Abstract
Background
ChAdOx1-S (Covishield™/Vaxzervria, AstraZeneca) and BBV152 (Covaxin) SARS-CoV-2 vaccines are proven to be safe and effective, but rare complications have been reported.
Objective
To describe reports of central nervous system (CNS) demyelination following ChAdOx1-S and BBV152 vaccinations.
Methods & Results
We report 29 (17 female; mean 38 years) cases of CNS demyelination; twenty-seven occurred in temporal association with ChAdOx1-S vaccine; two in association with BBV152 vaccine. Eleven patients had presentation with myelitis, six patients developed optic neuritis, five had acute demyelinating encephalomyelitis, three presented with brainstem demyelination, and four had multiaxial involvement. Myelin oligodendrocyte glycoprotein (MOG) antibodies were positive in ten patients. One patient with ADEM and tumefactive demyelinating lesions died after a prolonged intensive care unit stay and superimposed infection. As compared to the control group (87); the postvaccinial cases were found to have a significantly higher mean age, presence of encephalopathy (p value:0.0007), CSF pleocytosis (p value: 0.0094) and raised CSF protein (p value: 0.0062).
Conclusions
It is difficult to establish a causal relationship between vaccination and neurological adverse events such as demyelination. The temporal association with the vaccination and the presence of MOG antibodies raises the possibility of an immunogenic process triggered by the vaccine in susceptible individuals.
Keywords: MOG, SARS-CoV-VACCINE, COVID-19, CNS DEMYELINATION
Abbreviations: SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus-2; CNS, Central Nervous System; MOG, Myelin Oligodendrocyte Glycoprotein; ADEM, Acute Disseminated Encephalomyelitis; CSF, Cerebrospinal Fluid; COVID-19, Coronavirus Disease 2019; AEFI, Adverse Events Following Immunization; CVST, Cerebral Venous Sinus Thrombosis; VITT, Vaccine Induced Thrombotic Thrombocytopenia; GBS, Guillain-Barre Syndrome; ON, Optic Neuritis; ATM, Acute Transverse Myelitis; NMO, Neuromyelitis Optica; MRI, Magnetic Resonance Imaging; VEP, Visual Evoked Potential; BAER, Brainstem Auditory Evoked Response; SSEP, Somatosensory Evoked Potential; ANA, Antinuclear Antibody; CRP, C-Reactive Protein
1. Introduction
COVID-19, caused by novel beta-coronavirus, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has become the biggest health concern worldwide. The world is striving to combat the pandemic with the available medical facilities and recently developed vaccines. As of December 21 2021, 828,519,766 (59.18%) of the Indian population have received at least the first dose, while 554,958,415 (39.64%) have completed full vaccination of one of the three vaccines: ChAdOx1-S (Covishield™/Vaxzervria, Astra-Zeneca), BBV152 (Covaxin) and Gam-COVID-Vac (Sputnik V)(https://www.cowin.gov.in/) Though these were proven to be safe and effective in randomised controlled trials (Voysey et al., al.,2021; https://www.bharatbiotech.com; Logunov et al., al.,2021) very rare, but significant adverse events following immunisation (AEFI) have been detected in post-market release surveillance. amongst neurological complications, the association with ChAdOx1-S vaccine has been confirmed for cerebral venous sinus thrombosis (CVST) due to vaccine-induced thrombotic thrombocytopenia (VITT) (Alam W.,2021); however, isolated reports of Guillain Barre Syndrome (GBS) (Maramattom et al., al.,2021; Waheed et al., al.,2021) and central nervous system (CNS) demyelination have been published. We have recently observed CNS demyelination ranging from optic neuritis (ON), acute transverse myelitis (ATM), Acute demyelinating encephalomyelitis (ADEM) and brainstem demyelination in the immediate post-vaccination period. Hereby, we report on 29 patients with different neurological manifestations of CNS demyelination presenting within six weeks of vaccination against SARS-CoV-2 reported from a tertiary university hospital from India over a period from May to December 2021.
2. Patients and methods
All patients were evaluated prospectively at neurological emergency and outpatient services at a tertiary-care university hospital in south India from 15th May 2021 to 8th December 2021. The capture and reporting of the patients’ data are covered by the institutional ethics approval already in place for the Multiple Sclerosis and Allied Demyelination Registry maintained at the institute. The inclusion criteria comprised: a) receipt of a SARS-CoV-2 vaccine, either first or second dose, within the past 42 days (according to World Health Organization Global Advisory Committee on Vaccine safety- WHO GACVS)(https://vaccine-safety-training.org/frequency-and-severity.html) b) No recent history of COVID-19 infection within the past 3 months; and c) Evidence of CNS demyelination based on clinical and radiological features. The exclusion criterion was presence of other precipitating factors besides SARS-CoV-2 vaccine as a cause for demyelination in the last 3 months. Patients fulfilling these criteria had the following data captured: demographic profile, clinical features as evaluated by a consultant neurologist, the type of COVID-19 vaccine, investigations including hemogram, biochemical parameters, CSF (cerebrospinal fluid) analysis, serum and/or CSF neuromyelitis optica (NMO) antibodies, myelin oligodendrocyte glycoprotein (MOG) antibodies (testing done with IgG1), magnetic resonance imaging (MRI) of the brain and/or spine, evoked potentials [visual evoked potentials (VEP), brainstem auditory evoked response (BAER), somatosensory evoked potential (SSEP)] and ancillary investigations to exclude alternative aetiologies: serum antinuclear antibodies (ANA) profile, C-reactive protein (CRP), and antineutrophil cytoplasmic antibodies (ANCA). Two separate authors (NM, BS) applied the criteria for labelling causality in neurological AEFI independently as mentioned previously (Butler et al., al.,2021). The authors were blinded with respect to assessment of causality of vaccines for demyelination.
2.1. Statistical analysis
Data were expressed as descriptive statistics, such as Mean ± SD for continuous variables, frequency and percentage for categorical variables. The data was normally distributed for analysis. For quantitative variables- independent samples t- test were used and for proportions variables chi square test employed and a p value < 0.05 considered statistically significant.
3. Results
One hundred sixteen patients with CNS demyelination were assessed during the study period, of whom 29 (Table 1 ) met the inclusion criteria for postvaccinial demyelination and the rest were taken as controls (supplementary table) as either they were already diagnosed with one of the disorders with CNS demyelination or were not vaccinated within six weeks of the demyelination. The 29 post-vaccination demyelination patients were predominantly young females (17 females,12 males with mean age of 37.86±12.34 years),and majority of them (n = 17,58.6%) hailed from Karnataka district. The most common clinical presentations were paraparesis with sensory disturbances and sphincter dysfunction in eleven patients (37.9%), blurred vision in six (20.7%), features of disseminated encephalomyelitis in five (17.2%), multiaxial involvement in four (13.9%) and brainstem involvement in three (10.3%) patients. Only one patient (Patient 7) had history of SARS-CoV-2 infection, nine months prior to vaccination.
Table 1.
Clinical Characteristics of 29 Patients with Central Nervous System Demyelination following SARS-CoV-2 Vaccination.
| No | Age (years) / Gender | Presenting Complaints | Total Duration of Illness | Type of Vaccine/ Dosing | Duration between the dose and first neurological symptom | Examination finding | Investigations | Treatment | Diagnosis |
|---|---|---|---|---|---|---|---|---|---|
| 1. | 29/F | Headache, Rt eye blurring of vision | 15 days | ChAdOx1 nCoV- 19 / 1st dose | 11 days | Rt: eye RAPD, VA – Rt: hand movement close to face; Lt - 6/6 | CSF: 0 cells, P:18 mg/dl, G: 61 mg/dl Serum and CSF OCB absent ANA, ANCA, RA factor, CRP -negative Serum MOG- positive VEP: Rt - absent waveform, Lt – normal MRI brain: T2 /FLAIR hyperintensity of long intraorbital segment of Rt optic nerve with contrast enhancement | Inj. MP 1 gm x 5 days 1 cycle of LVPP T. Prednisolone 40 mg OD followed by tapering doses | MOG-antibody –associated Rt Optic neuritis |
| 2. | 26/F | Bl calf pain, backache, Bl LL weakness & decreased sensation below D6 level | 11 days | BBV152 / 1st dose | 11 days | Quadriparesis with paradoxical breathing, Power- Bilateral upper limb between MRC grade 2–3, lower limb MRC grade 0, decreased sensation below D6, DTRs- 2+ in upper limb, absent in lower limb, plantars equivocal | CSF: 207 cells -polymorphic predominant, P: 95.8 mg/dl, G: 50 mg/dl, ANA profile- PCNA strongly positive; CRP – positive ANCA, RA factor -negative Serum NMO-MOG - negative SSEP- absent waveforms, MRI: Long segment T2/FLAIR hyperintensity from C2- L1 with post contrast enhancement, axial section showing H-shaped involvement | Inj. MP 1 gm x 5 days 5 cycles of LVPP T. Prednisolone 40 mg OD followed by tapering doses | Acute Transverse myelitis - LETM |
| 3. | 54/F | Progressive quadriparesis followed by altered sensorium | 1 month 12 days | ChAdOx1 nCoV- 19 / 1st dose | 14 days | Drowsy, not opening eyes, bl UL flexion posturing, quadriparesis with 2/5 power in UL and 0/5 power in LL. | CSF: 8 cells- lymphocytic predominant, P:77 mg/dl, G:98 mg/dl ANA, ANCA, CRP -negative Serum NMO-MOG- negative MRI brain: T2/FLAIR hyperintensities in the corpus callosum, bl periventricular and subcortical white matter, infratentorial region with patchy contrast enhancement | Inj. MP 1 gm x 5 days 5 cycles of LVPP Inj. Iv Ig 100 g T. Prednisolone 40 mg OD followed by tapering doses | ADEM |
| 4. | 44/M | Imbalance on walking, hiccups, vomiting, urinary retention, double vision | 12 days | ChAdOx1 nCoV- 19 / 1st dose | 7 days | Lt VA: 6/9, Rt – 6/6. spastic quadriparesis, bilateral cerebellar signs in UL | CSF: 130 cells- lymphocytic predominant, P: 38 mg/dl, G: 63 mg/dl, ANA, ANCA -negative Serum and CSF MOG- Strongly positive, MRI: T2 hyperintensities in the cervical and dorsal cord and conus | Inj. MP 1 gm x 5 days 5 cycles of LVPP T. Prednisolone 40 mg OD | MOG-antibody –associated – LETM |
| 5. | 50/F | Bl feet paraesthesias with LL weakness. | 3 weeks | ChAdOx1 nCoV- 19 / 1st dose | 28 days | Bl finger extensor weakness, Lt LL decreased distal vibration sense with spasticity in Bl LL | CSF: 2 cells - lymphocytic predominant, P:28 mg/dl, G:87 mg/dl ANA profile- PCNA weakly positive ANCA -negative, Serum NMO-MOG -negative, NCS –normal MRI Spine: focal cervical syrinx (C7-T1). demyelination across C6 | I/V MP-5 days T. Prednisolone 40 mg OD T. Amitriptyline 25 mg OD | Acute Transverse myelitis |
| 6. | 39/M | Rt eye pain followed by blurring of vision | 20 days | ChAdOx1 nCoV- 19 / 1st dose | 14 days | RT eye-RAPD, Rt VA: Finger counting at 2 m Visual field- right inferonasal quadrant involvement | ANA, ANCA, APLA -negative, Serum MOG- positive, VEP- bl prolonged (Right-132 ms, left-115 ms) MRI: T2 /FLAIR hyperintensity of long intraorbital segment of Rt optic nerve with contrast enhancement | Inj. MP 1 gm x 5 days T. Prednisolone 40 mg OD | MOG-antibody –associated Rt Optic neuritis |
| 7. | 54/M | Left eye blurring of vision | 3 weeks | ChAdOx1 nCoV- 19 / 1st dose | 14 days | VA: Bl 6/12, Lt eye RAPD present, Rt eye-normal pupillary reaction. | ANA profile anti Jo1 −1+ positive, ANCA,VDRL-negative, VEP: Rt- 127 ms, Lt-absent waveform Serum MOG –Strongly positive MRI brain and spine: T2/FLAIR hyperintensity in Rt pons | Inj. MP 1 gm x 5 days T. Prednisolone 40 mg OD | MOG associated optic neuritis |
| 8. | 34/M | Rt eye blurring of vision | 2 weeks | ChAdOx1 nCoV- 19 / 1st dose | 1 day | Rt eye- non reactive pupil, VA-perception of light present, Lt eye VA −6 /18 | CSF: 2 cells – lymphocyte, P: 26 mg/dl, G: 65 mg/dl ANA profile, ANCA,VDRL, RA factor, CRP-negative Serum and CSF NMO-MOG – negative VEP- absent waveform on Rt side MRI: Rt optic nerve tortuosity with prominent perioptic sheath and fat stranding | Inj. MP 1 gm x 5 days T. Prednisolone 40 mg OD | Rt eye optic neuritis |
| 9. | 35/F | Progressive paraparesis followed by altered sensorium | 8 days | ChAdOx1 nCoV- 19 / 1st dose | 9 days | Conscious, confused, VA: Bl 6/9, Bl LL paraparesis with power 1/5, DTRs- 3+ in upper limb, 2+ in lower limb, plantars- left extensor, right equivocal | CSF: 58 cells -lymphocytes P: 47.4 mg/dl, G: 106 mg/dl CRP- positive ANA profile, ANCA, VDRL, RA factor-negative Serum MOG-positive VEP, BERA, SSEP – normal MRI: T2/FLAIR hyperintensities in mid brain, pons, left MCP, bl posterior internal capsule, thalamus, bl centrum semiovale and LETM from cervical cord to conus | Inj. MP 1 gm x 5 days T. Prednisolone 40 mg OD | MOG-antibody –associated ADEM |
| 10. | 20/F | Double vision | 2 weeks | ChAdOx1 nCoV- 19 / 1st dose | 3 days | VA: Bl 6/6, Rt eye adduction restriction, Lt eye restriction in all gazes, fundus normal | CRP- Negative, ANA profile, ANCA -negative Serum NMO-MOG – negative MRI brain: Multiple discrete T2/FLAIR hyperintensities in pericallosal, callososeptal, periventricular, and fronto parietal regions | Inj. MP 1 gm x 5 days T. Prednisolone 40 mg OD | Brainstem syndrome |
| 11. | 31/M | Bladder disturbances followed by progressive numbness of whole body and LL weakness | 5 days | ChAdOx1 nCoV- 19 / 1st dose | 14 days | Lower limb spasticity, paraparesis with power 1/5, decreased sensations by 70% below L1, plantars extensor, UL DTRs-3+ and LL 2+ | CSF: 370 cells - polymorphic predominant, P: 174 mg/dl, G: 168 mg/dl ANA profile, ANCA,VDRL, RA factor, CRP-negative Serum and CSF NMO-MOG – negative VEP and BERA- normal, SSEP of Lt. LL prolonged (55.9 ms) MRI: long segment cervico-dorsal T2/FLAIR hyperintensity with subtle enhancement | Inj. MP 1 gm x 5 days T. Prednisolone 40 mg OD 7 cycles of LVPP Inj. Rituximab 1 gm (1st dose) | Acute Transverse myelitis - LETM |
| 12. | 20/F | Rt UL paraesthesias followed by paraparesis & altered sensorium | 2 days | BBV152 / 1st dose | 1 day | VA: Bl 6/6. LL proximal weakness (3/5), distal 4/5, DTRs- 3+, Rt LL −50% decreased sensation, Plantars Equivocal | CSF: 8 cells - lymphocytic predominant,P:24.9 mg/dl, G:61 mg/dl ANA profile, ANCA,VDRL, RA factor, CRP -negative Serum and CSF NMO-MOG negative, CSF OCB – Positive VEP, BERA, SSEP- normal MRI: few juxtacortical and short segment cervical T2/FLAIR hyperintensity at C5 level with subtle enhancement | Inj. MP 1 gm x 5 days T. Prednisolone 40 mg OD 5 cycles of LVPP | ADEM |
| 13. | 45/F | Bilateral (Rt followed by Lt) eye blurring of vision | 6 weeks | ChAdOx1 nCoV- 19 / 1st dose | 21 days | VA: Rt 6/12, Lt hand movement perception, Lt RAPD present, Rt eye-normal pupillary reaction, Lt upper limb spasticity and extensor plantar | CSF: 2 cells - lymphocytic predominant, P: 52.3 mg/dl, G: 95 mg/dl CSF OCB- positive ANA profile, ANCA, RA factor, CRP-negative Serum MOG panel- strongly positive VEP: Bl waveform absent, BERA AND SSEP-Normal MRI brain and spine-T2/FLAIR short segment hyperintensity with enhancement of bilateral optic nerves, Rt optic nerve tortuous | Inj. MP 1 gm x 5 days T. Prednisolone 40 mg OD 3 cycles of LVPP | MOG associated optic neuritis |
| 14. | 33/F | Fever, vomiting followed by altered sensorium and persistent paraesthesias below mid thoracic level | 4 weeks | ChAdOx1 nCoV- 19 / 1st dose | 14 days | VA: Rt 6/12, Lt 6/9, Bl normal pupillary reaction, no other focal deficits | CSF: 105 cells - lymphocytic predominant, P: 28.12 mg/dl, G: 70.4 mg/dl Serum MOG –Strongly positive MRI brain: T2/FLAIR hyperintensity in Bl fronto parietal region, no enhancement | Inj. MP 1 gm x 5 days T. Prednisolone 40 mg OD Inj. Acyclovir 500 mg TID (treated outside) | MOG- associated ADEM |
| 15. | 53/F | Bl LL numbness, tingling paraesthesias & urinary disturbances | 12 days | ChAdOx1 nCoV- 19 / 2nd dose | 1 day | Tone and Power normal, Touch and pain sensation reduced by 75% below T4, Vibration sense reduced upto T4, plantars Bl equivocal, DTRs-UL 2+ and LL 3+ | CSF: 6 cells - lymphocytic predominant, P: 54.2 mg/dl, G: 77 mg/dl ANA, ANCA,VDRL, RA factor, CRP-negative ACE- 31.4 U/L Paraneoplastic panel:Anti – recoverin 2+ VEP-prolonged bl 123 ms, BERA, SSEP -normal Serum NMO MOG –negative MRI brain and spine: T2/FLAIR hyperintensity at Bl subcortical, periventricular deep white matter, insula, cerebellar hemispheres, brainstem, short segment expansile T2 hyperintensities are noted at C5,6,7 & D6–7 levels | Inj. MP 1 gm x 5 days T. Prednisolone 40 mg OD | Acute Transverse myelitis – LETM |
| 16. | 38/M | Giddiness, double vision, imbalance while walking, right eye blurring of vision followed by headache | 20 days | ChAdOx1 nCoV- 19 / 2nd dose | 6 days | VA:Rt 6/9, Lt 6/6, Bl normal pupillary reaction, Bl gaze evoked horizontal and torsional nystagmus, DTRs: 3+, plantars Bl extensor. | CSF: 6 CELLS; P: 67.8 mg/dl, G: 81 mg/dl ANA, ANCA,VDRL, RA factor, CRP-negative ACE- 20.7 U/L VEP-prolonged, BERA, SSEP -normal Serum NMO MOG –negative MRI brain and spine: T2/FLAIR hyperintensity in left MCP, right corona radiate with no contrast enhancement | Inj. MP 1 gm x 5 days T. Prednisolone 40 mg OD | CNS demyelination |
| 17 | 30/M | Sequential blurring of vison in both eyes | 11 days | ChAdOx1 nCoV- 19 / 1st dose | 14 days | VA: Rt eye-absent perception of light, Lt eye-2/60 Fundi: Bl Disc oedema | CSF: 4 cells – 50% lymphocytes, P:26.8 mg/dl, G:108 mg/dl, OCBs-positive ANA profile and ANCA -negative, Serum NMO-MOG -negative, VEP-Bl not recordable, BERA and SSEP-Normal MRI brain: subcortical hyperintense foci in Bl cerebral hemispheres MRI Optic nerves:Right>left intraneural hyperintensities in intraorbital segments | Inj MP 1 gm x 5 days 5 cycles of LVPP Inj Rituximab | Bilateral optic neuritis |
| 18 | 30/F | Paraesthesias over both palms followed by development of girdle like sensation over waist and electric shock like sensation on flexion of neck | 90 days | ChAdOx1 nCoV- 19 / 1st dose | 15 days | VA-6/6 Bl, Cranial nerves and motor examination-normal Sensory examination-40% decreased sensation to touch over both palms, Romberg's-negative | CSF: 4 cells,P-36 mg/dl, G: 60 mg/dl, OCB positive CRP-positive,ESR-68 mm/hr ANA, ANCA,VDRL, RA factor-negative, Vitamin B12, homocysteine-normal, ACE- 24.2 U/L Evoked potentials -normal Serum NMO MOG –negative MRI brain and spine: single focus of T2/flair hyperintensity in selenium of corpus callosum, short segment hyperintensity in cervical cord along C3. | Inj MP 1 gm x 5 days 3 cycles of LVPP T. MMF (1.5 gm/day) | ATM - Cervical cord demyelination |
| 19 | 36/M | Bl LL tingling and paraesthesias followed by development of motor weakness and urinary disturbances | 20 days | ChAdOx1 nCoV- 19/2nd dose | 32 days | VA: Rt (aphakia): PL present, left: 6/9. Cranial nerves-normal Upper limbs: motor and sensory examination-normal Lower limbs: hypotonia, power: hip joint: Bl 1/5, Knee joint: Bl 0/5, Ankle joint: Bl 1/5, DTRs;absent in lower limbs. Sensory level at D4 | CSF: 720 cells – 80% lymphocytes P: 144.4 mg/dl, G: 50 mg/dl ANA, ANCA,VDRL, RA factor, CRP-negative, ACE- 60.9 U/L Serum NMO-negative Serum MOG-Strongly positive MRI brain: hyperintensities along bilateral trigeminal nerves in pons MRI spine: long segment spinal cord involvement from obex till conus | Inj MP 1 gm x 7 days 5 cycles of LVPP | MOG associated LETM |
| 20 | 27/F | Ill-defined pain followed by weakness in left upper and lower limb, followed by right lower limb involvement, requiring a person support to walk | 26 days | ChAdOx1 nCoV- 19 / 1st dose | 8 days | VA-6/6 Bl, Cranial nerves -normal Motor examination- grade I spasticity in left upper limb, mild pronator drift, DTRs brisk. Sensory examination-normal | CSF: clear, P: 27.7 mg/dl, G: 62 mg/dl ANA, ANCA,VDRL, RA factor, CRP-negative, ACE-normal EPs- Normal Serum NMO and MOG-negative MRI brain: multifocal discrete hyperintense T2/flair lesions in Bl periventricular white matter with few lesions showing peripheral diffusion restriction and contrast enhancement. MRI spine-normal | Inj MP 1 gm x 5 days T. Prednisolone 40 mg OD | CNS demyelination |
| 21 | 60/M | Acute onset tingling paraesthesias and motor weakness in left upper and lower limb, followed by behavioural and memory disturbances | 34 days | ChAdOx1 nCoV- 19 / 2nd dose | 14 days | MMSE-27/30 Cranial nerves-VA:R-6/6, l- 6/9, nystagmus present Motor system-Power: normal,DTRs-brisk | CSF: 9 cells – 90% lymphocytes, P:68.3 mg/dl, G:132 mg/dl, OCBs-negative ANA,ANCA,B12,Homocysteine,VDRL-negative,ACE-normal Serum NMO and MOG -negative, VEP-normal MRI brain: multiple focal lesions in right pons, midbrain, medial temporal lobes, splenium of corpus callosum, high parietal lobe with tumefaction and peripheral enhancement | Inj MP 1 gm x 5 days T. Prednisolone 40 mg OD T. MMF(1 gm) | ADEM |
| 22 | 23/F | Burning paraesthesias in right palm associated with numbness and motor weakness followed by burning sensation in right foot over next 7 days | 41 days | ChAdOx1 nCoV- 19 / 2nd dose | 7 days | VA-6/6 Bl Cranial nerves-normal Motor system-normal Sensory system-decreased vibration along distal right upper and lower limb joints | CRP- 23 mg/dl ANA-negative Serum NMO and MOG-negative CSF-OCB negative MRI brain-T2/flair hyperintensities adjacent to right frontal horn, ependymal margins of bilateral lateral ventricles MRI spine-short segment hyperintensities at C2-C3,C5,D4 | Inj MP 1 gm x 5 days T. Prednisolone 40 mg OD | Cervical cord myelopathy |
| 23 | 40/M | Blurring of vision from left eye followed by acute urinary retention and right eye vision loss | 77 days | ChAdOx1 nCoV- 19 / 1st dose | 10 days | VA- 6/18 Bl Cranial, motor and sensory examination-normal | CSF: 8 cells – 100% lymphocytes, P:32 mg/dl, G:68 mg/dl,OCB-positive ANA,ANCA,VDRL -negative, Serum MOG -positive MRI brain: T2 Hyperintensities in pons, bilateral thalami, right frontal cortex MRI spine-longitudinally extensive myelitis from C4-D3 | Inj MP 1 gm x 5 days T. Prednisolone 60 mg OD T. MMF (2 gm) | MOG associated Opticomyelopathy |
| 24 | 45/M | H/o fever accompanied by urinary retention and difficulty in walking progressing to altered sensorium | 5 days | ChAdOx1 nCoV- 19 / 1st dose | 10 days | VA-6/6 BL Cranial nerves-normal Motor system-Tone and power normal in upper limbs LL-hypotonia, grade-0 power with hyporeflexia, plantars mute | CSF: 44 cells – 44% lymphocytes, P:90.9 mg/dl, G:68 mg/dl, rabies CSF PCR-Negative VEP-l-141,R-129,BERA-normal, N20-normal, P37–40(mildly prolonged), ANA-U1RNP-1+,C-ANCA-, Serum MOG – strongly positive S.NMO—Negative MRI of brain and spine-hyperintensities in brainstem, cervicodorsal cord and supratentorial regions with central cord swelling | INJ MP-5 days, LVPP 3 CYCLES TAB WYSOLONE 40 MG TAB MMF 1.5 GM | MOG-ADEM |
| 25 | 34/F | H/o recurrent vomiting and hiccups progressing to imbalance while walking | 60 days | ChAdOx1 nCoV- 19 / 2nd dose | 36 days | Cranial nerves: Right gaze evoked nystagmus, rest normal Motor examination::Tone and power normal, DTRs brisk BL Sensory examination: pseudoathetosis Left>Right,, Romberg's positive, Tandem gait impaired | CSF-1 cell,P-15,3 mg/dl,−63 mg/dl,OCB Negative ESR-46 mm/hr Serum NMO-weakly positive Serum MOG-negative ANA:Ro-52 1+,ANCA-negative MRI brain:T2 hyperintensity in dorsal aspect of medulla | I/V MP-5 days LVPP-3 cycles Tab Wysolone 40 mg Inj Rituximab | Area postrema syndrome - Aquaporin 4 positive NMO |
| 26 | 31/M | H/o progressive upper and lower limb tingling f/b difficulty in walking, urinary urgency, and constipation | 17 days | ChAdOx1 nCoV- 19 / 1st dose | 42 days | Cranial nerves-normal UL motor examination-normal, LL power-4/5,brisk DTRs, extensor plantars Sensory level at T4 | CSF: 32 cells – 100% lymphocytes, P:49.2 mg/dl, G:74 mg/dl ANA,ANCA,VDRL -negative, Serum NMO and MOG -negative MRI brain: T2 Hyperintensities in cervicomedullary junction, right frontal subcortical region MRI spine-cervical cord HI C2-C5,also in dorsal cord | I/V MP-5 days LVPP-4 cycles Tab Wysolone 40 mg Tab MMF 1.5 gm | ATM – acute transverse myelitis |
| 27 | 52/F | H/o progressive slurring of speech with right upper limb and lower limb weakness, followed by appearance of swallowing difficulty | 51 days | ChAdOx1 nCoV- 19 / 1st dose | 35 days | Spastic anarthria+ Gaze restricted left>right Right facial weakness Motor examination-hypotonic right upper and lower limb with 0/5 power, left sided power-5/5,BL DTRs brisk and plantars extensor | CSF-2 CELLS,P-40.5 mg/dl,G-56 mg/dl ESR-18,CRP-POSITIVE ANA,ANCA-Negative, VDRL-Negative S.NMO and MOG-Negative MRI brain:tumefactive demyelination in left frontal hemisphere with insular involvement along with left more than right midbrain involvement | I/V MP-5 days LVPP-4 cycles Tab Wysolone 40 mg Inj Rituximab | ADEM - Tumefactive demyelination |
| 28 | 65/F | H/o urinary retention followed by numbness and weakness of both hands and blurring of vision of right eye | 30 days | ChAdOx1 nCoV- 19 / 1st dose | 42 days | V/A-R- hand movements close to face,L-6/18 UL: motor examination normal LL: Power-0/5 DTRs absent in LL Sensory level:T6 | CSF-17 CELLS,P-49 mg/dl,G-59 mg/dl ESR-97 ANA,ANCA-Negative, VDRL-Negative S.NMO-Strongly positive S.MOG-Negative VEP-R-Not recordable, l-Normal SSEP-LL absent MRI brain: few hyperintensities in frontal subcortical white matter MRI Spine: D2-D11 hyperintensity with patchy contrast enhancement and bright spotty areas | LVPP – 3 cycles I/V MP-5 days Tab Wysolone 40 mg Tab MMF 1.5 gm | LETM - Aquaporin 4 positive NMO |
| 29 | 20/F | H/o tingling in tips of right hand followed by progressive imbalance while walking | 24 days | ChAdOx1 nCoV- 19 / 2nd dose | 39 days | V/A-6/6 BL Motor examination: Tone increased in right upper limb and lower limb Power - 5/5 in all 4 limbs DTRs: normal Plantar right extensor and left flexor Sensory system- Pain and touch decreased by 10 percent in right upper and lower limb JPS normal Vibration normal Romberg positive Gait ataxic | CSF- 4 CELLS,P-23 mg/dl,G-111 mg/dl,CSF- OCB+ ANA-,ANCA-,CRP-13 mg/dl,,EBV-IGG+ S.NMO and MOG-Negative MRI brain: hyperintensities in BL juxtacortical, subcortical, periventricular white matter, anterior temporal lobes as well as infratentorial regions including pons, MCP and medulla MRI Spine: short segment lesions in cervical and dorsal spine | I/V MP-5 days Tab Wysolone 40 mg Inj Rituximab | Cervical myelopathy - MS |
Abbreviations: No: number; F: female; Rt: right; RAPD: Relative afferent pupillary defect; VA: visual acuity; Lt: left; CSF: cerebrospinal fluid; P: protein; mg/dl= milligrams per decilitres; G: glucose; ANA: antinuclear antibodies; ANCA: antineutrophil cytoplasmic antibodies; RA: rheumatoid factor; CRP: C -reactive protein; MOG: myelin oligodendrocyte glycoprotein; OCB: oligoclonal band; VEP: visual evoked potential; MRI: magnetic resonance imaging; T2/ FLAIR: T2 weighted/ Fluid- attenuated inversion recovery; Inj.: Injection; MP: Methylprednisolone; LVPP: large volume plasmapheresis; T: tablet; OD: omne in die; once daily; Bl: bilateral; LL: lower limbs; D: Dorsal cord level; MRC: Medical research council; DTRs: deep tendon reflexes; PCNA: proliferating cell nuclear antigen; NMO: Neuromyelitis optica; SSEP: somatosensory evoked potential; C: cervical cord level; L: lumbar cord level; LETM: longitudinally extensive transverse myelitis; UL: upper limbs; Iv Ig: Intravenous immunoglobulin; ADEM: Acute disseminated encephalomyelitis; M: male; NCS: nerve conduction studies; APLA: Antiphospholipid antibodies; ms: milliseconds; VDRL: venereal disease research laboratory test; BERA: Brain Evoked Response Auditory; MCP: middle cerebellar peduncles: TID: ter in die; thrice daily; ACE: angiotensin-converting enzyme; BD: bis in die twice daily; MS: Multiple Sclerosis.
Twenty-seven patients received ChAdOx1-S vaccine, while two were vaccinated with BBV152. Most patients (n = 22,75.9%) developed the symptoms after the first vaccination dose and none of them had prior history of demyelination. The timing of presentation for neurological symptoms after vaccine exposure ranged from 1 to 42 days. The patients presented at a mean of 16.3 days after vaccination with majority of them (45%) presenting in the second week (8–14 days) of vaccine exposure. Based on the criteria by Butler et al. (Butler et al., al.,2021) for causality assessment in neurological AEFI, all our cases were judged to have a probable causality label. This is in view of temporal association of symptoms occurring within 6 weeks of administration of COVID-19 vaccination without an alternative reason.
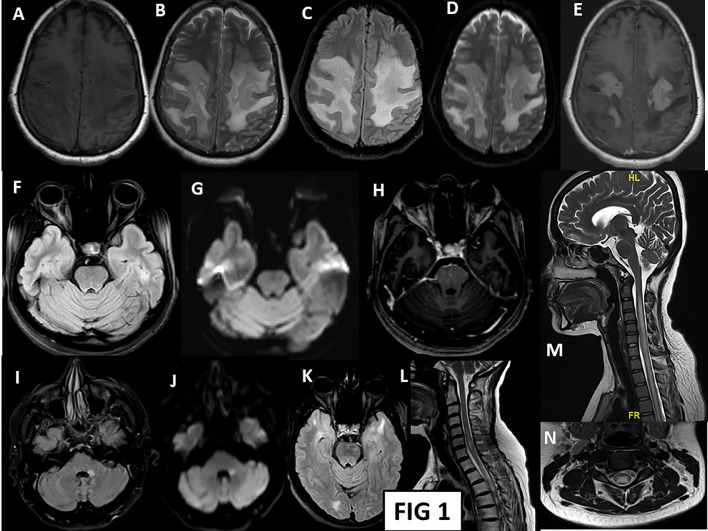
The most prevalent antibody in our series of probable postvaccinial cases was Myelin oligodendrocyte glycoprotein (MOG) antibody. The method employed for MOG testing at our centre is a cell-based immunoassay using transfected cell lines with full length human MOG for in vitro determination of IgG antibodies against this antigen. Our laboratory assessment of MOG antibody included its semiquantitative estimation based on assessment of intensity on immunofixation. Ten of our patients (34.5%) were positive for this antibody, out of which four of them presented with ON, three with ADEM, two had longitudinally extensive myelitis (LETM) and one presented with simultaneous ON and myelitis. Two patients were diagnosed with aquaporin 4 positive NMOSD (Neuromyelitis Optica Spectrum Disorder) in this postvaccinial period while one patient was diagnosed with Mc Donald's definite MS. Rest of the patients were seronegative for both AQP4 as well as MOG antibodies. The patients were also worked up for other secondary causes of demyelination. ANA profile showed antibody positivity in four patients; the antibodies were anti proliferating cell nuclear antigen (PCNA) (2 patients) and anti Jo-1 (antihistidyl transfer RNA [t-RNA] synthetase) (1 patient) and U1RNP(U1 Ribonucleoprotein) (1 patient). Paraneoplastic profile was done in one patient, and it was positive for anti-recoverin antibody with no evidence of malignancy on computed tomography of the chest and abdomen. CSF examination was performed in 26 patients. The CSF white cell count ranged from 0 to 720 cells/µl (0–5 cells/µl in ten patients (normal range); 5–50 cells/µl in nine patients; ≥ 51 cells/µl in six patients; excluded traumatic CSF of Patient 16). Thirteen patients had normal CSF protein (15–45 mg/dl); the mean protein of those with an elevated result was 82.04 mg/dl (excluded traumatic CSF of Patient 16). Imaging (Fig. 1 ) showed involvement of optic nerves in all patients presenting with optic neuritis. T2-Flair hyperintensities affecting the brain parenchyma and spinal cord were observed in seven and four patients respectively. There were ten patients showing lesions both in brain as well as spinal areas.
Fig 1.
A-E: MRI brain axial images of patient 3: shows T1 hypo to isointense (A), T2/FLAIR (B,C) hyperintensity lesion in bilateral frontoparietal parasagittal region. Diffusion images (D) shows restriction at periphery, same region showing T1 post contrast (E) patchy as well as open ring enhancement suggestive of tumefactive demyelination. F,G,H: MRI Brain axial images of patient 1: T2 FLAIR (F) sequence showing right optic nerve hyperintensity throughout the intraorbital segment with anterior-most portion showing diffusion restriction (G). The intraorbital segment shows T1 post contrast enhancement (H). I,J: MRI Brain axial images of patient 16: T2 Flair hyperintense (I) left middle cerebellar peduncle lesion with diffusion restriction (J). K, L: MRI brain (K) and spine (L) of patient 16: T2/FLAIR hyperintense lesions in bilateral anterior temporal poles (right more than left), occipital juxtacortical white matter. Spinal cord showing T2 hyperintense short segment lesion at C6 cervical cord. M,N: MRI spine (M, N) of patient 2: T1 isointense and T2 hyperintense (M) long segment hyperintensity extending from C2 to lower dorsal cord with no post contrast enhancement. Axial section (N) at C3 level showing central cord T2 hyperintensity –H Pattern.
28 out of 29 patients received intravenous methylprednisolone followed by oral steroids. Plasmapheresis was used in fifteen (51.72%) patients in view of either inadequate improvement with steroids or contraindications to their use such as presence of infected bed sore(patient 28). Furthermore one patient received intravenous immunoglobulin and five each received mycophenolate mofetil and rituximab due to persistent disabilities and incomplete recovery with steroids and plasmapheresis. Significant improvement was seen in most (96.5%) of the patients with medical management over 4-week period. One patient of ADEM (Patient 3) with tumefactive demyelinating lesions remained critically ill, requiring invasive ventilation, and died after a prolonged intensive care unit stay and superimposed infection (she could not be treated with rituximab or other immunosuppressants in view of persistent sepsis). Inadequate improvement with immunomodulation and the superimposed infection may have contributed to the severe illness and fatality in this patient. Two patients received a second ChAdOx1-S vaccine dose while taking 20 mg of oral prednisone and did not show neurological worsening. One patient received an alternative BBV152 vaccination after an original ChAdOx1-S vaccine dose without any adverse events.
Majority of patients in the control group had the diagnosis of MS (48 out of 87;55.1%) followed by Aquaporin-4-positive NMOSD (11 out of 87;12.6%). Ten patients in the control group were positive for antibody against MOG, out of which one developed the neurological symptoms during the period of active COVID infection. A total of 22 patients in the control group had a history of prior COVID infection, five of whom reported worsening/new neurological symptoms within 90 days of the infection, one patient was detected to be asymptomatic positive after she presented with a neurological relapse, and another one had onset of neurological symptoms during the period of active COVID infection (found to be positive for MOG antibody). 44 patients in the control group reported to be vaccinated against COVID-19. A history of worsening/relapse was present in three patients each within 6 weeks and 6–12 weeks of vaccination and in another six patients beyond the three-month period (supplementary table). As compared to the control group, the postvaccinial cases were found to have a significantly higher mean age (37.86 years of cases as compared to 30.82 years in controls). The difference in clinical features was remarkable for the presence of encephalopathy features in the postvaccinial group (20.7% in postvaccinial group vs 2.3% in the control group) (p value:0.0007). The type of vaccine received did not influence the incidence of neurological adverse effects observed when matched for the control population. Further on, we observed that a higher proportion of postvaccinial cases had CSF pleocytosis (60% in postvaccinial group and 31.1% in the control group) (p value: 0.0094) and raised CSF protein (48% in postvaccinial group and 18.7% in the control group) (p value: 0.0062) as compared to controls. The proportion of patients with MOG antibody positivity were also higher in the postvaccinial group (Table 2 ).
Table 2.
Comparison of Demographic, Clinical and Laboratory Parameters amongst The Postvaccinial Demyelination Cases And Control Population During The Same Period.
| CASES (N = 29) | CONTROLS (N = 87) | p-value | |
|---|---|---|---|
| Age (mean±SD) | 37.86 ± 12.34 | 30.82 ± 11.81 | 0.0069 |
| Gender, females (%) | 17 (58.6%) | 61 (70.1%) | 0.2553 |
| Optic nerve involvement at presentation | 9 (31%) | 21 (24.1%) | 0.4641 |
| Encephalopathy features | 6 (20.7%) | 2 (2.3%) | 0.0007 |
| Patients vaccinated with ChAdOx1 NCoV-19 | 27 (93.1%) | 34 out of 44 vaccinated controls (77.3%) | 0.0766 |
| CSF pleocytosis | 15 out of 25(60%) | 19 out of 61 (31.1%) | 0.0094 |
| Raised CSF protein | 12 out of 25(48%) | 11 out of 59 (18.7%) | 0.0062 |
| CSF oligoclonal bands | 6 out of 10(60%) | 32 out of 47(68%) | 0.6293 |
| ANA positivity | 6 out of 29(20.7%) | 12 out of 74(16.2%) | 0.5903 |
| CRP positivity | 6 out of 19(31.5%) | 7 out of 30(23.3%) | 0.5304 |
| MOG positivity | 10 out of 29 (34.5%) | 10 out of 69(14.5%) | 0.0257 |
| Aquaporin-4 positivity | 2 out of 29(6.9%) | 11 out of 69(15.9%) | 0.2325 |
Abbreviations: N: Number; SD: Standard deviation; CSF: Cerebrospinal fluid; ANA: Antinuclear antibody; CRP: C-Reactive Protein; MOG: Myelin Oligodendrocyte Glycoprotein.
4. Discussion
We report on 29 cases of CNS demyelination occurring within six weeks of administration of SARS-CoV-2 vaccine; 27 of them occurred in temporal association with ChAdOx1-S vaccine, while two of them were in association with BBV152 vaccine. Each patient was investigated extensively to rule out other causes of demyelination. Ten (34.5%) of them were found to be positive for MOG antibodies. The association of MOG antibodies with COVID-19 vaccines has not been reported previously, though there are reports of MOG associated myelitis and optic neuritis with COVID-19 infection (Zhou et al., al.,2020;https://www.aao.org/editors-choice/covid-19-linked-to-mog-antibody-associated-optic-n).Post vaccination MOG positivity has been reported with other vaccines such as tetanus, measles, Japanese encephalitis and rubella vaccines (Azumagawa et al., al.,2016; Kumar et al., al.,2020). However in these reports there were other associated inciting factors as well that could have precipitated MOG positive status like Chlamydophila pneumoniae subclinical infection and pregnancy. Most MOG-associated demyelination has been reported without any preceding event/illness though a handsome proportion has also been found to be associated with post-infectious demyelination following Epstein–Barr virus, herpes simplex virus and Borrelia infections (Wynford-Thomas et al., al.,2019) or following vaccination (Kumar et al., al.,2020).The role of MOG antibodies in these conditions is not clear, whether they are pathogenic or represents an epiphenomenon. The other antibodies found in this cohort were PCNA antibodies in two of our patients, and anti JO-1 and U1RNP each positive in other two patients. The association of these antibodies following vaccination has not been reported in literature. Antibodies against PCNA are normally found in sera from patients with chronic Hepatitis B (HBV) and Hepatitis C (HCV) infection(Hsu et al., al.,2006).Anti Jo-1 positive status is commonly seen post-vaccination (Vleugels RA and Callen JP.,2009) (Toplak N. and Avčin T.,2015).Anti-U1 RNP has been previously observed likewise with influenza vaccination as well. Whether seropositivity for these extractable nuclear antigens represents a heightened immune response in already susceptible individuals, or are contributory towards the disease pathogenesis, is a question that needs addressal in larger experimental studies.
Most of our postvaccinial patients responded to steroids while nearly half of them required additional rescue therapy in the form of plasmapheresis in view of inadequate improvement. This cohort had a single (3.4%) mortality in a patient with tumefactive demyelination, who did not respond to immunotherapy and subsequently died due to sepsis.
Post-vaccination acute demyelinating encephalomyelitis accounts for 5–10% of all cases of post-vaccine serious neurological events (Huynh et al., al.,2008).The overall incidence of post-vaccination ADEM is estimated to be with higher risk following immunization against measles. Other common causes of post-vaccination ADEM include vaccines against the varicella zoster, rubella, smallpox, and influenza viruses (Huynh et al., al.,2008). Apart from ADEM, optic neuritis has also been reported most commonly in association with vaccines (Karussis D and Petrou P.,2014) such as measles, rubella, hepatitis A and B, influenza, pneumococcal vaccine, BCG. Post vaccination myelitis has been previously reported in literature with influenza, pneumococcal, measles, rubella, Japanese encephalitis, and others. In this study, we have found all these three types of demyelination and 34.5% of them had associated positive MOG antibodies.
The exact pathophysiological mechanism in post-vaccine demyelination is not clearly identified. In combating the COVID-19 pandemic many different types of vaccines have been developed with varying mechanisms of action. Four major vaccine mechanisms have been explored against COVID-19 virus: DNA-based vaccines, mRNA-based vaccines, protein-based vaccines, and inactivated virus. We had 27 out of 29 patients who had received ChAdOX1-S vaccine in the postvaccine cohort. ChAdOX1-S is a recombinant vaccine developed by AstraZeneca and the University of Oxford which utilises non-replicating viral vector in form of replication deficient simian adenovirus containing genes for full length structural spike protein of SARS-CoV2(Mascellino et al., al.,2021). While BBV152 vaccine is a whole virion inactivated Vero cell vaccine which is developed in collaboration with Indian Council of Medical Research (ICMR)-National Institute of Virology (NIV).The virus in this vaccine has been inactivated using beta-propiolactone(Ella et al., al.,2021). Hence, ChAdOX1-S and BBV152 vaccine either use a modified Chimpanzee virus or inactivated whole SARS-CoV2 virion itself to generate a protective immune response, by offering a sufficient antigenic stimulus that can drive the host B and T cell responses without causing the disease(Mascellino et al., al.,2021).The prevalent mRNA vaccines (Pfizer and Moderna, amongst others) employ the utilization of mRNA sequence from the SARS-CoV2 virus that is able to code for the spike protein of the virus and hence build an immunogenic response in the vaccine recipients. In a recent systematic review, postvaccinial demyelinating events were reported with all of the approved COVID-19 vaccines, though the highest number of demyelinating events were reported with mRNA vaccines followed by viral vector and inactivated vaccines (Ismail II and Salama S.,2022). Pathogenetically, whether this immune response gets aberrant or autoreactive, or some other factors are potentially causative for the autoimmune demyelination is a subject of considerable interest. Immune adjuvants included in the vaccine preparations that aim to enhance the immune responses have been incriminated as one of the main mechanism. This phenomenon has been described as ‘autoimmune or inflammatory syndrome induced by adjuvants (ASIA).’ The other concept responsible for the immunopathogenesis is molecular mimicry-based on shared structural similarities due to shared amino acid sequences or similar conformational structures between the vaccine and self-antigen (Karussis D and Petrou P.,2014;Schattner A.,2005).The pathogenesis for MOG associated post-vaccine demyelination is postulated to be similar molecular mimicry mechanism, stimulation of autoreactive T-cell clones, enhanced antigen expression and possible epitope spread (Wynford-Thomas et al., al.,2019).
The COVID-19 pandemic has led us to witness various neurological manifestations of the virus. The pathogenesis resulting in various neurological manifestations can be due to a variety of mechanisms, such as immune mediated, hypoxic injury, coagulation abnormalities and direct invasion by virus (Zhao et al., 2020; Solomon et al., 2020; Song et al., 2020). There are many studies reporting COVID-19 associated demyelination (Artemiadis et al., al.,2021; Garg et al., al.,2021; Khandelwal et al., al.,2021; Moreno-Escobar et al., al.,2021; Zanin et al., al.,2020). Likewise, the development phase of our vaccine armamentarium against this virus saw a handful of demyelinating events during the clinical trial phases. A case of transverse myelitis was reported during pre-approval clinical trial where myelitis developed 14 days after 2nd dose of ChAdOx1-S COVID-19 vaccine. Two other cases of transverse myelitis that occurred during clinical trials in relation to ChAdOx1-S vaccine was attributed to underlying multiple sclerosis in one patient and the other patient had possible association with meningococcal vaccine (Voysey et al., al.,2021). An update on AstraZeneca Vaccine Analysis in United Kingdom of spontaneous reports received between January 2021 to July 2021 revealed ten cases of ADEM, 18 cases of demyelination and four cases of NMOSD (https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1009454/COVID-19_AstraZeneca_Vaccine_Analysis_Print_DLP_28.07.2021.pdf)
Post-marketing, till date, there have been three solitary case reports of post-vaccine demyelination in relation to COVID-19 vaccination, including one report from India. A case of COVID-19 vaccine (ChAdOX1-S) associated myelitis from India has been reported that improved with steroids (Singh MH et al.,2021). A case of ADEM was reported in a patient from China following SARS-CoV-2 (Vero Cells, Beijing Institute of Biological Products Co., Ltd., Beijing, China) vaccination and longitudinally extensive transverse myelitis (LETM) following ChAdOX1-S, AstraZeneca vaccine from Germany (Pagenkopf C and Südmeyer M.,2021). Our study is the largest series of CNS demyelination in temporal association with COVID-19 vaccine and exclusion of alternative aetiologies.
Vaccination in India began on January 16, 2021; initially vaccines were administered to all health care workers. The vaccination program then expanded to cover front line workers (police, paramilitary forces, sanitation workers, and disaster management volunteers) since February 2021. The next phase, since March 2021, included citizens above the age of 60 years and people above the age of 45 with comorbidities. Adults above 18 years of age were provided vaccination after third week of June 2021. From June 18 to July 17, 4.53 million doses were administered daily, the highest for any 30-day period since the vaccination drive started (https://www.thehindu.com/data/data-covid-19-vaccination-rate-improved-in-all-states-between-june-july-2021/article35509004.ece).The stepwise rollout in people of different backgrounds and ages, and fluctuations in numbers vaccinated, may hamper accuracy of our basic incidence calculation, as may the potential for cases to not have been reported, but at one case per 10 million population per year, these serious potential side effects appear very rare in comparison to the beneficial effects of the vaccine in combating the pandemic.
There are some limitations in our study. Since this was a chart review of the control population, we cannot flawlessly estimate the occurrence of COVID-19 infection in our population. We also did not have data for COVID antibody testing prior to vaccination in the cases presenting with postvaccinial demyelination. Another limitation of our study is the lack of facility of quantitative assessment of MOG antibody titres at our centre, though the semiquantitative assessment was still possible, thus categorising results into positive and strongly positive samples.
5. Conclusion
In conclusion, while it is difficult to establish a causal relationship between vaccination and neurological adverse events such as demyelination, neurologists and physicians should be aware of this potential rare adverse event. The temporal association with the vaccination, the disproportionate number of patients affected following different vaccine brands, and the presence of MOG antibodies in a substantial proportion of these individuals raises the possibility of an immunogenic process triggered by the vaccine in susceptible individuals. However, these are rare occurrences, with very low incidence, and so we urge this information not to be misquoted as it would result in vaccine hesitancy and subject to misinformation by the social media.
Availability of data and material
The anonymised data of each patient are available with unique alphanumeric code, that will be shared if required by the authors.
Author contributions
Netravathi M: Study concept, design, Data acquisition and analysis
Kamakshi Dhamija: Study concept, design, Data acquisition and analysis
Manisha Gupta: Study concept, design, Data acquisition and analysis
Arina Tamborska: Study concept, design, Data analysis
Nalini A: Study concept, design, Data acquisition
VV Holla: Study concept, design, Data acquisition
Nitish LK: Study concept, design, Data acquisition
Deepak Menon: Study concept, design, Data acquisition
Pal PK: Study concept, design, Data acquisition
Seena V: Study concept, design, Data acquisition
Yadav R: Study concept, design, Data acquisition
Ravindranadh M: Study concept, design, Data acquisition
Arshad F: Study concept, design, Data acquisition
Saini J: Study concept, design, Data acquisition
Mahadevan A: Study concept, design, Data acquisition
Tom Solomon: Study concept, design, Data acquisition and analysis
Bhagteshwar Singh: Study concept, design, Data acquisition and analysis
All authors: Drafting large portions of the manuscript, figures. All authors have critically reviewed and approved the manuscript.
Web references
-
1
CoWIN [Internet]. [cited 2021 Jul 18]. Available from: https://www.cowin.gov.in/
-
2
https://www.bharatbiotech.com/images/press/barat-biotech-bbv152-covaxin-phase3-final-analysis-03July2021.pdf
-
3
MODULE 1 – Adverse events: Frequency and severity - WHO Vaccine Safety Basics. Available from: https://vaccine-safety-training.org/frequency-and-severity.html
-
4
COVID-19 linked to MOG antibody-associated optic neuritis and myelitis. American Academy of Ophthalmology. 2020. Available from: https://www.aao.org/editors-choice/covid-19-linked-to-mog-antibody-associated-optic-n
-
5
https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1,009,454/COVID-19_AstraZeneca_Vaccine_Analysis_Print_DLP_28.07.2021.pdf
-
6
Radhakrishnan V, Sen S. Data | COVID-19 vaccination rate improved in all States between June-July 2021. The Hindu. 2021 Jul 24; Available from: https://www.thehindu.com/data/data-covid-19-vaccination-rate-improved-in-all-states-between-june-july-2021/article35509004.ece
Declaration of Competing Interest
None
None of the authors have any financial disclosure or Conflicts of interest.
Acknowledgements
1. ICMR: ICMR project Biomarker discovery in seronegative active NMO. Is/33/16/2019-TF/Rare/BMS
2. Patients for making us understand the illness better
References
- Alam W. COVID-19 vaccine-induced immune thrombotic thrombocytopenia: a review of the potential mechanisms and proposed management. Sci. Prog. 2021;104(2) doi: 10.1177/00368504211025927. 368504211025927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artemiadis A., Liampas A., Hadjigeorgiou L., Zis P. Myelopathy associated with SARS-COV-2 infection. a systematic review. Neurol. Res. 2021;43(8):633–641. doi: 10.1080/01616412.2021.1915078. [DOI] [PubMed] [Google Scholar]
- Azumagawa K., Nomura S., Shigeri Y., Jones L.S., Sato D.K., Nakashima I., et al. Post-vaccination MDEM associated with MOG antibody in a subclinical Chlamydia infected boy. Brain Dev. 2016;38(7):690–693. doi: 10.1016/j.braindev.2016.02.004. [DOI] [PubMed] [Google Scholar]
- Butler M., Tamborska A., Wood G.K., Ellul M., Thomas R.H., Galea I., et al. Considerations for causality assessment of neurological and neuropsychiatric complications of SARS-CoV-2 vaccines: from cerebral venous sinus thrombosis to functional neurological disorder. J. Neurol. Neurosurg. Psychiatry. 2021;92:1144–1151. doi: 10.1136/jnnp-2021-326924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ella R., Vadrevu K.M., Jogdand H., Prasad S., Reddy S., Sarangi V., et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: a double-blind, randomised, phase 1 trial. Lancet Infect. Dis. 2021;21(5):637–646. doi: 10.1016/S0140-6736(21)02000-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg R.K., Paliwal V.K., Gupta A. Spinal cord involvement in COVID-19: a review. J. Spinal Cord. Med. 2021;0(0):1–15. doi: 10.1080/10790268.2021.1888022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu T.-.C., Tsay G.J., Chen T.-.Y., Liu Y.-.C., Tzang B.-.S. Anti-PCNA autoantibodies preferentially recognize C-terminal of PCNA in patients with chronic hepatitis B virus infection. Clin. Exp. Immunol. 2006;144(1):110–116. doi: 10.1111/j.1365-2249.2006.03046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh W., Cordato D.J., Kehdi E., Masters L.T., Dedousis C. Post-vaccination encephalomyelitis: literature review and illustrative case. J. Clin. Neurosci. 2008;15(12):1315–1322. doi: 10.1016/j.jocn.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail I.I., Salama S. A systematic review of cases of CNS demyelination following COVID-19 vaccination. J. Neuroimmunol. 2022;362(577765) doi: 10.1016/j.jneuroim.2021.577765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karussis D., Petrou P. The spectrum of post-vaccination inflammatory CNS demyelinating syndromes. Autoimmun. Rev. 2014;13(3):215–224. doi: 10.1016/j.autrev.2013.10.003. [DOI] [PubMed] [Google Scholar]
- Khandelwal K., Puranik M., Gupta V., Khandelwal G., PK Dave, Hirve M. COVID-19 associated acute demyelination masquerading as stroke: a case report. Egyptian J. Radiol. Nuclear Med. 2021;52(1):32. doi: 10.1186/s43055-021-00410-7. [DOI] [Google Scholar]
- Kumar N., Graven K., Joseph N.I., Johnson J., Fulton S., Hostoffer R., et al. Case Report: postvaccination Anti–Myelin Oligodendrocyte Glycoprotein Neuromyelitis Optica Spectrum Disorder. Int. J. MS Care. 2020;22(2):85–90. doi: 10.7224/1537-2073.2018-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logunov D.Y., Dolzhikova I.V., Shcheblyakov D.V., Tukhvatulin A.I., Zubkova O.V., Dzharullaeva A.S., et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet North Am. Ed. 2021;397(10275):671–681. doi: 10.1016/S0140-6736(21)00234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maramattom B.V., Krishnan P., Paul R., Padmanabhan S., Nampoothiri S.C.V., Syed A.A., et al. Guillain-Barré Syndrome following ChAdOx1-S/nCoV-19 Vaccine. Ann. Neurol. 2021;90(2):312–314. doi: 10.1002/ana.26143. [DOI] [PubMed] [Google Scholar]
- Mascellino M.T., Di Timoteo F., M De Angelis, A Oliva. Overview of the Main Anti-SARS-CoV-2 Vaccines: mechanism of Action, Efficacy and Safety. Infect. Drug Resist. 2021;14:3459–3476. doi: 10.2147/IDR.S315727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Escobar M.C., Kataria S., Khan E., Subedi R., Tandon M., Peshwe K., et al. Acute transverse myelitis with Dysautonomia following SARS-CoV-2 infection: a case report and review of literature. J. Neuroimmunol. 2021;353(577523) doi: 10.1016/j.jneuroim.2021.577523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagenkopf C., Südmeyer M. A case of longitudinally extensive transverse myelitis following vaccination against Covid-19. J. Neuroimmunol. 2021;358(577606) doi: 10.1016/j.jneuroim.2021.577606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schattner A. Consequence or coincidence? The occurrence, pathogenesis, and significance of autoimmune manifestations after viral vaccines. Vaccine. 2005;23(30):3876–3886. doi: 10.1016/j.vaccine.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Singh Malhotra H., Gupta P., Prabhu V., Kumar Garg R., Dandu H., Agarwal V. COVID-19 vaccination-associated myelitis. QJM: Int. J. Med. 2021;114(8):591–593. doi: 10.1093/qjmed/hcab069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon I.H., Normandin E., Bhattacharyya S., Mukerji S.S., Keller K., Ali A.S., et al. Neuropathological Features of Covid-19. N. Engl. J. Med. 2020;383(10):989–992. doi: 10.1056/NEJMc2019373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song E., Zhang C., Israelow B., Lu-Culligan A., Prado A.V., Skriabine S., et al. 06. bioRxiv; 2020. (Neuroinvasion of SARS-CoV-2 in Human and Mouse Brain). (169946) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toplak N., Avčin T. DOI. John Wiley & Sons, Ltd; 2015. Autoantibodies Induced by Vaccine; pp. 93–102. (Vaccines and Autoimmunity). [DOI] [Google Scholar]
- Vleugels R.A., Callen J.P. In: Dermatological Signs of Internal Disease (Fourth Edition) editors. Callen JP, Jorizzo JL, Bolognia JL, Piette WW, Zone JJ, editors. London: W.B. Saunders; 2009. Chapter 2 - Dermatomyositis; pp. 11–19.https://www.sciencedirect.com/science/article/pii/B9781416061113000082 p. [Google Scholar]
- Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet North Am. Ed. 2021;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waheed S., Bayas A., Hindi F., Rizvi Z., Espinosa P.S. Neurological Complications of COVID-19: guillain-Barre Syndrome Following Pfizer COVID-19 Vaccine. Cureus. 2021;13(2):e13426. doi: 10.7759/cureus.13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynford-Thomas R., Jacob A., Tomassini V. Neurological update: MOG antibody disease. J. Neurol. 2019;266(5):1280–1286. doi: 10.1007/s00415-018-9122-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanin L., Saraceno G., Panciani P.P., Renisi G., Signorini L., Migliorati K., et al. Acta Neurochir (Wien); 2020. SARS-CoV-2 Can Induce Brain and Spine Demyelinating Lesions; pp. 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H., Shen D., Zhou H., Liu J., Chen S. Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol. 2020;19(5):383–384. doi: 10.1016/S1474-4422(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S., Jones-Lopez E.C., Soneji D.J., Azevedo C.J., Patel V.R. Myelin Oligodendrocyte Glycoprotein Antibody–Associated Optic Neuritis and Myelitis in COVID-19. J. Neuroophthalmol. 2020;40(3):398–402. doi: 10.1016/S1474-4422(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The anonymised data of each patient are available with unique alphanumeric code, that will be shared if required by the authors.