Abstract
Diabetes is a chronic metabolic disease and cerebral ischemia is a serious complication of diabetes. Antidiabetic therapy mitigates this complication but increases the risk of exposure to recurrent hypoglycemia (RH). We showed previously that RH exposure increases ischemic brain damage in insulin-treated diabetic (ITD) rats. The present study evaluated the hypothesis that increased intra-ischemic acidosis in RH-exposed ITD rats leads to pronounced post-ischemic hypoperfusion via activation of acid-sensing (proton-gated) ion channels (ASICs). Streptozotocin-diabetic rats treated with insulin were considered ITD rats. ITD rats were exposed to RH for five days and were randomized into PcTx1 (ASIC1a inhibitor), APETx2 (ASIC3 inhibitor), or vehicle groups. Transient global cerebral ischemia was induced overnight after RH. Cerebral blood flow was measured using laser Doppler flowmetry. Ischemic brain injury in hippocampus was evaluated using histopathology. Post-ischemic hypoperfusion in RH-exposed rats was of greater extent than that in control rats. Inhibition of ASICs prevented RH-induced increase in the extent of post-ischemic hypoperfusion and ischemic brain injury. Since ASICs activation-induced store-operated calcium entry (SOCE) play a role in vascular tone, next we tested if acidosis activates SOCE via activating ASICs in vascular smooth muscle cells (VSMCs). We observed that SOCE in VSMCs at lower pH is ASIC3 dependent. The results show the role of ASIC in post-ischemic hypoperfusion and increased ischemic damage in RH-exposed ITD rats. Understanding the pathways mediating exacerbated ischemic brain injury in RH-exposed ITD rats may help lower diabetic aggravation of ischemic brain damage.
Keywords: Cerebral blood flow, Psalmotoxin1, APETx2, store operated calcium entry, acidosis, vascular smooth muscle cells
Introduction
Cerebral ischemia is a pathological condition marked by severe reduction in cerebral blood flow, resulting in gross metabolic derangements associated with cerebral hypoxia (Hossmann 1997). Post-ischemic reperfusion results in initial reactive hyperemia (transient increase in cerebral blood flow) followed by a relatively sustained period of delayed post-ischemic hypoperfusion (Ginsberg et al. 1978; Hossmann et al. 1973; Levy et al. 1979). Pharmacological agents decrease cerebral ischemia-induced neurological deficits and hippocampal CA1 neuronal death by lowering the extent of post-ischemic hypoperfusion (Lin et al. 2010; Steen et al. 1983). Besides, an increase in the extent of ischemic brain injury is seen with a progressive increase in post-ischemic hypoperfusion caused by longer duration of ischemia (Matsumoto et al. 1990). Thus, post-ischemic hypoperfusion plays an important role in ischemic brain injury (Hossmann 1997; Hosomi et al. 2007; Hossmann and Zimmermann 1974).
Diabetes is a chronic metabolic disease affecting 425 million people worldwide (International Diabetes Federation 2018), and cerebral ischemia-induced brain injury is prevalent in diabetic subjects (Almdal et al. 2004; Jorgensen et al. 1994; Kissela et al. 2005; Ottenbacher et al. 2004). Mortality associated with ischemic heart disease and stroke is one of the most serious outcomes of diabetes (Mozaffarian et al. 2016; Morrish et al. 2001). Heart disease-induced mortality was higher than that produced by stroke in diabetic subjects (Centers for Disease Control and Prevention 2011). Available anti-diabetic therapy increases the risk of hypoglycemia in diabetics (Cryer 2007; Van den Berghe et al. 2006; van den Berghe et al. 2001; Yuan et al. 2015). Continuous blood glucose monitoring studies showed that the patients with type 1 diabetes daily experience hypoglycemia for a period of 1–1.5 hours (Tamborlane et al. 2008). Anti-diabetic therapy in patients with type 2 diabetes also increases the risk of hypoglycemia (Donnelly et al. 2005; Gehlaut et al. 2015). Severe hypoglycemia exerts detrimental effects on brain energetics and also induced injury as discussed previously (Suh et al. 2007; Vannucci and Vannucci 2001; Languren et al. 2013). We have previously shown that prior exposure to recurrent hypoglycemia (RH) of moderate intensity worsens ischemic brain damage in diabetic rats (Dave et al. 2011b; Shukla et al. 2018) and oxygen/glucose deprivation-induced damage in hippocampal organotypic slices (Dave et al. 2011a). In addition, we previously observed that intra-ischemic acidosis mediates ischemic brain damage in RH-exposed ITD rats (Rehni et al. 2018). However, the mechanisms by which increased ischemic acidosis increases ischemic brain injury in RH-exposed ITD rats are unknown. Low pH during acidosis activates acid-sensing (proton-gated) ion channels (ASICs) (Waldmann et al. 1997). ASICs are expressed in the neurons and non-neuronal cells in the brain and cerebral vasculature (Alvarez de la Rosa et al. 2003; Kellenberger and Schild 2015; L. H. Lin et al. 2014; Nakamura et al. 2009; Meng et al. 2009). ASICs are proposed to mediate acidosis-induced cell death in vitro (Li et al. 2010; Sherwood et al. 2011) and ischemic brain injury in vivo (Sherwood et al. 2011; Xiong et al. 2004). Store-operated calcium entry into pulmonary VSMCs via ASIC results in acute hypoxic pulmonary vasoconstriction (Jernigan et al. 2012; Jernigan et al. 2009; Nitta et al. 2014).
Therefore, we hypothesized that increased intra-ischemic acidosis in RH-exposed ITD rats leads to pronounced post-ischemic hypoperfusion by activation of ASICs. The potential role of ischemic acidosis-induced ASIC activation in mediating RH-induced increase in ischemic brain injury in treated diabetic rats is unknown. Therefore, during the present investigation, we tested this hypothesis. Considering higher mortality in diabetics due to heart disease compared to stroke, we employed a rodent model of global cerebral ischemia in the present study (Centers for Disease Control and Prevention 2011).
Materials and methods
Animals
Experiments on animals were conducted as per the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health and protocols approved by the Animal Care and Use Committee of the University of Miami.
Induction of Diabetes
Male Wistar rats (Charles River Laboratories International, Inc, Wilmington, MA) were made diabetic using a single intraperitoneal dose of streptozotocin (58 mg ˟ kg−1) (Sigma-Aldrich, St Louis, MO). Streptozotocin solution was made in citrate buffer immediately before administration. Induction of diabetes was confirmed by measuring blood glucose levels in samples obtained from tail pricking and use of a portable glucose meter between 9 AM and noon (FreeStyle Freedom, Abbott Diabetes Care Inc, Alameda, CA) (Dave et al. 2011b). After induction of diabetes, blood glucose levels were monitored twice a week. The data shown in Figure 1B for groups of diabetic animals are last readings of untreated diabetes; i.e., levels of blood glucose just prior to insulin pellet implantation. Animals having blood glucose levels >310 mg × dl−1 after streptozotocin injection were considered diabetic.
Figure 1.
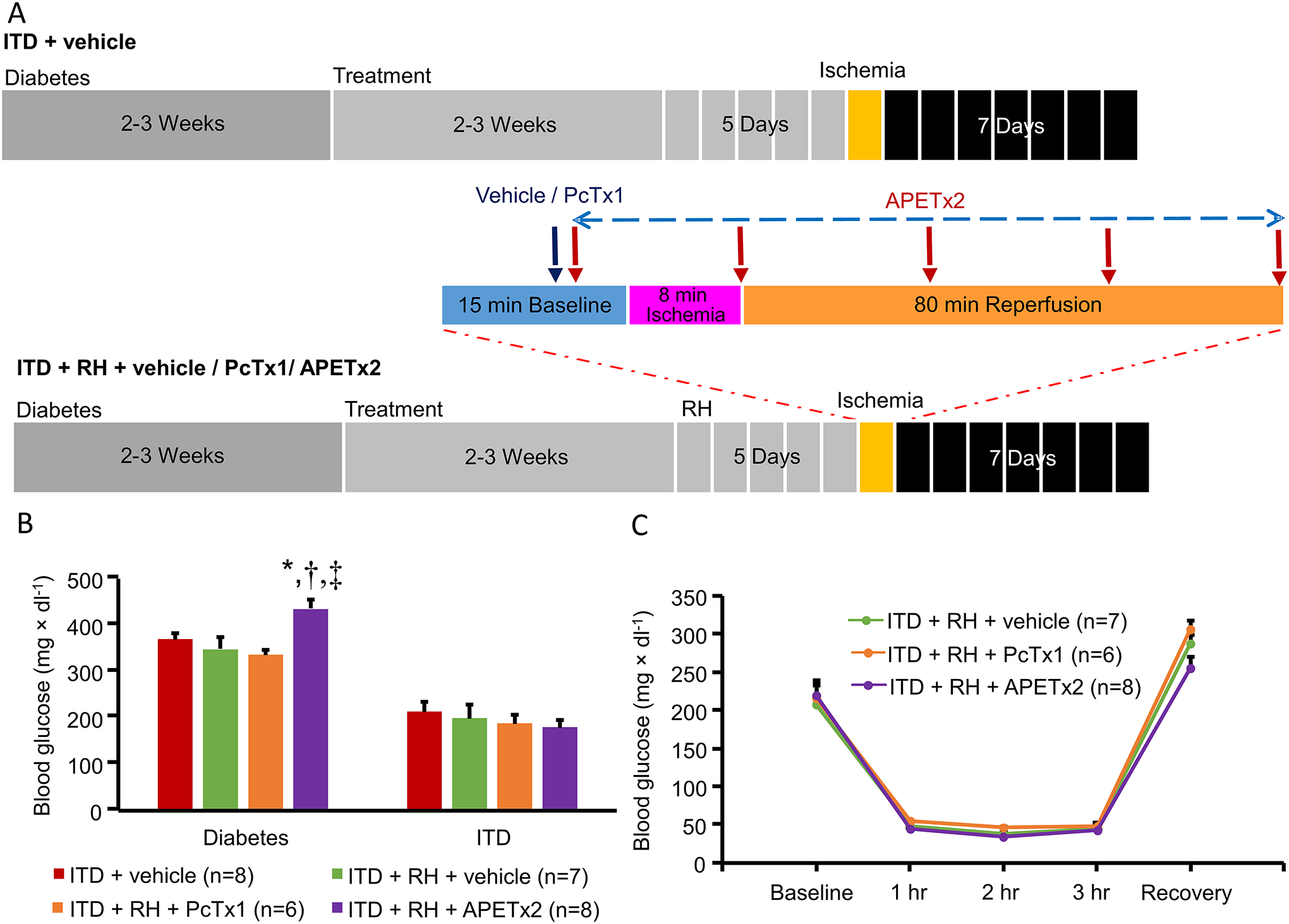
A) Synopsis of time course and experimental design of the study. All animals were subjected to cerebral ischemia and were euthanized at 7 days of reperfusion for histological analysis. B) Blood glucose levels after diabetes induction and insulin treatment, and C) Blood glucose levels before and during induction of hypoglycemia and after recovery. ITD + vehicle, ITD + RH + vehicle, ITD + RH + PcTx1, and ITD + RH + APETx2. * p<0.05 vs ITD + vehicle, † p<0.05 vs ITD + RH + vehicle and, ‡ p<0.05 ITD + RH + PcTx1.
Insulin Treatment
Approximately two to three weeks after the induction of diabetes, insulin pellet(s) (Linplant; LinShin, Toronto, Canada) were implanted subcutaneously to correct diabetic hyperglycemia. The same frequency of monitoring blood glucose levels was continued post-insulin pellet implantation. During this period, if the levels of blood glucose were out of the target range, the amount of implanted insulin pellet(s) was/were adjusted. These animals were considered as “insulin-treated diabetic rats” (Dave et al. 2011b). The data shown in Figure 1B for ITD groups are the last blood glucose levels recorded at the time of cerebral ischemia surgery.
Induction of Recurrent Hypoglycemia
RH exposure was carried out after two to three weeks of insulin treatment. At the time of hypoglycemia induction, food was removed from the cage to avoid the fluctuations in blood glucose levels during the period of hypoglycemia. Hypoglycemia was induced using a supplementary injection of insulin (Novolog Insulin aspart, Novo Nordisk, A/S, Bagsvaerd, Denmark). An episode of moderate hypoglycemia was induced for a period of 3 hours every day for 5 consecutive days. Blood glucose levels were monitored (as explained above) immediately prior to additional insulin dose and after every 1 hour until the completion of intended 3-hour period of hypoglycemia. A subcutaneous injection of dextrose and replacement of food was done to correct hypoglycemia. Euglycemia was ascertained by measuring blood glucose levels 30 minutes post-dextrose administration. Hypoglycemia was defined by the blood glucose levels below 70 mg × dl−1 (ADA Workgroup on Hypoglycemia 2005). The data shown in Figure 1C for ITD + RH groups are the blood glucose values of treated diabetic rats immediately before, during, and after recovery from hypoglycemia.
Induction of Global Cerebral Ischemia
Global cerebral ischemia was induced overnight after completion of the last episode of RH. Rats were anesthetized with isoflurane in a mixture containing 33% oxygen and 67% nitrous oxide, paralyzed and artificially ventilated. Physiological parameters (body temperature, head temperature, blood pH, partial pressure of carbon dioxide : pCO2 in blood, partial pressure of oxygen: pO2 in blood and mean arterial blood pressure: MABP) were monitored and maintained within normal range. An incision was made in the anterior neck region and the carotid arteries were isolated from the adjacent tissue. Ligatures (Polyethylene-10 tubing) were placed around the carotid arteries and secured with a flexible bilumen tube. Global cerebral ischemia was induced by tightening the carotid ligatures on the common carotid arteries with concurrent hypotension (blood pressure of ~50 mmHg was maintained by controlled outflow of blood using a syringe connected to a cannulated femoral artery). At the end of eight minutes of cerebral ischemia, the carotid ligatures were loosened and removed, and the withdrawn blood was injected back into the body circulation. The arteries were physically examined to confirm reflow of blood. The skin was sutured back, and appropriate post-operative care was provided.
Assessment of Cerebral Blood Flow
To quantify cerebral blood flow, a burr hole of 2 mm2 area was made over the right aspect of the skull 1.5 to 3.0 mm posterior and 1.5 to 4.0 mm lateral to the bregma. Using a stereotaxic apparatus, a laser Doppler blood flow probe was mounted onto the right cortex. Cerebral blood flow was measured by laser Doppler flowmetry using a fiber-optic probe (PeriFlux System 5000, PF 5010 - LDPM unit, Perimed, Järfälla, Sweden) (Della-Morte et al. 2011). Cerebral blood flow was recorded continuously at a frequency of 0.3 Hertz from 30 minutes before the onset of cerebral ischemia to 80 minutes of reperfusion using PeriSoft for Windows software.
Administration of ASIC inhibitors
For injection in the two lateral ventricles, two burr holes of 2 mm2 area were made over the left and right aspect of the skull 0.5 mm to 1.0 mm posterior and 1.0 mm and 2.0 mm lateral to the bregma. Using a stereotaxic apparatus, the needle of the syringe containing the treatment solution was inserted into the lateral ventricles of both hemispheres (3.5 mm deep). Psalmotoxin1 (PcTx1) (ASIC1a inhibitor, 0.75 ng per ventricle over 5 min) was injected as a solution in Ringer’s solution (147 mM NaCl, 4 mM KCl, and 1.3 mM CaCl2) ten to fifteen minutes before the onset of cerebral ischemia (Pignataro et al. 2007). APETx2 (ASIC3 inhibitor, 75 ng per ventricle over 5 min - repeated every 20 min), dissolved in Ringer’s solution, was injected from ten to fifteen minutes before the onset of cerebral ischemia to 80 minutes of reperfusion. Based on the sum of total volume of the rat brain ventricles and of cerebrospinal fluid (CSF), and flow rate of CSF (Davson and Segal 1970; Levinger 1971; Pardridge 2011; Tajima et al. 1993; Davson 1969), we estimated that the i.c.v. injections of PcTx1 and APETx2 used in the study are expected to result in an approximate concentration of 3.55 nM and 366 nM in CSF, respectively. These concentrations were 3–5 times the IC50 concentration of PcTx1 and APETx2 (Diochot et al. 2004).
Histological Assessment
After 7 days of reperfusion, animals were anesthetized with isoflurane, sternotomy was performed, the apex of the left cardiac ventricle was incised, a polyethylene catheter was inserted through the ventricle into the root of the aorta and was then ligated in place. The tip of the right atrium was incised to permit egress of the perfusate. The rats were then transcardially perfused (at a pressure of 120 mm Hg) with saline until the blood was completely washed out of the body. This was followed by perfusion with a mixture of formaldehyde, glacial acetic acid and methanol (in a ratio of 1:1:8) for 18–20 minutes, and brain samples were then isolated. Ten μm thick coronal sections (200 μm apart) of processed brain samples were collected from 2.8 to 4.0 mm posterior to bregma. Hematoxylin and eosin staining was performed on the sections. Evaluation was carried out using a Nikon microscope (Nikon Microphot-SA; Nikon Corporation, Tokyo, Japan), and a computer system (MCID Elite 6.0 software; InterFocus Imaging Ltd., Cambridge, UK). Ischemic brain damage was computed in terms of the number of normal neurons on CA1 hippocampus at a magnification of 40×. Normal neurons were counted at fields in sequence along the medial to lateral part of the CA1 hippocampus on both sides on three consecutive sections containing hippocampus at the level of ~ −3.6, ~ −3.8, and ~ −4.0 mm from bregma. The total count of neurons was added from the two brain hemispheres and the resulting values obtained from three sequential slides were averaged to compute the number of normal neurons in CA1 hippocampus.
Experimental Protocol
Rats were randomly assigned to various treatment groups in the following experiment (Figure 1 A):
Experiment 1: The effect of prior exposure of RH on post-ischemic hypoperfusion and ischemic brain injury in ITD rats. Groups included ITD + vehicle (control), and ITD + RH + vehicle.
Experiment 2: The effect of ASIC inhibition on RH-induced increase in post-ischemic hypoperfusion and ischemic brain injury in ITD rats. Groups included ITD + vehicle (control), ITD + RH + vehicle (control), ITD + RH + PcTx1, and ITD + RH + APETx2. Animals employed in the experiment 1 served as control animals for experiment 2.
Measurement of store-operated calcium entry (SOCE) in vascular smooth muscle cells
Freshly grown A7r5 cells (from rat aorta; CRL-1444: American type culture collection, Manassas, VA) were loaded with the Ca2+-sensitive fluorescent indicator Fluo-4AM (8 μM) (Life Technologies, Carlsbad, CA) in the presence of pluronic acid (0.05%) in calcium medium (135 mM NaCl, 5.9 mM KCl, 1.5 mM CaCl2, 1.2 mM MgCl2, 11.5 mM glucose and 11.6 mM HEPES, pH 7.4) and incubated for 40 minutes at 37°C and then for an additional 20 minutes at room temperature (Brueggemann et al. 2005; Jernigan et al. 2009). SOCE was measured at three pH values (6.0, 6.5, and 7.4) using Fluo-4 AM loaded cells superfused with Ca2+-free medium (135 mM NaCl, 5.9 mM KCl, 1.2 mM MgCl2, 11.5 mM glucose and 11.6 mM HEPES) with respective pH containing 50 μM diltiazem (Enzo Life Sciences, Farmingdale, NY) (to prevent Ca2+ entry through L-type voltage-gated Ca2+ channels), 10 μM cyclopiazonic acid (Enzo Life Sciences, Farmingdale, NY) (the sarco/endoplasmic reticulum Ca2+-ATPase inhibitor, to deplete intracellular Ca2+ stores and prevent Ca2+ reuptake), and 3 mM EGTA (to chelate any residual Ca2+). The changes in [Ca2+]i (SOCE) were quantified in terms of change in Fluo-4 AM fluorescence (Ex: 488 / Em: 520 nm) from baseline upon repletion of extracellular Ca2+ (1.5 mM) in the presence of diltiazem and cyclopiazonic acid over 30 min. Experiments were performed in triplicate for 6 observations in the presence of PcTx-1 (21.30 nM), APETx2 (300 nM), or vehicle control.
Statistical Analysis
Statistical analysis was carried out by Graph Pad prism software version 5. Statistically significant outlier data points were identified by Grubbs’ test and excluded from additional analysis. Animals having levels of blood glucose outside the expected range, and measures of physiological parameters during surgical procedures to be outside the normal range, were excluded from the study. When data from more than two groups was compared then one-way ANOVA followed by post hoc Tukey’s test for multiple comparisons was used. Comparison between two groups was done using Student’s t-test. P value <0.05 was considered statistically significant. The results are expressed as mean ± SEM.
Results
The details of study design are presented in Figure 1A. We observed a minor yet significant difference in blood glucose levels prior to insulin pellet implantation in the APETx2 treatment group when compared to ITD + vehicle control, ITD + RH + vehicle control and PcTx1 treatment groups. However, post-insulin treatment blood glucose values were not statistically different among all experimental groups (Figure 1B). Blood glucose levels, in ITD groups, were maintained slightly above euglycemia to avoid any unwanted hypoglycemia. No statistically significant differences in blood glucose levels were observed during hypoglycemia in all RH-exposed ITD groups (Figure 1C). Physiological parameters such as body weight, body temperature, head temperature, pCO2, pO2 and MABP were measured. A minor yet significantly lower value of blood pCO2 level was observed in the ITD + RH + APETx2 group during ischemia when compared to the respective values in ITD + vehicle and ITD + RH + vehicle groups. However, there was no other statistically significant difference between the physiological parameters in all other experimental groups (Table 1).
Table 1:
Physiological parameters.
| Group | Time Point | Body Weight (g) | Body Temperature (°C) | Head Temperature (°C) | pH | pCO2 (mmHg) | pO2 (mmHg) | MABP (mmHg) |
|---|---|---|---|---|---|---|---|---|
| ITD + vehicle (n=8) | Before | 352 ± 8 | 37.0 ± 0.0 | 36.8 ± 0.1 | 7.43 ± 0.02 | 38 ± 1 | 120 ± 4 | 97 ± 2 |
| During | 37.0 ± 0.0 | 36.6 ± 0.1 | 7.34 ± 0.02 | 50 ± 2 | 115 ± 5 | 50 ± 0 | ||
| After | 37.0 ± 0.0 | 36.8 ± 0.1 | 7.44 ± 0.01 | 35 ± 2 | 136 ± 8 | 101 ± 3 | ||
| ITD + RH + vehicle (n=7) | Before | 353 ± 9 | 37.0 ± 0.0 | 36.8 ± 0.1 | 7.47 ± 0.04 | 38 ± 1 | 118 ± 6 | 103 ± 6 |
| During | 36.9 ± 0.1 | 36.6 ± 0.1 | 7.34 ± 0.01 | 45 ± 2 | 118 ± 7 | 49 ± 1 | ||
| After | 37.0 ± 0.0 | 36.7 ± 0.1 | 7.43 ± 0.01 | 39 ± 1 | 115 ± 6 | 100 ± 7 | ||
| ITD + RH + PcTx1 (n=6) | Before | 339 ± 6 | 36.9 ± 0.1 | 36.4 ± 0.1 | 7.40 ± 0.02 | 37 ± 2 | 134 ± 8 | 103 ± 4 |
| During | 36.8 ± 0.1 | 36.5 ± 0.2 | 7.27 ± 0.02 | 53 ± 3 | 124 ± 6 | 49 ± 0 | ||
| After | 36.9 ± 0.1 | 36.5 ± 0.2 | 7.38 ± 0.02 | 36 ± 2 | 142 ± 9 | 113 ± 5 | ||
| ITD + RH + APETx2 (n=8) | Before | 353 ± 19 | 37.1 ± 0.1 | 36.6 ± 0.1 | 7.47 ± 0.01 | 37 ± 1 | 132 ± 9 | 103 ± 1 |
| During | 36.9 ± 0.1 | 36.5 ± 0.1 | 7.44 ± 0.01 | 34 ± 2*, † | 126 ± 8 | 49 ± 0 | ||
| After | 37.4 ± 0.1 | 36.9 ± 0.1 | 7.47 ± 0.01 | 34 ± 1 | 141 ± 5 | 113 ± 2 |
p<0.05 vs ITD + vehicle control.
p<0.05 vs ITD + RH + vehicle control.
RH exposure increases post-ischemic hypoperfusion in ITD rats via ASIC activation
Because we have previously observed that intra-ischemic acidosis in RH-exposed ITD rats is of greater extent, we hypothesized that increased intra-ischemic acidosis in RH-exposed ITD rats leads to pronounced post-ischemic hypoperfusion via ASICs. Animals belonging to the ITD + vehicle and the ITD + RH + vehicle groups demonstrated hyperemia immediately after ischemia. However, the extent of cerebral ischemia-induced hypoperfusion was greater (25% to 46%) in RH-exposed ITD rats from 3 to 4 minutes of ischemia and 23 to 62 minutes of reperfusion (p<0.05) when compared to ITD rats (Figure 2). These data suggest that prior RH exposure leads to severe post-ischemic hypoperfusion in ITD rats.
Figure 2:
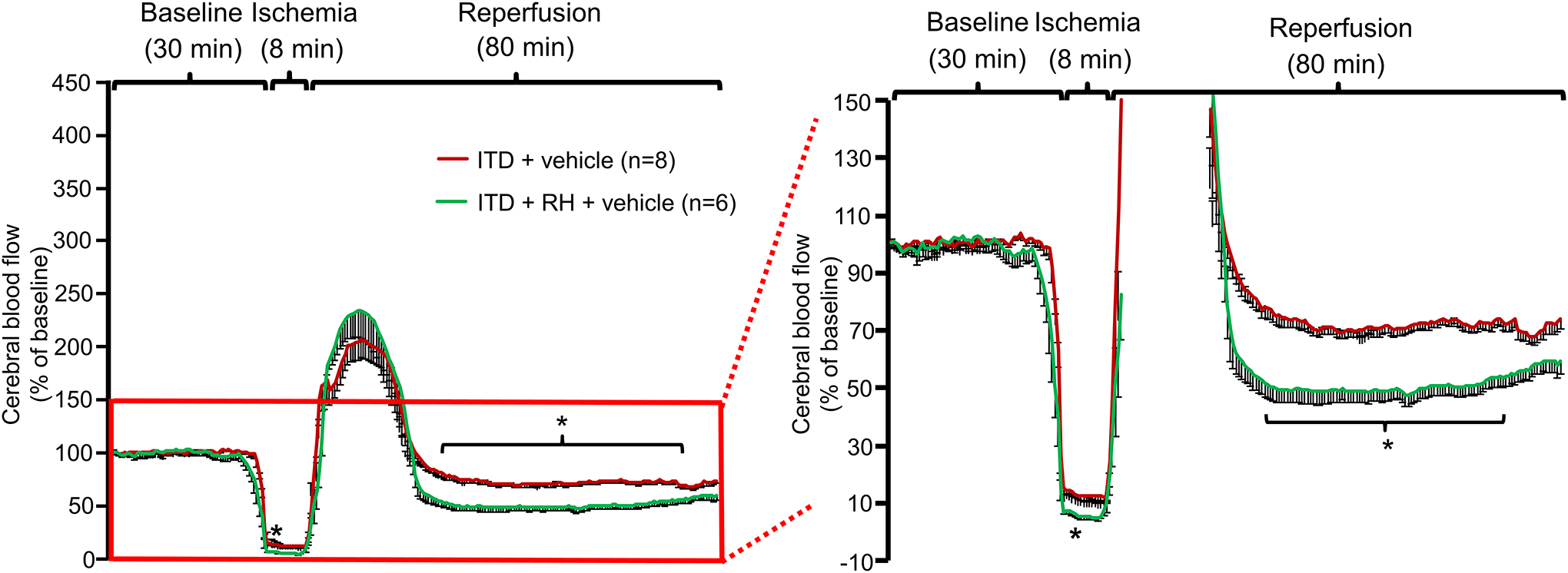
The effect of prior RH exposure to ITD rats on ischemia-induced decrease in percentage change in cerebral blood flow. Percentage change in cerebral blood flow versus time curve of rats belonging to (1) ITD + RH + vehicle and (2) ITD + vehicle. * p<0.05 vs ITD + Vehicle.
To evaluate the potential role of ASIC activation in increased post-ischemic hypoperfusion in RH-exposed ITD rats, next we sought to evaluate the effect of a selective ASIC1a inhibitor PcTx1 (Escoubas et al. 2003; Escoubas et al. 2000; Salinas et al. 2006) and the ASIC3 inhibitor APETx2 (Diochot et al. 2004; Karczewski et al. 2010), on ischemia-induced hypoperfusion in brains of RH-exposed ITD rats. We did not investigate the role of ASIC2 as it is activated at a much lower pH (pH0.5 = 4.9), which is not normally observed during cerebral ischemia, when compared with ASIC1a (pH0.5 = 6.8) and ASIC3 (pH0.5 = 6.6) (Benson et al. 2002; Wemmie et al. 2006). PcTx1 treatment to RH-exposed ITD rats significantly decreased (46 to 95%) the extent of post-ischemic hypoperfusion when compared to ITD + RH + vehicle control rats from 3 to 7 and 22 to 80 minutes after cerebral ischemia (p<0.05) (Figure 3). Further, APETx2 treatment to RH-exposed ITD rats also significantly decreased (27 to 54%) the extent of post-ischemic hypoperfusion when compared to its respective vehicle control group from 2 to 4 and 25 to 66 minutes after cerebral ischemia (p<0.05) (Figure 3). Overall, our results indicate that prior exposure of ITD rats to RH leads to severe post-ischemic cerebral hypoperfusion, possibly via ASIC1a and ASIC3 activation.
Figure 3:
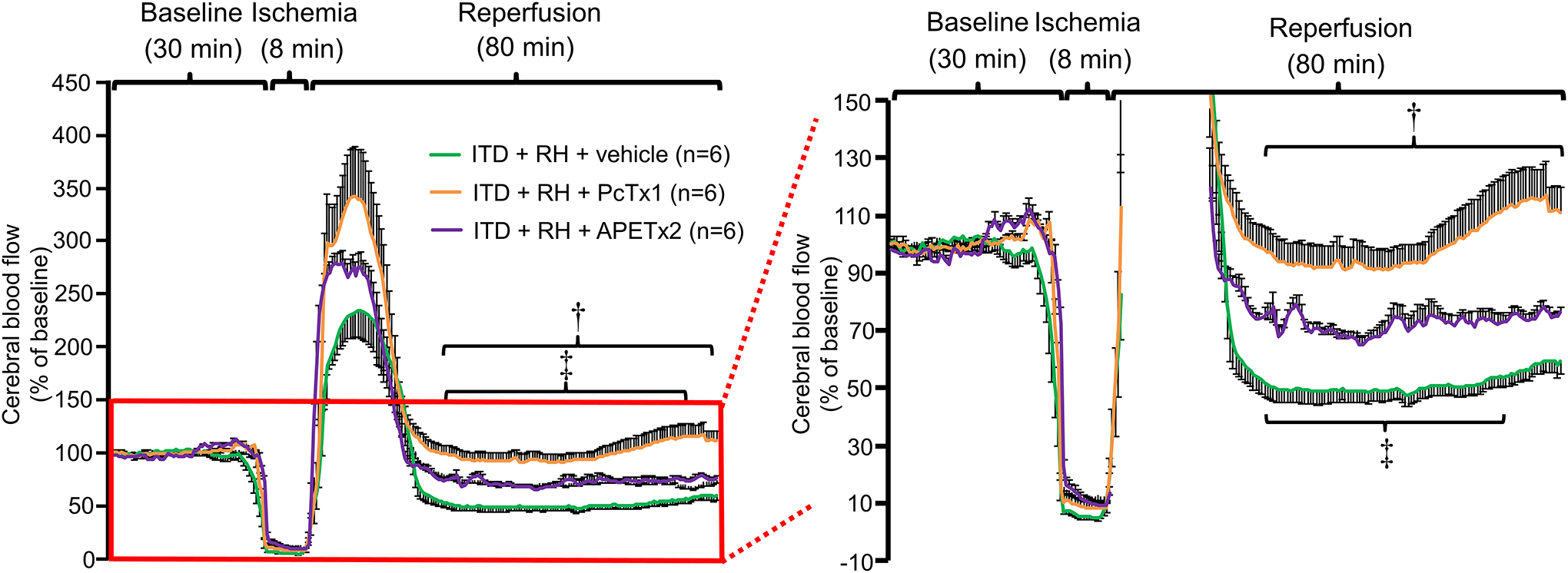
The effect of PcTx1 and APETx2 on ischemia-induced decrease in percentage change in cerebral blood flow in ITD rats subjected to RH. Percentage change in cerebral blood flow versus time curve of rats belonging to ITD + RH + vehicle, ITD + RH + PcTx1, and ITD + RH + APETx2 groups. ITD + RH + vehicle trace is the same as in Figure 2. † p<0.05, ITD + RH + PcTx1 vs ITD + RH + vehicle and, ‡ p<0.05, ITD + RH + APETx2 vs ITD + RH + vehicle.
ASIC inhibition attenuates RH-induced ischemic brain injury in ITD rats
To address the question if ASIC-dependent severe hypoperfusion in RH-exposed ITD rats is responsible for exacerbation of ischemic brain damage, we evaluated the effect of ASIC inhibition on the extent of ischemic brain injury in these rats. We quantified the degree of ischemic brain injury in RH-exposed ITD rats treated with either PcTx1, APETx2, or vehicle. The number of normal neurons in CA1 hippocampus in RH-exposed ITD rats was significantly lower (58%, p<0.05) than in ITD control rats. PcTx1 as well as APETx2 treatments prevented RH-induced increase in ischemic damage in CA1 hippocampus as the number of normal neurons were higher in these groups by 56 (p<0.01) and 62% (p<0.01) when compared to vehicle control group, respectively (Figure 4). This result demonstrates that decreasing post-ischemic hypoperfusion in RH-exposed ITD rats by ASIC inhibition prevents exacerbated ischemic brain injury in RH-exposed rats.
Figure 4:
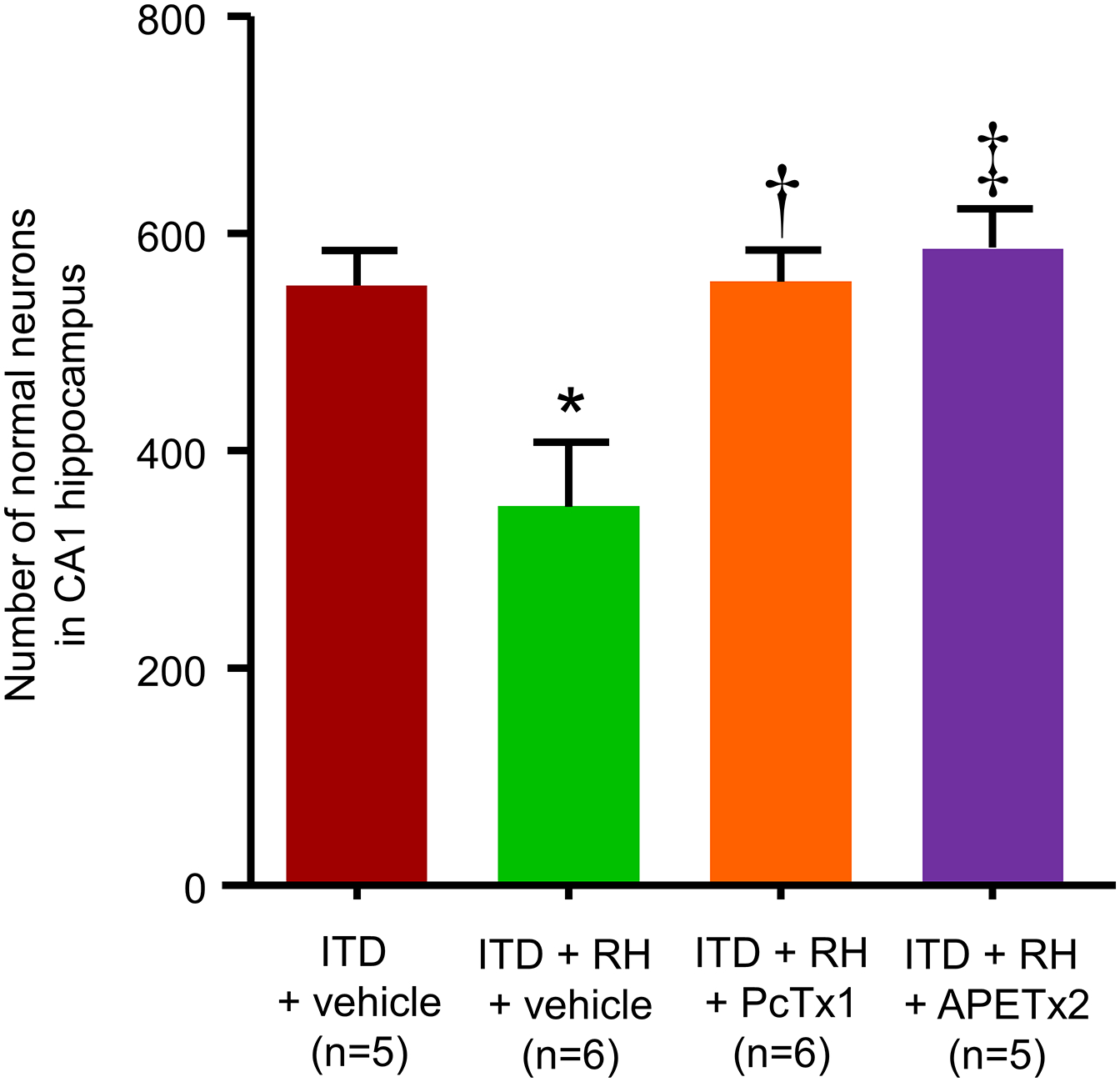
The effect of PcTx1 and APETx2 on RH-induced increase in ischemic damage in hippocampus of ITD rats. The numbers of normal neurons in CA1 hippocampus of rats belonging to the ITD + vehicle, ITD + RH + vehicle, ITD + RH + PcTx1, and ITD + RH + APETx2 groups are shown. * p<0.05 ITD + vehicle vs ITD + RH + vehicle, † p<0.05 ITD + RH + PcTx1 vs ITD + RH + vehicle, and ‡ p<0.05 ITD + RH + APETx2 vs ITD + RH + vehicle.
Acidosis leads to SOCE in vascular smooth muscle cells via ASIC3 activation
Because earlier studies demonstrated the role of ASIC-dependent SOCE in VSMCs in causing pulmonary vasoconstriction (Jernigan et al. 2012; Jernigan et al. 2009; Nitta et al. 2014), we next determined if increased intra-ischemic acidosis leads to SOCE in VSMCs via activation of ASICs. This hypothesis was tested in vitro using VSMCs. We determined the contributions of ASICs in SOCE at different pH by measuring SOCE in presence and absence of ASIC1a or ASIC3 inhibitors. We observed that SOCE in presence of APETx2 was lower by 40% (p<0.05) when measured at pH 6.0 (Figure 5A). However, SOCE at pH 6.5 and 7.4 was not affected in the presence of APETx2. We also did not observe any such effect of ASIC1a inhibition on SOCE at the three pH values that were tested (Figure 5B). Therefore, our observations indicate that SOCE during pronounced ischemic acidosis is ASIC3- and not ASIC1a-dependent.
Figure 5:
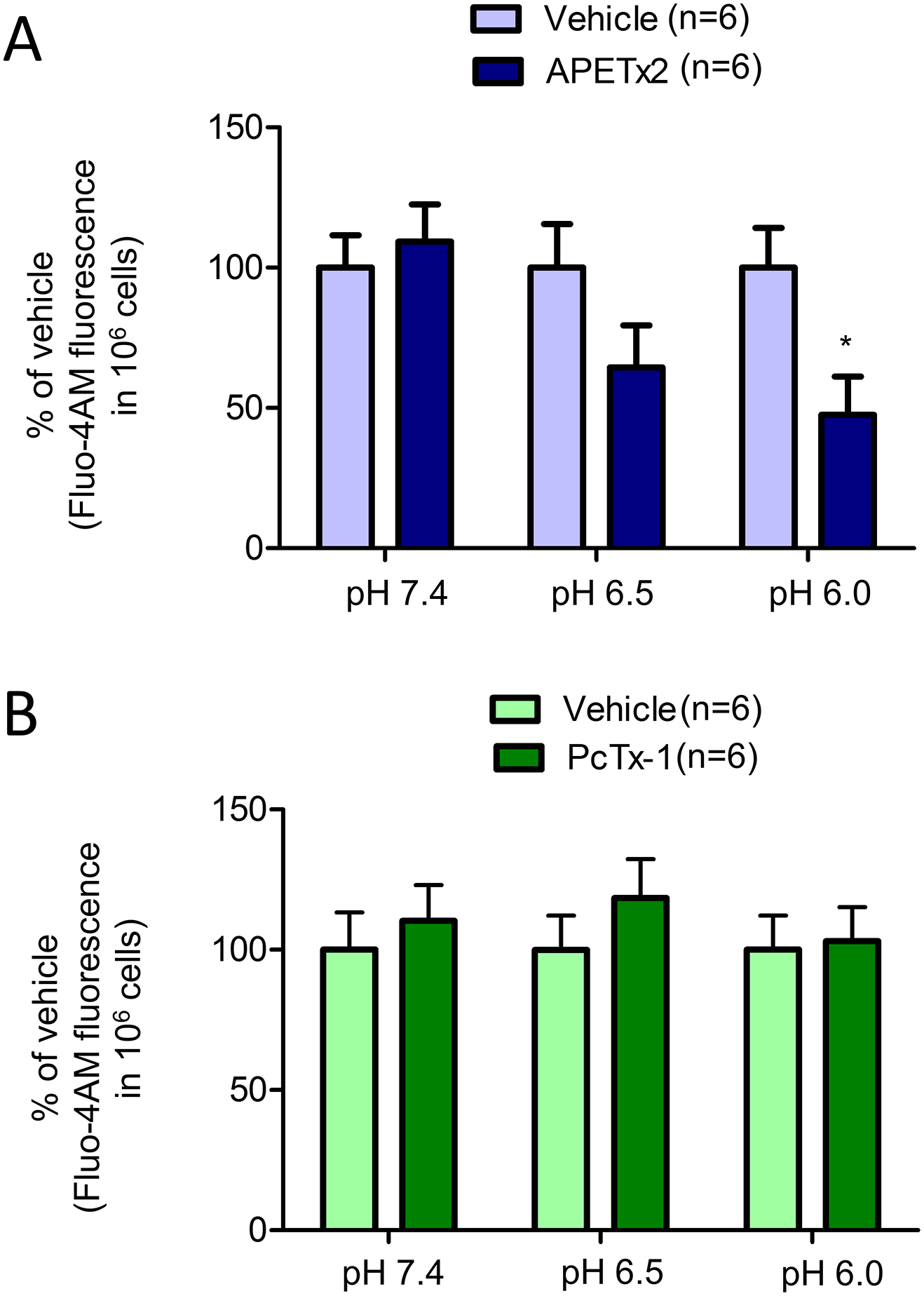
The role of (A) ASIC3, and (B) ASIC1a in acidosis-induced SOCE in VSMCs. SOCE at pH 7.4, 6.5, and 6.0 in presence of vehicle, APETx2, or PcTx1. *, p<0.05 vs respective vehicle control.
Discussion
Cerebral ischemia causes substantial mortality and morbidity in the population worldwide and diabetes causes a further increase in ischemic brain injury (Benjamin et al. 2017; Kissela and Air 2006; Morrish et al. 2001; Preis et al. 2009). Therapeutic options available to treat diabetic hyperglycemia are unable to ensure tight round–the-clock maintenance of euglycemia, and unavoidably result in transient episodes of hypoglycemia (Nathan et al. 1993; Leese et al. 2003). Repeated episodes of hypoglycemia inhibit counter-regulatory mechanisms regulating blood glucose levels (Parekh 2009; McCrimmon et al. 2005; Mandal and Briski 2018) and thus increases the risk of RH (Cryer 2013; Leese et al. 2003). We have previously shown that prior exposure to RH aggravates ischemic brain damage in treated diabetic rats (Dave et al. 2011b; Shukla et al. 2018). However, the mechanism responsible for RH-induced exacerbation of ischemic brain injury in diabetic rats is not well understood. We earlier demonstrated the role of ischemic acidosis in mediating RH-induced increase in ischemic brain injury in ITD rats (Rehni et al. 2018). However, the downstream mechanisms responsible for the pronounced ischemic acidosis-induced increase in brain injury in RH-exposed ITD rats are unknown. ASIC1a, ASIC2a and ASIC3 are expressed in VSMCs (Grifoni et al. 2008). ASIC1 are expressed in the central nervous system (Alvarez de la Rosa et al. 2003) and cerebral vasculature (Lin et al. 2014; Nakamura et al. 2009). A study has shown the presence of ASIC3 in many parts of the rat brain (Meng et al. 2009). An earlier study also confirmed the presence of ASIC3 in brain (including the hippocampus) using ASIC3 knockout mice (Drew et al. 2004; Wu et al. 2010). In addition, ASIC3 are also expressed in human brain (Babinski et al. 1999; Delaunay et al. 2012). In the present study, we showed that prior exposure of ITD rats to RH causes ASIC1a- and ASIC3-dependent post-ischemic hypoperfusion, which in turn, increases ischemic brain damage. Our results corroborate earlier findings that hypoglycemia in combination with ischemia causes profound post-ischemic hypoperfusion and decreases hypercapnic reactivity in neonates (Kim et al. 1994). We studied the effect of ischemia on hippocampus, which is a vulnerable part of the brain (Bartsch et al. 2015; Kirino and Sano 1984; Petito et al. 1987; Schmidt-Kastner 2015; Schmidt-Kastner and Freund 1991; Schmidt-Kastner et al. 1990). However, future studies are needed to evaluate the effect of ASIC inhibition on ischemic damage in other parts of the brain in RH-exposed ITD rats.
Low pH during acidosis activates ASICs (Waldmann 2001; Waldmann et al. 1997). ASICs are located in the VSMCs in the cerebral vasculature and are responsible for facilitation of myogenic response and VSMC migration (Chung et al. 2010; Drummond et al. 2004; Grifoni et al. 2006; Grifoni et al. 2008; Jernigan and Drummond 2005; Lin et al. 2014). Post-ischemic hypoperfusion is a characteristic phenomenon of ischemia-reperfusion injury (Ginsberg et al. 1978; Hossmann et al. 1973; Levy et al. 1979; Snyder et al. 1975). Preventing cerebral ischemia-induced vasoconstriction, by MEK1/2 inhibition, lowers ischemic brain damage (Johansson et al. 2014). ASICs are extensively distributed in the nervous system and are also known to induce neuronal depolarization in response to drop in pH (Kellenberger and Schild 2002; Lingueglia 2007; Waldmann 2001). An ischemic acidosis-related increase in extracellular protons trigger neuronal necroptosis via ASIC activation (Wang et al. 2015). Overall, the activation of ASIC sensitive proton-gated calcium currents and vascular changes may play a significant role in mediating acidosis-induced neuronal death (Sherwood et al. 2011) and ischemic brain damage (Xiong et al. 2004). Although prior exposure to RH leads to increased ischemia-induced acidosis and increased ischemic damage, the participation of ASIC in RH-induced increased ischemic damage was not known. We observed that both ASIC1a and ASIC3 inhibitors attenuated the cerebral blood flow deficits and brain injury after ischemia in RH-exposed ITD animals. Our results suggest that severe hypoperfusion observed after global cerebral ischemia in ITD animals exposed to RH may be due to the activation of ASICs.
ASICs are known to induce vasoconstriction in cerebral vessels (Chung et al. 2010; Drummond et al. 2004; Jernigan and Drummond 2005; Lin et al. 2014). Contraction in the VSMCs results from calcium influx through the cell membrane, intracellular calcium release, and increased sensitization of calcium-dependent contractile proteins (Avila-Medina et al. 2018). ASIC activation is proposed to cause vasoconstriction by mediating SOCE (Jernigan et al. 2009). Our current data shows that exposure to acidic pH leads to ASIC3-mediated SOCE in VSMCs. Therefore, it is plausible that increased ischemic acidosis may elicit vasoconstrictive effects on cerebral vessels via ASIC3-dependent SOCE in RH-exposed ITD rats. We did not observe any impact of ASIC1a inhibition on SOCE in the VSMCs at two acidic pH levels tested. However, we did observe lower ischemic damage in ASIC1a inhibitor-treated RH-exposed ITD rats. ASICs are expressed on brain neurons as well as central and peripheral immune cells (Kellenberger and Schild 2015). Calcium influx in neurons is known as one of the central mechanisms regulating cell death (Wojda et al. 2008). Although ASIC currents are Na+-selective, ASIC1a present in the cell membrane have an additional low permeability to Ca2+ ions as well (Kellenberger and Schild 2015; Sherwood et al. 2011; Xu et al. 2018; Zuo et al. 2018) and their blockade produces protective effects in several neurodegenerative diseases including stroke (Pignataro et al. 2007; Xiong et al. 2004; Hu et al. 2011; Wong et al. 2008; Arias et al. 2008). It is plausible that ASIC1a on neurons may also participate in RH-induced increase in ischemic damage. Moreover, the role of acidosis (Combs et al. 1990; Kuyama et al. 1994; Nagao et al. 1996; Rehni et al. 2018) and, ASIC activation (Pignataro et al. 2007; Xiong et al. 2004) in mediating ischemic brain injury is well established. Since we did not observe impact of PcTx1 on SOCE, but observed lower extent of cerebral ischemia-induced hypoperfusion and lower cerebral ischemic damage in PcTx1-treated group, we hypothesize that ASIC1 activation in the neurons may indirectly regulate post-ischemic hypoperfusion in the brain. Future studies to understand neuronal control of post-ischemic cerebral blood flow in RH-exposed ITD rats are warranted.
Besides presence of homomeric ASICs in brain, ASIC1a/2 heteromers are also present on the brain cells (Askwith et al. 2004; Sherwood et al. 2011). Heteromeric ASIC1a/2 have lower and higher proton sensitivities as compared to homomeric ASIC1a and ASIC2a, respectively (Hesselager et al. 2004; Joeres et al. 2016). Proton sensitivity of heteromeric ASIC2a/3 is higher than that of ASIC2a, ASIC2b and ASIC3 (Baron et al. 2001; Benson et al. 2002). Heteromeric ASIC1a/ASIC2a are not inhibited by PcTx1 while heteromeric ASIC2b/1a are inhibited by PcTx1 (Escoubas et al. 2000). The inhibitory effect of PcTx1 on ASIC1a/ASIC3 channels is controversial (Escoubas et al. 2000; Gregory et al. 2018). However, PcTx1 slows the kinetics of desensitization, recovery of desensitization, and inhibits pH dependent steady-state desensitization of ASIC1a/2/3 heteromeric channels (Gregory et al. 2018). The literature suggests that the effect of ASIC inhibitors observed in our studies may be due to inhibition of homomeric ASICs and its impact on properties of heteromeric ASICs. Future studies are required to determine the role of these ASIC heteromers in mediating pronounced ischemic acidosis induced increase in brain injury in ITD rats previously exposed to RH. ASIC2 is upregulated after global cerebral ischemia and may contribute to ischemia-induced neuronal injury (Jiang et al. 2017; Johnson et al. 2001). Earlier study also demonstrated the role of ASIC2a in surface trafficking of ASIC1a in brain (Harding et al. 2014; Jiang et al. 2017). However, as mentioned above, we did not evaluate contribution of ASIC2 in our experimental conditions as ASIC2 are activated at much lower pH (usually not observed during cerebral ischemia) (Benson et al. 2002; Wemmie et al. 2006). The role of ASIC2, if any, in RH exposure-induced increased ischemic damage remains to be evaluated. Our proof-of-concept study demonstrates the neuroprotective effect of ASIC inhibition on RH-induced increase in ischemic brain injury. Further rigorous preclinical evaluation following the Stroke Therapy Academic Industry Roundtable (STAIR) recommendations may help test the therapeutic potential of inhibitors used in the present study (Stroke Therapy Academic Industry Roundtable 1999).
Therefore, we conclude that severe post-ischemic hypoperfusion is responsible for RH-induced aggravation of ischemic brain damage, possibly mediated through the activation of ASICs. Nevertheless, further understanding of the mechanisms leading to ASIC-dependent changes in cerebral blood flow, and other non-vascular alterations in the brain that cause RH-related increase in ischemic brain injury in ITD rats is required. More understanding on how increased extent of ischemia/reperfusion-induced cerebral hypoperfusion leads to increased damage is also needed. Our study demonstrates that post-ischemic acidosis increases ischemic brain injury in RH-exposed ITD rats via ASIC activation mediated post-ischemic hypoperfusion. In addition, our data implicates that ASIC modulation may serve as an ameliorative approach for diabetes-induced increase in ischemic brain damage.
Acknowledgement:
This work was supported by National Institutes of Health grant NS073779 and American Heart Association grant 18POST34070061. The funding agencies were not involved in study design, in the collection, analysis and interpretation of data, in the writing of the report, and in the decision to submit the article for publication. We thank Dr. Brant Watson for critical reading of this manuscript.
Footnotes
Compliance with Ethics Requirements:All institutional and national guidelines for the care and use of laboratory animals were followed.
Conflicts of interest:
The authors declare that they have no conflict of interest.
References
- ADA Workgroup on Hypoglycemia (2005). Defining and reporting hypoglycemia in diabetes: a report from the American Diabetes Association Workgroup on Hypoglycemia. Diabetes Care, 28(5), 1245–1249, doi: 10.2337/diacare.28.5.1245. [DOI] [PubMed] [Google Scholar]
- Almdal T, Scharling H, Jensen JS, & Vestergaard H (2004). The independent effect of type 2 diabetes mellitus on ischemic heart disease, stroke, and death: a population-based study of 13,000 men and women with 20 years of follow-up. Arch Intern Med, 164(13), 1422–1426, doi: 10.1001/archinte.164.13.1422. [DOI] [PubMed] [Google Scholar]
- Alvarez de la Rosa D, Krueger SR, Kolar A, Shao D, Fitzsimonds RM, & Canessa CM (2003). Distribution, subcellular localization and ontogeny of ASIC1 in the mammalian central nervous system. J Physiol, 546(Pt 1), 77–87, doi: 10.1113/jphysiol.2002.030692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias RL, Sung ML, Vasylyev D, Zhang MY, Albinson K, Kubek K, et al. (2008). Amiloride is neuroprotective in an MPTP model of Parkinson’s disease. Neurobiol Dis, 31(3), 334–341, doi: 10.1016/j.nbd.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Askwith CC, Wemmie JA, Price MP, Rokhlina T, & Welsh MJ (2004). Acid-sensing ion channel 2 (ASIC2) modulates ASIC1 H+-activated currents in hippocampal neurons. J Biol Chem, 279(18), 18296–18305, doi: 10.1074/jbc.M312145200. [DOI] [PubMed] [Google Scholar]
- Avila-Medina J, Mayoral-Gonzalez I, Dominguez-Rodriguez A, Gallardo-Castillo I, Ribas J, Ordonez A, et al. (2018). The Complex Role of Store Operated Calcium Entry Pathways and Related Proteins in the Function of Cardiac, Skeletal and Vascular Smooth Muscle Cells. Front Physiol, 9, 257, doi: 10.3389/fphys.2018.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babinski K, Le KT, & Seguela P (1999). Molecular cloning and regional distribution of a human proton receptor subunit with biphasic functional properties. J Neurochem, 72(1), 51–57, doi: 10.1046/j.1471-4159.1999.0720051.x [DOI] [PubMed] [Google Scholar]
- Baron A, Schaefer L, Lingueglia E, Champigny G, & Lazdunski M (2001). Zn2+ and H+ are coactivators of acid-sensing ion channels. J Biol Chem, 276(38), 35361–35367, doi: 10.1074/jbc.M105208200. [DOI] [PubMed] [Google Scholar]
- Bartsch T, Dohring J, Reuter S, Finke C, Rohr A, Brauer H, et al. (2015). Selective neuronal vulnerability of human hippocampal CA1 neurons: lesion evolution, temporal course, and pattern of hippocampal damage in diffusion-weighted MR imaging. J Cereb Blood Flow Metab, 35(11), 1836–1845, doi: 10.1038/jcbfm.2015.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. (2017). Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation, 135(10), e146–e603, doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson CJ, Xie J, Wemmie JA, Price MP, Henss JM, Welsh MJ, et al. (2002). Heteromultimers of DEG/ENaC subunits form H+-gated channels in mouse sensory neurons. Proc Natl Acad Sci U S A, 99(4), 2338–2343, doi: 10.1073/pnas.032678399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brueggemann LI, Markun DR, Barakat JA, Chen H, & Byron KL (2005). Evidence against reciprocal regulation of Ca2+ entry by vasopressin in A7r5 rat aortic smooth-muscle cells. Biochem J, 388(Pt 1), 237–244, doi: 10.1042/BJ20041360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2011). National Diabetes Fact Sheet: National Estimates and General Information on Diabetes and Prediabetes in the United States, 2011. In US Department of Health and Human Services (Ed.). Atlanta, GA: Centers for Disease Control and Prevention. [Google Scholar]
- Chung WS, Farley JM, Swenson A, Barnard JM, Hamilton G, Chiposi R, et al. (2010). Extracellular acidosis activates ASIC-like channels in freshly isolated cerebral artery smooth muscle cells. Am J Physiol Cell Physiol, 298(5), C1198–1208, doi: 10.1152/ajpcell.00511.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combs DJ, Dempsey RJ, Maley M, Donaldson D, & Smith C (1990). Relationship between plasma glucose, brain lactate, and intracellular pH during cerebral ischemia in gerbils. Stroke, 21(6), 936–942, 10.1161/01.STR.21.6.936.. [DOI] [PubMed] [Google Scholar]
- Cryer PE (2007). Hypoglycemia, functional brain failure, and brain death. J Clin Invest, 117(4), 868–870, doi: 10.1172/JCI31669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryer PE (2013). Hypoglycemia-associated autonomic failure in diabetes. Handb Clin Neurol, 117, 295–307, doi: 10.1016/B978-0-444-53491-0.00023-7. [DOI] [PubMed] [Google Scholar]
- Dave KR, Pileggi A, & Raval AP (2011a). Recurrent hypoglycemia increases oxygen glucose deprivation-induced damage in hippocampal organotypic slices. Neurosci Lett, 496(1), 25–29, doi: 10.1016/j.neulet.2011.03.079. [DOI] [PubMed] [Google Scholar]
- Dave KR, Tamariz J, Desai KM, Brand FJ, Liu A, Saul I, et al. (2011b). Recurrent hypoglycemia exacerbates cerebral ischemic damage in streptozotocin-induced diabetic rats. Stroke, 42(5), 1404–1411, doi: 10.1161/STROKEAHA.110.594937. [DOI] [PubMed] [Google Scholar]
- Davson H (1969). The Cerebrospinal Fluid. In Lajtha A (Ed.), Handbook of Neurochemistry: Volume II: Structural Neurochemistry (pp. 23–48). Boston, MA: Springer US. [Google Scholar]
- Davson H, & Segal MB (1970). The effects of some inhibitors and accelerators of sodium transport on the turnover of 22Na in the cerebrospinal fluid and the brain. J Physiol, 209(1), 131–153, doi: doi: 10.1113/jphysiol.1970.sp009159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaunay A, Gasull X, Salinas M, Noel J, Friend V, Lingueglia E, et al. (2012). Human ASIC3 channel dynamically adapts its activity to sense the extracellular pH in both acidic and alkaline directions. Proc Natl Acad Sci U S A, 109(32), 13124–13129, doi: 10.1073/pnas.1120350109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della-Morte D, Raval AP, Dave KR, Lin HW, & Perez-Pinzon MA (2011). Post-ischemic activation of protein kinase C epsilon protects the hippocampus from cerebral ischemic injury via alterations in cerebral blood flow. Neurosci Lett, 487(2), 158–162, doi: 10.1016/j.neulet.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diochot S, Baron A, Rash LD, Deval E, Escoubas P, Scarzello S, et al. (2004). A new sea anemone peptide, APETx2, inhibits ASIC3, a major acid-sensitive channel in sensory neurons. EMBO J, 23(7), 1516–1525, doi: 10.1038/sj.emboj.7600177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly LA, Morris AD, Frier BM, Ellis JD, Donnan PT, Durrant R, et al. (2005). Frequency and predictors of hypoglycaemia in Type 1 and insulin-treated Type 2 diabetes: a population-based study. Diabet Med, 22(6), 749–755, doi: 10.1111/j.1464-5491.2005.01501.x. [DOI] [PubMed] [Google Scholar]
- Drew LJ, Rohrer DK, Price MP, Blaver KE, Cockayne DA, Cesare P, et al. (2004). Acid-sensing ion channels ASIC2 and ASIC3 do not contribute to mechanically activated currents in mammalian sensory neurones. J Physiol, 556(Pt 3), 691–710, doi: 10.1113/jphysiol.2003.058693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond HA, Gebremedhin D, & Harder DR (2004). Degenerin/epithelial Na+ channel proteins: components of a vascular mechanosensor. Hypertension, 44(5), 643–648, doi: 10.1161/01.HYP.0000144465.56360.ad. [DOI] [PubMed] [Google Scholar]
- Escoubas P, Bernard C, Lambeau G, Lazdunski M, & Darbon H (2003). Recombinant production and solution structure of PcTx1, the specific peptide inhibitor of ASIC1a proton-gated cation channels. Protein Sci, 12(7), 1332–1343, doi: 10.1110/ps.0307003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escoubas P, De Weille JR, Lecoq A, Diochot S, Waldmann R, Champigny G, et al. (2000). Isolation of a tarantula toxin specific for a class of proton-gated Na+ channels. J Biol Chem, 275(33), 25116–25121, doi: 10.1074/jbc.M003643200. [DOI] [PubMed] [Google Scholar]
- Gehlaut RR, Dogbey GY, Schwartz FL, Marling CR, & Shubrook JH (2015). Hypoglycemia in Type 2 Diabetes--More Common Than You Think: A Continuous Glucose Monitoring Study. J Diabetes Sci Technol, 9(5), 999–1005, doi: 10.1177/1932296815581052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg MD, Budd MW, & Welsh FA (1978). Diffuse cerebral ischemia in the cat: I. Local blood flow during severe ischemia and recirculation. Ann Neurol, 3(6), 482–492, doi: 10.1002/ana.410030605. [DOI] [PubMed] [Google Scholar]
- Gregory NS, Gautam M, Benson CJ, & Sluka KA (2018). Acid Sensing Ion Channel 1a (ASIC1a) Mediates Activity-induced Pain by Modulation of Heteromeric ASIC Channel Kinetics. Neuroscience, 386, 166–174, doi: 10.1016/j.neuroscience.2018.06.033. [DOI] [PubMed] [Google Scholar]
- Grifoni SC, Gannon KP, Stec DE, & Drummond HA (2006). ENaC proteins contribute to VSMC migration. Am J Physiol Heart Circ Physiol, 291(6), H3076–3086, doi: 10.1152/ajpheart.00333.2006. [DOI] [PubMed] [Google Scholar]
- Grifoni SC, Jernigan NL, Hamilton G, & Drummond HA (2008). ASIC proteins regulate smooth muscle cell migration. Microvasc Res, 75(2), 202–210, doi: 10.1016/j.mvr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding AM, Kusama N, Hattori T, Gautam M, & Benson CJ (2014). ASIC2 subunits facilitate expression at the cell surface and confer regulation by PSD-95. PLoS One, 9(4), e93797, doi: 10.1371/journal.pone.0093797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselager M, Timmermann DB, & Ahring PK (2004). pH Dependency and desensitization kinetics of heterologously expressed combinations of acid-sensing ion channel subunits. J Biol Chem, 279(12), 11006–11015, doi: 10.1074/jbc.M313507200. [DOI] [PubMed] [Google Scholar]
- Hosomi N, Ohyama H, Ichihara S, Takahashi T, Naya T, & Kohno M (2007). Relation of postischemic delayed hypoperfusion and cerebral edema after transient forebrain ischemia. J Stroke Cerebrovasc Dis, 16(3), 103–108, doi: 10.1016/j.jstrokecerebrovasdis.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Hossmann KA (1997). Reperfusion of the brain after global ischemia: hemodynamic disturbances. Shock, 8(2), 95–101, doi: 10.1097/00024382-199708000-00004. [DOI] [PubMed] [Google Scholar]
- Hossmann KA, Lechtape-Gruter H, & Hossmann V (1973). The role of cerebral blood flow for the recovery of the brain after prolonged ischemia. Z Neurol, 204(4), 281–299, doi: 10.1001/archneur.1973.00490300037004. [DOI] [PubMed] [Google Scholar]
- Hossmann KA, & Zimmermann V (1974). Resuscitation of the monkey brain after 1 h complete ischemia. I. Physiological and morphological observations. Brain Res, 81(1), 59–74, doi: 10.1016/0006-8993(74)90478-8. [DOI] [PubMed] [Google Scholar]
- Hu R, Duan B, Wang D, Yu Y, Li W, Luo H, et al. (2011). Role of acid-sensing ion channel 1a in the secondary damage of traumatic spinal cord injury. Ann Surg, 254(2), 353–362, doi: 10.1097/SLA.0b013e31822645b4. [DOI] [PubMed] [Google Scholar]
- International Diabetes Federation (2018). http://www.diabetesatlas.org/key-messages.html (Retrieved on August 25th, 2018).
- Jernigan NL, & Drummond HA (2005). Vascular ENaC proteins are required for renal myogenic constriction. Am J Physiol Renal Physiol, 289(4), F891–901, doi: 10.1152/ajprenal.00019.2005. [DOI] [PubMed] [Google Scholar]
- Jernigan NL, Herbert LM, Walker BR, & Resta TC (2012). Chronic hypoxia upregulates pulmonary arterial ASIC1: a novel mechanism of enhanced store-operated Ca2+ entry and receptor-dependent vasoconstriction. Am J Physiol Cell Physiol, 302(6), C931–940, doi: 10.1152/ajpcell.00332.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan NL, Paffett ML, Walker BR, & Resta TC (2009). ASIC1 contributes to pulmonary vascular smooth muscle store-operated Ca(2+) entry. Am J Physiol Lung Cell Mol Physiol, 297(2), L271–285, doi: 10.1152/ajplung.00020.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang N, Wu J, Leng T, Yang T, Zhou Y, Jiang Q, et al. (2017). Region specific contribution of ASIC2 to acidosis-and ischemia-induced neuronal injury. J Cereb Blood Flow Metab, 37(2), 528–540, doi: 10.1177/0271678X16630558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joeres N, Augustinowski K, Neuhof A, Assmann M, & Grunder S (2016). Functional and pharmacological characterization of two different ASIC1a/2a heteromers reveals their sensitivity to the spider toxin PcTx1. Sci Rep, 6, 27647, doi: 10.1038/srep27647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson SE, Larsen SS, Povlsen GK, & Edvinsson L (2014). Early MEK1/2 inhibition after global cerebral ischemia in rats reduces brain damage and improves outcome by preventing delayed vasoconstrictor receptor upregulation. PLoS One, 9(3), e92417, doi: 10.1371/journal.pone.0092417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MB, Jin K, Minami M, Chen D, & Simon RP (2001). Global ischemia induces expression of acid-sensing ion channel 2a in rat brain. J Cereb Blood Flow Metab, 21(6), 734–740, doi: 10.1097/00004647-200106000-00011. [DOI] [PubMed] [Google Scholar]
- Jorgensen H, Nakayama H, Raaschou HO, & Olsen TS (1994). Stroke in patients with diabetes. The Copenhagen Stroke Study. Stroke, 25(10), 1977–1984, doi: 10.1161/01.STR.25.10.1977. [DOI] [PubMed] [Google Scholar]
- Karczewski J, Spencer RH, Garsky VM, Liang A, Leitl MD, Cato MJ, et al. (2010). Reversal of acid-induced and inflammatory pain by the selective ASIC3 inhibitor, APETx2. Br J Pharmacol, 161(4), 950–960, doi: 10.1111/j.1476-5381.2010.00918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellenberger S, & Schild L (2002). Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Rev, 82(3), 735–767, doi: 10.1152/physrev.00007.2002. [DOI] [PubMed] [Google Scholar]
- Kellenberger S, & Schild L (2015). International Union of Basic and Clinical Pharmacology. XCI. structure, function, and pharmacology of acid-sensing ion channels and the epithelial Na+ channel. Pharmacol Rev, 67(1), 1–35, doi: 10.1124/pr.114.009225. [DOI] [PubMed] [Google Scholar]
- Kim YB, Gidday JM, Gonzales ER, Shah AR, & Park TS (1994). Effect of hypoglycemia on postischemic cortical blood flow, hypercapnic reactivity, and interstitial adenosine concentration. J Neurosurg, 81(6), 877–884, doi: 10.3171/jns.1994.81.6.0877. [DOI] [PubMed] [Google Scholar]
- Kirino T, & Sano K (1984). Selective vulnerability in the gerbil hippocampus following transient ischemia. Acta Neuropathol, 62(3), 201–208, doi: 10.1007/bf00691853. [DOI] [PubMed] [Google Scholar]
- Kissela B, & Air E (2006). Diabetes: impact on stroke risk and poststroke recovery. Semin Neurol, 26(1), 100–107, doi: 10.1055/s-2006-933313. [DOI] [PubMed] [Google Scholar]
- Kissela BM, Khoury J, Kleindorfer D, Woo D, Schneider A, Alwell K, et al. (2005). Epidemiology of ischemic stroke in patients with diabetes: the greater Cincinnati/Northern Kentucky Stroke Study. Diabetes Care, 28(2), 355–359, doi: 10.2337/diacare.28.2.355. [DOI] [PubMed] [Google Scholar]
- Kuyama H, Kitaoka T, Fujita K, & Nagao S (1994). The effect of alkalizing agents on experimental focal cerebral ischemia. Acta Neurochir Suppl (Wien), 60, 325–328, doi: 10.1007/978-3-7091-9334-1_87. [DOI] [PubMed] [Google Scholar]
- Languren G, Montiel T, Julio-Amilpas A, & Massieu L (2013). Neuronal damage and cognitive impairment associated with hypoglycemia: An integrated view. Neurochem Int, 63(4), 331–343, doi: 10.1016/j.neuint.2013.06.018. [DOI] [PubMed] [Google Scholar]
- Leese GP, Wang J, Broomhall J, Kelly P, Marsden A, Morrison W, et al. (2003). Frequency of severe hypoglycemia requiring emergency treatment in type 1 and type 2 diabetes: a population-based study of health service resource use. Diabetes Care, 26(4), 1176–1180, doi: 10.2337/diacare.26.4.1176. [DOI] [PubMed] [Google Scholar]
- Levinger IM (1971). The cerebral ventricles of the rat. J Anat, 108(Pt 3), 447–451. [PMC free article] [PubMed] [Google Scholar]
- Levy DE, Van Uitert RL, & Pike CL (1979). Delayed postischemic hypoperfusion: a potentially damaging consequence of stroke. Neurology, 29(9 Pt 1), 1245–1252, doi: 10.1212/WNL.29.9_Part_1.1245 [DOI] [PubMed] [Google Scholar]
- Li M, Inoue K, Branigan D, Kratzer E, Hansen JC, Chen JW, et al. (2010). Acid-sensing ion channels in acidosis-induced injury of human brain neurons. J Cereb Blood Flow Metab, 30(6), 1247–1260, doi: 10.1038/jcbfm.2010.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HW, Defazio RA, Della-Morte D, Thompson JW, Narayanan SV, Raval AP, et al. (2010). Derangements of post-ischemic cerebral blood flow by protein kinase C delta. Neuroscience, 171(2), 566–576, doi: 10.1016/j.neuroscience.2010.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin LH, Jin J, Nashelsky MB, & Talman WT (2014). Acid-sensing ion channel 1 and nitric oxide synthase are in adjacent layers in the wall of rat and human cerebral arteries. J Chem Neuroanat, 61–62, 161–168, doi: 10.1016/j.jchemneu.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingueglia E (2007). Acid-sensing ion channels in sensory perception. J Biol Chem, 282(24), 17325–17329, doi: 10.1074/jbc.R700011200. [DOI] [PubMed] [Google Scholar]
- Mandal SK, & Briski KP (2018). Hindbrain Dorsal Vagal Complex AMPK Controls Hypothalamic Gluco-regulatory Transmitter and Counter-Regulatory Hormone Responses to Hypoglycemia. Brain Res Bull, 44, 171–179. doi: 10.1016/j.brainresbull.2018.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Hatakeyama T, Morimoto K, & Yanagihara T (1990). Cerebral blood flow and neuronal damage during progressive cerebral ischemia in gerbils. Stroke, 21(10), 1470–1477, doi: 10.1161/01.STR.21.10.1470. [DOI] [PubMed] [Google Scholar]
- McCrimmon RJ, Evans ML, Fan X, McNay EC, Chan O, Ding Y, et al. (2005). Activation of ATP-sensitive K+ channels in the ventromedial hypothalamus amplifies counterregulatory hormone responses to hypoglycemia in normal and recurrently hypoglycemic rats. Diabetes, 54(11), 3169–3174, doi: 10.2337/diabetes.54.11.3169. [DOI] [PubMed] [Google Scholar]
- Meng QY, Wang W, Chen XN, Xu TL, & Zhou JN (2009). Distribution of acid-sensing ion channel 3 in the rat hypothalamus. Neuroscience, 159(3), 1126–1134, doi: 10.1016/j.neuroscience.2009.01.069. [DOI] [PubMed] [Google Scholar]
- Morrish NJ, Wang SL, Stevens LK, Fuller JH, & Keen H (2001). Mortality and causes of death in the WHO Multinational Study of Vascular Disease in Diabetes. Diabetologia, 44 Suppl 2, S14–21, doi: 10.1007/PL00002934. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. (2016). Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation, 133(4), e38–360, doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- Nagao S, Kitaoka T, Fujita K, Kuyama H, & Ohkawa M (1996). Effect of tris-(hydroxymethyl)-aminomethane on experimental focal cerebral ischemia. Exp Brain Res, 111(1), 51–56, doi: 10.1007/BF00229555. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Kamouchi M, Kitazono T, Kuroda J, Shono Y, Hagiwara N, et al. (2009). Amiloride inhibits hydrogen peroxide-induced Ca2+ responses in human CNS pericytes. Microvasc Res, 77(3), 327–334, doi: 10.1016/j.mvr.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, Davis M, et al. (1993). The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med, 329(14), 977–986, doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- Nitta CH, Osmond DA, Herbert LM, Beasley BF, Resta TC, Walker BR, et al. (2014). Role of ASIC1 in the development of chronic hypoxia-induced pulmonary hypertension. Am J Physiol Heart Circ Physiol, 306(1), H41–52, doi: 10.1152/ajpheart.00269.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottenbacher KJ, Ostir GV, Peek MK, & Markides KS (2004). Diabetes mellitus as a risk factor for stroke incidence and mortality in Mexican American older adults. J Gerontol A Biol Sci Med Sci, 59(6), M640–645, doi: 10.1093/gerona/59.6.M640. [DOI] [PubMed] [Google Scholar]
- Pardridge WM (2011). Drug transport in brain via the cerebrospinal fluid. Fluids Barriers CNS, 8(1), 7, doi: 10.1186/2045-8118-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh B (2009). Mechanisms of the blunting of the sympatho-adrenal response: a theory. Curr Diabetes Rev, 5(2), 79–91, doi: 10.2174/157339909788166846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petito CK, Feldmann E, Pulsinelli WA, & Plum F (1987). Delayed hippocampal damage in humans following cardiorespiratory arrest. Neurology, 37(8), 1281–1286. [DOI] [PubMed] [Google Scholar]
- Pignataro G, Simon RP, & Xiong ZG (2007). Prolonged activation of ASIC1a and the time window for neuroprotection in cerebral ischaemia. Brain, 130(Pt 1), 151–158, doi: 10.1093/brain/awl325. [DOI] [PubMed] [Google Scholar]
- Preis SR, Hwang SJ, Coady S, Pencina MJ, D’Agostino RB Sr., Savage PJ, et al. (2009). Trends in all-cause and cardiovascular disease mortality among women and men with and without diabetes mellitus in the Framingham Heart Study, 1950 to 2005. Circulation, 119(13), 1728–1735, doi: 10.1161/CIRCULATIONAHA.108.829176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehni AK, Shukla V, Perez-Pinzon MA, & Dave KR (2018). Acidosis mediates recurrent hypoglycemia-induced increase in ischemic brain injury in treated diabetic rats. Neuropharmacology, 135, 192–201, doi: 10.1016/j.neuropharm.2018.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas M, Rash LD, Baron A, Lambeau G, Escoubas P, & Lazdunski M (2006). The receptor site of the spider toxin PcTx1 on the proton-gated cation channel ASIC1a. J Physiol, 570(Pt 2), 339–354, doi: 10.1113/jphysiol.2005.095810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Kastner R (2015). Genomic approach to selective vulnerability of the hippocampus in brain ischemia-hypoxia. Neuroscience, 309, 259–279, doi: 10.1016/j.neuroscience.2015.08.034. [DOI] [PubMed] [Google Scholar]
- Schmidt-Kastner R, & Freund TF (1991). Selective vulnerability of the hippocampus in brain ischemia. Neuroscience, 40(3), 599–636, doi: 10.1016/0306-4522(91)90001-5. [DOI] [PubMed] [Google Scholar]
- Schmidt-Kastner R, Ophoff BG, & Hossmann KA (1990). Pattern of neuronal vulnerability in the cat hippocampus after one hour of global cerebral ischemia. Acta Neuropathol, 79(4), 444–455, doi: 10.1007/bf00308722. [DOI] [PubMed] [Google Scholar]
- Sherwood TW, Lee KG, Gormley MG, & Askwith CC (2011). Heteromeric acid-sensing ion channels (ASICs) composed of ASIC2b and ASIC1a display novel channel properties and contribute to acidosis-induced neuronal death. J Neurosci, 31(26), 9723–9734, doi: 10.1523/JNEUROSCI.1665-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla V, Fuchs P, Liu A, Cohan CH, Dong C, Wright CB, et al. (2018). Recurrent Hypoglycemia Exacerbates Cerebral Ischemic Damage in Diabetic Rats via Enhanced Post-Ischemic Mitochondrial Dysfunction. Transl Stroke Res, 10(1), 78–90. doi: 10.1007/s12975-018-0622-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JV, Nemoto EM, Carroll RG, & Safar P (1975). Global ischemia in dogs: intracranial pressures, brain blood flow and metabolism. Stroke, 6(1), 21–27, doi: 10.1161/01.STR.6.1.21. [DOI] [PubMed] [Google Scholar]
- Steen PA, Newberg LA, Milde JH, & Michenfelder JD (1983). Nimodipine improves cerebral blood flow and neurologic recovery after complete cerebral ischemia in the dog. J Cereb Blood Flow Metab, 3(1), 38–43, doi: 10.1038/jcbfm.1983.4. [DOI] [PubMed] [Google Scholar]
- Stroke Therapy Academic Industry Roundtable (1999). Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke, 30(12), 2752–2758, doi: 10.1161/01.STR.30.12.2752Stroke. [DOI] [PubMed] [Google Scholar]
- Suh SW, Hamby AM, & Swanson RA (2007). Hypoglycemia, brain energetics, and hypoglycemic neuronal death. Glia, 55(12), 1280–1286, doi: 10.1002/glia.20440. [DOI] [PubMed] [Google Scholar]
- Tajima A, Hans FJ, Livingstone D, Wei L, Finnegan W, DeMaro J, et al. (1993). Smaller local brain volumes and cerebral atrophy in spontaneously hypertensive rats. Hypertension, 21(1), 105–111. 10.1161/01/HYP.21.1.105 [DOI] [PubMed] [Google Scholar]
- Tamborlane WV, Beck RW, Bode BW, Buckingham B, Chase HP, Clemons R, et al. (2008). Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med, 359(14), 1464–1476, doi: 10.1056/NEJMoa0805017. [DOI] [PubMed] [Google Scholar]
- Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, et al. (2006). Intensive insulin therapy in the medical ICU. N Engl J Med, 354(5), 449–461, doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, et al. (2001). Intensive insulin therapy in critically ill patients. N Engl J Med, 345(19), 1359–1367, doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- Vannucci RC, & Vannucci SJ (2001). Hypoglycemic brain injury. Semin Neonatol, 6(2), 147–155, doi: 10.1053/siny.2001.0044. [DOI] [PubMed] [Google Scholar]
- Waldmann R (2001). Proton-gated cation channels--neuronal acid sensors in the central and peripheral nervous system. Adv Exp Med Biol, 502, 293–304, doi: 10.1007/978-1-4757-3401-0_19 [DOI] [PubMed] [Google Scholar]
- Waldmann R, Champigny G, Bassilana F, Heurteaux C, & Lazdunski M (1997). A proton-gated cation channel involved in acid-sensing. Nature, 386(6621), 173–177, doi: 10.1038/386173a0. [DOI] [PubMed] [Google Scholar]
- Wang YZ, Wang JJ, Huang Y, Liu F, Zeng WZ, Li Y, et al. (2015). Tissue acidosis induces neuronal necroptosis via ASIC1a channel independent of its ionic conduction. Elife, 4, doi: 10.7554/eLife.05682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wemmie JA, Price MP, & Welsh MJ (2006). Acid-sensing ion channels: advances, questions and therapeutic opportunities. Trends Neurosci, 29(10), 578–586, doi: 10.1016/j.tins.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Wojda U, Salinska E, & Kuznicki J (2008). Calcium ions in neuronal degeneration. IUBMB Life, 60(9), 575–590, doi: 10.1002/iub.91. [DOI] [PubMed] [Google Scholar]
- Wong HK, Bauer PO, Kurosawa M, Goswami A, Washizu C, Machida Y, et al. (2008). Blocking acid-sensing ion channel 1 alleviates Huntington’s disease pathology via an ubiquitin-proteasome system-dependent mechanism. Hum Mol Genet, 17(20), 3223–3235, doi: 10.1093/hmg/ddn218. [DOI] [PubMed] [Google Scholar]
- Wu WL, Lin YW, Min MY, & Chen CC (2010). Mice lacking Asic3 show reduced anxiety-like behavior on the elevated plus maze and reduced aggression. Genes Brain Behav, 9(6), 603–614, doi: 10.1111/j.1601-183X.2010.00591.x. [DOI] [PubMed] [Google Scholar]
- Xiong ZG, Zhu XM, Chu XP, Minami M, Hey J, Wei WL, et al. (2004). Neuroprotection in ischemia: blocking calcium-permeable acid-sensing ion channels. Cell, 118(6), 687–698, doi: 10.1016/j.cell.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Xu Y, Jiang YQ, Li C, He M, Rusyniak WG, Annamdevula N, et al. (2018). Human ASIC1a mediates stronger acid-induced responses as compared with mouse ASIC1a. FASEB J, 32(7), 3832–3843, doi: 10.1096/fj.201701367R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J, Liu T, Zhang X, Si Y, Ye Y, Zhao C, et al. (2015). Intensive Versus Conventional Glycemic Control in Patients with Diabetes During Enteral Nutrition After Gastrectomy. J Gastrointest Surg, 19(8), 1553–1558, doi: 10.1007/s11605-015-2871-7. [DOI] [PubMed] [Google Scholar]
- Zuo Z, Smith RN, Chen Z, Agharkar AS, Snell HD, Huang R, et al. (2018). Identification of a unique Ca(2+)-binding site in rat acid-sensing ion channel 3. Nat Commun, 9(1), 2082, doi: 10.1038/s41467-018-04424-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


