Abstract
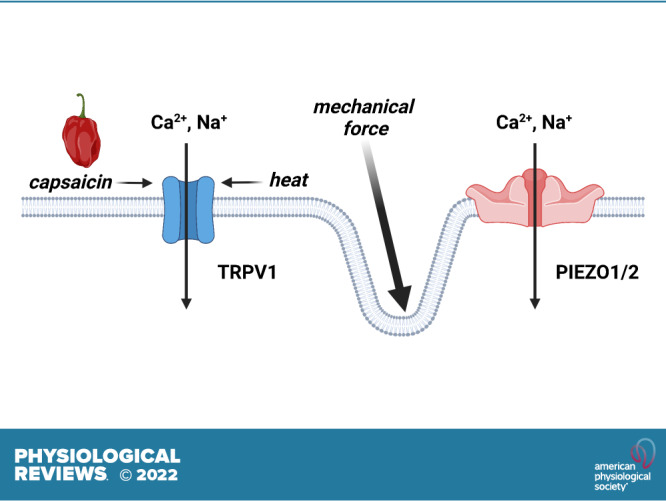
Keywords: Nobel Prize, Piezo channels, pressure, temperature, TRP channels
1. INTRODUCTION
The ability to detect, process, and react to light, sound, temperature, pressure, and other environmental signals is a necessary and defining characteristic of life. Elucidating the mechanistic basis of these essential processes has occupied many outstanding scientists for centuries. The 2021 Nobel Prize in Physiology or Medicine was awarded to David Julius and Ardem Patapoutian to honor their discovery of the fundamental sensors of temperature and pressure. Contrary to reports in the popular press, their breakthroughs are not about the conscious perception of pain, heat, and touch. Instead, the impact of their findings transcends the sensory nervous system and gets to the essence of how all cells sense rapid changes in their internal and external environment, shifting the paradigm of how we think about biological signaling in all tissues. The heart of their work is the molecular cloning and characterization of two new types of ion channels: thermally sensitive transient receptor potential (TRP) channels and pressure-sensing Piezo channels.
2. TEMPERATURE-SENSING TRP CHANNELS
Life on Earth can exist at temperatures between −15°C and 122°C, and all organisms detect and adapt to changes in temperature. In mammals, noxious heat is perceived by peripheral pain-sensing (nociceptive) neurons, but the primary mechanism of temperature detection remained unknown until the late 1990s. The Julius laboratory took up this question and based their seminal investigation on the observation that capsaicin, a substance found in chili peppers, produces a burning sensation when topically applied. Reasoning that the receptors for capsaicin and heat could be one and the same, they screened a cDNA library constructed from dorsal root ganglion neurons to isolate a novel gene that, when expressed in a heterologous system (HEK cells), produced cation currents and Ca2+ influx in response to capsaicin (1). The identified gene was found to encode a novel Ca2+-permeable, nonselective cation channel that was ultimately named transient receptor potential (TRP) vanilloid 1 (TRPV1). Notably, the Julius team showed that moderate heat (>40°C) also activated recombinant TRPV1 channels expressed in HEK cells (or Xenopus oocytes), demonstrating for the first time that TRPV1 is a fundamental sensor of pain-inducing heat. The same group subsequently used comparative genomic DNA sequence analysis to discover the closely related channel, TRPV2 (2). TRPV2 channels are insensitive to capsaicin but are activated at higher temperatures (∼45°C–53°C) than those required for TRPV1 activity. A third fundamental study from the Julius laboratory used an expression cloning strategy to discover that a member of the melastatin (M) TRP subfamily, TRPM8, is activated by menthol as well as by cool temperatures (∼8°C–28°C) (3). At about the same time, the Patapoutian laboratory identified and characterized TRPM8 using in silico analysis of genomic DNA databases (4). Shortly thereafter, Patapoutian and his team also discovered a fourth temperature-sensing channel, TRP ankyrin 1 (TRPA1), using a bioinformatics approach (5). TRPA1 is activated by noxious cold temperatures (<10°C) that are lower than the threshold of TRPM8, although some studies suggest that the thermosensitivity of TRPA1 is species specific. TRPV3 channels, also first described by the Patapoutian group, are activated by warm temperatures above 35°C (6). The physiological significance of thermo-sensing by TRP channels has been demonstrated with knockout mice; for example, TRPM8-knockout mice lose the ability to distinguish between warm (20°C) and cool (15°C) temperatures. Together, these studies conclusively show that specific sets of TRP channels detect temperatures ranging from <10°C to >50°C.
How are TRP channels activated by changes in temperature at the molecular level? Studies using Xenopus oocyte expression and cell-free systems (7) provide substantial evidence that temperature acts directly on TRPV1 channels themselves and not through second messenger pathways. The other thermosensitive TRP channels are thought to operate in this way. All TRP channels are expressed as polypeptide subunits with six-transmembrane pore-forming segments (reminiscent of voltage-dependent K+ channels) and large intracellular amino and carboxy termini containing diverse regulatory domains. Functional TRP channels are formed by the assembly of four subunits (FIGURE 1A).
FIGURE 1.
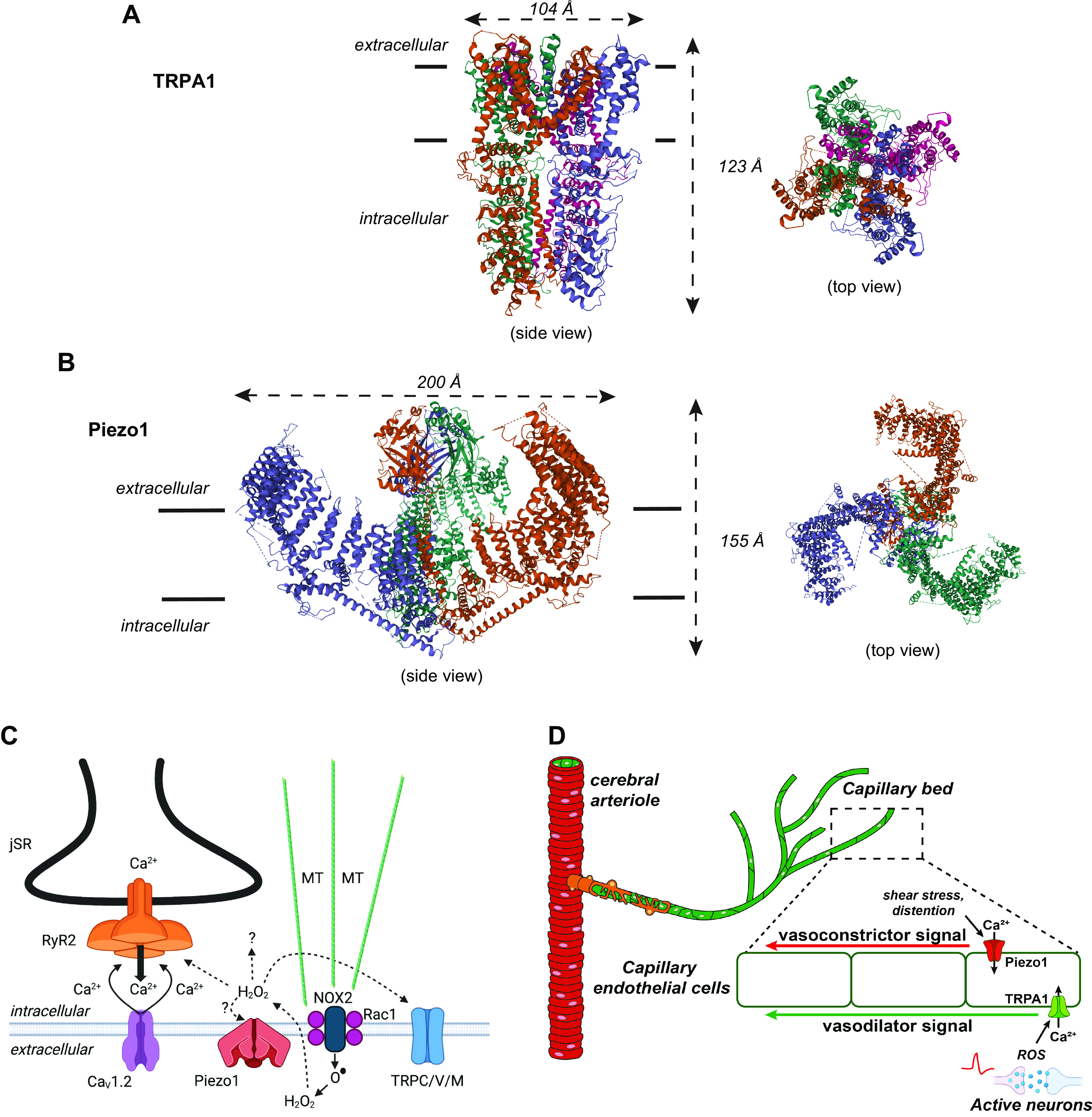
Structure and organization of the Nobel detection systems. A: transient receptor potential (TRP) ankyrin 1 (TRPA1): 3-dimensional (3-D) molecular structure: side or transverse view (left) and top view: outside-in (right). The 4-fold symmetry of the TRPA1 channel is shown in the top view. The TRPA1 structure was originally reported by Paulsen et al. (28). B: Piezo1: 3-D molecular structure: side or transverse view (left) and top view: outside-in (right). The top view shows the unusual triskelion or three-legged shape, which bows through the extracellular side of the plasma membrane, as is evident in the side view. The Piezo1 structure was originally reported by Ge et al. (29). C: TRP and Piezo channels in the X-ROS signaling pathway: hybrid, chemomechanical signaling in heart, skeletal muscle, bone, cancer. Diagram shows elements of X-ROS signaling in the heart. Stretch-dependent signaling in the heart is enabled through microtubule (MT) connections with membrane structures, presumably with the Rac1 subunit of NADPH-oxidase-2 ensemble (NOX-2) to generate extracellular superoxide (), which is rapidly dismuted to H2O2 and can enter the cell through water channels where local oxidation occurs. Piezo1 was recently shown to contribute to X-ROS signaling possibly activated by sarcolemmal membrane distortions or reactive oxygen species (ROS). Piezol may conduct Ca2+ to activate Ca2+ sparks from the junctional sarcoplasmic reticulum (jSR). Multiple intracellular ROS targets include diverse TRP channels, ryanodine receptors (RyR2), diverse kinases, and other proteins. This ROS signaling is local and rapidly reversible. D: complementary roles of TRPA1 and Piezo1 channels during neurovascular coupling: ROS produced by active brain neurons stimulates TRPA1 channels on capillary endothelial cells to initiate a propagating signal that dilates the upstream arteriole and increases regional blood flow. Subsequent activation of Piezo1 channels by increased shear stress and/or capillary distension may orchestrate a counterbalancing propagating signal that re-constricts the upstream arteriole to terminate the increase in blood flow. C was created with BioRender, with permission.
Considerable effort has been devoted to mutagenesis studies designed to identify “temperature-sensing domains” encoded by thermally sensitive TRP channels. Unexpectedly, this approach revealed that multiple regions encompassing nearly the entire length of TRP subunit proteins can affect temperature sensitivity. New insight into this question was provided by a recent cryo-electron microscopy (cryo-EM) study comparing high-resolution structures of TRPV3 channels in the thermally closed (4°C) and heat-activated state (8), which suggested a two-step process for heat activation of TRPV3. In the first step, lipid withdrawal from the vanilloid binding site pocket occurs in association with large-scale conformational changes in multiple regions, including the S2-S3 linker domain and intracellular termini, leading to temperature-dependent sensitization. The second step involves opening of sensitized channels in response to heat in association with further structural changes, including a widening of the pore and a shift in the position of the TRP domain. These findings are largely congruent with the outcomes of mutagenesis studies in showing that temperature sensing in TRPV3 is a broadly distributed function, although further investigations of this kind will be needed to confirm that this temperature-sensing mechanism is fundamental to all thermally sensitive TRP channels.
3. MECHANICAL-SENSING PIEZO CHANNELS
Virtually all prokaryotic and eukaryotic cells can generate electrical signals in response to mechanical stress. The mechanisms conferring mechanosensitivity are well understood and seemingly simple in bacteria, where mechanosensitive channels open in response to increases in membrane tension, activating adaptive signaling cascades (9). For decades, researchers searched in vain for a similar mechanosensitive channel in eukaryotes.
This all changed in 2010 with Patapoutian and colleagues’ seminal publication in Science (10) reporting the identification of Piezo1 and Piezo2 as mechanosensitive channels in eukaryotes. The effort by Patapoutian and his colleagues was nothing short of a tour de force. Their work began with the identification of a cell line that produced large membrane currents in response to mechanical stress. Using microarray analysis, Patapoutian’s team looked for the gene, narrowing their search to proteins that spanned the membrane at least twice. They then generated small interfering RNAs (siRNAs) targeting each gene, introduced them into cells one by one, and tested the cells for currents. They targeted more than 70 different genes before they got a “hit”—a noticeable reduction in current that was not evident in controls using randomized siRNA sequences. The sequence encoding the channel was identified as the gene Piezo1. Once they had demonstrated the specific gene in the mouse neural crest-derived N2A cell line that produced the mechano-activated currents, they cloned and sequenced the corresponding mouse gene. Armed with this information and mRNA expression profile data, they also cloned the closely related Piezo2 gene from dorsal root ganglion neurons (10). Piezo2 channels were found to be the central mechnotransducer of Merkel cells, a specialized type of cell that is responsible for the exquisite tactile sensitivity of rodent whiskers and human fingertips (11).
Mouse Piezo channels turned out to be large (>2,500 amino acids) molecules that are not homologous with other ion channels or proteins. Subsequent analyses showed that Piezo1 is a nonselective cation channel that responds to pressure (10–40 mmHg)-induced deformations of the lipid bilayer induced through an increase in their open probability. Piezo1 and Piezo2 channels, the only two known members of the Piezo family, both exhibit rapid (i.e., ms) voltage-dependent inactivation and a reversal potential near 0 mV. Some studies suggest that the kinetics of Piezo channels may be context- and stimulus dependent (12). Cryo-EM imaging (4.8-Å resolution) revealed that Piezo1 has a trimeric stoichiometry, and the fully assembled channel displays a “propeller-like” structure (FIGURE 1B). The central pore of the channels is flanked by three domains called “blades” that are formed by a set of helical bundles. The arc-shaped blades of Piezo1 are proposed to act as the channel’s mechanosensory domains. To date, the only known small molecule capable of selectively regulating Piezo1 activity is the synthetic compound Yoda1, which presumably acts by lowering the channel’s mechanical threshold for activation and slowing inactivation (13, 14). There are no known selective pharmacological regulators of Piezo2. Clustering of Piezo1 channels in response to Yoda1 (15), likely through a stochastic self-assembly mechanism (16, 17), is vital for amplifying the Ca2+ and electrical signals of these channels. Piezo channels play a critical role in multiple physiological processes, including sensing changes in blood flow in endothelial cells, response to touch, and serotonin release from the gut.
4. ION CHANNELS AS FUNDAMENTAL SENSORS
The sensory functions of ion channels highlighted by the Julius and Patapoutian laboratories are more far-reaching than the detection of temperature and pressure in peripheral neurons: the ability to detect and respond to changes in the extracellular environment is critical for all cells. The specialized properties of the diverse TRP superfamily allow these channels to do much of the heavy lifting. TRP channels are infamously polymodal and are directly activated by many endogenous and extracellular substances. For example, in addition to sensing cold temperatures, TRPA1 channels are activated by toxic environmental compounds, aromatic dietary molecules, and substances generated by reactive oxygen species (ROS) (18). The activity of TRP channels can also be affected by classic intracellular signaling molecules, such as the minor membrane phospholipid phosphatidylinositol 4,5-bisphosphate (PIP2) and the potent second messenger diacylglycerol (DAG). Because of these properties, downstream TRP channels are indirectly activated or inhibited by signaling process set in motion by numerous G protein-coupled receptors (GPCRs) and other cell surface receptors and thus play a broad role in physiology and pathophysiology.
5. HYBRID SIGNALING IS THE RULE
The single-channel sensors/transducers discovered by Julius and Patapoutian and discussed above are not always complete sensing systems. Instead, Piezo and/or TRP channels are critical components of multimolecular sensor-effectors signaling modules in many cell types.
One example of such organization that appears to be widely distributed is X-ROS signaling. The X-ROS pathway was first identified in the heart (19), where it links the stretching of cardiac myocytes to enhanced Ca2+ signaling that is dependent on the generation of local reactive oxygen species (ROS). The work by Julius and Patapoutian paved the way to understanding the important X-ROS signaling pathway, which appears to be a general chemo-mechanical signaling mechanism found not only in heart but also in skeletal muscle (20), bone (21), and oncogene-transformed breast cells (22).
X-ROS signaling generally involves a Piezo and/or a TRP channel along with other critical components. The essential parts of X-ROS signaling include a microtubule network linked to Rac1 within the NADPH-oxidase-2 ensemble (NOX-2) in the plasma membrane (see FIGURE 1C). In heart, when myocytes are stretched, NOX-2 is activated through microtubules to produce extracellular superoxide (), which is dismuted to extracellular H2O2. The extracellular H2O2 can oxidize local extracellular targets and can cross the plasma membrane through water channels to oxidize multiple local intracellular targets within the myocyte. The intracellular targets include various channels (e.g., the type 2 ryanodine receptor RyR2, Piezo1), kinases, and other proteins. The stretch-dependent increase in local ROS can oxidize nearby proteins, including Piezo and TRP channels. Thus, the actions of X-ROS signaling depend on the effects of these oxidations. In the heart, X-ROS signaling normally serves to “tune” Ca2+ signaling under physiological conditions and also contributes to Ca2+-dependent arrhythmogenicity under pathological conditions (19). The identity and effect of the oxidized targets are under active investigation, as are the details of Piezo1 involvement (23).
X-ROS is not the only signaling pathway tied to Piezo and TRP channels. For example, the sensory ion channels discovered by Patapoutian and Julius also encode a component of the neurovascular coupling response (FIGURE 1D). Neurovascular coupling refers to the ensemble of physiological processes that link regional increases in brain metabolic activity to localized elevations in cerebral blood flow. A recently discovered element of this vital response is the brain’s extensive capillary network, which functions as a sensory web that detects increases in neuronal activity (24). Inwardly rectifying K+ (KIR) channels on brain capillary endothelial cells were the first molecular detectors identified in this pathway. KIR channels are activated by increases in extracellular K+ released during neuronal action potentials and orchestrate the rapid dilation of upstream arterioles to increase blood flow locally. TRP channels are vitally important for vascular function (25). Notably, TRPA1 channels on brain capillary endothelial cells were recently reported to be critical for neurovascular coupling (26), acting by detecting ROS-derived lipid peroxidation products and subsequently initiating intercellular Ca2+ signals that, like KIR-mediated electrical signals, also propagate (albeit somewhat more slowly). Available evidence suggests that vasodilation orchestrated by capillary TRPA1 channels may be necessary for maintaining regional blood flow increases during prolonged brain activity. The link between these studies and the work of Patapoutian and colleagues becomes more explicit with a recent report that inserts Piezo1 into the mix (27). This preliminary study showed that Piezo1 channels are present and functional in brain capillary endothelial cells and appear to contribute to cerebral blood flow regulation in vivo, perhaps by providing an “off signal” in response to mechanical deformation and shear stress imparted by increased red blood cell flux through capillaries.
6. CONCLUSIONS
The broad impact of the Nobel Prize awarded to Dr. David Julius and Dr. Ardem Patapoutian appropriately recognizes their achievements while placing them in perspective for those outside the field. For physiologists, the award serves as a celebration of elegant and thoughtful work that has excited, encouraged, and delighted all. When juxtaposed against current questions, it also makes clear how much remains to be done. Their findings have provided key mechanistic insights into how channels in specific cells respond to stretch and temperature changes. They also draw attention to the more complex responses of hybrid systems that include the elements unveiled by Patapoutian and Julius as part of an intricate dance. Enormous challenges lie ahead in the effort to more completely understand how cells, organs, and organisms are capable of detecting, processing, and reacting to light, sound, temperature, pressure, stretch, and other physical changes. For all scientists, this Nobel Prize represents an important landmark along the way.
GRANTS
The authors’ research programs are supported by grants from the National Heart, Lung, and Blood Institute (R35HL155008 to S.E.; 1R01HL144071 to L.F.S., R01 HL142290 to W.J.L.), the National Institute of General Medical Science (P20GM130459 to S.E.); the National Institute of Neurological Disorders and Stroke (RF1NS110044 and R33NS115132 to S.E.); and the National Institutes of Health common fund (OT2OD026580 to L.F.S.).
DISCLOSURES
S. Earley is an associate editor for Physiological Reviews and was not involved and did not have access to information regarding the peer-review process or final disposition of this article. An alternate editor oversaw the peer-review and decision-making process for this article. No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.E., L.F.S., and W.J.L. prepared figures; S.E., L.F.S., and W.J.L. drafted manuscript; S.E., L.F.S., and W.J.L. edited and revised manuscript; S.E. and L.F.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The graphical abstract and FIGURE 1C were created with BioRender, with permission.
REFERENCES
- 1.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389: 816–824, 1997. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 2.Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature 398: 436–441, 1999. doi: 10.1038/18906. [DOI] [PubMed] [Google Scholar]
- 3.McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 416: 52–58, 2002. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- 4.Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, Patapoutian A. A TRP channel that senses cold stimuli and menthol. Cell 108: 705–715, 2002. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- 5.Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 112: 819–829, 2003. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 6.Peier AM, Reeve AJ, Andersson DA, Moqrich A, Earley TJ, Hergarden AC, Story GM, Colley S, Hogenesch JB, McIntyre P, Bevan S, Patapoutian A. A heat-sensitive TRP channel expressed in keratinocytes. Science 296: 2046–2049, 2002. doi: 10.1126/science.1073140. [DOI] [PubMed] [Google Scholar]
- 7.Cao E, Cordero-Morales JF, Liu B, Qin F, Julius D. TRPV1 channels are intrinsically heat sensitive and negatively regulated by phosphoinositide lipids. Neuron 77: 667–679, 2013. doi: 10.1016/j.neuron.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nadezhdin KD, Neuberger A, Trofimov YA, Krylov NA, Sinica V, Kupko N, Vlachova V, Zakharian E, Efremov RG, Sobolevsky AI. Structural mechanism of heat-induced opening of a temperature-sensitive TRP channel. Nat Struct Mol Biol 28: 564–572, 2021. doi: 10.1038/s41594-021-00615-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cox CD, Bavi N, Martinac B. Bacterial mechanosensors. Annu Rev Physiol 80: 71–93, 2018. doi: 10.1146/annurev-physiol-021317-121351. [DOI] [PubMed] [Google Scholar]
- 10.Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, Dubin AE, Patapoutian A. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 330: 55–60, 2010. doi: 10.1126/science.1193270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ikeda R, Cha M, Ling J, Jia Z, Coyle D, Gu JG. Merkel cells transduce and encode tactile stimuli to drive Abeta-afferent impulses. Cell 157: 664–675, 2014. doi: 10.1016/j.cell.2014.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Del Marmol JI, Touhara KK, Croft G, MacKinnon R. Piezo1 forms a slowly-inactivating mechanosensory channel in mouse embryonic stem cells. Elife 7: e33149, 2018. doi: 10.7554/eLife.33149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Botello-Smith WM, Jiang W, Zhang H, Ozkan AD, Lin YC, Pham CN, Lacroix JJ, Luo Y. A mechanism for the activation of the mechanosensitive Piezo1 channel by the small molecule Yoda1. Nat Commun 10: 4503, 2019. doi: 10.1038/s41467-019-12501-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Syeda R, Xu J, Dubin AE, Coste B, Mathur J, Huynh T, Matzen J, Lao J, Tully DC, Engels IH, Petrassi HM, Schumacher AM, Montal M, Bandell M, Patapoutian A. Chemical activation of the mechanotransduction channel Piezo1. Elife 4: e07369, 2015. doi: 10.7554/eLife.07369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ridone P, Pandzic E, Vassalli M, Cox CD, Macmillan A, Gottlieb PA, Martinac B. Disruption of membrane cholesterol organization impairs the activity of PIEZO1 channel clusters. J Gen Physiol 152: e201912515, 2020. doi: 10.1085/jgp.201912515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sato D, Hernández-Hernández G, Matsumoto C, Tajada S, Moreno CM, Dixon RE, O’Dwyer S, Navedo MF, Trimmer JS, Clancy CE, Binder MD, Santana LF. A stochastic model of ion channel cluster formation in the plasma membrane. J Gen Physiol 151: 1116–1134, 2019. doi: 10.1085/jgp.201912327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dixon RE, Navedo MF, Binder MD, Santana LF. Mechanisms and physiological implications of cooperative gating of ion channels clusters. Physiol Rev. In press. doi: 10.1152/physrev.00022.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Talavera K, Startek JB, Alvarez-Collazo J, Boonen B, Alpizar YA, Sanchez A, Naert R, Nilius B. Mammalian transient receptor potential TRPA1 channels: from structure to disease. Physiol Rev 100: 725–803, 2020. doi: 10.1152/physrev.00005.2019. [DOI] [PubMed] [Google Scholar]
- 19.Prosser BL, Ward CW, Lederer WJ. X-ROS signaling: rapid mechano-chemo transduction in heart. Science 333: 1440–1445, 2011. doi: 10.1126/science.1202768. [DOI] [PubMed] [Google Scholar]
- 20.Khairallah RJ, Shi G, Sbrana F, Prosser BL, Borroto C, Mazaitis MJ, Hoffman EP, Mahurkar A, Sachs F, Sun Y, Chen YW, Raiteri R, Lederer WJ, Dorsey SG, Ward CW. Microtubules underlie dysfunction in Duchenne muscular dystrophy. Sci Signal 5: ra56, 2012. doi: 10.1126/scisignal.2002829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lyons JS, Joca HC, Law RA, Williams KM, Kerr JP, Shi G, Khairallah RJ, Martin SS, Konstantopoulos K, Ward CW, Stains JP. Microtubules tune mechanotransduction through NOX2 and TRPV4 to decrease sclerostin abundance in osteocytes. Sci Signal 10: eaan5748, 2017. doi: 10.1126/scisignal.aan5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pratt SJ, Lee RM, Chang KT, Hernández-Ochoa EO, Annis DA, Ory EC, Thompson KN, Bailey PC, Mathias TJ, Ju JA, Vitolo MI, Schneider MF, Stains JP, Ward CW, Martin SS. Mechanoactivation of NOX2-generated ROS elicits persistent TRPM8 Ca2+ signals that are inhibited by oncogenic KRas. Proc Natl Acad Sci USA 117: 26008–26019, 2020. doi: 10.1073/pnas.2009495117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang F, Yin K, Wu K, Zhang M, Wang S, Cheng H, Zhou Z, Xiao B. The mechanosensitive Piezo1 channel mediates heart mechano-chemo transduction. Nat Commun 12: 869, 2021. doi: 10.1038/s41467-021-21178-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Longden TA, Dabertrand F, Koide M, Gonzales AL, Tykocki NR, Brayden JE, Hill-Eubanks D, Nelson MT. Capillary K+-sensing initiates retrograde hyperpolarization to increase local cerebral blood flow. Nat Neurosci 20: 717–726, 2017. doi: 10.1038/nn.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Earley S, Brayden JE. Transient receptor potential channels in the vasculature. Physiol Rev 95: 645–690, 2015. doi: 10.1152/physrev.00026.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thakore P, Alvarado MG, Ali S, Mughal A, Pires PW, Yamasaki E, Pritchard HA, Isakson BE, Tran CH, Earley S. Brain endothelial cell TRPA1 channels initiate neurovascular coupling. Elife 10: e63040, 2021. doi: 10.7554/eLife.63040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harraz OF, Klug NR, Senatore A, Koide M, Nelson MT. Piezo1 is a mechanosensor channel in CNS capillaries (Abstract). J Gen Physiol 154: e2021ecc12, 2022. doi: 10.1085/jgp.2021ecc12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paulsen CE, Armache JP, Gao Y, Cheng Y, Julius D. Structure of the TRPA1 ion channel suggests regulatory mechanisms. Nature 520: 511–517, 2015. doi: 10.1038/nature14367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ge J, Li W, Zhao Q, Li N, Chen M, Zhi P, Li R, Gao N, Xiao B, Yang M. Architecture of the mammalian mechanosensitive Piezo1 channel. Nature 527: 64–69, 2015. doi: 10.1038/nature15247. [DOI] [PubMed] [Google Scholar]


