Abstract
Background:
Cystic fibrosis related diabetes (CFRD) is associated with pulmonary decline and compromised nutritional status. Emerging data suggest that CFTR dysfunction may play a direct role in the pathogenesis of CFRD; however, studies investigating the effect of CFTR modulators on glycemic outcomes in patients with cystic fibrosis (CF) have shown mixed results. The impact of elexacaftor-tezacaftor-ivacaftor (ETI) on glycemic control is currently unknown. Our objective was to investigate the effect of ETI initiation on glycemia in adults with CF using continuous glucose monitoring (CGM).
Methods:
In this prospective observational study, 34 adults with CF and at least one F508del CFTR mutation wore CGM sensors for 14 days prior to starting ETI and again 3–12 months after ETI initiation. Hypoglycemia symptoms were queried at each visit, and most recent anthropometric measures and spirometry data were obtained by chart review.
Results:
Twenty-three participants completed the study. Compared to baseline, average glucose (AG), standard deviation (SD), % time >200 mg/dL, and peak sensor glucose decreased with ETI treatment, and % time in target range 70–180 mg/dL increased. Improvements in glycemic parameters were most notable in individuals with CFRD. There was no significant change in CGM-measured or self-reported hypoglycemia before and after ETI initiation.
Conclusion:
Initiation of ETI in adults with CF was associated with improvement CGM-derived measures of hyperglycemia and glycemic variability with no effect on hypoglycemia. Further studies are needed to investigate underlying etiology of these changes and the long-term impact of ETI on glycemic control in patients with CF.
Keywords: Continuous glucose monitoring, cystic fibrosis related diabetes, CFTR modulator, elexacaftor-tezacaftor-ivacaftor
1. INTRODUCTION:
As life expectancy for patients with cystic fibrosis (CF) improves, there has been an increased prevalence of non-pulmonary complications, most notably CF-related diabetes (CFRD). CFRD affects roughly 20% of adolescents and 35–50% of adults with CF and is associated with increased exacerbation frequency, decreased pulmonary function, lower weight, complications post-transplant, and earlier mortality [1–5].
The etiology of CFRD is likely multifactorial, related to islet cell dysfunction and β-cell loss from pancreatic exocrine obstruction and zygomen autodigestion, as well as increased insulin resistance from inflammatory cytokines and stress hormones. Although controversial, some studies in vitro and in animal models suggest that defective cystic fibrosis transmembrane conductance regulator (CFTR) protein is expressed in β-cells and has a direct role in the pathogenesis of CFRD [3,6–8]. Evidence of impaired β-cell function, most notably via decreased first-phase insulin secretion and relative proinsulin accumulation, has been observed even in younger individuals with stable pulmonary disease and nutritional status [3]. Early glycemic abnormalities have also been noted in infants and toddlers with CF, prior to the expected development of significant pancreatic damage and islet cell loss [4,5].
CFTR modulators are a group of targeted therapeutics aimed at correcting and/or potentiating the dysfunctional CFTR protein. Ivacaftor significantly improves percent predicted forced expiratory volume in 1 second (FEV1), exacerbation frequency, nutritional status, and quality of life measures in patients with CFTR gating mutations including G551D [9,10]. Several small studies have shown a beneficial effect of ivacaftor on glycemia in patients with this mutation [10–14]. The combination of ivacaftor with lumacaftor or tezacaftor was approved for patients homozygous for F508del mutation; however, pulmonary and glycemic benefits have been less significant [15–18]. Recent clinical trials of elexacaftor-tezacaftor-ivacaftor (ETI) showed substantial improvements in pulmonary and nutritional outcomes in patients with ≥1 copy of the F508del mutation [19]. The effects of ETI on glycemic status in patients with CF are currently unknown.
Continuous glucose monitors (CGM) use minimally invasive subcutaneous sensors to measure interstitial glucose levels every 5–15 minutes, providing comprehensive glycemic data over the course of the day and night. CGM has been validated in patients with CF and has been shown to detect early glycemic variability otherwise missed on 2-hour oral glucose tolerance testing (OGTT) [4,20–23]. We previously found that CGM-derived measures of hyperglycemia and glycemic variability correlate with important CF outcomes including BMI and pulmonary function [24]. However, few studies have used CGM to investigate the effect of CFTR modulators on glycemia.
We conducted a prospective, single-center observational study in adults with CF with and without CFRD as they started treatment with ETI, using CGM to capture changes in glycemic measures over time in the real-world setting. We hypothesized that ETI would improve average glucose (AG) and other CGM markers of hyperglycemia and glycemic variability before and after ETI initiation.
2. STUDY DESIGN AND METHODS
2.1. Study Population
Thirty-four adults with CF and at least one F508del mutation who were planning to start ETI therapy were recruited from a large academic CF center. Participants both with and without a diagnosis of CFRD were included in the study to capture a wide range of glycemia. For participants with pre-existing CFRD, the diagnosis of diabetes was confirmed by chart review based on criteria established by the American Diabetes Association and Cystic Fibrosis Foundation [25]. Exclusion criteria included current pregnancy and age <18 or >70 years. The study was approved by the Boston Children’s Hospital Institutional Review Board (Boston, MA). Written informed consent was obtained from all participants.
2.2. Clinical Assessments
Study visits took place at the time of concurrent clinical care. Baseline visits were scheduled within three months of expected ETI initiation, including up to the time of drug initiation. Due to research closures and safety concerns related to the COVID-19 pandemic, follow-up visits initially scheduled for three months after ETI initiation were delayed to up to 12 months, and some follow-up visits were completed virtually. Participants were queried at each visit regarding medical history, baseline and interval CF exacerbations and hospitalizations, medication use (including oral glucocorticoids, insulin, and CFTR modulators), the frequency of hypoglycemia symptoms over the preceding 12 weeks, and physical activity (Modified Activity Questionnaire, MAQ) [26]. Additional data collected by chart review included CFTR genotype, pancreatic insufficiency, most recent hemoglobin A1c (HbA1c) levels, anthropometric measures, and spirometry results (FEV1 and forced vital capacity [FVC]).
2.3. CGM Procedures
Blinded CGM sensors (Freestyle Libre Pro, Abbott Laboratories, Illinois) were placed at each study visit. Participants wore each sensor for 14 days, then mailed the sensor to the research staff for data capture. Participants already using the Dexcom G6 or Freestyle Libre CGM used their own sensors (n=8). These subjects were not blinded to their own data, as it was being used for day-to-day diabetes management. For participants who completed follow-up visits virtually, CGM sensors were mailed to their home address and self-inserted with study physician guidance via video conferencing.
Primary CGM measures of interest included AG, % time in range 70–180 mg/dL, hypoglycemia measures (% time <70 mg/dL, % time <54 mg/dL), hyperglycemia measures (% time >140 mg/dL, % time >200 mg/dL, % time >250 mg/dL), and measures of glycemic variability (standard deviation [SD], and coefficient of variation [CV]).
2.4. Statistical Analyses:
Statistical analysis was performed using STATA (version 16, 2019; College Station, TX: StataCorp LLC). Normality was assessed for all variables using the Shapiro-Wilk test. Baseline characteristics were compared between participants divided by glycemic category (CFRD vs non-CFRD) and between participants who did and did not complete the study using independent t-tests or Mann-Whitney U tests for normally and non-normally distributed data, respectfully. Categorical variables were compared using chi square tests. Analyses examining change over time were limited to participants who completed the follow-up visit (n=23). Changes in CGM measures, hypoglycemic symptoms, and other clinical outcomes before and after starting ETI were analyzed using Wilcoxon signed rank tests, followed by subgroup analyses evaluating within-group change in CGM measures in those with and without CFRD. Comparison of changes in CGM measures between those with and without CFRD were performed using independent t-tests or Mann-Whitney U tests. The correlations between absolute changes in CGM-derived glycemic measures and FEV1, BMI, and length of time between study visits were assessed using Pearson and Spearman correlation analysis for normally and non-normally distributed data, respectively.
2.5. Sample size considerations
Significant participant drop-out occurred due to the COVID-19 pandemic. Original sample size considerations were based on data collected in the Massachusetts General Hospital Glycemic Measures Project, an observational study that collected CGM data in adults with CF at two time points over a 3-month period [24]. In a subset of participants with available data from this study, the mean change in AG over three months was 8.5±10.6 mg/dL. Using these data, a sample size of 16 would be required to achieve a power of 80% at a two-sided alpha level of 0.05 to detect a significant difference. We doubled this estimate to account for the possibility of drop-out, lost data, and for pre-planned subgroup analyses. Therefore, the final number of subjects in this study (n=23) was adequate for assessment of our primary outcome in the entire sample, though power was limited for subgroup analyses.
3. RESULTS:
Baseline clinical characteristics of enrolled participants (n=34) are summarized in Table 1. All participants had a history of exocrine pancreatic insufficiency and at least one copy of the F508del mutation. Approximately half (44%) of participants were previously taking other CFTR modulators. Seventeen participants (50%) had pre-existing CFRD. Participants with CFRD had a higher rate of prior modulator use compared to those without CFRD (64.7% vs 23.5%, p=0.016). Apart from HbA1c values and insulin usage, participants with and without CFRD did not significantly differ (Table 1). Of the 17 participants with CFRD, 15 (88%) were using insulin, including two on insulin pumps. The two participants with CFRD not using insulin had mild CFRD with significant endogenous β-cell function and preferred to manage their diabetes with intensive dietary modification.
Table 1.
Baseline Characteristics
| Total (n=34) | Non-CFRD (n=17) | CFRD (n=17) | p-value | |
|---|---|---|---|---|
| Age (years) | 30.6 ± 1.5 | 28.8 ± 2.0 | 32.4 ± 2.2 | 0.248 |
| Female | 19 (56%) | 12 (71%) | 7 (41%) | 0.084 |
| Race | ||||
| - White | 33 (97%) | 16 (94%) | 17 (100%) | 0.317 |
| - Black | 0 | 0 | 0 | |
| - Asian | 0 | 0 | 0 | |
| - Native Hawaiian or Pacific Islander | 0 | 0 | 0 | |
| - American Indian or Alaskan Native | 1 (3%) | 1 (6%) | 0 | |
| - Other | 0 | 0 | 0 | |
| Ethnicity | ||||
| - Hispanic | 0 | 0 | 0 | |
| - Non-Hispanic | 34 (100%) | 17 (100%) | 17 (100%) | |
| Genotype | 0.084 | |||
| - F508del homozygous | 15 (44%) | 5 (29%) | 10 (59%) | |
| - F508del heterozygous | 19 (56%) | 12 (71%) | 7 (41.2%) | |
| Pancreatic insufficiency | 34 (100%) | 17 (100%) | 17 (100%) | |
| BMI (kg/m2) | 23.8 ± 0.6 | 24.0 ± 0.9 | 23.7 0.9 | 0.798 |
| FEV1 (% predicted) | 75 ± 4 | 80 ± 5 | 70 ± 6 | 0.240 |
| FVC (% predicted) | 88 ± 3.5 | 93.1 ± 3.8 | 82.9 ± 5.8 | 0.155 |
| HbA1c (%) | 6.3 ± 0.3 | 5.3 ± 0.1 | 7.3 ± 0.4 | <0.0001 |
| Hospitalizations in preceding year | 1 (0–7) | 0 (0–7) | 1 (0–2) | 0.385 |
| Prior modulator use | 15 (44%) | 4 (23.5%) | 11 (65%) | 0.016 |
| - Ivacaftor | 1 (3%) | 0 | 1 (6%) | |
| - Ivacaftor-lumacaftor | 3 (9%) | 1 (6%) | 2 (12%) | |
| - Ivacaftor-tezacaftor | 10 (29%) | 3 (17.6%) | 7 (41%) | |
| - Prior ETI trial agent | 1 (9%) | 0 | 1 (6%) |
Data displayed as mean ±SE or n (%) unless otherwise indicated.
CFRD, cystic fibrosis related diabetes; BMI, body mass index kg/m2; FEV1, forced expiratory volume; FVC, forced vital capacity; HbA1c, hemoglobin A1c; ETI, elexacaftor/tezacaftor/ivacaftor
Ten participants were either lost to follow-up or unwilling to complete the study virtually, and one participant died during the study period due to unrelated pulmonary complications. The rate of prior modulator use was significantly higher in participants who completed the study (56.5% vs 18.2%, p=0.035). There were otherwise no significant differences between participants who did or did not complete the study including age, gender, genotype, BMI, FEV1, HbA1c, and CFRD status (data not shown).
In those who completed the study, baseline CGM data were collected a median 2.6 weeks prior to ETI initiation (range 0–17.6 weeks). CGM data collection overlapped with ETI initiation in one subject. Follow-up CGM data were collected a median 7.1 months post-ETI initiation (range 3–11 months). All participants confirmed taking ETI at the follow-up visit with no significant lapses in therapy or modified dosing. None of the participants reported hospitalization or CF exacerbation within the three months preceding their final visit.
Table 2 shows glycemic and clinical outcomes before and after ETI initiation, and Figure 1 displays the absolute change in CGM measures from baseline to follow-up. CGM-derived AG, SD, % time 70–180 mg/dL, % time >200 mg/dL, peak sensor value, FEV1 and FVC significantly improved with ETI treatment. No significant change was detected in measures of hypoglycemia (% time <54 mg/dL and % time <70 mg/dL), MAQ score, weight, or BMI (Table 2). The prevalence of symptomatic hypoglycemia at baseline and follow-up did not significantly differ (60% vs 70% of participants, p=0.67), and the reported rate of symptomatic hypoglycemic episodes over the prior three months did not change with ETI treatment (1.2 vs 1.3 episodes per week, p=0.72).
Table 2.
Changes in Glycemic Measures Pre- and Post-ETI Initiation.
| Total Cohort N=23 | Non-CFRD N=9 | CFRD N=14 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre-ETI | Post-ETI | p-value | Pre-ETI | Post-ETI | p-value | Pre-ETI | Post-ETI | p-value | |
| CGM sensor wear time (days) | 12.9 ± 0.4 | 12.4 ± 0.5 | 0.398 | 13.0 ± 0.6 | 12.2 ± 0.9 | 0.572 | 12.9 ± 0.6 | 12.5 ± 0.6 | 0.553 |
| AG (mg/dL) | 136 ± 9 | 124 ± 8 | 0.018 | 96 ± 7 | 94 ± 6 | 0.314 | 162 ± 10 | 144 ± 10 | 0.033 |
| SD (mg/dL) | 46 ± 4 | 39 ± 4 | 0.001 | 28 ± 3 | 25 ± 2 | 0.095 | 57 ± 5 | 49 ± 4 | 0.008 |
| CV (%) | 32.6 ± 1.7 | 30.6 ± 1.4 | 0.117 | 29.1 ± 2.1 | 26.4 ± 2.0 | 0.069 | 34.9 ± 2.4 | 33.3 ± 1.7 | 0.433 |
| % Time 70–180 mg/dL | 67.5 ± 4.6 | 75.1 ± 4.0 | 0.040 | 73.4 ± 7.5 | 77.6 ± 6.2 | 0.953 | 63.6 ± 5.7 | 73.5 ± 5.3 | 0.011 |
| % Time >140 mg/dL | 35.3 ± 6.9 | 28.6 ± 5.8 | 0.074 | 9.2 ± 3.7 | 8.7 ± 3.8 | 0.953 | 54.8 ± 7.8 | 43.6 ± 7.2 | 0.071 |
| % Time >200 mg/dL | 16.4 ± 4.1 | 9.7 ± 2.6 | 0.006 | 2.0 ± 1.0 | 1.1 ± 0.7 | 0.028 | 26.4 ± 5.4 | 15.2 ± 3.6 | 0.023 |
| % Time >250 mg/dL | 7.7 ± 4.6 | 2.4 ± 2.1 | 0.057 | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.356 | 13.3 ± 3.5 | 7.9 ± 3.4 | 0.065 |
| % Time <70 mg/dL | 8.9 ± 3.6 | 9.0 ± 2.9 | 0.212 | 18.2 ± 8.3 | 17.8 ± 6.4 | 0.235 | 2.9 ± 1.0 | 3.4 ± 0.9 | 0.660 |
| % Time <54 mg/dL | 3.1 ± 1.4 | 1.6 ± 0.8 | 0.778 | 6.8 ± 3.4 | 2.5 ± 1.8 | 0.550 | 0.3 ± 0.2 | 0.5 ± 0.2 | 0.228 |
| Peak sensor value (mg/dL) | 306 ± 21 | 280 ± 20 | 0.045 | 225 ± 18 | 210 ± 15 | 0.270 | 361 ± 23 | 328 ± 24 | 0.100 |
| Weight (kg) | 66.9 ± 2.4 | 69.6 ± 2.6 | 0.123 | 65.0 ± 2.7 | 64.1 ± 3.1 | 0.414 | 67.7 ± 3.6 | 73.3 ± 3.6 | 0.045 |
| BMI (kg/m2) | 24.1 ± 0.7 | 24.5 ± 0.7 | 0.240 | 24.2 ± 1.1 | 23.2 ± 1.1 | 0.153 | 24.1 ± 1.0 | 25.4 ± 0.9 | 0.082 |
| FEV1 (% predicted) | 79 ± 5 | 91 ± 5 | <0.0001 | 86 ± 6 | 98 ± 6 | 0.005 | 75 ± 7 | 86 ± 6 | 0.001 |
| FVC (% predicted) | 92 ± 4 | 99 ± 5 | 0.0005 | 98 ± 6 | 109 ± 6 | 0.014 | 88 ± 6 | 91 ± 7 | 0.021 |
Data are displayed as mean ± SEM. P-values represent within group changes from baseline to follow-up.
Bolded values signify p values <0.05
CFRD, cystic fibrosis related diabetes; ETI, elexacaftor/tezacaftor/ivacaftor; CGM, continuous glucose monitor; AG, average glucose; SD, standard deviation; CV, coefficient of variation; BMI, body mass index kg/m2; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity s
Figure 1. Changes in CGM Measures Before and After ETI Initiation.
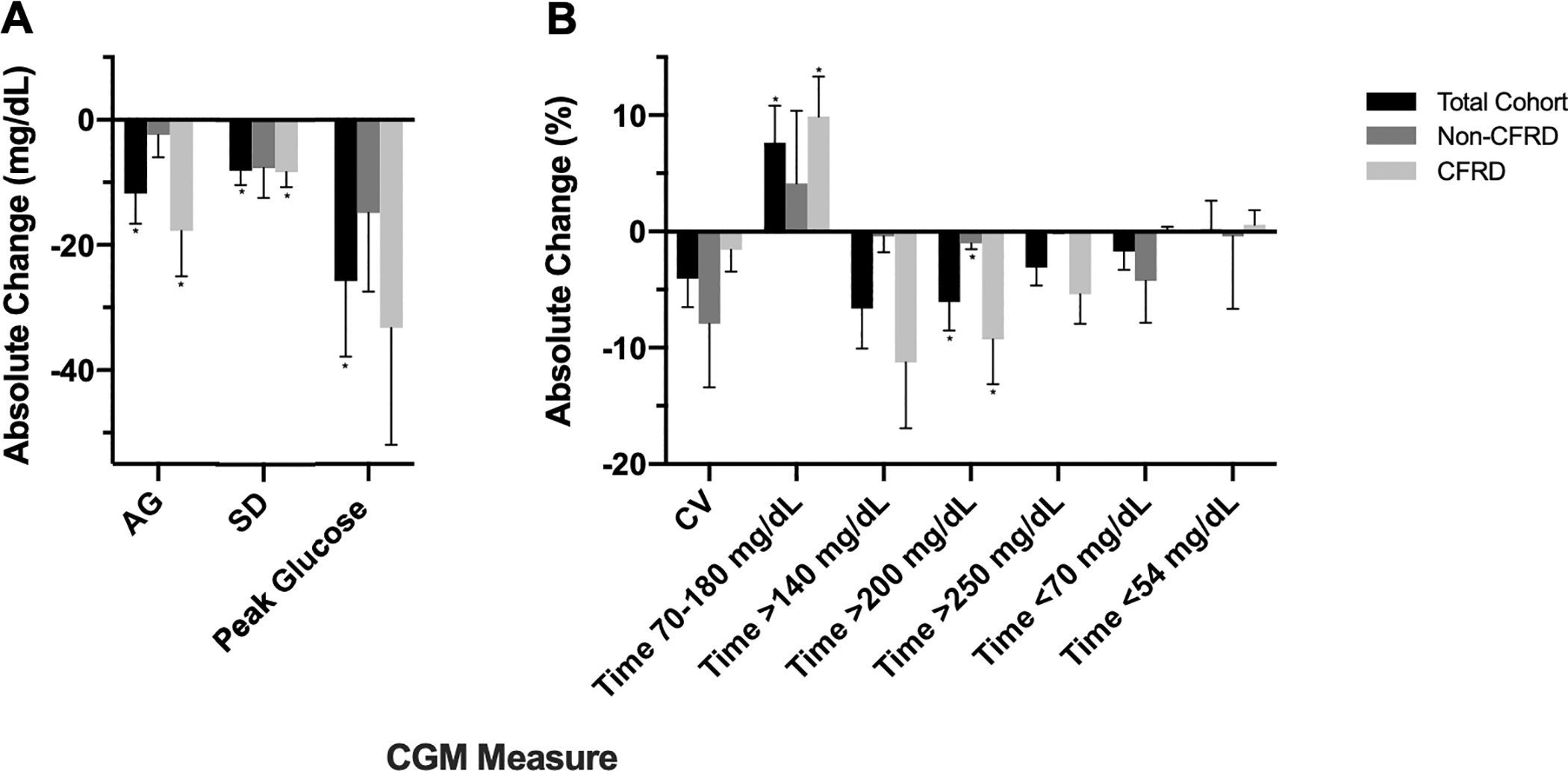
Changes in CGM measures before and after ETI-initiation are displated for a.) AG, SD, and peak glucose reported in mg/dL, and b) CV and time spent in glycemic ranges reported in percentages. Data are displayed as mean ± SEM.
* p value <0.05 for within group change from baseline to follow-up; no significant differences in glycemic changes were detected between the CFRD vs non-CFRD groups.
CGM, continuous glucose monitor; CFRD, cystic fibrosis related diabetes; ETI, elexacaftor/tezacaftor/ivacaftor; AG, average glucose; SD, standard deviation; CV, coefficient of variation
In subgroup analyses evaluating those with and without CFRD, both groups had significant improvements in % time >200 mg/dL and in spirometry measures (Table 2). Participants with CFRD (n=14) also had significant improvements in AG, SD, and % time 70–180 mg/dL, and weight (Table 2 and Figure 1). Neither group had significant change in MAQ score. Insulin total daily dose did not significantly change after ETI initiation in participants with CFRD. There were no significant differences in change in CGM measures from baseline to follow-up between patients with and without CFRD (Figure 1), though there was a trend toward a greater decrease in AG in those with CFRD (−18±7 mg/dL vs −2±4 m/dL, p=0.06).
On univariate correlation analyses in all participants, change in FEV1 was inversely correlated with change in SD (r=−0.60, p=0.01) with a trend noted between FEV1 change and % time >200 mg/dL change (r=−0.48, p=0.051). Changes in all other CGM measures were not significantly associated with changes in weight, BMI, or FEV1 (p>0.05 for all). The time between study visits also did not correlate with change in CGM measures (p>0.05 for all).
4. DISCUSSION:
In this prospective observational study, ETI initiation in adults with CF was associated with significant improvements in CGM-derived measures of AG, hyperglycemia, and glycemic variability, with no change in hypoglycemia detected by CGM or reported symptoms. Glycemic improvements with ETI were noted in participants both with and without CFRD and were most notable in those with CFRD. A decrease in CGM-measured SD was correlated with an increase in FEV1, suggesting that those with the greatest pulmonary benefits with ETI also had the most improvement in glycemic variability.
To our knowledge, this is the first prospective study investigating the impact of ETI initiation on dysglycemia in patients with CF. Prior studies investigating the effects of other CFTR modulators on glycemic control have shown mixed results. Ivacaftor has been associated with improved dysglycemia in patients with CF with gating mutations. A pilot study in patients ages 6–52 years with the G551D mutation reported improved insulin response to oral and IV glucose loading in four of the five participants after starting ivacaftor [12]. A prospective observational study in 24 patients with gating mutations found a small but significant reduction in HbA1c after six months of ivacaftor (median 42.5 vs 39.5 mmol/mol, p= 0.004) [27]. In contrast, studies investigating the effect of lumacaftor-ivacaftor on dysglycemia in patients with F508del mutations have shown inconsistent results. A prospective study of nine youth with homozygous F508del CF found no significant improvements in HbA1c, OGTT, or CGM data after 29 weeks of lumacaftor-ivacaftor [17]. Although one study in 40 children and adults with CF reported significant improvement in abnormal glucose tolerance after one year of ivacaftor/lumacaftor treatment [28], another found lumacaftor/ivacaftor therapy did not improve insulin secretion or glucose tolerance at 3, 6, and 12 months of treatment [29]. Published data suggest that ETI leads to substantially greater improvements in pulmonary function and nutritional status in patients with one or two copies of F508del than previous modulators [19], and this greater efficacy may explain in part the more pronounced glycemic benefits we observed with ETI compared to studies with other modulators.
The specific mechanisms underlying the glycemic improvements observed in our study are unclear and likely multifactorial. Although the pathophysiology of CFRD has been attributed primarily to the effects of CFTR dysfunction on the exocrine pancreas causing islet dysfunction and β-cell loss, there are controversial data suggesting that CFTR may also be expressed in pancreatic β-cells and play a direct role in insulin secretion. Similar to the sulfonylurea receptor, a key mediator of β-cell function and insulin signaling, CFTR is a member of the ABCC subfamily of ATP-binding cassette transporter proteins [3]. Some though not all studies have identified CFTR expression in β-cells, and data investigating the effects of inhibiting or augmenting CFTR on insulin secretion are conflicting [6–8]. If CFTR is expressed in β-cells and CFTR dysfunction contributes to insulin deficiency, then improving CFTR activity with CFTR modulators may enhance β-cell function and insulin secretion. At the same time, indirect effects of CFTR modulation on β-cell function may also occur via relief of islet inflammation, enhanced incretin secretion, and/or improved exocrine function and paracrine signaling [3]. In addition, insulin resistance may be improved by reduced systemic inflammation and infections and increased physical activity, though in our study physical activity score did not change after ETI initiation.
In subgroup analyses, participants with CFRD had significant within-group glycemic improvements in CGM measures of AG, hyperglycemia, and glycemic variability after starting ETI. The % time in target range 70–180 mg/dL increased to over 70% (the recommended treatment goal for patients with type 1 and 2 diabetes [30]) suggesting that starting ETI allowed participants to achieve recommended diabetes therapy goals. Interestingly, these glycemic improvements were noted despite no change in total daily dose of insulin before and after starting ETI. Participants without CFRD also had a reduction in time spent in hyperglycemic range >200 mg/dL, suggesting that glycemic improvements with ETI also occur in those without diabetes, though the small number in this group (n=9) limited the power to detect changes in other CGM measures.
Hypoglycemia is a reported side effect of CFTR modulators, and there have been anecdotal reports of an increase in incidence of hypoglycemia with ETI in clinical use. In this study, we did not find an increase in time spent in hypoglycemic ranges <70 mg/dL and <54 mg/dL or in participant report of hypoglycemic symptoms. However, we did not capture CGM data shortly after ETI was started and may have missed hypoglycemia occurring during this period.
These real-world results showing glycemic benefits of ETI have important implications in the post-modulator era. Because early hyperglycemia may negatively impact clinical outcomes in patients with CF, ETI may have not only direct benefits on pulmonary function and nutritional status but also indirect benefits by improving glycemia and reducing diabetes-related complications and morbidity with time. Emerging data are suggesting favorable trends in prevalence of CFRD in patients with CF and gating mutations treated with ivacaftor over five years [10]. This, in combination with our findings, raises the promising possibility that ETI initiation could improve dysglycemia and delay progression to CFRD, particularly as ETI receives FDA approval in younger children. This study highlights the importance of larger, long-term prospective studies investigating the impact on ETI on dysglycemia and CFRD prevalence in this patient population.
The strengths of this study include the prospective nature of data collection and the use of CGM for comprehensive characterization of glycemic changes over time. However, this study was limited by a relatively small number of participants in relatively good baseline health recruited from a single center. The timing of the COVID-19 pandemic significantly widened our follow-up time range and impacted study retention, which could have increased the variability in the glycemic results; however, there was no correlation between glycemic changes and length of time between visits, and the number of participants was still adequate to detect significant changes in glycemic measures. Because many follow-up visits were completed virtually, weight and spirometry measurements were missing in multiple participants, and spirometry measures included both in-clinic and home spirometer results. In addition, the inclusion of a control group of patients not receiving ETI could have potentially strengthened the study design; however, this was not feasible given the narrow recruitment window after FDA approval of ETI and the limited number of potential patients who did not qualify for this medication. We did not obtain OGTT or direct measures of insulin secretion at baseline and follow-up, and few participants without previously diagnosed CFRD had a recent OGTT to characterize their baseline glycemic status. Similarly, we did not obtain biochemical markers of illness severity, such as inflammatory markers, which may have been useful in assessing the etiology of participants’ glycemic improvements. Because of the observational design, ETI was managed by the participants’ clinical providers, and assessment of compliance was limited to participants’ self-report. In addition, insulin total daily dose was obtained by participant recall, potentially limiting the accuracy of these results. Participants consumed their free-living diets while enrolled; therefore, we did not account for any dietary changes (e.g. altered carbohydrate content) that may alter glycemic excursions and variability. Finally, CGM glucose data were only available at two distinct 14-day periods for each participant. Continuous CGM data collection throughout the entire study may have provided more comprehensive glycemic data and provided insight into the timing of participants’ glycemic improvements.
5. CONCLUSION:
ETI initiation in adults with CF was associated with improvement CGM-derived measures of hyperglycemia and glycemic variability. These findings occurred across a range of glycemic abnormalities, particularly in participants with underlying CFRD. ETI did not have a significant effect on CGM-measured or subjectively-reported hypoglycemia. While the pathophysiology of dysglycemia in CF and the mechanism of ETI’s glycemic benefits are still being investigated, the possibility of CFTR having a direct role in β-cell function remains intriguing. Further studies are needed to understand the etiology of these glycemic benefits and to investigate the long-term impact of ETI on dysglycemia and progression to CFRD.
Highlights.
Elexacaftor/tezacaftor/ivacaftor (ETI) was associated with improved glycemia.
The frequency of hypoglycemia did not change with treatment.
Further studies are needed to understand the implications of these findings.
ACKNOWLEDGMENTS
The authors thank the patients and the clinical and research teams of the Boston Children’s Hospital and Brigham and Women’s Hospital CF Center.
Declaration of Competing Interest
Dr. Putman reports grants from Vertex Pharmaceuticals and the Cystic Fibrosis Foundation, outside the submitted work. Dr. Sawicki reports personal fees from Vertex Pharmaceuticals, outside the submitted work. Dr. Uluer reports grants from the Cystic Fibrosis Foundation and serves an advisory board for Vertex Pharmaceuticals and as an unpaid board member for the Cystic Fibrosis Research Institute. Dr. Kennedy reports grants from the Cystic Fibrosis Foundation. The other authors have nothing to disclose.
Funding Statement.
This study was funded by the Pediatric Endocrine Society Rising Star Award and NIH NIDDK T32 (5T32DK007699). Funding sources had no role in the design or reporting of this study.
Abbreviations:
- AG
Average Glucose
- CV
coefficient of variation
- CGM
continuous glucose monitoring
- CF
cystic fibrosis
- CFRD
cystic fibrosis related diabetes
- CFTR
cystic fibrosis transmembrane conductance regulator
- ETI
elexacaftor-tezacaftor-ivacaftor
- FEV1
forced expiratory volume in 1 second
- FVC
forced vital capacity
- HbA1c
hemoglobin A1c
- OGTT
oral glucose tolerance test
- TIR
time in range
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- [1].Cystic Fibrosis Foundation. 2019. Patient Registry Annual Data Report. Bethesda, Maryland: 2019. [Google Scholar]
- [2].Moran A, Pekow P, Grover P, Zorn M, Slovis B, Pilewski J, et al. Insulin Therapy to Improve BMI in Cystic Fibrosis-Related Diabetes Without Fasting Hyperglycemia Results of the Cystic Fibrosis Related Diabetes Therapy Trial THE CYSTIC FIBROSIS RELATED DIABETES THERAPY STUDY GROUP* 2009. 10.2337/dc09-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Norris AW, Ode KL, Merjaneh L, Sanda S, Yi Y, Sun X, et al. Survival in a bad neighborhood: pancreatic islets in cystic fibrosis. J Endocrinol 2019. 10.1530/JOE-18-0468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Prentice BJ, Ooi CY, Strachan RE, Hameed S, Ebrahimkhani S, Waters SA, et al. Early glucose abnormalities are associated with pulmonary inflammation in young children with cystic fibrosis. J Cyst Fibros 2019. 10.1016/j.jcf.2019.03.010. [DOI] [PubMed] [Google Scholar]
- [5].Ode KL, Frohnert B, Laguna T, Phillips J, Holme B, Regelmann W, et al. Oral glucose tolerance testing in children with cystic fibrosis. Pediatr Diabetes 2010;11:487–92. 10.1111/j.1399-5448.2009.00632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hart NJ, Aramandla R, Poffenberger G, Fayolle C, Thames AH, Bautista A, et al. Cystic fibrosis-related diabetes is caused by islet loss and inflammation. JCI Insight 2018;3. 10.1172/jci.insight.98240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ntimbane T, Mailhot G, Spahis S, Rabasa-Lhoret R, Kleme ML, Melloul D, et al. CFTR silencing in pancreaticβ-cells reveals a functional impact on glucose-stimulated insulin secretion and oxidative stress response. Am J Physiol - Endocrinol Metab 2016;310:E200–12. 10.1152/ajpendo.00333.2015. [DOI] [PubMed] [Google Scholar]
- [8].Sun X, Yi Y, Xie W, Liang B, Winter MC, He N, et al. CFTR influences beta cell function and insulin secretion through non-cell autonomous exocrine-derived factors. Endocrinology 2017;158:3325–38. 10.1210/en.2017-00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ramsey BW, Davies J, McElvaney NG, Tullis E, Bell SC, Dřevínek P, et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med 2011;365:1663–72. 10.1056/NEJMoa1105185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Volkova N, Moy K, Evans J, Campbell D, Tian S, Simard C, et al. Disease progression in patients with cystic fibrosis treated with ivacaftor: Data from national US and UK registries. J Cyst Fibros 2020;19:68–79. 10.1016/j.jcf.2019.05.015. [DOI] [PubMed] [Google Scholar]
- [11].Hayes D, McCoy KS, Sheikh SI. Resolution of cystic fibrosis-related diabetes with ivacaftor therapy. Am J Respir Crit Care Med 2014;190:590–1. 10.1164/rccm.201405-0882LE. [DOI] [PubMed] [Google Scholar]
- [12].Bellin MD, Laguna T, Leschyshyn J, Regelmann W, Dunitz J, Billings J, et al. Insulin Secretion Improves in Cystic Fibrosis Following Ivacaftor Correction of CFTR: A Small Pilot Study n.d. 10.1111/pedi.12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Tsabari R, Elyashar HI, Cymberknowh MC, Breuer O, Armoni S, Livnat G, et al. CFTR potentiator therapy ameliorates impaired insulin secretion in CF patients with a gating mutation. J Cyst Fibros 2016;15:e25–7. 10.1016/j.jcf.2015.10.012. [DOI] [PubMed] [Google Scholar]
- [14].Christian F, Thierman A, Shirley E, Allen K, Cross C, Jones K. Sustained Glycemic Control With Ivacaftor in Cystic Fibrosis–Related Diabetes. J Investig Med High Impact Case Reports 2019;7. 10.1177/2324709619842898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Flume PA, Liou TG, Borowitz DS, Li H, Yen K, Ordoñez CL, et al. Ivacaftor in subjects with cystic fibrosis who are homozygous for the F508del-CFTR mutation. Chest 2012;142:718–24. 10.1378/chest.11-2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wainwright CE, Elborn JS, Ramsey BW. Lumacaftor-Ivacaftor in Patients with Cystic Fibrosis Homozygous for Phe508del CFTR. N Engl J Med 2015;373:1783–4. 10.1056/NEJMc1510466. [DOI] [PubMed] [Google Scholar]
- [17].Li A, Vigers T, Pyle L, Zemanick E, Nadeau K, Sagel SD, et al. Continuous glucose monitoring in youth with cystic fibrosis treated with lumacaftor-ivacaftor. J Cyst Fibros 2019. 10.1016/j.jcf.2018.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Thomassen JC, Mueller MI, Alejandre Alcazar MA, Rietschel E, van Koningsbruggen-Rietschel S. Effect of Lumacaftor/Ivacaftor on glucose metabolism and insulin secretion in Phe508del homozygous cystic fibrosis patients. J Cyst Fibros 2018. 10.1016/j.jcf.2017.11.016. [DOI] [PubMed] [Google Scholar]
- [19].Taylor-Cousar JL, Mall MA, Ramsey BW, McKone EF, Tullis E, Marigowda G, et al. Clinical development of triple-combination CFTR modulators for cystic fibrosis patients with one or two F508del alleles. ERJ Open Res 2019;5:00082–2019. 10.1183/23120541.00082-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Dobson L, Sheldon CD, Hattersley AT. Conventional measures underestimate glycaemia in cystic fibrosis patients. Diabet Med 2004;21:691–6. 10.1111/j.1464-5491.2004.01219.x. [DOI] [PubMed] [Google Scholar]
- [21].Leclercq A, Gauthier B, Rosner V, Weiss L, Moreau F, Constantinescu AA, et al. Early assessment of glucose abnormalities during continuous glucose monitoring associated with lung function impairment in cystic fibrosis patients. J Cyst Fibros 2014. 10.1016/j.jcf.2013.11.005. [DOI] [PubMed] [Google Scholar]
- [22].O’Riordan SMP, Hindmarsh P, Hill NR, Matthews DR, George S, Greally P, et al. Validation of continuous glucose monitoring in children and adolescents with cystic fibrosis: A prospective cohort study. Diabetes Care 2009;32:1020–2. 10.2337/dc08-1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Frost F, Dyce P, Nazareth D, Malone V, Walshaw MJ. Continuous glucose monitoring guided insulin therapy is associated with improved clinical outcomes in cystic fibrosis-related diabetes. J Cyst Fibros 2018. 10.1016/j.jcf.2018.05.005. [DOI] [PubMed] [Google Scholar]
- [24].Kevin Scully, Martin K, Ruazol M, Marchetti P, Larkin M, Zheng H, et al. The Relationship Between Hemoglobin A1c and Average Glucose from Continuous Glucose Monitoring (CGM) in Adults with CF. North Am. Cyst. Fibros. Conf., Phoenix: 2020. [Google Scholar]
- [25].Moran A, Brunzell C, Cohen RC, Katz M, Marshall BC, Onady G, et al. Clinical care guidelines for cystic fibrosis-related diabetes: A position statement of the American Diabetes Association and a clinical practice guideline of the Cystic Fibrosis Foundation, endorsed by the Pediatric Endocrine Society. Diabetes Care 2010;33:2697–708. 10.2337/dc10-1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kriska AM, Bennett PH. An epidemiological perspective of the relationship between physical activity and NIDDM: from activity assessment to intervention. Diabetes Metab Rev 1992;8:355–72. [DOI] [PubMed] [Google Scholar]
- [27].Banerjee A, Brennan AL, Horsley AR, Manchester B. PROSPECTIVE EXAMINATION OF THE EFFECTS OF IVACAFTOR ON GLYCAEMIC HEALTH n.d. 10.1136/thoraxjnl-2014-206260.324. [DOI]
- [28].Misgault B, Chatron E, Reynaud Q, Touzet S, Abely M, Melly L, et al. Effect of one-year lumacaftor–ivacaftor treatment on glucose tolerance abnormalities in cystic fibrosis patients. J Cyst Fibros 2020;19:712–6. 10.1016/j.jcf.2020.03.002. [DOI] [PubMed] [Google Scholar]
- [29].Moheet A, Beisang D, Zhang L, Sagel SD, VanDalfsen JM, Heltshe SL, et al. Lumacaftor/ivacaftor therapy fails to increase insulin secretion in F508del/F508del CF patients. J Cyst Fibros 2021;20:333–8. 10.1016/j.jcf.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Beck RW, Bergenstal RM, Riddlesworth TD, Kollman C, Li Z, Brown AS, et al. Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care 2019;42:400–5. 10.2337/dc18-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]


