Abstract
Dynamic chloride (Cl−) regulation is critical for synaptic inhibition. In mature neurons, Cl− influx and extrusion are primarily controlled by ligand-gated anion channels (GABAA and glycine receptors) and the potassium chloride cotransporter K+-Cl− cotransporter 2 (KCC2), respectively. Here, we report for the first time, to our knowledge, a presence of a new source of Cl− influx in striatal neurons with properties similar to chloride voltage-gated channel 1 (ClC-1). Using whole cell patch-clamp recordings, we detected an outwardly rectifying voltage-dependent current that was impermeable to the large anion methanesulfonate (MsO−). The anionic current was sensitive to the ClC-1 inhibitor 9-anthracenecarboxylic acid (9-AC) and the nonspecific blocker phloretin. The mean fractions of anionic current inhibition by MsO−, 9-AC, and phloretin were not significantly different, indicating that anionic current was caused by active ClC-1-like channels. In addition, we found that Cl− current was not sensitive to the transmembrane protein 16A (TMEM16A; Ano1) inhibitor Ani9 and that the outward Cl− rectification was preserved even at a very high intracellular Ca2+ concentration (2 mM), indicating that TMEM16B (Ano2) did not contribute to the total current. Western blotting and immunohistochemical analyses confirmed the presence of ClC-1 channels in the striatum mainly localized to the somata of striatal neurons. Finally, we found that 9-AC decreased action potential firing frequencies and increased excitability in medium spiny neurons (MSNs) expressing dopamine type 1 (D1) and type 2 (D2) receptors in the brain slices, respectively. We conclude that ClC-1-like channels are preferentially located at the somata of MSNs, are functional, and can modulate neuronal excitability.
Keywords: chloride homeostasis; ClC-1, chloride voltage-gated channel 1; dopamine type 1 and type 2 receptors; TMEM16A, transmembrane protein 16A (Ano1); striatal medium spiny neurons
INTRODUCTION
Chloride (Cl−) homeostasis is one of the key elements regulating neuronal excitability. Low intracellular Cl− concentration ([Cl−]i) in mature neurons sets up a negative Cl− equilibrium potential (ECl) that is lower than the resting membrane potential (1). A more negative ECl promotes synaptic inhibition via activation of γ-aminobutyric acid type A receptors (GABAARs) or glycine receptors (GlyRs)—anion channels permeable for Cl− and, to a lesser degree, carbonate (), resulting in hyperpolarizing current caused by Cl− entry (2). Cl− homeostasis is defined by several key elements, including routes of Cl− entry and extrusion. In mature neurons, the extrusion is controlled by K+-Cl− cotransporter 2 (KCC2)—a potassium-chloride cotransporter that uses the K+ gradient to remove Cl− (3, 4). In most models of intracellular synaptic homeostasis, Cl− entry is caused by activation of postsynaptic GABAARs and GlyRs (2). However, for active neurons, Cl− regulation is more complex and dynamic. ECl is not only statically defined by initial [Cl−]i and the rates of Cl− extrusion and entry but also depends on the subcellular/spatial distribution of Cl− channels, excitatory and/or inhibitory synaptic inputs, and, thus, membrane potential (for details, see Refs. 2, 5, and 6). Such dynamic regulation of Cl− equilibrium better explains how a hyperpolarizing inhibitory (“shunting”) Cl− current activation, especially in the absence of adequate compensation by KCC2-mediated Cl− extrusion, can lead to the accumulation of intracellular Cl− and, thus, a more positive ECl. Thus, the increased ECl can be observed as GABAARs activity-dependent disinhibition, and in some pathological situations, it may even result in excitation (2, 7–9). Indeed, KCC2 loss-of-function can compromise the dynamic regulation of [Cl−]i and has been shown to be involved in several neuropathological situations, which makes KCC2 a promising therapeutic target (2, 10–17). In addition, intracellular Cl− may act as a second messenger to regulate the expression of α3-α1- and δ-containing GABAARs, responsible for phasic and tonic inhibition, respectively (18).
An understanding of Cl− homeostasis is incomplete without accounting for all possible routes of Cl− entry into neurons. Importantly, Cl− entry considerations should not be limited to GABAARs and GlyRs, as new findings suggesting that neurons may express a variety of Cl− channels have been gaining acceptance. Specifically, Ca2+-dependent transmembrane protein 16B (TMEM16B; Ano2 gene) channels have been identified in neurons in the cortex, hippocampus, cerebellum, central lateral amygdala, and retina (19–22), and these channels can modulate GABAergic transmission (20, 21). The presence of voltage-dependent, ClC family channels in neurons has also been documented (23). Although the detection and characterization of inwardly rectifying ClC-2 channels have been shown in selective brain regions (23), the extent to which neurons express outwardly rectifying ClC-1 channels, which are widely expressed by skeletal muscle cells, remains controversial (23, 24). The main evidence for ClC-1 channel expression in neurons comes from the exomic sequencing of variant channel subunit genes in individuals with a clinically diagnosed idiopathic epilepsy, complemented by the identification of ClC-1 transcripts in specific brain regions from human autopsy specimens and mouse thalamus and cortex by RT-PCR, in situ hybridization, and immunoblotting (24). Several concerns were raised regarding the validity of the experimental approach (23). Specifically, even with expression data, the lack of electrophysiological and pharmacological evidence makes it difficult to judge whether ClC-1 channels are truly skeletal muscle specific or are also functional in neurons. Thus, it is not known whether ClC-1 could be a significant contributing factor to intracellular Cl− homeostasis, and excitability in neurons.
Electrophysiological, pharmacological, Western blotting, immunohistochemical, and next-generation sequencing techniques and approaches were used in the present study to confirm for the first time, to our knowledge, that ClC-1-like channels are expressed in striatal neurons and that they are critical regulators of action potential firing in striatal medium spiny neurons (MSNs). We conclude that these channels may be important Cl− entry sources in neurons and may contribute to dynamic regulation of Cl− homeostasis, synaptic input and output control, and neuronal excitability.
MATERIALS AND METHODS
Animals
All mice used in our experiments were housed in the Virginia Commonwealth University School of Medicine animal facility with unrestricted access to food and water and 12:12-h light-dark cycle (lights off at 18:00 h). Four different strains of mice were used to assess the expression and function of chloride channels in the striatum. Timed pregnant imprinting control region (CD1) mice were obtained from Charles River Laboratories (Wilmington, MA) for striatal neuronal and mixed-glial cocultures. B6.Cg-Tg(Drd1a-tdTomato)6Calak/J line 6 mice (Cat. No. 016204; The Jackson Laboratory; Bar Harbor, ME) and Drd2-eGFP (Cat. No. 036931-UCD; Mutant Mouse Resource and Research Centers) were used for slice electrophysiology experiments to obtains recordings from dopamine D1-receptor-expressing (D1) and dopamine D2-receptor-expressing (D2) MSNs, respectively. Tat transgenic (Tat-tg) mice express the HIV-1 tat transgene (HIV-1 Tat1-86) under the control of a doxycycline (DOX)-activated tetracycline (tet)-on expression system driven by a glial fibrillary acidic protein (GFAP) and (25) were generated in the vivarium of Virginia Commonwealth University. Control mice with the rtTa transgene, but without the tat transgene (Tat−), were used for the mixed glial cocultures and immunohistochemistry. The use of mice in this study was approved by the Virginia Commonwealth University Animal Care and Use Committee (IACUC Protocol Nos. AM10161 and AM10175), and all experiments were conducted in accordance with the National Institutes of Health (NIH Publication No. 85-23) ethical guidelines.
Neuronal-Mixed Glia Coculture
Neuronal-mixed glial cocultures were prepared in two steps. In the first step, mixed glia were isolated from the striata of 0- to 1-day-old Tat− pups (26, 27). Brains were cleaned of meninges, and striata were dissected, minced, and incubated with trypsin (2.5 mg/mL) and DNase (0.15 mg/mL) in serum-free medium for 30 min at 37°C. The trypsinized tissues were centrifuged for 5 min at 1,800 rpm, resuspended in Dulbecco’s modified Eagle cell culture medium containing 10% fetal bovine serum (FBM), triturated, and filtered through a 100-µm-diameter pore, nylon mesh Cell Strainer (Greiner Bio-One, Monroe, NC). The mixed glia were centrifuged for 5 min at 1,800 rpm, resuspended in FBM, and filtered through a 40-µm-diameter pore, nylon mesh Cell Strainer (Greiner) yielding mixed glia with nominal numbers of neurons that do not survive under our culture conditions (28). Mixed glia were plated in glass-bottom, 35-mm-diameter dishes covered with Geltrex (Gibco, ThermoFisher Scientific, Waltham, MA) at ∼75,000 cells/dish density. In the second step, 7–8 days later, striatal neurons were prepared from embryonic day 15–17 CD1 pups. Striata were dissected, minced, and digested with trypsin (2.5 mg/mL) and DNase (0.15 mg/mL) in Neurobasal Medium (Invitrogen, Waltham, MA) supplemented with B-27 additives (Invitrogen), l-glutamine (0.5 mM; Invitrogen), glutamate (25 μM; Sigma-Aldrich, Inc., St. Louis, MO), and a penicillin-streptomycin mixture (Invitrogen; 30 min, 37°C). The digested tissues were centrifuged for 5 min, resuspended, triturated, and filtered with a 70-µm-diameter pore mesh nylon filter. The mixture of cells was centrifuged again for 5 min, resuspended, triturated, and filtered with a 70-µm-diameter pore mesh nylon filter. The final product contained nominal glial contamination (27). Neurons were added to the mixed glial cultures at a density of ∼25,000 cells per dish. Cocultures were maintained in Neurobasal Medium (Invitrogen) supplemented with B-27 additives (Invitrogen), l-glutamine (0.5 mM; Invitrogen), glutamate (25, 12.5, and 0 μM, days 1–4, 5–6, and 7–9, respectively; Sigma-Aldrich, Inc., St. Louis, MO), and antibiotic mixture (Invitrogen) at 37°C in humidified 5% CO2:95% air for 7–8 days.
Neuronal Culture
Cultures enriched in striatal neurons were prepared identically as described earlier but plated on poly-l-lysine-coated glass-bottom 35-mm dishes without underlying glia. These cultures were used for immunocytochemical study and for the leak-subtracted voltage-dependent step current recordings.
Electrophysiology of Dissociated Striatal Neurons
Electrophysiological recordings from striatal neurons cocultured with mixed glia were performed 7–8 days after neurons were plated. All data were obtained by performing whole cell patch-clamp recordings in voltage-clamp mode using a HEKA double-patch-clamp EPC10 USB amplifier (HEKA Instruments, Inc, Holliston, MA) at a sampling frequency of 20 kHz and low-frequency filtering at 2.9 kHz. Series resistance was compensated at 80%. Pipette resistance ranged from 2.8 to 6.9 MΩ. We utilized borosilicate glass with a filament (Item No. BF150-86-10, Sutter Instruments, Novato, CA) to fabricate patch-clamp pipettes using a Sutter P-1000 micropipette puller (Sutter Instruments). Neurons were visualized by using a Zeiss Axio Observer.D1 inverted microscope (Carl Zeiss Microscopy LLC, White Plains, NY). Neurons were approached with pipettes attached to the S-probe of the patch-clamp amplifier mounted on a stainless-steel MP-845S micromanipulator operated by the TRIO MPC-100 controller (TRIO MPC-145 System, Sutter Instruments). The system provided exceptional mechanical stability permitting prolonged recordings. Data were collected at room (21–23°C) temperature. Extracellular (bath) solution usually consisted of the following (in mM): 150 N-methyl-d-glucamine-Cl (NMDG-Cl), 2 CaCl2, 2 MgCl2, 0.1 LaCl3, 0.0006 tetrodotoxin (TTX), 10 HEPES, pH 7.4 adjusted with NMDG-OH. Low extracellular Cl− solution contained (in mM) 150 NMDG-methanesulfonate, 2 CaCl2, 2 MgCl2, 0.1 LaCl3, 0.0006 TTX, 10 HEPES, pH7.4 adjusted with NMDG-OH. The intracellular (pipette) solution contained the following (in mM): 150 NMDG-methanesulfonate, 5 NMDG2-EGTA, 4.04 CaCl2 (300 nM free Ca2+ concentration), 10 HEPES, pH 7.4 adjusted with NMDG-OH. For symmetrical Cl− patch-clamp recordings, the pipette solution consisted of the following (in mM): 150 NMDG-Cl, 2 CaCl2, 2 MgCl2, 0.1 LaCl3, 10 HEPES, pH 7.4 adjusted with NMDG-OH, and the extracellular solution was the same as the pipette solution supplemented with 600 nM TTX. Cells were held at −70 mV, and Cl− current was evoked by applying voltage stimuli in the form of a ramp from −100 to +100 mV with a climbing rate of 1 mV/ms. The ramp stimuli were applied as time series at 5-s interstimulus intervals. For leak-subtracted patch-clamp recordings, we used a standard built-in P/8 method of the scaled leak and capacitive current subtraction (PatchMaster V2, HEKA). The leak current was held at −110 mV, and maximum and minimum scaled leak pulses were −62.5 mV and −117 mV, respectively. Hundred-millisecond-long step currents were elicited by stepping from the resting membrane potential of −70 mV to a voltage in a range from −100 to +120 mV with 10-mV increment delivered every 3 s. Step current amplitudes were measured as average values for the last 10 ms of the step duration. Step currents were followed by 100-ms-long repolarizing step to −50 mV to evoke tail currents characteristic for channels closing. Tail current amplitude was measured 0.3 ms later after stepping to −50 mV by averaging current values during the next 0.7 ms of the recording. Normalized tail currents were obtained by dividing the tail current amplitude values by one obtained at +120 mV step.
Striatal Slice Preparation
Adult male mice expressing D1-tdTomato- or D2-eGFP-labeled MSNs were anesthetized using 4% isoflurane for at least 3 min and maintained on isoflurane throughout the process of transcardial perfusion. Mice were transcardially perfused with calcium-free sucrose cutting medium containing the following (in mM): 3 KCl, 4.12 MgSO4, 1.2 NaH2PO4, 206 sucrose, 25 NaHCO3, and 25 glucose aerated using a 5% CO2 and 95% O2 mixture. Brains were extracted and cut coronally on a Leica VT1200 S vibratome in 350-μm-thick slices while submerged in aerated sucrose cutting medium held at 1–3°C by an external cooling apparatus (Huber mini chiller, Raleigh, NC). Slices were kept in the aerated artificial cerebrospinal fluid (ACSF) solution at 37°C for 30 min and were then maintained at room temperature.
Electrophysiology of Neurons in Striatal Slices
Patch-clamp recordings were obtained from D1 and D2 dorsolateral striatal MSNs. Brain slices were placed into a recording chamber mounted on a Zeiss Axio Examiner.A1 upright, fixed-stage microscope. The recording chamber was constantly perfused with ACSF solution containing (in mM) 125 NaCl, 3 KCl, 1.2 CaCl2, 1.2 MgSO4, 1.2 NaH2PO4, 25 NaHCO3, and 25 glucose and aerated with a gas mixture of 5% CO2-95% O2. The speed of perfusion varied from 3.8 mL/min to 4.5 mL/min. D1-tdTomato-labeled neurons were visualized using Prizmatix Ultra High-Power LED system with filter cubes for red wavelengths (607AF75 nm excitation, 650DRLP dichroic, 695AF55 nm emission; filter set XF140-2, Omega Optical) or for green wavelengths (470/40 nm excitation, 495 dichroic, 525/50 nm emission; filter set 38, Cat. No. 000000-1031-346; Zeiss) and a DAGE-MTI IR 1000 monochrome camera. Patch-clamp pipettes (8.0–8.5 MΩ) were pulled from borosilicate glass with a filament (Item No. BF150-86-10, Sutter Instruments, Novato, CA) on a Narishige PC-10 two-step puller (Narishige International USA, Inc, Amityville, NY). Pipettes were backfilled with intracellular solution containing the following (in mM): 140 K-gluconate, 10 KCl, 1 NaCl, 1 CaCl2, 1 MgCl2 4 Mg-ATP, 5 EGTA, 10 HEPES, pH7.4. Neurons were approached with glass pipettes fixed in a patch-clamp amplifier headstage mounted on Sutter MPC-200-ROE micromanipulator. Whole cell current-clamp recordings were obtained by using a MultiClamp 700B amplifier coupled with an Axon Digidata 1550 A digital-analog converter and pClamp 11 software (Molecular Devices, San Jose, CA). Action potentials were evoked by injecting −100- to 600-pA currents at 25-pA increments with each step lasting 500 ms and recorded in current-clamp mode. All recordings were done at 32°C.
Immunoblotting
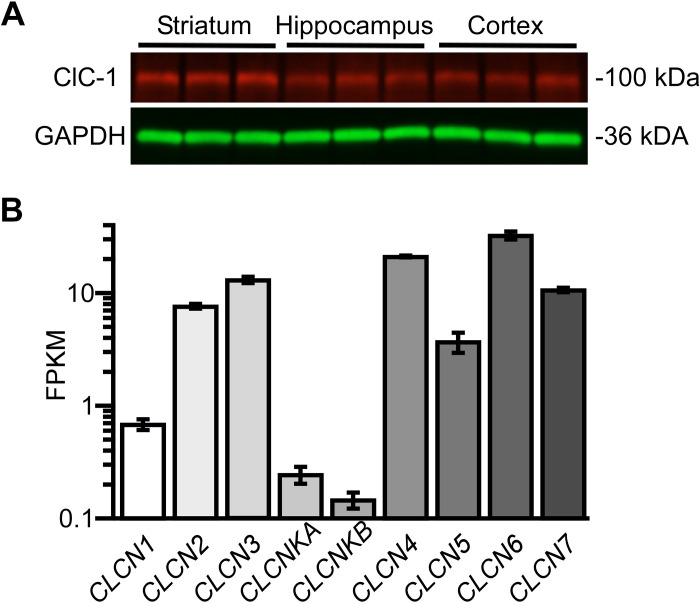
ClC-1 proteins were examined by immunoblotting of striatal, hippocampal, and cortical samples from CD1 mice (2 mo old, n = 3). Striata, hippocampi, and cortices were freshly harvested and homogenized with RIPA buffer (Cat. No. R0278, Sigma-Aldrich, Inc.), including a protease/phosphatase inhibitor cocktail (Cat. No. 78446, Thermo Fisher Scientific). Precellys 24 tissue homogenizer (Bertin Corp., Rockville, MD) and ceramic bead-containing tubes (Cat. No. CK14, Bertin Corp.) were used to homogenize the striatal tissue samples. Homogenized tissue lysates were centrifuged and stored at −80°C until use. The protein concentration of each sample was measured using a BCA protein assay kit (Pierce, Rockford, IL). Striatal, hippocampal, and cortical lysates of 30 μg were loaded per well onto 4–20% Tris·HCl Ready Gels (Cat. No. 5671094, Bio-Rad Laboratories, Hercules, CA). Precision Plus Protein Dual Color Standards [Cat. No. 1610374 Bio-Rad; molecular weight (MW) range = 10–250 kDa] were loaded onto a separate lane to verify the protein transfer and as molecular weight standards. Proteins were transferred to activated PVDF membranes (Cat. No. 1620264, Bio-Rad). A rabbit anti-CLCN1 antibody (1:1,000, Abcam, Cat. No. ab189857) was used to probe the blots for ClC-1 channel presence. Antibodies against glyceraldehyde 3-phosphate dehydrogenase (GAPDH, 1:2,000, Abcam, Cat. No. ab8245) detected GAPDH as an internal standard to normalize protein loading. F(ab')2-goat anti-rabbit IgG (H + L) cross-adsorbed, Alexa Fluor 647 (Thermo Fisher Scientific, Cat. No. A-21246) and goat anti-mouse IgG (H + L) cross-adsorbed, Alexa Fluor 488 (Thermo Fisher Scientific, Cat. No. A-11029) conjugated secondary antibodies were applied to visualize each protein band. Protein bands were detected on a ChemiDoc Imaging System and their intensity was analyzed by Image Lab Software (Bio-Rad). In addition to published reports, we confirmed that the anti-CLC-1 antibody 1) displayed a discrete band at the appropriate molecular weight (∼100 kDa; Fig. 5) and 2) that CLC-1 immunofluorescence was no longer detectable after the CLC-1 antibody was preabsorbed with the same peptide used to generate the antigenic response (Fig. 6). Other antibodies (NeuN and MAP2) used in this study have been exhaustively validated by us (29, 30), other investigators, and their unique and well-established cellular/subcellular and cell-type specific patterns of immunoreactivity.
Tissue Processing
Male Tat− mice ∼120 days old were anesthetized with 4% isoflurane for >3 min and maintained on isoflurane anesthesia until transcardial perfusion with 4% paraformaldehyde was achieved. Fixed brains were removed and placed in 4% PFA in PBS overnight and then washed 3 × 2 h with PBS the next day. Brains were then transferred to 10% sucrose solution overnight, moved to a 20% sucrose solution for 24 h, and embedded in Tissue-Tek O.C.T. compound (Sakura Finetek, Torrance, CA). Brains were sectioned in the coronal plane at a thickness of 18 μm. A Leica CM1850 cryostat was used to cut the sections (Leica Biosystems, Buffalo Grove, IL). Sections were mounted on slides (Fisherbrand Tissue Path Superfrost Plus Gold) and stored at −80°C until use.
Immunohistochemistry
Slides stored at −80°C were briefly warmed to room temperature for ∼5 min. All rinses were conducted at room temperature and rinsed in PBS 3 × 10 min, unless noted otherwise. Tissue sections were outlined with a PAP pen (Thermo-Fisher) and gently rehydrated with 1× PBS. Tissue was pretreated with 50%, 70%, and then 50% ethanol in 1× PBS for 20 min each; permeabilized for 30 min in 0.1% Tween-20 and 0.1% BSA in 1× PBS; blocked in 1% normal goat serum in 1× PBS (30 min, RT); and then incubated with rabbit anti-CLCN1 (Cat. No. ab189857; Abcam; 1:250) and mouse anti-MAP2 (Cat. No. ab11267; Abcam; 1:500) or NeuN (Cat. No. MAB377; Millipore; 1:200) primary antibodies in blocking solution overnight at 4°C. Sections were washed and incubated in fluorescently conjugated secondary antibodies (goat anti-rabbit Alexa Fluor 594, Cat. No. A11037; Invitrogen; 1:500; goat anti-mouse Alexa Fluor 488, Cat. No. A11001; Invitrogen; 1:500) for 45 min. Negative controls were obtained by 1) omitting primary anti-CLCN1 and NeuN antibodies and 2) preabsorbing anti-CLCN1 antibodies with recombinant human CLCN1 protein (Cat. No. ab158131, 1:50, Abcam). Slices were washed and Hoechst 33342 nuclear stain (Cat. No. H3570, Invitrogen, 1:20,000) was applied for 5 min. After a final rinse, slides were mounted in ProLong Gold Antifade reagent (Invitrogen, Cat. No. P36930). Stacks of ∼9 images (at 63×) were taken in the striatum using a Zeiss LSM 700 confocal microscope with Zen 2.3 software (Zeiss Inc., Thornwood, NY). Z-stacks of optical sections were processed using Image J.
Immunocytochemistry
After 7 days in culture, dissociated striatal neurons were fixed in 4% paraformaldehyde and stored at +8°C until use. Neurons were permeabilized (0.1% Triton X-100, 1% normal goat serum) for 15 min. After being blocked for 1 h (1% normal goat serum, 0.1% BSA), primary rabbit anti-CLCN1 (Cat. No. ab189857; Abcam; 1:250) and mouse anti-MAP2 (Cat. No. ab11267; Abcam; 1:500) antibodies were used to label ClC-1 and MAP2 antigenicity in neurons, after which nuclei were identified by 5-min application of nuclear Hoechst 33342 stain (1:20,000). After a final 3 × 10 min rinse with PBS, stacks of ∼9 images (at 63×) were taken using a Zeiss LSM 700 confocal microscope with Zen 2.3 software (Zeiss Inc.). Z-stacks of optical sections were processed using Image J.
Next-Generation Sequencing
RNA was isolated from freshly harvested striatal tissue with the miRNeasy Mini Kit (Qiagen, Hilden Germany) as per the manufacturer’s directions. After passing quality controls, RNA samples were used for preparation of sequencing libraries via the polyA selection approach; three biological replicates were performed. RNA sequencing was conducted by GENEWIZ (South Plainfield, NJ) using the Illumina HiSeq, PE 2x150 platform. ENSEMBLE IDs for each gene were translated to the gene name using https://www.biotools.fr/mouse/ensembl_symbol_converter, and gene expression levels were presented as fragments per kilobase exon per million mapped fragments (FPKM). FPKM values for n = 3 mice per treatment group were averaged and presented on a logarithmic scale using Prism 9 software (GraphPad Software, San Diego, CA).
Offline Data Analyses and Statistics
Electrophysiological recording analyses were performed using Igor Pro 8 (WaveMetrics, Lake Oswego, OR), Clampfit 10.7 (Molecular Devices, San Jose, CA), Prism 8 (GraphPad Software, San Diego, CA), and Microsoft Office Excel 2016 (Microsoft Corporation, Redmond, WA) software suites.
To characterize the Cl− current in the cultured neurons in time series, we measured the Cl− current amplitude at the end of each ramp voltage stimulus at +100 mV (I100 mV). The effects of extracellular Cl− replacement with methanesulfonate (MsO−), zero Cl− transmembrane gradient, and Cl− channel-specific and nonspecific blockers were evaluated as a fraction of I100 mV change.
To describe the effect of ClC-1 channel blocker 9-anthracenecarboxylic acid (9-AC) on current injection-evoked action potentials in striatal MSNs, we measured the frequency of action potentials at each injected current step. The frequency of firing was evaluated using two approaches. Using the first approach, we calculated the number of action potentials generated during the current injection and normalized it to the duration of the stimulus (0.5 s). Using a second approach, we measured the time between action potential peaks, calculated the average interpeak duration, and determined the firing frequency as a reciprocal to the average interpeak duration. This is especially advantageous if neurons stop firing after a few action potentials. Combined, both calculations provide a more comprehensive assessment of ClC-1-dependent alterations in the action potential firing characteristics.
Statistical significance (P < 0.05) was determined using a Student’s paired two-tailed t test for within-cell comparisons. A one-sample t test was used for within-group statistical analyses. One-way analysis of variance and Tukey’s post hoc test were used to assess differences among three independent groups.
RESULTS
Cl− Current in Cultured Striatal Neurons Is Voltage Dependent and Outwardly Rectifying
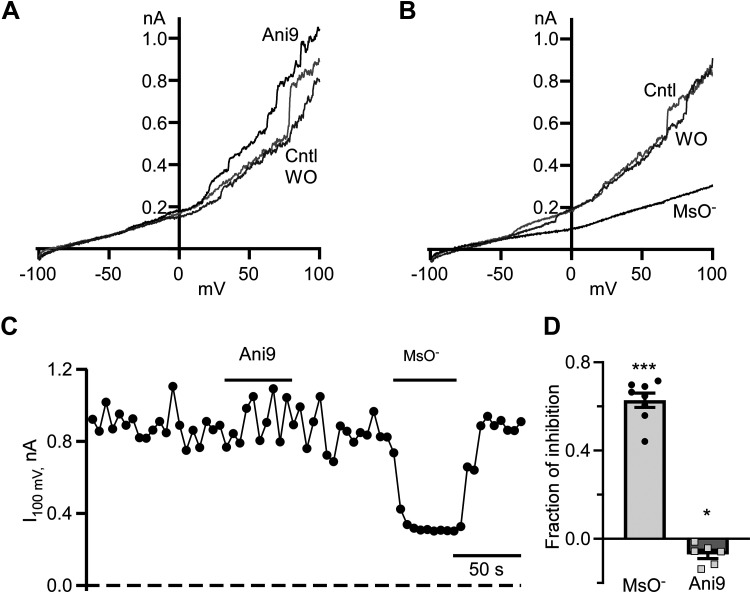
Our understanding of Cl− channels and their roles in neurons tends to overemphasize well-described ionotropic GABAARs and GlyRs. We tested whether other types of Cl− channels might be operational in striatal neurons. To isolate Cl− currents in whole cell patch-clamp recordings, we used extracellular and intracellular solutions favoring Cl− conductance only (see materials and methods). By applying voltage ramps from −100 to +100 mV, we observed a nonlinear current with progressively larger values at higher depolarizing voltages (Fig. 1, A and B). To confirm that the nonlinear current is mainly anionic, we replaced 95% of extracellular Cl− with nonpermeable methanesulfonate anion (MsO−). MsO− caused a robust and reversible decrease in the current amplitude (Fig. 1, B–D). The fraction of current decrease measured at +100 mV was 0.63 ± 0.03 (n = 7, P < 0.0001, one-sample t test). This finding strongly supported the idea that Cl− contributes significantly to the whole cell current in striatal neurons. We also found that applications of 10 µM Ani9, a specific blocker of the Ca2+-dependent Cl− TMEM16A channel (31), did not inhibit the current measured at the end of the voltage ramp. Instead, Ani9 caused a small, but nevertheless statistically significant, increase in current (Fig. 1, A–D). The fraction of current increase was only 0.07 ± 0.02 (n = 6, P < 0.05, one-sample t test).
Figure 1.
Cl− currents in striatal neurons in mixed neuronal-glial coculture. A: representative voltage ramp-evoked current traces were recorded in control (Cntl), at the end of 10 µM Ani9 application (Ani9), and after Ani9 washout (WO). B: typical voltage ramp-evoked current traces were recorded in controls (Cntl), at the end of extracellular Cl− replacement with methanesulfonate (MsO−), and after MsO− washout (WO). C: time course of a current measured at the end of voltage ramp at +100 mV (I100 mV). Durations of Ani9 application and Cl− replacement with MsO− are shown as horizontal lines. Currents were measured every 5 s. D: fraction of inhibition of I100 mV in the presence of MsO− and Ani9. ***P < 0.001, n = 8, one-sample t test, *P < 0.05, n = 6, one-sample t test.
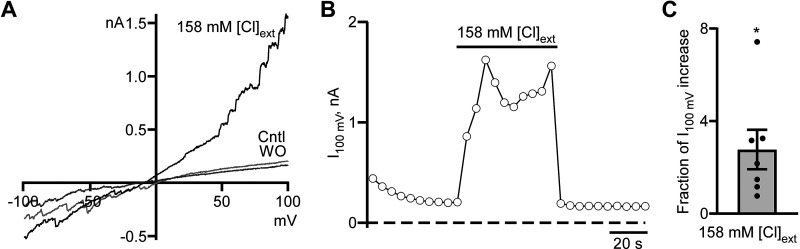
The Cl− current we detected in whole cell configuration appeared to be both voltage dependent and outwardly rectifying, implying that functional voltage-dependent Cl− channels are expressed by MSNs in vitro. To confirm this, we conducted a series of experiments, which started in the presence of low Cl− levels extracellularly and high Cl− concentrations in the recording pipette. The extracellular Cl− concentration was then increased for ∼50 s to match the intracellular Cl− concentration using the same solution as in the pipette except that tetrodotoxin was added to the solution, which resulted in a fast and reversible increase in the whole cell current that was more prominent at higher depolarizing voltages (Fig. 2). The fraction of current increase measured at the end of the ramp (+100 mV) was 2.77 ± 0.85 (n = 7, P < 0.05, one-sample t test; Fig. 2C). The detection of increased voltage-dependent Cl− current recorded in symmetrical Cl− solutions strongly suggests that at least one of the major contributors to it is a voltage-dependent Cl− channel that belongs to the ClC channel/transporter family (23). Since the pipette solution had a high concentration of Ca2+ (2 mM), it should lead to the loss of the outward rectification and current linearization if TMEM16B (Ano2) channels contribute significantly to the whole cell Cl− current (32). We never observed linearization in our experiments. Thus, we conclude that TMEM16B channels did not contribute to the whole cell current.
Figure 2.
Cl− current in striatal neurons in mixed neuronal-glial coculture is voltage dependent. A: Representative voltage ramp-evoked current traces were recorded in control (Cntl), at the end of symmetrical Cl− solution application (158 mM [Cl]ext), and washout (WO). B: time course of Cl− current measured at the end of voltage ramp at +100 mV (I100 mV). The duration of symmetrical Cl− solution (158 mM [Cl]ext) application is shown as a horizontal line. Currents were measured every 5 s. C: the fraction of I100 mV increase in symmetrical Cl− solution. *P < 0.05, n = 7, one-sample t test.
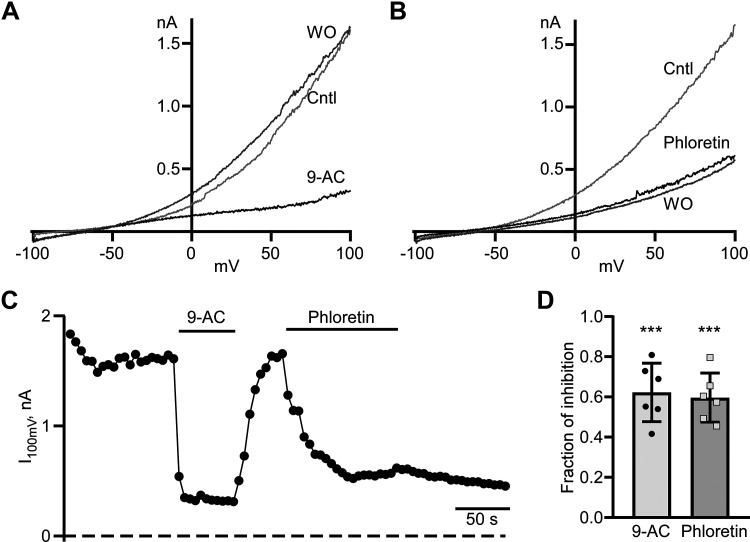
Voltage-Dependent Cl− Current Is Sensitive to 9-AC and Phloretin
The voltage-dependent Cl− currents we observed in striatal neurons were similar but not identical to previously identified currents in wild-type and mutant ClC-1 channels from skeletal muscle heterologously expressed in Xenopus laevis oocytes (33, 34). We used 9-AC, a ClC-1 inhibitor, to determine the extent to which ClC-1 channels contribute to voltage-dependent Cl− currents in cultured striatal neurons. We found that the application of 1 mM 9-AC triggered the rapid and reversible inhibition of the Cl− current (Fig. 3, A, C, and D) resulting in a fractional current decrease at +100 mV of 0.62 ± 0.6 (n = 6, P < 0.001, one-sample t test; Fig. 3D). Another nonselective Cl− channel inhibitor, phloretin, irreversibly decreased Cl− current to a similar degree, with fractional inhibition of 0.60 ± 0.05 (n = 6, P < 0.0001, one-sample t test; Fig. 3D). Statistical analysis of the fractional inhibition of the Cl− current at +100 mV caused by extracellular Cl− replacement with MsO− or of the inhibitory action of the ClC-1 blocker 9-AC or the nonspecific Cl− channel blocker phloretin revealed no significant differences between these three groups [F(2,17) = 0.1318; P = 0.8744, one-way ANOVA with Tukey’s post hoc test]. Together, our results indicate that ClC-1-like channels are likely to be major contributors to voltage-dependent Cl− currents in striatal neurons.
Figure 3.
Cl−current is sensitive to ClC-1 inhibitor 9-AC and nonspecific blocker phloretin. A: representative voltage ramp-evoked current traces were recorded in control (Cntl), at the end 1 mM 9-AC application (9-AC), and washout (WO). B: typical voltage ramp-evoked current traces were recorded in control (Cntl), at the end of extracellular 0.3 mM phloretin application (phloretin), and washout (WO). C: time course of a current measured at the end of voltage ramp at +100 mV (I100 mV). Durations of 9-AC and phloretin application are shown as horizontal lines. Currents were measured every 5 s. D: fraction of inhibition of I100 mV in the presence of 9-AC and phloretin. ***P < 0.001, n = 6, one-sample t test.
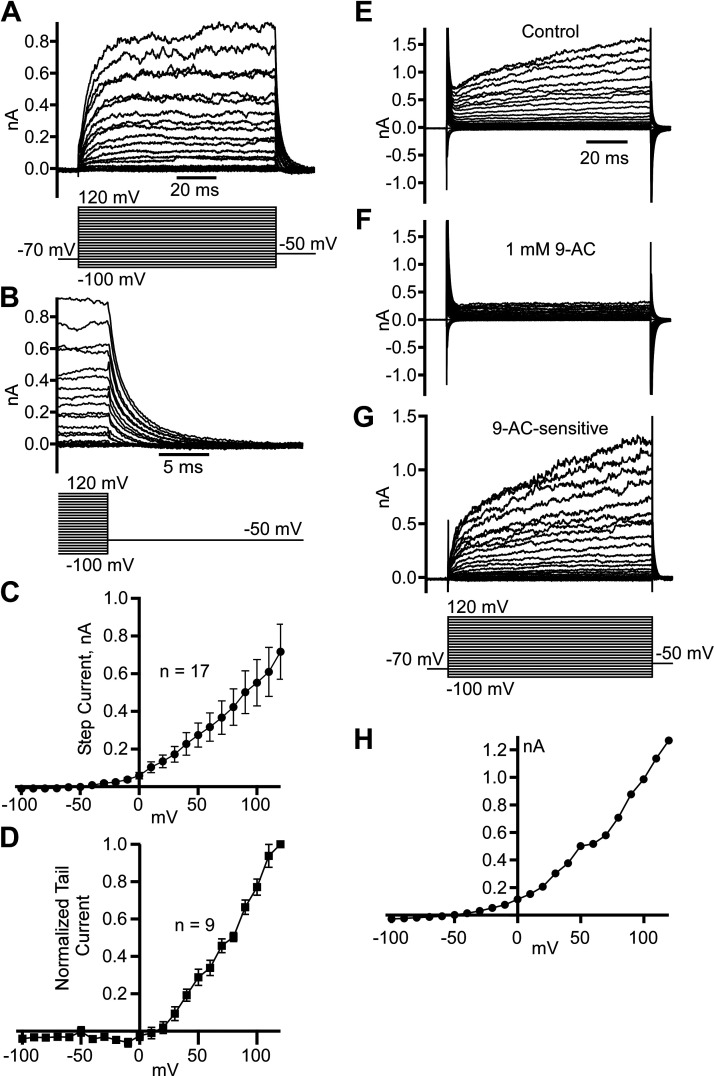
Leak-Subtracted Voltage-Dependent Current Shows a Reversal Potential below −50 mV as Expected for Cl− Channels
To isolate pure voltage-dependent current, we used the P/8 method for leak and capacitive current subtraction for whole cell patch-clamp recordings from dissociated striatal neurons (Fig. 4). The isolated step currents demonstrated outward rectification similar to what we observed with ramp-induced currents above and with a threshold of activation ranging from −50 to −40 mV (Fig. 4, A and C). The step currents demonstrated fast activation followed by steady-state noninactivating current typical for ClC channels (34). On repolarization to −50 mV, we observed a fast decay of outward tail currents (Fig. 4B), and the direction of the current indicated that the reversal potential is below −50 mV, as expected for Cl− current recordings at our experimental conditions. Interestingly, tail currents normalized to the tail current measured at +120 mV demonstrated an activation threshold at +10 mV and no expected saturation at high voltages (Fig. 4D). To confirm our findings, we also isolated voltage-dependent currents by recording step and tail currents before (Fig. 4E) and after 1 mM 9-AC application (Fig. 4F) and subtracted them to find 9-AC-sensitive current (Fig. 4G). The subtracted current voltage dependence (Fig. 4H) was similar to that obtained using the P/8 subtraction method (Fig. 4, A–C).
Figure 4.
Leak-subtracted voltage-dependent Cl− current demonstrates a predicted reversal potential below −50 mV. A: examples of leak-subtracted (P/8 method) step currents caused by voltage steps in a range from −100 to +120 mV. B: the same as in A recordings magnify the last 5 ms of step currents and outward tail currents caused by the −50 mV step. C: step current voltage dependence (means ± SE) obtained from 17 neurons. D: normalized tail current voltage dependence (means ± SE) obtained from 9 neurons. Examples of step current traces obtained at conditions of no leak subtraction in control (E), after 1 mM 9-AC application (F), and 9-AC sensitive (G) obtained by subtracting corresponding traces in F from E. H: 9-AC-sensitive step current voltage dependence measured at the end of the step currents in G.
ClC-1-Like Channels Are Expressed in the Plasma Membrane of Striatal Neurons
Next-generation sequencing confirmed the presence of all ClC channel/transporter family members in murine striatum (Fig. 5B). To further confirm that ClC-1-like channels are expressed in striatal neurons, Western blotting analysis identified the presence of this channel at the expected molecular weight marker (∼100 kDa) in murine striatal lysates, as well as in hippocampal and cerebral cortical lysates (Fig. 5A).
Figure 5.
ClC-1 channel antigenicity and mRNA were detected in murine striatum. A: CLC-1 channel antigenicity was detected at ∼100 kDa in the striatum, hippocampus, and cerebral cortex by immunoblotting. CLC-1 channel antigenicity was detected at ∼100 kDa in the striatum with anti-ClC-1 primary and Alexa Fluor 647-conjugated secondary antibodies, whereas the loading control (GAPDH) is detected by an Alexa Fluor 488-conjugated secondary antibody at ∼36 kDa. B: next-generation sequencing shows that ClC family gene (ClCN1, ClCN 2, ClCN3, ClCNKA, ClCNKB, ClCN4, ClCN5, ClCN6, ClCN7) transcripts were detected in the striatum. FPKM, fragments per kilobase of transcript per million mapped reads.
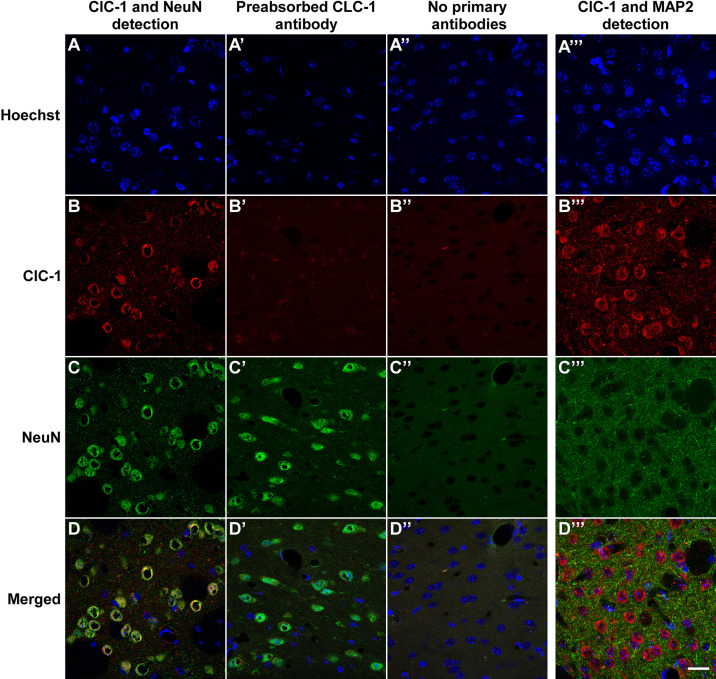
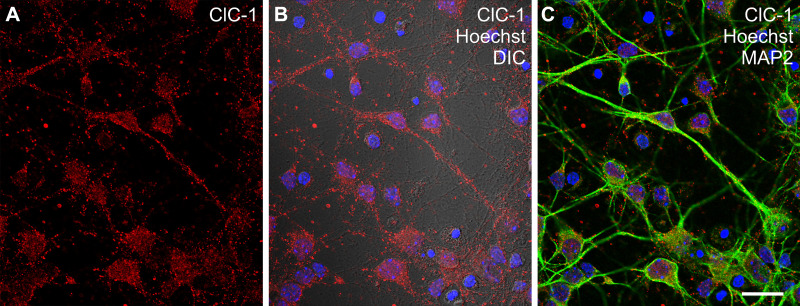
Since the striatal lysates contain some nonneural tissue, such as vascular endothelium, associated smooth muscle, and meningeal tissue, we performed immunohistochemical detection of ClC-1 antigenicity in striatal tissue sections (Fig. 6) and in dissociated MSNs in vitro (Fig. 7). Our immunohistochemical findings clearly demonstrated that ClC-1-like channels are localized in striatal neurons (Figs. 6 and 7). Moreover, ClC-1-like channels were predominantly localized to NeuN- or MAP2- immunoreactive neuronal perikarya (Fig. 6) and, to a lesser extent, displayed infrequent, punctate foci along MAP2-immunopositive dendrites (Figs. 6 and 7). Together, the biochemical, morphological, and electrophysiological data support the hypothesis that ClC-1-like channels are expressed by striatal neurons and that they can generate a substantial outward current, especially at higher depolarizing potentials.
Figure 6.
Pronounced immunohistochemical detection of ClC-1 channel expression in the striatum. Columns show the detection of primary ClC-1 and NeuN immunofluorescence (A), controls in which primary anti-ClC-1 antibodies were preabsorbed with ClC-1 blocking peptide (A′), controls in which primary anti-ClCN1 and NeuN antibodies were excluded (A′′), and the detection of primary ClC-1 and MAP2 immunofluorescence (A′′′). Sections were counterstained with Hoechst 33342 to visualize cell nuclei in the striatum. B, B′, B′′, and B′′′: ClC-1-specific immunofluorescence was preferentially associated with the cell bodies of MSNs; ClC-1 antigenicity was detected in dendrites and perhaps elsewhere confirming its presence in the striatum. NeuN (C, C′, and C′′) and MAP2 (C′′′) markers localize immunofluorescence to soma and dendrites, respectively. D, D′, D′′, and D′′′: merged images confirm soma localization of neuronal ClC-1 channels. Scale bar = 20 µm. MSNs, medium spiny neurons.
Figure 7.
Immunocytochemical detection of ClC-1-like channel antigenicity in dissociated striatal neurons. A: ClC-1 channel immunofluorescence (red) was readily detected in MSNs in vitro. B: Differential interference contrast (DIC) microscopy revealed ClC-1 immunofluorescence in association with both large- and fine-diameter neuritic processes. C: ClC-1 antigenicity was colocalized with MAP2 (green fluorescence) immunoreactive perikarya and dendrites of MSNs, and the cell cultures were additionally counterstained with Hoechst 33342 to reveal cell nuclei (blue fluorescence). Scale bar = 20 μm. MSNs, medium spiny neurons.
9-AC-Sensitive Cl− Currents Differentially Affect the Excitability of D1- and D2-Receptor-Expressing Medium Spiny Neurons
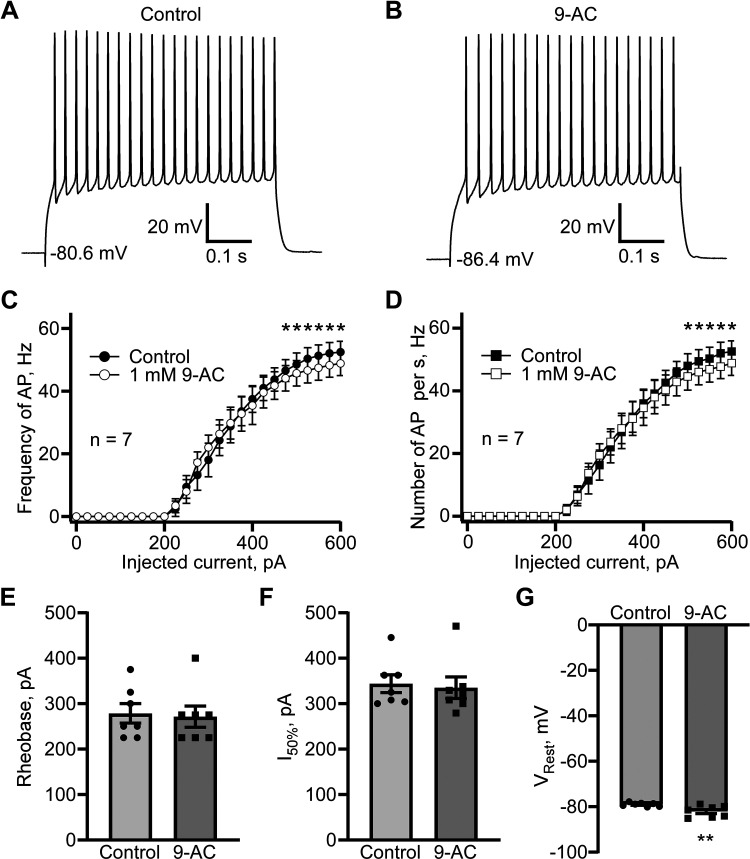
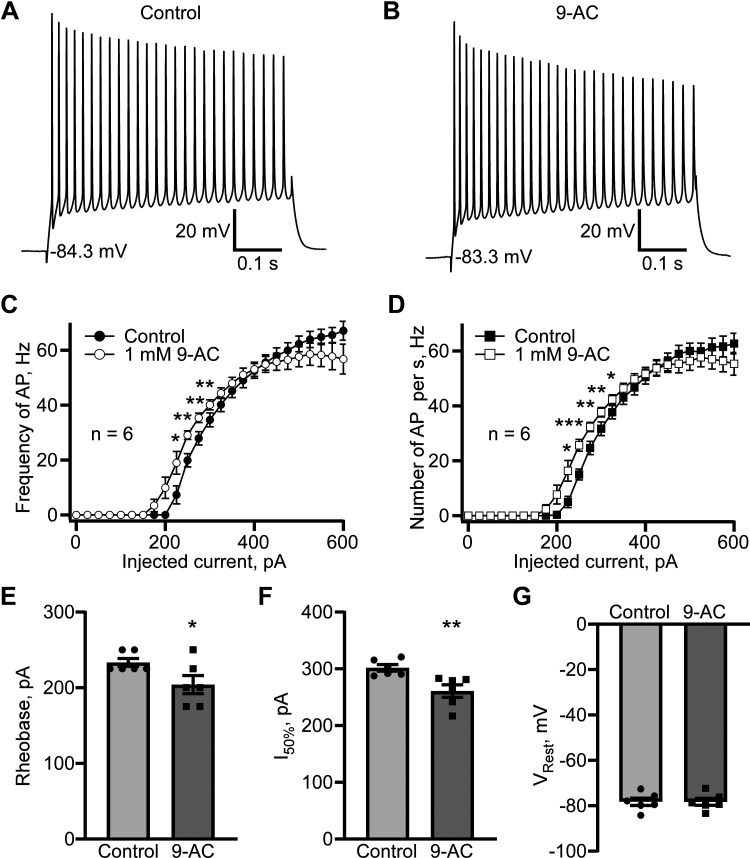
Because of their biophysical similarities to ClC-1, including their voltage-dependent activation profile and fast activation and slower deactivation (33, 34), ClC-1-like channels may cause action potentials to repolarize more rapidly. Specifically, ClC-1-like channels may be critical for accelerating the action potential repolarization phase and, therefore, maintain higher firing frequencies. Striatal mouse brain slices were used to confirm this hypothesis. Current-clamp recordings from D1 MSNs revealed that perfusing the slices with ClC-1 inhibitor 9-AC (1 mM, 5 min) reduced the firing frequency at higher stimulating currents (≥500 pA) on average ∼ 8.5%–9.7% (Fig. 8, A–D). This result provides strong support for our hypothesis that ClC-1-like channels are critical regulators of action potential repolarization. There were no effects of 1 mM 9-AC on rheobase (Fig. 8E) or the current (I50%) needed to trigger an action potential frequency 50% of the maximum (Fig. 8F). Instead, 1 mM 9-AC caused a minor but significant hyperpolarization of the resting membrane potential, VRest, of 3.0 ± 0.7 mV (n = 7, P < 0.01, paired t test; Fig. 8G). Given that the Cl− equilibrium potential was −75.1 mV, which resulted in an inward depolarizing Cl− current at resting membrane potentials in controls, we cannot exclude the possibility of a persistent, residual Cl− current sensitive to 9-AC that contributes slightly, albeit significantly, to the resting membrane potential. In D2 MSNs, the effect of 1 mM 9-AC application was different. We observed a leftward shift in action potential firing versus injected current dependence with the statistically significant increase in the firing frequency caused by 9-AC at the 225 pA (n = 6, P < 0.05, paired t test), 250 pA (n = 6, P < 0.01, paired t test), 275 pA (n = 6, P < 0.01, paired t test), and 300 pA (n = 6, P < 0.01, paired t test) current injections (Fig. 9C). The number of action potentials normalized to the duration of stimulation was significantly increased by 9-AC at 225 pA (n = 6, P < 0.05, paired t test), 250 pA (n = 6, P < 0.001, paired t test), 275 pA (n = 6, P < 0.01, paired t test), 300 pA (n = 6, P < 0.01, paired t test), and 325 pA (n = 6, P < 0.05, paired t test; Fig. 9D). We did not observe a significant difference in action potential firing rates at high injected currents (Fig. 9, C and D). The leftward shift of D2 MSNs firing frequency can be perfectly illustrated by a statistically significant decrease in rheobase (n = 6, P < 0.05) and I50% (n = 6, P < 0.01, paired t test; Fig. 9, E and F). Unlike in D1 MSNs, 9-AC did not affect the resting membrane potential in D2 MSNs.
Figure 8.
ClC-1 inhibitor 9-AC causes a reduction in action potential firing in striatal D1 MSN in the brain slices. Examples of action potential firing evoked by 600 pA current injection in control (A) and after 5 min perfusion with 9-AC (1 mM; B). Action potential firing evaluated as a firing frequency measured as a reciprocal value of mean interaction potential duration (C) and as several action potentials normalized to the stimulus duration (D). *P < 0.05, n = 7, paired t test. E: rheobase (means ± SE) obtained in control and in the presence of 9-AC (1 mM), P > 0.05, not significantly different, paired t test. F: current causing 50% of action potential firing (I50%, pA, means ± SE) calculated in control and in the presence of 9-AC (1 mM), P > 0.05, not significantly different, paired t test. G: resting membrane potential (Vrest, mV, means ± SE) obtained in control and in the presence of 1 mM 9-AC, P < 0.01, n = 7, paired t test. MSN, medium spiny neuron.
Figure 9.
The ClC-1 inhibitor 9-AC causes a leftward shift in action potential firing vs. injected current dependence in striatal D2 MSN in the brain slices. Examples of action potential firing evoked by 600 pA current injection in control (A) and after perfusing 5 min with 9-AC (1 mM; B). Action potential firing evaluated as a firing frequency measured as a reciprocal value of mean interaction potential duration (C) and as several action potentials normalized to the stimulus duration (D). Total of n = 6 recordings analyzed. Statistically significant differences at *P < 0.05, **P < 0.01, and ***P < 0.001 were obtained using Student’s paired t test analysis. E: rheobase (means ± SE) obtained in control and in the presence of 1 mm 9-AC is significantly different (n = 6, *P <0.05, paired t test). F: current causing 50% of action potential firing (I50%, pA, means ± SE) calculated in control and in the presence of 1 mm 9-AC is significantly different (n = 6, **P <0.01, paired t test). G: resting membrane potential (Vrest, mV, means ± SE) obtained in control and in the presence of 1 mM 9-AC not significantly different (n = 6, P > 0.05, paired t test). MSN, medium spiny neuron.
DISCUSSION
Here we demonstrate for the first time, to our knowledge, the presence of voltage-dependent Cl− channels in striatal neurons with properties similar to, but not identical to, skeletal muscle-specific ClC-1 channels (23, 33, 34). For these reasons, we refer to the channel we describe in MSNs as “ClC-1-like.” The Cl− current in MSNs is outwardly rectifying and voltage dependent and sensitive to both the ClC-1 inhibitor 9-AC and the nonspecific Cl− channel blocker phloretin. We also demonstrate the presence of ClC-1-like channels in striatal neurons, including MSNs, by immunoblotting and immunocytochemistry. Interestingly, ClC-1 immunofluorescence is primarily located in the cell body and, to a lesser extent, in dendrites, which likely uniquely impacts the biophysical properties of MSNs (Electrophysiological and Pharmacological Properties of Cl- Currents in Striatal Neurons). We conclude that ClC-1-like channels are present and functional in striatal neurons. These channels may be important modulators of the responsiveness of MSNs to glutamatergic (e.g., corticostriatal and thalamo-cortico-striatal) and dopaminergic (e.g., nigrostriatal) afferents.
Electrophysiological and Pharmacological Properties of Cl− Currents in Striatal Neurons
Our results showed that whole cell currents were reversibly decreased when 95% of the extracellular Cl− was replaced with the large anion MsO− or following incubation in the ClC-1- inhibitor 9-AC or the nonspecific blocker phloretin in neuronal-mixed glia cocultures. The effects of both 9-AC and phloretin were statistically similar to replacing Cl− with MsO−. Importantly, the Cl− current was outwardly rectifying and voltage dependent.
Voltage-dependent Cl− currents were isolated using the P/8 method of the leak and capacitive current subtraction. The generated step currents differed somewhat from wild-type and mutant ClC-1 (34). The tail currents were outward at −50 mV, confirming that ECl is below −50 mV, as we expected in our experiments for Cl− channels. The normalized tail current voltage dependence that was supposed to serve as a measure of the open probability voltage dependence, however, showed an activation threshold at +10 mV. Although we expected it to be much more negative, even below −50 mV, this paradoxical result could be explained by several contributing factors. These include (i) an inward gating current caused by voltage-gated Ca2+ and K+ deactivation gating charge movement interfering with the outward tail current; (ii) a rapid and undetectable deactivation of Cl− current at weak depolarizations; and (iii) the accuracy and precision of the leak subtraction at less-than-ideal patch-clamp conditions (e.g., neuron-related space clamp issues, the presence of multiple types of alternative voltage-gated channels) (e.g., neuron-related space clamp issues, the presence of multiple types of other voltage-gated channels). Nevertheless, normalized tail currents showed that the maximum open probability is expected to be higher than +120 mV, which sets striatal neuronal voltage-dependent Cl− channel apart from known ClC channels. We have also detected a 9-AC-sensitive step current similar to one obtained with P/8 subtraction. Unfortunately, this method requires exceptionally stable recordings, as one we used to illustrate in Fig. 4, E–H. The subtraction also doubles the noise, which decreases the feasibility of this approach considerably.
The inhibitory action of 9-AC supported the idea that Cl− currents were caused by the activation of ClC-1 channels. However, the voltage dependence of the current observed in striatal MSNs differed somewhat from the properties of ClC-1 in skeletal muscle cells or immature myotubes (35, 36), ClC-1-expressing Xenopus laevis oocytes (33, 34, 37), or HEK293 cells (36). Specifically, the voltage dependence of the Cl− current we describe in MSNs had a strong rightward shift and failed to display a saturating Cl− current at the end of the voltage ramps as is expected based on the known step-current voltage dependence of ClC-1 channels (33, 35, 36, 38). The reasons for the discrepancy may result from differing experimental conditions, including differences in 1) extracellular and intracellular solutions (surface charge screening effect), 2) holding potentials, and/or 3) in the parameters of the stimulus protocol. However, a speculative, but likely, reason for the disparity may reflect neuron-specific alterations in ClC-1 channel gating that necessitate a high degree of depolarization for ClC-1 activation and/or contamination from endosomal/vesicular ClC transporter Cl− currents, such as ClC-3, ClC-4, and ClC-5 (23), which also require a high degree of depolarization for channel activation. Myotonia congenita mutations in ClC-1 channels can alter the activation gating of these channels over a wide range of voltages until there is strong outward rectification with limited or no saturation at strong depolarizations (23, 33, 34) and their properties are almost identical to what we observe in the present study. Specifically, the human ClC-1 mutation I290M displays no current saturation at positive potentials because of a ∼70-mV rightward shift in the activation voltage dependence (34). Other mutations occurring in the D5–D6 transmembrane domain and cytoplasmic loop between D5 and D6 cause a rightward shift in ClC-1 channel activation (33). Thus, it is possible that altered gating of neuronal ClC-1 channels is a result of unidentified cytoplasmic modification or via the expression of a novel splice variant encoding the D5–D6 region.
We found that phloretin could irreversibly inhibit Cl− currents. It is known that phloretin is an efficient, although nonselective, inhibitor of ClC-3 channels (39) and that the half-maximum activation voltage (V1/2) of ClC-3 is about +75 mV (40). Similarly, ClC-4 and ClC-5 are also strong, outwardly rectifying Cl− transporters with very high V1/2 (40). If ClC-3 is also found in the synaptic vesicles (41, 42), it may potentially traffic to the plasma membrane during neurotransmitter release and may modulate (albeit subtly) the net Cl− current. Indeed, a sudden jump in current amplitude was observed in some recordings, which could be explained by an unexpected availability of a group of open channels (see Fig. 1, A–C). Since ClC-3, ClC-4, and ClC-5 become open under very high depolarizing conditions, we feel their contribution to the whole cell Cl− current in striatal neurons would be minor under physiological conditions.
The differences between the inhibitory effects of 9-AC and phloretin are not readily explained by a sensitivity of ClC-1 channels to phloretin or by complex interactions between ClC-1 and ClC-3 channels. To our knowledge, there is no published evidence that phloretin inhibits ClC-1. Assuming this is correct, we speculate that ClC-1 and ClC-3 channels interact either via convergent downstream signaling or possibly through the formation of hetero/oligomeric molecular complexes. Therefore, the observed heteromeric current does not fit completely the activation profile of ClC-1 and ClC-3 and is sensitive to 9-AC and phloretin. It is known that ClC-1 and ClC-2 channels can form heterodimers and that closely related ClC-3, ClC-4, and ClC-5 transporters can also form heterodimers (for review, see Ref. 23).
Finally, we cannot exclude the involvement of a voltage-gated Cl− channel in neurons with a similar sensitivity to 9-AC and ClC-1 antibodies. Accordingly, additional detailed studies of neuronal voltage-dependent Cl− channel expression and function are warranted.
At this juncture, we can exclude several potential candidates. Strong outward rectification, almost identical to the properties observed here, is one of the characteristics of Ca2+-dependent Cl− channels TMEM16A (Ano1) and TMEM16B (Ano2) (32, 43, 44). We found that TMEM16A-specific inhibitor Ani9 increased Cl− current on a small but statistically significant fraction. To our knowledge, there are no known TMEM16B channel-specific inhibitors commercially available to verify if these channels contribute to the total whole cell Cl− current in MSNs. Nevertheless, in a series of experiments with high intracellular Cl− concentration and 2 mM CaCl2 in the pipette solution, high cytoplasmic Ca2+ concentration failed to result in a loss of TMEM16B current outward rectification (see Fig. 2) (32). Thus, TMEM16A and TMEM16B channels did not contribute to Cl− current in MSNs.
Outward rectification of anion current is a signature of pannexin-1 channels, which are responsible for ATP transport from the cytoplasm into the extracellular space (45). Although they are present in neurons (46), we do not think that these channels were the major contributors to the whole cell Cl− current since pannexin-1 is not sensitive to 9-AC (47).
Finally, it is worth noting that the first author recently reported similar Cl− current voltage dependence in urinary bladder detrusor smooth muscle (DSM) cells (48, 49). However, based on other properties, it is unlikely that both striatal neurons and DSM cells have the same type of Cl− channels. In DSM, the Cl− current was always mechanosensitive. Extracellular solution flow caused an increase in the current amplitude, which we rarely observed in the cultured neurons. In addition, Cl− current activation in DSM appeared to be detected at stronger depolarizing membrane potentials. The molecular identity of DSM Cl− channels remains unknown.
ClC-1 Transcripts and Protein Are Detected in Striatal Neurons
Our findings indicate that ClC-1-like channels are expressed in striatal MSNs. ClC-1 channels were identified in the brain in one study and proposed to be critical controllers of neuronal excitability (24). These investigators found nonsynonymous single nucleotide polymorphisms in the CLCN1 gene in 152 individuals with sporadic epilepsy of unknown origin at a threefold higher frequency than in healthy controls (24). They also detected CLCN1 mRNA and protein in specific regions of human postmortem and murine brain regions (24). Although this study is reported to have limitations (23), publicly available data in the Allen Brain Institute database provide additional evidence of robust CLCN1 expression in the brain, including the striatum, using in situ hybridization (Allen Mouse Brain Atlas, Allen Institute; https://mouse.brain-map.org/gene/show/12507). Our immunostaining results are in line with the idea that ClC-1 channels can be expressed in the brain and indicate that they are found in, at least, D1-expressing MSNs in the striatum. The channels were mostly localized at the plasma membrane of the soma of neurons and nominally distributed in dendrites, a spatial distribution that suggests ClC-1 may be critical for regulating high-frequency action potentials. ClC-1 may also be a major source of intracellular Cl− replenishment in the soma of neurons but likely affects intracellular Cl− homeostasis differently in dendrites (2, 6). For this reason, we predict that ClC-1-like channels preferentially regulate action potentials in MSNs at higher frequencies and may be even more critical during action potential bursting activity. Although ClC-1 appeared to be expressed at lower levels in the dendrites than in the soma, the presence of ClC-1 in dendrites may have a profound effect on synaptic integration and/or plasticity if the larger surface-to-volume ratio is considered. It is clear that differences in the subcellular site(s) of ClC-1 localization would result in dramatic differences in the channels’ ability to regulate neuronal activity and that a differential distribution of the channels on different neuronal types may be a key factor in regulating intrastriatal microcircuitry.
Role of Voltage-Dependent Cl− Channels in Neurons
To confirm the importance of 9-AC-sensitive Cl− channels in neuronal excitability, we demonstrated that 9-AC reduced the frequency of firing in D1 MSNs at strong depolarizing current injections and produced a leftward shift in firing frequency versus injected current dependence in D2 MSNs. In D1 MSNs, the observed effect was expected because at higher firing frequencies, ClC-1-like channels remain in the open state longer as a result of robust, voltage-dependent activation gating (33) and a larger driving force defined by ECl. Thus, the 9-AC effect is expected to be most prominent at stronger depolarizations and high Cl− currents. By contrast, in D2 MSNs, the 9-AC effect is likely due to the modulation of presynaptic inputs. Assuming D2 MSNs express ClC-1-like channels at low levels (no statistically significant effect of 9-AC at the largest injected currents), and the neurons projecting to D2 MSNs express ClC-1-like channels at high levels, the inhibition of these channels should increase their sensitivity to the injected current.
Although the detailed mechanisms of differential effects caused by 9-AC in D1 and D2 neurons are not known, we hypothesize that neuronal ClC-1-like channels can be one of many critical factors defining neuron types and their activity patterns in the striatum.
We also found that 9-AC slightly, but significantly, lowered resting membrane potential. At our experimental conditions, Cl− equilibrium potential was above the resting membrane potential. Thus, a small fraction of Cl− current at the resting membrane potential should cause a small depolarization, and this depolarization should be reversed by 9-AC. In D2 MSNs, we observed no significant differences in the resting membrane potential between control and 9-AC-treated groups. Collectively, this and the aforementioned findings suggest that D2 MSNs express ClC-1-like channels at low or inconsistent levels.
Unlike other neuron types, D1 and D2 MSNs have extremely negative resting membrane potentials and must partially depolarize to reach a subthreshold plateau before they can fire (50). Depending on [Cl−]i, and, thus, the Cl− equilibrium potential, even small, persistent Cl− currents may facilitate or restrict MSNs from reaching a subthreshold plateau. The former is possible when the Cl− equilibrium potential is higher than the resting membrane potential, under conditions when [Cl−]i is high. The latter is likely when [Cl−]i is low, resulting in a Cl− equilibrium potential below the resting membrane potential. Thus, intracellular Cl− replenishment via GABAARs, GlyRs, ClC-1, or other voltage-dependent Cl− channels must be effectively counterbalanced by KCC2 transporter activity.
Role of Voltage-Dependent Neuronal Cl− Channels in Disease
ClC-1 channels have been linked to idiopathic epilepsy (24). We speculate that disruptions in Cl− homeostasis include voltage-dependent Cl− channels and may contribute to the pathophysiology of some CNS disorders. For example, although mutations in ClC-1 channels may not cause epilepsy, their altered function could enable other causal factors. Thus, ClC-1 channel dysfunction may contribute to deficits in KCC2 function caused by HIV-1 Tat or associated with epilepsy or cognitive deficits linked to Huntington’s disease (10, 13–15, 17).
To conclude, we demonstrate for the first time, to our knowledge, that there are functional voltage-dependent Cl− currents in striatal neurons and that neuronal ClC-1-like channels at least partially contribute to the Cl− current. More detailed studies, including loss-of-function and gain-of-function strategies, are necessary to further confirm the presence and role of ClC-1-like channels in neurons, including their regional and cell-type-specific patterns of expression and their involvement in dynamic Cl− regulation and in neuropathology.
GRANTS
This research was supported by the National Institutes of Health, National Institute on Drug Abuse (NIDA) Grants R01 DA034231 (to K.F.H. and P.E.K.), R01 DA045588, and R01 DA018633 (to K.F.H) and the pilot grant project for the P30 Center Grant on Drug Abuse Research (to V.Y.) funded via the NIH NIDA P30 Grant P30 DA033934 (Principal Investigator Dr. W.L. Dewey), F32DA053163 (to S.R.N), and R01 MH107507 (to A.R.M.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
V.Y., A.R.S.L., S.R.N., Y.K.H., A.R.M., P.E.K., and K.F.H. conceived and designed research; V.Y., A.R.S.L., S.R.N., Y.K.H., and M.G.M. performed experiments; V.Y., A.R.S.L., S.R.N., Y.K.H., and M.G.M. analyzed data; V.Y., A.R.S.L., S.R.N., Y.K.H., A.R.M., and K.F.H. interpreted results of experiments; V.Y., A.R.S.L., Y.K.H., and M.G.M. prepared figures; V.Y. drafted manuscript; V.Y., A.R.S.L., S.R.N., Y.K.H., M.G.M., A.R.M., P.E.K., and K.F.H. edited and revised manuscript; V.Y., A.R.S.L., S.R.N., Y.K.H., M.G.M., A.R.M., P.E.K., and K.F.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Heather Herman and Tao Tian for superb technical assistance.
REFERENCES
- 1.Ebihara S, Shirato K, Harata N, Akaike N. Gramicidin-perforated patch recording: GABA response in mammalian neurones with intact intracellular chloride. J Physiol 484: 77–86, 1995. doi: 10.1113/jphysiol.1995.sp020649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doyon N, Vinay L, Prescott SA, De Koninck Y. Chloride regulation: a dynamic equilibrium crucial for synaptic inhibition. Neuron 89: 1157–1172, 2016. doi: 10.1016/j.neuron.2016.02.030. [DOI] [PubMed] [Google Scholar]
- 3.Payne JA, Stevenson TJ, Donaldson LF. Molecular characterization of a putative K-Cl cotransporter in rat brain. A neuronal-specific isoform. J Biol Chem 271: 16245–16252, 1996. doi: 10.1074/jbc.271.27.16245. [DOI] [PubMed] [Google Scholar]
- 4.Payne JA. Functional characterization of the neuronal-specific K-Cl cotransporter: implications for [K+]o regulation. Am J Physiol Cell Physiol 273: C1516–C1525, 1997. doi: 10.1152/ajpcell.1997.273.5.C1516. [DOI] [PubMed] [Google Scholar]
- 5.Johansson S, Yelhekar TD, Druzin M. Commentary: chloride regulation: a dynamic equilibrium crucial for synaptic inhibition. Front Cell Neurosci 10: 182, 2016. doi: 10.3389/fncel.2016.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Currin CB, Trevelyan AJ, Akerman CJ, Raimondo JV. Chloride dynamics alter the input-output properties of neurons. PLoS Comput Biol 16: e1007932, 2020. doi: 10.1371/journal.pcbi.1007932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huguenard JR, Alger BE. Whole-cell voltage-clamp study of the fading of GABA-activated currents in acutely dissociated hippocampal neurons. J Neurophysiol 56: 1–18, 1986. doi: 10.1152/jn.1986.56.1.1. [DOI] [PubMed] [Google Scholar]
- 8.Gulledge AT, Stuart GJ. Excitatory actions of GABA in the cortex. Neuron 37: 299–309, 2003. doi: 10.1016/s0896-6273(02)01146-7. [DOI] [PubMed] [Google Scholar]
- 9.Hewitt SA, Wamsteeker JI, Kurz EU, Bains JS. Altered chloride homeostasis removes synaptic inhibitory constraint of the stress axis. Nat Neurosci 12: 438–443, 2009. doi: 10.1038/nn.2274. [DOI] [PubMed] [Google Scholar]
- 10.Barbour AJ, Nass SR, Hahn YK, Hauser KF, Knapp PE. Restoration of KCC2 membrane localization in striatal dopamine D2 receptor-expressing medium spiny neurons rescues locomotor deficits in HIV Tat-transgenic mice. ASN Neuro 13: 17590914211022089, 2021. doi: 10.1177/17590914211022089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lorenzo LE, Godin AG, Ferrini F, Bachand K, Plasencia-Fernandez I, Labrecque S, Girard AA, Boudreau D, Kianicka I, Gagnon M, Doyon N, Ribeiro-da-Silva A, De Koninck Y. Enhancing neuronal chloride extrusion rescues α2/α3 GABAA-mediated analgesia in neuropathic pain. Nat Commun 11: 869, 2020. doi: 10.1038/s41467-019-14154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang BL. The expanding therapeutic potential of neuronal KCC2. Cells 9: 240, 2020. doi: 10.3390/cells9010240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dargaei Z, Bang JY, Mahadevan V, Khademullah CS, Bedard S, Parfitt GM, Kim JC, Woodin MA. Restoring GABAergic inhibition rescues memory deficits in a Huntington's disease mouse model. Proc Natl Acad Sci USA 115: E1618–E1626, 2018. doi: 10.1073/pnas.1716871115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen L, Wan L, Wu Z, Ren W, Huang Y, Qian B, Wang Y. KCC2 downregulation facilitates epileptic seizures. Sci Rep 7: 156, 2017. doi: 10.1038/s41598-017-00196-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelley MR, Cardarelli RA, Smalley JL, Ollerhead TA, Andrew PM, Brandon NJ, Deeb TZ, Moss SJ. Locally reducing KCC2 activity in the hippocampus is sufficient to induce temporal lobe epilepsy. EBioMedicine 32: 62–71, 2018. doi: 10.1016/j.ebiom.2018.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delpire E. Advances in the development of novel compounds targeting cation-chloride cotransporter physiology. Am J Physiol Cell Physiol 320: C324–C340, 2021. doi: 10.1152/ajpcell.00566.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kahle KT, Khanna AR, Duan J, Staley KJ, Delpire E, Poduri A. The KCC2 cotransporter and human epilepsy: getting excited about inhibition. Neuroscientist 22: 555–562, 2016. doi: 10.1177/1073858416645087. [DOI] [PubMed] [Google Scholar]
- 18.Succol F, Fiumelli H, Benfenati F, Cancedda L, Barberis A. Intracellular chloride concentration influences the GABAA receptor subunit composition. Nat Commun 3: 738, 2012. doi: 10.1038/ncomms1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang WC, Xiao S, Huang F, Harfe BD, Jan YN, Jan LY. Calcium-activated chloride channels (CaCCs) regulate action potential and synaptic response in hippocampal neurons. Neuron 74: 179–192, 2012. doi: 10.1016/j.neuron.2012.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang W, Schmelzeisen S, Parthier D, Frings S, Möhrlen F. Anoctamin calcium-activated chloride channels may modulate inhibitory transmission in the cerebellar cortex. PLoS One 10: e0142160, 2015. doi: 10.1371/journal.pone.0142160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li KX, He M, Ye W, Simms J, Gill M, Xiang X, Jan YN, Jan LY. TMEM16B regulates anxiety-related behavior and GABAergic neuronal signaling in the central lateral amygdala. eLife 8, 2019. doi: 10.7554/eLife.47106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stöhr H, Heisig JB, Benz PM, Schöberl S, Milenkovic VM, Strauss O, Aartsen WM, Wijnholds J, Weber BH, Schulz HL. TMEM16B, a novel protein with calcium-dependent chloride channel activity, associates with a presynaptic protein complex in photoreceptor terminals. J Neurosci 29: 6809–6818, 2009. doi: 10.1523/JNEUROSCI.5546-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jentsch TJ, Pusch M. CLC chloride channels and transporters: structure, function, physiology, and disease. Physiol Rev 98: 1493–1590, 2018. doi: 10.1152/physrev.00047.2017. [DOI] [PubMed] [Google Scholar]
- 24.Chen TT, Klassen TL, Goldman AM, Marini C, Guerrini R, Noebels JL. Novel brain expression of ClC-1 chloride channels and enrichment of CLCN1 variants in epilepsy. Neurology 80: 1078–1085, 2013. doi: 10.1212/WNL.0b013e31828868e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bruce-Keller AJ, Turchan-Cholewo J, Smart EJ, Geurin T, Chauhan A, Reid R, Xu R, Nath A, Knapp PE, Hauser KF. Morphine causes rapid increases in glial activation and neuronal injury in the striatum of inducible HIV-1 Tat transgenic mice. Glia 56: 1414–1427, 2008. doi: 10.1002/glia.20708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.El-Hage N, Gurwell JA, Singh IN, Knapp PE, Nath A, Hauser KF. Synergistic increases in intracellular Ca2+, and the release of MCP-1, RANTES, and IL-6 by astrocytes treated with opiates and HIV-1 Tat. Glia 50: 91–106, 2005. doi: 10.1002/glia.20148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zou S, Fitting S, Hahn YK, Welch SP, El-Hage N, Hauser KF, Knapp PE. Morphine potentiates neurodegenerative effects of HIV-1 Tat through actions at μ-opioid receptor-expressing glia. Brain 134: 3616–3628, 2011. doi: 10.1093/brain/awr281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Podhaizer EM, Zou S, Fitting S, Samano KL, El-Hage N, Knapp PE, Hauser KF. Morphine and gp120 toxic interactions in striatal neurons are dependent on HIV-1 strain. J Neuroimmune Pharmacol 7: 877–891, 2012. doi: 10.1007/s11481-011-9326-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hahn YK, Masvekar RR, Xu R, Hauser KF, Knapp PE. Chronic HIV-1 Tat and HIV reduce Rbfox3/NeuN: evidence for sex-related effects. Curr HIV Res 13: 10–20, 2015. doi: 10.2174/1570162x13666150311163733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fitting S, Knapp PE, Zou S, Marks WD, Bowers MS, Akbarali HI, Hauser KF. Interactive HIV-1 Tat and morphine-induced synaptodendritic injury is triggered through focal disruptions in Na+ influx, mitochondrial instability, and Ca2+ overload. J Neurosci 34: 12850–12864, 2014. doi: 10.1523/JNEUROSCI.5351-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seo Y, Lee HK, Park J, Jeon DK, Jo S, Jo M, Namkung W. Ani9, a novel potent small-molecule ANO1 inhibitor with negligible effect on ANO2. PLoS One 11: e0155771, 2016. doi: 10.1371/journal.pone.0155771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cenedese V, Betto G, Celsi F, Cherian OL, Pifferi S, Menini A. The voltage dependence of the TMEM16B/anoctamin2 calcium-activated chloride channel is modified by mutations in the first putative intracellular loop. J Gen Physiol 139: 285–294, 2012. doi: 10.1085/jgp.201110764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kubisch C, Schmidt-Rose T, Fontaine B, Bretag AH, Jentsch TJ. ClC-1 chloride channel mutations in myotonia congenita: variable penetrance of mutations shifting the voltage dependence. Hum Mol Genet 7: 1753–1760, 1998. doi: 10.1093/hmg/7.11.1753. [DOI] [PubMed] [Google Scholar]
- 34.Pusch M, Steinmeyer K, Koch MC, Jentsch TJ. Mutations in dominant human myotonia congenita drastically alter the voltage dependence of the CIC-1 chloride channel. Neuron 15: 1455–1463, 1995. doi: 10.1016/0896-6273(95)90023-3. [DOI] [PubMed] [Google Scholar]
- 35.Lueck JD, Rossi AE, Thornton CA, Campbell KP, Dirksen RT. Sarcolemmal-restricted localization of functional ClC-1 channels in mouse skeletal muscle. J Gen Physiol 136: 597–613, 2010. doi: 10.1085/jgp.201010526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lueck JD, Mankodi A, Swanson MS, Thornton CA, Dirksen RT. Muscle chloride channel dysfunction in two mouse models of myotonic dystrophy. J Gen Physiol 129: 79–94, 2007. doi: 10.1085/jgp.200609635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steinmeyer K, Ortland C, Jentsch TJ. Primary structure and functional expression of a developmentally regulated skeletal muscle chloride channel. Nature 354: 301–304, 1991. doi: 10.1038/354301a0. [DOI] [PubMed] [Google Scholar]
- 38.Imbrici P, Altamura C, Pessia M, Mantegazza R, Desaphy JF, Camerino DC. ClC-1 chloride channels: state-of-the-art research and future challenges. Front Cell Neurosci 9: 156, 2015. doi: 10.3389/fncel.2015.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsuda JJ, Filali MS, Volk KA, Collins MM, Moreland JG, Lamb FS. Overexpression of CLC-3 in HEK293T cells yields novel currents that are pH dependent. Am J Physiol Cell Physiol 294: C251–C262, 2008. doi: 10.1152/ajpcell.00338.2007. [DOI] [PubMed] [Google Scholar]
- 40.Guzman RE, Grieschat M, Fahlke C, Alekov AK. ClC-3 is an intracellular chloride/proton exchanger with large voltage-dependent nonlinear capacitance. ACS Chem Neurosci 4: 994–1003, 2013. doi: 10.1021/cn400032z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salazar G, Love R, Styers ML, Werner E, Peden A, Rodriguez S, Gearing M, Wainer BH, Faundez V. AP-3-dependent mechanisms control the targeting of a chloride channel (ClC-3) in neuronal and non-neuronal cells. J Biol Chem 279: 25430–25439, 2004. doi: 10.1074/jbc.M402331200. [DOI] [PubMed] [Google Scholar]
- 42.Stobrawa SM, Breiderhoff T, Takamori S, Engel D, Schweizer M, Zdebik AA, Bösl MR, Ruether K, Jahn H, Draguhn A, Jahn R, Jentsch TJ. Disruption of ClC-3, a chloride channel expressed on synaptic vesicles, leads to a loss of the hippocampus. Neuron 29: 185–196, 2001. doi: 10.1016/s0896-6273(01)00189-1. [DOI] [PubMed] [Google Scholar]
- 43.Segura-Covarrubias G, Aréchiga-Figueroa IA, De Jesús-Pérez JJ, Sanchez-Solano A, Pérez-Cornejo P, Arreola J. Voltage-dependent protonation of the calcium pocket enable activation of the calcium-activated chloride channel anoctamin-1 (TMEM16A). Sci Rep 10: 6644, 2020. doi: 10.1038/s41598-020-62860-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cruz-Rangel S, De Jesús-Pérez JJ, Contreras-Vite JA, Pérez-Cornejo P, Hartzell HC, Arreola J. Gating modes of calcium-activated chloride channels TMEM16A and TMEM16B. J Physiol 593: 5283–5298, 2015. doi: 10.1113/JP271256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J 25: 5071–5082, 2006. doi: 10.1038/sj.emboj.7601378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang Y, Grinspan JB, Abrams CK, Scherer SS. Pannexin1 is expressed by neurons and glia but does not form functional gap junctions. Glia 55: 46–56, 2007. doi: 10.1002/glia.20435. [DOI] [PubMed] [Google Scholar]
- 47.Ma W, Hui H, Pelegrin P, Surprenant A. Pharmacological characterization of pannexin-1 currents expressed in mammalian cells. J Pharmacol Exp Ther 328: 409–418, 2009. doi: 10.1124/jpet.108.146365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yarotskyy V, Malysz J, Petkov GV. Extracellular pH and intracellular phosphatidylinositol 4,5-bisphosphate control Cl- currents in guinea pig detrusor smooth muscle cells. Am J Physiol Cell Physiol 317: C1268–C1277, 2019. doi: 10.1152/ajpcell.00189.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yarotskyy V, Malysz J, Petkov GV. Properties of single-channel and whole cell Cl- currents in guinea pig detrusor smooth muscle cells. Am J Physiol Cell Physiol 316: C698–C710, 2019. doi: 10.1152/ajpcell.00327.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wickens JR, Wilson CJ. Regulation of action-potential firing in spiny neurons of the rat neostriatum in vivo. J Neurophysiol 79: 2358–2364, 1998. doi: 10.1152/jn.1998.79.5.2358. [DOI] [PubMed] [Google Scholar]