Abstract
Background:
Despite the prevalence and negative impact of posttraumatic stress disorder (PTSD), there are few medications approved by the United States Food and Drug Administration (FDA) for treatment, and approved medications do not work well enough. We leveraged large-scale electronic health record data to identify existing medications that may be repurposed as PTSD treatments.
Methods:
We constructed a mechanistic tree of all FDA-approved medications and used the tree-based scan statistic to identify medications associated with greater than expected levels of clinically meaningful improvement in PTSD symptoms using electronic health record data from the US Department of Veterans Affairs. Our cohort included patients with a diagnosis of PTSD who had repeated symptom measurements using the PTSD Checklist over a 20-year period (N=168,941). We calculated observed numbers based on patients taking each drug or mechanistically related class of drugs and the expected numbers based on the tree as a whole.
Results:
Medications typically used to treat PTSD, such as the FDA-approved agent sertraline, were associated with improvement in PTSD symptoms, but the effects were small. Several, but not all, direct-acting antivirals used in the treatment of hepatitis C virus demonstrated a strong association with PTSD improvement. The finding was robust to a sensitivity analysis excluding patients who received established PTSD treatments including trauma-focused psychotherapy concurrent with their hepatitis treatment.
Conclusions:
Our exploratory approach both demonstrated findings that are consistent with what is known about pharmacotherapy for PTSD and uncovered a novel class of medications that may improve PTSD symptoms.
Keywords: Posttraumatic Stress Disorder, Statistical Scanning, Electronic Health Record, Pharmacology, Patient Reported Outcomes Measurement, Veterans
INTRODUCTION
Despite the high prevalence and negative impact of Posttraumatic Stress Disorder (PTSD; e.g., 1, 2, 3), there are few effective pharmacologic treatments for PTSD and even these effective medications do not work well enough (4, 5). In 2017, the VA PTSD Psychopharmacology Work Group published a statement in Biological Psychiatry describing key issues that are leading to a PTSD “pharmacotherapy crisis (6).” First, antidepressants, the most prescribed pharmacologic agents for PTSD, are associated with remission in fewer than half of the patients who take them. Currently, only sertraline and paroxetine are approved for the treatment of PTSD by the US Food and Drug Administration (FDA). The treatment guideline jointly issued by the VA and DoD include two additional antidepressant medications, fluoxetine and venlafaxine, that have similarly limited effectiveness (7). Second, there have been no new drugs approved by the FDA for the treatment of PTSD in almost two decades. The research and development of new drugs for PTSD has been limited, and current development may take many years to complete, resulting in a challenge to the field of how to proceed with identifying effective medications for PTSD treatment right now.
Leaders from the National Institute of Mental Health (NIMH) responded to the VA PTSD Psychopharmacology Work Group statement by noting that VA electronic health record (EHR) data provides “an unparalleled opportunity” to address the PTSD pharmacotherapy crisis (8). Their response highlighted the enormous potential of using large-scale EHR data to identify existing medications for other conditions that may also be effective medications for PTSD (8). There are several reasons why VA data is particularly well-suited to the discovery of novel pharmaceutical treatments for PTSD. First, PTSD is a common diagnosis among VA patients and, further, VA patients with PTSD have a high degree of medical comorbidity and receive a wide variety of medications to address these illnesses (9, 10). Second, the VA maintains national registry data sources capturing longitudinal patient care, including patient demographics, procedural and diagnostic codes, and pharmacy data. Notably, the VA’s EHR repository contains data sources not typically available in large administrative datasets, including clinical note text and structured data from standardized mental health symptom assessments, allowing for the ability to identify the concurrent delivery of psychosocial treatments and examine changes in disorder severity over time.
Accordingly, the present study leveraged large-scale VA EHR data to identify existing pharmaceutical agents commonly prescribed for a multitude of indications that are incidentally associated with improvements in PTSD symptoms. We adapted a tree-based scan statistic to identify medications and mechanistic classes of medications that are associated with PTSD symptom improvement.
METHODS AND MATERIALS
Study Sample and Data Sources
We conducted a nested case-control study within a previously established retrospective cohort that included all VA users with a clinical diagnosis of PTSD (International Classification of Diseases [ICD]-9: 309.81, ICD-10: F43.1x) from October 1, 1999 through September 30, 2019 (11). The data source contains information on services use, clinical diagnoses, filled prescriptions, and patient-reported outcome measures (PROMs) for PTSD using the PTSD Checklist (PCL) for these patients. This study was approved by the Veterans Institutional Review Board of Northern New England.
The goal of the current study was to evaluate any filled medications associated with PTSD symptom improvement, and as such, the analytic sample for this study included only patients with PROMs that were at least 30 days apart. We selected 30 days to allow for medications to be taken for at least one full course (typically 28 days). We excluded patients with PROMs more than 365 days apart as these are unlikely to provide an accurate assessment of PTSD symptom change between the two time points. For patients who had two overlapping PROM intervals, we chose those closest to 84 days in length to emulate typical PTSD medication efficacy trials (12). When a patient had two or more overlapping intervals at exactly 84 days, we chose the historically earliest interval. If patients had multiple non-overlapping intervals, all were included. Patients who met our selection criteria for PTSD in the original cohort and had at least one PROM pair where they started a new medication (defined below) constituted our overall analytic sample for the current study.
PTSD Improvement Cases and No Improvement Controls
In order to maximize the number of patients with available symptomatic measurement, we integrated two different versions of a PROM for PTSD into a single dataset (11). The two PROMs were the PCL versions aligned to the Diagnostic and Statistical Manual of Mental Disorders (DSM), version IV and Version 5 (13, 14), which we will heretofore call the PCL-IV and the PCL-5 (15, 16). Validation work shows a correlation of 0.87 between PCL versions in a large sample of Veterans (17). We used a validated crosswalk (ICC = 0.96) to convert all values to PCL-5 scoring (18). We defined PTSD improvement cases by a clinically meaningful improvement, which was a decrease of 15 points or more between assessment points (19). Because this improvement criterion was calculated using the Jacobson and Truax Reliable Change Index (1.96 times the standard error of the difference in change, which is the “distribution of change scores that would be expected if no actual change had occurred”; 20), we used a corollary that changes of 7 points or less could be due to measurement error. Thus, we defined the no PTSD improvement control group as worsening of symptoms or reductions of up to 7 points on the PCL. Improvements between 8 and 14 points were of uncertain clinical significance and were therefore excluded.
Identification and Classification of New Medications
Our predictors of interest were initiation of medications for any indication. Candidate agents included those that were started in the interval between PCL pairs and continued for at least a 28-day supply. When patients started multiple medications between a given PCL pair, they contributed information for each of the medications in the tree. To ensure we were examining new medications, any agent prescribed in the six months prior to the first PCL score was excluded. All medications were classified by mechanism of action to create a phylogenic tree using ChEMBL, the European Bioinformatics Institute’s open-source repository of bioactivity data (21). Using the ChEMBL database, we grouped medications by mechanistic class and used these classes to create hierarchical tree structures for use. Individual medication trees connected at higher level branch points, thus creating one overarching phylogeny of all FDA-approved medications grouped according to mechanism. We then transformed the resulting “Pharm Tree” (Table S1) into a log file for input into data mining software called TreeScan (described below).
Analytic Method: TreeScan
As this study was exploratory in nature, we used an innovative method to identify medications associated with cases of PTSD symptom improvement. The tree-based scan statistic (TreeScan) is data mining software that scans variables that exist in a natural hierarchy for associations with a dichotomous outcome, while adjusting for the multiple testing inherent in the large number of evaluated potential associations (22, 23). In TreeScan, “leaves” represent the finest granular level of data and, in this application, corresponded to individual medications (e.g., clonidine). Groups of closely related “leaves” called “branches” represented higher-level drug mechanisms (e.g., alpha-2 adrenergic receptor agonists). TreeScan works by calculating a log likelihood ratio based on the number of observed and expected number of events for each leaf of the tree, as well as for each branch consisting of multiple, related leaves (23). The cuts that maximize the log likelihood ratio are identified as potential associations. We calculated expected cases based on the overall distribution of cases in the entire tree. To obtain the number of expected cases for each medication, we multiplied the proportion of patients who had improved PTSD symptoms among all patients in the study by the number of patients who received each medication (population of the node). We calculated the ratio of observed to expected cases as a relative estimate of effect.
Medications with multiple mechanisms were grouped according to each individual mechanism, therefore one drug could appear in multiple trees. For example, trazodone (the medication most often prescribed to VA patients with PTSD; 6), is associated with three mechanisms in ChEMBL (i.e., serotonin 2a receptor antagonism, serotonin 2c receptor antagonism, serotonin transporter inhibition) and appears in three different branches with other medications that share the same mechanisms. It is possible that an association with improvement in PTSD symptoms could be observed at the level of the leaf representing an individual medication or at a higher-level branch representing a mechanism. The alerting threshold for TreeScan is based on a multiplicity-adjusted P value (24), which we set at 10%. Therefore, only outcome nodes with P < 0.1 were considered statistical alerts.
Sensitivity analyses
To assess the possibility that changes in PTSD symptoms could be attributed to established interventions, we identified the subset of patients in our analytic cohort who did not receive concurrent evidence-based treatment (EBT) for PTSD to determine if the patterns of observed results in the overall analytic sample were consistent within this group. We defined EBT as evidence-based psychotherapies (EBPs) and evidence-based antidepressants (EBAs) as recommended by the VA-DoD clinical practice guideline (CPG) and commonly used in routine practice (25, 26). While the 2017 VA-DoD CPG endorses many EBP protocols (27), VA implementation efforts over the last 20 years have focused on two protocols including prolonged exposure (PE) and cognitive processing therapy (CPT; 28). We previously identified the provision of PE and CPT in the parent cohort (11). To identify provision of EBAs, we identified filled prescriptions of fluoxetine, sertraline, paroxetine, or venlafaxine. To ensure patients had current PTSD, we repeated all analyses within the subset of patients who had a baseline PCL of 38 or higher, as well as a clinical PTSD diagnosis within 30 days of the baseline PCL score. A score of 50 or higher on the PCL-IV has been frequently used as a case definition for military-related PTSD (9, 29), and this is equivalent to a PCL-5 score of 36-38 (18, 30).
RESULTS
There were 192,912 PCL pairs that met our inclusion criteria, representing 168,941 patients (Table 1). There was a median of 89 days between the baseline and follow-up PCL, with 25% of intervals spanning less than 79 days and 25% of intervals spanning more than 141 days. Cases and controls were similar in terms of demographic and diagnostic characteristics with standardized mean differences of well below 0.1 (Table 2). Cases were more likely to have received EBT and had higher baseline PCL scores. When we restricted the cohort to those with no EBT receipt, there were 75,266 PCL pairs representing 69,453 patients. When we restricted the cohort to those with a baseline PCL of 38 or higher as well as a PTSD diagnosis within 30 days, there were 134,289 PCL pairs representing 119,372 patients overall and 47,778 PCL pairs representing 44,400 patients in the subgroup with no EBT receipt.
Table 1.
Cohort Selection
| Patients | PCL Pairs | ||
|---|---|---|---|
| Overall Cohort | |||
| A | How many patients had any diagnosis of PTSD between 10/1/99 and 9/30/19? | 2,098,389 | N/A |
| B | How many of (A) had at least 1 PCL Score? | 852,438 | N/A |
| C | How many of (B) had at least 1 additional PCL score within 30 to 365 days of the first? | 353,847 | 6,158,258 |
| D | How many of (C) do not include overlapping PCL pairs within the same patient? | 353,847 | 480,372 |
| E | How many of (D) had at least one concurrent drug trial lasting at least 28 days? | 192,006 | 223,048 |
| F | How many of (E) met the criteria to be considered a case* or control**? | 168,941 | 192,912 |
|
| |||
| Sensitivity Analysis Cohorts | |||
| G | How many of (F) did not coincide with any instance of EBT? | 69,453 | 75,266 |
| H | How many of (F) had a baseline PCL score of 38 or higher and a PTSD diagnosis within 30 days of baseline? | 119,372 | 134,289 |
| I | How many of (H) did not coincide with any instance of EBT? | 44,400 | 47,778 |
PTSD=Posttraumatic Stress Disorder, PCL=PTSD Checklist, EBT=Evidence-Based Treatment for PTSD
Case: PCL improvement of 15 points or more
Control: PCL improvement of 7 points or less or PCL worsening
Table 2.
Characteristics of the Sample
| Overall N=192,912 | Cases* n=43,402 | Controls** n=149,510 | SMD | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Variable | % | n | % | n | % | n | |
| Demographic Characteristics at Baseline | |||||||
| Age (M±SD) | 44.24 | 14.01 | 44.13 | 14.00 | 44.27 | 14.01 | 0.010 |
| Female | 14.33 | 27,641 | 15.88 | 6,892 | 13.88 | 20,749 | 0.056 |
| White Non-Hispanic | 60.01 | 115,757 | 61.00 | 26,477 | 59.72 | 89,280 | 0.026 |
| Black Non-Hispanic | 20.99 | 40,501 | 20.94 | 9,087 | 21.01 | 31,414 | 0.002 |
| Hispanic | 11.36 | 21,924 | 10.74 | 4,663 | 11.55 | 17,261 | 0.025 |
| Post-9/11 Era Veteran | 58.72 | 113,276 | 56.37 | 24,465 | 59.40 | 88,811 | 0.061 |
| Vietnam Era Veteran | 18.36 | 35,412 | 18.11 | 7,861 | 18.43 | 27,551 | 0.008 |
| Combat Exposure | 63.50 | 122,508 | 60.87 | 26,419 | 64.27 | 96,089 | 0.070 |
| Military Sexual Trauma Exposure | 16.26 | 31,364 | 17.79 | 7,722 | 15.81 | 23,642 | 0.053 |
|
| |||||||
| Comorbidities in the Two Years Prior to Baseline | |||||||
| Depressive Disorders | 66.75 | 128,763 | 67.80 | 29,427 | 66.44 | 99,336 | 0.029 |
| Anxiety Disorders | 39.62 | 76,425 | 40.87 | 17,737 | 39.25 | 58,688 | 0.033 |
| Bipolar Disorders | 8.05 | 15,528 | 9.12 | 3,960 | 7.74 | 11,568 | 0.050 |
| Psychotic Disorders | 4.31 | 8,315 | 4.73 | 2,054 | 4.19 | 6,261 | 0.026 |
| Personality Disorders | 6.77 | 13,058 | 7.29 | 3,164 | 6.62 | 9,894 | 0.026 |
| Substance Use Disorders | 33.99 | 65,573 | 36.89 | 16,010 | 33.15 | 49,563 | 0.078 |
|
| |||||||
| Concurrent Evidence Based PTSD Treatment | |||||||
| Any Sessions of EBP for PTSD | 26.48 | 51,092 | 38.57 | 16,740 | 22.98 | 34,352 | 0.343 |
| Any Prescriptions for EBA for PTSD | 46.12 | 88,974 | 47.06 | 20,423 | 45.85 | 68,551 | 0.024 |
| Any EBA or EBP for PTSD | 60.98 | 117,646 | 68.62 | 29,784 | 58.77 | 87,862 | 0.206 |
|
| |||||||
| PTSD Checklist (PCL) Information | |||||||
| Baseline Score (M±SD) | 49.02 | 16.40 | 56.05 | 12.70 | 46.98 | 16.78 | 0.610 |
| Baseline Score 38+ | 76.10 | 146,812 | 91.21 | 39,589 | 71.72 | 107,223 | 0.518 |
| PTSD Diagnosis within 30 Days of Baseline PCL | 89.48 | 172,619 | 91.85 | 39,865 | 88.79 | 132,754 | 0.104 |
| PTSD Diagnosis within 30 Days of Baseline PCL and Baseline Score of 38+ | 69.61 | 134,289 | 84.20 | 36,544 | 65.38 | 97,745 | 0.444 |
SMD=Standardized Mean Difference, PTSD=Posttraumatic Stress Disorder, EBP=Evidence-Based Psychotherapy, EBA=Evidence-Based Antidepressants
Case: PCL improvement of 15 points or more
Control: PCL improvement of 7 points or less or PCL worsening
Medications Associated with Improvement in PTSD Symptoms
Among all PCL pairs, TreeScan identified 25 unique associations between medications and improvements in PTSD symptoms. Although medications were grouped according to mechanism of action in the TreeScan hierarchy, we organized the resulting medication signals according to clinical indication for ease of interpretation (Table 3). Sertraline, which is FDA-approved for the treatment PTSD, was prescribed widely but had a weak overall association with symptom improvement (ratio of observed to expected [O:E]=1.16; p=0.001). Other medications commonly prescribed for PTSD and related mood disorders like citalopram, fluoxetine, trazodone, and lithium had similarly small associations with PTSD symptom improvement. Prazosin, which is commonly prescribed for PTSD-related nightmares also had a similar effect (O:E=1.10; P=0.001). Atypical antipsychotics have fallen in and out of favor as treatments for PTSD (31), and we observed several small associations at the branch level for these medications (e.g., the serotonin 2C receptor antagonist branch; O:E=1.04; P=0.01).
Table 3.
Medications Associated with Improvement in PTSD,* Among All Patients in the Overall Sample
| TreeScan Node | OBS | EXP | O:E | P |
|---|---|---|---|---|
| Antivirals | ||||
| Hepatitis C Virus NS3/4A Protease Inhibitor | 43 | 24 | 1.77 | 0.02 |
| 1Glecaprevir | 33 | 16 | 2.09 | 0.005 |
| Hepatitis C Virus NS5A Protein Inhibitor | 100 | 64 | 1.57 | 0.001 |
| 1Pibrentasvir | 33 | 16 | 2.09 | 0.005 |
| Velpatasvir | 24 | 11 | 2.10 | 0.04 |
|
| ||||
| Antinsychotics | ||||
| Serotonin 2A (5-HT2A) Receptor Antagonist | 10511 | 10102 | 1.04 | 0.001 |
| Serotonin 2C (5-HT2C) Receptor Antagonist | 9630 | 9281 | 1.04 | 0.01 |
|
| ||||
| Addiction Treatments | ||||
| Opioid (Mu/Kappa/Delta) Receptor Antagonist | 1031 | 815 | 1.27 | 0.001 |
| Naloxone | 426 | 312 | 1.37 | 0.001 |
| Naltrexone | 605 | 504 | 1.20 | 0.001 |
|
| ||||
| Supplements | ||||
| Folic Acid | 567 | 445 | 1.27 | 0.001 |
| Thiamine | 444 | 351 | 1.27 | 0.001 |
| Cholecalciferol | 2647 | 2470 | 1.07 | 0.02 |
|
| ||||
| Mood Disorder Treatments | ||||
| Serotonin Transporter Inhibitor | 17417 | 16196 | 1.09 | 0.001 |
| Sertraline | 4659 | 4025 | 1.16 | 0.001 |
| Citalopram | 1653 | 1508 | 1.10 | 0.01 |
| Fluoxetine | 1716 | 1571 | 1.09 | 0.02 |
| Trazodone | 4308 | 4061 | 1.06 | 0.005 |
| Lithium | 304 | 246 | 1.24 | 0.02 |
|
| ||||
| Sleep Disorder Treatments | ||||
| Adrenergic Alpha1 Receptor Antagonist | 5279 | 4960 | 1.07 | 0.001 |
| Prazosin | 4659 | 4259 | 1.10 | 0.001 |
| Melatonin Receptor Agonist | 1326 | 1190 | 1.12 | 0.005 |
| Melatonin | 1295 | 1161 | 1.12 | 0.006 |
|
| ||||
| Cognitive Stimulants | ||||
| Nicotine | 2371 | 2109 | 1.13 | 0.001 |
| Atomoxetine | 104 | 74 | 1.40 | 0.08 |
PTSD=Posttraumatic Stress Disorder; OBS = Observed Cases; EXP = Expected Cases; O:E = Observed Cases over Expected Cases
Improved by 15 or more points (compared to 7 points or less or PCL worsening) on the PTSD Checklist.
Glecaprevir/Pibrentasvir = Mavyret (fixed-dose tablet of Glecaprevir 100mg and Pibrentasvir 40mg)
Several drugs commonly used in detoxification and addiction treatment demonstrated associations. Two opioid antagonists, naloxone (O:E=1.37; P=0.001) and naltrexone (O:E=1.20; P=0.001), were associated with improvements in PTSD symptoms. Similarly, folic acid (O:E=1.27; P=0.001) and thiamine (O:E=1.27; P=0.001) were associated with improvements in PTSD symptoms. The drugs with the strongest associations with PTSD symptom improvement were Hepatitis C Virus (HCV) Direct Acting Antivirals (DAA) including glecaprevir (O:E=2.09; P=0.005), pibrentasvir (O:E=2.09; P=0.005), and velpatasvir (O:E=2.10, P=0.04). Of note, glecaprevir and pibrentasvir demonstrated associations that were identical in magnitude, which is explained by the fact that they are co-prescribed under the brand name Mavyret.
Sensitivity Analyses
Among the subset of PCL pairs that did not include EBT (no sessions of EBP, no prescriptions for EBA), TreeScan identified 19 unique associations representing cuts at the leaf or branch level (Table 4). Again, the strongest associations were HCV DAAs including Mavyret (O:E=3.09; P=0.01), indicating that the associations detected using TreeScan in the overall sample were not due to the effect of established PTSD interventions. Results were similar when we restricted the cohort to those with a baseline PCL of 38 or higher as well as a PTSD diagnosis of 30 days, with O:E ratios for Mavyret of 2.21 (P=0.001) in the overall group and 3.52 (P=0.001) in the subgroup with no EBT.
Table 4.
Medications Associated with Improvement in PTSD,* Among Patients in the Overall Sample without Any Concurrent Evidence-Based Treatment
| TreeScan Node | OBS | EXP | O:E | P |
|---|---|---|---|---|
| Antivirals | ||||
| Hepatitis C Virus NS3/4A Protease Inhibitor | 19 | 7 | 2.65 | 0.01 |
| 1Glecaprevir | 14 | 5 | 3.09 | 0.01 |
| Hepatitis C Virus NS5A Protein Inhibitor | 41 | 21 | 1.99 | 0.003 |
| 1Pibrentasvir | 14 | 5 | 3.09 | 0.01 |
|
| ||||
| Antinsychotics | ||||
| Serotonin 2A (5-HT2A) Receptor Antagonist | 3616 | 3225 | 1.14 | 0.001 |
| Serotonin 2C (5-HT2C) Receptor Antagonist | 3285 | 2953 | 1.12 | 0.001 |
| Dopamine D2 Receptor Antagonist | 819 | 690 | 1.19 | 0.001 |
| Quetiapine | 460 | 371 | 1.24 | 0.001 |
|
| ||||
| Addiction Treatments | ||||
| Opioid (Mu/Kappa/Delta) Receptor Antagonist | 306 | 242 | 1.27 | 0.003 |
|
| ||||
| Supplements | ||||
| Folic Acid | 185 | 134 | 1.38 | 0.001 |
| Thiamine | 146 | 106 | 1.38 | 0.01 |
|
| ||||
| Mood Disorder Treatments | ||||
| Serotonin Transporter Inhibitor | 3631 | 3227 | 1.14 | 0.001 |
| Citalopram | 1039 | 814 | 1.28 | 0.001 |
| Escitalopram | 558 | 474 | 1.18 | 0.01 |
| Trazodone | 1272 | 1157 | 1.1 | 0.05 |
| Lithium | 127 | 92 | 1.38 | 0.03 |
| Mirtazapine | 849 | 755 | 1.13 | 0.05 |
|
| ||||
| Sleep Disorder Treatments | ||||
| Adrenergic Alpha1 Receptor Antagonist | 1593 | 1418 | 1.13 | 0.001 |
| Prazosin | 1375 | 1170 | 1.18 | 0.001 |
OBS = Observed Cases; EXP = Expected Cases; O:E = Observed Cases over Expected Cases
Evidence-Based Treatment for PTSD included prolonged exposure, cognitive processing therapy, fluoxetine, sertraline, paroxetine, and venlafaxine.
Improved by 15 or more points (compared to 7 points or less or PCL worsening) on the PTSD Checklist.
Glecaprevir/Pibrentasvir = Mavyret (fixed-dose tablet of Glecaprevir 100mg and Pibrentasvir 40mg)
Illustrating Agents with Associations and Related Agents
To illustrate the mechanistic hierarchy of TreeScan and aid in conceptualization of these results, we recreated a simplified phylogenic tree containing the branches (mechanistic classes) and leaves (individual drugs) for which we identified improvement in PTSD symptoms in our overall results. The tree is not drawn to scale or all inclusive; it only represents a small cross-section of medications within a particular mechanistic class that demonstrated associations in our overall TreeScan models. The phylogenic tree highlights HCV DAAs (Figure 1) with three main branches (mechanisms): HCV nonstructural protein 3/4A (NS3/4A) protease inhibitors, nonstructural protein 5B (NS5B) RNA-dependent RNA polymerase inhibitors, and nonstructural protein 5A (NS5A) inhibitors. Cuts (branches and leaves) identified by TreeScan in our overall analysis are highlighted in blue. The figure legend outlines common combinations of DAAs listed in this tree.
Figure 1.
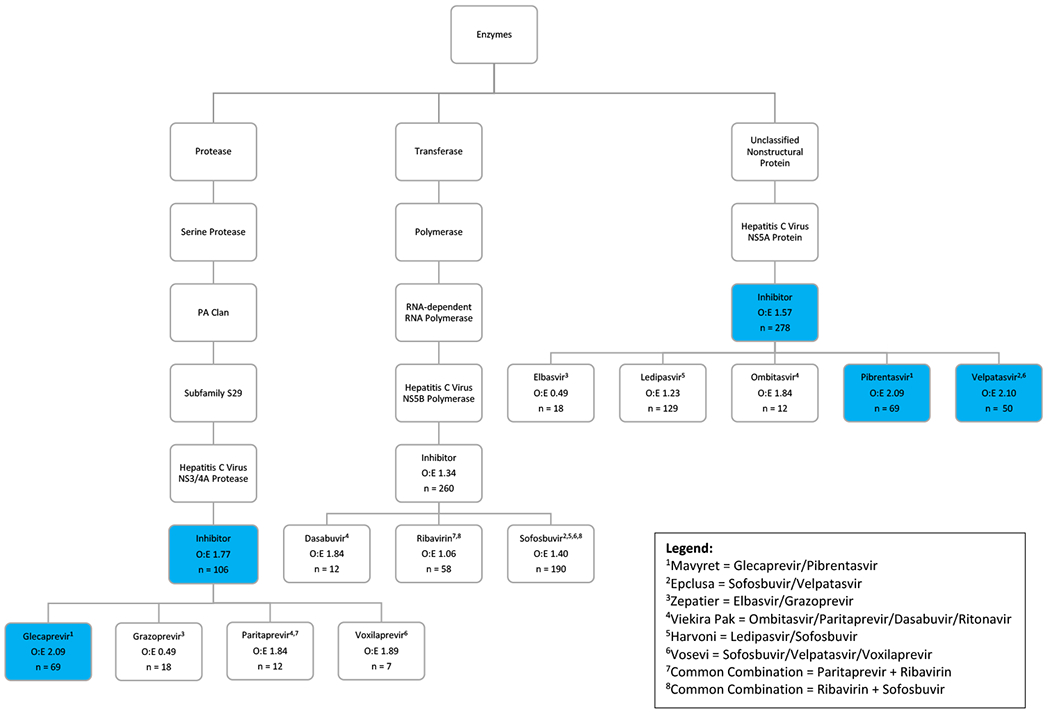
Mechanistic TreeScan Results for Antiviral Medications: Associations with Improvement in PTSD Symptoms. O:E = Observed Cases over Expected Case. Statistical alerts (P < 0.1) are highlighted in blue.
Similar to glecaprevir and pibrentasvir (Mavyret), grazoprevir and elbasvir are listed in separate branches but demonstrate effects that are identical in magnitude because they are prescribed together as Zepatier. However, sofosbuvir can be combined with four different DAAs listed in this tree, explaining the relatively large number of observations for this leaf. Sofosbuvir was most commonly prescribed with ledipasvir as Harvoni, which did not generate a statistical alert (O:E=1.23, P > 0.1). Among the three mechanistic branches, the NS3/4A protease inhibitors (O:E=1.77, P=0.02) demonstrated the largest effect size followed by the NS5A protein inhibitors (O:E=1.57, P=0.001). Within the NS5A inhibitor branch, both pibrentasvir and velpatasvir had strong associations with improvement in PTSD symptoms. Velpatasvir is prescribed in combinations including Epclusa (velpatasvir and sofosbuvir) and Vosevi (velpatasvir, sofosbuvir, and voxilaprevir), and there were no associations for other medications prescribed along with velpatasvir. This introduces the possibility that the NS5A inhibitor branch drives the DAA finding and the signal for the NS3/4A protease inhibitor branch is incidental, as glecaprevir is always prescribed with the NS5A inhibitor pibrentasvir.
DISCUSSION
This study is the first large-scale exploration of existing medications that may have potential efficacy for the treatment of PTSD using a population-based sample. There has been limited advancement in the pharmacologic treatment options for PTSD over the last two decades, and the findings from this study are an initial step towards future critical innovation in this area. To address this issue, we used a novel exploratory method called TreeScan. Although the application of any novel exploratory method can have limitations, several features of our results indicate that TreeScan worked as intended. As expected, we identified that the serotonin transport inhibitors commonly used and recommended for the treatment of PTSD improved PTSD symptoms in this study. We also observed improvements associated with medications known to enhance the effects of serotonin transport inhibitors, such as lithium (32). However, consistent with the existing PTSD literature and clinical experience, the effects we observed were small. Similarly, although prazosin is often prescribed to treat nightmares associated with PTSD, the effect on overall PTSD symptoms was limited. Previous research has identified a strong association between PTSD and substance use disorders (SUD), necessitating the development of treatments that address both conditions (33). Not surprisingly, medications closely associated with alcohol detoxification, like folic acid and thiamine, and addiction treatments, like naloxone and naltrexone, were associated with small improvements in PTSD symptoms. This is especially clear in the case of naloxone, which is prescribed as an emergency kit and assigned as a 30-day supply in the ordering system, but only taken in the case of an overdose. It is possible that possessing this potentially life-saving tool results in a substantial decrease in anxiety. Further, supplements like folic acid and thiamine are commonly initiated as part of initial detoxification management and continued orally as part of aftercare. Therefore, it is likely that we are observing more general effects of patients initiating substance abuse treatment than a direct effect of opioid antagonism.
Consistent with the goals of this study, we found a previously unknown association between use of several non-psychotropic medications and improvement in PTSD symptoms. Our sensitivity analyses indicate that these improvements are not due to concurrent receipt of established PTSD treatments. The strongest associations came from the DAAs. These agents have revolutionized the care of HCV; in contemporary practice they provide a cure for HCV infections in most cases (34, 35). These DAAs are most commonly prescribed as combinations to avoid antiviral resistance (36). There is growing evidence that HCV has direct effects on the brain, leading to neuropsychiatric symptoms (37). As opposed to interferon-containing HCV treatment regimens that were known to cause or worsen depression (38, 39), available studies suggest that DAA treatment without interferon does not cause or worsen depression and may lead to improvements in depression and related mental health symptoms (40–43). To our knowledge no prior study has evaluated the effect of DAA treatment on PTSD symptoms or compared the effects of various DAA combinations on mental health symptoms. If HCV is contributing to PTSD symptoms, some DAAs may be more effective than others because they are better at clearing HCV virus in the brain. However, the most prescribed combinations in our analysis, Harvoni and Mavyret, are both highly effective for HCV, but appear to have drastically different effects on PTSD symptoms. This finding introduces the possibility that several DAAs may improve PTSD symptoms through a mechanism unrelated to clearance of HCV.
Although the mechanism of action of individual DAAs at the hepatocyte has recently been explored (36), relatively little is known about their off-target effects. However, there are several mechanisms which could explain behavioral effects, including PTSD symptom improvement for these medications. First, pibrentasvir falls into a class of drugs called phenylpiperidines (44), which includes synthetic opioids (45). Disruptions in the endogenous opioid system has been postulated in the pathophysiology of PTSD (46), and there are high rates of opioid use disorders and pain disorders in the population we studied (47). Second, pibrentasvir inhibits NS5A, a non-structural HCV protein that binds to and inactivates protein kinase R (PKR; 48). PKR has a central role in cellular response to stress. Thus, pibrentasvir inhibition of NS5A or an unknown cellular homologue may result in modified PKR activity leading to alterations in gene expression profiles across diverse cell types. As PKR is activated by pro-inflammatory cytokines that disrupt the blood brain barrier, PKR has been linked with neuroinflammation and neurodegenerative disease, and inhibition of PKR may therefore exert a neuroprotective effect (49). PKR inactivation has been associated with enhanced memory and learning in animal models (50). As PTSD symptom improvement has been conceptualized as new learning, these medications may promote symptom improvement through this enhanced learning (51). However, the differential pattern of PTSD response among NS5A inhibitors suggests that other unknown factors such as blood brain barrier permeability are also relevant. Finally, it remains within the realm of possibility that some form of viral illness is involved in at least some cases of PTSD and DAAs may have differential effects on that as-yet-unidentified pathogen.
This exploratory study was designed as a preliminary step aimed at identifying novel medications associated with PTSD symptom improvement. Our most promising finding for the effect of Mavyret on PTSD symptoms was based on a very small sample size and as such our results should be viewed with caution and must be replicated in studies using more rigorous causal methods that address several possible sources of bias and in larger samples. First, it is possible that the PTSD symptom improvement we observed is due to improvements, and ultimately a cure, of HCV among persons on these medications. Contemporary DAA combinations have over 90% effectiveness in curing HCV among VA patients (52), and yet we did not observe that they are all associated with PTSD symptom improvement in equal measure. Thus, our initial results do not indicate that our PTSD symptom improvement finding is due to HCV symptom improvement, but it is very important for future work to examine this possibility explicitly. Second, it is likely that patients were nonrandomly assigned to DAA treatment in clinical practice. For example, it is possible that differences in the FDA approval dates for each DAA combination could result in cohort effects whereby chronically ill patients received the first medications to be approved. This could lead to differences in age, race, gender, liver disease severity, PTSD symptom severity, and treatment history among patients receiving different DAA combinations. Future work should account for these factors, particularly in light of the small sample upon which this finding is based in the current study. Third, it is possible that other treatments besides the EBTs we examined could account for the differential pattern of PTSD symptom response. While these treatments, such as atypical antipsychotics and antidepressants inadequately studied for PTSD treatment, had very small associations in this study, future work should examine their possible contribution. While many of these factors can be accounted for using retrospective data to emulate a clinical trial, only a prospective trial could answer the question of whether these agents would improve PTSD symptoms in patients without HCV. However, further retrospective analysis may help indicate whether this approach is likely to be effective and safe, as well as which agent to choose.
In addition to the possible sources of bias noted above, there are several limitations related to our application of TreeScan. First, this work was designed to detect potential indications of PTSD treatment effectiveness rather than eliminate candidate treatments. It is possible that we may have missed effective medications due to a lack of PCL data in some clinical scenarios. Second, our phylogenic tree could be missing mechanisms that are unknown or unrecognized by ChEMBL, decreasing our ability to detect aggregated effects at key branch points. While ChEMBL incorporates a wide variety of data sources (53), we openly share our “Pharm Tree” (Table S1) and research teams wishing to build on our work might consider incorporation of data from similar platforms such as DrugBank (44).
In conclusion, our exploratory approach both demonstrated findings consistent with what is known about pharmacotherapy for PTSD and uncovered a novel medication that may improve PTSD symptoms. Those novel medications demonstrated larger association with PTSD improvement than standard PTSD treatments typically used in clinical practice. Because these novel medications do not have known effects on typical monoamines associated with PTSD treatments (i.e., serotonin and norepinephrine), this association would indicate a gap either in our understanding of the psychotropic effects of some DAAs or in the pathophysiology of PTSD. Additional work to eliminate sources of potential bias in our finding is indicated, and if robust, this finding could point the way to additional novel pharmacologic treatment options for PTSD.
Supplementary Material
Table 5.
Standardized Morbidity Ratio for Medications Associated with Improvement in PTSD,* among all Patients with a Baseline PCL Score of 38 or Higher as well as a PTSD Diagnosis within 30 Days
| TreeScan Node | OBS | EXP | O:E | P |
|---|---|---|---|---|
| Antivirals | ||||
| Hepatitis C Virus NS3/4A Protease Inhibitor | 36 | 20 | 1.78 | 0.03 |
| 1Glecaprevir | 30 | 14 | 2.21 | 0.001 |
| Hepatitis C Virus NS5A Protein Inhibitor | 84 | 51 | 1.65 | 0.001 |
| 1Pibrentasvir | 30 | 14 | 2.21 | 0.001 |
| Velpatasvir | 21 | 10 | 2.17 | 0.02 |
|
| ||||
| Addiction Treatments | ||||
| Opioid (Mu/Kappa/Delta) Receptor Antagonist | 912 | 723 | 1.26 | 0.001 |
| Naloxone | 376 | 279 | 1.35 | 0.001 |
| Naltrexone | 536 | 444 | 1.21 | 0.001 |
|
| ||||
| Supplements | ||||
| Folic Acid | 469 | 391 | 1.20 | 0.003 |
| Thiamine | 377 | 304 | 1.24 | 0.001 |
| Cholecalciferol | 2238 | 2070 | 1.08 | 0.008 |
|
| ||||
| Mood Disorder Treatments | ||||
| Serotonin Transporter Inhibitor | 14605 | 13719 | 1.08 | 0.001 |
| Citalopram | 1315 | 1202 | 1.10 | 0.04 |
| Sertraline | 3909 | 3434 | 1.14 | 0.001 |
| Trazodone | 3610 | 3396 | 1.07 | 0.008 |
|
| ||||
| Sleep Disorder Treatments | ||||
| Melatonin Receptor Agonist | 1158 | 1058 | 1.10 | 0.09 |
| Melatonin | 1131 | 1033 | 1.10 | 0.09 |
|
| ||||
| Cognitive Stimulants | ||||
| Nicotine | 2007 | 1736 | 1.16 | 0.001 |
| Atomoxetine | 92 | 65 | 1.41 | 0.05 |
PTSD=Posttraumatic Stress Disorder; OBS = Observed Cases; EXP = Expected Cases; O:E = Observed Cases over Expected Cases
Improved by 15 or more points (compared to 7 points or less or PCL worsening) on the PTSD Checklist.
Glecaprevir/Pibrentasvir = Mavyret (fixed-dose tablet of Glecaprevir 100mg and Pibrentasvir 40mg)
Table 6.
Standardized Mortality Ratio for Medications Associated with Improvement in PTSD,* among Patients with a Baseline PCL Score of 38 or Higher as well as a PTSD Diagnosis within 30 Days and without Any Concurrent Evidence-Based Treatment
| TreeScan Node | OBS | EXP | O:E | P |
|---|---|---|---|---|
| Antivirals | ||||
| Hepatitis C Virus NS3/4A Protease Inhibitor | 16 | 6 | 2.85 | 0.004 |
| 1Glecaprevir | 14 | 4 | 3.52 | 0.001 |
| Hepatitis C Virus NS5A Protein Inhibitor | 32 | 15 | 2.21 | 0.001 |
| 1Pibrentasvir | 14 | 4 | 3.52 | 0.001 |
|
| ||||
| Antinsychotics | ||||
| Serotonin 2A (5-HT2A) Receptor Antagonist | 2903 | 2656 | 1.10 | 0.001 |
| Serotonin 2C (5-HT2C) Receptor Antagonist | 2634 | 2417 | 1.10 | 0.001 |
|
| ||||
| Addiction Treatments | ||||
| Opioid (Mu/Kappa/Delta) Receptor Antagonist | 256 | 205 | 1.25 | 0.02 |
|
| ||||
| Mood Disorder Treatments | ||||
| Serotonin Transporter Inhibitor | 2841 | 2574 | 1.12 | 0.001 |
| Citalopram | 785 | 632 | 1.25 | 0.001 |
| Trazodone | 992 | 893 | 1.12 | 0.03 |
|
| ||||
| Cognitive Stimulants | ||||
| Nicotine | 610 | 522 | 1.17 | 0.005 |
OBS = Observed Cases; EXP = Expected Cases; O:E = Observed Cases over Expected Cases
Evidence-Based Treatment for PTSD included prolonged exposure, cognitive processing therapy, fluoxetine, sertraline, paroxetine, and venlafaxine.
Improved by 15 or more points (compared to 7 points or less or PCL worsening) on the PTSD Checklist.
Glecaprevir/Pibrentasvir = Mavyret (fixed-dose tablet of Glecaprevir 100mg and Pibrentasvir 40mg)
KEY RESOURCES TABLE
| Resource Type | Specific Reagent or Resource | Source or Reference | Identifiers | Additional Information |
|---|---|---|---|---|
| Add additional rows as needed for each resource type | Include species and sex when applicable. | Include name of manufacturer, company, repository, individual, or research lab. Include PMID or DOI for references; use “this paper” if new. | Include catalog numbers, stock numbers, database IDs or accession numbers, and/or RRIDs. RRIDs are highly encouraged; search for RRIDs at https://scicrunch.org/resources. | Include any additional information or notes if necessary. |
| Antibody | This study does not include antibody data. | Not applicable | Not applicable | Not applicable |
| Bacterial or Viral Strain | This study does not include Bacterial or Viral Strain data. | Not applicable | Not applicable | Not applicable |
| Biological Sample | This study does not include Biological Sample. | Not applicable | Not applicable | Not applicable |
| Cell Line | This study does not include Biological Sample. | Not applicable | Not applicable | Not applicable |
| Chemical Compound or Drug | This study does not include Biological Sample. | Not applicable | Not applicable | Not applicable |
| Commercial Assay Or Kit | This study does not include Biological Sample. | Not applicable | Not applicable | Not applicable |
| Deposited Data; Public Database | VA Corporate Data Warehouse | Corporate Data Warehouse (CDW) (va.gov) | Not applicable | Not applicable |
| Genetic Reagent | This study does not include Genetic Reagent. | Not applicable | Not applicable | Not applicable |
| Organism/Strain | This study does not include Organism/Strain. | Not applicable | Not applicable | Not applicable |
| Peptide, Recombinant Protein | This study does not include Peptide, Recombinant Protein. | Not applicable | Not applicable | Not applicable |
| Recombinant DNA | This study does not include Recombinant DNA. | Not applicable | Not applicable | Not applicable |
| Sequence-Based Reagent | This study does not include Sequence-Based Reagent. | Not applicable | Not applicable | Not applicable |
| Software; Algorithm | TreeScan | TreeScan - Software for the Tree-Based Scan Statistic | Not applicable | Not applicable |
| Transfected Construct | This study does not include Transfected Construct. | Not applicable | Not applicable | Not applicable |
| Other | Pharm Tree for Tree Scan | Online Supplemental Table S1 | Not applicable | Not applicable |
ACKNOWLEDGEMENTS:
This work was supported by an award to Drs. Gradus and Shiner from the National Institute of Mental Health (R01MH121397). The cohort used for this study was developed through an award to Dr. Shiner from the Department of Defense (PR160206). The sponsors had no role in the study design, methods, analysis, and interpretation of results or in the preparation of the manuscript and the decision to submit it for publication. This work has not been published previously.
FINANCIAL DISCLOSURE:
Dr. Shiner was the Principal Investigator on a Cooperative Research and Development Agreement between the Veterans Educational and Research Association of Northern New England, Inc., the United States Department of Veterans Affairs, and Otsuka Pharmaceutical Development and Commercialization, Inc. Dr. Huybrechts has been an investigator on research grants awarded to Brigham and Women’s Hospital from Eli Lilly and Company and Takeda Pharmaceuticals. All other authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES:
- 1.Gradus JL, Antonsen S, Svensson E, Lash TL, Resick PA, Hansen JG (2015): Trauma, comorbidity, and mortality following diagnoses of severe stress and adjustment disorders: a nationwide cohort study. Am J Epidemiol. 182:451–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoge CW, Terhakopian A, Castro CA, Messer SC, Engel CC (2007): Association of posttraumatic stress disorder with somatic symptoms, health care visits, and absenteeism among Iraq war veterans. Am J Psychiatry. 164:150–153. [DOI] [PubMed] [Google Scholar]
- 3.Pietrzak RH, Goldstein RB, Southwick SM, Grant BF (2011): Prevalence and Axis I comorbidity of full and partial posttraumatic stress disorder in the United States: results from Wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions. J Anxiety Disord. 25:456–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shiner B, Leonard CE, Gui J, Cornelius SL, Schnurr PP, Hoyt JE, et al. (2020): Comparing Medications for DSM-5 PTSD in Routine VA Practice. J Clin Psychiatry. 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shiner B, Westgate CL, Gui J, Maguen S, Young-Xu Y, Schnurr PP, et al. (2018): A Retrospective Comparative Effectiveness Study of Medications for Posttraumatic Stress Disorder in Routine Practice. J Clin Psychiatry. 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krystal JH, Davis LL, Neylan TC, M AR, Schnurr PP, Stein MB, et al. (2017): It Is Time to Address the Crisis in the Pharmacotherapy of Posttraumatic Stress Disorder: A Consensus Statement of the PTSD Psychopharmacology Working Group. Biol Psychiatry. 82:e51–e59. [DOI] [PubMed] [Google Scholar]
- 7.Bernardy NC, et al. VA/DoD Clinical Practice Guideline for the Management of Posttraumatic Stress Disorder and Acute Stress Disorder. 2017, Washington, DC: United States Departments of Veterans Affairs and Defense. [Google Scholar]
- 8.Gordon JA, Borja SE, Tuma FK (2017): A Collaborative Psychopharmacology Research Agenda for Posttraumatic Stress Disorder. Biol Psychiatry. 82:460–461. [DOI] [PubMed] [Google Scholar]
- 9.Shiner B, Drake RE, Watts BV, Desai RA, Schnurr PP (2012): Access to VA services for returning veterans with PTSD. Mil Med. 177:814–822. [DOI] [PubMed] [Google Scholar]
- 10.Shiner B, Westgate CL, Bernardy NC, Schnurr PP, Watts BV (2017): Anticonvulsant Medication Use in Veterans With Posttraumatic Stress Disorder. J Clin Psychiatry. 78:e545–e552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shiner B, Levis M, Dufort VM, Patterson OV, Watts BV, DuVall SL, et al. (2021): Improvements to PTSD quality metrics with natural language processing. J Eval Clin Pract. [DOI] [PubMed] [Google Scholar]
- 12.Watts BV, Schnurr PP, Mayo L, Young-Xu Y, Weeks WB, Friedman MJ (2013): Meta-analysis of the efficacy of treatments for posttraumatic stress disorder. J Clin Psychiatry. 74:e541–550. [DOI] [PubMed] [Google Scholar]
- 13.APA (2000): Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision: DSM-IV-TR. Arlington, VA: American Psychiatric Association. [Google Scholar]
- 14.APA (2013): Diagnostic and Statistical Manual of Mental Disorders: Fifth Edition (DSM-5). Washington, D.C.: American Psychiatric Association. [Google Scholar]
- 15.Blevins CA, Weathers FW, Davis MT, Witte TK, Domino JL (2015): The PTSD Checklist for DSM-5 (PCL-5): Development and Initial Psychometric Evaluation. J Trauma Stress. 28:489–498. [DOI] [PubMed] [Google Scholar]
- 16.Weathers FW, Litz BT, Herman DS, Huska JA, Keane TM (1993): The PTSD Checklist (PCL): Reliability, validity, and diagnostic utility. Trauma, Coping, and Adaptation. San Antonio, Texas: International Society for Traumatic Stress Studies, 9th Annual Meeting. [Google Scholar]
- 17.Bovin MJ, Marx BP, Weathers FW, Gallagher MW, Rodriguez P, Schnurr PP, et al. (2016): Psychometric properties of the PTSD Checklist for Diagnostic and Statistical Manual of Mental Disorders-Fifth Edition (PCL-5) in veterans. Psychol Assess. 28:1379–1391. [DOI] [PubMed] [Google Scholar]
- 18.Moshier SJ, Lee DJ, Bovin MJ, Gauthier G, Zax A, Rosen RC, et al. (Under review): An empirical crosswalk for the PTSD Checklist: Translating DSM-IV to DSM-5 [DOI] [PMC free article] [PubMed]
- 19.Lee DJ, Bovin MJ, Weathers FW, Schnurr PP, Sloan DM, Marx BP (2019): Reliable Change Index and Clinically Significant Margins for the CAPS-5 and PCL-5 among Veterans. Boston, MA: International Society for Traumatic Stress Studies, 35th Annual Meeting. [Google Scholar]
- 20.Jacobson NS, Truax P (1991): Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol. 59:12–19. [DOI] [PubMed] [Google Scholar]
- 21.Bento AP, Gaulton A, Hersey A, Beilis LJ, Chambers J, Davies M, et al. (2014): The ChEMBL bioactivity database: an update. Nucleic Acids Res. 42:D1083–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kulldorff M, Fang Z, Walsh SJ (2003): A tree-based scan statistic for database disease surveillance. Biometrics. 59:323–331. [DOI] [PubMed] [Google Scholar]
- 23.Kulldorff M, Dashevsky I, Avery TR, Chan AK, Davis RL, Graham D, et al. (2013): Drug safety data mining with a tree-based scan statistic. Pharmacoepidemiol Drug Saf. 22:517–523. [DOI] [PubMed] [Google Scholar]
- 24.Huybrechts KF, Kulldorff M, Hernandez-Diaz S, Bateman BT, Zhu Y, Mogun H, et al. (2021): Active Surveillance of the Safety of Medications Used During Pregnancy. Am J Epidemiol. 190:1159–1168. [DOI] [PubMed] [Google Scholar]
- 25.Shiner B, Leonard C, Gui J, Cornelius S, Gradus JL, Schnurr PP, et al. (2021): Measurement Strategies for Evidence-Based Antidepressants for Posttraumatic Stress Disorder Delivery: Trends and Associations with Patient-Reported Outcomes. Adm Policy Ment Health. 48:70–87. [DOI] [PubMed] [Google Scholar]
- 26.Shiner B, Westgate CL, Gui J, Cornelius S, Maguen SE, Watts BV, et al. (2020): Measurement Strategies for Evidence-Based Psychotherapy for Posttraumatic Stress Disorder Delivery: Trends and Associations with Patient-Reported Outcomes. Adm Policy Ment Health. 47:451–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.The Management of Posttraumatic Stress Disorder Work Group (2017): VA/DoD Clinical Practice Guideline for the Management of Posttraumatic Stress Disorder and Acute Stress Disorder. Washington, DC: US Departments of Veterans Affairs and Defense. [Google Scholar]
- 28.Rosen CS, Matthieu MM, Wiltsey Stirman S, Cook JM, Landes S, Bernardy NC, et al. (2016): A Review of Studies on the System-Wide Implementation of Evidence-Based Psychotherapies for Posttraumatic Stress Disorder in the Veterans Health Administration. Adm Policy Ment Health. 43:957–977. [DOI] [PubMed] [Google Scholar]
- 29.Terhakopian A, Sinaii N, Engel CC, Schnurr PP, Hoge CW (2008): Estimating population prevalence of posttraumatic stress disorder: an example using the PTSD checklist. J Trauma Stress. 21:290–300. [DOI] [PubMed] [Google Scholar]
- 30.Hoge CW, Riviere LA, Wilk JE, Herrell RK, Weathers FW (2014): The prevalence of post-traumatic stress disorder (PTSD) in US combat soldiers: a head-to-head comparison of DSM-5 versus DSM-IV-TR symptom criteria with the PTSD checklist. Lancet Psychiatry. 1:269–277. [DOI] [PubMed] [Google Scholar]
- 31.Huang ZD, Zhao YF, Li S, Gu HY, Lin LL, Yang ZY, et al. (2020): Comparative Efficacy and Acceptability of Pharmaceutical Management for Adults With Post-Traumatic Stress Disorder: A Systematic Review and Meta-Analysis. Front Pharmacol. 11:559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stamm TJ, Adli M, Kirchheiner J, Smolka MN, Kaiser R, Tremblay PB, et al. (2008): Serotonin transporter gene and response to lithium augmentation in depression. Psychiatr Genet. 18:92–97. [DOI] [PubMed] [Google Scholar]
- 33.Sofuoglu M, Rosenheck R, Petrakis I (2014): Pharmacological treatment of comorbid PTSD and substance use disorder: Recent progress. Addictive Behaviors. 39:428–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Backus LI, Belperio PS, Shahoumian TA, Loomis TP, Mole LA (2017): Real-world effectiveness and predictors of sustained virological response with all-oral therapy in 21,242 hepatitis C genotype-1 patients. Antivir Ther. 22:481–493. [DOI] [PubMed] [Google Scholar]
- 35.Ioannou GN, Beste LA, Chang MF, Green PK, Lowy E, Tsui JI, et al. (2016): Effectiveness of Sofosbuvir, Ledipasvir/Sofosbuvir, or Paritaprevir/Ritonavir/Ombitasvir and Dasabuvir Regimens for Treatment of Patients With Hepatitis C in the Veterans Affairs National Health Care System. Gastroenterology. 151:457–471 e455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zając M, Muszalska I, Sobczak A, Dadej A, Tomczak S, Jelińska A (2019): Hepatitis C – New drugs and treatment prospects. European Journal of Medicinal Chemistry. 165:225–249. [DOI] [PubMed] [Google Scholar]
- 37.Iriana S, Curry MP, Afdhal NH (2017): Neurologic Manifestations of Hepatitis C Virus Infection. Clin Liver Dis. 21:535–542. [DOI] [PubMed] [Google Scholar]
- 38.Machado MO, Oriolo G, Bortolato B, Kohler CA, Maes M, Solmi M, et al. (2017): Biological mechanisms of depression following treatment with interferon for chronic hepatitis C: A critical systematic review. J Affect Disord. 209:235–245. [DOI] [PubMed] [Google Scholar]
- 39.Udina M, Castellvi P, Moreno-Espana J, Navines R, Valdes M, Forns X, et al. (2012): Interferon-induced depression in chronic hepatitis C: a systematic review and meta-analysis. J Clin Psychiatry. 73:1128–1138. [DOI] [PubMed] [Google Scholar]
- 40.Gallach M, Vergara M, da Costa JP, Miquel M, Casas M, Sanchez-Delgado J, et al. (2018): Impact of treatment with direct-acting antivirals on anxiety and depression in chronic hepatitis C. PLoS One. 13:e0208112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hahn D, Stokes CS, Kaiser R, Meyer MR, Lammert F, Gruenhage F (2018): Antidepressant effects of direct-acting antivirals against hepatitis C virus-Results from a pilot study. Eur J Clin Invest. 48:e13024. [DOI] [PubMed] [Google Scholar]
- 42.Kesen O, Kani HT, Yanartas O, Aykut UE, Gok B, Gunduz F, et al. (2019): Evaluation of depression, anxiety and quality of life in hepatitis C patients who treated with direct acting antiviral agents. Turk J Gastroenterol. 30:801–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pericot-Valverde I, Heo M, Niu J, Norton BL, Akiyama MJ, Agyemang L, et al. (2020): Declines in Depressive Symptoms Among People who Inject Drugs Treated With Direct-Acting Antivirals While on Opioid Agonist Therapy. Open Forum Infect Dis. 7:ofaa380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, et al. (2018): DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 46:D1074–D1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Elbaridi N, Kaye AD, Choi S, Urman RD (2017): Current Concepts of Phenylpiperidine Derivatives Use in the Treatment of Acute and Chronic Pain. Pain Physician. 20:Se23–se31. [PubMed] [Google Scholar]
- 46.van der Kolk BA (1994): The body keeps the score: memory and the evolving psychobiology of posttraumatic stress. Harv Rev Psychiatry. 1:253–265. [DOI] [PubMed] [Google Scholar]
- 47.Shiner B, Leonard Westgate C, Bernardy NC, Schnurr PP, Watts BV (2017): Trends in Opioid Use Disorder Diagnoses and Medication Treatment Among Veterans With Posttraumatic Stress Disorder. J Dual Diagn. 13:201–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.He Y, Tan SL, Tareen SU, Vijaysri S, Langland JO, Jacobs BL, et al. (2001): Regulation of mRNA translation and cellular signaling by hepatitis C virus nonstructural protein NS5A. J Virol. 75:5090–5098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gal-Ben-Ari S, Barrera I, Ehrlich M, Rosenblum K (2019): PKR: A Kinase to Remember. Frontiers in Molecular Neuroscience. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu PJ, Huang W, Kalikulov D, Yoo JW, Placzek AN, Stoica L, et al. (2011): Suppression of PKR promotes network excitability and enhanced cognition by interferon-γ-mediated disinhibition. Cell. 147:1384–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fonzo GA, Goodkind MS, Oathes DJ, Zaiko YV, Harvey M, Peng KK, et al. (2021): Amygdala and Insula Connectivity Changes Following Psychotherapy for Posttraumatic Stress Disorder: A Randomized Clinical Trial. Biol Psychiatry. 89:857–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Belperio PS, Chartier M, Ross DB, Alaigh P, Shulkin D (2017): Curing Hepatitis C Virus Infection: Best Practices From the U.S. Department of Veterans Affairs. Ann Intern Med. 167:499–504. [DOI] [PubMed] [Google Scholar]
- 53.Mendez D, Gaulton A, Bento AP, Chambers J, De Veij M, Felix E, et al. (2019): ChEMBL: towards direct deposition of bioassay data. Nucleic Acids Res. 47:D930–D940. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


