Abstract
Synthetic cells are engineered vesicles that can mimic one or more salient features of life. These features include directed localization, sense-and-respond behavior, gene expression, metabolism, and high stability. In nanomedicine, many of these features are desirable capabilities of drug delivery vehicles but are difficult to engineer. In this focus article, we discuss where synthetic cells offer unique advantages over nanoparticle and living cell therapies. We review progress in the engineering of the above life-like behaviors and how they are deployed in nanomedicine. Finally, we assess key challenges synthetic cells face before being deployed as drugs and suggest ways to overcome these challenges.
Graphical Abstract

Introduction
Living cells offer many impressive capabilities that nanoparticle engineers often seek to imitate. These include directed localization (e.g. chemotaxis), sense-and-respond behavior, gene expression, metabolism, and high chemical and serum stability. Recent years have seen considerable advances in the bottom-up engineering of synthetic cells. Though several aspects of this technology are still in their formative stages, this field has made substantial headway into the realm of clinical applications.
The phrase “synthetic cell” has been used widely and requires disambiguation. Here, we use “synthetic cell” to mean an aqueous compartment bounded by either a polymer or lipid membrane that comprises molecular machinery sufficient to mimic one or more of the above features of living cells. Moreover, in the context of medicine, these features can induce desirable therapeutic outcomes. Synthetic cells can range from 100 nm to 10’s of μm in size. However, most synthetic cell studies work at scales >1μm due to the technical difficulty of generating nano-sized vesicles. Synthetic cells can be built from defined, synthetic components (“bottom-up”) or a combination of synthetic and cell-derived components (“semi-synthetic”). Here, we consider “top-down” engineering or harnessing of extant living cells a distinct technology and beyond the scope of this review.
The above definition of synthetic cells (SCs) emphasizes the compartmentalization of an aqueous interior. This is because physical segregation of an aqueous core enables many of the functions of cellular life, including aqueous biochemistry involved in everything from gene expression to enzymatic reactions. The presence of a distinct amphiphilic membrane structure is also key, as it enables signal transduction, selective transport, and the anchoring of functional moieties on and within the membrane. The aqueous interior and amphiphilic membrane distinguishes SCs from solid polymer nanoparticles, lipid nanoparticles and micelles with hydrophobic interiors, which cannot perform many of these functions that are critical to living system.
In terms of complexity, synthetic cells can be thought of as an intermediate between passive nanoparticle drug delivery systems (e.g. liposomes) and engineered living cell therapies (e.g. Chimeric Antigen Receptor T cells or synthetic beta cells for diabetes treatment (Chen et.al. 2018)). Compared to nanoparticles, SCs generally have many more unique components. This greater complexity makes them more capable than nanoparticles but also more difficult to manufacture and control. Compared to living cells, SCs are much simpler and well-defined. Despite the incredible progress of modern cell biology, cells are still in large part black boxes -- they are incompletely understood and therefore inherently unpredictable, especially when faced with the wide diversity of environments in human physiology. Hence, engineered living cells present inherent risks when deployed as therapeutics. SCs, on the other han d, are assembled bottom-up from known components and are therefore better defined and more predictable. Additionally, SCs will not replicate (unless they are programmed to). Therefore, SCs can offer considerable safety advantages over living cells.
SCs can also perform tasks that living cells cannot (Figure 1). For example, SCs can utilize non-natural and toxic molecules to a degree not possible in extant living cells (Martin et al. 2018) . Non-natural amino acids have been shown to endow proteins with valuable properties such as longer half-lives and enabling biochemistry (Wei Gao et al. 2019; H.-N. Chang et al. 2015) . Highly toxic molecules that would otherwise kill living cells can also be produced with in vitro transcription/translation (IVTT) systems encapsulated in SCs (Orth et al. 2011; Salehi et al. 2016; Dondapati et al. 2018) . Composing SCs of other exotic chemistry can therefore endow them with unique advantages in terms of stability as well as the functions they can enact inside the body.
Figure 1:
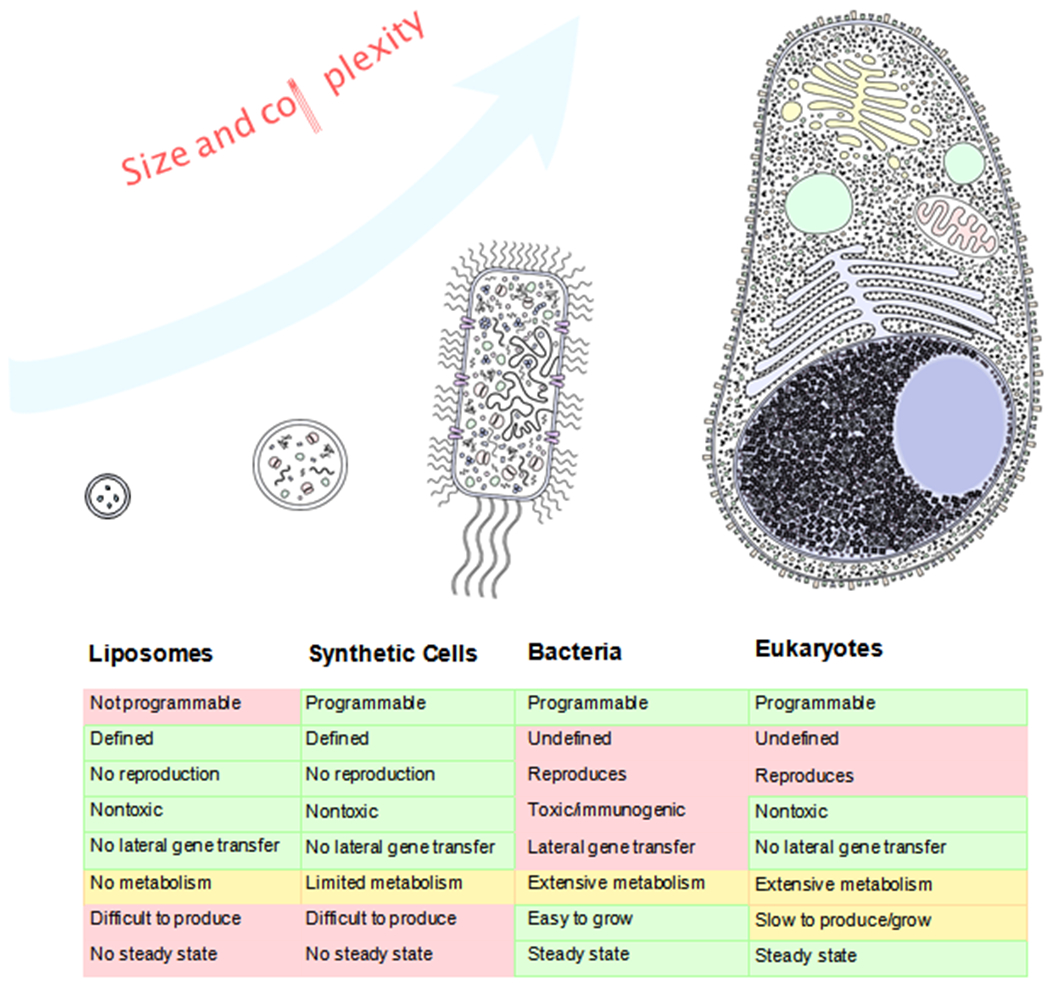
Comparison of Synthetic Cells to Other Drug Delivery Systems. Synthetic cells tend to fall between liposomes and living cells in terms of complexity. Their capabilities extend beyond those of liposomes but cannot match the sustained, nuanced behaviors of living cells. This relative simplicity also endows them with advantages, such as complete programmability and zero risk of uncontrolled replication.
Additionally, while manufacturing of SCs presents its own challenges (more below), it promises certain advantages over the manufacturing of engineered living cell therapies. Autologous cell therapies currently require extensive handling. The process of purifying, engineering, amplifying, and re-administering autologous cell therapies makes their manufacture extraordinarily expensive and time consuming(R. K. Iyer et al. 2018) . Synthetic cells, on the other hand, could in theory be generated at centralized facilities, lyophilized or otherwise preserved, and shipped to hospitals, much like current biotherapeutics. Their manufacturing is scalable via microfluidics and can be generalized across different indications and compositions. At the same time, their manufacture at a smaller scale would still allow customization for personalized medicine.
Here, we review how the capabilities of synthetic cells can be leveraged for therapeutic effect. First, we describe the structure and components of SCs and review some of the manufacturing approaches developed to date. We then discuss how the life-like processes that can be engineered in synthetic cells -- directed localization, sense & respond behavior, gene expression, metabolism -- can offer unique therapeutic strategies. Finally, we discuss both the technical and regulatory hurdles that challenge the development of SC therapeutics.
Composition & Structure
Synthetic cells mainly comprise a membrane and an internal payload. In the context of drug delivery, the membrane has several key functions. First, it protects the payload from destabilizing factors in the external environment. Second, it prevents the payload from getting out too quickly and triggering physiological responses (e.g. anaphylaxis). Third, it concentrates the payload in the interior, enabling critical biochemistry or the delivery of the payload to its target at a high concentration. Lastly, the membrane itself allows for the anchoring of membrane proteins or other moieties that further stabilize or functionalize the SC.
The composition of the membrane is critical to the SC’s stability and function and must be carefully considered. The membrane may comprise phospholipids, proteins(Huang et al. 2014) , polymers(Kuiper et al. 2008) , peptides(Fatouros et al. 2014) , colloids(S. Sun et al. 2016) , virus membrane or capsids(H. Liu et al. 2015; Y. Wang et al. 2020), or other amphiphilic molecules as well as small molecules such as cholesterol that have a stabilizing effect(Briuglia et al. 2015) . As such, the membrane composition can be tuned to endow the SC with varying stability in different environments. This, in effect, enables the SC to “sense” the environment (see below). The composition of the membrane can also affect the function of embedded proteins(Elmore and Dougherty 2003) . During formation of liposomes, the inner and outer leaflets can be produced separately, making their composition unique (Doktorova et al. 2018; de Matos et al. 2019) . This enables unique functions to be incorporated at the interior and exterior of the membrane.
Inside a patient, SCs experience diverse, challenging environments. Once injected, a unique protein corona will form around the SC(Francia et al. 2020) . The composition of the protein corona is dynamic and depends on many variables, including the membrane composition, charge, zeta potential, and size, among others(Baimanov, Cai, and Chen 2019; Giulimondi et al. 2019; Pattipeiluhu et al. 2020) . Undecorated SCs are typically recognized as non-self and cleared by the reticuloendothelial system (RES), mainly in the liver and spleen(Sercombe et al. 2015) . Once opsonized, the SCs are recognized by macrophages and cleared via phagocytosis(Sercombe et al. 2015) . SCs can also be damaged via enzyme activity, which further reduces their half-life. The use of PEG to shield the outside of the liposome from the surrounding matrix has been a highly successful strategy in lengthening nanoparticle half-lives in vivo(Maruyama et al. 1992; Photos et al. 2003) . Many strategies have been developed to improve on current shielding effects(Boyer and Zasadzinski 2007; He et al. 2019; Gulati, Stewart, and Steinmetz 2018) .
The interior of the SC contains the payload, which can be drug or aqueous solutions that further endow the SC with function. In the simplest case, the compartment houses only a small molecule drug to be delivered to the site of action. In one of the most complex cases, the compartment houses an in vitro transcription/translation (IVTT) reaction mixture that can generate genetically encoded RNA or protein(Silverman, Karim, and Jewett 2020) . In some cases, SCs can house entire particles, vesicles(N.-N. Deng et al. 2017) , or condensates(Niederholtmeyer, Chaggan, and Devaraj 2018; S. Liu et al. 2020) that can further endow the SC with functions such as sensing, metabolism(Leduc et al. 2007) and movements (more below)(Siton-Mendelson and Bernheim-Groswasser 2016) .
A variety of methods have been developed to manufacture synthetic cells. These include thin-film hydration(H. Zhang 2017), reverse emulsion(Pautot, Frisken, and Weitz 2003; Huang et al. 2013; Thompson, Williams, and Armes 2015), electroemulsion(Angelova and Dimitrov 1986), and others(Fatouros et al. 2014; Y. Hu and Qiu 2019; H. Liu et al. 2015). Recently, microfluidic platforms and other techniques have been developed to improve the efficiency, speed, and reliability of SC generation: electroformation and hydration(Girard et al. 2004), extrusion(Dittrich et al. 2006) , hydrodynamic focusing(Jahn et al. 2004), pulsed jetting(Funakoshi, Suzuki, and Takeuchi 2007; Kamiya et al. 2016; Gotanda et al. 2018) , double emulsion templating(Shum et al. 2008), transient membrane ejection(Matosevic and Paegel 2011), droplet emulsion transfer(Ota, Yoshizawa, and Takeuchi 2009), reverse emulsion (cDICE)(Abkarian, Loiseau, and Massiera 2011), droplet-supported dGUV (dsGUV)(Weiss et al. 2018; Haller et al. 2018), octanol-assisted liposome assembly (OLA)(Deshpande et al. 2016), wedge splitting(Deshpande et al. 2018) , and toroidal mixing(Webb et al. 2020). This rapid innovation suggests that many hurdles to microfluidic manufacturing may soon be overcome, which could prove catalytic to the field(Shah et al. 2020) . The synthetic cells that result from these processes tend to range from 500 nm to several tens of microns in size. The larger the particle, the faster it is cleared from the body, so therapeutic nanoparticles are generally made as small as possible, on the order of 10–100 nm(Hoshyar et al. 2016) . The more complicated the SC composition, the more difficult the manufacturing process becomes and places considerable technical and cost constraints on SC therapeutics.
Applications of synthetic cells
How synthetic cells are utilized in nanomedicine is determined by their capabilities (Figure 2 ). Life-like capabilities that can be engineered include directed localization, sense-and-respond behavior, gene expression, metabolism, and high in vivo stability. Applications of these properties range from diagnostics to therapeutics.
Figure 2: Life-like Functions of Synthetic Cells in Nanomedicine.
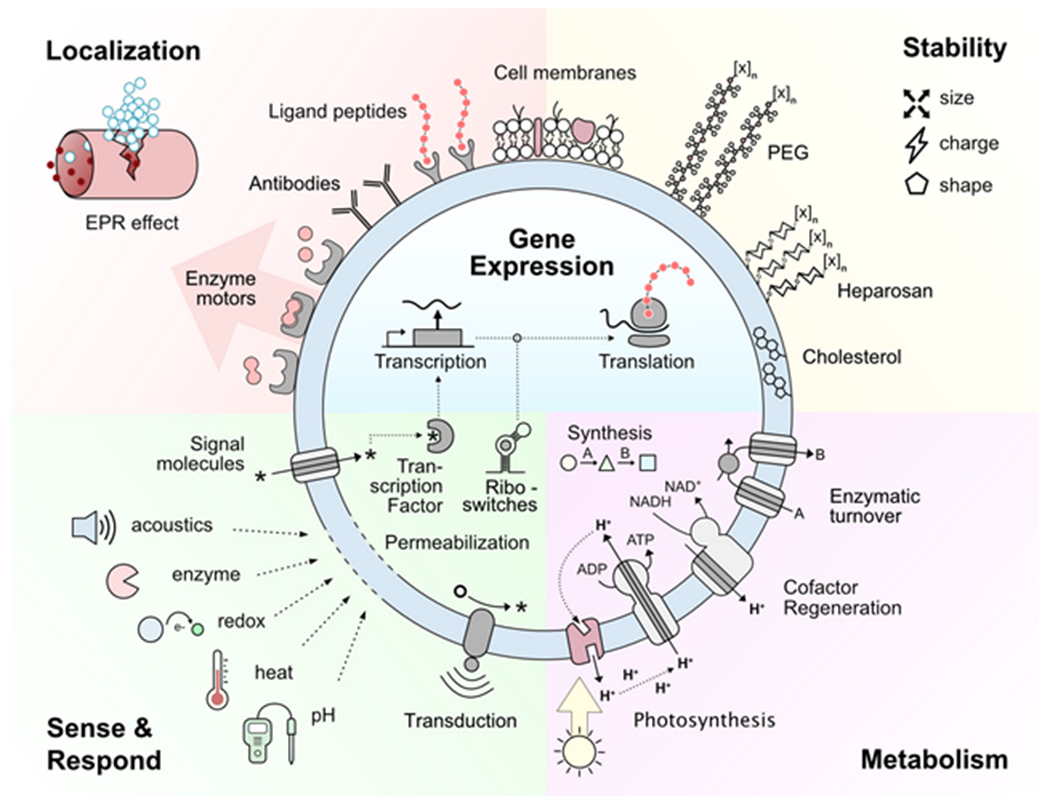
Synthetic cells are engineered with various chemical tools to mimic one or more functions of living cells. The physical properties of the SCs affect both their stability and localization through the EPR effect.
Directed Localization
A key strategy of living things is to move towards resources that benefit them. Similarly, there is great value in engineering synthetic cells that move to or localize at a target site. This physically concentrates SC’s activity at the target and reduces off-target toxicity. Targeted localization can be accomplished either actively (by energy-consuming movement) or passively (by increasing their affinity to the target tissue).
Significant progress has been made in endowing SCs with active chemotactic systems. While liposomes have been used to study natural systems of cell locomotion, these systems have proven difficult to employ due to their complexity(Pontani et al. 2009; Siton-Mendelson and Bernheim-Groswasser 2016) . Recently, relatively simple synthetic systems that leverage biophysical principles have provided more traction(Gentile et al. 2020) . Conjugation of enzymes to the surface of liposomes has been shown to endow them with the ability to move up or even down a pH or metabolite gradient(Ghosh et al. 2019; Somasundar et al. 2019; Hortelão et al. 2020) . This strategy has also proven effective in polymersomes(J. Wang et al. 2020) . Battaglia and colleagues created polymersomes with asymmetrically thick membranes that use encapsulated enzymes to generate a motor force(Joseph et al. 2017) . Another type of polymersome used a platinum nanoparticle to catalyze peroxide oxidation to chemotax towards neutrophils(Peng et al. 2015) . Other mechanisms for directed chemotaxis include the conjugation of complementary DNA oligomers or light-activated protein binders to a surface(Pan et al. 2019; Bartelt et al. 2018) . However, such strategies are more difficult to implement in vivo. Another method for directed localization is the conjugation of nanoparticles to living cells as “cellular backpacks”(Jones et al. 2017; Klyachko et al. 2017; Layek et al. 2018; Xie et al. 2017), but this does not require life-like behavior from the nanoparticle itself.
Passive targeting can be accomplished by increasing the affinity of SCs to the target tissue while relying on diffusion and circulation to do the work of carrying them to the tissue. In these cases, the surface of SCs can be modified with polymers, protein ligands, or antibodies that increase their targeting specificity(Leo et al. 2018; J. Cao et al. 2018; Kim, Niidome, and Lee 2019). When multiple targeting moieties are used, the specificity of the targeting increases (Khoshtinat Nikkhoi et al. 2018; Qu et al. 2014; Gray, Li, and Brown 2013; S. Oliveira et al. 2010; P. Guo et al. 2019) . While implementing these strategies, engineers must carefully consider the conjugation chemistry and orientation of the targeting molecules.
The physical properties of synthetic cells can also passively target them to specific tissues. For example, the small size of nanoparticles tends to concentrate them in tissues in which the enhanced permeability and retention (EPR) effect is observed, such as tumors and inflamed or otherwise damaged tissue(van den Hoven et al. 2011; Lobatto et al. 2015; Allen and Cullis 2013; Lammers et al. 2012) . However, recent work has shown that the EPR effect is dynamic and depends greatly on the composition and structure of the tissue(J. Fang, Islam, and Maeda 2020; Natfji et al. 2017; Danhier 2016). As another example, the strong positive charge of cationic lipid nanoparticles leads them to be taken up by the liver at high efficiencies(Witzigmann et al. 2020) . This has made cationic lipid nanoparticles excellent delivery vehicles for nucleic acid-based gene therapies such as Patisiran(Adams et al. 2018) .
Another approach to achieve localization is to coat nanoparticles with natural membranes(Esteban-Fernández de Ávila et al. 2018) . For example, nanoparticles decorated with red blood cells (RBC) membranes can target macrophages(Wan et al. 2018). To target cancer cells, nanoparticles can be decorated with cell membranes of platelets(Sarkar et al. 2013). Platelet membranes can be additionally labeled with anti-CD22 monoclonal antibodies to precisely deliver drugs to tumor cells(P. Xu et al. 2017; Q. Hu et al. 2015). As an alternative anti-cancer approach, one can use cancer cell membranes to coat nanoparticles that will be presented to antigen-presenting cells and promote anticancer immune response (R. H. Fang et al. 2014).
Sense and Respond
Living organisms can sense key environmental cues and subsequently respond with the appropriate action. Such seemingly “smart” behaviors are key to survival and are a highly desirable function in nanomedicines for several reasons. First, they enable more precise delivery to target tissues. Second, they enable more “analog” responses in which the output can be titrated to the strength of the input signal, thereby avoiding undue toxicity in neighboring tissue. The landscape of such “smart” sensing in vesicles has been extensively reviewed elsewhere(Abraham, Mao, and Tan 2018; Leduc et al. 2007; Majumder and Minko 2020; Torchilin 2014).
Sense-and-response functions can be facilitated directly via changes in SC membrane structure. Membranes have been composed of molecules sensitive to temperature(Yatvin et al. 1978; Needham et al. 2000; Tagami, Ernsting, and Li 2011; Ta et al. 2014; F. Liu et al. 2015; Xi et al. 2020; Jose et al. 2019), light(Miranda and Lovell 2016; Carter et al. 2014; D. Luo et al. 2016; Peyret et al. 2017; Enzian et al. 2020) , magnetism(Babincová et al. 2002; Amstad et al. 2011; H. Guo et al. 2015; H. Oliveira et al. 2013; Geilich et al. 2017), acoustics(Shekhar et al. 2017; Z. Deng et al. 2016; Rwei et al. 2017), pH(Naziris et al. 2017; Abri Aghdam et al. 2019; Leo et al. 2018), redox states(X. Yin et al. 2017; Chi et al. 2017; Mirhadi et al. 2020) , and enzymes(Thamphiwatana et al. 2014; Haas et al. 2015) . By combining these materials, the membrane can sometimes be made sensitive to multiple types of stimuli(Tran et al. 2017; S. Feng et al. 2019) , enabling even more precise targeting. The major limitation of these materials is that few respond to the specific molecules of interest, such as certain cell surface receptors or cancer metabolites. For this, more specific sensors such as protein receptors are required.
Sensing can also be accomplished by membrane-embedded amphiphiles or proteins that are sensitive to specific molecules or conditions. These can transduce detected signals into the interior of the SC through various mechanisms(Langton 2020) . Protein and peptide pores that respond to osmotic pressure, heat, pH, and electrical potential by creating selective and nonselective pores have been shown to work in liposomes(Louhivuori et al. 2010; Kisovec et al. 2017; Garamella et al. 2019; Aimon et al. 2011; Yanagisawa et al. 2011; Kreir et al. 2008). One group developed a membrane-spanning amphiphile that responds to changes in pH or protein unbinding by localizing at the interior leaflet of the liposome and inducing catalysis that results in the release of drug(Langton et al. 2017; Ding, Williams, and Hunter 2019) . Several bacterial 2-component systems(Ravikumar et al. 2017) have been functionally reconstituted in liposomes(Sanowar and Le Moual 2005; Ito et al. 2009; Jung, Tjaden, and Altendorf 1997; Pflüger et al. 2018), though none of them have been used to induce downstream protein production. Additionally, few other natural or engineered protein transducers (e.g. the SynNotch receptor),Morsut et al. 2016) have been successfully tested in synthetic cells( . This speaks to the sensitivity and complexity of many membrane-bound protein transduction systems and the difficulty with which they can be implemented in synthetic systems.
Another strategy is to embed nonspecific, permanently open pores into the membrane to enable passage of small molecule signals that then stimulate activity inside the SC. To that end, Staphylococcus aureus α-hemolysin has become a favorite tool for synthetic cell engineers due to its ability to spontaneously insert into a wide diversity of membranes and form nonspecific pores. This has been used to allow nutrients(Noireaux and Libchaber 2004) , chemical inducers (e.g. IPTG)(Lentini et al. 2014) , and other small molecules(Wu et al. 2011; Soga et al. 2020) to traffic the SC. Furthermore, SNAREs and DNA oligos have been used to facilitate SC fusions(Schuette et al. 2004; W. Xu et al. 2015) and thereby deliver molecular messengers into the interior of the target SC. Once inside the SC, these messengers can then induce catalysis or even protein production via gene expression.
Transcription factors, RNA riboswitches, and enzymes can also act as sensors inside the SC compartment. These sensors are limited to sensing the interior of the SC, so only molecules that can pass through the membrane or pore can be detected. Enzymes will detect their substrates, and the resulting reactions can change the ambient conditions, such as the pH(Peters, Nijemeisland, and van Hest 2015) . Various transcription factors and riboswitches that are sensitive to the presence of diverse small molecules(Salehi et al. 2017; X. Liu et al. 2020; L. Zhang, Guo, and Lu 2020; Dwidar et al. 2019) and even light(P. Zhang et al. 2020; Schroeder et al. 2012) can be used to control transcription and translation in SCs (see below). Combining sensor signals into transcriptional logic can enable powerful programming of Boolean behaviors(S. Iyer et al. 2013; Shis et al. 2014; Adamala et al. 2017) . Communication between SCs and natural living cells has also been engineered and can give rise to complex interactions between synthetic and natural populations(Lentini et al. 2014, 2017) . Furthermore, the communication that results from mass exchange and sensing among SCs enables the formation of complex multicellular “synthetic tissue” (Niederholtmeyer, Chaggan, and Devaraj 2018; Villar, Graham, and Bayley 2013; Aufinger and Simmel 2018; Ding, Williams, and Hunter 2019; T.-Y. D. Tang et al. 2018; Schwarz-Schilling et al. 2016; Toda et al. 2018; Adamala et al. 2017) . Transcriptional and translational responses to chemicals, however, are relatively slow compared to other sensing mechanisms. To program rapid responses such as those needed for a SC-based bionic jellyfish(Nawroth et al. 2012) (ref), electromechanical sensors and actuators still need to be developed.
Gene Expression
Another powerful capability of synthetic cells is their ability to express genes. Producing protein or small molecules in situ is advantageous in drug delivery when the drug is unstable, needs to be titrated or is so reactive that it would kill living cells. Through the action of encapsulated in vitro transcription/translation (IVTT) reaction mixture, SCs can produce RNA, proteins, or even small molecule drugs in situ. For this, nucleic acids encoding the desired gene must be co-encapsulated. IVTT reaction mixtures may include purified cell extract(Z. Z. Sun et al. 2013; Kwon and Jewett 2015) or defined mixtures of recombinant protein such as the PURE system(Y. Shimizu et al. 2001; Yoshihiro Shimizu and Ueda 2010; Lavickova and Maerkl 2019) . Gene expression can be induced when a triggering signal is sensed (see above). RNA that is produced could serve as a diagnostic signal that can be detected by sequencing RNA extracted from whole blood(Pös et al. 2018) . Translated proteins can be enzymes that together comprise a metabolic pathway that generates a small molecule drug(Dudley, Anderson, and Jewett 2016; Grubbe et al. 2020) . SCs can also synthesize membrane proteins that will spontaneously insert into membranes and act as uptake signals to target cells .(Kaneda et al. 2009; Lu et al. 2019)
Previously, in situ protein production could only be accomplished by living cells. These were delivered into the body encapsulated in polymer membranes, where they could survive a long time while ameliorating chronic conditions such as diabetes(Lim and Sun 1980; Soon-Shiong et al. 1994; Y. Sun et al. 1996; Calafiore et al. 1999; de Vos, Hamel, and Tatarkiewicz 2002), neurological diseases(Bloch et al. 2004), haemophilia(Basic, Vacek, and Sun 1996), or cancer(Löhr et al. 2001) and are reviewed elsewhere(Thomas Ming Swi Chang 2005, 2019) . Recent development of synthetic cells that encapsulate IVTT reactions offers an alternative approach to encapsulated whole cells. Schroeder and colleagues pioneered SC therapy by demonstrating that liposomes containing IVTT could be used to synthesize anti-cancer proteins inside tumors. In this work, they showed that synthetic cells producing Pseudomonas exotoxin A killed most cancer cells in culture and caused robust apoptosis when injected into 4T1 tumors in mice(Krinsky et al. 2018).
Beyond its utility in direct therapeutic intervention, cell-free gene expressions has enabled other biomedical technologies and novel research tools. For instance, the high stability of freeze-dried IVTT reactions has enabled the development of on-demand biotherapeutic manufacturing platforms(Pardee et al. 2016; Adiga et al. 2020, 2018; Jaroentomeechai et al. 2018). Another example is liposome display, a technology that uniquely enables in vitro selection and directed evolution of protein pores(Fujii et al. 2014; Uyeda et al. 2016). Membrane-bound IVTT protein production can also control the orientation of integral membrane proteins(Ando et al. 2018; Ohta et al. 2016). Challenges these technologies still face include relatively low titers of protein produced, reproduction of critical post-translational modifications, and the limitations on controlling the insertion and orientation of membrane proteins. Nonetheless, due to the close ties between the fields of cell-free biochemistry and synthetic cell engineering, each will doubtless benefit from the other’s continued advancement.
Metabolism
Living cells maintain their functions through active metabolism. This allows them to act against entropy repeatedly or continuously over extended periods of time, a behavior that is challenging to engineer in nanoparticle drug delivery systems. SCs, however, can be loaded with complex biochemistry that can mimic many of the metabolic processes that living cells perform. These include production of energy molecules such as ATP, the regeneration of essential cofactors, or chemical transformation of target metabolites. The value of this is both to sustain the therapeutic function of the SC as well as directly metabolize toxic metabolites.
By actively generating ATP or other energy molecules, SCs can maintain a sustained response instead of generating only a short burst of activity from the ATP encapsulated during production. Several different approaches have been taken to endow SCs with the ability to generate ATP. A common strategy is to create a proton gradient that can then be used by ATP synthase to drive ATP production. To generate the proton gradient, light-activated bacteriorhodopsin(Choi and Montemagno 2005; Dhir et al. 2018; Z. Chen et al. 2019) or other proton pumping systems(X. Feng et al. 2016; Steinberg-Yfrach et al. 1998; Cladera et al. 1996; Altamura et al., n.d.) can be embedded in the membrane. The resulting light-dependent ATP synthesis can then drive IVTT protein production(Berhanu, Ueda, and Kuruma 2019)or other ATP-dependent processes. Though this approach has been fruitful, it is challenging to implement in vivo because light only penetrates a few millimeters into the skin(Sabino et al. 2016). Instead, ATP synthesis can be driven via catabolic chemistry on ambient energy-rich molecules(Jewett and Swartz 2004; Calhoun and Swartz 2005; Biner et al. 2020; Caschera and Noireaux 2015). The feedstocks for these pathways are also substantially cheaper than the high-energy molecules used in some batch reactions(Calhoun and Swartz 2007).
Regeneration of cofactors such as nicotinamide adenine dinucleotide phosphate (NADPH) is often essential to maintain biochemical reactions. This can be accomplished by encapsulating enzymes that catalyze the regenerating reaction(Meeuwissen et al. 2011). Hirst and colleagues recently demonstrated sustained ATP synthesis by coupling ATP synthase to NADH oxidation(Biner et al. 2020). Integrating novel ways to import or regenerate cofactors and other reagents is critical for longer sustained reactions in synthetic cells.
Chemically transforming metabolites can provide vital therapeutic benefits. Vesicles that contain enzymes have been developed for therapeutic application since the 1960s. Seminal work by Thomas Chang demonstrated that compartmentalized enzymes could provide therapeutic effects in animals lacking normal enzyme activity(T. M. S. Chang and Poznansky 1968) . Since then, therapeutic encapsulations of urease(Cattaneo and Chang 1991; Gu and Chang 1990; Lvov et al. 2001; Miele et al. 2020) , catalase(R. Zhang et al. 2017; Shi et al. 2020) , superoxide dismutase(Riedl et al. 2005; Shazeeb, Feula, and Bogdanov 2014; Niesman, Johnson, and Penn 1997) , β-galactosidase(Rao, Chawan, and Veeramachaneni 1994) , bacterial DNA repair enzymes(Berardesca et al. 2012; D. Yarosh et al. 1996; D. B. Yarosh, Rosenthal, and Moy 2019), alcohol oxidases(Pratsinis et al. 2017; C. Lizano et al. 1998; Whitmire, Chambers, and Dillon 1991), glucose oxidase(S. Liu et al. 2020) among others, have opened doors to novel therapies. Many of these formulations aim to remove membrane-permeable metabolites from the body. Pratsinis, et al. employed liposomes bearing either alcohol oxidase or catalase in their membranes in peritoneal dialysis to remove ethanol from the blood of rats(Pratsinis et al. 2017). This work follows older efforts in which alcohol dehydrogenase and aldehyde dehydrogenase are encapsulated together to break down ethanol in vivo. In these systems, another enzyme (e.g. malate dehydrogenase) is used to regenerate the NAD+ cofactor required to maintain the oxidation reaction, highlighting the importance of cofactor regeneration to maintaining high catabolic rates(Campbell and Chang 1978; T. M. S. Chang 1987; Carmen Lizano, Teresa Pérez, and Pinilla 2001).
Encapsulated enzymes provide value to an impressive diversity of indications. A recent clinically tested example include Lipoxysan, a transdermal liposomal encapsulation of superoxide dismutase, which was recently tested in Peyronie’s disease in Phase 2 clinical trials (Riedl et al. 2005). Mann and colleagues demonstrated that complex assemblies of glucose oxidase-containing coacervate and hemoglobin-containing red blood cell-derived membranes were able to generate nitric oxide in vivo, inducing vasodilation(S. Liu et al. 2020) . An example in which SCs serve to aid in diagnosis is in the work by Molina and colleagues. In this work, the authors generated different SCs containing mixtures of three or more different enzymes. Based on the metabolites present, SCs would generate different colored products. Incubating these SCs in urine aided in the diagnosis of pre-diabetic states in patients(Courbet et al. 2018) .
Multilamellar liposomes, vesosomes, and different species of liposomes can work together to control reactions. Incompatible enzymes can be separated in defined compartments allowing the spatial organization and segregation of the multistep tandem reaction(Klermund, Poschenrieder, and Castiglione 2017) . Different liposomes containing varied enzymes can be connected via ɑ-haemolysin channels . Polymer SCs containing two distinct populations of enzyme-encapsulating vesicles have been demonstrated to function inside living cells(Godoy-Gallardo et al. 2017). To prevent the deactivation of catalysts in water or avoid unwanted cross-reactions, catalysts are often site-isolated in nanopockets or separately stored in compartments. These examples show that control of the localization of enzymes within an SC can be as valuable to the SCs function as the enzyme activity itself.
High stability
One of the most desirable characteristics of living cells is their ability to remain intact in the blood for long periods of time. Nanoparticles, on the other hand, are typically less stable and are rapidly cleared by the reticuloendothelial (RES) system(Sercombe et al. 2015). This is typically due to both their physical nature (large, spherical, stiff objects are more quickly removed from the blood) and to the fact that they do not display proteins that mark the nanoparticle as the body’s own cell(F. Chen et al. 2017; Vu et al. 2019; Zahednezhad et al. 2019).
Numerous strategies have been taken to endow nanoparticles with longer half-lives in blood. The decoration of the particles with polyethylene glycol (PEG) is perhaps the most successful “stealth” strategy. PEGylation, however, is falling out of favor due to the production and presence of anti-PEG antibodies(L. Yin et al. 2015), PEG tissue accumulation(Lane et al. 2017; Rippe et al. 2019) , evidence of lack of PEG degradation, potentially creating vacuoles(Baumann et al. 2014; Ivens et al. 2015) , and alteration of enzyme activity(Leuzzi et al. 2016). As such, alternatives to PEG such as heparosan are being developed(Lane et al. 2017; Rippe et al. 2019).
Decoration of particles with membranes derived from living cells is a powerful strategy to shield nanoparticles. This “semi-synthetic” approach, pioneered by Hu and colleagues, has proven highly versatile(C-M J. Hu et al. 2011). All manner of cell membranes and cell membrane proteins have been used to coat nanoparticles and SCs(Corbo et al. 2017; Liang et al. 2018; Che-Ming J. Hu et al. 2013; Weiwei Gao et al. 2015; L. Luo et al. 2017; J. Tang et al. 2017; H. Cao et al. 2016; Pitchaimani, Nguyen, and Aryal 2018). This effectively shields the SCs from the RES and endows them with some of the signaling properties of the cells from which their borrowed membranes derive. As mentioned above, this strategy also enables targeting.
Challenges Facing Synthetic Cell Therapeutics
The field of synthetic cell engineering is relatively new, and many challenges still remain to be solved or even identified. These challenges include need for molecular tools, integration of disparate technologies, difficult manufacturing, and regulatory frameworks that disfavor complex drug formulations (Figure 3 ).
Figure 3:
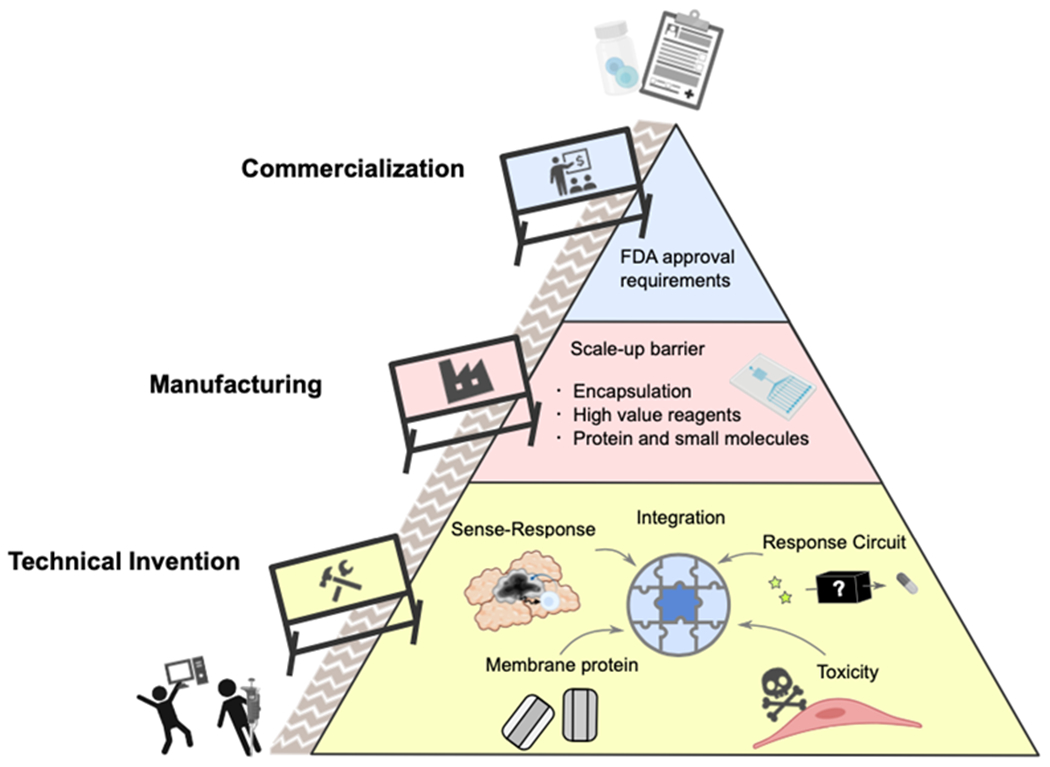
Barriers to Synthetic Cell use in nanomedicine. Most fundamentally, new innovations and solutions are needed to endow SCs with modular functions that can perform reliably in physiological conditions. Next, production and cost barriers need to be addressed. Finally, therapeutic SCs will encounter regulatory hurdles that may require adapting current frameworks.
To coordinate release of therapeutic agents at the right location (e.g. at a tumor), more robust sense & respond mechanisms are needed. Currently, there is a relative lack of sensors that activate SCs in response to specific molecules, such as membrane proteins overexpressed on cancer cells. Such tools are now readily available in living cell therapies, such as CAR-T cells. Transferring natural membrane transduction systems to synthetic membranes is difficult due to their size, complexity and requirements for post-translational modification and membrane insertion machinery. While bacterial transduction systems are smaller and relatively more robust proteins, they are likely immunogenic and cannot recognize eukaryotic membrane proteins. To enable reliable sensing of eukaryotic molecular markers of disease, a concerted effort is needed to engineer membrane transduction systems that function specifically in SCs.
A similar problem is the limited repertoire of trans-membrane channels available in synthetic cell systems. Living cells tightly control the flow of molecules in and out of the cell through channel proteins or membrane budding mechanisms. SCs, however, currently lack most of these systems. This is reflected in the prolific use of ɑ-hemolysin, a simple and robust bacterial toxin that enables passive transport of small molecules. Only a few active transporters have been demonstrated in synthetic cells, including proteins as complex as ATP synthase. If more reliable membrane transporters could be identified and engineered to spontaneously insert into the membrane in the desired orientation, it would greatly broaden the sensing and delivery repertoire of SCs.
An issue for the entire field of synthetic cell engineering, including all therapeutic applications of this technology, is the integration of subsystems into one robust entity. As described in earlier sections of this review, many SC subsystems have been developed to demonstrate specific functionalities. Because each subsystem is engineered ad hoc, it is often difficult to reconcile their diverse chemistries and structures. To address this, several approaches can be taken. First, engineers in the field could standardize the chemical and structural framework in which they develop SCs. To encourage this, funding agencies could require adherence to these frameworks when it is sensible. Second, subsystems could be engineered with integration in mind by reporting subsystem performance across a variety of contexts. Lastly, computational models rooted in empirical data could be developed to guide the integration, much like what was done to guide the integration of synthetic genetic circuits (Nielsen et al. 2016).
To use synthetic cells as human therapeutics, other issues will need to be solved as well, like the toxicity of cell-free IVTT systems. While defined systems such as PURE contain mostly purified proteins, cell-derived fractions of ribosomes still contain some amount of endotoxins (i.e. lipopolysaccharides), which are highly pyrogenic. So far, only direct tumor injection was demonstrated as a method for localizing synthetic cells into a solid tumor in mice (Krinsky et al. 2018), and there is a relative lack of available data on half-lives and dose-dependent toxicity of synthetic cell formulations in animals.
As therapeutic applications of synthetic cells progress through foundational research and commercial R&D pipelines, the field will need to face technical challenges related to scaling up production of those novel therapeutics. Among those challenges, two areas present the most well-defined focus points: the compartment and the chemicals inside it.
Scaling up manufacturing of lipid vesicles to create membrane encapsulating synthetic cells will require progress in current microfluidic technology, or perhaps development of entirely new class of liposome formation technologies. Synthetic cells are typically larger than liposome drug delivery vehicles (single microns vs tens of nanometers in diameter), and enzymes encapsulated inside synthetic cells can not be encapsulated via remote loading used for some liposomal drugs. This creates a need for a whole new class of reliable, reproducible and scalable encapsulation techniques.
Similarly, producing large amounts of proteins and small molecules needed to provide therapeutic quantities of synthetic cells might require adjustment in supply chains. Production of cell-free protein expression systems is already scalable to 100-liter reaction volumes (Zawada et al. 2011), but availability of certain high value reagents remains a limiting step. We as a field anxiously await the invention of “PURE that makes PURE” -- a cell-free IVTT reaction that can make every one of its own functional components and need little more than raw material as feedstock -- as an idealized solution to the scaling problem.
Once synthetic cell technologies pass into animal testing, the need to fulfill FDA approval requirements will become critical. Currently, there are few guidelines for developing therapies as molecularly complex as synthetic cells . FDA approval is granted either for drugs with precisely controlled chemical composition, or for natural cell therapeutics. Synthetic cells, being made from synthetic components but not being descended from known living cells, may require a new framework by which such systems are evaluated . Uniformity of the formulation will remain critical, putting pressure on the above mentioned supply chain and scalability of membrane formulations. The FDA is already aware of the needs that might arise with progress of novel therapies using untested chassis, facilitating development of new oversight rules through the FDA Emerging Technologies Program. (Center for Drug Evaluation and Research 2019) It will be critical that the synthetic cell community works closely with regulatory agencies to develop a supply pipeline and draft new frameworks for the testing and eventual deployment of those therapies for patient use.
Discussion & Outlook
Many have noted in recent years that as increased resources are devoted to development of drugs, ever fewer result in approved therapies. (Scannell et al. 2012) This phenomenon, known as Eroom’s law (the reverse of Moore’s law), highlights the need for new therapeutic modalities and approaches. Here we describe how synthetic cells offer a unique, engineerable platform for achieving a range of valuable therapeutic behaviors. These offer to augment existing means of drug delivery as well as tools for research and drug discovery pipelines. Though significant technical progress has been made in mimicking several advantageous features of living systems, the integration of these features remains a challenge, as do the development and manufacturing of such systems. Given recent progress, the vision of a synthetic cell that can identify and ameliorate disease in a programmable manner without adding risk of adverse effects looks less like a moonshot than an inevitable next step for medicine.
Funding Information
The authors were supported by the NIH grant 5R01MH114031-02, NSF grants 1840301 1844313 and John Templeton Foundation grant 61184.
References
- Abkarian Manouk, Loiseau Etienne, and Massiera Gladys. 2011. “Continuous Droplet Interface Crossing Encapsulation (cDICE) for High Throughput Monodisperse Vesicle Design.” Soft Matter 7 (10): 4610–14. 10.1039/C1SM05239J. [DOI] [Google Scholar]
- Abraham Tanishq, Mao Michelle, and Tan Cheemeng. 2018. “Engineering Approaches of Smart, Bio-Inspired Vesicles for Biomedical Applications.” Physical Biology 15 (6): 061001. 10.1088/1478-3975/aac7a2. [DOI] [PubMed] [Google Scholar]
- Aghdam Abri, Marjan Roya Bagheri, Mosafer Jafar, Baradaran Behzad, Hashemzaei Mahmoud, Baghbanzadeh Amir, de la Guardia Miguel, and Mokhtarzadeh Ahad. 2019. “Recent Advances on Thermosensitive and pH-Sensitive Liposomes Employed in Controlled Release.” Journal of Controlled Release: Official Journal of the Controlled Release Society 315 (December): 1–22. 10.1016/j.jconrel.2019.09.018. [DOI] [PubMed] [Google Scholar]
- “Activation of the Bacterial Sensor Kinase PhoQ by Acidic pH.” 2007. Molecular Cell 26 (2): 165–74. 10.1016/j.molcel.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Adamala Katarzyna P., Martin-Alarcon Daniel A., Guthrie-Honea Katriona R., and Boyden Edward S.. 2017. “Engineering Genetic Circuit Interactions within and between Synthetic Minimal Cells.” Nature Chemistry 9 (5): 431–39. 10.1038/nchem.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams David, Gonzalez-Duarte Alejandra, O’Riordan William D., Yang Chih-Chao, Ueda Mitsuharu, Kristen Arnt V., Tournev Ivailo, et al. 2018. “Patisiran, an RNAi Therapeutic, for Hereditary Transthyretin Amyloidosis.” The New England Journal of Medicine 379 (1): 11–21. 10.1056/NEJMoa1716153. [DOI] [PubMed] [Google Scholar]
- Adiga Rajani, Mustafa Al-Adhami, Abhay Andar, Borhani Shayan, Brown Sheniqua, Burgenson David, Cooper Merideth A., et al. 2018. “Point-of-Care Production of Therapeutic Proteins of Good-Manufacturing-Practice Quality.” Nature Biomedical Engineering 2 (9): 675–86. 10.1038/s41551-018-0259-1. [DOI] [PubMed] [Google Scholar]
- Adiga Rajani, Andar Abhay, Borhani Shayan, Burgenson David, Deldari Sevda, Frey Douglas, Ge Xudong, et al. 2020. “Manufacturing Biological Medicines on Demand: Safety and Efficacy of Granulocyte Colony-stimulating Factor in a Mouse Model of Total Body Irradiation.” Biotechnology Progress. 10.1002/btpr.2970. [DOI] [PubMed] [Google Scholar]
- Aimon Sophie, Manzi John, Schmidt Daniel, Jose Antonio Poveda Larrosa, Patricia Bassereau, and Toombes Gilman E. S.. 2011. “Functional Reconstitution of a Voltage-Gated Potassium Channel in Giant Unilamellar Vesicles.” PloS One 6 (10): e25529. 10.1371/journal.pone.0025529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen Theresa M., and Cullis Pieter R.. 2013. “Liposomal Drug Delivery Systems: From Concept to Clinical Applications.” Advanced Drug Delivery Reviews 65 (1): 36–48. 10.1016/j.addr.2012.09.037. [DOI] [PubMed] [Google Scholar]
- Altamura Emiliano, Albanese Paola, Marotta Roberto, Milano Francesco, Fiore Michele, Trotta Massimo, Stano Pasquale, and Mavelli Fabio. n.d. “Light-Driven ATP Production Promotes mRNA Biosynthesis inside Hybrid Multi-Compartment Artificial Protocells.” 10.1101/2020.02.05.933846. [DOI] [Google Scholar]
- Amstad Esther, Kohlbrecher Joachim, Müller Elisabeth, Schweizer Thomas, Textor Marcus, and Reimhult Erik. 2011. “Triggered Release from Liposomes through Magnetic Actuation of Iron Oxide Nanoparticle Containing Membranes.” Nano Letters 11 (4): 1664–70. 10.1021/nl2001499. [DOI] [PubMed] [Google Scholar]
- Ando Mitsuru, Schikula Shun, Sasaki Yoshihiro, and Akiyoshi Kazunari. 2018. “Proteoliposome Engineering with Cell-Free Membrane Protein Synthesis: Control of Membrane Protein Sorting into Liposomes by Chaperoning Systems.” Advancement of Science 5 (10): 1800524. 10.1002/advs.201800524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelova Miglena I., and Dimitrov Dimiter S.. 1986. “Liposome Electroformation.” Faraday Discussions of the Chemical Society 81 (0): 303–11. 10.1039/DC9868100303. [DOI] [Google Scholar]
- Aufinger Lukas, and Simmel Friedrich C.. 2018. “Artificial Gel-Based Organelles for Spatial Organization of Cell-Free Gene Expression Reactions.” Angewandte Chemie 57 (52): 17245–48. 10.1002/anie.201809374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babincová M, Cicmanec P, Altanerová V, Altaner C, and Babinec P. 2002. “AC-Magnetic Field Controlled Drug Release from Magnetoliposomes: Design of a Method for Site-Specific Chemotherapy.” Bioelectrochemistry 55 (1–2): 17–19. 10.1016/s1567-5394(01)00171-2. [DOI] [PubMed] [Google Scholar]
- Baimanov Didar, Cai Rong, and Chen Chunying. 2019. “Understanding the Chemical Nature of Nanoparticle-Protein Interactions.” Bioconjugate Chemistry 30 (7): 1923–37. 10.1021/acs.bioconjchem.9b00348. [DOI] [PubMed] [Google Scholar]
- Bartelt Solveig M., Jan Steinkühler, Dimova Rumiana, and Seraphine V. Wegner. 2018. “Light-Guided Motility of a Minimal Synthetic Cell.” Nano Letters. 10.1021/acs.nanolett.8b03469. [DOI] [PubMed] [Google Scholar]
- Basic Doris, Ivan Vacek, and Anthony M. Sun. 1996. “Microencapsulation and Transplantation of Genetically Engineered Cells: A New Approach to Somatic Gene Therapy.” Artificial Cells, Blood Substitutes, and Biotechnology. 10.3109/10731199609117437. [DOI] [PubMed] [Google Scholar]
- Baumann Andreas, Tuerck Dietrich, Prabhu Saileta, Dickmann Leslie, and Sims Jennifer. 2014. “Pharmacokinetics, Metabolism and Distribution of PEGs and PEGylated Proteins: Quo Vadis?” Drug Discovery Today. 10.1016/j.drudis.2014.06.002. [DOI] [PubMed] [Google Scholar]
- Berardesca Enzo, Bertona Marco, Altabas Karmela, Altabas Velimir, and Emanuele Enzo. 2012. “Reduced Ultraviolet-Induced DNA Damage and Apoptosis in Human Skin with Topical Application of a Photolyase-Containing DNA Repair Enzyme Cream: Clues to Skin Cancer Prevention.” Molecular Medicine Reports 5 (2): 570–74. 10.3892/mmr.2011.673. [DOI] [PubMed] [Google Scholar]
- Berhanu Samuel, Ueda Takuya, and Kuruma Yutetsu. 2019. “Artificial Photosynthetic Cell Producing Energy for Protein Synthesis.” Nature Communications 10 (1): 1325. 10.1038/s41467-019-09147-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biner Olivier, Fedor Justin G., Yin Zhan, and Hirst Judy. 2020. “Bottom-Up Construction of a Minimal System for Cellular Respiration and Energy Regeneration.” ACS Synthetic Biology 9 (6): 1450–59. 10.1021/acssynbio.0c00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch J, Bachoud-Lévi AC, Déglon N, Lefaucheur JP, Winkel L, Palfi S, Nguyen JP, et al. 2004. “Neuroprotective Gene Therapy for Huntington’s Disease, Using Polymer-Encapsulated Cells Engineered to Secrete Human Ciliary Neurotrophic Factor: Results of a Phase I Study.” Human Gene Therapy 15 (10): 968–75. 10.1089/hum.2004.15.968. [DOI] [PubMed] [Google Scholar]
- Boyer Cecile, and Zasadzinski Joseph A.. 2007. “Multiple Lipid Compartments Slow Vesicle Contents Release in Lipases and Serum.” ACS Nano 1 (3): 176–82. 10.1021/nn7002025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briuglia Maria-Lucia, Rotella Chiara, McFarlane Amber, and Lamprou Dimitrios A. 2015. “Influence of Cholesterol on Liposome Stability and on in Vitro Drug Release.” Drug Delivery and Translational Research 5 (3): 231–42. 10.1007/s13346-015-0220-8. [DOI] [PubMed] [Google Scholar]
- Calafiore R, Basta G, Luca G, Boselli C, Bufalari A, Bufalari A, Cassarani MP, Giustozzi GM, and Brunetti P. 1999. “Transplantation of Pancreatic Islets Contained in Minimal Volume Microcapsules in Diabetic High Mammalians.” Annals of the New York Academy of Sciences 875 (June): 219–32. 10.1111/j.1749-6632.1999.tb08506.x. [DOI] [PubMed] [Google Scholar]
- Calhoun Kara A., and Swartz James R.. 2005. “Energizing Cell-Free Protein Synthesis with Glucose Metabolism.” Biotechnology and Bioengineering 90 (5): 606–13. 10.1002/bit.20449. [DOI] [PubMed] [Google Scholar]
- ———. 2007. “Energy Systems for ATP Regeneration in Cell-Free Protein Synthesis Reactions.” Methods in Molecular Biology 375: 3–17. 10.1007/978-1-59745-388-2_1. [DOI] [PubMed] [Google Scholar]
- Campbell J, and Chang TMS. 1978. “Microencapsulated Multi-Enzyme Systems as Vehicles for the Cyclic Regeneration of Free and Immobilized Coenzymes.” Enzyme Engineering. 10.1007/978-1-4757-5163-5_42. [DOI] [Google Scholar]
- Cao Haiqiang, Dan Zhaoling, He Xinyu, Zhang Zhiwen, Yu Haijun, Yin Qi, and Li Yaping. 2016. “Liposomes Coated with Isolated Macrophage Membrane Can Target Lung Metastasis of Breast Cancer.” ACS Nano 10 (8): 7738–48. 10.1021/acsnano.6b03148. [DOI] [PubMed] [Google Scholar]
- Cao Jing, Li Chong, Wei Xiaohui, Tu Meiqing, Zhang Yan, Xu Fengwei, and Xu Yuhong. 2018. “Selective Targeting and Eradication of LGR5 Cancer Stem Cells Using RSPO-Conjugated Doxorubicin Liposomes.” Molecular Cancer Therapeutics 17 (7): 1475–85. 10.1158/1535-7163.MCT-17-0694. [DOI] [PubMed] [Google Scholar]
- Carter Kevin A., Shao Shuai, Hoopes Matthew I., Luo Dandan, Ahsan Bilal, Grigoryants Vladimir M., Song Wentao, et al. 2014. “Porphyrin–phospholipid Liposomes Permeabilized by near-Infrared Light.” Nature Communications. 10.1038/ncomms4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caschera Filippo, and Noireaux Vincent. 2015. “A Cost-Effective Polyphosphate-Based Metabolism Fuels an All E. Coli Cell-Free Expression System.” Metabolic Engineering. 10.1016/j.ymben.2014.10.007. [DOI] [PubMed] [Google Scholar]
- Cattaneo MV, and Chang TM. 1991. “The Potential of a Microencapsulated Urease-Zeolite Oral Sorbent for the Removal of Urea in Uremia.” ASAIO Transactions / American Society for Artificial Internal Organs 37 (2): 80–87. https://www.ncbi.nlm.nih.gov/pubmed/1649615. [PubMed] [Google Scholar]
- “Cell-Free Expression and Assembly of ATP Synthase.” 2011. Journal of Molecular Biology 413 (3): 593–603. 10.1016/j.jmb.2011.08.055. [DOI] [PubMed] [Google Scholar]
- Center for Drug Evaluation, and Research. 2019. “Emerging Technology Program.” October 10, 2019. https://www.fda.gov/about-fda/center-drug-evaluation-and-research-cder/emerging-technology-program.
- Chang Hao-Nan, Liu Bei-Yuan, Qi Yun-Kun, Zhou Yang, Chen Yan-Ping, Pan Kai-Mai, Li Wen-Wen, et al. 2015. “Blocking of the PD-1/PD-L1 Interaction by a D-Peptide Antagonist for Cancer Immunotherapy.” Angewandte Chemie 54 (40): 11760–64. 10.1002/anie.201506225. [DOI] [PubMed] [Google Scholar]
- Chang Thomas Ming Swi. 2005. “Therapeutic Applications of Polymeric Artificial Cells.” Nature Reviews Drug Discovery. 10.1038/nrd1659. [DOI] [PubMed] [Google Scholar]
- . 2019. “ARTIFICIAL CELL Evolves into Nanomedicine, Biotherapeutics, Blood Substitutes, Drug Delivery, Enzyme/gene Therapy, Cancer Therapy, Cell/stem Cell Therapy, Nanoparticles, Liposomes, Bioencapsulation, Replicating Synthetic Cells, Cell Encapsulation/scaffold, Biosorbent/immunosorbent Haemoperfusion/plasmapheresis, Regenerative Medicine, Encapsulated Microbe, Nanobiotechnology, Nanotechnology.” Artificial Cells, Nanomedicine, and Biotechnology 47 (1): 997–1013. 10.1080/21691401.2019.1577885. [DOI] [PubMed] [Google Scholar]
- Chang TMS 1987. “[7] Recycling of NAD(P) by Multienzyme Systems Immobilized by Microencapsulation in Artificial Cells.” Immobilized Enzymes and Cells, Part C. 10.1016/s0076-6879(87)36009-4. [DOI] [PubMed] [Google Scholar]
- Chang TMS, and Poznansky MJ. 1968. “Semipermeable Microcapsules Containing Catalase for Enzyme Replacement in Acatalasaemic Mice.” Nature. 10.1038/218243a0. [DOI] [PubMed] [Google Scholar]
- Chen Fangfang, Wang Guankui, Griffin James I., Brenneman Barbara, Banda Nirmal K., Holers V. Michael, Backos Donald S., Wu Linping, Seyed Moein Moghimi, and Simberg Dmitri. 2017. “Complement Proteins Bind to Nanoparticle Protein Corona and Undergo Dynamic Exchange in Vivo.” Nature Nanotechnology 12 (4): 387–93. 10.1038/nnano.2016.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Zhaowei, Gleiciani De Queiros Silveira, Ma Xuedan, Xie Yunsong, Wu Yimin A., Barry Edward, Rajh Tijana, Fry H. Christopher, Laible Philip D., and Rozhkova Elena A.. 2019. “Light‐Gated Synthetic Protocells for Plasmon‐Enhanced Chemiosmotic Gradient Generation and ATP Synthesis.” Angewandte Chemie. 10.1002/ange.201813963. [DOI] [PubMed] [Google Scholar]
- Chen Zhaowei, Jinqiang Wang, Wujin Sun, Edikan Archibong, Anna R Kahkoska, Xudong Zhang, Yue Lu, Frances S Ligler, John B Buse, and Zhen Gu. 2018. “Synthetic Beta Cells for Fusion-Mediated Dynamic Insulin Secretion.” Nature Chemical Biology 14 (1): 86–93. 10.1038/nchembio.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi Yingying, Yin Xuelei, Sun Kaoxiang, Feng Shuaishuai, Liu Jinhu, Chen Daquan, Guo Chuanyou, and Wu Zimei. 2017. “Redox-Sensitive and Hyaluronic Acid Functionalized Liposomes for Cytoplasmic Drug Delivery to Osteosarcoma in Animal Models.” Journal of Controlled Release: Official Journal of the Controlled Release Society 261 (September): 113–25. 10.1016/j.jconrel.2017.06.027. [DOI] [PubMed] [Google Scholar]
- Choi Hyo-Jick, and Montemagno Carlo D.. 2005. “Artificial Organelle: ATP Synthesis from Cellular Mimetic Polymersomes.” Nano Letters. 10.1021/nl051896e. [DOI] [PubMed] [Google Scholar]
- Cladera J, Rigaud JL, Bottin H, and Duñach M. 1996. “Functional Reconstitution of Photosystem I Reaction Center from Cyanobacterium Synechocystis Sp PCC6803 into Liposomes Using a New Reconstitution Procedure.” Journal of Bioenergetics and Biomembranes 28 (6): 503–15. 10.1007/BF02110440. [DOI] [PubMed] [Google Scholar]
- Corbo Claudia, Molinaro Roberto, Taraballi Francesca, Naama E Toledano Furman, Hartman Kelly A., Sherman Michael B., Enrica De Rosa, Dickson K Kirui, Francesco Salvatore, and Ennio Tasciotti. 2017. “Unveiling the in Vivo Protein Corona of Circulating Leukocyte-like Carriers.” ACS Nano. 10.1021/acsnano.7b00376. [DOI] [PubMed] [Google Scholar]
- Courbet Alexis, Amar Patrick, Fages François, Renard Eric, and Molina Franck. 2018. “Computer‐aided Biochemical Programming of Synthetic Microreactors as Diagnostic Devices.” Molecular Systems Biology. 10.15252/msb.20188441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danhier F 2016. “To Exploit the Tumor Microenvironment: Since the EPR Effect Fails in the Clinic, What Is the Future of Nanomedicine?” Journal of Controlled Release: Official Journal of the Controlled Release Society 244 (Pt A): 108–21. 10.1016/j.jconrel.2016.11.015. [DOI] [PubMed] [Google Scholar]
- Deng Nan-Nan, Yelleswarapu Maaruthy, Zheng Lifei, and Huck Wilhelm T. S.. 2017. “Microfluidic Assembly of Monodisperse Vesosomes as Artificial Cell Models.” Journal of the American Chemical Society 139 (2): 587–90. 10.1021/jacs.6b10977. [DOI] [PubMed] [Google Scholar]
- Deng Zhiting, Xiao Yang, Pan Min, Li Fei, Duan Wanlu, Meng Long, Liu Xin, Yan Fei, and Zheng Hairong. 2016. “Hyperthermia-Triggered Drug Delivery from iRGD-Modified Temperature-Sensitive Liposomes Enhances the Anti-Tumor Efficacy Using High Intensity Focused Ultrasound.” Journal of Controlled Release: Official Journal of the Controlled Release Society 243 (December): 333–41. 10.1016/j.jconrel.2016.10.030. [DOI] [PubMed] [Google Scholar]
- Deshpande Siddharth, Caspi Yaron, Meijering Anna E. C., and Dekker Cees. 2016. “Octanol-Assisted Liposome Assembly on Chip.” Nature Communications 7 (January): 10447. 10.1038/ncomms10447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande Siddharth, Willem Kasper Spoelstra, Marleen van Doorn, Jacob Kerssemakers, and Cees Dekker. 2018. “Mechanical Division of Cell-Sized Liposomes.” ACS Nano 12 (3): 2560–68. 10.1021/acsnano.7b08411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhir Satarupa, Salahub Sumalee, Anu Stella Mathews Surjith Kumar Kumaran, Montemagno Carlo D., and Abraham Sinoj. 2018. “Light-Induced ATP Driven Self-Assembly of Actin and Heavy-Meromyosin in Proteo-Tubularsomes as a Step toward Artificial Cells.” Chemical Communications 54 (42): 5346–49. 10.1039/c8cc02691b. [DOI] [PubMed] [Google Scholar]
- Ding Yudi, Williams Nicholas H., and Hunter Christopher A.. 2019. “A Synthetic Vesicle-to-Vesicle Communication System.” Journal of the American Chemical Society 141 (44): 17847–53. 10.1021/jacs.9b09102. [DOI] [PubMed] [Google Scholar]
- Dittrich Petra S., Heule Martin, Renaud Philippe, and Manz Andreas. 2006. “On-Chip Extrusion of Lipid Vesicles and Tubes through Microsized Apertures.” Lab on a Chip 6 (4): 488–93. 10.1039/b517670k. [DOI] [PubMed] [Google Scholar]
- Doktorova Milka, Heberle Frederick A., Eicher Barbara, Standaert Robert F., Katsaras John, London Erwin, Pabst Georg, and Marquardt Drew. 2018. “Preparation of Asymmetric Phospholipid Vesicles for Use as Cell Membrane Models.” Nature Protocols 13 (9): 2086–2101. 10.1038/s41596-018-0033-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dondapati Srujan Kumar, Doreen A. Wüstenhagen, Eckhard Strauch, and Stefan Kubick. 2018. “Cell-Free Production of Pore Forming Toxins: Functional Analysis of Thermostable Direct Hemolysin from Vibrio Parahaemolyticus.” Engineering in Life Sciences 18 (2): 140–48. 10.1002/elsc.201600259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley Quentin M., Anderson Kim C., and Jewett Michael C.. 2016. “Cell-Free Mixing of Escherichia Coli Crude Extracts to Prototype and Rationally Engineer High-Titer Mevalonate Synthesis.” ACS Synthetic Biology 5 (12): 1578–88. 10.1021/acssynbio.6b00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwidar Mohammed, Seike Yusuke, Kobori Shungo, Whitaker Charles, Matsuura Tomoaki, and Yokobayashi Yohei. 2019. “Programmable Artificial Cells Using Histamine-Responsive Synthetic Riboswitch.” Journal of the American Chemical Society 141 (28): 11103–14. 10.1021/jacs.9b03300. [DOI] [PubMed] [Google Scholar]
- Elmore Donald E., and Dougherty Dennis A.. 2003. “Investigating Lipid Composition Effects on the Mechanosensitive Channel of Large Conductance (MscL) Using Molecular Dynamics Simulations.” Biophysical Journal 85 (3): 1512–24. 10.1016/S0006-3495(03)74584-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enzian Paula, Schell Christian, Link Astrid, Malich Carina, Pries Ralph, Wollenberg Barbara, and Rahmanzadeh Ramtin. 2020. “Optically Controlled Drug Release from Light-Sensitive Liposomes with the New Photosensitizer 5,10-DiOH.” Molecular Pharmaceutics 17 (8): 2779–88. 10.1021/acs.molpharmaceut.9b01173. [DOI] [PubMed] [Google Scholar]
- Esteban-Fernández de Ávila Berta, Gao Weiwei, Karshalev Emil, Zhang Liangfang, and Wang Joseph. 2018. “Cell-Like Micromotors.” Accounts of Chemical Research 51 (9): 1901–10. 10.1021/acs.accounts.8b00202. [DOI] [PubMed] [Google Scholar]
- Fang Jun, Islam Waliul, and Maeda Hiroshi. 2020. “Exploiting the Dynamics of the EPR Effect and Strategies to Improve the Therapeutic Effects of Nanomedicines by Using EPR Effect Enhancers.” Advanced Drug Delivery Reviews, June. 10.1016/j.addr.2020.06.005. [DOI] [PubMed] [Google Scholar]
- Fang Ronnie H., Hu Che-Ming J., Luk Brian T., Gao Weiwei, Copp Jonathan A., Tai Yiyin, O’Connor Derek E., and Liangfang Zhang. 2014. “Cancer Cell Membrane-Coated Nanoparticles for Anticancer Vaccination and Drug Delivery.” Nano Letters 14 (4): 2181–88. 10.1021/nl500618u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatouros Dimitrios G., Lamprou Dimitrios A., Urquhart Andrew J., Yannopoulos Spyros N., Vizirianakis Ioannis S., Zhang Shuguang, and Koutsopoulos Sotirios. 2014. “Lipid-like Self-Assembling Peptide Nanovesicles for Drug Delivery.” ACS Applied Materials & Interfaces 6 (11): 8184–89. 10.1021/am501673x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Shuaishuai, Wu Zi-Xin, Zhao Ziyan, Liu Jinhu, Sun Kaoxiang, Guo Chuanyou, Wang Hongbo, and Wu Zimei. 2019. “Engineering of Bone- and CD44-Dual-Targeting Redox-Sensitive Liposomes for the Treatment of Orthotopic Osteosarcoma.” ACS Applied Materials & Interfaces 11 (7): 7357–68. 10.1021/acsami.8b18820. [DOI] [PubMed] [Google Scholar]
- Feng Xiyun, Jia Yi, Cai Peng, Fei Jinbo, and Li Junbai. 2016. “Coassembly of Photosystem II and ATPase as Artificial Chloroplast for Light-Driven ATP Synthesis.” ACS Nano 10 (1): 556–61. 10.1021/acsnano.5b05579. [DOI] [PubMed] [Google Scholar]
- Francia Valentina, Schiffelers Raymond M., Cullis Pieter R., and Witzigmann Dominik. 2020. “The Biomolecular Corona of Lipid Nanoparticles for Gene Therapy.” Bioconjugate Chemistry 31 (9): 2046–59. 10.1021/acs.bioconjchem.0c00366. [DOI] [PubMed] [Google Scholar]
- Fujii Satoshi, Matsuura Tomoaki, Sunami Takeshi, Nishikawa Takehiro, Kazuta Yasuaki, and Yomo Tetsuya. 2014. “Liposome Display for in Vitro Selection and Evolution of Membrane Proteins.” Nature Protocols 9 (7): 1578–91. 10.1038/nprot.2014.107. [DOI] [PubMed] [Google Scholar]
- Funakoshi Kei, Suzuki Hiroaki, and Takeuchi Shoji. 2007. “Formation of Giant Lipid Vesiclelike Compartments from a Planar Lipid Membrane by a Pulsed Jet Flow.” Journal of the American Chemical Society 129 (42): 12608–9. 10.1021/ja074029f. [DOI] [PubMed] [Google Scholar]
- Gao Wei, Cho Eunhee, Liu Yingying, and Lu Yuan. 2019. “Advances and Challenges in Cell-Free Incorporation of Unnatural Amino Acids Into Proteins.” Frontiers in Pharmacology 10 (May): 611. 10.3389/fphar.2019.00611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Weiwei, Fang Ronnie H., Thamphiwatana Soracha, Luk Brian T., Li Jieming, Angsantikul Pavimol, Zhang Qiangzhe, Hu Che-Ming J., and Zhang Liangfang. 2015. “Modulating Antibacterial Immunity via Bacterial Membrane-Coated Nanoparticles.” Nano Letters 15 (2): 1403–9. 10.1021/nl504798g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garamella Jonathan, Majumder Sagardip, Liu Allen P., and Noireaux Vincent. 2019. “An Adaptive Synthetic Cell Based on Mechanosensing, Biosensing, and Inducible Gene Circuits.” ACS Synthetic Biology 8 (8): 1913–20. 10.1021/acssynbio.9b00204. [DOI] [PubMed] [Google Scholar]
- Geilich Benjamin M., Gelfat Ilia, Sridhar Srinivas, van de Ven Anne L., and Webster Thomas J.. 2017. “Superparamagnetic Iron Oxide-Encapsulating Polymersome Nanocarriers for Biofilm Eradication.” Biomaterials 119 (March): 78–85. 10.1016/j.biomaterials.2016.12.011. [DOI] [PubMed] [Google Scholar]
- Gentile Kayla, Somasundar Ambika, Bhide Ashlesha, and Sen Ayusman. 2020. “Chemically Powered Synthetic ‘Living’ Systems.” Chem. 10.1016/j.chempr.2020.08.010. [DOI] [Google Scholar]
- Ghosh S, Mohajerani F, Son S, Velegol D, Butler PJ, and Sen A. 2019. “Motility of Enzyme-Powered Vesicles.” Nano Letters 19 (9). 10.1021/acs.nanolett.9b01830. [DOI] [PubMed] [Google Scholar]
- Girard Philippe, Pécréaux Jacques, Lenoir Guillaume, Falson Pierre, Rigaud Jean-Louis, and Bassereau Patricia. 2004. “A New Method for the Reconstitution of Membrane Proteins into Giant Unilamellar Vesicles.” Biophysical Journal 87 (1): 419–29. 10.1529/biophysj.104.040360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulimondi Francesca, Digiacomo Luca, Pozzi Daniela, Palchetti Sara, Vulpis Elisabetta, Capriotti Anna Laura, Chiozzi Riccardo Zenezini, et al. 2019. “Interplay of Protein Corona and Immune Cells Controls Blood Residency of Liposomes.” Nature Communications 10 (1): 3686. 10.1038/s41467-019-11642-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godoy-Gallardo Maria, Labay Cédric, Trikalitis Vasileios D., Kempen Paul J., Larsen Jannik B., Andresen Thomas L., and Hosta-Rigau Leticia. 2017. “Multicompartment Artificial Organelles Conducting Enzymatic Cascade Reactions inside Cells.” ACS Applied Materials & Interfaces 9 (19): 15907–21. 10.1021/acsami.6b16275. [DOI] [PubMed] [Google Scholar]
- Gotanda Masahide, Kamiya Koki, Osaki Toshihisa, Fujii Satoshi, Misawa Nobuo, Miki Norihisa, and Takeuchi Shoji. 2018. “Sequential Generation of Asymmetric Lipid Vesicles Using a Pulsed-Jetting Method in Rotational Wells.” Sensors and Actuators. B, Chemical 261 (May): 392–97. 10.1016/j.snb.2018.01.149. [DOI] [Google Scholar]
- Gray Bethany Powell, Li Shunzi, and Brown Kathlynn C.. 2013. “From Phage Display to Nanoparticle Delivery: Functionalizing Liposomes with Multivalent Peptides Improves Targeting to a Cancer Biomarker.” Bioconjugate Chemistry 24 (1): 85–96. 10.1021/bc300498d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubbe William S., Rasor Blake J., Krüger Antje, Jewett Michael C., and Karim Ashty S.. 2020. “Cell-Free Styrene Biosynthesis at High Titers.” Metabolic Engineering 61 (September): 89–95. 10.1016/j.ymben.2020.05.009. [DOI] [PubMed] [Google Scholar]
- Gu Kang Fu, and Chang Thomas Ming Swi. 1990. “Conversion of Ammonia or Urea into Essential Amino Acids, L-Leucine, L-Valine, and L-Isoleucine Using Artificial Cells Containing an Immobilized Multienzyme System and Dextran-NAD.” Journal of Molecular Catalysis. 10.1016/0304-5102(90)85228-a. [DOI] [PubMed] [Google Scholar]
- Gulati Neetu M., Stewart Phoebe L., and Steinmetz Nicole F.. 2018. “Bioinspired Shielding Strategies for Nanoparticle Drug Delivery Applications.” Molecular Pharmaceutics 15 (8): 2900–2909. 10.1021/acs.molpharmaceut.8b00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Hongyan, Chen Wansong, Sun Xiaoyi, Liu You-Nian, Li Juan, and Wang Jianxiu. 2015. “Theranostic Magnetoliposomes Coated by Carboxymethyl Dextran with Controlled Release by Low-Frequency Alternating Magnetic Field.” Carbohydrate Polymers 118 (March): 209–17. 10.1016/j.carbpol.2014.10.076. [DOI] [PubMed] [Google Scholar]
- Guo Peng, Yang Jiang, Liu Daxing, Huang Lan, Fell Gillian, Huang Jing, Moses Marsha A., and Auguste Debra T.. 2019. “Dual Complementary Liposomes Inhibit Triple-Negative Breast Tumor Progression and Metastasis.” Science Advances 5 (3): eaav5010. 10.1126/sciadv.aav5010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas Simon, Hain Nicole, Raoufi Mohammad, Handschuh-Wang Stephan, Wang Tao, Jiang Xin, and Schönherr Holger. 2015. “Enzyme Degradable Polymersomes from Hyaluronic Acid-Block-Poly(ε-Caprolactone) Copolymers for the Detection of Enzymes of Pathogenic Bacteria.” Biomacromolecules 16 (3): 832–41. 10.1021/bm501729h. [DOI] [PubMed] [Google Scholar]
- Haller Barbara, Kerstin Göpfrich Martin Schröter, Janiesch Jan-Willi, Platzman Ilia, and Spatz Joachim P.. 2018. “Charge-Controlled Microfluidic Formation of Lipid-Based Single- and Multicompartment Systems.” Lab on a Chip 18 (17): 2665–74. 10.1039/c8lc00582f. [DOI] [PubMed] [Google Scholar]
- He Haisheng, Lu Yi, Qi Jianping, Zhu Quangang, Chen Zhongjian, and Wu Wei. 2019. “Adapting Liposomes for Oral Drug Delivery.” Acta Pharmaceutica Sinica. B 9 (1): 36–48. 10.1016/j.apsb.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hortelão Ana C., Sonia García‐Jimeno Mary Cano‐Sarabia, Tania Patiño Daniel Maspoch, and Sanchez Samuel. 2020. “LipoBots: Using Liposomal Vesicles as Protective Shell of Urease‐Based Nanomotors.” Advanced Functional Materials. 10.1002/adfm.202002767. [DOI] [Google Scholar]
- Hoshyar Nazanin, Gray Samantha, Han Hongbin, and Bao Gang. 2016. “The Effect of Nanoparticle Size on in Vivo Pharmacokinetics and Cellular Interaction.” Nanomedicine 11 (6): 673–92. 10.2217/nnm.16.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoven Jolanda M. van den, Van Tomme Sophie R., Metselaar Josbert M., Nuijen Bastiaan, Beijnen Jos H., and Storm Gert. 2011. “Liposomal Drug Formulations in the Treatment of Rheumatoid Arthritis.” Molecular Pharmaceutics. 10.1021/mp2000742. [DOI] [PubMed] [Google Scholar]
- Huang Xin, Li Mei, Green David C., Williams David S., Patil Avinash J., and Mann Stephen. 2013. “Interfacial Assembly of Protein-Polymer Nano-Conjugates into Stimulus-Responsive Biomimetic Protocells.” Nature Communications 4: 2239. 10.1038/ncomms3239. [DOI] [PubMed] [Google Scholar]
- Huang Xin, Patil Avinash J., Li Mei, and Mann Stephen. 2014. “Design and Construction of Higher-Order Structure and Function in Proteinosome-Based Protocells.” Journal of the American Chemical Society 136 (25): 9225–34. 10.1021/ja504213m. [DOI] [PubMed] [Google Scholar]
- Hu Che-Ming J., Fang Ronnie H., Copp Jonathan, Luk Brian T., and Zhang Liangfang. 2013. “A Biomimetic Nanosponge That Absorbs Pore-Forming Toxins.” Nature Nanotechnology 8 (5): 336–40. 10.1038/nnano.2013.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C-MJ, Zhang L, Aryal S, Cheung C, Fang RH, and Zhang L. 2011. “Erythrocyte Membrane-Camouflaged Polymeric Nanoparticles as a Biomimetic Delivery Platform.” Proceedings of the National Academy of Sciences. 10.1073/pnas.1106634108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Quanyin, Sun Wujin, Qian Chengen, Wang Chao, Bomba Hunter N., and Gu Zhen. 2015. “Anticancer Platelet-Mimicking Nanovehicles.” Advanced Materials 27 (44): 7043–50. 10.1002/adma.201503323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Yumiao, and Qiu Liyan. 2019. “Polymersomes: Preparation and Characterization.” In Pharmaceutical Nanotechnology: Basic Protocols, edited by Weissig Volkmar and Elbayoumi Tamer, 247–65. New York, NY: Springer New York. 10.1007/978-1-4939-9516-5_17. [DOI] [Google Scholar]
- Ito Yoko, Nakagawa Shoko, Komagata Ayako, Masao Ikeda-Saito Yoshitsugu Shiro, and Nakamura Hiro. 2009. “Heme-Dependent Autophosphorylation of a Heme Sensor Kinase, ChrS, from Corynebacterium Diphtheriae Reconstituted in Proteoliposomes.” FEBS Letters 583 (13): 2244–48. 10.1016/j.febslet.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Ivens Inge A., Achanzar William, Baumann Andreas, Brändli-Baiocco Annamaria, Cavagnaro Joy, Dempster Maggie, Depelchin B. Olympe, et al. 2015. “PEGylated Biopharmaceuticals: Current Experience and Considerations for Nonclinical Development.” Toxicologic Pathology 43 (7): 959–83. 10.1177/0192623315591171. [DOI] [PubMed] [Google Scholar]
- Iyer Rohin K., Bowles Paul A., Kim Howard, and Dulgar-Tulloch Aaron. 2018. “Industrializing Autologous Adoptive Immunotherapies: Manufacturing Advances and Challenges.” Frontiers of Medicine 5 (May): 150. 10.3389/fmed.2018.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer Sukanya, Karig David K., Norred S. Elizabeth, Simpson Michael L., and Doktycz Mitchel J.. 2013. “Multi-Input Regulation and Logic with T7 Promoters in Cells and Cell-Free Systems.” PloS One 8 (10): e78442. 10.1371/journal.pone.0078442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn Andreas, Vreeland Wyatt N., Gaitan Michael, and Locascio Laurie E.. 2004. “Controlled Vesicle Self-Assembly in Microfluidic Channels with Hydrodynamic Focusing.” Journal of the American Chemical Society 126 (9): 2674–75. 10.1021/ja0318030. [DOI] [PubMed] [Google Scholar]
- Jaroentomeechai Thapakorn, Stark Jessica C., Natarajan Aravind, Glasscock Cameron J., Yates Laura E., Hsu Karen J., Mrksich Milan, Jewett Michael C., and DeLisa Matthew P.. 2018. “Single-Pot Glycoprotein Biosynthesis Using a Cell-Free Transcription-Translation System Enriched with Glycosylation Machinery.” Nature Communications 9 (1): 2686. 10.1038/s41467-018-05110-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewett Michael C., and Swartz James R.. 2004. “Mimicking the Escherichia Coli Cytoplasmic Environment Activates Long-Lived and Efficient Cell-Free Protein Synthesis.” Biotechnology and Bioengineering 86 (1): 19–26. 10.1002/bit.20026. [DOI] [PubMed] [Google Scholar]
- Jones R. Brad, Mueller Stephanie, Kumari Sudha, Vrbanac Vlad, Genel Shy, Tager Andrew M., Allen Todd M., Walker Bruce D., and Irvine Darrell J.. 2017. “Antigen Recognition-Triggered Drug Delivery Mediated by Nanocapsule-Functionalized Cytotoxic T-Cells.” Biomaterials 117 (February): 44–53. 10.1016/j.biomaterials.2016.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jose Anup, Ninave Kunal Manoj, Karnam Sriravali, and Venuganti Venkata Vamsi Krishna. 2019. “Temperature-Sensitive Liposomes for Co-Delivery of Tamoxifen and Imatinib for Synergistic Breast Cancer Treatment.” Journal of Liposome Research 29 (2): 153–62. 10.1080/08982104.2018.1502315. [DOI] [PubMed] [Google Scholar]
- Joseph Adrian, Contini Claudia, Cecchin Denis, Nyberg Sophie, Ruiz-Perez Lorena, Gaitzsch Jens, Fullstone Gavin, et al. 2017. “Chemotactic Synthetic Vesicles: Design and Applications in Blood-Brain Barrier Crossing.” Science Advances 3 (8): e1700362. 10.1126/sciadv.1700362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung K, Tjaden B, and Altendorf K. 1997. “Purification, Reconstitution, and Characterization of KdpD, the Turgor Sensor of Escherichia Coli.” The Journal of Biological Chemistry 272 (16): 10847–52. 10.1074/jbc.272.16.10847. [DOI] [PubMed] [Google Scholar]
- Kamiya Koki, Kawano Ryuji, Osaki Toshihisa, Akiyoshi Kazunari, and Takeuchi Shoji. 2016. “Cell-Sized Asymmetric Lipid Vesicles Facilitate the Investigation of Asymmetric Membranes.” Nature Chemistry 8 (9): 881–89. 10.1038/nchem.2537. [DOI] [PubMed] [Google Scholar]
- Kaneda Makoto, Nomura Shin-Ichiro M., Ichinose Shizuko, Kondo Satoshi, Nakahama Ken-Ichi, Akiyoshi Kazunari, and Morita Ikuo. 2009. “Direct Formation of Proteo-Liposomes by in Vitro Synthesis and Cellular Cytosolic Delivery with Connexin-Expressing Liposomes.” Biomaterials 30 (23–24): 3971–77. 10.1016/j.biomaterials.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Nikkhoi Khoshtinat, Shahryar Fatemeh Rahbarizadeh, Ahmadvand Davoud, and Moghimi Seyed Moein. 2018. “Multivalent Targeting and Killing of HER2 Overexpressing Breast Carcinoma Cells with Methotrexate-Encapsulated Tetra-Specific Non-Overlapping Variable Domain Heavy Chain Anti-HER2 Antibody-PEG-Liposomes: In Vitro Proof-of-Concept.” European Journal of Pharmaceutical Sciences: Official Journal of the European Federation for Pharmaceutical Sciences 122 (September): 42–50. 10.1016/j.ejps.2018.06.019. [DOI] [PubMed] [Google Scholar]
- Kim Min Woo, Niidome Takuro, and Lee Ruda. 2019. “Glycol Chitosan-Docosahexaenoic Acid Liposomes for Drug Delivery: Synergistic Effect of Doxorubicin-Rapamycin in Drug-Resistant Breast Cancer.” Marine Drugs 17 (10). 10.3390/md17100581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisovec Matic, Rezelj Saša, Knap Primož, Mojca Cajnko Miša, Caserman Simon, Flašker Ajda, Žnidaršič Nada, et al. 2017. “Engineering a pH Responsive Pore Forming Protein.” Scientific Reports. 10.1038/srep42231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klermund Ludwig, Poschenrieder Sarah T., and Castiglione Kathrin. 2017. “Biocatalysis in Polymersomes: Improving Multienzyme Cascades with Incompatible Reaction Steps by Compartmentalization.” ACS Catalysis. 10.1021/acscatal.7b00776. [DOI] [Google Scholar]
- Klyachko Natalia L., Polak Roberta, Haney Matthew J., Zhao Yuling, Gomes Neto Reginaldo J., Hill Michael C., Kabanov Alexander V., Cohen Robert E., Rubner Michael F., and Batrakova Elena V.. 2017. “Macrophages with Cellular Backpacks for Targeted Drug Delivery to the Brain.” Biomaterials 140 (September): 79–87. 10.1016/j.biomaterials.2017.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreir Mohamed, Farre Cecilia, Beckler Matthias, George Michael, and Fertig Niels. 2008. “Rapid Screening of Membrane Protein Activity: Electrophysiological Analysis of OmpF Reconstituted in Proteoliposomes.” Lab on a Chip 8 (4): 587–95. 10.1039/b713982a. [DOI] [PubMed] [Google Scholar]
- Krinsky N, Kaduri M, Zinger A, Shainsky-Roitman J, Goldfeder M, Benhar I, Hershkovitz D, and Schroeder A. 2018. “Synthetic Cells Synthesize Therapeutic Proteins inside Tumors.” Advanced Healthcare Materials 7 (9). 10.1002/adhm.201701163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper Suzanne M., Nallani Madhavan, Vriezema Dennis M., Cornelissen Jeroen J. L. M., van Hest Jan C. M., Nolte Roeland J. M., and Rowan Alan E.. 2008. “Enzymes Containing Porous Polymersomes as Nano Reaction Vessels for Cascade Reactions.” Organic & Biomolecular Chemistry 6 (23): 4315–18. 10.1039/b811196k. [DOI] [PubMed] [Google Scholar]
- Kwon Yong-Chan, and Jewett Michael C.. 2015. “High-Throughput Preparation Methods of Crude Extract for Robust Cell-Free Protein Synthesis.” Scientific Reports 5 (March): 8663. 10.1038/srep08663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammers Twan, Kiessling Fabian, Hennink Wim E., and Storm Gert. 2012. “Drug Targeting to Tumors: Principles, Pitfalls and (pre-) Clinical Progress.” Journal of Controlled Release: Official Journal of the Controlled Release Society 161 (2): 175–87. 10.1016/j.jconrel.2011.09.063. [DOI] [PubMed] [Google Scholar]
- Lane Rachel S., Haller F. Michael, Chavaroche Anais A. E., Almond Andrew, and DeAngelis Paul L.. 2017. “Heparosan-Coated Liposomes for Drug Delivery.” Glycobiology 27 (11): 1062–74. 10.1093/glycob/cwx070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langton Matthew J. 2020. “Engineering of Stimuli-Responsive Lipid-Bilayer Membranes Using Supramolecular Systems.” Nature Reviews Chemistry. 10.1038/s41570-020-00233-6. [DOI] [PubMed] [Google Scholar]
- Langton Matthew J., Scriven Lorel M., Williams Nicholas H., and Hunter Christopher A.. 2017. “Triggered Release from Lipid Bilayer Vesicles by an Artificial Transmembrane Signal Transduction System.” Journal of the American Chemical Society 139 (44): 15768–73. 10.1021/jacs.7b07747. [DOI] [PubMed] [Google Scholar]
- Lavickova Barbora, and Maerkl Sebastian J.. 2019. “A Simple, Robust, and Low-Cost Method To Produce the PURE Cell-Free System.” ACS Synthetic Biology 8 (2): 455–62. 10.1021/acssynbio.8b00427. [DOI] [PubMed] [Google Scholar]
- Layek Buddhadev, Sadhukha Tanmoy, Panyam Jayanth, and Prabha Swayam. 2018. “Nano-Engineered Mesenchymal Stem Cells Increase Therapeutic Efficacy of Anticancer Drug Through True Active Tumor Targeting.” Molecular Cancer Therapeutics 17 (6): 1196–1206. 10.1158/1535-7163.MCT-17-0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leduc Philip R., Wong Michael S., Ferreira Placid M., Groff Richard E., Haslinger Kiryn, Koonce Michael P., Lee Woo Y., et al. 2007. “Towards an in Vivo Biologically Inspired Nanofactory.” Nature Nanotechnology 2 (1): 3–7. 10.1038/nnano.2006.180. [DOI] [PubMed] [Google Scholar]
- Lentini Roberta, Noël Yeh Martín Michele Forlin, Belmonte Luca, Fontana Jason, Cornella Michele, Martini Laura, et al. 2017. “Two-Way Chemical Communication between Artificial and Natural Cells.” ACS Central Science 3 (2): 117–23. 10.1021/acscentsci.6b00330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentini Roberta, Santero Silvia Perez, Chizzolini Fabio, Cecchi Dario, Fontana Jason, Marchioretto Marta, Bianco Cristina Del, et al. 2014. “Integrating Artificial with Natural Cells to Translate Chemical Messages That Direct E. Coli Behaviour.” Nature Communications. 10.1038/ncomms5012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leo Vincenzo De, Vincenzo De Leo Francesco Milano, Mancini Erminia, Comparelli Roberto, Giotta Livia, Nacci Angelo, et al. 2018. “Encapsulation of Curcumin-Loaded Liposomes for Colonic Drug Delivery in a pH-Responsive Polymer Cluster Using a pH-Driven and Organic Solvent-Free Process.” Molecules. 10.3390/molecules23040739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuzzi Vincenzo, Rossi Luigia, Gabucci Claudia, Nardecchia Francesca, and Magnani Mauro. 2016. “Erythrocyte-Mediated Delivery of Recombinant Enzymes.” Journal of Inherited Metabolic Disease 39 (4): 519–30. 10.1007/s10545-016-9926-0. [DOI] [PubMed] [Google Scholar]
- Liang Hongxia, Huang Ke, Su Teng, Li Zhenhua, Hu Shiqi, Dinh Phuong-Uyen, Wrona Emily A., et al. 2018. “Mesenchymal Stem Cell/Red Blood Cell-Inspired Nanoparticle Therapy in Mice with Carbon Tetrachloride-Induced Acute Liver Failure.” ACS Nano 12 (7): 6536–44. 10.1021/acsnano.8b00553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim F, and Sun A. 1980. “Microencapsulated Islets as Bioartificial Endocrine Pancreas.” Science. 10.1126/science.6776628. [DOI] [PubMed] [Google Scholar]
- Liu Fei, Kozlovskaya Veronika, Medipelli Srikanth, Xue Bing, Ahmad Fahim, Saeed Mohammad, Cropek Donald, and Kharlampieva Eugenia. 2015. “Temperature-Sensitive Polymersomes for Controlled Delivery of Anticancer Drugs.” Chemistry of Materials. 10.1021/acs.chemmater.5b03048. [DOI] [Google Scholar]
- Liu Hanqing, Tu Zhigang, Feng Fan, Shi Haifeng, Chen Keping, and Xu Ximing. 2015. “Virosome, a Hybrid Vehicle for Efficient and Safe Drug Delivery and Its Emerging Application in Cancer Treatment.” Acta Pharmaceutica 65 (2): 105–16. 10.1515/acph-2015-0019. [DOI] [PubMed] [Google Scholar]
- Liu Songyang, Zhang Yanwen, Li Mei, Xiong Li, Zhang Zijian, Yang Xiaohai, He Xiaoxiao, Wang Kemin, Liu Jianbo, and Mann Stephen. 2020. “Enzyme-Mediated Nitric Oxide Production in Vasoactive Erythrocyte Membrane-Enclosed Coacervate Protocells.” Nature Chemistry 12 (12): 1165–73. 10.1038/s41557-020-00585-y. [DOI] [PubMed] [Google Scholar]
- Liu Xiangyang, Silverman Adam D., Alam Khalid K., Iverson Erik, Lucks Julius B., Jewett Michael C., and Raman Srivatsan. 2020. “Design of a Transcriptional Biosensor for the Portable, On-Demand Detection of Cyanuric Acid.” ACS Synthetic Biology 9 (1): 84–94. 10.1021/acssynbio.9b00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizano Carmen, Pérez M. Teresa, and Pinilla Montserrat. 2001. “Mouse Erythrocytes as Carriers for Coencapsulated Alcohol and Aldehyde Dehydrogenase Obtained by Electroporation.” Life Sciences. 10.1016/s0024-3205(01)00991-2. [DOI] [PubMed] [Google Scholar]
- Lizano C, Sanz S, Luque J, and Pinilla M. 1998. “In Vitro Study of Alcohol Dehydrogenase and Acetaldehyde Dehydrogenase Encapsulated into Human Erythrocytes by an Electroporation Procedure.” Biochimica et Biophysica Acta 1425 (2): 328–36. 10.1016/s0304-4165(98)00085-3. [DOI] [PubMed] [Google Scholar]
- Lobatto Mark E., Calcagno Claudia, Millon Antoine, Senders Max L., Fay Francois, Robson Philip M., Ramachandran Sarayu, et al. 2015. “Atherosclerotic Plaque Targeting Mechanism of Long-Circulating Nanoparticles Established by Multimodal Imaging.” ACS Nano 9 (2): 1837–47. 10.1021/nn506750r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löhr M, Hoffmeyer A, Kröger J, Freund M, Hain J, Holle A, Karle P, et al. 2001. “Microencapsulated Cell-Mediated Treatment of Inoperable Pancreatic Carcinoma.” The Lancet 357 (9268): 1591–92. 10.1016/s0140-6736(00)04749-8. [DOI] [PubMed] [Google Scholar]
- Louhivuori Martti, Risselada H. Jelger, van der Giessen Erik, and Marrink Siewert J.. 2010. “Release of Content through Mechano-Sensitive Gates in Pressurized Liposomes.” Proceedings of the National Academy of Sciences of the United States of America 107 (46): 19856–60. 10.1073/pnas.1001316107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Mei, Zhao Xiaoyun, Xing Haonan, Liu Hui, Lang Lang, Yang Tianzhi, Xun Zhe, Wang Dongkai, and Ding Pingtian. 2019. “Cell-Free Synthesis of Connexin 43-Integrated Exosome-Mimetic Nanoparticles for siRNA Delivery.” Acta Biomaterialia 96 (September): 517–36. 10.1016/j.actbio.2019.07.006. [DOI] [PubMed] [Google Scholar]
- Luo Dandan, Carter Kevin A., Razi Aida, Geng Jumin, Shao Shuai, Giraldo Daniel, Sunar Ulas, Ortega Joaquin, and Lovell Jonathan F.. 2016. “Doxorubicin Encapsulated in Stealth Liposomes Conferred with Light-Triggered Drug Release.” Biomaterials 75 (January): 193–202. 10.1016/j.biomaterials.2015.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Lan, Tang Junnan, Nishi Kodai, Yan Chen, Dinh Phuong-Uyen, Cores Jhon, Kudo Takashi, Zhang Jinying, Li Tao-Sheng, and Cheng Ke. 2017. “Fabrication of Synthetic Mesenchymal Stem Cells for the Treatment of Acute Myocardial Infarction in Mice.” Circulation Research 120 (11): 1768–75. 10.1161/CIRCRESAHA.116.310374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lvov Yuri, Antipov Alexei A., Mamedov Arif, Möhwald Helmuth, and Sukhorukov Gleb B.. 2001. “Urease Encapsulation in Nanoorganized Microshells.” Nano Letters. 10.1021/nl0100015. [DOI] [Google Scholar]
- Majumder Joydeb, and Minko Tamara. 2020. “Multifunctional and Stimuli-Responsive Nanocarriers for Targeted Therapeutic Delivery.” Expert Opinion on Drug Delivery, October, 1–23. 10.1080/17425247.2021.1828339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin Rey W., Des Soye Benjamin J., Kwon Yong-Chan, Kay Jennifer, Davis Roderick G., Thomas Paul M., Majewska Natalia I., et al. 2018. “Cell-Free Protein Synthesis from Genomically Recoded Bacteria Enables Multisite Incorporation of Noncanonical Amino Acids.” Nature Communications 9 (1): 1203. 10.1038/s41467-018-03469-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama K, Yuda T, Okamoto A, Kojima S, Suginaka A, and Iwatsuru M. 1992. “Prolonged Circulation Time in Vivo of Large Unilamellar Liposomes Composed of Distearoyl Phosphatidylcholine and Cholesterol Containing Amphipathic Poly(ethylene Glycol).” Biochimica et Biophysica Acta 1128 (1): 44–49. 10.1016/0005-2760(92)90255-t. [DOI] [PubMed] [Google Scholar]
- Matosevic Sandro, and Paegel Brian M.. 2011. “Stepwise Synthesis of Giant Unilamellar Vesicles on a Microfluidic Assembly Line.” Journal of the American Chemical Society 133 (9): 2798–2800. 10.1021/ja109137s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matos Maria B. C. de, Miranda Bárbara S., Nuari Yudha Rizky, Storm Gert, Leneweit Gero, Schiffelers Raymond M., and Kok Robbert J.. 2019. “Liposomes with Asymmetric Bilayers Produced from Inverse Emulsions for Nucleic Acid Delivery.” Journal of Drug Targeting 27 (5–6): 681–89. 10.1080/1061186X.2019.1579819. [DOI] [PubMed] [Google Scholar]
- Meeuwissen Silvie A., Rioz-Martínez Ana, de Gonzalo Gonzalo, Fraaije Marco W., Gotor Vicente, and van Hest Jan C. M.. 2011. “Cofactor Regeneration in Polymersome Nanoreactors: Enzymatically Catalysed Baeyer–Villiger Reactions.” Journal of Materials Chemistry. 10.1039/c1jm12407b. [DOI] [Google Scholar]
- Miele Ylenia, Medveczky Zsófia, Holló Gábor, Tegze Borbála, Imre Derényi Zoltán Hórvölgyi, Altamura Emiliano, Lagzi István, and Rossi Federico. 2020. “Self-Division of Giant Vesicles Driven by an Internal Enzymatic Reaction.” Chemical Science. 10.1039/c9sc05195c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda Dyego, and Lovell Jonathan F.. 2016. “Mechanisms of Light‐induced Liposome Permeabilization.” Bioengineering & Translational Medicine. 10.1002/btm2.10032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirhadi Elaheh, Mashreghi Mohammad, Mahdi Faal Maleki Seyedeh Hoda Alavizadeh, Arabi Leila, Badiee Ali, and Jaafari Mahmoud Reza. 2020. “Redox-Sensitive Nanoscale Drug Delivery Systems for Cancer Treatment.” International Journal of Pharmaceutics 589 (September): 119882. 10.1016/j.ijpharm.2020.119882. [DOI] [PubMed] [Google Scholar]
- Morsut Leonardo, Roybal Kole T., Xiong Xin, Gordley Russell M., Coyle Scott M., Thomson Matthew, and Lim Wendell A.. 2016. “Engineering Customized Cell Sensing and Response Behaviors Using Synthetic Notch Receptors.” Cell. 10.1016/j.cell.2016.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natfji Az Alddien, Ravishankar Divyashree, Osborn Helen M. I., and Greco Francesca. 2017. “Parameters Affecting the Enhanced Permeability and Retention Effect: The Need for Patient Selection.” Journal of Pharmaceutical Sciences 106 (11): 3179–87. 10.1016/j.xphs.2017.06.019. [DOI] [PubMed] [Google Scholar]
- Nawroth Janna C., Lee Hyungsuk, Feinberg Adam W., Ripplinger Crystal M., McCain Megan L., Grosberg Anna, Dabiri John O., and Parker Kevin Kit. 2012. “A Tissue-Engineered Jellyfish with Biomimetic Propulsion.” Nature Biotechnology 30 (8): 792–97. 10.1038/nbt.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naziris Nikolaos, Pippa Natassa, Meristoudi Anastasia, Pispas Stergios, and Demetzos Costas. 2017. “Design and Development of pH-Responsive HSPC:CH-PAA Chimeric Liposomes.” Journal of Liposome Research 27 (2): 108–17. 10.3109/08982104.2016.1166512. [DOI] [PubMed] [Google Scholar]
- Needham David, Anyarambhatla Gopal, Kong Garheng, and Dewhirst Mark W.. 2000. “A New Temperature-Sensitive Liposome for Use with Mild Hyperthermia: Characterization and Testing in a Human Tumor Xenograft Model.” Cancer Research 60 (5): 1197–1201. https://cancerres.aacrjournals.org/content/60/5/1197.abstract. [PubMed] [Google Scholar]
- Niederholtmeyer Henrike, Chaggan Cynthia, and Devaraj Neal K.. 2018. “Communication and Quorum Sensing in Non-Living Mimics of Eukaryotic Cells.” Nature Communications 9 (1): 5027. 10.1038/s41467-018-07473-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen Alec A. K., Der Bryan S., Shin Jonghyeon, Vaidyanathan Prashant, Paralanov Vanya, Strychalski Elizabeth A., Ross David, Densmore Douglas, and Voigt Christopher A.. 2016. “Genetic Circuit Design Automation.” Science 352 (6281): aac7341. 10.1126/science.aac7341. [DOI] [PubMed] [Google Scholar]
- Niesman MR, Johnson KA, and Penn JS. 1997. “Therapeutic Effect of Liposomal Superoxide Dismutase in an Animal Model of Retinopathy of Prematurity.” Neurochemical Research 22 (5): 597–605. 10.1023/a:1022474120512. [DOI] [PubMed] [Google Scholar]
- Noireaux Vincent, and Libchaber Albert. 2004. “A Vesicle Bioreactor as a Step toward an Artificial Cell Assembly.” Proceedings of the National Academy of Sciences of the United States of America 101 (51): 17669–74. 10.1073/pnas.0408236101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta Naoki, Kato Yasuhiko, Watanabe Hajime, Mori Hirotada, and Matsuura Tomoaki. 2016. “In Vitro Membrane Protein Synthesis inside Sec Translocon-Reconstituted Cell-Sized Liposomes.” Scientific Reports 6 (November): 36466. 10.1038/srep36466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira Hugo, Pérez-Andrés Encarnación, Thevenot Julie, Sandre Olivier, Berra Edurne, and Lecommandoux Sébastien. 2013. “Magnetic Field Triggered Drug Release from Polymersomes for Cancer Therapeutics.” Journal of Controlled Release: Official Journal of the Controlled Release Society 169 (3): 165–70. 10.1016/j.jconrel.2013.01.013. [DOI] [PubMed] [Google Scholar]
- Oliveira Sabrina, Schiffelers Raymond M., van der Veeken Joris, van der Meel Roy, Vongpromek Ranitha, van Bergen En Henegouwen Paul M. P., Storm Gert, and Roovers Rob C.. 2010. “Downregulation of EGFR by a Novel Multivalent Nanobody-Liposome Platform.” Journal of Controlled Release: Official Journal of the Controlled Release Society 145 (2): 165–75. 10.1016/j.jconrel.2010.03.020. [DOI] [PubMed] [Google Scholar]
- Orth Joachim H. C., Schorch Björn, Boundy Sam, Richard Ffrench-Constant Stefan Kubick, and Aktories Klaus. 2011. “Cell-Free Synthesis and Characterization of a Novel Cytotoxic Pierisin-like Protein from the Cabbage Butterfly Pieris Rapae.” Toxicon: Official Journal of the International Society on Toxinology 57 (2): 199–207. 10.1016/j.toxicon.2010.11.011. [DOI] [PubMed] [Google Scholar]
- Ota Sadao, Yoshizawa Satoko, and Takeuchi Shoji. 2009. “Microfluidic Formation of Monodisperse, Cell-Sized, and Unilamellar Vesicles.” Angewandte Chemie 48 (35): 6533–37. 10.1002/anie.200902182. [DOI] [PubMed] [Google Scholar]
- Pan Jing, Du Yancheng, Qiu Hengming, Upton Luke R., Li Feiran, and Choi Jong Hyun. 2019. “Mimicking Chemotactic Cell Migration with DNA Programmable Synthetic Vesicles.” Nano Letters 19 (12): 9138–44. 10.1021/acs.nanolett.9b04428. [DOI] [PubMed] [Google Scholar]
- Pardee Keith, Slomovic Shimyn, Nguyen Peter Q., Jeong Wook Lee Nina Donghia, Burrill Devin, Ferrante Tom, et al. 2016. “Portable, On-Demand Biomolecular Manufacturing.” Cell 167 (1): 248–59.e12. 10.1016/j.cell.2016.09.013. [DOI] [PubMed] [Google Scholar]
- Pattipeiluhu Roy, Crielaard Stefan, Iris Klein-Schiphorst Bogdan I. Florea, Kros Alexander, and Campbell Frederick. 2020. “Unbiased Identification of the Liposome Protein Corona Using Photoaffinity-Based Chemoproteomics.” ACS Central Science 6 (4): 535–45. 10.1021/acscentsci.9b01222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pautot Sophie, Frisken Barbara J., and Weitz DA. 2003. “Production of Unilamellar Vesicles Using an Inverted Emulsion.” Langmuir: The ACS Journal of Surfaces and Colloids 19 (7): 2870–79. 10.1021/la026100v. [DOI] [Google Scholar]
- Peng Fei, Tu Yingfeng, van Hest Jan C. M., and Wilson Daniela A.. 2015. “Self-Guided Supramolecular Cargo-Loaded Nanomotors with Chemotactic Behavior towards Cells.” Angewandte Chemie 54 (40): 11662–65. 10.1002/anie.201504186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters Ruud J. R. W., Nijemeisland Marlies, and van Hest Jan C. M.. 2015. “Reversibly Triggered Protein-Ligand Assemblies in Giant Vesicles.” Angewandte Chemie 54 (33): 9614–17. 10.1002/anie.201502920. [DOI] [PubMed] [Google Scholar]
- Peyret Ariane, Ibarboure Emmanuel, Tron Arnaud, Louis Beauté Ruben Rust, Sandre Olivier, McClenaghan Nathan D., and Lecommandoux Sebastien. 2017. “Polymersome Popping by Light‐Induced Osmotic Shock under Temporal, Spatial, and Spectral Control.” Angewandte Chemie International Edition. 10.1002/anie.201609231. [DOI] [PubMed] [Google Scholar]
- Pflüger Tobias, Hernández Camila F., Lewe Philipp, Frank Fabian, Mertens Haydyn, Svergun Dmitri, Baumstark Manfred W., Lunin Vladimir Y., Jetten Mike S. M., and Andrade Susana L. A.. 2018. “Signaling Ammonium across Membranes through an Ammonium Sensor Histidine Kinase.” Nature Communications 9 (1): 164. 10.1038/s41467-017-02637-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Photos Peter J., Bacakova Lucie, Discher Bohdana, Bates Frank S., and Discher Dennis E.. 2003. “Polymer Vesicles in Vivo: Correlations with PEG Molecular Weight.” Journal of Controlled Release: Official Journal of the Controlled Release Society 90 (3): 323–34. 10.1016/s0168-3659(03)00201-3. [DOI] [PubMed] [Google Scholar]
- Pitchaimani Arunkumar, Nguyen Tuyen Duong Thanh, and Aryal Santosh. 2018. “Natural Killer Cell Membrane Infused Biomimetic Liposomes for Targeted Tumor Therapy.” Biomaterials. 10.1016/j.biomaterials.2018.01.018. [DOI] [PubMed] [Google Scholar]
- Pontani Léa-Laetitia, van der Gucht Jasper, Salbreux Guillaume, Heuvingh Julien, Joanny Jean-François, and Sykes Cécile. 2009. “Reconstitution of an Actin Cortex inside a Liposome.” Biophysical Journal 96 (1): 192–98. 10.1016/j.bpj.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pös Ondrej, Orsolya Biró Tomas Szemes, and Nagy Bálint. 2018. “Circulating Cell-Free Nucleic Acids: Characteristics and Applications.” European Journal of Human Genetics: EJHG 26 (7): 937–45. 10.1038/s41431-018-0132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratsinis Anna, Zuercher Stefanie, Forster Vincent, Fischer Eric J., Luciani Paola, and Leroux Jean-Christophe. 2017. “Liposome-Supported Enzymatic Peritoneal Dialysis.” Biomaterials 145 (November): 128–37. 10.1016/j.biomaterials.2017.08.016. [DOI] [PubMed] [Google Scholar]
- Qu Boyi, Li Xiaocen, Guan Mei, Li Xun, Hai Li, and Wu Yong. 2014. “Design, Synthesis and Biological Evaluation of Multivalent Glucosides with High Affinity as Ligands for Brain Targeting Liposomes.” European Journal of Medicinal Chemistry 72 (January): 110–18. 10.1016/j.ejmech.2013.10.007. [DOI] [PubMed] [Google Scholar]
- Rao DR, Chawan CB, and Veeramachaneni R. 1994. “LIPOSOMAL ENCAPSULATION OF ?-GALACTOSIDASE: COMPARISON OF TWO METHODS OF ENCAPSULATION AND IN VITRO LACTOSE DIGESTIBILITY.” Journal of Food Biochemistry. 10.1111/j.1745-4514.1994.tb00500.x. [DOI] [Google Scholar]
- Ravikumar Sambandam, Baylon Mary Grace, Park Si Jae, and Choi Jong-Il. 2017. “Engineered Microbial Biosensors Based on Bacterial Two-Component Systems as Synthetic Biotechnology Platforms in Bioremediation and Biorefinery.” Microbial Cell Factories 16 (1): 62. 10.1186/s12934-017-0675-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedl Claus R., Sternig Peter, Gallé Günter, Langmann Florian, Vcelar Brigitta, Vorauer Karola, Wagner Andreas, Katinger Hermann, and Pflüger Heinz. 2005. “Liposomal Recombinant Human Superoxide Dismutase for the Treatment of Peyronie’s Disease: A Randomized Placebo-Controlled Double-Blind Prospective Clinical Study.” European Urology. 10.1016/j.eururo.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Rippe Marlène, Stefanello Talitha F., Kaplum Vanessa, Britta Elizandra A., Garcia Francielle P., Poirot Robin, Companhoni Mychelle V. P., Nakamura Celso V., Szarpak-Jankowska Anna, and Auzély-Velty Rachel. 2019. “Heparosan as a Potential Alternative to Hyaluronic Acid for the Design of Biopolymer-Based Nanovectors for Anticancer Therapy.” Biomaterials Science 7 (7): 2850–60. 10.1039/C9BM00443B. [DOI] [PubMed] [Google Scholar]
- Rwei Alina Y., Paris Juan L., Wang Bruce, Wang Weiping, Axon Christopher D., María Vallet-Regí Robert Langer, and Kohane Daniel S.. 2017. “Ultrasound-Triggered Local Anaesthesia.” Nature Biomedical Engineering 1 (August): 644–53. 10.1038/s41551-017-0117-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabino Caetano P., Deana Alessandro M., Yoshimura Tania M., da Silva Daniela F. T., França Cristiane M., Hamblin Michael R., and Ribeiro Martha S.. 2016. “The Optical Properties of Mouse Skin in the Visible and near Infrared Spectral Regions.” Journal of Photochemistry and Photobiology B: Biology. 10.1016/j.jphotobiol.2016.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi Amin S. M., Tang Miriam J. Shakalli, Smith Mark T., Hunt Jeremy M., Law Robert A., Wood David W., and Bundy Bradley C.. 2017. “Cell-Free Protein Synthesis Approach to Biosensing hTRβ-Specific Endocrine Disruptors.” Analytical Chemistry 89 (6): 3395–3401. 10.1021/acs.analchem.6b04034. [DOI] [PubMed] [Google Scholar]
- Salehi Amin S. M., Smith Mark Thomas, Bennett Anthony M., Williams Jacob B., Pitt William G., and Bundy Bradley C.. 2016. “Cell-Free Protein Synthesis of a Cytotoxic Cancer Therapeutic: Onconase Production and a Just-Add-Water Cell-Free System.” Biotechnology Journal 11 (2): 274–81. 10.1002/biot.201500237. [DOI] [PubMed] [Google Scholar]
- Sanowar Sarah, and Moual Hervé Le. 2005. “Functional Reconstitution of the Salmonella Typhimurium PhoQ Histidine Kinase Sensor in Proteoliposomes.” Biochemical Journal 390 (Pt 3): 769–76. 10.1042/BJ20050060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar Sounik, Aftab Alam Mohammed, Shaw Jyoti, and Dasgupta Anjan Kr. 2013. “Drug Delivery Using Platelet Cancer Cell Interaction.” Pharmaceutical Research 30 (11): 2785–94. 10.1007/s11095-013-1097-1. [DOI] [PubMed] [Google Scholar]
- Scannell Jack W., Blanckley Alex, Boldon Helen, and Warrington Brian. 2012. “Diagnosing the Decline in Pharmaceutical R&D Efficiency.” Nature Reviews. Drug Discovery 11 (3): 191–200. 10.1038/nrd3681. [DOI] [PubMed] [Google Scholar]
- Schroeder Avi, Goldberg Michael S., Kastrup Christian, Wang Yingxia, Jiang Shan, Joseph Brian J., Levins Christopher G., Kannan Sneha T., Langer Robert, and Anderson Daniel G.. 2012. “Remotely Activated Protein-Producing Nanoparticles.” Nano Letters 12 (6): 2685–89. 10.1021/nl2036047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuette Christina G., Hatsuzawa Kiyotaka, Margittai Martin, Stein Alexander, Riedel Dietmar, Petra Küster Marcelle König, Seidel Claus, and Jahn Reinhard. 2004. “Determinants of Liposome Fusion Mediated by Synaptic SNARE Proteins.” Proceedings of the National Academy of Sciences of the United States of America 101 (9): 2858–63. 10.1073/pnas.0400044101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz-Schilling M, Aufinger L, Mückl A, and Simmel FC. 2016. “Chemical Communication between Bacteria and Cell-Free Gene Expression Systems within Linear Chains of Emulsion Droplets.” Integrative Biology: Quantitative Biosciences from Nano to Macro 8 (4): 564–70. 10.1039/c5ib00301f. [DOI] [PubMed] [Google Scholar]
- Sercombe Lisa, Veerati Tejaswi, Moheimani Fatemeh, Wu Sherry Y., Sood Anil K., and Hua Susan. 2015. “Advances and Challenges of Liposome Assisted Drug Delivery.” Frontiers in Pharmacology 6 (December): 286. 10.3389/fphar.2015.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah Sanket, Dhawan Vivek, Holm René, Nagarsenker Mangal S., and Perrie Yvonne. 2020. “Liposomes: Advancements and Innovation in the Manufacturing Process.” Advanced Drug Delivery Reviews, July. 10.1016/j.addr.2020.07.002. [DOI] [PubMed] [Google Scholar]
- Shazeeb Mohammed Salman, Feula Giancarlo, and Bogdanov Alexei. 2014. “Liposome-Encapsulated Superoxide Dismutase Mimetic: Theranostic Potential of an MR Detectable and Neuroprotective Agent.” Contrast Media & Molecular Imaging. 10.1002/cmmi.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhar Himanshu, Bader Kenneth B., Huang Shenwen, Peng Tao, Huang Shaoling, McPherson David D., and Holland Christy K.. 2017. “In Vitrothrombolytic Efficacy of Echogenic Liposomes Loaded with Tissue Plasminogen Activator and Octafluoropropane Gas.” Physics in Medicine and Biology. 10.1088/1361-6560/62/2/517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Chao, Li Mingle, Zhang Zhen, Yao Qichao, Shao Kun, Xu Feng, Xu Ning, et al. 2020. “Catalase-Based Liposomal for Reversing Immunosuppressive Tumor Microenvironment and Enhanced Cancer Chemo-Photodynamic Therapy.” Biomaterials 233 (March): 119755. 10.1016/j.biomaterials.2020.119755. [DOI] [PubMed] [Google Scholar]
- Shimizu Y, Inoue A, Tomari Y, Suzuki T, Yokogawa T, Nishikawa K, and Ueda T. 2001. “Cell-Free Translation Reconstituted with Purified Components.” Nature Biotechnology 19 (8): 751–55. 10.1038/90802. [DOI] [PubMed] [Google Scholar]
- Shimizu Yoshihiro, and Ueda Takuya. 2010. “PURE Technology.” Methods in Molecular Biology 607: 11–21. 10.1007/978-1-60327-331-2_2. [DOI] [PubMed] [Google Scholar]
- Shis David L., Hussain Faiza, Meinhardt Sarah, Swint-Kruse Liskin, and Bennett Matthew R.. 2014. “Modular, Multi-Input Transcriptional Logic Gating with Orthogonal LacI/GalR Family Chimeras.” ACS Synthetic Biology 3 (9): 645–51. 10.1021/sb500262f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shum Ho Cheung, Lee Daeyeon, Yoon Insun, Kodger Tom, and Weitz David A.. 2008. “Double Emulsion Templated Monodisperse Phospholipid Vesicles.” Langmuir: The ACS Journal of Surfaces and Colloids 24 (15): 7651–53. 10.1021/la801833a. [DOI] [PubMed] [Google Scholar]
- Silverman Adam D., Karim Ashty S., and Jewett Michael C.. 2020. “Cell-Free Gene Expression: An Expanded Repertoire of Applications.” Nature Reviews. Genetics 21 (3): 151–70. 10.1038/s41576-019-0186-3. [DOI] [PubMed] [Google Scholar]
- Siton-Mendelson Orit, and Bernheim-Groswasser Anne. 2016. “Toward the Reconstitution of Synthetic Cell Motility.” Cell Adhesion & Migration 10 (5): 461–74. 10.1080/19336918.2016.1170260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soga Naoki, Ota Akira, Nakajima Kota, Watanabe Rikiya, Ueno Hiroshi, and Noji Hiroyuki. 2020. “Monodisperse Liposomes with Femtoliter Volume Enable Quantitative Digital Bioassays of Membrane Transporters and Cell-Free Gene Expression.” ACS Nano 14 (9): 11700–711. 10.1021/acsnano.0c04354. [DOI] [PubMed] [Google Scholar]
- Somasundar A, Ghosh S, Mohajerani F, Massenburg LN, Yang T, Cremer PS, Velegol D, and Sen A. 2019. “Positive and Negative Chemotaxis of Enzyme-Coated Liposome Motors.” Nature Nanotechnology 14 (12). 10.1038/s41565-019-0578-8. [DOI] [PubMed] [Google Scholar]
- Soon-Shiong P, Heintz RE, Merideth N, Yao QX, Yao Z, Zheng T, Murphy M, et al. 1994. “Insulin Independence in a Type 1 Diabetic Patient after Encapsulated Islet Transplantation.” The Lancet. 10.1016/s0140-6736(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Steinberg-Yfrach Gali, Rigaud Jean-Louis, Durantini Edgardo N., Moore Ana L., Gust Devens, and Moore Thomas A.. 1998. “Light-Driven Production of ATP Catalysed by F0F1-ATP Synthase in an Artificial Photosynthetic Membrane.” Nature. 10.1038/33116. [DOI] [PubMed] [Google Scholar]
- Sun Shiyong, Li Mei, Dong Faqin, Wang Shengjie, Tian Liangfei, and Mann Stephen. 2016. “Chemical Signaling and Functional Activation in Colloidosome-Based Protocells.” Small 12 (14): 1920–27. 10.1002/smll.201600243. [DOI] [PubMed] [Google Scholar]
- Sun Y, Ma X, Zhou D, Vacek I, and Sun AM. 1996. “Normalization of Diabetes in Spontaneously Diabetic Cynomologus Monkeys by Xenografts of Microencapsulated Porcine Islets without Immunosuppression.” Journal of Clinical Investigation. 10.1172/jci118929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Zachary Z., Hayes Clarmyra A., Shin Jonghyeon, Caschera Filippo, Murray Richard M., and Noireaux Vincent. 2013. “Protocols for Implementing an Escherichia Coli Based TX-TL Cell-Free Expression System for Synthetic Biology.” Journal of Visualized Experiments: JoVE, no. 79 (September): e50762. 10.3791/50762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagami Tatsuaki, Ernsting Mark J., and Li Shyh-Dar. 2011. “Optimization of a Novel and Improved Thermosensitive Liposome Formulated with DPPC and a Brij Surfactant Using a Robust in Vitro System.” Journal of Controlled Release. 10.1016/j.jconrel.2011.05.020. [DOI] [PubMed] [Google Scholar]
- Tang Junnan, Shen Deliang, Caranasos Thomas George, Wang Zegen, Vandergriff Adam C., Allen Tyler A., Hensley Michael Taylor, et al. 2017. “Therapeutic Microparticles Functionalized with Biomimetic Cardiac Stem Cell Membranes and Secretome.” Nature Communications 8 (January): 13724. 10.1038/ncomms13724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang T-Y Dora, Cecchi Dario, Fracasso Giorgio, Accardi Davide, Coutable-Pennarun Angelique, Mansy Sheref S., Perriman Adam W., Ross Anderson JL, and Mann Stephen. 2018. “Gene-Mediated Chemical Communication in Synthetic Protocell Communities.” ACS Synthetic Biology 7 (2): 339–46. 10.1021/acssynbio.7b00306. [DOI] [PubMed] [Google Scholar]
- Ta Terence, Bartolak-Suki Elizabeth, Park Eun-Joo, Karrobi Kavon, McDannold Nathan J., and Porter Tyrone M.. 2014. “Localized Delivery of Doxorubicin in Vivo from Polymer-Modified Thermosensitive Liposomes with MR-Guided Focused Ultrasound-Mediated Heating.” Journal of Controlled Release: Official Journal of the Controlled Release Society 194 (November): 71–81. 10.1016/j.jconrel.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thamphiwatana Soracha, Gao Weiwei, Pornpattananangkul Dissaya, Zhang Qiangzhe, Fu Victoria, Li Jiayang, Li Jieming, Obonyo Marygorret, and Zhang Liangfang. 2014. “Phospholipase A2-Responsive Antibiotic Delivery via Nanoparticle-Stabilized Liposomes for the Treatment of Bacterial Infection.” Journal of Materials Chemistry. B, Materials for Biology and Medicine 2 (46): 8201–7. 10.1039/C4TB01110D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson Kate L., Williams Mark, and Armes Steven P.. 2015. “Colloidosomes: Synthesis, Properties and Applications.” Journal of Colloid and Interface Science 447 (June): 217–28. 10.1016/j.jcis.2014.11.058. [DOI] [PubMed] [Google Scholar]
- Toda Satoshi, Blauch Lucas R., Tang Sindy K. Y., Morsut Leonardo, and Lim Wendell A.. 2018. “Programming Self-Organizing Multicellular Structures with Synthetic Cell-Cell Signaling.” Science 361 (6398): 156–62. 10.1126/science.aat0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torchilin Vladimir P. 2014. “Multifunctional, Stimuli-Sensitive Nanoparticulate Systems for Drug Delivery.” Nature Reviews. Drug Discovery 13 (11): 813–27. 10.1038/nrd4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran Tuan Hiep, Hanh Thuy Nguyen Nam Van Le, Phuong Tran Thi Thu, Lee Jong Seong, Ku Sae Kwang, Choi Han-Gon, Yong Chul Soon, and Kim Jong Oh. 2017. “Engineering of Multifunctional Temperature-Sensitive Liposomes for Synergistic Photothermal, Photodynamic, and Chemotherapeutic Effects.” International Journal of Pharmaceutics 528 (1–2): 692–704. 10.1016/j.ijpharm.2017.06.069. [DOI] [PubMed] [Google Scholar]
- Uyeda Atsuko, Nakayama Shintaro, Kato Yasuhiko, Watanabe Hajime, and Matsuura Tomoaki. 2016. “Construction of an in Vitro Gene Screening System of the E. Coli EmrE Transporter Using Liposome Display.” Analytical Chemistry. 10.1021/acs.analchem.6b02308. [DOI] [PubMed] [Google Scholar]
- Villar Gabriel, Graham Alexander D., and Bayley Hagan. 2013. “A Tissue-like Printed Material.” Science 340 (6128): 48–52. 10.1126/science.1229495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos P. de, Hamel AF, and Tatarkiewicz K. 2002. “Considerations for Successful Transplantation of Encapsulated Pancreatic Islets.” Diabetologia 45 (2): 159–73. 10.1007/s00125-001-0729-x. [DOI] [PubMed] [Google Scholar]
- Vu Vivian P., Gifford Geoffrey B., Chen Fangfang, Benasutti Halli, Wang Guankui, Groman Ernest V., Scheinman Robert, Saba Laura, Moghimi Seyed Moein, and Simberg Dmitri. 2019. “Immunoglobulin Deposition on Biomolecule Corona Determines Complement Opsonization Efficiency of Preclinical and Clinical Nanoparticles.” Nature Nanotechnology. 10.1038/s41565-018-0344-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Jiamian, Toebes B. Jelle, Plachokova Adelina S., Liu Qian, Deng Dongmei, Jansen John A., Yang Fang, and Wilson Daniela A.. 2020. “Self-Propelled PLGA Micromotor with Chemotactic Response to Inflammation.” Advanced Healthcare Materials. 10.1002/adhm.201901710. [DOI] [PubMed] [Google Scholar]
- Wang Yang, Uchida Masaki, Waghwani Hitesh Kumar, and Douglas Trevor. 2020. “Synthetic Virus-like Particles for Glutathione Biosynthesis.” ACS Synthetic Biology, November. 10.1021/acssynbio.0c00368. [DOI] [PubMed] [Google Scholar]
- Wan Xue, Zhang Shi, Wang Feng, Fan Wei, Wu Chenxi, Mao Kuirong, Wang Hongda, Hu Zheng, Yang Yong-Guang, and Sun Tianmeng. 2018. “Red Blood Cell-Derived Nanovesicles for Safe and Efficient Macrophage-Targeted Drug Delivery in Vivo.” Biomaterials Science 7 (1): 187–95. 10.1039/c8bm01258j. [DOI] [PubMed] [Google Scholar]
- Webb Cameron, Forbes Neil, Roces Carla B., Anderluzzi Giulia, Lou Gustavo, Abraham Suraj, Ingalls Logan, et al. 2020. “Using Microfluidics for Scalable Manufacturing of Nanomedicines from Bench to GMP: A Case Study Using Protein-Loaded Liposomes.” International Journal of Pharmaceutics 582 (May): 119266. 10.1016/j.ijpharm.2020.119266. [DOI] [PubMed] [Google Scholar]
- Weiss Marian, Frohnmayer Johannes Patrick, Benk Lucia Theresa, Haller Barbara, Janiesch Jan-Willi, Heitkamp Thomas, Börsch Michael, et al. 2018. “Sequential Bottom-up Assembly of Mechanically Stabilized Synthetic Cells by Microfluidics.” Nature Materials 17 (1): 89–96. 10.1038/nmat5005. [DOI] [PubMed] [Google Scholar]
- Whitmire DR, Chambers RP, and Dillon AR. 1991. “Multi-Enzyme Catalyzed Rapid Ethanol Lowering in Vitro.” Alcoholism, Clinical and Experimental Research 15 (5): 804–7. 10.1111/j.1530-0277.1991.tb00604.x. [DOI] [PubMed] [Google Scholar]
- Witzigmann Dominik, Kulkarni Jayesh A., Leung Jerry, Chen Sam, Cullis Pieter R., and van der Meel Roy. 2020. “Lipid Nanoparticle Technology for Therapeutic Gene Regulation in the Liver.” Advanced Drug Delivery Reviews, July. 10.1016/j.addr.2020.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Yao, Ma Liang, Cheley Stephen, Bayley Hagan, Cui Qiang, and Chapman Edwin R.. 2011. “Permeation of Styryl Dyes through Nanometer-Scale Pores in Membranes.” Biochemistry 50 (35): 7493–7502. 10.1021/bi2006288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Zhiwei, Su Yixue, Kim Gloria B., Selvi Erhan, Ma Chuying, Aragon-Sanabria Virginia, Hsieh Jer-Tsong, Dong Cheng, and Yang Jian. 2017. “Immune Cell-Mediated Biodegradable Theranostic Nanoparticles for Melanoma Targeting and Drug Delivery.” Small 13 (10): 1603121. 10.1002/smll.201603121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Laishun, Li Chenglong, Wang Yuandou, Gong Yanling, Su Feng, and Li Suming. 2020. “Novel Thermosensitive Polymer-Modified Liposomes as Nano-Carrier of Hydrophobic Antitumor Drugs.” Journal of Pharmaceutical Sciences 109 (8): 2544–52. 10.1016/j.xphs.2020.05.006. [DOI] [PubMed] [Google Scholar]
- Xu Peipei, Zuo Huaqin, Zhou Rongfu, Wang Fan, Liu Xu, Ouyang Jian, and Chen Bing. 2017. “Doxorubicin-Loaded Platelets Conjugated with Anti-CD22 mAbs: A Novel Targeted Delivery System for Lymphoma Treatment with Cardiopulmonary Avoidance.” Oncotarget 8 (35): 58322–37. 10.18632/oncotarget.16871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Weiming, Wang Jing, Rothman James E., and Pincet Frédéric. 2015. “Accelerating SNARE-Mediated Membrane Fusion by DNA-Lipid Tethers.” Angewandte Chemie 54 (48): 14388–92. 10.1002/anie.201506844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa Miho, Iwamoto Masayuki, Kato Ayako, Yoshikawa Kenichi, and Oiki Shigetoshi. 2011. “Oriented Reconstitution of a Membrane Protein in a Giant Unilamellar Vesicle: Experimental Verification with the Potassium Channel KcsA.” Journal of the American Chemical Society. 10.1021/ja2040859. [DOI] [PubMed] [Google Scholar]
- Yarosh Daniel B., Rosenthal Amanda, and Moy Ronald. 2019. “Six Critical Questions for DNA Repair Enzymes in Skincare Products: A Review in Dialog.” Clinical, Cosmetic and Investigational Dermatology 12 (August): 617–24. 10.2147/CCID.S220741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarosh D, Klein J, Kibitel J, Alas L, O’Connor A, Cummings B, Grob D, et al. 1996. “Enzyme Therapy of Xeroderma Pigmentosum: Safety and Efficacy Testing of T4N5 Liposome Lotion Containing a Prokaryotic DNA Repair Enzyme.” Photodermatology, Photoimmunology & Photomedicine 12 (3): 122–30. 10.1111/j.1600-0781.1996.tb00188.x. [DOI] [PubMed] [Google Scholar]
- Yatvin MB, Weinstein JN, Dennis WH, and Blumenthal R. 1978. “Design of Liposomes for Enhanced Local Release of Drugs by Hyperthermia.” Science 202 (4374): 1290–93. 10.1126/science.364652. [DOI] [PubMed] [Google Scholar]
- Yin Lichen, Chen Yongbing, Zhang Zhonghai, Yin Qian, Zheng Nan, and Cheng Jianjun. 2015. “Biodegradable Micelles Capable of Mannose-Mediated Targeted Drug Delivery to Cancer Cells.” Macromolecular Rapid Communications. 10.1002/marc.201400650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Xuelei, Chi Yingying, Guo Chuanyou, Feng Shuaishuai, Liu Jinhu, Sun Kaoxiang, and Wu Zimei. 2017. “Chitooligosaccharides Modified Reduction-Sensitive Liposomes: Enhanced Cytoplasmic Drug Delivery and Osteosarcomas-Tumor Inhibition in Animal Models.” Pharmaceutical Research 34 (10): 2172–84. 10.1007/s11095-017-2225-0. [DOI] [PubMed] [Google Scholar]
- Zahednezhad Fahimeh, Saadat Maryam, Valizadeh Hadi, Parvin Zakeri-Milani, and Behzad Baradaran. 2019. “Liposome and Immune System Interplay: Challenges and Potentials.” Journal of Controlled Release: Official Journal of the Controlled Release Society 305 (July): 194–209. 10.1016/j.jconrel.2019.05.030. [DOI] [PubMed] [Google Scholar]
- Zawada James F., Yin Gang, Steiner Alexander R., Yang Junhao, Naresh Alpana, Roy Sushmita M., Gold Daniel S., Heinsohn Henry G., and Murray Christopher J.. 2011. “Microscale to Manufacturing Scale-up of Cell-Free Cytokine Production--a New Approach for Shortening Protein Production Development Timelines.” Biotechnology and Bioengineering 108 (7): 1570–78. 10.1002/bit.23103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Hongwei. 2017. “Thin-Film Hydration Followed by Extrusion Method for Liposome Preparation.” In Liposomes: Methods and Protocols, edited by Gerard G. M. D’Souza, 17–22. New York, NY: Springer New York. 10.1007/978-1-4939-6591-5_2. [DOI] [PubMed] [Google Scholar]
- Zhang Liyuan, Guo Wei, and Lu Yuan. 2020. “Advances in Cell‐Free Biosensors: Principle, Mechanism, and Applications.” Biotechnology Journal. 10.1002/biot.202000187. [DOI] [PubMed] [Google Scholar]
- Zhang Peng, Yang Junzhu, Cho Eunhee, and Lu Yuan. 2020. “Bringing Light into Cell-Free Expression.” ACS Synthetic Biology 9 (8): 2144–53. 10.1021/acssynbio.0c00211. [DOI] [PubMed] [Google Scholar]
- Zhang Rui, Song Xuejiao, Liang Chao, Yi Xuan, Song Guosheng, Chao Yu, Yang Yu, Yang Kai, Feng Liangzhu, and Liu Zhuang. 2017. “Catalase-Loaded Cisplatin-Prodrug-Constructed Liposomes to Overcome Tumor Hypoxia for Enhanced Chemo-Radiotherapy of Cancer.” Biomaterials. 10.1016/j.biomaterials.2017.05.025. [DOI] [PubMed] [Google Scholar]


