPV: 20.8% | NPV: 93.8% | |
NPV, negative predictive value; PPV, positive predictive value.
Discussion
Parathyroid glands are crucial to maintaining normal serum calcium levels. Therefore, it is critical during thyroidectomies for the surgeon to have accurate knowledge of the parathyroid glands' state of vascularity to help preserve function postoperatively. We propose that LSCI can provide this information.
In this study, an average parathyroid speckle contrast value of 0.186 was found to have the highest sensitivity and specificity in separating patients with normal from patients with low postoperative PTH levels. Overall, 87.5% of patients with low POD1 PTH had average parathyroid speckle contrast greater than 0.186, while 84.4% of patients with normal POD1 PTH had average parathyroid speckle contrast less than 0.186. This value was taken to be the threshold for adequate parathyroid vascularization, that is, a parathyroid gland with speckle contrast below 0.186 was considered to be vascularized.
Requiring one such parathyroid gland for normal postoperative function produced much higher combined positive and negative predictive values than requiring two parathyroid glands, suggesting that only one gland is needed. All the patients who had no parathyroids with speckle contrast below 0.186 had low POD1 PTH, while POD1 PTH was low for only 5 of the 24 patients who had less than two vascularized glands.
The calculations were also performed on groups determined by the number of parathyroid glands identified because the actions a surgeon might take in a case where they identified four parathyroid glands could differ from those in a case where only one was identified. The predictive values were consistently higher for nmin = 1; regardless of whether one, two, or three parathyroid glands were identified during the surgery, only one gland needed to have speckle contrast below 0.186. A possible exception exists in the cases where four parathyroid glands were identified. However, it should be noted that the positive predictive value of requiring one vascularized gland in these cases could not be assessed as all these patients had at least one vascularized gland. We nevertheless expect that it would be as high as in the other groups—if all four parathyroid glands have been identified and none are vascularized, the patient will most likely have low postoperative PTH levels.
In a study using ICG angiography to evaluate parathyroid glands after thyroid surgery, Fortuny et al. reported that postoperative PTH levels were normal for all patients who had at least one well-vascularized parathyroid gland (evaluated by ICG fluorescence intensity) (39). In a similar but slightly different vein, our study found that all patients who had no vascularized parathyroid glands had low PTH levels on POD1. However, not all patients who did have one vascularized gland had normal postoperative PTH levels. Overall, 7% of patients who had at least one vascularized gland still had low postoperative PTH. An exception is Patient 8 in Table 2, who had favorable assessments of their parathyroid glands by both LSCI and the surgeon. Although PTH was below the normal range on POD1, it recovered to normal within days whereas recovery took weeks to months in the other patients. Therefore, it is possible that the transient PTH drop in this patient was due to postoperative swelling or other effects, rather than loss of parathyroid function.
Overall, 8.3% of patients experienced temporary hypoparathyroidism compared with the median reported rate of 27% (12). Additionally, while the rate of permanent hypoparathyroidism was 1.4%, it usually reported as up to 5% (2,3) or higher (5). Incidence of hypoparathyroidism after thyroid surgery is largely dependent on surgeon experience and specialization, and at a high-volume institution, there are less likely to be postsurgical complications. The three surgeons involved in this study are high-volume surgeons who perform over a hundred thyroidectomies annually, pointing to one limitation of this study—there were not many patients who experienced a negative outcome.
Furthermore, in some patients, not all parathyroid glands may have been evaluated. While it is possible for a patient to naturally have more or less than four parathyroid glands, 84–87% have four (53). Hence, for 47 of the 72 patients in this study, there could have been parathyroid glands that were hidden and therefore not evaluated. It could be argued that these hidden glands were protected and therefore largely responsible for the patients' normal postoperative outcomes. To overcome this limitation, future studies need to evaluate all four parathyroid glands. In four cases, a parathyroid gland was not easily accessible for imaging with the current device. Finally, this study was entirely reliant on the visual assessment of the surgeon to identify the parathyroid glands.
Nevertheless, LSCI is also highly promising because it can be performed simultaneously with NIRAF imaging and work is already underway to develop clinical devices utilizing both techniques (54–56). Specific points for consideration and discussion on discrepancies between LSCI and surgeon assessment can be found in the Supplementary Discussion section in the Supplementary Data.
In summary, LSCI is a promising approach for label-free intraoperative assessment of parathyroid gland vascularity. It is quantitative, real-time, label-free and does not interfere with NIRAF detection for localization of parathyroid glands. Based on this data set, a total thyroidectomy patient who does not have at least one vascularized parathyroid gland by LSCI will likely suffer hypoparathyroidism, while the vast majority of those who do will have normal PTH levels. Use of LSCI in thyroid surgery has the potential to help reduce long-term postsurgical hypoparathyroidism by providing surgeons with objective and accurate assessment of parathyroid gland vascularity.
Supplementary Material
Acknowledgments
We wish to express our gratitude to the operating room staff and patients recruited at the VUMC for their accommodation and participation in the study.
Authors' Contributions
E.A.M. was involved in conceptualization, study design, performing measurements in the operating room, preparing the original article, and editing the final article. G.T. and A.M.-J. were involved in conceptualization, study design, and editing the final article. N.B. and S.L.R. were involved in performing measurements in the operating room and editing the final article. C.C.S. was involved in study design, performing measurements in the operating room, and editing the final article.
Author Disclosure Statement
The authors declare no financial or commercial conflict of interest.
Funding Information
This study was financially supported by the National Institutes of Health under Grant number 1R01CA212147-01A1.
Supplementary Material
References
- 1. Naveh-Many T 2005 Development of parathyroid glands. In: Molecular Biology of the Parathyroid. Landes Bioscience/Eurekah.com, Georgetown, TX, pp 1–2. [Google Scholar]
- 2. Ritter K, Elfenbein D, Schneider DF, Chen H, Sippel RS. 2015. Hypoparathyroidism after total thyroidectomy: incidence and resolution. J Surg Res 197:348–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cannizzaro MA, Lo Bianco S, Picardo MC, Provenzano D, Buffone A. 2017. How to avoid and to manage post-operative complications in thyroid surgery. Updates Surg 69:211–215. [DOI] [PubMed] [Google Scholar]
- 4. McHenry CR, Speroff T, Wentworth D, Murphy T. 1994. Risk factors for postthyroidectomy hypocalcemia. Surgery 116:641–647; discussion 647–648. [PubMed] [Google Scholar]
- 5. Dedivitis RA, Aires FT, Cernea CR. 2017. Hypoparathyroidism after thyroidectomy: prevention, assessment and management. Curr Opin Otolaryngol Head Neck Surg 25:142–146. [DOI] [PubMed] [Google Scholar]
- 6. Tredici P, Grosso E, Gibelli B, Massaro MA, Arrigoni C, Tradati N. 2011. Identification of patients at high risk for hypocalcemia after total thyroidectomy. Acta Otorhinolaryngol Ital 31:144–148. [PMC free article] [PubMed] [Google Scholar]
- 7. Demeester-Mirkine N, Hooghe L, Van Geertruyden J, Maertelaer V. 1992. Hypocalcemia after thyroidectomy. Arch Surg 127:854–858. [DOI] [PubMed] [Google Scholar]
- 8. Baldassarre RL, Chang DC, Brumund KT, Bouvet M. 2012. Predictors of hypocalcemia after thyroidectomy: results from the nationwide inpatient sample. ISRN Surg 2012:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sanabria A, Kowalski LP, Tartaglia F. 2018. Inferior thyroid artery ligation increases hypocalcemia after thyroidectomy: a meta-analysis. Laryngoscope 128:534–541. [DOI] [PubMed] [Google Scholar]
- 10. Edafe O, Balasubramanian SP. 2017. Incidence, prevalence and risk factors for post-surgical hypocalcaemia and hypoparathyroidism. Gland Surg 6 (Suppl 1):S59–S68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. American College of Surgeons 2015. Total thyroidectomy complication rates and costs are lower if surgeon performs 25 or more cases yearly. Available at https://www.facs.org/media/press-releases/2015/sosa (accessed February 13, 2021).
- 12. Edafe O, Antakia R, Laskar N, Uttley L, Balasubramanian SP. 2014. Systematic review and meta-analysis of predictors of post-thyroidectomy hypocalcaemia. Br J Surg 101:307–320. [DOI] [PubMed] [Google Scholar]
- 13. Paras C, Keller M, White L, Phay J, Mahadevan-Jansen A. 2011. Near-infrared autofluorescence for the detection of parathyroid glands. J Biomed Opt 16:067012. [DOI] [PubMed] [Google Scholar]
- 14. McWade MA, Paras C, White LM, Phay JE, Mahadevan-Jansen A, Broome JT. 2013. A novel optical approach to intraoperative detection of parathyroid glands. Surgery 154:1371–1377; discussion 1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McWade MA, Sanders ME, Broome JT, Solórzano CC, Mahadevan-Jansen A. 2016. Establishing the clinical utility of autofluorescence spectroscopy for parathyroid detection. Surgery 159:193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thomas G, McWade MA, Paras C, et al. 2018. Developing a clinical prototype to guide surgeons for intraoperative label-free identification of parathyroid glands in real time. Thyroid 28:1517–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Thomas G, McWade MA, Nguyen JQ, Sanders ME, Broome JT, Baregamian N, Solórzano CC, Mahadevan-Jansen A. 2019. Innovative surgical guidance for label-free real-time parathyroid identification. Surgery 165:114–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Thomas G, Squires MH, Metcalf T, Mahadevan-Jansen A, Phay JE 2019 Imaging or fiber probe-based approach? Assessing different methods to detect near infrared autofluorescence for intraoperative parathyroid identification. J Am Coll Surg 229:596.e3–608.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McWade MA, Paras C, White LM, Phay JE, Solórzano CC, Broome JT, Mahadevan-Jansen A. 2014. Label-free intraoperative parathyroid localization with near-infrared autofluorescence imaging. J Clin Endocrinol Metab 99:4574–4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim SW, Song SH, Lee HS, Noh WJ, Oak C, Ahn Y-C, Lee KD. 2016. Intraoperative real-time localization of normal parathyroid glands with autofluorescence imaging. J Clin Endocrinol Metab 101:4646–4652. [DOI] [PubMed] [Google Scholar]
- 21. Ladurner R, Sommerey S, Arabi N Al, Hallfeldt KKJ, Stepp H, Gallwas JKS. 2017. Intraoperative near-infrared autofluorescence imaging of parathyroid glands. Surg Endosc 31:3140–3145. [DOI] [PubMed] [Google Scholar]
- 22. Kim SW, Lee HS, Ahn YC, et al. 2018. Near-infrared autofluorescence image-guided parathyroid gland mapping in thyroidectomy. J Am Coll Surg 226:165–172. [DOI] [PubMed] [Google Scholar]
- 23. Dip F, Falco J, Verna S, Prunello M, Loccisano M, Quadri P, White K, Rosenthal R. 2019. Randomized controlled trial comparing white light with near-infrared autofluorescence for parathyroid gland identification during total thyroidectomy. J Am Coll Surg 228:744–751. [DOI] [PubMed] [Google Scholar]
- 24. De Leeuw F, Breuskin I, Abbaci M, Casiraghi O, Mirghani H, Ben Lakhdar A, Laplace-Builhé C, Hartl D. 2016. Intraoperative near-infrared imaging for parathyroid gland identification by auto-fluorescence: a feasibility study. World J Surg 40:2131–2138. [DOI] [PubMed] [Google Scholar]
- 25. Kahramangil B, Dip F, Benmiloud F, Falco J, de La Fuente M, Verna S, Rosenthal R, Berber E. 2018. Detection of parathyroid autofluorescence using near-infrared imaging: a multicenter analysis of concordance between different surgeons. Ann Surg Oncol 25:957–962. [DOI] [PubMed] [Google Scholar]
- 26. Kose E, Kahramangil B, Aydin H, Donmez M, Berber E. 2019. Heterogeneous and low-intensity parathyroid autofluorescence: patterns suggesting hyperfunction at parathyroid exploration. Surgery 165:431–437. [DOI] [PubMed] [Google Scholar]
- 27. U.S. Food and Drug Administration (FDA) FDA permits marketing of two devices that detect parathyroid tissue in real-time during surgery | FDA. Available at https://www.fda.gov/news-events/press-announcements/fda-permits-marketing-two-devices-detect-parathyroid-tissue-real-time-during-surgery (accessed October 4, 2020).
- 28. Serra C, Silveira L, Canudo A. 2020. Identification of inadvertently removed parathyroid glands during thyroid surgery using autofluorescence. Gland Surg 9:893–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Serra C, Canudo A, Silveira L. 2020. Intraoperative identification of parathyroid glands by autofluorescence on total thyroidectomy—does it really reduces post-operative hypocalcemia? Surg Pract Sci 2:100011. [Google Scholar]
- 30. Papavramidis TS, Chorti A, Tzikos G, et al. 2021. The effect of intraoperative autofluorescence monitoring on unintentional parathyroid gland excision rates and postoperative PTH concentrations—a single-blind randomized-controlled trial. Endocrine 72:546–552. [DOI] [PubMed] [Google Scholar]
- 31. Kim YS, Erten O, Kahramangil B, Aydin H, Donmez M, Berber E. 2020. The impact of near infrared fluorescence imaging on parathyroid function after total thyroidectomy. J Surg Oncol 122:973–979. [DOI] [PubMed] [Google Scholar]
- 32. Sierra M, Herrera MF, Herrero B, Jiménez F, Sepúlveda J, Lozano RR, Gamino R, González O, Correa-Rotter R. 1998. Prospective biochemical and scintigraphic evaluation of autografted normal parathyroid glands in patients undergoing thyroid operations. Surgery 124:1005–1010. [DOI] [PubMed] [Google Scholar]
- 33. Lo CY, Tam SC. 2001. Parathyroid autotransplantation during thyroidectomy: documentation of graft function. Arch Surg 136:1381–1385. [DOI] [PubMed] [Google Scholar]
- 34. Ahmed N, Aurangzeb M, Muslim M, Zarin M. 2013. Routine parathyroid autotransplantation during total thyroidectomy: a procedure with predictable outcome. J Pakistan Med Assoc 63:190–193. [PubMed] [Google Scholar]
- 35. Kuriloff DB, Kizhner V. 2010. Parathyroid gland preservation and selective autotransplantation utilizing topical lidocaine in total thyroidectomy. Laryngoscope 120:1342–1344. [DOI] [PubMed] [Google Scholar]
- 36. Ander S, Johansson K, Smeds S. 1997. In situ preservation of the parathyroid glands during operations on the Thyroid. Eur J Surg 163:33–37. [PubMed] [Google Scholar]
- 37. Ezzat W, Fathey H, Fawaz S, El-Ashri A, Youssef T, Othman H. 2011. Intraoperative parathyroid hormone as an indicator for parathyroid gland preservation in thyroid surgery. Swiss Med Wkly 141: w13299. [DOI] [PubMed] [Google Scholar]
- 38. Chang T-P, Palazzo F, Tolley N, Constantinides V, Yang G-Z, Darzi A. 2014. Vascularity assessment of parathyroid glands using confocal endomicroscopy: towards an intraoperative imaging tool for real-time in situ viability assessment. Eur J Surg Oncol 40:S3. [Google Scholar]
- 39. Fortuny JV, Belfontali V, Sadowski SM, Karenovics W, Guigard S, Triponez F. 2016. Parathyroid gland angiography with indocyanine green fluorescence to predict parathyroid function after thyroid surgery. Br J Surg 103:537–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vidal Fortuny J, Karenovics W, Triponez F, Sadowski SM. 2016. Intra-operative indocyanine green angiography of the parathyroid gland. World J Surg 40:2378–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Demarchi MS, Karenovics W, Bédat B, Triponez F. 2020. Intraoperative autofluorescence and indocyanine green angiography for the detection and preservation of parathyroid glands. J Clin Med 9:830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vidal Fortuny J, Sadowski SM, Belfontali V, Guigard S, Poncet A, Ris F, Karenovics W, Triponez F. 2018. Randomized clinical trial of intraoperative parathyroid gland angiography with indocyanine green fluorescence predicting parathyroid function after thyroid surgery. Br J Surg 105:350–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zaidi N, Bucak E, Yazici P, Soundararajan S, Okoh A, Yigitbas H, Dural C, Berber E. 2016. The feasibility of indocyanine green fluorescence imaging for identifying and assessing the perfusion of parathyroid glands during total thyroidectomy. J Surg Oncol 113:775–778. [DOI] [PubMed] [Google Scholar]
- 44. Gálvez-Pastor S, Torregrosa NM, Ríos A, et al. 2019. Prediction of hypocalcemia after total thyroidectomy using indocyanine green angiography of parathyroid glands: a simple quantitative scoring system. Am J Surg 218:993–999. [DOI] [PubMed] [Google Scholar]
- 45. Razavi AC, Ibraheem K, Haddad A, Saparova L, Shalaby H, Abdelgawad M, Kandil E. 2019. Efficacy of indocyanine green fluorescence in predicting parathyroid vascularization during thyroid surgery. Head Neck 41:3276–3281. [DOI] [PubMed] [Google Scholar]
- 46. Chu W, Chennamsetty A, Toroussian R, Lau C. 2017. Anaphylactic shock after intravenous administration of indocyanine green during robotic partial nephrectomy. Urol Case Rep 12:37–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lang BHH, Wong CKH, Hung HT, Wong KP, Mak KL, Au KB. 2017. Indocyanine green fluorescence angiography for quantitative evaluation of in situ parathyroid gland perfusion and function after total thyroidectomy. Surgery 161:87–95. [DOI] [PubMed] [Google Scholar]
- 48. Fercher AF, Briers JD. 1981. Flow visualization by means of single-exposure speckle photography. Opt Commun 37:326–330. [Google Scholar]
- 49. Boas DA, Dunn AK. 2010. Laser speckle contrast imaging in biomedical optics. J Biomed Opt 15:011109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mannoh EA, Thomas G, Solórzano CC, Mahadevan-Jansen A. 2017. Intraoperative assessment of parathyroid viability using laser speckle contrast imaging. Sci Rep 7:14798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Julián MT, Balibrea JM, Granada ML, Moreno P, Alastrué A, Puig-Domingo M, Lucas A. 2013. Intact parathyroid hormone measurement at 24 hours after thyroid surgery as predictor of parathyroid function at long term. Am J Surg 206:783–789. [DOI] [PubMed] [Google Scholar]
- 52. Anastasiou OE, Yavropoulou MP, Papavramidis TS, Tzouvara C, Triantafyllopoulou K, Papavramidis S, Yovos JG. 2012. Secretory capacity of the parathyroid glands after total thyroidectomy in normocalcemic subjects. J Clin Endocrinol Metab 97:2341–2346. [DOI] [PubMed] [Google Scholar]
- 53. Mohebati A, Shaha AR. 2012. Anatomy of thyroid and parathyroid glands and neurovascular relations. Clin Anat 25:19–31. [DOI] [PubMed] [Google Scholar]
- 54. Mannoh EA, Luo M, Thomas G, Solórzano CC, Mahadevan-Jansen A 2019 A combined autofluorescence and laser speckle contrast imaging system for parathyroid surgical guidance (Conference Presentation). In: Mahadevan-Jansen, A (ed) Advanced Biomedical and Clinical Diagnostic and Surgical Guidance Systems XVII. SPIE, San Francisco, CA, USA. [Google Scholar]
- 55. Oh E, Kim WW, Nam S-H, Cheon GW, Ning B, Cha J. 2020. Development of a portable imager for intraoperative real-time localization of parathyroid glands. In: Mahadevan-Jansen, A (ed) Advanced Biomedical and Clinical Diagnostic and Surgical Guidance Systems XVIII. SPIE, San Francisco, CA, USA. [Google Scholar]
- 56. Mannoh EA, Parker LB, Thomas G, Solórzano CC, Mahadevan-Jansen A. 2021. Development of an imaging device for label-free parathyroid gland identification and vascularity assessment. J Biophotonics 14:e202100008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.