Abstract
The prevalence of major depressive disorder (MDD) is highest in young adulthood, an effect that has been magnified by the COVID-19 pandemic. Importantly, individuals with MDD are at a greater risk of developing cardiovascular disease (CVD). Accumulating evidence supports immune system dysregulation as a major contributor to the elevated CVD risk in older adults with MDD; however, whether this is present in young adults with MDD without comorbid disease remains unclear. Interestingly, recent data suggest augmented T-cell mitochondrial reactive oxygen species (T-cell mitoROS) as a potent driver of immune dysregulation in animal models of psychiatric disease. With this background in mind, we tested the hypothesis that young adults with MDD would have augmented T-cell mitoROS and circulating proinflammatory cytokines compared with healthy young adults without MDD (HA). Whole blood was drawn from 14 young adults with MDD (age: 23 ± 2 yr) and 11 HA (age: 22 ± 1 yr). T-cell mitoROS (MitoSOX red; total: CD3+, T-helper: CD4+, T cytotoxic: CD8+) and serum cytokines were assessed by flow cytometry. Total T-cell mitoROS was significantly greater in adults with MDD compared with HA [median: 14,089 arbitrary units (AU); median: 1,362 AU, P = 0.01]. Likewise, both T-helper and T-cytotoxic cell mitoROS were significantly greater in adults with MDD compared with HA (both: P < 0.05). There were no differences in circulating cytokines between groups (all cytokines: P > 0.05). Collectively, these findings suggest that elevated T-cell mitoROS may represent an early marker of immune system dysregulation in young, otherwise healthy, adults with MDD.
NEW & NOTEWORTHY To our knowledge, we provide the first evidence of augmented T-cell mitochondrial reactive oxygen species (T-cell mitoROS) in young, otherwise healthy adults with MDD. Although the elevated T-cell mitoROS did not correspond to a proinflammatory profile, these findings suggest that elevated T-cell mitoROS may be an early marker of immune system dysregulation in young adults with MDD.
Keywords: cytokines, depression, immune system dysregulation, inflammation, neural-immune regulation
INTRODUCTION
Major depressive disorder (MDD) is one of the most common mental illnesses worldwide and is a leading cause of disability and overall burden of disease (1). Notably, the prevalence is increasing in young adulthood, an effect that the COVID-19 pandemic has further magnified (2). This is alarming given the accumulating evidence implicating depression as a major risk factor for cardiovascular disease (CVD) and mortality (3). Data support immune system alterations via upregulation of proinflammatory pathways as a prominent mediator of the bidirectional relation between depression and comorbid CVD (4). Importantly, the majority of these data come from older adults with MDD and whether an inflammatory phenotype is present in young adults with MDD before the onset of CVD and other comorbidities remains unclear (5). Furthermore, the underlying molecular mechanisms causing immune system dysregulation in MDD remain to be determined.
A growing body of research implicates immune dysregulation via T cells (i.e., CD3+) of the adaptive immune system as a central mediator of cardiovascular-related diseases (6–9). Although these cells are essential for immunity, alterations in their phenotype and function can promote a proinflammatory state (10). For example, data from animal models of hypertension and atherosclerosis have demonstrated that T cells play a critical role in the development of disease by inducing peripheral vascular inflammation and dysfunction (7, 11). Proinflammatory T-cytotoxic cells appear to be particularly involved in mediating these effects (12). Notably, accumulating evidence also specifically implicates alterations in T cell function in humans diagnosed with MDD (13–17). Mitochondrial dysfunction plays a key role in promoting alterations in T cell function (18), an effect that has been recently observed in animal models of mood and anxiety disorders (13, 19–21). Furthermore, these data suggest that mitochondria-derived reactive oxygen species (mitoROS) in T cells are mechanistically involved in promoting a proinflammatory phenotype (21). To our knowledge, no study has explored whether this pathway is involved in mediating an inflammatory phenotype in human depression.
With this background in mind, we sought to quantify T-cell mitoROS and inflammatory cytokine profiles in unmedicated young adults with MDD but otherwise healthy. We hypothesized that young adults with MDD would have augmented T-cell mitoROS and circulating proinflammatory cytokines compared with healthy young nondepressed adults.
METHODS
Study Population
Fourteen nonmedicated young, otherwise healthy adults with MDD (male: n = 4; female: n = 10; age: 22 ± 3 yr) and 11 healthy young adults with no evidence or history of psychiatric disease (HA; male: n = 6; female: n = 5; age: 23 ± 6 yr) participated. Participants were recruited from the University of Texas at Arlington and surrounding areas. All participants underwent a structured diagnostic clinical interview, the Mini‐International Neuropsychiatric Interview (MINI; administered in-person by J.L.G.) (22), to determine the presence or absence of major psychiatric illness defined by standard Diagnostic and Statistical Manual of Mental Disorders (DSM-5) diagnostic criteria (23). Results were reviewed with a psychiatrist (E.F.S.) as part of a best estimate diagnostic process (24), consistent with our previous studies (25, 26). Individuals diagnosed with MDD were excluded for comorbid psychiatric disorders (e.g., schizophrenia, bipolar disorder, psychosis, posttraumatic stress disorder, and panic disorder), active suicidal ideation, or current use of psychopharmacological medications. All participants filled out a comprehensive health history questionnaire and were deemed free of autoimmune, cardiovascular, renal, and metabolic disease. In addition, participants were not using any tobacco products, were nonobese (body mass index: < 30 kg/m2), and were not taking prescription medication, except for hormonal contraceptives. These data were collected during the COVID-19 pandemic. Extensive screening was performed to ensure that no recent COVID-19 infection or any related symptoms were present. Total weekly habitual physical activity was estimated using the validated international physical activity questionnaire (IPAQ) long form (27). All female participants completed a urine pregnancy test to confirm the absence of pregnancy. Participants were asked to abstain from alcohol, exercise, and any medications for 24 h and caffeine and food for 12 h before study visit. Verbal and written informed consent were voluntarily obtained before participation and only after explaining study benefits, procedures, and risks. All experimental procedures conformed to the guidelines set forth by the Declaration of Helsinki and were approved by The Institutional Review Board at The University of Texas at Arlington (2019-0266). These data were collected as part of a larger clinical trial (NCT04838262).
Depressive Symptom Severity
All participants completed the National Institutes of Health Patient-Reported Outcomes Measurement Information System (PROMIS; emotional distress–depression, short form) (28) and patient health questionnaire‐9 (PHQ-9) (29) as previously described (25). Both indexes provide a valid and sensitive index of depressive symptomology (28, 29) and have been previously used by our laboratory (25). The depressive symptom severity measures were self-assessments. Participants completed these surveys during the study visit on a laboratory-provided tablet (iPad), administered by laboratory personnel. Briefly, the PROMIS assessed the emotional manifestations of depression over the 7 days immediately preceding participation (28). The raw PROMIS scores were converted into T-scores, which can be compared with the United States general population scores. A T-score of 50 represents the population mean, whereas a T-score above 60 indicates the presence of depressive symptoms that fall one standard deviation above the general population mean. The PHQ-9 assessed emotional and somatic depressive symptom severity based on the diagnostic criteria for DSM‐5 depressive disorders (23) over the 14 days immediately preceding participation. Calculated PHQ-9 scores can range from 0 to 27, with scores of 5, 10, 15, and 20 representing mild, moderate, moderately severe, and severe depression, respectively.
T-Cell Mitochondrial Reactive Species Assessment
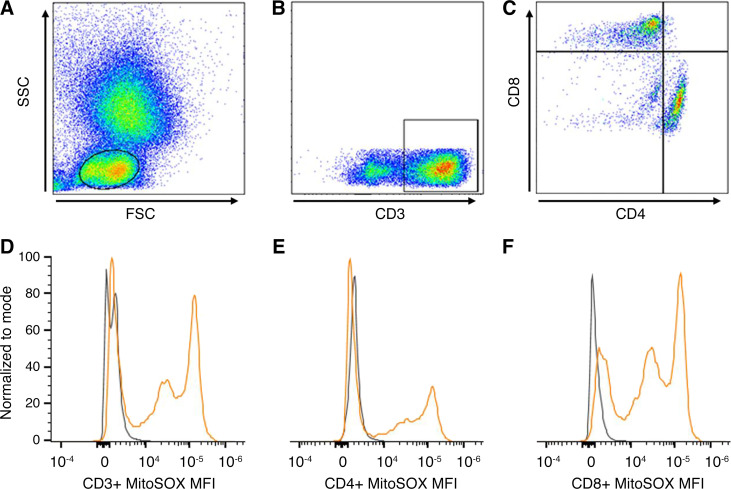
Peripheral blood was obtained using EDTA-coated tubes. Peripheral leukocytes were freshly isolated from whole blood following red blood cell lysis with ammonium chloride potassium buffer and prepared for further processing. Viable leukocytes were manually counted under the microscope using Trypan blue and a glass hemacytometer. The cell suspension concentration was adjusted to 1 × 106/100 µL by dilution with phosphate-buffered saline (PBS). T cell populations were determined by intracellular antibody staining using the following anti-human antibodies: FITC-CD3, Tonbo No. 35–0038 (0.5 µg in 100 µL; total T cells), PerCP-Cy5.5-CD4, Tonbo No. 65-0048 (0.08 µg in 100 µL; T helper), and PE-Cy7-CD8, Tonbo No. 60–0086 (0.04 µg in 100 µL; T cytotoxic). All antibodies were from Tonbo biosciences (San Diego, CA). Antibody concentrations were predetermined by separate titration experiments. T-cell mitochondrial ROS production was assessed by MitoSOX red (Thermo Fisher Scientific No. M36008, Waltham, MA), a validated and commonly used fluorogenic dye for the detection of mitochondrial superoxide in live cells (21, 30). We recorded a standardized 100,000 cells/sample in singlets on a two-laser Cytek Northern Lights Spectral Cytometer using the SpectroFlo real-time unmixing acquisition software. Resultant flow cytometry data were analyzed using the FlowJo software (Becton, Dickinson, and Co.; 2021). Figure 1, top, shows an example of gating strategy used to isolate lymphocytes (Fig. 1A), total T cells (Fig. 1B), and T-helper and T-cytotoxic subpopulations (Fig. 1C).
Figure 1.
The top panel shows an example of flow cytometry gating strategy to isolate lymphocytes (A), which can be differentiated further into T cells by their CD3 positivity (B). From the total T cells, T-helper cells can be identified by their CD4 positivity (C) and T-cytotoxic cells from their CD8 positivity (C). The bottom panel shows sample flow cytometry plots representing the median MitoSOX fluorescence intensity (MFI) of one healthy nondepressed adult (gray) and one otherwise healthy young adult with major depressive disorder (orange) in total T cell (CD3+; D), T-helper cells (CD4+; E), and T-cytotoxic cells (CD8+; F).
Cytokine Assessment
Whole blood was obtained in serum collection tubes and allowed to clot for 30 min before separating serum by centrifugation (10 min at 1,900 relative centrifugal force and 4°C). Serum was immediately removed and aliquoted into polypropylene tubes for storage at −80°C until further processing. On the day of the assay, serum was thawed to room temperature, and 11 cytokines and two chemokines were detected in duplicates using the Biolegend LEGENDplex Human Essential Immune Response panel bead-based immunoassay performed according to the manufacturer’s instructions (31). Cytokines were assessed on a two-laser BD LSR II flow cytometer and analyzed using the LEGENDplex Data Analysis Software Suite according to the manufacturer’s instructions (31). We were unable to obtain cytokine measures for one HA.
Data and Statistical Analyses
Participant’s characteristics data are presented as means ± SD and were analyzed using an unpaired Student’s t test. Fisher’s exact test was used to evaluate sex ratio between groups. Individual T-cell mitoROS data was quantified by subtracting each sample’s respective unstained control to remove background fluorescence and presented as the median fluorescence intensity [MFI; arbitrary units (AU)], allowing assessment of the shift in fluorescence intensity of the cell population (100,000 cells/sample). Cytokine and chemokine data are presented as the predicted median concentration of each cytokine and chemokine (pg/mL) calculated by the LEGENDplex Data Analysis Software Suite (standard curves: <10% coefficient of variation; intra-assay: <10% coefficient of variation) (31). Personnel conducting flow cytometry analyses were blinded to grouping. Group comparison for the T-cell mitoROS, cytokine, and chemokine data was performed using the Mann–Whitney U test due to these data violating the Gaussian normal distribution assumption. Associations were analyzed using Spearman rank correlation. A priori significance was set at P < 0.05, and exact P values are reported on individual graphs. GraphPad Prism version 9 was used to perform all statistical analyses.
RESULTS
General
No differences in anthropometric measures, blood pressure, or heart rate were found between HA and MDD groups (Table 1; all: P > 0.05). In addition, indices of metabolic health were not different between HA and MDD groups and within normal ranges (glucose: 90 ± 10 mg/dL, 88 ± 6 mg/dL; total cholesterol: 179 ± 25 mg/dL, 171 ± 30 mg/dL; both: P > 0.05). Likewise, no group differences were found in T cell characteristics (Table 1; all: P > 0.05) and T cell count/mL of blood (P = 0.92). As expected, depressive symptom severity as assessed by the PROMIS (raw and T-scores), and PHQ-9 were significantly greater in young adults with MDD (Table 1; both: P < 0.001).
Table 1.
Participant characteristics
| HA | MDD | P Value | |
|---|---|---|---|
| n (men/women) | 11 (6/5) | 14 (4/10) | 0.24 |
| Body weight, kg | 73 ± 19 | 64 ± 15 | 0.19 |
| Height, cm | 169.6 ± 13.6 | 166.7 ± 8.8 | 0.52 |
| Body mass index, kg/m2 | 25.0 ± 3.0 | 22.8 ± 4.0 | 0.16 |
| Total physical activity, min/day | 231 ± 333 | 273 ± 203 | 0.71 |
| Cardiovascular | |||
| SBP, mmHg | 115 ± 11 | 114 ± 9 | 0.73 |
| DBP, mmHg | 74 ± 7 | 74 ± 6 | 0.79 |
| MAP, mmHg | 87 ± 8 | 87 ± 7 | 0.99 |
| HR, beats/min | 76 ± 12 | 72 ± 10 | 0.36 |
| Depression severity | |||
| PROMIS, raw score | 15 ± 6 | 24 ± 6 | 0.0004* |
| PROMIS, T score | 52 ± 7 | 62 ± 6 | 0.0008* |
| PHQ-9, AU | 2 ± 2 | 9 ± 6 | 0.0013* |
| T cells | |||
| %CD3+, lymphocytes | 61 ± 12 | 67 ± 8 | 0.16 |
| %CD4+, CD3+ | 50 ± 7 | 50 ± 11 | 0.87 |
| %CD8+, CD3+ | 37 ± 5 | 34 ± 8 | 0.27 |
| CD4-to-CD8 ratio | 1.38 ± 0.39 | 1.60 ± 0.69 | 0.36 |
Values are represented as means ± SD; n, number of participants. AU, arbitrary units; SBP, systolic blood pressure; DBP, diastolic blood pressure; HA, healthy nondepressed young adults; HR, heart rate; MAP, mean arterial pressure; MDD, major depressive disorder; PHQ-9, patient health questionnaire (symptom severity: 0–4, minimal; 5–9, mild; 10–14, moderate; 15–19, moderately severe; 20–27, severe); PROMIS, patient-reported outcome measurement information system; SBP, systolic blood pressure. All data, except sex ratios (Fisher’s exact test), were analyzed using Student’s unpaired sample t test. *P < 0.05, significantly greater in MDD compared with HA.
T-Cell mitoROS
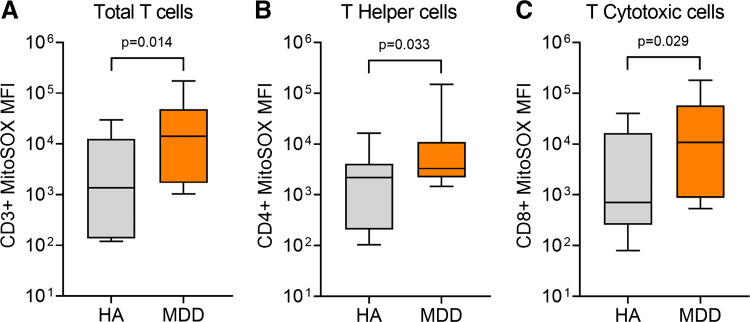
Figure 1 (bottom) shows the median MitoSOX MFI signal for total T cells (Fig. 1D), T-helper cells (CD4+; Fig. 1E), and T-cytotoxic cells (CD8+; Fig. 1F) overlayed in one HA (gray) and one adult with MDD (orange). Group summary data show that total T-cell mitoROS was significantly greater in adults with MDD compared with HA (P = 0.014, Fig. 2A). Likewise, T-helper cell mitoROS was significantly greater in MDD compared with HA (P = 0.033, Fig. 2B). Finally, similar results were seen in T-cytotoxic cells with mitoROS being significantly greater in MDD compared with HA (P = 0.029, Fig. 2C). There was a significant positive association between total T-cell mitoROS and depressive symptom severity assessed by the PROMIS (r = 0.368, P = 0.035) but not the PHQ-9 (r = 0.194, P = 0.176).
Figure 2.
Box (median, 25th and 75th percentiles) and whisker (5th and 95th percentiles) plot showing group differences in MitoSOX median fluorescence intensity (MFI) between healthy nondepressed adults in gray plots [HA, n = 11 (6 males/5 females)] and otherwise healthy young adults with major depressive disorder in orange plots [MDD, n = 14 (4 males/10 females)] for total T cell (CD3+; A), T-helper (CD4+; B), and T-cytotoxic (CD8+; C) cells. Data were analyzed using the Mann–Whitney U test.
Circulating Immune Markers
There were no group differences in any markers of inflammation (Table 2; all: P > 0.05). Specifically, there were no differences in proinflammatory cytokines (IL-1β, IL-2, IL-6, IL-8, IL17A, TNF-α, IFNγ, IL-12p70, TGF-β), anti-inflammatory cytokines (IL-4 and IL-10), or chemokines (MCP-1 and IP-10). Likewise, there were no significant associations between depressive symptom severity and any inflammatory markers (all: P > 0.05).
Table 2.
Serum cytokine and chemokine profile (pg/mL)
| HA | MDD | P Value | |
|---|---|---|---|
| n (men/women) | 10 (6/4) | 14 (4/10) | 0.21 |
| Proinflammatory cytokines | |||
| IL-1β | 4.41 (0.78, 10.55) | 1.31 (0.69, 5.70) | 0.28 |
| IL-2 | 1.33 (0.46, 16.09) | 0.79 (0.43, 9.91) | 0.38 |
| IL-6 | 5.93 (0.66, 19.76) | 6.46 (0.44, 58.14) | 0.47 |
| IL-8 | 2.76 (0.72, 9.95) | 1.14 (0.66, 7.63) | 0.27 |
| IL-17A | 4.40 (0.59, 16.13) | 0.68 (0.44, 11.90) | 0.22 |
| TNF-α | 7.23 (0.95, 37.77) | 1.44 (0.64, 26.08) | 0.31 |
| IFNγ | 20.26 (0.45, 64.16) | 3.70 (0.42, 76.26) | 0.34 |
| IL-12p70 | 1.25 (0.77, 3.56) | 0.91 (0.52, 9.83) | 0.40 |
| TGF-β1, free active | 30.88 (3.57, 99.89) | 3.57 (3.57, 34.04) | 0.09 |
| Anti-inflammatory cytokines | |||
| IL-4 | 5.34 (0.68, 120.50) | 0.97 (0.68, 60.40) | 0.42 |
| IL-10 | 5.20 (0.65, 10.99) | 1.57 (0.60, 22.19) | 0.59 |
| Chemokines | |||
| MCP-1 | 127.90 (102.70, 222.00) | 205.20 (87.15, 256.50) | 0.51 |
| IP-10 | 128.30 (108.00, 269.00) | 139.90 (53.99, 253.20) | 0.67 |
Values (in pg/mL) are medians (25th percentile, 75th percentile); n, number of participants. HA, healthy nondepressed young adults; IFNγ, interferon-γ; IL-1β, interleukin-1β; IP-10 interferon γ-induced protein 10; MCP-1, monocyte chemoattractant protein-1; MDD, major depressive disorder; TNF-α, tumor necrosis factor-α; TGF-β1, transforming growth factor-β1. All data, except sex ratios (fisher’s exact test), were analyzed using Mann–Whitney U test. P > 0.05, no significant difference found in any of the assessed markers.
DISCUSSION
The primary novel findings of this study were that T cells from young, otherwise healthy, adults with MDD exhibit augmented mitoROS production. Moreover, elevated mitoROS in T-helper and T-cytotoxic cells suggests that increased T-cell mitoROS is evident in both T cell subtypes. In contrast to our hypothesis, there were no group differences in circulating cytokines and chemokines. Finally, we found that depressive symptom severity was positively associated with total T-cell mitoROS but not with any inflammatory markers. Collectively, these findings suggest that T-cell mitoROS may be an early marker of immune system dysregulation in young adults with MDD.
Our findings align with an emerging body of evidence suggesting a role for immune dysregulation via T cells in rodent models of mood and anxiety disorders (19, 21, 32). Interestingly, a recent study demonstrated reduced T-cell mitochondrial respiratory capacity in young adults with MDD compared with their nondepressed counterparts (17). Extending this, our novel findings demonstrate that T-cell mitoROS is augmented in adults with MDD. Elevated mitoROS is a potent signal transducer provoking a shift in pro- and anti-inflammatory cytokine profiles (33, 34). Indeed, Case et al. (34) demonstrated using mouse splenocytes that augmented T-cell mitoROS increased proinflammatory and decreased anti-inflammatory cytokine production in both T-helper and T-cytotoxic cells. Importantly, this pathway appears to be upregulated in animal models of mood and anxiety disorders (19, 21, 32). That is, induced depression-like behavior in mice was linked to elevated T-cell mitoROS and proinflammatory cytokine expression (21), suggesting that altered redox regulation in T cells may mechanistically contribute to increased inflammation-related CVD.
Despite marked increases in T-cell mitoROS in young adults with MDD, circulating concentrations of proinflammatory cytokines and chemokines were not elevated. Although this was somewhat surprising given the data from rodent models showing T-cell mitoROS induced elevation in proinflammatory cytokine release (33), it is important to note that circulating cytokines are highly dynamic and stem from multiple sources (e.g., T cells, macrophages, endothelial cells, etc.). Thus, our findings do not exclude the possibility that T cells in adults with MDD release more cytokines. Moreover, although there are data demonstrating elevated circulating proinflammatory cytokines in older adults with MDD, the evidence in young adults is unclear (5), an inconsistency that has been attributed to the fact that young, otherwise healthy, individuals have had a limited duration of depression. In this regard, the cumulative effect of the illness likely becomes more pronounced with a longer duration of depression. Considered collectively, the findings of the present study suggest that increased T-cell mitoROS may be an early indicator of immune alterations in MDD. It is also important to note that inflammation is only one mechanism contributing to the elevated CVD risk in MDD. As an example, decreased expression of brain-derived neurotrophic factor has been suggested to contribute to both CVD and MDD risk (35), highlighting another area for future study.
Depressive symptom severity may further modulate T-cell mitoROS and inflammation in young adults. Although we did not find any associations between inflammatory markers and depressive symptomology, increased depressive symptom severity was positively related to greater total T-cell mitoROS. Notably, this positive correlation supports and extends previous work showing a link between increased severity of depression and reductions in mitochondrial function in peripheral blood mononuclear cells (36) and T cells (17). Interestingly, this relation was only evident when using the PROMIS to assess depressive symptomology and not when using the PHQ-9. Because the PROMIS is an index of the emotional distress of depression, whereas the PHQ-9 encompasses both the emotional and somatic symptoms of depression consistent with the DSM-5 diagnostic criteria (23), the lack of relation with the PHQ-9 as an index of disease severity may indicate that negative emotions more strongly modulate T-cell mitoROS. Further characterization of depressive symptoms using other available assessment instruments (e.g., Montgomery-Åsberg Depression Rating Scale and Hamilton Depression Rating Scale) (37, 38) should be considered in future studies. In terms of examining the effects of depression on T-cell mitoROS and inflammation, we recruited unmedicated otherwise healthy individuals with MDD to reduce the potential confounding influences of other variables (e.g., medications for depression, obesity, comorbid disease, etc.,). Thus, additional studies including participants with more severe depression and comorbid factors associated with MDD are warranted.
Although T-cell mitoROS mediates intracellular signaling for normal T cell activation and function, excess mitoROS can damage DNA, proteins, and lipid membranes and can be detrimental to T cell function (39, 40). Importantly, excess cellular mitoROS has been implicated in the pathology of several diseases (39) and there is strong evidence linking T cells to vascular dysfunction in comorbid diseases associated with MDD (7–9, 11, 12). The mechanisms responsible for an augmented T-cell mitoROS in young adults with MDD are unknown; however, a few possibilities warrant discussion. First, recent data from animal models of psychiatric disease suggest that an impaired neural-immune interaction may be involved. Indeed, Moshfegh et al. (21) demonstrated that enhanced norepinephrine release corresponded to augmented T-cell mitoROS and proinflammatory cytokine production in mice exhibiting symptoms of depression and anxiety. These functional data are corroborated by in vitro data from the same group showing that norepinephrine directly stimulates T-cell mitoROS, thereby increasing the production of proinflammatory cytokines (34). Although no studies have investigated circulating norepinephrine or muscle sympathetic nerve activity in young adults with MDD, there is some evidence for elevated sympathetic activity in middle-aged and older adults with MDD (41–43). However, whether norepinephrine plays a direct role in increasing T-cell mitoROS in humans has yet to be determined. Second, a reduced antioxidant capacity is also a plausible mediator of an augmented mitoROS production given its important role in maintaining redox balance. Indeed, previous studies suggest reduced total antioxidant capacity measured in serum from adults with MDD (44–46), highlighting this as a possibility. Third, metabolic (e.g., mitochondrial bioenergetics) and phenotypic (e.g., mitochondrial mass) changes in T cell subsets could also contribute to an increased mitoROS production (19, 47). Future studies are needed to more clearly elucidate the underlying mechanisms mediating augmented T-cell mitoROS in MDD.
Although circulating inflammatory cytokines were not elevated, the novel findings of the present investigation demonstrate pronounced increases in T-cell mitoROS, from both T cell subtypes, in young otherwise healthy adults with MDD. These data add to the growing body of literature indicating alterations in T-cell mitochondrial health in MDD (13, 17, 19–21). Because increased T-cell mitoROS may be an early marker of immune dysregulation that has wide-ranging deleterious consequences for cardiovascular health, future studies investigating the mechanistic underpinnings, as well as the functional impact, are warranted.
GRANTS
This work was funded by the National Institute of Health Grants MH123928 (to J.L.G.), HL133414 (to J.L.G.), AG060395 (to D.W.T.), and AG061271 (to D.W.T.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.-K.G., P.J.F., D.W.T., and J.L.G. conceived and designed research; A.-K.G. and D.W.T. performed experiments; A.-K.G. analyzed data; A.-K.G., A.M.D., E.F.S., P.J.F., D.W.T., and J.L.G. interpreted results of experiments; A.-K.G. prepared figures; A.-K.G. drafted manuscript; A.-K.G., A.M.D., E.F.S., P.J.F., D.W.T., and J.L.G. edited and revised manuscript; A.-K.G., A.M.D., E.F.S., P.J.F., D.W.T., and J.L.G. approved final version of manuscript.
REFERENCES
- 1.World Health Organization. Depression and Other Common Mental Disorders: Global Health Estimates. Geneva: World Health Organization, 2017. [Google Scholar]
- 2.Santomauro DF, Mantilla Herrera AM, Shadid J, Zheng P, Ashbaugh C, Pigott DM, et al. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet 398: 1700–1712, 2021. doi: 10.1016/S0140-6736(21)02143-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Correll CU, Solmi M, Veronese N, Bortolato B, Rosson S, Santonastaso P, Thapa-Chhetri N, Fornaro M, Gallicchio D, Collantoni E, Pigato G, Favaro A, Monaco F, Kohler C, Vancampfort D, Ward PB, Gaughran F, Carvalho AF, Stubbs B. Prevalence, incidence and mortality from cardiovascular disease in patients with pooled and specific severe mental illness: a large-scale meta-analysis of 3,211,768 patients and 113,383,368 controls. World Psychiatry 16: 163–180, 2017. [Erratum in World Psychiatry 17: 120, 2018]. doi: 10.1002/wps.20420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iwata M, Ota KT, Duman RS. The inflammasome: pathways linking psychological stress, depression, and systemic illnesses. Brain Behav Immun 31: 105–114, 2013. doi: 10.1016/j.bbi.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toenders YJ, Laskaris L, Davey CG, Berk M, Milaneschi Y, Lamers F, Penninx BWJH, Schmaal L. Inflammation and depression in young people: a systematic review and proposed inflammatory pathways. Mol Psychiatry, 2021. doi: 10.1038/s41380-021-01306-8. [DOI] [PubMed] [Google Scholar]
- 6.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II–induced hypertension and vascular dysfunction. J Exp Med 204: 2449–2460, 2007. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trott DW, Harrison DG. The immune system in hypertension. Adv Physiol Educ 38: 20–24, 2014. doi: 10.1152/advan.00063.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elkhatib SK, Case AJ. Autonomic regulation of T-lymphocytes: implications in cardiovascular disease. Pharmacol Res 146: 104293, 2019. doi: 10.1016/j.phrs.2019.104293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trott DW, Islam MT, Buckley DJ, Donato AJ, Dutson T, Sorensen ES, Cai J, Gogulamudi VR, Phuong TTT, Lesniewski LA. T lymphocyte depletion ameliorates age-related metabolic impairments in mice. GeroScience 43: 1331–1347, 2021. doi: 10.1007/s11357-021-00368-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwartz DM, Burma AM, Kitakule MM, Luo Y, Mehta NN. T cells in autoimmunity-associated cardiovascular diseases. Front Immunol 11: 588776, 2020. doi: 10.3389/fimmu.2020.588776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olsen F. Transfer of arterial hypertension by splenic cells from DOCA-salt hypertensive and renal hypertensive rats to normotensive recipients. Acta Pathol Microbiol Scand C 88: 1–5, 1980. doi: 10.1111/j.1699-0463.1980.tb00065.x. [DOI] [PubMed] [Google Scholar]
- 12.Trott DW, Thabet SR, Kirabo A, Saleh MA, Itani H, Norlander AE, Wu J, Goldstein A, Arendshorst WJ, Madhur MS, Chen W, Li C-I, Shyr Y, Harrison DG. Oligoclonal CD8+ T cells play a critical role in the development of hypertension. Hypertension 64: 1108–1115, 2014. doi: 10.1161/HYPERTENSIONAHA.114.04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller AH. Depression and immunity: a role for T cells? Brain Behav Immun 24: 1–8, 2010. doi: 10.1016/j.bbi.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kronfol Z, Silva J Jr, Greden J, Dembinski S, Gardner R, Carroll B. Impaired lymphocyte function in depressive illness. Life Sci 33: 241–247, 1983. doi: 10.1016/0024-3205(83)90382-x. [DOI] [PubMed] [Google Scholar]
- 15.Schleifer SJ, Keller SE, Siris SG, Davis KL, Stein M. Depression and immunity: lymphocyte function in ambulatory depressed patients, hospitalized schizophrenic patients, and patients hospitalized for herniorrhaphy. Arch Gen Psychiatry 42: 129–133, 1985. doi: 10.1001/archpsyc.1985.01790250023003. [DOI] [PubMed] [Google Scholar]
- 16.Schleifer SJ, Bartlett JA, Keller SE, Eckholdt HM, Shiflett SC, Delaney BR. Immunity in adolescents with major depression. J Am Acad Child Adolesc Psychiatry 41: 1054–1060, 2002. doi: 10.1097/00004583-200209000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Gamradt S, Hasselmann H, Taenzer A, Brasanac J, Stiglbauer V, Sattler A, Sajitz-Hermstein M, Kierszniowska S, Ramien C, Nowacki J, Mascarell-Maricic L, Wingenfeld K, Piber D, Ströhle A, Kotsch K, Paul F, Otte C, Gold SM. Reduced mitochondrial respiration in T cells of patients with major depressive disorder. iScience 24: 103312, 2021. doi: 10.1016/j.isci.2021.103312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ledderose C, Bao Y, Lidicky M, Zipperle J, Li L, Strasser K, Shapiro NI, Junger WG. Mitochondria are gate-keepers of T cell function by producing the ATP that drives purinergic signaling. J Biol Chem 289: 25936–25945, 2014. doi: 10.1074/jbc.M114.575308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Medina-Rodriguez EM, Lowell JA, Worthen RJ, Syed SA, Beurel E. Involvement of innate and adaptive immune systems alterations in the pathophysiology and treatment of depression. Front Neurosci 12: 547, 2018. doi: 10.3389/fnins.2018.00547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fan K-Q, Li Y-Y, Wang H-L, Mao X-T, Guo J-X, Wang F, Huang L-J, Li Y-N, Ma X-Y, Gao Z-J, Chen W, Qian D-D, Xue W-J, Cao Q, Zhang L, Shen L, Zhang L, Tong C, Zhong J-Y, Lu W, Lu L, Ren K-M, Zhong G, Wang Y, Tang M, Feng X-H, Chai R-J, Jin J. Stress-induced metabolic disorder in peripheral CD4+ T cells leads to anxiety-like behavior. Cell 179: 864–879.e19, 2019. doi: 10.1016/j.cell.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Moshfegh CM, Elkhatib SK, Collins CW, Kohl AJ, Case AJ. Autonomic and redox imbalance correlates with T-lymphocyte inflammation in a model of chronic social defeat stress. Front Behav Neurosci 13: 103, 2019. doi: 10.3389/fnbeh.2019.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59, Suppl 20: 22–33, 1998. [PubMed] [Google Scholar]
- 23.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (5th ed.). American Psychiatric Publishing, 2013. [Google Scholar]
- 24.Kosten TA, Rounsaville BJ. Sensitivity of psychiatric diagnosis based on the best estimate procedure. Am J Psychiatry 149: 1225–1227, 1992. doi: 10.1176/ajp.149.9.1225. [DOI] [PubMed] [Google Scholar]
- 25.Darling AM, Richey RE, Akins JD, Saunders EFH, Matthew Brothers R, Greaney JL. Cerebrovascular reactivity is blunted in young adults with major depressive disorder: the influence of current depressive symptomology. J Affect Disord 295: 513–521, 2021. doi: 10.1016/j.jad.2021.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greaney JL, Koffer RE, Saunders EFH, Almeida DM, Alexander LM. Self‐reported everyday psychosocial stressors are associated with greater impairments in endothelial function in young adults with major depressive disorder. J Am Heart Assoc 8: e010825, 2019. doi: 10.1161/JAHA.118.010825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wanner M, Probst-Hensch N, Kriemler S, Meier F, Autenrieth C, Martin BW. Validation of the long international physical activity questionnaire: influence of age and language region. Prev Med Rep 3: 250–256, 2016. doi: 10.1016/j.pmedr.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi SW, Schalet B, Cook KF, Cella D. Establishing a common metric for depressive symptoms: linking the BDI-II, CES-D, and PHQ-9 to PROMIS depression. Psychol Assess 26: 513–527, 2014. doi: 10.1037/a0035768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA 282: 1737–1744, 1999. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 30.Mukhopadhyay P, Rajesh M, Haskó G, Hawkins BJ, Madesh M, Pacher P. Simultaneous detection of apoptosis and mitochondrial superoxide production in live cells by flow cytometry and confocal microscopy. Nat Protoc 2: 2295–2301, 2007. doi: 10.1038/nprot.2007.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lehmann JS, Zhao A, Sun B, Jiang W, Ji S. Multiplex cytokine profiling of stimulated mouse splenocytes using a cytometric bead-based immunoassay platform. J Vis Exp 56440, 2017. doi: 10.3791/56440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Avitsur R, Stark JL, Dhabhar FS, Sheridan JF. Social stress alters splenocyte phenotype and function. J Neuroimmunol 132: 66–71, 2002. doi: 10.1016/s0165-5728(02)00310-7. [DOI] [PubMed] [Google Scholar]
- 33.Naik E, Dixit VM. Mitochondrial reactive oxygen species drive proinflammatory cytokine production. J Exp Med 208: 417–420, 2011. doi: 10.1084/jem.20110367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Case AJ, Roessner CT, Tian J, Zimmerman MC. Mitochondrial superoxide signaling contributes to norepinephrine-mediated T-lymphocyte cytokine profiles. PLoS One 11: e0164609, 2016. doi: 10.1371/journal.pone.0164609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hashimoto K. Sigma-1 receptor chaperone and brain-derived neurotrophic factor: emerging links between cardiovascular disease and depression. Prog Neurobiol 100: 15–29, 2013. doi: 10.1016/j.pneurobio.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 36.Karabatsiakis A, Böck C, Salinas-Manrique J, Kolassa S, Calzia E, Dietrich DE, Kolassa IT. Mitochondrial respiration in peripheral blood mononuclear cells correlates with depressive subsymptoms and severity of major depression. Transl Psychiatry 4: e397, 2014. doi: 10.1038/tp.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry 134: 382–389, 1979. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 38.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 23: 56–62, 1960. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yarosz EL, Chang C-H. The role of reactive oxygen species in regulating T cell-mediated immunity and disease. Immune Netw 18: e14, 2018. doi: 10.4110/in.2018.18.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Case AJ, McGill JL, Tygrett LT, Shirasawa T, Spitz DR, Waldschmidt TJ, Legge KL, Domann FE. Elevated mitochondrial superoxide disrupts normal T cell development, impairing adaptive immune responses to an influenza challenge. Free Radic Biol Med 50: 448–458, 2011. doi: 10.1016/j.freeradbiomed.2010.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Veith RC, Lewis N, Linares OA, Barnes RF, Raskind MA, Villacres EC, Murburg MM, Ashleigh EA, Castillo S, Peskind ER, Pascualy M, Halter JB. Sympathetic nervous system activity in major depression: basal and desipramine-induced alterations in plasma norepinephrine kinetics. Arch Gen Psychiatry 51: 411–422, 1994. doi: 10.1001/archpsyc.1994.03950050071008. [DOI] [PubMed] [Google Scholar]
- 42.Barton DA, Dawood T, Lambert EA, Esler MD, Haikerwal D, Brenchley C, Socratous F, Kaye DM, Schlaich MP, Hickie I, Lambert GW. Sympathetic activity in major depressive disorder: identifying those at increased cardiac risk? J Hypertens 25: 2117–2124, 2007. doi: 10.1097/HJH.0b013e32829baae7. [DOI] [PubMed] [Google Scholar]
- 43.Scalco AZ, Rondon MU, Trombetta IC, Laterza MC, Azul JB, Pullenayegum EM, Scalco MZ, Kuniyoshi FH, Wajngarten M, Negrão CE, Lotufo-Neto F. Muscle sympathetic nervous activity in depressed patients before and after treatment with sertraline. J Hypertens 27: 2429–2436, 2009. doi: 10.1097/HJH.0b013e3283310ece. [DOI] [PubMed] [Google Scholar]
- 44.Baek S-E, Lee G-J, Rhee C-K, Rho D-Y, Kim D-H, Huh S, Lee S-K. Decreased total antioxidant activity in major depressive disorder patients non-responsive to antidepressant treatment. Psychiatry Investig 13: 222–226, 2016. doi: 10.4306/pi.2016.13.2.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taene A, Khalili-Tanha G, Esmaeili A, Mobasheri L, Kooshkaki O, Jafari S, Shokouhifar A, Sarab GA. The association of major depressive disorder with activation of NLRP3 inflammasome, lipid peroxidation, and total antioxidant capacity. J Mol Neurosci 70: 65–70, 2020. doi: 10.1007/s12031-019-01401-0. [DOI] [PubMed] [Google Scholar]
- 46.Bajpai A, Verma AK, Srivastava M, Srivastava R. Oxidative stress and major depression. J Clin Diagn Res 8: CC04–CC07, 2014. doi: 10.7860/JCDR/2014/10258.5292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chávez MD, Tse HM. Targeting mitochondrial-derived reactive oxygen species in T cell-mediated autoimmune diseases. Front Immunol 12: 703972, 2021. doi: 10.3389/fimmu.2021.703972. [DOI] [PMC free article] [PubMed] [Google Scholar]