Abstract
Prepubertal obesity (PPO) has emerged as a major health problem over the past few decades and is a risk factor for the development of proteinuria. The current study investigated whether the development of renal injury in the obese SSLepR mutant strain occurs before puberty. When determining the temporal changes in serum sex hormones in female and male SS and SSLepR mutant rats between 4 and 10 wk of age, we only observed significant increases in estradiol and testosterone levels in female and male SS rats at 10 wk of age than at 4 wk of age. The results suggest that studying both strains between 4 and 8 wk of age is appropriate to study the effects of PPO on renal injury in this model. Proteinuria was significantly higher in SSLepR mutant rats as opposed to the values observed in SS rats at 8 wk of age, and we did not observe any sex differences in proteinuria in either strain. The kidneys from the SSLepR mutant rats displayed significant glomerular and tubular injury and renal fibrosis versus the values measured in SS rats without any sex differences. Overall, we observed increased immune cell infiltration in the kidneys from SSLepR mutant rats compared with SS rats. Interestingly, female SSLepR mutant rats displayed significant increases in not only M1 macrophages (proinflammatory) but also M2 macrophages (anti-inflammatory) versus male SSLepR mutant rats. These results suggest the SSLepR mutant rat may be a useful model to study early progression of obesity-related renal injury before the onset of puberty.
Keywords: obesity, renal disease, sex, SSLepR mutant strain, M1 and M2 macrophages
INTRODUCTION
Childhood/prepubertal obesity (PPO) is a major public health problem in the United States and has dramatically increased over the last few decades (1, 2). PPO is associated with major clinical consequences, including cardiovascular disease such as diabetes and hypertension, which are the two leading causes for renal disease (3, 4). With the rising prevalence of obesity in children, studies examining how obesity, before the occurrence of diabetes/hyperglycemia and/or hypertension, contributes to the development of proteinuria and renal injury in children are lacking. Therefore, there is a critical need to understand the early mechanisms involved in the early development and progression of renal disease associated with PPO. Moreover, the mechanisms involved remain unclear due to a lack of obese animal models that mimic the progression of renal disease in this unique population. The current obese rodent models do not develop renal disease before the development of diabetes and hypertension or before the onset of puberty (5). Previous studies have indicated that the prepubescent stage in a rat is between 3 and 10 wk of age in various models of renal disease including stroke-prone spontaneous hypertensive (SPSHR) and Dahl salt-sensitive (SS) rats (6, 7). Recently, we reported that the obese SS leptin receptor mutant (SSLepR mutant) rat exhibits proteinuria and progressive renal disease before 8 wk of age independent of hyperglycemia and increases in arterial pressure (8, 9). This suggests that the SSLepR mutant rat may be a useful model to study the mechanisms involved in the early development and progression of obesity-induced renal injury before the onset of puberty.
Patients suffering from diabetes- and/or hypertension-induced renal disease have some degree of macrophages and T-cell infiltration into the kidney (10–12). Mattson and colleagues have reported that the development of hypertension-induced renal injury in SS rats is associated with the renal infiltration of macrophages and T cells (10, 13–16). Macrophages are critically involved in the pathogenesis of renal injury, repair, and fibrosis by secreting ROS, growth factors and proinflammatory cytokines in various experimental models of renal disease (17, 18). Macrophages can be polarized and divided into two distinct phenotypes: M1-macrophages (classical; proinflammatory) and M2-macrophages (alternative; anti-inflammatory) (17, 19–21). M1-macrophages induce renal inflammation and fibrosis whereas M2-macrophages protect against renal inflammation and fibrosis. We recently observed increased renal macrophage infiltration in SSLepR mutant rats compared with SS rats, and the depletion of macrophages reduced the early progression of renal injury in SSLepR mutant rats (22). However, we did not determine the composition of the macrophages. Therefore, the goals of the current study were 1) to confirm that the development of renal injury occurs before elevations in sex hormones, and 2) to determine the composition of the renal macrophages during the progression of renal injury in female and male lean SS and obese SSLepR mutant rats during the prepubescent stage.
METHODS
General
Experiments were performed on 132 male and female SS and SSLepR mutant rats between 4 and 10 wk of age (69 males and 63 females). Both strains were obtained from our in-house colony of heterozygous SSLepR mutant rats that were originally created from the Medical College of Wisconsin using zinc figure nuclease technology (8). Genotyping was performed by the Molecular and Genomics Facility at the University of Mississippi Medical Center. The rats had free access to food and water throughout the study. They were fed a 1% NaCl diet (TD8640; Harlan Laboratories, Madison, WI) to minimize the development of hypertension. The rats were housed in the Laboratory Animal Facility at the University of Mississippi Medical Center that was approved by the American Association for the Accreditation of Laboratory Animal Care, and all protocols were approved by the University of Mississippi Medical Center Institutional Animal Care and Use Committee.
Protocol 1: Comparison of the Temporal Changes in Sex Steroidal Hormones in Female and Male SS and SSlepR mutant Rats
Experiments were performed on female and male SS and SSLepR mutant rats at 4, 6, 8, and 10 wk of age to represent a normal time course in which rats would progress to puberty. At each time period, the rats were anesthetized with 2% isoflurane, and the abdominal cavity was opened to collect blood from the aorta. Serum sex steroidal hormone concentrations of estradiol and testosterone were measured by ELISAs (Crystal Chem, IL; estradiol and testosterone).
Protocol 2: Characterization of the Early Development of Renal Injury in Female and Male SS and SSlepR mutant Rats before the Onset of Puberty
These experiments were performed in 4-wk-old female and male SS and SSLepR mutant rats. Telemetry transmitters (Model HD-S10, Data Sciences International, St. Paul, MN) were implanted in the carotid artery and the unit was placed under the skin on the back at 4 wk of age for the measurement of mean arterial pressure (MAP). After 3 days of recovery, MAP was recorded for 3 consecutive days to obtain a baseline MAP at 4 wk of age. Then, the rats were weighed and placed in metabolic cages for an overnight urine collection to determine proteinuria using the Bradford method (Bio-Rad Laboratories; Hercules, CA), and a blood sample was collected from the tail vein for the measurement of blood glucose levels (glucometer from Bayer HealthCare; Mishawaka, IN). Every 2 wk, MAP, proteinuria, and blood glucose levels were measured until the rats reached 8 wk of age. To determine tubular injury, urine concentrations of kidney injury molecule-1 (KIM-1; Abcam; Waltham, MA) and neutrophil gelatinase-associated lipocalin (NGAL; Abcam; Waltham, MA) were measured, and renal function was determined by creatinine clearance (BioAssay Systems; Hayward, CA) at the 8 wk time period. At the end of the study, rats were put under anesthesia, and a vacutainer was placed in the abdominal aorta below the renal arteries to collect a final blood sample to measure plasma cholesterol (Cayman Chemical Company, Ann Arbor, MI), triglycerides (Cayman Chemical Company, Ann Arbor, MI), insulin (Mercodia Rat Insulin ELISA, Uppsala, Sweden), and creatinine concentrations. Next, the kidneys were perfused with saline until they visibly appeared pale. The kidneys were collected and weighed. The right kidney was fixed in a 10% buffered formalin solution for histology, and the left kidney was used to measure the infiltration of immune cells by flow cytometry as previously described (22).
Immune Cell Isolation from the Kidney
Immune cells in the kidney were isolated as described previously (22). Briefly, the left kidney was minced and incubated in RPMI-1640 media containing 0.1% collagenase and 10 µg/mL DNAse I and was later homogenized. The solution was then filtered through sequences of 100-, 70-, and 40-µm cell strainers. Mononuclear cells were separated by Percoll density gradient centrifugation at 400 g for 30 min at room temperature, and then the pellet was washed and resuspended in 1 mL FACS buffer. Next, the cells were counted on an Automated Cell Counter and Image Cytometer (Nexcelom Bioscience, Lawrence, MA).
Flow Cytometry
Mononuclear cells were stained with viobility 405/520 fixable dyes (1:50 dilution; Miltenyi Biotec, Auburn, CA) for 20 min at 4°C to differentiate the dead cells from the live cells. The macrophage panel was established with the following antibodies: anti-rat CD68-APC (1:10 dilution; Miltenyi Biotec, Auburn, CA), anti-rat iNOS (1:100 dilution; Abcam, Cambridge, MA)/goat anti-rabbit Alexa Fluor405 (1:100 dilution; Abcam, Cambridge, MA) for M1 macrophages, and anti-rat CD163-FITC (1:50 dilution; Bio-Rad) for M2 macrophages. The lymphocyte panel was established with the following antibodies: anti-rat CD45-VioBlue (1:10 dilution; Miltenyi Biotec, Auburn, CA) for B cells, anti-rat CD45R-PE-Vio770 (1:10 dilution; Miltenyi Biotec, Auburn, CA), anti-rat CD3-FITC (1:50 dilution; Miltenyi Biotec, Auburn, CA) for T cells, anti-rat CD4-APC-Vio770 (1:10 dilution; Miltenyi Biotec, Auburn, CA) for helper T cells, and anti-rat CD8a-PerCp-Vio700 (1:50 dilution; Miltenyi Biotec, Auburn, CA) for cytotoxic T cells. Flow cytometry was performed on the Miltenyi MACSQuant Analyzer 10 (Miltenyi Biotec, Auburn, CA), and data were analyzed using FlowLogic software (Miltenyi Biotec, Auburn, CA). The gating strategies are shown in Fig. 1.
Figure 1.
Flow cytometry gating strategies for lymphocytes (A) and macrophages (B) in female and male Dahl salt-sensitive (SS) and obese SS leptin receptor mutant (SSLepR mutant) rats at 8 wk of age. A: after gating for the mononuclear cell population using forward scatter (FSC) and side scatter (SSC), dead cells were excluded using viobility staining, and doublets were excluded. CD45+ staining was used to gate for total lymphocytes. From this population, gates were placed for CD3− cells and CD3+ T cells. CD45R+ B cells were identified in the CD3− cell population, and CD4+ T helper and CD8+ cytotoxic T subsets were identified within the CD3+ T-cell population. B: similar to A, live, singlet CD45+/CD3− cells were gated. Within this population CD68+/iNOS+ M1 macrophages and CD68+/CD163+ M2 macrophages were identified.
Renal Histopathology
Paraffin kidney sections were prepared from half of the kidneys collected from SS and SSLepR mutant rats at each time point. Kidney sections were cut into 3-µm sections and stained with periodic acid-Schiff (PAS) and Masson’s trichrome. Thirty glomeruli per PAS section were scored in a blinded fashion to determine glomerular injury by scoring in a blinded fashion on a 0–4 scale with 0 representing a normal glomerulus, 1 representing a 25% of loss, 2 representing a 50% loss, 3 representing a 75% loss, and 4 representing >75% loss of capillaries in the tuft (8, 23). To determine the degree of renal fibrosis, 10 representative images per section from each animal were captured using a Nikon Eclipse 55i microscope equipped with a Nikon DS-Fi1 color camera (Nikon, Melville, NY). We analyzed for the percentage of the image stained blue (primarily collagen) and thresholded for the blue staining in the Masson’s trichrome-stained sections using NIS-Elements D 3.0 software (8, 23). Next, we used those same thresholding parameters for the blue staining for each image per rat in the study to measure renal fibrosis.
Statistical Analysis
The data are presented as means ± SE. Statistical analysis was performed using GraphPad Prism 8 (GraphPad Software, San Diego, CA). The significance of differences in between strains and sex values for a single timepoint was determined by a two-way ANOVA followed by a Holm–Sidak’s multiple-comparisons test. The time course changes in sex hormone and metabolic and cardiovascular parameter values from baseline, between strains, and sex were determined using three-way ANOVA followed by a Tukey’s multiple-comparisons test. P values of <0.05 were considered significantly different.
RESULTS
Protocol 1: Comparison of the Temporal Changes in Sex Steroidal Hormones in Female and Male SS and SSlepR mutant Rats
Temporal changes in plasma estradiol and testosterone levels.
The time course of changes in the levels of estradiol and testosterone in female and male SS and SSLepR mutant rats are presented in Table 1. At baseline, plasma estradiol and testosterone levels were similar in all groups. At 10 wk of age, the levels of estradiol in female SS rats were significantly higher from baseline (25 ± 5 and 9 ± 2 ng/mL, respectively) and compared with their male counterparts (12 ± 2 ng/mL) and female SSLepR mutant rats (5 ± 1 ng/mL). We did not observe any changes in the levels of estradiol in both male SS and SSLepR mutant rats throughout the study. Similar to estradiol, the levels of testosterone were only significantly elevated from baseline to 10 wk of age in male SS rats (0.6 ± 0.2 and 3.7 ± 0.6 ng/mL, respectively). Moreover, testosterone levels were increased more than twofold in male SS rats compared with female SS rats at 6 wk of age and remained higher throughout the study. Interestingly, we observed a significant increase in testosterone levels in male SSLepR mutant rats versus the values measured in female SSLepR mutant rats at 6 wk of age. However, this difference was lost after 8 wk of age.
Table 1.
Temporal changes in sex hormones in female and male Dahl salt-sensitive (SS) and SS leptin receptor mutant (SSLepR mutant) rats
| Strain and Sex | Estradiol, ng/mL |
|||
|---|---|---|---|---|
| 4 Week | 6 Week | 8 Week | 10 Week | |
| SS female | 9 ± 2 | 11 ± 5 | 13 ± 3 | 25 ± 5* |
| SS male | 13 ± 1 | 14 ± 1 | 9 ± 2 | 12 ± 2# |
| SSLepR mutant female | 9 ± 1 | 7 ± 2 | 7 ± 1 | 5 ± 1† |
| SSLepR mutant male | 12 ± 1 | 10 ± 2 | 10 ± 1 | 12 ± 1 |
| Strain and Sex | Testosterone, ng/mL |
|||
|---|---|---|---|---|
| 4 Week | 6 Week | 8 Week | 10 Week | |
| SS female | 0.34 ± 0.06 | 0.34 ± 0.08 | 0.56 ± 0.07 | 0.46 ± 0.05 |
| SS male | 0.61 ± 0.17 | 1.25 ± 0.17# | 1.75 ± 0.28# | 3.62 ± 0.62*# |
| SSLepR mutant female | 0.42 ± 0.24 | 0.17 ± 0.04 | 0.26 ± 0.04 | 0.34 ± 0.04 |
| SSLepR mutant male | 0.43 ± 0.10 | 1.10 ± 0.22# | 0.65 ± 0.06 | 0.59 ± 0.12† |
Values are means ± SE. *P < 0.05 vs. baseline within the same stain and sex, †P < 0.05 vs. SS rats within the same sex, and #P < 0.05 vs female rats within the same strain. (n = 5–8/group at each time period).
Protocol 2: Characterization of the Early Development of Renal Injury in Female and Male SS and SSlepRmutant Rats before the Onset of Puberty
Comparison of metabolic parameters in female and male SS and SSLepR mutant rats.
Body weight and the levels of glucose, insulin, cholesterol, and triglycerides in female and male SS and SSLepR mutant rats at 8 wk of age are presented in Table 2. We observed significant increases in body weight, insulin, cholesterol, and triglyceride levels in the SSLepR mutant strain compared with the SS strain. Moreover, we did not detect any sex differences in body weight and plasma insulin and cholesterol levels in SS and SSLepR mutant rats. However, triglycerides levels were significantly higher in female SSLepR mutant rats compared with male SSLepR mutant rats (507 ± 52 vs. 323 ± 35 mg/dL, respectively). Similar to our previous studies, there was no difference in blood glucose levels in SS and SSLepR mutant strains (8, 9, 22).
Table 2.
Comparison of metabolic parameters in female and male Dahl salt-sensitive (SS) and SS leptin receptor mutant (SSLepR mutant) rats at 8 wk of age
| Metabolic Parameters |
SS |
SSLepR mutant |
||
|---|---|---|---|---|
| Female | Male | Female | Male | |
| Body weight, g | 234 ± 30 | 313 ± 19 | 328 ± 24† | 380 ± 18† |
| Glucose, mg/dL | 106 ± 4 | 97 ± 2 | 104 ± 4 | 102 ± 8 |
| Insulin, ng/dL | 0.73 ± 0.04 | 0.77 ± 0.04 | 3.48 ± 0.40† | 3.54 ± 0.39† |
| Cholesterol, mg/dL | 89 ± 4 | 94 ± 5 | 173 ± 22† | 172 ± 19† |
| Triglyceride, mg/dL | 58 ± 7 | 86 ± 10 | 507 ± 52† | 323 ± 35†# |
Values are means ± SE. †P < 0.05 vs. SS rats within the same sex, and #P < 0.05 vs. female rats within the same strain. (n = 7–12/group in each parameter).
Temporal changes in MAP and proteinuria.
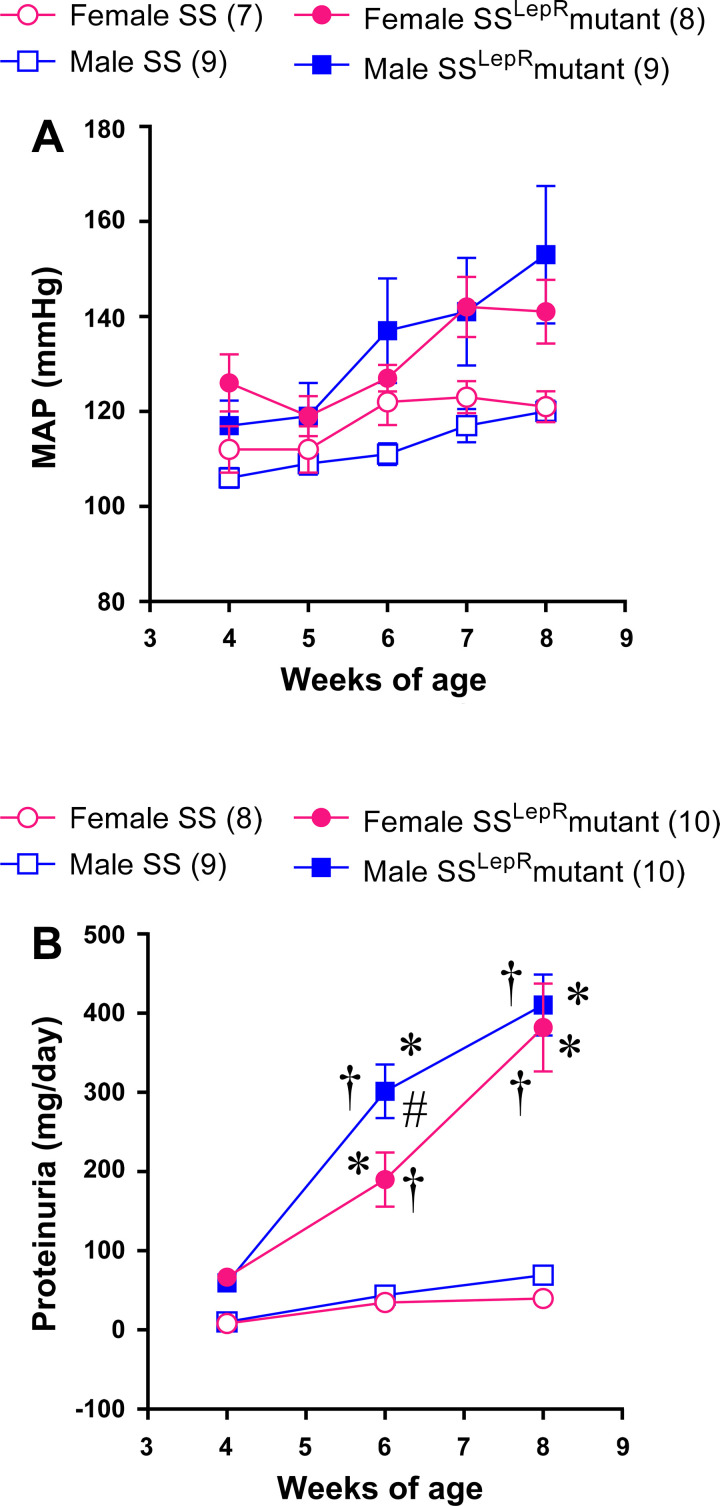
The time course of changes in MAP and proteinuria in female and male SS and SSLepR mutant rats are presented in Fig. 2. At 4 wk of age, MAP was similar in all groups (Fig. 2A). We did not observe any difference in MAP between female and male SS rats throughout the study. Although there was tendency for MAP to increase over the course of the study in female and male SSLepR mutant rats ranging from mild to moderate hypertensive, these values did not reach statistical significance compared with female and male SS rats. When examining sex differences in proteinuria, there was a trend for proteinuria to be elevated in SSLepR mutant rats compared with SS rats at 4 wk of age (Fig. 2B). Over the course of the study, proteinuria was significantly higher in SSLepR mutant rats than in SS rats, but the progression of proteinuria was delayed in female SSLepR mutant rats compared with male SSLepR mutant rats.
Figure 2.
Temporal changes in mean arterial pressure (MAP) (A) and proteinuria (B) in female and male Dahl salt-sensitive (SS) rats and obese SS leptin receptor mutant (SSLepR mutant) rats at 8 wk of age. Numbers of rats studied (n = 7–10/group). Values are means ± SE. Temporal changes in mean arterial pressure and protein excretion were compared between and within strains using three-way ANOVA followed by a Tukey’s multiple-comparisons test. *A significant difference from the corresponding value within the same strain at baseline; †significant difference from the corresponding value in SS rats within the same sex, and #significant difference from the corresponding value in female rats within the same strain.
Glomerular and tubular injury and renal function.
Representative images of glomerular pathology and corresponding analyses of glomerular injury and the measurements of tubular injury and renal function (creatinine clearance; Ccr) in female and male SS and SSLepR mutant rats are represented in Fig. 3. The kidneys from both female and male SSLepR mutant rats exhibited increased mesangial expansion and glomerular injury compared with their SS counterparts (Fig. 3, A and C). In Fig. 3, B and D, renal fibrosis (% blue staining) was markedly increased in the kidneys from both female and male SSLepR mutant rats. In Fig. 3, E and F, KIM-1 and NGAL excretion, markers of tubular injury, were significantly increased in both female and male SSLepR mutant rats compared with their SS littermates. Similarly, when measuring renal function via Ccr, Ccr was more than twofold higher in SSLepR mutant rats compared with SS rats (Fig. 3G). However, we did not detect any sex differences in glomerular and tubular injury, renal fibrosis, and Ccr in SS and SSLepR mutant rats before puberty.
Figure 3.
Representative images and analyses of renal histopathology, markers of tubular injury, and renal function in female and male Dahl salt-sensitive (SS) and obese SS leptin receptor mutant (SSLepR mutant) rats at 8 wk of age. A: periodic acid-Schiff staining. B: Masson’s trichrome staining. C: glomerular injury score. D: renal fibrosis (% blue staining). E: kidney injury molecule-1 (KIM-1). F: neutrophil gelatinase-associated lipocalin (NGAL). G: renal function (creatinine clearance). Numbers of rats studied (n = 6–15/group). Values are means ± SE. Temporal changes in mean arterial pressure and protein excretion were compared between and within strains using a three-way ANOVA followed by the Holm–Sidak test. The significance of the difference in mean values for a single time point was determined by a two-way ANOVA followed by Holm–Sidak’s multiple comparisons test. *A significant difference from the corresponding value within the same strain at baseline; †a significant difference from the corresponding value in SS rats within the same sex.
Measurement of immune cell infiltration.
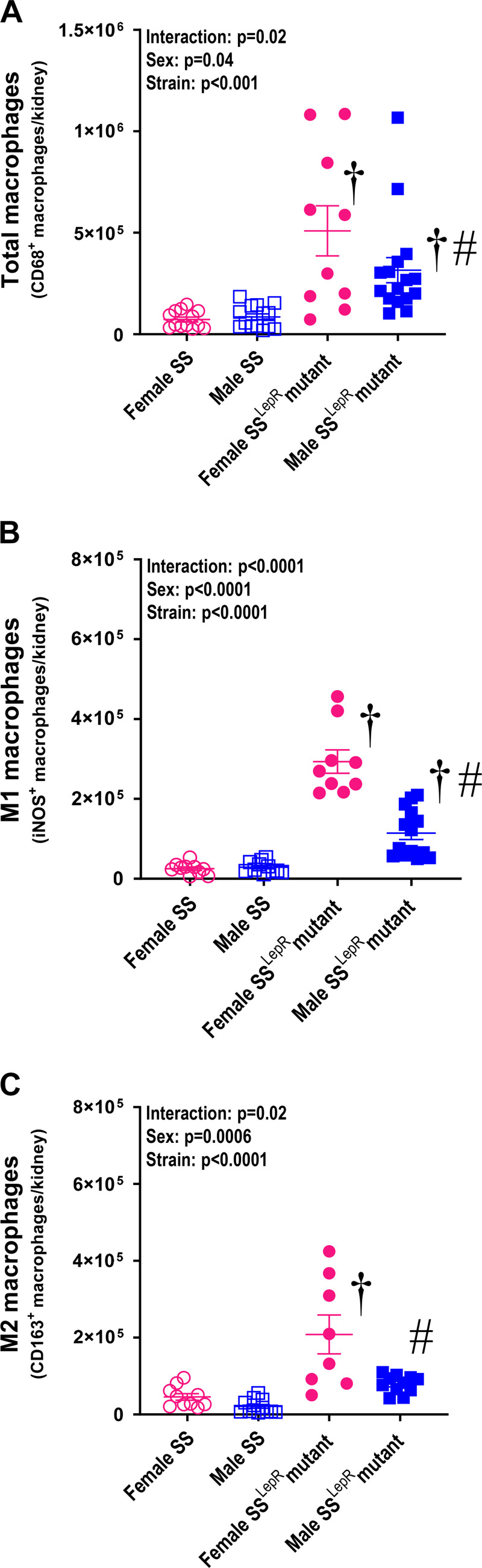
Measurement of renal macrophage and lymphocyte infiltration in female and male SS and SSLepR mutant rats are presented in Fig. 4 and Table 3. We observed a significant increase in total renal and M1 macrophage infiltration in SSLepR mutant rats compared with SS rats (Fig. 4, A and B). Interestingly, total and M1 macrophages were reduced in the kidneys from male SSLepR mutant rats compared with female SSLepR mutant rats. Renal M2 macrophages were significantly higher in female SSLepR mutant compared with female and male SS rats and their male SSLepR mutant counterparts (Fig. 4C). We observed a significant increase in renal leukocytes, B cell, and total T-cell infiltration in SSLepR mutant rats compared with SS rats (Table 3). Although we did not observe any sex differences in renal leukocyte and total T-cell infiltration in SS and SSLepR mutant rats, B cells were markedly higher in female SSLepR mutant rats compared with their male SSLepR mutant counterparts. Helper T cells were more than sixfold and 15-fold higher in male SS and female SSLepR mutant rats than in female SS rats, respectively. The infiltration of helper T cells were increased in male SSLepR mutant rats compared with the values measured in male SS rats, but it did not reach statistical significance. There was a tendency for cytotoxic T cells to be elevated in SSLepR mutant rats compared with SS rats, but the results did not reach statistical significance.
Figure 4.
Comparison of renal macrophage infiltration in female and male Dahl salt-sensitive (SS) and obese SS leptin receptor mutant (SSLepR mutant) rats at 8 wk of age. A: total macrophages (CD68+ cells). B: M1 macrophages (CD68+ and iNOS+ cells). C: M2 macrophages (CD68+ and CD163+ cells). Numbers of rats studied (n = 11–14/group). Values are means ± SE. The significance of the difference in mean values for a single time point was determined by a two-way ANOVA followed by Holm–Sidak’s multiple comparisons test. †A significant difference from the corresponding value in SS rats within the same sex; #a significant difference from the corresponding value in female rats within the same strain.
Table 3.
Comparison of renal lymphocytes infiltration in the kidneys of female and male Dahl salt-sensitive (SS) and SS leptin receptor mutant (SSLepR mutant) rats at 8 wk of age
| Immune Cells(Per Kidney) | SS |
SSLepR mutant |
||
|---|---|---|---|---|
| Female | Male | Female | Male | |
| Leukocytes (CD45+) | 291,718 ± 114,532 | 230,421 ± 29,976 | 1,728,789 ± 342,577† | 2,091,599 ± 392,688† |
| B lymphocytes (CD45+/CD45R+) | 83,566 ± 45,931 | 54,703 ± 8,598 | 800,721 ± 203,864† | 225,977 ± 90,908†# |
| Total T Cells (CD45+/CD3+) | 5,938 ± 1,842 | 29,334 ± 6,676 | 150,246 ± 37,028† | 150,506 ± 44,856† |
| Helper T Cells (CD45+/CD3+/CD4+) | 3,005 ± 1,076 | 19,015 ± 4,482 | 47,430 ± 11,505† | 48,749 ± 14,902 |
| Cytotoxic T Cells (CD45+/CD3+/CD8a+) | 496 ± 160 | 4,959 ± 2,059 | 14,060 ± 4,311 | 21,575 ± 10,671 |
Values are means ± SE. †P < 0.05 vs. SS rats within the same sex, and #P < 0.05 vs. female rats within the same strain. (n = 11–14/group in each parameter).
DISCUSSION
PPO has increased at an alarming rate, and recent studies have reported increases in the presence of proteinuria in obese children (4, 24–26). With PPO on the rise, there is an urgent need to understand the early underlying mechanisms that contribute to the future risk of CKD later in life. The mechanisms involved remain unclear due to a lack of obese animal models that mimic the progression of renal disease in this unique population. Although most obese rodent models develop some form of renal disease after puberty, the onset of renal injury usually occurs after the development of hypertension, diabetes, and/or age-related mechanisms (5). We previously reported that the SSLepR mutant rat displays progressive proteinuria and renal injury as early as 6 wk of age that was associated with increased macrophage infiltration (8, 9, 22, 23). However, we did not determine whether the development of renal injury occurred before the SSLepR mutant rats reached puberty nor did we determine the composition of the renal macrophages (M1 vs. M2). Therefore, the current study investigated whether the development of renal injury occurs before puberty and determined whether there were differences in the infiltration of M1 and M2 macrophages in female and male SSLepR mutant rats. We observed that the development of progressive renal injury occurs before elevations in plasma estradiol and testosterone levels in female and male SSLepR mutant rats. Similar to our previous studies, SSLepR mutant rats developed some characteristics of metabolic syndrome (increased body weight, dyslipidemia, and hyperinsulinemia) without hyperglycemia and elevations in arterial pressure when compared with their lean counterparts. Throughout the study, proteinuria was significantly higher in the SSLepR mutant rats compared with SS rats. Although the progression of proteinuria was delayed in female SSLepR mutant rats compared with their male counterparts, by the end of the study proteinuria was comparable in female versus male SSLepR mutant rats. We also observed that SSLepR mutant rats exhibited increased glomerular filtration rate (GFR) or renal function via Ccr compared with SS rats. Moreover, the degree of renal injury was more prominent in the SSLepR mutant rats compared with SS rats without any sex differences. When measuring immune cells, we observed marked increases in T cells, B cells, macrophages in SSLepR mutant rats versus values measured in SS rats. Interestingly, macrophages (total, M1, and M2) and B cells were significantly increased in the kidneys from female SSLepR mutant rats compared with male SSLepR mutant rats. These results suggest the obese SSLepR mutant strain may be a useful model to study early progression of renal injury before the onset of puberty and to understand underlying proinflammatory mechanisms that may contribute to renal disease associated with obesity.
One of the major findings in the current study is puberty for SS rats is around 10 wk of age. One previous study described the prepubertal stage for SS rats was between 3 and 9 wk of age (7). However, these studies did not measure sex hormone levels to confirm whether the rats had reached puberty. In the current study, we observed both, estradiol and testosterone, to be significantly elevated in SS rats after 10 wk of age compared with 4 wk of age. In contrast, we did not detect any increases in sex hormones in SSLepR mutant rats during the study, which is contradictory to what occurs in children with obesity. Freedman et al. (27) reported that obesity during childhood leads to advanced puberty. One explanation on why we did not observe elevations in sex hormones in SSLepR mutant rats could be the lack of leptin signaling. Leptin is a key hormone in energy homeostasis and has been demonstrated to play a major role in the development of puberty by having a direct effect on the production of sex hormones (28). These results indicate that SS rats reach puberty around 9–10 wk of age and suggest that studying renal disease associated with prepubertal obesity before 10 wk of age may be appropriate in SS and SSLepR mutant rats.
Studies examining the impact of sex on the development of obesity-induced renal injury are limited, especially during the prepubescent stage. Before this study, we did not fully explore the impact of sex on the progression of metabolic and cardiovascular disease in these obese rats during the prepubescent stage (8, 9, 22). Previous reports have demonstrated that female rats are protected from metabolic and cardiovascular disorders before menopause (29, 30). However, in the current study, we did not find any sex differences in the rise in arterial pressure or progression of renal injury between female and male SS and SSLepR mutant rats. Similarly, recent reports demonstrate that arterial pressure is markedly elevated in SS rats fed a high-fat (HF) diet when compared with SS rats fed a control diet without displaying any sex differences (31, 32). Furthermore, Fernandes et al. (31) observed that the development of renal injury was significantly reduced in female SS rats versus male SS rats fed a HF diet, which is in contrast to the current study. One interesting finding that we did observe was the delay in the progression of proteinuria at 6 wk of age in female SSLepR mutant rats compared with male SSLepR mutant rats. However, by the end of the study, proteinuria in female SSLepR mutant rats is similar to their male obese counterparts. This may be due to the potential delay in weight gain in female SSLepR mutant rats compared with male SSLepR mutant rats, which may have had an impact on GFR. One of the key factors that influences proteinuria is GFR, in which increased proteinuria is proportional to elevations in GFR during the early stages of renal disease associated with obesity. In the current study, body weight and GFR (Ccr) are similar in female and male SSLepR mutant rats by 8 wk of age. To support this claim, previous studies have demonstrated that weight loss significantly reduces GFR (33–37). These results suggest that there are no major sex differences when examining the progression of obesity-induced renal injury before puberty in SSLepR mutant rats.
The infiltration of immune cells has been associated with renal disease in both preclinical and clinical studies. Moreover, Mattson and colleagues have reported extensively that the development of renal injury in SS rats is associated with the infiltration of immune cells including T cells, B cells, monocytes, and macrophages (10, 13–16). However, these studies focused strictly on hypertension-induced renal disease in SS rats fed high-salt (HS) diet and did not determine whether there were sex differences. Studies characterizing the infiltration of immune cells during the development of renal injury associated with obesity are limited. In the current study, the kidneys from female and male SSLepR mutant rats had increased total lymphocytes, T cells, B cells, and macrophages versus female and male SS rats. However, the most intriguing observation was the increased infiltration of total macrophages in the female SSLepR mutant rats compared with their obese male counterparts. We would have predicted that total and M1 macrophages would be increased in the male SSLepR mutant rats compared with female SSLepR mutant rats. In addition, M1 and M2 macrophages were elevated in female SSLepR mutant rats versus the other groups in the study. In contrast, Fernandes et al. (31) observed increased total macrophages in male SS rats compared with female rats fed a HF diet for more than 17 wk. The differences between the two studies can be attributed to the differences in age and the type of obesity (HF diet vs. genetic—lack of leptin signaling). In the current study, the elevations in renal total, M1, and M2 macrophages in female SSLepR mutant rats versus their obese male counterparts can be potentially explained by the early delay in renal injury at 6 wk of age (i.e., proteinuria) observed in the female SSLepR mutant rats. The polarization and the amount of macrophages in the kidney may have been influenced by the delay, but this needs further investigation. Furthermore, we observed a tendency for increased cytotoxic T cells in male versus female SSLepR mutant rats, which may indicate that the early progression of renal injury may be T-cell driven in male SSLepR mutant rats and macrophage driven in female SSLepR mutant rats. Another finding was increased B cells in the kidneys from SSLepR mutant rats compared with lean SS rats. B cells have been implicated to play a significant role in the pathology of lupus nephritis (38–41), but their role in the progression of renal injury associated with obesity remains unclear. Taken together, these results demonstrate that the early progression of obesity-induced renal injury is associated with increased immune cell infiltration. Moreover, future studies are needed to target the pathways of these immune cells during renal injury related to obesity.
The overall goal of the current study was to investigate whether the development of renal injury in the obese SSLepR mutant strain occurs before puberty. Although the results confirm that the SSLepR mutant strain does develop renal injury before puberty, there are some limitations of the study that should be noted. One limitation is that we did not make sure that female rats were on similar estrus cycles, which may have had an effect on the plasma levels of the sex hormones. However, individual data from female rats were closely related. An additional limitation is not performing time course measurements on the infiltration of immune cells during progression of renal injury. This could have explained why we observed differences between female and male SSLepR mutant rats, since the progression of proteinuria was delayed in the female SSLepR mutant rats. Future studies will be designed to consider these limitations.
Translational Perspective
Obesity is associated with the two most common causes of renal disease, hypertension, and diabetes. However, renal dysfunction starts before the development of hypertension and diabetes. Moreover, obese children are at great risk factor of developing proteinuria in childhood and CKD later in life. In the current study, we confirmed the obese SSLepR mutant strain develops renal disease before puberty independent of diabetes and significant elevations in arterial pressure. In addition, we observed a delay in progressive proteinuria in female versus male SSLepR mutant rats. Although there were minimal sex differences in the SSLepR mutant strain, it represents an ample model to study mechanisms involved in the progression of renal disease associated with PPO. From the current study, immune cell and inflammatory pathways may be playing a significant role in the development and progression of renal injury in the obese SSLepR mutant rat. In support of this suggestion, we recently observed that depleting macrophages during this unique time period slowed the progression of proteinuria and renal injury in SSLepR mutant rats (22). Therefore, further studies examining these inflammatory pathways during PPO are needed to develop novel therapeutic targets for the treatment of renal disease in this distinct population.
GRANTS
This work was supported by the National Institutes of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (NIH/NIDDK) Grant DK109133 (to J.M.W.), the National Heart, Lung and Blood Institute of the National Institutes of Health (NIH/NHLBI) Grant HL151407 (to D.C.C.). The work performed through the UMMC Molecular and Genomics Facility is supported, in part, by funds from the NIGMS, including Mississippi INBRE (P20GM103476), Obesity, Cardiorenal and Metabolic Diseases-COBRE (P20GM104357), and Mississippi Center of Excellence in Perinatal Research (MS-CEPR)-COBRE (P20GM121334).
DISCLAIMERS
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
B.P., C.A.S., U.S.E., and J.M.W. conceived and designed research; B.P., C.A.S., U.S.E., A.K.B., O.K.T., J.C.M., S.F., S.V.S., D.C.C., and J.M.W. performed experiments; B.P., C.A.S., U.S.E., A.K.B., O.K.T., J.C.M., S.F., S.V.S., D.C.C., and J.M.W. analyzed data; B.P., C.A.S., U.S.E., A.K.B., O.K.T., J.C.M., S.F., S.V.S., D.C.C., and J.M.W. interpreted results of experiments; B.P., C.A.S., U.S.E., A.K.B., J.C.M., S.V.S., D.C.C., and J.M.W. prepared figures; B.P., C.A.S., U.S.E., A.K.B., S.F., D.C.C., and J.M.W. drafted manuscript; B.P., C.A.S., U.S.E., A.K.B., and J.M.W. edited and revised manuscript; B.P., C.A.S., U.S.E., A.K.B., O.K.T., J.C.M., S.F., S.V.S., D.C.C., and J.M.W. approved final version of manuscript.
REFERENCES
- 1.Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of obesity among adults and youth: United States, 2011-2014. NCHS Data Brief : 1–8, 2015. [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Lawman HG, Fryar CD, Kruszon-Moran D, Kit BK, Flegal KM. Trends in obesity prevalence among children and adolescents in the United States, 1988-1994 through 2013-2014. JAMA 315: 2292–2299, 2016. doi: 10.1001/jama.2016.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanyaolu A, Okorie C, Qi X, Locke J, Rehman S. Childhood and adolescent obesity in the United States: a public health concern. Glob Pediatr Health 6: 2333794X19891305, 2019. doi: 10.1177/2333794X19891305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burgert TS, Dziura J, Yeckel C, Taksali SE, Weiss R, Tamborlane W, Flegal KM. Microalbuminuria in pediatric obesity: prevalence and relation to other cardiovascular risk factors. Int J Obes (Lond) 30: 273–280, 2006. doi: 10.1038/sj.ijo.0803136. [DOI] [PubMed] [Google Scholar]
- 5.McPherson KC, Shields CA, Poudel B, Fizer B, Pennington A, Szabo-Johnson A, Thompson WL, Cornelius DC, Williams JM. Impact of obesity as an independent risk factor for the development of renal injury: implications from rat models of obesity. Am J Physiol Renal Physiol 316: F316–F327, 2019. doi: 10.1152/ajprenal.00162.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakaya H, Sasamura H, Hayashi M, Saruta T. Temporary treatment of prepubescent rats with angiotensin inhibitors suppresses the development of hypertensive nephrosclerosis. J Am Soc Nephrol 12: 659–666, 2001. doi: 10.1681/ASN.V124659. [DOI] [PubMed] [Google Scholar]
- 7.Nakaya H, Sasamura H, Mifune M, Shimizu-Hirota R, Kuroda M, Hayashi M, Saruta T. Prepubertal treatment with angiotensin receptor blocker causes partial attenuation of hypertension and renal damage in adult Dahl salt-sensitive rats. Nephron 91: 710–718, 2002. doi: 10.1159/000065035. [DOI] [PubMed] [Google Scholar]
- 8.McPherson KC, Taylor L, Johnson AC, Didion SP, Geurts AM, Garrett MR, Williams JM. Early development of podocyte injury independently of hyperglycemia and elevations in arterial pressure in nondiabetic obese Dahl SS leptin receptor mutant rats. Am J Physiol Renal Physiol 311: F793–F804, 2016. doi: 10.1152/ajprenal.00590.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McPherson KC, Shields CA, Poudel B, Johnson AC, Taylor L, Stubbs NA, Nichols A, Cornelius DC, Garrett MR, Williams JM. Altered renal hemodynamics is associated with glomerular lipid accumulation in obese Dahl salt-sensitive leptin receptor mutant rats. Am J Physiol Renal Physiol 318: F911–F921, 2020. doi: 10.1152/ajprenal.00438.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mattson DL. Infiltrating immune cells in the kidney in salt-sensitive hypertension and renal injury. Am J Physiol Renal Physiol 307: F499–F508, 2014. doi: 10.1152/ajprenal.00258.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.You H, Gao T, Cooper TK, Brian Reeves W, Awad AS. Macrophages directly mediate diabetic renal injury. Am J Physiol Renal Physiol 305: F1719–F1727, 2013. doi: 10.1152/ajprenal.00141.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng Z, Zheng F. Immune cells and inflammation in diabetic nephropathy. J Diabetes Res 2016: 1841690, 2016. doi: 10.1155/2016/1841690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fehrenbach DJ, Abais-Battad JM, Dasinger JH, Lund H, Mattson DL. Salt-sensitive increase in macrophages in the kidneys of Dahl SS rats. Am J Physiol Renal Physiol 317: F361–F374, 2019. doi: 10.1152/ajprenal.00096.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alsheikh AJ, Dasinger JH, Abais-Battad JM, Fehrenbach DJ, Yang C, Cowley AW Jr, Mattson DL. CCL2 mediates early renal leukocyte infiltration during salt-sensitive hypertension. Am J Physiol Renal Physiol 318: F982–F993, 2020. doi: 10.1152/ajprenal.00521.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fehrenbach DJ, Dasinger JH, Lund H, Zemaj J, Mattson DL. Splenocyte transfer exacerbates salt-sensitive hypertension in rats. Exp Physiol 105: 864–875, 2020. doi: 10.1113/EP088340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fehrenbach DJ, Mattson DL. Inflammatory macrophages in the kidney contribute to salt-sensitive hypertension. Am J Physiol Renal Physiol 318: F544–F548, 2020. doi: 10.1152/ajprenal.00454.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao Q, Wang Y, Harris DC. Macrophage heterogeneity, phenotypes, and roles in renal fibrosis. Kidney Int Suppl (2011) 4: 16–19, 2014. doi: 10.1038/kisup.2014.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adamiec-Mroczek J, Oficjalska-Młyńczak J. Assessment of selected adhesion molecule and proinflammatory cytokine levels in the vitreous body of patients with type 2 diabetes–role of the inflammatory-immune process in the pathogenesis of proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol 246: 1665–1670, 2008. doi: 10.1007/s00417-008-0868-6. [DOI] [PubMed] [Google Scholar]
- 19.Hesketh M, Sahin KB, West ZE, Murray RZ. Macrophage phenotypes regulate scar formation and chronic wound healing. Int J Mol Sci 18: 1545, 2017. doi: 10.3390/ijms18071545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rőszer T. Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediators Inflamm 2015: 816460, 2015. doi: 10.1155/2015/816460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 25: 677–686, 2004. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 22.Poudel B, Shields CA, Brown AK, Ekperikpe U, Johnson T, Cornelius DC, Williams JM. Depletion of macrophages slows the early progression of renal injury in obese Dahl salt-sensitive leptin receptor mutant rats. Am J Physiol Renal Physiol 318: F1489–F1499, 2020. doi: 10.1152/ajprenal.00100.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spires D, Poudel B, Shields CA, Pennington A, Fizer B, Taylor L, McPherson KC, Cornelius DC, Williams JM. Prevention of the progression of renal injury in diabetic rodent models with preexisting renal disease with chronic endothelin A receptor blockade. Am J Physiol Renal Physiol 315: F977–F985, 2018. doi: 10.1152/ajprenal.00182.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Savino A, Pelliccia P, Chiarelli F, Mohn A. Obesity-related renal injury in childhood. Horm Res Paediatr 73: 303–311, 2010. doi: 10.1159/000308161. [DOI] [PubMed] [Google Scholar]
- 25.Fowler SM, Kon V, Ma L, Richards WO, Fogo AB, Hunley TE. Obesity-related focal and segmental glomerulosclerosis: normalization of proteinuria in an adolescent after bariatric surgery. Pediatr Nephrol 24: 851–855, 2009. doi: 10.1007/s00467-008-1024-6. [DOI] [PubMed] [Google Scholar]
- 26.Csernus K, Lanyi E, Erhardt E, Molnar D. Effect of childhood obesity and obesity-related cardiovascular risk factors on glomerular and tubular protein excretion. Eur J Pediatr 164: 44–49, 2005. doi: 10.1007/s00431-004-1546-2. [DOI] [PubMed] [Google Scholar]
- 27.Freedman DS, Wang J, Maynard LM, Thornton JC, Mei Z, Pierson RN, Dietz WH, Horlick M. Relation of BMI to fat and fat-free mass among children and adolescents. Int J Obes (Lond) 29: 1–8, 2005. doi: 10.1038/sj.ijo.0802735. [DOI] [PubMed] [Google Scholar]
- 28.Garcia-Mayor RV, Andrade MA, Rios M, Lage M, Dieguez C, Casanueva FF. Serum leptin levels in normal children: relationship to age, gender, body mass index, pituitary-gonadal hormones, and pubertal stage. J Clin Endocrinol Metab 82: 2849–2855, 1997. doi: 10.1210/jc.82.9.2849. [DOI] [PubMed] [Google Scholar]
- 29.Murphy E, Lagranha C, Deschamps A, Kohr M, Nguyen T, Wong R, Sun J, Steenbergen C. Mechanism of cardioprotection: what can we learn from females? Pediatr Cardiol 32: 354–359, 2011. doi: 10.1007/s00246-010-9877-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silbiger SR. Raging hormones: gender and renal disease. Kidney Int 79: 382–384, 2011. doi: 10.1038/ki.2010.474. [DOI] [PubMed] [Google Scholar]
- 31.Fernandes R, Garver H, Harkema JR, Galligan JJ, Fink GD, Xu H. Sex differences in renal inflammation and injury in high-fat diet-fed dahl salt-sensitive rats. Hypertension 72: e43–e52, 2018. doi: 10.1161/HYPERTENSIONAHA.118.11485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor LE, Gillis EE, Musall JB, Baban B, Sullivan JC. High-fat diet-induced hypertension is associated with a proinflammatory T cell profile in male and female Dahl salt-sensitive rats. Am J Physiol Heart Circ Physiol 315: H1713–H1723, 2018. doi: 10.1152/ajpheart.00389.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Navarro-Díaz M, Serra A, Romero R, Bonet J, Bayés B, Homs M, Pérez N, Bonal J. Effect of drastic weight loss after bariatric surgery on renal parameters in extremely obese patients: long-term follow-up. J Am Soc Nephrol 17: S213–S217, 2006. doi: 10.1681/ASN.2006080917. [DOI] [PubMed] [Google Scholar]
- 34.Serpa Neto A, Bianco Rossi FM, Dal Moro Amarante R, Alves Buriti N, Cunha Barbosa Saheb G, Rossi M. Effect of weight loss after Roux-en-Y gastric bypass, on renal function and blood pressure in morbidly obese patients. J Nephrol 22: 637–646, 2009. [PubMed] [Google Scholar]
- 35.Friedman AN, Moe S, Fadel WF, Inman M, Mattar SG, Shihabi Z, Quinney SK. Predicting the glomerular filtration rate in bariatric surgery patients. Am J Nephrol 39: 8–15, 2014. doi: 10.1159/000357231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saliba J, Kasim NR, Tamboli RA, Isbell JM, Marks P, Feurer ID, Ikizler A, Abumrad NN. Roux-en-Y gastric bypass reverses renal glomerular but not tubular abnormalities in excessively obese diabetics. Surgery 147: 282–287, 2010. doi: 10.1016/j.surg.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chagnac A, Weinstein T, Herman M, Hirsh J, Gafter U, Ori Y. The effects of weight loss on renal function in patients with severe obesity. J Am Soc Nephrol 14: 1480–1486, 2003. doi: 10.1097/01.ASN.0000068462.38661.89. [DOI] [PubMed] [Google Scholar]
- 38.Chang A, Henderson SG, Brandt D, Liu N, Guttikonda R, Hsieh C, Kaverina N, Utset TO, Meehan SM, Quigg RJ, Meffre E, Clark MR. In situ B cell-mediated immune responses and tubulointerstitial inflammation in human lupus nephritis. J Immunol 186: 1849–1860, 2011. doi: 10.4049/jimmunol.1001983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Obrișcă B, Sorohan B, Tuță L, Ismail G. Advances in lupus nephritis pathogenesis: from bench to bedside. Int J Mol Sci 22: 3766, 2021. doi: 10.3390/ijms22073766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shen Y, Sun C-Y, Wu F-X, Chen Y, Dai M, Yan Y-C, Yang C-D. Association of intrarenal B-cell infiltrates with clinical outcome in lupus nephritis: a study of 192 cases. Clin Dev Immunol 2012: 967584, 2012. doi: 10.1155/2012/967584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steinmetz OM, Velden J, Kneissler U, Marx M, Klein A, Helmchen U, Stahl RA, Panzer U. Analysis and classification of B-cell infiltrates in lupus and ANCA-associated nephritis. Kidney Int 74: 448–457, 2008. doi: 10.1038/ki.2008.191. [DOI] [PubMed] [Google Scholar]